Introduction
Cervical cancer (CaCx) progression may be related to
the persistent presence of high-risk human papillomavirus (HPV)
infection-derived oncoproteins E5, E6 and E7 (1,2). E6
can abrogate p53-induced apoptosis by inducing p53 degradation
(3). Apoptosis can be triggered by
one of the two following major mechanisms: binding of death ligands
to death receptors (DRs) in the extrinsic pathway or cytotoxicity
that initiates the intrinsic 'mitochondrial' pathway (4). Binding ligands in the extrinsic
pathway include tumour necrosis factor α (TNFα), Fas (CD95/APO1)
ligand and TNF-related apoptosis inducing ligand (TRAIL). However,
TNFα not only activates caspase-8 in the extrinsic apoptosis
pathway but also promotes tumour development via chronic
inflammation. Thus, the selective inhibition of TNF-induced
extrinsic apoptosis would be required for inflammation-associated
tumour growth (5).
Connexins (Cxs) have gap junction (GJ)
channel-dependent, hemichannel-dependent and GJ-independent pathway
functions in the apoptotic process (6). Indeed, different types of Cxs may
possess various functions. Cx43 and Cx40, but not Cx37, promote
apoptosis via the transfer of pro-apoptotic signals between HeLa
cells through gap junctions (7).
The traditional viewpoint mainly supports GJs as a tumour
suppressors (8). However, there is
no consensus regarding the function of GJs composed of Cx32 in
bystander effects. On the one hand, Cx32 downregulation contributes
to hepatocellular carcinoma proliferation and metastasis (9), and the inhibition of GJ function or
its component Cx32 significantly decreases TNFα hepatotoxicity
(10). On the other hand, Cx32
expression also confers protective effects, which is the opposite
of the effects of Cx26 in irradiated HeLa cells (11,12).
Aside from its GJ function, little is known regarding the
non-junctional functions of Cx32 in CaCx. As GJ components, Cxs
also play roles outside the GJs, and the function of non-junctional
Cxs appears to be contradictory in some reports. For example, in
glioblastoma multiforme, Cx43 may be a biomarker for predicting the
survival of patients with methylguanine methyl
transferase-independent temozolomide resistance (13). However, Cx43 plays a pro-apoptotic
role in cisplatin-induced auditory cell death in both junctional
and non-junctional conditions (14). Cx26 and Cx43 may play important
roles in CaCx carcinogenesis (15,16),
but the function of Cx32 in CaCx has rarely been investigated. Our
previous study (17) demonstrated
that, relative to controls, Cx32 was upregulated and
cytoplasmically localized in CaCx specimens. Cx32 expression was
correlated with an advanced FIGO staging, differentiation and
increased tumour size, while non-junctional Cx32 prevented
intrinsic apoptosis induced by streptonigrin in human CaCx cells by
promoting the EGFR, ERK and STAT3 signalling pathways. As CaCx is
highly related to HPV infections and may be related to
inflammation-associated tumour growth, we investigated whether Cx32
is a key regulator of TNF-related inflammation-associated tumour
growth in this study.
Because the role of Cx32 in the extrinsic apoptosis
pathway is still unclear in CaCx, we investigated the impact of
Cx32 on this pathway when induced by TNFα or TRAIL in this study.
Constitutive activation of nuclear factor κB (NF-κB)-dependent
pathways is a hallmark of cancer. Abnormal NF-κB activation
provides resistance to malignant cells, and the NF-κB signalling
pathway is also a critical factor for apoptosis induced by TNFα or
TRAIL. Therefore, we examined whether the NF-κB pathway was a
target of Cx32 via treatment with various inhibitors (18).
Materials and methods
Materials
18α-glycyrrhetinic acid (18α-GA), oleamide, dimethyl
sulfoxide (DMSO), Hoechst 33258, 2-aminoethoxydiphenyl-borate
(2-APB), and anti-β-tubulin, anti-β-actin mouse IgG, anti-PCNA and
secondary antibodies were acquired from Sigma-Aldrich (St. Louis,
MO, USA). Anti-Cx32 antibodies were from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Anti-P65 (NF-κB), histone H3, matrix
metalloproteinase (MMP)-9, MMP-2, c-inhibition of apoptosis (IAP)1,
X-linked inhibitor of apoptosis (XIAP) and cleaved-caspase-3
antibodies were obtained from Cell Signaling Technology (Danvers,
MA, USA). SC75741, JSH-23 and afatinib were obtained from Selleck
Chemicals (Houston, TX, USA). TNFα reagent was from PeproTech
(Rocky Hill, NJ, USA), and TRAIL was acquired from Sino Biological
(Beijing, China). FITC-conjugated goat anti-rabbit secondary
antibodies were from Abbkine. Hygromycin B, G418 and doxycycline
(Dox) were obtained from Calbiochem (San Diego, CA, USA). Annexin
V-FITC apoptosis detection kits were from Biotool (Houston, TX,
USA). Cycloheximide (CHX) was obtained from DingGuo (Guang Zhou,
China). Lipofectamine™ 2000 and calcein-AM (acetoxymethyl ester)
were acquired from Invitrogen (Carlsbad, CA, USA). All other
reagents were from Sigma unless stated otherwise.
Clinical tissue samples
The clinical tissue samples were obtained from the
Xinjiang Medical University-Affiliated Tumour Hospital. Cervical
tissue samples were resected during surgery. The use of these
clinical samples was allowed by the ethics committee of Xinjiang
Medical University Affiliated Tumour Hospital.
Cell lines and cell cultures
Human CaCx cell lines (C-33A cell line) were
purchased from the American Type Culture Collection (Manassas, VA,
USA). As previously described and characterized (19), another stable Cx32-transfected HeLa
cell line (HeLa-Cx32) was under the control of a bidirectional
tetracycline-inducible promoter. In these cells, Cx32 expression
was induced by doxycycline (1 µg/ml) exposure for ~48 h. The
positive cells were screened with 100 µg/ml G418 sulfate and
200 µg/ml hygromycin B in DMEM supplemented with 10% foetal
bovine serum (FBS). C-33A cells were grown in MEM supplemented with
10% FBS, antibiotics and glutamine. Low-density culturing was
adopted to study the non-junctional function of Cx32 in apoptosis.
The cells were seeded in wide dishes (150 mm), which provided
enough distance among cells such that adjacent cells were prevented
from forming GJs.
GJ functional assay
Gap junction intracellular communication (GJIC)
function was assessed with a 'parachute' dye-coupling assay as
described by Goldberg et al (20). After cells were cultured to
confluence in 12-well plates, 5 µM calcein-AM was added to
donor cells for 30 min at 37°C. Then, the cells were rinsed,
trypsinized and seeded onto the receiver cells at a 1:150
donor/receiver ratio. Donor cells can be observed due to
calcein-AM. If GJIC function is normal, calcein-AM from donor cells
can be intracellularly transferred into the receiver cells. Both
the donor cells and the monolayer of receiver cells were incubated
for 4 h at 37°C. Then, the average amount of receiver cells
containing calcein-AM per donor cell was observed with a
fluorescence microscope (Olympus IX71, Tokyo, Japan). The level of
GJIC was measured based on the average amount of dye in the
receiver cells.
Apoptosis assay
Briefly, ~1–2×105 cells per well were
seeded in 6-well plates. Before adding the apoptosis-inducing
reagent to the cells, HeLa-Cx32 or C-33A cells were divided into
several groups (HeLa-Cx32 were incubated with or without
doxycycline for 48 h and C-33A cells were treated with Cx32 siRNA
or non-specific siRNA for 48 h). HeLa-Cx32 cells were incubated
with TNFα (100 ng/ml) or TRAIL (20 ng/ml) for 24 h, while the C-33A
cells were incubated with TNFα (100 ng/ml) plus CHX (1
µg/ml). After the cells were washed twice with PBS, they
were trypsinized and harvested. Next, the cells were centrifuged
and resuspended in binding buffer. After the cells were stained
with Annexin V-FITC and propidium iodide (PI) for 15 min at room
temperature in the dark, the samples were swiftly analysed in a
flow cytometer. The early or late apoptosis rate was used for the
analysis in Expo32 software.
Cx32 siRNA interference experiments
After the cells had grown to 30–50% confluence,
non-specific siRNA (negative control) or the Cx32 siRNA (50 nM,
Ribbon, Guangzhou, China) and Lipofectamine™ 2000 were added
together and mixed. Then, the mixture was added to the cells in
each well according to the manufacturer's protocol. After the cells
were incubated with the siRNAs for 48 h, TNFα (TNFα+CHX for C-33A
cells) was added to induce apoptosis.
The sequences of the synthetic Cx32 siRNAs are as
follows: siCx32_1, 5′-CCGGCATTCTACTGCCATT-3′; siCx32_2,
5′-GGCTCACCAGCAACACATA-3′; and siCx32_3, 5′-GCAACAGCGTTTGCTATGA-3′.
Among them, the inhibitory effects of siCx32_3 were the best, and
it was chosen for use in the remaining experiments.
Cell viability measurement
Cells were plated in 96-well plates and treated with
drugs at various concentrations for 24 h. Cell Counting Kit-8
(CCK8) reagent was added to cells and allowed to react for a 3–4 h
reaction, and the optical density was measured at a wavelength of
450 nm using a microplate reader (Bio-Tek Instruments). In a second
method, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was added to cells for a 3–4-h reaction, and DMSO was
used to dissolve the sediment. The optical density was measured at
a wavelength of 490 nm for the MTT method. The normalized cell
survival rate was measured from the optical density based on the
CCK-8 and/or MTT results.
Western blot analysis
Cell plates were placed on ice after tissue or cells
were washed three times with PBS. Tissues or cells were lysed in
lysis buffer [150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM
Na3VO4, 2.5 mM sodium pyrophosphate, 1%
Triton X-100, 1 mM β-glycerophosphate, 20 mM Tris-HCl (pH 7.4) and
protease inhibitors (1:1,000)] for ≥30 min. After scratching,
collection and ultrasonication, the lysates were centrifuged at
12,000 rcf for 30 min at 4°C, and the supernatants were collected.
Proteins concentrations were measured with a BCA protein assay kit
(Thermo Fisher, MA, USA). Nucleoproteins were collected using
nuclear and cytoplasmic extraction reagents (Thermo Scientific)
following the manufacturer's instructions (21). The same amount of each sample (20
µg) was separated via SDS-PAGE and transferred to a
nitrocellulose membrane. Before antibody blotting, 5% milk was used
to block the membranes for 1 h. Monoclonal antibodies including
those for Cx32 (1:1,000), P65 (1:1,000), histone H3 (1:1,000),
MMP-9 (1:1,000), MMP-2 (1:1,000), c-IAP1 (1:1,000), XIAP (1:1,000),
cleaved-caspase-3 (1:1,000), β-actin (1:10,000) and β-tubulin
(1:10,000), were incubated with the membrane overnight at 4°C. The
next day, the membranes were incubated with HRP-conjugated
secondary antibodies at room temperature for 1–2 h. After being
washed with TBST, immunopositive bands in the membrane were
detected and visualized with Western Lightning chemiluminescence
reagents (Thermo Fisher). ImageJ software was used to analyse the
western blotting band density data. The ratio of the target protein
to the respective loading control (e.g., tubulin) was calculated,
and the mean of the ratios from the control bands were normalized
as '100'.
Statistical analysis
All of the experiments had a minimum of three
replicates. The data represent the mean ± standard error (SE) and
were analysed using SPSS 16.0 software. Statistical significance
(P<0.05) was determined via a one-way ANOVA (>2 groups) or
Student's t-test (2 groups). Non-parametric data were analysed
using two independent sample tests. Pearson's correlation analysis
was used to analyzed the correlation between Cx32 and NF-κB
expression and GraphPad Prism 6.0 software was used to create the
histograms and scatter plots. In the figures, asterisk (*)
represents P<0.05 compared to the corresponding group.
Results
Overexpression of Cx32 suppressed the
extrinsic apoptosis of HeLa cells regardless of whether GJ function
was inhibited
Similar to the research methods used for studying
the role of Cx32 in endogenous apoptosis, we first used HeLa-Cx32
cells in which the expression of Cx32 could be controlled by Dox to
investigate the function of Cx32 in exogenous apoptosis. We
detected the expression of Cx32 after HeLa-Cx32 or HeLa wild-type
cells were incubated with Dox. The results showed that Dox could
induce Cx32 expression in HeLa-Cx32 cells but not in HeLa wild-type
cells (Fig. 1A). Reports have
shown that 2-APB, 18α-GA and oleamide can effectively inhibit GJIC
(22). According to our previous
study, 2-APB, 18α-GA and oleamide were used as GJ inhibitors in our
experiments (23,24). To eliminate the interference of
tool drugs on apoptosis and help choose a suitable working
concentration, we detected the survival rate of cells using CCK-8
and MTT assays. Based on the results, we used inhibitors at the
following concentrations which showed no significant cytotoxicity:
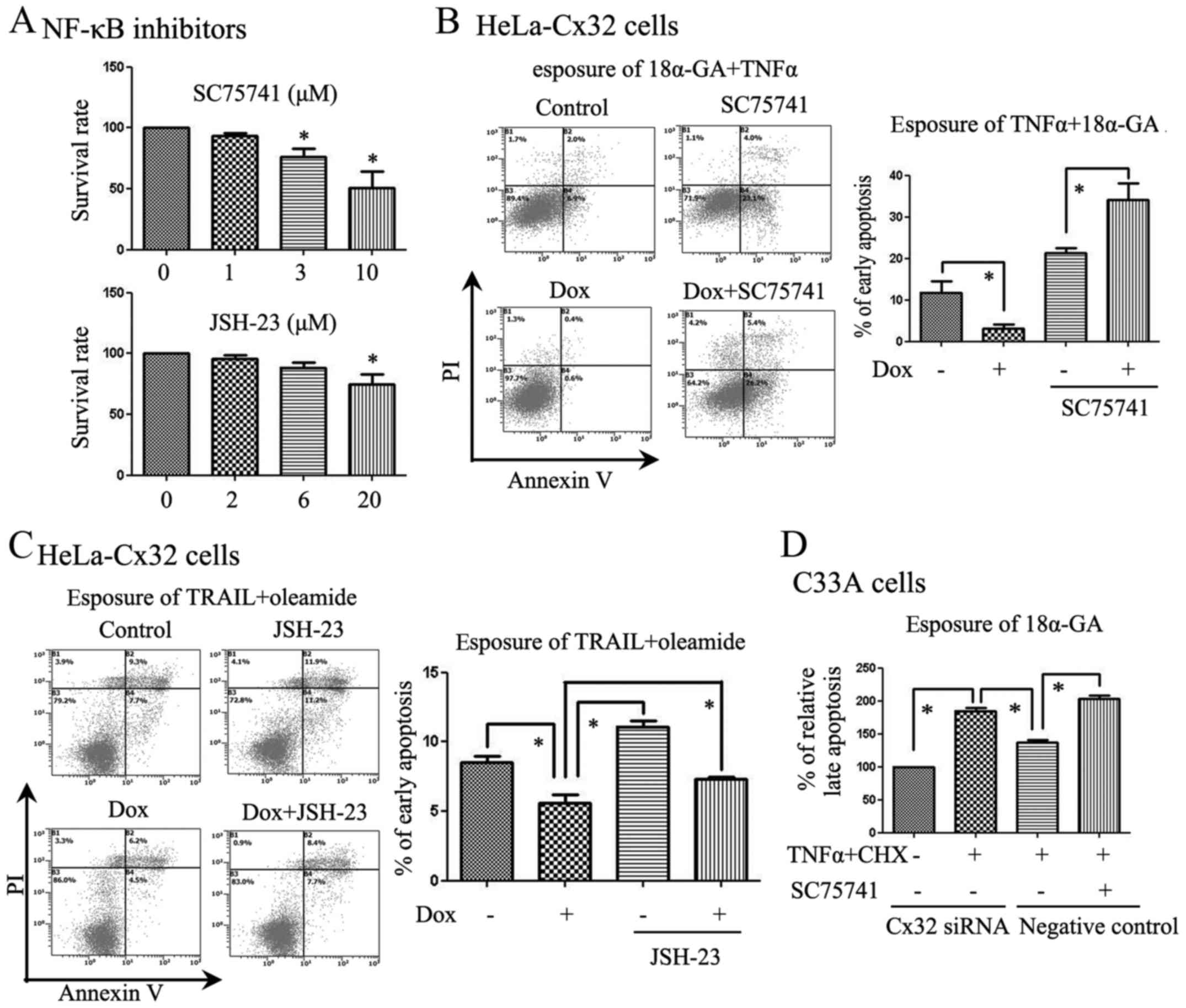
18α-GA (10 µM), oleamide (25 µM), SC75741 (1
µM) and JSH-23 (2 µM) (Figs. 1B and 4A). We confirmed in our parachute assay
that Dox could enhance GJ function, but that 2-APB, 18α-GA and
oleamide were able to inhibit it (Fig.
1C).
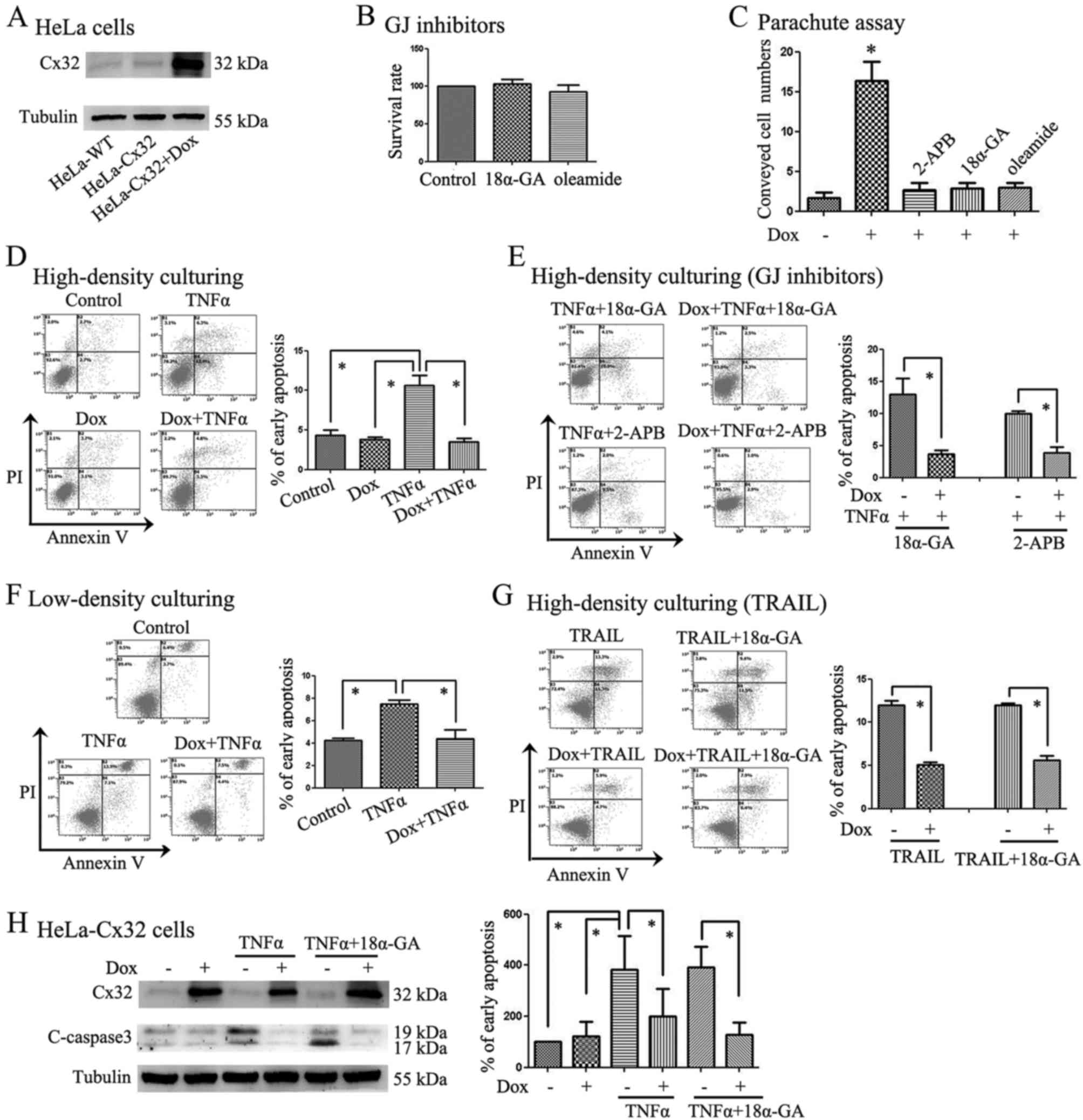 | Figure 1Cx32 expression was controlled in
HeLa-Cx32 cells and its impact on apoptosis induced by TNFα or
TRAIL was detected. (A) Cx32 expression was induced in wild-type
HeLa cells (HeLa-WT) or HeLa-Cx32 cells after 48-h Dox treatment
(n=3). (B) CCK-8 assay results showed that 18α-GA (10 µM)
and oleamide (25 µM) displayed no significant cytotoxicity
to cells (n=5). (C) A parachute dye was used in HeLa-Cx32 cells,
and Dox was used to induce Cx32 expression. The results showed that
GJs were inhibited by 2-APB, 18α-GA and oleamide (n=3). (D) Under
high-density culture conditions, Cx32 overexpression suppressed
TNFα-induced apoptosis (n=4). (E) GJs were inhibited by 2-APB or
18α-GA, and these GJ inhibitors did not change the Cx32
anti-apoptotic functions (n=3–4). (F) Low-density culturing showed
that the Cx32 anti-apoptotic functions were still present without
GJ formation (n=4). (G) Aside from TNFα, TRAIL (20 ng/ml) was used
to induce apoptosis. High Cx32 expression induced by Dox inhibited
the apoptosis induced by TRAIL (n=3). (H) HeLa-Cx32 cells were
divided into 6 groups: control group, Dox group, TNFα group,
Dox+TNFα group, TNFα+18α-GA group, and Dox+TNFα+18α-GA group.
Expression of cleaved-caspase-3, an executor of apoptosis, was
detected via western blotting. The results were consistent with
apoptosis detection via flow cytometry with Annexin V-FITC (n=3).
*P<0.05 with respect to the control or corresponding
group. |
Following treatment with TNFα or TRAIL, the early
apoptosis rate of HeLa-Cx32 cells in the different groups was
analysed. In the subsequent experiment, HeLa-Cx32 cells were
divided into the following groups, with the total culture time of
each group being equal: control group (incubated with solvent), Dox
group (incubated with Dox for 48 h), TNFα group (treated with TNFα
for 24 h), Dox+TNFα group (cells were incubated with Dox for 48 h
and treated with TNFα for another 24 h); TNFα+18α-GA group (18α-GA
was added 2 h before treatment with TNFα); and Dox+TNFα+18α-GA
group (incubated with Dox for 48 h and 18α-GA was then added 2 h
before treatment with TNFα for another 24 h). The incubation times
for the other GJ inhibitors, such as 2-APB and oleamide, were
identical to that used for 18α-GA. The division of the TRAIL groups
was similar to that of the TNFα group.
In Fig. 1D, the
HeLa-Cx32 cells were divided into 4 groups: the control group, Dox
group, TNFα group, and Dox+TNFα group. The result showed that the
apoptosis rate of HeLa-Cx32 cells in the TNFα group was much higher
than in the other groups (Fig.
1D). To confirm that Cx32 function was related with GJIC in
this instance, low-density culturing or GJ inhibitors, such as
2-APB and 18α-GA, were used (Fig. 1E
and F). 2-APB or 18α-GA was added to inhibit GJs before TNFα
was added to the cells. The results showed that the anti-apoptosis
function of Cx32 was present even when 2-APB or 18α-GA was added
(Fig. 1E). An additional method
(low-density culturing) was used to ensure that the distance among
cells was great enough so that they could not form GJs. In these
low density cultures, Cx32 prevented apoptosis induced by TNFα
(Fig. 1F), which was consistent
with the GJ inhibitor results (Fig.
1E). Aside from TNFα, we also utilized a stronger apoptosis
inducer, TRAIL, when repeating the experiment, and the results were
similar to those obtained for TNFα (Fig. 1G). Low-density culturing and GJ
inhibitors such as 2-APB and 18α-GA did not change the
anti-apoptotic functions of Cx32, indicating that the
anti-apoptosis effect of Cx32 may not be related to GJIC. To
further explore the extent of apoptosis, we also used western
blotting to detect the expression of cleaved-caspase-3, which is an
executioner caspase in apoptosis. HeLa-Cx32 cells were divided into
the following 6 groups: control group, Dox group, TNFα group,
Dox+TNFα group, TNFα+18α-GA group, and Dox+TNFα+18α-GA group. The
results showed that the changes in cleaved-caspase-3 expression
levels were consistent with the extent of apoptosis detected via
flow cytometry using Annexin V-FITC (Fig. 1H). After a 48-h incubation with
Dox, Cx32 expression was induced, and apoptosis rates were lower in
the high-Cx32-expression groups.
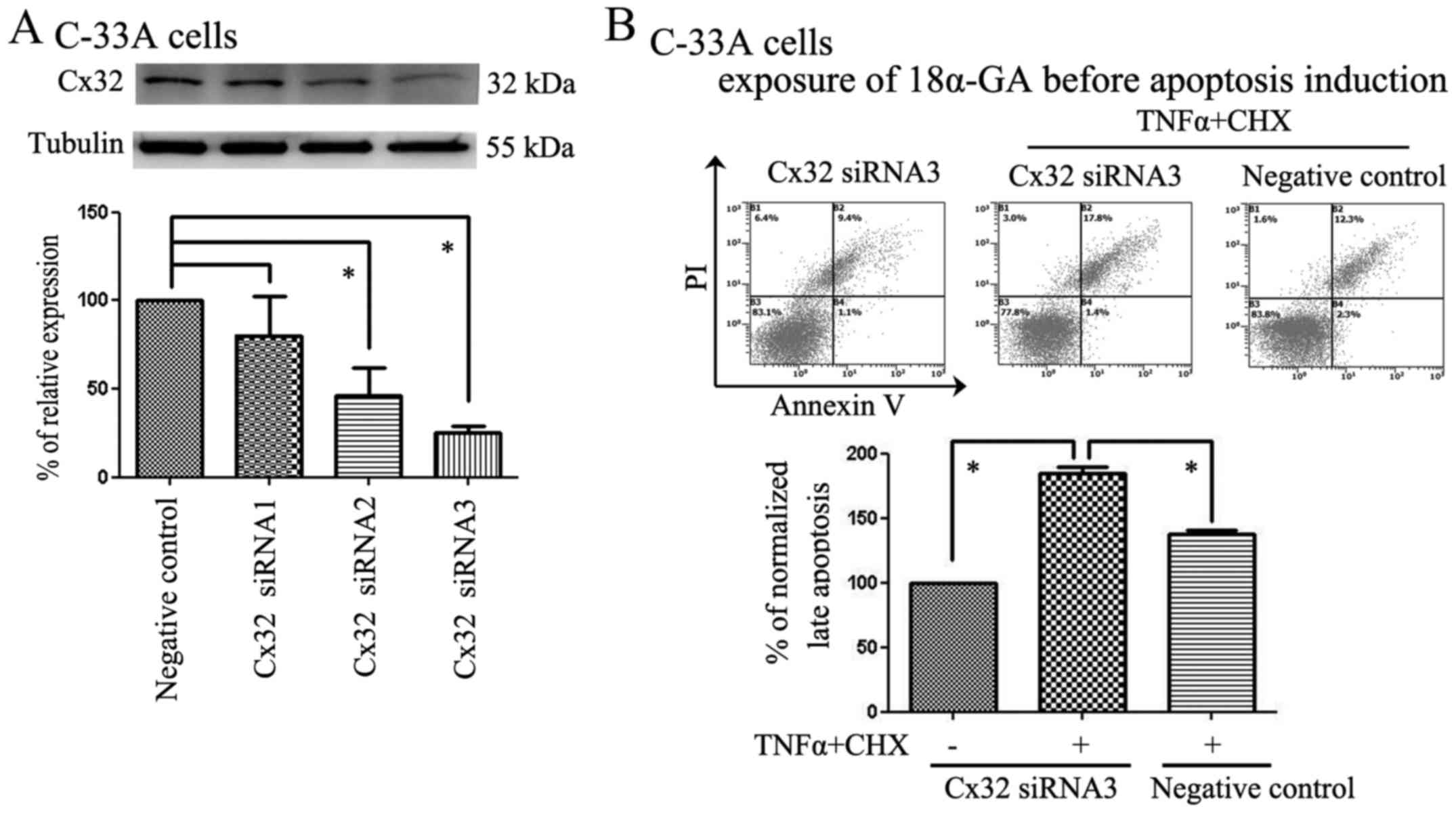
siRNA knockdown of endogenous Cx32
expression in C-33A cells reduced the anti-apoptotic effect of
Cx32
We also used C-33A cells as a model to further
explore the role of Cx32 in apoptosis via negative regulation.
According to our results, C-33A cells natively expressed Cx32;
thus, we chose these cells for the Cx32 interference experiment. In
our study, non-specific siRNAs were used as a negative control (NC)
and three Cx32 siRNAs (S1, S2 and S3) were used to knock down Cx32
expression. S2 and S3 were the most efficient, and the S3 fraction
was therefore used in our further experiments (Fig. 2A). As the apoptosis-inducing
ability of TNFα is limited based on our experimental results, we
decided to use TNFα (50 ng/ml) and CHX (1 µg/ml) in
combination to induce apoptosis in C-33A cells, which has been
demonstrated in previous reports (25). After using Cx32 siRNA3 to knock
down Cx32 expression in C-33A cells, all of the groups were
incubated with 18α-GA, and some of the groups were incubated with
TNFα plus CHX to induce apoptosis. Consistent with the results from
the HeLa-Cx32 experiments, the apoptosis rates in the Cx32
knockdown groups were much higher than those in the non-specific
siRNA groups after cotreatment with TNFα plus CHX (Fig. 2B).
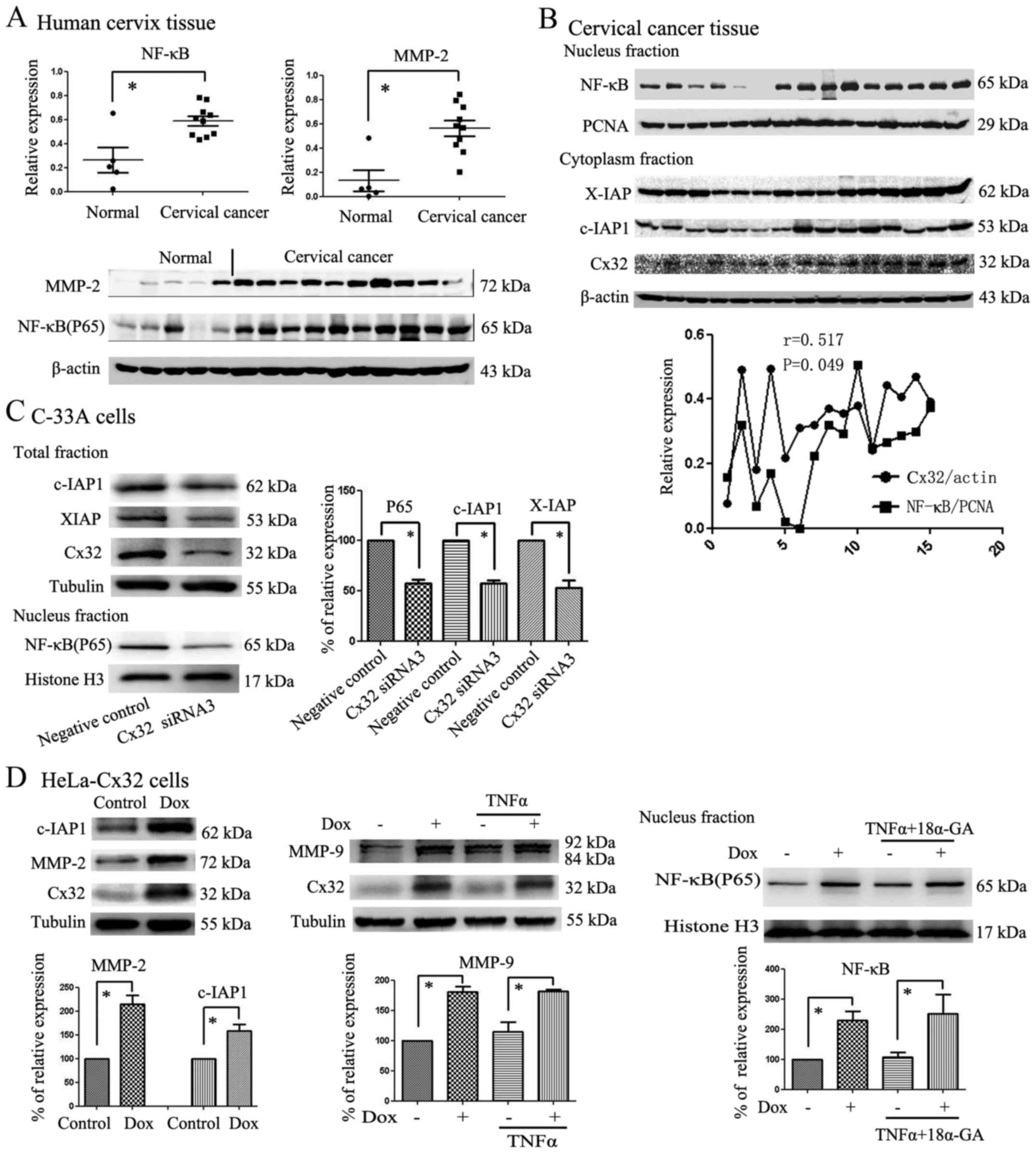
Cx32 expression upregulates NF-κB and
activates its downstream effectors MMPs, c-IAP1 and XIAP
Our previous study showed that Cx32 expression was
obviously higher in CaCx clinical tissue samples than in normal
samples. Because NF-κB acts as an important factor in the extrinsic
apoptosis pathway, we examined the expression of NF-κB and MMP-2 in
CaCx clinical tissue samples to determine the effects of Cx32 on
the NF-κB signalling pathway. Metalloproteases (MMPs) and c-IAP1
are downstream in the NF-κB signalling pathway. In total, 15
samples were used to detect NF-κB and MMP-2 expression. Some of the
samples were from CaCx tissue with high Cx32 expression, and others
were from para-CaCx tissue with low Cx32 expression. Under these
circumstances, we found that NF-κB and MMP-2 expression was
obviously higher in CaCx samples than in para-CaCx samples
(Fig. 3A).
Based on the variation in NF-κB and MMP-2 levels in
human cervical samples, we investigated the expression of Cx32,
NF-κB and its target proteins in CaCx samples. Nucleoproteins and
cytoplasm proteins from 15 CaCx tissue samples were collected and
then the expression of NF-κB, Cx32, XIAP and c-IAP1 were detected
by western blotting. The results showed that Cx32 expression was
correlated with expression of NF-κB (r=0.517, P=0.049) and the
variation tendency of XIAP and c-IAP1 coincided with NF-κB
variation (Fig. 3B). Then we
continued to explore the Cx32 and NF-κB expression in vitro.
After Cx32 expression was reduced by Cx32 siRNA3, the expression of
P65 in the nucleus and total XIAP and c-IAP1 expression in C-33A
cells also decreased (Fig. 3C).
Consistent with the results in the C-33A cells, our results showed
that Cx32 not only regulated the expression of P65 (NF-κB) in the
nucleus of HeLa-Cx32 cells but also regulated total MMP-9, MMP-2
and c-IAP1 expression in HeLa-Cx32 cells (Fig. 3D). Similar results were observed
for MMP-2, MMP-9 and c-IAP1, which are also NF-κB target
proteins.
Cx32 exerts an inhibitory effect on
extrinsic apoptosis via the NF-κB pathway in CaCx cells
Based on the above results, we next used NF-κB
inhibitors as tools to study the effects of Cx32. As an inhibitor
of NF-κB, SC75741 specifically inhibits NF-κB-mediated signalling
on a transcriptional level (18).
Before apoptosis was induced, we added SC75741 or JSH-23 to the
cells. We added SC75741 to two of the groups and found that it
reduced the anti-apoptotic functions of Cx32 in response to TNFα
(Fig. 4B). Moreover, JSH-23,
another NF-κB inhibitor, changed the anti-apoptotic functions of
Cx32 in response to TRAIL when using oleamide (25 µM) to
inhibit GJs (Fig. 4C). SC75741
also reversed the anti-apoptotic functions of Cx32 in C-33A cells
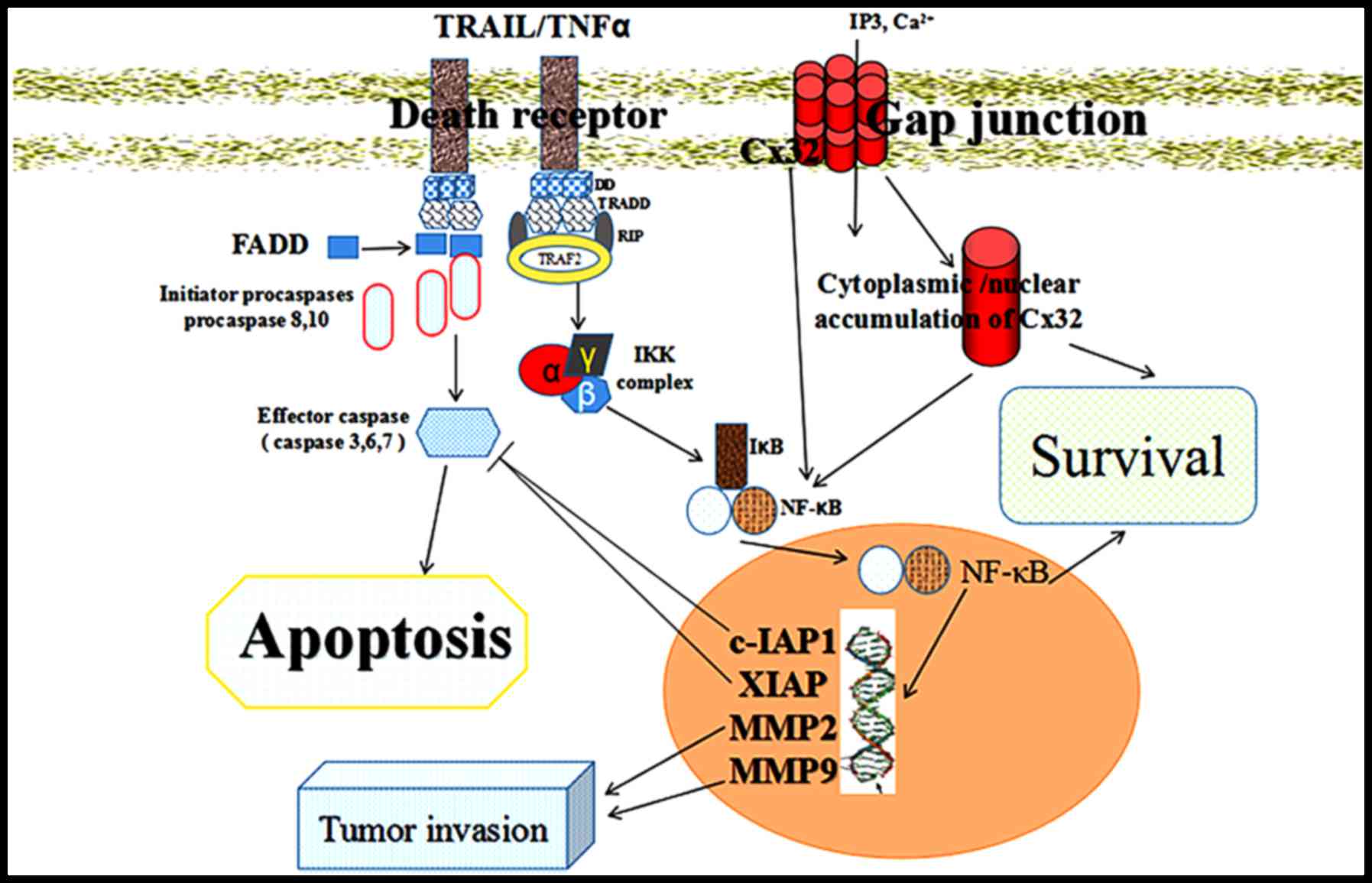
(Fig. 4D). We have summarized the
targets of Cx32 in a diagram to show its functions and downstream
signalling pathways (NF-κB, c-IAP1, and MMP-2) in the extrinsic
apoptotic pathway of CaCx cells (Fig.
5).
Discussion
Our data indicate that Cx32, whether in GJs or not,
is critical for active NF-κB signalling in CaCx cells. Due to the
complexity of GJ-dependent and GJ-independent effects in apoptosis,
we focused on the non-junctional functions of Cx32 in CaCx in this
study. To study these non-junctional functions in apoptosis, 2-APB,
18α-GA, oleamide and low-density culturing were utilized to block
the effects of GJIC on apoptosis.
There are two major apoptosis pathways, the
intrinsic and extrinsic pathways. The extrinsic apoptosis pathway
depends on death ligands, which include TNFα, FasL and TRAIL, and
their binding to DRs (26). TNFα
leads to an increase in apoptosis by upregulating the
transcriptional factor FoxO1, which leads to the increased
expression of apoptotic genes (27). TNFα can not only induce an
inflammatory response but can also induce apoptosis via cotreatment
with protein synthesis inhibitors, such as CHX (28). Because the apoptosis rates induced
by TNFα were low in our study, we added CHX to promote apoptosis in
C33A cells (25). Our data show
that TRAIL is a more sensitive cytokine at lower concentrations
than TNFα. In fact, TRAIL is a selective cytokine that induces
tumour cell apoptosis while sparing normal cells (29,30).
To validate the universal functions of Cx32 in extrinsic apoptosis,
we used both TNFα and TRAIL to induce apoptosis in our study.
CaCx progression is related to EGFR, MMPs and
cyclooxygenase-2 (COX-2) overexpression (31,32).
TNFα-induced NF-κB activation was not prevented by EGFR or Src
inhibition, suggesting that TNFα exerts both EGFR-dependent and
EGFR-independent effects (33).
Activation of NF-κB is a central event in the responses of normal
cells to inflammatory signals, such as those caused by HPV
infection. Abnormal constitutive activation of NF-κB is important
for the survival of most cancer cells, and the activation of NF-κB
and EGFR can be connected by SOS1 (34). In the apoptosis pathway induced by
TNFα or TRAIL, NF-κB acts as a key factor for cell survival
(35). NF-κB, especially the P65
(RelA) subunit, can induce the expression of anti-apoptotic genes,
such as IAP genes, thus inhibiting tumour cell apoptosis. XIAP,
c-IAP1 and survivin are very important members of the IAP family
(36). c-IAP1 and c-IAP2 suppress
TNFα-stimulated cell death by preventing the formation of the TNF
receptor 1 (TNFR1) pro-apoptotic signalling complex (37). Overexpression of XIAP and c-IAP1
are associated with drug resistance in cancer cells and reduce
patient survival after chemotherapy and radiotherapy. Our data show
that c-IAP1 expression is related to Cx32 expression. This result
demonstrates a relationship between Cx32 and c-IAP1, which may
account for the anti-apoptotic effect of Cx32 in CaCx cells.
NF-κB can be activated by many different stimuli
including pro-inflammatory cytokines (e.g., TNFα, IL-1),
lipopolysaccharides, and viral proteins (38). After activation, the transcription
factor NF-κB can translocate into the nucleus and bind the promoter
of immunoglobulin κ-chains in B-cells. The downregulation of Cx43
expression induced by high glucose levels activates NF-κB in
glomerular mesangial cells, leading to renal inflammation (39). Inflammation caused by HPV is an
important factor for CaCx progression. However, whether Cx32 is
related to inflammation requires further study. In our study, we
found that high Cx32 expression levels promoted P65 (RelA)
expression in the nucleus. After we added SC75741 or JSH-23 to
inhibit NF-κB, the anti-apoptotic effects of Cx32 in response to
TNFα and TRAIL vanished or weakened. The exact mechanisms for why
non-junctional Cx32 is required in the NF-κB signalling pathway
remain unclear.
HPV, like several other viruses, has developed a
method for evading the TNF-mediated host immune response (40). One of the key attributes of
HPV-induced cervical malignant transformation is HPV E2 gene
disruption. E2 may regulate NF-κB and STAT3 activation in the
presence of TNFα, with survival implications for HPV-infected cells
(41). KIAA1199 is an oncogenic
protein induced by HPV infection that connects the NF-κB and EGFR
oncogenic cascades and transmits pro-survival and invasive signals
(42). CaCx cell lines display
important differences with respect to Cx32 and HPV. HeLa and SiHa
cells are HPV-positive and Cx32-negative, while C-33A cells are
HPV-negative and Cx32-positive. Therefore, whether HPV and Cx32 are
independent factors in CaCx could also be a question for further
investigation.
The existence of a link between Cx43 or Cx26 and MMP
expression has been demonstrated in various tumour cells (43,44).
As a marker for tumour invasion and progression, MMPs are key
proteins for the development of tumours and the associated
microenvironment (45). In our
study, we detected MMP-9 and MMP-2 expression after controlling
Cx32 in HeLa-Cx32 cells and found that they had positive
correlations with or without TNFα incubation. Perhaps there may be
a balance between GJ-associated and non-junctional Cx32 in
apoptosis. Thus, the anti- or pro-apoptotic effects of GJs and/or
Cx32 play important roles in maintaining this balance depending on
their concentration, intensity, or the type of apoptosis inducer.
However, although the participation of nuclear Cx proteins in
controlling cell-death-related gene expression, partly via
Cx-responsive elements (CxRE) has been suggested (46,47),
the exact mechanism regarding how Cx32 modulates NF-κB requires
further investigation.
In conclusion, by promoting NF-κB, its downstream
signalling pathway components (e.g., c-IAP1, and MMP-2) and
aberrant nuclear localization, high Cx32 expression levels result
in anti-apoptotic functions in the extrinsic apoptotic pathway in
CaCx, independent of the Cx32 function in GJs. This study
demonstrates that non-junctional Cx32 serves as a novel regulator
of exogenous apoptosis in CaCx cells, which may provide new
insights into the roles of Cx32 in CaCx progression and
microenvironment formation.
Acknowledgments
This study was supported in part by the Joint Fund
of the National Nature Science Foundation of China (contract no.
U1303221), the National Natural Science Foundation of China
(contract nos. 81373439 and 81473234), and a grant for the
construction of technique plate for evaluation of the
pharmacodynamics of new drugs in Xinjiang from the Department of
Science and Technology of Guangdong province (contract no.
2014A020209032).
References
|
1
|
Lagunas-Martínez A, Madrid-Marina V and
Gariglio P: Modulation of apoptosis by early human papillomavirus
proteins in cervical cancer. Biochim Biophys Acta. 1805:6–16.
2010.
|
|
2
|
de Freitas AC, Coimbra EC and Leitão Mda
C: Molecular targets of HPV oncoproteins: Potential biomarkers for
cervical carcinogenesis. Biochim Biophys Acta. 1845:91–103.
2014.PubMed/NCBI
|
|
3
|
Muench P, Probst S, Schuetz J, Leiprecht
N, Busch M, Wesselborg S, Stubenrauch F and Iftner T: Cutaneous
papilloma-virus E6 proteins must interact with p300 and block
p53-mediated apoptosis for cellular immortalization and
tumorigenesis. Cancer Res. 70:6913–6924. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parrish AB, Freel CD and Kornbluth S:
Cellular mechanisms controlling caspase activation and function.
Cold Spring Harb Perspect Biol. 5:a86722013. View Article : Google Scholar
|
|
5
|
Han J, Soletti RC, Sadarangani A, Sridevi
P, Ramirez ME, Eckmann L, Borges HL and Wang JY: Nuclear expression
of β-catenin promotes RB stability and resistance to TNF-induced
apoptosis in colon cancer cells. Mol Cancer Res. 11:207–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carette D, Gilleron J, Chevallier D,
Segretain D and Pointis G: Connexin a check-point component of cell
apoptosis in normal and physiopathological conditions. Biochimie.
101:1–9. 2014. View Article : Google Scholar
|
|
7
|
Kameritsch P, Khandoga N, Pohl U and
Pogoda K: Gap junctional communication promotes apoptosis in a
connexin-type-dependent manner. Cell Death Dis. 4:e5842013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao XY, Li QQ, Gao YF, Zhou HH, Liu ZQ and
Jin WL: Gap junction as an intercellular glue: Emerging roles in
cancer EMT and metastasis. Cancer Lett. 381:133–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao B, Zhao W, Wang Y, Xu Y, Xu J, Tang
K, Zhang S, Yin Z, Wu Q and Wang X: Connexin32 regulates hepatoma
cell metastasis and proliferation via the p53 and Akt pathways.
Oncotarget. 6:10116–10133. 2015. View Article : Google Scholar :
|
|
10
|
Chen ZY, Wang R, Huang F, Yuan DD and Li
SR: Inhibition of gap junctions relieves the hepatotoxicity of
TNF-α. Genet Mol Res. 14:11896–11904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, de Toledo SM, Hu G, Hei TK and
Azzam EI: Connexins and cyclooxygenase-2 crosstalk in the
expression of radiation-induced bystander effects. Br J Cancer.
111:125–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Autsavapromporn N, De Toledo SM, Jay-Gerin
JP, Harris AL and Azzam EI: Human cell responses to ionizing
radiation are differentially affected by the expressed connexins. J
Radiat Res (Tokyo). 54:251–259. 2013. View Article : Google Scholar
|
|
13
|
Murphy SF, Varghese RT, Lamouille S, Guo
S, Pridham KJ, Kanabur P, Osimani AM, Sharma S, Jourdan J, Rodgers
CM, et al: Connexin 43 inhibition sensitizes chemoresistant
glioblastoma cells to temozolomide. Cancer Res. 76:139–149. 2016.
View Article : Google Scholar :
|
|
14
|
Kim YJ, Kim J, Kim YS, Shin B, Choo OS,
Lee JJ and Choung YH: Connexin 43 acts as a proapoptotic modulator
in cisplatin-induced auditory cell death. Antioxid Redox Signal.
25:623–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao YW, Lu TC, Pan XL, Li F, Zhong HH, Sun
Y, Jiang JF and Li L: Correlation of expression of connexin to
growth and progression of cervical carcinoma in situ. Chin J
Cancer. 24:567–572. 2005.In Chinese.
|
|
16
|
Aasen T, Graham SV, Edward M and Hodgins
MB: Reduced expression of multiple gap junction proteins is a
feature of cervical dysplasia. Mol Cancer. 4:312005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Y, Lai Y, Ge H, Guo Y, Feng X, Song
J, Wang Q, Fan L, Peng Y, Cao M, et al: Non-junctional Cx32
mediates anti-apoptotic and pro-tumor effects via epidermal growth
factor receptor in human cervical cancer cells. Cell Death Dis.
8:e27732017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ehrhardt C, Rückle A, Hrincius ER,
Haasbach E, Anhlan D, Ahmann K, Banning C, Reiling SJ, Kühn J,
Strobl S, et al: The NF-κB inhibitor SC75741 efficiently blocks
influenza virus propagation and confers a high barrier for
development of viral resistance. Cell Microbiol. 15:1198–1211.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu D, Fan L, Xu C, Liu Z, Zhang Y, Liu L,
Wang Q and Tao L: GJIC Enhances the phototoxicity of
photofrin-mediated photodynamic treatment by the mechanisms related
with ROS and Calcium pathways. J Biophotonics. 8:764–774. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldberg GS, Bechberger JF and Naus CC: A
pre-loading method of evaluating gap junctional communication by
fluorescent dye transfer. Biotechniques. 18:490–497.
1995.PubMed/NCBI
|
|
21
|
Zhu M, Du J, Liu AD, Holmberg L, Chen SY,
Bu D, Tang C and Jin H: L-cystathionine inhibits oxidized low
density lipoprotein-induced THP-1-derived macrophage inflammatory
cytokine monocyte chemoattractant protein-1 generation via the
NF-κB pathway. Sci Rep. 5:104532015. View Article : Google Scholar
|
|
22
|
Juszczak GR and Swiergiel AH: Properties
of gap junction blockers and their behavioural, cognitive and
electrophysiological effects: Animal and human studies. Prog
Neuropsychopharmacol Biol Psychiatry. 33:181–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Qin SK, Wu Q, Wang ZS, Zheng RS,
Tong XH, Liu H, Tao L and He XD: Connexin-dependent gap junction
enhancement is involved in the synergistic effect of sorafenib and
all-trans retinoic acid on HCC growth inhibition. Oncol Rep.
31:540–550. 2014. View Article : Google Scholar :
|
|
24
|
He B, Tong X, Wang L, Wang Q, Ye H, Liu B,
Hong X, Tao L and Harris AL: Tramadol and flurbiprofen depress the
cytotoxicity of cisplatin via their effects on gap junctions. Clin
Cancer Res. 15:5803–5810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi Z, Shen L, Zhou H, Jiang Y, Lan L, Luo
L and Yin Z: Phosphorylation of heat shock protein 27 antagonizes
TNF-α induced HeLa cell apoptosis via regulating TAK1
ubiquitination and activation of p38 and ERK signaling. Cell
Signal. 26:1616–1625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai X, Zhang J, Arfuso F, Chinnathambi A,
Zayed ME, Alharbi SA, Kumar AP, Ahn KS and Sethi G: Targeting
TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural
products as a potential therapeutic approach for cancer therapy.
Exp Biol Med (Maywood). 240:760–773. 2015. View Article : Google Scholar
|
|
27
|
Zhang B, Gui L, Zhu L, Zhao X, Yang Y and
Li Q: Forkhead box protein O1 mediates apoptosis in a cancer
cervical cell line treated with the antitumor agent tumor necrosis
factor-α. Genet Mol Res. 14:7446–7454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Du F and Wang X: TNF-alpha induces
two distinct caspase-8 activation pathways. Cell. 133:693–703.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oikonomou E and Pintzas A: The TRAIL of
oncogenes to apoptosis. Biofactors. 39:343–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sadarangani A, Kato S, Espinoza N, Lange
S, Llados C, Espinosa M, Villalón M, Lipkowitz S, Cuello M and Owen
GI: TRAIL mediates apoptosis in cancerous but not normal primary
cultured cells of the human reproductive tract. Apoptosis.
12:73–85. 2007. View Article : Google Scholar
|
|
31
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
32
|
Soonthornthum T, Arias-Pulido H, Joste N,
Lomo L, Muller C, Rutledge T and Verschraegen C: Epidermal growth
factor receptor as a biomarker for cervical cancer. Ann Oncol.
22:2166–2178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kakiashvili E, Dan Q, Vandermeer M, Zhang
Y, Waheed F, Pham M and Szászi K: The epidermal growth factor
receptor mediates tumor necrosis factor-alpha-induced activation of
the ERK/GEF-H1/RhoA pathway in tubular epithelium. J Biol Chem.
286:9268–9279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De S, Dermawan JK and Stark GR: EGF
receptor uses SOS1 to drive constitutive activation of NFκB in
cancer cells. Proc Natl Acad Sci USA. 111:11721–11726. 2014.
View Article : Google Scholar
|
|
35
|
Flusberg DA and Sorger PK: Surviving
apoptosis: Life-death signaling in single cells. Trends Cell Biol.
25:446–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi XP, Han T, Li YX, Long XY and Li WZ:
Simultaneous silencing of XIAP and survivin causes partial
mesenchymal-epithelial transition of human pancreatic cancer cells
via the PTEN/PI3K/Akt pathway. Mol Med Rep. 12:601–608. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Varfolomeev E and Vucic D: (Un)expected
roles of c-IAPs in apoptotic and NFkappaB signaling pathways. Cell
Cycle. 7:1511–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sethi G, Shanmugam MK, Ramachandran L,
Kumar AP and Tergaonkar V: Multifaceted link between cancer and
inflammation. Biosci Rep. 32:1–15. 2012. View Article : Google Scholar
|
|
39
|
Xie X, Lan T, Chang X, Huang K, Huang J,
Wang S, Chen CX, Liu P and Huang H: Connexin43 mediates NF-κB
signalling activation induced by high glucose in GMCs: Involvement
of c-Src. Cell Commun Signal. 11:382013. View Article : Google Scholar
|
|
40
|
Filippova M, Song H, Connolly JL, Dermody
TS and Duerksen-Hughes PJ: The human papillomavirus 16 E6 protein
binds to tumor necrosis factor (TNF) R1 and protects cells from
TNF-induced apoptosis. J Biol Chem. 277:21730–21739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prabhavathy D, Prabhakar BN and
Karunagaran D: HPV16 E2-mediated potentiation of NF-κB activation
induced by TNF-α involves parallel activation of STAT3 with a
reduction in E2-induced apoptosis. Mol Cell Biochem. 394:77–90.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shostak K, Zhang X, Hubert P, Göktuna SI,
Jiang Z, Klevernic I, Hildebrand J, Roncarati P, Hennuy B, Ladang
A, et al: NF-κB-induced KIAA1199 promotes survival through EGFR
signalling. Nat Commun. 5:52322014. View Article : Google Scholar
|
|
43
|
Yano T and Yamasaki H: Regulation of
cellular invasion and matrix metalloproteinase activity in HepG2
cell by connexin 26 transfection. Mol Carcinog. 31:101–109. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lamiche C, Clarhaut J, Strale PO, Crespin
S, Pedretti N, Bernard FX, Naus CC, Chen VC, Foster LJ, Defamie N,
et al: The gap junction protein Cx43 is involved in the
bone-targeted metastatic behaviour of human prostate cancer cells.
Clin Exp Metastasis. 29:111–122. 2012. View Article : Google Scholar
|
|
45
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kardami E, Dang X, Iacobas DA, Nickel BE,
Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S and Spray DC: The
role of connexins in controlling cell growth and gene expression.
Prog Biophys Mol Biol. 94:245–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Decrock E, Vinken M, De Vuyst E, Krysko
DV, D'Herde K, Vanhaecke T, Vandenabeele P, Rogiers V and Leybaert
L: Connexin-related signaling in cell death: To live or let die?
Cell Death Differ. 16:524–536. 2009. View Article : Google Scholar : PubMed/NCBI
|