Introduction
Colon cancer is one of the top three most common
types of cancer worldwide, and is associated with high levels of
morbidity and mortality (1).
Despite the development of colorectal endoscopy, the incidence of
colon cancer has continued to rise. Numerous therapies are
currently available for the treatment of colon cancer; however, the
associated mortality rate remains largely unimproved. Therefore,
the pathogenic mechanisms underlying the development and
progression of colon cancer require further elucidation, in order
to develop better therapeutic strategies.
Sirtuin 6 (SIRT6) is a member of the nicotinamide
adenine dinucleotide positivity-dependent class III deacetylase
sirtuin family, which is involved in various signaling pathways
that regulate gene transcription and glucose homeostasis (2-4). In
cancer research, SIRT6 is considered of great importance in the
development and progression of numerous types of cancer (5,6).
SIRT6 has been reported to suppress liver cancer tumorigenesis in
mice (7-9). Notably, SIRT6 has been revealed to
act as an oncogene in skin and prostate cancer (10,11).
These previous findings suggest that its function may be
tissue-dependent; however, the expression of SIRT6 in patients with
colon cancer and its association with clinical features remain to
be fully elucidated. Furthermore, the mechanisms underlying the
function of SIRT6 in colon cancer and its dysregulation remain to
be elucidated.
MicroRNAs (miRNAs/miRNAs) are a large family of
short single-stranded endogenous and non-coding RNAs, which contain
19-22 nucleotides. miRNAs can affect gene expression at the
transcriptional or post-transcriptional level by interacting with
the 3′-untranslated region (UTR) of target gene mRNAs (12,13).
Increasing bioinformatics evidence and subsequent functional assays
have revealed that miRNAs serve key roles in numerous biological
processes in several types of cancer, including apoptosis and
proliferation (14). With the
development of bioinformatics, differentially expressed miRNAs in
colon cancer tissues compared with in adjacent tissues have been
detected (15); however, with the
exception of miR-34a, miRNAs that regulate SIRT6 expression remain
unknown (16).
In the present study, the expression levels of SIRT6
were detected in colon cancer tissues and adjacent tissues by
immunohistochemistry (IHC), and the correlation between SIRT6 and
clinical features was investigated. Cell counting kit-8 (CCK-8) and
apoptotic assays were used to explore the effects of SIRT6 on
proliferation and apoptosis. Using bioinformatics analysis, the
present study identified miR-34c-5p as the most likely miRNA to
interact with SIRT6 mRNA. Subsequently, it was confirmed that
miR-34c-5p could bind to the 3′-UTR of SIRT6 mRNA, using human
colon cancer cell lines RKO and HCT116. Furthermore, the ectopic
expression of miR-34c-5p promoted tumor cell proliferation and
inhibited apoptosis of colon cancer cells via activation of the
Janus kinase 2 (JAK2)/signal transducer and activator of
transcription 3 (STAT3) signaling pathway, which was consistent
with the effects of SIRT6 downregulation.
Materials and methods
Tissue chip and IHC
The tissue chip (HColA180Su09) used in the present
study comprised 100 colon cancer tissues and 80 corresponding
adjacent colon tissues (1.5 cm away from the resection edge of the
tumor; no residual tumor confirmed), and was purchased from
Shanghai Outdo Biotech Co., Ltd. (Shanghai, China). The patients
were operated on between May 2006 and May 2007, and the last
follow-up was July 2015. All patients were pathologically diagnosed
with colon cancer and did not receive treatment prior to surgery.
The details of tissue chip production, including information
regarding informed consent and ethics committee approval, and the
IHC protocol were previously elaborated by Ding et al
(17). The antibody used for IHC
was as follows: Anti-SIRT6 (dilution 1:100; cat. no. 13572-1-AP;
Proteintech, Rosemont, IL, USA). The solvent [antibody diluent
(Dako, Glostrup, Denmark)] of the primary antibody as a negative
control. In the present study, three tissue points, which failed to
be stained, and their matched tissues were excluded.
Immunostaining was evaluated by two
pathologists who were blinded to the clinical information
The proportion of positive cells was categorized as
follows: <25%, score 1; 26-50%, score 2; 51-75%, score 3;
76-100%, score 4. In addition, the extent of staining was scored as
follows: No staining, 1; light yellow staining, 2; light brown
staining, 3; brown staining, 4. The average of the two scores was
used to define low expression (average <6) or high expression
(average ≥6).
Bioinformatics analysis
The miRNA microarray dataset GSE35982, using the
GPL14767 microarray platform, was downloaded from the National
Center for Biotechnology Information Gene Expression Omnibus (GEO;
http://www.ncbi.nlm.nih.gov/geo/). The
experimental design was described by Fu et al (15). The known human miRNAs registered in
miRBase (Release 21; http://www.mirbase.org/) were used to analyze the
differential expression of miRNAs with limma package (18); if numerous probes corresponded to
one gene, their expression values were treated as averages. |log
fold change|>1.5 and P<0.05 were used to determine
significant differentially expressed miRNAs between cancer and
matched adjacent tissues. The miRNAs possibly targeting SIRT6 were
predicted using miRanda (http://www.microrna.org/microrna/home.do) and
MicroCosm Targets (http://www.ebi.ac.uk/enrightsrv/microcosm/htdocs/targets/v5/).
Cell culture
The human colon cancer cell lines RKO, HT-29, SW620,
COLO 205 and HCT116 were purchased from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). All cells were grown
in Roswell Park Memorial Institute-1640 medium supplemented with
10% fetal bovine serum (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 100 U/ml penicillin + 100 μg/ml
streptomycin. Cells were cultured at 37°C in an incubator
containing 5% CO2. To suppress the JAK2/STAT3 signaling
pathway, cells were treated with 50 μM AG490 (a JAK2 protein
tyrosine kinase inhibitor, dissolved in dimethyl sulfoxide)
(Selleck Chemicals, Houston, TX, USA) 1 day post-transfection for
at least 24 h at 37°C. The solvent of AG490 (1 μl), dimethyl
sulfoxide, was used to treat the negative control group.
Synthetic RNA oligonucleotides and
transient transfection
miRNA inhibitors, miRNA mimics, negative control
(NC), SIRT6 small interfering (si)RNA (si-SIRT6), NC-siRNA, SIRT6
overexpression plasmid vector with pcDNA 3.1 and its control
plasmid vector were all obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The sequences of oligonucleotides were follow:
miRNA mimics, 5′-AGGCAGUGUAGUUAGCUGAUUGC-3′ and
5′-AAUCACUAACCACACGGCCAGG-3′; miRNA inhibitors,
5′-GCAAUCAGCUAACUACACUGCCU-3′; miRNA NC,
5′-CAGUACUUUUGUGUAGUACAA-3′; si-SIRT6, 5′-TCATGACCCGGCTCATGAA-3′;
NC-siRNA, 5′-TCACCCATCGGTACGTGAA-3′. Briefly, RKO and HCT116 cells
(200×103 cells/well) in 6-well plates were transfected
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
oligonucleotide doses used were the largest doses suggested by the
manufacturer's protocols. All transfections were transient; the
cells were not harvested for subsequent assays until 48 h
post-transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. miRNA
RT-qPCR was performed using Hairpin-it miRNAs qPCR Quantitation kit
(Shanghai GenePharma Co., Ltd.) according to the manufacturer's
protocol. For mRNA RT-qPCR, RT was performed using the PrimeScript
RT Reagent kit and RT-qPCR was conducted using the QuantiTect
SYBR-Green PCR kit (both from Takara Biotechnology Co., Ltd.,
Dalian, China) on an ABI 7500 Fast System Thermocycler (Thermo
Fisher Scientific, Inc.), according to the manufacturers'
protocols. qPCR for mRNA expression was conducted as follows:
Initial denaturation at 95°C for 30 sec, followed by 40 cycles of
annealing at 95°C for 5 sec and extension at 60°C for 30 sec. qPCR
for miRNA expression was conducted as follows: Initial denaturation
at 95°C for 3 min, followed by 40 cycles of annealing at 95°C for
12 sec and extension at 62°C for 60 sec. The relative miRNA
expression of each gene was normalized to U6 RNA levels, and the
relative mRNA expression levels were normalized to GAPDH mRNA
expression. Triplicate reactions were performed, and the data were
analyzed using the 2−ΔΔCq method (19). The primers were synthesized by
Shanghai GenePharma Co., Ltd. The primer sequences were as follows:
miR-34c-5p, forward, 5′-tgccagttagtagcccagaagcaa-3′ and reverse,
5′-tgatgtgccagggaagaaagccta-3′; SIRT6, forward,
5′-gcgtgtggagtatttggatgac-3′ and reverse,
5′-agtgtgatgatggtgaggatgg-3′; and GAPDH, forward,
5′-ttctacaatgagctgcgtgtggct-3′ and reverse,
5′-tagcacagcctggatagcaacgta-3′. The U6 primers were purchased from
Sangon Biotech Co., Ltd. (Shanghai, China) and the primers were:
Forward, 5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′. For comparison, the relative expression
levels of the blank group were set to 1.
Protein extraction and western blot
analysis
Colon cancer cells were washed twice with PBS and
lysed with radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Beijing, China). Protein concentrations
were determined using a bicinchoninic acid protein kit (Thermo
Fisher Scientific, Inc.). Total protein (40 μg) was
separated by 10% SDS-PAGE and proteins were transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% defatted milk in Tris-buffered saline containing 0.5%
Tween-20 at room temperature for 2 h. The membranes were then
incubated with primary antibodies, including anti-SIRT6 (ab191385;
dilution 1:2,000), anti-phosphorylated-JAK2 (ab32101; dilution
1:2,000), anti-JAK2 (ab108596; dilution 1:5,000),
anti-phosphorylated-STAT3 (ab76315; dilution 1:3,000), anti-STAT3
(ab68153; dilution 1:1,000), anti-tubulin (ab6046; dilution 1:500)
and anti-GAPDH (ab9485; dilution 1:1,000) (all from Abcam,
Cambridge, MA, USA), at 4°C overnight. Finally, the membranes were
washed and incubated with the horseradish peroxidase-conjugated
secondary antibody (ab6721; dilution 1:5,000; Abcam) for 2 h at
room temperature. The protein bands were detected using an
electrochemiluminescence substrate (Thermo Fisher Scientific, Inc.)
via a chemiluminescence method (LAS-3000; Fujifilm Corporation,
Tokyo, Japan). The details of western blot analysis of the
JAK2/STAT3 signal pathway are clearly described by Feng et
al (20).
Dual-luciferase assays
Cells (2-4×105 cells/well) were seeded
into 6-well plates and cultured overnight. The cells were then
transfected with the control vector, wild-type or mutant SIRT6
luciferase plasmids (1 μg wild-type or mutant plasmids per
well; Shanghai GeneChem Co., Ltd., Shanghai, China) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The wild-type plasmid contained the predicted
binding site of miR-34c-5p, whereas the mutant plasmid contained a
mutated sequence of the predicted binding site of miR-34c-5p
(5′-CATCAGATCACCGATCCACGCC-3′). In addition, cells were
cotransfected with miR-34c-5p mimics, inhibitors or NC. Relative
luciferase activities were standardized to the activity of the
Renilla luciferase reporter gene and were assessed 24 h
following cotransfection. Firefly and Renilla luciferase
activities were quantified using a dual-luciferase assay system
(Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol. For the ease of comparison, the luciferase
values of cells transfected with NC + control vector were set to
1.
Cell proliferation assays
Cell proliferation was measured using CCK-8
(Beyotime Institute of Biotechnology). Post-transfection, cells
were seeded in 96-well plates at a density of 3-5×103
cells/well. CCK-8 (10 μl/well) was added at various time
points (0, 1, 2 and 3 days) and incubated at 37°C for 2 h. To
estimate the number of viable cells, the absorbance of each well
was detected at 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The assays were performed
in triplicate to reduce random errors.
Colony formation assays
The colony formation assays were used to estimate
in vitro tumorigenicity. A total of 200 cells were seeded
into 6-well plates post-transfection. The cells were cultured for 7
days at 37°C until most of the single colonies contained >50
cells. Subsequently, the cells were washed with PBS, fixed with 5
ml 100% paraformaldehyde for 15 min and stained with 0.1% crystal
violet for 30 min at room temperature. The number of colonies was
counted under a light microscope.
Cell apoptosis analysis
Flow cytometric analysis of apoptosis was performed
using an Annexin V/fluorescein isothiocyanate (FITC) and propidium
iodide (PI) apoptosis detection kit (BD Biosciences, San Jose, CA,
USA) following transfection for 48 h. The cells were harvested
using trypsin, washed twice with cold PBS and suspended in binding
buffer. The cell suspension (300 μl) was incubated with
Annexin V/FITC (5 ml) in a light-resistant container at room
temperature for 15 min. Finally, PI (2 ml) was added 5 min prior to
detection using a flow cytometer (Bio-Rad Laboratories, Inc.). Each
experiment was performed at least three times.
Statistical analysis
The difference in SIRT6 expression between cancerous
and adjacent tissues was analyzed by χ2 test. Overall
survival was analyzed by Kaplan-Meier analysis and log-rank test.
Univariate analysis and multivariate survival analysis were
performed using a Cox regression model. The association between
SIRT6 and clinical characteristics was explored using Spearman rank
correlation. Other data are presented as the means ± standard
deviation, and were analyzed by Student's t-test when comparing two
groups or two-way analysis of variance followed by
Student-Newman-Keuls-q test. Statistical analyses were processed
using SPSS 23.0 software (IBM Corporation, Armonk, NY, USA) and
GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference. Bioinformatics analysis was processed using R program
version 3.2.2 (R Foundation for Statistical Computing, Vienna,
Austria; http://www.r-project.org/).
Results
SIRT6 is downregulated in colon cancer
tissues
SIRT6 expression was assessed in cancerous and
adjacent tissues by IHC, as shown in Fig. 1 and Table I. SIRT6 was revealed to be
predominantly expressed in the nucleus and was significantly
overexpressed in adjacent tissues compared with in cancerous
tissues (P<0.05). The association between SIRT6 and clinical
features, such as TNM stage and pathological grade, are shown in
Table II; the tissue points that
failed to be stained were excluded. The results indicated that the
expression of SIRT6 was negatively associated with T stage, which
is mainly affected by proliferation and apoptosis (P<0.05).
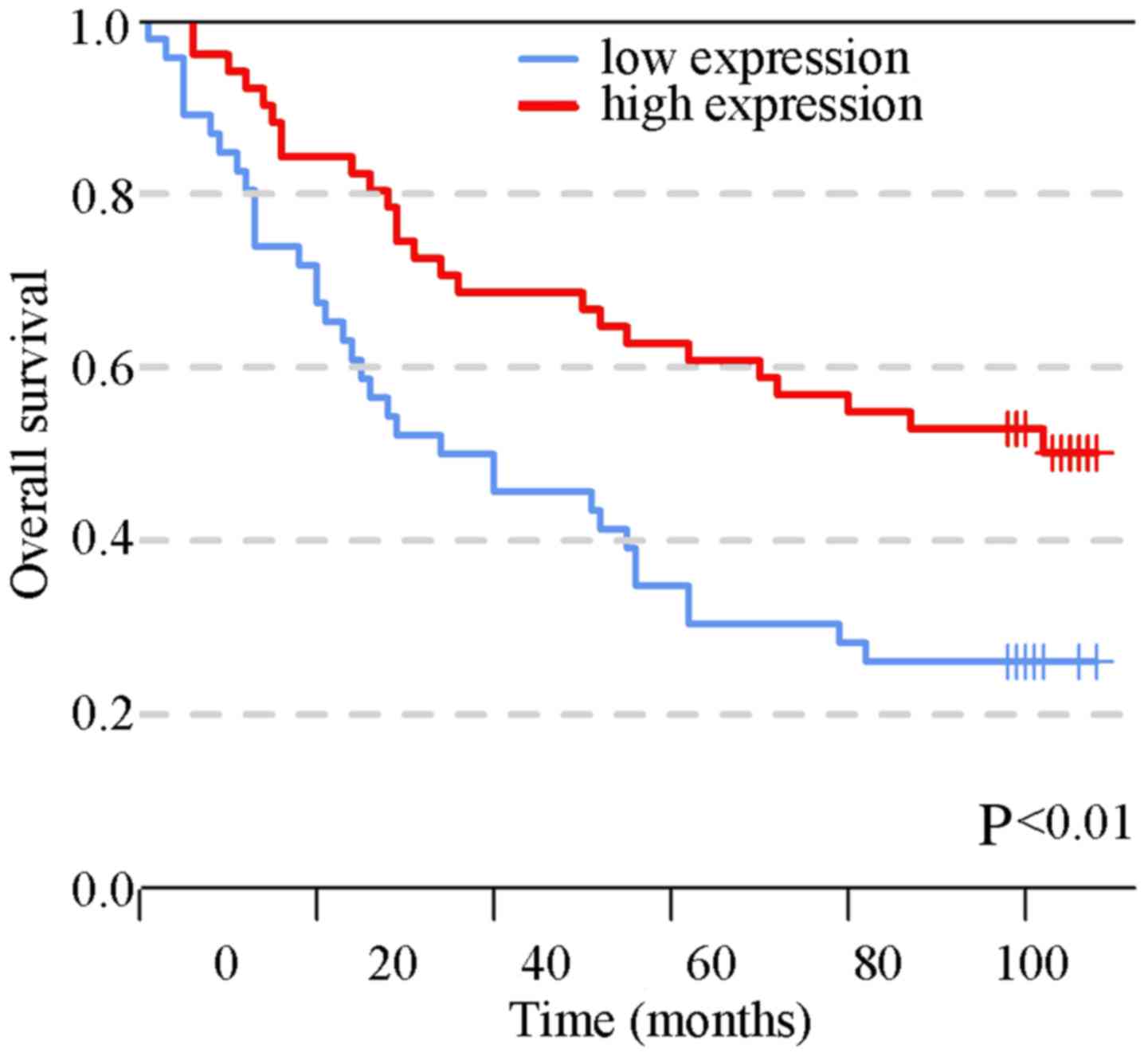
Furthermore, univariate survival analysis was conducted and the
results demonstrated that the patients with high SIRT6 expression
had a better prognosis relative to those with a lower expression of
SIRT6 (Fig. 2; 26.1 vs. 50.2%,
P<0.01). In addition, N stage, M stage, TNM stage, age and grade
were associated with the prognosis of colon cancer. To determine
whether SIRT6 is an independent prognostic factor, these variables,
which were associated with prognosis, were included in a
multivariate Cox regression analysis; the results demonstrated that
SIRT6 expression was a protective independent prognostic factor. In
addition, age and grade were isolated factors harmful to the
prognosis of patients with colon cancer (Table III).
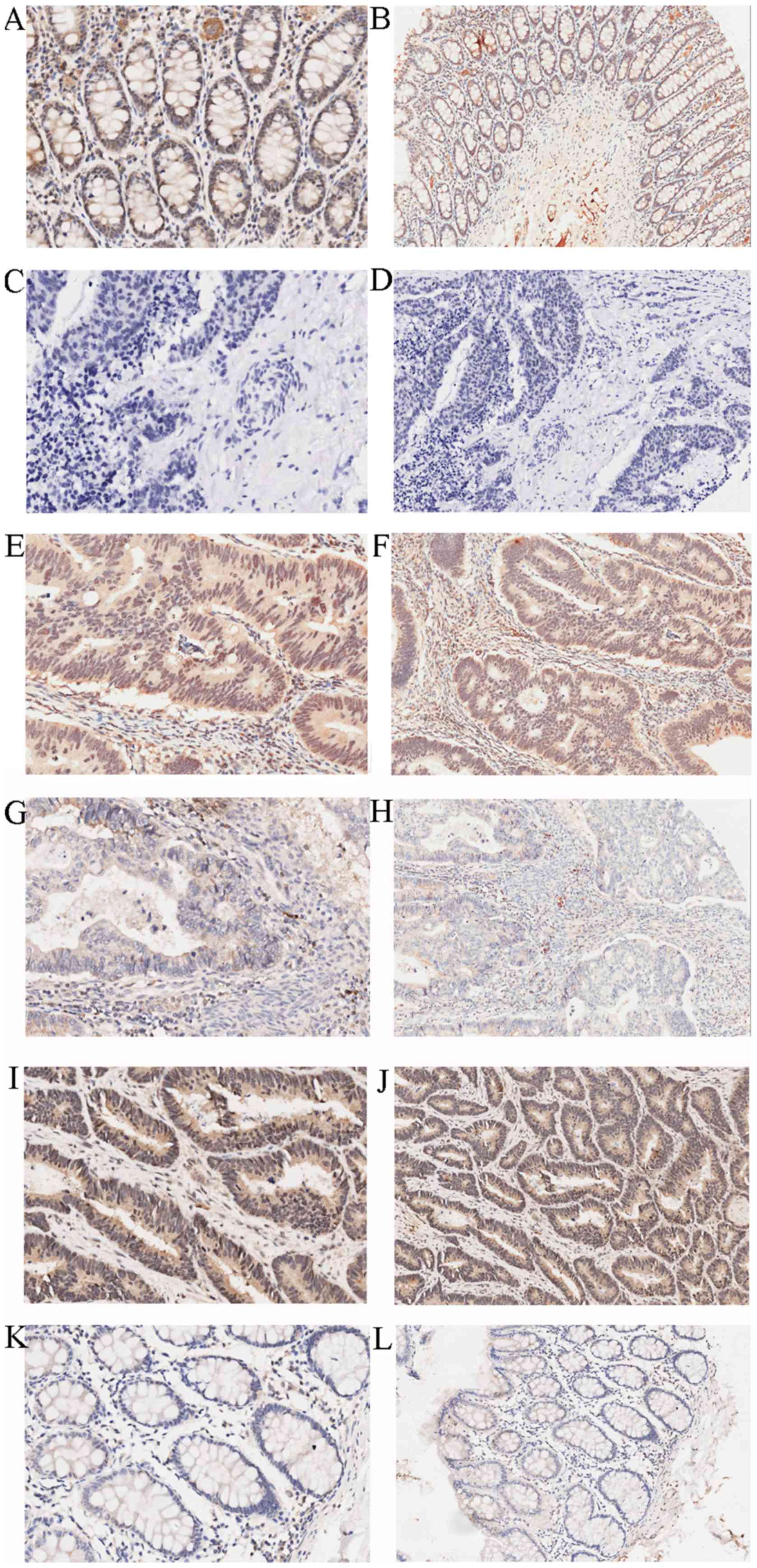 | Figure 1Immunohistochemical staining of
SIRT6. High expression of SIRT6 in adjacent colon tissues: (A)
Magnification, ×200; (B) magnification, ×100. Negative control: (C)
Magnification, ×200; (D) magnification, ×100. High expression of
SIRT6 in colon cancer tissues: (E) Magnification, ×200; (F)
magnification, ×100. Low expression of SIRT6 in colon cancer
tissues: (G) Magnification, ×200; (H) magnification, ×100. High
expression of SIRT6 in adjacent tissues: (I) Magnification, ×200;
(J) magnification, ×100. Low expression of SIRT6 in adjacent
tissues: (K) Magnification, ×200; (L) magnification, ×100. SIRT6,
sirtuin 6. |
 | Table IDifferential expression of SIRT6 in
paired cancerous and adjacent tissues. |
Table I
Differential expression of SIRT6 in
paired cancerous and adjacent tissues.
| Tissue | n | SIRT6 expression
| χ2
value | P-value |
|---|
| High | Low |
|---|
| Cancerous | 77 | 37 | 40 | 5.1970 | 0.02a |
| Adjacent | 77 | 51 | 26 | | |
 | Table IICorrelation between SIRT6 expression
and clinical features. |
Table II
Correlation between SIRT6 expression
and clinical features.
| Characteristic | SIRT6 expression
| Total | rs | P-value |
|---|
| High | Low |
|---|
| Sex | | | | −0.141 | 0.168 |
| Female | 26 | 17 | 43 | | |
| Male | 25 | 29 | 54 | | |
| Age (years) | | | | −0.024 | 0.822 |
| ≤70 | 25 | 17 | 42 | | |
| >70 | 22 | 28 | 50 | | |
| Null | | | 5 | | |
| Grade | | | | −0.113 | 0.272 |
| I | 3 | 4 | 7 | | |
| II | 32 | 21 | 53 | | |
| III | 16 | 21 | 37 | | |
| T stage | | | | −0.241 | 0.02a |
| T1 | 1 | 0 | 1 | | |
| T2 | 5 | 0 | 5 | | |
| T3 | 38 | 35 | 73 | | |
| T4 | 5 | 9 | 14 | | |
| Null | | | 4 | | |
| N stage | | | | −0.164 | 0.113 |
| N0 | 34 | 24 | 58 | | |
| N1 | 15 | 12 | 27 | | |
| N2 | 2 | 8 | 10 | | |
| Null | | | 2 | | |
| M stage | | | | −0.188 | 0.065 |
| M0 | 51 | 43 | 94 | | |
| M1 | 0 | 3 | 3 | | |
| TNM stage | | | | −0.168 | 0.104 |
| I | 4 | 1 | 5 | | |
| II | 30 | 23 | 53 | | |
| III | 17 | 17 | 34 | | |
| IV | 0 | 3 | 3 | | |
| Null | | | 2 | | |
 | Table IIIUnivariate and multivariate survival
analyses. |
Table III
Univariate and multivariate survival
analyses.
| Variable | Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sirtuin 6
expression | 0.475 | 0.282–0.799 | 0.005a | 0.531 | 0.301–0.936 | 0.029a |
| Sex | 1.052 | 0.627–1.763 | 0.849 | | | |
| Age (years) | 1.955 | 1.132–3.373 | 0.016a | 2.399 | 1.297–4.437 | 0.005a |
| T stage | 1.540 | 0.861–2.754 | 0.146 | | | |
| N stage | 2.233 | 1.553–3.213 | <0.001a | 1.808 | 0.823–3.971 | 0.140 |
| M stage | 13.908 | 3.680–46.626 | <0.001a | 1.972 | 0.424–9.173 | 0.387 |
| TNM stage | 2.790 | 1.756–4.431 | <0.001a | 1.136 | 0.441–2.926 | 0.791 |
| Grade | 2.664 | 1.653–4.296 | <0.001a | 2.033 | 1.221–3.386 | 0.006a |
SIRT6 inhibits colon cancer cell
proliferation and promotes apoptosis
Thorough western blot analysis, it was demonstrated
that SIRT6 was highly expressed in RKO cells and lowly expressed in
HCT116 cells (Fig. 3A). To explore
the role of SIRT6 in colon cancer, HCT116 and RKO cells were
transfected with si-SIRT6, NC-siRNA, SIRT6 overexpression plasmid
vector or control plasmid vector. SIRT6 was overexpressed in the
HCT116 cell line and was knocked down in the RKO cell line.
Transfection efficiency was confirmed, as shown in Fig. 3B and C. The results of CCK-8 assays
demonstrated that overexpression of SIRT6 significantly inhibited
cell viability, whereas SIRT6 knockdown had the opposite effect
(Fig. 3D; P<0.05). Furthermore,
through apoptotic analysis, upregulation of SIRT6 was revealed to
markedly increase cell apoptosis, whereas SIRT6 knockdown
suppressed it (Fig. 3E and F). On
the basis of colony formation, enhancing the expression of SIRT6
significantly reduced the colony-forming ability of cells, whereas
SIRT6 knockdown exhibited the opposite effects (Fig. 3G and H).
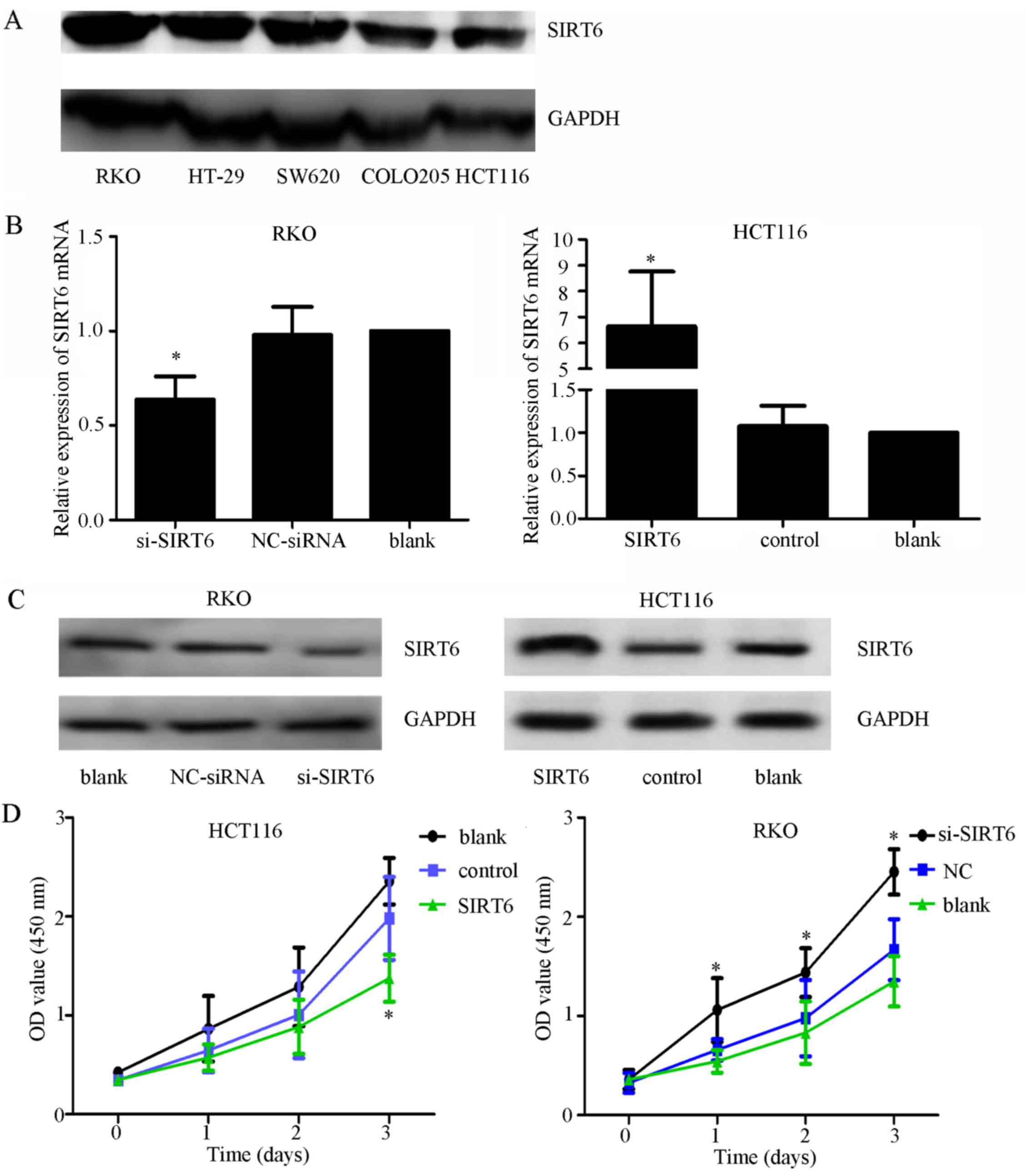 | Figure 3Effects of SIRT6 on colon cancer cell
lines. (A) Expression of SIRT6 in different colon cancer cell
lines. The RKO cell line had the highest expression and the HCT116
cell line had the lowest expression. (B) mRNA expression levels of
SIRT6 after silencing or overexpression were evaluated by
quantitative polymerase chain reaction to confirm the efficiency of
transfection. si-SIRT6 markedly downregulated the expression of
SIRT6 mRNA in RKO cells, and the SIRT6 overexpression plasmid
vector increased the expression of SIRT6 in HCT116 cells. (C)
Expression of SIRT6 after silencing or overexpression was assessed
by western blot analysis, to confirm the efficiency of
transfection. si-SIRT6 markedly downregulated the expression of
SIRT6 in RKO cells, and the SIRT6 overexpression plasmid vector
increased the expression of SIRT6 in HCT116 cells. (D) SIRT6
overexpression inhibited cell growth compared with in the control
groups, whereas silencing SIRT6 promoted cell proliferation
compared with in the control groups; *P<0.05 vs. the
NC, or control or blank groups. (E and F) Annexin V-FITC/PI was
used to detect apoptosis after silencing SIRT6 in RKO cells or
overexpressing SIRT6 in HCT116 cells. Cell apoptosis was elevated
in the SIRT6 group and decreased in the si-SIRT6 group compared
with in the control groups; *P<0.05 vs. the NC-siRNA,
control or blank groups. (G and H) Colony formation assays
indicated that silencing SIRT6 enhanced the colony-forming ability
of cells, whereas overexpression of SIRT6 exhibited the opposite
effect; *P<0.05 vs. the NC-siRNA, control or blank
groups. FITC, fluorescein isothiocyanate; NC, negative control; OD,
optical density; PI, propidium iodide; si/siRNA, small interfering
RNA; SIRT6, sirtuin 6. |
JAK2/STAT3 signaling is suppressed in
colon cancer cells in response to SIRT6 overexpression
Previous study has revealed that the JAK2/STAT3
signaling pathway is highly activated in colon cancer, and
contributes to proliferation, migration, invasion, cell cycle
progression and angiogenesis (21-24).
The association between SIRT6 and JAK2/STAT3 has been confirmed in
other cancers; however, to the best of our knowledge, it has not
been reported in colon cancer (20,25).
Therefore, the present study aimed to determine the effects of
SIRT6 overexpression on the JAK2/STAT3 signaling pathway. The
results indicated that SIRT6 overexpression markedly reduced the
phosphorylation of JAK2 and STAT3 compared with in the control
cells, whereas silencing SIRT6 increased the phosphorylation of
JAK2 and STAT3 (Fig. 4A). These
results indicated that SIRT6 may suppress proliferation and induce
apoptosis via inactivation of the JAK2/STAT3 signaling pathway in
colon cancer cells.
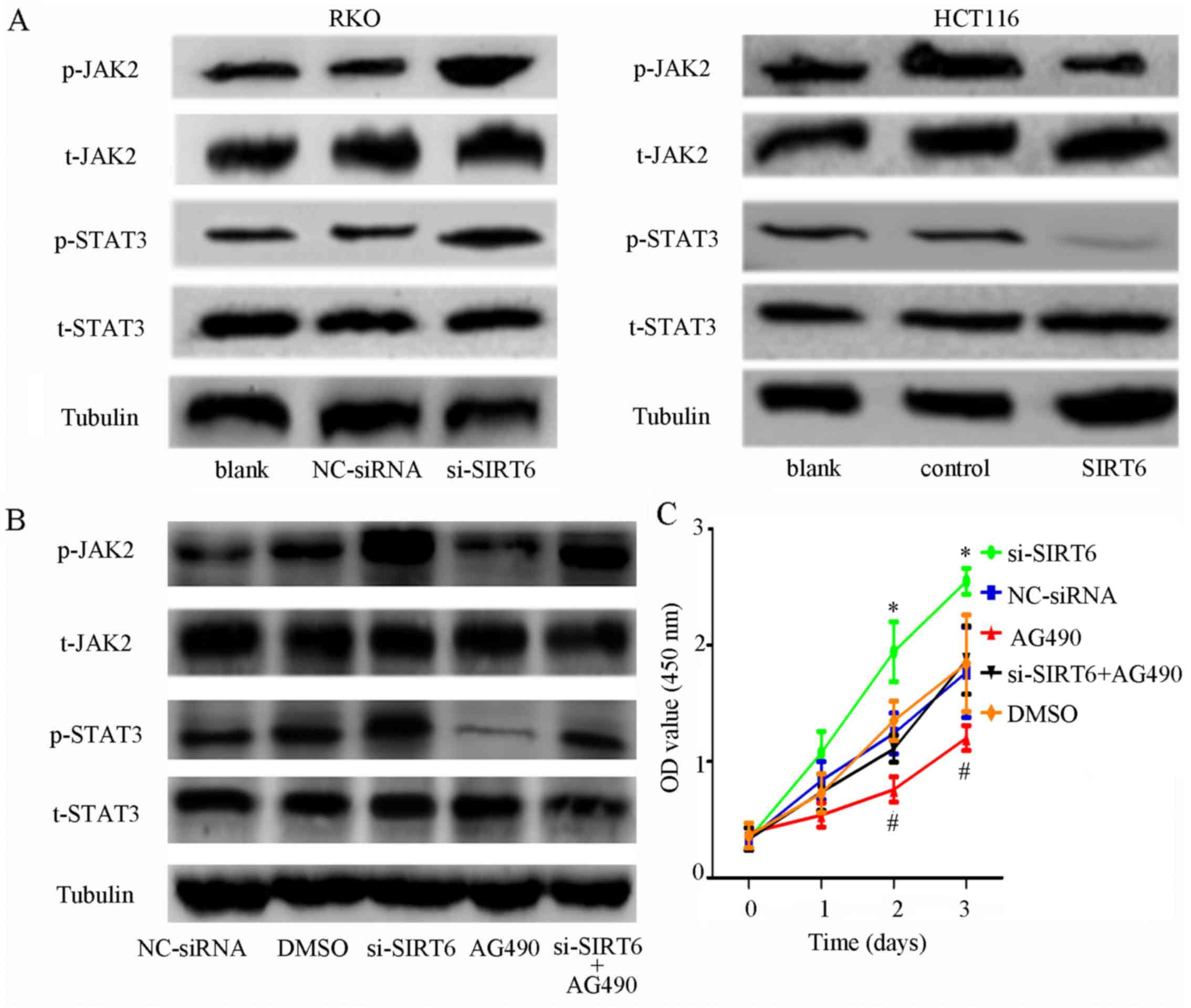 | Figure 4SIRT6 overexpression inhibits the
JAK2/STAT3 signaling pathway in colon cancer cells. (A)
Phosphorylation of JAK2 and STAT3 was increased in the si-SIRT6
group and inhibited in the SIRT6 group compared with the other
groups. (B) Phosphorylation of JAK2 and STAT3 was activated by
SIRT6 knockdown and inhibited by AG490 in RKO cell lines. However,
transfection with si-SIRT6 abolished the effects of AG490, which
further confirmed that SIRT6 may regulate the JAK2/STAT3 signaling
pathways. (C) Proliferation rate was higher in the si-SIRT6 group
and lower in the si-SIRT6 + AG490 group. The
proliferation-inhibiting effects of AG490 were attenuated by
si-SIRT6; *P<0.05, si-SIRT6 group vs. the other
groups; #P<0.05, AG490 group vs. the other groups.
(D) AG490 induced apoptosis of RKO cells, whereas si-SIRT6 exerted
the opposite effect. However, the effects of AG490 were reversed by
si-SIRT6; *P<0.05 vs. other groups;
#P<0.05 vs other groups. (E) Colony-forming ability
was increased by SIRT6 knockdown and decreased by AG490. The
effects of AG490 could be eliminated by SIRT6 knockdown;
*P<0.05 vs. the other groups; #P<0.05
vs. the other groups. DMSO, dimethyl sulfoxide; FITC, fluorescein
isothiocyanate; JAK2, Janus kinase 2; NC, negative control; OD,
optical density; p-, phosphorylated; PI, propidium iodide;
si/siRNA, small interfering RNA; SIRT6, sirtuin 6; STAT3, signal
transducer and activator of transcription 3; t, total. |
The JAK2 inhibitor AG490 was used to further confirm
whether SIRT6 may serve a role in the JAK2/STAT3 signaling pathway.
RKO cells transfected with si-SIRT6 were treated with AG490. As
shown in Fig. 4B, AG490 markedly
decreased the expression levels of phosphorylated (p)-JAK2 and
p-STAT3, whereas the inhibitory effects were abolished by si-SIRT6.
Furthermore, SIRT6 downregulation promoted the proliferation of
cells and inhibited apoptosis, whereas the JAK2 inhibitor exhibited
the opposite effects. Furthermore, the effects of AG490 on cell
proliferation and apoptosis were counteracted by SIRT6 knockdown
(Fig. 4C–E).
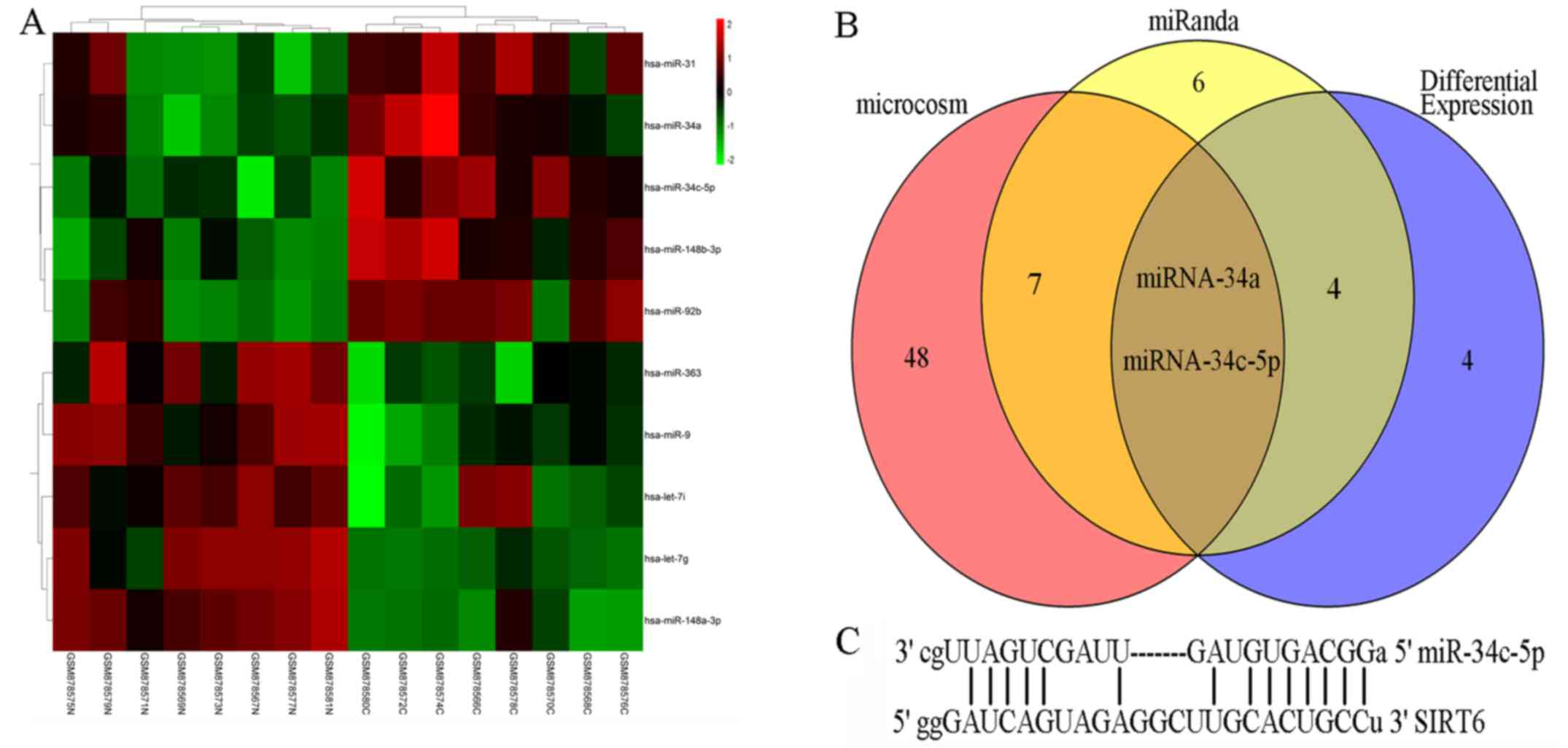
Bioinformatics analysis
miRNAs are major regulators of gene expression. To
explore why SIRT6 is downregulated in colon cancer, the present
study determined whether miRNAs may account for it. The results
from a miRNA microarray analysis suggested that 10 miRNAs were
significantly differentially expressed in colon cancer (Fig. 5A). Subsequently, miRNAs that
possibly targeted SIRT6 were predicted by miRanda and MicroCosm.
Notably, miR-34a and miR-34c-5p were not only dysregulated in colon
cancer but were also predicted to potentially target the 3′-UTR of
SIRT6 (Fig. 5B). Furthermore, the
interaction between miR-34a and SIRT6 mRNA has been reported by
previous studies (10,16). The possible binding sites between
SIRT6 mRNA and miR-34c-5p are shown in Fig. 5C.
SIRT6 is a direct target of
miR-34c-5p
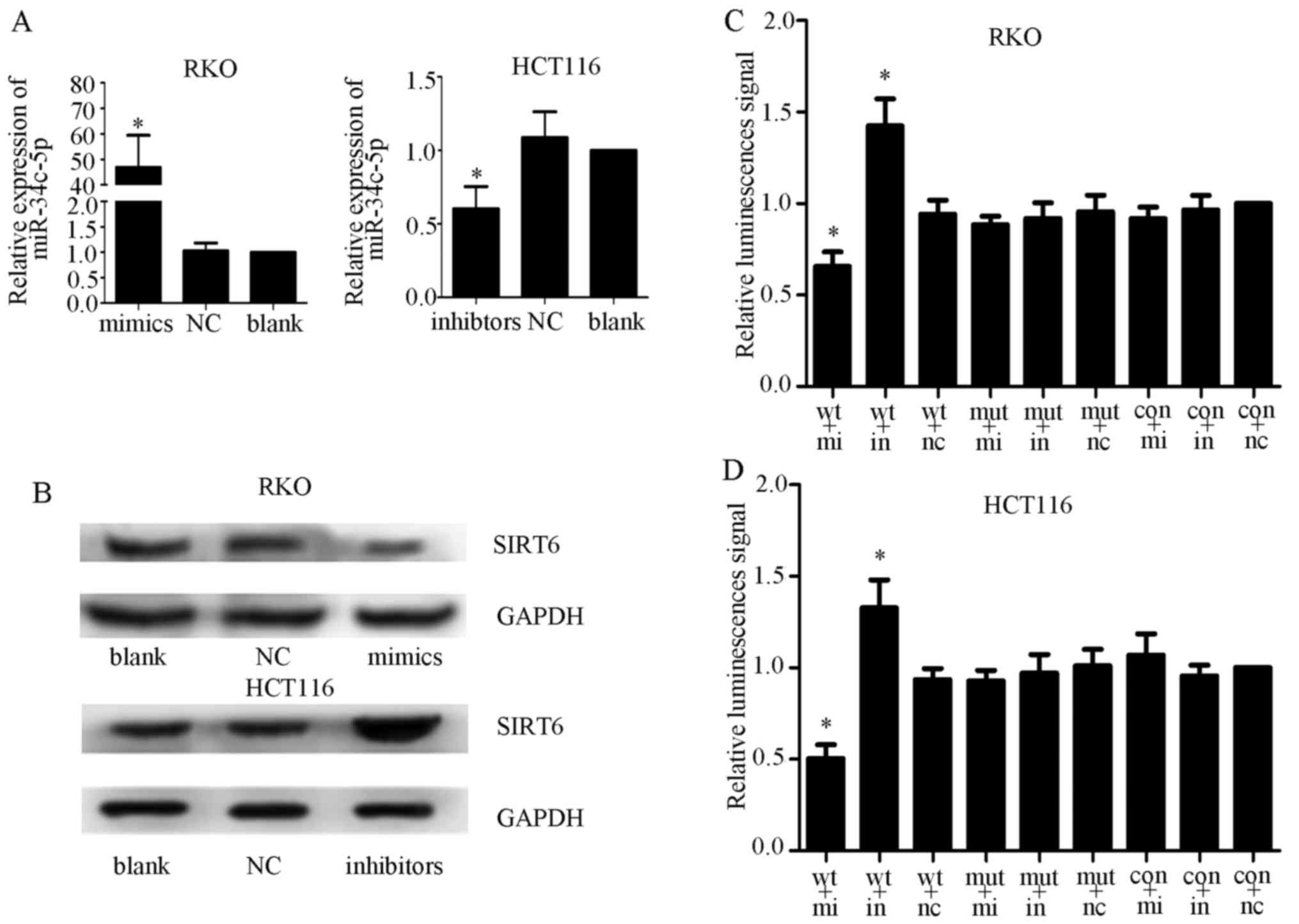
A previous study reported that the expression levels
of miR-34c-5p were increased in colorectal cancer tissues (26). To determine whether miR-34c-5p
could interact with the 3′-UTR of SIRT6, miR-34c-5p and NC mimics
and inhibitors were transfected into RKO and HCT116 cell lines. The
transfection efficiency was measured by qPCR (Fig. 6A) and western blot analysis. The
results demonstrated that miR-34c-5p overexpression inhibited the
expression of SIRT6, whereas miR-34c-5p knockdown exhibited the
opposite effect, thus indicating that miR-34c-5p may be a specific
miRNA that targets SIRT6 in colon cancer cells (Fig. 6B). Subsequently, luciferase
reporter assays were performed to determine whether miR-34c-5p acts
on the 3′-UTR of SIRT6 mRNA. Control, wild-type and mutant vectors,
alongside Renilla luciferase reporter genes, were
transfected into colon cancer cells together with miR-34c-5p
mimics, inhibitors or NC. The results demonstrated that relative
luciferase activity was significantly decreased in the mimics +
wild-type group and increased in the inhibitors + wild-type group.
However, no significant alteration in relative luminescence
intensities was detected in the other groups (Fig. 6C and D). These findings suggested
that miR-34c-5p may bind directly to the 3′-UTR of SIRT6 mRNA and
suppress SIRT6 expression in colon cancer cells.
Elevated expression of miR-34c-5p
facilitates proliferation and inhibits apoptosis of colon cancer
cells
To further confirm whether the functions of
miR-34c-5p are contrary to SIRT6, miR-34c-5p mimics, inhibitors and
NC were transfected into cells, and CCK-8 assays were performed.
The results indicated that transfection with miR-34c-5p mimics
significantly facilitated cell growth, whereas transfection with
miR-34c-5p inhibitors had the opposite effect, compared with in the
control groups (Fig. 7A;
P<0.05). To explore the mechanism underlying the effects of
miR-34c-5p on promoting cell proliferation, apoptosis and colony
formation assays were performed on cells transfected with
miR-34c-5p mimics, inhibitors or NC. Flow cytometry suggested that
the percentage of apoptotic cells was significantly lower in the
mimic group compared with in the control groups, whereas the levels
of cell apoptosis were elevated in cells exposed to inhibitors
(Fig. 7B and C; P<0.05). The
results of colony formation assays indicated that miR-34c-5p
inhibitors weakened colony-forming ability (Fig. 7D and E). These findings suggested
that increased miR-34c-5p expression had tumor-promoting effects in
colon cancer cells, which is in accordance with SIRT6
knockdown.
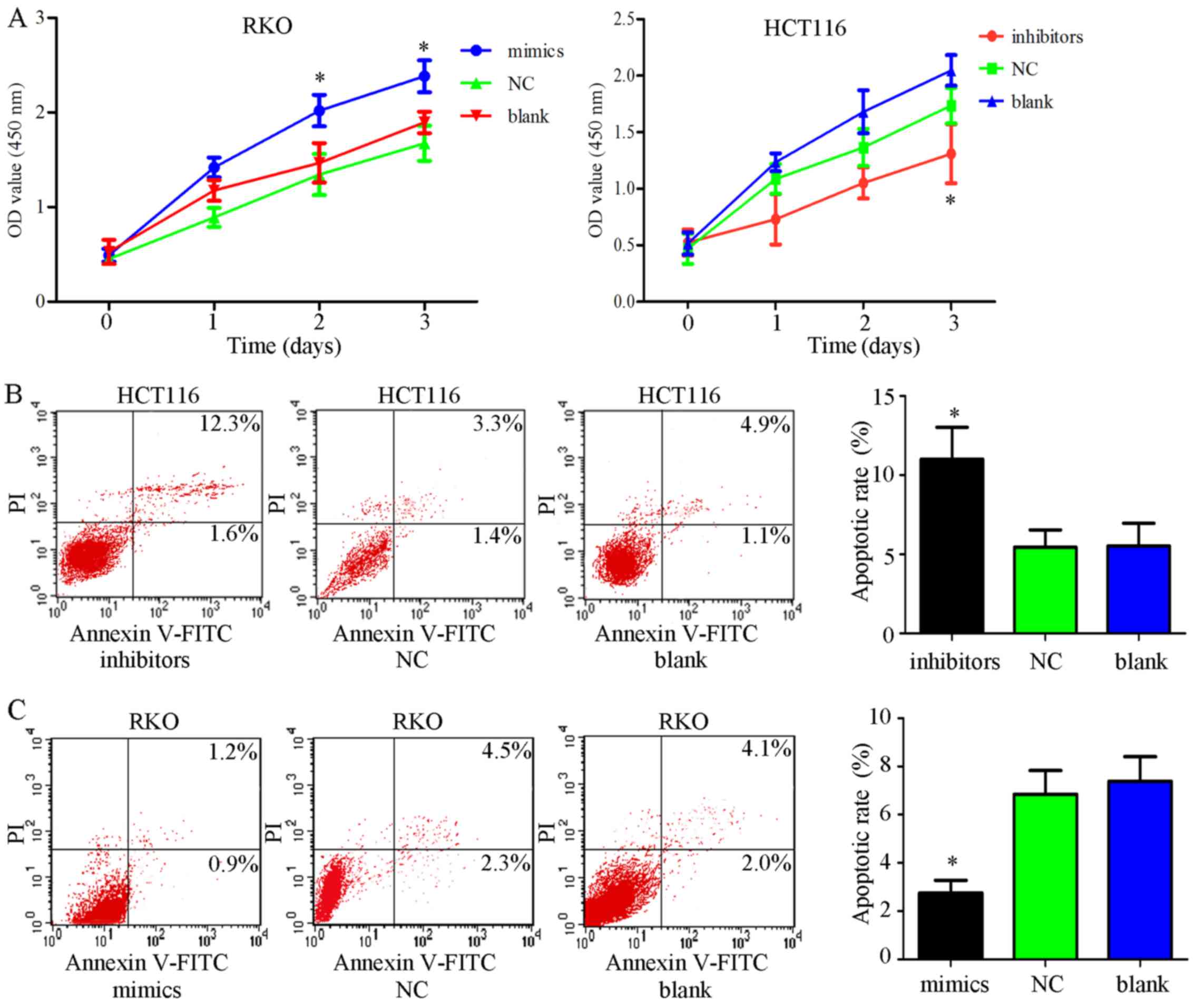 | Figure 7Effects of miR-34c-5p on colon cancer
cells. (A) Cell viability was determined using Cell Counting kit-8
assays post-transfection with mimics or inhibitors in RKO or HCT116
cell lines. miR-34c-5p mimics facilitated cell growth compared with
in the control groups, whereas miR-34c-5p inhibitors suppressed
cell viability; *P<0.05 vs. other groups. (B and C)
Annexin V-FITC/PI was employed to estimate apoptosis
post-transfection with miR-34c-5p mimics in RKO cells or miR-34c-5p
inhibitors in HCT116 cells. The results indicated that cell
apoptotic rate was decreased in the mimic group and increased in
the inhibitor group compared with in the control groups;
*P<0.05 vs. other groups. (D and E) Compared with in
the other groups, transfection with miR-34c-5p inhibitors inhibited
colony formation, whereas miR-34c-5p mimics enhanced it;
*P<0.05 vs. other groups. (F) JAK2 and STAT3
phosphorylation was promoted by mimics and decreased by inhibitors
compared with in the other groups. miR-34c-5p may promote cell
growth and inhibit apoptosis via the JAK2/STAT3 pathways. FITC,
fluorescein isothiocyanate; JAK2, Janus kinase 2; miR, microRNA;
NC, negative control; OD, optical density; p-, phosphorylated; PI,
propidium iodide; SIRT6, sirtuin 6; STAT3, signal transducer and
activator of transcription 3; t, total. |
JAK2/STAT3 signaling pathway is activated
in colon cancer cells by miRNA-34c-5p
As aforementioned, miRNA-34c-5p may promote
proliferation and inhibit apoptosis by silencing SIRT6. However,
whether miR-34c-5p exerts its functions via the JAK2/STAT3
signaling pathway remains to be elucidated. Therefore, the present
study altered the expression of miR-34c-5p, in order to observe its
effects on the JAK2/STAT3 signaling pathway. The results
demonstrated that the JAK2/STAT3 signaling pathway was activated in
the mimic group compared with in the NC and blank groups.
Conversely, the phosphorylation of JAK2 and STAT3 was decreased in
the inhibitor group compared with in the NC and blank groups
(Fig. 7F). These data indicated
that miRNA-34c-5p may exert tumor-promoting effects via activation
of the JAK2/STAT3 signaling pathway.
Discussion
The SIRT family is involved in numerous biological
processes, including apoptosis and aging (27-29).
As a member of the SIRT family, SIRT6 serves versatile roles in
human cancer. SIRT6 acts as a cancer suppressor gene in pancreatic
cancer by controlling Lin-28 homolog B (30), and promotes cytokine production and
migration in pancreatic cancer cells by regulating Ca2+
responses (31). Decreased
expression of SIRT6 promotes tumor cell growth and is closely
correlated with the poor prognosis of ovarian cancer, gastric
cancer and glioma (20,25,32).
In non-small cell lung cancer, SIRT6 can suppress cell
proliferation via the inhibition of Twist1, and astragaloside IV
can sensitize non-small cell lung cancer cells to gefitinib by
upregulating SIRT6 (33,34). However, some studies have reported
opposite findings. Azuma et al and Bai et al
demonstrated that high SIRT6 expression is associated with poor
prognosis and reduced chemosensitivity, and may promote cancer cell
metastasis and invasion via the extracellular signal-regulated
kinase (ERK)1/2/matrix metalloproteinase 9 pathway (35,36).
Notably, SIRT6 is upregulated and contributes to the progression of
papillary thyroid cancer and hepatocellular carcinoma via numerous
mechanisms (37-39). Similar to non-small cell lung
cancer, the role of SIRT6 in hepatocellular carcinoma is
controversial. It has previously been reported that SIRT6
suppresses hepatocellular carcinoma cell growth by inhibiting the
ERK signaling pathway (40).
However, studies focusing on the role of SIRT6 in the pathogenesis
and prognosis of colon cancer are very limited. The majority of
studies regarding SIRT6 and colon cancer have focused on the role
of SIRT6 as a mediator at the cellular level, suggesting that SIRT6
may have an inhibitory effect on colon cancer (41-43).
In the present study, the correlation between SIRT6
and the clinical features of colon cancer were explored by tissue
chip and IHC. The results demonstrated that SIRT6 was predominantly
expressed in the nucleus, and its expression in colon cancer was
significantly lower than in adjacent tissues. The correlation
suggested that SIRT6 was negatively associated with T stage, and
the prognosis of patients with colon cancer was significantly
increased in those with high SIRT6 expression. Furthermore, Cox
multivariate regression analysis indicated that SIRT6 expression
was a protective independent prognostic factor.
T stage is mainly affected by proliferation and
apoptosis. Since a correlation was detected between T stage and
SIRT6 expression, the present study aimed to determine whether
SIRT6 affected proliferation and apoptosis of colon cancer cells.
RKO cells were revealed to possess a high expression of SIRT6,
whereas HCT116 cells possessed low SIRT6 expression; these cell
lines were selected for subsequent analyses. Using HCT116 cells in
which SIRT6 overexpression was induced and RKO cells in which SIRT6
expression was knocked down, it was demonstrated that SIRT6
significantly attenuated proliferation and increased the levels of
cell apoptosis. Furthermore, the results demonstrated that SIRT6
may inactivate the JAK2/STAT3 signaling pathway, which has
previously been reported to induce the expression of B-cell
lymphoma 2 (Bcl-2) and Bcl-extra large, and inhibit apoptosis
(21,25,44).
The present study indicated that SIRT6 may act as a tumor
suppressor gene in colon cancer, which may affect tumor size by
regulating proliferation and apoptosis via the JAK2/STAT3 signaling
pathway.
The present study demonstrated that the expression
levels of SIRT6 were markedly decreased in cancer tissues;
therefore, the present study aimed to determine the possible
mechanisms underlying its downregulation. miRNAs are a type of
small, non-coding RNA that regulate the expression of genes.
Therefore, the present study investigated whether miRNAs are
responsible for the dysregulation of SIRT6 in colon cancer.
miRanda, MicroCosm and GEO databases were used to predict the
miRNAs that may participate in SIRT6 regulation. The results
indicated that miR-34c-5p may interact with the mRNA of SIRT6.
miRNA-34c-5p serves various roles in numerous types
of cancer; miRNA-34c-5p can affect the drug resistance of ovarian,
gastric and lung cancers (45-47).
In addition, miRNA-34c-5p has been reported to be associated with
recurrence in laryngeal squamous cell carcinoma, and the
development and radioresistance of nasopharyngeal carcinoma
(48-50). Furthermore, miR-34c-5p is involved
in the proliferation, apoptosis and invasion of endometrial
carcinoma, glioma cells and cervical cancer (51-53).
However, the biological function of miRNA-34c-5p and its target
genes in colon cancer remains to be elucidated.
Using dual-luciferase reporter assays, the present
study confirmed that SIRT6 is a target of miR-34c-5p, and increased
expression of miR-34c-5p may be responsible for the downregulation
of SIRT6 in colon cancer. Furthermore, the overexpression of
miRNA-34c-5p resulted in a marked reduction in cell apoptosis and
enhanced proliferation via activation of the JAK2/STAT3 pathway.
Conversely, knockdown of miRNA-34c-5p exhibited the opposite
effects, which is consistent with silencing SIRT6 or ectopic
overexpression of SIRT6.
To the best of our knowledge, the present study is
the first to report the relationship between SIRT6 and the clinical
features of colon cancer, and to explain the effects of miRNA on
SIRT6 dysregulation. The present study also explored the biological
function of miRNA-34c-5p and its mechanism in colon cancer.
However, the patients involved in the present study may not be
enough to draw a definite conclusion that SIRT6 is associated with
prognosis and T stage. More patients with different stages of
cancer and their follow-up data should be included in future
studies. In addition, the present study only demonstrated that
miRNA-34c-5p may promote proliferation and inhibit cell apoptosis
through silencing SIRT6 and activating the JAK2/STAT3 signaling
pathway; however, the in-depth mechanisms underlying how SIRT6
affects the JAK2/STAT3 signaling pathway and biological functions
should be further investigated in in vitro and in
vivo experiments.
In conclusion, the present study demonstrated that
SIRT6 downregulation, partly due to the upregulation of miR-34c-5p,
may promote colon cancer growth via activation of the JAK2/STAT3
signaling pathway, and SIRT6 is likely a biomarker for predicting
the prognosis of colon cancer. Therefore, focusing on the
miR-34c-5p/SIRT6/JAK2/STAT3 regulatory axis may be considered a
promising strategy for the treatment of colon cancer.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by the Liaoning
Province Natural Science Foundation (grant nos. 201602293 and
201705400360).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
NL, FR and KL designed the research, analyzed the
data, and wrote and revised the manuscript. DM, YC and HL performed
the research. All authors have read and approved the final version
of this manuscript.
[4] Ethics
approval and consent to participate
The human samples in the tissue chip were obtained
from the National Human Genetic Resources Sharing Service Platform
(2005KDA21300). All clinical samples described in the present study
were obtained from patients who had provided written informed
consent.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lombard DB, Schwer B, Alt FW and
Mostoslavsky R: SIRT6 in DNA repair, metabolism and ageing. J
Intern Med. 263:128–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sebastián C, Satterstrom FK, Haigis MC and
Mostoslavsky R: From sirtuin biology to human diseases: An update.
J Biol Chem. 287:42444–42452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tennen RI and Chua KF: Chromatin
regulation and genome maintenance by mammalian SIRT6. Trends
Biochem Sci. 36:39–46. 2011. View Article : Google Scholar
|
|
5
|
Michishita E, McCord RA, Berber E, Kioi M,
Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL,
Barrett JC, et al: SIRT6 is a histone H3 lysine 9 deacetylase that
modulates telomeric chromatin. Nature. 452:492–496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ran LK, Chen Y, Zhang ZZ, Tao NN, Ren JH,
Zhou L, Tang H, Chen X, Chen K, Li WY, et al: SIRT6 overexpression
potentiates apoptosis evasion in hepatocellular carcinoma via
BCL2-associated X protein-dependent apoptotic pathway. Clin Cancer
Res. 22:3372–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sebastián C, Zwaans BM, Silberman DM,
Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber
D, et al: The histone deacetylase SIRT6 is a tumor suppressor that
controls cancer metabolism. Cell. 151:1185–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Min L, Ji Y, Bakiri L, Qiu Z, Cen J, Chen
X, Chen L, Scheuch H, Zheng H, Qin L, et al: Liver cancer
initiation is controlled by AP-1 through SIRT6-dependent inhibition
of survivin. Nat Cell Biol. 14:1203–1211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marquardt JU, Fischer K, Baus K, Kashyap
A, Ma S, Krupp M, Linke M, Teufel A, Zechner U, Strand D, et al:
Sirtuin-6-dependent genetic and epigenetic alterations are
associated with poor clinical outcome in hepatocellular carcinoma
patients. Hepatology. 58:1054–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lefort K, Brooks Y, Ostano P, Cario-André
M, Calpini V, Guinea-Viniegra J, Albinger-Hegyi A, Hoetzenecker W,
Kolfschoten I, Wagner EF, et al: A miR-34a SIRT6 axis in the
squamous cell differentiation network. EMBO J. 32:2248–2263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Xie QR, Wang B, Shao J, Zhang T,
Liu T, Huang G and Xia W: Inhibition of SIRT6 in prostate cancer
reduces cell viability and increases sensitivity to
chemotherapeutics. Protein Cell. 4:702–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu J, Tang W, Du P, Wang G, Chen W, Li J,
Zhu Y, Gao J and Cui L: Identifying microRNA-mRNA regulatory
network in colorectal cancer by a combination of expression profile
and bioinformatics analysis. BMC Syst Biol. 6:682012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dotto GP and Karine L: miR-34a/SIRT6 in
squamous differentiation and cancer. Cell Cycle. 13:1055–1056.
2014. View
Article : Google Scholar :
|
|
17
|
Ding W, Hu W, Yang H, Ying T and Tian Y:
Prognostic correlation between MTA2 expression level and colorectal
cancer. Int J Clin Exp Pathol. 8:7173–7180. 2015.PubMed/NCBI
|
|
18
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Feng J, Yan PF, Zhao HY, Zhang FC, Zhao WH
and Feng M: SIRT6 suppresses glioma cell growth via induction of
apoptosis, inhibition of oxidative stress and suppression of
JAK2/STAT3 signaling pathway activation. Oncol Rep. 35:1395–1402.
2016. View Article : Google Scholar
|
|
21
|
Xu JH, Zhang C, Tang B, Hao YX, Chen J,
Liu T and Cui H: Effect of JAK2/STAT3/vimentin signaling pathway on
proliferation and migration of human colon cancer cells. Zhonghua
Wei Chang Wai Ke Za Zhi. 13:282–285. 2010.In Chinese. PubMed/NCBI
|
|
22
|
Lu YM, Chen W, Zhu JS, Chen WX and Chen
NW: Eriocalyxin B blocks human SW1116 colon cancer cell
proliferation, migration, invasion, cell cycle progression and
angiogenesis via the JAK2/STAT3 signaling pathway. Mol Med Rep.
13:2235–2240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H,
Fan Z, Cai J and Li Q: Berberine inhibits invasion and metastasis
of colorectal cancer cells via COX-2/GE2 mediated JAK2/STAT3
signaling pathway. PLoS One. 10:e01234782015. View Article : Google Scholar
|
|
24
|
Chae IG, Kim DH, Kundu J, Jeong CH, Kundu
JK and Chun KS: Generation of ROS by CAY10598 leads to inactivation
of STAT3 signaling and induction of apoptosis in human colon cancer
HCT116 cells. Free Radic Res. 48:1311–1321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou J, Wu A, Yu X, Zhu J and Dai H: SIRT6
inhibits growth of gastric cancer by inhibiting JAK2/STAT3 pathway.
Oncol Rep. 38:1059–1066. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kara M, Yumrutas O, Ozcan O, Celik OI,
Bozgeyik E, Bozgeyik I and Tasdemir S: Differential expressions of
cancer-associated genes and their regulatory miRNAs in colorectal
carcinoma. Gene. 567:81–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawahara TL, Michishita E, Adler AS,
Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang
HY, et al: SIRT6 links histone H3 lysine 9 deacetylation to
NF-kappaB-dependent gene expression and organismal life span. Cell.
136:62–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao C, Kim HS, Lahusen T, Wang RH, Xu X,
Gavrilova O, Jou W, Gius D and Deng CX: SIRT6 deficiency results in
severe hypoglycemia by enhancing both basal and insulin-stimulated
glucose uptake in mice. J Biol Chem. 285:36776–36784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HS, Xiao C, Wang RH, Lahusen T, Xu X,
Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, et al:
Hepatic-specific disruption of SIRT6 in mice results in fatty liver
formation due to enhanced glycolysis and triglyceride synthesis.
Cell Metab. 12:224–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kugel S, Sebastián C, Fitamant J, Ross KN,
Saha SK, Jain E, Gladden A, Arora KS, Kato Y, Rivera MN, et al:
SIRT6 Suppresses Pancreatic Cancer through Control of Lin28b. Cell.
165:1401–1415. 2016. View Article : Google Scholar :
|
|
31
|
Bauer I, Grozio A, Lasigliè D, Basile G,
Sturla L, Magnone M, Sociali G, Soncini D, Caffa I, Poggi A, et al:
The NAD+-dependent histone deacetylase SIRT6 promotes
cytokine production and migration in pancreatic cancer cells by
regulating Ca2+ responses. J Biol Chem. 287:40924–40937.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang G, Liu Z, Qin S and Li K: Decreased
expression of SIRT6 promotes tumor cell growth correlates closely
with poor prognosis of ovarian cancer. Eur J Gynaecol Oncol.
36:629–632. 2015. View Article : Google Scholar
|
|
33
|
Han Z, Liu L, Liu Y and Li S: Sirtuin
SIRT6 suppresses cell proliferation through inhibition of Twist1
expression in non-small cell lung cancer. Int J Clin Exp Pathol.
7:4774–4781. 2014.PubMed/NCBI
|
|
34
|
Dai PC, Liu DL, Zhang L, Ye J, Wang Q,
Zhang HW, Lin XH and Lai GX: Astragaloside IV sensitizes non-small
cell lung cancer cells to gefitinib potentially via regulation of
SIRT6. Tumour Biol. 39:10104283176975552017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Azuma Y, Yokobori T, Mogi A, Altan B,
Yajima T, Kosaka T, Onozato R, Yamaki E, Asao T, Nishiyama M, et
al: SIRT6 expression is associated with poor prognosis and
chemosensitivity in patients with non-small cell lung cancer. J
Surg Oncol. 112:231–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai L, Lin G, Sun L, Liu Y, Huang X, Cao
C, Guo Y and Xie C: Upregulation of SIRT6 predicts poor prognosis
and promotes metastasis of non-small cell lung cancer via the
ERK1/2/MMP9 pathway. Oncotarget. 7:40377–40386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qu N, Hu JQ, Liu L, Zhang TT, Sun GH, Shi
RL and Ji QH: SIRT6 is upregulated and associated with cancer
aggressiveness in papillary thyroid cancer via BRAF/ERK/Mcl 1
pathway. Int J Oncol. 50:1683–1692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tao NN, Ren JH, Tang H, Ran LK, Zhou HZ,
Liu B, Huang AL and Chen J: Deacetylation of Ku70 by SIRT6
attenuates Bax-mediated apoptosis in hepatocellular carcinoma.
Biochem Biophys Res Commun. 485:713–719. 2017. View Article : Google Scholar
|
|
39
|
Lee N, Ryu HG, Kwon JH, Kim DK, Kim SR,
Wang HJ, Kim KT and Choi KY: SIRT6 Depletion Suppresses Tumor
Growth by Promoting Cellular Senescence Induced by DNA Damage in
HCC. PLoS One. 11:e01658352016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang ZG and Qin CY: Sirt6 suppresses
hepatocellular carcinoma cell growth via inhibiting the
extracellular signal regulated kinase signaling pathway. Mol Med
Rep. 9:882–888. 2014. View Article : Google Scholar
|
|
41
|
Rizzo A, Iachettini S, Salvati E, Zizza P,
Maresca C, D'Angelo C, Benarroch-Popivker D, Capolupo A, Del Gaudio
F, Cosconati S, et al: SIRT6 interacts with TRF2 and promotes its
degradation in response to DNA damage. Nucleic Acids Res.
45:1820–1834. 2017. View Article : Google Scholar :
|
|
42
|
Penrose H, Heller S, Cable C, Makboul R,
Chadalawada G, Chen Y, Crawford SE and Savkovic SD: Epidermal
growth factor receptor mediated proliferation depends on increased
lipid droplet density regulated via a negative regulatory loop with
FOXO3/Sirtuin6. Biochem Biophys Res Commun. 469:370–376. 2016.
View Article : Google Scholar
|
|
43
|
Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q,
Kong S, Ye J, Gao B and Fang D: USP10 antagonizes c-Myc
transcriptional activation through SIRT6 stabilization to suppress
tumor formation. Cell Reports. 5:1639–1649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Qiu W, Zhang G, Xu S, Gao Q and
Yang Z: MicroRNA-204 targets JAK2 in breast cancer and induces cell
apoptosis through the STAT3/BCl-2/survivin pathway. Int J Clin Exp
Pathol. 8:5017–5025. 2015.PubMed/NCBI
|
|
45
|
Tung SL, Huang WC, Hsu FC, Yang ZP, Jang
TH, Chang JW, Chuang CM, Lai CR and Wang LH: miRNA-34c-5p inhibits
amphiregulin-induced ovarian cancer stemness and drug resistance
via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis.
6:e3262017. View Article : Google Scholar :
|
|
46
|
Catuogno S, Cerchia L, Romano G, Pognonec
P, Condorelli G and de Franciscis V: miR-34c may protect lung
cancer cells from paclitaxel-induced apoptosis. Oncogene.
32:341–351. 2013. View Article : Google Scholar
|
|
47
|
Wu H, Huang M, Lu M, Zhu W, Shu Y, Cao P
and Liu P: Regulation of microtubule-associated protein tau (MAPT)
by miR-34c-5p determines the chemosensitivity of gastric cancer to
paclitaxel. Cancer Chemother Pharmacol. 71:1159–1171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li G, Qiu Y, Su Z, Ren S, Liu C, Tian Y
and Liu Y: Genome-wide analyses of radioresistance-associated miRNA
expression profile in nasopharyngeal carcinoma using next
generation deep sequencing. PLoS One. 8:e844862013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo Z, Zhang L, Li Z, Li X, Li G, Yu H,
Jiang C, Dai Y, Guo X, Xiang J, et al: An in silico analysis of
dynamic changes in microRNA expression profiles in stepwise
development of nasopharyngeal carcinoma. BMC Med Genomics. 5:32012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Re M, Çeka A, Rubini C, Ferrante L, Zizzi
A, Gioacchini FM, Tulli M, Spazzafumo L, Sellari-Franceschini S,
Procopio AD, et al: MicroRNA-34c-5p is related to recurrence in
laryngeal squamous cell carcinoma. Laryngoscope. 125:E306–E312.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li F, Chen H, Huang Y, Zhang Q, Xue J, Liu
Z and Zheng F: miR-34c plays a role of tumor suppressor in HEC 1-B
cells by targeting E2F3 protein. Oncol Rep. 33:3069–3074. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu Z, Wu Y, Tian Y, Sun X, Liu J, Ren H,
Liang C, Song L, Hu H, Wang L, et al: Differential effects of
miR-34c-3p and miR-34c-5p on the proliferation, apoptosis and
invasion of glioma cells. Oncol Lett. 6:1447–1452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
López JA and Alvarez-Salas LM:
Differential effects of miR-34c-3p and miR-34c-5p on SiHa cells
proliferation apoptosis, migration and invasion. Biochem Biophys
Res Commun. 409:513–519. 2011. View Article : Google Scholar : PubMed/NCBI
|