Introduction
Breast cancer is one of the most frequent
malignancies in women, leading to >300,000 mortalities annually
worldwide (1). Despite recent
improvement in the mortality rate through drastic treatment
strategies, including radiotherapy and chemotherapy, the patient
survival rate has not improved owing to tumor relapse and
metastasis (2). A growing body of
evidence suggests that cancer recurrence and metastasis may be
driven by a subpopulation of cells within the tumor termed cancer
stem cells (CSCs) or cancer-initiating cells (3). As with normal stem cells, these cells
have the ability to self-renew and differentiate into multiple
lineages; therefore, they are considered to be responsible for
tumorigenesis, therapeutic resistance, metastasis and recurrence
(4). Stem-like breast cancer cells
were first identified by Al-Hajj et al (5), who identified that a subpopulation of
cells from human breast tumors and cluster of differentiation
(CD)44+CD24−ESA+ pleural effusion
cells of patients are responsible for breast cancer tumorigenicity.
CD44+CD24− breast cancer stem cells (BCSCs)
may be induced by promoting epithelial-mesenchymal transition via
suppression of epithelial (E-)cadherin by short hairpin RNA,
ectopic expression of an E-cadherin transcriptional suppressor such
as Snail or Twist or treatment with transforming growth factor-β
(6). BCSCs may be enriched by
growth factor-enriched serum-free non-adherent sphere culture of
primary cancer cells and established cell lines, including MCF7
breast cancer cells (7). Since
CSCs are considered to be the primary reason for therapeutic
failure, these cells are considered a candidate therapeutic
target.
One of the hallmarks of CSCs is their resistance to
therapy (3). Preclinical data
indicate that BCSCs are more resistant to ionizing radiation (IR)
compared with serum-cultured normal cancer cells. The
radioresistance in BCSCs is based on their decreased production of
reactive oxygen species in response to IR owing to enhanced
expression of free radical-scavenging proteins (8,9).
Additionally, upregulation of Notch ligand expression followed by
activation of the Notch pathway by IR enriches BCSCs in MCF7 cells
and maintains stemness in these cells (8). A previous study demonstrated that
increased survival of MCF7 BCSCs in response to IR is mediated by
downregulation of the senescence pathway, not apoptosis (10). Thus, targeting BCSCs may be a
promising way to increase radiotherapeutic effectiveness in breast
cancer.
The POU-domain transcription factor octamer-binding
transcription factor 4 (Oct4) is one of the master regulators of
maintenance of embryonic stem cells along with sex-determining
region Y-box 2 (Sox2) and Nanog, and one of the key transcription
regulators of stem cell pluripotency (11). Oct4 is expressed in various
malignant tumor tissues and cell lines, including non-small cell
lung cancer, liver cancer and glioma lines (12–14).
Oct4 is also expressed in breast cancer tissues and BCSCs (15), and is associated with poorly
differentiated high-grade estrogen receptor-negative tumors
(16). Oct4 confers
chemoresistance on liver cancer cells via protein kinase B
(Akt)-mediated upregulation of ATP-binding cassette transporter G2
(ABCG2) (13). Additionally, Oct4
promotes colony formation of glioma cells (14), whereas Oct4 suppression leads to
the induction of apoptosis in breast cancer cells (15). However, the function of Oct4 in the
response of tumor cells to IR is poorly understood.
In the present study, the function of Oct4 was
investigated in radioresistance of breast cancer cells. Using
mammosphere and radioresistant cells derived from MCF7 cells,
results indicated that radioresistance of breast cancer cells is
associated with Oct4 expression. Mammosphere and radioresistant
cells expressed an increased level of Oct4, and overexpression of
Oct4 increased radioresistance of MCF7 cells. Importantly, Oct4
expression suppressed IR-induced premature senescence by enhancing
IL-24 production through signal transducer and activator of
transcription 3 (STAT3) and nuclear factor κB (NF-κB) signaling
pathways, which are associated with IR resistance of breast cancer
cells.
Materials and methods
Reagents
Antibodies against Oct4 (cat. no. sc-5279), c-Myc
(cat. no. sc-40), interleukin (IL)-24 (cat. no. sc-22769),
Krüppel-like factor 4 (cat. no. sc-20691), p53 (cat. no. sc-126),
phosphorylated (p)-p53 (Ser15) (cat. no. sc-101762), p21 (cat. no.
sc-397), p27 (cat. no. sc-527), p16 (cat. no. sc-486), Sirt1 (cat.
no. sc-15404) and β-actin (cat. no. sc-47778) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies
against Bmi-1 (cat. no. 05-637), Nanog (cat. no. AB9220),
phosphorylated histone H2AX (γ-H2AX; cat. no. 05-636) and Notch1
(cat. no. MAB5352) were from EMD Millipore (Billerica, MA, USA).
Antibodies against p-extracellular-signal-regulated kinase 1/2
(cat. no. 4695), p-Akt (Ser473) (cat. no. 9271), STAT3
(cat. no. 9139), p-STAT3 (Ser727) (cat. no. 9134),
p-STAT3 (Tyr705) (cat. no. 9145), p-c-Jun N-terminal
kinase (cat. no. 2361), p-retinoblastoma protein
(Ser807/811) (cat. no. 9308), non-p-β-catenin (cat. no.
8814), inhibitor of NF-κB (IκB) (cat. no. 4814) and p-IκB (cat. no.
2859) were obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Anti-Sox2 (cat. no. MAB2018) was from R&D Systems,
Inc. (Minneapolis, MN, USA). Specific inhibitors of STAT3 (WP1066)
and NF-κB (Bay 11-7082) were purchased from Calbiochem; Merck KGaA
(Darmstadt, Germany).
Cell culture
Human breast cancer cells (MCF7 cells) (ATCC,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium (DMEM) (Welgen, Daegu, Korea) supplemented with 10% fetal
bovine serum (FBS; JR Scientific, Inc., Woodland, CA, USA) and 1%
penicillin/streptomycin at 37°C in a 5% CO2 incubator.
For collecting mammospheres, cells were trypsinized and cultured at
37°C in serum-free DMEM/Ham's F12 (Cellgro; Corning Incorporated,
Corning, NY, USA) with epidermal growth factor (EGF) and basic
fibroblast growth factor (bFGF) (R&D Systems, Inc.) (10 ng/ml
each). Mammospheres were collected following three passages of
culture.
Sphere-forming and soft-agar clonogenic
assays
For the sphere-forming assay, a single-cell
suspension prepared by trypsinization was seeded in serum-free
DMEM/Ham's F12 with EGF and bFGF (10 ng/ml each) at a density of
1×103 cells/ml. After 10 days of culture, spheres were
attached to the plate by adding FBS (10%), stained using Diff-Quick
solution (Sysmex Corporation, Kobe, Japan) and counted by eye. For
the soft-agar clonogenic assay, cells were resuspended in the same
volume of 0.7% agar with 10% FBS and plated onto the bottom agar
layer (1:1 mixture of 1% agar and 2X DMEM/Ham's F12 with 10% FBS).
After 14 days of incubation, colonies in five random fields/well
were counted under a brightfield microscope (magnification,
×100).
Invasion and migration assays
Matrigel invasion assays were performed using
Transwell chambers (Costar; Corning Incorporated) precoated with
Matrigel (100 µg/cm2). Cells (105
cells in 100 µl serum-free DMEM) were seeded into the upper
part of each chamber, whereas the lower compartments were filled
with 600 µl DMEM with 1% FBS. Following incubation at 37°C
for 18 h, non-invading cells on the upper surface of the filter
were removed using a cotton swab, and the invading cells on the
lower surface of the filter were fixed and stained using a
Diff-Quick kit (Sysmex Corporation), according to the
manufacturer's protocol. Invasiveness was determined by counting
cells in five brightfield microscopic fields/well (magnification,
×100), and the extent of invasion was expressed as the mean number
of cells/microscopic field. Transwell migration assays were
performed using a similar procedure to that used for the invasion
assay, except that the lower surfaces of filters were coated with
gelatin (100 µg/cm2) and upper surfaces were not
coated.
Western blot analysis
Cells (2×106) were lysed in lysis buffer.
Following a brief sonication, the lysates were clarified by
centrifugation at 12,000 g for 15 min at 4°C, and protein content
was determined using the Bradford method. An aliquot (25 µg
protein/lane) of total protein was separated by SDS-PAGE (10 or 12%
gel) for 2 h at 80 V, blotted onto a nitrocellulose membrane (0.2
µm pore size; GE Healthcare, Chicago, IL, USA) for 1.5 h at
100 V. The membrane was blocked with 3% BSA in TBST [20 mmol/l
Tris-HCl (pH 7.6), 137 mmol/l NaCl and 0.01% Tween-20] for 1 h at
room temperature followed by incubation with primary antibody
(1:1,000) for 2 h at room temperature. Following extensive washing
with TBST, the membrane was probed with secondary antibody [rabbit
immunoglobulin G (IgG) heavy and light chain antibody (cat. no.
A120-101P) or mouse IgG heavy and light chain antibody (cat. no.
A90-116P); 1:20,000; Bethyl Laboratories, Inc., Montgomery, TX,
USA] conjugated with horseradish peroxidase for 1 h at room
temperature. Following washing three times with TBST for 15 min,
immunoblots were visualized using enhanced chemiluminescence (GE
Healthcare), according to the manufacturer's protocol.
Reverse transcription-polymerase chain
reaction (RT-PCR)
High-quality total RNA was isolated from cells
(1×105) using TRIsure™ (Bioline; Meridian Bioscience
Inc., London, UK), according to the manufacturer's protocol. cDNA
was synthesized using a SentiFAST™ cDNA synthesis kit (cat. no.
BIO-65054; Bioline; Meridian Bioscience Inc.). PCR for IL-6
(149-bp), IL-8 (194-bp), IL-24 (429-bp) and 18S (433-bp) was
performed as described previously (22). Oligonucleotide primer sequences
were as follows: IL-6, 5′-ACTCACCTCTTCAGAACGAATTG-3′ (forward) and
5′-CCATCTTTGGAAGGTTCAGGTTG-3′ (reverse); IL-8,
5′-TTTTGCCAAGGAGTGCTAAAGA-3′ (forward) and
5′-AACCCTCTGCACCCAGTTTTC-3′ (reverse); IL-24,
5′-GACTTTAGCCAGCAGACCCTT-3′ (forward) and
5′-GGTTGCAGTTGTGACACGAT-3′ (reverse); 18S,
5′-AAACGGCTACCACATCCAAG-3′ (forward) and 5′-CGCTCCCAAGATCCAACTAC-3′
(reverse). The PCR products were analyzed on a 2% agarose gel,
stained with ethidium bromide (Mbiotech, Inc., Hanam, Korea), and
visualized under UV light using an image analyzer (ChemiDoc XRS;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunocytochemistry and γ-H2AX focus
assay
Cells (1×105) were seeded in a chamber
slide with DMEM supplemented with 10% FBS. The following day, the
cells were fixed with 4% paraformaldehyde for 20 min and washed
with PBS three times. Subsequently, the cells were incubated in
blocking solution (5% BSA and 0.5% Triton X-100 in PBS) for 1 h at
room temperature. The cells were stained with primary antibodies
(against Oct4 and γ-H2AX) in blocking solution (1:100) for 2 h and
washed with PBS three times. Subsequently, the cells were incubated
with Alexa Fluor 488-labeled goat anti-rabbit and Alexa Fluor
594-labeled goat anti-mouse secondary antibodies (both 1:1,000;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1
h. Nuclei were stained using Vectashield mounting medium with DAPI)
(Vector Laboratories, Inc., Burlingame, CA, USA) for 5 min at room
temperature and stained cells were viewed using a confocal
laser-scanning microscope (magnification, ×100). γ-H2AX foci were
determined in ≥50 cells.
Colony formation assay and selection of
IR-resistant (IRR) cells
For determining IR resistance, 500 cells were seeded
in 60-mm dishes and were exposed to γ-rays from a 137Cs
γ-ray source (Atomic Energy of Canada, Korea Institute of
Radiological and Medical Sciences, Seoul, Korea) at a dose rate of
3.81 Gy/min. After 7 days of incubation, the culture dishes were
washed with PBS and the cells were stained with 0.05% crystal
violet in 20% methanol. Following three washes with distilled
water, colonies were counted by eye. For collection of IRR cells,
cells (500 cells/60-mm dish) were exposed to IR at 5 or 7 Gy and
cultured for 21 days. Emerging colonies were collected as IRR
cells.
Transduction of adenoviruses and
transfection of small interfering RNA (siRNA)
Adenoviral-Oct4 and control virus
(adenoviral-luciferase) were a gift from Dr Hee-Choong Kwon (Korea
Institute of Radiological and Medical Sciences). MCF7 cells
(5×105) were infected with Adenoviruses (multiplicity of
infection, 100) in the presence of 8 µl/ml polybrene. Cells
(5×105) were transfected with a pool of siRNAs against
Oct4 (5′-AUCACGUAAAGCUAGAAA-3′ and 5′-GGGAUCAUUUCUAGCUUU-3′;
Integrated DNA Technologies, Coralville, IA, USA) and Stealth™ RNAi
Negative Control Duplexes (Invitrogen; Thermo Fisher Scientific,
Inc.) at a final concentration of 50 nM using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.).
Cell-cycle and Annexin V-propidium iodide
(PI) assays
For the Annexin V-PI assay, cells (5×105)
were cultured in 60-mm dishes and exposed to IR (6 Gy). Cell death
was quantified using an Annexin V-FITC Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol. For the cell-cycle assay, cells
(5×105) were suspended in 100% ice-cold ethanol at −20°C
overnight. The cells were washed with PBS and stained with
cell-cycle solution (50 µg/ml PI, 10 µg/ml
ribonuclease A and 0.05% Triton X-100 in PBS) in the dark for 40
min. Following centrifugation at 200 × g for 5 min at 4°C, the
cells were resuspended in PBS and analyzed using a FACSCalibur flow
cytometer (BD Biosciences).
Senescence-associated β-galactosidase
(SA-β-gal) staining
Cells (1×104) were seeded in a 35-mm dish
and were exposed to IR (4 or 6 Gy). After 4 days of incubation, the
cells were washed with PBS and fixed with 2% formaldehyde (prepared
in PBS) for between 4 and 5 min. The cells were washed with PBS
twice, stained with SA-β-gal staining solution [NaCl,
MgCl2 and X-gal in dimethylformamide, citric acid/sodium
phosphate buffer (pH 6.0), potassium ferrocyanide and potassium
ferricyanide] and incubated at 37°C for between 12 and 16 h.
Following incubation, the cells were washed with PBS twice and blue
colonies were counted under an inverted brightfield microscope
(magnification, ×100).
Cytokine array-base analysis
Cytokine array-base analysis was performed using a
Human Cytokine Array kit (cat. no. ARY022; R&D Systems, Inc.),
according to the manufacturer's protocol.
Fluorescence-activated cell sorting
(FACS)
Cells (2×105) were seeded in 60-mm dishes
and were exposed to IR (4 or 6 Gy). After 4 days of incubation, the
cells were washed with PBS and treated with bafilomycin A1 (100 nM)
in fresh culture medium. After 1 h of incubation,
5-dodecanoylaminofluorescein di-D-galactopyranoside
(C12FDG) solution (1 µl/ml) was added to the
plate, and the cells were incubated further for between 2 and 3 h.
The solution in the plate was removed and the cells were washed
twice with PBS. The cells were harvested and analyzed by flow
cytometry, according to a previously published experimental
protocol (17).
Statistical analysis
The mean ± standard deviation of at least three
independent experiments was calculated with reference to a control.
Data analysis was performed using two-tailed Student's t-test to
compare two experimental groups or an analysis of variance to
compare ≤3 groups. Means were compared using an independent-samples
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Mammosphere and radioresistant cells
exhibit increased Oct4 expression compared with parental breast
cancer cells
Since a number of studies have demonstrated that
BCSCs are enriched in mammospheres and are the cause of IR
resistance of MCF7 cells (16,18,19),
it was first examined whether mammosphere-cultured breast cancer
cells exhibit resistance to IR and a stem-like cell character. As
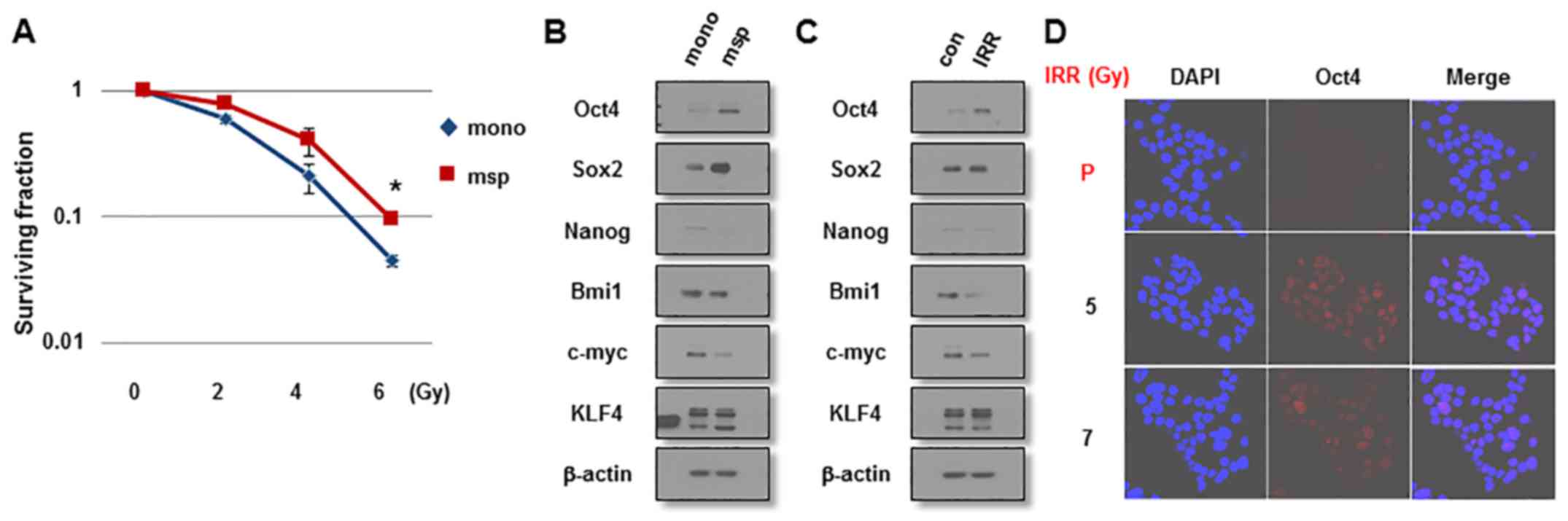
presented in Fig. 1A,
mammosphere-cultured MCF7 cells were more resistant to IR exposure
compared with monolayer MCF7 cells cultured in serum-enriched
medium as indicated by clonogenic survival assays. Mammosphere
cells expressed increased levels of the stemness-associated
factors, including Oct4 and Sox2, compared with monolayer cells
(Fig. 1B). To investigate the
involvement of these factors in the radioresistance of breast
cancer cells, MCF7 cells were exposed to IR (6 Gy) and were allowed
to regrow for 3 weeks. In line with these results, IRR cells
expressed increased levels of Oct4, but not of Sox2 or other
stemness-related factors, compared with parental cells (Fig 1C). The increased Oct4 expression was
confirmed by immunocytochemical staining of IRR MCF7 cells
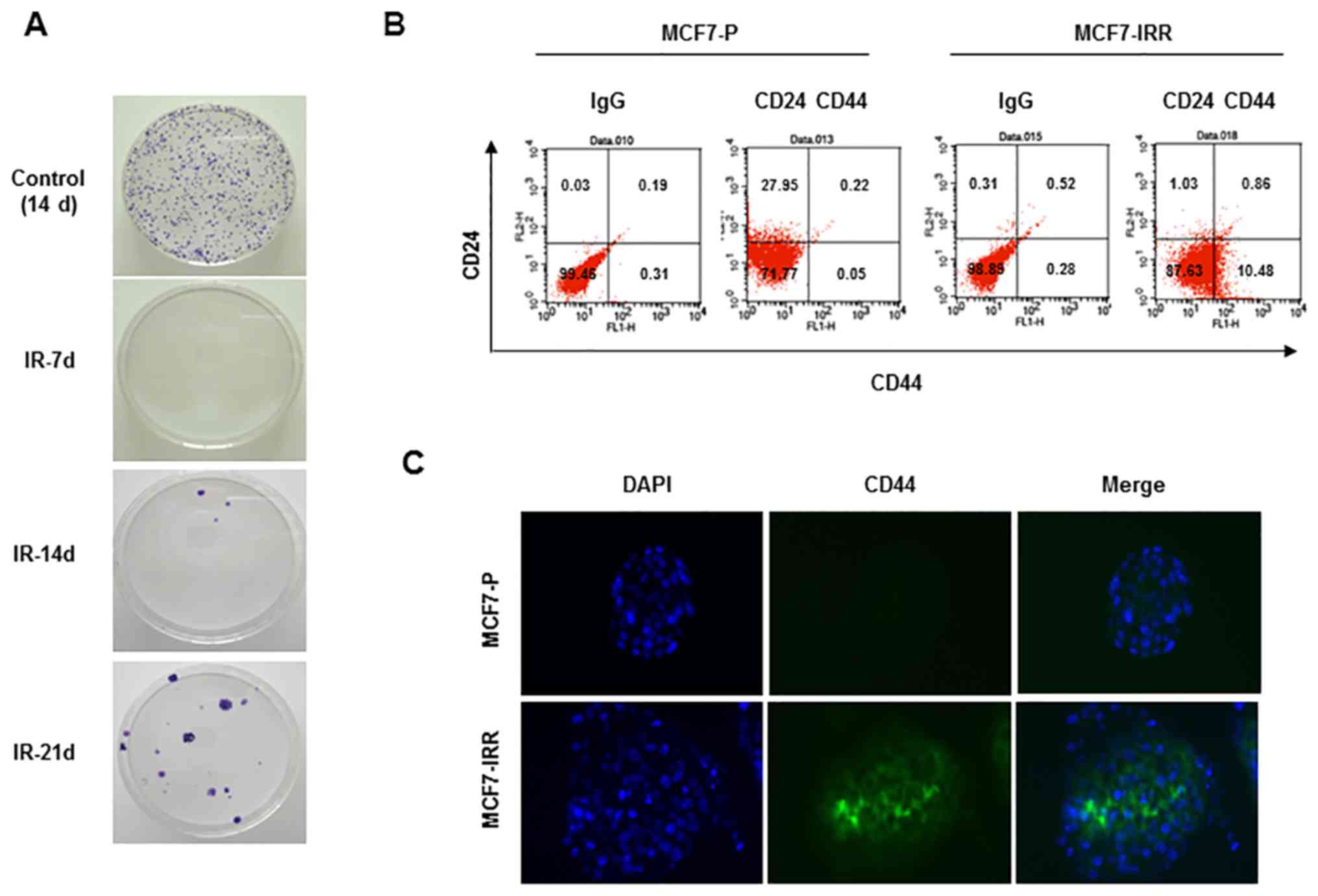
(Fig. 1D). IRR MCF7 colonies began
to emerge in the second week and were collected in the third week
(Fig. 2A). Collected cells
exhibited a larger CD44+CD24− cell fraction
compared with parental cells, indicating that IRR cells exhibited
BCSC traits (Fig. 2B).
CD44+ IRR MCF7 cells were also confirmed using
immunocytochemistry (Fig. 2C). On
the basis of these results, it was decided to investigate the
function of Oct4 in stemness and IR resistance of breast cancer
cells.
Overexpression of Oct4 enhances stemness
and IR resistance of breast cancer cells
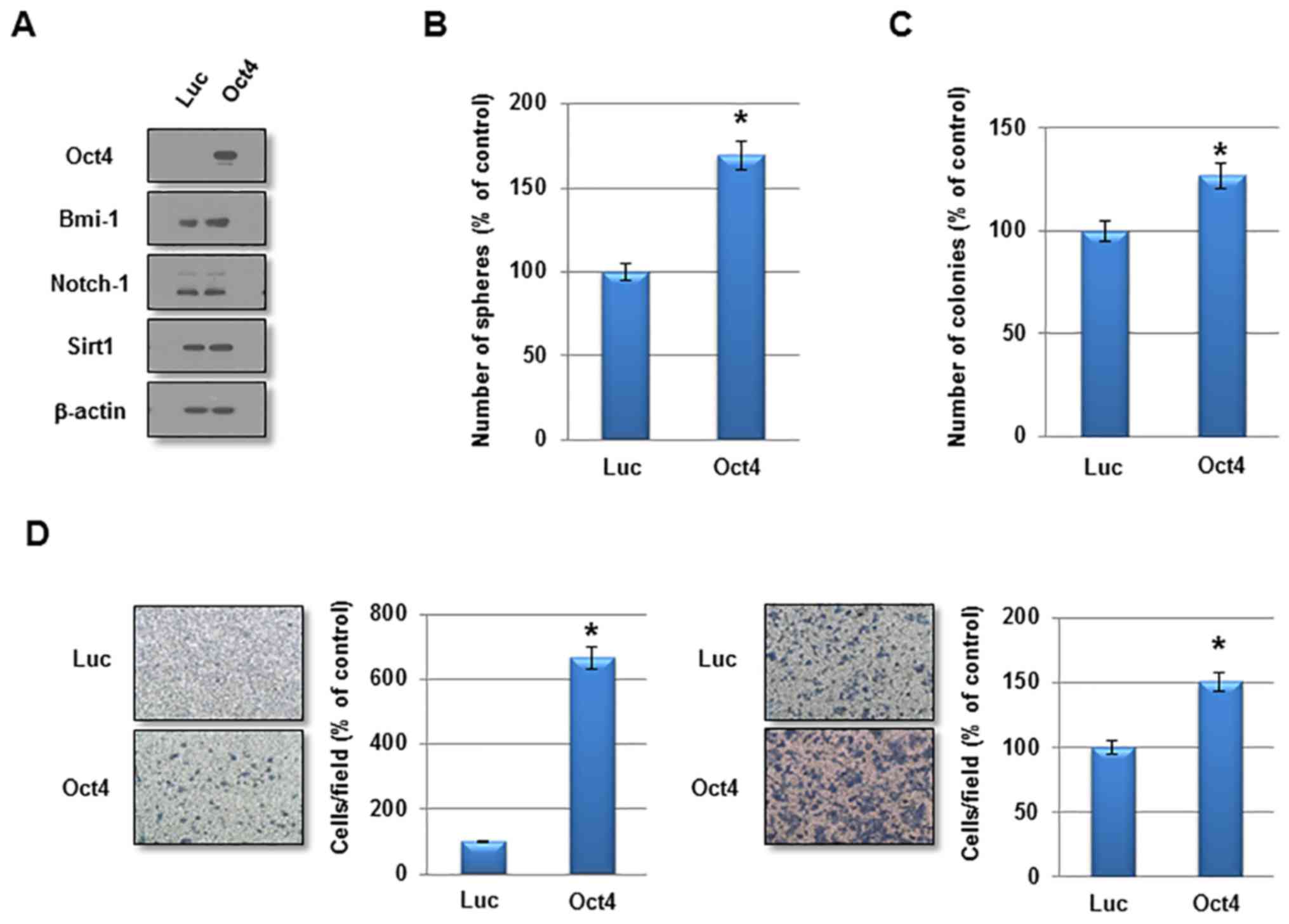
To investigate the function of Oct4 in the
self-renewal activity and IR resistance of breast cancer cells,
adenovirus harboring Oct4 was introduced into MCF7 cells.
Overexpression of Oct4 was confirmed by western blotting (Fig. 3A). Other stem cell-associated
factors were not altered significantly by Oct4 overexpression
(Fig. 3A). In a sphere-forming
assay, overexpression of Oct4 significantly enhanced mammosphere
formation in MCF7 cells (Fig. 3B).
Additionally, Oct4 promoted clonogenicity in soft agar and enhanced
the invasive and migratory activities in MCF7 cells (Fig. 3C and D). Importantly, introduction
of Oct4 significantly increased clonogenic survival upon IR
exposure in MCF7 cells (Fig. 4A).
To confirm the specific function of Oct4 in IR resistance of breast
cancer cells, siRNA against Oct4 (siOct4) was introduced into
Oct4-overexpressing MCF7 cells (Fig.
4B) and decreased clonogenic survival following IR observed in
these cells (Fig. 4C). Next, it
was determined whether Oct4 is involved in regulating the DNA
damage response induced by IR in breast cancer cells.
Immunocytochemical analysis revealed that focus formation of γ-H2AX
following IR exposure was decreased in Oct4-overexpressing MCF7
cells compared with in vector control cells (Fig. 4D), which was identified to be a
significant difference (Fig. 4E).
Taken together, these results suggested that Oct4 serves a crucial
function in self-renewal activity and IR resistance by decreasing
DNA damage in breast cancer cells.
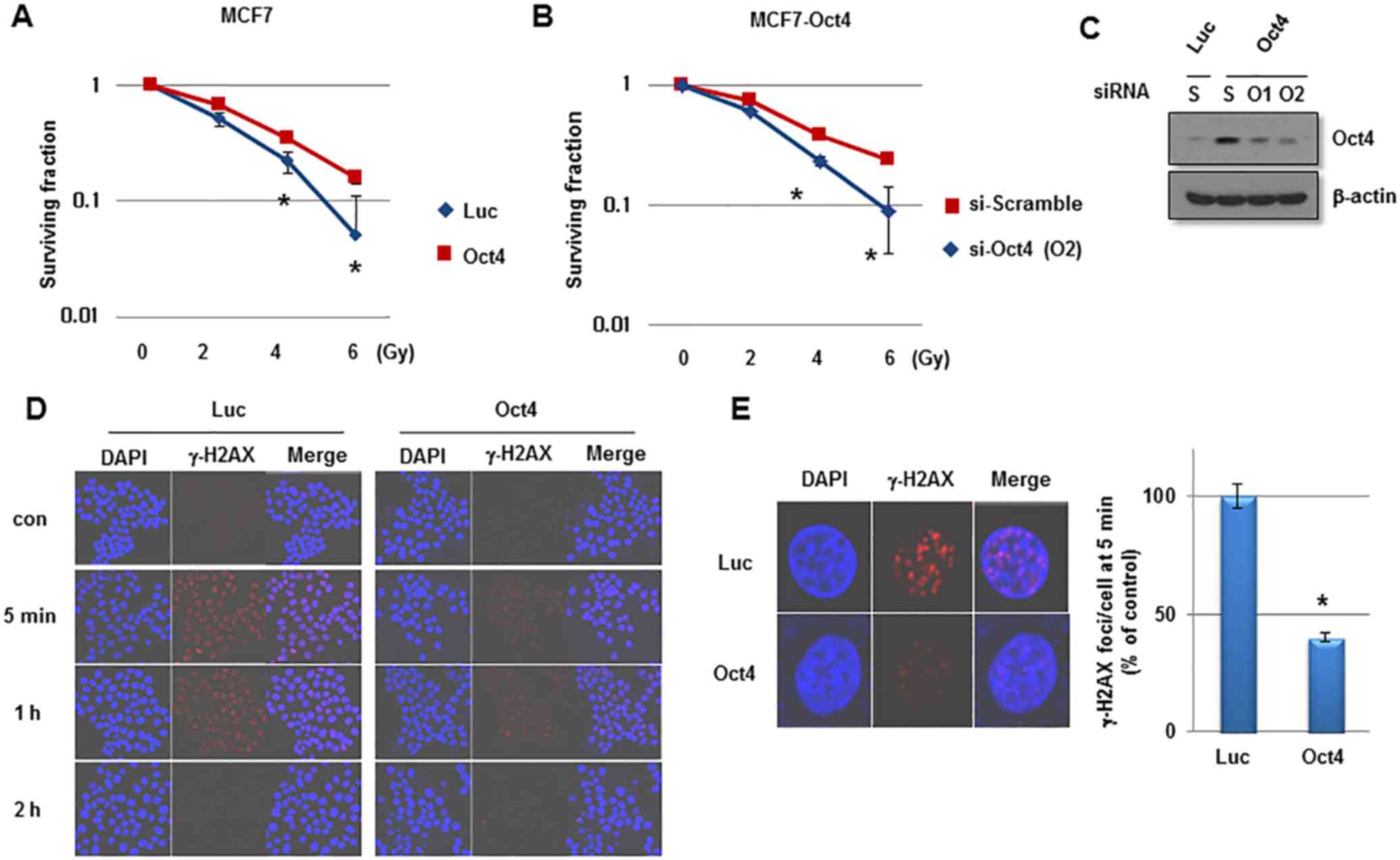 | Figure 4Enhanced IR resistance by Oct4
overexpression in breast cancer cells. Clonogenic survival assays
of MCF7 cells (A) infected with adenoviral-Luc and adenoviral-Oct4,
and (B) transfected with scramble or Oct4-targeted siRNA. (C)
Western blot analysis of Oct4 in MCF7 cells transfected with
scramble or Oct4-targeted siRNA. (D) Immunocytochemical staining of
γ-H2AX foci of MCF7 cells transduced with Luc and Oct4 following IR
exposure (6 Gy) with anti-γ-H2AX at the indicated time-points. (E)
Representative image of γ-H2AX foci (left) and quantification of
foci/cell at 5 min after IR (right). *P<0.05. IR,
ionizing radiation; Oct4, octamer-binding transcription factor 4;
siRNA, small interfering RNA; γ-H2AX, phosphorylated histone H2AX;
Luc, luciferase; con, control; si-Scramble, scramble siRNA;
si-Oct4, Oct4-targeted siRNA; S, scramble; O1/2, Oct4-targeted
siRNA 1/2. |
Oct4 decreases IR-induced premature
senescence in breast cancer cells
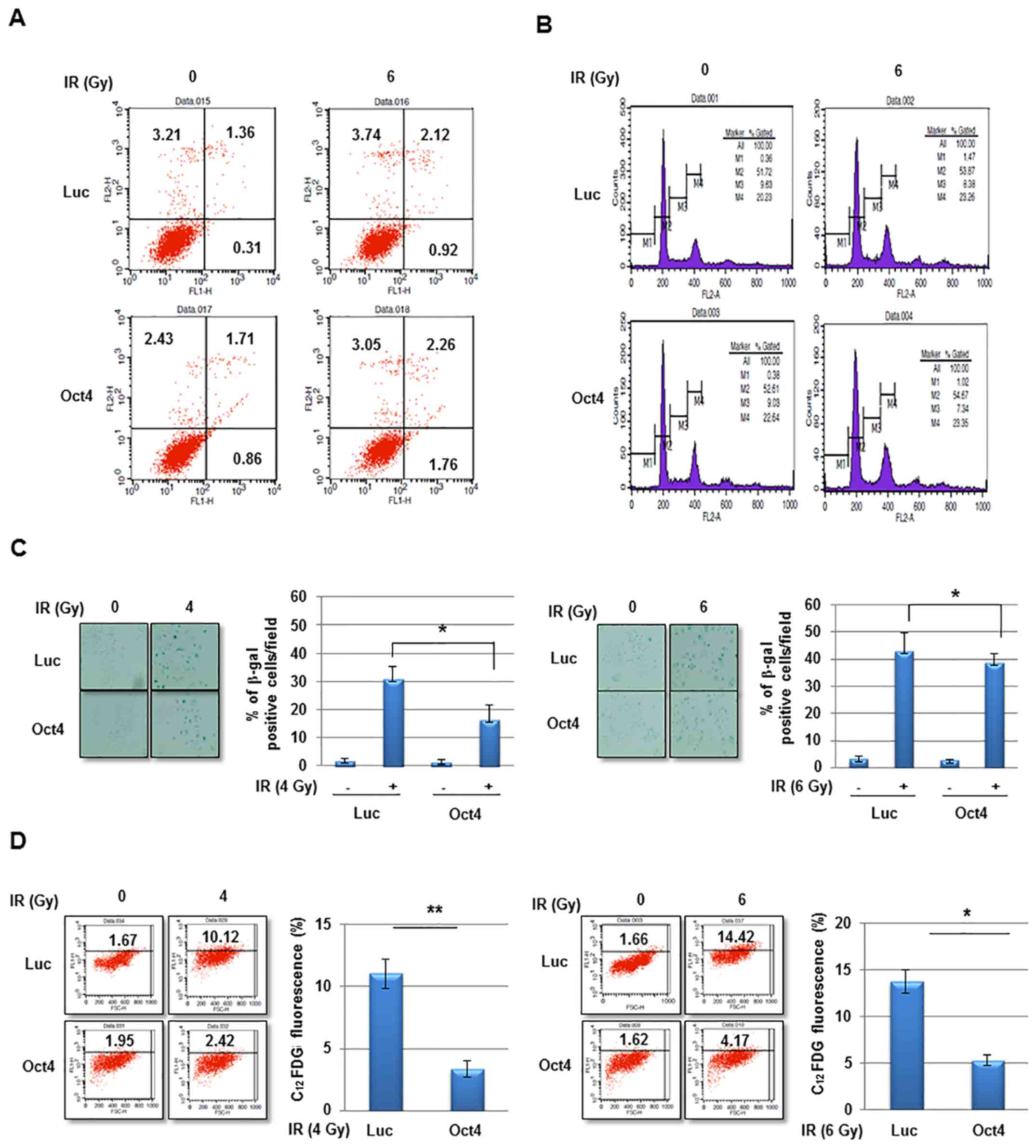
The potential mechanism of Oct4-mediated IR
resistance of breast cancer cells was then investigated. IR
exposure did not markedly induce cell death and did not affect
cell-cycle distribution in MCF7 cells (Fig. 5A and B). However, exposure to IR at
4 and 6 Gy significantly increased senescence in MCF7 cells as
revealed using the SA-β-gal assay and FACS (C12FDG
fluorescence) analysis, which was significantly suppressed by
overexpression of Oct4 (Fig. 5C and
D). Whereas the Oct4-mediated suppression of IR-induced
premature senescence was more evident at 4 Gy compared with at 6 Gy
in the SA-β-gal assay (Fig. 5C),
FACS analysis revealed similar suppressive effects under the two
conditions (Fig. 5D). These
results indicated that Oct4-mediated IR resistance is achieved by
suppression of IR-induced premature senescence rather than by
apoptosis or cell-cycle arrest.
STAT3 and NF-κB signaling is required for
Oct4-mediated inhibition of IR-induced senescence in MCF7
cells
The next aim was to elucidate the signaling crucial
for Oct4-mediated suppression of IR-induced premature senescence in
breast cancer cells. Among the pivotal signaling molecules for
cancer cell survival, STAT3 and NF-κB were commonly activated in
mammosphere, IRR and Oct4-overexpressing MCF7 cells as was revealed
by the enhanced phosphorylation of Tyr705 in STAT3 and
of Ser32/36 in IκB (Fig.
6A). IR exposure of Oct4-overexpressing cells further increased
Oct4 expression, and STAT3 (Tyr705) and IκB
phosphorylation (Fig. 6B). To
examine the involvement of STAT3 and NF-κB signaling in
Oct4-mediated suppression of IR-induced premature senescence, the
cells were pretreated with the STAT3-specific inhibitor WP1066
prior to IR. Pretreatment with WP1066 marginally affected
IR-induced senescence in control cells; however, it significantly
enhanced Oct4-mediated suppression of IR-induced senescence as
determined using the SA-β-gal assay and FACS analysis (Fig. 7A and B). In addition,
siRNA-mediated downregulation of STAT3 (Fig. 7C) inhibited the suppression of
IR-induced senescence (Fig. 7D and
E). Finally, pretreatment with the NF-κB-specific inhibitor Bay
11-7082 prevented Oct4-mediated suppression of IR-induced
senescence (Fig. 7F and G). Taken
together, these results indicated that Oct4-mediated inhibition of
IR-induced premature senescence in MCF7 cells is achieved via
activation of the STAT3 and NF-κB signaling pathways.
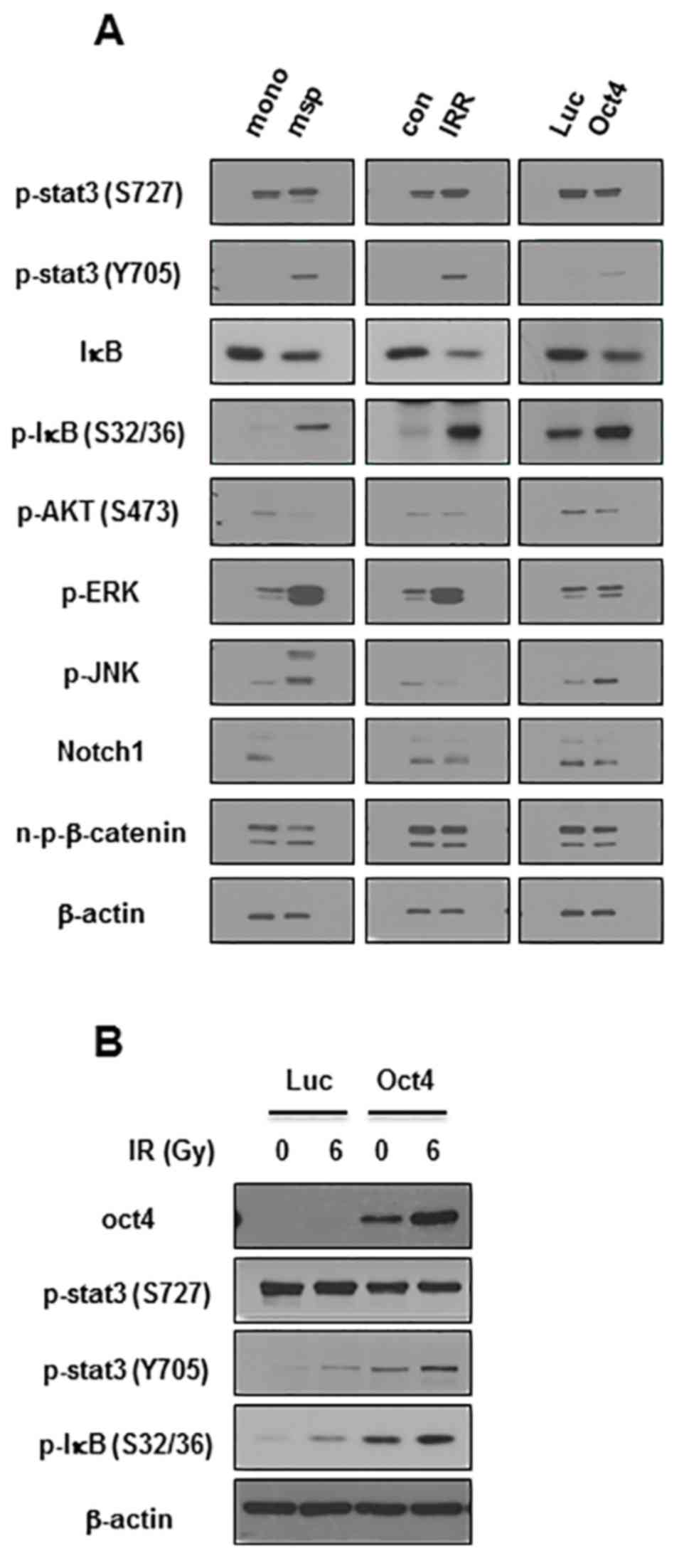 | Figure 6Increased activation of STAT3 and
NF-κB in mammosphere, IRR, and Oct4-overexpressing breast cancer
cells. (A) Western blot analysis of MCF7 cells with indicated
antibodies. (B) Western blot analysis of MCF7 cells transduced with
Luc and Oct4 following IR exposure (6 Gy). STAT3, signal transducer
and activator of transcription 3; NF-κB, nuclear factor κB; Oct4,
octamer-binding transcription factor 4; mono, serum-containing
monolayer culture; msp, serum-free mammosphere culture; con,
parental control cells; IRR, IR-resistant cells regrown following 6
Gy of IR exposure; Luc, luciferase; p-, phosphorylated; IκB,
inhibitor of NF-κB; AKT, protein kinase B; ERK,
extracellular-signal-regulated kinase; JNK, c-Jun N-terminal
kinase; n-p-β-catenin, non-phosphorylated-β-catenin. |
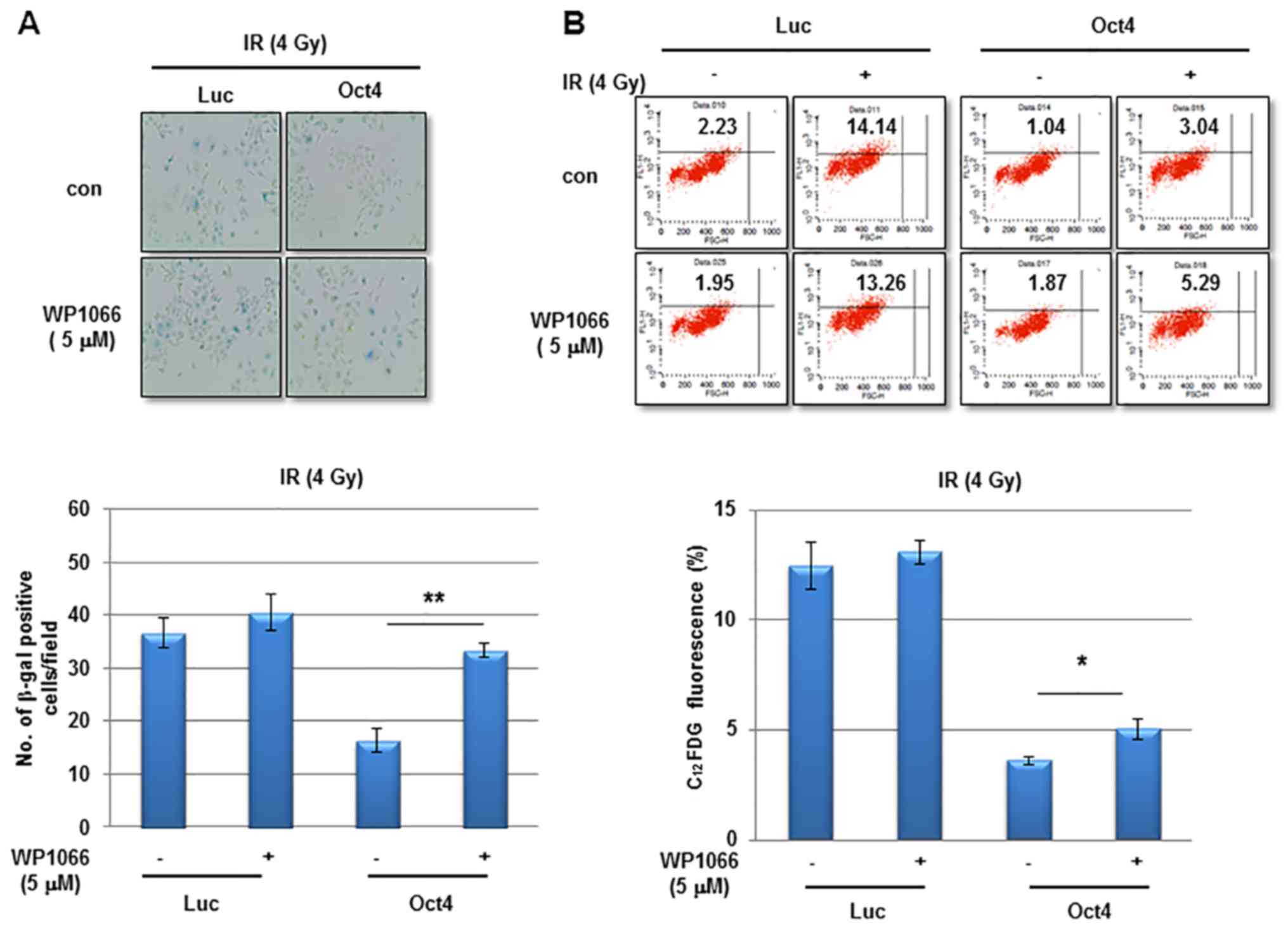 | Figure 7Involvement of STAT3 and NF-κB
signaling in Oct4-mediated suppression of IR-induced senescence.
(A) Representative images of SA-β-gal assay (upper panel) and
quantification (lower panel) and (B) flow cytometric assays (upper
panel) and quantification (lower panel) of IR-induced senescence in
MCF7 cells transduced with adenoviral-Luc and adenoviral-Oct4 3
days after exposure to 4 Gy of IR. Cells were pretreated with or
without STAT3-specific inhibitor WP1066 (5 µM). (C) Western
blot analysis of MCF7 cells transfected with sicon, siSTAT3-1 or -3
with the indicated antibodies. SA-β-gal assays (D) and flow
cytometric assays (E) of MCF7 cells transfected with siSTAT3-1 and
-3. SA-β-gal assays (F) and flow cytometric assays (G) of MCF7
cells treated with NF-κB-specific inhibitor Bay 11-7082 (5 and 10
µM) prior to IR exposure. *P<0.05,
**P<0.01. STAT3, signal transducer and activator of
transcription 3; NF-κB, nuclear factor κB; Oct4, octamer-binding
transcription factor 4; SA-β-gal, senescence-associated
β-galactosidase; IR, ionizing radiation; siSTAT3, small interfering
RNA against STAT3; sicon, control small interfering RNA; Luc,
luciferase; con, parental control; C12FDG,
5-dodecanoylaminofluorescein di-D-galactopyranoside. |
Oct4 augments IL-24 expression through
the STAT3 and NF-κB signaling pathways
The next aim was to identify the effector molecule
for Oct4-mediated inhibition of IR-induced senescence in MCF7
cells. Cytokine array-base analysis revealed that expression of
IL-24, along with that of IL-6, C-reactive protein and SDF-1, was
increased in Oct4-overexpressing cells (Fig. 8A). Among these proteins, IL-24
expression was commonly enhanced in mammosphere, IRR and
Oct4-overexpressing cells compared with control cells as indicated
using RT-PCR (Fig. 8B). The
increase in IL-24 levels by Oct4 was confirmed by western blot
analysis (Fig. 8C). IL-24
expression was increased further by IR in these cells (Fig. 8D). Treatment with STAT3 inhibitor
and transfection of siRNA against STAT3 suppressed basal and
Oct4-induced IL-24 expression (Fig. 8E
and F). In addition, RT-PCR and western blot analysis
demonstrated that NF-κB inhibition by Bay 11-7082 treatment also
inhibited IL-24 expression (Fig. 8G
and H). Taken together, these results suggested that IL-24 is
an effector molecule of the Oct4-mediated STAT3 and NF-κB signaling
axis.
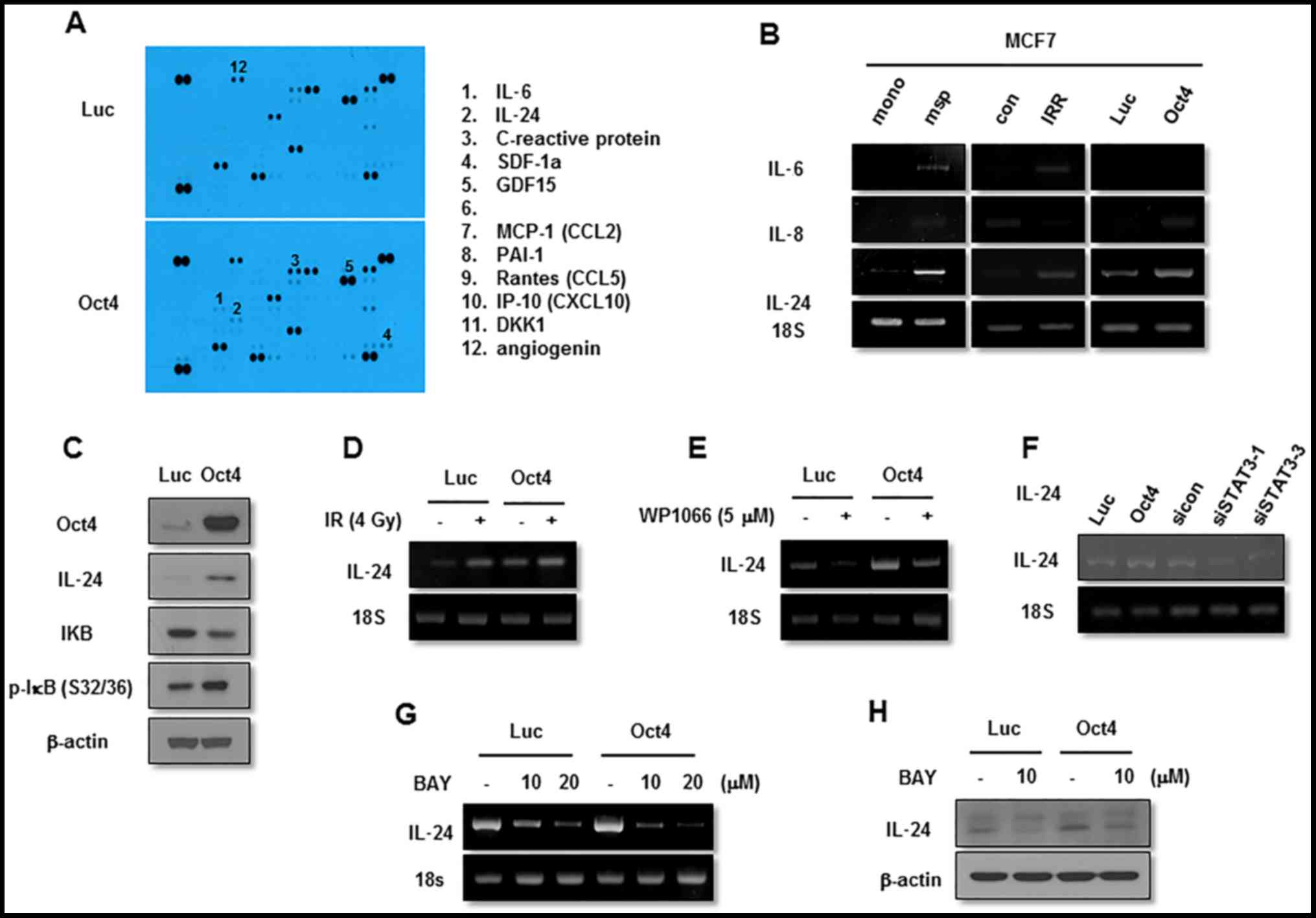 | Figure 8Function of IL-24 in Oct4-mediated
suppression of IR-induced senescence. (A) Cytokine array of MCF7
cells. RT-PCR analysis of MCF7 cells (B) cultured as indicated with
the indicated primers. (C) Western blot analysis of adenoviral-Luc-
and adenoviral-Oct4-transduced MCF7 cells. RT-PCR analysis of MCF7
cells (D) exposed to 4 Gy of IR, (E) treated with STAT3 inhibitor
WP1066 (5 µM), (F) transfected with siRNA-targeting STAT3 or
(G) treated with NF-κB inhibitor Bay 11-7082 (10 and 20 µM)
following transduction with adenoviral-Luc or adenoviral-Oct4. (H)
Western blot analysis of adenoviral-Luc- and
adenoviral-Oct4-transduced MCF7 cells pretreated with Bay 11-7082
(10 µM) with the indicated antibodies. IL, interleukin;
Oct4, octamer-binding transcription factor 4; RT-PCR, reverse
transcription-polymerase chain reaction; IR, ionizing radiation;
siRNA, small interfering RNA; STAT3, signal transducer and
activator of transcription 3; NF-κB, nuclear factor κB; IκB,
inhibitor of NF-κB; SDF-1α, stromal cell-derived factor 1α; GDF15,
growth differentiation factor 15; MCP-1, monocyte chemoattractant
protein 1; CCL, CC chemokine ligand; PAI-1, plasminogen activator
1; Rantes, regulated on activation, normal T-cell expressed and
secreted; IP-10, interferon γ-induced protein 10; CXCL10, CXC
cytokine ligand 10; DKK1, dickkopf-related protein 10; msp,
mammosphere; mono, monolayer-cultured; IRR, IR-resistant; Luc,
luciferase; siSTAT3, small interfering RNA against STAT3; sicon,
control small interfering RNA; p-, phosphorylated. |
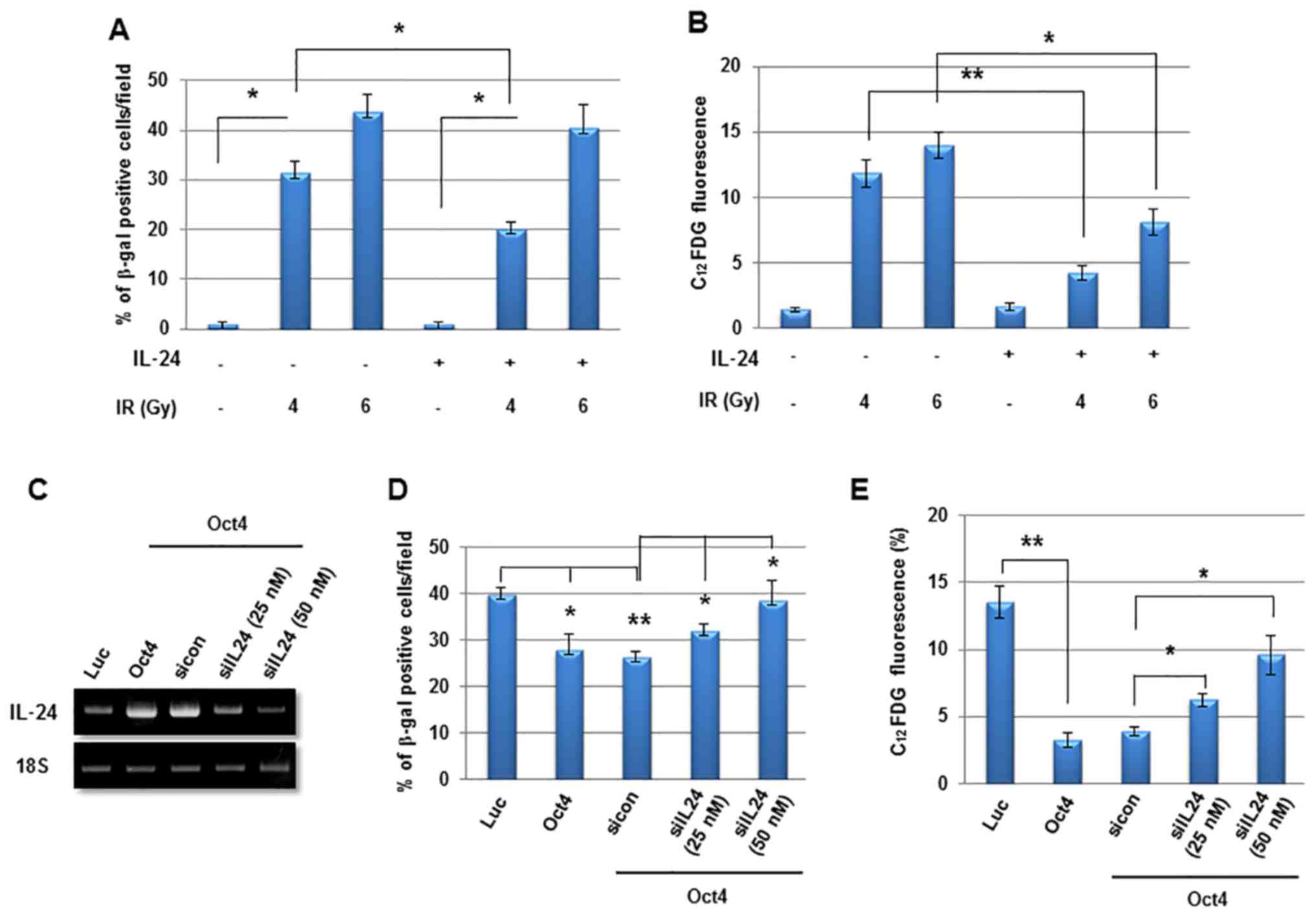
IL-24 serves a critical function in
Oct4-mediated suppression of IR-induced senescence
Finally, to examine the function of IL-24 in
Oct4-mediated suppression of IR-induced senescence further, MCF7
cells were treated with recombinant human IL-24 and it was
identified to significantly suppress IR-induced senescence
(Fig. 9A and B). In contrast,
siRNA-mediated suppression of IL-24 expression (Fig. 9C) significantly restored IR-induced
senescence in Oct4-overexpressing MCF7 cells (Fig. 9D and E). These results indicated
that Oct4-mediated suppression of IR-induced premature senescence
is achieved through IL-24 production via the STAT3 and NF-κB
signaling pathways.
Discussion
It has been identified previously that cancer cells
are hierarchically organized in heterogeneous populations and
contain minor subpopulations of stem-like cancer cells that are
markedly capable of initiating tumor growth and resistance to
conventional therapy, including IR (3). As accelerated relapse of cancer
following sublethal therapy is thought to be caused by CSCs,
eliminating CSCs may be a major step in improving the treatment of
cancer. Consistent with this concept, the function of Oct4 was
investigated in the maintenance of stemness and resistance to IR in
breast cancer cells. It was identified that sphere-cultured cells
were more resistant to IR exposure and exhibited increased Oct4
expression compared with monolayer-cultured breast cancer cells. In
addition, IRR MCF7 cells exhibited more marked Oct4 expression
compared with parental cells. Ectopic expression of Oct4 conferred
IR resistance on breast cancer cells, whereas siOct4 reversed this
effect. Oct4-mediated IR resistance was not due to the induction of
apoptosis or cell-cycle arrest, but to IR-induced premature
senescence. Oct4 expression enhanced the activation of STAT3 and
NF-κB, and siRNA-mediated or pharmacological inhibition of these
signaling molecules inhibited Oct4-mediated suppression of
IR-induced senescence. Finally, the inhibition of IR-induced
senescence by the activation of Oct4-mediated STAT3 and Oct4/NF-κB
signaling was achieved through IL-24 production. These results
suggested that the embryonic stem cell factor Oct4 is a stemness
factor for BCSCs with IR-resistance capacity that acts through
suppressing IR-induced premature senescence and it thus may be
considered a potential target for eliminating CSCs in this
devastating type of cancer.
Expression of Oct4 is thought to be a putative
marker for CSCs in various types of cancer, including breast cancer
(12–14). A number of studies have addressed
the function of Oct4 in the maintenance of stemness and therapeutic
resistance. In drug-resistant liver cancer cells, Oct4 mRNA
expression is increased and overexpression of Oct4 results in
chemoresistance of liver cancer cells via the upregulation of ABCG2
(14). Consistent with the results
of the present study, CD133+ lung cancer cells maintain
stemness and drug resistance by upregulating Oct4 expression
(12). However, the function of
Oct4 in IR resistance of CSCs is not well understood. The results
of the present study indicated that ectopic expression of Oct4
enhanced IR resistance in breast cancer cells by suppressing
IR-induced premature senescence. Thus, it is highly likely that the
higher IR resistance of BCSCs enriched in mammospheres compared
with that of differentiated monolayer-cultured cells is, at least
in part, due to enhanced Oct4 expression.
In human epidermal growth factor receptor 2-positive
breast cancer cells, activation of the STAT3 signaling pathway is
crucial for radioresistance (18–20).
Additionally, IR-induced NF-κB activation confers radioresistance
on breast cancer cells (21–23).
In the present study, STAT3 and NF-κB activation was common in
mammosphere, radioresistant and Oct4-overexpressing MCF7 cells, and
blockade of these molecules with specific siRNAs or through
pharmacological inhibition sensitized these cells to IR-induced
senescence. Therefore, the results of the present study suggest
that Oct4-mediated activation of STAT3 and NF-κB signaling confers
IR resistance on breast cancer cells.
IL-24, also known as melanoma
differentiation-associated gene-7, is a secretory cytokine of the
IL-10 family. Following identification in differentiated melanoma,
it was demonstrated to serve a critical function in tumor
inhibition (24–26). IL-24 induces cancer cell death by
augmenting endoplasmic reticulum stress and mitochondrial
dysfunction (27,28). It also stimulates autophagy in
various cancer cells (29). In
addition, IL-24 suppresses tumor angiogenesis directly by
inhibiting tumor blood vessel formation and/or indirectly by
suppressing the production of angiogenic growth factors, such as
vascular endothelial growth factor, bFGF and IL-8 (30). Furthermore, IL-24 inhibits cancer
cell invasion and metastasis by downregulating
metastasis-associated genes (31).
The present study identified another function of IL-24 in the
suppression of IR-induced premature senescence. Ectopic expression
of Oct4 increased IL-24 production in breast cancer cells, and
IL-24 production was increased further by IR exposure in these
cells. Although IL-24 reportedly enhances the IR effect in cancer
cells (32,33), this appeared to be an effect of the
adenoviral expression system. However, the results of the present
study indicated that moderate expression of IL-24 was able to
suppress IR-induced premature senescence in cancer cells.
In summary, the results of the present study provide
the first evidence that Oct4-mediated STAT3 and NF-κB signaling
serves an important function in resistance to IR by suppressing
IR-induced premature senescence in breast cancer cells. Thus,
controlling Oct4 is a promising approach for efficient suppression
or elimination of BCSCs, and may be beneficial in radiotherapy for
breast cancer.
Abbreviations:
|
BCSC
|
breast cancer stem cell
|
|
CSC
|
cancer stem cell
|
|
IR
|
ionizing radiation
|
|
IRR
|
IR-resistant
|
|
Oct4
|
octamer-binding transcription factor
4
|
|
Sox2
|
sex-determining region Y-box 2
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
IL
|
interleukin
|
|
NF-κB
|
nuclear factor κB
|
|
IκB
|
inhibitor of NF-κB
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the Korea
Institute of Radiological and Medical Sciences, funded by the
Ministry of Science, ICT and Future Planning, Republic of Korea
(grant nos. 1711045557, 1711045538 and 1711045554/50531-2017) and
by the Basic Science Research Program Grant (grant no.
NRF-2017R1A2B4003233) from the National Research Foundation of
Korea, funded by the Ministry of Education, Science and Technology,
Republic of Korea.
Availability of data and materials
The analyzed datasets generated in the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
JYK and JCK conducted experiments and data
acquisition. JYL was involved in conceptualization interim
discussions. MJP designed the present study and wrote the
manuscript. All authors critically reviewed the manuscript content
and agree with the submission of the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parkin DM, Pisani P and Ferlay J:
Estimates of the worldwide incidence of 25 major cancers in 1990.
Int J Cancer. 80:827–841. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating
cells to radiation. J Natl Cancer Inst. 98:1777–1785. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AND, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karimi-Busheri F, Rasouli-Nia A, Mackey JR
and Weinfeld M: Senescence evasion by MCF-7 human breast
tumor-initiating cells. Breast Cancer Res. 12:R312010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YC, Hsu HS, Chen YW, Tsai TH, How CK,
Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu
P, Chen KK, Lopez JP, Poon RTP and Fan ST: Octamer 4 (Oct4)
mediates chemotherapeutic drug resistance in liver cancer cells
through a potential Oct4-AKT-ATP-binding cassette G2 pathway.
Hepatology. 52:528–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y,
Cao X, Ling EA and Hao A: Oct4 is expressed in human gliomas and
promotes colony formation in glioma cells. Glia. 57:724–733. 2009.
View Article : Google Scholar
|
|
15
|
Hu T, Liu S, Breiter DR, Wang F, Tang Y
and Sun S: Octamer 4 small interfering RNA results in cancer stem
cell-like cell apoptosis. Cancer Res. 68:6533–6540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Debacq-Chainiaux F, Erusalimsky JD,
Campisi J and Toussaint O: Protocols to detect
senescence-associated beta-galactosidase (SA-betagal) activity, a
biomarker of senescent cells in culture and in vivo. Nat Protoc.
4:1798–1806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duru N, Fan M, Candas D, Menaa C, Liu HC,
Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, et al:
HER2-associated radioresistance of breast cancer stem cells
isolated from HER2-negative breast cancer cells. Clin Cancer Res.
18:6634–6647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JS, Kim HA, Seong MK, Seol H, Oh JS,
Kim EK, Chang JW, Hwang SG and Noh WC: STAT3-survivin signaling
mediates a poor response to radiotherapy in HER2-positive breast
cancers. Oncotarget. 7:7055–7065. 2016.PubMed/NCBI
|
|
20
|
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang
Y, Deng J, Margolick JB, Liotta LA, Petricoin E III and Zhang Y:
Activation of the PTEN/mTOR/STAT3 pathway in breast cancer
stem-like cells is required for viability and maintenance. Proc
Natl Acad Sci USA. 104:16158–16163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmed KM, Zhang H and Park CC: NF-κB
regulates radioresistance mediated by β1-integrin in
three-dimensional culture of breast cancer cells. Cancer Res.
73:3737–3748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Veuger SJ, Hunter JE and Durkacz BW:
Ionizing radiation-induced NF-kappaB activation requires PARP-1
function to confer radioresistance. Oncogene. 28:832–842. 2009.
View Article : Google Scholar
|
|
23
|
Kunigal S, Lakka SS, Joseph P, Estes N and
Rao JS: Matrix metalloproteinase-9 inhibition down-regulates
radiation-induced nuclear factor-kappa B activity leading to
apoptosis in breast tumors. Clin Cancer Res. 14:3617–3626. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang H, Su ZZ, Lin JJ, Goldstein NI,
Young CS and Fisher PB: The melanoma differentiation associated
gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA.
93:9160–9165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su ZZ, Madireddi MT, Lin JJ, Young CS,
Kitada S, Reed JC, Goldstein NI and Fisher PB: The cancer growth
suppressor gene mda-7 selectively induces apoptosis in human breast
cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad
Sci USA. 95:14400–14405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saeki T, Mhashilkar A, Swanson X, Zou-Yang
XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et
al: Inhibition of human lung cancer growth following
adenovirus-mediated mda-7 gene expression in vivo. Oncogene.
21:4558–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lebedeva IV, Su ZZ, Sarkar D, Kitada S,
Dent P, Waxman S, Reed JC and Fisher PB: Melanoma differentiation
associated gene-7, mda-7/interleukin-24, induces apoptosis in
prostate cancer cells by promoting mitochondrial dysfunction and
inducing reactive oxygen species. Cancer Res. 63:8138–8144.
2003.PubMed/NCBI
|
|
28
|
Yacoub A, Hamed HA, Allegood J, Mitchell
C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus
WC, et al: PERK-dependent regulation of ceramide synthase 6 and
thioredoxin play a key role in mda-7/IL-24-induced killing of
primary human glioblastoma multiforme cells. Cancer Res.
70:1120–1129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park MA, Yacoub A, Sarkar D, Emdad L,
Rahmani M, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, et
al: PERK-dependent regulation of MDA-7/IL-24-induced autophagy in
primary human glioma cells. Autophagy. 4:513–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramesh R, Mhashilkar AM, Tanaka F, Saito
Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L,
et al: Melanoma differentiation-associated gene 7/interleukin
(IL)-24 is a novel ligand that regulates angiogenesis via the IL-22
receptor. Cancer Res. 63:5105–5113. 2003.PubMed/NCBI
|
|
31
|
Ramesh R, Ito I, Gopalan B, Saito Y,
Mhashilkar AM and Chada S: Ectopic production of MDA-7/IL-24
inhibits invasion and migration of human lung cancer cells. Mol
Ther. 9:510–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su ZZ, Lebedeva IV, Sarkar D, Emdad L,
Gupta P, Kitada S, Dent P, Reed JC and Fisher PB: Ionizing
radiation enhances therapeutic activity of mda-7/IL-24: Overcoming
radiation- and mda-7/IL-24-resistance in prostate cancer cells
overexpressing the anti-apoptotic proteins bcl-xL or bcl-2.
Oncogene. 25:2339–2348. 2006. View Article : Google Scholar
|
|
33
|
Yacoub A, Mitchell C, Lebedeva IV, Sarkar
D, Su ZZ, McKinstry R, Gopalkrishnan RV, Grant S, Fisher PB and
Dent P: mda-7 (IL-24) Inhibits growth and enhances radiosensitivity
of glioma cells in vitro via JNK signaling. Cancer Biol Ther.
2:347–353. 2003. View Article : Google Scholar : PubMed/NCBI
|