Introduction
Papillary thyroid cancer (PTC) and follicular
thyroid cancer (FTC) are the most common types of thyroid cancer,
accounting for 94% of all cases (1). Most of them are differentiated and
have a good prognosis with a 10-year survival rate of >92%
(2). But among clinical staging
criteria, metastatic lymphadenopathy is one of the best predictors
of a poor prognosis, recurrence and motility (3–5) as
it likely reflects aggressive primary tumor biology (6,7)
(seer.cancer.gov/statfacts/html/oralcav.html). So
exploring the mechanism of lymph node metastasis will deepen our
understanding of malignant characteristics of some differentiated
thyroid cancer.
Anoctamin family (ANO, also known as
TMEM16) contains 10 members which are identified as putative
intracellular calcium activated chloride channels (8,9). It
has been reported that some members of anoctamin family are
overexpressed in cancer (10),
moreover, overexpression of ANO1 and ANO6 can
increase cancer cell migration (11,12).
Anoctamin5 (ANO5), also referred to as TMEM16E, is
one member of the anoctamin family. Somatic mutation or
microdeletion of ANO5 usually results in muscular dystrophy
(13–18), but its exact role in tumorigenesis
and cancer progression is still not clear.
We first evaluated the expression profile of this
family in thyroid cancer by mining the public GEO database. We
discovered and proved the downregulation of ANO5 expression
in thyroid cancer. Thereafter, we revealed that downregulation of
ANO5 is negatively associated with lymph node metastasis and
inhibition of ANO5 promotes the migration and invasion of
thyroid cancer cells. In addition, we also found that lower
ANO5 expression was positively associated with
JAK/STAT3 pathway which is well-known to be activated during
cancer metastasis (19,20). The present results provided novel
insight into ANO5's function in thyroid cancer
metastasis.
Materials and methods
Tissue specimens and cell lines
Thyroid cancer tissue samples used in this study
were harvested from Shanghai Tenth People's Hospital between
November, 2013 and December, 2015. Written informed consent from
all patients was obtained. Thyroid cancer cell lines TPC-1 and
FTC-133 were obtained by Dr Lei Ye in Rui-Jin Hospital. TPC-1 cells
were maintained in RPMI-1640 medium and FTC-133 cells were cultured
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from HyClone, Logan, UT, USA) 100
U/ml penicillin, and 100 µg/ml streptomycin.
Antibodies and reagents
The target antibodies were purchased from Abcam.
RNA extraction and real-time PCR
Total RNA was extracted with TRIzol®
(Invitrogen, Garlsbad, CA, USA), following the manufacturer's
protocol. The OD260/OD280 ratio of RNA ranged from 1.8 to 2.0.
Reverse transcription was performed in 20 µl reaction volume
with 2 µg of RNA using M-MLv reverse transcriptase kit
(Takara, Otsu, Japan). Quantitative real-time PCR was carried out
using aBI 7900 Detection system with the SYBR Premix Ex Taq™
(Takara). Primer sequences specific to 38 genes and housekeeping
gene GaDPH are listed in Table
I.
 | Table IReal-time PCR primers utilized in
this study. |
Table I
Real-time PCR primers utilized in
this study.
| Genes | Sequences |
|---|
| ADH1B | F:
CCCGGAGAGCAACTACTGC |
| R:
AACCAGTCGAGAATCCACAGC |
| ANO5 | F:
TTTTGGAAACAACGACAAGCCA |
| R:
ACCATACTGGTGACGACAAGAG |
| BMP2 | F:
ACCCGCTGTCTTCTAGCGT |
| R:
TTTCAGGCCGAACATGCTGAG |
| CDH16 | F:
GTCCCTAGAGCCTATCCACCT |
| R:
TGCATTCACTTCAAAGGGTCC |
| CLCNKB | F:
GCCCTCCTTCTATGATGGCAC |
| R:
CCTGCCCTTGGTGACAGTG |
| DLG2 | F:
CCTCTACGTCAGAGCCATGTT |
| R:
ATCGGGCACGTTCCTTTCTTT |
| DPP6 | F:
CTACGCCGCCATCAATGATTC |
| R:
GGGATAGTGGTAGGGCTTCAC |
| DPY19L2 | F:
CTTCCAGTTCGTCCGTAATTCC |
| R:
TCTCCCGTTCCAAAGATGAGAG |
| EDN3 | F:
GGGACTGTGAAGAGACTGTGG |
| R:
AGACACACTCCTTGTCCTTGTA |
| ERBB4 | F:
GTCCAGCCCAGCGATTCTC |
| R:
AGAGCCACTAACACGTAGCCT |
| ESRRG | F:
GCCCTCACTACACTGTGTGAC |
| R:
CCTGCTAATTTGGACTGGTCTT |
| FABP4 | F:
ACTGGGCCAGGAATTTGACG |
| R:
CTCGTGGAAGTGACGCCTT |
| FHL1 | F:
AAGAACCGCTTCTGGCATGAC |
| R:
CCCCTTGTACTCCACGTTTTG |
| FOS | F:
CCGGGGATAGCCTCTCTTACT |
| R:
CCAGGTCCGTGCAGAAGTC |
| GHR | F:
CCATTGCCCTCAACTGGACTT |
| R:
AATATCTGCATTGCGTGGTGC |
| GNA14 | F:
GAGCGATGGACACGCTAAGG |
| R:
TCCTGTCGTAACACTCCTGGA |
| GPM6A | F:
ATTCCCTATGCCTCTCTGATTGC |
| R:
GCCATCTCAAAGTAGGTTTGCAG |
| HGD | F:
ATTTACACCGAGTTTGGCAAGA |
| R:
GGTCTCCTCAAAGACATCTATGC |
| ITPR1 | F:
ATTGCTGGGGACCGTAATCC |
| R:
TCCAATGTGACTCTCATGGCA |
| KIAA1324 | F:
GGAGCTTCATGCCTGCAAAGA |
| R:
CATCAAACCGAATGCCTGTGC |
| LIFR | F:
TGGAACGACAGGGGTTCAGT |
| R:
GAGTTGTGTTGTGGGTCACTAA |
| LRP2 | F:
GTTCAGATGACGCGGATGAAA |
| R:
TCACAGTCTTGATCTTGGTCACA |
| PID1 | F:
CGTGGAGTGCGAGAGCAAG |
| R:
CTGGGAAACCTCTTCGGAGGA |
| PLA2R1 | F:
TAAATCGGTTCTGACCCTGGA |
| R:
GCCACCGTAAGGAAACGAG |
| PTHLH | F:
AAGGTGGAGACGTACAAAGAGC |
| R:
CAGAGCGAGTTCGCCGTTT |
| RYR2 | F:
CATCGAACACTCCTCTACGGA |
| R:
GGACACGCTAACTAAGATGAGGT |
| SLC26A4 | F:
TGGTGGGATCTGTTGTTCTGA |
| R:
GGATCTGCCAAGTACCTCACT |
| SLC26A7 | F:
GTGACCCAAGGATTGGCCTTT |
| R:
GGCAACATGATGTCCCATTCC |
| SLC4A4 | F:
GGGTGCCCTGACTGAAGTTC |
| R:
GGTCGTGCCTGTCTTTTGCT |
| TFF3 | F:
CCAAGCAAACAATCCAGAGCA |
| R:
GCTCAGGACTCGCTTCATGG |
| TMEM171 | F:
AACCGCTAAACGAGACAGACA |
| R:
ACACAATCCCACAAGCACAATC |
| TMPRSS3 | F:
TGGAAGGGTCACTACGCAAAT |
| R:
AGTGGTGTAATGCAGTCACCT |
| TNFRSF11B | F:
GCGCTCGTGTTTCTGGACA |
| R:
AGTATAGACACTCGTCACTGGTG |
| TPO | F:
GCCAACAAGCGGAGTGATTG |
| R:
GGGCAGCATGTAAGGGAGAC |
| TPPP | F:
AGGGGTGACGAAAGCCATC |
| R:
CGGACACATAGCCTGACTCG |
| WSCD2 | F:
AAACCTGTGCGCTTCTTTACC |
| R:
GTACCTGCGAGCAATGCTTGA |
| KLK7 | F:
TAATGACCTCATGCTCGTGAAGC |
| R:
CAGCCGGAGACAGTACAGG |
| GaPDH | F:
CTGGGCTACACTGAGCACC |
| R:
AAGTGGTCGTTGAGGGCAATG |
Protein extraction and western blot
analysis
Total cellular proteins were extracted using cell
lysis buffer containing 50 mM Tris-HCl (pH 6.8), 2% SDS, 10%
glycerol, 10% 2-mercaptoethanol, and protease inhibitor cocktail
(Sigma, St. Louis, MO, USA). Then protein concentration was
determined using the BCA kit (Thermo Fisher Scientific, Waltham,
MA, USA). Protein (30 µg) was subjected to electrophoresis
by SDS-PAGE on the 10% gel and then transferred to a polyvinylidene
difluoride (PVDF) membrane. The membrane was blocked with 5% bovine
serum albumin (BSA) and 0.1% Tween-20 in PBS for 2 h at room
temperature. After incubation with the appropriate primary antibody
overnight at 4°C with anti-ANO5 (1:500), anti-STAT3
(1:1,000), anti-p727-STAT3 (1:1,000) (all from Abcam), and
anti-GAPDH (1:3,000; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) antibodies, membranes were washed and incubated with
the IRDye 800CW secondary antibodies for 1 h at room temperature.
The labeled protein bands were detected using the Odyssey Infrared
Imaging system (Li-COR Biosciences, Lincoln, NE, USA). GADPH
was used as a loading control.
Construction of ANO5 expression
vectors
Human full-length ANO5 cDNA (GenBank
accession no. NM_001142649.1) was acquired from normal thyroid
tissues. Primers for PCR amplification were designed as follows:
forward, 5′-ATA TCT AGA ATG GGC GAC CCG GAT CTC CTG GAA G-3′ and
reverse, 5′-ACG CGG CCG CTT AGA GTG TTG ATT TAG CCA GCT G-3′. The
PCR product was subcloned into the pCDH vector (Systembioscience,
Inc.) and verified by restriction digestion and DNA sequencing.
Lentivirus production
All recombinant lentiviruses were produced by
transfecting HEK293T cells according to standard protocols. In
brief, sub-confluent HEK293T cells were co-transfected with 4
µg pCDH-ANO5 plasmid vector, 3 µg PLP1, 3
µg PLP2 and 2 µg PLP-VSVG in 50 µl
Lipofectamine 2000. The medium was changed after 16 h and
supernatant was harvested 72 h later.
siRNA synthesis
One siRNA against ANO5 was chemically
synthesized and the sequences were listed as follows: siRNA-1269,
5′-GCU GUA GUU GGC UUA GCU UTT-3′; siRNA-2487, 5′-GCU CAU AGC AUA
GGU GUU UTT-3′. The non-targeting nucleotides were used as a
negative control siRNA-NC, 5′-UUC UCC GAA CGU GUC ACG UTT-3′.
In vitro migration and invasion
assays
Cell migration/invasion assays were performed using
24-well Transwells (6.5 mm pore size, Costar), coated without
(migration) or with (invasion) Matrigel. TPC-1 and FTC-133 cells
were starved in serum-free media for 12 h, trypsinized and washed
three times in RPMI-1640/DMEM containing 0.1% BSA. Cells
(1×104) were seeded into the upper chamber, and 600
µl medium containing 10% FBS was placed in the lower chamber
at 37°C in 5% CO2. After 48 h incubation, Matrigel and
cells remaining in the upper chamber were removed. Cells on the
lower surface of the membrane were fixed in 4% paraformaldehyde and
stained with Coomassie Brilliant Blue, photographed and counted
under a dissecting microscope. Every experiment was repeated three
times.
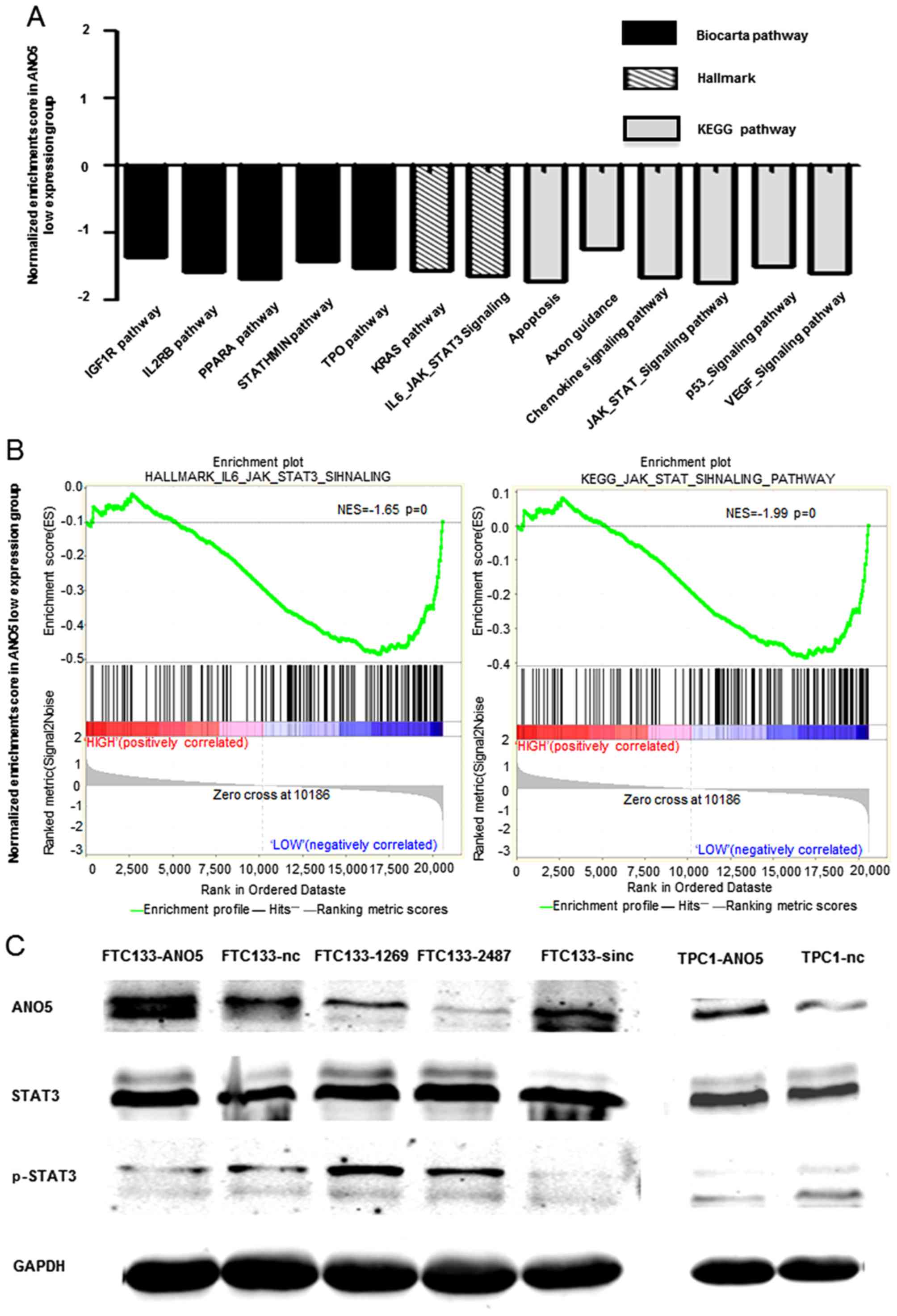
Gene set enrichment analysis (GSEA)
GSEA was carried out using GSEA software according
to literature (21,22). Firstly we classified thyroid cancer
samples (GSE3678) into ANO5 high expression and ANO5
low expression group according to ANO5 expression,
subsequently three gene sets including KEGG, Hallmark and BioCarta
were chosen to conduct GSEA.
Statistical analysis
The statistical difference of quantitative variables
was evaluated with Student's t-test using GraphPad Prism 5 software
and R ×64 3.2.2 software, p<0.05 was considered statistically
significant.
Results
ANO5 is downregulated in thyroid
cancer
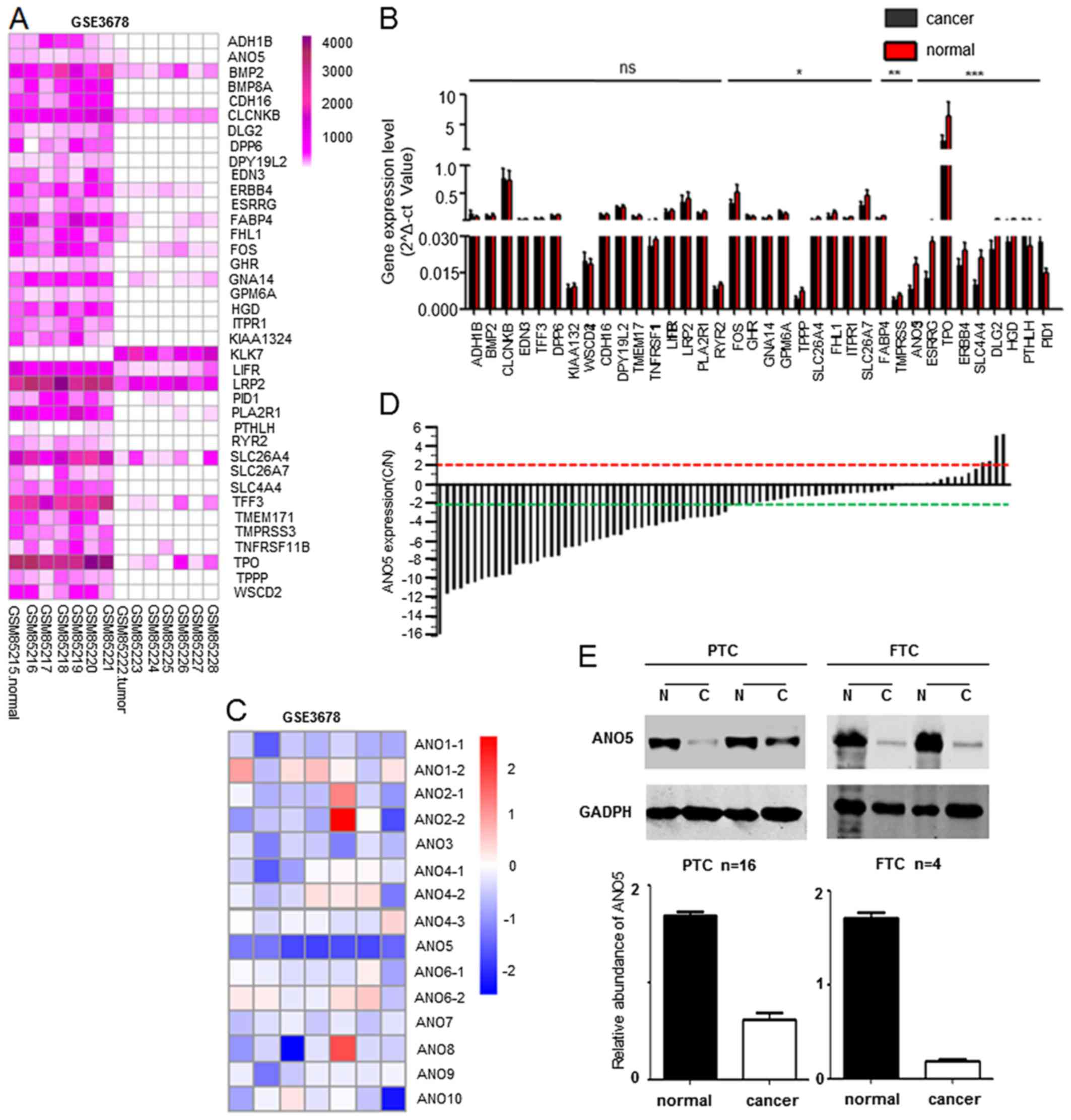
In order to find differentially expressed genes in
thyroid cancer and adjacent normal tissue, a GEO dataset (GSE3678)
which contains seven cancerous and seven normal tissues was chosen.
A total of 38 genes were discovered to display at least 3-fold
alterations (Fig. 1A).
Furthermore, we evaluated expression of these 38 genes in clinical
thyroid cancer tissues and adjacent noncancerous tissues by
real-time PCR (Fig. 1B). We found
that the expression of some genes including TPO (23), ANO5, ERBB4 (24) and SLC4A4 (25) genes were in accordance with the
results of GEO gene expression atlas (Fig. 1A), then we focused on ANO5
and its family through biological information analysis and previous
literature studies. In order to investigate the role of anoctamin
family in thyroid cancer progression, we first measured the
expression profile of this family in thyroid cancer tissue samples
by mining a public database (GSE3678). Interestingly, only
ANO5 was significantly downregulated in thyroid cancer
compared to adjacent noncancerous tissues (Fig. 1C). Subsequently we confirmed that
69.5% (57/82) thyroid cancer showed up to 2-fold downregulation of
ANO5 by real-time PCR assay (Fig. 1D). Similarly, western blot assay
also proved that ANO5 is downregulated in PTC and follicular
thyroid cancer compared to adjacent noncancerous tissues (Fig. 1E). Collectively, these data
revealed that ANO5 expression is significantly downregulated
in thyroid cancer.
Downregulation of ANO5 is positively
associated with lymph node metastasis of thyroid cancer
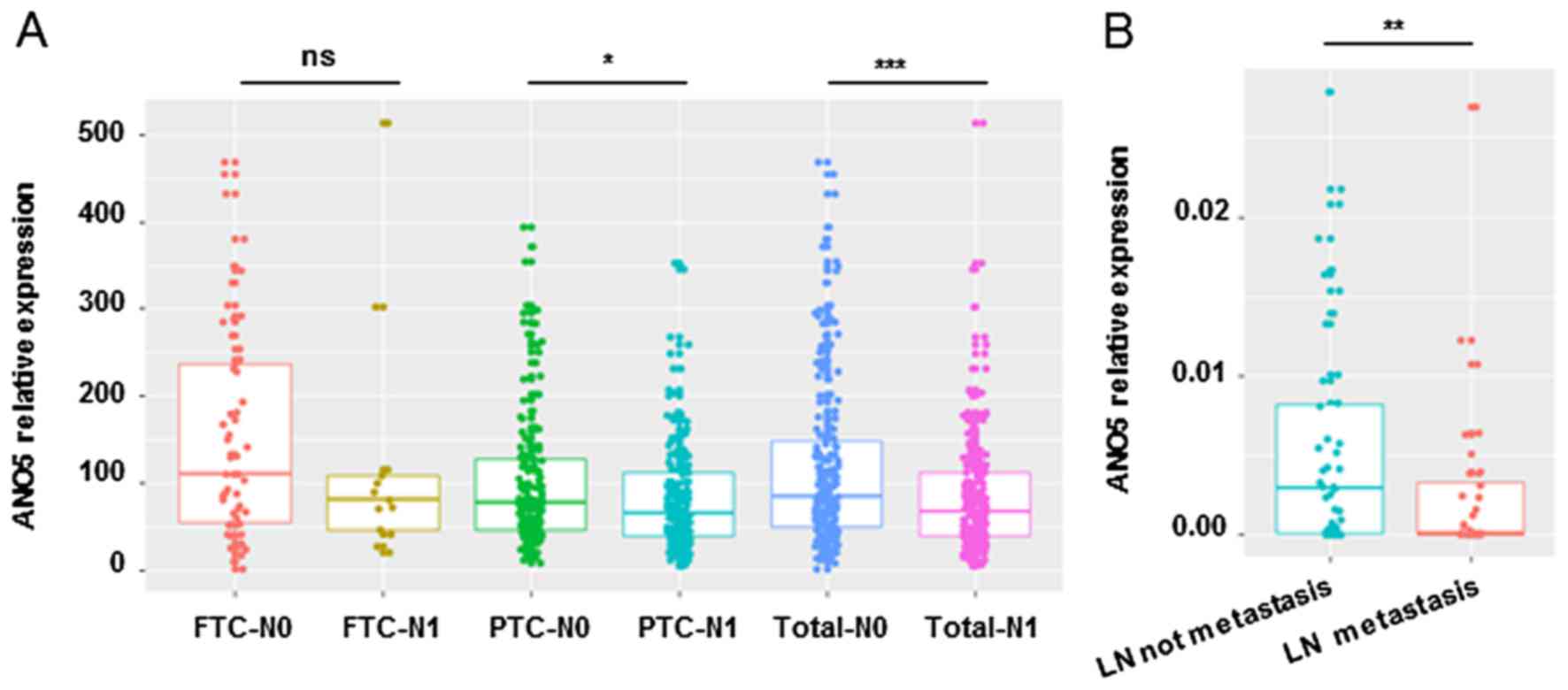
In order to explore the correlationship between
downregulation of ANO5 and clinical characteristics, we
analyzed RNA-seq data of thyroid cancer from TCGA database. We
found that ANO5 expression is significantly associated with
lymph node stage (N0 or N1, p=0.01) and neoplasm histologic type
(follicular or classical/papillary, p=6.97E-05) (Table II), ANO5 expression levels
in thyroid cancer with lymph node metastasis is lower than that
without lymph node metastasis (Fig.
2A). Real-time PCR also confirmed the downregulation of
ANO5 in thyroid cancer with lymph node metastasis compared
to that without lymph node metastasis (Fig. 2B). In addition, we proved, as shown
in Table III, ANO5
expression was significantly associated with lymph node metastasis
(lymph node negative vs. lymph node positive, p=0.0038,
χ2=8.376) in our in-house samples. There are no
significant association between ANO5 expression and other
tumor characteristics, such as age, gender, size, Hashimoto
background or multifocal disease (Table III). In order to carry out
cellular and functional experiments, we next evaluated ANO5
expression in thyroid cancer cell lines, including FTC-133 and
TPC-1, by real-time PCR, data show that ANO5 is undetectable
in PTC cell line (Fig. 3A). In
total, these findings indicate that downregulation of ANO5
is positively associated with lymph node metastasis of thyroid
cancer.
 | Table IIThe correlation of ANO5 expression
with clinical characteristics of thyroid cancer from TCGA. |
Table II
The correlation of ANO5 expression
with clinical characteristics of thyroid cancer from TCGA.
| Clinical
characteristic | No. of
patients | ANO5
| p-value |
|---|
| High | Low |
|---|
| Age (years) | | | | |
| ≥60 | 117 | 65 | 52 | |
| <60 | 379 | 183 | 196 | 0.169 |
| Gender | | | | |
| Male | 134 | 62 | 72 | |
| Female | 362 | 186 | 176 | 0.312 |
| Recurrence | | | | |
| Yes | 46 | 22 | 24 | |
| No | 436 | 215 | 221 | 0.848 |
| Overall survival
(month) | | | | |
| ≥60 | 97 | 41 | 56 | |
| <60 | 399 | 207 | 192 | 0.090 |
| Neoplasm histologic
type | | | | |
|
Classical/usual | 354 | 161 | 193 | |
| Follicular | 100 | 68 | 32 | 6.97E-05 |
| Tall cell | 35 | 16 | 19 | 0.990 |
| Tumor stage | | | | |
| T1 | 141 | 71 | 70 | |
| T2 | 164 | 86 | 78 | 0.717 |
| T3 | 167 | 79 | 88 | 0.594 |
| T4 | 22 | 10 | 12 | 0.669 |
| Metastasis
stage | | | | |
| M0 | 276 | 136 | 140 | |
| M1 | 9 | 5 | 4 | 0.711 |
| Lymph node
stage | | | | |
| N0 | 224 | 122 | 102 | |
| N1 | 222 | 94 | 128 | 0.010 |
 | Table IIIThe correlation of ANO5 expression
with clinical characteristics of PTC. |
Table III
The correlation of ANO5 expression
with clinical characteristics of PTC.
| Clinical
characteristic | No. of
patients | ANO5 expression
(ct)
| p-value | χ2 |
|---|
| >30 | ≤30 |
|---|
| Age (years) | | | | | |
| <45 | 32 | 17 | 15 | 0.3244 | 0.971 |
| ≥45 | 50 | 21 | 29 | | |
| Gender | | | | | |
| Male | 25 | 14 | 11 | 0.2456 | 1.349 |
| Female | 57 | 26 | 33 | | |
| Microcarcinoma | | | | | |
| Yes | 33 | 16 | 17 | 0.6846 | 0.165 |
| No | 49 | 26 | 23 | | |
| Hashimoto's
thyroiditis | | | | | |
| Yes | 43 | 20 | 23 | 0.8415 | 0.04 |
| No | 39 | 19 | 20 | | |
| Multifocal | | | | | |
| Yes | 29 | 13 | 16 | 0.2356 | 1.407 |
| No | 53 | 31 | 22 | | |
| LN metastasis | | | | | |
| Yes | 32 | 22 | 10 | 0.0038 | 8.376 |
| No | 50 | 18 | 32 | | |
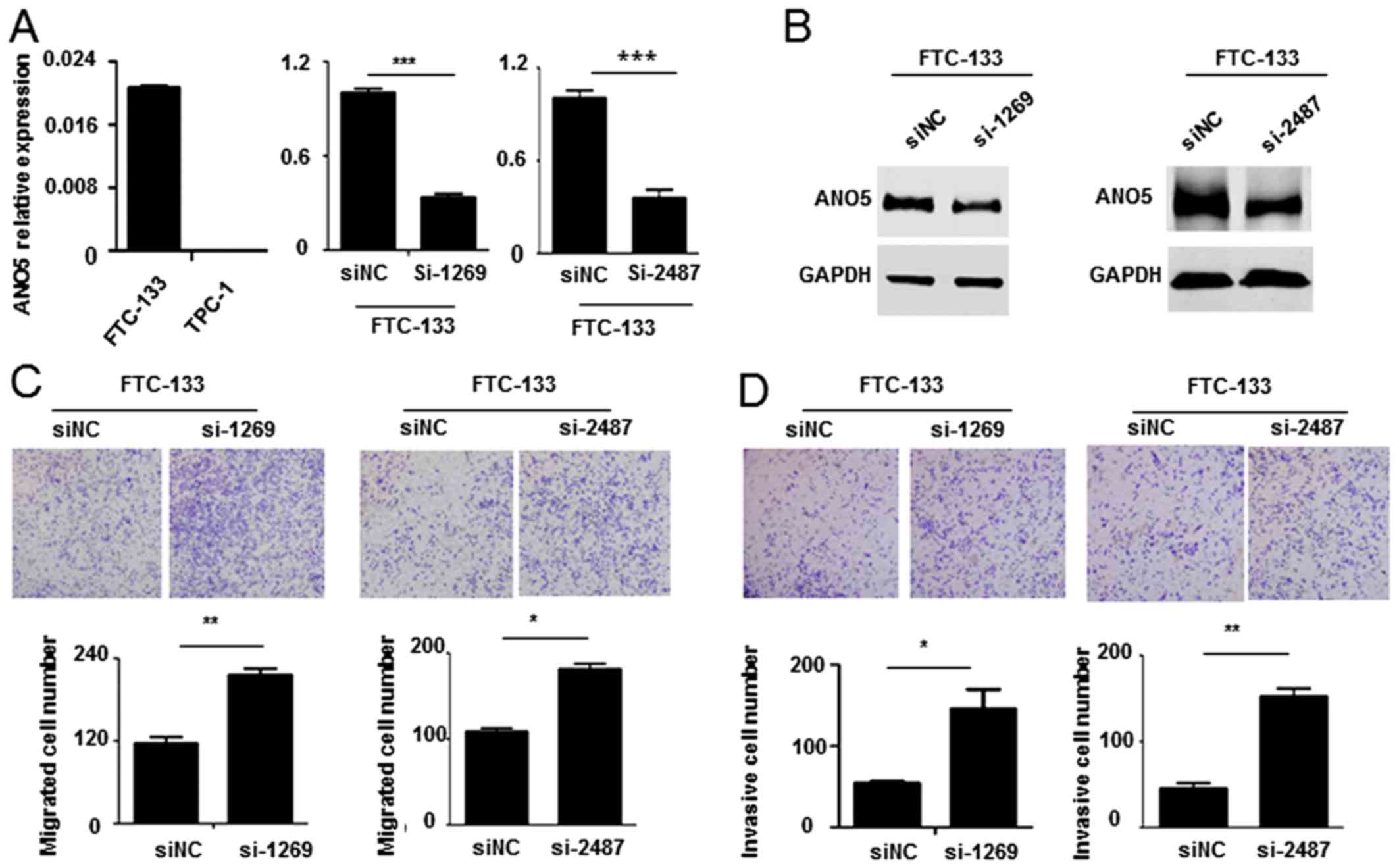
Knockdown of ANO5 promotes FTC-133 cell
migration and invasion
To understand whether ANO5 knockdown affects
thyroid cancer cells migration and invasion, we synthesized siRNA
against ANO5 (siANO5) and transfected FTC-133 cells
which have higher ANO5 expression. Real-time PCR and western
blot results indicate that siRNA significantly decreased
ANO5 expression (Fig. 3A and
B). Cell migration assay showed that knockdown of ANO5
increased the migrated cell number (Fig. 3C). Meanwhile, inhibition of
ANO5 also promoted FTC-133 cell invasion (Fig. 3D). Our results prove that knockdown
of ANO5 promotes FTC-133 cell migration and invasion.
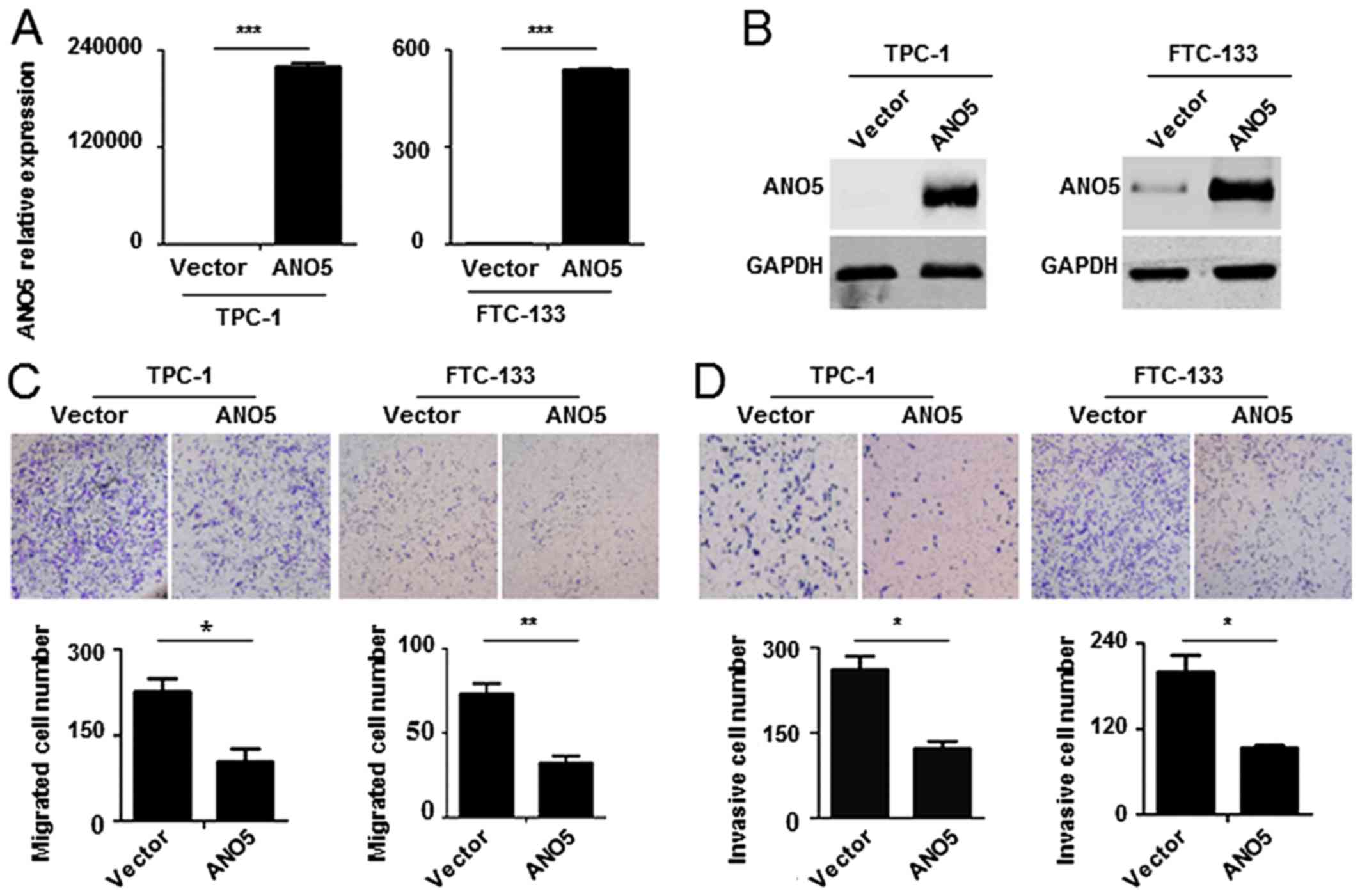
Overexpression of ANO5 inhibits FTC-133
and TPC-1 cell migration and invasion
Next we detect the effect of ANO5
overexpression on thyroid cancer cell migration and invasion.
Lentivirus expressing ANO5 was constructed and TPC-1 and
FTC-133 cells infected. Real-time PCR and western blotting proved
that ANO5 successfully expressed in TPC-1 and FTC-133 cells
(Fig. 4A and B). Cell migration
and invasion assays showed that ectopic expression of ANO5
decreased the invasive and migrated cell number of TPC-1 and
FTC-133 cells (Fig. 4C and D).
Collectively these data demonstrate that overexpression of
ANO5 inhibits FTC-133 and TPC-1 cell migration and
invasion.
ANO5 activates the JAK/STAT3 pathway in
thyroid cancer
To explore the mechanism by which ANO5
regulates thyroid cancer cells migration and invasion, we carried
out GSEA using public datasets (GSE3678). We found that lower
ANO5 expression was negatively associated with some
important signaling pathways such as TPO, KRAS,
p53 and VEGF (Fig.
5A). In addition, the results also showed that lower
ANO5 expression was positively associated with
JAK/STAT3 pathway which is well-known to be activated during
cancer metastasis (19,20) (Fig.
5B). Western blot results indicated that overexpression of
ANO5 suppressed phosphorylation of STAT3 but
silencing of ANO5 increased the phosphorylation of
STAT3 (Fig. 5C).
Collectively these data demonstrated that ANO5 can regulate
JAK/STAT3 signaling pathway in thyroid cancer.
Discussion
Most of thyroid cancers are well differentiated and
have good prognosis, but lymph node metastasis usually increase the
risk of recurrence and mortality (3–5). In
this study, for the first time, we identified that ANO5 gene
was documented to be expressed in 7 papillary thyroid carcinoma
samples. Moreover, TCGa databases showed that expression of
ANO5 in PTC with LN metastases (n=166) is lower than those
without LN metastases (n=185) (Fig.
2B). We found ANO5 is downregulated in thyroid cancer
tissues including PTC and FTC (Fig.
1E), downregulation of ANO5 promotes thyroid cancer cell
migration and invasion (Fig. 3C and
D), while overexpression of ANO5 has the opposite effect
(Fig. 4C and D). Identifying the
molecular events that regulate thyroid cancer metastasis holds
promise for developing more effective prevention for human thyroid
cancer. One of the major signaling pathways that is aberrantly
activated and is critical for thyroid tumor metastasis is the
JAK/STAT3 pathway by GSEA (Fig.
5B). The phosphorylated STAT3 protein can translocate into the
nucleus, where it activates the transcription of various genes that
regulate vital cellular functions, including cell proliferation and
metastasis (26). These data
suggested a relationship between ANO5 and JAK/STAT3
pathway activation, but it remained to be determined if the
JAK/STAT3 pathway is required for thyroid cancer metastasis.
These data indicated that ANO5 is a potential tumor
suppressor gene in thyroid cancer, and downregulation of
ANO5 participates in lymph node metastasis. Thus, the
functional effect of ANO5 in PTC metastasis and has
potential clinical value for developing gene therapy to treat PTC
and subsequent lymph node or distant metastases and improving
prognosis.
Other than ANO5, some ANO family
members such as ANO1-4 and ANO6-10 have been reported
to be related to tumors. Previous studies reported that
ANO1, another member of anoctamin family, is upregulated in
gastrointestinal stromal tumors (27) and head and neck squamous cell
carcinomas (28), inhibition of
ANO1 can suppresses tumor invasion (29–32).
We measured ANO5 expression in PTCs using a public database,
and found that in contrast to ANO1 expression, ANO5
is downregulated in cancer tissues (Fig. 1C). Additional study of ANO5
revealed that it negatively regulate lymph node metastases of PTC,
a role opposing that of ANO1 in other tumors (33,34).
It has been reported that overexpression of ANO6 also
increase cancer cell migration (12). ANO7 has been reported to
participate in the development of breast (35) and prostate (36) cancers, the monoantiboby targeting
the extracellular regions of ANO7 has a potential application for
immunotherapy (37). ANO5
itself functions as a Cl− channel, but its activation
requires higher Ca2+ concentration than other aNO
members (38). Thus, some
antagonism may exist among different aNO family members with
respect to tumors, and this warrants further study.
In conclusion, we identified that ANO5 is
downregulated in thyroid cancer and downregulation of ANO5
promotes thyroid cancer cell migration and invasion. In addition,
we found that the expression level of ANO5 was correlated
with activation of JAK/STAT3 pathway in thyroid cancer,
suggesting a potential application of ANO5 as a biomarker.
Altogether, our results demonstrate that targeting JAK/STAT3
pathway, using siRNA knockdown of ANO5, effectively promote
lymph node metastasis of thyroid cancer, therefore, could be a
potential novel therapeutic approach for treating lymph thyroid
cancer. Thus, we confirmed that ANO5 is a novel potential
biomarker of thyroid cancer and its expression correlates with
lymph node metastasis. To further uncover the effect of ANO5
on proliferation and cell cycle and the detailed molecular
mechanism of lymph node metastasis of thyroid cancer is necessary
for clinical gene therapy in the future.
Acknowledgments
We would like to thank Lei Ye (Rui Jin Hospital) for
providing us with TPC-1 and FTC-133 cells. This study was supported
by the grants from the National Natural Science Foundation of China
(grant nos. 81472501 and 81502197).
References
|
1
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilliland FD, Hunt WC, Morris DM and Key
CR: Prognostic factors for thyroid carcinoma. A population-based
study of 15,698 cases from the Surveillance, Epidemiology and End
Results (SEER) program 1973–1991. Cancer. 79:564–573. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Podnos YD, Smith D, Wagman LD and
Ellenhorn JD: The implication of lymph node metastasis on survival
in patients with well-differentiated thyroid cancer. Am Surg.
71:731–734. 2005.
|
|
4
|
Lundgren Cl, Hall P, Dickman PW and
Zedenius J: Clinically significant prognostic factors for
differentiated thyroid carcinoma: a population-based, nested
case-control study. Cancer. 106:524–531. 2006. View Article : Google Scholar
|
|
5
|
Wada N, Suganuma N, Nakayama H, Masudo K,
Rino Y, Masuda M and Imada T: Microscopic regional lymph node
status in papillary thyroid carcinoma with and without
lymphadenopathy and its relation to outcomes. Langenbecks Arch
Surg. 392:417–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Myers JN, Greenberg JS, Mo V and Roberts
D: Extracapsular spread. A significant predictor of treatment
failure in patients with squamous cell carcinoma of the tongue.
Cancer. 92:3030–3036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allen CT, Law JH, Dunn GP and Uppaluri R:
Emerging insights into head and neck cancer metastasis. Head Neck.
35:1669–1678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian Y, Schreiber R and Kunzelmann K:
Anoctamins are a family of Ca2+-activated Cl−
channels. J Cell Sci. 125:4991–4998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartzell HC, Yu K, Xiao Q, Chien LT and Qu
Z: anoctamin/TMEM16 family members are Ca2+-activated
Cl− channels. J Physiol. 587:2127–2139. 2009. View Article : Google Scholar
|
|
10
|
Galindo BE and Vacquier VD: Phylogeny of
the TMEM16 protein family: Some members are overexpressed in
cancer. Int J Mol Med. 16:919–924. 2005.PubMed/NCBI
|
|
11
|
Liu F, Cao QH, Lu DJ, Luo B, Lu XF, Luo RC
and Wang XG: TMEM16a overexpression contributes to tumor invasion
and poor prognosis of human gastric cancer through TGF-β signaling.
Oncotarget. 6:11585–11599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jacobsen KS, Zeeberg K, Sauter DR, Poulsen
KA, Hoffmann EK and Schwab A: The role of TMEM16A (ANO1) and
TMEM16F (ANO6) in cell migration. Pflugers Arch. 465:1753–1762.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bolduc V, Marlow G, Boycott KM, Saleki K,
Inoue H, Kroon J, Itakura M, Robitaille Y, Parent L, Baas F, et al:
Recessive mutations in the putative calcium-activated chloride
channel anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular
dystrophies. Am J Hum Genet. 86:213–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Penttilä S1, Palmio J, Suominen T, Raheem
O, Evilä A, Muelas Gomez N, Tasca G, Waddell LB, Clarke NF, Barboi
A, et al: Eight new mutations and the expanding phenotype
variability in muscular dystrophy caused by ANO5. Neurology.
78:897–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Magri F, Del Bo R, D'angelo MG, Sciacco M,
Gandossini S, Govoni A, Napoli L, Ciscato P, Fortunato F, Brighina
E, et al: Frequency and characterisation of anoctamin 5 mutations
in a cohort of Italian limb-girdle muscular dystrophy patients.
Neuromuscul Disord. 22:934–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wahbi K, Béhin A, Bécane HM, Leturcq F,
Cossée M, Laforêt P, Stojkovic T, Carlier P, Toussaint M, Gaxotte
V, et al: Dilated cardiomyopathy in patients with mutations in
anoctamin 5. Int J Cardiol. 168:76–79. 2013. View Article : Google Scholar
|
|
17
|
Marconi C, Brunamonti Binello P, Badiali
G, Caci E, Cusano R, Garibaldi J, Pippucci T, Merlini A, Marchetti
C, Rhoden KJ, et al: a novel missense mutation in ANO5/TMEM16E is
causative for gnathodiaphyseal dyplasia in a large Italian
pedigree. Eur J Hum Genet. 21:613–619. 2013. View Article : Google Scholar :
|
|
18
|
Lahoria R, Winder TL, Lui J, Al-Owain MA
and Milone M: Novel ANO5 homozygous microdeletion causing myalgia
and unprovoked rhabdomyolysis in an arabic man. Muscle Nerve.
50:610–613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen W, Liang W, Wu J, Kowolik CM, Buettner
R, Scuto A, Hsieh MY, Hong H, Brown CE, Forman SJ, et al: Targeting
JAK1/STAT3 signaling suppresses tumor progression and metastasis in
a peritoneal model of human ovarian cancer. Mol Cancer Ther.
13:3037–3048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelialmesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES, et al: Gene set enrichment analysis: a
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu BH, Chen H, Cai CM, Fang JZ, Wu CC,
Huang LY, Wang L and Han ZG: Epigenetic silencing of JMJD5 promotes
the proliferation of hepatocellular carcinoma cells by
downregulating the transcription of CDKN1a 686. Oncotarget.
7:6847–6863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho YA, Kong SY, Shin A, Lee J, Lee EK,
Lee YJ and Kim J: Biomarkers of thyroid function and autoimmunity
for predicting high-risk groups of thyroid cancer: a nested
case-control study. BMC Cancer. 14:873–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang H, Liu M, Yan X, Zhou Y, Wang W,
Wang X, Fu Z, Wang N, Zhang S, Wang Y, et al: miR-193a-3p functions
as a tumor suppressor in lung cancer by downregulating ERBB4. J
Biol Chem. 290:926–940. 2015. View Article : Google Scholar
|
|
25
|
Kim HS, Kim DH, Kim JY, Jeoung NH, Lee IK,
Bong JG and Jung ED: Microarray analysis of papillary thyroid
cancers in Korean. Korean J Intern Med. 25:399–407. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: a leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
West RB, Corless CL, Chen X, Rubin BP,
Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R,
et al: The novel marker, DOG1, is expressed ubiquitously in
gastrointestinal stromal tumors irrespective of KIT or PDGFRa
mutation status. Am J Pathol. 165:107–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carles A, Millon R, Cromer A, Ganguli G,
Lemaire F, Young J, Wasylyk C, Muller D, Schultz I, Rabouel Y, et
al: Head and neck squamous cell carcinoma transcriptome analysis by
comprehensive validated differential display. Oncogene.
25:1821–1831. 2006. View Article : Google Scholar
|
|
29
|
Liu W, Lu M, Liu B, Huang Y and Wang K:
Inhibition of Ca(2+)-activated Cl(−) channel aNO1/TMEM16A
expression suppresses tumor growth and invasiveness in human
prostate carcinoma. Cancer Lett. 326:41–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia L, Liu W, Guan L, Lu M and Wang K:
Inhibition of calcium-activated chloride channel ANO1/TMEM16A
suppresses tumor growth and invasion in human lung cancer. PLoS
One. 10:e01365842015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G,
Zhong L, Ma Z, Zheng J, Fang X, et al: Inhibition of TMEM16A
expression suppresses growth and invasion in human colorectal
cancer cells. PLoS One. 9:e1154432014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng L, Yang J, Chen H, Ma B, Pan K, Su C,
Xu F and Zhang J: Knockdown of TMEM16A suppressed MAPK and
inhibited cell proliferation and migration in hepatocellular
carcinoma. Onco Targets Ther. 9:325–333. 2016.PubMed/NCBI
|
|
33
|
Shiwarski DJ, Shao C, Bill A, Kim J, Xiao
D, Bertrand CA, Seethala RS, Sano D, Myers JN, Ha P, et al: To
'grow' or 'go': TMEM16a expression as a switch between tumor growth
and metastasis in SCCHN. Clin Cancer Res. 20:4673–4688. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sauter DRP, Novak I, Pedersen SF, Larsen
EH and Hoffmann EK: ANO1 (TMEM16A) in pancreatic ductal
adenocarcinoma (PDAC). Pflugers Arch. 467:1495–1508. 2015.
View Article : Google Scholar :
|
|
35
|
Li Y, Wang X, Vural S, Mishra NK, Cowan KH
and Guda C: Exome analysis reveals differentially mutated gene
signatures of stage, grade and subtype in breast cancers. PLoS One.
10:e01193832015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohsenzadegan M, Shekarabi M, Madjd Z,
Asgari M, Abolhasani M, Tajik N and Farajollahi MM: Study of NGEP
expression pattern in cancerous tissues provides novel insights
into prognostic marker in prostate cancer. Biomarkers Med.
9:391–401. 2015. View Article : Google Scholar
|
|
37
|
Das S, Hahn Y, Walker DA, Nagata S,
Willingham MC, Peehl DM, Bera TK, Lee B and Pastan I: Topology of
NGEP, a prostate-specific cell:cell junction protein widely
expressed in many cancers of different grade level. Cancer Res.
68:6306–6312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duran C, Qu Z, Osunkoya AO, Cui Y and
Hartzell HC: ANOs 3–7 in the anoctamin/Tmem16 Cl−
channel family are intracellular proteins. Am J Physiol Cell
Physiol. 302:C482–C493. 2012. View Article : Google Scholar
|