Introduction
Hepatocellular carcinoma (HCC), as the most common
type of liver cancer, and is still listed as one of the leading
causes of cancer-related mortality worldwide, mainly due to its
association with severe cirrhosis induced by alcohol, hepatitis B
and hepatitis C infection (1). The
incidence of hepatocellular carcinoma in China has been the highest
over the past years, accounting for >50% of the global incidence
of hepatocellular carcinoma (2).
Despite great efforts of basic and clinical investigations focusing
on hepatocellular carcinoma, our knowledge of the molecular
pathology of this malignant solid tumor remains limited. The
diagnosis of hepatocellular carcinoma has mainly relied on a blood
test and the application of several imaging modalities, such as
sonography and computer tomography, and confirmation by tissue
biopsy (3). Biomarker-based
accurate diagnosis and targeting therapy has become one of the
major promising innovations in current medicine (4). Previous studies have characterized
alpha-fetoprotein (AFP) (5),
carbohydrate antigen 19-9 (CA 19-9) (6), carcinoembryonic antigen (CEA)
(7) and cancer antigen 125 (CA125)
(8) as diagnostic biomarkers for
hepatocellular carcinoma (9),
demonstrating the great prospect of biomarker application in liver
cancer diagnosis and treatment. The identification of novel
biomolecules closely associated with hepatocellular carcinoma
initiation and progression may promote the development of novel
diagnostic and therapeutic methods.
Circular RNAs (circRNAs) refer to the group of newly
identified endogenous RNA molecules that can form covalently closed
continuous loops (10,11), which were first identified early in
1976 from the RNA virus (12).
Previously, this particular type of circRNA structure did not raise
great interest among the research community and has long been
regarded as an unusable byproduct of accidental errors in
post-transcriptional RNA processing (12,13).
Benefitting from the great progress in new sequencing technology,
the function of circRNA molecules has been re-evaluated with the
identification of large numbers of such molecules from various
species associated with pleiotropic physiological processes
(10,13–15).
For instance, the revelation of the simultaneous existence of
circRNAs from more than hundreds of gene transcripts by sequencing
a number of normal human cells and cancerous cell lines has
indicated that this particular RNA molecule structure produced by
non-canonical RNA splicing may be a prevalent and important form of
gene expression in human cells (16). Recent studies have indicated that
circRNAs can function as miRNA sponges and RNA binding proteins
(RBPs) to modulate gene expression, and can also be translated into
protein or small peptides with specific functions (10,11,13,14,17,18).
As a critical link in the gene expression regulatory network,
circRNA molecules have been shown to be involved in various
pathological processes, including neurodegenerative and
cardiovascular diseases, as well as in human cancers of different
organs (10,14,19).
circRNAs are also involved in the pathology of human
liver cancers. A recent system biology study identified 127
differentially expressed circRNAs in liver cancer cells, among
which the top 5 candidates were further investigated and were shown
to be able to significantly distinguish malignant liver cells by
forming a complex circRNA-miRNA-mRNA network associated with
various signaling pathways, such as p53 and cell cycle-related
cellular regulation (20). In
addition, many novel circRNA molecules have recently been found to
be linked to hepatitis B-related hepatocellular carcinoma and other
liver cancer types, demonstrating the great potential of circRNAs
as novel biomarkers for liver cancer diagnosis and candidate
targets for anticancer drug development (21–25).
However, considering the complexity of hepatocellular carcinoma
initiation and progression, our understanding of the roles of
circRNAs in liver cancer remains largely unexplored.
Cyclin-dependent kinase 13 (CDK13) functions
as one of the five transcription-regulating kinases in human cells
by forming the Cdk13/Cyclin K complex to regulate transcription and
pre-mRNA molecule processing (26). Specifically, the overexpression of
CKD13 has been shown to alter pre-RNA splicing (27). Moreover, CDK13 has been
identified as a novel component of the perinucleolar compartment
(PNC), which is a subnuclear structure found mainly in cancer cells
and is closely associated with metastatic capacity (27). More importantly, a genomic analysis
using a single-nucleotide polymorphism (SNP) chip revealed that the
copy number of CDK13 was frequently amplified in primary
hepatocellular carcinoma and was also closely associated with the
onset age in patients with HCC the disease (28), suggesting a potential role for
CDK13 in liver cancer pathology. Recent large-scale sequencing
studies have revealed that the CDK13 transcript could form a
circRNA in various human tissues and cells (29–32).
However, the specific role and underlying mechanisms of action of
CDK13-related circRNAs in the pathology of liver cancer
remain unknown.
Recently, researchers have reported that the
occurrence of liver cancer is related to certain signaling
pathways. These relevant signaling pathways include the
Ras/Raf/Mek/Erk pathway and PI3K/Akt/mTOR pathways (33). Tang et al found out that
miR-125 suppresses the PI3K/AKT pathway to inhibit migration and
invasion in liver cancer (34). In
addition, the JAK/STAT signaling pathway is also recognized in
liver cancer as it unregulates the tumor tissues (35). Both PI3K/AKT and JAK/STAT signaling
play a significant role in cell growth and proliferation (36,37),
which is related to the progression of liver cancers. Moreover, the
PI3K and JAK/STAT signaling pathways participate in tumor
occurrence, development and infiltration in various types of
cancer, such as breast cancer, nasopharyngeal carcinoma and
colorectal cancer (38–40). However, research on circRNAs and
these two signaling pathways is limited.
In the present study, we report the functional
characterization of a novel circRNA, circCDK13 (hsa_circ_0001699),
transcribed from the human CDK13 gene in liver cancer cells
and tissues (29). The expression
and regulatory function of circCDK13 in liver cancer cell cycle
progression and downstream signaling pathways, as well as its in
vitro tumorigenic capacity, were also investigated, providing
direct evidence of the association of circCDK13 in the progression
of liver cancer.
Materials and methods
Clinical tissues, cell lines, and
animals
The liver cancer tissues used in this study were
collected from 62 patients with hepatocellular carcinoma who were
hospitalized at the Third Affiliated Hospital of Sun Yat-sen
University (Guangzou, China), after obtaining written informed
consent signed by each patient from February, 2015 to February,
2016 (Table I). The sample
collection processes and following analysis were approved by the
Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen
University prior to the surgery. The clinical samples were
confirmed by experienced clinical pathologists before further
analysis. The liver cancer cell lines, HepG2, Huh7 and SK-Hep1 were
obtained from the Cell Bank of Chinese Academy of Sciences
(Shanghai, China). The cell lines, MHCC97-H, MHCC97-L and SMCC7721,
and the normal liver cell line, L-02, were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
MHCC-LM3 cells were provided by the Liver Cancer Institute of
Zhongshan Hospital, Fudan University (Shanghai, China). The liver
cancer cells were cultured in DMEM supplemented with 0.1% fetal
bovine serum (FBS) at 37°C with 5% CO2. For the
tumorigenesis assay, we used 12 BALB/c nude mice (4 weeks old,
female, weighing 14–16 g) obtained from the Shanghai Laboratory
Animal Center, Chinese Academy of Sciences (Shanghai, China). The
mouse were housed in a controlled environment (temperature,
20–26°C; humidity, 40–70%; 12-h light/dark cycle). The mouse
experiments in this study was performed under the Affidavit of the
Approval of Animal Ethical and Welfare of the Laboratory Animal
Center of the Third Affiliated Hospital of Sun Yat-sen
University.
 | Table ISummary of the patient
characteristics. |
Table I
Summary of the patient
characteristics.
| Case | Sex | Age (years) | Diagnosis | Grade | Tumor number | Tumor size |
|---|
| 1 | M | 58 | HCC | II | 1 | >3 cm |
| 2 | M | 53 | HCC | II | >1 | >3 cm |
| 3 | M | 34 | HCC | III | >1 | >3 cm |
| 4 | M | 53 | HCC | II | >1 | >3 cm |
| 5 | F | 70 | HCC | II | 1 | >3 cm |
| 6 | M | 40 | HCC | II | >1 | >3 cm |
| 7 | M | 54 | HCC | II | >1 | >3 cm |
| 8 | M | 42 | HCC | II–III | 1 | >3 cm |
| 9 | M | 43 | HCC | II | 1 | >3 cm |
| 10 | M | 28 | HCC | II | 1 | >3 cm |
| 11 | M | 68 | HCC | II | 1 | >3 cm |
| 12 | F | 53 | HCC | II | 1 | >3 cm |
| 13 | F | 28 | HCC | II | >1 | >3 cm |
| 14 | M | 62 | HCC | II | 1 | >3 cm |
| 15 | M | 28 | HCC | II | >1 | >3 cm |
| 16 | M | 43 | HCC | II–III | >1 | >3 cm |
| 17 | M | 75 | HCC | II | 1 | >3 cm |
| 18 | M | 35 | HCC | I | 1 | >3 cm |
| 19 | M | 32 | HCC | II | 1 | >3 cm |
| 20 | M | 41 | HCC | III | 1 | >3 cm |
| 21 | M | 42 | HCC | II | 1 | >3 cm |
| 22 | M | 66 | HCC | II | >1 | >3 cm |
| 23 | M | 54 | HCC | II | 1 | ≤3 cm |
| 24 | M | 64 | HCC | II | >1 | >3 cm |
| 25 | M | 62 | HCC | I | >1 | >3 cm |
| 26 | M | 43 | HCC | II | 1 | >3 cm |
| 27 | M | 59 | HCC | II | >1 | >3 cm |
| 28 | M | 61 | HCC | II | >1 | >3 cm |
| 29 | M | 36 | HCC | III | 1 | >3 cm |
| 30 | M | 34 | HCC | II | 1 | >3 cm |
| 31 | M | 34 | HCC | II | >1 | >3 cm |
| 32 | M | 57 | HCC | III | 1 | >3 cm |
| 33 | M | 76 | HCC | III | 1 | >3 cm |
| 34 | M | 72 | HCC | I | >1 | >3 cm |
| 35 | F | 46 | HCC | II | >1 | >3 cm |
| 36 | M | 64 | HCC | III | 1 | >3 cm |
| 37 | M | 29 | HCC | II | 1 | >3 cm |
| 38 | M | 50 | HCC | II | 1 | ≤3 cm |
| 39 | M | 52 | HCC | III | 1 | >3 cm |
| 40 | M | 43 | HCC | II | 1 | >3 cm |
| 41 | F | 67 | HCC | III | >1 | >3 cm |
| 42 | M | 47 | HCC | II | 1 | ≤3 cm |
| 43 | M | 31 | HCC | II | 1 | >3 cm |
| 44 | M | 66 | HCC | I | >1 | >3 cm |
| 45 | M | 54 | HCC | II | >1 | ≤3 cm |
| 46 | M | 42 | HCC | II | >1 | >3 cm |
| 47 | M | 59 | HCC | II | 1 | >3 cm |
| 48 | M | 65 | HCC | I | 1 | >3 cm |
| 49 | M | 45 | HCC | III | >1 | ≤3 cm |
| 50 | M | 58 | HCC | I | 1 | >3 cm |
| 51 | M | 53 | HCC | II | >1 | >3 cm |
| 52 | M | 53 | HCC | II | >1 | >3 cm |
| 53 | M | 68 | HCC | II | 1 | >3 cm |
| 54 | M | 35 | HCC | I | 1 | >3 cm |
| 55 | M | 43 | HCC | II | 1 | >3 cm |
| 56 | M | 71 | HCC | III | 1 | >3 cm |
| 57 | M | 38 | HCC | II | >1 | ≤3 cm |
| 58 | M | 61 | HCC | III | 1 | >3 cm |
| 59 | M | 58 | HCC | II | 1 | ≤3 cm |
| 60 | F | 41 | HCC | III | >1 | >3 cm |
| 61 | M | 51 | HCC | II | >1 | >3 cm |
| 62 | M | 62 | HCC | II | >1 | >3 cm |
RNA extraction, Sanger sequencing and
reverse transcription-quantitavie PCR (RT-qPCR)
Total RNA samples from the hepatocellular carcinoma
tissues and the cell lines were extracted using the PureLink™ RNA
Mini kit (cat. no. 12183018A; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following the manufacturer's instructions. The
tissues were fully homogenized in liquid nitrogen, and the fine
powder was used for RNA extraction. The synthesis of cDNA with ~2
µg of total RNA was completed using the High-Capacity cDNA
Reverse Transcription kit (cat. no. 4368813; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Quantitative (real-time) polymerase chain reaction (qPCR) was
carried out to determine the relative gene transcript or circular
RNA levels using the SYBR Select Master Mix kit (cat. no. 4472908;
Applied Biosystems, Foster City, CA, USA) following the
manufacturer's instructions. For the quantification of circNA
levels, divergent primers were designed, and opposite-directed
pairs of primers were used to assess the gene transcript level. The
GAPDH gene was used as the internal control. Each
quantitative analysis was performed at least 3 times for
statistical analysis. For the identification of the ligation site
of circCDK13, the PCR products were analyzed by Sanger sequencing.
Briefly, the purified double-stranded cDNA was repaired at the end
and added poly-A ends and then ligated. The PCR method was then
used for the amplification of the cDNA. The library of cDNA was
then detected using Agilent 2100 system software and quantified by
qPCR. The library was then sequenced using the illumina Hiseq 2000
platform (Illumina, San Diego, CA, USA). The sequences of the
primers used in this study were as follows: CDK13 exon 5
forward, 5′-AGATGCTTTGGATTTCAAGAAGG-3′; CDK13 exon 2
reverse, 5′-TGACGACTTCTGGATCTAGACAATC-3′; CDK13 exon 6
forward, 5′-CCACTTACACCAAGCATAGGA-3′; CDK13 exon 9 reverse,
5′-CTTCTTTATCTTCAGGCAGCAT-3′; and GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′; and reverse,
5′-GACAAGCTTCCCGTTCTCAG-3′.
Plasmid construction and cell
transfection
The establishment of liver cancer cells
overexpressing circRNA was conducted according to the protocols
described in a previous study (41). Briefly, the cDNA of circCDK13 was
synthesized by the Nuclee Biotechnology Company (Guangzhou, China)
and cloned into the lentiviral expression vector, pLVX-IRESneo
(Clontech Laboratories Inc., San Francisco, CA, USA; pLVX-IRESneo
was used as the control vector). The cDNA sequence encoding exons
2–5 of the CDK13 gene, together with the additional sequence
500 bp upstream and 300 bp downstream of the non-linear splice
site, were used in this study. Following construction, direct
sequencing was performed to verify the sequences. Subsequently, the
construct was used to transfect the liver cancer cells with
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). The
expression of circular RNA in transfected cells was confirmed by
RT-qPCR using specific primers pairs. At 24 h following
transfection, the following experiments were carried out.
MTS assay
The proliferative potential of the liver cancer
cells was determined using the
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt) (MTS) assay using the MTS Assay (Cell Proliferation)
(Colorimetric) kit (cat. no. ab197010; Abcam, Cambridge, UK)
according to the manufacturer's instructions. Briefly, the cells
transfected with lentiviral expression vectors were counted under a
microscope, and 100 µl cell suspension was seeded into each
well of a 96-well plate (1×104 cells/well) and cultured
at 37°C with a suitable level of CO2. Following
adherence, 10 µl MTS solution were added at 24, 48 and 72 h.
The absorbance at 490 nm (OD490) was measured using a
spectrophotometer (ELx800; BioTek Instruments, Inc., Winooski, VT,
USA) following culture at 37°C for 4 h. At least 3 biological and
technical replicates were analyzed.
Cell cycle assay
The effects of the circRNA on cell cycle progression
were evaluated by measuring the percentages of cells in the G0/G1,
M and G2 phases of the cell cycle using the Cell Cycle kit (cat.
no. F559763; Forevergen, Guangzhou, China) following the
manufacturer's instructions. Briefly, the liver cancer cells were
washed 3 times with PBS solution, suspended by digestion with
trypsin, and washed and collected by centrifugation at 300 × g for
1 min. After cell counting, 1×106 cells were fixed with
70% ice ethanol at −20°C overnight, collected by centrifugation at
500 × g for 5 min, and then washed twice with PBS solution. The
cell pellets were mixed and resuspended in 500 µl cell cycle
staining solution, cultured at 37°C for 30 min, and finally
detected by flow cytometry (FACSCalibur flow cytometer; BD
Biosciences, San Jose, CA, USA). To evaluate statistical
significance, at least 3 biological and technical replicates were
performed for this assay.
Cell migration assay
The Transwell culture system was used to determine
the effects of the circRNA overexpression on the liver cancer cell
migratory capacity. The cultured liver cancer cells transfected
with the expression plasmids were digested into a single-cell
suspension with trypsin and counted under a microscope (IM-35; Carl
Zeiss Inc., Thornwood, NY, USA). The cell density was adjusted to
1×106/ml using serum-free medium. The upper chambers
were filled with a 100 µl cell suspension, and the lower
chambers were filled with complete medium containing 10% FBS. The
cells were then cultured at 37°C for 24 h in a humidified
atmosphere with 5% CO2. The cells in the lower chambers
were then fixed with 4% paraformaldehyde for 10 min, washed once
with PBS solution, stained with crystal violet for 10 min, washed
again with PBS solution, and finally counted and photographed with
a Leica DC300F digital microscope (Leica Camera AG, Solms,
Germany). Three biological replicates were performed for the
statistical analysis of the cell proliferative capacity.
Cell invasion assay
The invasion capacity of the liver cancer cells was
also measured using the Transwell culture system. Specifically, a
layer of Matrigel Basement Membrane Matrix (BD Biocoat; BD
Biosciences, Bedford, MA, USA) was laid on the inner sides of the
Transwell chambers according to the manufacturer's instructions, to
mimic the in vivo extracellular matrix environment. The
liver cancer cells were suspended by trypsin digestion, and the
cell density was determined by counting under a microscope and
diluted to 1×106/ml. The upper and lower chambers were
filled with a 100 µl cell suspension and 600 µl
culture medium, respectively, and then maintained at 37°C for 24 h.
The cancer cells in the lower chambers were then fixed with 4%
paraformaldehyde for 15 min, washed once with PBS solution, stained
with 1% crystal violet solution for 10 min, washed again with PBS
solution, and finally counted and photographed. Three biological
replicates were evaluated for statistical analysis.
Microarray analysis
The expression patterns of the liver cancer cells
overexpressing circCDK13 were analyzed by microarray assay as
previously described (42).
Briefly, total RNA samples were extracted from the liver cancer
cells using the PicoPure™ RNA Isolation kit (cat. no. KIT0204;
Applied Biosystems) following the manufacturer's instructions. cDNA
synthesis was carried out using the Agilent in situ
Hybridization Kit plus (Agilent Technologies, Wilmington, DE, USA).
The complementary double-stranded cDNA labeled with Cy3 or Cy5 was
then hybridized and subjected to microarray analysis using Agilent
Human G4131F 4×44 K. The spot intensities were then determined and
analyzed using ImaGene 8.0 software and Nexus Expression software
(BioDiscovery, El Segundo, CA, USA). Gene spots showing differences
exceeding 1.5-fold were defined as differentially expressed genes
and selected for subsequent clustering analysis. The heatmap and
enrichment analysis of the signaling pathways were completed by
searching against the Koyto Encyclopedia of Genes and Genomes
(KEGG) database (www.genome.jp/kegg/pathway.html).
Western blot analysis
The abundances of several major signaling components
were measured by western blot analysis as previously described
(42). After the cells were washed
with PBS solution, total proteins were lysed in RIPA buffer (50 mM
Tris HCl, pH 7.4; 150 mM NaCl; 1% NP-40; 0.5% sodium deoxycholate),
extracted from the liver cancer cells and boiled with sample
loading buffer at 100°C for 5 min. BCA assay was used to quantify
the total protein. Approximately 30 mg proteins was loaded and
separated by 12% SDS-PAGE, transferred onto PVDF membranes, and
incubated with 5% lipid-free milk solution, primary antibodies and
secondary antibodies. The blots were finally developed with ECL
solution (Amersham™) and exposed to film. The primary antibodies
used in this study included anti-AKT1/2/3 (cat. no. ab106693),
anti-AKT (phospho; cat. no. ab106693), anti-STAT3 (cat. no.
ab68153), anti-STAT3 (phospho Y705) (cat. no. ab76315) and
anti-GAPDH (cat. no. ab8245) antibodies, which were purchased from
Abcam. The secondary antibody horseradish peroxidase-conjugated
goat anti-mouse or anti-rabbit secondary antibodies (cat. no.
115-035-044) were purchased from Jackson ImmunoResearch (West
Grove, PA, USA). For statistical quantification, 3 biological
repeats were performed, and GAPDH was used as the internal
standard.
Tumorigenicity assay
The liver cancer cells in logarithmic growth phase
were collected by washing with PBS solution and digestion with
trypsin, and resuspended in PBS solution. The cell density was
adjusted to 5×107/ml. Nude mice were subcutaneously
injected with 100 µl Sk-hep-1 NC or Sk-hep-1 circCDK13 cell
suspension (6 mice in each group) into the left armpit. The tumor
volumes were calculated each week using the following formula:
Tumor volume (mm3) = tumor length × tumor width × tumor
width/2. The tumors were finally weighed and photographed 45 days
later.
Statistical analysis
Significant differences between multiple groups were
analyzed by the one-way ANOVA with the Least Significant Difference
(LSD) test, and differences between 2 groups were analyzed by the
Student's t-test using the SPSS 18.0 software package. A
significant difference was defined by a P-value <0.05.
Results
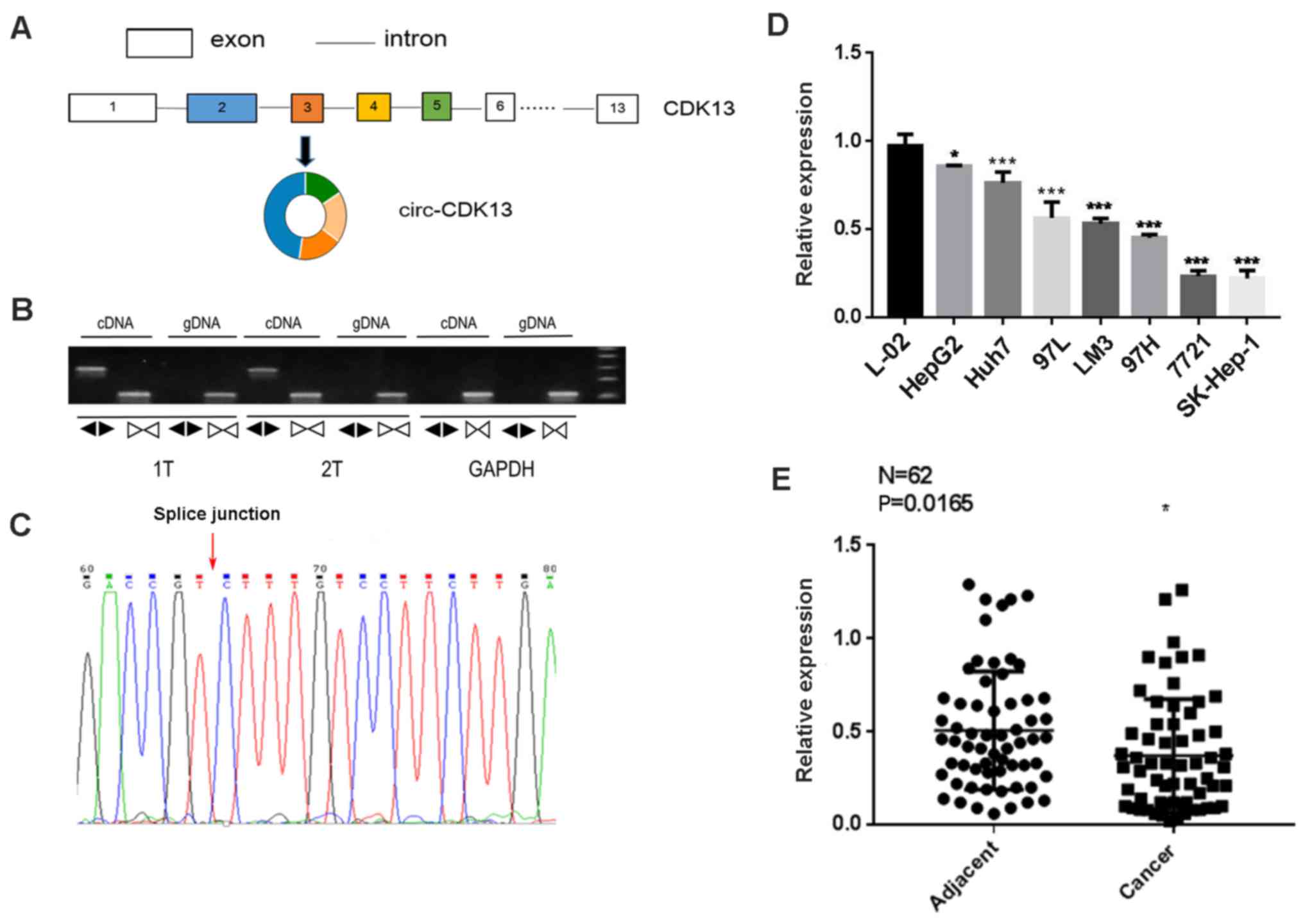
circCDK13 expression is decreased in
liver cancer
By bioinformatics searches against the circBase
database (29) and several
large-scale investigations using deep sequencing (30–32),
the circRNA circCDK13 was produced from 4 exons encoded by
CDK13, as shown in Fig. 1A.
The ligation of the second and fifth exons of the CDK13
transcript formed the circular structure of circCDK13 (Fig. 1A). For the investigation of the
circCDK13 in the context of liver cancer, the expression of
circCDK13 in the cancer tissues from 2 patients with hepatocellular
carcinoma was examined by RT-qPCR with 2 different primer pairs
targeting the genomic DNA and circRNA, respectively. Our results
revealed the circular form of CDK13 RNA, circCDK13, in the
clinical samples from these 2 patients with hepatocellular
carcinoma (Fig. 1B). The ligation
site of circCDK13 was identified by Sanger sequencing of the PCR
products, further confirming the presence of the circular from of
circCDK13 (Fig. 1C). Subsequently,
we measured the expression levels of circCDK13 in 7 liver cancer
cell lines, and found that the circCDK13 levels in these cancerous
cells were all significantly lower than those in the control L-02
cells, supporting the potential involvement of circCDK13 in
liver cancer progression (Fig.
1D). The decrease in circCDK13 expression was also
observed in a large number of patients with hepatocellular
carcinoma by RT-qPCR (Fig. 1E).
The observed expression levels of circCDK13 in both liver cancer
tissues and cell lines suggested that circCDK13 might play
regulatory roles during the initiation and development of
hepatocellular carcinoma.
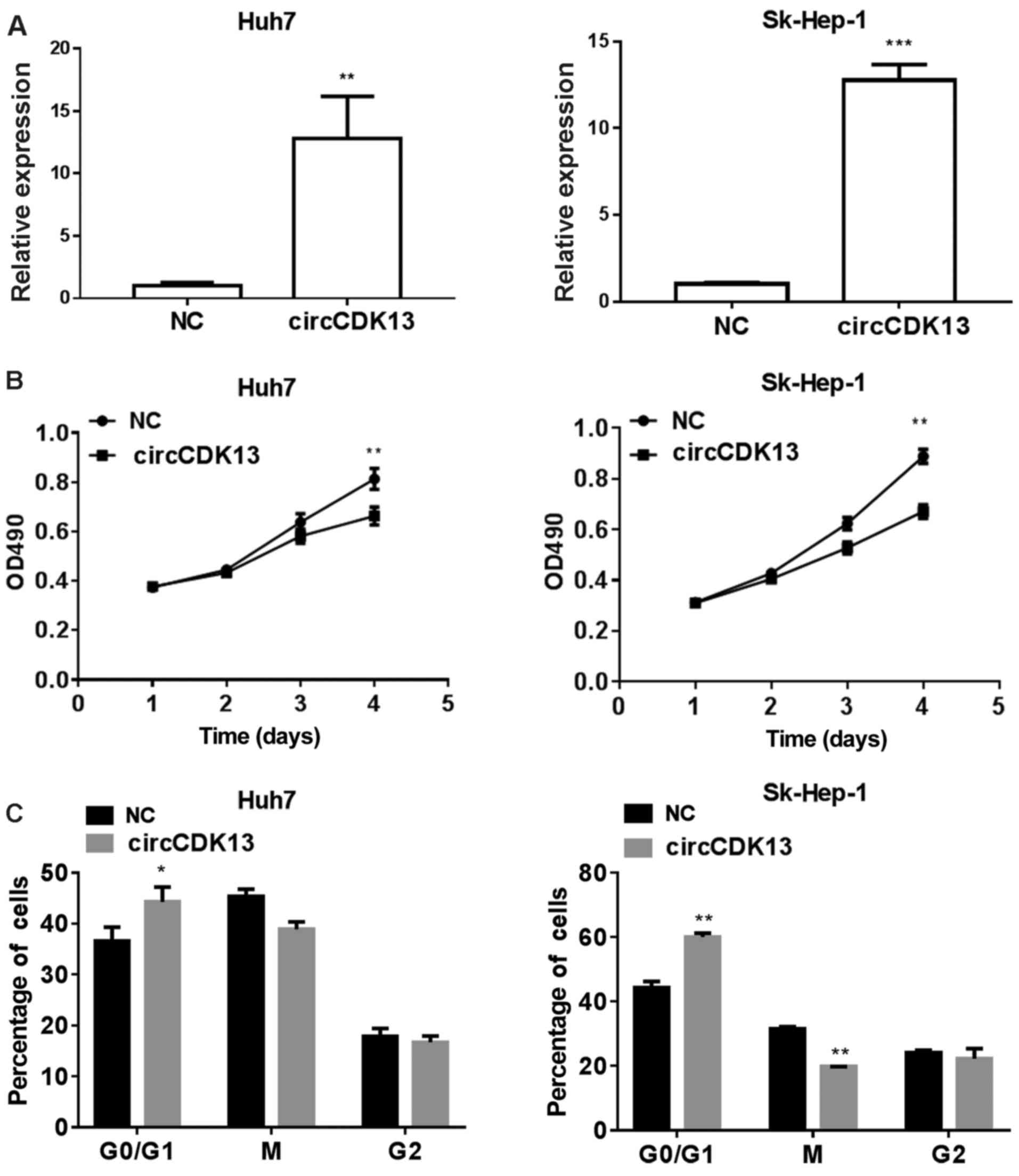
Overexpression of circCDK13 suppresses
cancer cell proliferation and cell cycle progression
To investigate the possible roles of circCDK13
during liver cancer development, we established SK-Hep-1 and Huh7
cells overexpressing circCDK13 using the lentiviral expression
system as described in the Materials and methods. According to the
results shown in Fig. 1D, the
expression of circCDK13 differed significantly between the
different cell lines. The expression of circCDK13 in the HepG2 and
Huh7 cells was higher than that in the other cells, while the
expression of circCDK13 was lowest in the SK-Hep-1 cells. Thus, the
HepG2, Huh7 and SK-Hep-1 were the candidate cells. However, after
screening, we found that the invasive ability of the HepG2 cells
was weak, and these cells were thus not suitable for following
experiments. Hence, the Huh7 and SK-Hep-1 cells were selected for
use in further experiments. By RT-qPCR analysis, we observed that
the expression levels of circCDK13 in the SK-Hep1 and Huh7 cells
were markedly elevated following transfection with the
overexpression plasmid compared with the negative control (Fig. 2A). Moreover, the proliferation
rates of both the SK-Hep1 and Huh7 cells overexpressing circCDK13
were significantly lower than those of the control group (Fig. 2B), indicating the role of circCDK13
in regulating liver cancer cell proliferation during liver cancer
development. Additionally, the percentages of liver cancer cells in
the G0/G1, M and G2 phases of the cell cycle were analyzed by flow
cytometry, and we observed that a greater number of
circCDK13-overexpressing liver cancer cells compared with the
negative controls were in the G0/G1 phase of the cell cycle, while
the number of cells in the M phase was decreased by circCDK13
overexpression (Fig. 2C). These
results indicated that the expression of circCDK13 played an
important role in the pathology of liver cancer by modulating cell
proliferation and cell cycle progression.
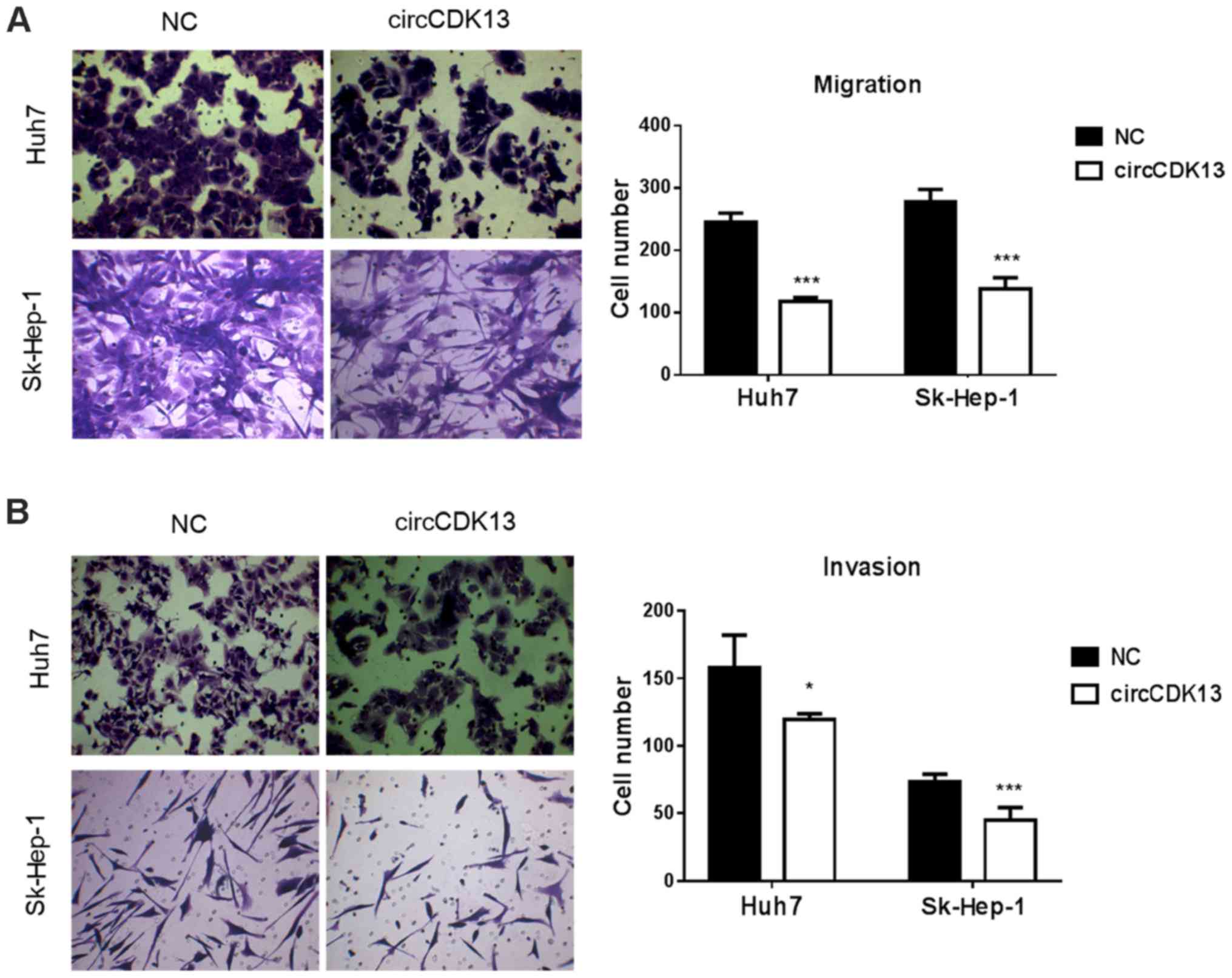
circCDK13 inhibits liver cancer cell
migration and invasion
The dysregulation of cell migration and aberrant
cell invasion are characteristics of liver cancer. For the further
verification of the role of circCDK13 in the pathological processes
of liver cancer, the migratory and invasive capacity of the
SK-Hep-1 and Huh7 cells overexpressing circCDK13 were evaluated in
this study. As shown in Fig. 3A,
the results of Transwell culture assay revealed that the increase
in circCDK13 expression markedly suppressed the migratory rates of
both the SK-Hep1 and Huh7 cells compared with those of the control
group (Fig. 3A). Similar to the
migration assay, we found that the invasive capacity of the
SK-Hep-1 and Huh7 cells overexpressing circCDK13 was also
significantly suppressed compared with the control group (Fig. 3B). In both the migration and
invasion assays, the Sk-Hep-1 cells overexpressing circCDK13
exhibited a highly significant decrease in cell numbers compared
with the control group (Fig. 3).
The markedly suppressed migratory and invasive abilities of the
human liver cancer cells directly confirmed that this newly
identified circRNA molecule is as an essential factor for liver
cancer development as it suppresses cell migration and invasion, as
well as cell proliferation and cell cycle progression.
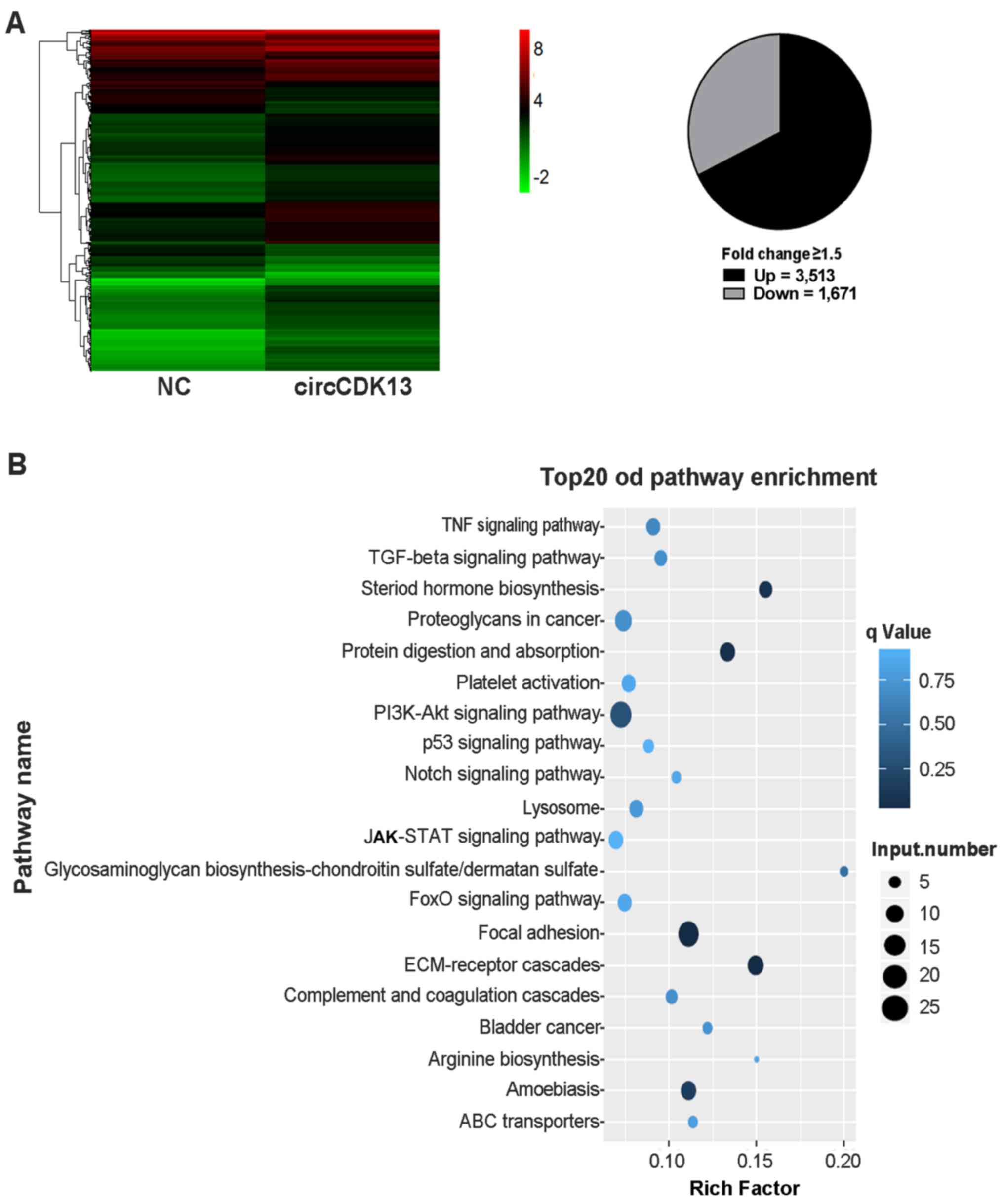
circCDK13 overexpression regulates
JAK/STAT and PI3K/AKT signaling
To investigate the molecular mechanisms underlying
the effects of circCDK13 on liver cancer cell proliferation,
migration and invasion, transcriptome analysis using a microarray
assay was performed to compare the differences in expressional
profiles between the liver cancer cells overexpressing circCDK13
and the control group. In total, >4,000 genes were found to be
significantly differentially expressed in the liver cancer cells
overexpressing circCDK13 compared with the negative control,
including 3,513 upregulated and 1,671 downregulated genes (Fig. 4A). Moreover, our KEGG pathway
analysis revealed that these differentially expressed genes were
significantly enriched in the JAK/STAT, PI3K/AKT and focal adhesion
signaling pathways (Fig. 4B).
Specifically, transcriptome analysis revealed that several genes in
the JAK/STAT and PI3K/AKT pathways were differentially expressed in
the liver cancer cells with an enhanced circCDK13 expression
(Fig. 5A). The results from
transcriptome and bioinformatics analysis strongly indicated that
the functions of circCDK13 in regulating liver cancer cell
proliferation, migration and invasion were mediated by multiple
signaling pathways. Furthermore, the levels of phosphorylated AKT
(p-AKT) and phosphorylated STAT3 (p-STAT3) proteins were markedly
downregulated in both the Huh7 and SK-Hep-1 cells overexpressing
circCDK13, directly confirming that the JAK/STAT and PI3K/AKT
pathways were regulated by circCDK13 in liver cancer cells
(Fig. 5B). These results revealed
that circCDK13 regulates various signaling pathways in the liver
cancer pathological processes, and JAK/STAT and PI3K/AKT may be two
of the major mediating signaling pathways.
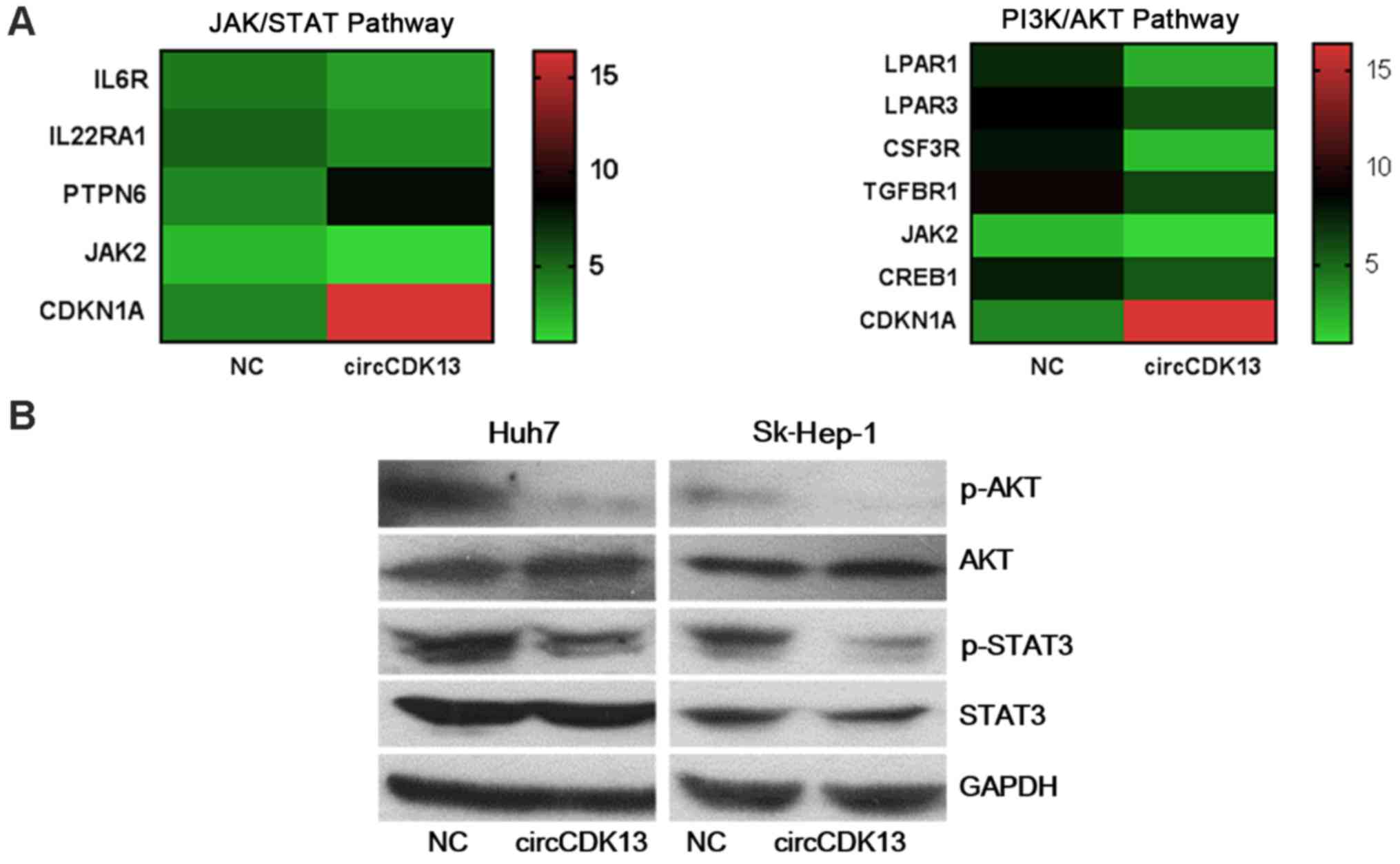 | Figure 5Regulation of JAK/STAT and PI3K/AKT
by circCDKA13 in liver cancer cells. (A) Expression levels of major
genes in the AK/STAT and PI3K/AKT signaling pathways were altered
in response to circCDK13 overexpression induced by transfection
with circCDK13 overexpression plasmid. The changes in gene
expression were detected by transcriptome analysis. NC, negative
control; circCDK13, circular CDK13 overexpression plasmid; IL6R,
interleukin-6 receptor; IL22RA1, interleukin-22 receptor alpha 1;
PTPN6, protein tyrosine phosphatase, non-receptor type 6; JAK,
Janus kinase; CDKN1A, cyclin-dependent kinase inhibitor 1A; LPAR1,
lysophosphatidic acid receptor 1; LPAR3, lysophosphatidic acid
receptor 3; CSF3R, colony-stimulating factor 3 receptor; TGFBR1,
transforming growth factor-β type I receptor; CREB1,
cAMP-responsive element-binding protein 1. (B) Changes in the
protein abundance of major JAK/STAT and PI3K/AKT signaling
components induced by circCDK13 overexpression. The overexpression
of circCDK13 downregulated the expression levels of phosphorylated
AKT (p-AKT) and STAT3 (p-STAT3). The expression levels of total
AKT, p-AKT, total STAT3 and p-STAT3 were determined by western blot
analysis. GAPDH was used as an internal control. |
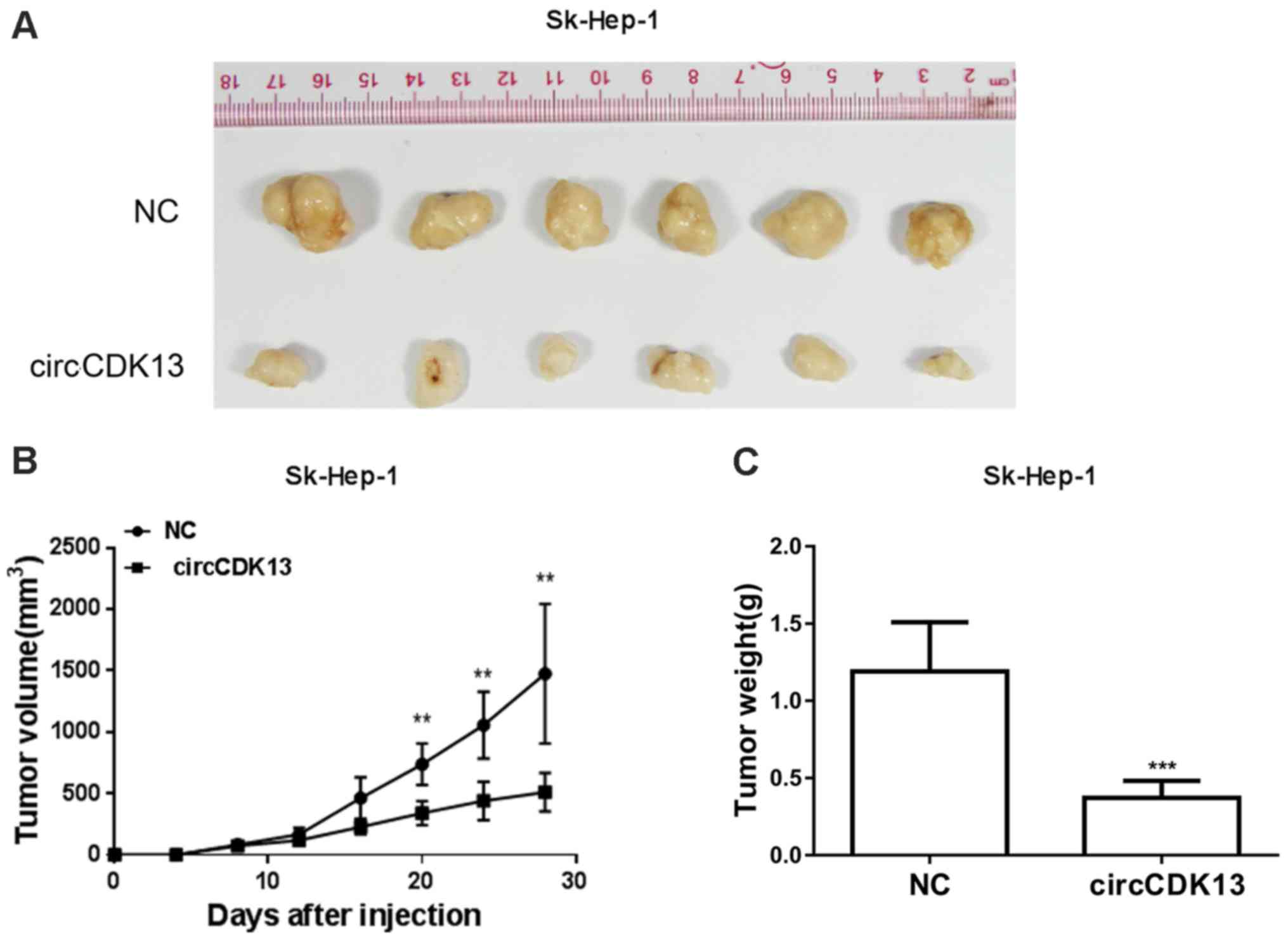
circCDK13 suppresses the tumorigenicity
of liver cancer cells
For further confirmation of the role of circCDK13 in
liver cancer progression, a tumorigenicity assay was carried out by
transplanting Sk-Hep-1 cells overexpressing circCDK13 or negative
control cells into nude mice. The tumors formed from the liver
cancer cells overexpressing circCDK13 were much smaller than those
derived from the negative control group (Fig. 6A). Consistently, the tumor volumes
in the experimental groups with an elevated circCDK13 expression
were also significantly smaller compared with those in the negative
control (Fig. 6B). Additionally,
markedly decreased tumor weights were observed in the experimental
group of mice transplanted with liver cancer cells overexpressing
circCDK13 (Fig. 6C). The
significantly suppressed tumor-forming capacity of the liver cancer
cells overexpressing circCDK13 strongly supports our hypothesis
that circCDK13 functions as a novel circRNA molecule associated
with liver cancer cell proliferation and migration during liver
cancer development and progression.
Discussion
The re-discovery of circRNAs as revalently expressed
macromolecules with significant physiological and pathological
importance has opened a new and broad research field for the study
of malignant diseases. In light of progress being made in research
in recent years, it has become widely accepted that circRNAs may be
explored as biomarkers for various human cancers (10,14,19).
Facing the critical situation of liver cancer prevention and
treatment, the application of circRNA molecules closely associated
with liver cancer progression may be a promising strategy for the
early diagnosis and novel therapeutic reagent screening. Recent
large-scale sequencing endeavors have identified a large number of
circRNAs from various human tissues and cells (30–32),
and a public circRNA database is also available (29), providing a valuable research
foundation for our understanding of the function of circRNAs. More
importantly, verification and functional characterization are
urgently required to disclose the specific expression, function,
regulation and underlying mechanisms of these novel players under
different physiological and pathological contexts. circCDK13
(hsa_circ_0001699) from the CDK13 gene was detected by
several sequencing analyses in the mammalian brain (31), CD19+ B cells (30), and several normal and malignant
human cells, such as K562, Nhek and HepG2 cells (32). However, the expression of circCDK13
must be verified in a case study, and little is known about its
function and underlying mechanisms of action. In the present study,
the expression of circCDK13 in human liver cancer cells and tissues
was first verified by RT-qPCR, which confirmed the prevalent
existence of this circular molecular during liver cancer
progression.
To investigate the function of circCDK13 in liver
cancer, liver cancer cells overexpressing circCDK13 by lentiviral
transfection were established. The overexpression of circCDK13
significantly repressed the proliferative, migratory and invasive
capacities of the liver cancer cells, and also altered cell cycle
progression, directly revealing the function of circCDK13 in
regulating liver cancer development. The role of circCDK13 is
similar to that of another newly identified circRNA,
hsa_circ_0004018, which has also been shown to be suppressed in
hepatocellular carcinoma tissues (24). Of note, the expression of
hsa_circ_0001649 has also been shown to be significantly
downregulated in hepatocellular carcinoma tissues and correlated
with tumor size (22).
Additionally, the circRNA microarray has been applied for the
large-scale identification of hepatocellular carcinoma-related
circular RNAs, and >200 differential circRNAs were identified in
tissues from patients with hepatocellular carcinoma (25). The number of differentially
expressed circRNAs associated with liver cancer progression has
been rapidly increasing, and the functional characterization of
these macromolecules in the context of liver cancer merits great
efforts. The revelation of the cellular functions of a single
circRNA in liver cancer is the first step, and accumulating
knowledge concerning the large number of circRNAs during the
pathology of liver cancer would provide important insight into the
complex regulatory networks underlying the initiation and
development of liver cancer. Our findings regarding the function of
circCDK13 in cell behaviors, together with the markedly suppressed
tumor size during tumorigenesis, as shown in Fig. 6, directly demonstrates that this
novel circRNA acts as a potential suppressor of liver cancer.
The majority of recent studies examining circRNAs
have focused on the expression patterns of circRNAs and cellular
functions. The mechanisms of these circRNAs among the complex
cellular signaling networks remain largely unexplored. In the
present study, a microarray analysis was performed to explore the
signaling pathways mediating the regulatory role of circCDK13. Many
key components of the JAK/STAT and PI3K/AKT pathways were found to
be differentially expressed and regulated by circCDK13 in liver
cancer cells. The JAK/STAT signaling pathway has been established
as very critical regulatory machinery for cytokine-dependent gene
expression and cellular development and survival (43), and it is closely associated with
many types of cancer (44).
PI3K/AKT signaling, which controls cell growth, survival, cell
cycle progression and other cellular functions, is also frequently
altered in human cancers and is associated with the development of
resistance to chemotherapy (45).
The alteration of these signaling pathways by circCDK13
overexpression herein suggested that the cancer-inhibiting roles of
circCDK13 may be mediated by these well-known cellular pathways.
From the results of cell transcriptome sequencing, it was
demonstrated that circCDK13 upregulates the JAK/STAT signaling
pathway upstream suppressor genes (PTPN6). According to the
mechanisms of sponging, the miR-135b-5p binding site is in
circCDK13. A previous study reported that LZTS1 expression was
inhibited by miR-135b-5p (46) and
it suppressed HCC cell proliferation by inhibiting the activation
of the PI3K/AKT pathway (47).
Hence, circCDK13 may upregulate the relevant gene expression of the
signaling pathway through sponge miRNAs to inhibit these two
signaling pathways. In addition, a large number of genes involved
in other cellular signaling pathways were also differentially
expressed by the overexpression of circCDK13, inferring that this
circRNA molecule could carry out its tumor suppressor role by
modulating various signaling cascades in human cells. The
large-scale transcriptome analysis of gene expression profiles
regulated by a circRNA in this study also provides valuable clues
for the investigations of other circRNAs associated with liver
cancer.
It should also be noted that the direct targets of
circCDK13 in liver cancer cells were not characterized in the
present study. As reported, circRNAs may carry out their biological
roles as sponges (11), and the
future identification of target miRNAs would greatly enhance our
understanding of their tumor suppressor functions. Furthermore, the
great potential of circCDK13 to regulate cell proliferation and
migration may also exist in other developmental processes and
diseases, considering the ubiquitous expression of circCDK13 in
various human tissues and cell lines (29). The study of circCDK13 in distinct
physiological and pathological processes associated with cell
proliferation and migration would broaden our knowledge of the
functions of circRNAs.
In conclusion, we reported herein the expression of
a novel circRNA, circCDK13, in liver cancer tissues and cell lines.
The overexpression of circCDK13 suppresses liver cancer cell
proliferation, migration, invasion and tumorigenesis, possibly
mediated via the JAK/STAT and PI3K/AKT pathways. Our results
characterize circCDK13 as a suppressive factor of liver cancer, and
thus it may be explored as a biomarker for liver cancer diagnosis
and treatment.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 31600710 and 81172193).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
QL and YBL design the research and draft the
manuscript. JWC and CRZ conducted the experiments. JC and XL
performed the data analysis. MSH critically reviewed and revised
the manuscript and approved the final version to be published. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The liver cancer tissues used in this study were
collected from patients with hepatocellular carcinoma after
obtaining written informed consent signed by each patient. The
sample collection processes and following analysis were approved by
the Ethics Committee of the Third Affiliated Hospital of Sun
Yat-sen University prior to the surgery. The mouse experiments in
this study was performed under the Affidavit of the Approval of
Animal Ethical and Welfare of the Laboratory Animal Center of the
Third Affiliated Hospital of Sun Yat-sen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clark T, Maximin S, Meier J, Pokharel S
and Bhargava P: Hepatocellular carcinoma: Review of epidemiology,
screening, imaging diagnosis, response assessment, and treatment.
Curr Probl Diagn Radiol. 44:479–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsueh CT, Liu D and Wang H: Novel
biomarkers for diagnosis, prognosis, targeted therapy and clinical
trials. Biomark Res. 1:12013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu JB, Qi FZ, Xu G, Chen GF, Qin LX and
Zhang JH: Value of alpha-fetoprotein and clinical characteristics
in patients with liver neoplasm. Neoplasma. 61:218–224. 2014.
View Article : Google Scholar
|
|
6
|
Prieto De Paula JM, Mayor Toranzo E,
Gallardo Borge L and Franco Hidalgo S: Small-cell lung cancer and
elevated CA 19.9 tumor marker levels. Arch Bronconeumol.
48:385–386. 2012.In Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HX, Liu DD, Jin BJ, Wang YW, Liu Q,
Duan RB, Zhao P and Ma MX: Changes of serum trace elements, AFP,
CEA, SF, T3, T4 and IGF-II in different periods of rat liver
cancer. Chin J Cancer Res. 23:301–305. 2011. View Article : Google Scholar
|
|
8
|
Zhang D, Yu M, Xu T and Xiong B:
Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of
colorectal liver metastasis in Chinese population.
Hepatogastroenterology. 60:1297–1301. 2013.PubMed/NCBI
|
|
9
|
Benowitz S: Liver cancer biomarkers
struggling to succeed. J Natl Cancer Inst. 99:590–591. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong Y, He D, Peng Z, Peng W, Shi W, Wang
J, Li B, Zhang C and Duan C: Circular RNAs in cancer: An emerging
key player. J Hematol Oncol. 10:22017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Yang J, Zhou P, Le Y, Zhou C, Wang
S, Xu D, Lin HK and Gong Z: Circular RNAs in cancer: Novel insights
into origins, properties, functions and implications. Am J Cancer
Res. 5:472–480. 2015.PubMed/NCBI
|
|
14
|
Wang Y, Mo Y, Gong Z, Yang X, Yang M,
Zhang S, Xiong F, Xiang B, Zhou M, Liao Q, et al: Circular RNAs in
human cancer. Mol Cancer. 16:252017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar :
|
|
16
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7:e307332012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Granados-Riveron JT and Aquino-Jarquin G:
The complexity of the translation ability of circRNAs. Biochim
Biophys Acta. 1859:1245–1251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greene J, Baird AM, Brady L, Lim M, Gray
SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, Function
and Role in Human Diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren S, Xin Z, Xu Y, Xu J and Wang G:
Construction and analysis of circular RNA molecular regulatory
networks In liver cancer. Cell Cycle. 16:2204–2211. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai F, Yano Y, Fukumoto T, Takebe A,
Tanaka M, Kuramitsu K, Anggorowati N, Rinonce HT, Widasari DI,
Saito M, et al: Quantification of pregenomic RNA and covalently
closed circular DNA in hepatitis B virus-related hepatocellular
carcinoma. Int J Hepatol. 2013:8492902013. View Article : Google Scholar
|
|
22
|
Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z,
Yang J, Fan J, Liu L and Qin W: Hsa_circ_0001649: A circular RNA
and potential novel biomarker for hepatocellular carcinoma. Cancer
Biomark. 16:161–169. 2016. View Article : Google Scholar
|
|
23
|
Shang X, Li G, Liu H, Li T, Liu J, Zhao Q
and Wang C: Comprehensive circular RNA profiling reveals that
hsa_circ_0005075, a new circular RNA biomarker, is involved in
hepatocellular carcinoma development. Medicine (Baltimore).
95:e38112016. View Article : Google Scholar
|
|
24
|
Fu L, Yao T, Chen Q, Mo X, Hu Y and Guo J:
Screening differential circular RNA expression profiles reveals
hsa_circ_0004018 is associated with hepatocellular carcinoma.
Oncotarget. 8:58405–58416. 2017.PubMed/NCBI
|
|
25
|
Huang XY, Huang ZL, Xu YH, Zheng Q, Chen
Z, Song W, Zhou J, Tang ZY and Huang XY: Comprehensive circular RNA
profiling reveals the regulatory role of the
circRNA-100338/miR-141-3p pathway in hepatitis B-related
hepatocellular carcinoma. Sci Rep. 7:54282017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greifenberg AK, Hönig D, Pilarova K,
Düster R, Bartholomeeusen K, Bösken CA, Anand K, Blazek D and Geyer
M: Structural and functional analysis of the Cdk13/Cyclin K
complex. Cell Rep. 14:320–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Even Y, Escande ML, Fayet C and Genevière
AM: CDK13, a kinase involved in Pre-mRNA splicing, is a component
of the perinucleolar compartment. PLoS One. 11:e01491842016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HE, Kim DG, Lee KJ, Son JG, Song MY,
Park YM, Kim JJ, Cho SW, Chi SG, Cheong HS, et al: Frequent
amplification of CENPF, GMNN and CDK13 genes in hepatocellular
carcinomas. PLoS One. 7:e432232012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glažar P, Papavasileiou P and Rajewsky N:
circBase: A database for circular RNAs. RNA. 20:1666–1670. 2014.
View Article : Google Scholar
|
|
30
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rybak-Wolf A, Stottmeister C, Glažar P,
Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss
R, et al: Circular RNAs in the mammalian brain are highly abundant,
conserved, and dynamically expressed. Mol Cell. 58:870–885. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thomas M: Molecular targeted therapy for
hepatocellular carcinoma. J Gastroenterol. 44(Suppl 19): 136–141.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang H, Li RP, Liang P, Zhou YL and Wang
GW: miR-125a inhibits the migration and invasion of liver cancer
cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol
Lett. 10:681–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilson GS, Tian A, Hebbard L, Duan W,
George J, Li X and Qiao L: Tumoricidal effects of the JAK inhibitor
Ruxolitinib (INC424) on hepatocellular carcinoma in vitro. Cancer
Lett. 341:224–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun ZJ, Chen G, Hu X, Zhang W, Liu Y, Zhu
LX, Zhou Q and Zhao YF: Activation of PI3K/Akt/IKK-α/NF-kappaB
signaling pathway is required for the apoptosis-evasion in human
salivary adenoid cystic carcinoma: Its inhibition by quercetin.
Apoptosis. 15:850–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Booz GW, Day JN and Baker KM: Interplay
between the cardiac renin angiotensin system and JAK-STAT
signaling: Role in cardiac hypertrophy, ischemia/reperfusion
dysfunction, and heart failure. J Mol Cell Cardiol. 34:1443–1453.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Levine DA, Bogomolniy F, Yee CJ, Lash A,
Barakat RR, Borgen PI and Boyd J: Frequent mutation of the PIK3CA
gene in ovarian and breast cancers. Clin Cancer Res. 11:2875–2878.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Or YY, Hui AB, Tam KY, Huang DP and Lo KW:
Characterization of chromosome 3q and 12q amplicons in
nasopharyngeal carcinoma cell lines. Int J Oncol. 26:49–56.
2005.
|
|
40
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,
2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces
tumor cell invasion in colorectal cancer cells. Neoplasia.
10:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015.
|
|
42
|
Simile MM, Latte G, Demartis MI, Brozzetti
S, Calvisi DF, Porcu A, Feo CF, Seddaiu MA, Daino L, Berasain C, et
al: Post-translational deregulation of YAP1 is genetically
controlled in rat liver cancer and determines the fate and
stem-like behavior of the human disease. Oncotarget. 7:49194–49216.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: New surprises in the Jak/Stat pathway.
Cell. 109(Suppl 1): S121–S131. 2002. View Article : Google Scholar
|
|
44
|
Arumuggam N, Bhowmick NA and Rupasinghe
HP: A Review: Phytochemicals targeting JAK/STAT signaling and IDO
expression in cancer. Phytother Res. 29:805–817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin CW, Chang YL, Chang YC, Lin JC, Chen
CC, Pan SH, Wu CT, Chen HY, Yang SC, Hong TM, et al: MicroRNA-135b
promotes lung cancer metastasis by regulating multiple targets in
the Hippo pathway and LZTS1. Nat Commun. 4:18772013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He Y and Liu X: The tumor-suppressor gene
LZTS1 suppresses hepatocellular carcinoma proliferation by
impairing PI3K/Akt pathway. Biomed Pharmacother. 76:141–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|