Introduction
Gastric cancer (GC) is one of the most common human
malignant diseases and the second leading cause of cancer mortality
worldwide (1). The reason for the
high fatality rate associated with this disease is that most cases
of GC are clinically detected only at an advanced stage with
distant metastasis. Metastasis is the spread of cancer cells from
their primary location to other parts of the body. Unfortunately,
once cancer becomes metastatic it cannot be treated effectively by
surgical and radiation therapies (2). Tumor metastasis is a complex process
that requires the integration of signaling events that occur in
distinct locations within the cell. However, little is known about
molecular mechanisms underlying tumor metastasis (3,4).
Therefore, a highly critical issue is to explore the molecular
mechanisms and to identify 'key' molecular markers related to
metastasis in gastric cancer, which will provide new targets for
intervention in the metastatic recurrence of gastric cancer
(5).
The recent discovery of altered adaptor proteins in
cancer has identified a fundamental change involved in cell
migration and invasion (6–8). Adaptor proteins are composed
exclusively of domains and motifs that mediate molecular
interactions, and can thereby link signaling proteins such as
activated cell-surface receptors to downstream effectors. Adaptor
proteins are emerging as important regulators of key signaling
events that control cellular behavior underlying many biological
and pathological processes (6).
Adaptor proteins accomplish this through their multiple functional
domains by bringing together and targeting protein-binding partners
to specific locations within cells. For example, APPL1 is a 709
amino acid, endosomal protein, containing a pleckstrin-homology
(PH) domain, phosphotyrosine-binding (PTB) domain and leucine
zipper motif. APPL1 interacts with 14 proteins, including
follicle-stimulating hormone receptor, deleted in colorectal
carcinoma (DCC), Rab5a and Akt2 (9). These membrane receptors and signaling
molecules take part in various signaling pathways to mediate
apoptosis, development (10), cell
proliferation, chromatin remodeling (11), DNA repair (12) and cell survival (13). Recently, emerging data suggest that
APPL1 also plays a key role in the regulation of cell migration
(14). However, the molecular
mechanism is not well understood.
APPL1 was originally identified as an Akt2-binding
protein in a yeast two-hybrid screening system and Akt2 is a known
regulator of cell migration (15).
Akt is a serine/threonine kinase that is activated downstream of
phosphatidylinositol 3-kinase. Subsequently, active Akt
phosphorylates its downstream effectors to regulate several
cellular processes, including cell growth, survival and
proliferation (16). Moreover,
there has recently been growing interest in the function of Akt in
the regulation of cell migration. Akt has been shown to stimulate
the migration of epithelial cells, fibroblasts and fibrosarcomas
(17,18).
Recent studies have indicated that APPL1 gene
amplification is common in breast (20), prostate cancer (7) and several cell lines, including
pancreatic carcinoma cells (12),
HCT116 and SW480 colorectal cancer cell lines (9). Importantly, the expression of APPL1
protein and mRNA were highly upregulated in gastric cancer. It was
also reported that the expression of APPL1 in GC was statistically
associated with depth of infiltration and lymph node metastasis
(19). The observation suggested
that expression of APPL1 had a role in tumor infiltration and
metastasis. However, little is known about the molecular mechanism
of APPL1 in tumor metastasis in gastric cancer. In the present
study, we investigated the expression of APPL1 protein in gastric
cancer and its direct effect on cell migration. We also showed that
APPL1 promoted cell migration via the Akt2 pathway using loss of
function assays. Our results suggest that APPL1 promotes invasion
and metastasis of gastric cancer cells and the underlying molecular
mechanism may facilitate Akt2 phosphorylation and activation of
downstream effectors.
Materials and methods
Retrieval of TCGA public data
Based on The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov/) public datasets, we
evaluated and analyzed APPL1 expression in gastric carcinoma.
Clinical information and gene expression profile data were
downloaded at the website of the UCSC cancer browser (http://xena.ucsc.edu/). The APPL1 mRNA expression
levels were measured experimentally using the Illumina HiSeq 2000
RNA Sequencing platform of the British Columbia Cancer Agency TCGA
genome characterization center. The results was shown as in
log2 RPKM (reads per kilobase of exon model per million
mapped reads), which approximates the relative abundance of APPL1
transcripts in different samples. The value of APPL1 expression of
the gastric carcinoma was compared with the value of adjacent
non-cancerous stomach tissues; the clinical data were then used to
analyze the association between the APPL1 expression and selected
clinical characteristics.
The expression of miR-145 in gastric carcinoma was
also downloaded from TCGA datasets, shown as in log2 RPM
(reads per million mapped reads). The correlation between miR-145
and APPL1 expression in GC tissues was analyzed by using the
Pearson's correlation coefficient.
Human gastric carcinoma tissue collection
and cell culture
Human gastric carcinoma and adjacent normal tissues
were consecutively collected between April 2013 and November 2015
at the Pathology Department of the First Affiliated Hospital of
Medical College, Xi'an Jiaotong University. No local or systemic
treatment was conducted prior to operation. Clinicopathological
data such as age and gender, as well as lymph node metastasis
status, lymphatic and venous invasion status, tumor stage, and pTNM
stage were obtained by reviewing their pathology records. Tumor
stage was determined according to the American Joint Committee on
Cancer (AJCC) staging criteria. A total of 32 paired samples were
performed to examine APPL1 expression, including 18 (56.3%) men and
14 (43.7%) women. The age distribution of the patients examined
ranged from 42 to 78 years of age. Informed consent was obtained
from each patient and the study was approved by the Institute
Research Ethics Committee at the Cancer Center of Xi'an Jiaotong
University.
Stomach adenocarcinoma cell lines (including AGS,
BGC-823, SGC-7901 and MKN-45) and a GES-1 cell line were obtained
from the Shanghai Genechem Co., Ltd. (Shanghai, China). The cells
were grown at 37°C in a 5% CO2 incubator, in RPMI-1640
culture media supplemented with fetal bovine serum (FBS).
RNA extraction and quantitative real-time
PCR
Total RNA was isolated from prepared gastric issues
and cell lines using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA), measured spectrophotometrically using NanoDrop (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). cDNA was synthesized by
using PrimeScript® RT reagent kit (DRR037A; Takara,
Dalian, China), according to the manufacturer's protocol.
Quantitative real-time PCR (qRT-PCR) was performed using the
SYBR-Green PCR kit (Takara), and was conducted in the IQ5 Optical
System real-time PCR machine. The relative expression of APPL1
genes was calculated with the 2−ΔΔCt method. The primers
used are listed in Table I.
 | Table IDNA primer and miRNA sequences. |
Table I
DNA primer and miRNA sequences.
| DNA/siRNA | Genes | Sequences |
|---|
| PCR primer | APPL1 | F:
5′-ACGGGCCCTCTAGACTCGAGCGCCACCATGCCGGGGATCGACAAGCTGCCC-3′ |
| R:
5′-AGTCACTTAAGCTTGGTACCGATGCTTCTGATTCTCTCTTCTTTCCTC-3′ |
| F:
5′-GCCCGCAGACAAGGTCTTTA-3′ |
| R:
5′-TGAGGTCAGGTGTGTTGCTG-3′ |
| β-actin | F:
5′-CCAACCGCGAGAAGATGA-3′ |
| R:
5′-CCAGAGGCGTACAGGGATAG-3′ |
| APPL1 3′-UTR | wt |
5′-CGACATAAAGATTTGAAACTGGAAC-3′ |
|
5′-TCGAGTTCCAGTTTCAAATCTTTATGTCGAGCT-3′ |
| mut |
5′-CGACATAAAGATTTGACGACGAAC-3′ |
|
5′-TCGAGTTCGTCGTCAAATCTTTATGTCGAGCT-3′ |
| siRNA | si-APPL1 | Top:
5′-GCUCAGAUAAGUUUCUUUA-3′ |
| Bottom:
5′-UAAAGAAACUUAUCUGAGC-3′ |
| NC | Top:
5′-AAUUCUCCGAACGUGUCACGU-3′ |
| Bottom:
5′-ACGUGACACGUUCGGAGAAUU-3′ |
Western blot analysis
Protein expression levels were assessed using
western blot analysis. In brief, total cell lysates from different
experiments were obtained by lysing the cells in RIPA buffer.
Following protein concentration assay, equal amounts of protein
were run on 10% SDS-PAGE gels and transferred to polyvinylidene
fluoride (PVDF) membranes. The membranes were blocked with 5%
non-fat milk for 1 h at room temperature, and incubated with the
various primary antibodies overnight at 4°C, including mouse
monoclonal antibody (mAb) anti-APPL1 (dilution 1:1,000; sc-271901;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit mAb
anti-E-cadherin (dilution 1:1,000; ab-133597; Abcam), rabbit mAb
anti-N-cadherin (dilution 1:5,000; ab76011; Abcam), rabbit mAb
anti-vimentin (dilution 1:1,000; ab-92547; Abcam), rabbit mAb
anti-Akt2 (dilution 1:1,000; #2964; Cell Signaling Technology,
Inc., Danvers, MA, USA), rabbit mAb anti-phospho-Akt2 (p-Akt2)
(dilution 1:1,000; #4060; Cell Signaling Technology), rabbit mAb
anti-MMP2 (dilution 1:1,000; ab92536; Abcam), rabbit mAb anti-MMP9
(dilution 1:1,000; ab76003; Abcam) and mouse mAb anti-β-actin
(dilution 1:5,000; 66009-1-Ig; Proteintech Group, Inc., Rosemont,
IL, USA). The PVDF membranes were then incubated with the
corresponding secondary antibodies [horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG, 1:2,000, SA00001-1;
Proteintech Group; HRP-conjugated goat anti-rabbit IgG, 1:2,000,
SA00001-2; Proteintech Group] for 1 h at room temperature and
washed three times with TBST. Finally, the membranes were incubated
with ECL (Pierce, Rockford, IL, USA) for chemiluminescence
detection. Luminescent signals were detected and recorded by
Syngene's G:BOX (Syngene, Cambridge, UK). Relative protein
expression of APPL1 was then normalized to β-actin levels in each
sample.
siRNA synthesis and transfection
Small interfering RNAs (siRNAs) were designed for
APPL1 gene silencing, and were synthesized by Shanghai GenePharma.
Scramble siRNA was used as negative control (NC siRNA). The
sequences are listed in Table I.
MNK-45 and AGS cells were cultured for 24 h in plates and the
siRNAs were transiently transfected into the cells using
jetPRIME® reagent (Polyplus-transfection) according to
the manufacturer's protocol.
Plasmid construction and
transfection
The APPL1 expression vector was constructed by
Shanghai Genechem. The full-length of APPL1 cDNA was generated by
PCR amplification (The primer sequences are listed in Table I). The PCR product was subcloned
into GV141 plasmid using the XhoI/KpnI restriction
sites. Proper construction of plasmids was verified by automated
sequencing. Transfections into BGC-823 and SGC-7901 cells were
carried out using jetPRIME® reagent, according to the
manufacturer's protocol.
Wound healing assay
A wound healing assay was performed to examine the
capacity for cell metastasis. Briefly, cells were cultured to 90%
confluence in 12-well plates. A 200-μl disposable pipette
tip was used to create a linear scratch wound. Photographs were
taken immediately after wound induction and following 48-h
incubation. The extent of wound closure was measured using ImageJ
software.
Transwell assay
Cells (2.0×104) in serum-free medium were
plated into the upper chamber (8 μm pore size; Millipore,
Billerica, MA, USA), and the bottom wells were filled with complete
medium. The cells were allowed to migrate across the membrane for
48 h. Following incubation, the cells were removed from the upper
surface of the filter by using a cotton swab. The migrant cells
that adhered to the bottom of the membrane were stained with 1%
crystal violet. Quantitative analysis of migration rates was
performed by solubilization of crystal violet and obtaining optical
density (OD) at 570 nm.
Bioinformatic analysis
Information about human miR-145 was registered and
obtained in miRBase (http://www.miRBase.org/). The prediction of miRNA
targets was acquired from TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/) public databases.
Dual-Luciferase reporter assay
The primary transcript of miR-145 was synthesized
and cloned into the pcDNA6.2GW/EmGFP vector using
EcoRI/HindIII restriction sites. The 3′-UTR of APPL1
containing miR-145 binding sites was cloned downstream of the
luciferase reporter in the pmirGLO Dual-Luciferase miRNA Target
Expression Vector (Promega, Madison, WI, USA). This
pmirGLO-APPL1-3′-UTR vector was cotransfected with
pcDNA6.2GW/EmGFP-miR-145 vector into BGC-823 cell lines. The
pmirGLO vector and a vector containing mutual binding site were
used as the control (The sequences are listed in Table I). The reporter gene assays were
performed 48 h post-transfection using the Dual-Luciferase reporter
assay system (Promega), according to the manufacturer's
instructions. The normalized firefly luciferase activity (firefly
luciferase activity/Renilla luciferase activity) for each
construct was compared with that of the pmirGLO vector control. All
experiments were performed at least three times.
Statistical analysis
Each experiment was repeated at least three times.
Data are presented as means ± SD. Unless otherwise indicated, the
statistical significance of differences between each pair of groups
was analyzed using a Student's t-test (two-tailed). All statistical
analyses were performed using GraphPad Prism version 7.0 for
Windows (Graphpad Software, Inc., San Diego, CA, USA).
Results
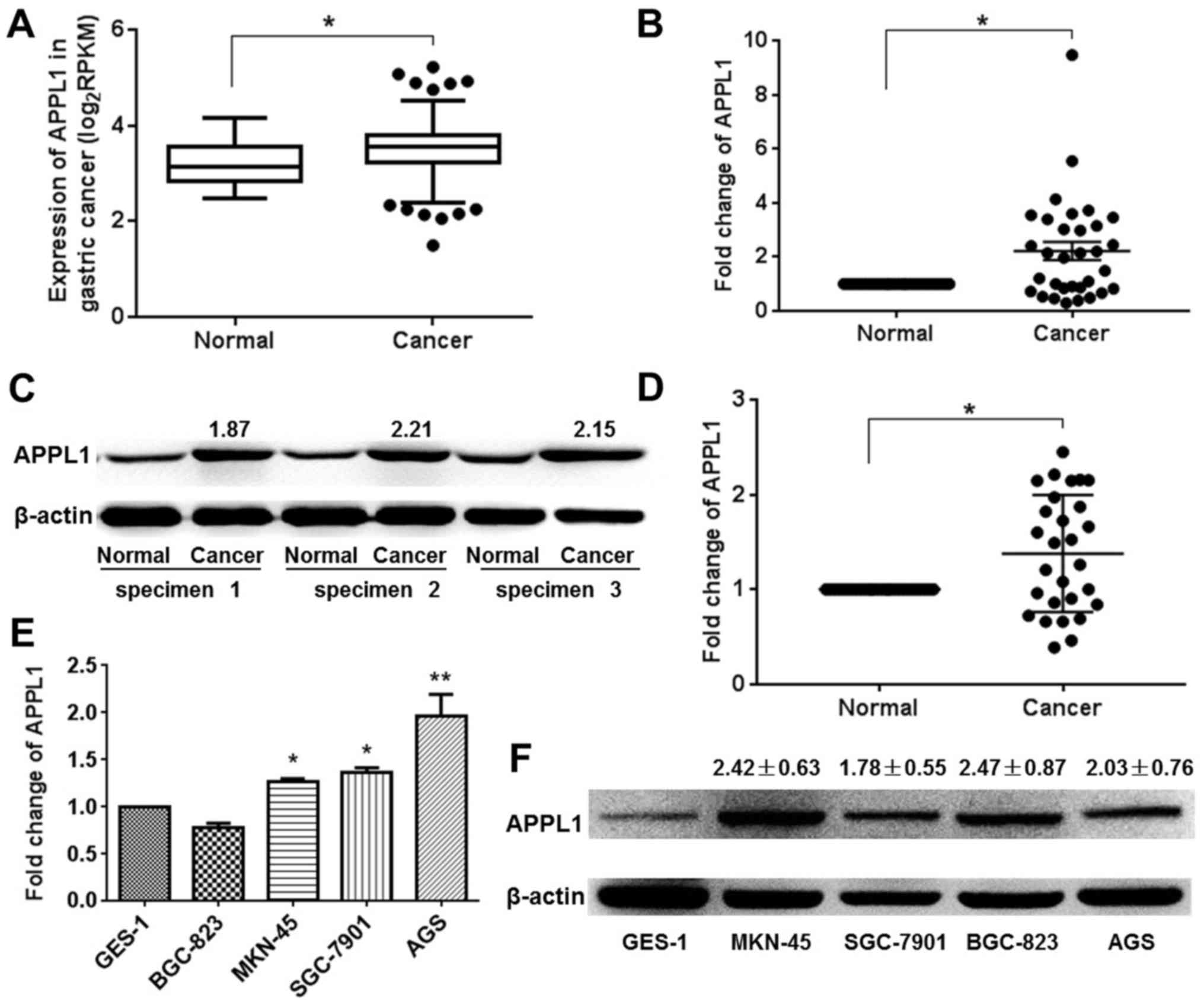
APPL1 is frequently highly expressed in
human gastric cancer tissue samples and cell lines
In order to evaluate and analyze APPL1 expression in
gastric carcinoma, an independent cohort of 417 patient specimens
(including 380 gastric carcinoma tissues and 37 adjacent normal
tissues) was downloaded from TCGA datasets. The results showed that
the mean expression value of APPL1 was increased in gastric
carcinoma tissues compared to adjacent normal tissues (3.52±0.025
vs. 3.19±0.007; P<0.05) (Fig.
1A). To validate APPL1 expression observed in the TCGA
datasets, we examined the expression of APPL1 in human gastric
cancer tissue samples collected from the Pathology Department of
our affiliated hospital using qRT-PCR and western blot analysis.
The results of qRT-PCR showed that most gastric cancer patients (21
of 32, 65.6%) showed higher APPL1 expression levels, compared with
corresponding adjacent normal tissues (Fig. 1B). The upregulation of APPL1
protein in gastric carcinoma was also detected by using western
blot analysis. Three representative western blot results were
selected and shown in Fig. 1C. The
quantitative statistical results showed there were 13 gastric
cancer patients with higher APPL1 expression levels (Fig. 1D). It was observed that the level
of APPL1 mRNA and protein were significant upregulated in four
gastric carcinoma cell lines, including MKN-45, SGC-7901, BGC-823
and AGS cell lines, compared with normal gastric mucosa epithelial
cell line GES-1 (Fig. 1E and F).
The results suggested that increased APPL1 expression was a
frequent event in human gastric cancer tissues, which was
interestingly consistent with the statistical results of APPL1
expression in TCGA datasets.
Association of APPL1 mRNA expression with
clinicopathological outcomes in GC
In order to investigate the role of increased
expression of APPL1 in gastric carcinoma, we analyzed the
association of APPL1 mRNA expression with the clinicopathological
outcomes. Based on qRT-PCR results, the association of APPL1
expression with the clinicopathological outcome of 32 patient
specimens was analyzed and listed in Table II. Since the sample size is
limited, there was no significant association between the
expression of APPL1 with clinicopathological features of patients
from our institute (P>0.05). In order to effectively enlarge the
sample size, we downloaded 190 patient specimens
clinicopathological outcomes from TCGA datasets. The results shown
here are based upon data generated by the TCGA datasets. The
selection for specimens with altered expression of APPL1 was
performed according to the methods previously reported (21). Tumors with APPL1 expression levels
greater than the 75th percentile (3.807) were defined as having
high APPL1 expression, whereas tumors that fell below the 25th
percentile (3.221) were defined as low APPL1-expressing tumors. As
shown in Table III, no
significant correlation was observed between APPL1 expression with
age, sex, pathologic stage, tumor stage, or lymph node status
(P>0.05). However, APPL1 expression was correlated with
metastasis stage (P=0.037), indicating that the high expression of
APPL1 may play an important role in gastric carcinoma
metastasis.
 | Table IIAssociation between the expression of
APPL1 with clinicopathological features in 32 patients collected by
ourselves. |
Table II
Association between the expression of
APPL1 with clinicopathological features in 32 patients collected by
ourselves.
| Variables | Patients
| Percentage
| P-value |
|---|
High
(n=21) | Low
(n=11) | High | Low |
|---|
| Sex | | | | | |
| Male | 13 | 5 | 61.90% | 45.45% | 0.3730 |
| Female | 8 | 6 | 38.10% | 54.55% | |
| Age (years) | | | | | |
| ≥60 | 19 | 8 | 90.48% | 72.73% | 0.1891 |
| <60 | 2 | 3 | 9.52% | 27.27% | |
| Pathologic
stage | | | | | |
| I+II | 14 | 6 | 66.67% | 54.55% | 0.8639 |
| III+IV | 7 | 5 | 33.33% | 45.45% | |
| Tumor stage | | | | | |
| T1/T2 | 7 | 4 | 33.33% | 36.36% | 0.6515 |
| T3/T4 | 14 | 7 | 66.67% | 63.64% | |
| Lymph node
status | | | | | |
| N0 | 6 | 4 | 28.57% | 36.36% | 0.3606 |
| N1/N2/N3 | 15 | 7 | 71.43% | 63.64% | |
| Metastasis
stage | | | | | |
| M0 | 15 | 10 | 71.43% | 90.91% | 0.2055 |
| M1 | 6 | 1 | 28.57% | 9.09% | |
 | Table IIIAssociation between the expression of
APPL1 with clinicopathological features in 190 patients based on
TCGA datasets. |
Table III
Association between the expression of
APPL1 with clinicopathological features in 190 patients based on
TCGA datasets.
| Variables | Patients
| Percentage
| P-value |
|---|
High
(n=95) | Low
(n=95) | High | Low |
|---|
| Sex | | | | | |
| Male | 58 | 62 | 61.1% | 65.3% | 0.5475 |
| Female | 37 | 33 | 38.9% | 34.7% | |
| Age (years) | | | | | |
| ≥60 | 67 | 62 | 73.6% | 66.0% | 0.2564 |
| <60 | 24 | 32 | 26.4% | 34.0% | |
| Pathologic
stage | | | | | |
| I+II | 41 | 47 | 45.6% | 50.0% | 0.5463 |
| III+IV | 49 | 47 | 54.4% | 50.0% | |
| Tumor stage | | | | | |
| T1/T2 | 21 | 31 | 22.6% | 32.6% | 0.1235 |
| T3/T4 | 72 | 64 | 77.4% | 67.4% | |
| Lymph node
status | | | | | |
| N0 | 30 | 36 | 32.6% | 37.9% | 0.4495 |
| N1/N2/N3 | 62 | 59 | 67.4% | 62.1% | |
| Metastasis
stage | | | | | |
| M0 | 89 | 80 | 93.7% | 84.2% | 0.0373 |
| M1 | 6 | 15 | 6.3% | 15.8% | |
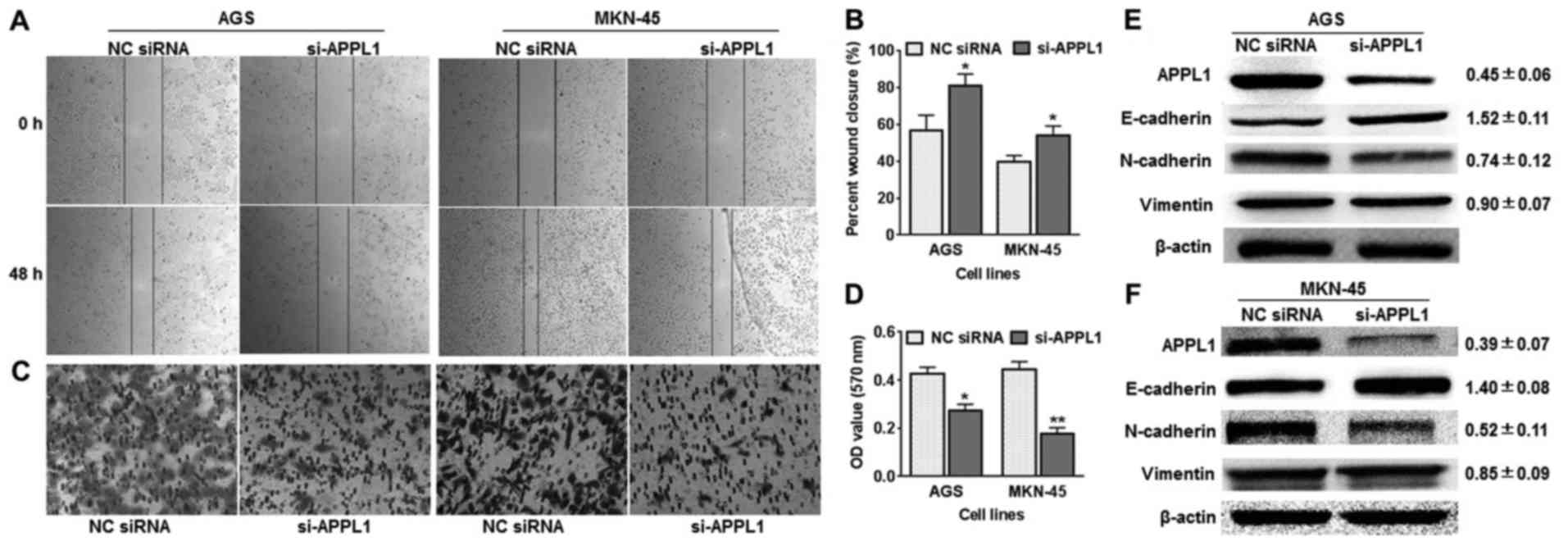
Silencing of APPL1 suppresses gastric
cancer cell migration
As shown in Table
III, the expression of the APPL1 gene was correlated with tumor
metastasis. We used a Transwell assay and a wound healing assay to
analyze the effect of APPL1 on tumor cell migration. Firstly, the
expression of the APPL1 gene was silenced using specifically
designed siRNAs in AGS and MKN-45 cells. In the wound healing
assay, AGS cells in the si-APPL1 group migrated more slowly than
the control group. Similar results were obtained in the MKN-45 cell
line (Fig. 2A and B). In the
Transwell assay, we stained the invaded cells to measure the
directional metastasis ability of the AGS and MNK-45 cells after
silencing APPL1 expression. The invasiveness of cells transfected
with si-APPL1 was dramatically decreased compared with the control
cells (Fig. 2C and D). Using
western blot analysis, the expression of mesenchymal markers was
detected in AGS and MKN-45 cells, including E-cadherin, N-cadherin
and vimentin. The results showed that APPL1 silencing dramatically
increased the expression of E-cadherin (1.52±0.11 and 1.40±0.08 in
AGS and MKN-45 cells, respectively), but attenuated the expression
of N-cadherin and vimentin (Fig. 2E
and F). These results support the hypothesis that APPL1 plays a
role in suppression of invasive cell migration and metastasis.
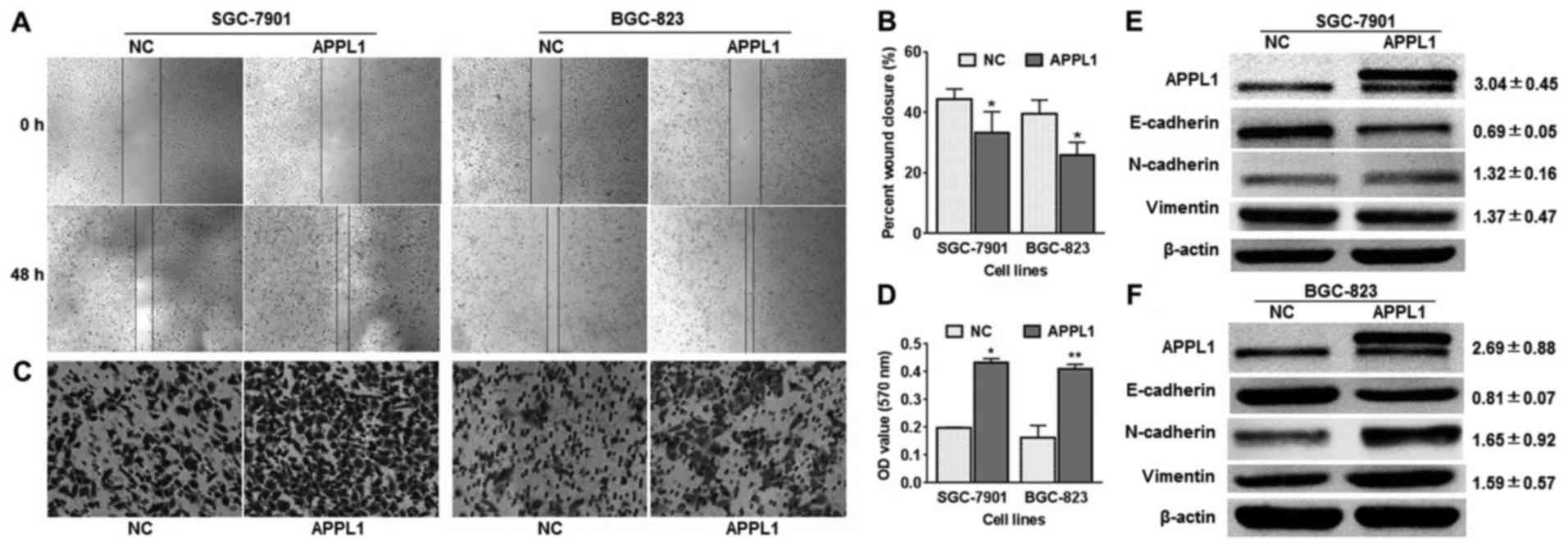
Overexpression of APPL1 facilitates
gastric cancer cell migration
Subsequently, we increased APPL1 expression in
BGC-823 and SGC-7901 cells by using a GV141 plasmid vector to
further verify the effects of APPL1 on GC cell metastasis. As the
overexpression vector carries other tags such as 3FLAG, the
generated exogenous APPL1 protein is a fusion protein with more
molecular weight than the endogenous protein. In addition, the
western blot results showed two bands appeared in overexpression
vector treated cells, which indicated that GV141 plasmid vector
effectively increased APPL1 protein expression in BGC-823 and
SGC-7901 cells. The results observed from Transwell and wound
healing assays showed that both BGC-823 and SGC-7901 cell lines
treated with APPL1 overexpression vector were distinctively more
migratory than control cells at 48 h after transfection (Fig. 3A–D). In accordance with these
observations, the results of western blot analysis showed that
overexpression of APPL1 reversed the previously observed changes in
mesenchymal markers expression (Fig.
3E and F). Among these, E-cadherin depressed 0.69±0.05-fold in
SGC-7901 cells.
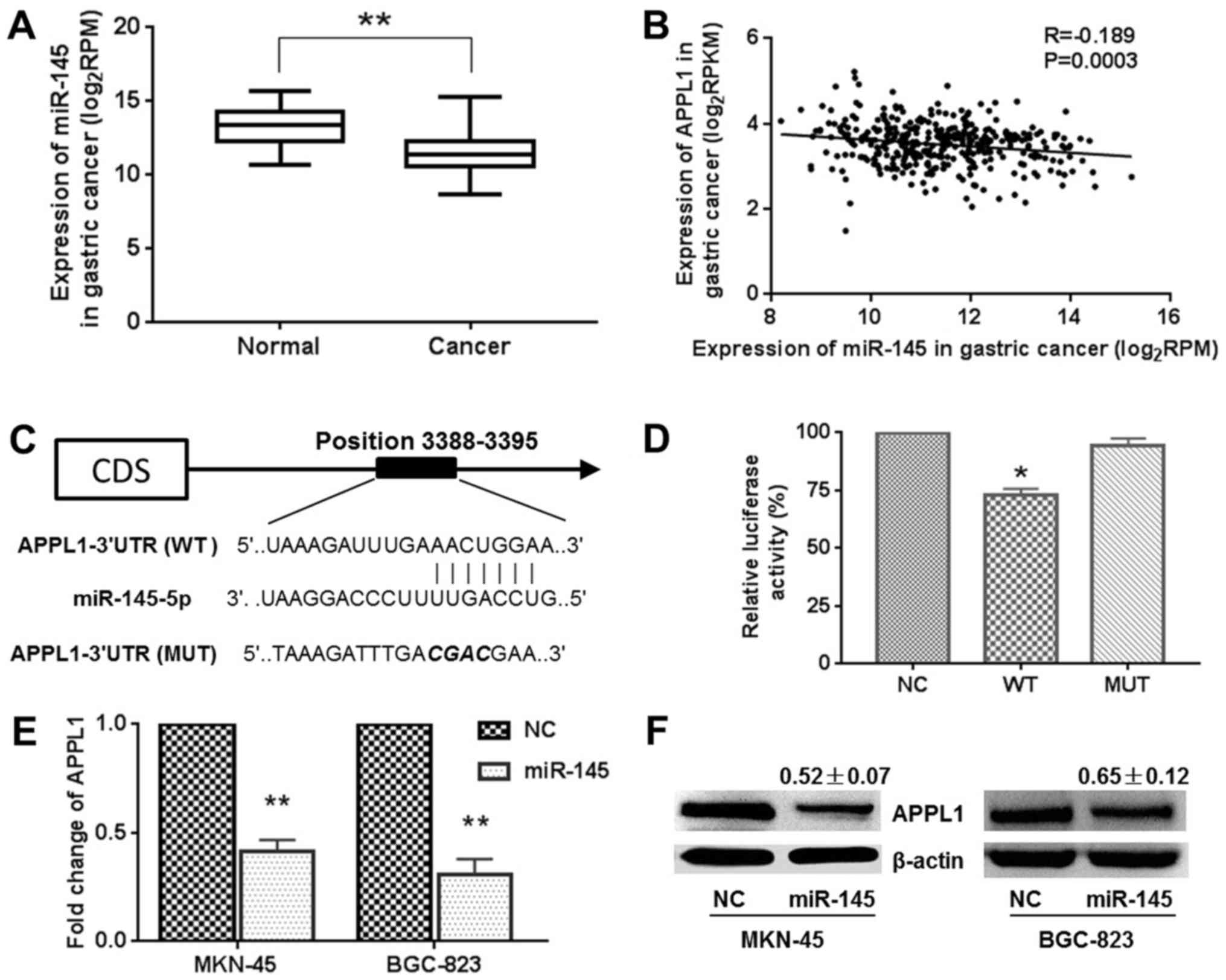
APPL1 is a target gene of miR-145
In order to uncover the mechanisms by which APPL1
mediates metastasis, we studied the upstream and downstream
regulation networks of the APPL1 gene. In our previous study, it
was reported that miR-145 expression was lower in gastric carcinoma
(22), which was consistent with
the statistical results of miR-145 expression based upon TCGA
datasets (Fig. 4A). The data
obtained from TCGA datasets also demonstrated that the expression
of miR-145 was negatively low correlation with that of APPL1 in
gastric cancer (R=−0.189) (Fig.
4B). In the TargetScan and miRanda databases, the region
complementary to the miR-145 seed region was found in the 3′-UTR of
APPL1 mRNA (Fig. 4C).
Dual-Luciferase reporter system containing wild-type (AACTG) or
mutant (CGAC) 3′-UTR of APPL1 was used to verify an interaction
between APPL1 and miR-145. As shown in Fig. 4D, miR-145/APPL1 wild-type
3′-UTR-transfected cells showed a significant reduction (~70.6%) of
luciferase activity. However, miR-145 failed to inhibit the
luciferase activity of the reporter vector containing mutant
binding sites, indicating that miR-145 may suppress gene expression
through its binding sequences at the 3′-UTR of APPL1. Furthermore,
a reduction of the APPL1 mRNA and protein expression levels was
observed in MKN-45 and BGC-823 cells transfected with
pcDNA6.2GW/EmGFP-miR-145 compared with control vector-transfected
cells (Fig. 4E and F). For
example, the APPL1 protein decreased 0.52±0.07- and 0.65±0.12-fold
in MKN-45 and BGC-823 cells, respectively. These results indicate
that miR-145 directly recognizes the 3′-UTR of APPL1 mRNA and
inhibits APPL1 translation.
APPL1 facilitate gastric cancer cell
migration via regulation of Akt2 phosphorylation
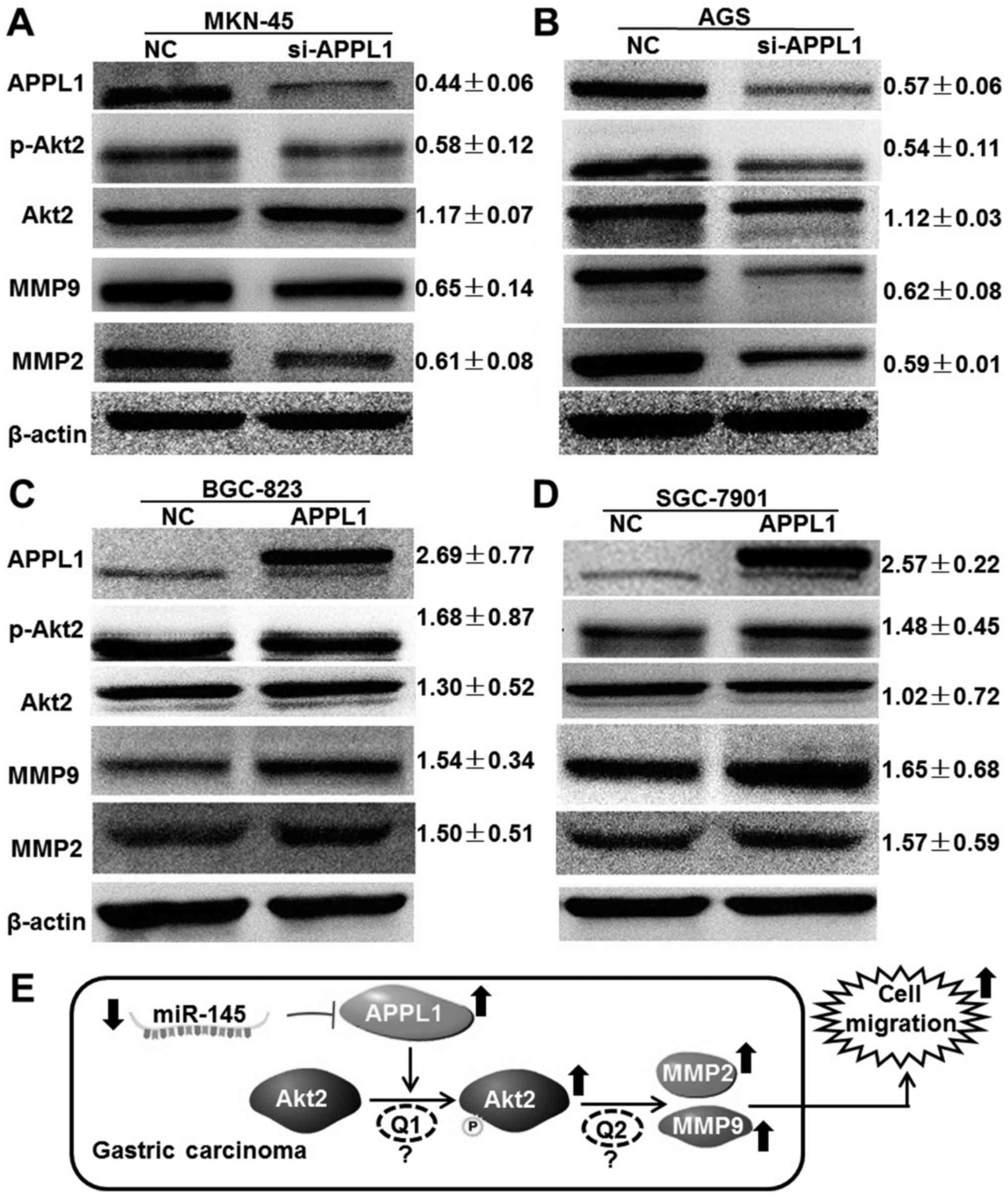
Because APPL1 was initially identified as an
Akt2-interacting protein in a yeast two-hybrid screen (15), we hypothesized that APPL1
facilitates gastric cancer cell migration via regulation of Akt2
phosphorylation. Using western blot analysis, the expression of
downstream molecules was demonstrated following changes in APPL1
expression. When APPL1 expression was silenced by siRNA in MKN-45
and AGS cells, we found that although the expression of Akt2 was
not affected, its phosphorylation level was significantly
suppressed (0.58±0.12 and 0.54±0.11 in MKN-45 and AGS cells,
respectively). The expression of downstream effectors of Akt2, MMP2
and MMP9 was also reduced (Fig. 5A and
B). Accordingly, APPL1 overexpression in BGC-823 and SGC-7901
cells not only promoted phosphorylation levels of Akt2 but also
increased the expression of MMP2 and MMP9 (Fig. 5C and D). Taken together, these
results suggest that APPL1 facilitates gastric cancer cell
migration by regulating Akt2 phosphorylation and downstream
effector expression.
Discussion
Metastasis is the predominant cause of
cancer-related mortality in gastric carcinogenesis and the
mechanism of tumor metastasis is a multifactorial process
associated with multiple genetic and epigenetic events. Previous
studies have shown that adaptor proteins promote invasion and
migration progression of some cancer types by altering early
endosome biogenesis, which could have important implications for
cancer cell biomarker release and intracellular signaling (3,7,8).
In the present study, we focused on changes in APPL1
expression in gastric cancer and the effect of APPL1 on cancer cell
metastasis. The results showed that APPL1 protein and mRNA were
upregulated both in gastric carcinoma tissue and cultured cell
lines, which were consistent with findings previously reported in
prostate (8), breast (20) and gastric cancer (19).
These results were also consistent with the
statistical analysis results of TCGA datasets (Fig. 1A). TCGA is a collaboration between
the National Cancer Institute (NCI) and the National Human Genome
Research Institute (NHGRI). The tumor and normal tissues from more
than 11,000 patients have been profiled, covering 37 types of
genetic and clinical data for 33 types of cancer (23). The TCGA research center has
published many studies identifying the mutations and dysregulations
associated with tumors in comparison to matched normal tissue
samples (24). When we focused on
stomach cancer, the gene expression profile and phenotype data of
417 stomach cancer specimens were downloaded by using UCSC's Xena.
We found that APPL1 in 380 gastric cancer tissues was significant
overexpression, compared to the average of 37 normal tissues
(3.52±0.025 vs. 3.19±0.007; P<0.05). More importantly, we
demonstrated that APPL1 expression was significantly correlated
with metastasis stage (P=0.037; Table III), indicating that APPL1 may
act as an oncogene facilitating metastasis of gastric carcinomas.
Since the size of patient specimens is limited, there was no
significant association between the expression of APPL1 with
clinicopathological features of gastric carcinoma patients
collected from our institute (P>0.05; Table II). In this way, TCGA represents a
rich resource for cancer prognostic studies. Besides identifying
differentially expressed genes, we have also shown that study of
clinicopathological features can be extended to the study of the
function of differentially expressed genes.
Metastasis is the spread of cancer cells from their
primary location to other parts of the body. Once cancer becomes
metastatic, it cannot be effectively treated by surgical and
radiation therapies (2). The
process by which malignant cells become migratory and invasive is
complex and requires overcoming barriers posed by constantly
changing microenvironments. First, cells need to escape the
surrounding matrix, adopting the phenotypes of mesenchymal cells,
known as the epithelial-mesenchymal transition (EMT) (25,26).
EMT is a process whereby tightly interacting and immotile
epithelial cells acquire the phenotype of loosely adherent and
motile mesenchymal cells, which facilitates invasion and metastasis
of tumors (25,26). Pathological EMT is associated with
E-cadherin repression, which has been shown to contribute to tumor
progression (27,28). The present study showed that APPL1
silencing dramatically increased the expression of E-cadherin, but
attenuated the expression of N-cadherin and vimentin (Fig. 2E and F). Conversely, overexpression
of APPL1 reversed the expression changes of these mesenchymal
markers (Fig. 3E and F). The
results indicated that APPL1 might promote invasion and metastasis
of gastric cancer cells by facilitating EMT.
Adaptor proteins are emerging as critical regulators
of multiple aspects of the migration process. APPL1 was initially
identified as an Akt2-interacting protein in a yeast two-hybrid
screen (15). APPL1 interacts only
with the inactive form of Akt2, anchoring it to the p110α subunit
of PI3K in the cytoplasm (15,29).
Akt2 is one of three closely related serine/threonine-protein
kinases, which mediates serine and/or threonine phosphorylation of
a range of downstream substrates. Over 100 substrates have been
reported; therefore, Akt2 could regulate many processes, including
metabolism, proliferation, cell survival, growth and metastasis
(25,30–32).
It has been established that Akt2 phosphorylation and activation is
very important for elevation of matrix metalloproteinases
(MMP2/MMP9), leading to the enhanced ability of migration and
invasion in bladder cancer (33).
Our results showed that APPL1 promotes Akt2 phosphorylation and
activation in gastric cancer cells (Fig. 5A–D), which is in agreement with the
findings of Mitsuuchi et al (15). In addition, activation of MMP2 and
MMP9 was also observed when cell migration increased, suggesting
that EMT was induced via cytoskeleton reorganization and activation
of E-Cadherin repressors (30,31,34,35).
In summary, the present study demonstrated that
expression of APPL1 protein and mRNA was upregulated in gastric
carcinoma tissues and cell lines. The expression of APPL1 in GC was
statistically associated with metastasis stage. Overexpression of
APPL1 promotes invasion and metastasis of gastric cancer cells and
the underlying molecular mechanism may facilitate EMT via Akt2
phosphorylation (Fig. 5E).
Unfortunately, the molecular mechanism is still not determined,
which was indicated by 'Q1' and 'Q2' in Fig. 5E. For example, the kinase activity
of APPL1 for Akt2 phosphorylation has not yet been confirmed by
experiment. The results of western blot analysis just indicated
that the phosphorylation level of Akt was increased. More
importantly, the Co-IP and GST pull-down assay are needed to
confirm the interaction between APPL1 and inactive Akt2, and the
recruitment of Akt2 from cytoplasm to cell membrane (15,29).
Furthermore, the activation of Akt2 results in the elevation of
MMP2/MMP9 via a unknown mechanism. It was reported that Akt2
phosphorylation and activation could play the regulatory role via
several signal pathways (TGF-β, Wnt/β-catenin, JAK2/STAT3, PI3K/Akt
and NF-κB) (2,32,36–38).
In this event, whether Akt2 directly regulates the expression of
MMP2/MMP9 through transcription factor NF-κB is worth further
study. Importantly, a previous study reported that protein levels
and phosphorylation levels of APPL1 were highly expressed in
tissues from human hepatocellular carcinoma and breast cancer
(3). In this study, we focused on
the regulation of APPL1 expression by miR-145, which is a
post-transcriptional regulation, no association with
phosphorylation modification. Thus, we expect to analyze the
phosphorylation levels of APPL1 in gastric cancer tissues and
investigate the function of activated APPL1 in gastric cancer cells
in future studies.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (no. 31400730) and the China
Postdoctoral Science Foundation (nos. 2014M562426 and
2015T81015).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheng S, Qiao M and Pardee AB: Metastasis
and AKT activation. J Cell Physiol. 218:451–454. 2009. View Article : Google Scholar
|
|
3
|
Ding Y, Cao Y, Wang B, Wang L, Zhang Y,
Zhang D, Chen X, Li M and Wang C: APPL1-mediating Leptin signaling
contributes to proliferation and migration of cancer cells. PLoS
One. 11:e01661722016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Broussard JA, Lin WH, Majumdar D, Anderson
B, Eason B, Brown CM and Webb DJ: The endosomal adaptor protein
APPL1 impairs the turnover of leading edge adhesions to regulate
cell migration. Mol Biol Cell. 23:1486–1499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu SH, Huang JZ, Xu ML, Yu G, Yin XF, Chen
D and Yan GR: ACK1 promotes gastric cancer epithelial-mesenchymal
transition and metastasis through AKT-POU2F1-ECD signalling. J
Pathol. 236:175–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deakin NO and Turner CE: Distinct roles
for paxillin and Hic-5 in regulating breast cancer cell morphology,
invasion, and metastasis. Mol Biol Cell. 22:327–341. 2011.
View Article : Google Scholar :
|
|
7
|
Johnson IR, Parkinson-Lawrence EJ, Keegan
H, Spillane CD, Barry-O'Crowley J, Watson WR, Selemidis S, Butler
LM, O'Leary JJ and Brooks DA: Endosomal gene expression: A new
indicator for prostate cancer patient prognosis? Oncotarget.
6:37919–37929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson IR, Parkinson-Lawrence EJ,
Shandala T, Weigert R, Butler LM and Brooks DA: Altered endosome
biogenesis in prostate cancer has biomarker potential. Mol Cancer
Res. 12:1851–1862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deepa SS and Dong LQ: APPL1: Role in
adiponectin signaling and beyond. Am J Physiol Endocrinol Metab.
296:E22–E36. 2009. View Article : Google Scholar :
|
|
10
|
Tan Y, You H, Wu C, Altomare DA and Testa
JR: Appl1 is dispensable for mouse development, and loss of Appl1
has growth factor-selective effects on Akt signaling in murine
embryonic fibroblasts. J Biol Chem. 285:6377–6389. 2010. View Article : Google Scholar :
|
|
11
|
Miaczynska M, Christoforidis S, Giner A,
Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG
and Zerial M: APPL proteins link Rab5 to nuclear signal
transduction via an endosomal compartment. Cell. 116:445–456. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hennig J, McShane MP, Cordes N and Eke I:
APPL proteins modulate DNA repair and radiation survival of
pancreatic carcinoma cells by regulating ATM. Cell Death Dis.
5:e11992014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schenck A, Goto-Silva L, Collinet C, Rhinn
M, Giner A, Habermann B, Brand M and Zerial M: The endosomal
protein Appl1 mediates Akt substrate specificity and cell survival
in vertebrate development. Cell. 133:486–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dadson K, Chasiotis H, Wannaiampikul S,
Tungtrongchitr R, Xu A and Sweeney G: Adiponectin mediated
APPL1-AMPK signaling induces cell migration, MMP activation, and
collagen remodeling in cardiac fibroblasts. J Cell Biochem.
115:785–793. 2014. View Article : Google Scholar
|
|
15
|
Mitsuuchi Y, Johnson SW, Sonoda G, Tanno
S, Golemis EA and Testa JR: Identification of a chromosome
3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts
with the oncoprotein-serine/threonine kinase AKT2. Oncogene.
18:4891–4898. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou GL, Tucker DF, Bae SS, Bhatheja K,
Birnbaum MJ and Field J: Opposing roles for Akt1 and Akt2 in
Rac/Pak signaling and cell migration. J Biol Chem. 281:36443–36453.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai JS, Song JG, Zhu CH, Wu K, Yao Y and
Li N: Expression of APPL1 is correlated with clinicopathologic
characteristics and poor prognosis in patients with gastric cancer.
Curr Oncol. 23:e95–e101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mauro L, Pellegrino M, De Amicis F,
Ricchio E, Giordano F, Rizza P, Catalano S, Bonofiglio D, Sisci D,
Panno ML, et al: Evidences that estrogen receptor α interferes with
adiponectin effects on breast cancer cell growth. Cell Cycle.
13:553–564. 2014. View
Article : Google Scholar
|
|
21
|
McNiel EA and Tsichlis PN: Analyses of
publicly available genomics resources define FGF-2-expressing
bladder carcinomas as EMT-prone, proliferative tumors with low
mutation rates and high expression of CTLA-4, PD-1 and PD-L1.
Signal Transduct Target Ther. 2:160452017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang S, Gao L, Yang Y, Tong D, Guo B, Liu
L, Li Z, Song T and Huang C: miR-145 mediates the antiproliferative
and gene regulatory effects of vitamin D3 by directly targeting
E2F3 in gastric cancer cells. Oncotarget. 6:7675–7685. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bass AJ, Thorsson V, Shmulevich I,
Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C,
Shen H, et al Cancer Genome Atlas Research Network: Comprehensive
molecular characterization of gastric adenocarcinoma. Nature.
513:202–209. 2014. View Article : Google Scholar :
|
|
24
|
Wong N, Khwaja SS, Baker CM, Gay HA,
Thorstad WL, Daly MD, Lewis JS Jr and Wang X: Prognostic microRNA
signatures derived from The Cancer Genome Atlas for head and neck
squamous cell carcinomas. Cancer Med. 5:1619–1628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adhes Migr. 9:317–324. 2015. View Article : Google Scholar
|
|
26
|
Irie HY, Pearline RV, Grueneberg D, Hsia
M, Ravichandran P, Kothari N, Natesan S and Brugge JS: Distinct
roles of Akt1 and Akt2 in regulating cell migration and
epithelial-mesenchymal transition. J Cell Biol. 171:1023–1034.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shin TH, Sung ES, Kim YJ, Kim KS, Kim SH,
Kim SK, Lee YD and Kim YS: Enhancement of the tumor penetration of
monoclonal antibody by fusion of a neuropilin-targeting peptide
improves the antitumor efficacy. Mol Cancer Ther. 13:651–661. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito T, Jones CC, Huang S, Czech MP and
Pilch PF: The interaction of Akt with APPL1 is required for
insulin-stimulated Glut4 translocation. J Biol Chem.
282:32280–32287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu J, Li X, Yang H, Chang R, Kong C and
Yang L: SIN1 promotes invasion and metastasis of hepatocellular
carcinoma by facilitating epithelial-mesenchymal transition.
Cancer. 119:2247–2257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang
W, Ren YB, Su B, Cao GW, Yang Y, et al: p28GANK overexpression
accelerates hepatocellular carcinoma invasiveness and metastasis
via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1α
pathways. Hepatology. 53:181–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Chen LH, Yuan YW, Li QS, Sun AM and
Guan J: Activation of AKT is associated with metastasis of
nasopharyngeal carcinoma. Tumour Biol. 33:241–245. 2012. View Article : Google Scholar
|
|
33
|
Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan
J, Wang X and Li L: CXCL5/CXCR2 axis promotes bladder cancer cell
migration and invasion by activating PI3K/AKT-induced upregulation
of MMP2/MMP9. Int J Oncol. 47:690–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji BC, Hsiao YP, Tsai CH, Chang SJ, Hsu
SC, Liu HC, Huang YP, Lien JC and Chung JG: Cantharidin impairs
cell migration and invasion of A375.S2 human melanoma cells by
suppressing MMP-2 and -9 through PI3K/NF-κB signaling pathways.
Anticancer Res. 35:729–738. 2015.PubMed/NCBI
|
|
35
|
Ahn JH, Choi YS and Choi JH: Leptin
promotes human endometriotic cell migration and invasion by
up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol
Hum Reprod. 21:792–802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sasaki T and Kuniyasu H: Significance of
AKT in gastric cancer (Review). Int J Oncol. 45:2187–2192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv Q, Hu JX, Li YJ, Xie N, Song DD, Zhao
W, Yan YF, Li BS, Wang PY and Xie SY: MiR-320a effectively
suppresses lung adenocarcinoma cell proliferation and metastasis by
regulating STAT3 signals. Cancer Biol Ther. 18:142–151. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hupalowska A, Pyrzynska B and Miaczynska
M: APPL1 regulates basal NF-κB activity by stabilizing NIK. J Cell
Sci. 125:4090–4102. 2012. View Article : Google Scholar : PubMed/NCBI
|