Introduction
Osteosarcoma is the most common primary malignant
bone tumor arising from long bones in children and adolescents
(1). During the past decades,
treatment strategies combining surgery, chemotherapy and in some
cases radiotherapy, have increased the 5-year survival rates of
patients with localized tumor from 20% to approximately 60%
(2). However, the prognosis of
patients with advanced osteosarcoma remains unfavorable due to
recurrence, distant metastasis and resistance to treatment
(3). Therefore, novel diagnostic
biomarkers and therapeutic targets for patients with osteosarcoma
are required.
Sex determining region Y-box protein (SOX)3 is a
member of SOX family transcription factors, which selectively
interact with the common target sequence (A/T)ACAA(A/T)G and
activate gene transcription. SOX3, together with SOX1, SOX2 and
SOX21, belongs to the subgroup of the SOX B family (4). SOX3 is believed to play a critical
role during embryonic development (5–7). In
recent years, evidence has been provided to suggest the involvement
of SOX3 in tumorigenesis. Xia et al reported that SOX3 was
able to induce the oncogenic transformation of chicken embryo
fibroblasts (8). Yang et al
and Cai et al indicated that SOX3 acted as an oncogene in
human epithelial ovarian cancer (9) and esophageal squamous cell carcinoma
(ESCC) (10). Although other
members of the SOX family, including SOX2 (11,12),
SOX9 (13) and SOX18 (14), have been found to be involved in
the development of osteosarcoma, the expression and biological
function of SOX3 in osteosarcoma remain unclear.
In this study, we demonstrated that SOX3 was
upregulated in osteosarcoma tissues in comparison with
non-cancerous bone cyst tissues. To determine whether SOX3 is
involved in the development and progression of osteosarcoma, we
carried out the functional characterization of SOX3 in human
osteosarcoma cell lines in which SOX3 was silenced. We further
investigated the mechanisms underlying the effects of SOX3
knockdown on osteosarcoma. Our results indicate that SOX3 may act
as an oncogene in osteosarcoma by regulating cell proliferation,
migration and invasion.
Materials and methods
Patients and tumor sample
preparations
This study was approved by the Ethics Committees of
Shanghai Sixth People's Hospital, Shanghai, China. Written consent
was obtained from all the enrolled patients for the use of tissue
specimens. A total of 70 patients with primary osteosarcoma and 20
patients with bone cysts admitted to Department of Orthopedic
Surgery, Shanghai Sixth People's Hospital were enrolled in this
study. All collected primary osteosarcoma and non-cancerous bone
cyst tissue samples were immediately frozen in liquid nitrogen and
stored at −80°C until use.
RNA extraction and RT-qPCR
TRIzol (Invitrogen, Carlsbad, CA, USA) was used to
extract total RNA according to the manufacturer's instructions.
Total RNA was then reverse transcribed with Moloney murine leukemia
virus reverse transcriptase (M-MLV RT; Promega, Madison, WI, USA).
Quantitative (real-time) PCR was carried out on an ABI 7300
real-time PCR machine (Applied Biosystems, Foster City, CA, USA).
The sequences of the primers used were as follows: SOX3, forward,
5′-TGAACTCAAGAACCCCGTAGG-3′ and reverse, 5′-GCTGCGTTCGCACTACTCT-3′;
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward,
5′-CACCCACTCCTCCACCTTTG-3′ and reverse, 5′-CCACCACCCTGTTGCTGTAG-3′.
The abundance of SOX3 mRNA was expressed relative to GAPDH
mRNA.
Cell culture, lentiviral production and
infection
The MG63 and U2OS osteosarcoma cells, and the 293T
cells were purchased from the American Type Culture Collection
(Rockville, MD, USA) and maintained in a humidified incubator at
37°C/5% CO2. The MG63 and 293T cells were grown in DMEM
medium with 10% fetal bovine serum (FBS), while the U2OS cells was
grown in RPMI-1640 medium (all from Invitrogen) with 10% FBS.
SOX3 shRNA (shSOX3) target sequence
(CAAGGAGTTAGTTAAATGC) and a scramble shRNA (shNC) was cloned into
the lentiviral vector PLKO.1 (Addgene, Cambridge, MA, USA).
Lentivirus was produced by transfecting the 293T cells with the
shRNA plasmids and packaging plasmids using Lipofectamine 2000
(Invitrogen) according to the manufacturer's instructions. The
viral supernatant was collected and filtered at 48–72 h following
transfection. The cells were infected with the viruses in the
presence of 8 µg/ml of Polybrene (Sigma, St. Louis, MO,
USA). A U2OS stable cell line was established using puromycin
(Sigma) selection.
Western blot analysis
Protein was extracted using ice-cold radio
immunoprecipitation assay buffer (Beyotime, Shanghai, China). Equal
amounts of protein from each sample were electrophoretically
resolved with sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and transferred onto nitrocellulose
membranes (Millipore, Bedford, MA, USA). After blocking with 5%
skim milk for 1 h at room temperature, the blots were probed with
specific primary antibodies at 4°C overnight. The protein
expression levels were determined by incubating the membranes with
horseradish peroxidase-conjugated secondary antibody (Beyotime) and
enhanced chemiluminescence reagent (Millipore). Each sample was
examined in triplicate and the protein expression levels were
expressed relative to GAPDH. Antibodies used included anti-SOX3
(Abcam, Cambridge, MA, USA; ab183606; 1:500), anti-cyclin D1
(Abcam; ab16663; 1:200), anti-Bax (Abcam; ab32503; 1:2,000),
anti-Bcl-2 (Abcam; ab692; 1:500), anti-Twist (Abcam; ab50581;
1:2,000), anti-matrix metalloproteinase-9 (MMP-9) (Abcam; ab119906;
1:500), anti-GAPDH (Cell Signaling Technology, Danvers, MA, USA;
#5174; 1:1,500), anti-Cdc25A (Cell Signaling Technology; #3652;
1:1,000), anti-proliferating cell nuclear antigen (PCNA) (Cell
Signaling Technology; #13110; 1:1,000), anti-Snail (Cell Signaling
Technology; #3879S; 1:1,000) and anti-E-cadherin (Cell Signaling
Technology; #14472; 1:1,000). Goat anti-mouse (A0216; 1:1,000) and
goat anti-rabbit (A0208; 1:1,000) secondary antibody (both from
Beyotime). The protein expression levels were determined by
incubating the membranes with horseradish peroxidase conjugated
secondary antibody (Beyotime) at room temperature for 1 h. Enhanced
chemiluminescence reagent (Millipore) was applied to examine the
protein expression. Western blot analysis was repeated three times
and quantification of the blots was performed by using ImageJ
software (NIH, Bethesda, MD, USA) with GAPDH as loading
control.
Cell proliferation assay
Cell proliferation was examined using the Cell
Counting kit-8 CCK-8; Beyotime) following the manufacturer's
instructions. Briefly, the MG63 or U2OS cells were plated in
96-well plates (3×103 cells/well). Following overnight
incubation, the cells were infected with shNC or shSOX3 lentivirus.
Cell proliferation was determined every 24 h with CCK-8 solution.
Each experiment was performed in triplicate.
Tumor xenograft model
All animal experiments were approved by the Ethics
Committee of the Department of Orthopedic Surgery, Shanghai Sixth
People's Hospital. A total of 12 female athymic nude mice (4 to 5
weeks old, 15–20 g) (SLAC Animal, Shanghai, China) was used in this
study. U2OS stable cells (2×106) were injected
subcutaneously into the left flanks (six mice were injected with
shNC-transfected cells and six mice were injected with
shSOX3-transfected cells. Tumors were measured every 3 days using a
Vernier caliper, and tumor volumes were calculated using the
following formula: V=0.5 × (length × width2). At 24 days
following implantation, all mice were sacrificed, and the tumors
were isolated, weighed and protein expression in the tumor samples
was examined by western blot analysis.
Cell cycle distribution analysis
At 24 h following infection, the cells were
harvested and washed with phosphate-buffered saline (PBS), followed
by fixation with ice-cold 70% ethanol at −20°C overnight. After
washing with PBS, the cells were resus-pended in PBS containing
0.05 mg/ml propidium iodide (PI; Sigma) and 100 U/ml RNase A in the
dark at room temperature for 30 min. Samples were analyzed for DNA
content on a flow cytometer (BD Biosciences, San Jose, CA,
USA).
Analysis of cell apoptosis
At 24 h following infection, the cells were
harvested and washed with PBS, followed by staining with the
Annexin V-fluorescein isothiocyanate (FITC)/PI apoptosis assay kit
(Beyotime) as instructed by the manufacturer. Samples were analyzed
for cell apoptosis on a flow cytometer. Cells undergoing early and
late apoptosis were defined by Annexin V+/PI−
staining and Annexin V+/PI+ staining,
respectively.
Cell migration and invasion assays
Cell migration assays were performed in Boyden
chambers (8-µm pore size; Corning, Corning, NY, USA). The
cells (5×104 cells/well) transfected with the shRNAs in
serum-free medium were seeded into the upper chamber. Medium with
10% FBS was added to the lower chamber. Following 24 h of
incubation, the cells in the upper chamber were completely removed
with a cotton swab. The cells attached to the bottom of the
membranes were fixed with 4% paraformaldehyde and stained with 0.5%
crystal violet. The migrated cells were counted in 5 randomly
selected fields (×200) under a microscope (Nikon, Tokyo,
Japan).
Cell invasion assays were performed in the same
manner as the migration assay, with the difference that the upper
chamber was pre-coated with 30 µl Matrigel (BD Biosciences).
Experiments were performed in triplicate.
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism software (version 6.0; GraphPad Prism Software, San Diego,
CA, USA). The Student's t-test was used for statistical analysis
between 2 independent groups. P-values <0.05 were considered to
indicate statistically significant differences.
Results
Upregulation of SOX3 in human
osteosarcoma tissues
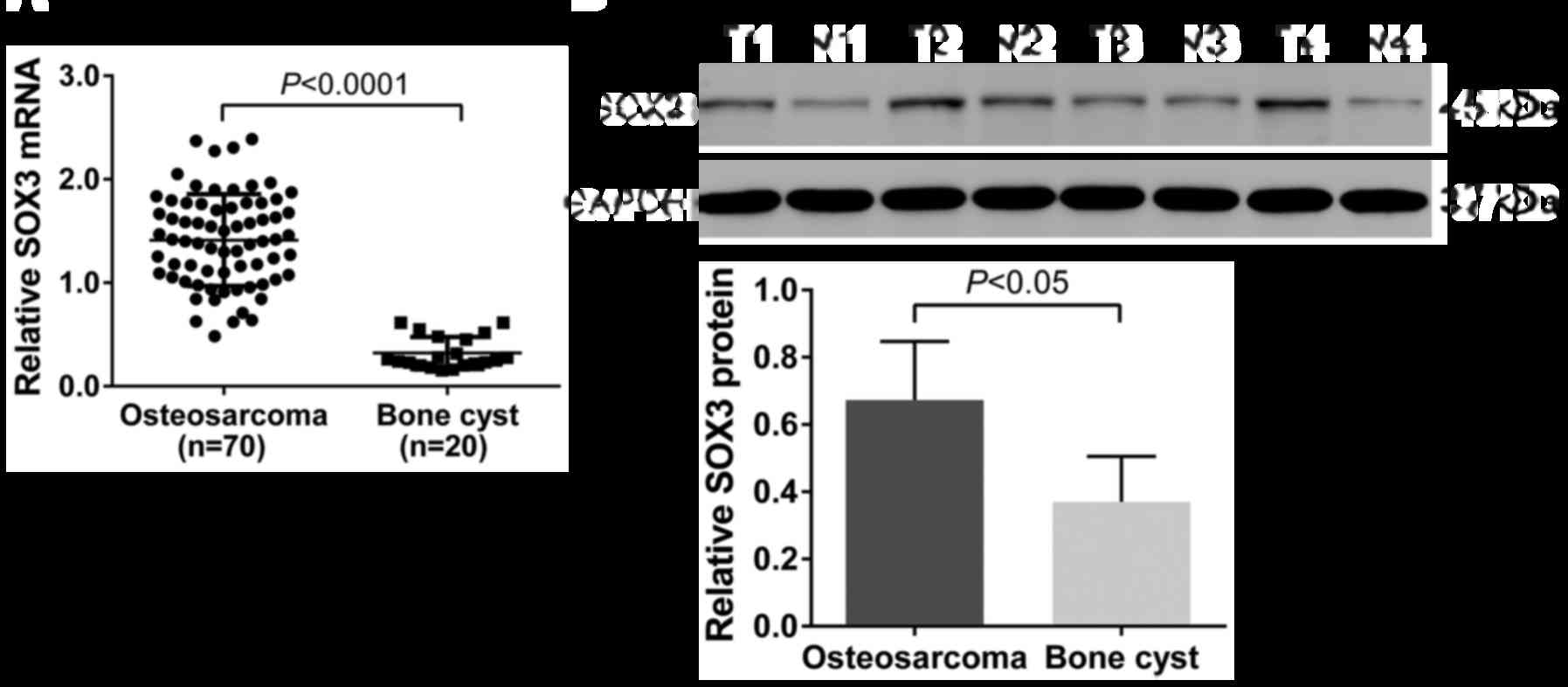
As shown in Fig.
1A, the relative level of SOX3 mRNA in the osteosarcoma tissues
(1.41±0.05) was significantly higher than that in non-cancerous
bone cyst tissues (0.32±0.03; P<0.0001). Similar results were
obtained at the protein level (osteosarcoma tissues, 0.67±0.09;
bone cyst tissues, 0.37±0.07; P<0.05; Fig. 1B).
SOX3 knockdown inhibits the proliferation
of osteosarcoma cells in vitro
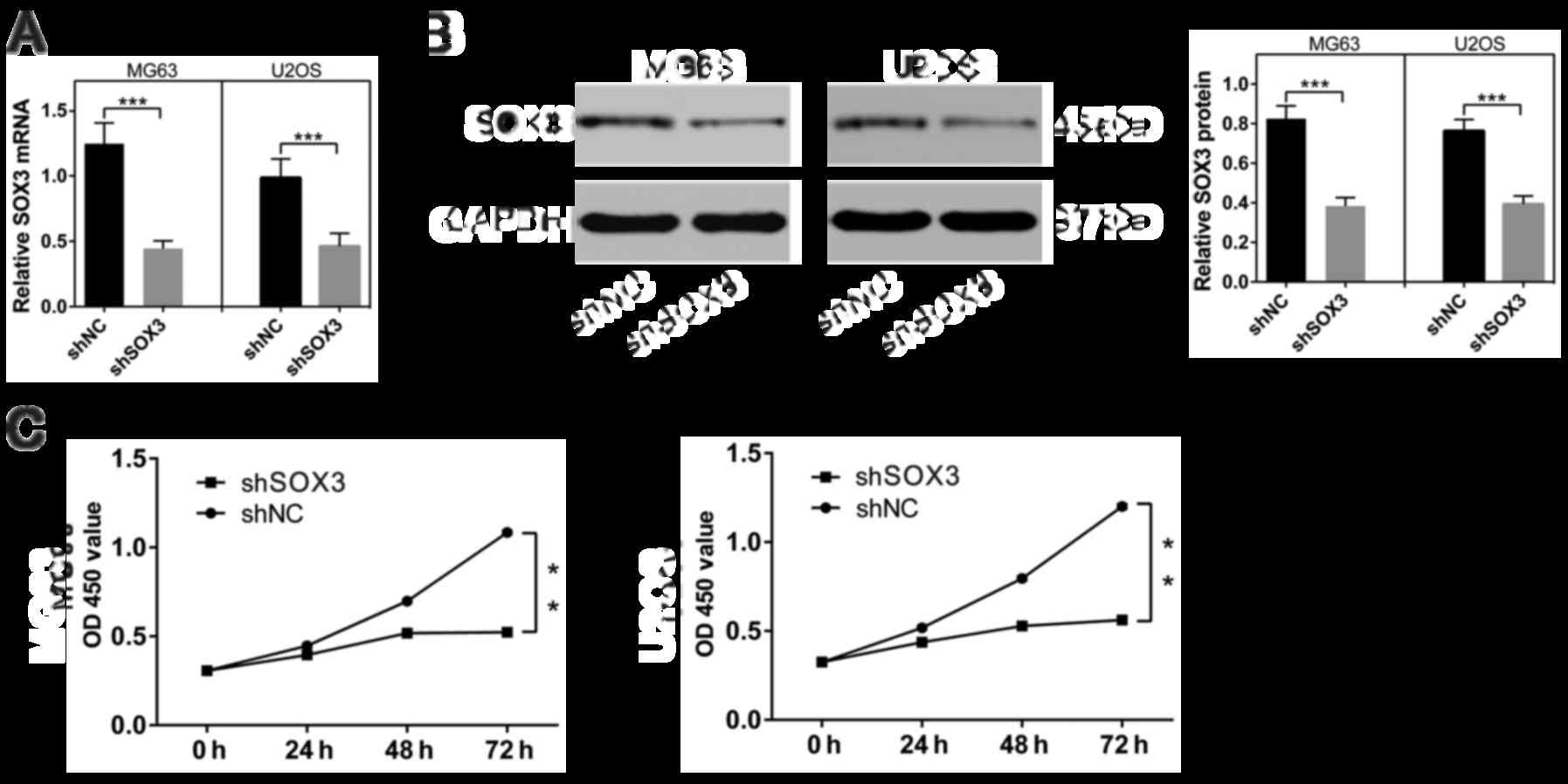
To perform thefunctional analysis of SOX3, we
knocked down SOX3 expression in two human osteosarcoma cells (MG63
and U2OS) by using a lentivirus system. At 48 h following
infection, the effects of shSOX3 on endogenous SOX3 expression were
evaluated. As shown in Fig. 2A and
B, SOX3 mRNA and protein expression was markedly inhibited due
to shSOX3 infection in both osteosarcoma cells (P<0.001).
To determine the proliferation rates following the
downregulation of SOX3 in the osteosarcoma cell lines, CCK-8 assays
were performed over a 3-day period. We found that both cell lines
in which SOX3 was silenced exhibited significant growth inhibition
compared with the shNC-infected cells (P<0.01; Fig. 2C).
SOX3 knockdown suppresses tumor growth in
a xenograft mouse model
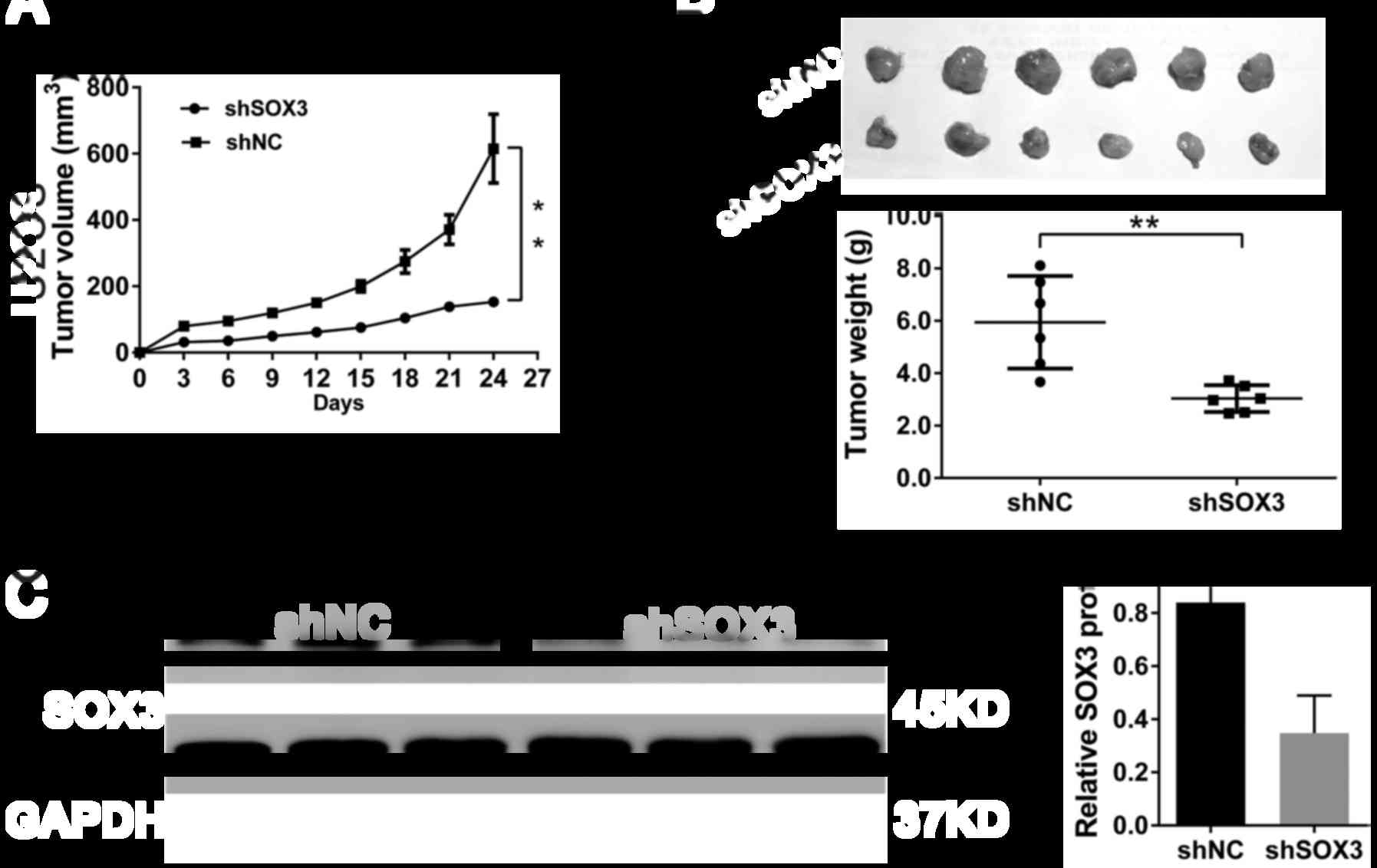
To investigate whether SOX3 knockdown in
osteosarcoma cells suppresses tumor growth in vivo, U2OS
cells stably transfected with shSOX3 or shNC were subcutaneously
implanted into nude mice. As shown in Fig. 3A, the growth rates of xenografts
formed from shSOX3 stably transfected cells were much slower than
those from shNC cells (P<0.01). After 24 days, the volumes and
weights of the tumors formed from shSOX3-transfefed cells were
significantly decreased compared with those of tumors derived from
shNC-transfected cells (P<0.01; Fig. 3B). Moreover, SOX3 protein
expression decreased by 58.5% in the xenografts formed from
shSOX3-transfected cells, as compared to the xenografts formed from
shNC-transfected cells (P<0.01; Fig. 3C). These data suggested that the
knockdown of SOX3 inhibited tumor growth in nude mice.
SOX3 knockdown induces G1 phase arrest of
osteosarcoma cells
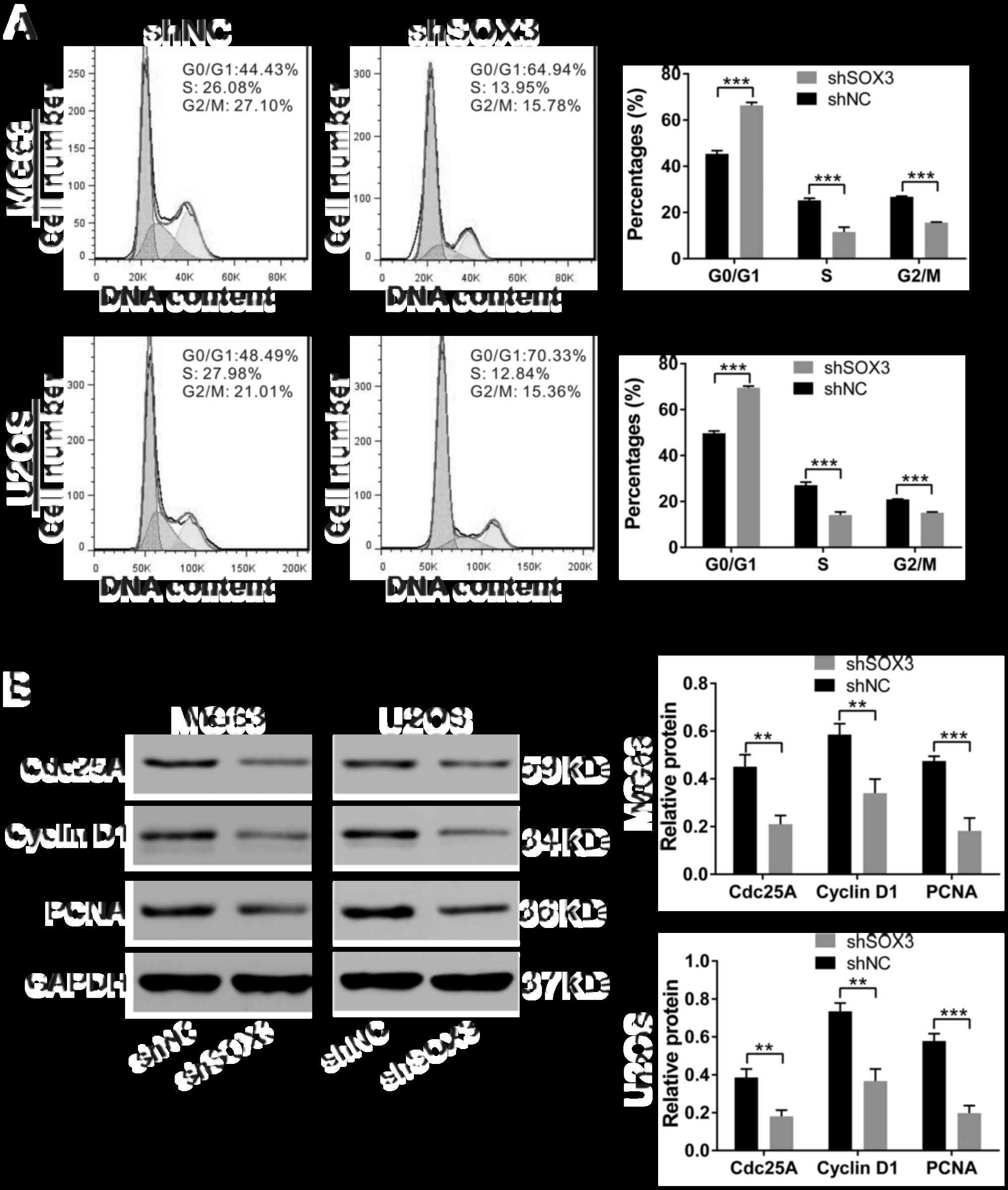
To clarify the mechanisms underlying the growth
inhibitory effects of the knockdown of SOX3 by shRNA on
osteosarcoma cell lines, cell cycle distribution was analyzed by
flow cytometry in the cells stained with PI (Fig. 4A). At 24 h following infection, a
higher proportion of shSOX3-infected cells was observed in the G1
phase (MG63, 66.34±1.29%; U2OS, 49.68±1.04%; P<0.001) in
comparison with that of shNC-infected cells (MG63, 45.40±1.39%;
U2OS, 69.52±0.72%). Concomitant decreases were observed in the
proportions of cells in S and G2/M phases.
To explore the potential molecular mechanisms
responsible for shSOX3-induced G1 arrest in osteosarcoma cells, we
explored the effects of shSOX3 on the expression of cell
cycle-regulated proteins. Western blot analysis revealed that the
protein levels of Cdc25A, cyclin D1 and PCNA were significantly
decreased in the shSOX3-infected cells, compared with the
shNC-infected cells (Fig. 4B).
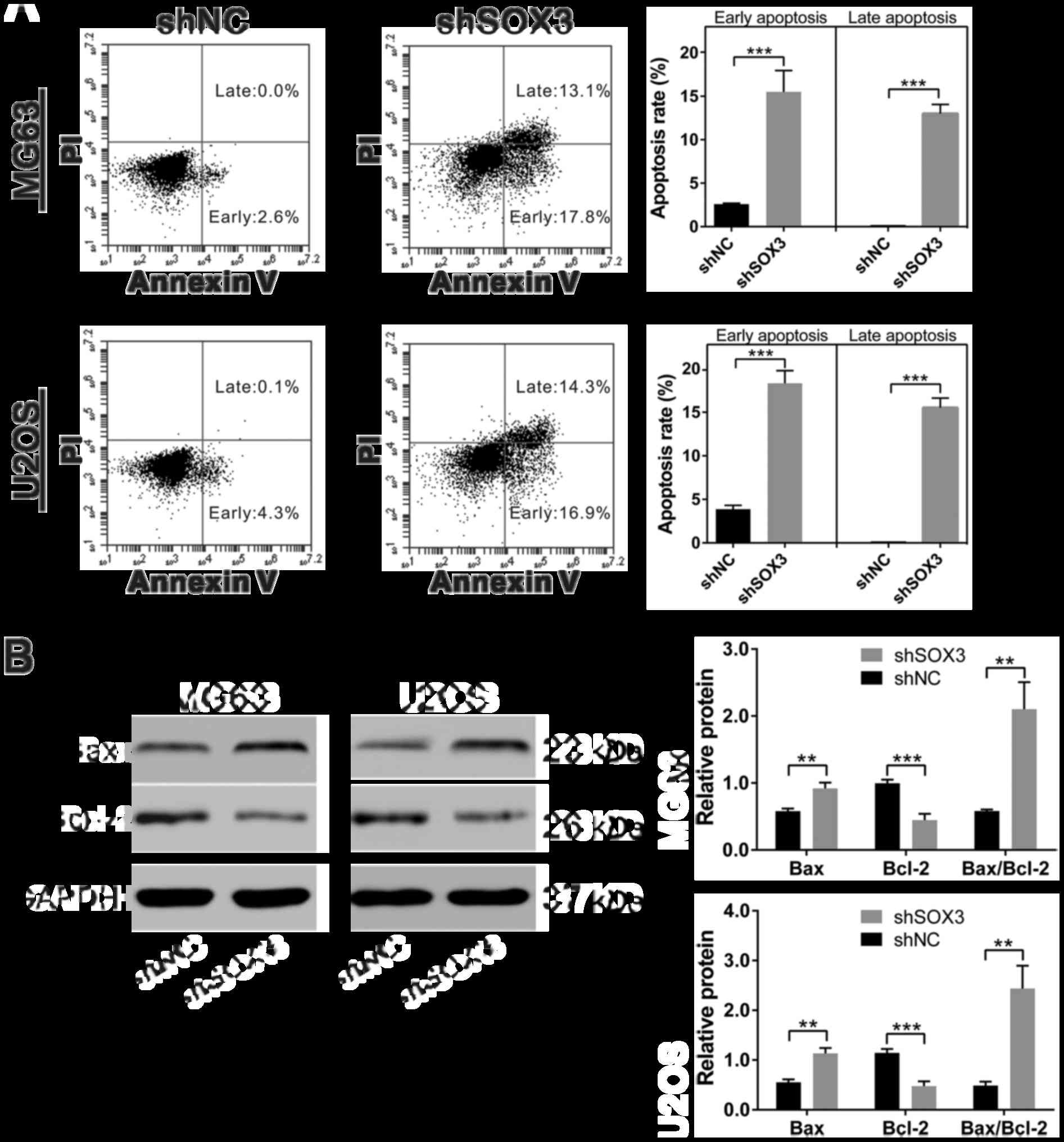
SOX3 knockdown induces the apoptosis of
osteosarcoma cells
To determine whether apoptosis occurred in
shSOX3-infected cells, we performed flow cytometry analysis on the
cells stained with Annexin V/PI. As shown in Fig. 5A, a significant increase in the
percentages of cells undergoing apoptosis was observed in the MG63
cells in which SOX2 was knocked down (15.50±2.46 and 13.00±1.05%
for early and late apoptosis, respectively) compared with the
shNC-infected cells (2.60±0.10 and 0.03±0.06% for early and late
apoptosis, respectively). Similar results were obtained in the U2OS
cells.
To explore the possible molecular mechanisms
responsible for the shSOX3-induced apoptosis of osteosarcoma cells,
we examined the effects of the silencing of SOX3 by shSOX3 on the
expression of cell apoptosis-related proteins. Western blot
analysis revealed that the protein levels of Bax, a pro-apoptotic
protein (15), were significantly
increased in the shSOX3-infected cells, compared with the
shNC-infected cells, whereas the expression of Bcl-2, an
anti-apoptotic protein (15), was
decreased in the shSOX3-infected cells compared with the control
cells (Fig. 5B).
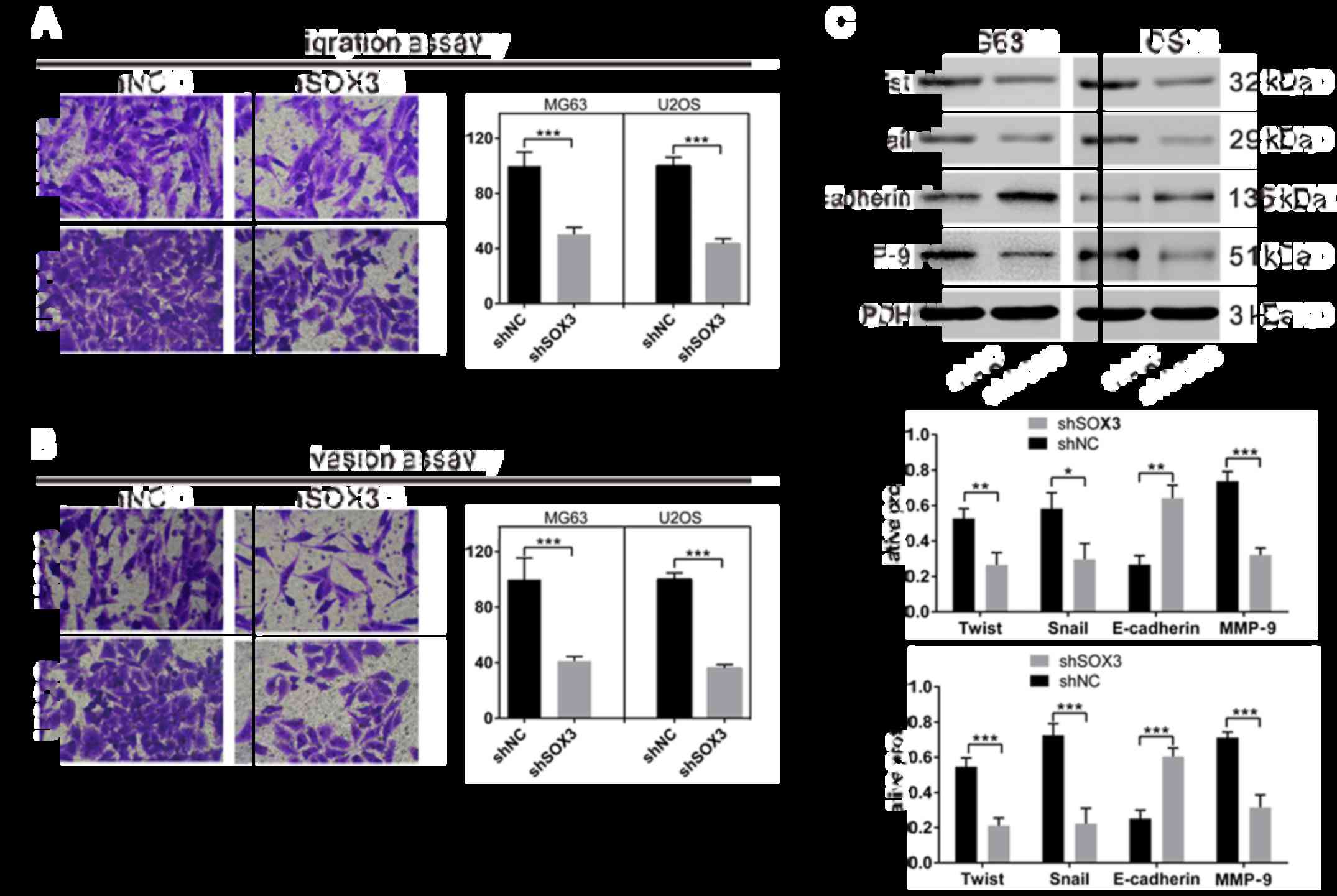
SOX3 knockdown suppresses the migration
and invasion of osteosarcoma cells
To determine whether SOX3 affects the migration and
invasion of osteosarcoma cells, Transwell assay was performed
(Fig. 6A). The shSOX3-infected
cells (both cell lines) exhibited a significant decrease in
migration and invasion compared with the shNC-infected cells.
It has been noted that epithelial-mesenchymal
transition (EMT) is a critical event during tumor invasion and
metastasis (16). The effect of
SOX3 knockdown on the expression of EMT-related proteins was also
explored. The protein levels of EMT-promoting proteins [Twist,
Snail and MMP-9 (17)] were
significantly decreased in the cells in which SOX3 was knocked
down, whereas the expression of the main factor of EMT [E-cadherin
(17)] was markedly elevated in
comparison with the shNC-infected cells.
Discussion
Previous studies have reported that SOX3 expression
is often increased in human esophageal squamous cell carcinoma
(ESCC) (18) and epithelial
ovarian cancer (9). In the current
study, we found that SOX3 expression was upregulated in
osteosarcoma, suggesting the oncogenic role of SOX3. Furthermore,
we performed the functional characterization of SOX3 in
osteosarcoma cell lines by lentivirus-mediated RNA interference.
Previous studies have suggested the promoting effect of SOX3 on the
proliferation of ESCC (10) and
epithelial ovarian cancer cell lines (9). In this study, we found that the
silencing of SOX3 inhibited cell proliferation, migration and
invasion. SOX3 plays an oncogenic role and may be a potential
target for osteosarcoma treatment.
In general, deregulated cell cycle and apoptosis are
main causes for uncontrolled proliferation (19). Several members of SOX family
proteins, including SOX2 (20),
SOX7 (21) and SOX21 (22), have been shown to regulate cell
cycle and apoptosis in diverse cell lines. In this study, flow
cytometric analysis revealed that SOX3 knockdown in osteosarcoma
cells induced G1 phase arrest and apoptosis. We demonstrated that
decreased protein levels of Cdc25A, cyclin D1, PCNA and Bcl-2, and
an increased protein level of Bax were associated with SOX3
knockdown. These results were consistent with the findings of G1
phase arrest and increased cell apoptosis observed in the
shSOX3-infected osteosarcoma cells. It has been reported that Src
(23) activates the transcription
of cyclin D1. Cyclin D1 (24) and
Cdc25A (25) are direct
TCF/β-catenin transcriptional targets. SOX3 targets Src kinase in
epithelial ovarian cancer cell lines (9), whereas the overexpression of
Xenopus SOX3 was found to inhibit β-catenin activity
(26,27). Thus, further studies are warranted
to whether SOX3 functions in the cell cycle through Src or Wnt
signals in osteosarcoma cells.
We further elucidated that SOX3 knockdown suppressed
cell migration and invasion. We demonstrated that SOX3 knockdown
led to decreased protein levels of Twist, Snail and MMP-9, and
increased protein level of E-cadherin. It has been reported that
other SOX family members, such as SOX2 and SOX4, promote cell
migration and invasion in breast, prostate and liver cancer cells
via regulating EMT (28–31). SOX family members have been shown
to regulate the expression of EMT-related protein, including Snail,
ZEB1, Twist and E-cadherin (28,32,33),
being consistent with our finding. In chicken embryo, SOX3 is found
to repress Snail expression (7).
The discrepancy between this finding and our data may be due to
different species and biology processes. Further studies are
required to clarify the mechanism how SOX3 regulates the expression
of EMT-related proteins.
In conclusion, we revealed that SOX3 expression was
frequently upregulated in osteosarcoma. Moreover, we also showed
that silencing of SOX3 expression inhibited cell proliferation
through cell cycle arrest and apoptosis in osteosarcoma cells, as
well as suppressed cell migration and invasion. To the best of our
knowledge, we have demonstrated, for the first time, SOX3 acts as
an oncogene in osteosarcoma, and SOX3 inhibitors or downstream
effectors may be interesting targets for osteosarcoma therapy.
Acknowledgments
Not applicable.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
YG and XY conceived and designed the study. YG, JY
and MT performed the experiments. YG, JY and XY wrote the
manuscript. All authors read and approved the manuscript.
[4] Ethics
approval and consent to participate
For the use of human sample, approval was obtained
by the Ethics Committees of Shanghai Sixth People's Hospital.
Written consent was obtained from all enrolled patients for the use
of tissue specimens. All animal experiments were approved by the
Ethics Committee of Department of Orthopedic Surgery, Shanghai
Sixth People's Hospital.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Rabinowicz R, Barchana M, Liphshiz I,
Futerman B, Linn S and Weyl-Ben-Arush M: Cancer incidence and
survival among children and adolescents in Israel during the years
1998 to 2007. J Pediatr Hematol Oncol. 34:421–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarkar A and Hochedlinger K: The sox
family of transcription factors: Versatile regulators of stem and
progenitor cell fate. Cell Stem Cell. 12:15–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alatzoglou KS, Azriyanti A, Rogers N, Ryan
F, Curry N, Noakes C, Bignell P, Hall GW, Littooij AS, Saunders D,
et al: SOX3 deletion in mouse and human is associated with
persistence of the craniopharyngeal canal. J Clin Endocrinol Metab.
99:E2702–E2708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Archer TC, Jin J and Casey ES: Interaction
of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Dev Biol.
350:429–440. 2011. View Article : Google Scholar :
|
|
7
|
Acloque H, Ocaña OH, Matheu A, Rizzoti K,
Wise C, Lovell-Badge R and Nieto MA: Reciprocal repression between
Sox3 and snail transcription factors defines embryonic territories
at gastrulation. Dev Cell. 21:546–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia Y, Papalopulu N, Vogt PK and Li J: The
oncogenic potential of the high mobility group box protein Sox3.
Cancer Res. 60:6303–6306. 2000.PubMed/NCBI
|
|
9
|
Yang J, Yuan D, Li J, Zheng S and Wang B:
miR-186 downregulates protein phosphatase PPM1B in bladder cancer
and mediates G1-S phase transition. Tumour Biol. 37:4331–4341.
2016. View Article : Google Scholar
|
|
10
|
Cai QY, Liang GY, Zheng YF, Tan QY, Wang
RW and Li K: Sox3 silencing inhibits metastasis and growth of
esophageal squamous cell carcinoma cell via Downregulating GSK-3β.
Int J Clin Exp Pathol. 9:2939–2949. 2016.
|
|
11
|
Basu-Roy U, Seo E, Ramanathapuram L, Rapp
TB, Perry JA, Orkin SH, Mansukhani A and Basilico C: Sox2 maintains
self renewal of tumor-initiating cells in osteosarcomas. Oncogene.
31:2270–2282. 2012. View Article : Google Scholar
|
|
12
|
Yang C, Hou C, Zhang H, Wang D, Ma Y,
Zhang Y, Xu X, Bi Z and Geng S: miR-126 functions as a tumor
suppressor in osteosarcoma by targeting Sox2. Int J Mol Sci.
15:423–437. 2013. View Article : Google Scholar
|
|
13
|
Zhu H, Tang J, Tang M and Cai H:
Upregulation of SOX9 in osteosarcoma and its association with tumor
progression and patients' prognosis. Diagn Pathol. 8:1832013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Z, Liu J, Wang J and Zhang F: SOX18
knockdown suppresses the proliferation and metastasis, and induces
the apoptosis of osteosarcoma cells. Mol Med Rep. 13:497–504. 2016.
View Article : Google Scholar
|
|
15
|
Oltval ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar
|
|
16
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li K, Wang RW, Jiang YG, Zou YB and Guo W:
Overexpression of Sox3 is associated with diminished prognosis in
esophageal squamous cell carcinoma. Ann Surg Oncol. 20(Suppl 3):
S459–S466. 2013. View Article : Google Scholar
|
|
19
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Otsubo T, Akiyama Y, Yanagihara K and
Yuasa Y: SOX2 is frequently downregulated in gastric cancers and
inhibits cell growth through cell-cycle arrest and apoptosis. Br J
Cancer. 98:824–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Huang S, Dong W, Li L, Feng Y,
Pan L, Han Z, Wang X, Ren G, Su D, et al: SOX7, downregulated in
colorectal cancer, induces apoptosis and inhibits proliferation of
colorectal cancer cells. Cancer Lett. 277:29–37. 2009. View Article : Google Scholar
|
|
22
|
Ferletta M, Caglayan D, Mokvist L, Jiang
Y, Kastemar M, Uhrbom L and Westermark B: Forced expression of
Sox21 inhibits Sox2 and induces apoptosis in human glioma cells.
Int J Cancer. 129:45–60. 2011. View Article : Google Scholar
|
|
23
|
Lee RJ, Albanese C, Stenger RJ, Watanabe
G, Inghirami G, Haines GK III, Webster M, Muller WJ, Brugge JS,
Davis RJ, et al: pp60(v-src) induction of cyclin D1 requires
collaborative interactions between the extracellular
signal-regulated kinase, p38, and Jun kinase pathways. A role for
cAMP response element-binding protein and activating transcription
factor-2 in pp60(v-src) signaling in breast cancer cells. J Biol
Chem. 274:7341–7350. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vijayakumar S, Liu G, Rus IA, Yao S, Chen
Y, Akiri G, Grumolato L and Aaronson SA: High-frequency canonical
Wnt activation in multiple sarcoma subtypes drives proliferation
through a TCF/β-catenin target gene, CDC25A. Cancer Cell.
19:601–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kormish JD, Sinner D and Zorn AM:
Interactions between SOX factors and Wnt/β-catenin signaling in
development and disease. Dev Dyn. 239:56–68. 2010.
|
|
27
|
Zorn AM, Barish GD, Williams BO, Lavender
P, Klymkowsky MW and Varmus HE: Regulation of Wnt signaling by Sox
proteins: XSox17 alpha/beta and XSox3 physically interact with
beta-catenin. Mol Cell. 4:487–498. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T,
Liu Y, Li X, Xiang R and Li N: SOX2 promotes tumor metastasis by
stimulating epithelial-to-mesenchymal transition via regulation of
WNT/β-catenin signal network. Cancer Lett. 336:379–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
SOX2 promotes the migration and invasion of laryngeal cancer cells
by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep.
31:2651–2659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
Overexpression of SOX2 promotes migration, invasion, and
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in laryngeal cancer Hep-2 cells. Tumour Biol. 35:7965–7973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jafarnejad SM, Wani AA, Martinka M and Li
G: Prognostic significance of Sox4 expression in human cutaneous
melanoma and its role in cell migration and invasion. Am J Pathol.
177:2741–2752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parvani JG and Schiemann WP: Sox4, EMT
programs, and the metastatic progression of breast cancers:
Mastering the masters of EMT. Breast Cancer Res. 15:R722013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guan Z, Zhang J, Wang J, Wang H, Zheng F,
Peng J, Xu Y, Yan M, Liu B, Cui B, et al: SOX1 Downregulates
β-catenin and reverses malignant phenotype in nasopharyngeal
carcinoma. Mol Cancer. 13:2572014. View Article : Google Scholar
|