Introduction
It is generally known that ovarian cancer is one of
the leading causes of cancer deaths in women and the second most
common gynecologic cancer (1).
Metastasis, which is mainly responsible for the high mortality of
ovarian cancer, includes direct spread, lymph node metastasis,
implantation metastasis and hematogenous metastasis. Of the several
ways of metastasis, lymph node metastasis is one of the most
valuable indicators of biological characteristics of patients
(2). Patients with positive lymph
node have a significantly shorter overall survival than
node-negative patients. Although the combined treatments of
surgery, radiotherapy, chemotherapy and endocrine therapy have
greatly improved the survival rate of ovarian cancer patients, the
treatment effect of patients with relapse or metastasis is still
not ideal. Molecular targeted therapy might provide a new means for
those hard-to-treat ovarian cancer patients. However, due to the
absence of well-defined molecular targets, more extensive genomic
and biological studies of ovarian cancer are required to better
understand the complexity of the disease and to develop effective
treatments.
SKOV3-PM4, a subline of human ovarian carcinoma cell
line with high directional lymphatic metastasis, was screened and
established after repeated in vivo passage with nude mice
and its characteristics were compared with the parental SKOV3 line
by our research group. An isobaric tag for relative and absolute
quantitation labelling followed by nano liquid
chromatography-matrix-assisted laser desorption ionization-time of
flight-tandem mass spectrometry was used to identify the
differential expression proteins between SKOV3 cell line and
SKOV3-PM4 cell line. Bioinformatics analysis revealed that
downregulation of SPARC is closely related to lymph node metastasis
in ovarian cancer (3,4).
SPARC, also known as 43K protein, osteonectin and
BM-40, is a calcium-binding glycoprotein first reported by Sage and
his colleagues in 1984 (5). SPARC
is involved in cell renewal, embryonic development, angiogenesis,
tissue remodeling and modulation of cell-matrix interactions
(6). Growing evidence suggests
that SPARC abnormally expresses and plays an important role in a
variety of cancers (7–10). VEGFs, mainly including VEGF-A,
VEGF-B, VEGF-C, VEGF-D and VEGF-E family members, considered as the
most effective pro-angiogenic factors secreted by tumor cells, also
play an vital role in tumor development, invasion and metastases
formation. A number of reports have shown that SPARC inhibits VEGF
expression during the formation of new blood vessel by which
indirectly restrain the development, growth, invasion and
metastasis of tumor cells (11–13).
Despite these advances, the relationship among
SPARC, VEGFs and lymph node metastasis in ovarian cancer has not
been completely investigated. In the present study, we increased
the expression of SPARC in SKOV3-PM4 cells by lentivirus-mediated
RNA overexpression, and then a series of experiments were performed
in vitro to clarify the role of SPARC in lymph node
metastasis of ovarian cancer. We found that overexpression of SPARC
could obviously attenuate SKOV3-PM4 cell proliferation, migration
and invasion. In addition, the expression of SPARC, VEGFs, D2-40
(vascular lymphatic marker) and CD34 (vascular endothelial marker)
in human ovarian malignant tumor tissue specimens were measured by
immunohistochemistry. The numbers of lymphatic microvessel and
microvessel formation were counted by D2-40 and CD34 antigen
staining, respectively. Our results showed that SPARC inhibited the
ovarian cancer metastasis of lymph nodes by decreasing the
expression level of VEGF-C and VEGF-D.
Materials and methods
Cell line and plasmids
SKOV3 cell line was purchased from the Cell
Resources Center of Shanghai Biological Sciences Institute.
SKOV3-PM4 cell line was established by our research group (3) and preserved at Medical Scientific
Research Center of Guangxi Medical University. HEK293T cells (Human
embryonic kidney cells expressing the large simian virus 40 T
antigen), Plasmid H1, Plasmid H2 and DH5α E. coli were
obtained from the Oncology Laboratory of Medical Scientific
Research Center of Guangxi Medical University.
Construction of the SPARC overexpression
lentiviral vector and virus packaging
To construct a plasmid expressing SPARC, the
sequence was amplifed with the primers (Table I) and they both contained
MluI and XhoI restriction enzyme sites. Polymerase
chain rection (PCR) was used to clone the SPARC gene using the
following reaction conditions: 95°C pre-denaturation for 5 min,
94°C denaturation for 30 sec, 57°C annealing for 30 sec, 72°C
extension for 30 sec for 30 cycles, 72°C extension for 10 min. The
PCR products were detected by a 1% agarose gel electrophoresis and
were sequenced by the Beijing Genomics Institute (Shenzhen, China).
The recombinant plasmids were transformed into DH5α E.
coli.
 | Table IqRT-PCR primers. |
Table I
qRT-PCR primers.
| Gene | Primer sequence | Annealing temperature
(°C) |
|---|
| GAPDH | Forward:
5′-GTCAAGGCTGAGAACGGGAA-3′ | 60 |
| Reverse:
5′-AAATGAGCCCCAGCCTTCTC-3′ | |
| SPARC | Forward:
5′-GCAGCAATGACAACAAGACCT-3′ | 60 |
| Reverse:
5′-ATTCGGTCAGCTCAGAGTCCA-3′ | |
| VEGF-A | Forward:
5′-AGGAGGGCAGAATCATCA-3′ | 62 |
| Reverse:
5′-AGATGTCCACCAGGGTCTC-3′ | |
| VEGF-B | Forward:
5′-GTACCCGAGCAGTCAGCT-3′ | 62 |
| Reverse:
5′-CCCTGTCTGGCTTCACAG-3′ | |
| VEGF-C | Forward:
5′-ACAGGCCAACCTCAACTCAA-3′ | 62 |
| Reverse:
5′-GTAGACGGACACACATGGAG-3′ | |
| VEGF-D | Forward:
5′-TCCCATCGGTCCACTAGGTT-3′ | 62 |
| Reverse:
5′-TGGTACTCTTCCCCAGCTCA-3′ | |
| VEGF-E | Forward:
5′-ATTCACAGCCCAAGGTTT-3′ | 61 |
| Reverse:
5′-AGCCCAAATCTTTCATCAA-3′ | |
Cell culture and transfection
SKOV3 cells and SKOV3-PM4 cells were cultured in
RPMI-1640 medium (Gibco, Gaithersburg, MD, USA), supplemented with
10% fetal bovine serum (FBS; Gibco) in a humidified incubator and
1% penicillin/streptomycin at 37°C with 5% CO2
atmosphere. SKOV3-PM4 cells were cultured in 6-well tissue culture
plates and were infected with lentivirus for 24 h. Then, the medium
was replaced with fresh complete medium and cultured for 48 h,
followed by selection with puromycin (2 μg/ml). Cells were
observed under fluorescence microscope to confirm that >80% of
cells were GFP-positive and the SPARC expression in the stably
transfected cells were examined by real-time PCR and western
blotting. The tranfected cells in the present study were divided
into 2 groups: group 1 (SKOV3-PM4-NC, cells transfected with empty
vectors), and group 2 (SKOV3-PM4-SPARC, cells transfected with
SPARC overexpressed RNA).
qRT-PCR for SPARC and VEGFs mRNA
expression
Total RNA of SPARC and VEGFs were isolated from
cultured cell lines using the TRIzol reagent (Invitrogen, Carlsbad,
CA, USA) according to the instructions of the manufacturer. The
primers (Table I) were designed by
Oligo 7.0 software and synthesized by Takara Biotechnology Co. Ltd.
(Dalian, China). The mRNA level of the target gene was quantified
by measuring the CT value to determine its relative expression. The
results are reported using the fold change in the gene expression
of the target genes relative to the internal control gene (GAPDH).
The mean-fold change in target gene expression was calculated as
2−ΔΔCT, where ΔΔCT = (CTTarget −
CTGAPDH)sample − (CTTarget −
CTGAPDH) control.
Western blotting for SPARC and VEGF
protein expression
The protein concentration was determined using the
BCA assay. One hundred micrograms of total protein was loaded into
each well and seperated by electrophoresis in a 10% SDS-PAGE gel.
The proteins were electro-transferred to polyvinylidene fluoride
(PVDF) membranes. After blocking with 5% non-fat milk in
phosphate-buffered saline (PBS) containing 0.5% Tween-20 (PBST),
the PVDF membranes were incubated overnight at 4°C with SPARC
(1:500; Cell Signaling Technology, Danvers, MA, USA), VEGF-A
(1:500; Abcam, Cambridge, MA, USA), VEGF-B (1:500; Abcam), VEGF-C
(1:500; Abcam), VEGF-D (1:500; Abcam), VEGF-E (1:500; Abcam) and
GAPDH (1:1,000; Abcam) primary monoclonal antibodies in PBST buffer
containing 0.1% Tween-20. The next day, the membranes were washed
and incubated with a secondary antibody conjugated with horseradish
peroxidase (HRP) (1:2,000; Abcam). The intensity of protein
staining was determined with ImageJ software.
Laser confocal microscopy for the
distribution of SPARC protein
Laser scanning confocal microscopy was used to
observe the distribution of SPARC protein in cells. Cells were
seeded in the glass base of the plate at 1×105 cells/ml
in RPMI-1640 with 10% FBS. After 24 h, supernatants were discarded
and the cells were fixed with methanol for 10 min. After washing
with PBS three times with PBS for 2 min each time, cells were
incubated with a SPARC primary antibody (1:500; Cell Signaling
Technology) for 2 h and then with fluorescence labeling secondary
antibodies (1:2,000; Abcam) for 2 h. The sections were
counterstained with DAPI. Localization and expression of SPARC
protein in cells were visualized and captured using laser confocal
microscopy (Nikon A1; Nikon, Tokyo, Japan).
Cell counting method and colony formation
test for cell proliferation
To determine the growth rate of cells after
lentiviral treatments, 104 cells were seeded in 24-well
plate in 100 μl RPMI-1640 with 10% FBS. The cells were
digested and counted under the microscopy for 7 days. Cells
(103) were added to 6-well plates with RPMI-1640
containing 10% FBS, and each cell group contained 3-wells. After
incubation at 37°C for 14 days, the cells were washed twice with
PBS and were stained with Giemsa solution. The number of colonies
containing >50 cells was counted under a microscope.
Transwell assay for cell migration and
invasion
Each Trans-well (BD Biosciences, Bedford, MA, USA)
was coated with 100 μl Matrigel (BD Biosciences). Cells were
adjusted to a density of of 5×104 cells/ml, resuspended
in 200 μl serum-free medium and seeded into the upper
chamber. Complete medium was added to the lower chamber as a
chemotactic factor. After 24-h incubation, cells remaining on the
upper surface were removed, and cells on the lower surface were
fixed, stained with Giemsa, then counted. Cell migration assay was
also performed using Transwells without Matrigel coating.
Immunohistochemistry for expression of
VEGF-C, VEGF-D, LVD and MVD in tissue specimens of human ovarian
cancer
Forty-seven human ovarian malignant tumor tissue
specimens were collected from patients who underwent surgical
treatment at the Cancer Hospital Affliated to Guangxi Medical
University (Nanning, China) from January 2008 to May 2013. None of
the patients received any therapies prior to surgery. Of the 47
ovarian cancer patients (mean age 45.0±3.1 years), 32 were serous
carcinoma and 15 non-serous carcinoma (including 8 mucinous
cystadenocarcinoma, 5 endometrioid carcinoma and 2 clear cell
carcinoma). Of all the patients, 27 had no lymph node metastasis
and 20 had lymph node metastasis. All human ovarian tissue
specimens were collected during the operation. Patients or their
family members' consent were obtained before the collection of
surgical materials and written informed consents were obtained from
all the patients. This study was approved by the Ethical Review
Committee of the Affliated Tumor Hospital of Guangxi Medical
University (Guangxi, China).
Tissue sections (4 μm) were cut, and
deparaffinized. The sections were incubated overnight at 4°C with
SPARC, VEGF-C, VEGF-D, D2-40 (1:100) and CD34 (1:100; Beijing
Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China)
primary antibody respectively and PBS was used as a negative
control.
Both cytoplasm and the membrance of nucleus appeared
brown-yellow were considered to be SPARC, VEGF-C and VEGF-D
positive expression. For the assessment of SPARC, VEGF-C and VEGF-D
staining, each tissue section was independently analyzed by two
pathologists in the double-blinded manner. According to the
proportion and the intensity of positive staining, the stained
cells were scored from 0 to 3 (0 point, 0% positive tumor cells; 1
point, 0–33.33%; 2 point, 33.33–66.67%; 3 point, 66.67–100%). While
the staining intensity was classified as a scale of 0 (no
staining), 1 (light yellow), 2 (yellowish brown) and 3 (brown). The
final staining score was calculated by adding together the
percentage and intensity scores, and the scores of 0, 1–3, 4–6 and
>7 were converted into four grades negative, weak, moderate and
strong, respectively.
Lymphatic microvessel and microvessels were
identified by immunostaining endothelial cells with D2-40 and CD34
monoclonal antibodies, respectively. LVD and MVD were assessed
according to the methods summarized by Van der Auwera et al
(14). The entire section was
scanned systematically at low magnification (×40) in order to
identify the most intense areas of neovascularization within the
tumor. The lymphatic microvessel and microvessels were counted at
high magnification (×200), and the average count of three fields
was calculated.
Statistical analysis
The data were analyzed with SPSS 16.0 software.
Enumeration data are presented as mean ± standard deviation, the
results from two groups were compared by t-test. χ2-test
was used to analyze the clinical data. Correlation analysis of
VEGF-C, VEGF-D with SPARC expression were studied using the
Spearman's rank correlation test. The correlation of SPARC
expression with LVD, MVD were analyzed using the Mann-Whitney U
test. P<0.05 were considered statistically significant.
Results
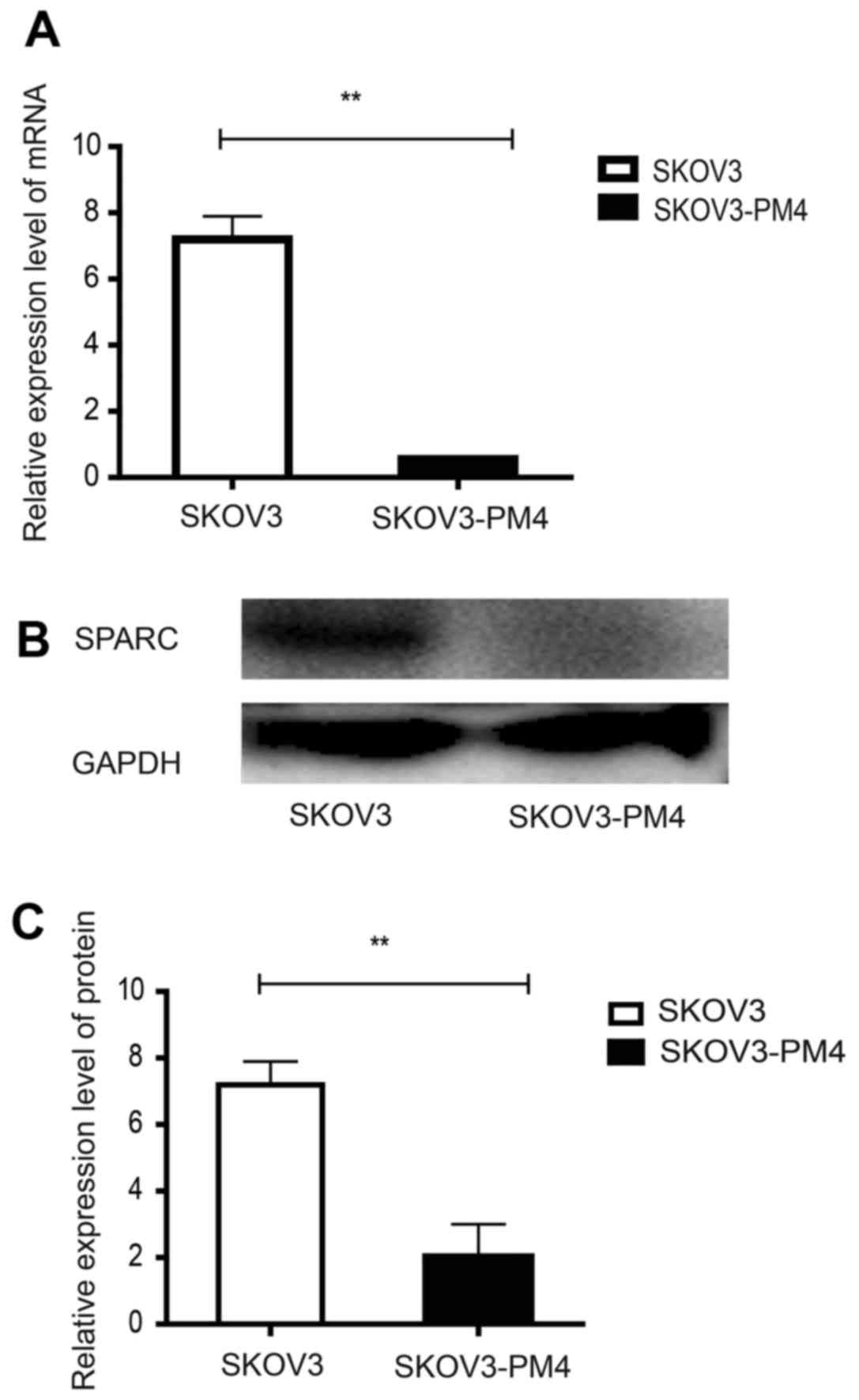
SPARC expression in SKOV3 and SKOV3-PM4
cells
To evaluate the expression of SPARC in SKOV3 cells
and SKOV3-PM4 cells, qRT-PCR and western blotting were performed.
As shown in Fig. 1, SKOV3-PM4
cells exhibited a significantly decreased SPARC expression in both
mRNA and protein level compared to SKOV3 cells (P<0.01).
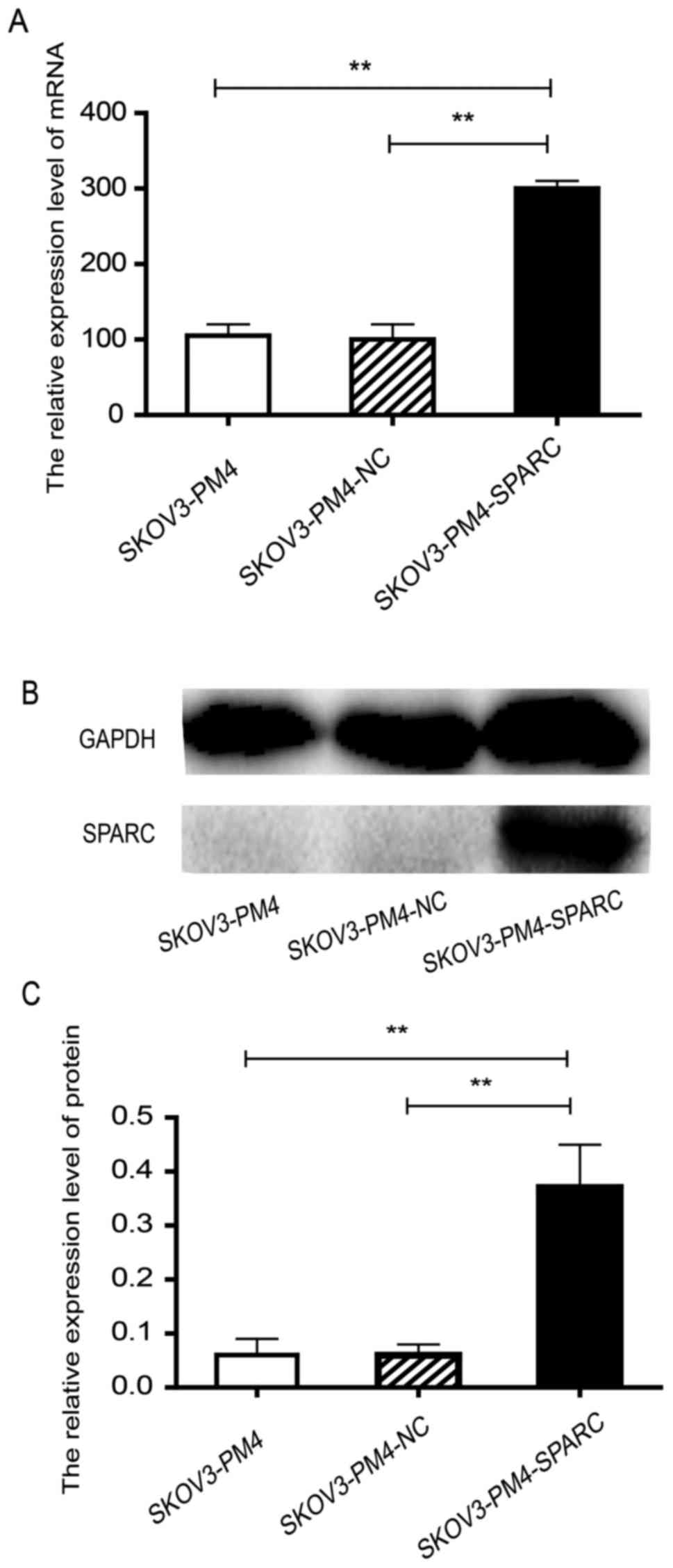
Construction of SPARC overexpression
SKOV3-PM4 cells
To functionally link SPARC expression with lymph
node metastasis, we transfected SKOV3-PM4 cells with SPARC
overexpressed lentivirus vector labelled with green fluorescent
protein. The transfected cells stably expressed green fluorescent
protein which could be observed using an inverted fluorescence
microscope.
SKOV3-PM4-SPARC cells expressed high-level SPARC,
much more than SKOV3-PM4 cells and SKOV3-PM4-NC cells at both mRNA
and protein levels (P<0.01; Fig.
2) The results showed that SPARC gene was successfully
overexpressed in SKOV3-PM4 cells.
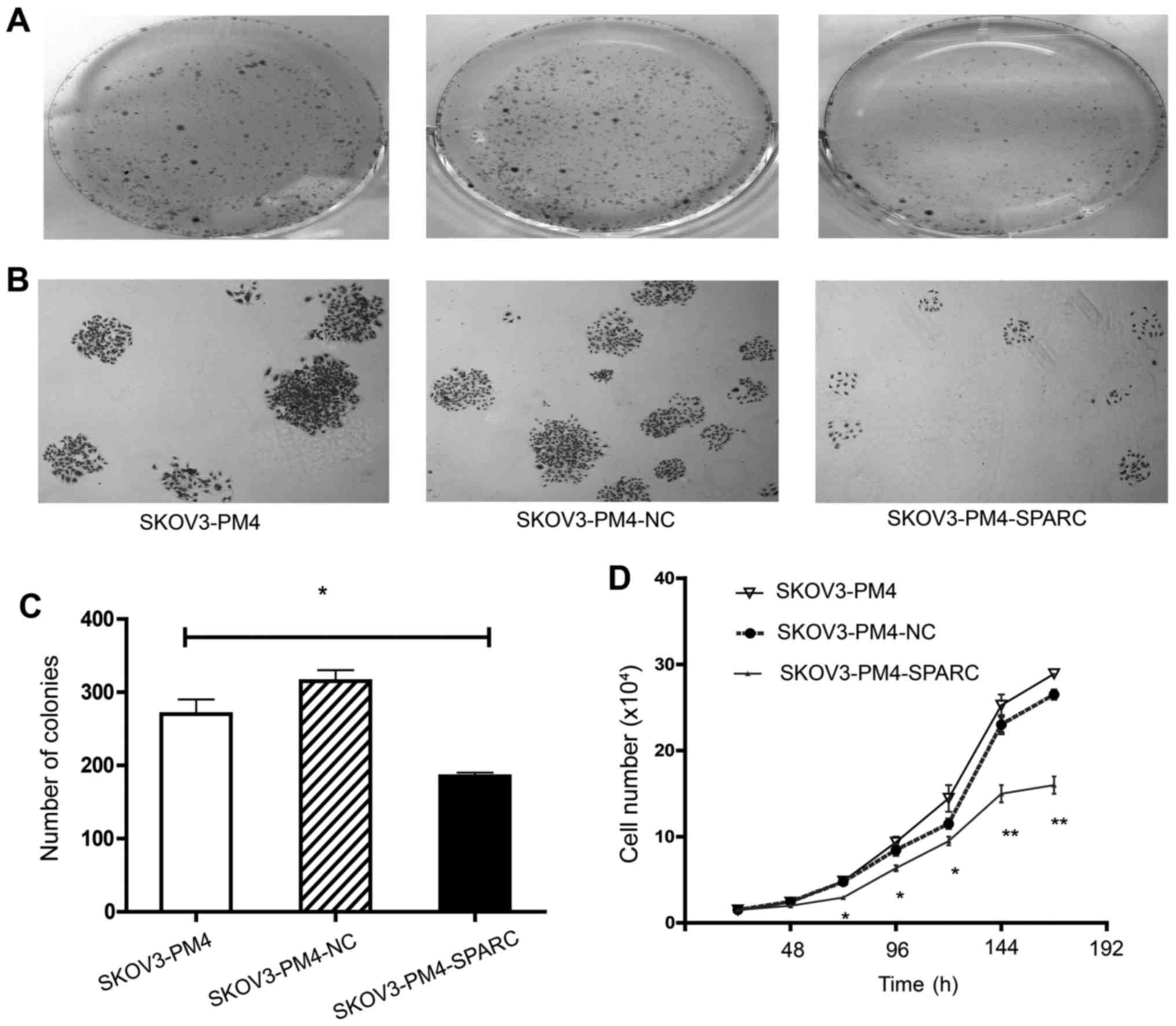
Proliferation of SKOV3-PM4 cells
The effect of overexpressed SPARC on the
proliferation of SKOV3-PM4 cells was investigated by soft agar
colony formation assay and cell proliferation assay. The ability of
colony formation of SKOV3-PM4-SPARC cells was markedly lower than
that of SKOV3-PM4 and SKOV3-PM4-NC cells (P<0.05; Fig. 3A–C). Differences of cell
proliferation were observed among SKOV3-PM4, SKOV3-PM4-NC and
SKOV3-PM4-SPARC cells from the second day to the seventh day after
cells seeding. The doubling time of cells was 37.20±1.87,
37.19±1.83 and 44.54±2.89 h, respectively (P<0.05). The growth
curves of cells showed that overexpression of SPARC significantly
reduced the proliferation of SKOV3-PM4 cells (Fig. 3D).
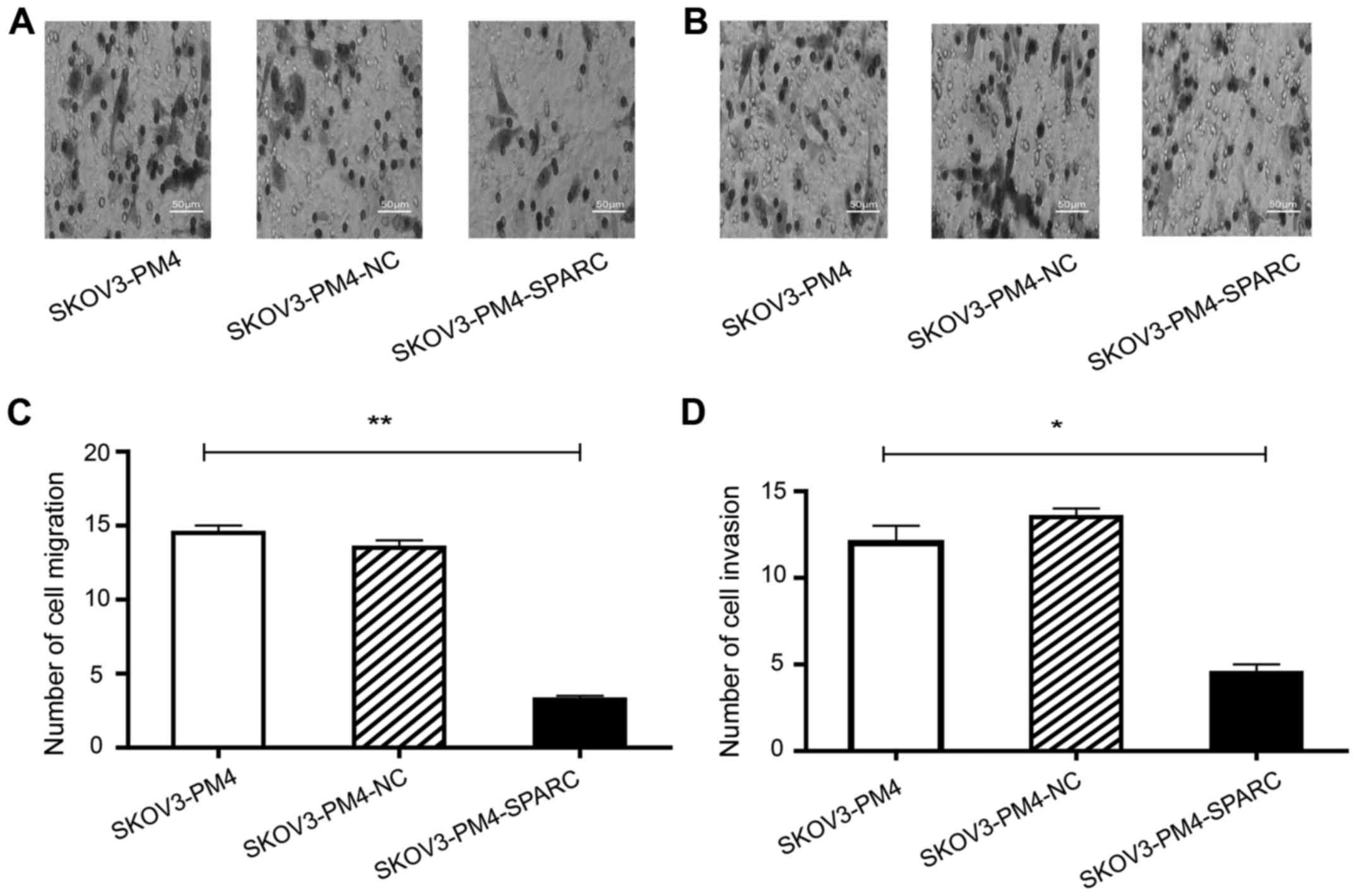
Migration and invasion of SKOV3-PM4
cells
To investigate the effects of SPARC on migration and
invasion of SKOV3-PM4 cells, Transwell assay without Matrigel and
Transwell invasion assay based on Matrigel were performed by using
the same cell number. The results showed that the migration
(Fig. 4A and C) and invasion
(Fig. 4B and D) ability of
SKOV3-PM4-SPARC cells was significantly inferior to that of
SKOV3-PM4 and SKOV3-PM4-NC cells.
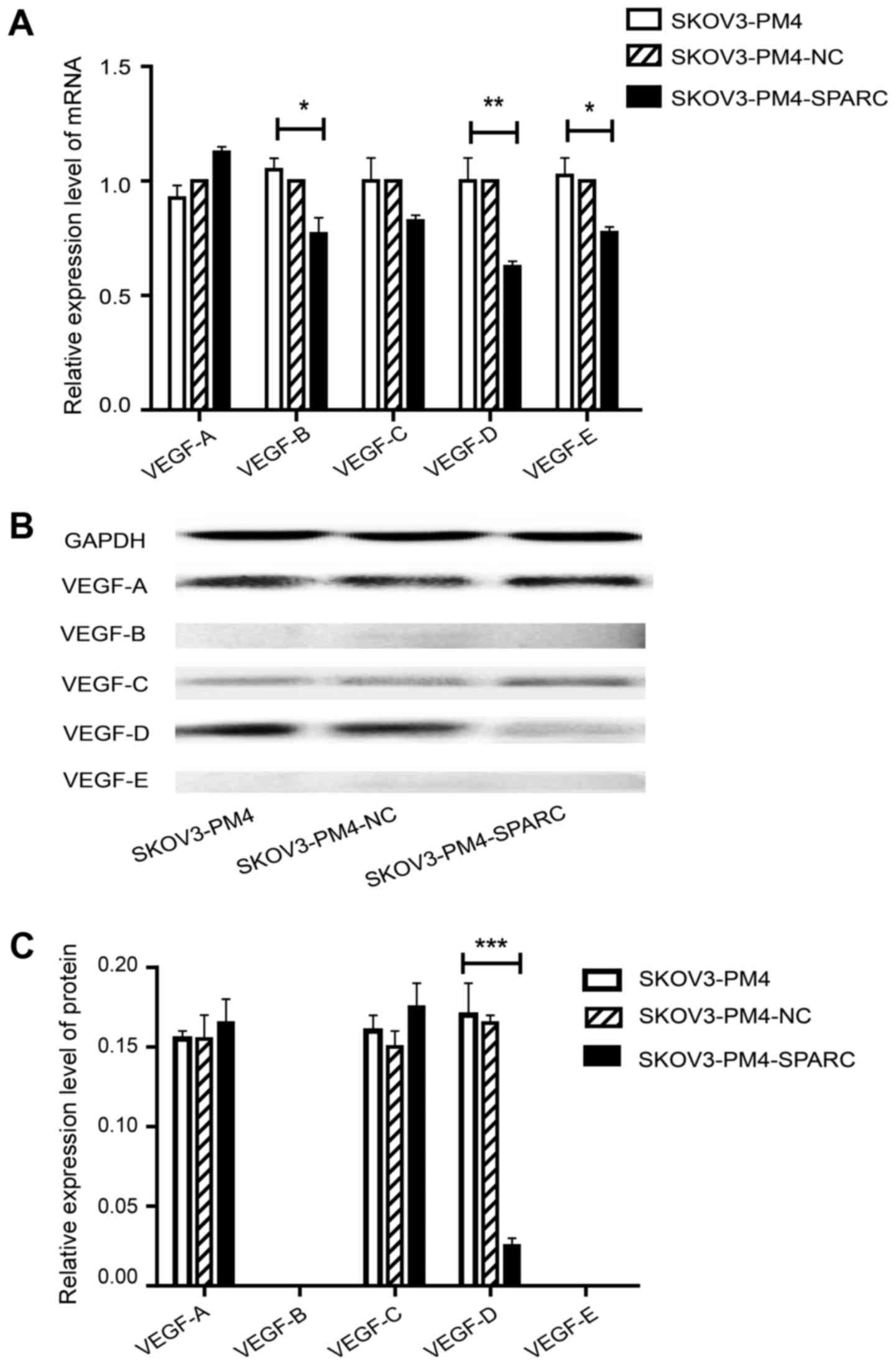
The relationship between SPARC
overexpression and VEGF family
To determine the relationship between SPARC
overexpression and VEGF family, the expression level of VEGF family
was evaluated by qRT-PCR and western blotting. As compared with
SKOV3-PM4 cells, the expression level of VEGF-D in SKOV3-PM4-SPARC
cells was markedly decreased while VEGF-A and VEGF-C had no
significant change at either mRNA or protein level. VEGF-B and
VEGF-E protein were not deteced in either SKOV3-PM4 or
SKOV3-PM4-SPARC cells whereas their mRNA expression level showed
significant differences between these two cell lines (Fig. 5).
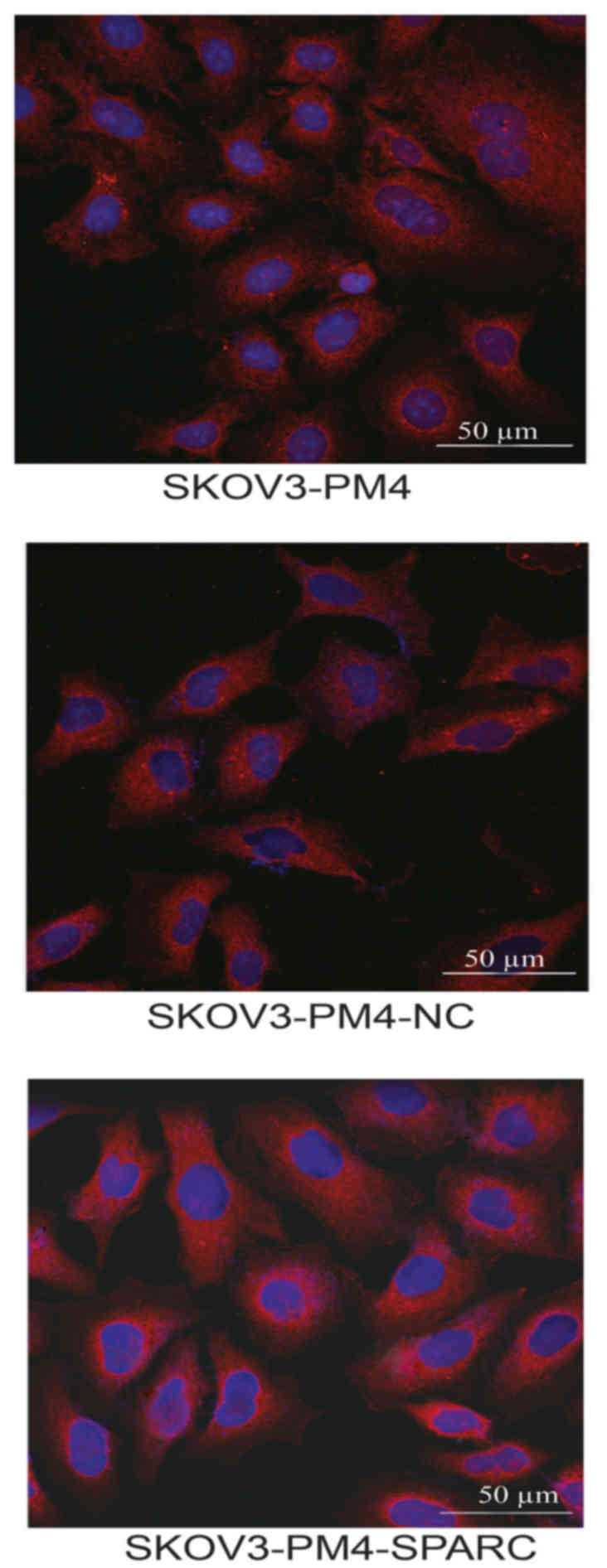
Distribution of SPARC protein were
observed by laser confocal microscopy
Distribution of SPARC protein in SKOV3-PM4,
SKOV3-PM4-NC and SKOV3-PM4-SPARC cells were observed under laser
confocal microscopy. SPARC proteins are mainly distributed in
cytoplasm region and membrane of the nucleus (Fig. 6).
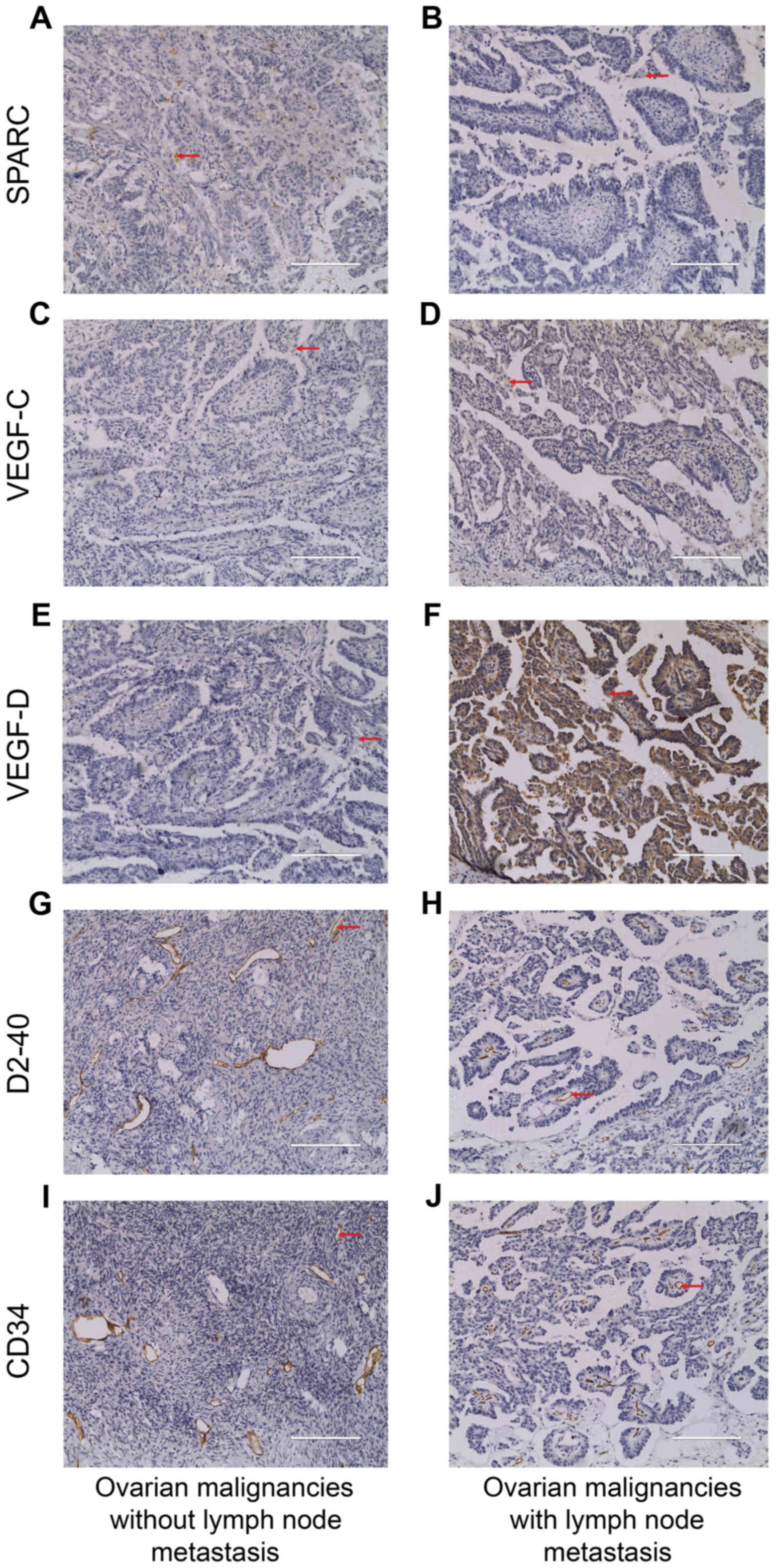
Expression of SPARC protein and VEGF
family in ovarian malignant tumor tissue
To evaluate the role of SPARC in lymph node
metastasis in ovarian cancer, we carried out immunohistochemistry
for SPARC, VEGF-C and VEGF-D in paraffin-embedded tissue sections
obtained from 47 cases of human ovarian malignant tumors.
Angiogenesis and lymphangiogenesis were evaluated by immunostaining
with CD34 and D2-40 antibodies. Consistent with the result observed
by laser confocal microscopy, SPARC expression was found not only
in the cytoplasm but also in the membrane of the nucleus of ovarian
cancer cells (Fig. 7A and B).
Localization of VEGF-C and VEGF-D expression were similar to SPARC
(Fig. 7C–F). The positive staining
of CD34 and D2-40 was brown-yellow, localized in the membrane of
vascular and lymphatic endothelial cells, respectively (Fig. 7G–J).
There was a significantly difference between the
expression of SPARC, VEGF-C and VEGF-D in the lymph node-involved
group compared to the lymph node non-involved group. As shown in
Table II, SPARC expression was
significantly lower in tissues with lymph node metastasis as
compared to tissues without lymph node metastasis, and was inversly
associated with the degree of malignancy. The expression of VEGF-C
and VEGF-D showed the opposite results. A negative correlation was
found between the expression of SPARC and VEGF-C (r=−0.394,
P=0.006) or VEGF-D (r=−0.337, P=0.021). LVD and MVD were
significantly higher in SPARC negative specimens than positive
specimens using Mann-Whitney U test (P<0.05) (Table III).
 | Table IIThe relationship among SPARC, VEGF-C
and VEGF-D expression in ovarian malignant tumor tissues with
clinicopathological parameters. |
Table II
The relationship among SPARC, VEGF-C
and VEGF-D expression in ovarian malignant tumor tissues with
clinicopathological parameters.
| Parameters | n | SPARC positive rate
(%) | P-value | VEGF-C positive
rate (%) | P-value | VEGF-D positive
rate (%) | P-value |
|---|
| FIGO staging |
| I–II | 9 | 9 (100) | 0.000c | 4 (44.4) | 0.013a | 2 (22.2) | 0.026a |
| III–IV | 38 | 7 (18.4) | | 33 (86.8) | | 25 (65.8) | |
| Lymph node
metastasis |
| Yes | 20 | 3 (15.0) | 0.039a | 20 (100) | 0.003b | 15 (75.0) | 0.027a |
| No | 27 | 13 (48.2) | | 17 (62.9) | | 12 (44.4) | |
 | Table IIIAnalysis of the correlation among
LVD, MVD and SPARC expression. |
Table III
Analysis of the correlation among
LVD, MVD and SPARC expression.
| Groups | n | LVD (D2-40) mean ±
SD | P-value | MVD (CD34) mean ±
SD | P-value |
|---|
| SPARC positive | 16 | 11.06±2.02 | 0.012 | 15.44±1.59 | 0.000 |
| SPARC negative | 31 | 12.7±1.77 | | 17.71±1.88 | |
Discussion
Tumor metastasis to regional lymph nodes is often
regarded as the first step of tumor dissemination, which precedes
the metastasis of the vascular system and is seen as a major
prognostic indicator of tumor progression. SPARC is a
calcium-binding protein that not only competitively binds to the
cell membrane surface growth factor receptor but also binds to
several residents of ECM to influence cell proliferation, adhesion,
migration, matrix degradation and angiogenesis (15,16).
Studies have shown that SPARC plays different roles in many types
of tumors including ovarian cancer and SPARC either lowly or highly
expressed in ovarian cancer (17–19).
Nevertheless, the specific contribution of SPARC in growth and
progression of ovarian cancer is largely unexplored.
The aim of the present study was to demonstrate that
SPARC might be an optional target for suppressing the lymph node
metastasis in ovarian cancer. In accordance with the study by Zhang
et al (12), our results
showed that SPARC inhibited ovarian cancer growth in vitro
as well, upregulation of SPARC expression suppressed the
proliferation, migration and invasion of SKOV3-PM4 cells. In
ovarian malignant tissues with lymph node metastasis, the
expression of SPARC was significantly lower as compared to tissues
without lymph node metastasis. SKOV3 cells expressed high-level
SPARC, much more than SKOV3-PM4 at both mRNA and protein levels.
These findings suggested that SPARC might be a crucial factor for
preventing ovarian cancer lymph node metastasis. However, the
underlying mechanisms of the SPARC involved in ovarian cancer
progression have not been clarified.
The metastasis of ovarian cancer is a multifactorial
and multistep process in which angiogenesis and lymphangiogenesis
are prerequisites. Angiogenesis, in which new blood vessels develop
through endothelial cell proliferation from extant vasculature,
provides an effcient way for tumor cells to leave their primary
site and enter the blood stream (20). Lymphangiogenesis is a complex
process that has received much attention as an important mediator
of tumor cell dissemination recently. It has been reported that
lymphangiogenesis promotes lymphatic metastases probably by
augmenting the potential entry point density of lymphatic vessels
for tumor cells entering the lymphatic system (21). Increasing evidence has revealed
that lymphangiogenic factors such as VEGF-C and VEGF-D induce
tumor-associated lymphatic vessel growth, enhancing the metastatic
spread of tumor cells to lymph nodes. Nevertheless, our knowledge
of the mechanisms that underlie lymphangiogenesis still lags far
behind that of angiogenesis. Therefore, further efforts are needed
for better understanding of lymphatic biology to develop more
effective treatments for ovarian cancer.
A differential expression of SPARC in tumor
neovascular endothelial cells compared with mature vascular
endothelial cells, suggesting that SPARC has been implicated in
angiogenesis (22). SPARC was
thought to be a key factor that inhibits the activity of VEGFs,
platelet derived growth factor (PDGF), and basic fibroblast growth
factor (bFGF). Studies reported that the expression of VEGFs highly
increased in colon cancer along with the decreased expression of
SPARC (23), and VEGF-D induced
tumor lymphangiogenesis and increase the lymphatic metastasis in
ovarian cancer (24). Through
suppressing the expression and secretion of VEGFs, SPARC inhibited
glioma growth by reducing tumor vascularity. In ovarian cancer
animal models, the absence of SPARC led to high expression of VEGF,
MMP2 and MMP9, indicating that SPARC modulates lymph node
metastasis and promotes the metastatic potential of ovarian cancers
through angiogenesis (25).
Microvessel density (MVD) and lymphatic microvessel
density (LVD) is now widely used to evaluate the angiogenesis and
lymphangiogenesis of tumors. In the present study, we detected the
expression level of SPARC and VEGFs in human ovarian malignancy
tissues by immunohistochemistry. It was revealed that SPARC
expression, which was inversely associated with the degree of
malignancy, had a negative correlation with VEGF-D expression, LVD
and MVD which were actually higher for advanced tumors than for
non-advanced tumors. We also observed overexpression of SPARC could
markedly downregulate the expression of VEGF-D at both mRNA and
protein level by qRT-PCR and western blot assay, implying that
repression of SPARC expression may upregulate VEGF-D expression,
causing the subsequent high count of MVD and LVD. Coincidentally,
it was reported that overexpression of SPARC inhibited
VEGF-mediated angiogenesis (12).
Another recent study confirmed that high-level expression of VEGF-D
could induce invasive and metastatic behavior of breast cancer,
while knock-down of it could inhibit lymphangiogenesis and
lymphatic metastasis (26).
Of note, a negative correlation was also found
between the expression of SPARC and VEGF-C in human ovarian
malignancy tissues, whereas in cultured cells, SPARC expression was
irrelevant to VEGF-C expresssion at both protein and mRNA level.
Given that SPARC plays a necessary part in cell proliferation and
adhesion, it is reasonable that cell culture conditions themselves
could modify the expression of SPARC. Several reports have
demonstrated that overexpression of VEGF-C can promote tumor
lymphangiogenesis resulting in tumor metastasis to the lymph nodes
in a variety of cancers and VEGF-C is associated with tumor
progression (27,28). Consistent with the results of our
immunohistochemistry study, numerous studies have shown that the
less expression of SPARC, the more expression of VEGF-C and the
more progression of tumor and vice versa (29–31).
Based on the above findings, it can be speculated that SPARC might
function as a tumor suppressor which inhibits angiogenesis and
lymphangiogenesis in ovarian cancer by reducing the expression of
VEGF-C and VEGF-D.
In summary, we have provided evidence that SPARC
influences lymph node metastasis by reducing the expression level
of VEGF-C and VEGF-D. On the basis of the results of this study,
SPARC might become a promising new therapeutic biomarker in ovarian
cancer. Targeting SPARC-mediated pathway might be a valid strategy
to understand how SPARC affects tumor metastasis and progress and
may result in the development of anti-angiogenesis or
anti-lymphangiogenesis therapy against ovarian cancer.
Acknowledgments
The present study was supported by grants obtained
from the National Natural Science Foundation of China (no.
81360502) and the Guangxi Natural Science Foundation (no.
2014GXNSFAA118225).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruan HY, Li DR, Li L, Guan X and Zhang W:
Establishment of human ovarian carcinoma cell lines with
directional highly lymphatic metastasis and study of their
biological characteristics. Zhonghua Fu Chan Ke Za Zhi. 42:482–486.
2007.In Chinese. PubMed/NCBI
|
|
4
|
Zhang XY, Yin FQ, Liu L, Gao T, Ruan HY,
Guan X, Lu YX and Li DR: Effects of HLEC on the secreted proteins
of epithelial ovarian cancer cells prone to metastasize to lymph
nodes. Cancer Biol Med. 10:221–226. 2013.PubMed/NCBI
|
|
5
|
Sage H, Johnson C and Bornstein P:
Characterization of a novel serum albumin-binding glycoprotein
secreted by endothelial cells in culture. J Biol Chem.
259:3993–4007. 1984.PubMed/NCBI
|
|
6
|
Bornstein P and Sage EH: Matricellular
proteins: Extracellular modulators of cell function. Curr Opin Cell
Biol. 14:608–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsiao YH, Lien HC, Hwa HL, Kuo WH, Chang
KJ and Hsieh FJ: SPARC (osteonectin) in breast tumors of different
histologic types and its role in the outcome of invasive ductal
carcinoma. Breast J. 16:305–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas R, True LD, Bassuk JA, Lange PH and
Vessella RL: Differential expression of osteonectin/SPARC during
human prostate cancer progression. Clin Cancer Res. 6:1140–1149.
2000.PubMed/NCBI
|
|
9
|
Sato T, Oshima T, Yamamoto N, Yamada T,
Hasegawa S, Yukawa N, Numata K, Kunisaki C, Tanaka K, Shiozawa M,
et al: Clinical significance of SPARC gene expression in patients
with gastric cancer. J Surg Oncol. 108:364–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan SK, Griffith OL, Tai IT and Jones SJ:
Meta-analysis of colorectal cancer gene expression profiling
studies identifies consistently reported candidate biomarkers.
Cancer Epidemiol Biomarkers Prev. 17:543–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chlenski A, Liu S, Guerrero LJ, Yang Q,
Tian Y, Salwen HR, Zage P and Cohn SL: SPARC expression is
associated with impaired tumor growth, inhibited angiogenesis and
changes in the extracellular matrix. Int J Cancer. 118:310–316.
2006. View Article : Google Scholar
|
|
12
|
Zhang JL, Chen GW, Liu YC, Wang PY, Wang
X, Wan YL, Zhu J, Gao HQ, Yin J, Wang W, et al: Secreted protein
acidic and rich in cysteine (SPARC) suppresses angiogenesis by
down-regulating the expression of VEGF and MMP-7 in gastric cancer.
PLoS One. 7:e446182012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yunker CK, Schultz C, Lemke N, Golembieski
WA, Nelson K, Ojetebbe N, Gutierrez JA, Mikkelsen T and Rempel SA:
SPARC suppresses and inversely correlates with VEGF expression and
secretion in SPARC-transfected U87MG cells and tumors and in
primary xenograft human gliomas. Cancer Res. 66:656. 2006.
|
|
14
|
Van der Auwera I, Cao Y, Tille JC, Pepper
MS, Jackson DG, Fox SB, Harris AL, Dirix LY and Vermeulen PB: First
international consensus on the methodology of lymphangiogenesis
quantification in solid human tumours. Br J Cancer. 95:1611–1625.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tai IT and Tang MJ: SPARC in cancer
biology: Its role in cancer progression and potential for therapy.
Drug Resist Updat. 11:231–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim NI, Kim GE, Lee JS and Park MH: In
phyllodes tumors of the breast expression of SPARC
(osteonectin/BM40) mRNA by in situ hybridization correlates with
protein expression by immunohistochemistry and is associated with
tumor progression. Virchows Arch. 470:91–98. 2017. View Article : Google Scholar
|
|
17
|
Bull Phelps SL, Carbon J, Miller A,
Castro-Rivera E, Arnold S, Brekken RA and Lea JS: Secreted protein
acidic and rich in cysteine as a regulator of murine ovarian cancer
growth and chemosensitivity. Am J Obstet Gynecol. 200:180 e1–7.
2009. View Article : Google Scholar
|
|
18
|
Socha MJ, Said N, Dai Y, Kwong J,
Ramalingam P, Trieu V, Desai N, Mok SC and Motamed K: Aberrant
promoter methylation of SPARC in ovarian cancer. Neoplasia.
11:126–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Wang M, Xi B, Xue J, He D, Zhang J
and Zhao Y: SPARC is a key regulator of proliferation, apoptosis
and invasion in human ovarian cancer. PLoS One. 7:e424132012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jendraschak E and Sage EH: Regulation of
angiogenesis by SPARC and angiostatin: Implications for tumor cell
biology. Semin Cancer Biol. 7:139–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura Y, Yasuoka H, Tsujimoto M,
Kurozumi K, Nakahara M, Nakao K and Kakudo K: Importance of lymph
vessels in gastric cancer: A prognostic indicator in general and a
predictor for lymph node metastasis in early stage cancer. J Clin
Pathol. 59:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller JR, Moon RT, Dev G, Cadigan KM,
Nusse R, Hart MJ, Santos RDL, Albert IN and Rubinfeld B: Genes
expressed in human tumor endothelium. Science. 289:1197–1202. 2000.
View Article : Google Scholar
|
|
23
|
Liang JF, Wang HK, Xiao H, Li N, Cheng CX,
Zhao YZ, Ma YB, Gao JZ, Bai RB and Zheng HX: Relationship and
prognostic significance of SPARC and VEGF protein expression in
colon cancer. J Exp Clin Cancer Res. 29:71–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du LC, Chen XC, Wang D, Wen YJ, Wang CT,
Wang XM, Kan B, Wei YQ and Zhao X: VEGF-D-induced draining
lymphatic enlargement and tumor lymphangiogenesis promote lymph
node metastasis in a xenograft model of ovarian carcinoma. Reprod
Biol Endocrinol. 12:14–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Said N, Socha MJ, Olearczyk JJ, Elmarakby
AA, Imig JD and Motamed K: Normalization of the ovarian cancer
microenvironment by SPARC. Mol Cancer Res. 5:1015–1030. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Majumder M, Tutunea-Fatan E, Xin X,
Rodriguez-Torres M, Torres-Garcia J, Wiebe R, Timoshenko AV,
Bhattacharjee RN, Chambers AF and Lala PK: Co-expression of α9β1
integrin and VEGF-D confers lymphatic metastatic ability to a human
breast cancer cell line MDA-MB-468LN. PLoS One. 7:e350942012.
View Article : Google Scholar
|
|
27
|
Hirakawa S, Brown LF, Kodama S, Paavonen
K, Alitalo K and Detmar M: VEGF-C-induced lymphangiogenesis in
sentinel lymph nodes promotes tumor metastasis to distant sites.
Blood. 109:1010–1017. 2007. View Article : Google Scholar
|
|
28
|
Kumar B, Chile SA, Ray KB, Reddy GE,
Addepalli MK, Kumar AS, Ramana V and Rajagopal V: VEGF-C
differentially regulates VEGF-A expression in ocular and cancer
cells; promotes angiogenesis via RhoA mediated pathway.
Angiogenesis. 14:371–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradshaw AD, Reed MJ, Carbon JG, Pinney E,
Brekken RA and Sage EH: Increased fibrovascular invasion of
subcutaneous polyvinyl alcohol sponges in SPARC-null mice. Wound
Repair Regen. 9:522–530. 2001. View Article : Google Scholar
|
|
30
|
Yan M, Schneider J, Gear R, Lu F, LaDow K,
Warshawsky D and Heffelfinger SC: Expression of angiogenic factors
is upregulated in DMBA-induced rat mammary pathologies.
Pathobiology. 71:253–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu H, Zhang S, Zhang R and Zhang L: The
role of VEGF-C/D and Flt-4 in the lymphatic metastasis of
early-stage invasive cervical carcinoma. J Exp Clin Cancer Res.
28:98–103. 2009. View Article : Google Scholar : PubMed/NCBI
|