Introduction
Myelodysplastic syndromes (MDS) are a diverse group
of disorders in which bone marrow stem and progenitor cells are
damaged. In the 'pro-leukemia state', these cells are usually
characterized by ineffective hematopoiesis, leading to peripheral
blood cytopenia and a high risk of progression to acute myeloid
leukemia (AML) in certain patients (1). Currently, the primary treatments for
patients with MDS, including low-dose chemotherapy and allogeneic
bone marrow hematopoietic stem cell transplantation, are
unsatisfactory. MDS is associated with high mortality as it
eventually progresses to AML, particularly in the cases of severe
cytopenia (2,3). Thus, elucidating the underlying
molecular mechanisms of MDS and identifying potential targets for
effective therapy is urgently required. Accumulating studies reveal
that chromosomal abnormalities and abnormal gene expression have
critical roles in the evolution and progression of MDS (4). In particular, patients with
extrachromosomal abnormalities, such as uniparental disomy (UPD),
have garnered attention from various research groups recently
(5,6).
UPD is the inheritance of two copies of a
chromosomal region from one parent, which may generate homozygous
alleles for a deleterious recessive variant. The current research
has demonstrated that certain regions of UPD are only discovered in
the myeloid cells of patients with MDS, and they are associated
with the occurrence and development of MDS and AML (7,8). As
one of seven genes identified in these regions of UPD by gene chip
technology, sperm-associated antigen 6 (SPAG6) had an evidently
higher expression in MDS patient-derived myeloid cells than the
healthy control group (9,10). SPAG6 is located in the chromosomal
region 10p12.2, and it was initially identified in human testis
tissue with the function of regulating germ cell maturation and
flagellar motility (11–13). Subsequent studies have unveiled
that SPAG6 is closely associated with certain malignant diseases,
including breast and lung cancer (14). Increasing evidence also indicated
that, as a novel cancer-testis antigen, SPAG6 exhibits aberrant
overexpression in patients with AML and displays normal levels when
the patients undergo continuous remission (9,10,15).
Thus, SPAG6 may represent a potential therapeutic target for
monitoring the evolution and progression of hematologic
malignancies (16). Since the
overexpression of SPAG6 has been identified in MDS patient-derived
myeloid cells, we hypothesize that SPAG6 may have a significant
role in the pathogenesis of MDS.
SKM-1 is an MDS cell line derived from a male
patient with MDS-transformed AML (17). Our previous studies indicated that
knockdown of SPAG6 by lentiviral infection significantly increased
the apoptosis of SKM-1 cells by inducing the activation of specific
caspases and prominent expression of tumor suppressor genes,
including p53 and phosphatase and tensin homolog (PTEN) (18). Our further studies demonstrated
that apoptosis initiated by SPAG6-silencing in SKM-1 cells was
mediated through the death receptor pathway by upregulating the
expression of Fas-associated via death domain in tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) signaling
(19). PTEN, as a negative
regulator of the phosphatidylionositol 3-kinase (PI3K)/protein
kinase B (AKT) pathway, has been demonstrated to be deleted or
inactivated in various solid tumors. PTEN also has been reported to
have a crucial role in regulating cellular viability though
apoptosis of the mitochondrial pathway (20,21),
not only in solid tumors but also in MDS and AML cells (22). However, the molecular mechanisms of
PTEN-induced apoptosis through the mitochondrial pathway have not
been intensively defined in SKM-1 cells with SPAG6 knockdown. In
the present study, whether SPAG6 silencing could induce PTEN
expression and regulate apoptosis through the PI3K/AKT pathway in
SKM-1 cells was determined. A mouse xenograft model was also
employed to further evaluate the effects of SPAG6 knockdown on
inducing tumor apoptosis in vivo.
Materials and methods
Cell culture and reagents
The MDS cell line SKM-1 was provided by Professor
Jianfeng Zhou of Tongji Medical College of Huazhong University of
Science and Technology (Wuhan, China). Cells were cultured in
RPMI-1640 basic medium (Corning Incorporated, Corning, NY, USA)
supplemented with heat-inactivated 10% fetal bovine serum
(Capricorn Scientific GmbH, Ebsdorfergrund, Germany) without
antibiotic. All cells were cultured at 37°C in a CO2
incubator with 5% CO2 and 95% humidified air. The
culture medium was changed every 2–3 days, and cells were
maintained without any significant cell morphological change or
biological response. LY294002 and z-VAD-fmk, purchased from Selleck
Chemicals (Houston, TX, USA), were used to treat cells
(5×104 cells) at 20 µM for 1 h.
Lentivirus production and infection
The SPAG6-targeting short hairpin RNA (shRNA)
lentiviral vector (SPAG6-shRNA) and control non-specific lentiviral
vector (NC-shRNA) were constructed as reported previously (23). The target sequence for SPAG6 shRNA
was 5′-TGATGCTAAATTGAAGCAT-3′ and that for nonsense negative
control (NC) was 5′-TTCTC CGAACGTGTCACGT-3′. Lentiviral particles
were produced by Shanghai GeneChem Co., Ltd. (Shanghai, China) by
transiently cotransfecting lentiviral vector and helper plasmids
(Addgene, Inc., Cambridge, MA, USA) into 293T cells (American Type
Culture Collection, Manassas, VA, USA), followed by concentrating
by ultracentrifugation for 2 h at 20,000 × g at 4°C. Lentiviral
plasmids were transfected into 293T cells (20 µg plasmids
per 10 cm tissue culture dishes) via FuGENE® HD
Transfection reagent (Promega Corporation, Madison, WI, USA),
following the protocol from Addgene. SKM-1 cells at the exponential
growth stage were plated in 6-well plates (5×104 cells
per well) and infected with SPAG6-shRNA or NC-shRNA lentivirus at a
multiplicity of infection of 20 in the presence of 5 µg/ml
polybrene. All cells were washed and resuspended in complete medium
at 10 h after infection. The infection efficiencies and apoptosis
rates of SKM-1 cells were evaluated by flow cytometry by detecting
the GFP and Annexin V staining on day 5 after lentiviral
infection.
Apoptosis assay by flow cytometry
Lentivirus-infected SKM-1 cells were collected,
washed twice with PBS, and then resuspended in 800 µl PBS.
Cell apoptosis rates were detected using Annexin V and
7-aminoactinomycin D (7-AAD) double-staining, according to the
manufacturer's instructions (BD Biosciences, San Jose, CA, USA).
Flow cytometry was performed on a FACSCalibur machine (BD
Biosciences) in the Life Science Department of Chongqing Medical
University (Chongqing, China), and data were analyzed by FlowJo 7.6
software (FlowJo LLC, Ashland, OR, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRIzol®
reagent in accordance with the manufacturer's protocol (Takara
Biotechnology Co., Ltd., Dalian, China). RNA (500 ng per sample)
was reverse transcribed at 42°C for 1 h into cDNA using the
PrimeScript™RT reagent kit (Takara Biotechnology Co., Ltd.). PCRs
were performed using a CFX-Connect Real-Time PCR system and
SYBRGreen (Bio-Rad Laboratories, Inc., Hercules, CA, USA) for a
total of 40 cycles, following standard assay procedures. The
cycling parameters were 95°C for 30 sec, then 40 cycles of 95°C for
5 sec and 60°C for 39 sec. The relative gene expression was
calculated by the 2−ΔΔCq method (24), and GAPDH was used as the
endogenous control gene. The PCR primer pairs were designed based
on the cDNA sequences (NCBI source: Homo sapiens), and the
primer sequences were as follows: SPAG6 forward,
5′-CCTTTCAGCTCTCAGTCAGGTTTC-3′ and reverse,
5′-TCTTCACGTTTCATCCTTGTCCTT-3′ (accession number: NM_012443.3);
PTEN forward, 5′-ACACGACGGGAAGACAAGTT-3′ and reverse,
5′-CTGGTCCTGGTATGAAGAATG-3′ (accession number: NM_000314.6); DNA
methyltransferase 1 (DNMT1) forward,
5′-CAGGCAGTTCAACACCCTCATC-3′ and reverse,
5′-GCTGAAGAAGCCGTCCCACT-3′ (accession number: NM_001130823.2);
GAPDH forward, 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′ (accession number: NM_002046.6).
Western blot analysis
Cells were lysed on ice for 30 min using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) supplemented with 1%
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology). The protein concentrations were determined using
the BCA Protein assay kit (Beyotime Institute of Biotechnology).
Protein samples were denatured by boiling following mixing with
loading buffer. A total of 50 µg of protein per lane was
separated by 8–12% sodium dodecylsulfate-polyacrylamide gel
electrophoresis (Bio-Rad Laboratories, Inc.) and transferred onto
polyvinylidene difluoride membranes. Following blocking for 2 h
with 5% skim milk, or with 5% bovine serum albumin (Biosharp,
Hefei, China) for phosphorylated proteins, at room temperature,
these membranes were incubated with specific antibodies overnight
at 4°C. Primary antibodies against the following proteins were
used: SPAG6, caspase-8, DNMT1 (cat. nos. ab155653, ab108333, and
ab188453, respectively; 1:1,000 dilution; all from Abcam,
Cambridge, UK); PTEN, AKT, phospho-AKT [P-AKT(S473)], apoptosis
regulator Bcl-2 (cat. nos. 9188T, 4685T, 4060T, and 3498T,
respectively; 1:1,000 dilution; Cell Signaling Technology, Inc.,
Danvers, MA, USA); induced myeloid leukemia cell differentiation
protein Mcl-1 (Mcl-1), caspase-9, cytochrome c (cat. nos.
sc-12756, sc-56076, and sc-13561, respectively; 1:500 dilution;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA); caspase-3 (cat.
no. YT0656; 1:500 dilution; ImmunoWay Biotechnology Company, Plano,
TX, USA); GAPDH (cat. no. AG019; 1:1,500 dilution; Beyotime
Institute of Biotechnology). Subsequently, the membranes were
washed with Tris-buffered saline Tween-20 (TBST) five times (5 min
each) and incubated with a goat anti-rabbit or goat anti-mouse
peroxidase-conjugated secondary antibody (cat. nos. A0216 and
A0208, respectively; 1:3,000 dilution; Beyotime Institute of
Biotechnology) for 1 h at room temperature. After washing with TBST
another five times (5 min each), the membranes were processed to
detect antigen signals with an enhanced chemiluminescence kit
(Advansta, Inc., Menlo Park, CA, USA). The protein expression
levels were analyzed using Vilber Fusion software (Fusion FX5
Spectra; Vilber Lourmat, Marne-la-Vallée, France).
Xenograft assays
Nonobese diabetic/severe combined immunodeficient
mice (NOD/SCID; 4–5 weeks old, female, with the average weight of
17.4 g) were purchased from Beijing HFK Bioscience Co., Ltd.
(Beijing, China) and housed in the animal breeding facilities at
the Laboratory Animal Center of Chongqing Medical University
(Chongqing, China). Mice were randomly divided into two groups (5
mice per group) and were inoculated subcutaneously in the right
flank with SKM-1 cells (1×107 cells per mouse) that were
stably infected with the SPAG6-shRNA or the NC-shRNA lentivirus. A
caliper was used for measuring the maximum lengths and widths of
the xenografts, and the formula 0.523 × length × width2
was used for calculating the tumor volumes, as reported previously
(25). The mice were monitored
every 3 days for signs of body weight loss and tumor growth. At 4
weeks after tumor cell inoculation, the mice were sacrificed by
cervical vertebrae dislocation, and tumor tissues were collected
for further analyses. All procedures involving experimental animals
were approved by the University Committee on the Use and Care of
Animals at Chongqing Medical University.
Immunohistochemical evaluation
Parts of each tumor tissue were fixed with 4%
polyoxymethylene for 8 h and embedded in wax, cut into
4-µm-thick slices. The immunohistochemical analyses were
detected using an assay kit (Beyotime Institute of Biotechnology).
Following deparaffinization (three times in xylene for 15 min,
three times in ethanol for 5 min, and three times in PBS for 3 min)
and permeabilization (freshly prepared 0.1% Triton X-100 in 0.1%
sodium citrate for 2 min on ice), the sections were first put into
boiled citrate solution (0.01 M, pH 6.0) for 15 min at 95°C. As the
temperature cooled, the sections were washed five times (3 min
each) in PBS and blocked with 10% goat serum (Beyotime Institute of
Biotechnology) for 1 h at room temperature. Following incubation
overnight with primary antibodies at 4°C, the sections were washed
five times (5 min each) in PBS, and incubated with biotinylated
secondary antibodies (1:1,000 dilution; cat. no. 610438, BD
Biosciences) for 1 h at 4°C. Primary antibodies against the
following proteins were used: SPAG6, poly(ADP-ribose) polymerase
(PARP; cat. nos. ab155653 and ab32138, respectively; 1:200
dilution; Abcam); cleaved-caspase-8 (Asp391; 1:200 dilution; Cell
Signaling Technology, Inc.); BH3-interacting domain death agonist
(BID; cat. no. YT0656; 1:50 dilution; ImmunoWay Biotechnology
Company). Then, the sections were washed five times (5 min each)
and then incubated with a streptavidin-peroxidase complex
(ready-to-use, Zymed; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 1 h at room temperature. Finally, the slides were
counterstained with hematoxylin (3 min at room temperature) and
detected using diaminobenzidine (5 min at room temperature) under a
light microscope.
Statistical analysis
All data are expressed as the mean ± standard
deviation, and the corresponding experiments were repeated with
technical replicates at least three times. One-way analysis of
variance was used to compare differences between multiple groups,
and a least significant difference post hoc test was used for
multiple comparisons for continuous variables. The Student's t-test
was used to analyze differences between two groups. Statistical
analysis was performed with SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Lentivirus-mediated knockdown of SPAG6
increases PTEN expression in SKM-1 cells
Initially, experiments were performed to demonstrate
that the knockdown of SPAG6 expression was achieved by
lentivirus-mediated delivery of shRNA against SPAG6 in SKM-1 cells.
At 5 days after viral infection, the expression of green
fluorescence protein and the high infection efficiencies in both
SPAG6-shRNA and control (NC-shRNA) virus-transduced SKM-1 cells
demonstrated that the lentiviral particles SPAG6-shRNA and NC-shRNA
were able to infect SKM-1 cells effectively (data not shown), as
previously reported (19).
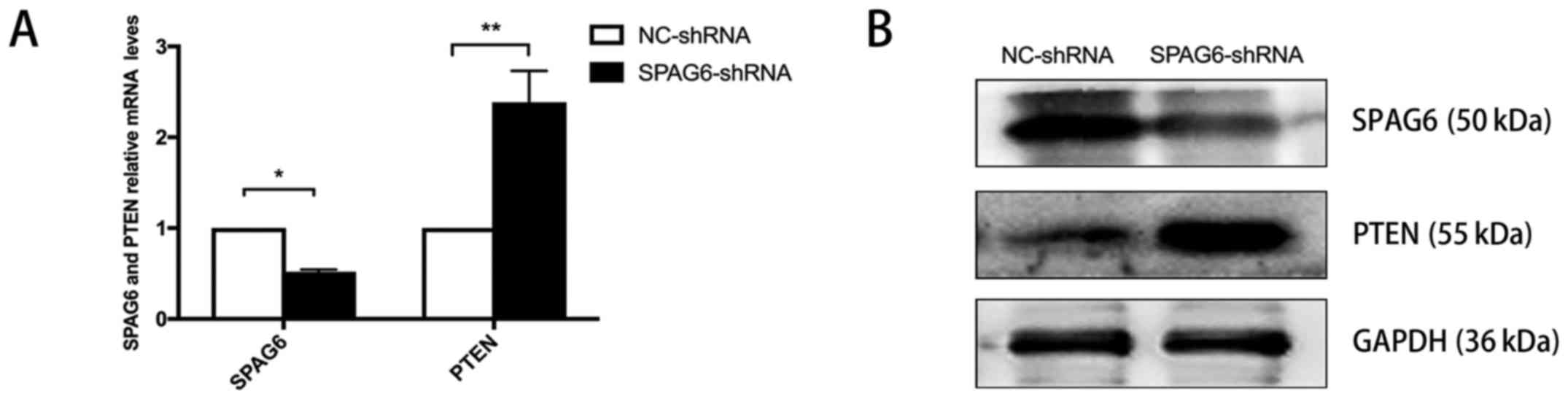
Subsequent RT-qPCR and western blot analyses also demonstrated that
SKM-1 cells infected with SPAG6-shRNA virus had significantly
decreased mRNA (Fig. 1A) and
protein (Fig. 1B) levels of SPAG6,
compared with NC-shRNA-infected cells. These results further
indicate that the cell model with downregulated SPAG6 expression
was successfully established. Consistent with our previous report
(18), knockdown of SPAG6
substantially increased the expression of PTEN at the mRNA
(Fig. 1A) and protein levels
(Fig. 1B) in SKM-1 cells. These
findings suggest that SPAG6 is involved in regulating PTEN
expression in vitro.
Knockdown of SPAG6 decreases AKT
phosphorylation and promotes apoptosis in SKM-1 cells
PTEN affects cellular apoptosis by negatively
regulating the mitochondrial apoptotic pathway via PI3K/AKT
signaling (26). A positive
association between PTEN expression and cell apoptosis was also
observed in the SKM-1 cells. As reported in a previous study
(19), the SPAG6-shRNA-transduced
SKM-1 cells exhibited substantially higher apoptosis rates than the
NC-shRNA-transduced SKM-1 cells by the Annexin V and 7-AAD staining
assays. To determine whether SPAG6 regulates apoptosis through the
PI3K/AKT pathway, the activation of AKT was detected. SKM-1 cells
with significantly decreased SPAG6 expression had markedly reduced
P-AKT, although the total AKT levels were not significantly
different to control SKM-1 cells (Fig.
2A and B). Subsequently, the expression of factors associated
with the mitochondrial apoptotic pathway was examined. As shown in
Fig. 2C and D, the protein levels
of Mcl-1 and Bcl-2 were lower in SPAG6-shRNA cells compared with
control cells, while the levels of caspase-9 and cytochrome
c were higher in the SPAG6-shRNA group than in the control
group. Taken together, the results indicated that SPAG6 regulated
apoptosis by activating the PTEN/PI3K/AKT-mediated mitochondrial
apoptotic pathway.
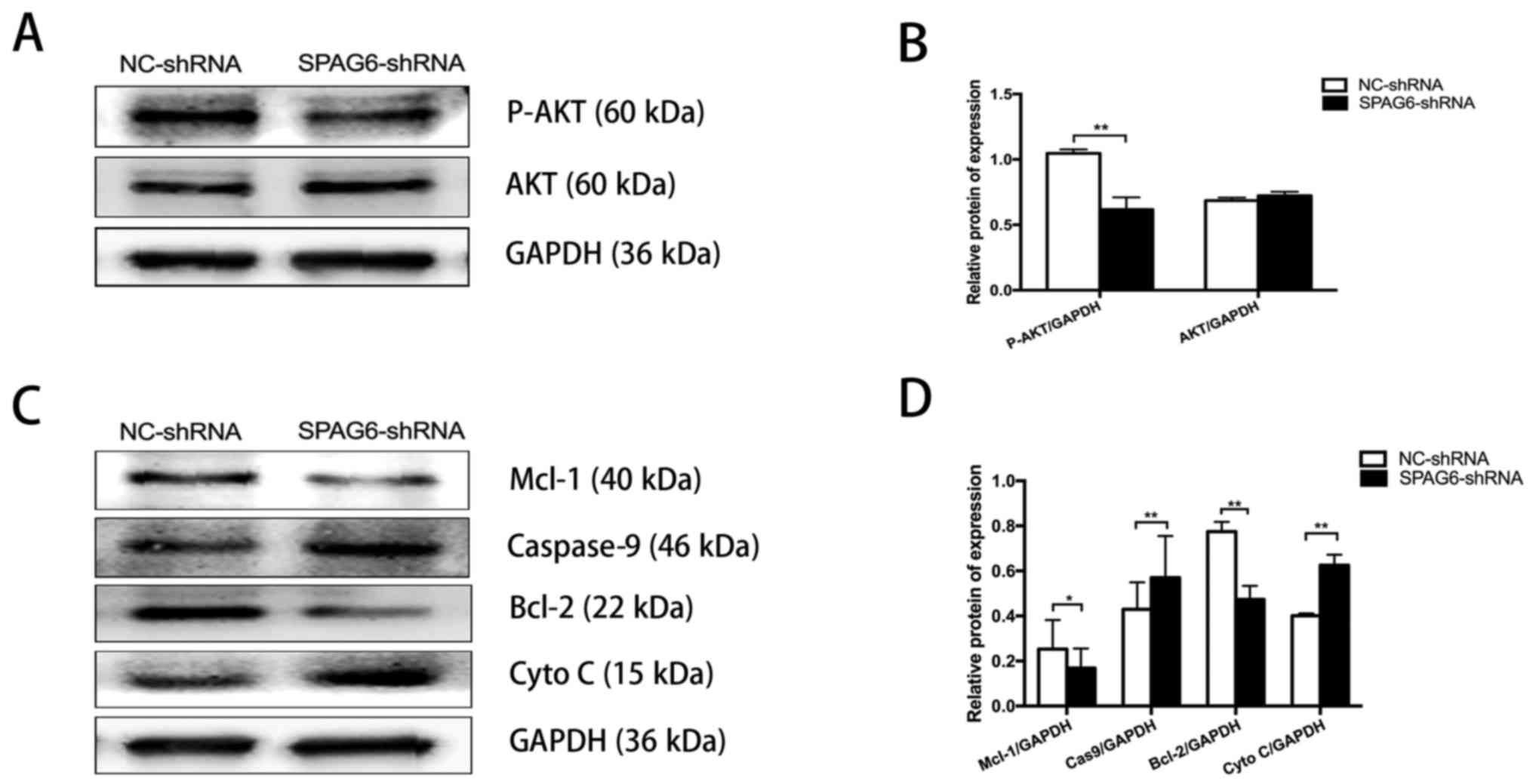 | Figure 2Knockdown of SPAG6 decreases AKT
phosphorylation and promoted apoptosis in SKM-1 cells. (A)
Representative western blot analysis of protein levels for total
AKT and phosphorylated AKT in SKM-1 cells with and without SPAG6
knockdown. (B) Densitometry of the P-AKT and AKT western blot
bands. (C) Representative western blot analysis of the Mcl-1,
Bcl-2, caspase-9 and Cyto C protein levels in SKM-1 cells with and
without SPAG6 knockdown. (D) Densitometry of the Mcl-1, Bcl-2,
caspase-9 and Cyto C western blot bands. GAPDH was used as a
loading control. Data are presented as the mean ± standard
deviation. *P<0.05; **P<0.01. P-AKT,
phospho-AKT; AKT, protein kinase B; NC, negative control; shRNA,
short hairpin RNA; SPAG6, sperm-associated antigen 6; Mcl-1,
induced myeloid leukemia cell differentiation protein Mcl-1; Bcl-2,
apoptosis regulator Bcl-2; Cyto C, cytochrome c. |
PI3K/AKT inhibition and SPAG6 silencing
synergistically promotes apoptosis in SKM-1 cells
To determine whether PTEN activation and AKT
inactivation were sufficient for induction of apoptosis in SKM-1
cells, the PI3K inhibitor LY294002. As shown in Fig. 3A and B, the apoptosis rates of
LY294002-treated and SPAG6-shRNA lentivirus-transduced SKM-1 cells
were significantly higher than that in the control cells. Notably,
cotreatment with LY294002 and SPAG6-shRNA markedly promoted
apoptosis, and these SKM-1 cells displayed a higher apoptotic rate
than any of the other groups. Western blot analysis indicated that
the PTEN level in cells infected with SPAG6-shRNA lentivirus alone
was higher than that in cells treated with LY294002 alone, although
it was significantly lower than that in cells cotreated with
LY294002 and SPAG6-shRNA lentivirus (Fig. 3C and D). Correspondingly, an
opposing pattern of changes for AKT phosphorylation was observed,
which was attributed to the negative regulation of AKT activity by
PTEN (Fig. 3C and D). Furthermore,
co-administration of LY294002 significantly enhanced SPAG6-shRNA
lentivirus-mediated Mcl-1 downregulation, caspase-9 and caspase-3
activation, and cytochrome c release in SKM-1 cells
(Fig. 3C and D). Taken together,
these findings suggest that PTEN-mediated AKT inactivation has a
critical role in enhancing apoptosis in SKM-1 cells with SPAG6
knockdown.
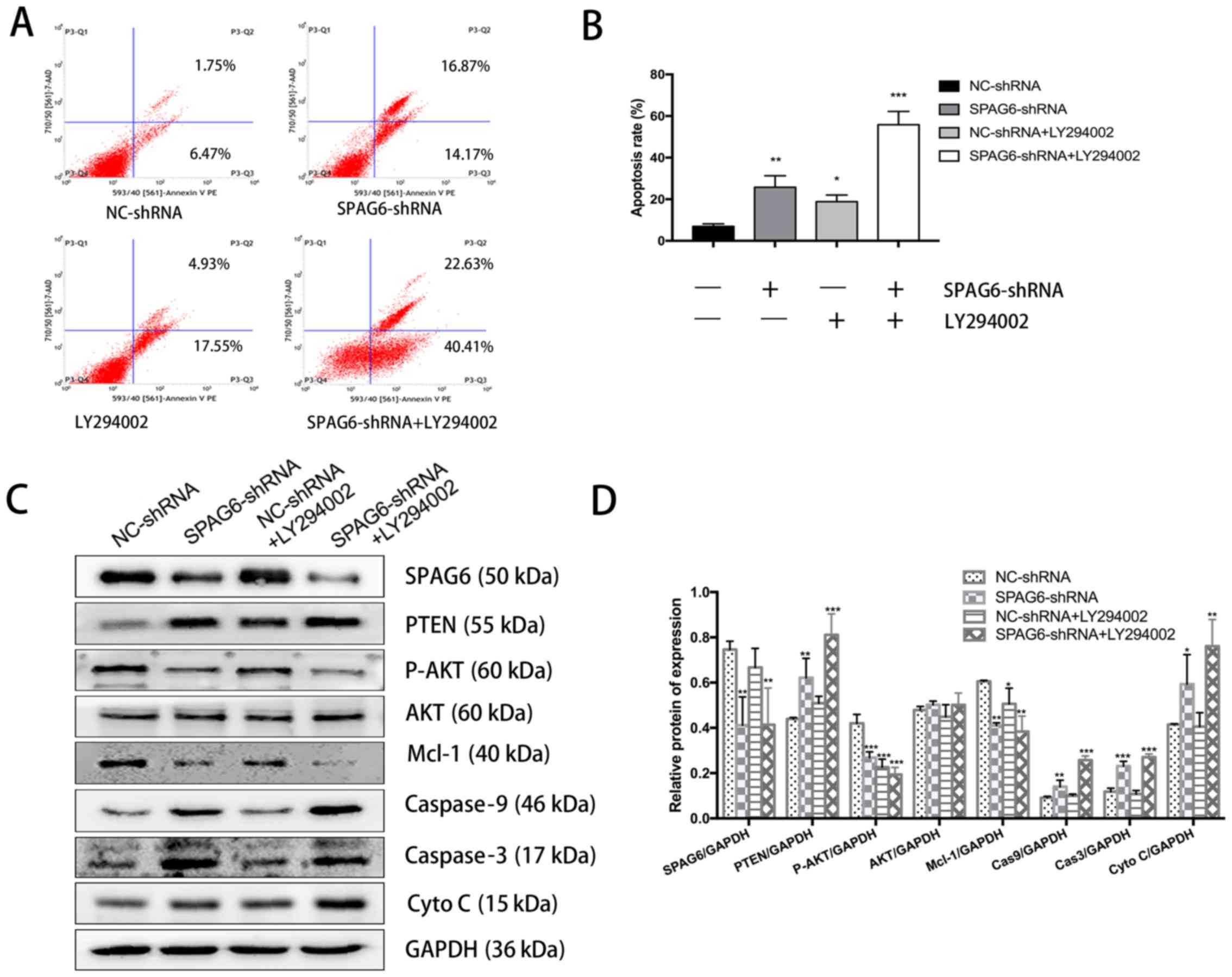 | Figure 3Inhibition of AKT by LY294002
enhances SPAG6 silencing-mediated apoptosis and PTEN activation.
SKM-1 cells were pretreated with 20 µM LY294002 for 1 h,
followed by SPAG6-targeted (SPAG6-shRNA) or control (NC-shRNA)
lentiviral infection. (A) Representative flow cytometry profile of
Annexin V and 7-aminoactinomycin D staining in SKM-1 cells with
different treatments. (B) Summary of the apoptosis rates in SKM-1
cells with different treatments. (C) Western blot analysis of the
SPAG6, PTEN, P-AKT, AKT, MCL-1, caspase-9, caspase-3, and
cytochrome c protein levels in SKM-1 cells with different
treatments. GAPDH was used as a loading control. (D) Summarization
of densitometry of the western blot bands. *P<0.05,
**P<0.01, ***P<0.001, vs. NC-shRNA
alone. All data are expressed as the mean ± standard deviation (n=3
for each group). NC, negative control; shRNA, short hairpin RNA;
SPAG6, sperm-associated antigen 6; PTEN, phosphatase and tensin
homolog; P-AKT, phospho-AKT; AKT, protein kinase B; Mcl-1, induced
myeloid leukemia cell differentiation protein Mcl-1; Cas, caspase;
Cyto C, cytochrome c. |
SPAG6 knockdown-induced PTEN upregulation
is independent of caspase activation
Apoptosis can be initiated through either death
receptor activation or the mitochondrial apoptotic pathway. The
cleavage/activation of caspase-8 and PARP also downregulate AKT
activity and indirectly activate the mitochondrial apoptotic
pathway (27). Thus, it is
possible that PTEN activation followed by dephosphorylation of AKT
in SKM-1 cells with SPAG6 knockdown is caused by caspase
activation. To elucidate the role of caspase activation in PTEN
activation, SKM-1 cells were treated with z-VAD-fmk, a
cell-permeant pan-caspase inhibitor, to block the caspase-dependent
apoptotic pathway prior to knockdown of SPAG6. Western blot
analysis demonstrated that although z-VAD-fmk slightly inhibited
the expression of total caspase-8/9, it did not block PTEN
activation in SKM-1 cells with silenced SPAG6 (Fig. 4A and B). In the presence of
z-VAD-fmk, knockdown of SPAG6 was still able to increase the level
of total caspase-8/9/3 compared with z-VAD-fmk + NC-shRNA
treatment, suggesting the progression of cell apoptosis. These data
clearly indicate that caspase activation is a consequence of SPAG6
silencing-mediated AKT inactivation, but not the cause of PTEN
activation in SKM-1 cells.
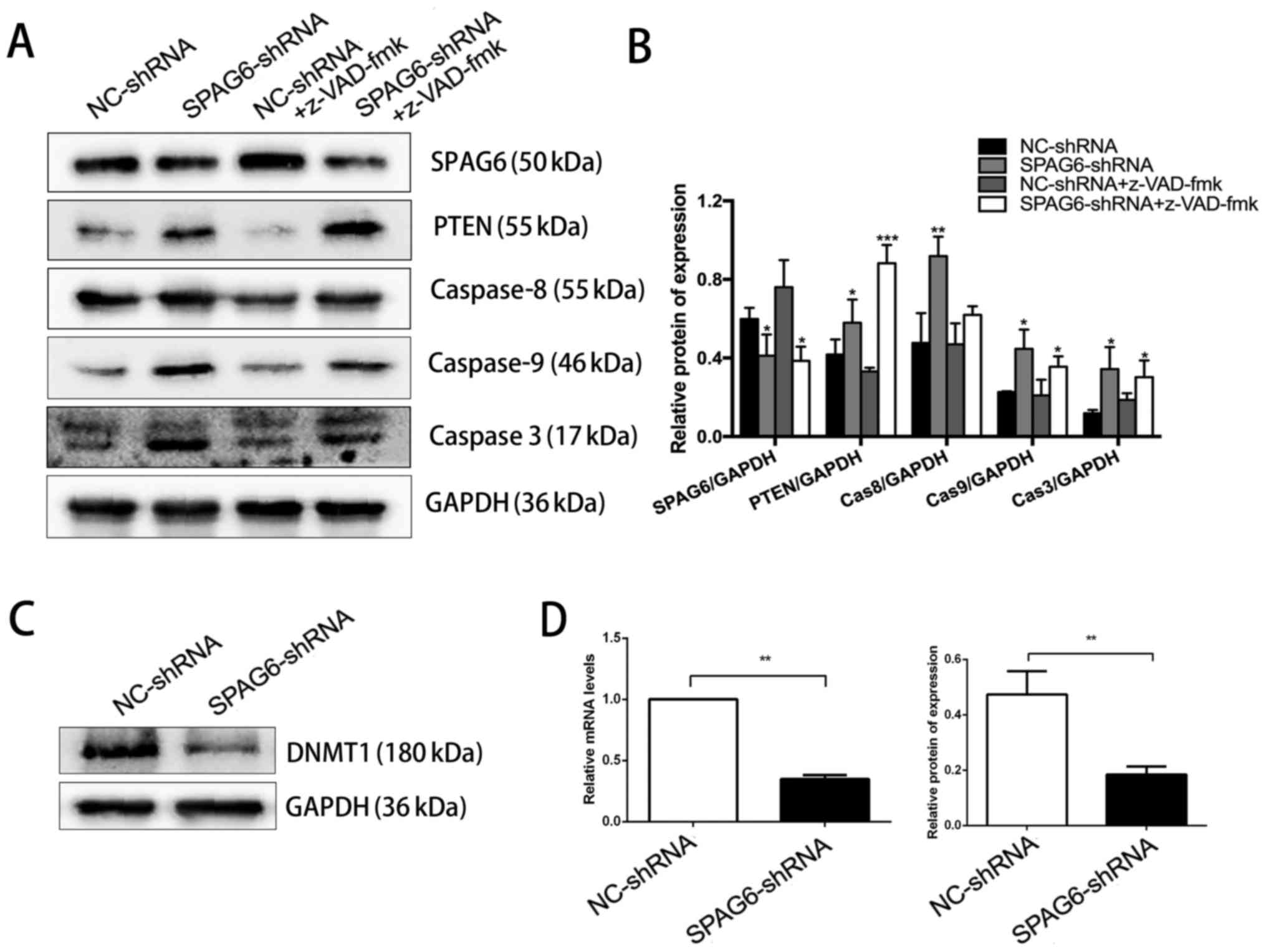 | Figure 4SPAG6 knockdown-induced PTEN
upregulation is caspase activation-independent and associated with
DNMT1 downregulation. SKM-1 cells were pretreated with z-VAD-fmk
(20 µM) for 1 h, followed by SPAG6-targeted (SPAG6-shRNA) or
control (NC-shRNA) lentiviral infection. (A) The protein levels of
SPAG6, PTEN, caspase-8, caspase-9, and caspase-3 in SKM-1 cells
with different treatments were determined by western blot analysis.
GAPDH was used as a loading control. (B) Summarization of
densitometry of the western blot bands. (C) Western blot analysis
for DNMT1 expression in SKM-1 cells with or without SPAG6
knockdown. GAPDH was used as a loading control. (D) Relative
expression of DNMT1 at both the mRNA (left) and protein (right)
levels. All data are presented as the mean ± standard (n=3 for each
group). *P<0.05, **P<0.01,
***P<0.001, vs. NC-shRNA alone. NC, negative control;
shRNA, short hairpin RNA; SPAG6, sperm-associated antigen 6; PTEN,
phosphatase and tensin homolog; Cas, caspase; DNMT1, DNA
methyltransferase 1. |
PTEN activation in SKM-1 cells with SPAG6
knockdown is associated with reduced expression of DNMT1
There is growing evidence indicating that DNA
methylation can silence tumor suppressors through high expression
of DNA methyltransferases (DNMTs) to facilitate tumorigenesis and
tumor development (28). To
explore whether DNMTs have a role in upregulating PTEN expression,
the expression level of DNMT1 was determined by RT-qPCR and western
blot analysis. Consistently, the mRNA and protein levels of DNMT1
were significantly decreased in SKM-1 cells infected with
SPAG6-shRNA lentivirus, compared to that in the control cells
(Fig. 4C and D). Thus, potentially
a positive association between SPAG6 and DNMT1 expression may
contribute to SPAG6 silencing-induced PTEN activation.
Knockdown of SPAG6 inhibits tumor growth
and induces apoptosis in a SKM-1 xenograft mouse model
To determine whether the in vitro findings
were applicable in vivo, NOD/SCID mice were inoculated with
SKM-1 cells with or without SPAG6 knockdown by subcutaneous
injection. Immunohistochemical analyses and western blotting were
performed to evaluate the morphological changes and induction of
apoptosis in tumor cells (Fig. 5).
The tumor sizes and tumor weights of the mice inoculated with SKM-1
cells pre-infected by SPAG6-shRNA lentivirus were significantly
less than those of the control mice (Fig. 5A). As reported in our previous
study (18), tumor sections from
the mice inoculated with SPAG6-shRNA-transduced SKM-1 cells showed
an apparently necrotic morphology, infiltration of inflammatory
cells, disordered and irregular tumor cell arrangement, and tumor
cells with an increased nuclear cytoplasmic ratio. In addition, the
number of dark brown-colored apoptotic cells from the SPAG6-shRNA
lentivirus group was significantly higher than that from the
NC-shRNA lentivirus group (18).
Furthermore, tumor cells with SPAG6 knockdown exhibited evident
signs of cell apoptosis, as indicated by significantly altered
signals from the results of immunohistochemical staining for
apoptosis-associated molecules, including BID, PARP, Bcl-2,
cleaved-caspase-8, and cleaved-caspase-3 between these two groups
(Fig. 5B). The western blot
results further substantiated the upregulation of pro-apoptotic
factors like cleaved-caspase-8, cleaved-caspase-3, PARP and BID,
and downregulation of the anti-apoptotic factor Bcl-2 in tumor
cells with SPAG6 knockdown (Fig. 5C
and D). Collectively, these findings support that that SPAG6
knockdown induced apoptosis of SKM-1 cells via the extrinsic and
intrinsic pathways during in vitro culturing and in the
in vivo xenograft mouse model.
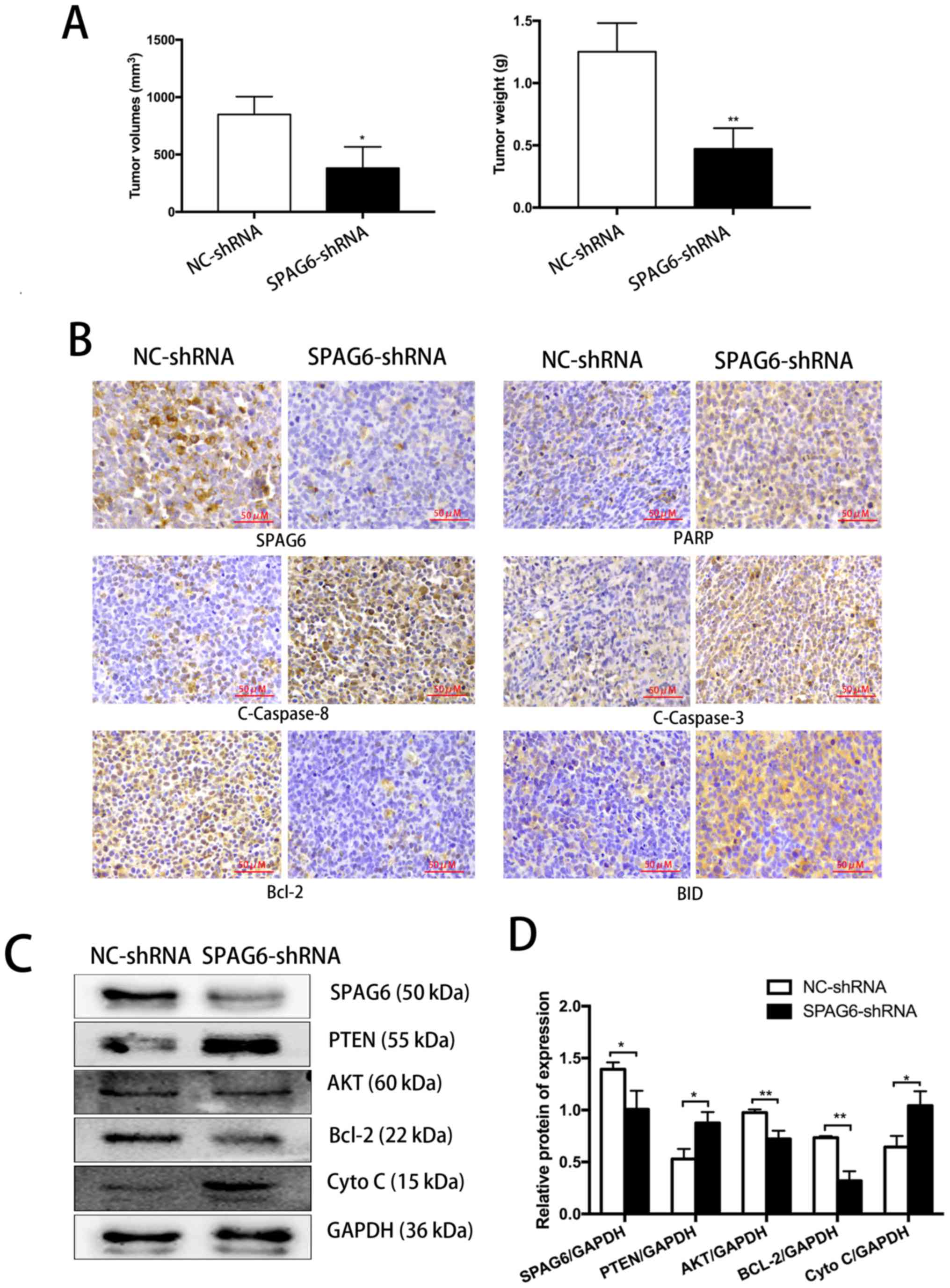 | Figure 5In a SKM-1 xenograft mouse model,
silencing of SPAG6 induces PTEN/PI3K/AKT signaling-mediated
apoptosis of tumor cells. (A) Comparison of the sizes and weights
of tumors from the mice inoculated with lentivirus pre-infected
SKM-1 cells at 4 weeks after inoculation. (B) Representative images
showing the immunohistochemistry staining results for SPAG6 and
apoptosis-associated molecules including PARP, Bcl-2, BID, cleaved
caspase-8, and cleaved caspase-3 (×20). (C) Western blot analysis
for the expression of SPAG6, PTEN, AKT, Bcl-2, and Cyto C in tumor
tissues from mice inoculated with SKM-1 cells that were
pre-infected with SPAG6-tageting shRNA or control shRNA lentivirus.
GAPDH was used as a loading control. (D) Summarization of
densitometry of the western blot bands. Data are representative
results from at least three mice per group. Data are presented as
the mean ± standard deviation (n=5 for each group in A; n=3 for
each group in D). *P<0.05, **P<0.01,
vs. NC-shRNA. NC, negative control; shRNA, short hairpin RNA;
SPAG6, sperm-associated antigen 6; PARP, poly(ADP-ribose)
polymerase; C-, cleaved-; BID, BH3-interacting domain death
agonist; PTEN, phosphatase and tensin homolog; Cyto C, cytochrome
c. |
Discussion
Despite a hypercellular or normal marrow, the
majority of patients with early-stage MDS exhibit peripheral blood
cytopenia, which has been ascribed to excessive programmed cell
death of hematopoietic cells (29,30).
However, the progression of MDS in the late stage may be attributed
to cellular over-proliferation and relatively reduced apoptosis by
abnormal gene expression or inactivation of certain tumor
suppressor genes (31). PTEN, a
well-established tumor suppressor that negatively regulates
PI3K/AKT signaling, has been reported to have low expression in,
and contribute to the development of, various malignancies,
including prostate, ovarian, and breast cancer (32–34).
The genetic abnormalities of PTEN have a crucial role in inducing
the disease progression from low-risk MDS to high-risk MDS, and
eventually to acute leukemia (35).
The formation and development of various malignant
tumors are largely associated with uncontrolled cell proliferation
and abnormal apoptosis. In general, the death receptor pathway and
the mitochondrial pathway have been reported to control programmed
cell death to maintain the balance of tissue homeostasis (36). Our previous study demonstrated that
silencing of SPAG6 promoted cell apoptosis through the death
receptor pathway by altering the TRAIL signals in the MDS cell line
SKM-1 (19). On the other hand,
decreased SPAG6 expression was associated with increased expression
of the tumor suppressor gene PTEN in SKM-1 cells. Additionally, the
expression of molecules in the PI3K/AKT pathway were detected,
which function as survival signals to support tumor growth.
Significant AKT dephosphorylation and evident signs of cell
apoptosis (MCL-1 downregulation, caspase-9 activation, cytochrome
c release) were observed following SPAG6 knockdown in SKM-1
cells. Thus, in addition to the TRAIL signaling pathway, the
PTEN/PI3K/AKT pathway also contributed to SPAG6 silencing-initiated
apoptosis in SKM-1 cells.
AKT, a serine-threonine kinase, is activated by PI3K
to interfere with cell survival via the apoptotic machinery. The
activity of PI3K can be antagonized by the tumor suppressor PTEN
through removal of the 3-phosphate from phosphati-dylinositol
3,4,5-trisphosphate (37,38). AKT regulates cell apoptosis by
directly or indirectly inducing pro-apoptotic or inhibiting
anti-apoptotic Bcl-2 family proteins and releasing cytochrome
c to initiate the mitochondrial pathway. SKM-1 cells
co-administered with the PI3K inhibitor LY294002 and SPAG6-shRNA
lentivirus exhibited a higher apoptosis rate than SKM-1 cells with
either single treatment. As in the LY294002 group, the treatment of
cells with SPAG6-shRNA lentivirus reduced AKT phosphorylation,
compared to the control group. The synergistic effect of LY294002
and SPAG6-shRNA on cell apoptosis was attributed to AKT
inactivation, which was also supported by superimposed elevation of
PTEN expression. This observation further substantiates that SPAG6
regulates PTEN activation and subsequent AKT phosphorylation to
control cell survival via a mitochondrial apoptotic pathway in
SKM-1 cells.
Our previous study also indicated that SPAG6
knockdown induced the activation of caspase-8, which facilitated
the cleavage of BID to form truncated BID, and mediated the
activation of caspase-3 and caspase-9 in SKM-1 cells (19). This raised the possibility that AKT
dephosphorylation might be the indirect consequence of caspase
activation-induced PTEN expression (27). To determine whether PTEN activation
is crucial for cell apoptosis though the PI3K/AKT pathway, we
treated SKM-1 cells with the broad caspase inhibitor z-VAD-fmk to
abrogate caspase-8-mediated cleavage/activation of BID and
caspase-9. However, the increased PTEN expression was not abrogated
following caspase inhibition. Furthermore, the observation of
increased caspase-9 protein in z-VAD-fmk-treated SPAG6 knockdown
cells suggested that PTEN-mediated AKT dephosphorylation promoted
apoptosis through the mitochondrial apoptotic pathway rather than
the caspase-dependent pathway. Furthermore, the investigations
using the xenograft mouse model reached the same conclusions as the
in vitro study.
Apoptosis can be triggered in a multistep pathway by
extracellular stimuli and intracellular signals. Several studies
have demonstrated that PTEN has a relatively low expression level
in myeloid cells of patients with MDS (39). The PI3K inhibitor LY294002 enhanced
PTEN activation and the apoptotic rate in SKM-1 cells, while the
caspase inhibitor z-VAD-fmk failed to prevent PTEN activation. A
potential explanation is that various signaling pathways may be
involved in regulating apoptosis. In addition, the expression of
PTEN is induced not only by caspase-dependent cleavage/activation
but also by other pathways, such as the nuclear factor-κB, c-Jun
N-terminal kinases and p53 pathways. Furthermore, growing evidence
demonstrates that inactivation of tumor suppressor genes is
associated with hypermethylation, rather than intra-genic mutations
(28).
From an epigenetics standpoint, DNA methylation may
suppress the expression of PTEN by DNMTs in human MDS (40). A recent study also indicate that
DNA methylation is involved in the transcriptional regulation of
SPAG6 in non-small cell lung cancer (41). However, the association between
SPAG6 expression and DNA methylation in MDS remains unreported. As
is well established, two different processes of methylation occur:
One is de novo methylation, which establishes the
methylation state, and DNMT3a and DNMT3b were identified as
predominantly responsible for this process in mammals; the other
method is maintenance methylation, which copies the pattern onto
daughter DNA strands following DNA replication, and DNMT1 was
identified as predominantly responsible for this process. The first
mammalian MT discovered was DNMT1, which is highly conserved among
eukaryotes (42,43). To the best of our knowledge, the
current study if the first to report that SPAG6 silencing decreases
DNMT1 expression and subsequently upregulates PTEN expression.
However, additional in-depth studies are required for further
elucidation of the mechanisms of SPAG6-mediated PTEN activation.
Furthermore, there is an inherent limitation using a cell line to
study the role of SPAG6 in cell apoptosis, as patients with MDS
undergo disease progression over time. As such, the current
xenograft mouse model may not accurately reflect the progression of
MDS. Therefore, a murine xenotransplant model is required for
future investigations on how SPAG6 impacts MDS disease
progression.
In summary, the data suggest that SPAG6 has a
critical role in the PTEN-mediated PI3K/AKT pathway to affect
apoptosis in the SKM-1 human MDS cell line. Knockdown of SPAG6 also
inhibits tumor growth of SKM-1 xenografts by inducing cell
apoptosis via extrinsic and intrinsic pathways in vivo.
Collectively, these data support that SPAG6 silencing induces PTEN
activation and AKT inactivation, which in turn results in cell
apoptosis in the mitochondrial pathway. Since the therapeutic
efficacy of current MDS treatments is not ideal in the clinic, the
findings of the current study suggest that SPAG6 might be a
promising putative molecular target for advancing the treatment of
MDS.
Acknowledgments
We thank Ms. Shaoqiu Jiang, Ms. Huan Yan, Ms. Hui
Gou, Ms. Tingting Li and Ms. Yao Ding (The First Affiliated
Hospital of Chongqing Medical University, Chongqing, China) for
their assistance. We thank the service provided by the Chongqing
Key Laboratory of Translational Medicine in Major Metabolic
Diseases (The First Affiliated Hospital of Chongqing Medical
University, Chongqing, China).
Funding
The Natural Science Foundation of Chongqing (grant
no. CSTC2013jjB0145) and the National Natural Sciences Foundation
of China (grant no. 81570109).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW, LL and XLi contributed to the conception of the
study. JY and LL performed the experiments data analyses and wrote
the manuscript. XLu and ZZ helped perform the analysis with
constructive discussions.
Ethics approval and consent to
participate
All applicable international, national, and/or
institutional guidelines for the care and use of animals were
followed. All procedures performed in studies involving animals
were in accordance with the ethical standards of the institution or
practice at which the studies were conducted and approved by the
University Committee on the Use and Care of Animals at Chongqing
Medical University (Chongqing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Greenberg PL, Stone RM, Al-Kali A, Barta
SK, Bejar R, Bennett JM, Carraway H, De Castro CM, Deeg HJ, DeZern
AE, et al: Myelodysplastic syndromes, version 2.2017, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw. 15:60–87.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li N, Chen Q, Gu J, Li S, Zhao G, Wang W,
Wang Z and Wang X: Synergistic inhibitory effects of deferasirox in
combination with decitabine on leukemia cell lines SKM-1, THP-1,
and K-562. Oncotarget. 8:36517–36530. 2017.PubMed/NCBI
|
|
3
|
Rose C, Brechignac S, Vassilief D, Pascal
L, Stamatoullas A, Guerci A, Larbaa D, Dreyfus F, Beyne-Rauzy O,
Chaury MP, et al GFM (Groupe Francophone des Myélodysplasies): Does
iron chelation therapy improve survival in regularly transfused
lower risk MDS patients? A multicenter study by the GFM (Groupe
Francophone des Myélodysplasies). Leuk Res. 34:864–870. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schanz J, Tüchler H, Solé F, Mallo M, Luño
E, Cervera J, Granada I, Hildebrandt B, Slovak ML, Ohyashiki K, et
al: New comprehensive cytogenetic scoring system for primary
myelodysplastic syndromes (MDS) and oligoblastic acute myeloid
leukemia after MDS derived from an international database merge. J
Clin Oncol. 30:820–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmad A and Iqbal MA: Significance of
genome-wide analysis of copy number alterations and UPD in
myelodysplastic syndromes using combined CGH - SNP arrays. Curr Med
Chem. 19:3739–3747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pfeifer D, Pantic M, Skatulla I, Rawluk J,
Kreutz C, Martens UM, Fisch P, Timmer J and Veelken H: Genome-wide
analysis of DNA copy number changes and LOH in CLL using
high-density SNP arrays. Blood. 109:1202–1210. 2007. View Article : Google Scholar
|
|
7
|
Wang L, Fidler C, Nadig N, Giagounidis A,
Della Porta MG, Malcovati L, Killick S, Gattermann N, Aul C,
Boultwood J, et al: Genome-wide analysis of copy number changes and
loss of heterozygosity in myelodysplastic syndrome with del(5q)
using high-density single nucleotide polymorphism arrays.
Haematologica. 93:994–1000. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bis DM, Schüle R, Reichbauer J, Synofzik
M, Rattay TW, Soehn A, de Jonghe P, Schöls L and Züchner S:
Uniparental disomy determined by whole-exome sequencing in a
spectrum of rare motoneuron diseases and ataxias. Mol Genet Genomic
Med. 5:280–286. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinbach D, Schramm A, Eggert A, Onda M,
Dawczynski K, Rump A, Pastan I, Wittig S, Pfaffendorf N, Voigt A,
et al: Identification of a set of seven genes for the monitoring of
minimal residual disease in pediatric acute myeloid leukemia. Clin
Cancer Res. 12:2434–2441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steinbach D, Bader P, Willasch A,
Bartholomae S, Debatin KM, Zimmermann M, Creutzig U, Reinhardt D
and Gruhn B: Prospective validation of a new method of monitoring
minimal residual disease in childhood acute myelogenous leukemia.
Clin Cancer Res. 21:1353–1359. 2015. View Article : Google Scholar
|
|
11
|
Neilson LI, Schneider PA, Van Deerlin PG,
Kiriakidou M, Driscoll DA, Pellegrini MC, Millinder S, Yamamoto KK,
French CK and Strauss JF III: cDNA cloning and characterization of
a human sperm antigen (SPAG6) with homology to the product of the
Chlamydomonas PF16 locus. Genomics. 60:272–280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Jones BH, Tang W, Moss SB, Wei Z,
Ho C, Pollack M, Horowitz E, Bennett J, Baker ME and Strauss JF
3rd: Dissecting the axoneme interactome: The mammalian orthologue
of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the
mammalian orthologue of Chlamydomonas PF16. Mol Cell Protezom.
4:914–923. 2005. View Article : Google Scholar
|
|
13
|
Sapiro R, Kostetskii I, Olds-Clarke P,
Gerton GL, Radice GL and Strauss JF III: Male infertility, impaired
sperm motility, and hydrocephalus in mice deficient in
sperm-associated antigen 6. Mol Cell Biol. 22:6298–6305. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lonergan KM, Chari R, Deleeuw RJ, Shadeo
A, Chi B, Tsao MS, Jones S, Marra M, Ling V, Ng R, et al:
Identification of novel lung genes in bronchial epithelium by
serial analysis of gene expression. Am J Respir Cell Mol Biol.
35:651–661. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mulaw MA, Krause A, Deshpande AJ, Krause
LF, Rouhi A, La Starza R, Borkhardt A, Buske C, Mecucci C, Ludwig
WD, et al: CALM/AF10-positive leukemias show upregulation of genes
involved in chromatin assembly and DNA repair processes and of
genes adjacent to the breakpoint at 10p12. Leukemia. 26:1012–1019.
2012. View Article : Google Scholar
|
|
16
|
Siliņa K, Zayakin P, Kalniņa Z, Ivanova L,
Meistere I, Endzeliņš E, Abols A, Stengrēvics A, Leja M, Ducena K,
et al: Sperm-associated antigens as targets for cancer
immunotherapy: Expression pattern and humoral immune response in
cancer patients. J Immunother. 34:28–44. 2011. View Article : Google Scholar
|
|
17
|
Nakagawa T, Matozaki S, Murayama T,
Nishimura R, Tsutsumi M, Kawaguchi R, Yokoyama Y, Hikiji K, Isobe T
and Chihara K: Establishment of a leultaemic cell line from a
patient with acquisition of chromosomal abnormalities during
disease progression in myelodysplastic syndrome. Br J Haematol.
85:469–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Wang L, Luo X, Chen L, Yang Z and
Liu L: SPAG6 silencing inhibits the growth of the malignant myeloid
cell lines SKM-1 and K562 via activating p53 and caspase
activation-dependent apoptosis. Int J Oncol. 46:649–656. 2015.
View Article : Google Scholar
|
|
19
|
Li X, Yang B, Wang L, Chen L, Luo X and
Liu L: SPAG6 regulates cell apoptosis through the TRAIL signal
pathway in myelodysplastic syndromes. Oncol Rep. 37:2839–2846.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao L, Shan Y, Liu B, Li Y and Jia L:
Functional screen analysis reveals miR-3142 as central regulator in
chemoresistance and proliferation through activation of the
PTEN-AKT pathway in CML. Cell Death Dis. 8:e28302017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scherr M and Eder M; M SMaE: Gene transfer
into hematopoietic stem cells using lentiviral vectors. Curr Gene
Ther. 2:45–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Storti P, Donofrio G, Colla S, Airoldi I,
Bolzoni M, Agnelli L, Abeltino M, Todoerti K, Lazzaretti M, Mancini
C, et al: HOXB7 expression by myeloma cells regulates their
pro-angiogenic properties in multiple myeloma patients. Leukemia.
25:527–537. 2011. View Article : Google Scholar
|
|
26
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JY and Widmann C: The RasGAP
N-terminal fragment generated by caspase cleavage protects cells in
a Ras/PI3K/Akt-dependent manner that does not rely on NFkappa B
activation. J Biol Chem. 277:14641–14646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rhee I, Bachman KE, Park BH, Jair KW, Yen
RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, et al:
DNMT1 and DNMT3b cooperate to silence genes in human cancer cells.
Nature. 416:552–556. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsoplou P, Kouraklis-Symeonidis A,
Thanopoulou E, Zikos P, Orphanos V and Zoumbos NC: Apoptosis in
patients with myelodysplastic syndromes: Differential involvement
of marrow cells in 'good' versus 'poor' prognosis patients and
correlation with apoptosis-related genes. Leukemia. 13:1554–1563.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parker JE and Mufti GJ: Excessive
apoptosis in low risk myelodysplastic syndromes (MDS). Leuk
Lymphoma. 40:1–24. 2000. View Article : Google Scholar
|
|
31
|
Kerbauy DB and Deeg HJ: Apoptosis and
antiapoptotic mechanisms in the progression of myelodysplastic
syndrome. Exp Hematol. 35:1739–1746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rodriguez M, Siwko S, Zeng L, Li J, Yi Z
and Liu M: Prostate-specific G-protein-coupled receptor
collaborates with loss of PTEN to promote prostate cancer
progression. Oncogene. 35:1153–1162. 2016. View Article : Google Scholar
|
|
33
|
Schöndorf T, Göhring UJ, Roth G, Middel I,
Becker M, Moser N, Valter MM and Hoopmann M: Time to progression is
dependent on the expression of the tumour suppressor PTEN in
ovarian cancer patients. Eur J Clin Invest. 33:256–260. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsutsui S, Inoue H, Yasuda K, Suzuki K,
Higashi H, Era S and Mori M: Reduced expression of PTEN protein and
its prognostic implications in invasive ductal carcinoma of the
breast. Oncology. 68:398–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nyåkern M, Tazzari PL, Finelli C, Bosi C,
Follo MY, Grafone T, Piccaluga PP, Martinelli G, Cocco L and
Martelli AM: Frequent elevation of Akt kinase phosphorylation in
blood marrow and peripheral blood mononuclear cells from high-risk
myelodysplastic syndrome patients. Leukemia. 20:230–238. 2006.
View Article : Google Scholar
|
|
36
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu X, Yan R, Cheng X, Song L, Zhang W, Li
K and Zhao S: The function of sperm-associated antigen 6 in
neuronal proliferation and differentiation. J Mol Histol.
47:531–540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee DW, Futami M, Carroll M, Feng Y, Wang
Z, Fernandez M, Whichard Z, Chen Y, Kornblau S, Shpall EJ, et al:
Loss of SHIP-1 protein expression in high-risk myelodysplastic
syndromes is associated with miR-210 and miR-155. Oncogene.
31:4085–4094. 2012. View Article : Google Scholar
|
|
40
|
Shu Y, Zhou X, Qi X, Liu S, Li K, Tan J,
Liu Z, Yu J, Zhang P and Zou L: β-Arrestin1 promotes the
self-renewal of the leukemia-initiating cell-enriched subpopulation
in B-lineage acute lymphoblastic leukemia related to DNMT1
activity. Cancer Lett. 357:170–178. 2015. View Article : Google Scholar
|
|
41
|
Altenberger C, Heller G, Ziegler B,
Tomasich E, Marhold M, Topakian T, Müllauer L, Heffeter P, Lang G,
End-Pfützenreuter A, et al: SPAG6 and L1TD1 are transcriptionally
regulated by DNA methylation in non-small cell lung cancers. Mol
Cancer. 16:12017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fuks F, Burgers WA, Brehm A, Hughes-Davies
L and Kouzarides T: DNA methyltransferase Dnmt1 associates with
histone deacetylase activity. Nat Genet. 24:88–91. 2000. View Article : Google Scholar
|
|
43
|
Hermann A, Gowher H and Jeltsch A:
Biochemistry and biology of mammalian DNA methyltransferases. Cell
Mol Life Sci. 61:2571–2587. 2004. View Article : Google Scholar : PubMed/NCBI
|