Introduction
Glioma is the most common primary intraparenchymal
brain tumor in the central nervous system (CNS), accounting for
approximately 7% of the death caused by cancer (1,2). It
is divided into grade I–IV with increasing severity by the World
Health Organization (WHO). Although significant improvements in
treatments for glioma, highly invasive WHO high-grade glioma
exhibits a relentless malignant progression, resistance to all
therapeutic approaches and ultimately leads to the patient's death
(3). There is an urgent need for
further research on the pathogenesis of glioma and improve the
outcome of patients.
As has been shown in many tumors, glioma stem cells
(GSCs), a small subpopulation of stem cells with enhanced
self-renewal capacity and a multilineage differentiation potential,
are believed to be the real driving force for tumor initiation,
progression and relapse (4–6).
GSCs give rise to a variety of proliferating and differentiated
cells that make up the tumor mass and play a key role in resistance
to radiotherapy and chemotherapy (7,8),
angiogenesis (9) and metastasis
(10). A recent study showed that
temozolomide (a mainstay of chemotherapy in high-grade glioma)
actually increased the proportion of GSCs by inducing conversion of
non-GSCs into GSCs (4). Thus, the
biology of GSCs should be explored in order to effectively enhance
the treatment in glioma (11).
Investigation of genetic mutations associated with
glioma showed that patients frequently carry isocitrate
dehydrogenase 1 (IDH1) mutation (12–14).
IDH1 are frequently mutated in WHO grade II and grade III glioma
and in secondary glioblastoma. Approximately 90% of the IDH1
mutation in glioma is R132H (15,16).
IDH1 mutation results in production and accumulation of the
metabolite D-2-hydroxyglutarate (D-2-HG) in IDH1 mutated cells,
which greatly impacts the cellular metabolism and contributes to
tumor pathogenesis (13,17,18).
IDH1 mutation is expected to be stable and homogenous among all
tumor cells within IDH1-mutated glioma. As a genetic mutation, it
is supposed to occur at the tumor precursor cell stage (19). However, the full effects of mutant
IDH1 on GSCs biology are not fully understood. Our previous study
suggested that the R132H mutation in IDH1 serves a tumor suppressor
function in human glioma cells by negatively regulating
Wnt/β-catenin signaling (20). The
vital role of the Wnt signaling pathway for the function of normal
and cancer stem cells is commonly accepted (21). One of the hallmarks of stem cells
is the ability to maintain long telomeres by function of the TERT
gene. TERT expression is directly enhanced by binding of β-catenin
to its promoter region and thereby links telomerase activity to Wnt
signaling (22). In the present
study, we investigated the role of IDH1-R132H in GSCs and detected
Wnt/β-catenin signaling expression to verify its role in regulating
the cellular properties of GSCs.
Materials and methods
Clinical samples and cell line
The samples used in the present study were obtained
from 172 patients newly diagnosed with glioma that underwent sample
resection without therapy between 2010 and 2015 at the Department
of Neurological Surgery, the Affiliated Hospital of Nantong
University. The collection of the glioma specimens was performed in
accordance with the protocols approved by the Ethics Committee of
the Affiliated Hospital of Nantong University. The clinical
demographic details of the 172 patients are outlined in Table I. Specimens were snapped frozen in
liquid nitrogen immediately after surgery and stored at −80°C until
use.
 | Table IGSCs marker and clinicopathological
parameters in 172 glioma specimens. |
Table I
GSCs marker and clinicopathological
parameters in 172 glioma specimens.
| Variable | Total | CD133 and nestin
| χ2 | P-value | r |
|---|
| Positive | Negative |
|---|
| Age (years) | | | | 3.276 | 0.070 | −0.138 |
| <40 | 32 | 12 | 20 | | | |
| ≥40 | 140 | 31 | 109 | | | |
| Sex | | | | 0.303 | 0.582 | −0.042 |
| Male | 110 | 29 | 81 | | | |
| Female | 62 | 14 | 48 | | | |
| Tumor location | | | | 4.067 | 0.397 | 0.035 |
| Frontal | 74 | 15 | 59 | | | |
| Parietal | 15 | 6 | 9 | | | |
| Occipital | 26 | 7 | 19 | | | |
| Temporal | 31 | 10 | 21 | | | |
| Unknown | 26 | 5 | 21 | | | |
| Surgery | | | | 0.062 | 0.969 | −0.009 |
| Biopsy | 24 | 6 | 18 | | | |
| Partial
resection | 26 | 7 | 19 | | | |
| Gross total
resection | 122 | 30 | 92 | | | |
| Tumor diameter
(cm) | | | | 2.005 | 0.157 | −0.108 |
| <4 | 117 | 33 | 84 | | | |
| ≥4 | 55 | 10 | 45 | | | |
| IDH1-mu | | | | | | |
| Positive | 48 | 6 | 42 | 5.548 | 0.018 | 0.180 |
| Negative | 124 | 37 | 87 | | | |
| TNM grade | | | | 17.128 | 0.001 | 0.274 |
| I | 15 | 3 | 12 | | | |
| II | 54 | 4 | 50 | | | |
| III | 53 | 15 | 38 | | | |
| IV | 50 | 21 | 29 | | | |
Human GSCs used in the present study were isolated
from glioblastoma patients with IDH1 mutation as previously
described (23,24). In brief, a few minutes after tumor
removal, tissues were dissociated into a cell suspension by 0.25%
trypsin digestion. Cells that passed through a 100 µm
strainer (Falcon, Oxnard, CA, USA) were cultured in the medium
composed of Dulbecco's modified Eagle's medium (DMEM) with 2% B27,
epidermal growth factor (EGF, 10 ng/ml) and basic fibroblast growth
factor (bFGF, 10 ng/ml). Cells were incubated at 37°C with 95% air,
5% CO2 and 100% humidity. Two weeks later, floating
primary tumor spheres were collected. The sphere cells were
harvested, dissociated into single cells and plated into a 24-well
plate for the subsphere-forming. Subspheres from one mother cell
were characterized by immunocytochemistry against CD133 and
nestin.
To induce differentiation of GSCs, the cells
(1×105 cells/ml) were plated onto poly-D-lysine coated
coverslips in 96-well plates containing DMEM with 10% fetal bovine
serum (FBS).
Tissue microarray immunohistochemistry
(TMA-IHC)
Immunohistochemistry was performed using protocols
described previously by Gurung et al (25). In brief, the TMA slides were
deparaffinised, rehydrated, washed and endogenous peroxidase was
blocked using Bond-III 'Dewax Protocol D' following the
manufacturer's instructions (Leica Biosystems, Newcastle, UK).
Epitope retrieval was achieved using Bond-III 'Protocol H1(30)' (Leica Biosystems). The slides were
incubated with antibodies against anti-IDH1-R132H (1:400; Dianova
GmbH, Hamburg, Germany), CD133 (1:500; Abcam, Cambridge, UK) or
nestin (1:500; Abcam) at room temperature (RT) for 1 h. Antibody
binding was detected using diaminobenzidine (DAB) with hematoxylin
counterstaining following Bond-max and Bond-x 'IHC protocol F'
(Leica Biosystems). The immunostaining results were interpreted
independently by two expert pathologists who were blinded to the
clinical data. The staining intensity was scored using the
following scale of four grades: 0, no staining; 1, weak staining;
2, moderate staining; and 3, strong staining. At least 5 areas of
each core were viewed and the proportion of cells in each core
staining positively was assigned a score (0-100%). A
semi-quantitative histopathology (H) score was obtained by
multiplying the staining intensity score with the percentage score
(0–300). An H-score higher than the median was considered
positive.
Generation of lentiviral constructs and
transfection
To over-express IDH1-wt, the coding sequence of IDH1
was amplified using the following primers: forward primer
5′-ATGTCCAAA AAAATCAGTGGCGG-3′ and reverse primer 5′-GTTTGGCC
TGAGCTAGTTTG-3′. This fragment was then sub cloned into the
lentiviral vector pLenti6.3-MCS-IRES2-EGFP vector (Invitrogen,
Carlsbad, CA, USA) using the restriction sites for BamHI and
AscI. To overexpress the R132H mutant form of IDH1, we
procured commercial synthetic sequences of IDH1 mutated at Arg 132,
and sub-cloned them into the pLenti6.3-MCS-IRES2-EGFP vector. An
empty vector (EV) was used as the control. The vectors and the
Packaging Mix were cotransfected into the 293FT cell line to
generate lentiviral stocks, which were used to transfect the target
cells.
Immunofluorescent staining
For immunofluorescent staining, cells were first
fixed in 4% PFA, followed by incubation in hydrogen peroxide,
blocking of non-specific antibody binding sites, and overnight
incubation with the following primary antibodieså: rat
anti-β-catenin (1:2,000; Abcam), mouse monoclonal anti-nestin
(1:600; Roche Diagnostics GmbH, Mannheim, Germany), rat anti-CD133
(1:600; Roche Diagnostics GmbH) and mouse monoclonal anti-MAP-2
(1:600; Roche Diagnostics GmbH). The following day, cells were
labeled secondary antibody with Alexa Fluor 568-conjugated goat
anti-rat (1:1,000; Molecular Probes, Eugene, OR, USA) or
488-conjugated goat anti-mouse (1:800; Invitrogen). Cell nuclei
were counter-stained with Hoechst 33342 for 30 min at RT.
Immunopositive cells were observed under a fluorescent
microscope.
Real-time PCR analysis
To determine the level of mRNA expression in cells,
the total RNA was extracted from 1×106 cells in each
group using UNIQ-10 Spin Column RNA Purified kit (Sangon Biotech
Co., Ltd., Shanghai, China). The first strand cDNA was synthesized
using RevertAid™ First Strand cDNA Synthesized kit (Fermentas,
Burlington, ON, Canada). First Strand cDNA was subsequently
subjected to Corbett RG-6000 PCR system (Qiagen, Dusseldorf,
German) using FastStart Universal SYBR-Green Master Mix (Roche,
Basel, Switzerland). The reactions were optimized by varying the
annealing temperatures from 50 to 55°C, the sense and anti-sense
primers were synthesized as follows: GAPDH 5′-GCAA
GTTCAACGGCACAG-3′ and 5′-GCCAGTAGACTCCACG ACAT-3′; CD133
5′-TTCTGCCTGTGTAACTTTGCA-3′ and R, 5′-TTGTTGTGCAACGTCTTCAAGTAT-3′;
nestin 5′-AG CGTTGGAACAGAGGTTGGA-3′ and 5′-TGTTTCCTCCCA
CCCTGTGTCT-3′; β-catenin 5′-ATTGAAGCTGAGGGAGC CAC-3′ and
5′-TCCTGGCCATATCCACCAGA-3′; LEF1 5′-AAATAAAGTGCCCGTGGTGC-3′ and
5′-CATGCCTTG TTTGGAGTTGACA-3′; TCF4 5′-CGGCGGTGGGGGGA TGAC-3′ and
5′-GGCCGCTTCTTCCAAACTTTCC-3′.
Western blot analysis
To determine the level of protein expression in
cells, aliquots of total protein (50 µg per lane) were
electrophoresed on a 12% SDS-polyacrylamide gradient gel and
transferred to nitrocellulose membranes (Millipore, Darmstadt,
Germany). Washed in rinse buffer at RT and incubated in blocking
buffer (5% fat-free milk in rinse buffer) for 30 min, the membranes
were incubated for 2 h at RT with the following primary antibodies:
anti-IDH1 R132H (1:500; Abcam;), anti-IDH1 (1:500; Dianova GmbH),
anti-β-catenin (1:4,000; Abcam), anti-LEF1 (1:500; Santa Cruz
Biotechnolgy, Santa Cruz, CA, USA), anti-TCF4 (1:200; Santa Cruz
Biotechnolgy), anti-nestin (1:600; Roche Diagnostics GmbH),
anti-CD133 (1:600; Roche Diagnostics GmbH) and anti-MAP-2 (1:800;
Invitrogen). The following day, they were incubated with the
HRP-conjugated secondary antibodies and visualized using the
enhanced chemiluminescence (ECL) system (Pierce Biotechnology Inc.,
Rockford, IL, USA). In addition, β-actin was used as a reference
protein.
Proliferation and apoptosis assay of
GSCs
The cells of EV, IDH1-wt and IDH1-mu groups were
cultured in the above mentioned medium. At day 7 in vitro
(DIV), some cell spheres of three groups were dissociated into
single-cell suspension mechanically by trypsin digestion. Collected
cells were washed once in cold phosphate-buffered saline (PBS).
Cells were analyzed using Cell Cycle and Apoptosis Analysis kit
(Beyotime Institute of Biotechnology, Haimen, China) by flow
cytometry (Epics XL; Beckman Coulter, Fullerton CA, USA), according
to the manufacturer's protocol.
Invasion and migration assays of
GSCs
To measure changes in cell migration, we performed
the Transwell migration assay using 24-well BD Matrigel invasion
chambers (BD Biosciences, San Jose, CA, USA) as previously
described (20). In brief,
5×104 cells were seeded into the upper well in DMEM
(without serum) and incubated for 24 h to allow the cells to
migrate into the lower well through an 8-µm pore membrane.
After 24 h, the latter were fixed and stained for easy
visualization and quantification. The average number of cells per
field was determined by counting the number of cells in six random
fields per well. To determine the invasive properties, cells were
plated into chambers coated with Matrigel, which were then inserted
into a 24-well plate and incubated for 48 h in DMEM and 10% FBS. At
the end of the assay, the cells that adhered to the lower well were
fixed, stained and visualized.
Statistical analysis
Statistical analysis was performed using statistics
package for social science 21.0 (SPSS 21.0). The group comparison
was analyzed using the independent t-test or one-way ANOVA.
Associations between positive rate of GSC markers and the
clinicopathological characteristics were analyzed using the
Pearson's chi-squared test and the correlation analysis was
compared using the Pearson test. Five-year overall survival (OS)
was estimated using the Kaplan-Meier method and the differences in
survival among groups were compared using the log-rank test.
P<0.05 was considered statistically significant.
Results
The number of GSCs was decreased in
patients with IDH1-R132H-mu and correlated with TNM stage and poor
overall survival
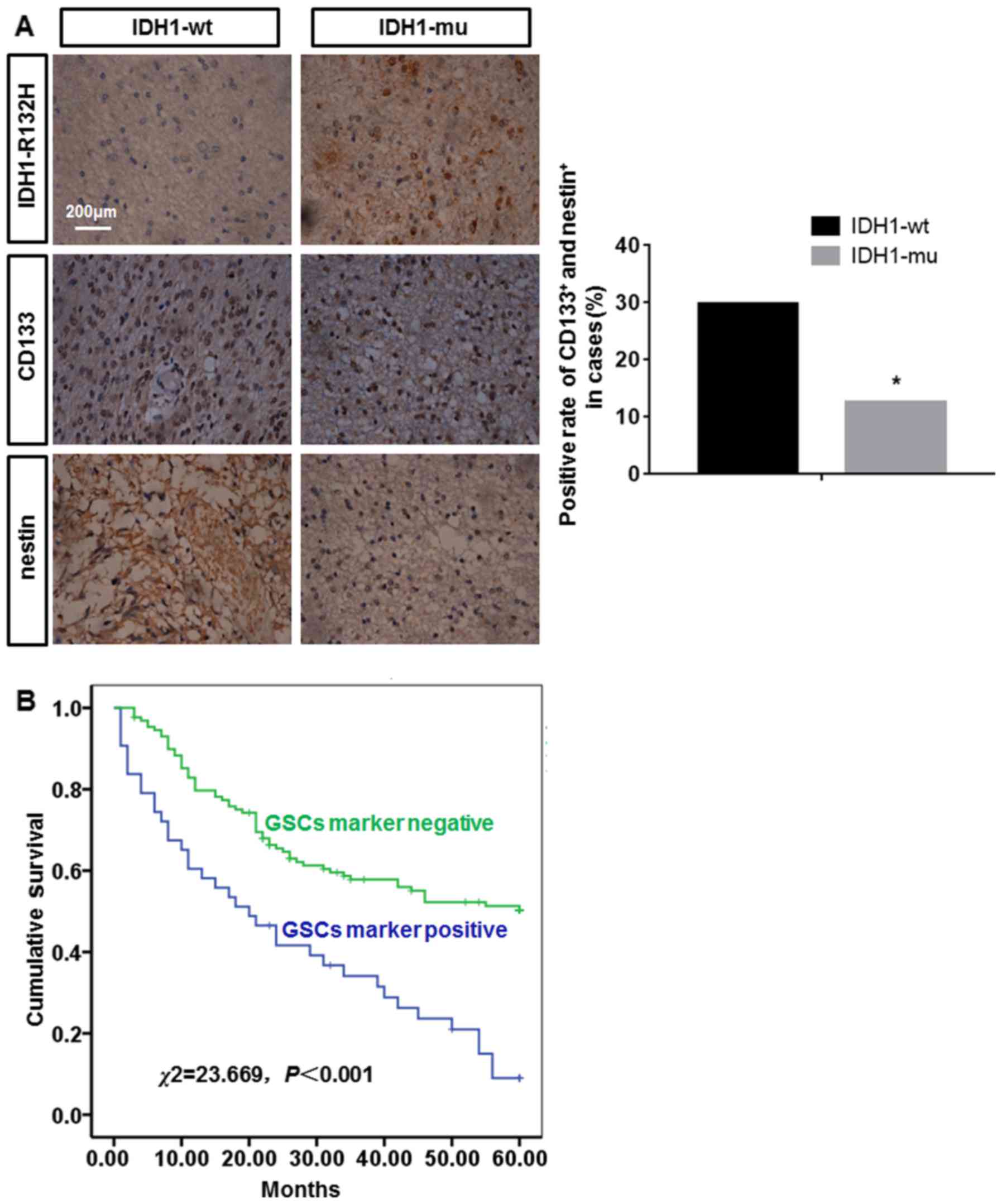
The results of TMA-IHC showed that there were 48
patients with IDH1-mu and 124 patients with IDH1-wt. GSCs in
patients with IDH1-mu or IDH1-wt were identified by CD133 and
nestin TMA-IHC. Many CD133+ and nestin+ cells
were found in the patients with IDH1-wt while a few
CD133+ and nestin+ cells in the patients with
IDH1-mu. The positive rate of GSCs in the patients with IDH1-wt was
more than that in the patients with IDH1-mu. The difference among
them was statistically significant (P<0.05) (Fig. 1A). The correlation between positive
rate of GSC markers and a series of clinicopathological
characteristics was conducted. However, the relationship between
the expression patterns of GSC markers and the histologic grade in
glioma was not analyzed in the present study because of the limited
samples of high histologic grade glioma. In the following study, we
will collect enough samples to carry out this analysis. As shown in
Table I, positive rate of GSC
marker was significantly correlated with tumor stage (r=0.274) and
IDH1 mutation (r=0.180). Five-year OS of patients was estimated
using the Kaplan-Meier. The median 5-year OS of patients with GSC
marker negative was 41 months (95% CI, 36.64–44.39 months). The
median 5-year OS of patients with GSCs marker positive was 26
months (95% CI, 19.28–31.89 months). The 5-year OS rate was
significantly lower for GSC marker positive patients than for GSCs
marker negative patients, the difference was statistically
significant (P<0.05) (Fig.
1B).
The expression of IDH1-R132H in
transfected GSCs in vitro
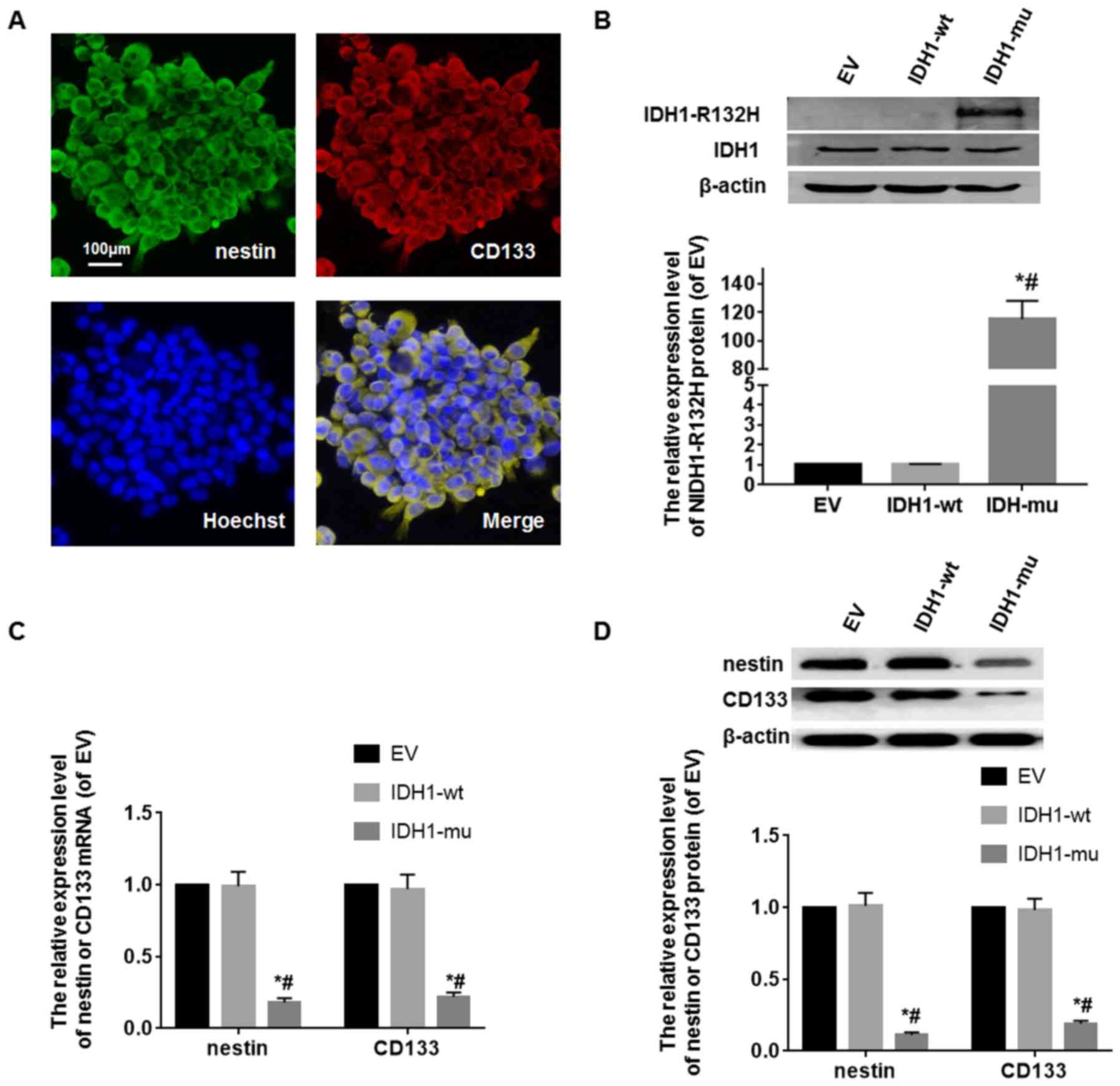
GSCs were identified by CD133 and nestin markers
in vitro. The cells were cultured in serum-free medium, many
cells formed floating primary tumor spheres. Subspheres from one
mother cell were CD133 and nestin double positive (Fig. 2A). The expression of IDH1-R132H in
GSCs was detected by western blot analysis. IDH1-R132H protein
level in IDH1-R132H modified GSCs was more than that in the EV and
IDH1-wt modified GSCs. The difference among them was statistically
significant (P<0.05) (Fig. 2B).
The expression of CD33 or nestin mRNA and protein in the
transfected GSCs were detected by RT-PCR and western blot analysis.
CD33 or nestin mRNA and protein level in IDH1-R132H modified GSCs
was lower than that in the EV and IDH1-wt modified GSCs (P<0.05)
(Fig. 2C and D).
IDH1-R132H mutation reduced the activity
of Wnt/β-catenin signaling in GSCs
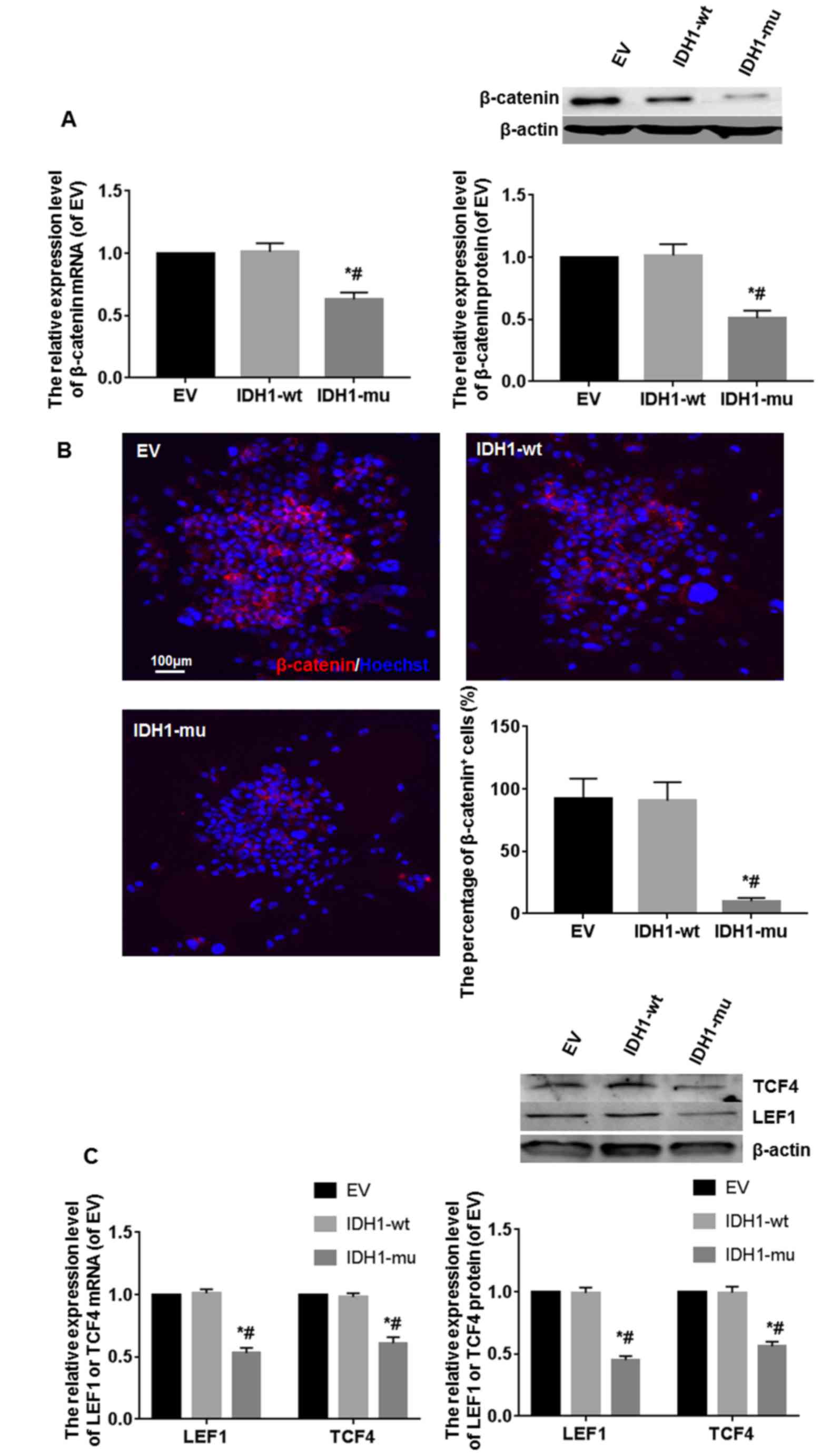
The β-catenin expression level was detected by
RT-PCR, western blot analysis and immunofluorescence. Compared with
IDH1-wt and EV groups, the mRNA and protein expression of β-catenin
in IDH1-mu group were significantly decreased. The difference among
them was statistically significant (P<0.05) (Fig. 3A). Many intense
β-catenin+ cells were found in the EV and IDH1-wt
groups, and a few weak β-catenin+ cells were found in
the IDH1-mu group. The difference among them was statistically
significant (P<0.05) (Fig. 3B).
To further analyze the level of Wnt/β-catenin signaling in the
GSCs, the concentration of the β-catenin co-actors and Wnt
signaling mediators TCF4 and LEF1 was measured by RT-PCR and
western blot analysis. The mRNA and protein expression levels of
TCF4 and LEF1 were downregulated in IDH1-mu group, compared with
IDH1-wt and EV groups. The difference among them was statistically
significant (P<0.05) (Fig.
3C).
IDH1-R132H mutation led to a less
aggressive phenotype in GSCs
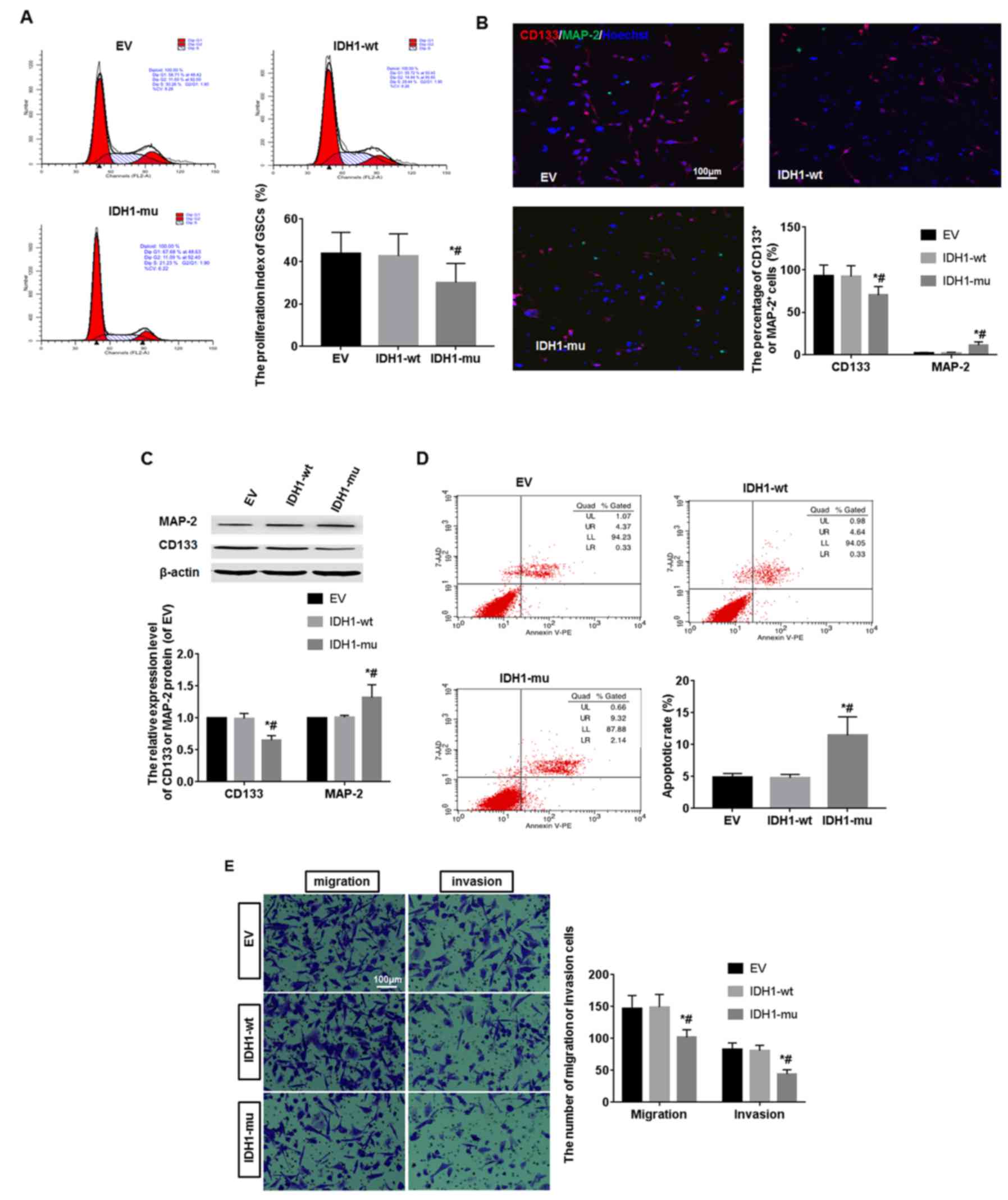
The proliferation ability of GSCs in 3 groups was
detected by flow cytometry. The result showed the proliferation
index (PI) of IDH1-mu group was 30.05±9.01, while the PI of EV and
IDH1-wt groups were 43.86±9.85 and 42.64±10.43, respectively. The
PI of IDH1-mu group was lower than that of other two groups
(P<0.05) (Fig. 4A). The
differentiation ability of GSCs in 3 groups was detected by
immunofluorescence and western blot analysis. Scarce
MAP-2+ neurons and many CD133+ cells were
found in the 3 groups. Compared with IDH1-wt and EV group, the
percentage of MAP-2+ neurons in IDH1-mu group was more
than that of the other two groups, but the percentage of
CD133+ cells in IDH1-mu group was less than that of the
other two groups (P<0.05) (Fig.
4B). Compared with IDH1-wt and EV groups, the CD133 protein
level was decreased and MAP-2 protein level was increased in the
IDH1-mu group (P<0.05) (Fig.
4C). The apoptotic level of GSCs in 3 groups was measured by a
flow cytometry assay. The percentage of apoptotic cells was
significantly higher in IDH1-mu group than that in IDH1-wt and EV
groups (P<0.05) (Fig. 4D). To
investigate the migration and invasion ability of GSCs, we assayed
the rate of cellular migration and invasion using Transwell assay
system. The migrated and invasive cells in IDH1-mu group obviously
decreased compared with other two groups (P<0.05) (Fig. 4E).
Discussion
Glioma is characterized by high morphological,
genetic and phenotypic heterogeneity. However, its origin and the
mechanisms which mediate its growth, recurrence and progression
were still not fully understood (24). Studies have shown that the marked
intratumoral heterogeneity, mirrored by the presence of distinct
subpopulations of cells showing different tumorigenic capabilities,
was one of the factors underlying tumor recurrence and poor
long-term survival (26). In
particular GSCs in glioma of different grades from both children
and adults, a small subpopulation of cells with stem-like
properties, such as self-renewal capacity and a multilineage
differentiation potential, are believed to be the real driving
force for glioma initiation, progression and recurrence (5). GSCs give rise to a variety of
proliferating and differentiated cells that make up the tumor mass
and play a key role in the resistance to radiotherapy and
chemotherapy (7,8), angiogenesis (9) and metastasis (10). However, its molecular signal
pathways are still unclear. As it has been shown in many studies,
another feature of GSCs is the aberrant activation of several
embryonic signaling pathways, such as Wnt signaling pathway to
regulate self-renewal, migration and differentiation of GSCs
(27–29).
Investigation of genetic mutations associated with
glioma showed that patients frequently carried IDH1 mutations
(12–14). Notably, the presence of the
IDH1-R132H mutation significantly improved prognosis and longer
progression-free and overall survival times in glioma patients
(14,30,31).
Studies have shown that patients with IDH1-wt displayed a worse
clinical outcome than those with IDH1-R132H. IDH1-R132H is now
being used as a predictor of better prognosis in glioma patients
(12). Our previous study showed
that the R132H mutation in IDH1 serves a tumor suppressor function
in human glioma cells by negatively regulating Wnt/β-catenin
signaling (20).
However, few studies have investigated the potential
mechanistic cross-talk among IDH1 mutation, GSCs and Wnt/β-catenin
signaling. In the present study, we first showed an inverse
correlation between the presence of the IDH1-R132H mutation and the
CD133+ or nestin+ cells in tumor samples
obtained from glioma patients. CD133 (a cell surface marker for
normal NSCs) and nestin (a cytoskeleton protein associated with
NSCs and progenitor cells in CNS development) are recommended for
the specific identification of GSCs (32-35).
The results showed GSCs in the patients with IDH1-R132H were less
than that in the patients with IDH1-wt. The positive rate of GSCs
was significantly correlated with TNM stage and poor overall
survival. To explore the relation between this mutation and GSCs,
we overexpressed IDH1-R132H in GSCs isolated from patients with
glioblastoma in vitro, as a control, we overexpressed either
the wild-type IDH1 or the empty vector. A comprehensive examination
of the cellular properties of the transfected GSCs in vitro
showed that IDH1-R132H overexpression led to decreased
proliferation, improved differentiation, inducing apoptosis and
reducing the ability to migrate and invade surrounding substrate.
At the molecular level, our data showed that the IDH1-R132H
mutation triggered a significant reduction in the activity of
Wnt/β-catenin signaling. To confirm this conclusion, the expression
of mediators, effectors and targets of canonical Wnt signaling,
including β-catenin, TCF4 and LEF1 were downregulated in GSC
overexpressing IDH1-R132H, as determined by RT-PCR and western blot
analysis.
In conclusion, the above data demonstrate that IDH1
mutation reduces the malignant progression of glioma by causing a
less aggressive phenotype in GSCs which involve the Wnt/β-catenin
signaling. IDH1 may serve as a potential therapeutic target for
glioma. However, further investigations are needed in order to
deeply understand the biology of GSCs.
Acknowledgments
This study was supported by Jiangsu Provincial Key
Medical Center, China Postdoctoral Science Foundation (grant no.
2015M581845), Postdoctoral Research Funding Plan in Jiangsu
Province (grant no. 1601039A), Nantong Science and Technology Plan
(grant no. MS32016016 and no. MS32015023), Young Medical Talent
Project in Jiangsu Province (grant no. QNRC2016691).
References
|
1
|
Furnari FB, Fenton T, Bachoo RM, Mukasa A,
Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al:
Malignant astrocytic glioma: Genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gladson CL, Prayson RA and Liu WM: The
pathobiology of glioma tumors. Annu Rev Pathol. 5:33–50. 2010.
View Article : Google Scholar :
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Auffinger B, Tobias AL, Han Y, Lee G, Guo
D, Dey M, Lesniak MS and Ahmed AU: Conversion of differentiated
cancer cells into cancer stem-like cells in a glioblastoma model
after primary chemotherapy. Cell Death Differ. 21:1119–1131. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133+ and CD133− glioblastoma-derived
cancer stem cells show differential growth characteristics and
molecular profiles. Cancer Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li
Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD and Rich JN: Stem
cell-like glioma cells promote tumor angiogenesis through vascular
endothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dey M, Ulasov IV, Tyler MA, Sonabend AM
and Lesniak MS: Cancer stem cells: The final frontier for glioma
virotherapy. Stem Cell Rev. 7:119–129. 2011. View Article : Google Scholar :
|
|
12
|
Uno M, Oba-Shinjo SM, Silva R, Miura F,
Clara CA, Almeida JR, Malheiros SM, Bianco AM, Brandt R, Ribas GC,
et al: IDH1 mutations in a Brazilian series of glioblastoma.
Clinics (Sao Paulo). 66:163–165. 2011. View Article : Google Scholar
|
|
13
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan W, Zhang W, You G, Bao Z, Wang Y, Liu
Y, Kang C, You Y, Wang L and Jiang T: Correlation of IDH1 mutation
with clinico-pathologic factors and prognosis in primary
glioblastoma: A report of 118 patients from China. PLoS One.
7:e303392012. View Article : Google Scholar
|
|
15
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sonoda Y, Kumabe T, Nakamura T, Saito R,
Kanamori M, Yamashita Y, Suzuki H and Tominaga T: Analysis of IDH1
and IDH2 mutations in Japanese glioma patients. Cancer Sci.
100:1996–1998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim
SH, Ito S, Yang C, Wang P, Xiao MT, et al: Oncometabolite
2-hydroxyglutarate is a competitive inhibitor of
α-ketoglutarate-dependent dioxygenases. Cancer Cell. 19:17–30.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chitneni SK: IDH1 Mutations in Glioma:
Considerations for radiotracer development. SM Radiol J.
2:10092016.PubMed/NCBI
|
|
20
|
Cui D, Ren J, Shi J, Feng L, Wang K, Zeng
T, Jin Y and Gao L: R132H mutation in IDH1 gene reduces
proliferation, cell survival and invasion of human glioma by
downregulating Wnt/β-catenin signaling. Int J Biochem Cell Biol.
73:72–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JI, Venteicher AS, Hong JY, Choi J,
Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al:
Telomerase modulates Wnt signalling by association with target gene
chromatin. Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He H, Niu CS and Li MW: Correlation
between glioblastoma stem-like cells and tumor vascularization.
Oncol Rep. 27:45–50. 2012.
|
|
24
|
Li Q, Qiao G, Ma J and Li Y:
Downregulation of VEGF expression attenuates malignant biological
behavior of C6 glioma stem cells. Int J Oncol. 44:1581–1588. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gurung PM, Veerakumarasivam A, Williamson
M, Counsell N, Douglas J, Tan WS, Feber A, Crabb SJ, Short SC,
Freeman A, et al: Loss of expression of the tumour suppressor gene
AIMP3 predicts survival following radiotherapy in muscle-invasive
bladder cancer. Int J Cancer. 136:709–720. 2015.
|
|
26
|
Dirks PB: Brain tumor stem cells: Bringing
order to the chaos of brain cancer. J Clin Oncol. 26:2916–2924.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Torres S, Lorente M, Rodríguez-Fornés F,
Hernández-Tiedra S, Salazar M, García-Taboada E, Barcia J, Guzmán M
and Velasco G: A combined preclinical therapy of cannabinoids and
temozolomide against glioma. Mol Cancer Ther. 10:90–103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheema TA, Kanai R, Kim GW, Wakimoto H,
Passer B, Rabkin SD and Martuza RL: Enhanced antitumor efficacy of
low-dose Etoposide with oncolytic herpes simplex virus in human
glioblastoma stem cell xenografts. Clin Cancer Res. 17:7383–7393.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Costello JF, Berger MS, Huang HS and
Cavenee WK: Silencing of p16/CDKN2 expression in human gliomas by
methylation and chromatin condensation. Cancer Res. 56:2405–2410.
1996.PubMed/NCBI
|
|
30
|
Nobusawa S, Watanabe T, Kleihues P and
Ohgaki H: IDH1 mutations as molecular signature and predictive
factor of secondary glioblastomas. Clin Cancer Res. 15:6002–6007.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie F, Tang JJ, Wang X, Liu YH and Mao Q:
Correlation between IDH1 mutation and prognosis in supratentorial
high-grade astrocytomas. Sichuan Da Xue Xue Bao Yi Xue Ban.
44:184–187. 1922013.In Chinese.
|
|
32
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
33
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen G, Shen F, Shi Z, Liu W, Hu W, Zheng
X, Wen L and Yang X: Identification of cancer stem-like cells in
the C6 glioma cell line and the limitation of current
identification methods. In Vitro Cell Dev Biol Anim. 44:280–289.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou XD, Wang XY, Qu FJ, Zhong YH, Lu XD,
Zhao P, Wang DH, Huang QB, Zhang L and Li XG: Detection of cancer
stem cells from the C6 glioma cell line. J Int Med Res. 37:503–510.
2009. View Article : Google Scholar : PubMed/NCBI
|