Introduction
Oral cancer is a rare disease accounting for <5%
of all malignancies worldwide (1).
Despite the fact that it is a rare type of cancer, it has been
shown that oral cancer is associated with the use of smokeless
tobacco in middle-aged individuals >40 years of age (2). The disruption of normal cell function
by smoking and alcohol consumption can cause oral cancer, and
moreover, there is a synergistic effect if smoking and drinking are
used at simultaneously (2). Oral
cancer includes squamous epidermal carcinoma, adenoid cystic
carcinoma, mucoepidermoid carcinoma and adenocarcinoma. Among
these, the main type of oral cancer is squamous cell carcinoma
(3). The prognosis of oral cancer
varies widely depending on the tumor-node-metastasis staging system
(4). If oral cancer is detected in
its earliest stage, the majority of patients have a high 5-year
survival rate (5). The therapeutic
strategies against oral cancer include surgery, radiation and
chemo-radiotherapy (4). Some
anticancer drugs used in oral cancer are highly toxic and
inefficient (6). The toxicity of
these drugs in normal cells has been one of the major obstacles to
successful cancer chemotherapy (6). Additionally, oral cancer still has
oral cancer-specific target molecules that have not yet been
discovered, despite the suggestion of promising targets, such as
cyclooxygenase and epidermal growth factor receptor, as well as
others (7). With the further
identification of target proteins, extensive research and the
development of specific tumor biomarkers are warranted for the
effective treatment of oral cancer.
It has been reported that there are many natural
products with anticancer effects (6,8).
Among these, oridonin (Ordn) is a bioactive entkaurane diterpenoid
found in Rabdosia rubescens (9). Rabdosia rubescens is also
known as Dong Ling Cao in traditional Chinese medicine, and has
been used in the treatment of stomach aches, pharyngitis, sore
throats and coughs (8). A recent
study indicated that Ordn exerts potent antioxidant,
anti-bacterial, anti-inflammatory, pro-apoptotic, anticancer and
neurological effects (10). In
addition, Rabdosia rubescens has long been used in China due
to its low toxicity and lack of side-effects (11). However, it has not yet been proven
whether or not Ordn can be effective used in the treatment of
cancer.
Reactive oxygen species (ROS) are by-products of
normal cellular metabolism during respiration processor organic
compounds and can be beneficial or harmful to cells, depending on
their concentrations (12). A
marked increase in ROS levels can cause oxidative stress and can
induce cell death, including apoptosis, autophagy and necrosis
(13). When cells are exposed to
ROS-induced stress, the mitogen-activated protein kinase (MAPK)
cascade is sequentially activated, mainly including growth
factor-regulated extracellular signal-related kinases (ERKs), c-jun
NH2-terminal kinases (JNKs) and p38 MAPKs (14). It has been demonstrated that
apoptosis induced by ROS is mediated by p38 and JNK activation
(15). MAPKs play an important in
the regulation of cellular processes, such as cell growth and
proliferation, differentiation and apoptosis (16).
Apoptosis is an important phenomenon in cell death
induced by anticancer drugs and contributes to the elimination of
unnecessary and unwanted cells via macrophages and neighboring
cells (17). Programmed cell death
is associated with characteristic morphological and biochemical
events (18). Endoplasmic
reticulum (ER) stress can activate specific apoptotic pathways to
eliminate severely damaged cells, in which protein folding defects
cannot be resolved (19). Various
ER stress inducers have consistently been shown to induce
CCAAT/enhancer-binding protein homologous protein (CHOP), and death
receptor (DR)4 and DR5 expression on cell surfaces (20). Under the apoptotic cascade, the
collapse of mitochondrial membrane potential (MMP) is a prominent
hallmark, indicating that the mitochondrial apoptotic pathway is
consequently activated (21).
Anticancer drugs may disrupt the mitochondria by increasing the
permeability of the outer mitochondrial membrane that may result in
the obstruction of intracellular ATP synthesis, and the release of
cytochrome c (cyto c) to the cytosol to form
apoptosomes and to boost a series of caspases (22). The ability of the mitochondria to
mediate apoptosis is tightly regulated by various related proteins
(23). As a result, specific
pro-apoptotic/anti-apoptotic proteins, such as p21, p27, myeloid
cell leukemia-1 (Mcl-1), survivin, truncated Bid (tBid) and Bax can
potentially determine the response of cancer cells to the apoptotic
signal (24,25).
However, whether or not Ordn exerts pro-apoptotic
effects probably through the modulation of the p38 and JNK
signaling pathways remains unclear. Therefore, the aim of the
present study was to investigate the antitumor effects of Ordn on
the oral cancer cell lines, HN22 and HSC4 cells, and to further
elucidate the molecular mechanism involved in its anti-neoplastic
activities.
Materials and methods
Reagents and antibodies
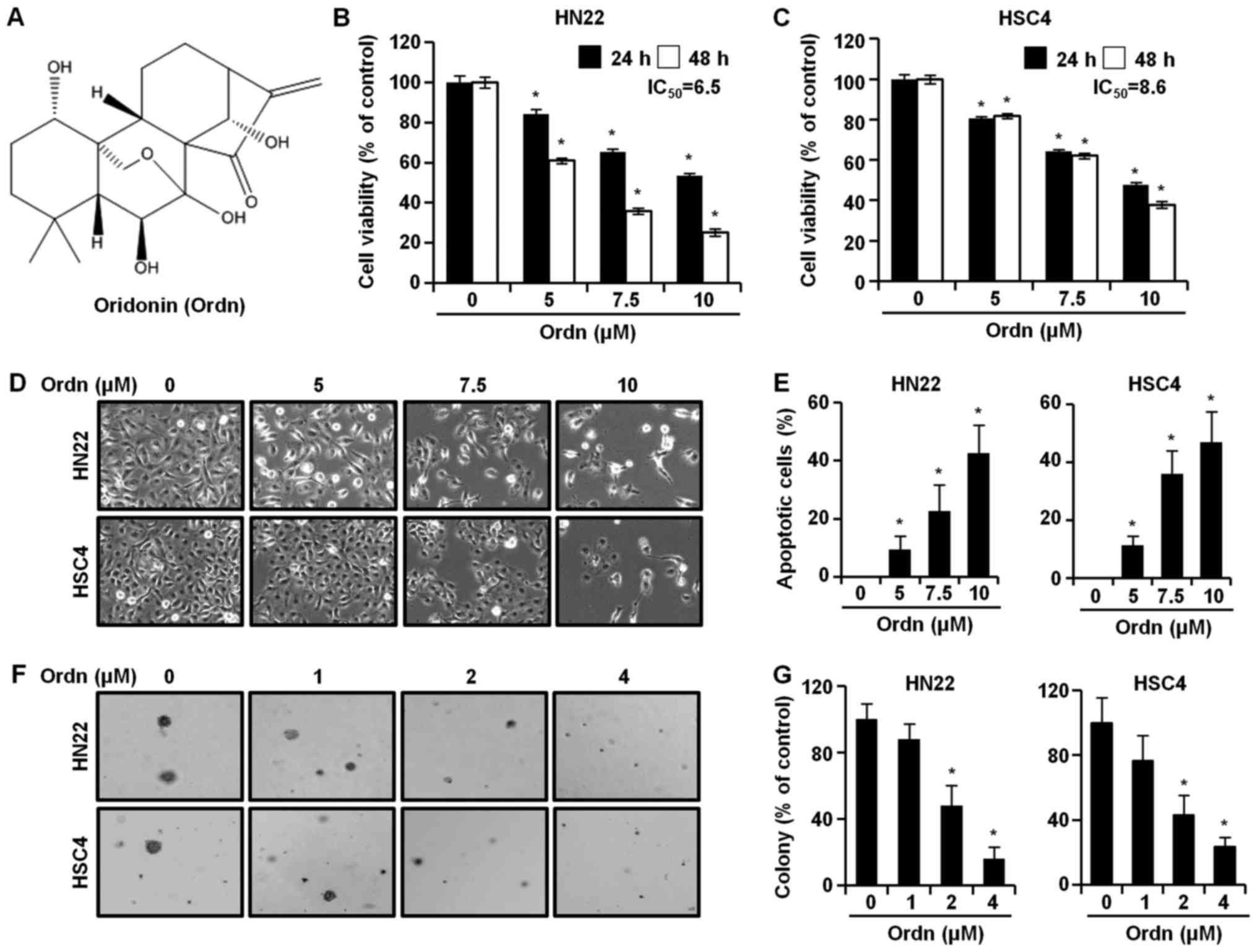
Ordn (chemical structure shown in Fig. 1A) was kindly provided by professor
Zigang Dong of China-US (Henan) Hormel Cancer Institute (Zhengzhou,
Henan, China). Dulbecco's modified Eagle's medium, fetal bovine
serum (FBS), trypsin, penicillin and streptomycin and
phosphate-buffered saline (PBS) were purchased from HyClone (Logan,
UT, USA). Antibodies against CHOP (sc-793), DR4 (sc-7863), DR5
(sc-166624), poly(ADP-Ribose) polymerase (PARP)-1 (sc-7150), p21
(sc-6246), p27 (sc-528), Mcl-1 (sc-819), survivin (sc-17779), Bax
(sc-493), cyto c (sc-13156), α-tubulin (sc-5286), cytochrome
c oxidase 4 (COX4; sc-69359), apoptotic protease activating
factor-1 (Apaf-1; sc-33870) and actin (sc-1615) were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The specific
antibodies to JNK (#9252), p-JNK (Thr183/Tyr185; #9251), p38
(#9212), p-p38 (Thr180/Tyr182; #9211) and tBid (#2002) were
obtained from Cell Signaling Technologies (Danvers, MA, USA). Basal
Medium Eagle, 4′-6-diamidino-2-phenylindole (DAPI),
N-acetyl-L-cysteine (NAC) and 3-(4,5-dimethylthiazol-2-yl)-2,5
diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich,
Inc. (St. Louis, MO, USA).
Cell culture
The HN22 (RRID:CVCL_5522) cell line has been
described previously (26), and
was provided by Dankook University (Cheonan, Korea). The HSC4
(RRID:CVCL_1289) cell line was obtained from the Human Science
Research Resources Bank (Osaka, Japan), and was provided by
Hokkaido University (Hokkaido, Japan). The cells were maintained in
Dulbecco's modified Eagle's medium containing 10% heat-inactivated
FBS and 100 U/ml each of penicillin and streptomycin at 37°C in a
5% CO2 incubator.
MTT assay
The HN22 (2 × 103/well) and HSC4 (2.5 ×
103/well) cells were seeded into 96-well plates.
Following incubation overnight, the adherent cells were exposed to
various concentrations (0, 5, 7.5 and 10 µM) of Ordn for 24
and 48 h. Following treatment, 30 µl of MTT solution (5
mg/ml) were added to each well followed by incubation for a further
2 h at 37°C. The supernatant was subsequently removed and DMSO was
then added to the cells. To solubilize the formazan, the 96-well
plates were gently mixed on a gyratory shaker for 5 min at 37°C.
The absorbance of the formazan solution was recorded at a
wavelength of 570 nm by Enspire Multimode Plate reader
(Perkin-Elmer, Akron, OH, USA). The viability results are expressed
as the IC50 mean values of 3 independent
experiments.
Anchorage-independent cell transformation
assay
The oral cancer cells suspended in Basal Medium
Eagle supplemented with FBS, gentamicin and L-glutamine were added
to 0.3% agar in a top layer over a base layer of 0.6% agar
containing Ordn (1, 2 and 4 µM). The cultures were
maintained at 37°C in a 5% CO2 incubator for 3 weeks,
and then the cell colonies were counted under a microscope (Olympus
Corporation, Tokyo, Japan).
DAPI staining
The number of cells undergoing apoptosis was
quantified after DAPI staining. Briefly, he HN22 and HSC4 cells
were treated with Ordn (5, 7.5 and 10 µM) for 48 h and then
harvested by trypsinization. The cells were washed a third time
with PBS and centrifuged at 850 × g for 5 min at 4°C. The cell
pellets were fixed in 100% methanol at room temperature for 30 min.
The cells were deposited on slides and stained with DAPI solution
in the dark. Subsequently, the DAPI-stained apoptotic cells was
observed under an Olymps IX79-DP73 fluorescence microscope (Olympus
Corporation).
Cell cycle analysis
The assay was performed using the Muse™ Cell Cycle
kit (MCH100106; Merck Millipore, Billerica, MA, USA) to measure the
DNA content at cell cycle stages (GO/G1, S and G2/M), as previously
described (27). Either the HN22
or the HSC4 cells were plated in a 6-well plate and treated with
Ordn at various concentrations (0, 5, 7.5 and 10 µM) for 48
h at 37°C. The cells were harvested and then suspended in cold PBS.
The cell pellets were fixed in cold 70% ethanol for overnight at
−20°C. After washing again with cold PBS, the cells were stained
with Muse™ Cell Cycle kit reagent. Following 30 min of incubation
at room temperature in the dark, the cell cycle distribution was
analyzed using the Muse™ cell analyzer flow cytometer (Merck
Millipore).
Annexin V staining
According to the manufacturer's instructions, the
assay was carried out using the Muse™ Annexin V and Dead Cell kit
(MCH100105; Merck Millipore). Briefly, the HN22 and HSC4 cells were
seeded in a 6-well plate and incubated at 37°C for 24 h. The cells
were treated with Ordn (0, 5, 7.5 and 10 µM), harvested,
washed twice with cold PBS and transferred to 1.5 ml
microcentrifuge tubes. Muse™ Annexin V and Dead Cell reagent was
then added to each tube, followed by incubation for a further 20
min at room temperature in the dark. The analyses of apoptotic
cells were carried out using the Muse™ cell analyzer.
Determination of ROS levels
The assay was performed using the Muse™ cell
Analyzer to determine oxidative stress induced by Ordn. The Muse™
Oxidative Stress Kit (MCH100111; Merck Millipore) allows for the
quantitative measurements of ROS levels in cells subjected to
oxidative stress. Following treatment with Ordn (0, 5, 7.5 and 10
µM), the HN22 and HSC4 cells were collected. Following
centrifugation (1,5 00 g, 5 min, room temperature), the cells were
resuspended in 1X assay buffer. Finally, 190 µl of Muse™
Oxidative Stress working solution was mixed with 10 µl of
the cell suspension and incubated at 37°C for 30 min prior to
analysis. Following incubation, the stained cells were examined
using the Muse™ cell analyzer.
Measurement of MMP
To examine the changes in mitochondrial
transmembrane potential at the early stages, MMP was measured using
the Muse™ Cell Analyzer with the Muse MitoPotential Assay kit
(MCH100110; Merck Millipore). The HN22 and HSC4 cells were seeded
on 6-well plates for 24 h and then treated with various
concentrations (0, 5, 7.5 and 10 µM) of Ordn for 48 h. The
harvested cells were washed with PBS and collected by
centrifugation at 1,500 × g for 5 min at room temperature.
Following centrifugation, the supernatant was removed and the cell
pellets were stained with the Muse™ MitoPotential working solution
for 20 min at 37°C. After the cells were stained with
7-aminoactinomycin D (7-AAD) for 5 min at room temperature, the
stained cells were examined using the Muse™ cell analyzer.
Multi-caspase assay
The assay was carried out using the Muse™
Multi-caspase assay kit (MCH100109; Merck Millipore) to assess the
activation of multiple caspases (caspase-1, -3, -4, -5, -6, -7, -8
and -9). Briefly, HN22 and HSC4 cells were seeded at 37°C in a
6-well plate for 24 h. After treatment with various doses of Ordn
(0, 5, 7.5 and 10 µM), the cells were washed in PBS and
resuspended in 1X caspase buffer. Muse™ Multi-Caspase reagent
working solution was added to the cells and kept incubated for 30
min at 37°C. One hundred and fifty microliters of Muse™ Caspase
7-AAD working solution was added in each tube and incubated for 5
min at room temperature. The data were analyzed using the Muse™
cell analyzer.
Western blot analysis
The cells were harvested and washed with cold PBS.
Cell lysates was carried out using RIPA lysis buffer and the lysate
was then subjected to centrifugation at 16,000 × g for ~30 min at
4°C. Total protein concentrations in the supernatant were
determined through calibration with BSA. Total protein extracts
were separated electrophoretically using 10, 12 or 15% SDS-PAGE
gels and transferred onto polyvinylidene fluoride membranes. After
the transfer, the membranes were blocked for ~2 h at room
temperature with skim milk. The membranes incubated overnight at
4°C with antibodies (all diluted 1:1,000) against CHOP, DR4, DR5,
PARP, C-PARP, p38, p-p38, JNK, p-JNK, p21, p27, Mcl-1, survivin,
tBid, Bax, cyto c, COX4, α-tubulin, Apaf-1 and actin. After
washing 5 times, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody [goat anti-rabbit IgG
(#31460, 1:6,000 dilution), goat anti-mouse IgG (#31430, 1:5,000
dilution) (both from Thermo Fisher Scientific, Waltham, MA, USA)
and donkey anti-goat IgG (sc-2020, 1:4,000 dilution; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. Immunoblotting
was performed using the ECL Plus Western blotting detection system
(Santa Cruz Biotechnology, Inc.) and then each protein was
quantified by ImageJ Instrument software.
Statistical analysis
The data are presented as the means ± SD.
Statistical analysis of the data was performed using the Prism 5.0
statistical package. The statistical significance of differences
among groups were analyzed using ANOVA and Fisher's Least
Significant Difference post hoc test. Mean values were considered
statistically significant at P<0.05. In the present study, the
data are representative of 3 independent experiments in
triplicate.
Results
Ordn inhibits the proliferation and
colony-forming ability of oral cancer cells
To examine the effects of Ordn on the viability of
the oral cancer cell lines, HN22 and HSC4, we performed MTT assay,
measuring the activity of mitochondrial dehydrogenases (28). We treated the cells with Ordn for
different periods of time (24 or 48 h) and various concentrations.
As a result, Ordn significantly decreased the viability of both the
HN22 (Fig. 1B) and HSC4 (Fig. 1C) cells in a dose- and
time-dependent manner. As shown in Fig. 1B and C, the IC50 values
of Ordn were determined from the dose-response curves of the HN22
and HSC4 cells, accounting for 6.5 and 8.6 µM in the HN22
and HSC4 cells, respectively. Treatment of the HN22 cells with Ordn
at 5, 7.5 and 10 µM for 48 h decreased cell viability to
60.97, 35.81 and 25.14% relative to that of the control,
respectively. Similarly, the viability of the HSC4 cells treated
with Ordn at 5, 7.5 and 10 µM for 48 h dose-dependently
decreased to 81.77, 62.02 and 37.81% relative to that of the
control, respectively. Morphological changes were examined under an
optical microscope following treatment of the oral cancer cells
with Ordn at concentrations of 0, 5, 7.5 and 10 µM for 48 h.
As shown in Fig. 1D, it was found
that the control cells exhibited normal cell shapes with a clear
outline and were spread evenly in the culture plates. Following 48
h of treatment with Ordn, a significant proportion of the oral
cancer cells became dislodged from the plates. In addition, the
remaining adherent oral cancer cells exhibited typical
morphological changes, such as cell shrinkage, floating and large
intercellular spacing. Ordn markedly suppressed colony formation in
both the HN22 and HSC4 cells in a concentration-dependent manner
(Fig. 1F and G). Ordn (2
µM) inhibited colony formation by 48 and 43.14% in the HN22
and HSC4 cells, respectively.
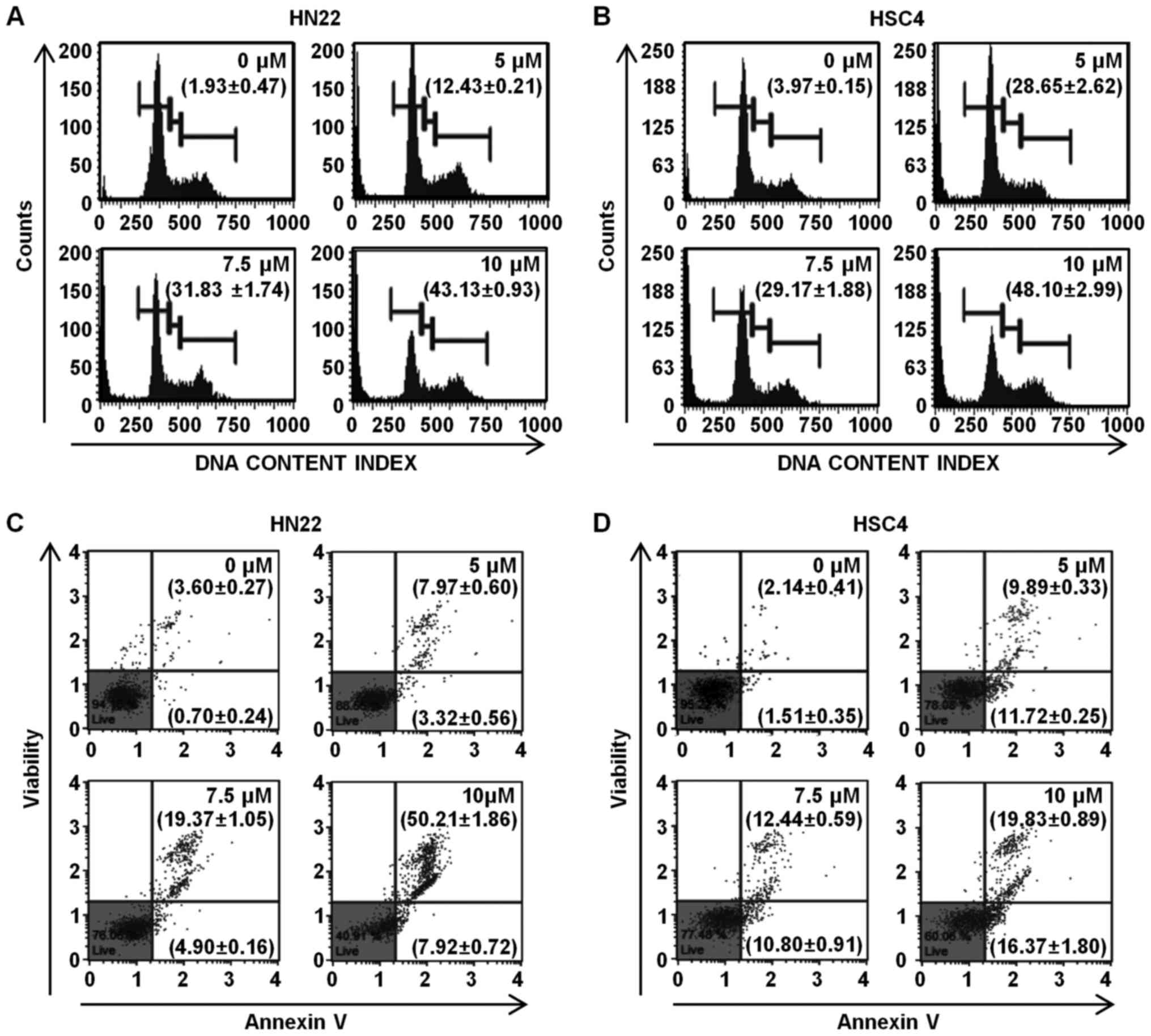
Ordn induces the apoptosis of HN22 and
HSC4 cells
To investigate chromatin condensation, fragmented
nuclei and nuclear shrinkage, the nuclei of the Ordn-treated cells
were observed after DAPI staining. The DAPI-stained nuclei of the
HN22 and HSC4 cells were observed using fluorescence microscopy.
The DAPI-stained cells were quantified and the apoptotic cell
numbers were assessed as means with standard deviation by the
graph. The results revealed apoptotic nuclei in the HN22 and HSC4
cells following Ordn treatment at concentrations of 5, 7.5 and 10
µM for 48 h. The percentage of apoptosis was increased to
9.34, 24.44 and 43.18% in the HN22 cells and 10.83, 26.58 and
50.71% in the HSC4 cells with the increasing concentrations of Ordn
at 5, 7.5 and 10 µM, respectively (Fig. 1E). To investigate the mechanisms
responsible for Ordn-induced apoptosis, the HN22 and HSC4 cells
were examined using the Cell Cycle kit and Annexin V and Dead cell
kit in Muse™ cell analyzer. The HN22 and HSC4 cells were treated
with 0, 5, 7.5 and 10 µM Ordn for 48 h. We found that Ordn
resulted in a significant concentration-dependent cell cycle arrest
in the sub-G1 phase. The cell cycle distribution in the sub-G1
phase was 1.93±0.47, 12.43±0.21, 31.83±1.74 and 43.13±0.93% in the
HN22 cells treated with Ordn at 0, 5, 7.5 and 10 µM,
respectively (Fig. 2A). The sub-G1
phase distribution in the HSC4 cells was 3.97±0.15, 28.65±2.62,
29.17±1.88 and 48.10±2.99% in the cells treated with Ordn at 0, 5,
7.5 and 10 µM, respectively (Fig. 2B). To examine cell apoptosis,
untreated or Ordn-treated HN22 and HSC4 cells were stained with
Annexin V/7-AAD. As shown in Fig. 2C
and D, treatment of the cells with Ordn at various
concentrations (0, 5, 7.5 and 10 µM) resulted in a
dose-dependent increase in the early and late apoptotic population
(4.3±0.4, 11.29±0.13, 24.27±0.99 and 58.13±1.88% in the HN22 cells,
and 3.65±0.06, 21.61±0.55, 23.24±1.47 and 36.2±2.64% in the HSC4
cells).
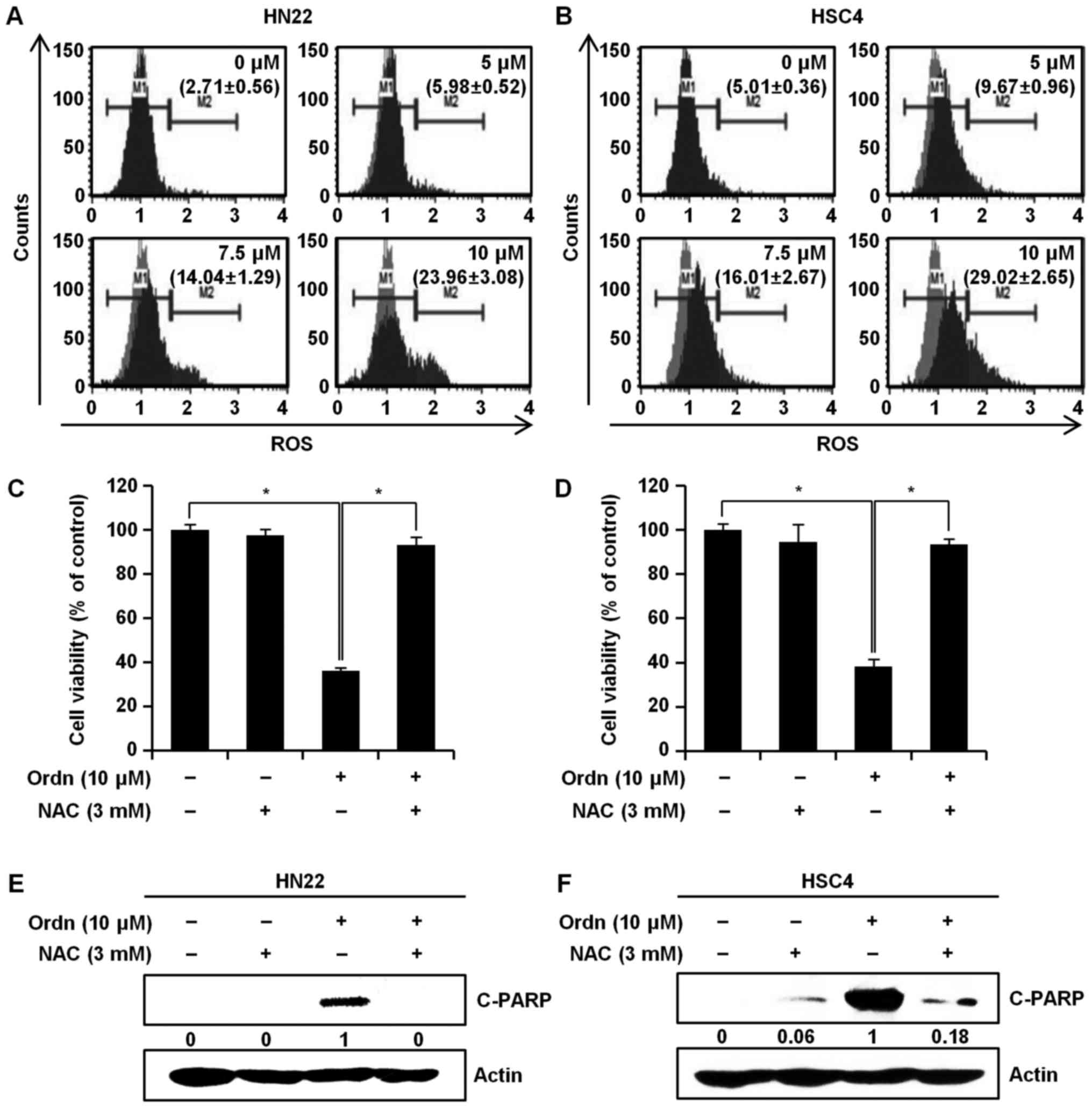
Ordn increases ROS generation
As reported previously, the increased generation of
ROS can induce cell apoptosis (12,29).
Thus, we measured the intracellular ROS levels using the Muse™ cell
analyzer with the Muse™ Oxidative Stress kit. As shown in Fig. 3A and B, a marked increase in ROS
levels was observed in the cells treated with Ordn at 0, 5, 7.5 and
10 µM for 48 h. In the HN22 cells, we observed a significant
increase in ROS production, of 2.71±0.56, 5.98±0.52, 14.04±1.29 and
23.96±3.08% (M2 phase of ROS positively stained cells) at Ordn
concentrations of 0, 5, 7.5 and 10 µM, respectively
(Fig. 3A). For the HSC4 cells, the
obtained results were 5.01±0.36, 9.67±0.96, 16.01±2.67 and
29.02±2.65% of the M2 phase cells, respectively (Fig. 3B). We then examined the protective
effects of NAC in the Ordn-treated HN22 and HSC4 cells. NAC is
widely used as a free radical scavenger (30). The cells were pretreated with 3 mM
NAC, followed by the addition of Ordn at 10 µM for 48 h. As
shown in Fig. 3C and D, the loss
of cell viability induced by Ordn was prevented by NAC. Moreover,
western blot analysis of the HN22 and HSC4 cells revealed that Ordn
enhanced the cleavage of PARP; however, pretreatment with NAC
reversed these effects (Fig. 3E and
F).
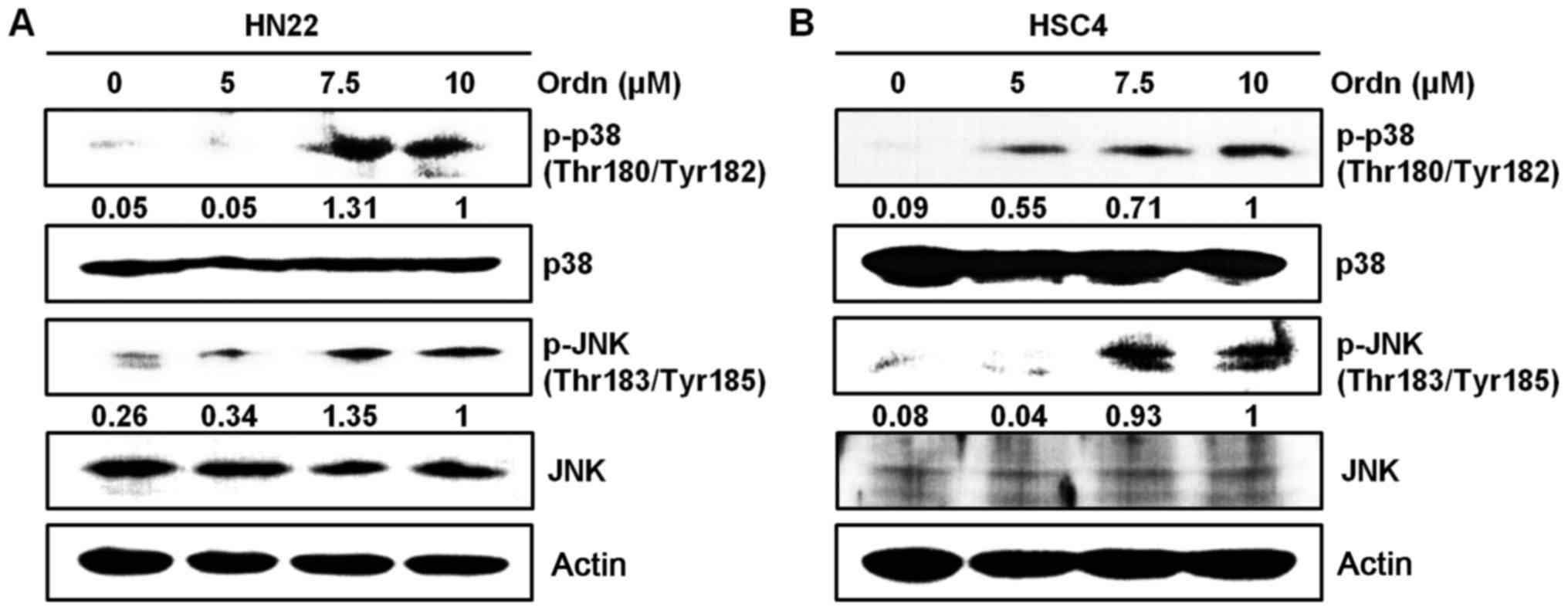
Ordn induces the apoptosis of oral cancer
cells via the ROS-related p38 and JNK pathways
The MAPK pathways are one of the numerous cascades
downstream of the ROS signaling pathway closely associated with
apoptosis, as previously reported (31). In this study, we carried out
western blot analysis to determine whether Ordn can induce the
activation of the MAPK signaling pathway. Therefore, we examined
the changes in the expression of proteins associated with the MAPK
pathway, including p38 and JNK in the oral cancer cells following
treatment with Ordn. The phosphorylation levels of p38 and JNK were
markedly increased in response to Ordn treatment in the HN22 and
HSC4 cells (Fig. 4).
Ordn regulates the factors related to the
apoptosis of oral cancer cells
A previous study provided evidence that ROS
generation is increased in ER stress (32). In addition, a close association has
been identified between DR4 and DR5 expression and ER stress
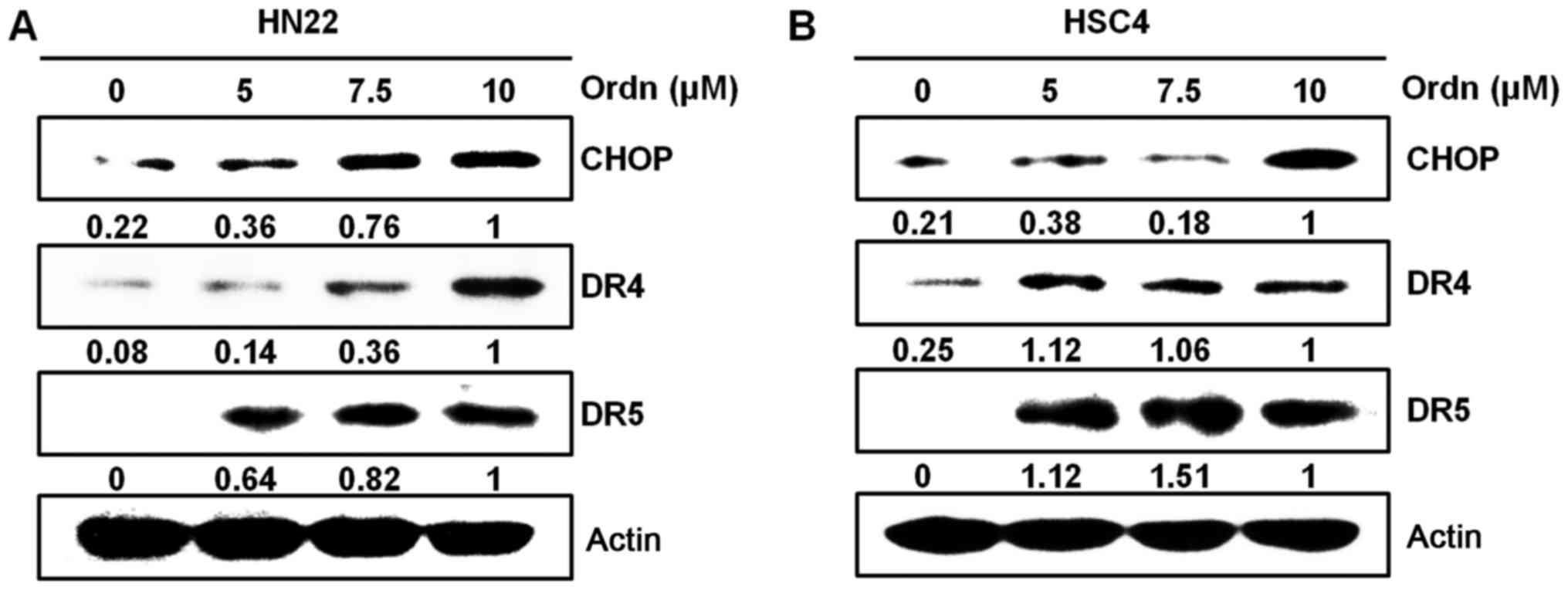
(20). As CHOP is an ER
stress-inducible transcription factor (20), in this study, we examined whether
Ordn treatment induces ER stress in the HN22 and HSC4 cells. Using
western blot analysis, we examined whether the CHOP, DR4 and DR5
protein levels were upregulated following treatment of the cells
with Ordn. Ordn treatment increased CHOP levels in the oral cancer
cells, preceding the upregulation of the DR4 and DR5 levels
(Fig. 5). To further characterize
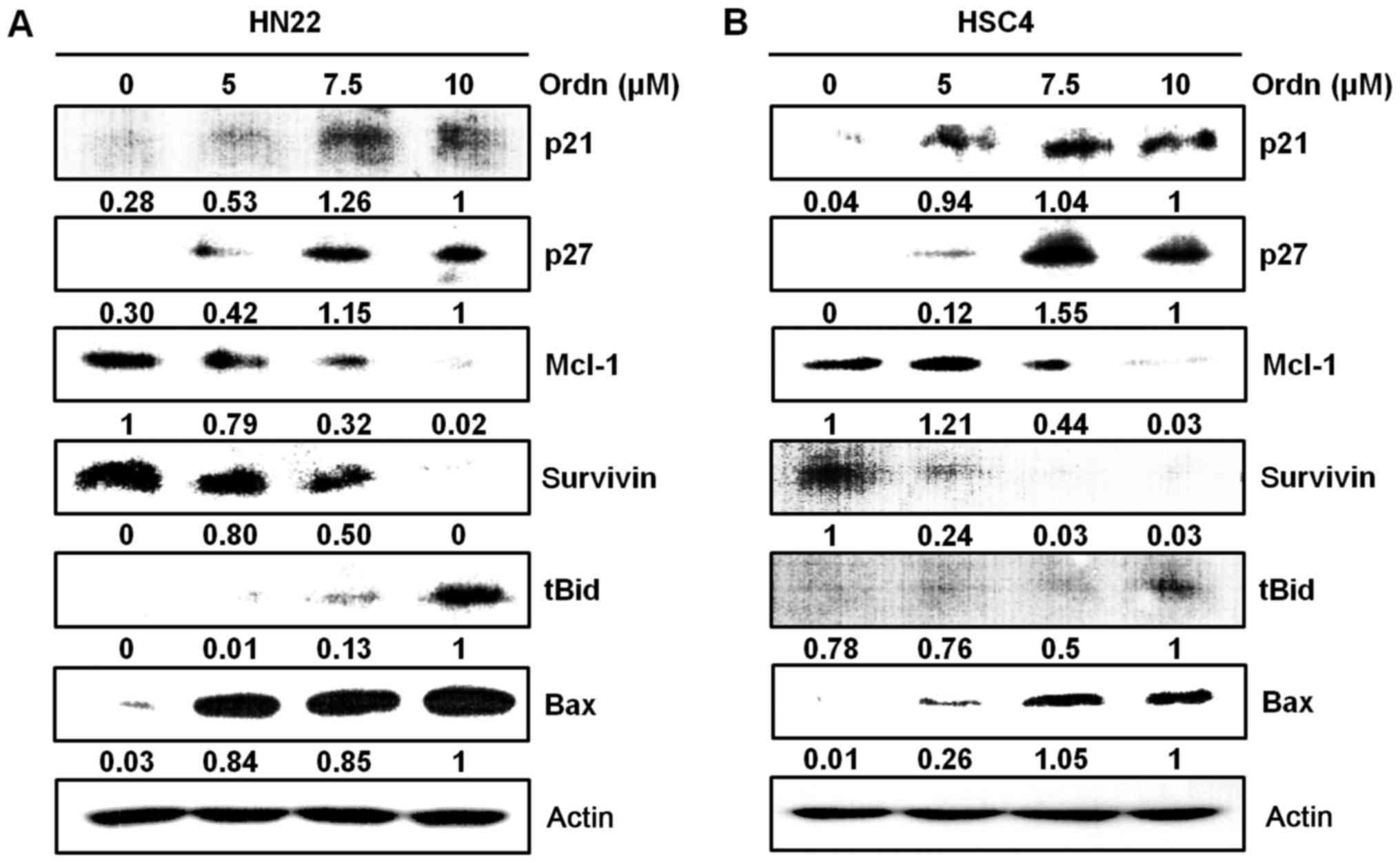
the molecular mechanisms responsible for Ordn-induced apoptosis,
the expression levels of cell cycle modulators (p21 and p27),
pro-apoptotic proteins (tBid and Bax) and anti-apoptotic proteins
(Mcl-1 and survivin) were detected in the HN22 and HSC4 cells
treated with Ordn. We found that Ordn decreased the expression of
Mcl-1 and survivin, and increased p21, p27, tBid and Bax expression
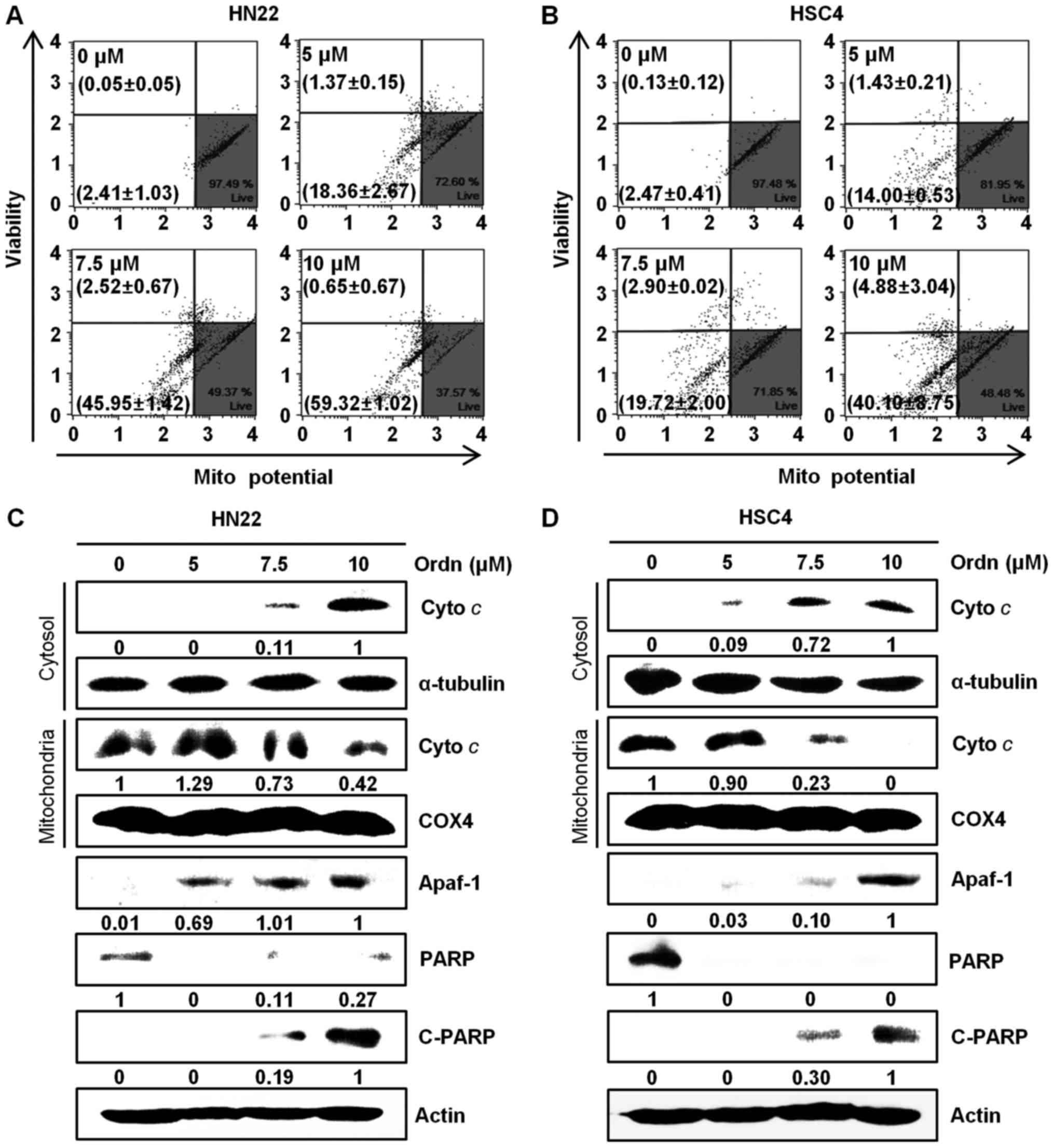
(Fig. 6). The Loss of
mitochondrial inner trans-membrane potential is a reliable
indicator of mitochondrial dysfunction (21). This phenomenon is associated with
the early stages of apoptosis (33). In this study, following treatment
of the cells with Ordn, the state of mitochondrial membranes was
assessed using a Muse™ cell analyzer. Due to the accumulated
fluorescent dye within inner membrane of intact mitochondria,
control cells emit a high fluorescence intensity (34). Treatment of the cells with Ordn at
a high concentration led to a decrease in fluorescence. Following
treatment with Ordn at concentrations of 0, 5, 7.5 and 10
µM, the percentage of depolarized HN22 cells was 2.41±1.03,
18.36±2.67, 45.95±1.42 and 59.32±1.02%, respectively (Fig. 7A). The HSC4 cells exhibited a
depolarized population of 2.47±0.41, 14.00±0.53, 19.72±2.00 and
40.10±8.75% at concentrations of 0, 5, 7.5 and 10 µM Ordn,
respectively (Fig. 7B).
Furthermore, we examined the changes in the expression of
downstream molecules that can occur after the loss of MMP. Firstly,
we analyzed the release of cyto c as an apoptosis-related
mitochondrial downstream molecule by western blot analysis. The
cyto c protein is the pro-apoptotic mitochondrial protein
located in the intermembrane space (35). Our data indicated that the amount
of cyto c in the cytoplasm increased as a result of
mitochondrial release in the HN22 and HSC4 cells treated with Ordn
(Fig. 7C and D). During the
apoptotic cascades, Apaf-1 and PARP play an important role
(35). Thus, in this study, the
expression levels of Apaf-1, PARP and cleaved PARP in the oral
cancer cells treated with Ordn were examined by western blot
analysis. As shown in Fig. 7C and
D, the expression levels of Apaf-1 and cleaved PARP were
increased significantly, whereas the expression of PARP was
decreased in the HN22 and HSC4 cells treated with Ordn. These
results indicated that Ordn regulated these proteins in a
concentration-dependent manner. It is well known that the release
of cyto c from the mitochondria can trigger a cascade of
caspases, which is associated with the final pathway of cell
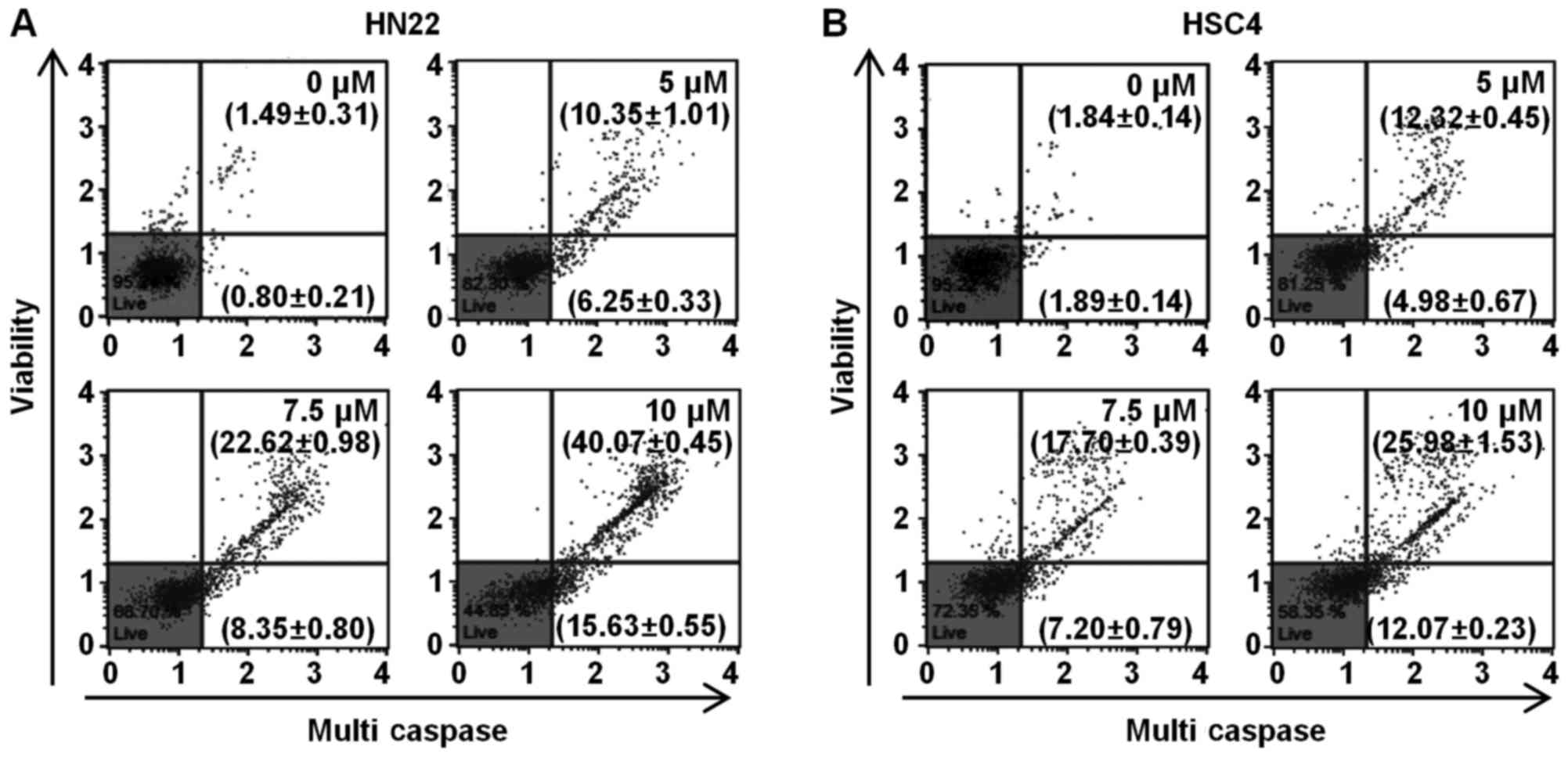
apoptosis (36). The Muse™
Multi-Caspase assay kit was used to detect the presence of multiple
caspases (caspase-1, -3, -4, -5, -6, -7, -8 and -9) apart from
caspase-2. To determine whether caspase plays a role in the
Ordn-mediated apoptosis of oral cancer cells, the HN22 and HSC4
cells were examined using the Muse™ cell analyzer after Muse™
Multi-Caspase Reagent and Muse™ Caspase 7-AAD staining. The number
represents the percentage of cells with caspase activity (lower
right quadrant), and cell population of caspase activity/dead cells
(upper right quadrant) in each condition. Multi-caspase activity
was activated in the HN22 and HSC4 cells depending on the
concentration of Ordn (Fig. 8).
The results indicated that the apoptosis of oral cancer cells was
induced by Ordn via the activation of caspases.
Discussion
The majority of patients with oral cancer have a
high 5-year survival rate if the disease is detected in its
earliest stage (37). Generally,
various targets, such as cyclooxygenase and epidermal growth factor
receptor have been suggested; however, oral cancer still has no
specific target molecule (4).
Currently, anticancer drugs used in the treatment of oral cancer
are highly toxic (6). Therefore,
further research and the development of specific tumor biomarkers
is warranted in order to enhance the efficacy of oral cancer
treatment. It has been reported that Ordn has an anti-inflammatory
activity (8). Thus, in this study,
we examined the anticancer effects of Ordn on HN22 and HSC4 oral
cancer cells, and also aimed to elucidate the underlying
mechanisms.
To assess the anticancer effects of Ordn on the OSCC
cells, we conducted MTT assay, which is widely used to detect cell
number, proliferation, cell viability, cell survival and toxicity
(38). We found that Ordn
significantly suppressed cell proliferation and the colony-forming
ability of both the HN22 and HSC4 cells in a dose-dependent manner
(Fig. 1). Cell viability was
further confirmed, based on the changes in cell morphological
features using a microscope (Fig.
1D). Additionally, cell apoptosis was further corroborated by
DAPI staining, propidium iodide staining and Annexin V/7-AAD
staining (Figs. 1E and 2). The growth inhibitory effects induced
by Ordn were associated with an increase in the sub-G1 apoptotic
population in the HN22 and HSC4 cells. Additionally, it was
suggested that Ordn may be associated with an increase in sub-G1
apoptotic population of OSCC cells through the p21 and p27
pathways, as Ordn increased the expression of p21 and p27, which
are cell cycle regulatory proteins (Fig. 6). Apoptosis is mediated in an
orchestrated manner by two major pathways that is mediated by death
receptors on the cell surface (extrinsic), and by mitochondria
(intrinsic) (39). Due to the
translocation of plasma membrane phosphatidylserine to the cell
surface outer leaflet, apoptotic cells can be identified via the
binding of Annexin V, which has a high affinity for
phosphatidylserine (40).
Furthermore, 7-AAD, a fluorescent DNA-binding agent, can
discriminate the cells that are alive, dead, or in the early or
late stages of apoptosis (41). In
this study, the oral cancer cells treated with Ordn were found to
become Annexin V-positive in a dose-dependent manner, as shown by
the rightward movement of the scatter plot compared with the
control cells (Fig. 2C and D).
Thus, Ordn can effectively induce the apoptosis of HN22 and HSC4
cells. It has been reported that CHOP directly regulates DR4 and
DR5 expression during cell apoptosis to link between ER stress and
DR5 expression (20). CHOP
upregulation precedes the increase in DR4 and DR5 levels (42). In the present study, it was
demonstrated that treatment of the oral cancer cells with Ordn
induced the expression of CHOP, DR4 and DR5 (Fig. 5). We provided some evidence that
Ordn triggers ER stress. A number of mechanisms have been proposed
to explain ROS-mediated apoptosis and MAPK activation (15). ROS are responsible for the
activation of the JNK and p38 pathways, and consequently lead to an
increase in the levels of other pro-apoptotic molecules in cells
(43). In this study, we evaluated
whether Ordn triggers intracellular ROS production and examined
whether ROS mediate JNK and p38 MAPK signaling. Our results were
quantitatively detected by MUSE™ to measure intracellular ROS
levels. We found that Ordn led to a significant increase in ROS
levels in a concentration-dependent manner (Fig. 3A and B). To verify the direct
effects of Ordn-induced ROS production during cell apoptosis, we
pretreated the cells with NAC, a ROS scavenger (44), prior to Ordn treatment. As shown in
Fig. 3C and D, pretreatment of the
cells with NAC significantly suppressed Ordn-induced apoptosis. In
addition, NAC attenuated the activation of the cleavage of PARP
(Fig. 3E and F). These findings
indicate that ROS play an important role in Ordn-induced oral
cancer cell apoptosis.
The MAPK signaling pathways are composed of several
sub-families of kinases, including p38, JNK (16). The subfamilies have been greatly
implicated in controlling cell proliferation, differentiation and
apoptosis (15). In this study, to
examine whether MAPK pathways are involved in Ordn-induced
apoptosis, we examined the activation of several protein kinases.
It was found that Ordn induced the phosphorylation of p38 and JNK
(Fig. 4). The results demonstrated
that Ordn-induced apoptosis probably occurs through the regulation
of p38 and JNK signaling pathways in the HN22 and HSC4 cells. Ordn
also altered the expression of specific
pro-apoptotic/anti-apoptotic targets, such as tBid, Bax, Mcl-1 and
survivin which are implicated in the apoptotic response (Fig. 6). Indeed, mitochondria metabolic
pathways play crucial roles in cell apoptosis (18). MMP is crucial for the proton
gradient across the mitochondria membrane and is lost due to the
opening of the mitochondrial permeability transition pore (21). The depolarization of the
mitochondrial membranes may lead to severe consequences, including
a decrease in ATP synthesis and the redistribution of pro-apoptotic
mitochondrial factors (21). The
results of this study indicated that Ordn induced a dose-dependent
collapse of MMP in both the HN22 and HSC4 cells (Fig. 7A and B). The loss of mitochondrial
transmembrane potential leads to the release of cyto c from
the intermembrane space into the cytosol, suggesting the
involvement of the mitochondrial pathway in cell apoptosis
(22). In this study, Ordn
treatment led to cyto c release, which was confirmed by
western blot analysis. In support of these findings, we observed
that the release of cyto c into the cytosol was clearly
associated with Apaf-1 and the cleavage of PARP (Fig. 7C and D). It is noteworthy that the
upregulation of cleaved PARP was observed, as PARP was considered
to be an important indicator of cell apoptosis (45). The release of cyto c can
lead to the activation of caspases, which are crucial effectors of
apoptosis and the end-point features of apoptosis (22). Caspases are frequently associated
with cleavage of a set of proteins, resulting in disassembly of the
cell (25). In the present study,
the sequential activation of multi-caspases was induced by Ordn
treatment, suggesting that caspases cascade functioned as crucial
effectors for the triggering of apoptotic machinery by Ordn in HN22
and HSC4 cells (Fig. 8).
In conclusion, it appears to be clear that Ordn
directly induces cell apoptosis probably through ROS generation and
MAPK signaling pathways. These results further support the
hypothesis that Ordn exerts anticancer and antioxidant effects on
oral cancer cells. Ordn appears to be a promising drug candidate
that can arrest the growth of oral cancer cells in the development
of future anti-oral cancer treatments. Therefore, further studies
using animal studies and clinical trials are warranted in order to
evaluate and validate the anticancer effects of Ordn.
Acknowledgments
Not applicable.
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
Ordn
|
oridonin
|
|
ROS
|
reactive oxygen species
|
|
DR
|
death receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERKs
|
extracellular signal-related
kinases
|
|
JNKs
|
c-jun NH2-terminal kinases
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
NAC
|
N-acetyl-L-cysteine
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5
diphenyltetrazolium bromide
|
|
MMP
|
mitochondrial membrane potential
|
|
7-AAD
|
7-aminoactinomycin D
|
|
DAPI
|
4′-6-diamidino-2-phenylindole
|
|
CHOP
|
CCAAT/enhancer-binding protein
homologous protein
|
|
Mcl-1
|
myeloid cell leukemia-1
|
|
tBid
|
truncated Bid
|
|
Apaf-1
|
apoptotic protease activating
factor-1
|
|
cyto c
|
cytochrome c
|
|
PARP
|
poly(ADP-Ribose) polymerase
|
Notes
[1]
Funding
This study was supported by grants (16182MFDS391)
from the Korean Ministry of Food and Drug Safety in 2017. This
study was also carried out with the support of the 'Cooperative
Research Program for Agriculture Science and Technology Development
(Project no. PJ012704012018)' project of the National Institute of
Animal Science, Rural Development Administration, Republic of
Korea. This research was also supported by grants (81572812) from
the National Natural Science Foundation of Science.
[2] Authors'
contributions
HNO, JHSe, JIC and JHSh contributed to the design
of the study and wrote the manuscript. HNO, JHSe, MHL, GY, SSC, KL,
HC, KBO, YSC, HK and ALH were responsible for data acquisition,
data analysis and interpretation. HNO, JIC and JHSh were
responsible for article revision. MHL, GY, SSC, KL, HC, KBO, YSC
and HK were responsible for data interpretation and methodology.
JIC and JHSh were responsible for funding acquisition and
supervision. All authors have read and approved the final version
of this manuscript.
[3] Availability
of data and materials
The analyzed datasets generated during the study
are available from the corresponding author on reasonable
request.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Schneider K, Roller M, Kalberlah F and
Schuhmacher-Wolz U: Cancer risk assessment for oral exposure to PAH
mixtures. J Appl Toxicol. 22:73–83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Awan KH and Patil S: Association of
smokeless tobacco with oral cancer - Evidence from the South Asian
Studies: A Systematic Review. J Coll Physicians Surg Pak.
26:775–780. 2016.PubMed/NCBI
|
|
3
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma - an update. CA Cancer J
Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
5
|
Brocklehurst P, Kujan O, O'Malley LA,
Ogden G, Shepherd S and Glenny AM: Screening programmes for the
early detection and prevention of oral cancer. Cochrane Database
Syst Rev. 11:CD0041502013.
|
|
6
|
Maggioni D, Biffi L, Nicolini G and
Garavello W: Flavonoids in oral cancer prevention and therapy. Eur
J Cancer Prev. 24:517–528. 2015. View Article : Google Scholar
|
|
7
|
Kao J, Sikora AT and Fu S: Dual EGFR and
COX-2 inhibition as a novel approach to targeting head and neck
squamous cell carcinoma. Curr Cancer Drug Targets. 9:931–937. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Z and Chen Y: Oridonin, a promising
antitumor natural product in the chemotherapy of hematological
malignancies. Curr Pharm Biotechnol. 15:1083–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding Y, Ding C, Ye N, Liu Z, Wold EA, Chen
H, Wild C, Shen Q and Zhou J: Discovery and development of natural
product oridonin-inspired anticancer agents. Eur J Med Chem.
122:102–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Owona BA and Schluesener HJ: Molecular
insight in the multifunctional effects of oridonin. Drugs R D.
15:233–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Han T, Liao J, Hu X, Xu S, Tian K,
Gu X, Cheng K, Li Z, Hua H, et al: Oridonin, a promising
ent-Kaurane diterpenoid lead compound. Int J Mol Sci.
17:172016.
|
|
12
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamogashira T, Fujimoto C and Yamasoba T:
Reactive oxygen species, apoptosis, and mitochondrial dysfunction
in hearing loss. BioMed Res Int. 2015:6172072015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darling NJ and Cook SJ: The role of MAPK
signalling pathways in the response to endoplasmic reticulum
stress. Biochim Biophys Acta. 1843:2150–2163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jalmi SK and Sinha AK: ROS mediated MAPK
signaling in abiotic and biotic stress- striking similarities and
differences. Front Plant Sci. 6:7692015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang G, Shi LZ and Chi H: Regulation of
JNK and p38 MAPK in the immune system: Signal integration,
propagation and termination. Cytokine. 48:161–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urra H, Dufey E, Lisbona F, Rojas-Rivera D
and Hetz C: When ER stress reaches a dead end. Biochim Biophys
Acta. 1833:3507–3517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tait SW and Green DR: Mitochondria and
cell signalling. J Cell Sci. 125:807–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: apoptosomes or mitochondria? Genes Cells. 3:697–707.
2998PubMed/NCBI
|
|
25
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cardinali M, Pietraszkiewicz H, Ensley JF
and Robbins KC: Tyrosine phosphorylation as a marker for aberrantly
regulated growth-promoting pathways in cell lines derived from head
and neck malignancies. Int J Cancer. 61:98–103. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darzynkiewicz Z: Cytometry of the cell
cycle: In search for perfect methodology for DNA content analysis
in tissue specimens. Cell Cycle. 9:3395–3396. 2010. View Article : Google Scholar
|
|
28
|
Twentyman PR and Luscombe M: A study of
some variables in a tetrazolium dye (MTT) based assay for cell
growth and chemo-sensitivity. Br J Cancer. 56:279–285. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong CH and Joo SH: Downregulation of
reactive oxygen species in apoptosis. J Cancer Prev. 21:13–20.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun SY: N-acetylcysteine, reactive oxygen
species and beyond. Cancer Biol Ther. 9:109–110. 2010. View Article : Google Scholar :
|
|
31
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways? J Signal
Transduct. 2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verfaillie T, Rubio N, Garg AD, Bultynck
G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A,
et al: PERK is required at the ER-mitochondrial contact sites to
convey apoptosis after ROS-based ER stress. Cell Death Differ.
19:1880–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castedo M, Ferri K, Roumier T, Métivier D,
Zamzami N and Kroemer G: Quantitation of mitochondrial alterations
associated with apoptosis. J Immunol Methods. 265:39–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poncet D, Boya P, Metivier D, Zamzami N
and Kroemer G: Cytofluorometric quantitation of apoptosis-driven
inner mitochondrial membrane permeabilization. Apoptosis.
8:521–530. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar
|
|
36
|
Monian P and Jiang X: Clearing the final
hurdles to mitochondrial apoptosis: Regulation post cytochrome c
release. Exp Oncol. 34:185–191. 2012.PubMed/NCBI
|
|
37
|
Montero PH and Patel SG: Cancer of the
oral cavity. Surg Oncol Clin N Am. 24:491–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stockert JC, Blázquez-Castro A, Cañete M,
Horobin RW and Villanueva A: MTT assay for cell viability:
Intracellular localization of the formazan product is in lipid
droplets. Acta Histochem. 114:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ilmarinen P, Moilanen E and Kankaanranta
H: Mitochondria in the center of human eosinophil apoptosis and
survival. Int J Mol Sci. 15:3952–3969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He Z, Sun Q, Liang YJ, Chen L, Ge YF, Yun
SF and Yao B: Annexin A5 regulates Leydig cell testosterone
production via ERK1/2 pathway. Asian J Androl. 18:456–461. 2016.
View Article : Google Scholar :
|
|
41
|
Balmer J, Zulliger R, Roberti S and
Enzmann V: Retinal cell death caused by sodium iodate involves
multiple caspase-dependent and caspase-independent cell-death
pathways. Int J Mol Sci. 16:15086–15103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stolfi C, Pallone F and Monteleone G:
Molecular targets of TRAIL-sensitizing agents in colorectal cancer.
Int J Mol Sci. 13:7886–7901. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bjørn ME and Hasselbalch HC: The role of
reactive oxygen species in myelofibrosis and related neoplasms.
Mediators Inflamm. 2015:6480902015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang F, Lau SS and Monks TJ: A dual role
for poly(ADP-ribose) polymerase-1 during caspase-dependent
apoptosis. Toxicol Sci. 128:103–114. 2012. View Article : Google Scholar : PubMed/NCBI
|