Introduction
Lung cancer (LC) is the leading cause of
cancer-related deaths worldwide (1), with 1.6 million new cases and 1.38
million deaths annually (2,3).
Lung cancer is a heterogeneous disease and a leading cause of
cancer-related death in most developed countries. On the basis of
differences in histology, LC is roughly divided into small-and
non-small cell LC (SCLC and NSCLC, respectively). The latter, which
accounts for approximately 85% of all LC malignancies and the
overall 5-year survival of patients with NSCLC, remains
approximately 15–20% (4,5).
MicroRNAs (miRNAs or miRs) are evolutionarily
conserved non-coding single-stranded RNA molecules of approximately
18–25 nucleotides. They negatively regulate gene expression at the
post-transcriptional level by either degrading the target mRNA or
inhibiting translation of the mRNA into functional protein
(6,7). miRNAs have indeed been shown to
regulate multiple hallmarks of cancer, for example, increased
proliferation and evasion of cell death (8,9).
Recently, altered expression of miRNAs have been found to be a
common feature of several cancers. An increasing number of reports
have implicated a role for miRNAs in lung cancer progression
(10,11). miRNAs are potential targets for
treating NSCLC carcinomas (12),
and research has focused on the diagnostic and prognostic potential
of different miRNAs in NSCLC. It is believed that miRNA expression
is important in NSCLC development (13,14).
Functional elucidation of miR-768-3p shed light on the specific
roles in carcinogenic events of various cancers. However, the
possible roles of miR-768-3p in pathogenic process and development
involved in human NSCLC have not been illuminated previously.
The aim of the present study was to investigate the
expression of miR-768-3p in human NSCLC and the possible effects of
miR-768-3p alteration on the biological behavior of human NSCLC
cells. Firstly, 83 patients attending the clinic of Kunming
Hospital (pathologically diagnosed as NSCLC), between June 2010 and
December 2011, were invited to participate in the study. Their
surgical tumor samples were obtained for qRT-PCR analysis. Human
NSCLC cell line, A549 and HCC4006, were employed and transfected
with either miR-768-3p mimics or miR-768-3p-antagomir. Transfection
efficiency was evaluated by qRT-PCR. The cell proliferation and
apoptosis fractions were also evaluated by MTT assay and flow
cytometry (FCM). The scratch wound assay and Transwell assays were
also employed to explore the effects of miR-768-3p on the in
vitro migration and invasion of NSCLC cells, respectively. We
also used nude mice bearing NSCLC xenograft to evaluate the
possible effects of miR-768-3p on NSCLC growth and proliferation
in vivo.
Materials and methods
Ethics approval and consent to
participate
The study related to humans was approved by The
Regional Committee for Research Ethics. The study complied with all
the relevant national regulations, institutional policies in
accordance with the tenets of the Helsinki Declaration.
Subject
The anonymized patients (n=83) diagnosed with NSCLC
within the period from June 2010 to December 2011 at the Hospital
(No. 2 People's Hospital of Kunming, Kunming, China), were employed
in the present study. All NSCLC patients had primary lung cancer
without any co-existing illness nor treatment, including drugs,
chemotherapy and radio-chemotherapy. All participating patients
voluntarily signed the informed consent. All cases were reviewed by
three independent pathologists in our hospital and the clinical
stages of NSCLC referenced to the criteria recently established by
the National Comprehensive Cancer Network (NCCN). The TNM stages of
tumors were determined according to the standard TNM classification
system of the International Union Against Cancer (7th edition)
(http://www.uicc.org/).
Sample preparation
For the present study, lung cancer and their
adjacent normal tissues were collected after video-assisted
fiberoptic bronchoscopy. The tissues were immediately stored at
−80°C until nucleic acids isolation.
Cell culture and treatment
The bronchoepithelial cell line BEAS-2B (human
bronchial epithelium), A549 and HCC4006 human NSCLC cell line was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM) with low glucose (GE Healthcare Life
Sciences, Vienna, Austria), supplemented with 10% fetal calf serum
(FCS; Sigma-Aldrich, Munich, Germany) and 100 U/ml penicillin, 100
μg/ml streptomycin and 2 mM Lglutamine (PAA Laboratories; GE
Healthcare Life Sciences). The cells were cultivated in cell
culture flasks (Falcon®; Becton-Dickinson Austria GmbH,
Schwechat, Austria) at 37°C in an atmosphere of 5% CO2
until the desired cell confluence for further experiments was
reached (15).
Upregulation or downregulation of miR-768-3p
expression in cell lines was achieved by transduction with
miR-768-3p-mimic (at a final concentration of 20 pmol/μl) or
miR-768-3p-antagomir (at a final concentration of 25
pmol/μl) by using Lipofectamine™ 2000 system (Invitrogen),
respectively. The miR-768-3p-mimic, miR-768-3p-antagomir and their
matched miR-NC were obtained from Guangzhou RiboBio Co., Ltd.
(miR-Rib™ miRNA; Guangzhou, China). These cell lines treated with
different reagents seeded in 6-well plates were harvested for
isolated RNAs 48 h after transfection.
Quantitative RT-PCR
Total RNA from serum and tissue samples was prepared
using a TRIzol and miRNeasy Mini kit (Qiagen) according to the
manufacturer's instruction.
Reverse transcription and real-time PCR
(RT-PCR) quantification of miRNA
cDNA was synthesized from total RNA using
gene-specific primers according to the TaqMan MicroRNA assay as per
the protocol of the manufacturer (Applied Biosystems, Foster City,
CA, USA). Quantitative PCR of miRNA was performed using an Applied
Biosystems 7300 Sequence Detection system. The 10 μl PCR
reaction contained 0.67 μl reverse transcription product, 1X
TaqMan Universal PCR Master Mix, and 1 μl of the primer and
probe mix, according to the TaqMan MicroRNA assay protocol (Applied
Biosystems) (16). The relative
expression levels of genes were determined using the
2−ΔΔCt analysis method. The snU6 served as control.
MTT assay
Cell viability was determined using the tetrazolium
salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay. Briefly, cells were plated into 96-well culture plates
at an optimal density of 5×103 cells/ml in 200 μl
of culture medium per well. After 24–96 h of culture, 20 μl
of 5 mg/ml MTT was added to each well and incubated at 37°C for 4
h. The medium was then gently aspirated and 150 μl of
dimethyl sulfoxide (DMSO) was added to each well to solubilize the
formazan crystals. The optical density of each sample was
immediately measured using a microplate reader (Bio-Rad
Laboratories, Hercules, CA, USA) at 490 nm.
Apoptosis assay
A propidium iodide (PI) and Annexin V-FITC-flow
cytometry assay (BD Biosciences) was used to detect the apoptosis
rate in the cells after various treatment transfection. Briefly,
cells were harvested in complete RPMI-1640 medium and centrifuged
at 1,000 rpm for 5 min. Each of the cell lines was washed with 1X
phosphate-buffered saline (PBS) and stained with 50 μg/ml PI
and Annexin V-FITC, following the manufacturer's instructions.
Western blot analysis
The protein expression of apoptosis-related proteins
in NSCLC cells was determined by using western blot analysis
according to the protocol described before. Briefly, cell samples
were lysed on ice for 30 min in CytoBuster Protein Extraction
Buffer (Novagen, Madison, WI, USA) and 50 μg of protein was
used for 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE). The protein was then transferred to a
nitrocellulose (NC) membrane and was sealed with Tris-buffered
saline Tween-20 (TBST) containing 5% non-fat milk powder. The
membrane was subsequently incubated with goat anti-human Fas
(1:1,000; Cell Signaling Technology, Danvers, MA, USA), FasL
(1:800; Santa Cruz Biotechnology, Santa Cruz, CA, USA) proteins,
and rabbit anti-human GAPDH (1:1,000; R&D Systems, Minneapolis,
MN, USA) at 4°C overnight, respectively. After washing in TBST, the
membrane was incubated with HPR conjugated secondary antibodies
(1:1,000) at 25°C, and the protein quantity was determined using
electrochemiluminescence (ECL) technique (BestBio, Oakland, CA,
USA). The results were photographed using the JS Gel Imaging System
(Shanghai Peiqing Science and Technology Co., Ltd., Shanghai,
China) and the gray density was calculated using SensiAnsys
software (Shanghai Peiqing Science and Technology).
Migration assays
Scratch wound assay employed to detect the migration
of the NSCLC cell lines before and after various reagent
treatments, as described before (17). Briefly, the A549 and HCC4006 cells
transfected with miR-768-3p-mimics, miR-768-3p-antagomir and
control miRNA-NC, at ~80% confluency were seeded onto 6-well plates
and incubated at 37°C for 24 h. Then, a vertical scratch wound was
made through the center of each well using a 10-μl pipette
tip. The cells were then washed three times with PBS to remove the
scratched cells, and fresh serum-free medium was transferred. After
12 h, the cells were examined by light microscopy at a
magnification of ×200 to determine the resealing of the cell
monolayer.
Cell invasion assays
We employed BioCoat Matrigel invasion chambers (BD
Biosciences, Bedford, MA, USA) to compare the effect of miR-768-3p
overexpression or knockdown on in vitro invasion of NSCLC
cells as previously described (16,18).
Briefly, for the invasion assay, Costar Transwell 8 μm
inserts were coated with 50 μg reduced serum Matrigel (BD
Biosciences). Invasion chambers were coated with Matrigel and
1×106 cells were added per chamber. Medium supplemented
with 10% fetal bovine setum (FBS) was used in the lower chamber.
Following incubation of cells that had invaded through the membrane
that were fixed and stained with crystal violet before the membrane
was removed and mounted on a slide for microscopic assessment.
Invasive cells were visualized at ×40 magnification and the number
of cells in five random fields was counted and an average
calculated.
Matrix metalloprotein-2/9 (MMP-2/9)
activity assay
The activity of migration associated matrix
metalloproteines, MMP-2 and MMP-9, were determined by QuickZyme
MMPs activity assay (QucikZyme BioSciences, Leiden, The
Netherlands) according to the manufacturer's protocols. Briefly,
after transfection, cells were washed with fresh medium and
replaced with serum-free medium. After additional 24 h, the medium
was collected and centrifuged at 10,000 × g for 10 min. Respective
supernatant was added to the 96-well strip coated with MMP-2
antibody or MMP-9 antibody and incubated at 4°C overnight. After
washing with wash buffer 3 times, 50 μl assay buffer was
added into the well, followed by adding 50 μl detection
reagent. After incubation at 37°C for 1 h, OD405 was measured with
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA)
(19).
NSCLC xenograft nude mouse model
To test the oncogenic phenotypes of miR-768-3p in
NSCLC, we established NSCLC xenograft nude mouse model. Forty-five
nude mice (BALB/c strain, 4–5 weeks old, 18–20 g) were purchased
from Beijing HFK Bioscience Co., Ltd. (Beijing, China). Mice were
housed and raised in the laboratory animal center of the Affiliated
Cancer Hospital of Kunming Medical University. Animal use and
treatment was approved by the Animal Ethics Committee of Kunming
Medical University. Mice were randomly assigned to each of the
following 9 groups (n=5): normal BEAS-2B-treated group; miR-768-3p
overexpression human NSCLC cell
(A549/HCC4006-miR-768-3p-mimics)-treated groups; miR-768-3p
functional deficient NSCLC cell
(A549/HCC4006-miR-768-3p-antagomir)-treated groups; matched control
groups (A549/HCC4006-mimics-NC and A549/HCC4006-antagomir-NC). As
described before, cells were harvested, digested and injected
intradermally into the left axilla of the nude mice (20,21).
After seeding, liquid absorption at the injection site, tumor
growth (volume and weight), and mouse survival were measured. Tumor
volume was measured on days 3, 5, 9, 13, 17, 21, 25 and 29
post-injection. The largest (a) and smallest diameters (b) of each
tumor were measured twice on days 3, 5, 9, 13, 17, 21, 25 and 29 to
estimate tumor volume (V) using the formula: V = 0.52 ×
a2 × b (20,21). On day 29, all mice were sacrificed
for tumor isolation. Then the tumor weight and volume were
evaluated. Mean tumor volumes were used to plot tumor growth curves
for each group of mice.
Statistical analysis
Data are presented as means ± standard deviation
(SD). The unpaired t-test was used for comparison between groups.
Multivariate logistic regression analysis was performed to evaluate
the association between miR-768-3p expressional level (low or
high), along with clinicopathological characteristics of NSCLC
including sex, age, smoking status, pathological type, cell grading
(well, moderate or poor differentiation), TNM stage, as well as
tumor size. The strength of association was measured using ORs with
95% CI. Logistic regression was used for ordinal data to estimate
adjusted ORs. The cofactors included in regression analysis were
sex, age, smoking status, along with various clinicopathological
parameters, including pathological type, cell grading, TNM stage,
as well as tumor size. Chi-square test and Fisher's exact test were
used to assess the survival curves in NSCLC patients with different
miR-768-3p expressional levels. The statistical significance of MTT
cell activity, apoptosis factions, invasion and migration, as well
as the associated protein levels and MMP2/9 activity among
miR-768-3p mimics, antagomir and the matched NC control groups was
determined using one-way ANOVA. Significance level was
predetermined to be P≤0.05 unless otherwise indicated. All analyses
were conducted with SPSS version 19.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
Abnormal expression of miR-768-3p
associated with NSCLC
The miR-768-3p RNA levels were detected in the tumor
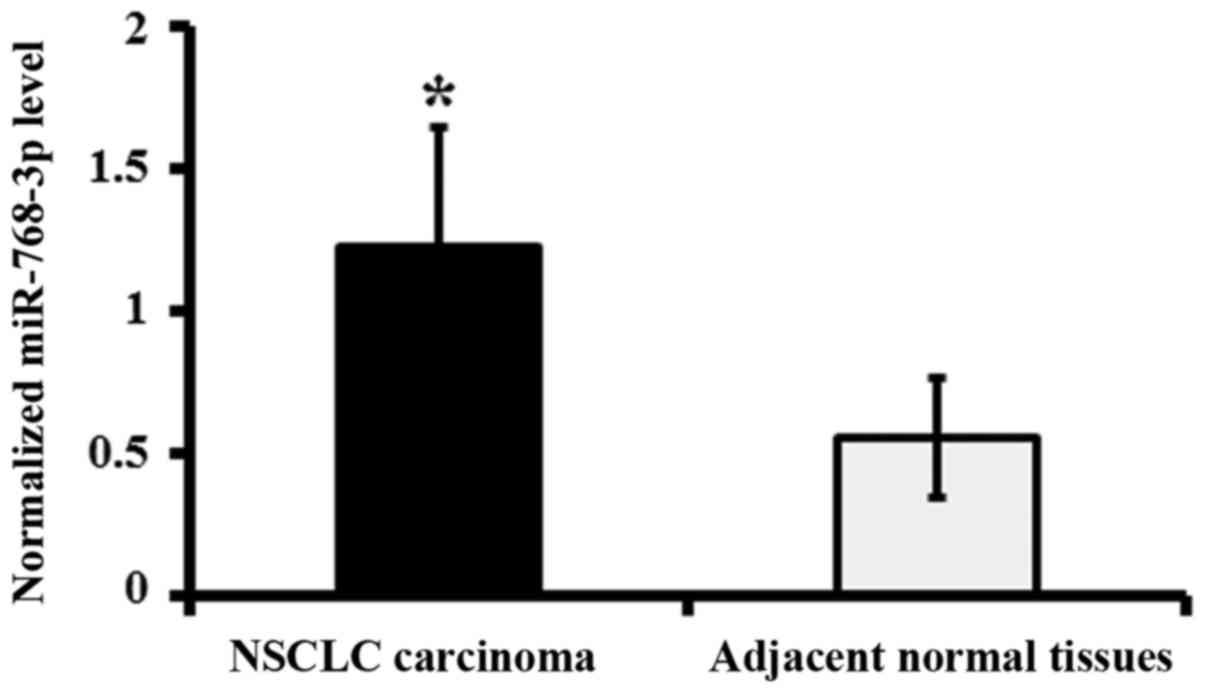
tissues of NSCLC patients by qRT-PCR analysis. The data showed that
the expression levels of miR-768-3p in NSCLC carcinoma were
significantly increased compared to that of adjacent normal tissues
(P<0.05; Fig. 1).
According to the miR-768-3p expression level, we
divided all the enrolled patients into two groups: Those with less
than or equal to median of miR-768-3p expression levels (low level)
and those with more than median of miR-768-3p expression levels
(high level). The median was used as cut-off (22).
The association between miR-768-3p status and
various clinicopathological characteristics results determined by
ORs revealed a positive trend for miR-768-3p level and sex (male,
P=0.0009), age (50–65, P=0.00012), smoking status (ever, P=0.0004),
pathological type (adenocarcinomas), cell grading (moderate,
P=0.00051; poor, P=0.002), TNM stage (I, P=0.001; II, P=0.001), as
well as tumor size (>3, P=0.0002; Table I).
 | Table IAnalysis of miR-768-3p expression in
tumors and clinical characteristics of NSCLC patients. |
Table I
Analysis of miR-768-3p expression in
tumors and clinical characteristics of NSCLC patients.
| miR-768-3p
expression (n=83)
|
|---|
| Low level n,
(%) | High level n,
(%) | Adjusted OR (95%
CI) |
|---|
| Sex | 18 | 65 | |
| Male | 8 (9.6) | 45 (54.2) | 36.89
(20.35–46.85) |
| Female | 10 (12) | 20 (24.2) | 3.18
(1.23–5.62) |
| Age (years) | | | |
| <50 | 8 (9.6) | 7 (8.4) | 1.68
(0.68–2.98) |
| 50–65 | 5 (6) | 54 (65.2) | 46.98
(29.66–58.47) |
| >65 | 5 (6) | 4 (4.8) | 1.36
(0.54–3.08) |
| Smoking status | | | |
| Never | 10 (12) | 18 (21.8) | 9.15
(3.64–15.22) |
| Ever | 8 (9.6) | 47 (56.6) | 39.88
(29.87–46.89) |
| Pathological
type | | | |
| Adeno- | 3 (3.6) | 41 (49.5) | 30.98
(21.36–43.68) |
| Squamous | 7 (8.4) | 16 (19.3) | 13.02
(9.65–23.87) |
| Large | 8 (9.6) | 8 (9.6) | 2.36
(1.11–3.82) |
|
Differentiation | | | |
| Poor | 5 (6) | 17 (20.4) | 21.88
(15.24–31.49) |
| Moderate | 6 (7.2) | 39 (47) | 39.72
(26.81–48.32) |
| Well | 7 (8.7) | 9 (10.7) | 1.58
(0.54–2.49) |
| TNM stage | | | |
| I | 6 (7.2) | 25 (30.1) | 24.33
(18.62–36.27) |
| II | 7 (8.7) | 26 (31.3) | 25.89
(17.12–32.66) |
| III | 5 (6) | 14 (16.7) | 10.33
(6.87–15.94) |
| Tumor size
(cm) | | | |
| ≤3 | 7 (8.4) | 25 (38.5) | 20.13
(12.53–32.83) |
| >3 | 8 (9.6) | 40 (61.5) | 44.62
(31.35–57.24) |
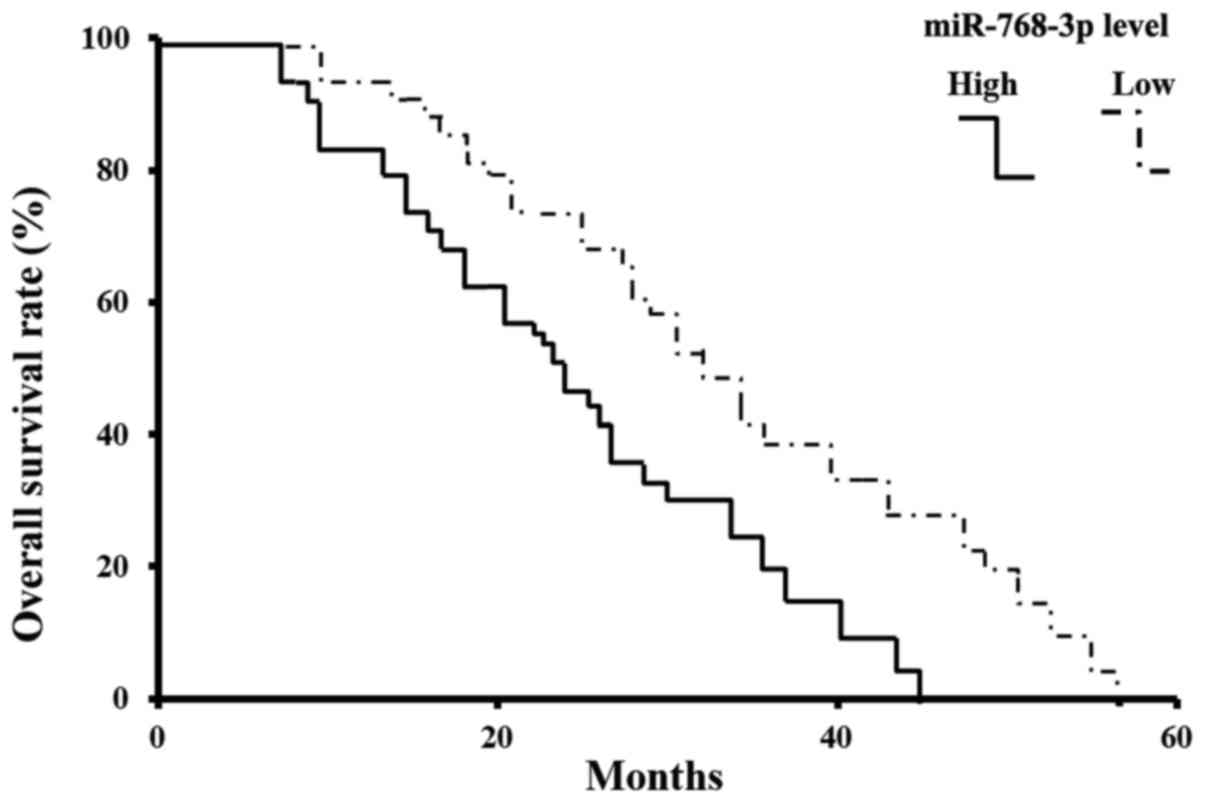
miR-768-3p is associated with survival
rate of NSCLC
The 60 months (5-year) survival rate of TNM stage 1
is >94%, while it is <10% in patients with TNM stage II-III.
Within a period of 60 months of the follow-up, 13 NSCLC related
deaths occurred. All of the deaths come from patients with
miR-768-3p positive tumors. Kaplan-Meier estimated the overall
survival rate based on tissue miR-768-3p expression in the patients
with a follow-up period of 5 years (Fig. 2). In the entire cohort, the overall
survival rate of patients with miR-768-3p low expressed tumors were
significantly higher than that of those with miR-768-3p positive
tumors (83.83 vs. 17.24%; log-rank test: χ2=22.96,
P=0.000002, P<0.05) (Fig.
2).
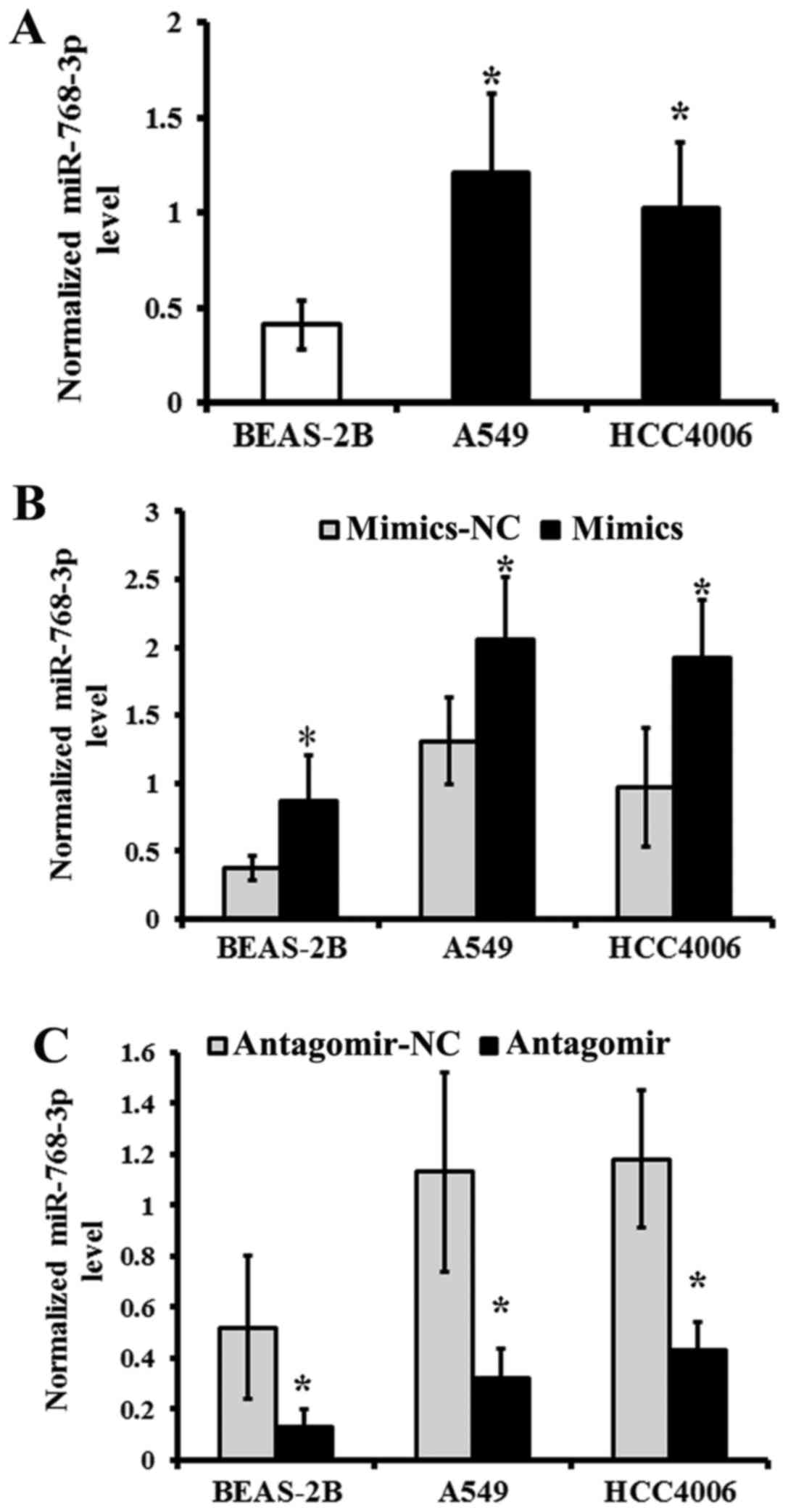
The expression of miR-768-3p in NSCLC
cell lines
The level of miR-768-3p was detected in BEAS-2B,
A549 and HCC4006 human NSCLC cell lines by qRT-PCR. BEAS-2B served
as normal control.
The data showed that miR-768-3p increased in either
A549 or HCC4006 human NSCLC cell lines, compared with that of
BEAS-2B cell line (P<0.05; Fig.
3A), respectively. After transduction with miR-768-3p mimics,
the expression of miR-768-3p was significantly upregulated in
BEAS-2B, A549 and HCC4006 human NSCLC cell lines than that of the
NC treated group, respectively (P<0.05; Fig. 3B).
Reversely, the expression of miR-768-3p was
significantly downregulated following transduction with miR-768-3p
antagomir in the BEAS-2B, A549 and HCC4006 NSCLC cell lines,
compared with that of the matched NC treated group, respectively
(P<0.05; Fig. 3C).
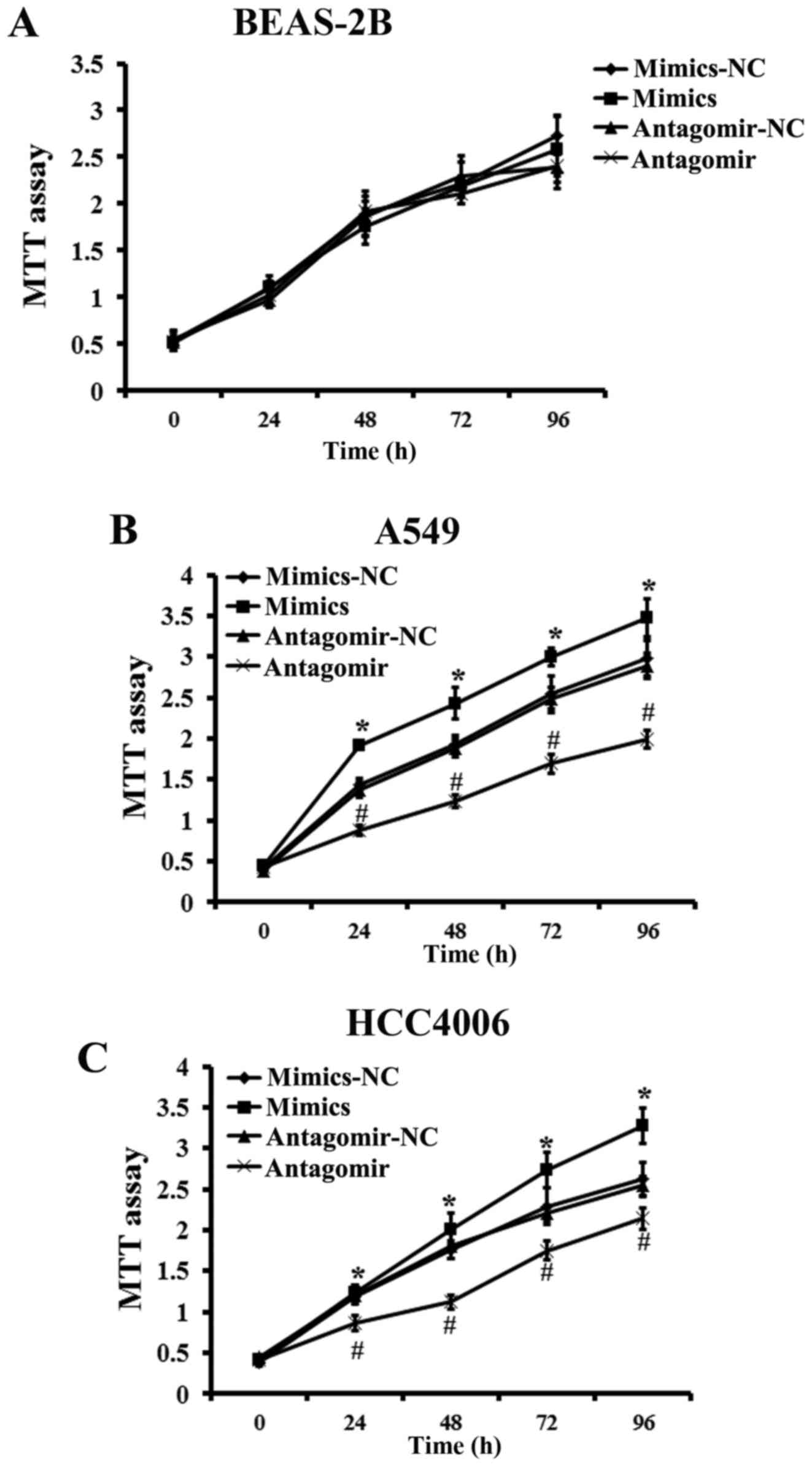
Effects of miR-768-3p on the
proliferation of NSCLC cell lines
We assessed the effect of miR-768-3p overexpression
or knockdown on the regulation of NSCLC cells viability. Treatment
with miR-768-3p-mimics significantly increased the cell
proliferation in either of A549 cells or HCC4006 cells, compared
with that of NC-treated group, respectively. MTT assay also showed
that miR-768-3p knockdown by miR-768-3p antagomir transduction
caused significantly decrease in cell viability of either A549 or
HCC4006 cell lines. However, the effects of neither miR-768-3p
mimics nor antagomir transduction were confirmed in BEAS-2B cells
(vs. NC treated control, P>0.05; Fig. 4).
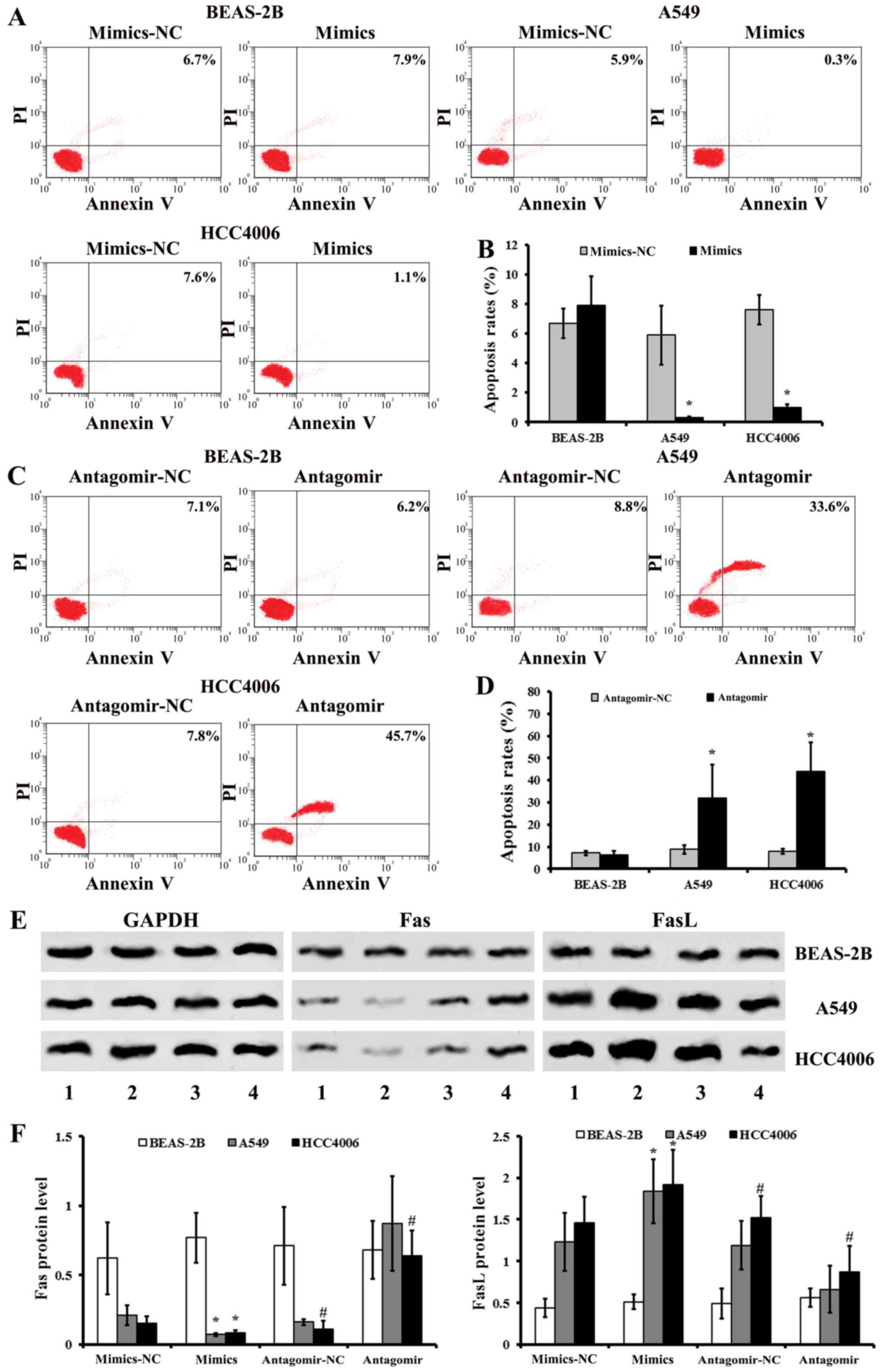
Alterations of apoptosis in NSCLC cells
after miR-768-3p regulation
The apoptosis rate of NSCLC after miR-768-3p
mimics/antagomir transduction was determined by FCM. There were
less apoptotic NSCLC cells in A549 and HCC4006 groups treated with
miR-768-3p mimics, when compared with that of the matched NC
treated groups, respectively (P<0.05; Fig. 5A and B). The data also showed that
there was a significant increase in the apoptosis rate in
miR-768-3p antagomir infected cells relative to the matched control
NC infected ones, respectively (P<0.05; Fig. 5C and D). However, there was no
significant different in the apoptosis of human normal BEAS-2B
cells (P>0.05; Fig. 5A–D).
The expression of apoptosis associated proteins Fas
and FasL were also determined in NSCLC cells after miR-768-3p
mimics/antagomir treatment. Consistence with the apoptosis assay,
compared with the matched NC control, miR-768-3p mimics induced
increase of FasL and decrease of Fas expressions, while miR-768-3p
antagomir decreased FasL and increased Fas levels, respectively
(Fig. 5E and F).
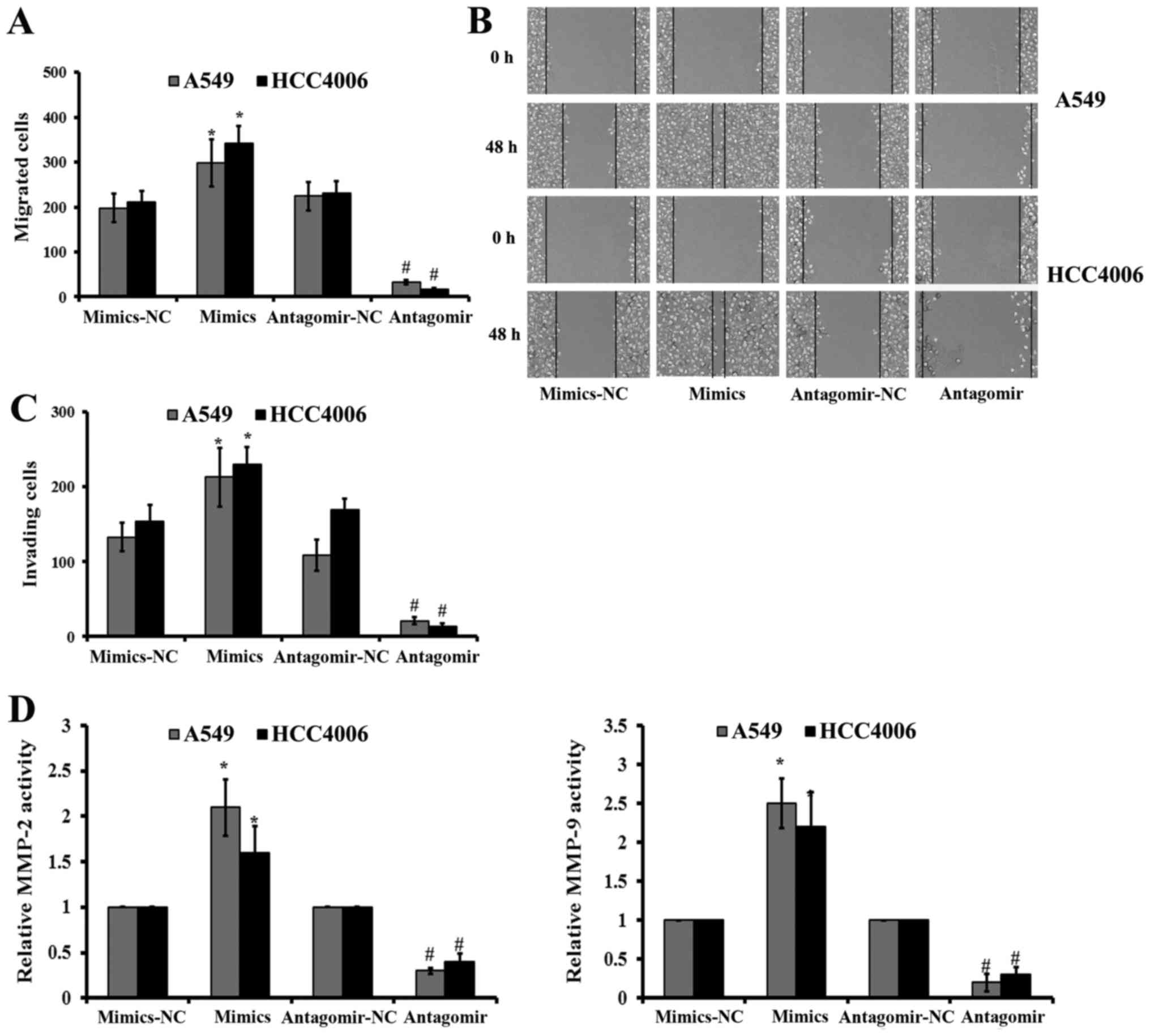
Effect of miR-768-3p on migration,
invasion and MMP activity in NSCLC cells
Following miR-768-3p regulation, we compared the
migration and invasion of NSCLC cells following miR-768-3p mimics,
miR-768-3p antagomir, as well as the matched control NC
transfection.
Scratch wound assay showed a faster migration of the
miR-768-3p mimics transfected NSCLC cells than the control
mimics-NC transfected ones (Fig. 6A
and B). There were significant reductions of crystal violet
stained NSCLC cells following miR-768-3p antagomir transfection, in
comparison with that of the control NC treated cells, respectively
(P<0.05; Fig. 6C).
Consistently, MMP-2 and MMP-9 activities were decreased in both
A549 and HCC4006 cells following miR-768-3p antagomir transduction
(Fig. 6D).
Reversely, transfection with miR-768-3p mimics
significantly increased the migration, invasion and the MMP-2/9
activities in both kinds of NSCLC cells, compared with that of the
matched mimics-NC transduced ones, respectively (P<0.05;
Fig. 6).
Effects of miR-768-3p on tumor formation
and growth
Tumor formation and growth were measured in mice
after injections of different NSCLC cells. Tumor growth was fastest
and neoplasms were larger in mice treated with
miR-768-3p-mimics-treated NSCLC cells compared with the other
groups 13 days post-injection (P<0.05; Fig. 7). Reversely, xenograft tumor formed
last and grew slowest in those injected with miR-768-3p-antagomir
transfected cells. Tumor sizes were also smaller in the miR-768-3p
deficient group than in the other groups between 13 and 29 days
post-injection (P<0.05). These results indicated that miR-768-3p
promoted proliferation and tumor formation of NSCLC xenografts in
the mouse model.
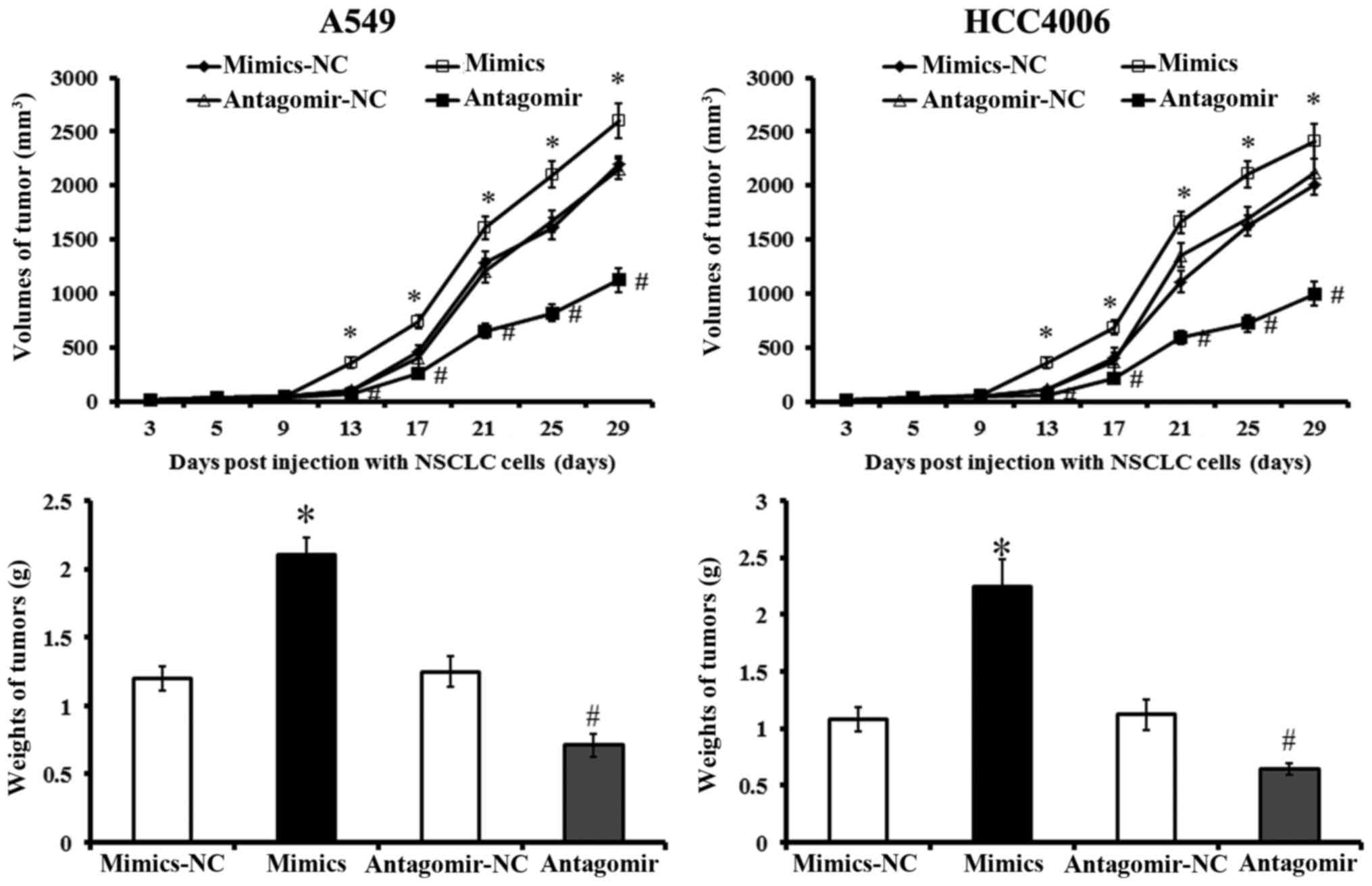 | Figure 7Tumor formation and growth in nude
mice after NSCLC xenografts. BEAS-2B, A549 and HCC4006 cells
transfected with miR-768-3p mimics, mimics-NC, antagomir or
antagomir-NC were injected into nude mice. Tumor size was measured
on days 3, 5, 9, 13, 17, 21, 25 and 29 post-injection,
respectively. Tumor volume growth curve was plotted. Tumor weights
were measured in different groups. *P<0.05 vs.
mimics-NC treated cells; #P<0.05 vs. antagomir-NC
treated cells. Values plotted are means ± SD (n=5). |
Discussion
The present study revealed that: i) miR-768-3p
significantly increased in tissues from NSCLC cases, compared with
that of adjacent normal control; ii) expression of miR-768-3p in
NSCLC tumor cells were associated with clinicopathological factors
of NSCLC, including tumor size, histological grade, lymph node
metastasis, as well as 5-year survival rate. The data also showed
that inhibiting miR-768-3p function by miR-768-3p-atagomir
infection caused significant increase in apoptosis and Fas levels,
decrease in FasL expression, cell viability, migration and
invasion, MMP-2 and MMP-9 activities of A549 and HCC4006 cells,
respectively. Moreover, miR-768-3p promoted proliferation and tumor
formation in nude mice bearing NSCLC xenograft tumor. These results
suggested that abnormal elevated miR-768-3p in NSCLC tumor and cell
lines played important role in NSCLC carcinogenic progression,
including apoptosis, migration and invasion. The targeting of
miR-768-3p may be a potential therapeutic strategy for the
treatment of NSCLC.
Aberrant miRNA expression profiles are frequently
observed in cancers (23,24) and many miRNAs are implicated in the
initiation and progression of cancer and represent potential
targets for anticancer treatment (25,26).
However, the possible role of miR-768-3p in NSCLC is still not
known. Evidence has shown that abnormal expression of miR-768-3p
was observed in various cancers, indicating its oncogenic features.
It was described that miR-768-3p were overexpressed in plasma of
patients with hepatocellular carcinoma (27) and downregulated in gastric and
thyroid tumors (28,29), respectively. Jian and his
colleagues (30) reported that in
human melanoma downregulation of miR-768-3p as a result of
activation of the mitogen-activated protein kinase kinase
(MEK)/extracellular signal-regulated kinase (ERK) pathway has an
important role in the upregulation of eIF4E and enhancement in
protein synthesis. We found that significantly increased miR-768-3p
in NSCLC cases were associated with clinicοpathological factors of
NSCLC. The following detection also revealed the elevated
miR-768-3p in NSCLC cells. The data suggested the oncogenic roles
of miR-768-3p involved in pathological events of NSCLC.
The data revealed that miR-768-3p inhibition caused
significantly decrease in cell viability and proliferation.
Induction of apoptosis highly affects cell proliferation. In
present study, flow cytometry with Annexin V/PI staining also
showed that miR-768-3p antagomir transduction induced apoptosis in
NSCLC cells, which was supported by increased expressions of Fas
and decreased expressions of FasL. Reversely, miR-768-3p mimics
significantly inhibited apoptosis and promoted FasL generation in
NSCLC cells. The Fas ligand, FasL played a key role in the
initiation of apoptotic pathway (31), and was shown that alterations in
Fas/FasL pathway within tumor cells could result in escape from
apoptosis and immune surveillance. In malignant cells, their
reduced capability to undergo apoptosis in response to some
physiological stimuli resulting in a significant survival advantage
and possibly contributing to tumorigenesis (32,33).
Recent evidence revealed that: i) the Fas-negative NSCLC patients
showed significantly lower survival rate than those with
Fas-positive ones; ii) FasL protein was increased in most NSCLC
cases (up to 89%) compared to normal lungs (34). Data demonstrated the reduced
membranous Fas expression as a mechanism of apoptotic resistance
was considered to play an important part of the pulmonary
carcinogenesis. Increased FasL expression is thought to be a basis
for the immune evasion in NSCLCs (34). Combined with the above, the present
study suggested that miR-768-3p played a role in the apoptotic
resistance of NSCLCs by Fas/FasL regulation.
Our results also suggested that abnormally elevated
miR-768-3p in NSCLC tumor and cell lines played an important role
in NSCLC carcinogenic progression, including migration and
invasion. NSCLC has a very poor prognosis and is often
characterized by aggressive local invasion, early metastasis and
poor response to chemotherapy (35). Consequently, targeting and
prevention of cancer cell metastasis is among the biggest hurdles
in clinical oncology (36).
Although a variety of metastasis-promoting genes have been recently
identified to be related to the metastasis of NSCLC, the molecular
mechanisms governing this metastasis process are still not
completely understood and the treatment efficiency of metastatic
NSCLC has not been significantly improved (37,38).
During metastasis, cancer cells rely heavily on cell-extracellular
matrix (ECM) interactions, cytoskeleton remodeling and gene
transcription (39). Taking into
account that MMPs such as MMP-2 and MMP-9 can be involved in the
development of several human malignancies, as degradation of
collagen IV in basement membrane and extracellular matrix
facilitates tumor progression, including invasion, metastasis, and
angiogenesis, we analyzed their activity (39). The data also revealed that
inhibiting miR-768-3p function leads to increased migration and
invasion rate, as well as MMP-2/9 activities in NSCLC cells, which
indicated that miR-768-3p was involved in metastatic process of
NSCLC by promoting NSCLC cells to migrate and invade.
In conclusion, the results demonstrated that
abnormal elevated miR-768-3p in both carcinoma tissues in NSCLC
cases and NSCLC cell lines, were associated with clinic
pathological factors of NSCLC. Further inhibition of miR-768-3p by
miR-768-3p antagomir transduction: i) significantly increased
apoptosis by Fas/FasL regulation; ii) promoted proliferation of
NSCLC both in vitro and in vivo; iii) increased
migration and invasion of NSCLC cells. Just as we expected,
transfection with miR-768-3p mimics reversed the effects described
above. Our data suggested that miR-768-3p played a role in the
apoptotic resistance of NSCLCs by Fas/FasL regulation, and it was
involved in metastatic process of NSCLC by promoting NSCLC cells to
proliferate, migrate and invade. The targeting of miR-768-3p may be
a potential therapeutic strategy for the future treatment of
NSCLC.
References
|
1
|
Jemal A, Thun MJ, Ries LA, Howe HL, Weir
HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, et al: Annual
report to the nation on the status of cancer, 1975–2005, featuring
trends in lung cancer, tobacco use, and tobacco control. J Natl
Cancer Inst. 100:1672–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutvágner G and Zamore PD: RNAi: Nature
abhors a double-strand. Curr Opin Genet Dev. 12:225–232. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ketting RF, Fischer SE, Bernstein E, Sijen
T, Hannon GJ and Plasterk RH: Dicer functions in RNA interference
and in synthesis of small RNA involved in developmental timing in
C. elegans. Genes Dev. 15:2654–2659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He L, Thomson JM, Hemann MT,
Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe
SW, Hannon GJ, et al: A microRNA polycistron as a potential human
oncogene. Nature. 435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang WC, Liu J, Xu X and Wang G: The role
of microRNAs in lung cancer progression. Med Oncol. 30:675–683.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito M, Schetter AJ, Mollerup S, Kohno T,
Skaug V, Bowman ED, Mathé EA, Takenoshita S, Yokota J, Haugen A, et
al: The association of microRNA expression with prognosis and
progression in early-stage, non-small cell lung adenocarcinoma: A
retrospective analysis of three cohorts. Clin Cancer Res.
17:1875–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malleter M, Jacquot C, Rousseau B,
Tomasoni C, Juge M, Pineau A, Sakanian V and Roussakis C: miRNAs, a
potential target in the treatment of non-small-cell lung
carcinomas. Gene. 506:355–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raponi M, Dossey L, Jatkoe T, Wu X, Chen
G, Fan H and Beer DG: MicroRNA classifiers for predicting prognosis
of squamous cell lung cancer. Cancer Res. 69:5776–5783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin PY, Yu SL and Yang PC: MicroRNA in
lung cancer. Br J Cancer. 103:1144–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koeck S, Amann A, Huber JM, Gamerith G,
Hilbe W and Zwierzina H: The impact of metformin and salinomycin on
transforming growth factor β-induced epithelial-to-mesenchymal
transition in non-small cell lung cancer cell lines. Oncol Lett.
11:2946–2952. 2016.PubMed/NCBI
|
|
16
|
Hou FQ, Lei XF, Yao JL, Wang YJ and Zhang
W: Tetraspanin 1 is involved in survival, proliferation and
carcinogenesis of pancreatic cancer. Oncol Rep. 34:3068–3076. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Z, Huang C, Lin G and Ye Y:
siRNA-induced TRAF6 knockdown promotes the apoptosis and inhibits
the invasion of human lung cancer SPC-A1 cells. Oncol Rep.
35:1933–1940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ouchi Y, Banno Y, Shimizu Y, Ando S,
Hasegawa H, Adachi K and Iwamoto T: Reduced adult hippocampal
neurogenesis and working memory deficits in the Dgcr8-deficient
mouse model of 22q11.2 deletion-associated schizophrenia can be
rescued by IGF2. J Neurosci. 33:9408–9419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santiago-Gómez A, Barrasa JI, Olmo N,
Lecona E, Burghardt H, Palacín M, Lizarbe MA and Turnay J:
4F2hc-silencing impairs tumorigenicity of HeLa cells via modulation
of galectin-3 and β-catenin signaling, and MMP-2 expression.
Biochim Biophys Acta. 1833:2045–2056. 2013. View Article : Google Scholar
|
|
20
|
Hu T, Chang YF, Xiao Z, Mao R, Tong J,
Chen B, Liu GC, Hong Y, Chen HL, Kong SY, et al: miR-1 inhibits
progression of high-risk papillomavirus-associated human cervical
cancer by targeting G6PD. Oncotarget. 7:86103–86116.
2016.PubMed/NCBI
|
|
21
|
Hu T, Zhang C, Tang Q, Su Y, Li B, Chen L,
Zhang Z, Cai T and Zhu Y: Variant G6PD levels promote tumor cell
proliferation or apoptosis via the STAT3/5 pathway in the human
melanoma xenograft mouse model. BMC Cancer. 22(13): 2512013.
View Article : Google Scholar
|
|
22
|
He Z, Xia Y, Liu B, Qi X, Li Z, Wang J,
Chen L and Chen Y: Down-regulation of miR-452 is associated with
poor prognosis in the non-small-cell lung cancer. J Thorac Dis.
8:894–900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: Progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Gao D and Huang L: In vivo
delivery of miRNAs for cancer therapy: challenges and strategies.
Adv Drug Deliv Rev. 81C:128–141. 2011.
|
|
27
|
Zhou J1, Yu L, Gao X, Hu J, Wang J, Dai Z,
Wang JF, Zhang Z, Lu S, Huang X, Wang Z, et al: Plasma microRNA
panel to diagnose hepatitis B virus-related hepatocellular
carcinoma. J Clin Oncol. 29:4781–4788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vriens MR, Weng J, Suh I, Huynh N,
Guerrero MA, Shen WT, Duh QY, Clark OH and Kebebew E: MicroRNA
expression profiling is a potential diagnostic tool for thyroid
cancer. Cancer. 118:3426–3432. 2012. View Article : Google Scholar
|
|
29
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroenterol
Hepatol. 24:652–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jian Q, An Q, Zhu D, Hui K, Liu Y, Chi S
and Li C: MicroRNA 340 is involved in UVB-induced dendrite
formation through the regulation of RhoA expression in melanocytes.
Mol Cell Biol. 34:3407–3420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Xu KP, Jiang D, Zhao J, Ge JF and
Zheng SY: Relationship of Fas, FasL, p53 and bcl-2 expression in
human non-small cell lung carcinomas. Int J Clin Exp Pathol.
8:13978–13986. 2015.
|
|
35
|
Forde PM and Ettinger DS: Targeted therapy
for non-small-cell lung cancer: Past, present and future. Expert
Rev Anticancer Ther. 13:745–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frisch SM, Schaller M and Cieply B:
Mechanisms that link the oncogenic epithelial-mesenchymal
transition to suppression of anoikis. J Cell Sci. 126:21–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi YH, Burdick MD, Strieter BA, Mehrad B
and Strieter RM: CXCR4, but not CXCR7, discriminates metastatic
behavior in non-small cell lung cancer cells. Mol Cancer Res.
12:38–47. 2014. View Article : Google Scholar :
|
|
38
|
Jin Y, Li F, Zheng C, Wang Y, Fang Z, Guo
C, Wang X, Liu H, Deng L, Li C, et al: NEDD9 promotes lung cancer
metastasis through epithelial-mesenchymal transition. Int J Cancer.
134:2294–2304. 2014. View Article : Google Scholar
|
|
39
|
Xu LJ, Wang YC, Lan HW, Li J and Xia T:
Grb2-associated binder-2 gene promotes migration of non-small cell
lung cancer cells via Akt signaling pathway. Am J Transl Res.
8:1208–1217. 2016.PubMed/NCBI
|