Introduction
Mineral dust-induced gene (mdig; which is also known
as MYC-induced nuclear antigen, mina53 and NO52) is a novel
tumor-related gene that has been discovered in the alveolar
macrophages of coal miners (1).
Many substances can induce mdig expression, such as arsenic
(2) and mineral dust (3), among others. It has been reported
that mdig is a proto-oncogene that is highly expressed in a variety
of tumor cells, where it not only serves as an independent factor
leading to tumor formation, but also as a promoter of tumor cell
proliferation (1,4–6).
Mdig/mina53 is a downstream target gene of the transcription factor
c-Myc, and is located on chromosome 3 (3q12.1). The full length
mdig gene is composed of 1,510 bases and contains 10 exons that
encode 465 amino acids, which amount to a 53-kDa nucleoprotein
(1,7). The mdig/mina53 protein contains a
conserved JumonjiC (Jmjc) domain that regulates the expression of
certain genes in histones through the action of demethylases
(8). In a study by Komiya et
al, NIH-3T3 cells transfected with mdig/mina53 were analyzed
using a gene chip technique, which indicated that 125 genes were
upregulated and 129 genes were downregulated as a result of mdig
overexpression; among the altered genes, 17 were associated with
growth factors, 12 were associated with cell proliferation and the
cell cycle, 59 were involved in cell invasion and metastasis, 34
were associated with transcriptional regulation, and 20 genes were
associated with metabolism (9). It
also has been observed that mdig plays an important role in the
regulation of cell invasion and metastasis. Our previous study
found a contradictory phenomenon: compared with a control group,
the expression of mdig in A549 cells increased the ability of the
cells to proliferate, while the invasive and metastatic properties
of the cells were significantly reduced (6), indicating that mdig can promote the
proliferation of tumor cells, but inhibit cell invasion and
metastasis; however, the exact mechanism underlying these
contradictory findings is not clear.
Epithelial-mesenchymal transition (EMT) is not only
a key mechanism in the formation of multi-germ layers and the
maintenance of tissue integrity during embryonic development, but
is also a key mechanism underlying the invasion and metastasis of
tumor cells, the formation of tumor stem cells and tumor resistance
(10–14). EMT in tumor cells is primarily
characterized by a decrease in intercellular connections, a
disappearance of cell polarity and an elongated spindle-like cell
morphology, which enables cells to detach and become mobile, and
ultimately promotes the occurrence of cell invasion and metastasis
(10,15,16).
The molecular mechanisms of EMT mainly manifest as a gradual loss
of epithelial cell markers (E-cadherin, ZO-1, and claudin-1), a
reduction in the expression of cell adhesion proteins (integrin β1,
and integrin β4), and an increase in the expression of mesenchymal
cell markers (N-cadherin, and vimentin) (10). Previous studies have confirmed that
the GSK-3β/β-catenin pathway is an important signal transduction
pathway in the regulation of EMT occurrence in cells. Notably,
GSK-3β has been reported to phosphorylate β-catenin, while
phosphorylation of GSK-3β (P-GSK-3β) inhibits its ability to
phosphorylate β-catenin (17,18);
non-phosphorylated β-catenin can then translocate into the nucleus
and promote the expression of the downstream transcription factors
snail, slug and ZEB1, thereby promoting the occurrence of EMT and
tumor cell invasion and metastasis (12,19).
The present study demonstrated that mdig can inhibit
the phosphorylation of GSK-3β (P-Ser9-GSK-3β) and promote the
phosphorylation and destabilization of β-catenin (P-Ser33, Ser37,
and Thr41-β-catenin), in order to suppress the expression of slug,
snail and ZEB1 and the occurrence of EMT, and thereby inhibit the
invasion and metastasis of non-small cell lung cancer (NSCLC).
Materials and methods
Cell culture
The human NSCLC cell line A549 was purchased from
the Chinese Academy of Sciences (Shanghai, China). A549 is a tumor
cell line originating from alveolar epithelium, with a typical
epithelial cell morphology and adherent growth. As a human lung
adenocarcinoma cell, A549 cell has been applied widely in EMT
study. The human umbilical vein endothelial cells (HUVECs) were
purchased from the Peking University Cancer Institute (Beijing,
China). HUVEC is an epithelial origin cell line, with a typical
epithelial cell morphology, cobblestone appearance with large dark
nuclei and adherent growth. This cell line is susceptible to
transfection and often used as a tool cell. The cells were cultured
in RPMI-1640 culture medium (Hyclone, USA) containing 10% fetal
bovine serum (FBS; Hyclone) in a 5% CO2 cell incubator
(Thermo Fisher Scientific, Inc., USA) at 37°C.
Lentivirus transfection
An mdig overexpression lentiviral vector (LV-mdig;
GenBank accession NM_032778), an empty control lentiviral vector
(vector), mdig silencing lentiviral vectors (LV-mdig-RNAi 1,
sequence: 5′-GGGTGATTTGTTGTACTTT-3′; LV-mdig-RNAi 2, sequence:
5′-AACGATTCAGTTTCACCAA-3′) and a control lentiviral vector (LV-con,
sequence: 5′-TTCTCCGAACGTGTCACGT-3′) were purchased from GeneChem
(Shanghai, China). The day before transfection, 5 ml
(5×104 cells/ml) of the target cells were inoculated
into a T25 flask (Corning, USA), and when cell confluence reached
30–50%, the cells were incubated with lentivirus concentrations
equivalent to the target cell infection index (MOI: A549–50,
HUVEC-20). After 16 h, the medium was replenished with 5 ml fresh
complete medium and the cells were incubated for a further 48 h.
The cells were subsequently analyzed under an inverted fluorescence
microscope (Observer A1; ZEISS, Germany), and the transfection
efficiency was expressed as the percentage of GFP-positive cells
identified with a GFP fluorescence module (excitation, BP470/40;
beam splitter, FT495; emission, BP525/50).
Transwell invasion assay
A matrix gel (Matrigel matrix; Corning) was diluted
at a 1:3 ratio and spread evenly onto the bottom of 24-well
Transwell inserts. A549 cells, and A549 cells transfected with
LV-con, LV-mdig and LV-mdig-RNAi were digested and counted during
the logarithmic growth phase, and a cell suspension
(1×106 cells/ml) was prepared with serum-free RPMI-1640
cell culture broth. Cell suspension (150 μl) was added to
each chamber in a 24-well Transwell plate (Corning), and 600
μl RPMI-1640 medium containing 20% FBS was added to each
lower chamber. Subsequently, the upper chambers were inserted into
the lower chambers and placed in a 5% CO2 incubator at
37°C for 24 h. The upper chambers were then removed and fixed in 4%
paraformaldehyde prior to crystal violet staining. Cells were
counted in randomly selected visual fields of an inverted
fluorescence microscope at ×400 magnification.
Transwell migration assay
A549 cells and A549 cells transfected with LV-con,
LV-mdig and LV-mdig-RNAi were digested and counted during the
logarithmic growth phase, and a cell suspension (5×105
cells/ml) was prepared with serum-free RPMI-1640 cell culture
broth. Cell suspension (150 μl) was added to each chamber in
a 24-well Transwell plate (Corning), and 600 μl RPMI-1640
medium containing 20% FBS was added to each lower chamber.
Subsequently, the upper chambers were inserted into the lower
chambers and placed in a 5% CO2 incubator at 37°C for 16
h. The upper chambers were then removed, fixed in 4%
paraformaldehyde and stained with crystal violet. Cells were
counted in randomly selected visual fields of an inverted
fluorescence microscope at ×400 magnification.
Wound healing assay
The day before the experiment, A549 cells
transfected with LV-con, LV-mdig and LV-mdig-RNAi were digested and
counted during the logarithmic growth phase. The cells
(5×105 cells/ml) were incubated in 6-well plates
(Corning) for 24 h, and then the cell monolayer was scratched with
a 200-μl pipette tip (Coring) positioned at a perpendicular
angle to the plate to keep the scratch width consistent. The medium
was subsequently removed and the cells were washed three times with
PBS (Hyclone), then incubated with serum-free RPMI-1640 medium in a
5% CO2 incubator at 37°C. After 0, 12 and 24 h, GFP
fluorescence was visualized and the cells were imaged with an
inverted fluorescence microscope. ImageJ software (ImageJ 1.51J8,
Wayne Rasband, National Institutes of Health, USA) was used to
measure the scratch area and calculate the percentage of scratch
healing, and the scratch healing areas of the different groups were
compared.
Western blotting
After 4 passages, total protein was extracted from
transfected cells with RIPA buffer containing 10% PMSF, and total
protein concentration was measured using a BCA Protein assay kit
(Thermo Fisher Scientific, Inc.). The protein samples (30
μg) were subjected to SDS-PAGE (Bio-Rad, USA) and then
transferred onto Immobilon-P PVDF membranes (0.45 and 0.22
μm), which were then blocked at room temperature for 2 h
with 5% non-fat dried milk. The membranes were subsequently washed
with TBST, then incubated with primary antibodies (rabbit mAbs)
against mina53 (#173573) (1:1,000; Abcam, USA), phospho-GSK-3β
(#5558), integrin β4 (#14803), integrin β1 (#9699), non-phospho
(active) β-catenin (non-phospho-Ser33/37/Thr41) (#8814), vimentin
(#5741), N-cadherin (#13116), claudin-1 (#13255), β-catenin
(#8480), ZO-1 (#8193), snail (#3879), anti-slug (#9585), ZEB1
(#3396), E-cadherin (#3195) and GAPDH (#5174) (1:1,000; Cell
Signaling Technology, USA) at 4°C overnight. After another TBST
wash, the membranes were incubated with secondary antibody (#7074)
(anti-rabbit IgG; 1:3,000, Cell Signaling Technology) at room
temperature for 2 h. Immunoreactive bands were detected with an ECL
western blotting system (Clarity Western ECL Substrate; Bio-Rad).
The gray scale densities of the bands were measured with ImageJ
software, and the density ratio of each protein band to that of
GAPDH was calculated and expressed as a percentage relative to the
normal control group.
Statistical analysis
The data were expressed as the mean ± standard
deviation (SD). Comparisons between groups were performed by a
one-way analysis of variance (ANOVA). P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed with SPSS 22.0 software for Windows (IBM
Corp., Armonk, NY, USA). All experiments were repeated ≥3
times.
Results
Construction of mdig-knockdown and
mdig-overexpressing A549 cell lines
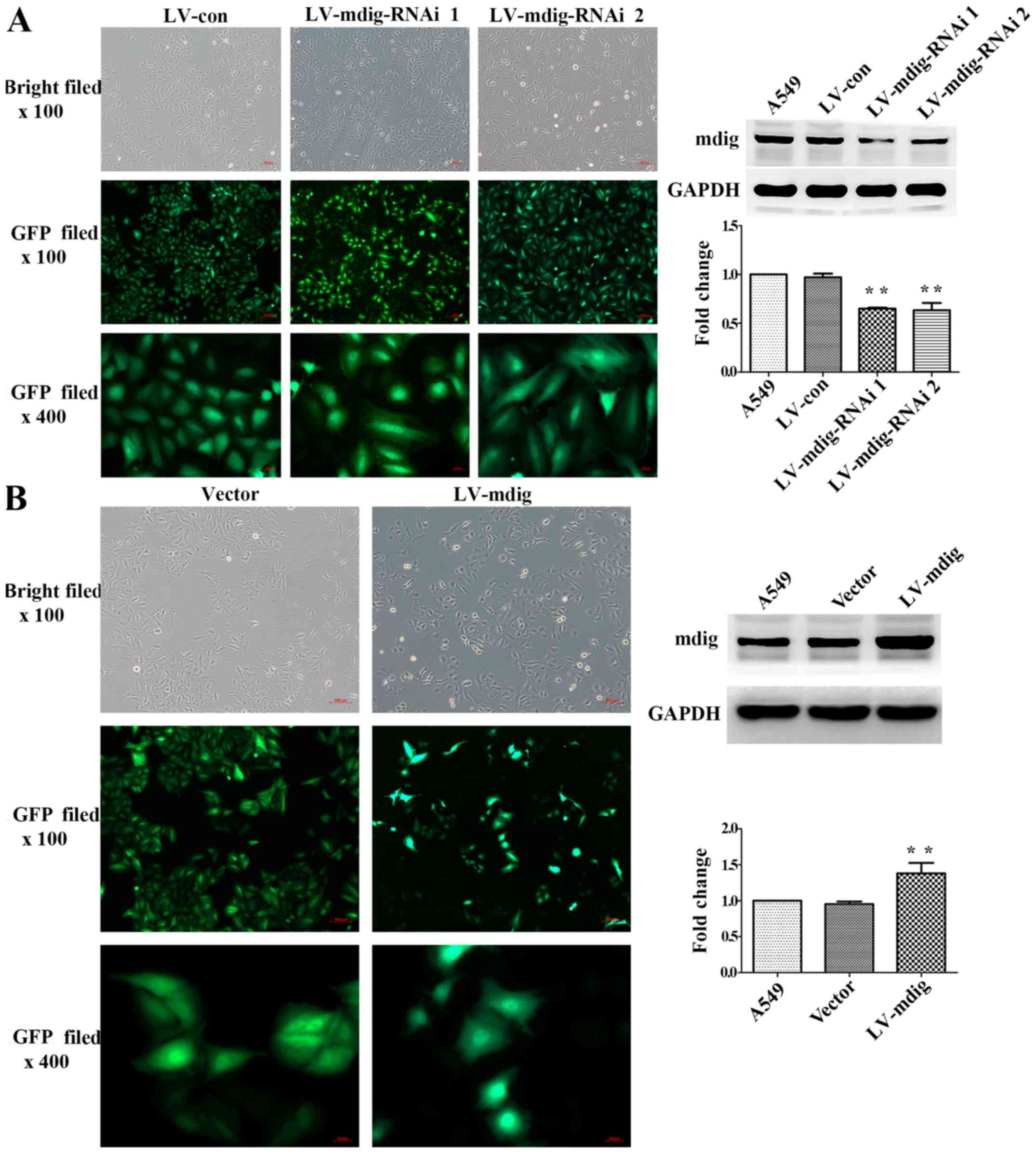
Fourth generation A549 cells transfected with
lentivirus were observed under the bright field of an inverted
fluorescence microscope at ×100 magnification. The same fields of
view were analyzed for GFP fluorescence at ×100 and ×400
magnification. All the cell groups exhibited a high cell viability
and high transfection efficiency (Fig.
1). In the mdig silencing experiment, cells in the LV-con group
exhibited a cobblestone-like cell morphology, while cells in the
LV-mdig-RNAi 1 and LV-mdig-RNAi 2 groups exhibited more elongated
spindle-like shapes. In addition, mdig protein expression was
significantly decreased in the RNAi groups when compared with
normal A549 cells and the LV-con group (P<0.01) (Fig. 1A). The LV-mdig-RNAi 1 group was
used in the subsequent Transwell assays. In A549 cells
overexpressing mdig, the morphology of cells in the LV-mdig group
appeared rounder than the cobblestone morphology of cells in the
vector group, and mdig protein expression was significantly
increased in the LV-mdig group when compared with normal A549 cells
and the vector group (P<0.01) (Fig.
1B).
Construction of an mdig-overexpressing
HUVEC line
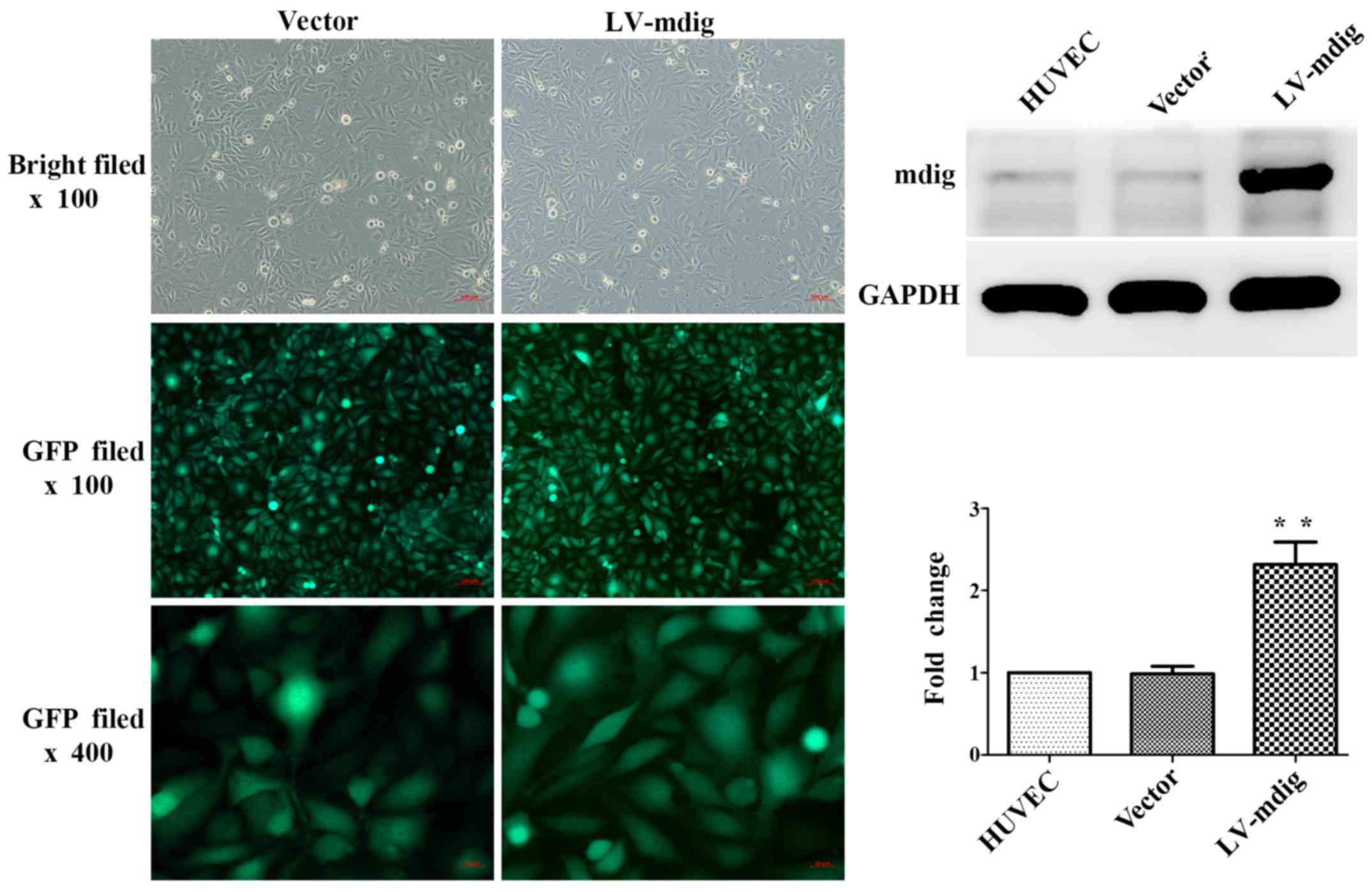
Inverted fluorescence microscopy was used to
visualize fourth generation HUVECs transfected with lentivirus as
above. All the cell groups exhibited a high cell viability status
and high transfection efficiency. There were no marked differences
in the morphologies of cells between the LV-mdig and vector groups.
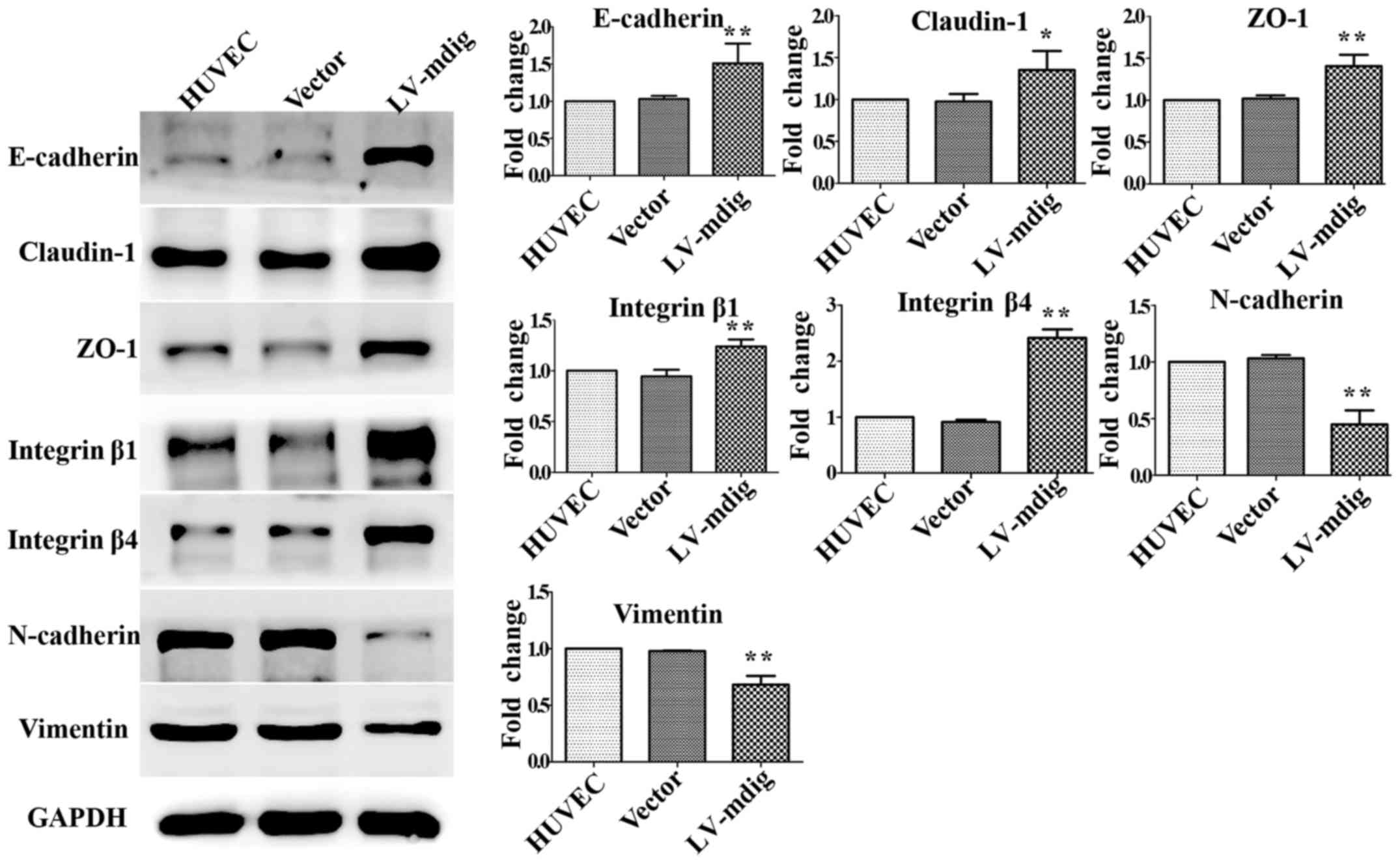
However, the expression of mdig protein in the LV-mdig group was
significantly upregulated when compared with the normal HUVEC and
vector groups (P<0.01) (Fig.
2).
Effect of mdig on the invasion and
migration of A549 cells
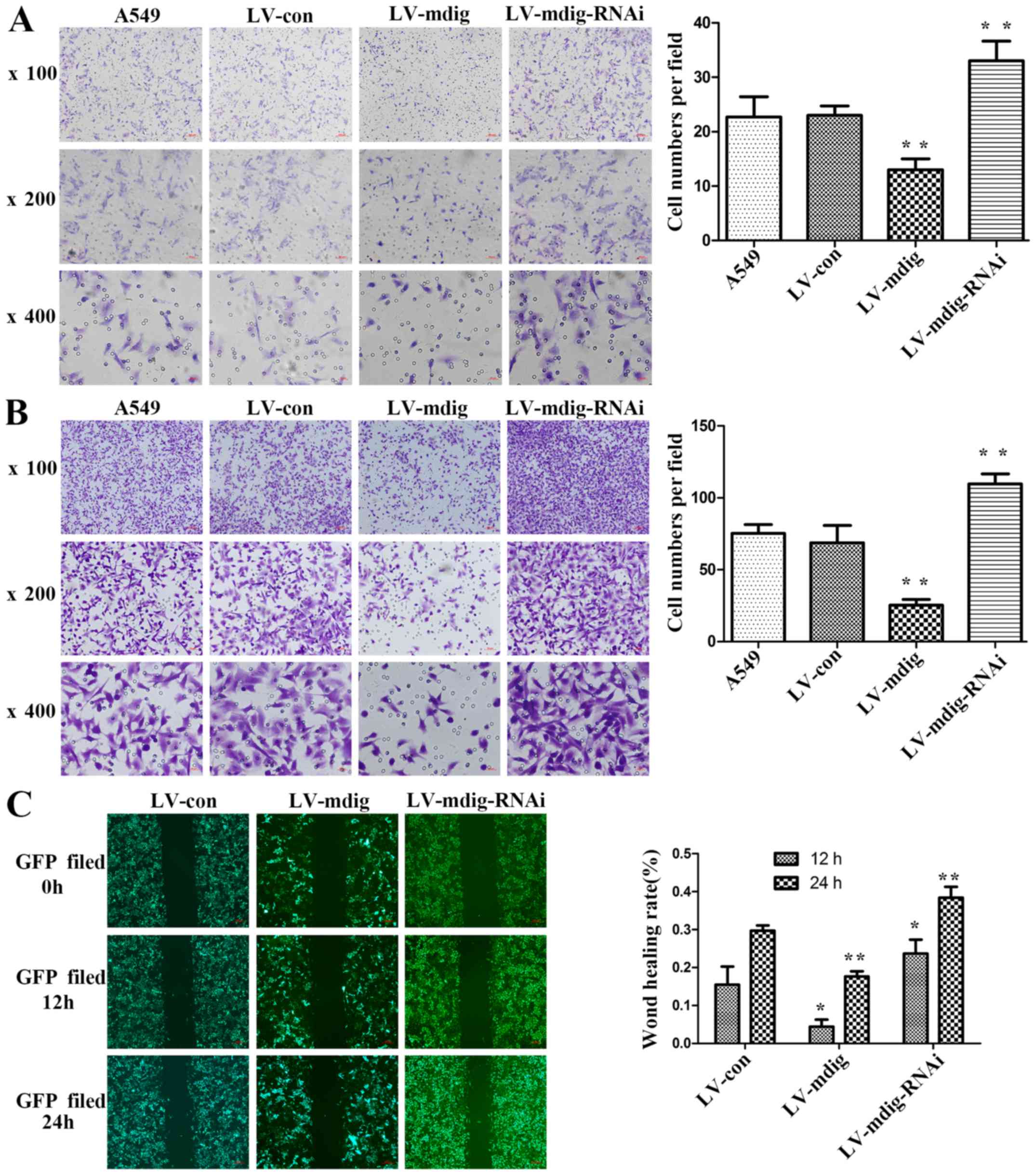
In this study, Transwell assays were performed to
determine the effect of mdig on the invasion and migration of A549
cells. In the invasion assay, there was no significant difference
in the number of cells in the matrix layer between the A549 and
LV-con groups. By contrast, the number of cells in the LV-mdig
group was significantly lower than that in the LV-con and A549
groups (P<0.01), and the number of cells in the LV-mdig-RNAi
group was significantly higher than that in the LV-con and A549
groups (P<0.01) (Fig. 3A). The
same results were obtained in the migration assay (Fig. 3B).
A scratch-wound assay was performed to further
verify the effect of mdig on the migratory ability of A549 cells.
In this assay, the LV-mdig group exhibited a significantly slower
healing speed than the LV-con group (P<0.05), while the
LV-mdig-RNAi group exhibited a significantly faster healing speed
than the LV-con group (P<0.05) (Fig. 3C).
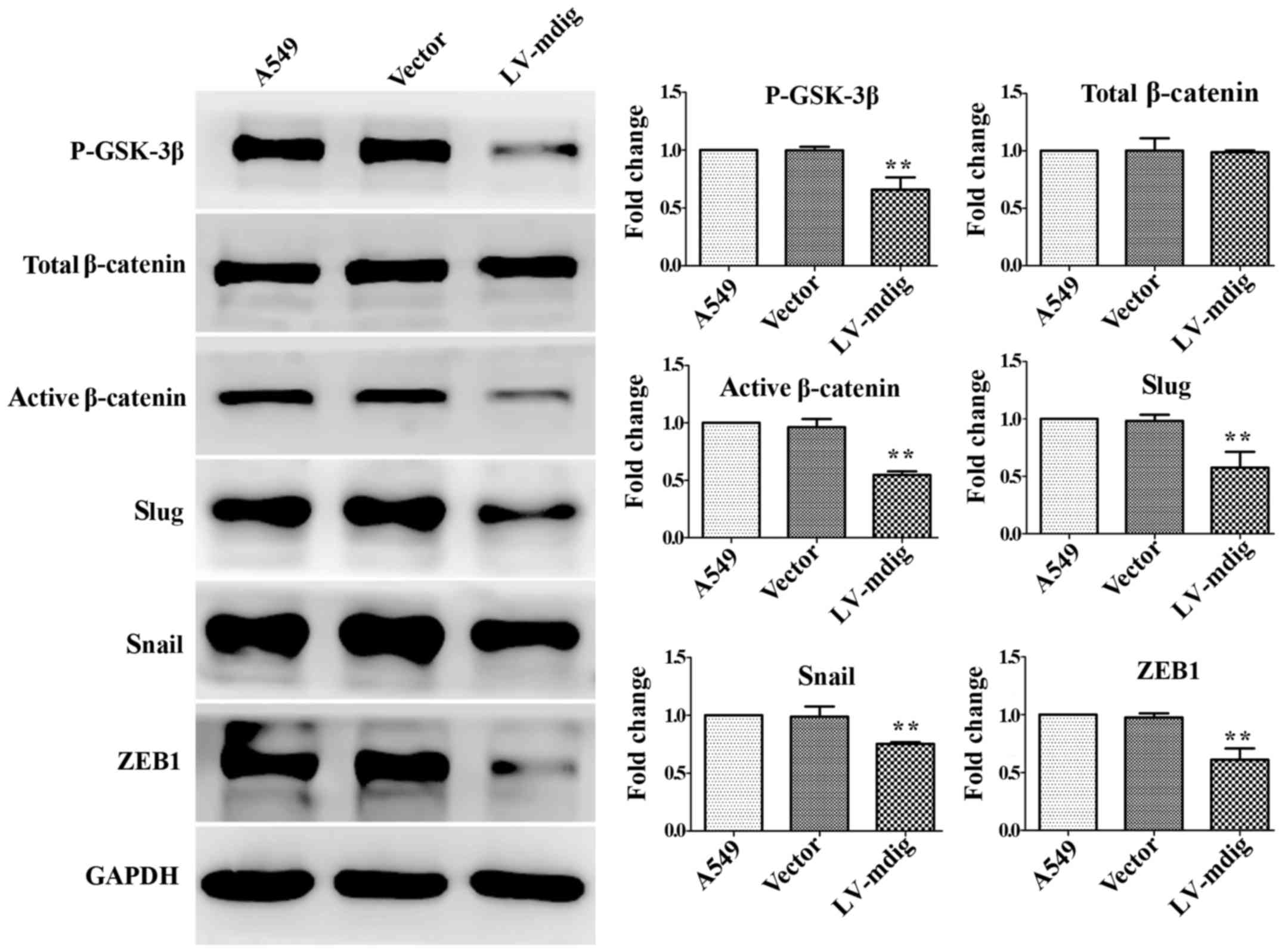
Mdig regulates the GSK-3β/β-catenin
signaling pathway
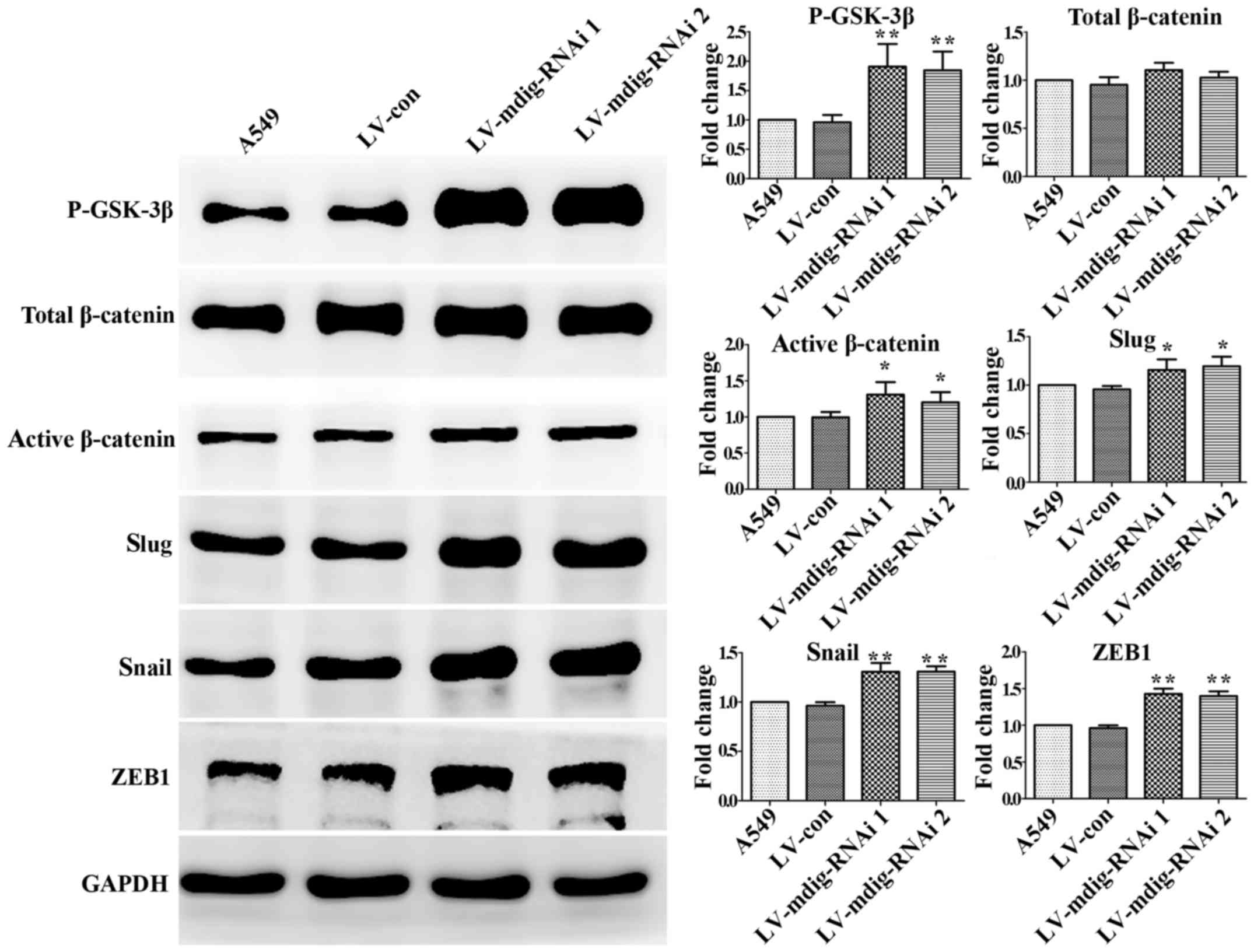
In the mdig-knockdown and mdig-overexpressing cell
lines, western blotting was performed to analyze the
GSK-3β/β-catenin signaling pathway and its downstream regulation of
EMT transcriptional regulators. In the mdig knockdown experiment,
the expression levels of P-GSK-3β, active β-catenin, slug, snail
and ZEB1 in the mdig-knockdown A549 cell group were significantly
higher than those in the normal A549 and LV-con groups (P<0.05),
while changes in the levels of total β-catenin were not significant
(P>0.05) (Fig. 4). In the mdig
overexpression experiment, the expression levels of P-GSK-3β,
active β-catenin, slug, snail and ZEB1 in mdig-overexpressing A549
cells were significantly lower than those in the normal A549 and
LV-con groups (P<0.01), while changes in the levels of total
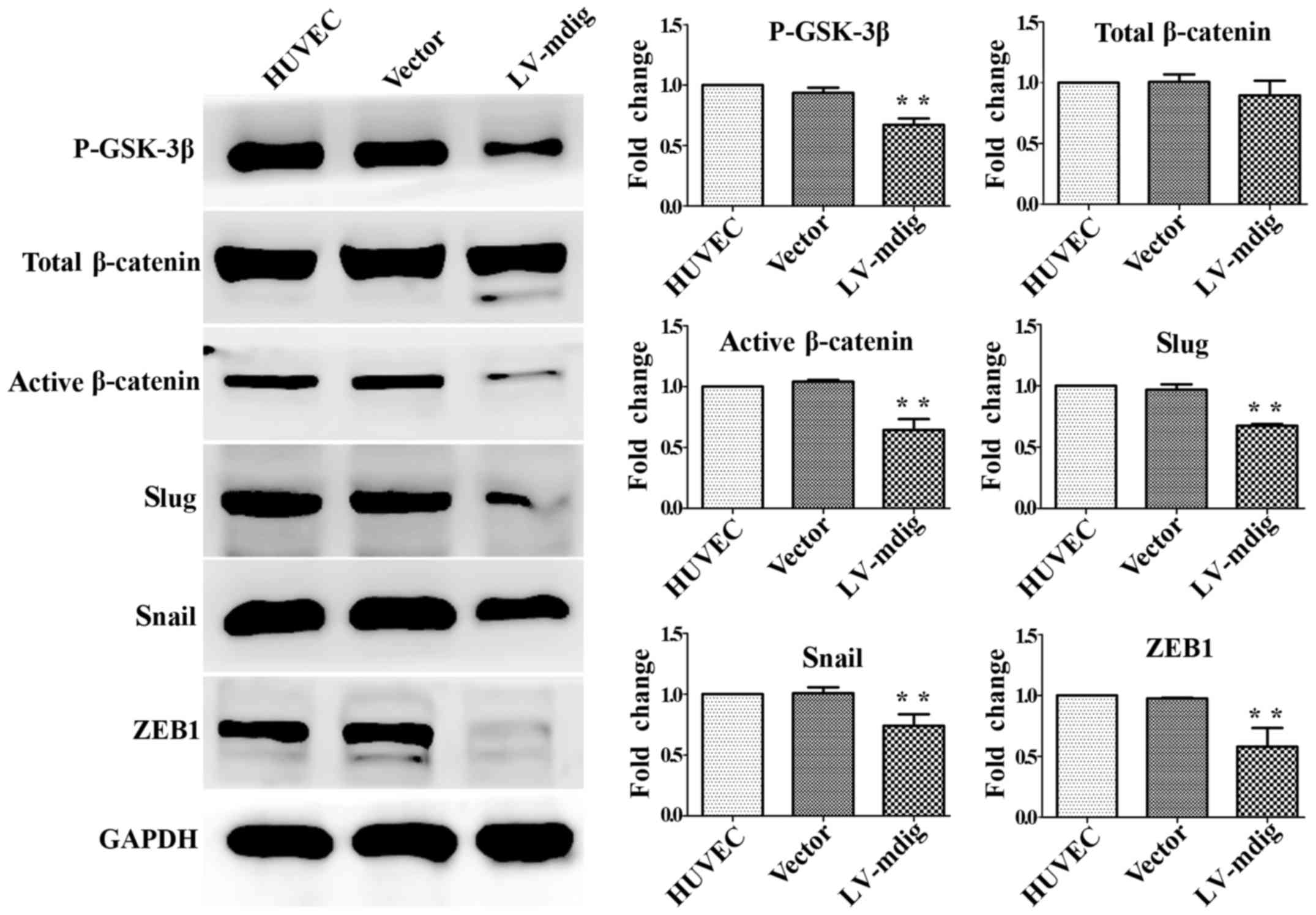
β-catenin were not significant (P>0.05) (Fig. 5). The same results were obtained
for mdig-overexpressing HUVECs (Fig.
6). These results show that mdig can inhibit the
phosphorylation of GSK-3β and thus promote the phosphorylation of
β-catenin. This may reduce the levels of active
(non-phosphorylated) β-catenin, leading to a decrease in its direct
promotion of the EMT-related factors snail, slug and ZEB1.
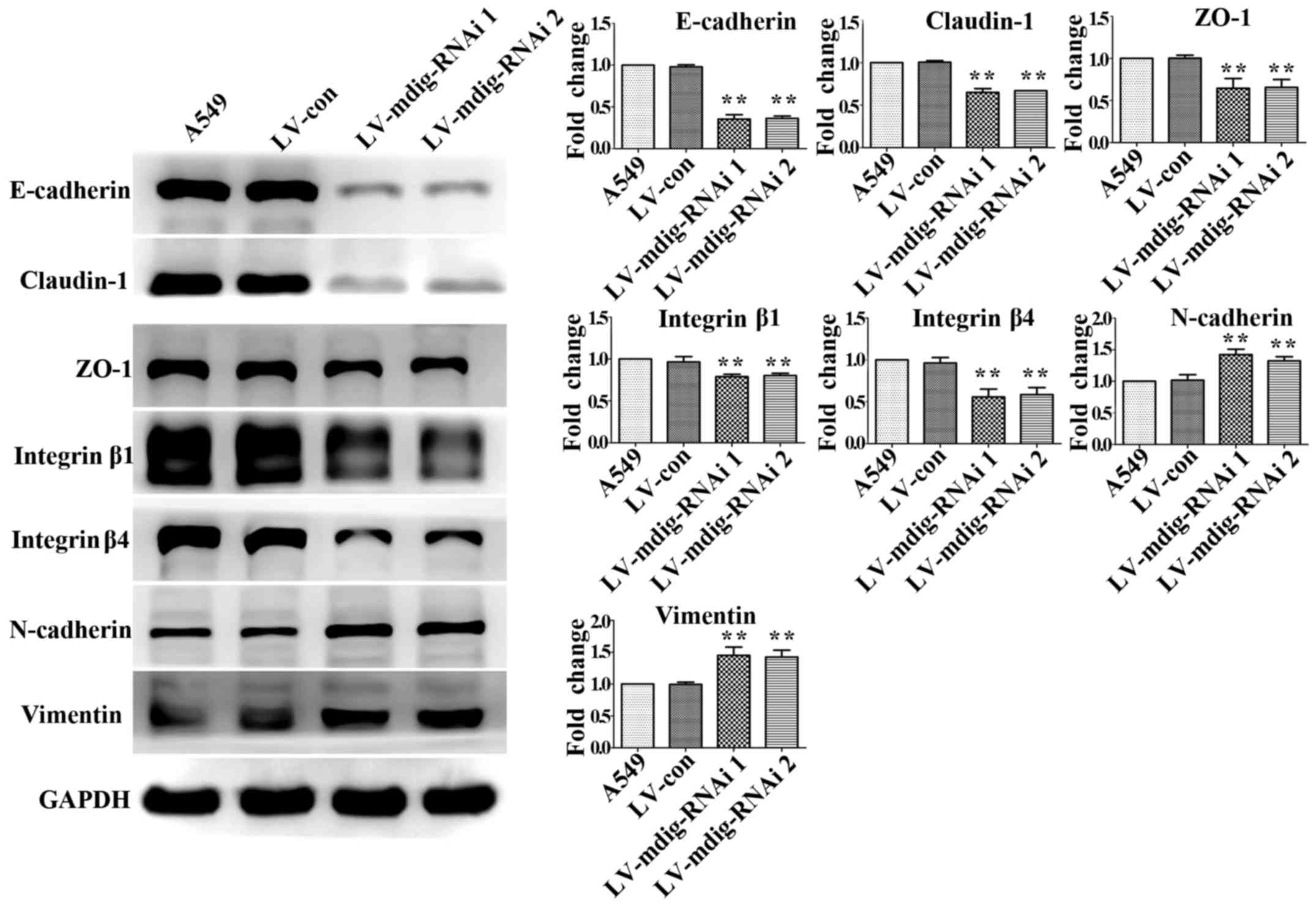
Mdig regulates major molecular markers of
EMT
Western blotting was used to detect the expression
of major EMT markers in the mdig-knockdown and mdig-overexpressing
cell lines. In the mdig knockdown experiment, the expression levels
of E-cadherin, claudin-1, ZO-1, integrin β1 and integrin β4 in
mdig-silenced A549 cells were significantly downregulated
(P<0.01), while those of N-cadherin and vimentin were
significantly upregulated (P<0.01), relative to the A549 and
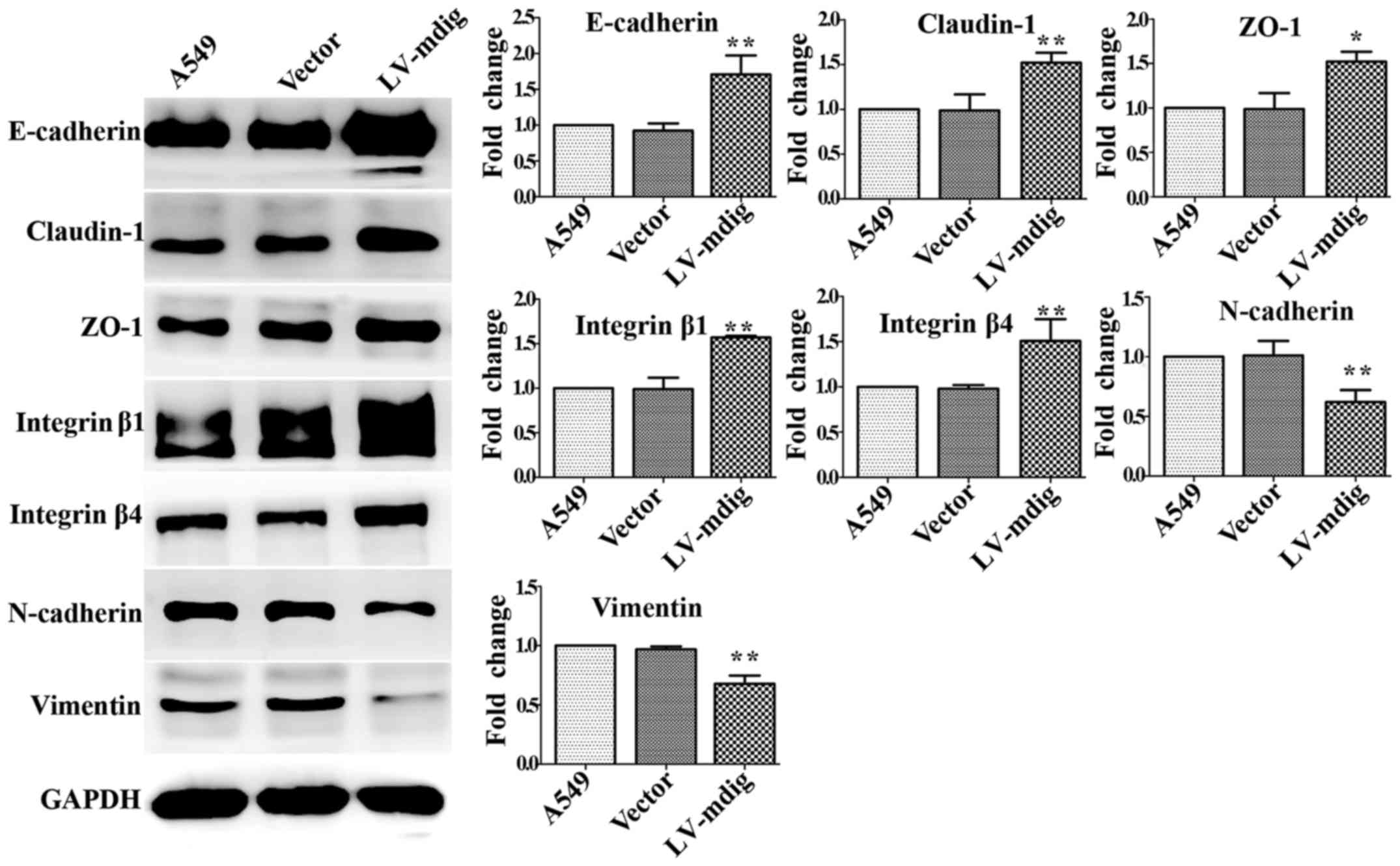
LV-con groups (Fig. 7). Opposite
results were obtained for mdig-overexpressing A549 cells; compared
with the A549 and LV-con groups, the expression levels of
E-cadherin, claudin-1, ZO-1, integrin β1 and integrin β4 were
significantly upregulated in mdig-overexpressing A549 cells
(P<0.01), while those of N-cadherin and vimentin were
significantly downregulated (P<0.01) (Fig. 8). The results for
mdig-overexpressing HUVECs were equivalent to those for
mdig-overexpressing A549 cells (Fig.
9); mdig overexpression was able to upregulate the epithelial
cell markers E-cadherin, claudin-1, ZO-1, integrin β1 and integrin
β4 (P<0.05), while downregulating the expression of the
mesenchymal cell markers N-cadherin and vimentin (P<0.01).
Discussion
Lung cancer has become one of the world's highest
ranked malignancies in terms of morbidity and mortality rates, and
thus is a serious threat to human health and quality of life.
Smoking, environmental pollution and occupational, physical and
chemical carcinogen exposure have all been identified as risk
factors of lung cancer (20,21).
It is presently established that the occurrence and development of
human lung cancer is due to a large number of genetic changes. Mdig
is a lung cancer-related gene that is highly expressed in lung
cancer tissues and most lung cancer cell lines, but not in normal
lung tissues, and can be induced by environmental stimuli in
alveolar macrophages (1). Previous
studies have confirmed that mdig is a proto-oncogene that exhibits
high expression in a variety of tumors, and serves key roles in
promoting tumor cell proliferation. The main reason for the high
mortality rate of cancer patients is due to the invasion and
metastasis of tumor cells; however, current research on mdig
regarding its potential regulation of cell invasion and metastasis
is limited. Our previous study found that the overexpression of
mdig in A549 cells significantly increased cell proliferation, but
significantly reduced cell invasion and migration (6). These data indicated that mdig can
promote tumor cell proliferation while inhibiting cell invasion and
metastasis; however, the exact mechanism underlying these
contradicting effects is not clear. The study also found that the
expression levels of mdig and the overall survival (OS) rate of
lung cancer patients were inversely related, with high expression
levels of mdig indicating a poor prognosis alongside the
stimulatory effects on cell proliferation. However, when patients
were classified according to the American Joint Committee on Cancer
(AJCC) staging system for lymph node metastasis status (N), we
noted that higher mdig expression only predicted a poorer OS rate
of patients with AJCC N0 (no regional lymph node metastasis) and
AJCC N1 (possible proximal lymph node metastasis), but not for
those with AJCC N2 (distant lymph node metastasis). Therefore,
although these findings were statistically insignificant, higher
mdig expression appears to predict a better, rather than poorer,
survival rate for AJCCN2 patients, which may support the findings
that mdig is an inhibitory factor for cell migration and invasion
(6). Komiya et al also
confirmed that mdig/mina53 may be a prognostic indicator of lung
cancer; they found that the expression of mdig was markedly
increased in early squamous cell carcinoma, and that NSCLC patients
positive for mdig/mina53 expression had a better prognosis than
mdig-negative patients, which indicated that mdig/mina53 may
inhibit tumor cell invasion and metastasis and promote apoptosis
(9). These findings are in
accordance with our previous study, and suggest that mdig has the
ability to inhibit tumor cell invasion and metastasis in NSCLC;
however, the corresponding molecular mechanism is not clear.
Tumor cell invasion and metastasis may occur to
varying degrees during the different stages of EMT. Notably, the
occurrence of EMT has been associated with NSCLC invasion and
translocation (10). Studies have
shown that morphological changes are induced by EMT in tumor cells;
when EMT occurs in A549 cells, the morphology of cells changes from
a cobblestone shape to an elongated spindle-like shape
characteristic of fibroblastoid cells (16). In order to investigate the
mechanism underlying the regulatory effects of mdig on the invasion
and metastasis of A549 cells, the present study constructed
mdig-silenced and mdig-overexpressing A549 cell lines. It was
observed that the morphology of mdig-silenced A549 cells changed
from a cobblestone shape to an elongated spindle-like shape (i.e.,
a fibroblastoid appearance), while the morphology of mdig-
overexpressing A549 cells became rounder in appearance compared
with the initial cobblestone shape, thus indicating that the
expression of mdig plays an important role in the morphological
changes of A549 cells. On the basis of the aforementioned findings,
Transwell assays were performed to determine the effects of mdig
silencing and overexpression on the invasion and migration of A549
cells. The results of the invasion experiment demonstrated that
mdig overexpression significantly decreased the number of cells
that invaded through the matrix and membrane, while mdig knockdown
significantly increased the number of cells when compared with the
normal A549 and control groups. Identical results were obtained
from the migration experiment. In order to further verify the
effect of mdig on the migratory ability of A549 cells, a
scratch-wound assay was performed. Compared with the control group,
the mdig overexpression A549 cell group exhibited a significantly
slower healing rate, and the mdig knockdown group exhibited a
significantly faster healing rate. Therefore, mdig overexpression
can inhibit the invasion and metastasis of A549 cells, and
silencing of mdig can increase the invasive and migratory
properties of cells, suggesting that mdig has the ability to
inhibit the invasion and metastasis of A549 cells.
In order to further explore the mechanism underlying
these inhibitory effects of mdig, this study examined the effect of
mdig on the expression of the GSK-3β/β-catenin signaling axis, the
downstream transcription factors snail, slug and ZEB1, and the
major molecular markers of EMT. The results showed that mdig could
inhibit the phosphorylation of GSK-3β at Ser9 and promote the
phosphorylation of β-catenin, which resulted in a decrease in the
active (non-phospho at Ser33, Ser37 and Thr41) form of β-catenin,
leading to a reduction in the direct promotion of slug, snail and
ZEB1. Our results indicated that the regulation of mdig on the
signaling pathway was mainly post-translational modification. Mdig
could also upregulate epithelial cell markers (E-cadherin,
claudin-1 and ZO-1) and mediate the expression of integrin β1 and
integrin β4, the key facilitators of extracellular matrix adhesion,
while downregulating mesenchymal cell markers (N-cadherin and
vimentin). These results suggested that mdig can promote the
phosphorylation of β-catenin by inhibiting the phosphorylation of
GSK-3β, in order to suppress the expression of slug, snail and ZEB1
and the occurrence of EMT, and thereby inhibit the invasion and
metastasis of NSCLC. The present study also used HUVEC cells to
verify the above molecular mechanisms, and the same conclusions
were obtained. Collectively, our results elucidated the molecular
mechanism underlying the inhibitory effects of mdig on the invasion
and metastasis of NSCLC by demonstrating a strong correlation
between the expression levels of mdig and the examined proteins in
tumor cells and normal cells, as observed previously (6,9), and
supported the clinical findings that mdig-positive NSCLC is
associated with a better prognosis than the mdig-negative form in
patients at the advanced stage because of mdig inhibiting invasion
and metastasis of NSCLC (22). The
present study focused on the regulation of tumor cell biological
behavior by mdig, and advanced our understanding of the underlying
regulatory mechanism regarding downstream target genes. However,
the correlation between the expression levels of mdig and the
examined proteins in patient samples and whether it is only through
GSK-3β/β-catenin pathway for mdig to affect the invasion and
migration of NSCLC need to be studied in the future. It should be
noted that positive mdig expression in other types of tumors, such
as breast cancer (23), liver
cancer (24), gastric cancer
(25,26), neuroblastoma (27), renal cell carcinoma (28), and esophageal squamous cell
carcinoma (29,30), is a poor prognostic indicator. Mdig
expression is not significantly associated with the prognosis of
some tumors, such as primary gingival squamous cell carcinoma
(31). However, in general, mdig
is considered to be an important factor associated with important
tumor-related genes, and not only exhibits different functions
during different tumor stages, but also in different tumor tissues
(32). Future research should
further explore the molecular mechanisms of mdig in tumor cell
behavior, and also focus on the value of mdig in the diagnosis,
staging, treatment and prognosis of tumors.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81472194) and by the Project
of Liaoning Distinguished Professor [grant no. (2013) 204] to
Hongwen Zhao.
References
|
1
|
Zhang Y, Lu Y, Yuan BZ, Castranova V, Shi
X, Stauffer JL, Demers LM and Chen F: The Human mineral
dust-induced gene, mdig, is a cell growth regulating gene
associated with lung cancer. Oncogene. 24:4873–4882. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun J, Yu M, Lu Y, Thakur C, Chen B, Qiu
P, Zhao H and Chen F: Carcinogenic metalloid arsenic induces
expression of mdig oncogene through JNK and STAT3 activation.
Cancer Lett. 346:257–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu K, Li L, Thakur C, Lu Y, Zhang X, Yi Z
and Chen F: Proteomic characterization of the World Trade Center
dust-activated mdig and c-myc signaling circuit linked to multiple
myeloma. Sci Rep. 6:363052016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma D, Guo D, Li W and Zhao H: Mdig, a lung
cancer-associated gene, regulates cell cycle progression through
p27(KIP1). Tumour Biol. 36:6909–6917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan XP, Dong WG, Zhang Q, Yang ZR, Lei XF
and Ai MH: Potential effects of Mina53 on tumor growth in human
pancreatic cancer. Cell Biochem Biophys. 69:619–625. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu M, Sun J, Thakur C, Chen B, Lu Y, Zhao
H and Chen F: Paradoxical roles of mineral dust induced gene on
cell proliferation and migration/invasion. PLoS One. 9:e879982014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuneoka M, Koda Y, Soejima M, Teye K and
Kimura H: A novel myc target gene, mina53, that is involved in cell
proliferation. J Biol Chem. 277:35450–35459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma
D, Yi Z and Chen F: Mdig de-represses H19 large intergenic
non-coding RNA (lincRNA) by down-regulating H3K9me3 and
heterochromatin. Oncotarget. 4:1427–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komiya K, Sueoka-Aragane N, Sato A,
Hisatomi T, Sakuragi T, Mitsuoka M, Sato T, Hayashi S, Izumi H,
Tsuneoka M, et al: Mina53, a novel c-Myc target gene, is frequently
expressed in lung cancers and exerts oncogenic property in NIH/3T3
cells. J Cancer Res Clin Oncol. 136:465–473. 2010. View Article : Google Scholar
|
|
10
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: Epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and beta-catenin.
Cells Tissues Organs. 179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cuyàs E, Corominas-Faja B and Menendez JA:
The nutritional phenome of EMT-induced cancer stem-like cells.
Oncotarget. 5:3970–3982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Godde NJ, Galea RC, Elsum IA and Humbert
PO: Cell polarity in motion: Redefining mammary tissue organization
through EMT and cell polarity transitions. J Mammary Gland Biol
Neoplasia. 15:149–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren ZX, Yu HB, Li JS, Shen JL and Du WS:
Suitable parameter choice on quantitative morphology of A549 cell
in epithelial-mesenchymal transition. Biosci Rep. 35:352015.
|
|
17
|
Yost C, Torres M, Miller JR, Huang E,
Kimelman D and Moon RT: The axis-inducing activity, stability, and
subcellular distribution of beta-catenin is regulated in Xenopus
embryos by glycogen synthase kinase 3. Genes Dev. 10:1443–1454.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komiya K, Sueoka-Aragane N, Sato A,
Hisatomi T, Sakuragi T, Mitsuoka M, Sato T, Hayashi S, Izumi H,
Tsuneoka M, et al: Expression of Mina53, a novel c-Myc target gene,
is a favorable prognostic marker in early stage lung cancer. Lung
Cancer. 69:232–238. 2010. View Article : Google Scholar
|
|
23
|
Thakur C, Lu Y, Sun J, Yu M, Chen B and
Chen F: Increased expression of mdig predicts poorer survival of
the breast cancer patients. Gene. 535:218–224. 2014. View Article : Google Scholar :
|
|
24
|
Huo Q, Ge C, Tian H, Sun J, Cui M, Li H,
Zhao F, Chen T, Xie H, Cui Y, et al: Dysfunction of IKZF1/MYC/MDIG
axis contributes to liver cancer progression through regulating
H3K9me3/p21 activity. Cell Death Dis. 8:e27662017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xing J, Wang K, Liu PW, Miao Q and Chen
XY: Mina53, a novel molecular marker for the diagnosis and
prognosis of gastric adenocarcinoma. Oncol Rep. 31:634–640. 2014.
View Article : Google Scholar
|
|
26
|
Ogasawara S, Komuta M, Nakashima O, Akiba
J, Tsuneoka M and Yano H: Accelerated expression of a Myc target
gene Mina53 in aggressive hepatocellular carcinoma. Hepatology Res.
40:330–336. 2010. View Article : Google Scholar
|
|
27
|
Fukahori S, Yano H, Tsuneoka M, Tanaka Y,
Yagi M, Kuwano M, Tajiri T, Taguchi T, Tsuneyoshi M and Kojiro M:
Immunohistochemical expressions of Cap43 and Mina53 proteins in
neuroblastoma. J Pediatr Surg. 42:1831–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishizaki H, Yano H, Tsuneoka M, Ogasawara
S, Akiba J, Nishida N, Kojiro S, Fukahori S, Moriya F, Matsuoka K,
et al: Overexpression of the myc target gene Mina53 in advanced
renal cell carcinoma. Pathol Int. 57:672–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teye K, Arima N, Nakamura Y, Sakamoto K,
Sueoka E, Kimura H and Tsuneoka M: Expression of Myc target gene
mina53 in subtypes of human lymphoma. Oncol Rep. 18:841–848.
2007.PubMed/NCBI
|
|
30
|
Tsuneoka M, Fujita H, Arima N, Teye K,
Okamura T, Inutsuka H, Koda K, Shirouzu K and Kimura H: Mina53 as a
potential prognostic factor for esophageal squamous cell carcinoma.
Clin Cancer Res. 10:7347–7356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuratomi K, Yano H, Tsuneoka M, Sakamoto
K, Kusukawa J and Kojiro M: Immunohistochemical expression of
Mina53 and Ki67 proteins in human primary gingival squamous cell
carcinoma. Kurume Med J. 53:71–78. 2006. View Article : Google Scholar
|
|
32
|
Thakur C and Chen F: Current understanding
of mdig/MINA in human cancers. Genes Cancer. 6:288–302.
2015.PubMed/NCBI
|