Introduction
The human epidermal growth factor receptor 3 (HER3
or ErbB3) has recently attracted attention as a candidate target
for anticancer therapy (1,2). HER3 is involved in the development of
a variety of cancer types such as prostate, breast, lung, and
colorectal, as well in the resistance towards tyrosine
kinase-targeted therapies (3,4).
HER3 has an inactive tyrosine kinase domain, therefore its
heterodimerization with other HER-family members is required for
activation and signalling (5). The
preferred partner for HER3 heterodimerization is HER2 and together
they form one of the most potent units in tumourigenesis that is
able to activate downstream signalling pathways, such as MAPK/MEK
and PI-3K/Akt (6). The role of
HER3 expression in resistance to anti-HER2 therapy in breast cancer
is well documented (2,3). Signalling by the HER2/HER3
heterodimer is also critical in hormone-refractory prostate cancer
and it was demonstrated that blocking of heterodimerization
inhibited the growth of hormone-refractory prostate cancer
xenografts (7). HER3 is expressed
in >50% of prostate cancers (PCa) and its expression is strongly
associated with disease progression, androgen resistance, and has
been linked to a less favourable prognosis (8,9).
HER3 is involved in PCa resistance to PI3K inhibiting therapies
(gefitinib, erlotinib and lapatinib), to HER1 and HER2 targeting
immunotherapy (cetuximab and trastuzumab), and to external
radiotherapy (10–12). Several therapeutic agents targeting
HER3 are currently in clinical development, including fully human
and humanized monoclonal antibodies (mAbs), bispecific mAbs, and
tyrosine kinase inhibitors (13).
Clinical evaluations have demonstrated that elevated expression of
HER3 or its ligand heregulin is associated with response to
HER3-targeting therapy (14).
Therefore, determination of HER3 expression level is necessary for
stratification of patients for HER3-targeting therapies.
Currently, molecular phenotyping of cancer relies
mostly on biopsy-based approaches. However, biopsies are invasive
and cannot be used repeatedly. Because of inter- and intratumoural
heterogeneity, the biopsy samples may not be representative of all
metastases, leading to false-negative findings and suboptimal
treatment of patients. In addition, HER3 expression often changes
in response to therapy (15). This
means that a sample from the primary tumour would not be
informative, which requires frequent sampling that is indeed
questionable in the clinics. Taken together, this complicates the
selection of appropriate therapy.
To overcome problems with the invasiveness of
biopsies, spatial and temporal heterogeneity of receptor expression
and to allow monitoring of changes in receptor expression over
time, radionuclide molecular imaging can be applied (16,17).
This method allows for serial investigations of the tyrosine kinase
receptor status before, during and after treatment. Molecular
imaging using radionuclides can therefore strongly contribute to
patient management by selecting eligible patients for a certain
treatment.
Two factors have to be taken into consideration to
reach high specificity and sensitivity in imaging of HER3
expression. First, even in the case of overexpression in tumours,
the expression level of HER3 is low, below 50,000 receptors/cell
(18). This means that a targeting
probe with a low picomolar affinity is required to get images with
appropriate contrast (19).
Second, there is endogenous expression of HER3 in several normal
tissues (http://www.proteinatlas.org). This
may be the reason for the modest imaging contrast of antibody-based
probes for radionuclide imaging of HER3 expression that have
previously been reported (20,21).
Affibody molecules are high-affinity scaffold
proteins with a molecular weight of ~7 kDa, which have demonstrated
their utility as a targeting moiety for imaging agents in oncology.
Affibody molecules with high affinity to several cancer-related
receptors (e.g. EGFR, HER2 and IGF-1R) have previously been
selected (22–25). It has been demonstrated in
preclinical and clinical studies that affibody molecules provide
high contrast imaging already a few hours after administration due
to the fast blood clearance of the unbound tracer and rapid tumour
penetration (26–29). Clinical data show that the
anti-HER2 affibody molecule ABY-025 is non-toxic and
non-immunogenic (27,28). Affibody molecules with low
picomolar affinity to HER3 have been generated recently (29). The tests performed after selection
demonstrated that the anti-HER3 affibody molecules bind selectively
to HER3, but not to 16 common serum proteins as well as
neutravidin, streptavidin, HER1, HER2 and HER4 (30). The anti-HER3 affibody molecules
also demonstrated cross-species reactivity with the murine HER3
counterpart, mErbB3 (31), and
murine models would therefore reflect the factors influencing the
distribution of the anti-HER3 affibody molecules in humans. In
preclinical therapy studies, treatment of mice with a construct
containing two anti-HER3 affibody molecules (600
μg/injection, 3 injections/week) up to 70 days was not
associated with any toxicity (32,33).
The feasibility of using the HER3-targeting affibody
molecule for in vivo imaging has been demonstrated using
technetium-99m (99mTc) label (31). Further development of the imaging
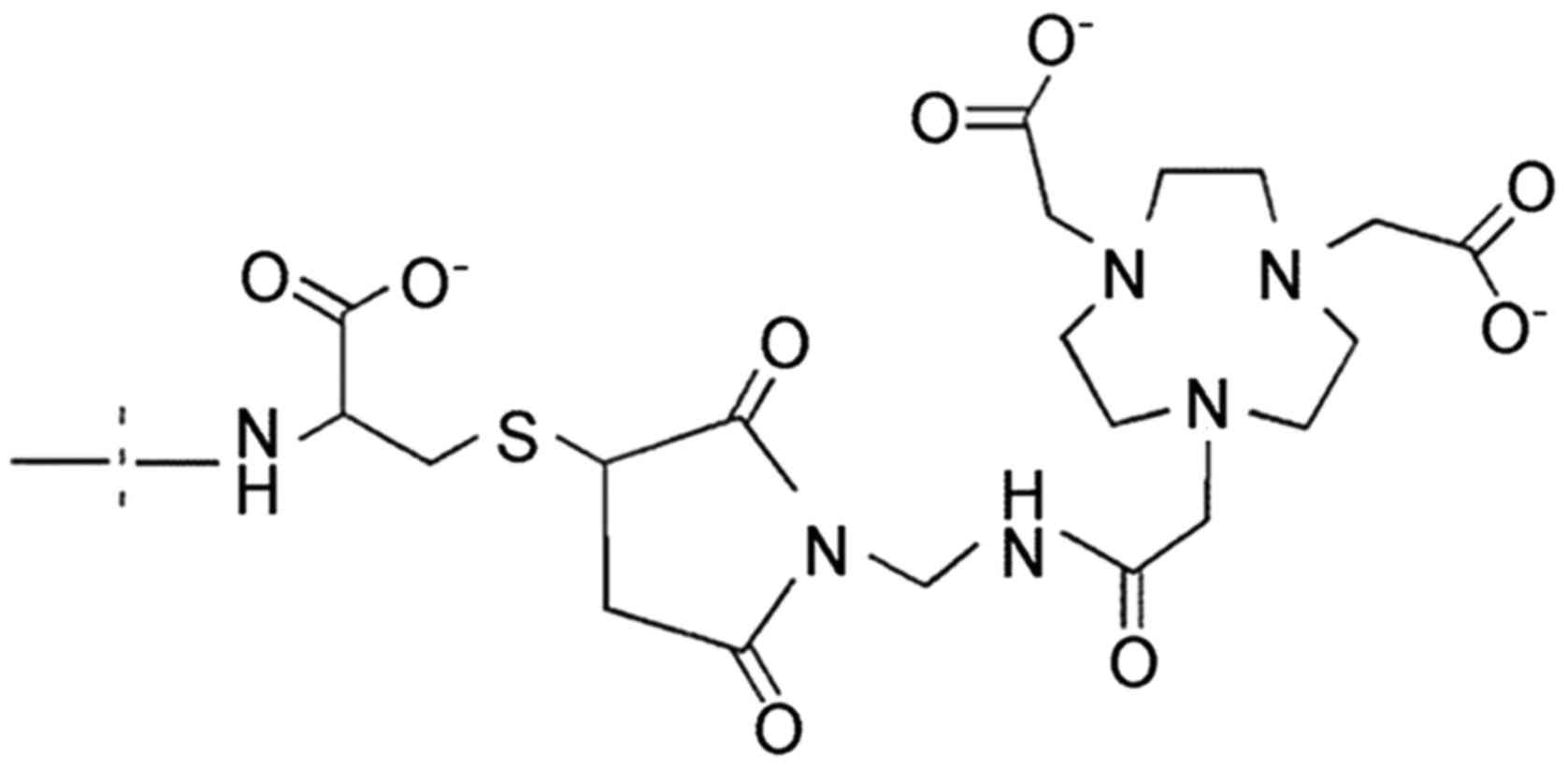
agent was performed by site-specific conjugation of a NOTA chelator
[2,2′,2″-(1,4,7-triazonane-1,4,7-triyl)triacetic acid] to a
C-terminal cysteine (Fig. 1) for
labelling with radio-metals: indium-111 (111In) for
single photon emission computed tomography (SPECT) (34), gallium-68 (68Ga)
(35) and 18F (via AlF
chemistry) (36) for positron
emission tomography (PET). Although 68Ga- and
18F-labelled affibody molecules provided adequate
imaging of HER3 expression in murine models at 1–3 h post injection
(p.i.), biodistribution data for 111In-ZHER3
demonstrated that imaging contrast could be further improved at
later time-points (34).
A possible reason for the observed increase of the
imaging contrast with time may be expression of mErbB3 (murine
counterpart of HER3) in a number of healthy tissues, particularly
in liver and intestines. Internalization of anti-HER3 affibody
molecules after their binding to the receptors is not rapid, and an
appreciable fraction remains bound to receptors on the cell surface
(35). Dissociation of these
surface-bound affibody molecules results in slower clearance of
affibody-bound radioactivity from blood and longer time is required
to reach maximum tumour-to-blood ratio.
Based on this information, our overall goal in this
study was to develop an affibody-based imaging agent to HER3 with
an extended imaging window. Taken into account that PET has certain
advantages over SPECT due to higher sensitivity, better resolution
and quantification accuracy (37),
we aimed to use a positron-emitting radionuclide with a half-life
permitting imaging at the day after injection. Cobalt-55
(55Co) is a positron-emitter with a half-life of 17.5 h
and positron abundancy of 76%, which can be produced using
low-energy cyclotrons. Its half-life allows performing imaging at
the day of injection as well as the next day. 55Co can
be produced using cyclotrons available in most PET facilities with
costs comparable to the production of copper-64, a positron-emitter
with 12.7 h half-life (38). Due
to its half-life, 55Co also can be distributed to
distant hospitals without cyclotrons. 55Co in ionic form
was earlier used for imaging of various diseases, such as multiple
sclerosis (39) and ischemic
stroke (40). For convenience in
preclinical experiments, a surrogate nuclide for 55Co,
i.e. 57Co (T1/2=271.6 days) could be used
(41). Recently, we demonstrated
that in vitro and in vivo data obtained using
57Co and 55Co were in good agreement
(42). Both anti-HER1 and
anti-HER2 affibody molecules have previously been successfully
labelled with radiocobalt using cyclic tetraaza chelator DOTA
(41,43). Both radiolabelled conjugates
demonstrated high stability of Co-DOTA complex in vivo. The
anti-HER2 affibody molecule labelled with radiocobalt had
significantly higher tumour-to-blood, tumour-to-lung and
tumour-to-muscle ratios than its counterpart labelled with
111In that should improve the overall imaging contrast
(41). Anti-HER1 affibody molecule
labelled with radiocobalt further demonstrated that tumour-to-blood
ratio increased three-fold between 3 and 24 h p.i. (43). Importantly, substitution of
68Ga by 57Co reduced hepatic uptake of
anti-HER1 affibody molecule >3-fold (43).
We hypothesized that imaging of HER3 expression
should be improved with time due to increased imaging contrast. To
prove this, we labelled an anti-HER3 affibody conjugate,
HEHEHE-Z08698-NOTA, with radiocobalt and investigated its in
vitro and in vivo properties.
Materials and methods
Materials
The cell lines for in vitro and in
vivo experiments were purchased from American Type Tissue
Culture Collection (ATCC via LGC Promochem, Borås, Sweden). The
prostate carcinoma (DU145) and colorectal carcinoma (LS174T) cell
lines were cultured in RPMI-1640 media supplemented with 10% fetal
bovine serum (FBS) and 1% PEST (penicillin 100 IU/ml, streptomycin
100 μg/ml) (all from Biochrom AG, Berlin, Germany) at 37°C
and 5% CO2. To detach cells, trypsin-EDTA (0.25%
trypsin, 0.02% EDTA in buffer; Biochrom AG) was used.
57Co was purchased from PerkinElmer Sweden (Upplands
Väsby, Sweden). The affibody molecule used in this study,
HEHEHE-Z08698-NOTA (further denoted ZHER3), was
produced, conjugated with NOTA, purified and characterized as it
was described previously (34).
The equilibrium dissociation constant of ZHER3 binding
to HER3 was 45 pM (34). All
chemicals were from Merck KGaA (Darmstadt, Germany). The buffer
(0.2 M ammonium acetate, pH 5.5) used for labelling procedure was
purified from metal contamination by Chelex 100 resin (Bio-Rad
Laboratories, Hercules, CA, USA). To measure the purity of the
labelled affibody molecules, radio instant thin-layer
chromatography (radio-ITLC, 150–771 Dark Green, Tec-Control
Chromatography strips from Biodex Medical Systems, Shirley, NY,
USA) was used [method was also validated by sodium dodecyl sulphate
polyacrylamide gel electrophoresis (SDS-PAGE)]. The distribution of
radioactivity along the chromatography strips and SDS gels was
measured by a Cyclone Storage Phosphor System (PerkinElmer,
Waltham, MA, USA). Radioactivity content in samples was measured
using automated γ-spectrometer 1480 WIZARD (PerkinElmer).
Obtained values are presented as an average with
standard deviation. In order to calculate significant differences
(p<0.05), data were assessed by an unpaired, two-tailed t-test
using GraphPad Prism (version 6 for windows; GraphPad Software, San
Diego, CA, USA).
Radiolabelling of ZHER3 with
57Co
Affibody conjugate ZHER3 (50 μg
diluted in 9.3 μl PBS) was mixed with 40.7 μl
ammonium acetate buffer, pH 5.5. The stock solution of
57Co (18 μl, 7 MBq) was added and mixture was
incubated at 60°C for 30 min. An aliquot of the labelled protein
was analysed by radio-ITLC eluted with 0.2 M citric acid, pH 2.0.
Free ionic 57Co migrates with the solvent front
(Rf=1), whereas radiolabelled affibody molecules
remain at the application point. Cross-validation of the ITLC
analytical system was performed by SDS-PAGE analysis (200 V, NuPAGE
4–16% Bis-Tris Gel; Invitrogen AB, Carlsbad, CA, USA). NAP-5
columns (GE Healthcare, Logan, UT, USA) equilibrated with PBS, were
used for purification of conjugate to ensure high purity according
to manufacturer's procedure. For stability test, samples of the
purified radiolabelled conjugate were diluted with 500-fold molar
excess of EDTA in PBS, control samples were diluted with equal
amount of PBS. Samples were incubated at room temperature for 1 h
and analysed by radio-ITLC.
In vitro specificity test and cellular
processing of 57Co-ZHER3
The specificity of 57Co-ZHER3
binding to the HER3-receptors and its cellular processing, were
studied using DU145 and LS174T cell lines. In vitro
specificity tests were performed in triplicates according to the
method described earlier (44).
For in vitro specificity test, cells were incubated for 2 h
with 0.1 nM solution of 57Co-ZHER3 or with 50
nM solutions of non-labelled ZHER3, or affibody
conjugate ZHER3-ABD-ZHER3, or seribantumab or
bevacizumab and 0.1 nM 57Co-ZHER3. Values of
cell-associated radioactivity were compared. In cellular processing
study, cells were continuously incubated with 0.1 nM solution of
57Co-ZHER3 and internalized and membrane
bound radioactivity were measured at predetermined time-points. The
membrane bound radioactivity was detached by incubating cells with
0.2 M glycine buffer containing 4 M urea, pH 2.0 for 5 min on ice.
The radioactivity that remained was considered as internalized and
was collected using 1 M sodium hydroxide.
In vivo studies
All animal experiments were planned and performed in
accordance with Swedish national legislation on laboratory animals'
protection and were approved by the local Ethics Committee for
Animal Research in Uppsala.
Female BALB/C nu/nu mice bearing DU145 or LS174T
xenografts were used to study the targeting properties and
biodistribution of radiolabelled affibody conjugate. Cells
[2×106 cells/mouse for LS174T in media and
5×106 for DU145 in matrigel/media (1/1)] were implanted
subcutaneously 10 to 15 days before the experiment. At the time of
the experiment, the weight of mice bearing LT174T xenografts was
23.1±0.8 g and the tumour weight was 0.40±0.07 g. In the case of
DU145 xenografts, the weight of mice was 19±1 g and the tumour
weight was 0.07±0.02 g.
Group of three to four mice received an intravenous
(i.v.) bolus injection of 2 μg of
57Co-ZHER3 (10 kBq/mouse) diluted in 100
μl PBS. The protein dose was adjusted using non-labelled
conjugate. Mice were sacrificed at 3 and 24 h p.i., by injection of
a lethal dose of anaesthesia [mixture of Ketalar (50 mg/ml, Pfizer)
and Rompun (20 mg/ml, Bayer)], which was followed by heart puncture
and exsanguination with a heparinized syringe. Samples of blood,
organs and tumours were collected and uptake of radioactivity in
tissues was measured. Tissue uptake was calculated as the
percentage of injected radioactivity per gram tissue (% ID/g).
Radioactivity uptake in the carcass and gastrointestinal tract with
content was calculated as % ID/whole sample.
In order to evaluate if the uptake of
57Co-ZHER3 in xenografts and
mErbB3-expressing organs (lung, liver, stomach, small intestines
and salivary gland) was receptor mediated, an in vivo
saturation text was performed. 57Co-ZHER3 (10
kBq/mouse) was injected i.v. with the protein dose being adjusted
to 70 μg per mouse by dilution with non-labelled affibody
molecule. Biodistribution was performed at 3 h p.i. as described
above.
After injections of radiolabelled conjugate
conditions of mice were controlled according to Guidelines for Pain
and Distress in Laboratory Animals from National Cancer Institute
(NIH, Bethseda, MD, USA) adopted by Uppsala University; controlled
parameters: exterior, general conditions, behaviour, stress, pain,
ataxia, appetite, sores and blistering, skin colour, eye
inflammation and porphyria. All injections were tolerated well.
Imaging studies
Xenografted mice were imaged at 3 and 24 h p.i. of 2
μg of 57Co-ZHER3. Two mice with LS174T
xenografts (800 kBq) were euthanized before imaging and the urinary
bladders were excised post-mortem. Each subject was imaged using
Triumph™ Trimodality System (Gamma Medica), an integrated
microSPECT/PET/CT platform. The computed tomography (CT)
acquisition: FOV, 80 mm; magnification, 1.48; one projection, 512
frames. SPECT acquisition: FOV, 80 mm; 5 pinhole collimators; 64
projections. CT raw files were reconstructed by filter back
projection (FBP). SPECT raw data was reconstructed by the FLEX™
SPECT software, which uses an ordered subset expectation
maximization (OSEM) iterative reconstruction algorithm. SPECT and
CT data were fused and analyzed using PMOD v3.508 (PMOD
Technologies Ltd., Zurich, Switzerland). One mouse bearing DU145
xenograft (1400 kBq) was imaged using nanoScan SPECT/CT (Mediso
Medical Imaging Systems, Budapest, Hungary). For the 3 h p.i.
imaging, the animal was placed under sevofluran anesthesia. At the
later time-point of 24 h p.i., the animal was euthanized. CT
acquisition: CT-energy peak of 50 keV, 670 μA, 480
projections, 2.29 min acquisition time. SPECT acquisition: energy
window, 109.89–134.31 keV, 110 projection, matrix of 256×256.
Totally 60 min scan time for the 3 h p.i. 180 min for the 24 h p.i.
CT raw files were reconstructed in real time using Nucline 2.03
Software (Mediso Medical Imaging Systems). SPECT raw data were
reconstructed using Tera-Tomo™ 3D SPECT reconstruction
technology.
Results
Labelling ZHER3 with
57Co
The affibody conjugate ZHER3
(HEHEHE-Z08698-NOTA) was labelled with 57Co with a yield
of 81±11% (n=6) as determined by radio-ITLC. The purity of
57Co-ZHER3 after size-exclusion purification
(NAP-5 column) was >99%. The specific activity was up to 0.7
MBq/μg. The radiocobalt label was stable under challenge
with 500-fold molar excess of EDTA.
In vitro specificity test and cellular
processing for 57Co-ZHER3
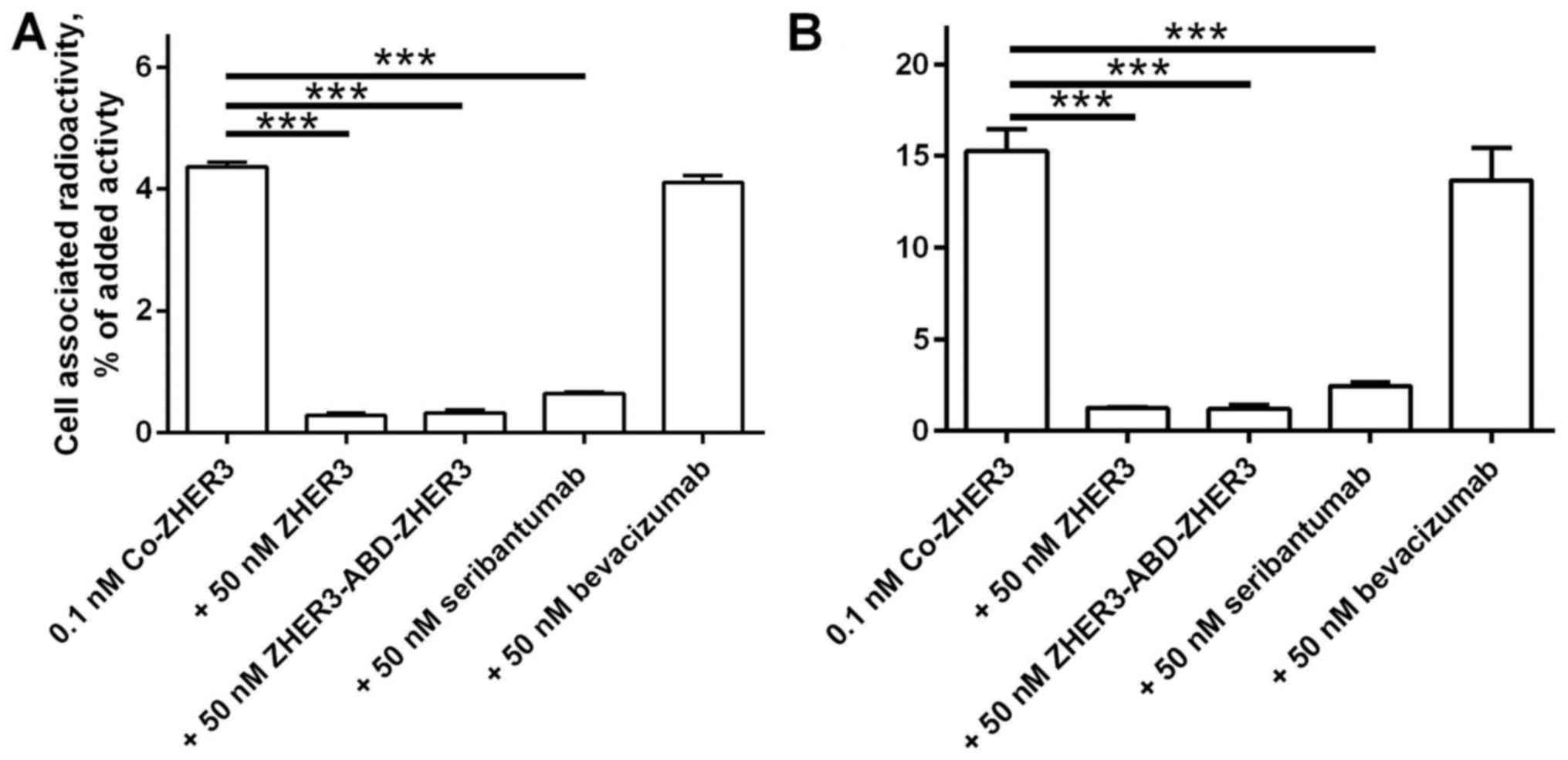
Pre-saturation of receptors with non-labelled
affibody molecule (Fig. 2)
resulted in a significant decrease (n=3, p<10−4) of
the cell-associated radioactivity. These data demonstrated
specificity of the conjugate binding to the HER3-receptors. Binding
of 57Co-ZHER3 to the cells was also
significantly decreased (n=3, p<10−4) by
pre-incubation with anti-HER3 mAb seribantumab (45) and an affibody conjugate
ZHER3-ABD-ZHER3 (32). Binding of radiolabelled conjugate
was not influenced by pre-saturation with non-HER3-targeting mAb,
bevacizumab (Fig. 2).
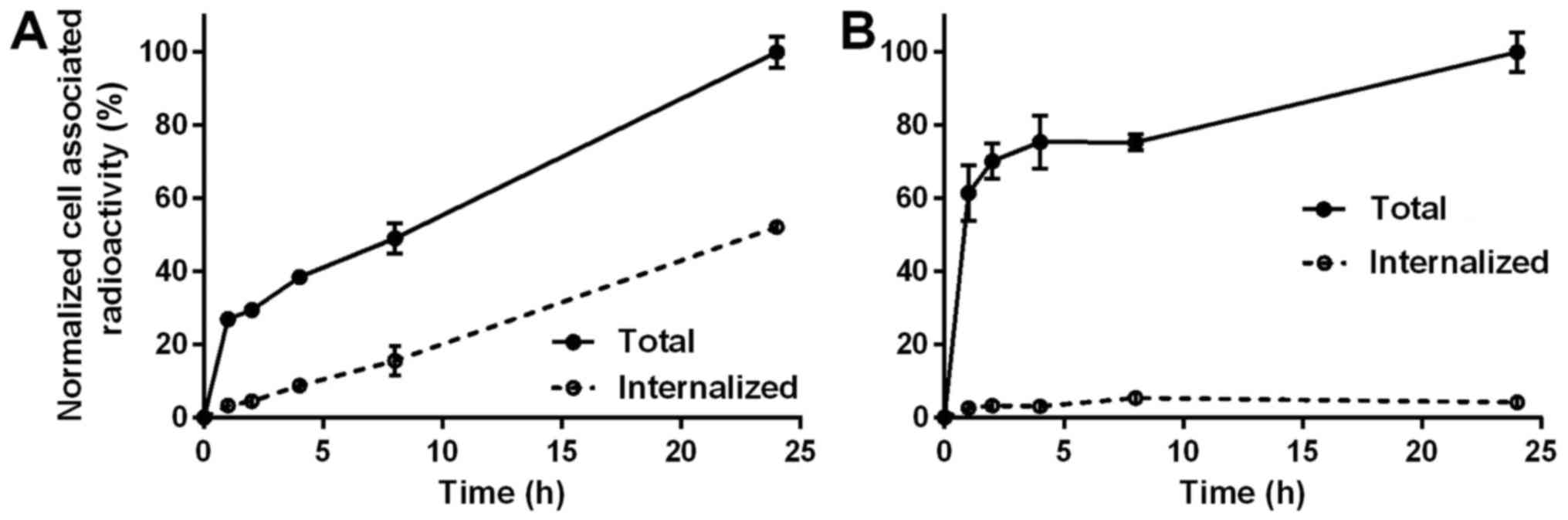
The pattern of cellular processing of
57Co-ZHER3 among the tested cell lines was
different (Fig. 3). For DU145
cells, cellular uptake of radioactivity and internalized fraction
constantly increased over time, and this pattern was similar to the
cellular processing of 99mTc-ZHER3 (31). Total cell associated radioactivity
for DU145 cells increased by 2.5-fold from 1 to 24 h during
continuous incubation and internalized fraction reached 50% at 24
h. For LS174T cells, a phase of rapid binding within the first hour
was followed by a more slow binding and the cell-associated
radioactivity increased only by 60% from 1 to 24 h. The
internalized fraction did not change markedly over time, and was
constantly at a level of 2.5–5% of total cell-associated
radioactivity.
In vivo experiments
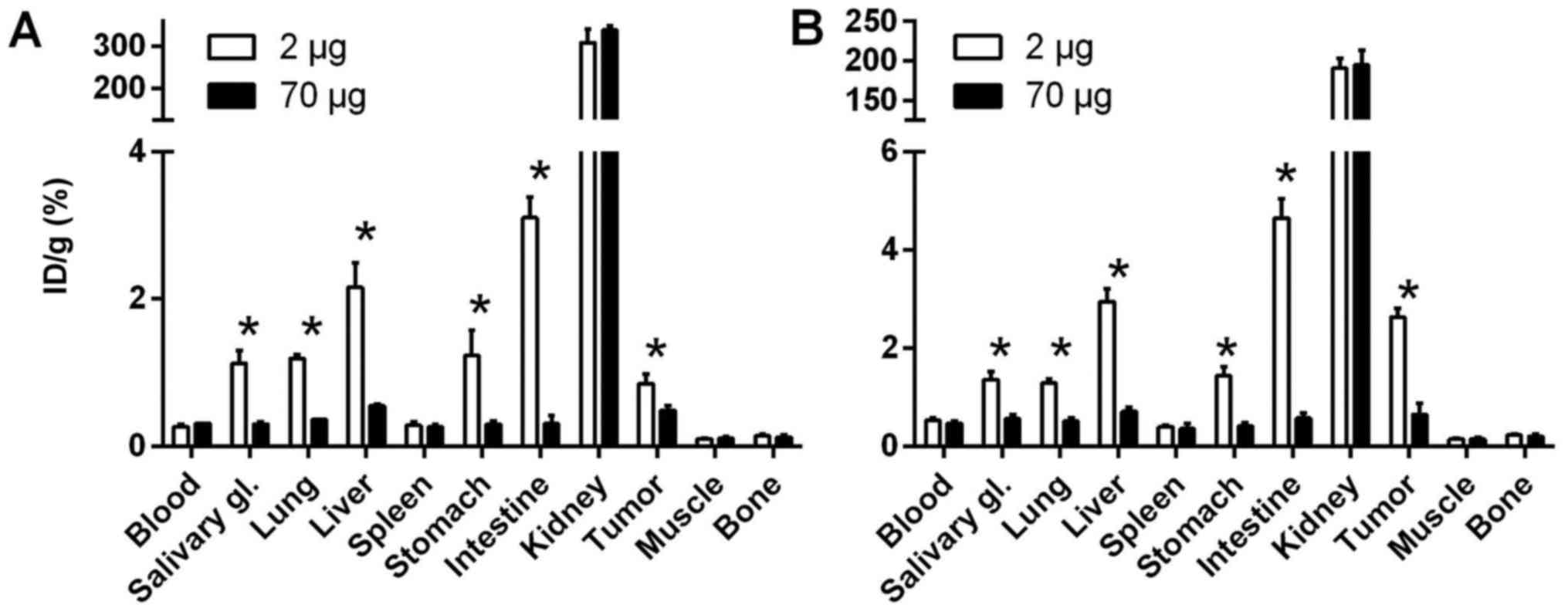
The HER3-mediated uptake of the
57Co-ZHER3 conjugate was demonstrated in both
DU145 and LS174T xenografts (Fig.
4). Co-injection of a high dose of non-labelled conjugate (70
μg) resulted in a significant (n=3–4, p<0.02) decrease in
tumour uptake. Saturation of receptors caused also a significant
decrease in radioactivity uptake in salivary glands, lung, liver,
stomach, small intestine (mErbB3-expressing organs) in comparison
with uptake after injection of 2 μg, indicating a specific
uptake of 57Co-ZHER3 (n=3–4, p<0.005).
Biodistribution of 57Co-ZHER3
at 3 and 24 h p.i. of 2 μg of labelled protein in Balb/c
nu/nu mice bearing DU145 or LS174T xenografts is presented in
Table I. The overall pattern of
radioactivity distribution in both tumour models was in good
agreement with previously published data for technetium-, indium-
and gallium-labelled variants (31,34–36).
Clearance of radioactivity from blood was rapid; at 3 h p.i. the
radioactivity concentration in blood was appreciably below 1% ID/g
and further decreased to 24 h p.i. A decrease in radioactivity
uptake with time was observed in almost all studied organs;
significant decrease was observed in blood, salivary glands, lungs,
liver, tumour and kidneys (n=3–4, p<0.05). Tumour uptake at 3 h
p.i. was 0.8±0.1% ID/g for DU145 xenografts and 2.6±0.2% ID/g for
LS174T xenografts, at 24 h p.i. radioactivity uptake in tumours
decreased by ~25%. In LS174T model tumour uptake of radioactivity
(1.9±0.7% ID/g) at 24 h p.i. significantly (n=4, p<0.0025)
exceeded the liver uptake (1.5±0.2% ID/g). The radioactivity uptake
after injection of 57Co-ZHER3 was the highest
in kidneys at both time-points, indicating that the excretion
pathway was mainly renal.
 | Table IBiodistribution of
57Co-ZHER3 in tumour-bearing Balb/c nu/nu
mice after i.v. injection of 2 μg of conjugate (presented as
%ID/g, gastrointestinal tract (GI) and carcass as %ID/sample).
Results are presented as average of 3–4 animals ± SD. |
Table I
Biodistribution of
57Co-ZHER3 in tumour-bearing Balb/c nu/nu
mice after i.v. injection of 2 μg of conjugate (presented as
%ID/g, gastrointestinal tract (GI) and carcass as %ID/sample).
Results are presented as average of 3–4 animals ± SD.
| DU145
| LS174T
|
|---|
| 3 hours | 24 hours | 3 hours | 24 hours |
|---|
| Blood | 0.27±0.02 | 0.096±0.007a | 0.53±0.05 | 0.23±0.01a |
| Tumour | 0.8±0.1 | 0.58±0.03a | 2.6±0.2 | 1.9±0.1a |
| Salivary
glands | 1.1±0.2 | 0.62±0.02a | 1.4±0.2 | 0.9±0.1a |
| Lung | 1.19±0.05 | 0.36±0.03a | 1.28±0.09 | 0.59±0.05a |
| Liver | 2.2±0.3 | 0.88±0.06a | 2.9±0.3 | 1.5±0.2a |
| Spleen | 0.28±0.04 | 0.27±0.04 | 0.39±0.05 | 0.40±0.03 |
| Stomach | 1.2±0.3 | 0.5±0.2a | 1.4±0.2 | 1.0±0.4 |
| Intestine | 3.1±0.3 | 1.6±0.5a | 4.7±0.4 | 2±1a |
| Kidney | 310±32 | 231±15a | 190±12 | 158±5a |
| Muscle | 0.099±0.009 | 0.068±0.008a | 0.14±0.01 | 0.13±0.02 |
| Bone | 0.14±0.03 | 0.09±0.01a | 0.23±0.02 | 0.22±0.02 |
| GI | 3.6±0.6 | 3±1 | 5.2±0.4 | 4±1 |
| Carcass | 6.3±0.1 | 2±2a | 8.7±0.6 | 5.5±0.7a |
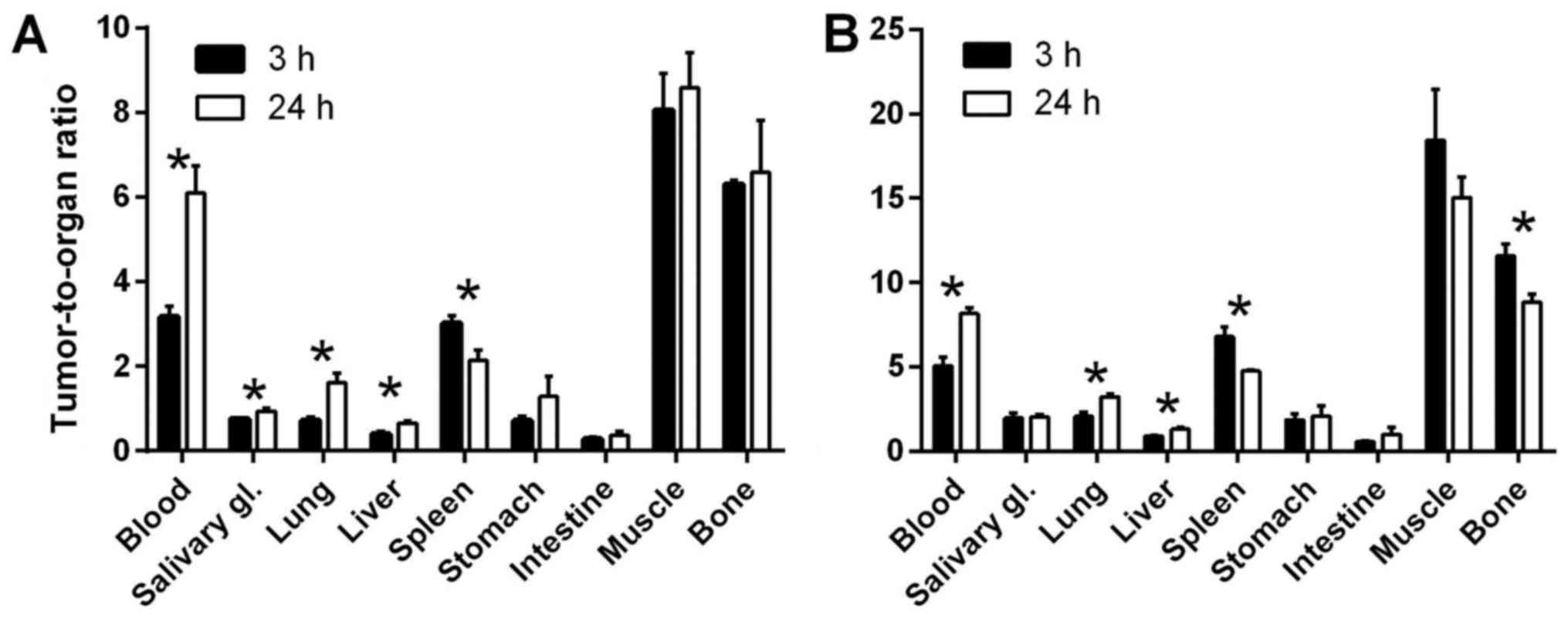
Because of good radioactivity retention in tumours
and significant decrease of radioactivity concentration in blood
over time, the tumour-to-blood ratios significantly increased with
time and reached 6.1±0.1 for DU145 models (n=3,
p<10−3) and 8.2±0.3 for LS174T (n=4,
p<10−4) at 24 h p.i. (Fig. 5). Also tumour-to-lung and
tumour-to-liver ratios significantly increased over time (n=3–4,
p<5×10−4 for DU145 and p<5×10−5 for
LS174T models). At 3 h p.i. for the DU145 model, tumour-to-muscle
ratio was 8.1±0.9 and tumour-to-bone was 6.30±0.10, and for LS174T
model, 18±3 and 11.6±0.7, respectively. However, at 24 h p.i. these
ratios decreased for LS174T model to 15±1 and 8.8±0.5, respectively
(significantly for bone, n=3–4, p<0.001).
Imaging studies
Images of xenograft-bearing mice injected with 2
μg of 57Co-ZHER3, were acquired 3 and
24 h p.i., and are presented in Fig.
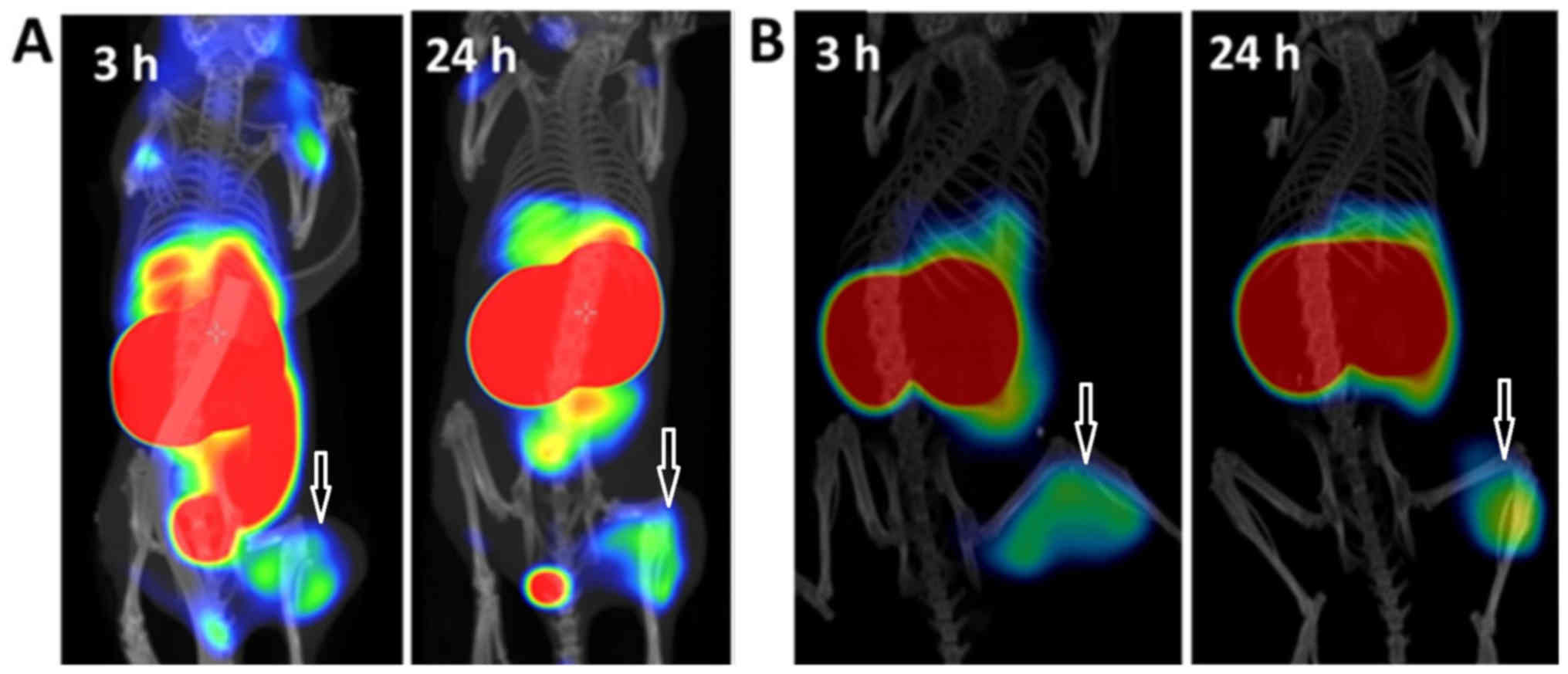
6. Images reflected the findings observed in the
biodistribution. The highest radioactivity accumulation was
observed in the kidneys, which also exceeded the uptake in any
other organs. Background radioactivity was low, which confirmed the
rapid blood clearance. Both xenografts were clearly visualized.
Radioactivity uptake in the liver and gastrointestinal (mErbB3
expressing area) was observed at 3 h p.i. and was more visible for
DU145 xenografts due to lower uptake of radioactivity in tumours.
At 24 h p.i., we could observe that the radioactivity accumulation
in liver and in gastrointestinal tract decreased in both models,
which also correlated with the biodistribution data. The images at
24 h p.i. were superior to images at 3 h for both xenograft models,
for LS174T the radioactivity uptake in tumour exceeded that in
liver.
Discussion
Patient stratification is a key issue for targeted
therapy. Clinical data demonstrated that high HER3 expression in
combination with low HER2 is a predictor for response to treatment
with an anti-HER3 antibody seribantumab (14). Thus, detection of elevated HER3
expression is decisive for therapy selection. Unlike HER1 and HER2
expression, the HER3 extracellular expression develops during the
course of the disease. Radionuclide molecular imaging may provide a
non-invasive solution for repetitive monitoring of HER3 status in
tumours. The experience with HER2 detection suggests that PET
imaging using affibody molecules is sensitive, specific and
reproducible (28).
This study demonstrated the feasibility of using a
radiocobalt-labelled anti-HER3 affibody molecule ZHER3
as a PET imaging agent. we showed that the binding of radiolabelled
affibody conjugate to its target, HER3, was preserved after
labelling with radiocobalt and was receptor-specific both in
vitro and in vivo. we also demonstrated that the
radiolabelled conjugate had a rapid binding to HER3-expressing
cells in vitro. Total cellular uptake of radioactivity
increased up to 24 h of continuous incubation. This pattern was in
good agreement with our recent observation that HER3 receptors are
constantly formed by cancer cells when incubated with anti-HER3
affibody molecules (32). Notably,
the internalized fraction was appreciably lower in the case of
57Co-ZHER3 binding to LS174T cells compared
to DU145 cells (Fig. 3). A similar
phenomenon has been observed for 68Ga-ZHER3,
where the internalization of bound anti-HER3 affibody molecules was
lowest for the LS174T cell line (35). This effect may be explained by
interaction of HER3 with other members of HER-family that are
expressed by cancer cells. The differences in internalisation
patterns of the same radiolabelled proteins by different cell lines
are an often-observed phenomenon, e.g. in the case of cMAb-U36
interaction with CD44v6 (45) and
ZHER2 affibody molecule with HER2 (41). The internalisation pattern of a
radiolabelled protein depends on various factors. Residualizing
properties of radiocatabolites, ability of the protein to trigger
internalisation after binding to its molecular target, and
interaction of the molecular target with other cell-surface
molecules are probably the main ones. Taken in account that the two
first mentioned factors should be independent on cell line, we
assume that interaction of HER3 with other receptors plays the main
role for differences observed in the internalisation patterns of
57Co-ZHER3 in the two cell lines. Previously,
it has been shown that a heterodimerization between HER1 and HER2
influenced the internalization rate of the HER1 complex with its
ligand by cells with different expression levels of these receptors
(46). It is known that HER3
heterodimerizes with other receptors of the HER-family (5). This can influence the probability of
particular formations of heterodimers and, in this way, cellular
processing of the HER3-(pseudo)ligand complex depending on cell
line. More detailed experiments are required to elucidate this
issue, which should be a focus of follow-up studies.
It has to be noted, that the difference in
internalization did not translate into a difference in retention of
radioactivity in xenografts in vivo. The tumour-associated
radioactivity for both DU145 and LS174T xenografts at 24 h p.i. was
~75% of radioactivity at 3 h p.i. (Table I). A very similar retention pattern
was observed previously for retention of
111In-ZHER3 in BT-474 breast cancer
xenografts (34).
The overall biodistribution pattern of
57Co-ZHER3 was in good agreement with the
published data for other radiolabelled variants of ZHER3
(31,34–36).
The biodistribution profile of the radiocobalt-labelled anti-HER3
affibody molecule was characterized by very fast blood clearance.
Radioactivity concentration in blood was below 0.5% ID/g at 3 h
p.i. and further decreased 2–2.5-fold with time. A significant
decrease of radioactivity accumulation with time was also observed
in the liver (>2-fold), which is a mErBb3 expressing organ
(n=3–4, p<0.01). Rapid blood clearance and low radioactivity
uptake in liver, bone and spleen [organs which accumulate free
cobalt(48)] indicates high in
vivo stability of the Co-NOTA complex.
An interesting observation in this study was that
the radiocobalt labelled affibody molecule provided the lowest
radioactivity uptake in liver at optimal imaging time compared with
all radiolabelled variants of ZHER3:08698 described
earlier. For comparison, liver radioactivity uptake was 5.5–7% ID/g
for 18F at 1 h p.i. (36), 2.5–5% ID/g for 68Ga at 3
h p.i. (35), 5% ID/g for
99mTc at 8 h p.i. (31), and 3–5% ID/g for 111In
at 24 h p.i. (34). At 24 h p.i.,
hepatic uptake for 57Co-ZHER3 was lower
(0.9–1.5% ID/g) than for 111In-ZHER3, and
radioactivity uptake in liver was below the uptake in LS174T
xenografts. This fact is important because liver is an organ where
metastases are frequently present. We can speculate that the
hepatic uptake is mediated by two mechanisms: one is
receptor-mediated and can therefore be saturated, and the other is
unspecific/off target binding, that may rely on the lipophilic and
charged moieties on the surface of the tracer. For a HER2-targeting
affibody molecule, it was demonstrated that a positively charged or
lipophilic moiety on the N and C termini of the affibody molecule
markedly increased the hepatic uptake (49). Divalent cobalt coordinated with the
NOTA-chelator has a neutrally charged complex in contrast to
positively charged complexes of trivalent metals (indium and
gallium). The observed decrease in hepatic uptake of radioactivity
in the present study supports the hypothesis that by reducing
positive charge, the off-target interactions of the anti-HER3
affibody conjugate could be decreased.
The biodistribution of
57Co-ZHER3 also demonstrated good
radioactivity retention in tumours over time (decrease of
radioactivity uptake was ~25% between 3 and 24 h p.i.), which
contributed to significantly increased tumour to non-tumour ratios
for blood and mErbB3-expressing organs (salivary glands, lungs and
liver) (Fig. 5) resulting in an
improved imaging contrast. Even though the contrast was sufficient
at 3 h p.i. with clearly visualized tumours, the radioactivity
uptake in organs with endogenous mErbB3 expression (liver and
intestines) was high at this time-point. However, at 24 h p.i.,
image contrast was improved due to better radioactivity retention
in tumours than in normal organs (Fig.
6).
Comparison of imaging properties of
57Co-ZHER3 with properties of
89Zr-labelled anti-HER3 monoclonal antibodies Mab#58
(21) and Rg7116 (20) is clearly in favour to
57Co-ZHER3. The monoclonal antibodies have a
tumour-to-blood ratio of ~1 at 4 days after injection. Even at 6
days, the tumour-to-blood ratio is not more than 3, which is less
than 57Co-ZHER3 provides already at 1 day
after injection. The reduction of the size of the imaging probe
compared with an antibody F(ab′)2 fragment improved
contrast and shortened time to reach maximum contrast (50,51).
At one day after injection,
64Cu-DOTA-mAb105-F(ab′)2 demonstrated
tumour-to-back-ground ratio comparable with tumour-to-blood for
57Co-ZHER3, however liver radioactivity
uptake of the F(ab′)2 probe exceeded the tumour uptake.
Very recently, after submission of this paper, the selection and
characterization of HER3-targeting undecapeptide HER3P1 labelled
with 68Ga was reported (52). Despite low affinity to HER3
(270±151 nM), this peptide was capable of visualising HER3
expression in murine models, however tumour-to-blood and
tumour-to-liver ratios were 2.5 and 0.7. It also has to be noted
that no information about cross-reactivity to mErbB3 was provided
for all anti-HER3 probes mentioned above. In development of an
imaging probe, the cross-reactivity to the murine counterpart of
the targeted receptor provides representative information on uptake
in organs with endogenous receptor expression.
High contrast images of HER3 expression in tumour
models obtained 24 h p.i. support our hypothesis that imaging of
HER3 expression should be improved with time. The radiocobalt
labelled anti-HER3 affibody molecule can be used for non-invasive
detection of HER3 expression in patients with suspected
HER3-mediated therapy resistance. Pre-selected patients have better
chance to benefit from the targeted therapy. As already mentioned,
therapeutic antibodies targeting HER3 are in different phases of
clinical development including phase III (patritumab) and phase II
(seribantumab, istiratumab (bispecific, HER3/IGF-1R), and
duligotumab (bispecific, HER3/EGFR) (2). Many more potential drug candidates
are in preclinical evaluation. Additionally, we have recently
demonstrated that an anti-HER3 affibody dimer fused with a domain
with high affinity to albumin,
ZHER3-ABD-ZHER3, inhibited HER3-induced
phosphorylation in vitro and delayed growth of HER3
expressing tumours in vivo (32,33).
The fact that 57Co-ZHER3 binds to the same
epitope as the anti-HER3 therapeutic agents seribantumab (47) and the affibody conjugate
ZHER3-ABD-ZHER3 (32) makes it appropriate to use this
conjugate to monitor receptor occupancy during therapy. The
complete inhibition of HER3-mediated signalling is required to
maximise therapeutic effect of anti-HER3 therapy (53). The non-invasive radionuclide
molecular imaging and the non-immunogenic character of affibody
molecules allow repetitive investigations, which was demonstrated
in clinic with the use of anti-HER2 affibody molecule ABY-025
labelled with 68Ga (28).
Taken into account our clinical experience with
imaging of HER2 expression in patients with breast cancer
metastases (27,28), we can expect that lesions with HER3
expression (normally corresponding to HER2 expression +) should be
visualized. This experience together with our published data on
relation between imaging contrast and injected protein dose
(35) also points to that fine
tuning of injected dose should be done on initial stage of clinical
study. Additionally, we are planning to investigate influence of
overall charge of metal-chelator complex on biodistribution of the
anti-HER3 affibody-based imaging probe. For example, exchange of
the neutral charge of the Ga-DOTA moiety to a negative charge of
Co-DOTA decreased the radioactivity uptake three-fold in liver of
an anti-HER1 affibody molecule (43).
Comparing results with earlier performed studies on
affibody molecules targeting HER3 using different radiolabels,
99mTc, 111In, 68Ga and
18F (31,34–36),
we can conclude that the radiocobalt label demonstrated the highest
tumour-to-liver ratio, as well as high tumour-to-muscle and
tumour-to-bone ratios. Taken together, we believe that using
radiocobalt as a label for anti-HER3 affibody molecules is a
promising approach, which contributes to a high imaging contrast
in vivo.
Acknowledgments
The molecular imaging work in this publication was
supported by the Wallenberg infrastructure for PET-MRI (WIPPET) at
SciLifeLab Pilot Facility for Preclinical PET-MRI, a Swedish
nationally available imaging platform at Uppsala University,
Sweden, financed by Knut and Alice Wallenberg Foundation
(SPECT/CT). This study was supported by the Swedish Cancer Society
[grants CAN2014/474 (A.O.), CAN2015/350 (V.T.) and CAN2016/463
(S.S.)], the Swedish Research Council [grants 2015-02509 (A.O.),
2015-02353 (V.T.) and 2012-05236 (S.S.)], the Swedish Agency for
Innovation VINNOVA [grant 2016-04060 (A.O.)] and the Wallenberg
Center for Protein Technology (S.S. and J.L.) which are
acknowledged for financial support.
References
|
1
|
Baselga J and Swain SM: Novel anticancer
targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malm M, Frejd FY, Ståhl S and Löfblom J:
Targeting HER3 using mono- and bispecific antibodies or alternative
scaffolds. MAbs. 8:1195–1209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Claus J, Patel G, Ng T and Parker PJ: A
role for the pseudokinase HER3 in the acquired resistance against
EGFR- and HER2-directed targeted therapy. Biochem Soc Trans.
42:831–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gala K and Chandarlapaty S: Molecular
pathways: HER3 targeted therapy. Clin Cancer Res. 20:1410–1416.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amin DN, Campbell MR and Moasser MM: The
role of HER3, the unpretentious member of the HER family, in cancer
biology and cancer therapeutics. Semin Cell Dev Biol. 21:944–950.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma J, Lyu H, Huang J and Liu B: Targeting
of erbB3 receptor to overcome resistance in cancer treatment. Mol
Cancer. 13:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mellinghoff IK, Vivanco I, Kwon A, Tran C,
Wongvipat J and Sawyers CL: HER2/neu kinase-dependent modulation of
androgen receptor function through effects on DNA binding and
stability. Cancer Cell. 6:517–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leung HY, Weston J, Gullick WJ and
Williams G: A potential autocrine loop between heregulin-alpha and
erbB-3 receptor in human prostatic adenocarcinoma. Br J Urol.
79:212–216. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soler M, Mancini F, Meca-Cortés O,
Sánchez-Cid L, Rubio N, López-Fernández S, Lozano JJ, Blanco J,
Fernández PL and Thomson TM: HER3 is required for the maintenance
of neuregulin-dependent and -independent attributes of malignant
progression in prostate cancer cells. Int J Cancer. 125:2565–2575.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poovassery JS, Kang JC, Kim D, Ober RJ and
Ward ES: Antibody targeting of HER2/HER3 signaling overcomes
heregulin-induced resistance to PI3K inhibition in prostate cancer.
Int J Cancer. 137:267–277. 2015. View Article : Google Scholar
|
|
11
|
Huang Z, Brdlik C, Jin P and Shepard HM: A
pan-HER approach for cancer therapy: Background, current status and
future development. Expert Opin Biol Ther. 9:97–110. 2009.
View Article : Google Scholar
|
|
12
|
Dote H, Cerna D, Burgan WE, Camphausen K
and Tofilon PJ: ErbB3 expression predicts tumor cell
radiosensitization induced by Hsp90 inhibition. Cancer Res.
65:6967–6975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kol A, Terwisscha van Scheltinga AG,
Timmer-Bosscha H, Lamberts LE, Bensch F, de Vries EG and Schröder
CP: HER3, serious partner in crime: Therapeutic approaches and
potential biomarkers for effect of HER3-targeting. Pharmacol Ther.
143:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JF, Ray-Coquard I, Selle F, Poveda AM,
Cibula D, Hirte H, Hilpert F, Raspagliesi F, Gladieff L, Harter P,
et al: Randomized phase II Trial of seribantumab in combination
with paclitaxel in patients with advanced platinum-resistant or
-refractory ovarian cancer. J Clin Oncol. 34:4345–4353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Cosimo S and Baselga J: Management of
breast cancer with targeted agents: Importance of heterogeneity.
[corrected]. Nat Rev Clin Oncol. 7:139–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tolmachev V, Stone-Elander S and Orlova A:
Radiolabelled receptor-tyrosine-kinase targeting drugs for patient
stratification and monitoring of therapy response: Prospects and
pitfalls. Lancet Oncol. 11:992–1000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pecking AP, Bellet D and Alberini JL:
Immuno-SPET/CT and immuno-PET/CT: A step ahead to translational
imaging. Clin Exp Metastasis. 29:847–852. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robinson MK, Hodge KM, Horak E, Sundberg
AL, Russeva M, Shaller CC, von Mehren M, Shchaveleva I, Simmons HH,
Marks JD, et al: Targeting ErbB2 and ErbB3 with a bispecific
single-chain Fv enhances targeting selectivity and induces a
therapeutic effect in vitro. Br J Cancer. 99:1415–1425. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tolmachev V, Tran TA, Rosik D, Abrahmsén
L, Sjöberg A and Orlova A: Tumor targeting using Affibody
molecules: An interplay of a target expression level, affinity and
binding site composition. J Nucl Med. 53:953–960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terwisscha van Scheltinga AG, Lub-de Hooge
MN, Abiraj K, Schröder CP, Pot L, Bossenmaier B, Thomas M,
Hölzlwimmer G, Friess T, Kosterink JG, et al: ImmunoPET and
biodistribution with human epidermal growth factor receptor 3
targeting antibody 89Zr-Rg7116. MAbs. 6:1051–1058. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan Q, Furukawa T, Tashiro T, Okita K,
Jin ZH, Aung W, Sugyo A, Nagatsu K, Endo H, Tsuji AB, et al:
Immuno-PET imaging of HER3 in a model in which HER3 signaling plays
a critical role. PLoS One. 10:e01430762015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tolmachev V, Rosik D, Wållberg H, Sjöberg
A, Sandström M, Hansson M, Wennborg A and Orlova A: Imaging of EGFR
expression in murine xenografts using site-specifically labelled
anti-EGFR 111In-DOTA-Z EGFR:2377 affibody molecule:
Aspect of the injected tracer amount. Eur J Nucl Med Mol Imaging.
37:613–622. 2010. View Article : Google Scholar
|
|
23
|
Orlova A, Magnusson M, Eriksson TL,
Nilsson M, Larsson B, Höidén-Guthenberg I, Widström C, Carlsson J,
Tolmachev V, Ståhl S, et al: Tumor imaging using a picomolar
affinity HER2 binding affibody molecule. Cancer Res. 66:4339–4348.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orlova A, Hofström C, Strand J, Varasteh
Z, Sandstrom M, Andersson K, Tolmachev V and Gräslund T:
[99mTc(CO)3]+-(HE)3-ZIGF1R:4551, a
new affibody conjugate for visualization of insulin-like growth
factor-1 receptor expression in malignant tumours. Eur J Nucl Med
Mol Imaging. 40:439–449. 2013. View Article : Google Scholar
|
|
25
|
Ståhl S, Gräslund T, Eriksson Karlström A,
Frejd FY, Nygren PÅ and Löfblom J: Affibody molecules in
biotechnological and medical applications. Trends Biotechnol.
35:691–712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahlgren S and Tolmachev V: Radionuclide
molecular imaging using affibody molecules. Curr Pharm Biotechnol.
11:581–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sörensen J, Sandberg D, Sandström M,
Wennborg A, Feldwisch J, Tolmachev V, Åström G, Lubberink M,
Garske-Román U, Carlsson J, et al: First-in-human molecular imaging
of HER2 expression in breast cancer metastases using the
111In-ABY-025 affibody molecule. J Nucl Med. 55:730–735.
2014. View Article : Google Scholar
|
|
28
|
Sörensen J, Velikyan I, Sandberg D,
Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M,
Lubberink M, Olofsson H, et al: Measuring HER2-receptor expression
in metastatic breast cancer using [68Ga]ABY-025 affibody
PET/CT. Theranostics. 6:262–271. 2016. View Article : Google Scholar
|
|
29
|
Malm M, Kronqvist N, Lindberg H,
Gudmundsdotter L, Bass T, Frejd FY, Höidén-Guthenberg I, Varasteh
Z, Orlova A, Tolmachev V, et al: Inhibiting HER3-mediated tumor
cell growth with affibody molecules engineered to low picomolar
affinity by position-directed error-prone PCR-like diversification.
PLoS One. 8:e627912013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kronqvist N, Malm M, Göstring L,
Gunneriusson E, Nilsson M, Höidén Guthenberg I, Gedda L, Frejd FY,
Ståhl S and Löfblom J: Combining phage and staphylococcal surface
display for generation of ErbB3-specific affibody molecules.
Protein Eng Des Sel. 24:385–396. 2011. View Article : Google Scholar
|
|
31
|
Orlova A, Malm M, Rosestedt M, Varasteh Z,
Andersson K, Selvaraju RK, Altai M, Honarvar H, Strand J, Ståhl S,
et al: Imaging of HER3-expressing xenografts in mice using a
(99mTc(CO)3-HEHEHE-ZHER3:08699 affibody
molecule. Eur J Nucl Med Mol Imaging. 41:1450–1459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bass TZ, Rosestedt M, Mitran B, Frejd FY,
Löfblom J, Tolmachev V, Ståhl S and Orlova A: In vivo evaluation of
a novel format of a bivalent HER3-targeting and albumin-binding
therapeutic affibody construct. Sci Rep. 7:431182017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orlova A, Bass T, Atterby C,
Gudmundsdotter L, Frejd FY, Löfblom J, Tolmachev V and Ståhl S:
Evaluating the therapeutic potential of a dimeric HER3-binding
affibody construct in comparison with a monoclonal antibody,
seribantumab. Affibody Molecules Targeting HER3 for Cancer Therapy.
Bass T: Royal Institute of Technology, School of Biotechnology;
Stockholm: 2017
|
|
34
|
Andersson KG, Rosestedt M, Varasteh Z,
Malm M, Sandström M, Tolmachev V, Löfblom J, Ståhl S and Orlova A:
Comparative evaluation of 111In-labeled NOTA conjugated
affibody molecules for visualization of HER3 expression in
malignant tumors. Oncol Rep. 34:1042–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosestedt M, Andersson KG, Mitran B,
Tolmachev V, Löfblom J, Orlova A and Ståhl S: Affibody-mediated PET
imaging of HER3 expression in malignant tumours. Sci Rep.
5:152262015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Da Pieve C, Allott L, Martins CD, Vardon
A, Ciobota DM, Kramer-Marek G and Smith G: Efficient [(18)F]AlF
radiolabeling of ZHER3:8698 affibody molecule for
imaging of HER3 positive tumors. Bioconjug Chem. 27:1839–1849.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fani M, André JP and Maecke HR:
68Ga-PET: A powerful generator-based alternative to
cyclotron-based PET radiopharmaceuticals. Contrast Media Mol
Imaging. 3:67–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thisgaard H, Olesen ML and Dam JH:
Radiosynthesis of 55Co- and 58mCo-labelled
DOTATOC for positron emission tomography imaging and targeted
radionuclide therapy. J Labelled Comp Radiopharm. 54:758–762. 2011.
View Article : Google Scholar
|
|
39
|
Jansen HM, Willemsen AT, Sinnige LG, Paans
AM, Hew JM, Franssen EJ, Zorgdrager AM, Pruim J, Minderhoud JM and
Korf J: Cobalt-55 positron emission tomography in
relapsing-progressive multiple sclerosis. J Neurol Sci.
132:139–145. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jansen HM, Pruim J, vd Vliet AM, Paans AM,
Hew JM, Franssen EJ, de Jong BM, Kosterink JG, Haaxma R and Korf J:
Visualization of damaged brain tissue after ischemic stroke with
cobalt-55 positron emission tomography. J Nucl Med. 35:456–460.
1994.PubMed/NCBI
|
|
41
|
Wållberg H, Ahlgren S, Widström C and
Orlova A: Evaluation of the radiocobalt-labeled
[MMA-DOTA-Cys61]-ZHER2:2395(-Cys) affibody
molecule for targeting of HER2-expressing tumors. Mol Imaging Biol.
12:54–62. 2010. View Article : Google Scholar
|
|
42
|
Mitran B, Thisgaard H, Rosenström U, Dam
JH, Larhed M, Tolmachev V and Orlova A: High contrast PET imaging
of GRPR expression in prostate cancer using cobalt-labeled bombesin
antagonist RM26. Contrast Media Mol Imaging. Aug 10–2017.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garousi J, Anderson KG, Dam JH, Olsen BB,
Mitran B, Orlova A, Buijs J, Ståhl S, Löfblom J, Thisgaard H, et
al: The use of radiocobalt as a label improves imaging of EGFR
using DOTA-conjugated affibody molecule. Sci Rep. 7:59612017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wållberg H and Orlova A: Slow
internalization of anti-HER2 synthetic affibody monomer
111In-DOTA-ZHER2:342-pep2: Implications for
development of labeled tracers. Cancer Biother Radiopharm.
23:435–442. 2008. View Article : Google Scholar
|
|
45
|
Nestor M, Sundström M, Anniko M and
Tolmachev V: Effect of cetuximab in combination with
alpha-radioimmunotherapy in cultured squamous cell carcinomas. Nucl
Med Biol. 38:103–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Björkelund H, Gedda L, Malmqvist M and
Andersson K: Resolving the EGF-EGFR interaction characteristics
through a multiple-temperature, multiple-inhibitor, real-time
interaction analysis approach. Mol Clin Oncol. 1:343–352. 2013.
View Article : Google Scholar
|
|
47
|
Schoeberl B, Faber AC, Li D, Liang MC,
Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al: An
ErbB3 antibody, MM-121, is active in cancers with ligand-dependent
activation. Cancer Res. 70:2485–2494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jansen HM, Knollema S, van der Duin LV,
Willemsen AT, Wiersma A, Franssen EJ, Russel FG, Korf J and Paans
AM: Pharmacokinetics and dosimetry of cobalt-55 and cobalt-57. J
Nucl Med. 37:2082–2086. 1996.PubMed/NCBI
|
|
49
|
Hofström C, Altai M, Honarvar H, Strand J,
Malmberg J, Hosseinimehr SJ, Orlova A, Gräslund T and Tolmachev V:
HAHAHA, HEHEHE, HIHIHI, or HKHKHK: Influence of position and
composition of histidine containing tags on biodistribution of
[(99m)Tc(CO)3](+)-labeled affibody molecules. J Med
Chem. 56:4966–4974. 2013. View Article : Google Scholar
|
|
50
|
Wehrenberg-Klee E, Turker NS, Chang B,
Heidari P and Mahmood U: Development of a HER3 PET probe for breast
cancer imaging. J Nucl Med. 55(Suppl 1): s5502014.
|
|
51
|
Wehrenberg-Klee E, Turker NS, Heidari P,
Larimer B, Juric D, Baselga J, Scaltriti M and Mahmood U:
Differential receptor tyrosine kinase PET imaging for therapeutic
guidance. J Nucl Med. 57:1413–1419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Larimer BM, Phelan N, Wehrenberg-Klee E
and Mahmood U: Phage display selection, in vitro characterization,
and correlative PET imaging of a novel HER3 peptide. Mol Imaging
Biol. Jul 21–2017.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Garrett JT, Olivares MG, Rinehart C,
Granja-Ingram ND, Sánchez V, Chakrabarty A, Dave B, Cook RS, Pao W,
McKinely E, et al: Transcriptional and posttranslational
up-regulation of HER3 (ErbB3) compensates for inhibition of the
HER2 tyrosine kinase. Proc Natl Acad Sci USA. 108:5021–5026. 2011.
View Article : Google Scholar : PubMed/NCBI
|