Introduction
Gastric cancer (GC) is one of the most common
carcinomas and the third major contributor to cancer mortality
worldwide (1). However, the
molecular mechanisms involved in the oncogenesis and progression of
GC have not yet been fully understood. Despite the fact that the
improvements in medical and surgical therapy have lowered the
mortality of GC during the past few decades, the 5-year survival
rate for patients with advanced GC remains unsatisfactory at less
than 10–25% (2,3). Thus, a detailed molecular
understanding of GC pathogenesis and selective therapeutic targets
for GC patients are urgently needed.
The histone acetyltransferases p300 and CBP are
large (300 kDa) modular proteins. p300 and CBP share 63% identical
amino acid sequences and have very similar functions. Consequently,
these histone acetyltransferases are together considered p300/CBP
(4). p300/CBP play crucial roles
in the cell cycle, DNA synthesis, cellular differentiation and
organ development (4–8). In solid tumours, such as human
prostate cancer (PCa), p300/CBP are upregulated and induce the gene
transcription of the androgen receptor (AR), which is a key PCa
promoter (9–11). However, the inhibition of p300/CBP
decreases the proliferation and invasive capacity of prostate
cancer cells (9–11). In the colon cancer cell line
HCT116, the absence of p300 increases the apoptosis in response to
different forms of DNA damage (12). Additionally, the p300/CBP genes are
upregulated in melanoma cell lines (13) and participate in the regulation of
melanocyte lineage-specific MITF transcription factor, which is
associated with antiapoptosis, angiogenisis and metastasis in
melanomas (14–16). In pancreatic cancer, p300 is also
involved in controlling the migratory and invasive behaviour of the
tumours and inactivation of p300 blocks the migration of pancreatic
cancer cells (17). Recently, p300
was reported to be involved in the epithelial to mesenchymal
transition (EMT) in the human GC cell line BGC-823 (18). Nevertheless, whether p300/CBP could
serve as potential antineoplastic targets for GC remains
unclear.
C646, a small molecule inhibitor of p300/CBP, exerts
antitumour activity in many cancer cell lines (11,19).
However, its effects on GC cells and the mechanisms underlying
these effects have not been extensively studied. In the present
study, we investigated the therapeutic effects of the HAT inhibitor
C646 on several GC cell lines and further explored the potential
mechanisms underlying its antitumour activity.
Materials and methods
Cell lines
The normal human gastric epithelial cell line GES-1
and the human GC cell lines SGC-7901 (moderately differentiated),
MKN45 (poorly differentiated), BGC-823 (undifferentiated) and KATO
III (signet-ring cell) were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). The human GC cell
line MGC-803 (poorly differentiated) was obtained from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). All the cell lines were cultured in RPMI-1640 medium
(Corning Inc., Corning, NY, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco, Carlsbad, CA, USA) and 1.0%
penicillin/streptomycin (Gibco) at 37°C in a humidified atmosphere
of 95% air and 5% CO2.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
The total RNA of each cell line was extracted using
TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's protocol. qRT-PCR was conducted as previously
described (20). The primer
sequences were as follows: c-Met, forward,
5′-TTTCAAATGGCCACGGGACAACA CA-3′ and reverse,
5′-TGGGCTGGGGTATAACATTCAA GA-3′; MMP7, forward,
5′-GATGGTAGCAGTCTAGGGAT TAACTTC-3′ and reverse,
5′-GGAATGTCCCATACCCAAA GAA-3′; MMP9, forward, 5′-CACGCAC
GACGTCTTCCA-3′ and reverse, 5′-AAGCGGTCCTGGCAGAAAT-3′ (Sangon
Biotech Co., Ltd., Shanghai, China). Gene copy number was
calculated using the ΔΔCt method.
Western blot analysis
The total protein from each cell line was lysed
using cell lysis buffer (Cell Signaling Technology, Danvers, MA,
USA), according to the manufacturer's instructions. Western
blotting was performed as previously described (21). Rabbit antibodies corresponding to
acetylated histone H3 (Ac-H3, acetyl K18) and total histone H3 were
purchased from Abcam (Cambridge, UK). Antibodies against c-Met,
total Akt, phospho-Akt (Ser473), PARP and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased
from Cell Signaling Technology.
Cell Counting kit-8 (CCK-8) assay
Normal gastric epithelial cells and 5 GC cell lines
were seeded in 96-well plates at 0.8–1.0×104 cells/well
(in 100 μl complete RPMI-1640). After attachment, the cells
were washed and replaced with fresh RPMI-1640 medium containing 1%
FBS. Thereafter, C646 (Selleck Chemicals, Houston, TX, USA) at the
required concentrations was added to the wells. Twenty-four hours
later, ~10 μl of CCK-8 dye was added to each well, and the
plate was incubated for 1–3 h. The optical density (OD) was
measured at 450 nm with a microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA).
Cell cycle assay and cell apoptosis
assay
Cells were plated in 6-well plates at
3×105 cells/well. Twenty-four hours later, the cells
were treated with C646 10 μmol/l for 6 h (for the cell cycle
assay) or 24 h (for the cell apoptosis assay). Then, cell cycle
assay and cell apoptosis assay were performed as previously
described (20).
Wound healing assay
After treatment with C646 (10 μmol/l) for 24
h, cells were equally seeded in a 12-well plate. After attachment,
a monolayer wound was introduced using a 10-μl pipette tip.
The cells were washed with phosphate-buffered saline (PBS) to
remove floating debris, and the vertical distance between both
sides of the wound in at least three distinct randomly selected
areas was measured at 0 and 24 h after wound injury using Image-Pro
Plus 6.0 software.
Transwell assay
Following treatment with C646 (10 μmol/l) for
24 h, 2.5×104 cells/well were seeded into 24-well
chambers (Corning) in 1% FBS medium and 10% FBS medium was added to
the lower wells. After 6 or 24 h of incubation, the cells remaining
in the upper chamber were carefully removed with cotton swabs.
Migrated cells on the bottom side of the membrane were stained with
crystal violet for 15 min. Migrated cells were counted in at least
5 randomly selected fields.
Statistical analyses
GraphPad Prism 6 (GraphPad Software, Inc., San
Diego, CA, USA) was used for all analyses. Continuous data are
expressed as the means ± standard deviations. Statistical analyses
were performed using a one-way ANOVA in cases in which multiple
comparisons were performed. Student's t-test was performed to
compare the mean differences between the two groups. P<0.05 was
considered significant. All experiments were performed in
triplicate.
Results
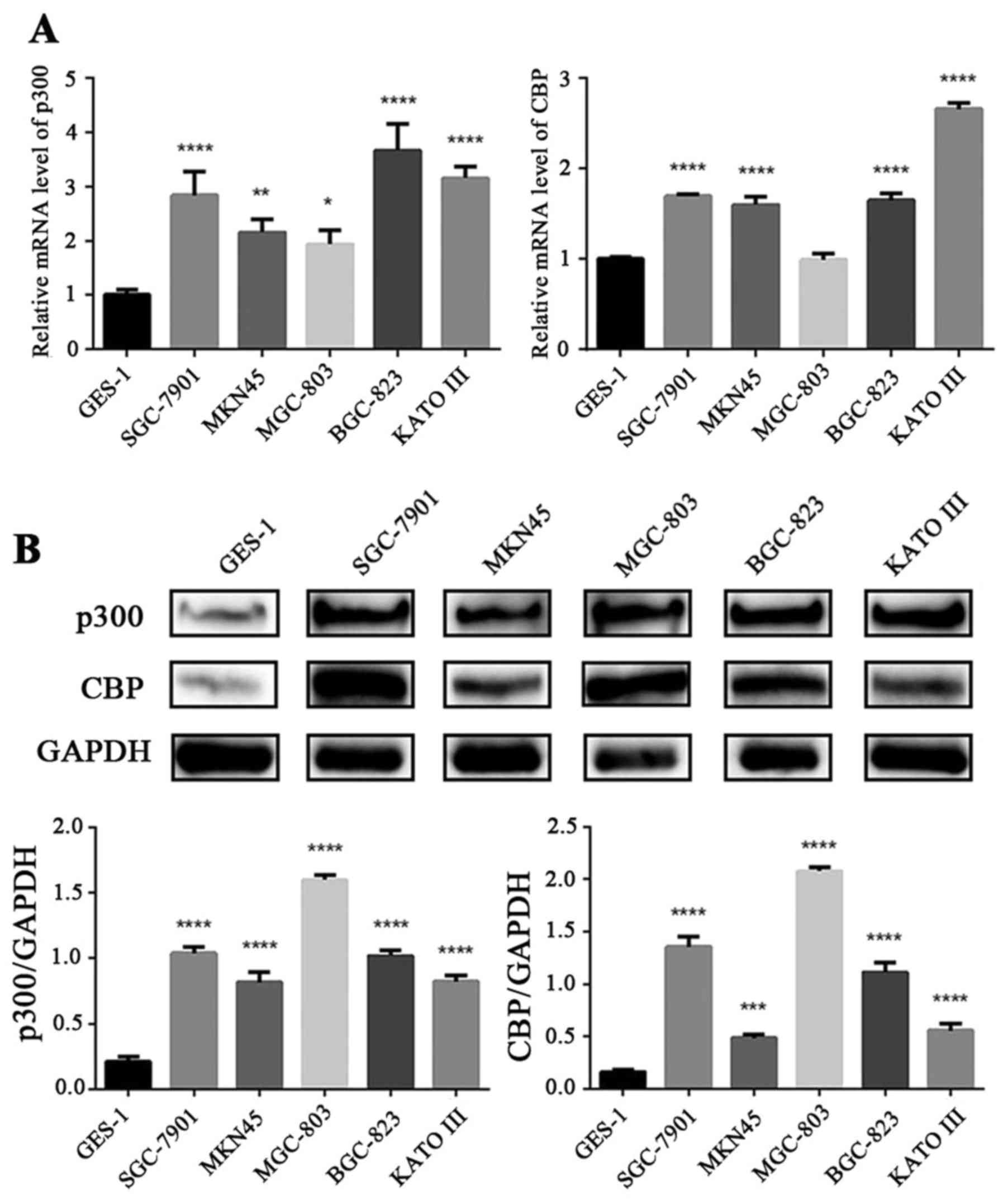
p300/CBP are upregulated in GC cells
compared with normal gastric epithelial cells
We examined the gene and protein expression of
p300/CBP in normal gastric epithelial cell line (GES-1) and 5 GC
cell lines (SGC-7901, MKN45, MGC-803, BGC-823 and KATO III) via
qRT-PCR and western blotting. As shown in Fig. 1A, the mRNA levels of p300
were highly expressed in the 5 GC cell lines compared with the
normal gastric epithelial cell line (P<0.05). Except for the
MGC-803 cell line, the CBP mRNA levels in the other four GC
cell lines were significantly higher than those in the GES-1 cells
(P<0.0001). The protein expression levels of p300/CBP in the 5
GC cell lines were significantly higher than those in the GES-1
cells (P<0.001) (Fig. 1B).
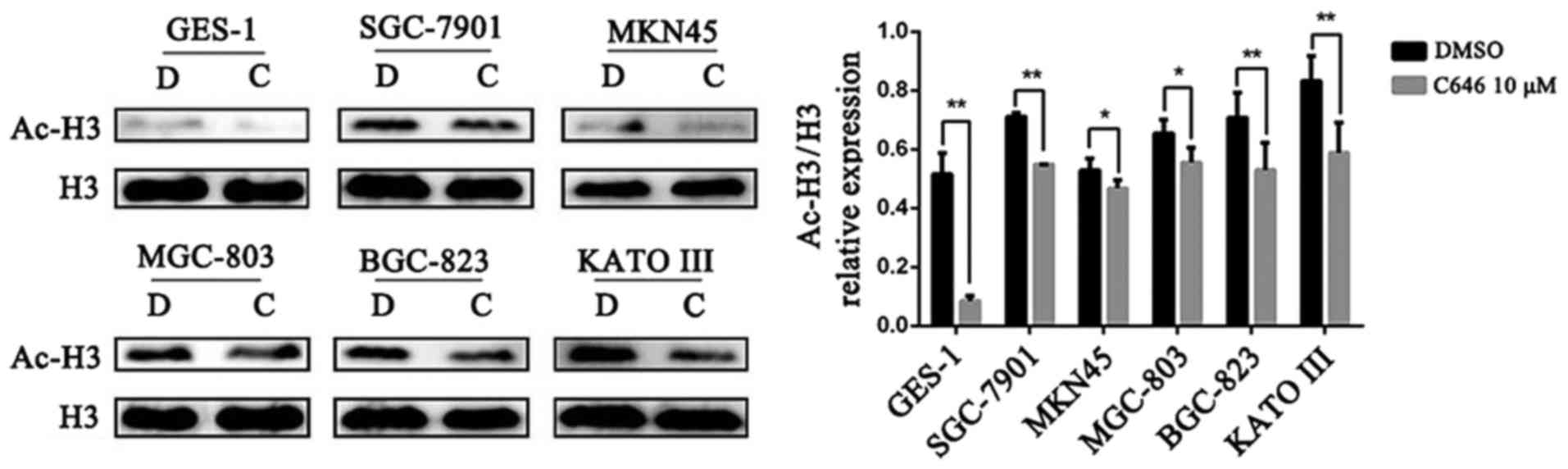
C646 reduces histone H3 acetylation
C646 is a selective inhibitor of p300/CBP, which
control the acetylation of histone H3 (22,23).
In the present study, the acetylation of histone H3 was detected to
verify the effect of C646 on normal gastric epithelial cells and GC
cells. All 6 cell lines were treated with dimethyl sulfoxide (DMSO)
or C646 10 μmol/l for 6 h. We found that C646 treatment
significantly reduced the levels of histone H3 acetylation in both
GC cells and normal gastric epithelial cells (P<0.05). In
addition, the basal acetylated histone H3 (Ac-H3) expression level
of the GES-1 cell line was lower than that of the GC cell lines
(Fig. 2).
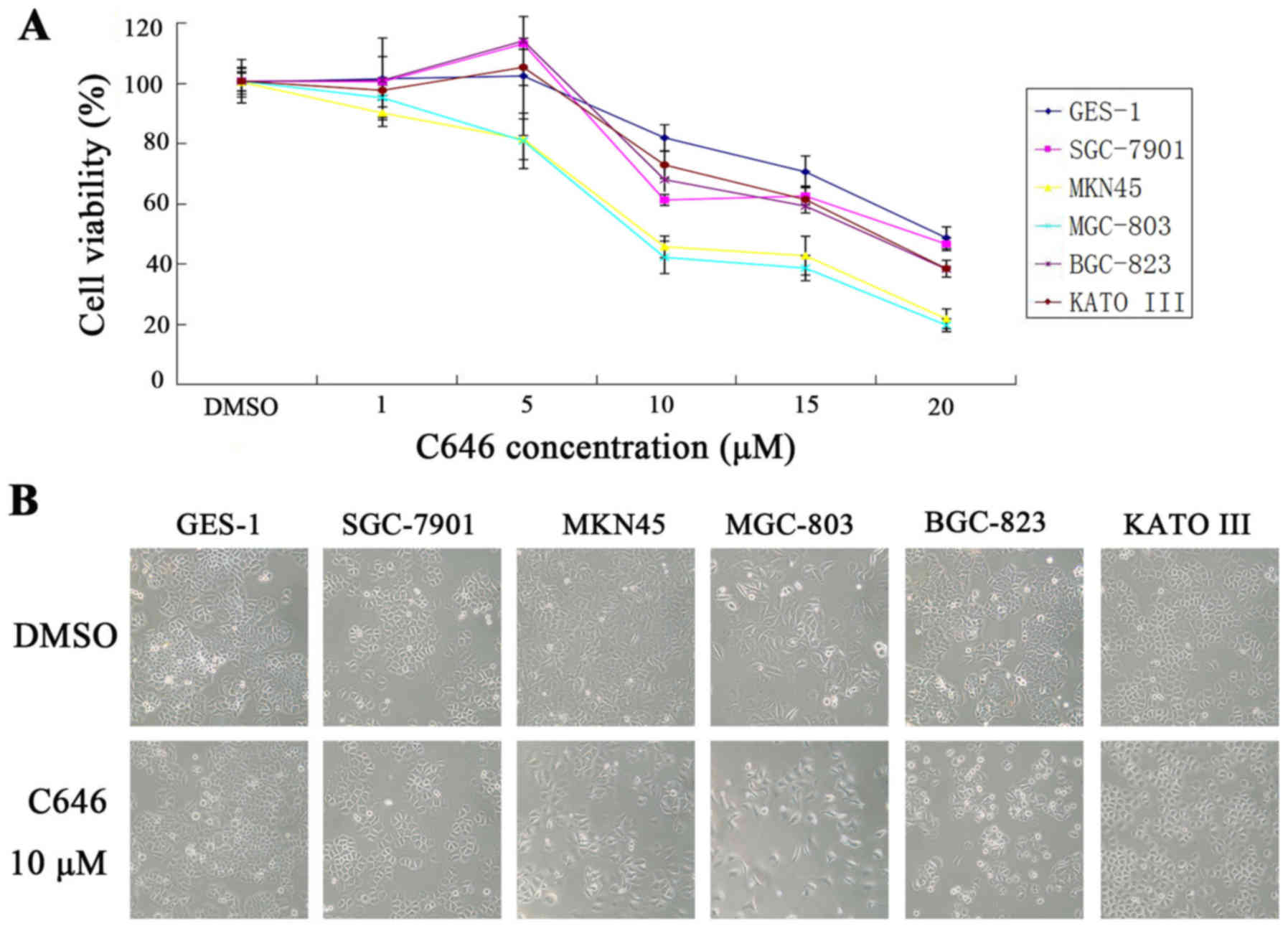
C646 inhibits cell viability and cell
cycle and promotes cell apoptosis in GC cells
As demonstrated above, p300/CBP were overexpressed
in 5 GC cell lines compared with normal gastric epithelial cells.
To investigate the effects of p300/CBP inhibition, both normal
gastric epithelial cells and GC cell lines were treated with an
increasing concentration of C646 (1, 5, 10, 15 and 20
μmol/l) for 24 h. As shown in Fig. 3A, the cell viability of GES-1 cells
was not significantly inhibited when the cells were treated with
C646 at 1, 5 or 10 μmol/l. However, when the cells were
treated with 15 or 20 μmol/l C646, we observed a significant
inhibitory effect on cell viability in GES-1 cells (P<0.05).
Regarding the GC cell lines, the cell viability of SGC-7901,
BGC-823 and KATO III showed no significant changes or even a slight
elevation after treatment with 1 or 5 μmol/l C646. However,
clear inhibition of cell viability was observed when these three GC
cell lines were treated with higher concentrations of C646
(P<0.01). Additionally, treatment with 10, 15 or 20
μmol/l C646 for 24 h showed a stronger inhibitory effect on
the cell viability of these three GC cell lines than that of the
GES-1 cell line; however, this results was not statistically
significant. Regarding the MKN45 and MGC-803 cell lines, C646
demonstrated strong inhibitory effects on cell viability at all 5
concentrations (P<0.05). In addition, the cell viability of the
MKN45 and MGC-803 cell lines decreased significantly compared with
that of the GES-1 cell line (P<0.05). Furthermore, the number of
apoptotic GC cells increased after treatment with C646 (Fig. 3B).
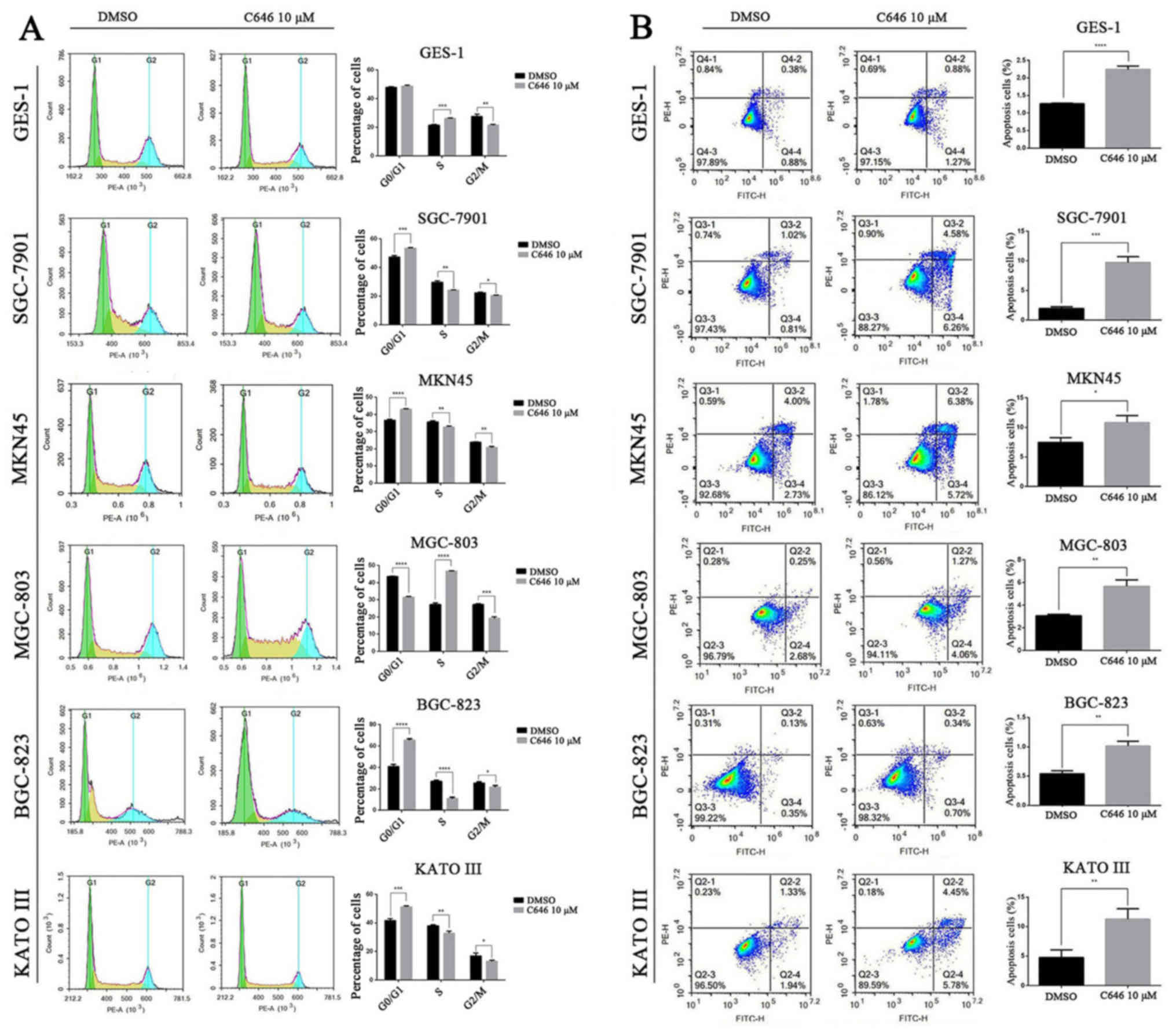
To further investigate the causes of cell viability
inhibition, we assessed the cell cycle and cell apoptosis using
flow cytometry. C646 (10 μmol/l) was added to the above 6
cell lines, and the cell cycle was evaluated after 6 h. As
displayed in Fig. 4A, the cell
cycle of normal gastric epithelial GES-1 cells and GC MGC-803 cells
was significantly blocked in S phase (P<0.001) and decreased
number of cells were in G2/M phase. However, the cell cycle of the
other 4 GC cell lines was significantly arrested in the G0/G1 phase
(P<0.001) and reduced numbers of cells were in the S and G2/M
phases (Fig. 4A). Annexin
V-FITC/PI staining was performed to detect the apoptotic cells of
the above 6 cell lines after C646 treatment (10 μmol/l for
24 h). As shown in Fig. 4B, C646
significantly promoted the cell apoptosis of both normal gastric
epithelial cell line and the 5 GC cell lines (P<0.05).
C646 prevents the migration and invasion
of GC cells
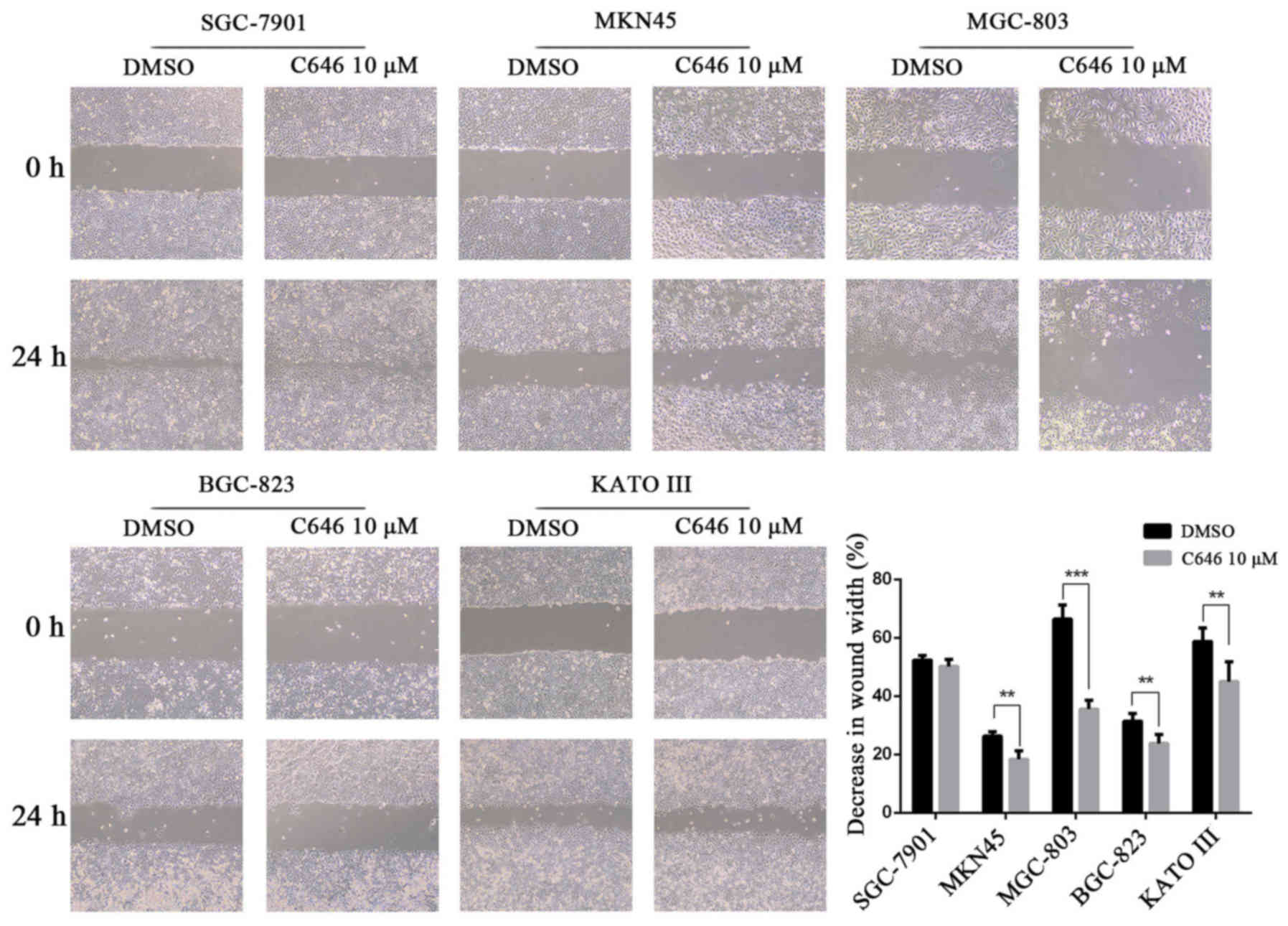
We performed a wound healing assay and found that
C646 significantly inhibited wound closure in the GC cells MKN45,
MGC-803, BGC-823 and KATO III (P<0.01). However, the wound
closure of SGC-7901 cells was not significantly affected by C646
compared with DMSO (Fig. 5). We
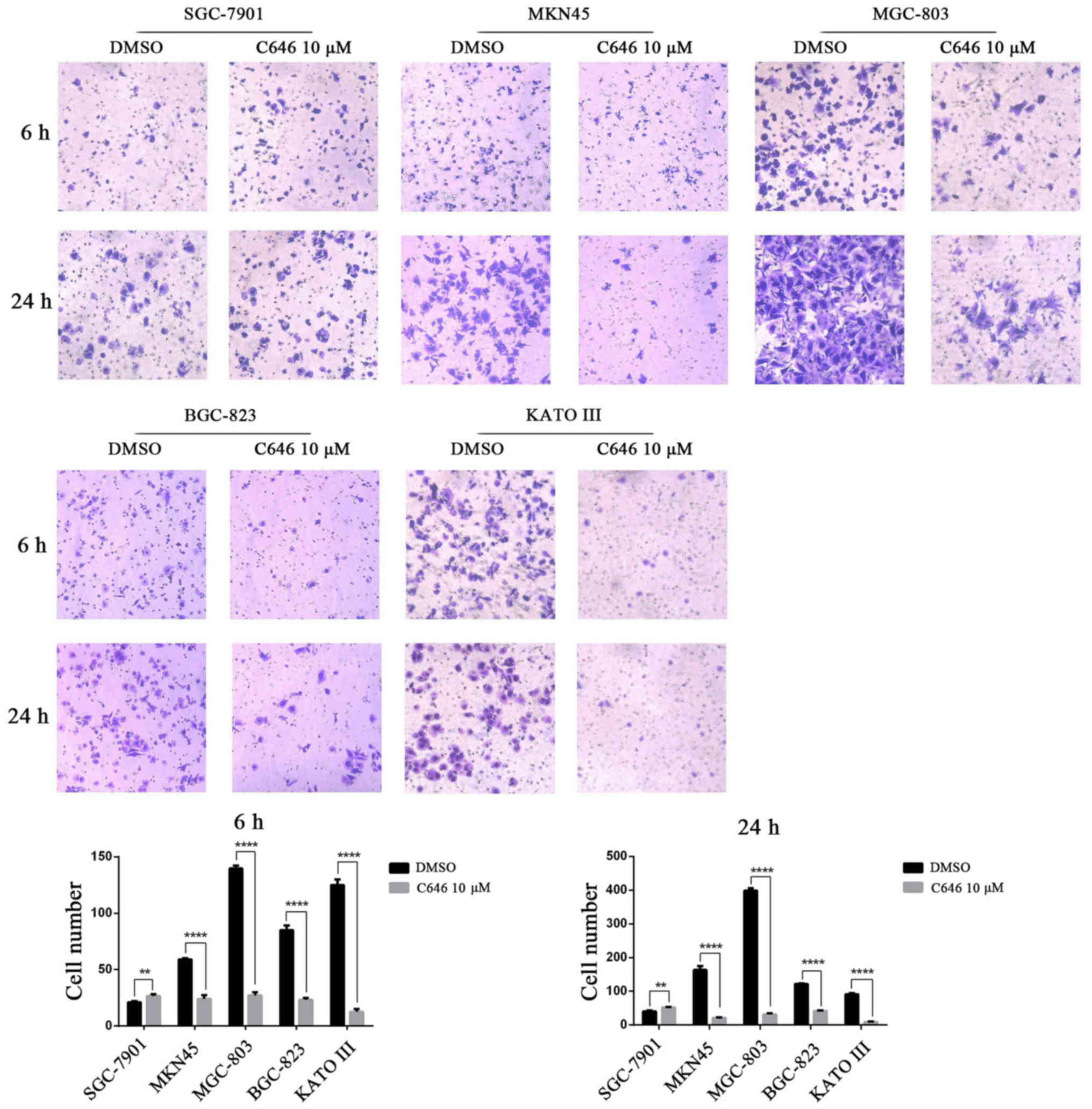
also performed a Transwell assay with the GC cell lines following
the administration of C646 10 μmol/l for 24 h. C646
treatment significantly reduced the number of invading cells in the
MKN45, MGC-803, BGC-823 and KATO III cell lines (P<0.0001),
whereas C646 treatment had the opposite effect in the SGC-7901 cell
line (P<0.001) (Fig. 6).
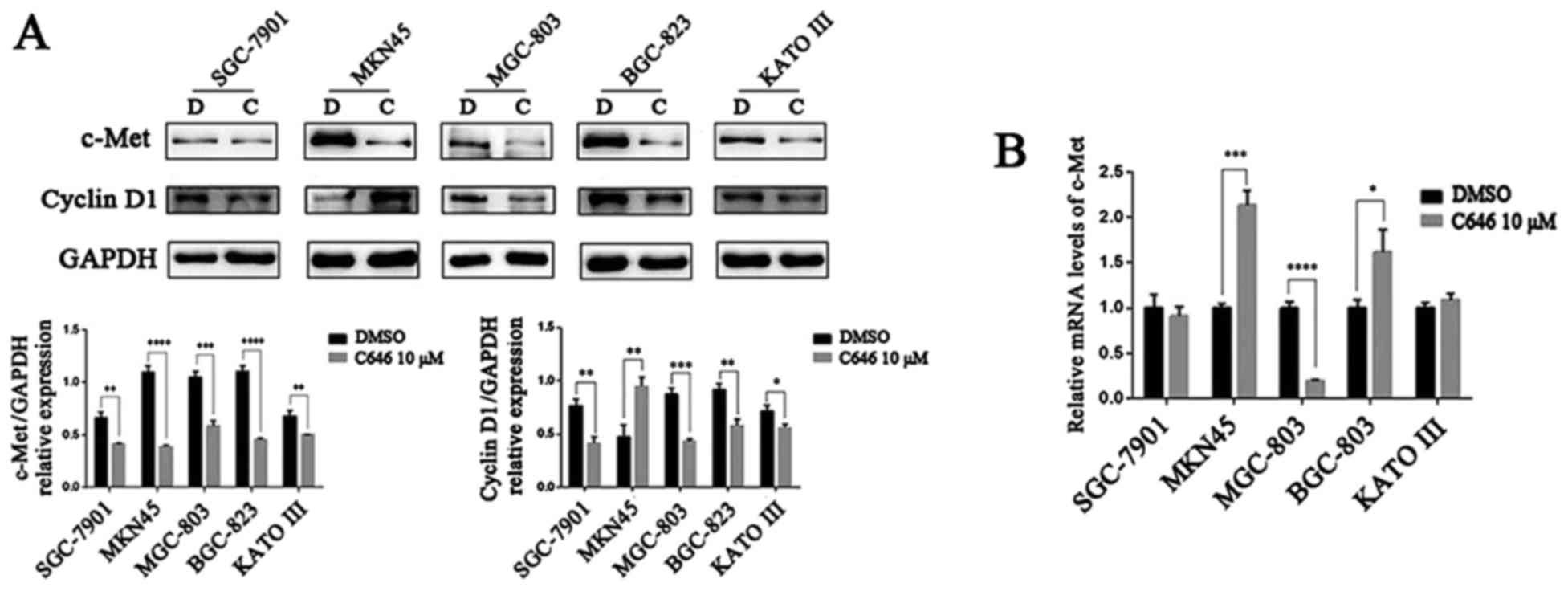
C646 suppresses the expression of c-Met,
cyclin D1, p-Akt, Bcl2, MMP7 and MMP9 in GC cells
According to previous studies, the tumourigenesis
and development of GC were closely related to the receptor tyrosine
kinase (RTK) c-Met (24–26). The activation of c-Met triggers
signal transduction through the MAPK or PI3K/Akt/mTOR signalling
pathways, which initiates cell proliferation and migration,
regulates the cell cycle and reduces cell apoptosis (24). Erk 1/2 and Akt are key members of
the MAPK and PI3K/Akt/mTOR signal pathways, respectively. cyclin D1
is a key protein that regulates the cell cycle. Accordingly, we
investigated the protein expression of c-Met, cyclin D1, Erk 1/2
and Akt in 5 GC cell lines after C646 treatment. Surprisingly, C646
dramatically inhibited the expression of c-Met protein (P<0.01)
(Fig. 7A). The mRNA level of
c-Met was consistent with its protein expression level in
MGC-803 cells, whereas no significant change or even an elevated
trend was detected in the other 4 GC cell lines (Fig. 7B). C646 (10 μmol/l, 6 h)
significantly downregulated cyclin D1 protein levels in the
SGC-7901, MGC-803, BGC-823 and KATO III cell lines (P<0.05), but
C646 treatment exhibited the opposite effect on cyclin D1
expression in MKN45 cells (Fig.
7A). We also observed a downregulation of total and
phosphorylated Akt protein levels in the 5 GC cell lines after C646
treatment. However, changes in total and phosphorylated Erk 1/2
levels showed the opposite trend (Fig.
8A).
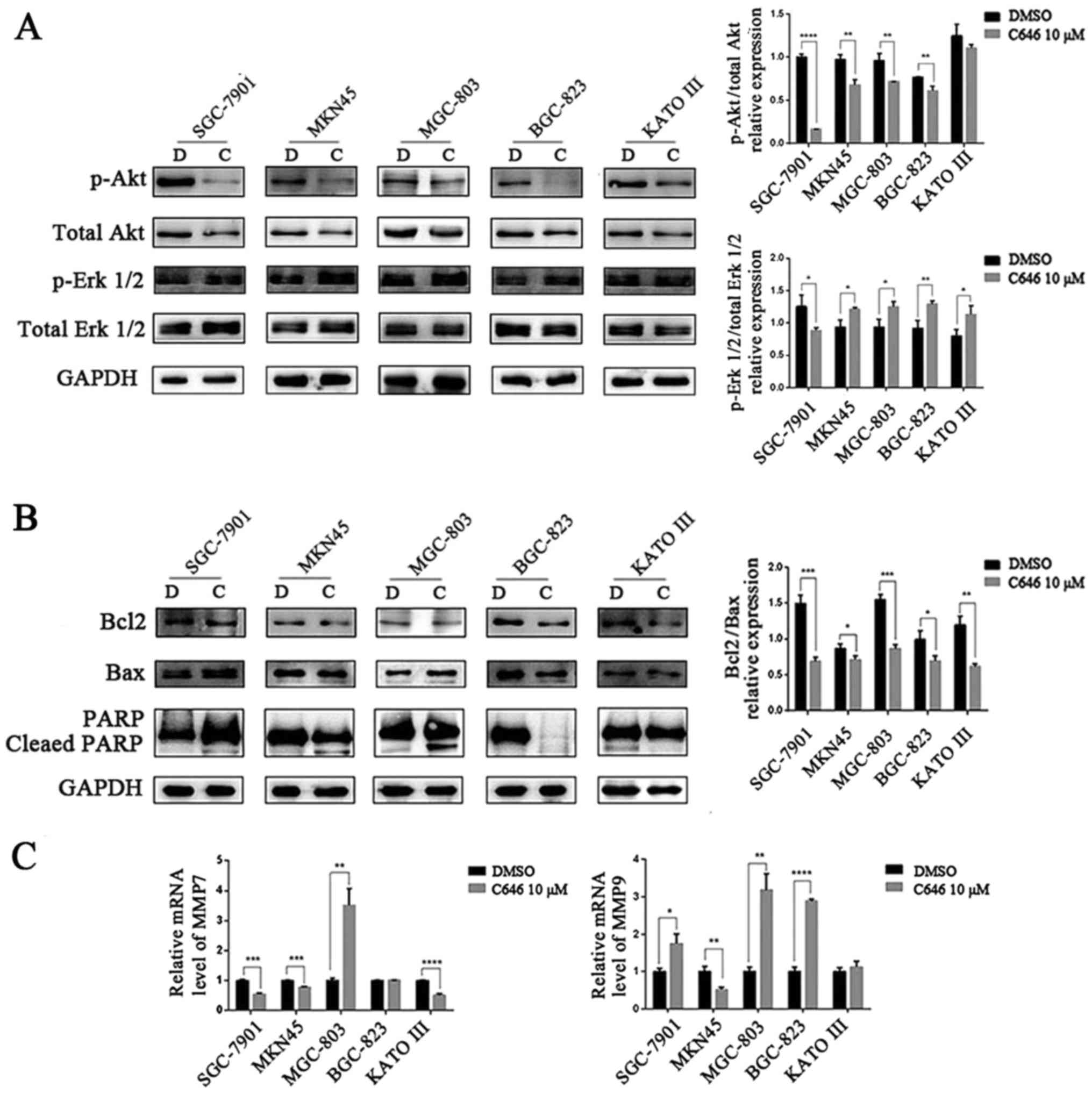 | Figure 8C646 activates the apoptotic pathway
in all 5 GC cell lines and inhibits MMP expression in some of the
GC cell lines. (A) After treatment with 10 μmol/l C646 for 6
h, the protein expression levels of p-Akt, total Akt, p-Erk 1/2 and
total Erk 1/2 in GC cell lines were detected by western blotting.
(B) GC cell lines were exposed to 10 μmol/l C646 for 6 h.
Western blotting was conducted to quantify the protein expression
levels of Bcl2, Bax and PARP. (C) After treatment with 10
μmol/l C646 for 24 h, qRT-PCR was performed to determine the
mRNA expression levels of MMP7 and MMP9 in GC cells.
The data shown are the means ± SD of three independent experiments
(*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. the DMSO
group). D, DMSO; C, C646. |
Bcl-2, Bax and PARP are apoptosis-related proteins.
In our experiments, we quantified the expression levels of these
three proteins in the 5 GC cell lines after C646 (10 μmol/l)
treatment for 6 h. C646 treatment decreased the ratio of Bcl-2/Bax
expression in all of the 5 GC cell lines (P<0.05). We also
detected cleaved PARP in the SGC-7901, MKN45 and MGC-803 cell lines
(Fig. 8B).
Finally, we measured the mRNA expression of matrix
metalloprotease 7 (MMP7) and matrix metalloprotease 9 (MMP9) by
qRT-PCR. As shown in Fig. 8C, in
the MKN45 and KATO III cell lines, the results were consistent with
an earlier study, and at least one of the two (MMP7 or
MMP9) mRNA levels decreased after C646 treatment. Regarding
the SGC-7901 cell line, the increased mRNA level of MMP9
could explain its enhanced invasive ability. However, in contrast
to previous results, the expression of MMPs mRNA was
increased in the MGC-803 and BGC-823 cell lines.
Discussion
The histone acetyltransferases p300/CBP play crucial
roles in several cell biology functions, such as the cell cycle,
DNA synthesis and cellular differentiation (5,7).
p300/CBP could acetylate histones and non-histone proteins to
suppress or promote tumourigenesis and malignancy (22,23).
p300/CBP are thought to contribute to the development of many
tumours, such as leukaemia, human prostate, colon, melanoma and
pancreatic cancer (11–13,17,27).
However, the differences in p300/CBP expression between normal
gastric epithelial cells and GC cells have rarely been reported. In
the present study, p300/CBP proteins were upregulated in 5 GC cell
lines, and the mRNA expression of p300/CBP was also
upregulated in GC cells (except MGC-803 cells) compared with normal
gastric epithelial cells, indicating that p300/CBP play important
roles in the tumourigenesis of GC.
To investigate the effects of p300/CBP on GC cell
lines, we used C646, a selective inhibitor of p300/CBP. C646
treatment supressed cell viability and induced cell apoptosis in
both normal gastric epithelial cells and the 5 GC cell lines. The
inhibition of cell viability in normal gastric epithelial cells by
C646 was not as strong as that of GC cells. We speculate that there
are two reasons for this outcome. First, both GC cells and normal
gastric epithelial cells express p300/CBP, but their expression
levels are lower in normal gastric epithelial cells than in GC
cells. Second, the expression level of Ac-H3 in GC cell lines is
higher than that in the GES-1 cell line, suggesting that p300/CBP
are excessively activated in GC cells. In addition, we observed
that C646 exhibited the strongest inhibitory effects toward poorly
differentiated GC cells (MKN45 and MGC-803).
In addition, C646 blocked the cell cycle in the
G0/G1 phase in SGC-7901, MKN45, BGC-823 and KATO III GC cells. This
outcome is consistent with a previous study (19). However, the cell cycle was arrested
in S phase in normal gastric epithelial GES-1 cells and GC MGC-803
cells, indicating that the cell cycle is differentially impacted by
C646 in different cell lines. The migration and invasion of most GC
cell lines were inhibited, but those biological functions were not
affected or even enhanced by C646 in the SGC-7901 cell line. The
disparate influences of C646 on the migration and invasion ability
of different GC cells may be related to the degree of cell
differentiation. SGC-7901 is a moderately differentiated GC cell
line, however, the other GC cells were poorly differentiated (MKN45
and MGC-803), undifferentiated (BGC-823) or classified as
signet-ring cancer cells (KATO III). C646 represents a potential
new drug for patients with GC, especially poorly differentiated GC.
However, for moderately differentiated GC, C646 was more likely to
promote tumourigenesis and progression.
c-Met, a receptor tyrosine kinase, plays a crucial
role in the tumourigenesis and development of GC (24–26).
We detected the protein levels of c-Met and its downstream
signalling molecules Akt and Erk 1/2. The protein expression of
c-Met, as well as that of total and phosphorylated Akt, was
downregulated after treatment with C646 in all 5 GC cell lines.
Moreover, C646 treatment led to decreased mRNA expression of
c-Met in MGC-803 cells. However, in the other 4 GC cell
lines, the c-Met mRNA expression levels were unchanged or
even elevated. MicroRNAs (miRNAs) have been reported to be involved
in regulating the expression of many cellular functional genes at
the post-transcriptional levels (1,28).
Studies have demonstrated that miRNAs inhibited the cell
proliferation, invasion and migration of GC cells by directly
targeting the c-Met gene and downregulating its expression
(29–31). Thus, we speculate that C646
regulates the expression of c-Met at the transcriptional level (for
MGC-803 cells) or posttranscriptional level (for SGC-7901, MKN45,
BGC-823 and KATO III cells) and further regulates cell
proliferation, migration, the cell cycle and apoptosis through the
Akt signalling pathway. The expression level of phosphorylated Erk
1/2 was found to be increased after C646 treatment in many of the
GC cell lines; however, the exact mechanism underlying this
phenomenon is unclear. Changes in p-Erk 1/2 expression under the
stimulation of some compounds has been reported within 1 h
(32,33). We suspect that the reversed
expression of p-Erk 1/2 observed in the present study may be
related to our experimental time-point.
p300/CBP can enhance the transcription of cyclin
D1 and promote G1-S cell cycle progression (34). To investigate whether the cell
cycle arrest of GC cell lines was associated with cyclin D1
expression, we performed a western blot assay. As expected, the
expression of cyclin D1 protein was inhibited by C646 in the GC
cell lines SGC-7901, MGC-803, BGC-823 and KATO III. Thus, C646
blocked the cell cycle in these 4 GC cell lines by inhibiting
cyclin D1 expression. Notably, C646 arrested the G0/G1 phase of the
MKN45 cell line but could not inhibit cyclin D1 expression,
suggesting that other cyclins may also participate in the
regulation of the cell cycle in different GC cells.
Bcl-2 is an important anti-apoptotic protein and Bax
is a crucial pro-apoptotic protein. Apoptotic activity can be
reflected by the ratio of Bcl-2/Bax (24). The nuclear enzyme poly(ADP-ribose)
polymerase (PARP) is a key modulator of the apoptotic pathway,
which can be proteolytically cleaved by caspase-3/7 when cell
apoptosis occurs (35). In the
present study, C646 treatment decreased the ratio of Bcl-2/Bax in
the 5 GC cell lines. In addition, cleaved PARP was discovered in
SGC-7901, MKN45 and MGC-803 cells. Consequently, C646 may induce
apoptosis of GC cells through different pathways.
As members of the matrix metalloproteinase family,
MMP7 and MMP9 are involved in tumour metastasis, and their
expression is associated with the invasion activity of cancer
cells. The expression of MMP7 and MMP9 mRNA was
detected in 5 GC cells after C646 treatment. For the SGC-7901,
MKN45 and KATO III cell lines, the MMP expression trends
could explain the previous migration and invasion results. While
C646 increased the mRNA expression of MMP7 and (or)
MMP9 in the BGC-823 and MGC-803 cell lines, the results are
inconsistent with previous reports. Tumour metastasis is a highly
complex process that is closely associated with EMT. He et
al (36) found that mesothelin
(MSLN) played an essential role in cell adhesion, migration and
invasion of many solid tumour cells (such as human lung cancer
cells and mesothelioma cells) by regulating EMT. In addition,
reports have revealed that MSLN is expressed in many GCs and
associated with lymphatic involvement, tumour invasion and the
5-year survival rate of GC patients (37–39).
We speculate that C646 might affect the migration and invasion of
MGC-803 and BGC-823 cells by regulating the expression of MSLN or
other MMPs that were not quantified in the present study.
In conclusion, p300/CBP might contribute to the
carcinogenesis and development of GC. The p300/CBP inhibitor C646
inhibited cell growth, blocked the cell cycle and promoted cell
apoptosis in GC cells by regulating the c-Met/Akt pathway and the
expression of Bcl2, PARP and cyclin D1. Additionally, C646
exhibited a potent inhibitory effect on migration and invasion in
poorly differentiated, undifferentiated or signetring GC cells.
Based on these results, C646 is a potential treatment for
multi-type (except the moderately differentiated type) GC.
Acknowledgments
The present study was supported by the Scientific
Research Foundation of the National Health and Family Planning
Commission of the People's Republic of China (WKJ-ZJ-1614). We are
very grateful to the State Key Laboratory for the Diagnosis and
Treatment of Infectious Diseases of the First Affiliated Hospital
of Zhejiang University for providing excellent technical
assistance.
References
|
1
|
Tan P and Yeoh KG: Genetics and molecular
pathogenesis of gastric adenocarcinoma. Gastroenterology.
149:1153–1162.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyer HJ and Wilke H: Treatment strategies
in gastric cancer. Dtsch Arztebl Int. 108:698–705.
7062011.PubMed/NCBI
|
|
3
|
Saka M, Morita S, Fukagawa T and Katai H:
Present and future status of gastric cancer surgery. Jpn J Clin
Oncol. 41:307–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giles RH, Peters DJ and Breuning MH:
Conjunction dysfunction: CBP/p300 in human disease. Trends Genet.
14:178–183. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roth SY, Denu JM and Allis CD: Histone
acetyltransferases. Annu Rev Biochem. 70:81–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shiama N: The p300/CBP family: Integrating
signals with transcription factors and chromatin. Trends Cell Biol.
7:230–236. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giordano A and Avantaggiati ML: p300 and
CBP: Partners for life and death. J Cell Physiol. 181:218–230.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan HM and La Thangue NB: p300/CBP
proteins: HATs for transcriptional bridges and scaffolds. J Cell
Sci. 114:2363–2373. 2001.PubMed/NCBI
|
|
9
|
Debes JD, Sebo TJ, Lohse CM, Murphy LM,
Haugen DA and Tindall DJ: p300 in prostate cancer proliferation and
progression. Cancer Res. 63:7638–7640. 2003.PubMed/NCBI
|
|
10
|
Linja MJ, Porkka KP, Kang Z, Savinainen
KJ, Jänne OA, Tammela TL, Vessella RL, Palvimo JJ and Visakorpi T:
Expression of androgen receptor coregulators in prostate cancer.
Clin Cancer Res. 10:1032–1040. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santer FR, Höschele PP, Oh SJ, Erb HH,
Bouchal J, Cavarretta IT, Parson W, Meyers DJ, Cole PA and Culig Z:
Inhibition of the acetyltransferases p300 and CBP reveals a
targetable function for p300 in the survival and invasion pathways
of prostate cancer cell lines. Mol Cancer Ther. 10:1644–1655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iyer NG, Chin SF, Ozdag H, Daigo Y, Hu DE,
Cariati M, Brindle K, Aparicio S and Caldas C: p300 regulates
p53-dependent apoptosis after DNA damage in colorectal cancer cells
by modulation of PUMA/p21 levels. Proc Natl Acad Sci USA.
101:7386–7391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin WM, Baker AC, Beroukhim R, Winckler W,
Feng W, Marmion JM, Laine E, Greulich H, Tseng H, Gates C, et al:
Modeling genomic diversity and tumor dependency in malignant
melanoma. Cancer Res. 68:664–673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garraway LA, Widlund HR, Rubin MA, Getz G,
Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J,
et al: Integrative genomic analyses identify MITF as a lineage
survival oncogene amplified in malignant melanoma. Nature.
436:117–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato S, Roberts K, Gambino G, Cook A,
Kouzarides T and Goding CR: CBP/p300 as a co-factor for the
Microphthalmia transcription factor. Oncogene. 14:3083–3092. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yajima I, Kumasaka MY, Thang ND, Goto Y,
Takeda K, Iida M, Ohgami N, Tamura H, Yamanoshita O, Kawamoto Y, et
al: Molecular Network associated with MITF in skin melanoma
development and progression. J Skin Cancer. 2011:7301702011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paladino D, Yue P, Furuya H, Acoba J,
Rosser CJ and Turkson J: A novel nuclear Src and p300 signaling
axis controls migratory and invasive behavior in pancreatic cancer.
Oncotarget. 7:7253–7267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han RF, Ji X, Dong XG, Xiao RJ, Liu YP,
Xiong J and Zhang QP: An epigenetic mechanism underlying
doxorubicin induced EMT in the human BGC-823 gastric cancer cell.
Asian Pac J Cancer Prev. 15:4271–4274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao XN, Lin J, Ning QY, Gao L, Yao YS,
Zhou JH, Li YH, Wang LL and Yu L: A histone acetyltransferase p300
inhibitor C646 induces cell cycle arrest and apoptosis selectively
in AML1-ETO-positive AML cells. PLoS One. 8:e554812013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu ML, Wang YM, Zhou XX, Yao HP, Zheng S,
Xiang Z and Ji F: An inhibitor of the acetyltransferases CBP/p300
exerts antineoplastic effects on gastrointestinal stromal tumor
cells. Oncol Rep. 36:2763–2770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Gu ML, Zhou XX, Ma H, Yao HP and
Ji F: Altered expression of ETV1 and its contribution to
tumorigenic phenotypes in gastrointestinal stromal tumors. Oncol
Rep. 32:927–934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedmann DR and Marmorstein R: Structure
and mechanism of non-histone protein acetyltransferase enzymes.
FEBS J. 280:5570–5581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Gural A, Sun XJ, Zhao X, Perna F,
Huang G, Hatlen MA, Vu L, Liu F, Xu H, et al: The leukemogenicity
of AML1-ETO is dependent on site-specific lysine acetylation.
Science. 333:765–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Yao X, Zhu M, Qian H, Jiang L, Lan
T, Wu M, Pang J and Chen Y: PKG II reverses HGF-triggered cellular
activities by phosphorylating serine 985 of c-Met in gastric cancer
cells. Oncotarget. 7:34190–34200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marano L, Chiari R, Fabozzi A, De Vita F,
Boccardi V, Roviello G, Petrioli R, Marrelli D, Roviello F and
Patriti A: c-Met targeting in advanced gastric cancer: An open
challenge. Cancer Lett. 365:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuse N, Kuboki Y, Kuwata T, Nishina T,
Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, et
al: Prognostic impact of HER2, EGFR, and c-MET status on overall
survival of advanced gastric cancer patients. Gastric Cancer.
19:183–191. 2016. View Article : Google Scholar
|
|
27
|
Katsumoto T, Yoshida N and Kitabayashi I:
Roles of the histone acetyltransferase monocytic leukemia zinc
finger protein in normal and malignant hematopoiesis. Cancer Sci.
99:1523–1527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu YF, Zhang L, Waye MM, Fu WM and Zhang
JF: MiR-218 mediates tumorigenesis and metastasis: Perspectives and
implications. Exp Cell Res. 334:173–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Zhang H, Wang X, Qu Y, Duan J, Liu
R, Deng T, Ning T, Zhang L, Bai M, et al: Direct targeting of HGF
by miR-16 regulates proliferation and migration in gastric cancer.
Tumour Biol. 37:15175–15183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei B, Huang QY, Huang SR, Mai W and Zhong
XG: MicroRNA-34a attenuates the proliferation, invasion and
metastasis of gastric cancer cells via downregulation of MET. Mol
Med Rep. 12:5255–5261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han C, Zhou Y, An Q, Li F, Li D, Zhang X,
Yu Z, Zheng L, Duan Z and Kan Q: MicroRNA-1 (miR-1) inhibits
gastric cancer cell proliferation and migration by targeting MET.
Tumour Biol. 36:6715–6723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji Z, Su J, Liu C, Wang H, Huang D and
Zhou X: Integrating genomics and proteomics data to predict drug
effects using binary linear programming. PLoS One. 9:e1027982014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kholodenko BN, Demin OV, Moehren G and
Hoek JB: Quantification of short term signaling by the epidermal
growth factor receptor. J Biol Chem. 274:30169–30181. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HR, Mitra J, Lee S, Gao SJ, Oh TK, Kim
MH, Ha T and Jung JU: Kaposi's sarcoma-associated herpesvirus viral
interferon regulatory factor 4 (vIRF4) perturbs the G1-S cell cycle
progression via deregulation of the cyclin D1 gene. J Virol.
90:1139–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao H, Wu B, Le Y and Zhu Z: Homeobox
protein VentX induces p53-independent apoptosis in cancer cells.
Oncotarget. 7:39719–39729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He X, Wang L, Riedel H, Wang K, Yang Y,
Dinu CZ and Rojanasakul Y: Mesothelin promotes
epithelial-to-mesenchymal transition and tumorigenicity of human
lung cancer and mesothelioma cells. Mol Cancer. 16:632017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ito T, Kajino K, Abe M, Sato K, Maekawa H,
Sakurada M, Orita H, Wada R, Kajiyama Y and Hino O: ERC/mesothelin
is expressed in human gastric cancer tissues and cell lines. Oncol
Rep. 31:27–33. 2014. View Article : Google Scholar
|
|
38
|
Einama T, Homma S, Kamachi H, Kawamata F,
Takahashi K, Takahashi N, Taniguchi M, Kamiyama T, Furukawa H,
Matsuno Y, et al: Luminal membrane expression of mesothelin is a
prominent poor prognostic factor for gastric cancer. Br J Cancer.
107:137–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baba K, Ishigami S, Arigami T, Uenosono Y,
Okumura H, Matsumoto M, Kurahara H, Uchikado Y, Kita Y, Kijima Y,
et al: Mesothelin expression correlates with prolonged patient
survival in gastric cancer. J Surg Oncol. 105:195–199. 2012.
View Article : Google Scholar
|