Introduction
Blood hypercoagulability is common in cancer
patients (1–3) with different risk of venous
thromboembolism (VTE) according to cancer type (4–6).
Cancer cells are directly involved in the pathogenesis of
thrombosis through the induction and amplification of thrombin
generation (7,8). According to the cell-based model of
blood coagulation, triggering of thrombin generation occurs by
direct contact of tissue factor (TF)-expressing cells with plasma
factors VII (FVII) and VIIa (FVIIa) which is amplified in the
presence of procoagulant phospholipids (PPL) provided by
cell-derived microparticles (9–11).
The TF/FVIIa complex generates activated factor X (FXa) that
induces the initial generation of trace amounts of thrombin leading
to activation of platelets, factor V (FV) and factor VIII (FVIII).
In the presence of ionized calcium, intrinsic tenase
(FIXa/FVIIIa/procoagulant phospholipids) and prothrombinase
(FXa/FVa/procoagulant phospholipids) lead to a burst of thrombin
generation (12–14). An alternative pathway of thrombin
generation involves activation of the contact system through FXII
activation (15,16). The contact system is usually
considered less important than the TF-pathway, but has attracted
recent interest, especially in the context of cancer-induced
hypercoagulability (17,18). Finally, some cancer cells express
the cancer procoagulant (CP) factor, a 68 kDa protease that
directly activates FX, which represents an additional pathway of
cancer-induced hypercoagulability (19,20).
We have recently shown that exposure of human plasma
to pancreatic adenocarcinoma (BXPC3) or breast cancer cells (MCF7)
can enhance thrombin generation (21). Of note, these findings are not
restricted to cells from solid tumors since Marchetti at al
reported that cells from hematological malignancies may also
enhance thrombin generation (22).
We herein characterize the mechanisms by which cancer cells trigger
blood coagulation using an original and validated experimental
system, to elucidate the role of the TF-pathway and the intrinsic
clotting system in thrombin generation. It is widely accepted that
the plasma microenvironment plays a key role in hypercoagulability.
Therefore, thrombin generation was assessed under different
experimental conditions with respect to the concentration of TF and
procoagulant phospholipids in the plasma as well as in plasma
selectively deficient in factor VII (FVII), factor XII (FXII),
factor XI (FXI), factor IX (FIX), factor VIII (FVIII), factor X
(FX), factor V (FV) and factor II (FII). We finally determined the
amounts of TF released by cancer cells in their local
microenvironment.
Materials and methods
Cell cultures
Different histological types of cancer cells derived
from pancreatic cancer (BXPC3), breast cancer (MDA-MB231, MCF7,
BT20), colon cancer (HT29, HCT-116) and ovarian cancer (IGROV1)
were characterized with respect to thrombin generation in human
plasma. We then selected two cells lines, MCF-7 and BXPC3, with
different capacity for thrombin generation. Both cell lines are
typical of their cancer origin, since pancreatic cancer is known
for its strong thromobogenic potential whereas the thrombogenic
potential of breast cancer is less prominent. BXPC3, a human
pancreatic adenocarcinoma cell line and MCF7 breast adenocarcinoma
cell line were obtained from American Type Culture Collection
(ATCC, Rockville, MD, USA).
Cells were expanded and cultured as described
elsewhere (21). A volume of 100
µl cell suspension (50 cells/µl) was placed in
96-well plates and cells were incubated for 24 h at 37°C in a 100%
humidified atmosphere with 5% CO2. After 24 h
incubation, BXPC3 cells reached 70% confluence and MCF7 cells 80%
confluence. Under these conditions, the two cancer cell lines
induced similar levels of thrombin generation. To rule out any
potential interference of the trypsin used to detach the cells on
thrombin generation, we compared thrombin generation for cells that
has been detached mechanically or by exposure to trypsin. We also
confirmed that the concentration of fetal bovine serum (ranging
from 1% to 10%) and the incubation time (ranging from 5 to 72 h)
did not significantly influence the procoagulant potential as
determined by thrombin generation. For the studied cancer cell
lines, a plateau effect on thrombin generation was observed at cell
numbers higher than 50 cells/µl. Cells were only used if the
apoptotic fraction was inferior to 2%.
Primary human umbilical vein cells (HUVECs) were
used to represent normal human cells. HUVECs were obtained from
Clonetics (San Diego, CA, USA) and cultured in endothelial cell
growth media EGM-2 (Clonetics) containing 2% fetal bovine serum and
supplements. Cells of 2nd passage were used for the experiments.
Cells were cultured in 25 cm2 culture flasks at 37°C in
a 100% humidified atmosphere with 5% CO2. Cells were
used for experiments at a confluence of 80%. For thrombin
generation, the same experimental protocol was used for the HUVECs
(50 cells/µl) as described above for BXPC3 and MCF7
cells.
Normal human plasma
Platelet-poor plasma (PPP) for thrombin generation
experiments was purchased from Diagnostica Stago, Gennevilliers,
France (ref. 00539). Plasma samples for thrombin generation
experiments in platelet-rich plasma (PRP) were obtained from
healthy volunteers, members of the laboratory staff, who had not
taken any medication during the previous 30 days. Blood samples
were taken by atraumatic antecubital venipuncture and collected in
siliconized vacutainer tubes (Becton Dickinson, Meylan, France)
containing buffered 0.13 M trisodium citrate (nine parts blood to
one part citrate solution, 3.8%). Platelet-rich plasma was prepared
after centrifugation of citrated whole blood for 10 min at 150 × g
at room temperature. After centrifugation, the supernatant PRP was
removed and the platelet count was adjusted to 150×109/l
by dilution with autologous PPP obtained after a further
centrifugation of the remaining blood for 15 min at 2,000 × g.
Specific TF activity and concentration of
alternatively spliced TF
BXPC3 and MCF7 cells were washed three times in PBS,
suspended in distilled water (at a final concentrations of 50 to
200 cells/µl) and incubated at 4°C for 30 min. Then, samples
were centrifuged for 30 min at 1000 × g, supernatants were
collected and kept frozen at −80°C until measurements of TF
activity.
Tissue factor activity (TFa) was measured in the
absence or presence of cells with the same normal plasma as used
for thrombin generation. Tissue factor activity was assessed with
an in-house chromogenic method as described elsewhere (23–25).
Alternatively spliced TF (asTF) was measured by a specific
enzyme-linked immunosorbent assay (elISA) as follows. The wells of
a micro-ELISA plate were coated with an anti-asTF monoclonal
antibody and the test samples, pre-diluted 1:1 with the sample
dilution buffer, were added to the coated well. The asTF molecules,
if present, will bind to the well. After a washing step to remove
unbound material, a specific anti-human astF peroxidase-conjugated
monoclonal antibody was added. The anti-asTF peroxidase binds to
the bound asTF. Following a washing step to remove excess anti-asTF
peroxidase, the bound enzyme peroxidase was determined using the
TMB substrate. Then, the color intensity was determined by
spectrometry at 450 nm.
Cancer procoagulant assay
The CP activity was determined using an in-house
chromogenic assay. The activity of CP was evaluated by measuring
the conversion of purified FX to FXa after incubation of cells in
the presence of CaCl2 (50 mmol/l in bis-Tris propane
buffer, pH 6.7) for 30 min at 37°C. Then, the FXa specific
chromogenic substrate MAPA-Gly-Arg-pNA (CBS-0244 from Diagnostica
Stago) was added and the amidolytic activity was measured. The
kinetics of color development was recorded at 405 nm for 30 min.
The CP activity was expressed as mUnits/ml (1 Unit = the amount of
enzyme needed to release 1 µmol p-nitroanilide from the
substrate in 1 min). A calibration curve was constructed using
increasing concentrations of Russell's viper venom (RVV); a serine
proteinase that activates FX (from Diagnostica Stago). The RVV was
used as the standard control to calibrate the assay. Thrombin
formation is totally inhibited by incubation of cell extracts with
1 mmol/l HgCL2. Results are expressed as mU/mg of
protein. The specific cysteine proteinase inhibitor E64 (Sigma, St.
Louis, MO, USA) was added to exclude possible non-specific
interactions.
Calibrated automated thrombogram
assay
In each well of the micro-plate, 80 µl of PPP
samples were mixed with saline (20 µl). Thrombin generation
was initiated by adding 20 µl triggering solution containing
CaCl2 (16.7 mM final concentration) and fluorogenic
substrate (Z-Gly-Gly-Arg-AMC, 417 µM final concentration).
Thrombin generation was assessed with the Calibrated Automated
Thrombogram assay (Thrombinoscope b.v., Maastricht, The
Netherlands) as described elsewhere (26). Among thrombogram parameters we
analyzed the mean rate index (MRI), which reflects the rate of the
propagation phase of thrombin generation [calculated by the formula
MRI = Peak / (ttPeak − lag-time)]. This parameter includes
lag-time, the time to Peak (ttPeak) and the Peak. As previously
shown, these parameters of the thrombogram, reflect the biological
activity of cancer cells on thrombin generation better than the
endogenous thrombin potential.
Procoagulant potential of cancer cells
assessed with the calibrated automated thrombogram assay
BXPC3 cells, MCF7 cells or HUVECs were added to the
wells of microtiter plates suitable for thrombin generation
assessment. Then, 80 µl of normal PPP were added to each
well and thrombin generation was assessed as described above. For
control experiments, the same procedure was used in the absence of
cells. Each result represents several independent experiments as
specified in the figure legends. In additional control experiments,
thrombin generation was assessed in plasma spiked with increasing
concentration of lymphocytes from healthy donors and was compared
to thrombin generation obtained after calcification of normal
plasma. No significant difference was found between the two
experimental procedures (data not shown). In preliminary
experiments, we also verified that the culture medium (containing
RPMI, glutamine, penicillin, streptomycin and fetal calf serum) did
not influence thrombin generation process of normal PPP. Thrombin
generation was initiated and recorded as described above.
Thrombin generation in the presence of an
anti-TF antibody
In separate experiments, 50 µl of a working
suspension of BXPC3 or MCF7 cells (100 cells/µl) were mixed
with an equal volume containing an anti-TF mouse monoclonal
antibody 4509 (American Diagnostica, neuville-sur-Oise, France) or
a murine anti-human TF9-10H10 monoclonal antibody (AbD Serotec,
Bio-Rad, Marnes-la-Coquette, France). Mouse IgG1 isotype (100
µg/ml) or saline were used in control experiments. Cells
were incubated with the anti-TF antibody or the isotype IgG1 for 15
min at 37°C and 20 µl of this suspension were mixed with 180
µl of PPP at a final concentration of 5 cells/µl. The
experimental conditions were defined after conducting preliminary
experiments on thrombin generation, with variable concentrations of
the cells and the anti-TF monocolonal antibody. Cells were used at
the lower active concentration in plasma and antibody was employed
at the concentration of 25 µg/ml. At this concentration the
anti-TF antibody completely inhibited the effect of high TF
concentrations on thrombin generation. To eliminate any
interactions of the phospholipid concentration, the impact of the
anti-TF antibody on thrombin generation was carried out in normal
PPP in the presence of MP-Reagent®.
Impact of exogenous TF and phospholipids
on the procoagulant potency of cancer cells
To evaluate the contribution of the microenvironment
on the procoagulant potency of cancer cells, thrombin generation
was assessed under the following experimental conditions: a) in the
presence of optimal concentrations of TF (5 pM) and procoagulant
phospholipids (4 µM) using the PPP-Reagent®
(27), b) in the presence of 1 pM
TF and 4 µM procoagulant phospholipids (PPP-Reagent low), c)
in the presence of 5 pM TF without any addition of exogenous
procoagulant phospholipids (PRP-Reagent), d) in the presence of 4
µM of procoagulant phospholipids without any exogenous TF
(MP-Reagent) and e) without any exogenous addition of TF and/or
procoagulant phospholipids which represents the baseline thrombin
generation triggered by cancer cells. For simplicity, the
comparisons of thrombin generation produced under the different
experimental systems were done on the basis of MRI, a parameter
stemming from an equation that includes the other major parameters
of thrombogram. The reagents PPP-Reagent, PRP-Reagent, MP-Reagent
were purchased from Diagnostica Stago.
Thrombin generation after inhibition of
the contact system of blood coagulation
To evaluate the impact of FXII activation by cancer
cells, PPP spiked with 20 µg/ml of corn trypsin inhibitor
(CTI, Merck Chemicals, Nottingham, UK) was incubated for 1 h at
37°C. Then, PPP was added into the micro-plate wells containing
BXPC3 and MCF7 cells and thrombin generation was assessed as
described above. Preliminary experiments showed that CTI
concentrations equal or higher that 20 µg/ml significantly
increased the lag-time as compared to the control. In contrast, no
significant differences were observed at CTI concentrations higher
than 20 µg/ml. No significant differences of thrombin
generation were found between plasma obtained from blood collected
in tubes prefilled with CTI and with addition of CTI after plasma
preparation (data not shown). To estimate the relative contribution
of TF and contact system activation by cancer cells on thrombin
generation, PPP spiked with 20 µg/ml of CTI and 25
µg/ml of anti-TF monoclonal antibody was incubated for 1 h
at 37°C.
Thrombin generation in clotting
factor-deficient plasma
Immunodepleted lyophilised plasma deficient of
clotting factors (FVII, FIX, FX, FII, FVIII, FXI, FXII) or protein
C were from Diagnostica Stago. The clotting factor-deficient plasma
was reconstituted according to the manufacturer's instructions.
Clotting factor deficient plasma and normal PPP were added in the
wells of the micro-plate containing cancer cells. Subsequently,
thrombin generation was assessed as described above.
Statistical analysis
Non-parametric Mann-Withney test was applied to
control changes in the thrombogram parameters in the presence or
absence of cancer cells as well as in the different experimental
condition described above. Results are shown as mean ± SD. SPSS
statistical software package was used for statistical analysis. The
inhibition of thrombin generation (TG) was calculated by the
formula inhibition of TG = (1 − TG cells / TG control) %. The upper
normal limit (UNL) for each parameter of the thrombograms was
defined for the control group as follows: UNL = mean ± 2SD.
Results
Procoagulant activity of BXPC3 and MCF7
cells
Addition of cancer cells to normal PPP was
accompanied by a significant increase of the Peak and MRI and a
reduction of the lag-time and ttPeak compared to cell-free PPP
(Table I). A similar effect was
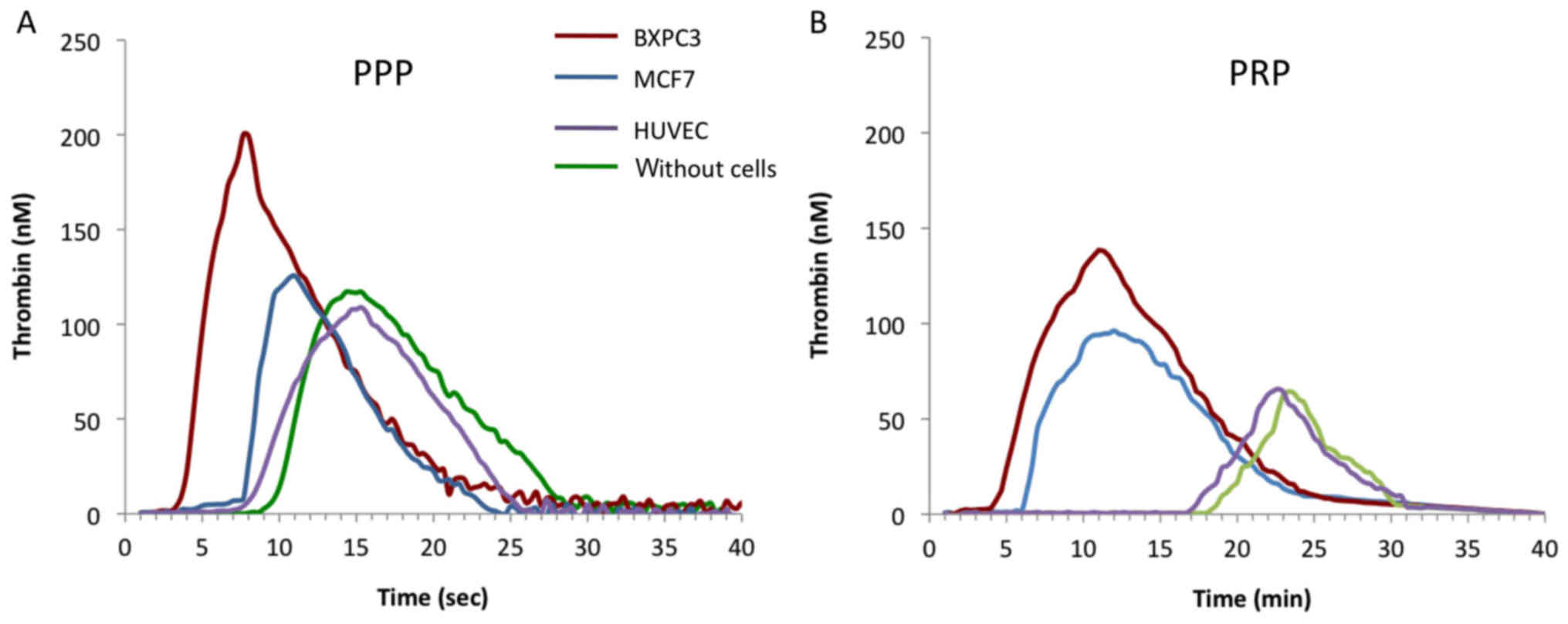
observed when the experiment was performed in PRP (Fig. 1A and B). We then selected two cells
lines, MCF-7 and BXPC3, with different capacity for thrombin
generation. Both cell lines are typical of their cancer of origin,
since pancreatic cancer is known for its strong thromobogenic
potential whereas the thrombogenic potential of breast cancer is
less prominent. The two types of cancer cells had different
influence on thrombin generation in normal human plasma. At equal
cell number, MCF7 had less procoagulant activity than BXPC3 cells.
In comparison, HUVECs had no significant influence on the
parameters of the thrombogram compared to cell-free PPP or PRP
(Fig. 1A and B).
 | Table IVariability in the procoagulant
activity of cancer cells on thrombin generation in normal human
plasma. |
Table I
Variability in the procoagulant
activity of cancer cells on thrombin generation in normal human
plasma.
| Cancer cell
lines | Lag-time (min) | tt-Peak (min) | Peak (nM) | MRI (nM/min) |
|---|
| MCF7 | 6.1±0.9 | 9.6±1.1 | 121±22 | 34±6 |
| MDA_MB-231 | 8.9±0.6 | 14.5±0.8 | 76±8 | 14±3 |
| BT20 | 2.3±0.4 | 6.0±1.1 | 129±6 | 35±8 |
| HT29 | 5.36±1.3 | 11.8±1.9 | 81±11 | 13±4 |
| HCT116 | 4.48±0.3 | 9.9±0.5 | 91±12 | 17±3 |
| IGROV1 | 2.5±1.4 | 7.1±2.4 | 95±10 | 22±7 |
| BXPC3 | 4.1±1.1 | 6.9±1.3 | 199±13 | 71±7 |
| Control | 9.6±1.2 | 16.3±1.5 | 118±9 | 17±4 |
Based on these results, further studies were
undertaken to elucidate the mechanistic basis for the
hypercoagulability of BXPC3 and MCF7 cells.
Tissue factor expression by BXPC3 and
MCF7 cells
Both BXPC3 and MCF7 cells expressed significantly
higher TFa levels compared to normal plasma (0.20±0.05 pM,
p<0.05) and to HUVECs (0.23±0.02 pM). However, BXPC3 expressed
significantly higher TFa levels compared to MCF7 cells (1.42±0.10
pM vs. 0.82±0.08 pM, respectively, p<0.05). The levels of TFa
increased with the number of cells (Table II). For all experimental
conditions, the levels of TFa produced by BXPC3 cells were
significantly higher than TFa expressed by the same number of MCF7
cells.
 | Table IILevels of TFa and asTF in the
supernatant of BXPC3 cells, MCF7 cells, HUVECs and in normal
plasma. |
Table II
Levels of TFa and asTF in the
supernatant of BXPC3 cells, MCF7 cells, HUVECs and in normal
plasma.
| Tissue factor
levels | PPP without
cells | 50,000
cells/µl
| 200,000
cells/µl
|
|---|
| HUVECs | BXPC3 | MCF7 | HUVEC | BXPC3 | MCF7 |
|---|
| TF (pM) | 0.20±0.05 | 0.23±0.02 | 1.42±0.1a,b,c | 0.82±0.08a,b | 0.26±0.02 | 2.65±0.05a,b,c | 1.21±0.05a,b |
| asTF (ng/ml) | 0.02±0.01 | 0.03±0.02 | 0.35±0.09a,b,c | 0.05±0.02b | 0.04±0.01 | 0.65±0.02a,b,c | 0.12±0.01a,b |
The levels of asTF were also significantly higher in
the presence of BXPC3 or MCF7 cells compared to normal plasma
(0.02±0.01 ng/ml; p<0.05) or in the presence of HUVECs
(0.03±0.02 ng/ml; p<0.05). The levels of asTF were significantly
increased in plasma incubated with BXPC3 cells compared to plasma
incubated with MCF7 cells (0.35±0.09 ng/ml vs. 0.05±0.02 ng/ml,
respectively; p<0.05) (Table
II). For all concentrations of BXPC3 or MCF7 cells, the levels
of asTF in plasma followed the concentrations of TFa.
Impact of anti-TF monoclonal antibodies
on thrombin generation induced by BXPC3 and MCF7 cells
The addition of anti-TF monoclonal antibodies to PPP
with BXPC3 or MCF7 cells significantly prolonged the lag-time and
ttPeak and decreased the MRI and the Peak of thrombin generation
compared to control experiments without anti-TF antibodies.
Noteworthy, the relative inhibition of thrombin generation was
higher for BXPC3 cells compared to MCF7 cells (Table III). In comparison, the anti-TF
antibodies did not have a significant effect on thrombin generation
in the presence of HUVECs.
 | Table IIIImpact of anti-TF antibodies (4509
and TF9-10H10) on thrombin generation by BXPC3 cells, MCF7 cells or
HUVECs in normal PPP (control) and in the presence of
MP-Reagent. |
Table III
Impact of anti-TF antibodies (4509
and TF9-10H10) on thrombin generation by BXPC3 cells, MCF7 cells or
HUVECs in normal PPP (control) and in the presence of
MP-Reagent.
| Parameters | Control
| BXPC3
| MCF7
| HUVEC
|
|---|
| PPP | Isotype IgG | 4509 | TF9-10H10 | PPP | Isotype IgG | 4509 | TF9-10H10 | PPP | Isotype IgG | 4509 | TF9-10H10 | PPP | Isotype IgG | Anti-TF | TF9–10H10 |
|---|
| Lagtime (min) | 8.8±0.7 | 9.9±0.7 | 10.8±1.2 | 11.7±0.9 | 4.1±0.7 | 4.2±0.1 | 6.9±0.3c | 9.6±1.1c | 5.5±0.3 | 5.5±0.2 | 6.6±0.3c | 7.9±0.4c | 7.2±0.8 | 7.4±0.7 | 8.1±0.6 | 8.7±0.6 |
| tt Peak (min) | 14.6±0.6 | 15.8±1.1 | 19.2±5.1 | 20.1±4.1 | 5.9±1.1 | 6.1±0.2 | 10.8±0.2c | 16.2±1.6c | 8.2±0.2 | 8.8±0.4 | 10.2±1.2b | 12.5±2.3c | 10.8±0.9 | 10.1±1.2 | 12.1±1.0 | 13.3±1.1 |
| Peak (nM) | 136±14 | 119±12 | 118±11 | 115.3±10 | 358±11 | 348±10 | 169±12c | 95.6±11c | 192±11 | 188±12 | 151±8a | 126±10b | 171±11 | 169±12 | 168±10 | 160±12 |
| MRI (nM/min) | 24±11 | 20±13 | 14±8 | 13±6 | 198±12 | 183±9 | 44±8c | 15±7c | 66±8 | 57±7 | 42±2b | 26±8b | 49±9 | 48±8 | 43±9 | 35±8 |
Expression of cancer procoagulant
activity by BXPC3 and MCF7 cells
Both BXPC3 and MCF7 cells expressed cancer
procoagulant activity, with MCF-7 cells expressing significantly
higher activity compared to BXPC3 cells (220±35 mU/mg vs. 60±15
mU/mg, respectively; p<0.05). Addition of the cysteine protease
inhibitor E64 resulted in significant inhibition of FXa activity in
the presence of both BXPC3 (91% inhibition) and MCF7 cells (84%
inhibition) (Table IV).
 | Table IVComparison of the expression of
cancer pro-coagulant (CP) activity by MCF7 and BXPC3 cells. |
Table IV
Comparison of the expression of
cancer pro-coagulant (CP) activity by MCF7 and BXPC3 cells.
| Cell lines | CP (mU/mg) | Inhibition by E64
(%) |
|---|
| MCF7 | 220±35 | 84 |
| BXPC3 | 60±15 | 91 |
Influence of contact phase activation on
thrombin generation triggered by BXPC3 and MCF7 cells
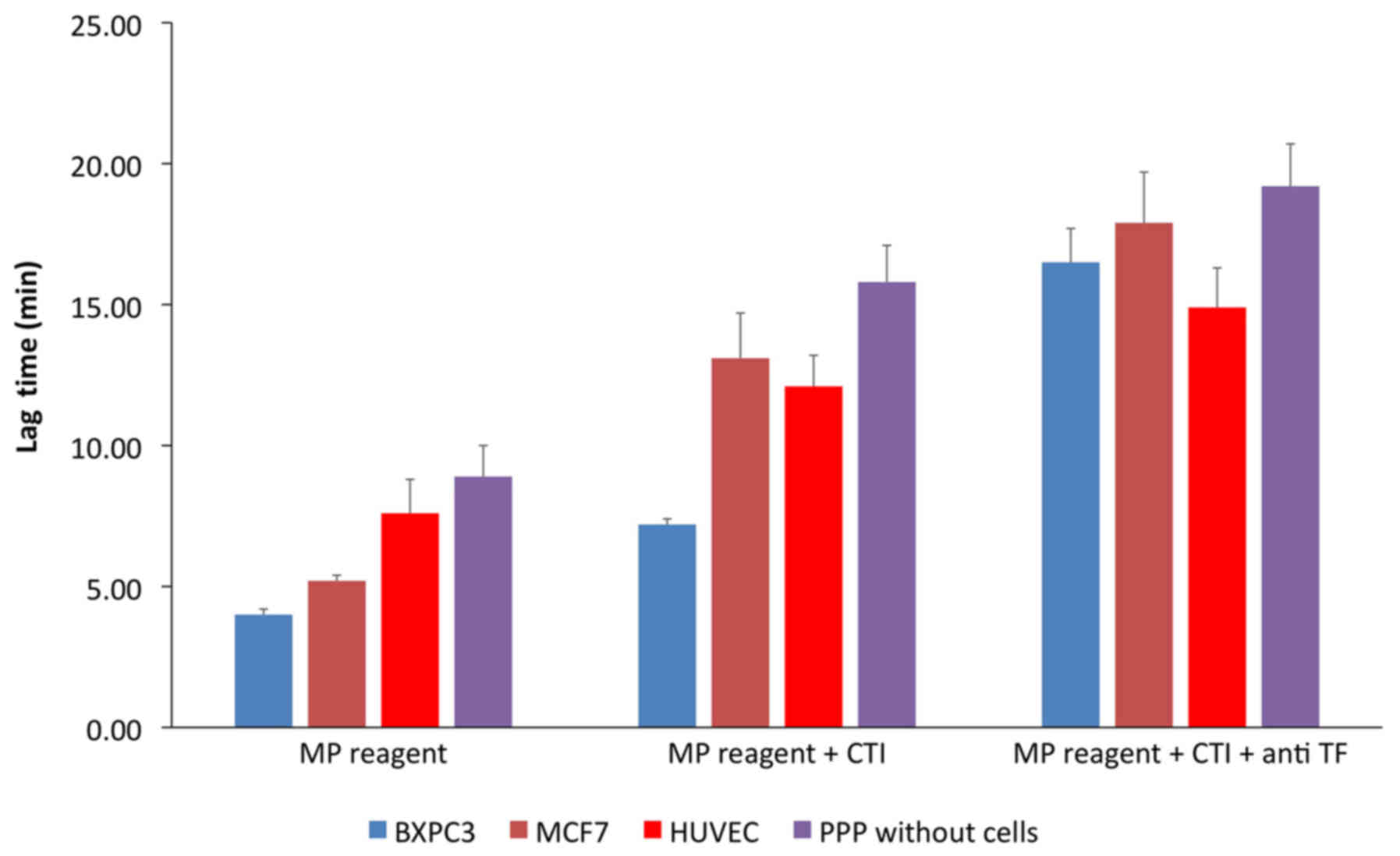
In the control experiment (PPP without cells) the
addition of CTI (corn trypsin inhibitor) resulted in prolongation
of the lag-time by 1.7±0.9-fold, compared to that observed in the
absence of CTI. In normal PPP with BXPC3 or MCF7 cells, addition of
CTI increased the lag-time by 1.8±1.3-fold and 2.6±1.2-fold,
respectively, compared to the same experiment without CTI (Fig. 2). The increase of the lag-time
induced by CTI was significantly less important in PPP with HUVECs
(1.6±1.1-fold). Concomitant addition of CTI and anti-TF increased
the lag-time 2.3-fold in the presence of BXPC3 cells, 1.3-fold in
the presence of MCF7, 1.2-fold in the presence of HUVECs, and
1.2-fold in cell-free PPP.
Thrombin generation triggered by BXPC3
and MCF7 cells in clotting factor-deficient plasma
The interaction of cancer cells with clotting
factors was investigated by assessing thrombin generation in plasma
deficient in specific clotting factors compared to normal plasma
(control experiment). In the presence of BXPC3 or MCF7 cells,
thrombin generation in plasma deficient for clotting factors VII,
IX, XII, XI, and VIII was significantly reduced compared to normal
plasma. Of note, the decrease of thrombin generation was different
for each type of clotting factor-deficient plasma (table V).
 | Table VParameters of thrombin generation
triggered with MP reagent by BXPC3 or MCF7 cells in the presence of
in normal PPP and in plasma selectively depleted of the indicated
clotting factors. |
Table V
Parameters of thrombin generation
triggered with MP reagent by BXPC3 or MCF7 cells in the presence of
in normal PPP and in plasma selectively depleted of the indicated
clotting factors.
| Parameters | Lag-time (min)
| tt-Peak (min)
| Peak (nM)
| MRI (nM/min)
|
|---|
| BXPC3 | MCF7 | BXPC3 | MCF7 | BXPC3 | MCF7 | BXPC3 | MCF7 |
|---|
| Normal PPP | 4.0±0.6 | 5.2±0.1 | 5.9±1.2 | 8.1±1.3 | 320±12.6 | 194±10 | 188±7 | 67±2 |
| FVII
deficiency | 11.2±0.9b | 9.1±0.2b | 20.1± 0.9b | 11.2±1.2a | 66±11b | 79±9b | 9±2b | 37±3b |
| FIX deficiency | 5.9±0.8a | 13±0.8b | 12.2±2.0a | 12.1±1.3a | 70±12b | 15±2b | 12±3b | 2.3±4b |
| FXII
deficiency | 11.2±0.2b | 8.3±1.5a | 14.3±0.8a | 16.3±1.4b | 192±8b | 88±7b | 64±8b | 13±2b |
| FXI deficiency | 9.3±1.2b | 10.3±1.2b | 12.5±2.4a | 14.8±1.6b | 145±10b | 58±6b | 48±3a | 14±5b |
| FVIII
deficiency | 4.8±1.2 | 5.1±0.9 | 13.1±1.2a | 17.2±2.1b | 86±7b | 30±3b | 11±6a | 2.8±3b |
| PC deficiency | 3.1± 0.9 | 4.9±1.1 | 4.4±0.8a | 6.2±1.2 | 410±14b | 380±12b | 310±15b | 314±14b |
In FVII-deficient plasma, the lag-time increased
2.7-fold in the presence of BXPC3 cells and 1.7-fold in the
presence of MCF7 cells compared to normal PPP (p<0.001). The
peak and MRI decreased by 79 and 95%, respectively, in the presence
of BXPC3 cells and by 59 and 45%, respectively, in the presence of
MCF7 cells (p<0.05).
In FXII-deficient plasma, the lag-time increased
2.7-fold for BXPC3 and 1.6-fold for MCF7 cells, compared to normal
PPP (p<0.05). The peak and the MRI decreased by 26% and 55%,
respectively, in the presence of BXPC3 and by 55% and 81%,
respectively, in the presence of MCF7 cells (p<0.05).
In FXI-deficient plasma, the lag-time increased
2.2-fold for BXPC3 and 1.9-fold for MCF7 cells compared to the
experiment with normal PPP (p>0.05). The peak and the MRI
decreased by 55 and 74%, respectively, in the presence of BXPC3 and
by 70 and 79%, respectively, in the presence of MCF7 cells
(p<0.05).
In FIX-deficient plasma, the lag-time increased
1.4-fold for BXPC3 and 2.5-fold for MCF7 cells compared to normal
PPP (p<0.05). The peak and the MRI decreased by 78 and 94%,
respectively, in the presence of BXPC3 and by 92 and 96%,
respectively, in the presence of MCF7 cells (p<0.05).
In FVIII deficient-plasma, the lag-time was not
modified by either BXPC3 or MCF7 cells, compared to normal PPP.
However, the peak and the MRI decreased by 73 and 94%,
respectively, in the presence of BXPC3 and by 84 and 96%,
respectively, in the presence of MCF7 cells (p<0.05).
In FX-, FV- and FII-deficient plasma, neither BXPC3
nor MCF7 cells induced any detectable thrombin generation. In
protein C-deficient plasma, the peak and the MRI increased 1.3- and
1.6-fold, respectively, in the presence of BXPC3 and 1.9- and
4.7-fold, respectively, in the presence of MCF7 cells (p<0.05).
The lag-time increased 0.7-fold for BXPC3 and 0.9-fold for MCF7
cells as compared to normal PPP (p<0.05).
Impact of tissue factor and procoagulant
phospholipids on the procoagulant capacity of HUVECs, BXPC3 and
MCF7 cells
For control experiments, thrombin generation varied
as a function of the presence of TF and procoagulant phospholipids
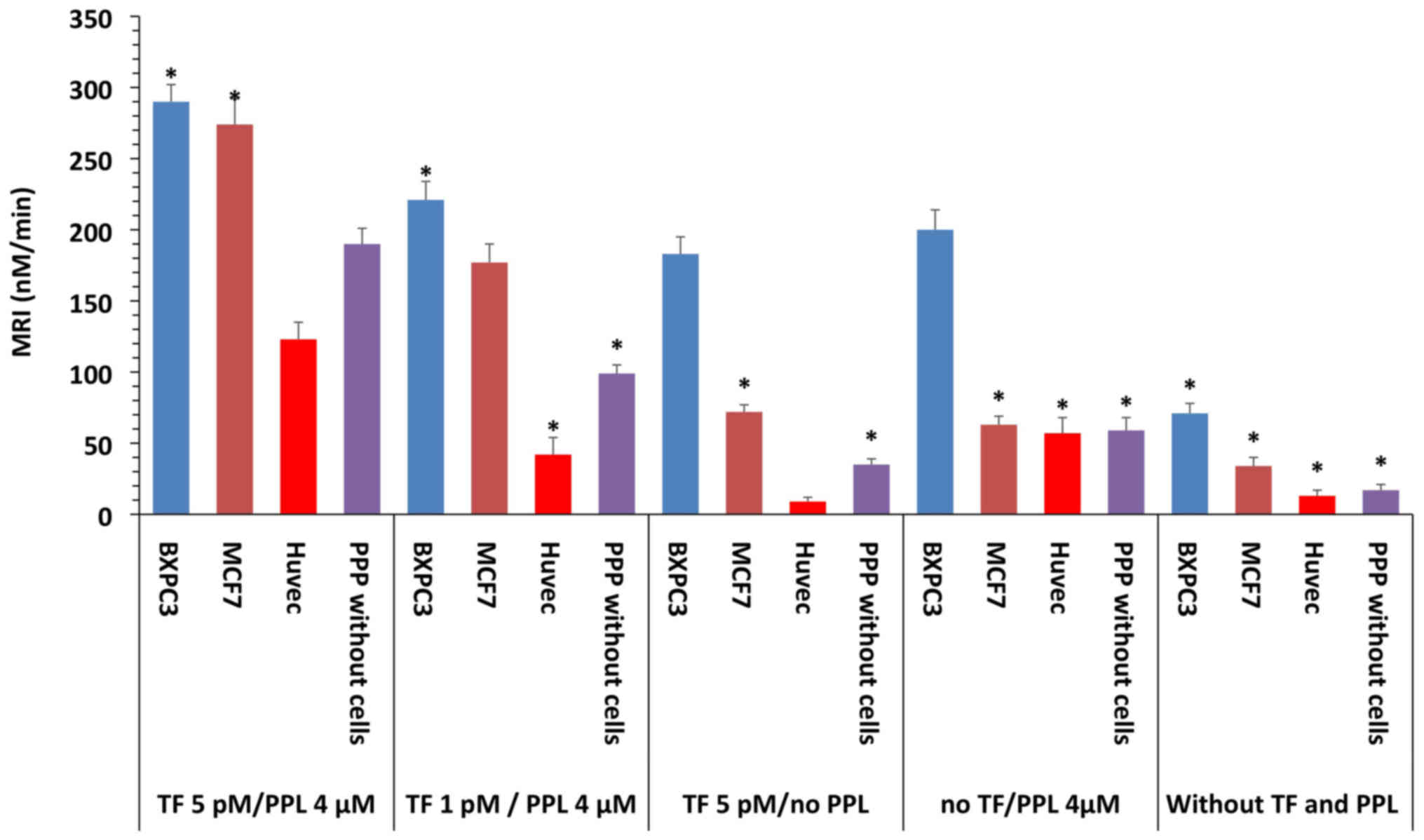
(PPL) and as a function of TF concentration as showed in Table VI and Fig. 3. In the presence of optimal TF
concentrations and procoagulant phospholipids (TF 5 pM/PPL 4
µM), BXPC3 and the MCF7 cells increased the MRI by 52 and
44%, respectively, compared to the MRI obtained in the presence of
TF (5 pM/PPL 4 µM) in PPP alone. Therefore, thrombin
generation was higher in the presence of BXPC3 or MCF7 cells
compared to the upper normal levels of normal plasma. When no TF
and procoagulant phospholipids were added to the plasma with BXPC3,
MCF7 or HUVECs, the MRI and peak were significantly lower compared
to TF (5 pM/PPL 4 µM). Both BXPC3 and MCF7 cells and HUVECs
induced significantly lower MRI with a reduction of 75 and 81%,
respectively, in the presence of BXPC3 and MCF7, and 90% for HUVECs
and the cell-free control (Table
VI).
 | Table VIThrombin generation in the presence
of BXPC3 cells, MCF7 cells and HUVECs triggered by different
conditions. |
Table VI
Thrombin generation in the presence
of BXPC3 cells, MCF7 cells and HUVECs triggered by different
conditions.
| Parameters | TF 5 pM/PPL 4
µM
| TF 1 pM/PPL 4
µM
| TF 5 pM/no PPL
|
|---|
| HUVEC | BXPC3 | MCF7 | PPP without
cells | HUVEC | BXPC3 | MCF7 | PPP without
cells | HUVEC | BXPC3 | MCF7 | PPP without
cells |
|---|
| Lagtime (min) | 2.3±0.6 | 2.1±0.2 | 2.5±0.4 | 2.9±0.7 | 4.5±0.7c | 2.6±0.8 | 2.8±1.1 | 3.9±0.8a | 6.1±0.1b | 3.7±0.8c | 4.7±0.6c | 5.8±1.0c |
| ttPeak (min) | 4.7±0.5 | 3.6±0.6 | 4.0±0.4 | 4.9±0.6 | 8.5±0.8c | 4.4±0.9 | 4.7±0.5b | 6.5±1.5a | 14.7±1.1c | 4.9±1.1a | 7.2±1.3c | 10.3±1.1c |
| Peak (nM) | 297±10 | 435±11 | 411±13 | 380±14 | 170±11c | 399±11c | 334±11c | 260±12c | 76±13b | 220±11c | 180±10c | 160±13c |
| MRI (nM/min) | 123±12 | 290±12 | 274±18 | 190±11 | 42±12c | 221±13c | 177±13c | 99±6c | 9±2c | 183±12c | 72±5c | 35±4c |
| No TF/PPL 4
µM
| Without TF and PPL
| | | | |
| Lagtime (min) | 7.2±0.8c | 4.0±0.7c | 5.2±0.9c | 8.8±1.5c | 8.6±1.1c | 4.1±1.1c | 6.1±0.9c | 9.6±1.2c | | | | |
| ttPeak (min) | 10.2±1.2c | 5.8±1.1a | 8.2±1.0c | 14.6±1.3c | 15.2±1.4c | 6.9±1.3c | 9.6±1.1c | 16.3±1.5c | | | | |
| Peak (nM) | 171±13c | 360±11c | 190±12c | 136±11c | 86±11c | 199±14c | 121±10c | 118±9c | | | | |
| MRI (nM/min) | 57±11c | 200±14c | 63±6c | 24±9c | 13±4c | 71±7c | 34±6c | 17±4c | | | | |
In plasma containing BXPC3 or MCF7 cells without
addition of TF but with optimal concentrations of procoagulant
phospholipids, the MRI significantly decreased compared to the same
experiment in the presence of TF (5 pM/PPL 4 µM). Both BXPC3
and MCF7 cells induced significantly lower thrombin generation
compared to that observed in the presence of TF (5 pM/PPL 4
µM) (Table VI). The MRI
decreased by 31% and 77%, respectively, in the presence of BXPC3
and MCF7, and 53 and 68% for HUVECs and the cell-free control,
respectively.
In BXPC3 or MCF7 containing plasma at optimal TF
concentrations but without any addition of procoagulant
phospholipids, the MRI significantly decreased compared to the same
experiment in the presence of TF (5 pM/PPL 4 µM). Both BXPC3
and MCF7 cells induced significantly less thrombin generation
compared to that observed in the presence of TF (5 pM/PPL4
µM) (Table VI). The MRI
decreased 36 and 73%, respectively, in the presence of BXPC3 and
MCF7 cells and 92 and 81% for HUVECs and the cell-free control,
respectively.
In BXPC3 or MCF7-containing plasma with optimal
concentrations of procoagulant phospholipids but with a suboptimal
concentration of TF, the MRI significantly decreased as compared to
TF (5 pM/PPL 4 µM). The MRI decreased 23 and 35%,
respectively, in the presence of BXPC3 and MCF7 cells and 65 and
47% for HUVEC cells and the cell-free control, respectively
(Table VI).
The impact of TF and procoagulant phospholipids on
the MRI in plasma with BXPC3 and MCF7 cells is further detailed in
Fig. 3.
Discussion
The present study addressed three issues associated
with cancer-induced hypercoagulability linked to thrombin
generation: a) identification of the principal procoagulant
elements expressed by cancer cells, b) evaluation of the relative
roles of the TF-pathway and the intrinsic pathway in the thrombin
generation process and c) contribution of the TF and procoagulant
phospholipids present in the plasma microenvironment.
It is well established that cancer cells express TF,
the major trigger of blood coagulation (28–30).
We have previously demonstrated that BXPC3 pancreatic
adenocarcinoma cells express significantly higher amounts of TF
compared to MCF7 breast cancer cells as well as by normal human
endothelial cells (HUVECs). The levels of TF expressed by cancer
cells have been correlated with their effect on thrombin generation
(21). In agreement, we herein
show that the activity of TF (TFa) expressed by BXPC3 cells was
significantly higher than that expressed by MCF7 cells. The number
of BXPC3 or MCF7 cells tend to correlate with the TFa which was not
the case for the HUVECs. The levels of asTF were also correlated
with the number of BXPC3 or MCF7 cells. The TF isoform described as
alternatively spliced TF (asTF) is expressed by both tumor cells
and tumor tissues from cancer patients (31). However, the procoagulant activity
of asTF has been debated (32–34).
We find that astF was expressed in abundant amounts by BXPC3 cells
while lower expression levels were observed for MCF7 cells and
HUVECs. The levels of asTF expressed by both types of cancer cells
increased in parallel with the number of cells and were correlated
with the release of TFa in the cellular environment. However, the
experimental design of the present study does not allow the
exploration of any potential relationship between the procoagulant
activity of cancer cells and the levels of asTF.
The stimulation of thrombin generation by BXPC3 and
MCF7 cells was partially reversed by the presence of a mono-clonal
anti-TF antibody, which was not the case for HUVECs. These findings
could be explained in, at least, two ways. First, the concentration
of the anti-TF antibody may not be sufficiently high to completely
neutralize TF activity. However, this hypothesis can be ruled out
since the concentrations of the TF antibody was sufficiently high
to completely inhibit thrombin generation in the presence of high
levels of TF.
Second, thrombin generation could also be driven by
alternative pathways independent of TF such as expression of cancer
procoagulant activity thereby resulting in activation of the
intrinsic system of blood coagulation. Both BXPC3 and MCF7 cells
expressed CP and directly activate FX in a cysteine-dependent
manner. However, the cancer procoagulant activity of MCF7 cells was
approximately 4-fold higher compared to BXPC3 cells. The capacity
of cancer cells to activate the intrinsic pathway of blood
coagulation was determined using CTI, which selectively inhibits
FXIIa (35,36). Inhibition of FXIIa resulted in a
significant prolongation of the initiation phase of thrombin
generation triggered by both BXPC3 and MCF7 cells. This experiment
clearly shows that both types of cancer cells could induce thrombin
generation via activation of FXII, with MCF7 cells being more
potent in this regard.
We also evaluated the role of each of the different
clotting factors on thrombin generation triggered by cancer cells.
The results show that FVII was of major importance for the
initiation and propagation phase of thrombin generation induced by
BXPC3 cells. The binding of factor VII to TF is considered to be
the principal pathway of FX activation during normal coagulation
(14). Thrombin generation
triggered by the BXPC3 cells in FVII-deficient plasma thrombin
could not be sustained, in contrast to thrombin generation
triggered by MCF7 cells, in full agreement with the capacity of
MCF7 to trigger thrombin generation via an alternative pathway
through activation of FXII. This was confirmed by experiments with
FXII-deficient plasma, where MCF7 mediated thrombin generation was
almost completely abrogated. FXII is activated following
prekallikrein and high molecular weight kininogen activation. FVII
and thrombin are also among the activators of FXII. In our
experimental conditions, in the presence of either BXPC3 or MCF7
cells, the levels of both FVII and FII were normal. Therefore, we
assume that when coagulation was triggered by MCF7 cells, the
deficiency of FXII interrupted the activation pathway leading to
FXIa generation and to intrinsic tenase formation. The experiments
with FXI-deficient plasma further supported the importance of the
intrinsic pathway activation by the MCF7 cells. In contrast, for
BXPC3 cells, the intrinsic pathway was clearly secondary compared
to TF. Thrombin generation triggered by both BXPC3 or MCF7 cells
was abrogated in FVIII- or FIX-deficient plasma confirming that the
formation of the intrinsic tenase is of major importance in the
amplification of the propagation phase of thrombin generation in
the presence of cancer cells. In addition, this experiment
demonstrated the weak procoagulant efficiency of the CP. In plasma
deficient of FX, FV or FII neither BXPC3 nor MCF7 cells, were able
to promote thrombin generation. Thus, the possibility of a
'prothrombinase-like' or a 'thrombin like' activity expressed by
the cancer cells was eliminated. Finally, the experiments with
protein C-deficient plasma showed that the down-regulation of
thrombin generation by the protein C pathway was significantly more
important for MCF7 cells as compared to BXPC3 cells.
The last part of the study revealed that the
procoagulant activity of cancer cells was necessary, but not
sufficient, to induce hypercoagulability; i.e. thrombin generation
higher than the upper normal limit, as defined by the addition of
physiologically relevant concentrations of TF and procoagulant
phospholipid to normal plasma (37,38).
Subsequently, we determined if the procoagulant efficiency of
cancer cells could be amplified by procoagulant elements in the
plasma. In plasma enriched with an optimal concentration of
procoagulant phospholipids, the presence of BXPC3 cells resulted in
near normalization of thrombin generation. In the presence of MCF7
cells and procoagulant phospholipids, thrombin generation remained
significantly lower compared to the normal levels under optimal
conditions. We also examined the impact of exogenous TF (without
any exogenous addition of procoagulant phospholipids) on thrombin
generation triggered by cancer cells. We found that procoagulant
phospholipids are essential for the enhancement of thrombin
generation induced by cancer cells even if the microenvironment is
rich in TF. Importantly, in contrast to cancer cells, normal
endothelial cells (HUVECs) had no effect on thrombin
generation.
We finally studied the impact of the joint presence
of TF and procoagulant phospholipids on thrombin generation
triggered by cancer cells. This experiment showed that
hypercoagulability induced by cancer cells is the resultant of the
combination of the procoagulant properties of cancer cells in
addition to the procoagulant elements of the plasma
microenvironment which consist of a) an optimum concentration of
procoagulant phospholipids and b) TF at concentrations higher than
1 pM.
In conclusion, we herein report that BXPC3 and MCF7
cancer cells trigger and enhance thrombin generation by both the
TF-pathway and the intrinsic pathway. Although the TF-pathway is
dominant, the intrinsic system should not be neglected since the
relative impact of the two pathways varies as a function of cancer
cell type. The formation of intrinsic tenase and prothrombinase
plays a major role for thrombin generation triggered by cancer
cells. To the best of our knowledge, the present study reports for
the first time that the inherent procoagulant properties of cancer
cells are necessary, but not sufficient, to induce
hypercoagulability since procoagulant elements of the
microenvironment, in particular TF and phospholipids, are necessary
elements for cancer-induced hypercoagulability.
Acknowledgments
The authors wish to acknowledge Ms. Hayat Mokrani
and Mr. Matthieu Gusse, for their skillful technical assistance.
The study was supported by an unrestricted grant from Stago France
and by the 'Association de Recherche sur la Thrombose et
l'Evaluation de son Risque'.
Glossary
Abbreviations
Abbreviations:
|
VTE
|
venous thromboembolism
|
|
TF
|
tissue factor
|
|
CP
|
cancer procoagulant
|
|
MCF7
|
Michigan Cancer Foundation 7 human
breast cancer cells
|
|
BXPC3
|
human pancreatic adenocarcinoma
cells
|
|
FVII
|
coagulation factor VII
|
|
FXII
|
coagulation factor XII
|
|
FXI
|
coagulation factor XI
|
|
FIX
|
coagulation factor IX
|
|
FVIII
|
coagulation factor VIII
|
|
FX
|
coagulation factor X
|
|
FV
|
coagulation factor V
|
|
FII
|
coagulation factor II
|
|
TFa
|
tissue factor activity
|
|
asTF
|
alternatively spliced TF
|
|
RVV
|
Russell's viper venom
|
|
PPP
|
platelet poor plasma
|
|
MRI
|
mean rate index
|
|
HUVECs
|
primary human umbilical vein
endothelial cells
|
|
CTI
|
corn trypsin inhibitor
|
|
TG
|
thrombin generation
|
|
UNL
|
upper normal limit
|
|
ttPeak
|
time to peak
|
|
PPL
|
procoagulant phospholipids
|
References
|
1
|
Trousseau A: Clinique Médicale de
l'Hôtel-Dieu de Paris. 3. 2nd edition. JB Baillière; Paris: pp.
654–712. 1865
|
|
2
|
Falanga A, Russo L and Milesi V: The
coagulopathy of cancer. Curr Opin Hematol. 21:423–429. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Thromboembolism is a leading cause of
death in cancer patients receiving outpatient chemotherapy. J
Thromb Haemost. 5:632–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chew HK, Wun T, Harvey D, Zhou H and White
RH: Incidence of venous thromboembolism and its effect on survival
among patients with common cancers. Arch Intern Med. 166:458–464.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prandoni P, Falanga A and Piccioli A:
Cancer and venous thromboembolism. Lancet Oncol. 6:401–410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AY and Levine MN: Venous
thromboembolism and cancer: Risks and outcomes. Circulation.
107(Suppl 1): I17–I21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caine GJ, Stonelake PS, Lip GY and Kehoe
ST: The hypercoagulable state of malignancy: Pathogenesis and
current debate. Neoplasia. 4:465–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Cicco M: The prothrombotic state in
cancer: Pathogenic mechanisms. Crit Rev Oncol Hematol. 50:187–196.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roberts HR, Hoffman M and Monroe DM: A
cell-based model of thrombin generation. Semin Thromb Hemost.
32(Suppl 1): 32–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin C, Mackman N and Key NS:
Microparticles and cancer. Pathophysiol Haemost thromb. 36:177–183.
2008. View Article : Google Scholar
|
|
11
|
Davila M, Amirkhosravi A, Coll E, Desai H,
Robles L, Colon J, Baker CH and Francis JL: Tissue factor-bearing
microparticles derived from tumor cells: Impact on coagulation
activation. J Thromb Haemost. 6:1517–1524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rauch U and Nemerson Y: Circulating tissue
factor and thrombosis. Curr Opin Hematol. 7:273–277. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Butenas S, Orfeo T and Mann KG: Tissue
factor activity and function in blood coagulation. Thromb Res.
122(Suppl 1): S42–S46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mackman N: The role of tissue factor and
factor VIIa in hemostasis. Anesth Analg. 108:1447–1452. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mutch NJ: Emerging roles for factor XII in
vivo. J Thromb Haemost. 9:1355–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Müller F and Renné T: Novel roles for
factor XII-driven plasma contact activation system. Curr Opin
Hematol. 15:516–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomassen MC, Heinzmann AC, Herfs L,
Hartmann R, Dockal M, Scheiflinger F, Hackeng TM and Rosing J:
Tissue factor-independent inhibition of thrombin generation by
tissue factor pathway inhibitor-α. J Thromb Haemost. 13:92–100.
2015. View Article : Google Scholar
|
|
18
|
Nickel KF, Ronquist G, Langer F, Labberton
L, Fuchs TA, Bokemeyer C, Sauter G, Graefen M, Mackman N, Stavrou
EX, et al: The polyphosphate-factor XII pathway drives coagulation
in prostate cancer-associated thrombosis. Blood. 126:1379–1389.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gordon SG and Mielicki WP: Cancer
procoagulant: A factor X activator, tumor marker and growth factor
from malignant tissue. Blood Coagul Fibrinolysis. 8:73–86. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Donati MB, Gambacorti-Passerini C, Casali
B, Falanga A, Vannotti P, Fossati G, Semeraro N and Gordon SG:
Cancer procoagulant in human tumor cells: Evidence from melanoma
patients. Cancer Res. 46:6471–6474. 1986.PubMed/NCBI
|
|
21
|
Gerotziafas GT, Galea V, Mbemba E,
Khaterchi A, Sassi M, Baccouche H, Prengel C, van Dreden P, Hatmi
M, Bernaudin JF, et al: Tissue factor over-expression by human
pancreatic cancer cells BXPC3 is related to higher prothrombotic
potential as compared to breast cancer cells MCF7. Thromb Res.
129:779–786. 2012. View Article : Google Scholar
|
|
22
|
Marchetti M, Diani E, Ten Cate H and
Falanga A: Characterization of the thrombin generation potential of
leukemic and solid tumor cells by calibrated automated
thrombography. Haematologica. 97:1173–1180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Dreden P, Rousseau A, Savoure A,
Lenormand B, Fontaine S and Vasse M: Plasma thrombomodulin
activity, tissue factor activity and high levels of circulating
procoagulant phospholipid as prognostic factors for acute
myocardial infarction. Blood Coagul Fibrinolysis. 20:635–641. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rousseau A, Favier R and Van Dreden P:
Elevated circulating soluble thrombomodulin activity, tissue factor
activity and circulating procoagulant phospholipids: New and useful
markers for pre-eclampsia? Eur J Obstet Gynecol Reprod Biol.
146:46–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schneider P, Van Dreden P, Rousseau A,
Kassim Y, Legrand E, Vannier JP and Vasse M: Increased levels of
tissue factor activity and procoagulant phospholipids during
treatment of children with acute lymphoblastic leukaemia. Br J
Haematol. 148:582–592. 2010. View Article : Google Scholar
|
|
26
|
Hemker HC, Giesen P, AlDieri R, Regnault
V, de Smed E, Wagenvoord R, Lecompte T and Béguin S: The calibrated
automated thrombogram (CAT): A universal routine test for hyper-
and hypocoagulability. Pathophysiol Haemost Thromb. 32:249–253.
2002. View Article : Google Scholar
|
|
27
|
Gerotziafas GT, Depasse F, Busson J,
Leflem L, Elalamy I and Samama MM: Towards a standardization of
thrombin generation assessment: the influence of tissue factor,
platelets and phospholipids concentration on the normal values of
Thrombogram-Thrombinoscope assay. Thromb J. 3:162005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tilley RE, Holscher T, Belani R, Nieva J
and Mackman N: Tissue factor activity is increased in a combined
platelet and microparticle sample from cancer patients. Thromb Res.
122:604–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Key NS, Chantrathammachart P, Moody PW and
Chang JY: Membrane microparticles in VTE and cancer. Thromb Res.
125(Suppl 2): S80–S83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zwicker JI, Liebman HA, Neuberg D, Lacroix
R, Bauer KA, Furie BC and Furie B: Tumor-derived tissue
factor-bearing microparticles are associated with venous
thromboembolic events in malignancy. Clin Cancer Res. 15:6830–6840.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bogdanov VY, Kirk RI, Miller C, Hathcock
JJ, Vele S, Gazdoiu M, Nemerson Y and Taubman MB: Identification
and characterization of murine alternatively spliced tissue factor.
J Thromb Haemost. 4:158–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Böing AN, Hau CM, Sturk A and Nieuwland R:
Human alternatively spliced tissue factor is not secreted and does
not trigger coagulation. J Thromb Haemost. 7:1423–1426. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szotowski B, Antoniak S and Rauch U:
Alternatively spliced tissue factor: A previously unknown piece in
the puzzle of hemostasis. Trends Cardiovasc Med. 16:177–182. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Censarek P, Bobbe A, Grandoch M, Schrör K
and Weber AA: Alternatively spliced human tissue factor (asHTF) is
not pro-coagulant. Thromb Haemost. 97:11–14. 2007.PubMed/NCBI
|
|
35
|
Spronk HM, Dielis AW, Panova-Noeva M, van
Oerle R, Govers-Riemslag JW, Hamulyák K, Falanga A and Cate HT:
Monitoring thrombin generation: Is addition of corn trypsin
inhibitor needed? Thromb Haemost. 101:1156–1162. 2009.PubMed/NCBI
|
|
36
|
van Veen JJ, Gatt A, Cooper PC, Kitchen S,
Bowyer AE and Makris M: Corn trypsin inhibitor in fluorogenic
thrombin-generation measurements is only necessary at low tissue
factor concentrations and influences the relationship between
factor VIII coagulant activity and thrombogram parameters. Blood
Coagul Fibrinolysis. 19:183–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parhami-Seren B, Butenas S, Krudysz-Amblo
J and Mann KG: Immunologic quantification of tissue factors. J
Thromb Haemost. 4:1747–1755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berntorp E and Salvagno GL:
Standardization and clinical utility of thrombin-generation assays.
Semin Thromb Hemost. 34:670–682. 2008. View Article : Google Scholar : PubMed/NCBI
|