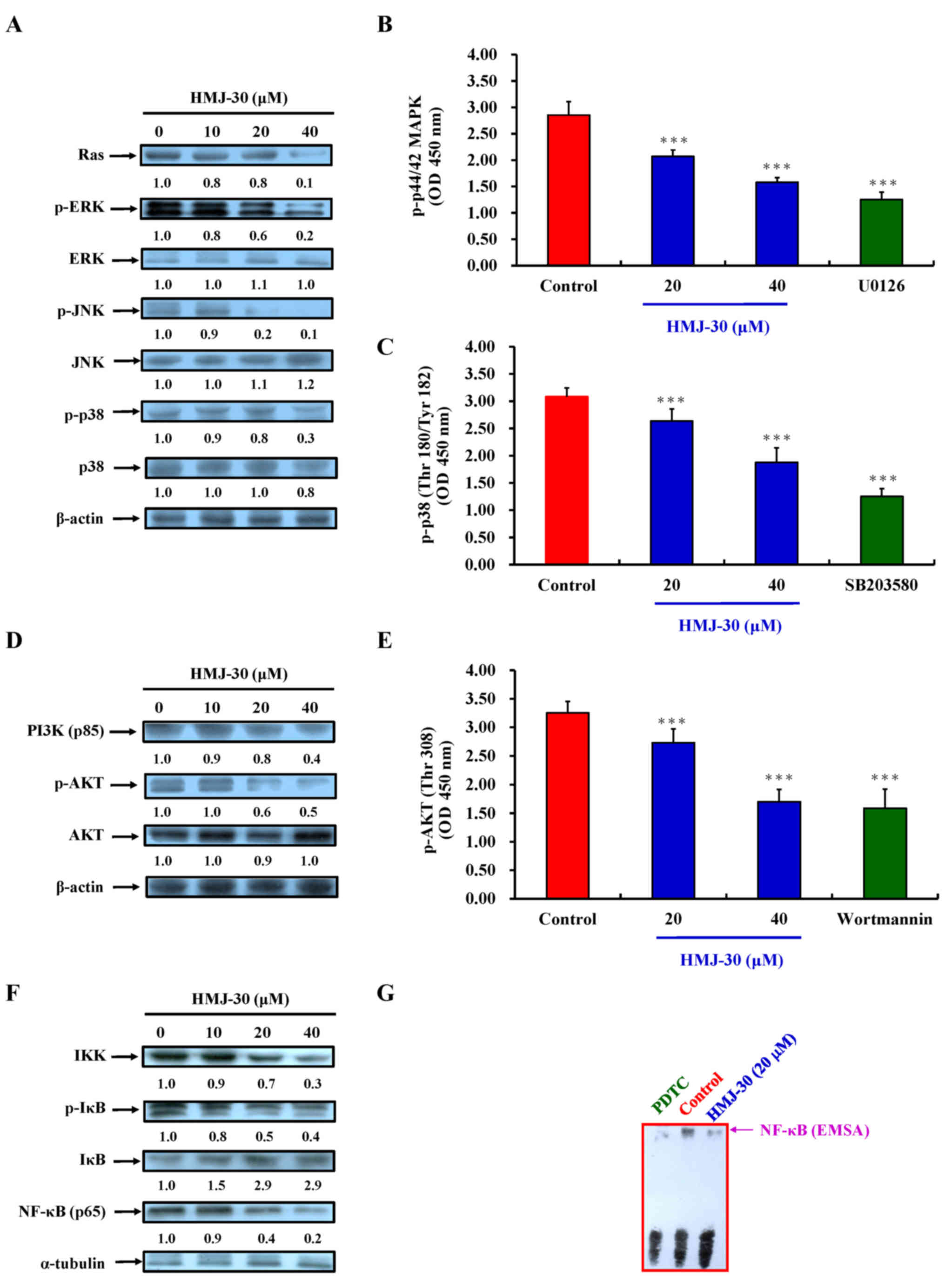
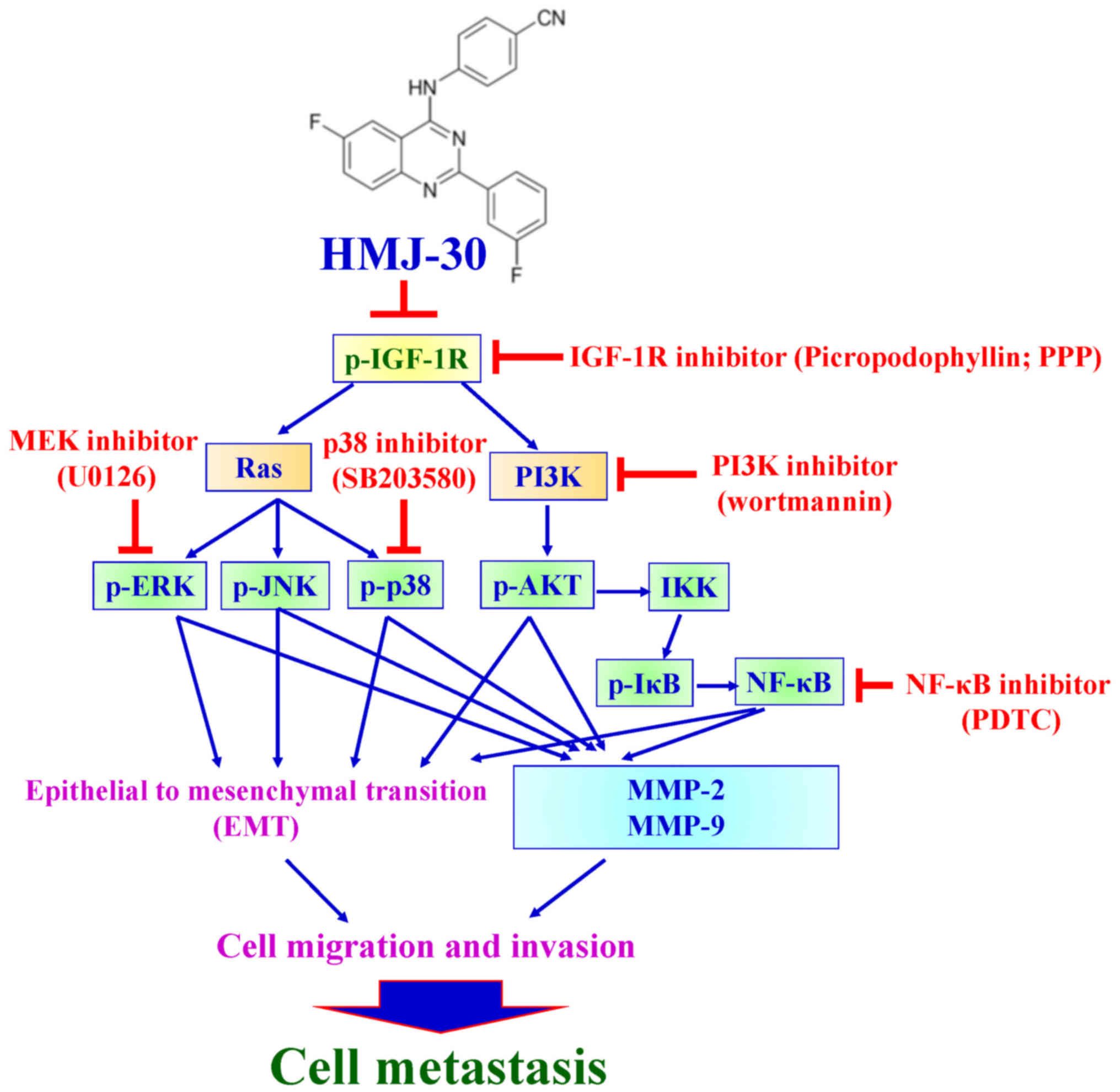
|
1
|
Arndt CA and Crist WM: Common
musculoskeletal tumors of childhood and adolescence. N Engl J Med.
341:342–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: Current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Day K and Gorlick R: Novel therapeutic
agents for osteosarcoma. Expert Rev Anticancer Ther. 9:511–523.
2009. View Article : Google Scholar
|
|
4
|
Wells A: Tumor invasion: Role of growth
factor-induced cell motility. Adv Cancer Res. 78:31–101. 2000.
View Article : Google Scholar
|
|
5
|
Duan Z, Choy E, Harmon D, Yang C, Ryu K,
Schwab J, Mankin H and Hornicek FJ: Insulin-like growth factor-I
receptor tyrosine kinase inhibitor cyclolignan picropodophyllin
inhibits proliferation and induces apoptosis in multidrug resistant
osteosarcoma cell lines. Mol Cancer Ther. 8:2122–2130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Gibson Wood W, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ullrich A, Gray A, Tam AW, Yang-Feng T,
Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, et
al: Insulin-like growth factor I receptor primary structure:
Comparison with insulin receptor suggests structural determinants
that define functional specificity. EMBO J. 5:2503–2512.
1986.PubMed/NCBI
|
|
8
|
Zwick E, Bange J and Ullrich A: Receptor
tyrosine kinase signalling as a target for cancer intervention
strategies. Endocr Relat Cancer. 8:161–173. 2001. View Article : Google Scholar
|
|
9
|
Rowlands MA, Gunnell D, Harris R, Vatten
LJ, Holly JM and Martin RM: Circulating insulin-like growth factor
peptides and prostate cancer risk: A systematic review and
meta-analysis. Int J Cancer. 124:2416–2429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurmasheva RT and Houghton PJ: IGF-I
mediated survival pathways in normal and malignant cells. Biochim
Biophys Acta. 1766:1–22. 2006.PubMed/NCBI
|
|
11
|
Conover CA: Insulin-like growth
factor-binding proteins and bone metabolism. Am J Physiol
Endocrinol Metab. 294:E10–E14. 2008. View Article : Google Scholar
|
|
12
|
Johnson LC: A general theory of bone
tumors. Bull N Y Acad Med. 29:164–171. 1953.PubMed/NCBI
|
|
13
|
Raile K, Höflich A, Kessler U, Yang Y,
Pfuender M, Blum WF, Kolb H, Schwarz HP and Kiess W: Human
osteosarcoma (U-2 OS) cells express both insulin-like growth
factor-I (IGF-I) receptors and insulin-like growth
factor-II/mannose-6-phosphate (IGF-II/M6P) receptors and synthesize
IGF-II: Autocrine growth stimulation by IGF-II via the IGF-I
receptor. J Cell Physiol. 159:531–541. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YH, Wang ZX, Qiu Y, Xiong J, Chen YX,
Miao DS and De W: Lentivirus-mediated RNAi knockdown of
insulin-like growth factor-1 receptor inhibits growth, reduces
invasion, and enhances radiosensitivity in human osteosarcoma
cells. Mol Cell Biochem. 327:257–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burrow S, Andrulis IL, Pollak M and Bell
RS: Expression of insulin-like growth factor receptor, IGF-1, and
IGF-2 in primary and metastatic osteosarcoma. J Surg Oncol.
69:21–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benini S, Baldini N, Manara MC, Chano T,
Serra M, Rizzi S, Lollini PL, Picci P and Scotlandi K: Redundancy
of autocrine loops in human osteosarcoma cells. Int J Cancer.
80:581–588. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kappel CC, Velez-Yanguas MC, Hirschfeld S
and Helman LJ: Human osteosarcoma cell lines are dependent on
insulin-like growth factor I for in vitro growth. Cancer Res.
54:2803–2807. 1994.PubMed/NCBI
|
|
18
|
Pinski J, Schally AV, Halmos G, Szepeshazi
K and Groot K: Somatostatin analog RC-160 inhibits the growth of
human osteosarcomas in nude mice. Int J Cancer. 65:870–874. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mansky PJ, Liewehr DJ, Steinberg SM,
Chrousos GP, Avila NA, Long L, Bernstein D, Mackall CL, Hawkins DS
and Helman LJ: Treatment of metastatic osteosarcoma with the
somatostatin analog OncoLar: Significant reduction of insulin-like
growth factor-1 serum levels. J Pediatr Hematol Oncol. 24:440–446.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yee D: Targeting insulin-like growth
factor pathways. Br J Cancer. 94:465–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boyer B, Vallés AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial - mesenchymal
and mesenchymal - epithelial transitions in carcinoma progression.
J Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi
K and Takahashi Y: Mesenchymal-epithelial transition during somitic
segmentation is regulated by differential roles of Cdc42 and Rac1.
Dev Cell. 7:425–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michael JP: Quinoline, quinazoline and
acridone alkaloids. Nat Prod Rep. 25:166–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang SH, Rait A, Pirollo KF, Zhou Q,
Yenugonda VM, Chinigo GM, Brown ML and Chang EH: Tumor-targeting
nanodelivery enhances the anticancer activity of a novel
quinazolinone analogue. Mol Cancer Ther. 7:559–568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plé PA, Green TP, Hennequin LF, Curwen J,
Fennell M, Allen J, Lambert-Van Der, Brempt C and Costello G:
Discovery of a new class of anilinoquinazoline inhibitors with high
affinity and specificity for the tyrosine kinase domain of c-Src. J
Med Chem. 47:871–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hennequin LF, Stokes ES, Thomas AP,
Johnstone C, Plé PA, Ogilvie DJ, Dukes M, Wedge SR, Kendrew J and
Curwen JO: Novel 4-anilinoquinazolines with C-7 basic side chains:
Design and structure activity relationship of a series of potent,
orally active, VEGF receptor tyrosine kinase inhibitors. J Med
Chem. 45:1300–1312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Li S, Hu YP, Wang J, Hauser J,
Conway AN, Vinci MA, Humphrey L, Zborowska E, Willson JK, et al:
Blockade of EGFR and ErbB2 by the novel dual EGFR and ErbB2
tyrosine kinase inhibitor GW572016 sensitizes human colon carcinoma
GEO cells to apoptosis. Cancer Res. 66:404–411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Obaid AM, Abdel-Hamide SG, El-Kashef
HA, Abdel-Aziz AA, El-Azab AS, Al-Khamees HA and El-Subbagh HI:
Substituted quinazolines, part 3. Synthesis, in vitro antitumor
activity and molecular modeling study of certain
2-thieno-4(3H)-quinazolinone analogs. Eur J Med Chem. 44:2379–2391.
2009. View Article : Google Scholar
|
|
31
|
Ciardiello F, Caputo R, Bianco R, Damiano
V, Pomatico G, De Placido S, Bianco AR and Tortora G: Antitumor
effect and potentiation of cytotoxic drugs activity in human cancer
cells by ZD-1839 (Iressa), an epidermal growth factor
receptor-selective tyrosine kinase inhibitor. Clin Cancer Res.
6:2053–2063. 2000.PubMed/NCBI
|
|
32
|
Lu CC, Chen HP, Chiang JH, Jin YA, Kuo SC,
Wu TS, Hour MJ, Yang JS and Chiu YJ: Quinazoline analog HMJ-30
inhibits angiogenesis: Involvement of endothelial cell apoptosis
through ROS-JNK-mediated death receptor 5 signaling. Oncol Rep.
32:597–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiu YJ, Hour MJ, Lu CC, Chung JG, Kuo SC,
Huang WW, Chen HJ, Jin YA and Yang JS: Novel quinazoline HMJ-30
induces U-2 OS human osteogenic sarcoma cell apoptosis through
induction of oxidative stress and up-regulation of ATM/p53
signaling pathway. J Orthop Res. 29:1448–1456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsai YF, Huang CW, Chiang JH, Tsai FJ, Hsu
YM, Lu CC, Hsiao CY and Yang JS: Gadolinium chloride elicits
apoptosis in human osteosarcoma U-2 OS cells through extrinsic
signaling, intrinsic pathway and endoplasmic reticulum stress.
Oncol Rep. 36:3421–3426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen HJ, Lin CM, Lee CY, Shih NC, Peng SF,
Tsuzuki M, Amagaya S, Huang WW and Yang JS: Kaempferol suppresses
cell metastasis via inhibition of the ERK-38-JNK and AP-1 signaling
pathways in U-2 OS human osteosarcoma cells. Oncol Rep. 30:925–932.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chueh FS, Chen YY, Huang AC, Ho HC, Liao
CL, Yang JS, Kuo CL and Chung JG: Bufalin-inhibited migration and
invasion in human osteosarcoma U-2 OS cells is carried out by
suppression of the matrix metalloproteinase-2, ERK, and JNK
signaling pathways. Environ Toxicol. 29:21–29. 2014. View Article : Google Scholar
|
|
37
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG, et al: Antitumor effects
of emodin on LS1034 human colon cancer cells in vitro and in vivo:
Roles of apoptotic cell death and LS1034 tumor xenografts model.
Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu SH, Hang LW, Yang JS, Chen HY, Lin HY,
Chiang JH, Lu CC, Yang JL, Lai TY, Ko YC, et al: Curcumin induces
apoptosis in human non-small cell lung cancer NCI-H460 cells
through ER stress and caspase cascade- and mitochondria-dependent
pathways. Anticancer Res. 30:2125–2133. 2010.PubMed/NCBI
|
|
39
|
Lee MR, Lin C, Lu CC, Kuo SC, Tsao JW,
Juan YN, Chiu HY, Lee FY, Yang JS and Tsai FJ: YC-1 induces
G0/G1phase arrest and mitochondria-dependent apoptosis in
cisplatin-resistant human oral cancer CAR cells. Biomedicine
(Taipei). 7:122017. View Article : Google Scholar
|
|
40
|
Yang JS, Lin CA, Lu CC, Wen YF, Tsai FJ
and Tsai SC: Carboxamide analog ITR-284 evokes apoptosis and
inhibits migration ability in human lung adenocarcinoma A549 cells.
Oncol Rep. 37:1786–1792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsai SC, Tsai MH, Chiu CF, Lu CC, Kuo SC,
Chang NW and Yang JS: AMPK-dependent signaling modulates the
suppression of invasion and migration by fenofibrate in CAL 27 oral
cancer cells through NF-κB pathway. Environ Toxicol. 31:866–876.
2016. View Article : Google Scholar
|
|
42
|
Hsu SC, Yang JS, Kuo CL, Lo C, Lin JP,
Hsia TC, Lin JJ, Lai KC, Kuo HM, Huang LJ, et al: Novel quinolone
CHM-1 induces apoptosis and inhibits metastasis in a human
osterogenic sarcoma cell line. J Orthop Res. 27:1637–1644. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS,
Wu SH and Chung JG: Benzyl isothiocyanate (BITC) inhibits migration
and invasion of human colon cancer HT29 cells by inhibiting matrix
metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC
and MAPK signaling pathway. J Agric Food Chem. 58:2935–2942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen HJ, Jiang YL, Lin CM, Tsai SC, Peng
SF, Fushiya S, Hour MJ and Yang JS: Dual inhibition of EGFR and
c-Met kinase activation by MJ-56 reduces metastasis of HT29 human
colorectal cancer cells. Int J Oncol. 43:141–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han J, Lee JD, Bibbs L and Ulevitch RJ: A
MAP kinase targeted by endotoxin and hyperosmolarity in mammalian
cells. Science. 265:808–811. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lai WW, Hsu SC, Chueh FS, Chen YY, Yang
JS, Lin JP, Lien JC, Tsai CH and Chung JG: Quercetin inhibits
migration and invasion of SAS human oral cancer cells through
inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling
pathways. Anticancer Res. 33:1941–1950. 2013.PubMed/NCBI
|
|
47
|
Yu CS, Huang AC, Yang JS, Yu CC, Lin CC,
Chung HK, Huang YP, Chueh FS and Chung JG: Safrole induces G0/G1
phase arrest via inhibition of cyclin E and provokes apoptosis
through endoplasmic reticulum stress and mitochondrion-dependent
pathways in human leukemia HL-60 cells. Anticancer Res.
32:1671–1679. 2012.PubMed/NCBI
|
|
48
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hour MJ, Tsai SC, Wu HC, Lin MW, Chung JG,
Wu JB, Chiang JH, Tsuzuki M and Yang JS: Antitumor effects of the
novel quinazolinone MJ-33: Inhibition of metastasis through the
MAPK, AKT, NF-κB and AP-1 signaling pathways in DU145 human
prostate cancer cells. Int J Oncol. 41:1513–1519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weroha SJ and Haluska P: IGF-1 receptor
inhibitors in clinical trials - early lessons. J Mammary Gland Biol
Neoplasia. 13:471–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hofmann F and García-Echeverría C:
Blocking the insulin-like growth factor-I receptor as a strategy
for targeting cancer. Drug Discov Today. 10:1041–1047. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Girnita A, Girnita L, del Prete F,
Bartolazzi A, Larsson O and Axelson M: Cyclolignans as inhibitors
of the insulin-like growth factor-1 receptor and malignant cell
growth. Cancer Res. 64:236–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sengupta N and MacDonald TT: The role of
matrix metalloproteinases in stromal/epithelial interactions in the
gut. Physiology (Bethesda). 22:401–409. 2007.
|
|
54
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
55
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors of metalloproteinases: Evolution, structure and
function. Biochim Biophys Acta. 1477:267–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wojtowicz-Praga SM, Dickson RB and Hawkins
MJ: Matrix metalloproteinase inhibitors. Invest New Drugs.
15:61–75. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hong KO, Kim JH, Hong JS, Yoon HJ, Lee JI,
Hong SP and Hong SD: Inhibition of Akt activity induces the
mesenchymal-to-epithelial reverting transition with restoring
E-cadherin expression in KB and KOSCC-25B oral squamous cell
carcinoma cells. J Exp Clin Cancer Res. 28:282009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jo M, Lester RD, Montel V, Eastman B,
Takimoto S and Gonias SL: Reversibility of epithelial-mesenchymal
transition (EMT) induced in breast cancer cells by activation of
urokinase receptor-dependent cell signaling. J Biol Chem.
284:22825–22833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Funasaka T, Hu H, Yanagawa T, Hogan V and
Raz A: Down-regulation of phosphoglucose isomerase/autocrine
motility factor results in mesenchymal-to-epithelial transition of
human lung fibrosarcoma cells. Cancer Res. 67:4236–4243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Reuben PM and Cheung HS: Regulation of
matrix metal-loproteinase (MMP) gene expression by protein kinases.
Front Biosci. 11:1199–1215. 2006. View
Article : Google Scholar
|
|
61
|
Sliva D: Signaling pathways responsible
for cancer cell invasion as targets for cancer therapy. Curr Cancer
Drug Targets. 4:327–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Clark IM, Swingler TE, Sampieri CL and
Edwards DR: The regulation of matrix metalloproteinases and their
inhibitors. Int J Biochem Cell Biol. 40:1362–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pontén J and Saksela E: Two established in
vitro cell lines from human mesenchymal tumours. Int J Cancer.
2:434–447. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li S, Zhang D, Yang L, Burnier JV, Wang N,
Lin R, Lee ER, Glazer RI and Brodt P: The IGF-I receptor can alter
the matrix metalloproteinase repertoire of tumor cells through
transcriptional regulation of PKC-{alpha}. Mol Endocrinol.
23:2013–2025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar
|
|
66
|
Berx G, Raspé E, Christofori G, Thiery JP
and Sleeman JP: Pre-EMTing metastasis? Recapitulation of
morphogenetic processes in cancer. Clin Exp Metastasis. 24:587–597.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wiggan O, Fadel MP and Hamel PA: Pax3
induces cell aggregation and regulates phenotypic
mesenchymal-epithelial interconversion. J Cell Sci. 115:517–529.
2002.PubMed/NCBI
|
|
68
|
Wiggan O, Shaw AE and Bamburg JR:
Essential requirement for Rho family GTPase signaling in Pax3
induced mesenchymal-epithelial transition. Cell Signal.
18:1501–1514. 2006. View Article : Google Scholar
|
|
69
|
Hsu YM, Chen YF, Chou CY, Tang MJ, Chen
JH, Wilkins RJ, Ellory JC and Shen MR: KCl cotransporter-3
down-regulates E-cadherin/beta-catenin complex to promote
epithelial-mesenchymal transition. Cancer Res. 67:11064–11073.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kim HJ, Litzenburger BC, Cui X, Delgado
DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM,
et al: Constitutively active type I insulin-like growth factor
receptor causes transformation and xenograft growth of immortalized
mammary epithelial cells and is accompanied by an
epithelial-to-mesenchymal transition mediated by NF-kappaB and
snail. Mol Cell Biol. 27:3165–3175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Graham TR, Zhau HE, Odero-Marah VA,
Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW and O'Regan
RM: Insulin-like growth factor-I-dependent up-regulation of ZEB1
drives epithelial-to-mesenchymal transition in human prostate
cancer cells. Cancer Res. 68:2479–2488. 2008. View Article : Google Scholar : PubMed/NCBI
|