Introduction
Despite recent advances in surgical techniques and
treatment options, gastric cancer (GC) remains the third most
common cause of cancer-related deaths worldwide (1). Approximately one-third of patients
with GC have locally advanced cancer or metastatic disease at the
time of diagnosis (2).
Tumor-node-metastasis (TNM) staging is the most-recognized
prognostic categorization for patients with GC; however, the
prognosis for patients with the same TNM stage can differ (2), and the current staging system cannot
conclusively predict patient outcomes. These findings highlight the
critical need to discover prognostic biomarkers that can identify
patients with GC who are at a high risk of developing disease
recurrence and who may benefit from aggressive treatment.
Furthermore, a better understanding of the molecular mechanisms
underlying metastasis is crucial for the development of novel
treatment strategies to improve the survival of patients with GC
with metastasis.
Colony-stimulating factor-1 (CSF-1) is a critical
hematopoietic growth factor involved in cell differentiation,
proliferation and activation via binding to its receptor,
c-fms/CSF-1 receptor (CSF-1R), expressed on microglia and
macrophages (3,4). Several studies have demonstrated that
the overexpression of CSF-1 and CSF-1R correlates significantly
with disease progression in various types of cancer (5,6).
Moreover, there is evidence to suggest that a CSF-1/CSF-1R
autocrine loop contributes to tumor invasiveness and metastasis in
breast, ovarian, lung and prostate cancer (7–14).
Previous studies by our group have demonstrated that
several metastasis-associated genes and oncogenic cytokines are
differentially expressed in advanced GC and can be used as
biomarkers for the prognosis and prediction of metastasis in
patients with GC (15–20). Although an increasing number of
studies have established the function of the CSF-1/CSF-1R axis in
other types of cancer, to date, and at least to the best of our
knowledge, there have been no systematic investigations of the
clinical significance of the CSF-1/CSF-1R axis and its potential
functional role in the development of human GC. Thus, in this
study, we investigated the expression profiles of CSF-1 and CSF-1R
in a large cohort of GC tissue specimens to clarify their clinical
significance as prognostic biomarkers in patients with GC and to
assess the functional role of the CSF-1/CSF-1R axis in GC
development.
Materials and methods
Patients and sample collection
Our study included 148 patients (118 males and 30
females) who underwent surgery for GC between 2000 and 2009 at Mie
University Hospital, Tsu, Japan. The criteria for inclusion
included the availability of cancer tissue samples with complete
clinical data and isolated RNA of sufficient quality for real-time
PCR. The mean patient age was 67 years (range, 18–90 years). No
patient received chemotherapy or radiotherapy prior to surgery and
no peri-operative mortalities were observed. The diagnosis of GC
was confirmed for all 148 patients based on clinicopathological
findings. All patients were classified according to the Japanese
Classification of Gastric Carcinoma (21): A total of 21 patients had stage I
disease, 40 had stage II, 43 had stage III and 44 had stage IV.
Distal or total gastrectomy with D2 lymphadenectomy was performed
in patients who underwent curative resection. Patients with liver,
peritoneal, or distant metastasis underwent palliative gastrectomy
with D1 lymphadenectomy. The mean follow-up time was 25 months
(range, 1–79 months). During the study period, 68 patients died due
to cancer-related causes. Tissue specimens were preserved
immediately following surgical resection in RNAlater Stabilization
Reagent (Qiagen, Chatsworth, CA, USA) and stored at −80°C until RNA
extraction. Written informed consent was obtained from each
patient, and the study was approved by the Institutional Review
Boards of Mie University (no. 2215).
Total RNA extraction, cDNA synthesis and
reverse transcription PCR (RT-PCR)
RNAlater-preserved surgical specimens were
homogenized with a Mixer Mill MM 300 homogenizer and tissue total
RNA was isolated using RNeasy Mini kits (both from Qiagen)
according to the manufacturer's instructions. cDNA was synthesized
from 5 µg total RNA with random hexamer primers and SuperScript III
Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA).
Real-time (quantitative) PCR and relative
mRNA expression analysis
Quantitative PCR (qPCR) following reverse
transcription (RT-qPCR) analysis was performed using the StepOne
Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), as
previously described (16). CSF-1,
CSF-1R, vascular endothelial growth factor A (VEGFA), Fms related
tyrosine kinase 1 (FLT1) and GAPDH mRNA expression levels were
measured using Power SYBR-Green Master Mix (Life Technologies,
Carlsbad, CA, USA). Primers for CSF-1, CSF-1R, VEGFA, FLT1 and
GAPDH were designed using Primer3 software (Biology Workbench
Version 3.2; San Diego Supercomputer Center, University of
California, San Diego, CA, USA). The following sequences were used:
CSF-1 forward, GGAGACCTCGTGCCAAATTA and reverse,
GGCATTGGGGGTGTTATCTC; CSF-1R forward, TGAGCAAGACCTGGACAAGGA and
reverse, CCATTGGTCAACAGCACGTTA; VEGFA forward, TCTTCAAGCCATCCTGTGTG
and reverse, CTATGTGCTGGCCTTGGTG; FLT1 forward,
CTGAAGGAAGGGAGCTCGTC and reverse, TCCCAGATTATGCGTTTTCC; and GAPDH
forward, GGAAGGTGAAGGTCGGAGTC and reverse, AATGAAGGGGTCATTGATGG. We
performed 40 cycles of amplification under the following
conditions: Denaturation at 95°C for 10 sec, annealing at 60°C for
10 sec and elongation at 72°C for 20 sec. Following amplification,
the products were subjected to a temperature gradient ranging from
68°C to 95°C at 0.2°C/sec under continuous fluorescence monitoring
to produce a melting curve of the products. Following proportional
background adjustment, the fit-point method was used to determine
the cycle in which the log-linear signal was distinguished from the
background, and that cycle number was used as a crossing-point
value. The expression levels of target transcripts and GAPDH were
evaluated using Applied Biosystems StepOne Software v2.1, and
quantified by the standard curve method, as previously described
(22).
Immunohistochemical (IHC) analysis
IHC analyses of CSF-1 and CSF-1R were performed on
the surgical specimens of primary GC using avidin-biotin-peroxidase
methods (DakoCytomation, Carpinteria, CA, USA) on formalin-fixed,
paraffin-embedded (FFPE) tissues sliced into sections of
2–3-µm width. Following deparaffinization and dehydration,
the specimens were brought to a boil in 10 mM sodium citrate buffer
for antigen unmasking. The specimens were then blocked and
incubated with primary antibodies overnight at 4°C. Antibodies were
detected using Envision reagents (Envision kit/HRP; DakoCytomation,
Glostrup, Denmark). The sections were incubated with primary goat
polyclonal antibodies against CSF-1 (1:50; sc-1324; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and CSF-1R (1:100; AP7604b;
Abgent, San Diego, CA, USA) followed by labeled streptavidin-biotin
(LASB2 kit/HRP), and then stained with 3,3′-diaminobenzidine (both
from DakoCytomation, Carpinteria, CA, USA). The sections were
counterstained with hematoxylin, dehydrated and mounted. Positive
and negative control samples using spleen specimens were examined
in parallel.
Immunofluorescence
Double immunofluorescence combined CSF-1 and CSF-1R.
The sections were incubated with the primary antibodies for CSF-1
and for CSF-1R (1:100, described above) overnight at 4°C. After
washing the FFPE sections 5 times for 5 min with distilled water,
Alexa Fluor® 488 donkey anti-goat IgG (1:500, A-11055;
Invitrogen, Renfrew, UK) and Dylight549 donkey anti-rabbit IgG
(1:1,000, 611–742–127; Rockland, Limerick, PA, USA) as secondary
antibodies, were incubated with the sections for 1 h at room
temperature. Nuclear staining was carried out with
4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; ProLong Gold
Antifade Reagent with DAPI; Invitrogen). Confocal images were
acquired using a IX71 inverted microscope with a DP70 digital
camera system (Olympus, Center Valley, PA, USA).
Cell lines
The human GC cell lines, MKN7 (intestinal type),
MKN45 (diffuse type), MKN74 (intestinal type), KATO III (diffuse
type) and NUGC3 (diffuse type) were obtained from the Cell Resource
Center for Biomedical Research, Tohoku University, Sendai, Japan.
These cell lines have been tested and authenticated at the Cell
Resource Center for Biomedical Research, Tohoku University. The
cells were maintained in RPMI-1640 medium supplemented with 10%
fetal bovine serum and antibiotics at 37°C in a 5% CO2
atmosphere.
Reagents recombinant human CSF-1
(rhCSF-1) was purchased from PeproTech (Rocky Hill, NJ, USA) and
prepared according to the manufacturer's instructions
The c-fms/CSF-1R tyrosine kinase inhibitor (14,23,24)
was purchased from Santa Cruz Biotechnology and stored at −20°C
before use in vitro. To investigate the association between
CSF-1 and CSF-1R, we used 5 µM of CSF-1R inhibitor. The
effects of rhCSF-1 (100 ng/ml) on the GC cell lines were compared
to those on the untreated cells, cells treated with CSF-1R
inhibitor or with cells pretreated with CSF-1R inhibitor for 2 h
followed by treatment with rhCSF-1.
Cell proliferation assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay (Sigma, St. Louis, MO, USA) was used to measure
cell proliferation, as previously described (16,25).
Each independent experiment was performed three times in
triplicate.
Cell migration scratch assays
Confluent GC cells were serum-deprived for 48 h and
a wound was generated using a sterile 200-µl pipette tip.
The cells were pre-incubated with or without reagents (CSF-1,
CSF-1R inhibitor), and wound closure was assessed using an Olympus
IX71 microscope (Olympus) at ×10 magnification, as previously
described (25).
Anoikis assays
Anoikis assays were performed in 6-well Costar
Ultra-Low Attachment multi-well plates (Corning Life Sciences,
Corning, NY, USA). GC cell lines were resus-pended at
5×105 cells/ml in RPMI-1640 medium containing
anoikis-enhancing or -inhibiting reagents (CSF-1, CSF-1R
inhibitor). Following the induction of anoikis with 24 h of
incubation, MTT assay was performed, as previously described
(15).
Statistical analysis
Results are expressed as the median ± interquartile
range, and all statistical analyses were performed using Medcalc
version 16.4.3 (Broekstraat 52, 9030; Mariakerke, Belgium).
Differences between groups were estimated using the Chi-squared
(χ2 test), Mann-Whitney U test and one-way ANOVA, as
appropriate. F-tests were used to assess the equality of variance
for comparable groups, and Scheffé test was used as a post hoc test
after ANOVA. Correlation coefficient tests were conducted for
statistical correlations. The Spearman's correlation coefficient
test was conducted for statistical correlations. For time-to-event
analyses, survival estimates were calculated using Kaplan-Meier
analysis, and groups were compared using the log-rank test.
Receiver operating characteristic (ROC) curves were established to
determine the cut-off values for the analysis of prognosis by
Youden's index. Overall survival (OS) was measured from the date
the patient underwent surgery to the date of death resulting from
any cause, or to the last known follow-up for patients that were
still alive. Disease-free survival (DFS) was measured from the date
the patient underwent curative surgery to the date of disease
recurrence, death from any cause (i.e., cancer-unrelated deaths
were not censored), or the final contact with the patient. For
assessment of the performance of prognostic markers for OS and DFS,
the power calculations were based on the detection difference of
0.05 between favorable and unfavorable prognosis groups. We
estimated that 126 and 88 patients (distributed equally between the
2 groups) were needed to achieve 80% power to substantiate >25
and 30% differences in prognostic and recurrent outcomes,
respectively, at a significance level of 0.05 using a two-sided
log-rank test. Our cohort of 148 patients with GC was therefore
more than adequate. The Cox proportional hazards model was used to
estimate hazard ratios (HRs) for death. Assumption of
proportionality was confirmed for the Cox proportional hazards
analyses by generating Kaplan-Meier survival curves (e.g., high vs.
low expression groups) and by ensuring that the two curves did not
intersect. Multivariate logistic regression models were used to
predict factors influencing lymph node and peritoneal metastasis.
Forced-entry regression was used to include these variables in all
multivariable equations to analyze whether each of the predictors
affected the outcome after adjusting for known confounders. All
P-values were two-sided, and those <0.05 were considered to
indicate statistically significant differences.
Results
High expression of CSF-1/CSF-1R is
associated with disease progression in patients with GC
To determine whether the expression status of CSF-1
and CSF-1R has clinical significance in patients with GC, we
analyzed the association between the expression patterns and
various clinicopathological factors (Table I). Expression profiling revealed
that the elevated expression of CSF-1 was significantly associated
with the presence of lymph node metastasis (P=0.03), peritoneal
metastasis (P=0.03) and the progression of TNM stage classification
(P=0.003) in patients with GC. Furthermore, the overexpression of
CSF-1R was significantly associated with the same factors for
disease progression and metastasis formation, such as an advanced T
category (P=0.002), lymph node metastasis (P=0.02), peritoneal
metastasis (P=0.02) and the progression of TNM stage classification
(P=0.005). Although the median values of both CSF-1 and CSF-1R in
patients with stage IV disease were decreased compared with their
median values in patients with stage III disease, scattergram
analyses revealed no significant differences between stage III and
stage IV GC as regards both CSF-1 and CSF-1R expression (Fig. 1A and B). Indeed, patients with
stage IV harbored various type of distant metastasis, including
hepatic metastasis, distant lymph node metastasis and peritoneal
metastasis, and the background of these patients may be influenced
by these findings in this study.
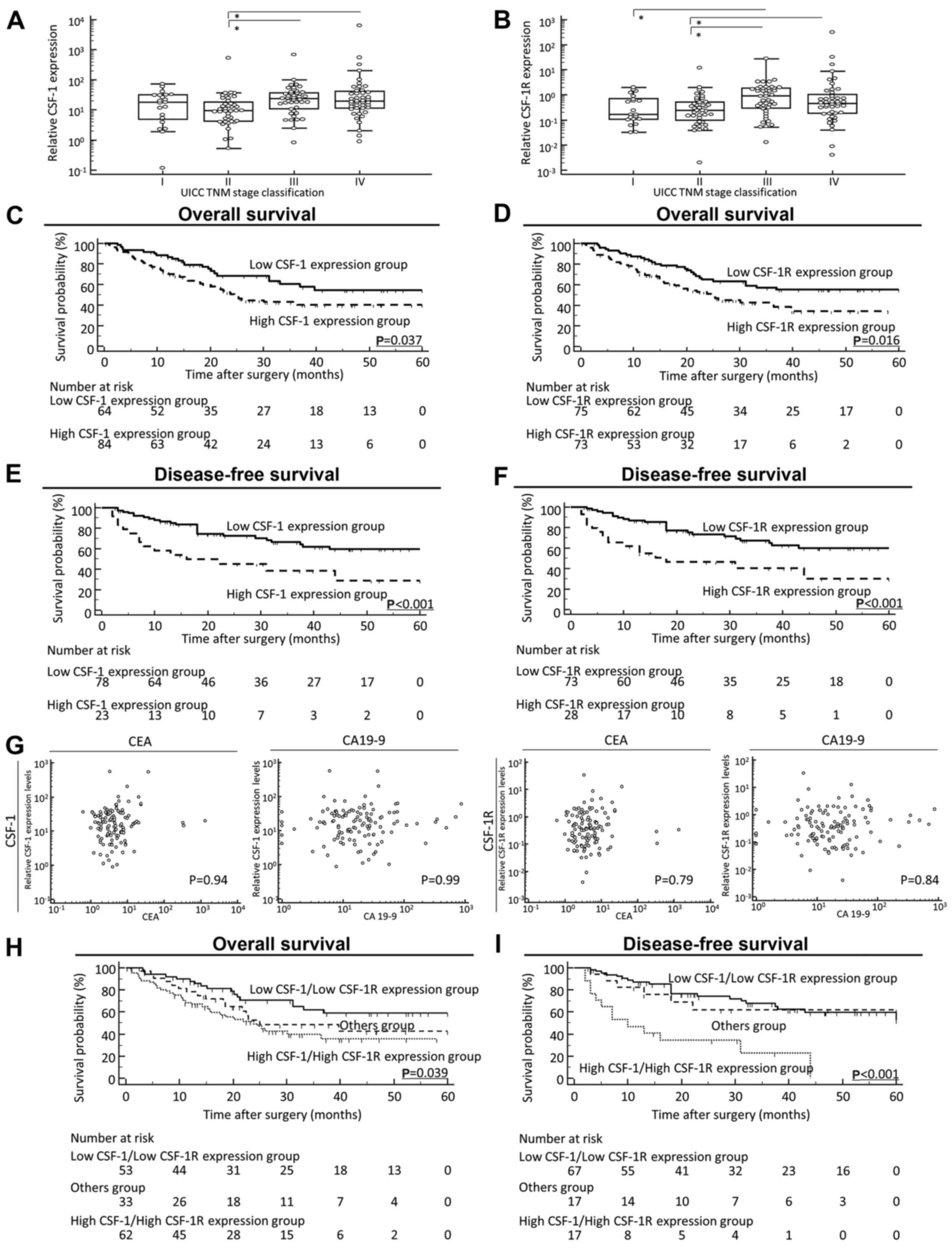 | Figure 1Prognostic value of the
colony-stimulating-factor-1(CSF-1)/CSF-1 receptor (CSF-1R)
expression status on the overall survival (OS) and disease-free
survival (DFS) of patients with gastric cancer (GC). (A and B)
Scattergrams of the CSF-1 and CSF-1R expression status according to
the Japanese Classification of Gastric Carcinoma in GC patients.
Although median values of both (A) CSF-1 and (B) CSF-1R in patients
with stage IV disease were decreased compared with their median
values in patients with stage III disease, scattergram analyses
revealed no significant difference between stage III and stage IV
GC as regards both CSF-1 and CSF-1R expression. (C and D)
Kaplan-Meier survival curves for the overall survival of patients
with GC based on the expression of (C) CSF-1 and (D) CSF-1R. The OS
rate of patients with GC with a high tumor expression of CSF-1 or
CSF-1R was significantly lower than that of patients with a low
tumor expression of CSF-1 or CSF-1R (CSF-1, P=0.037; CSF-1R,
P=0.016; log-rank test). (E and F) Kaplan-Meier survival curves for
the DFS of patients with GC based on the expression of (E) CSF-1
and (F) CSF-1R. It should be noted here that for disease-free
survival, patients with non-curative intent (stage IV) were not
included; thus, the patient numbers differ from those for OS. The
DFS rate of patients with GC with a high tumor expression of CSF-1
or CSF-1R was significantly lower than that of patients with a low
tumor expression of CSF-1 or CSF-1R (CSF-1, P<0.001; CSF-1R,
P<0.001; log-rank test). (G) Correlation between pre-operative
tumor marker levels and CSF-1 and CSF-1R expression levels in
primary tumors. Both the CSF-1 (left panel) and CSF-1R (right
panel) expression levels did not significantly correlate with
well-established tumor markers, such as CEA and CA19-9 in this
study cohort. (H) Kaplan-Meier survival curves for (H) OS and (I)
DFS of patients with GC based on the co-expression of CSF-1 and
CSF-1R. The 'Others group' included patients with a high CSF-1
expression or high CSF-1R expression. Co-expression status of CSF-1
and CSF-1R was significantly associated with a poor OS and DFS of
patients with GC (OS, P=0.039; DFS, P<0.001; log-rank test). All
statistical tests were two-sided. |
 | Table IClinicopathological variables and
CSF-1/CSF-1R expression in patients with gastric cancer. |
Table I
Clinicopathological variables and
CSF-1/CSF-1R expression in patients with gastric cancer.
| Variable | n |
CSF-1expression | P-value | CSF-1R
expression | P-value |
|---|
| Sex | | | | | |
| Male | 118 | 16.7±26.6 | 0.76 | 0.41±0.99 | 0.92 |
| Female | 30 | 20.3±25.4 | | 0.4±1.03 | |
| Age (years) | | | | | |
| <70a | 72 | 17.7±27.5 | 0.64 | 0.53±1.2 | 0.42 |
| ≥70 | 76 | 16.8±26.2 | | 0.34±0.76 | |
| Location | | | | | |
| Proximal | 64 | 16.7±21.0 | 0.74 | 0.38±0.89 | 0.63 |
| Distal | 84 | 18.8±27.7 | | 0.43±1.26 | |
| Histological
type | | | | | |
| Intestinal
type | 73 | 20.3±29.4 | 0.46 | 0.44±1.33 | 0.34 |
| Diffuse type | 75 | 17.2±20.1 | | 0.38±0.88 | |
| Tumor Size | | | | | |
| ≥5.5 cmb | 74 | 16.7±29.7 | 0.45 | 0.43±1.24 | 0.81 |
| <5.5 cm | 74 | 19.2±22.2 | | 0.38±0.99 | |
| Pathological T
category | | | | | |
| pT1/2 | 50 | 14.5±23.2 | 0.08 | 0.17±0.53 |
0.002c |
| pT3/4 | 98 | 18.5±27.7 | | 0.52±1.28 | |
| Lymph node
metastasis | | | | | |
| N0 | 42 | 12.2±21.7 | 0.03c | 0.25±0.47 | 0.02c |
| N1 | 106 | 18.8±26.8 | | 0.46±1.28 | |
| Peritoneal
metastasis | | | | | |
| P0 | 122 | 16.1±23.9 | 0.03c | 0.38±0.91 | 0.02c |
| P1 | 26 | 25.2±48.1 | | 0.54±1.75 | |
| Distant
metastasis | | | | | |
| M0 | 104 | 16.7±25.2 | 0.1 | 0.38±1.06 | 0.41 |
| M1 | 44 | 19.4±29.8 | | 0.46±0.88 | |
| UICC TNM
classification | | | | | |
| Stage I | 21 | 18.5±26.6 |
0.003c | 0.17±0.61 |
0.005c |
| Stage II | 40 | 9.58±13.4 | | 0.24±0.43 | |
| Stage III | 43 | 24.6±26.9 | | 0.91±1.57 | |
| Stage IV | 44 | 19.4±29.8 | | 0.46±0.88 | |
High expression of CSF-1/CSF-1R is
associated with recurrence and a poor outcome in patients with
GC
We then performed time-to-event analyses to evaluate
the prognostic relevance of CSF-1 and CSF-1R expression for OS and
DSF. The expression cut-off thresholds for CSF-1 and CSF-1R for
these analyses were determined from ROC curves with Youden's index.
Of note, the high expression of CSF-1 and CSF-1R was significantly
associated with a poor prognosis, compared with the low expression
groups, for both OS [CSF-1, P=0.037; CSF-1R, P=0.016; log-rank test
(Fig. 1C and D)] and DFS [CSF-1,
P<0.001; CSF-1R, P<0.001; log-rank test (Fig. 1E and F)]. Of note, both the CSF-1
and CSF-1R expression levels did not correlate significantly with
well-established tumor markers, such as CEA and CA19-9 in this
cohort (Fig. 1G). Furthermore, the
analysis of the prognostic value of these markers revealed that a
high expression of both CSF-1 and CSF-1R in the same tissue
(co-expression) was significantly associsated with a poor
prognosis, compared with patients with a low co-expression, for
both OS (P=0.039, log-rank test) and DFS (P<0.001, log-rank
test) (Fig. 1H and I). To
determine the value of high CSF-1/CSF-1R co-expression as a
predictive biomarker for disease recurrence and the prognosis of
patients with GC, we performed multivariate Cox regression
analysis. The data revealed that a high CSF-1/CSF-1R co-expression
was an independent prognostic factor for OS [HR, 1.38; 95%
confidence interval (CI), 1.02–1.88; P=0.038] in patients with GC
(Table IIA). In addition, an
advanced T category (HR, 4.01; 95% CI, 1.75–9.21; P=0.001), the
presence of lymph node metastasis (HR, 4.85; 95% CI, 1.57–14.9;
P=0.006) and a high CSF-1/CSF-1R co-expression (HR, 1.79; 95% CI,
1.21–2.67; P=0.004) were independent prognostic factors for DFS in
patients with GC (Table IIB).
 | Table IIMultivariate analysis for predictors
of overall survival and disease-free survival. |
Table II
Multivariate analysis for predictors
of overall survival and disease-free survival.
A, Multivariate
analysis for predictors of overall survival
|
|---|
| Variables | Univariate
| Multivariate
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male) | 0.78 | 0.44–1.38 | 0.39 | 1.3 | 0.78–2.17 | 0.32 |
| Age (≥70
years)a | 1.16 | 0.72–1.87 | 0.54 | 0.5 | 0.26–0.96 |
0.038d |
| Histological type
(intestinal type) | 0.97 | 0.6–1.56 | 0.89 | 0.98 | 0.59–1.62 | 0.94 |
| Tumor size (≥5.5
cm)b | 1.53 | 0.95–2.48 | 0.08 | 1.44 | 0.88–2.36 | 0.15 |
| T classification
(pT3/4) | 3.32 | 1.8–6.11 |
<0.001d | 1.54 | 0.58–4.07 | 0.39 |
| Vessel involvement
(present) | 3.96 | 1.59–9.86 |
0.003d | 5.95 | 1.5–23.6 |
0.011d |
| Lymphatic vessel
involvement (present) | 2.03 | 0.64–6.48 | 0.23 | 0.19 | 0.03–1.15 | 0.07 |
| Lymph node
metastasis (present) | 3.77 | 1.86–7.62 |
<0.001d | 1.99 | 0.78–5.13 | 0.15 |
| UICC TNM stage
classification (stage III/IV) | 4.92 | 2.67–9.07 |
<0.001d | 2.26 | 0.74–6.9 | 0.15 |
| High CSF-1/high
CSF-1R expressionc | 1.44 | 1.08–1.9 |
0.012d | 1.38 | 1.02–1.88 |
0.038d |
B, Multivariate
analysis for predictors of disease-free survival
|
|---|
| Variables | Univariate
| Multivariate
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male) | 1.47 | 0.58–3.78 | 0.42 | 0.49 | 0.17–1.44 | 0.49 |
| Age (≥70
years)a | 1.17 | 0.63–2.18 | 0.62 | 1.1 | 0.57–2.15 | 0.77 |
| Histological type
(intestinal type) | 1.3 | 0.7–2.42 | 0.4 | 1.54 | 0.8–2.96 | 0.19 |
| Tumor size (≥5.5
cm)b | 0.96 | 0.52–1.78 | 0.89 | 0.64 | 0.33–1.24 | 0.19 |
| T classification
(pT3/4) | 4.48 | 2.06–9.75 |
<0.001d | 4.01 | 1.75–9.21 |
0.001d |
| Vessel involvement
(present) | 2.56 | 1.07–6.12 |
0.035d | 2.11 | 0.59–7.5 | 0.25 |
| Lymphatic vessel
involvement (present) | 1.91 | 0.59–6.21 | 0.28 | 0.6 | 0.11–3.3 | 0.55 |
| Lymph node
metastasis (present) | 7.37 | 2.61–20.8 |
<0.001d | 4.85 | 1.57–14.9 |
0.006d |
| High CSF-1/high
CSF-1R expressionc | 2.08 | 1.44–3.01 |
<0.001d | 1.79 | 1.21–2.67 |
0.004d |
To further assess the clinical significance of the
CSF-1/CSF-1R expression status in GC tissues, we analyzed the
association of high CSF-1/CSF-1R co-expression with various
clinicopathological factors in patients with GC (Table III). A high co-expression was
significantly associated with a younger age (P=0.02), an advanced T
category (P=0.047), the presence of lymph node metastasis (P=0.017)
and with the progression of TNM stage classification (P=0.013).
 | Table IIIClinicopathological variables and
CSF-1/CSF-1R expression in gastric cancer patients. |
Table III
Clinicopathological variables and
CSF-1/CSF-1R expression in gastric cancer patients.
| Variable | n | High CSF-1/high
CSF-1R (n=62) | Othersb (n=33) | Low CSF-1/low
CSF-1R (n=53) | P-value |
|---|
| Sex | | | | | |
| Male | 118 | 48 | 27 | 43 | 0.61d |
| Female | 30 | 14 | 6 | 10 | |
| Age (years) | | | | | |
| <70a | 72 | 37 | 10 | 25 | 0.02d,e |
| ≥70 | 76 | 25 | 23 | 28 | |
| Location | | | | | |
| Proximal | 64 | 25 | 15 | 24 | 0.59d |
| Distal | 84 | 37 | 18 | 29 | |
| Histological
type | | | | | |
| Intestinal
type | 73 | 34 | 12 | 27 | 0.63d |
| Diffuse type | 75 | 28 | 21 | 26 | |
| Tumor size | | | | | |
| ≥5.5 cmc | 74 | 31 | 16 | 27 | 0.93d |
| <5.5 cm | 74 | 31 | 17 | 26 | |
| Pathological T
category | | | | | |
| pT1/2 | 50 | 15 | 13 | 22 |
0.047d,e |
| pT3/4 | 98 | 47 | 20 | 31 | |
| Lymph node
metastasis | | | | | |
| N0 | 42 | 12 | 9 | 21 |
0.017d,e |
| N1 | 106 | 50 | 24 | 32 | |
| Peritoneal
metastasis | | | | | |
| P0 | 122 | 48 | 26 | 48 | 0.068d |
| P1 | 26 | 14 | 7 | 5 | |
| Distant
metastasis | | | | | |
| M0 | 104 | 41 | 23 | 40 | 0.28d |
| M1 | 44 | 21 | 10 | 13 | |
| UICC TNM
classification | | | | | |
| Stage I | 21 | 8 | 5 | 8 |
0.013d,e |
| Stage II | 40 | 8 | 9 | 23 | |
| Stage III | 43 | 25 | 9 | 9 | |
| Stage IV | 44 | 21 | 10 | 13 | |
A high co-expression of CSF-1/CSF-1R is a
predictive factor for the presence of lymph node and peritoneal
metastasis in patients with GC
We found that the overexpression of the CSF-1/CSF-1R
axis was intimately associated with the presence of lymph node and
peritoneal metastasis in patients with GC (Tables I and III). Based on these findings, we
performed a multivariate logistic analysis to determine the
clinical significance of CSF-1/CSF-1R co-expression as a predictive
biomarker for metastasis (Table
IV). Notably, a high CSF-1/CSF-1R co-expression was shown to be
an independent predictive factor for both lymph node metastasis
[odds ratio (OR), 2.07; 95% CI, 1.29–3.33; P=0.003] and peritoneal
metastasis (OR, 2.27; 95% CI, 1.1–4.69; P=0.026).
 | Table IVMultivariate analysis for lymph node
metastasis and peritoneal metastasis. |
Table IV
Multivariate analysis for lymph node
metastasis and peritoneal metastasis.
A, Multivariate
analysis for lymph node metastasis.
|
|---|
| Variables | Univariate
| Multivariate
|
|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex (male) | 1.34 | 0.57–3,18 | 0.5 | 1.63 | 0.59–4.51 | 0.34 |
| Age (≥70
years)a | 1.61 | 0.78–3.31 | 0.19 | 1.62 | 0.69–3.77 | 0.26 |
| Histological type
(intestinal type) | 1.36 | 066–2.78 | 0.41 | 1.6 | 0.67–3.78 | 0.29 |
| Tumor size (≥5.5
cm)b | 1.14 | 0.56–2.33 | 0.71 | 1.15 | 0.48–2.74 | 0.76 |
| T classification
(pT3/4) | 2.66 | 1.27–5.57 | 0.01d | 2.18 | 0.91–5.27 | 0.08 |
| Vessel involvement
(present) | 6.52 | 2.66–16.0 |
<0.001d | 4.45 | 1.44–13.8 | 0.01d |
| Lymphatic vessel
involvement (present) | 8.08 | 2.03–32.2 |
0.003d | 2.26 | 0.41–12.4 | 0.35 |
| High CSF-1/high
CSF-1R expressionc | 1.93 | 1.27–2.93 |
0.002d | 2.07 | 1.29–3.33 |
0.003d |
B, Multivariate
analysis for peritoneal metastasis
|
|---|
| Variables | Univariate
| Multivariate
|
|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex (male) | 0.25 | 0.1–0.63 |
0.003d | 0.21 | 0.07–0.63 |
0.006d |
| Age (≥70
years)a | 1.13 | 0.48–2.64 | 0.78 | 1.07 | 0.39–2.9 | 0.9 |
| Histological type
(intestinal type) | 0.45 | 0.19–1.09 | 0.08 | 0.51 | 0.18–1.44 | 0.2 |
| Tumor size (≥5.5
cm)b | 2.65 | 1.07–6.56 |
0.035d | 2.19 | 0.76–6.28 | 0.14 |
| T classification
(pT3/4) | 4.8 | 1.37–16.9 |
0.014d | 3.37 | 0.78–4.5 | 0.1 |
| Vessel involvement
(present) | – | – | 0.99 | – | – | 0.99 |
| Lymphatic vessel
involvement (present) | – | – | 0.99 | – | – | 1 |
| Lymph node
metastasis (present) | 2.49 | 0.8–7.72 | 0.11 | 1.92 | 0.49–7.55 | 0.35 |
| High CSF-1/high
CSF-1R expressionc | 2.38 | 1.26–4.49 |
0.007d | 2.27 | 1.1–4.69 |
0.026d |
CSF-1 and CSF-1R are highly expressed in
cancer cells compared with cancer stromal cells or normal mucosa in
GC tissues
To confirm the pathological expression patterns of
CSF-1 and CSF-1R in the clinical specimens, we performed IHC
analysis of 10 primary GC tissues. Notably, both CSF-1 and CSF-1R
expression levels were mainly expressed in the cellular membrane of
GC cells, and little expression was detected in the cancer stroma
or adjacent normal mucosa (Fig.
2). From these results, we concluded that CSF-1 and CSF-1R were
overexpressed in GC cells compared with normal mucosa, suggesting
that this signaling axis may play a role in disease progression.
Therefore, we focused the remainder of our study on assessing the
biological function of the CSF-1/CSF-1R axis in gastric
neoplasia.
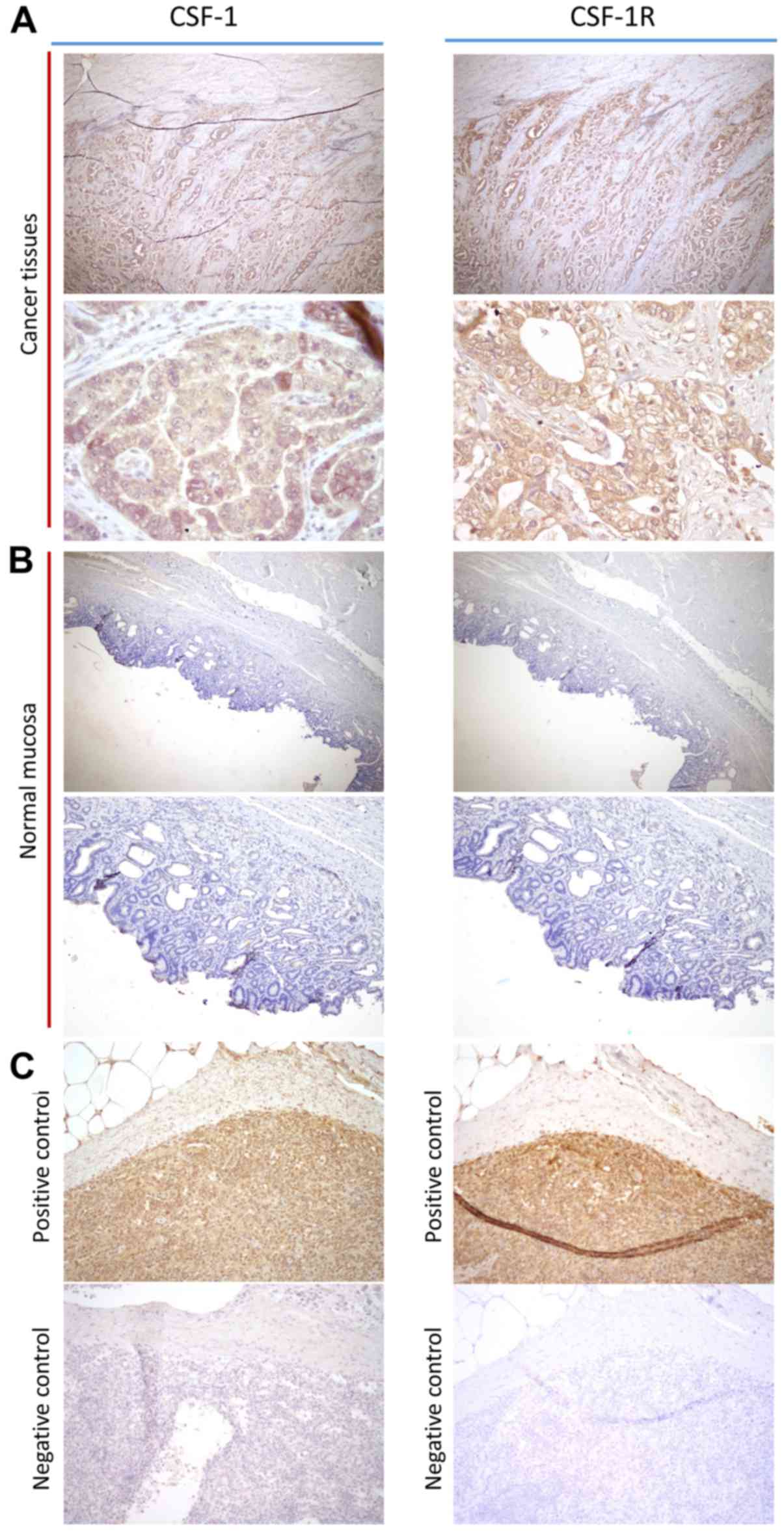 | Figure 2Immunohistochemical and
immunofluorescence analysis of colonystimulating-factor-1 (CSF-1)
and CSF receptor (CSF-1R) expression in gastric cancer (GC)
tissues. (A) CSF-1 and CSF-1R expression in cancer tissues (left
upper panel, CSF-1: magnification, ×100; left lower panel, CSF-1:
magnification, ×400; right upper panel, CSF-1R: magnification,
×100; right lower panel, CSF-1R: magnification, ×400). (B) CSF-1
and CSF-1R expression in adjacent normal mucosa (left upper panel,
CSF-1: magnification, ×100; left lower panel, CSF-1: magnification,
×200; right upper panel, CSF-1R: magnification, ×100; right lower
panel, CSF-1R: magnification, ×200). (C) Positive and negative
control of CSF-1 and CSF-1R staining in spleen (left upper panel,
CSF-1 positive control: magnification, ×200; left lower panel,
CSF-1 negative control: magnification, ×200; right upper panel,
CSF-1R positive control: magnification, ×200; right lower panel,
CSF-1R negative control: magnification, ×200). |
Inhibition of the CSF-1/CSF-1R axis
suppresses GC cell proliferation, migration and anoikis
resistance
As described above, we found that the overexpression
of CSF-1 and CSF-1R was associated with disease progression,
metastasis, DFS and OS in patients with GC, and this prompted us to
examine the functional role of this axis in the pathogenesis of GC.
For these analyses, we incubated human GC cell lines in
vitro with or without rhCSF-1 and/or a CSF-1R signaling
inhibitor. First, CSF-1 and CSF-1R expression in the MKN7, MKN45,
MKN74, KATO III and NUGC3 human GC cell lines was assessed by
RT-qPCR (Fig. 3A). Furthermore, to
clarify the localization of CSF-1 and CSF-1R expression in GC
cells, we carried out fluorescent immunocytochemistry using the
NUGC3 cells as both CSF-1 and CSF-1R were highly co-expressed in
these cells (Fig. 3A). Fluorescent
immunocytochemistry clearly revealed the co-expression of CSF-1 and
CSF-1R in the cellular membrane of the same GC cell, and
successfully verified the finding of gene expression in the GC cell
line (Fig. 3B). Based on the
findings of RT-qPCR, the MKN74 cells were subsequently selected for
use in the following experiments, since they lacked CSF-1
expression and exhibited the highest expression of CSF-1R.
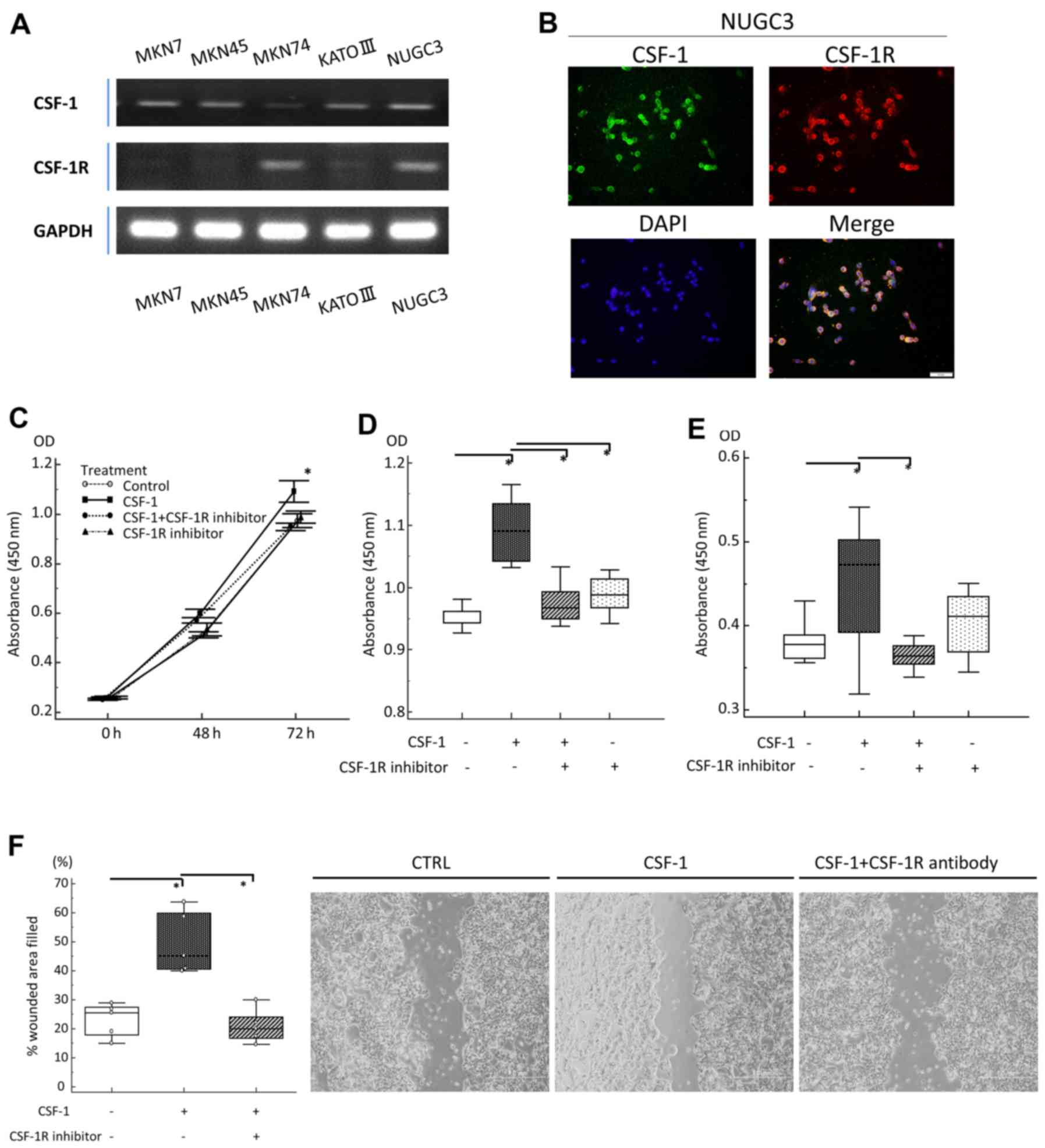 | Figure 3In vitro analyses of gastric
cancer (GC) cell lines treated with recombinant human
colony-stimulating-factor-1 (rhCSF-1) and a CSF receptor (CSF-1R)
inhibitor. (A) A semi-quantitative RT-qPCR analysis of CSF-1 and
CSF-1R transcripts in GC cell lines. (B) Immunofluorescent staining
of NUGC3 cells (right upper panel, CSF-1: magnification, ×200; left
upper panel, CSF-1R: magnification, ×200; right lower panel, DAPI:
magnification, ×200; left lower panel, Merge: magnification, ×200).
(C) Proliferation of MKN74 cells after 48 and 72 h of incubation.
Cells were incubated with CSF-1 (100 ng/ml), CSF-1R inhibitor (5
µM), or incubated with CSF-1R inhibitor (5 µM) prior
to the addition of CSF-1 (100 ng/ml). (D) Effect of CSF-1 and/or
CSF-1R inhibitor on MKN74 cell proliferation at 72 h, as assessed
by MTT assay. (E) Anoikis assay of MKN74 cells following treatment
with CSF-1 (100 ng/ml), CSF-1R inhibitor (5 µM), or
following incubation with CSF-1R inhibitor (5 µM) prior to
the addition of CSF-1 (100 ng/ml). After 18 h, the assay, the
number of viable cancer cells floating in low-attachment plates was
measured by MTT assay. (F) Migration scratch assay of MKN74 cells
following treatment with medium (negative control), CSF-1 (100
ng/ml), or following incubation with CSF-1R (5 µM) prior to
the addition of CSF-1 (100 ng/ml). |
The cells were pre-incubated with or without the
CSF-1R inhibitor and then exposed to CSF-1. After 72 h, the effects
on cell proliferation were analyzed by MTT assay. We observed a
significant increase in the proliferation of rhCSF-1-treated GC
cells compared with the untreated cells, and pre-incubation with
the CSF-1R inhibitor suppressed the effects of rhCSF-1, confirming
that the CSF-1-stimulated proliferation of GC cells occurred via
CSF-1R (Fig. 3C and D).
Anoikis is a form of apoptosis induced by the loss
of cell adhesion (26), and
resistance to anoikis is considered a necessary property of cancer
cells during dissemination and metastasis (27). As our data revealed that the
overexpression of CSF-1 and CSF-1R was an independent risk factor
for lymph node and peritoneal metastasis, we hypothesized that a
novel function of the CSF-1/CSF-1R axis may be to support
resistance to anoikis in the advanced stages of GC. To determine
whether the inhibition of the CSF-1/CSF-1R axis induces anoikis
resistance, we pre-incubated GC cells with or without the CSF-1R
inhibitor and then exposed them to rhCSF-1 in anchorage-independent
growth cultures using ultra-low attachment plates. We found that
rhCSF-1 treatment alone caused an increase in the number of
floating viable GC cells to a level significantly higher than that
observed in the control cultures. The effect of CSF-1 was blocked
in GC cells pretreated with the CSF-1R inhibitor (Fig. 3E). Furthermore, in wound-healing
assays, we observed an increase in the migration of rhCSF-1-treated
cells compared with untreated cells, and here as well, pretreatment
with the CSF-1R inhibitor suppressed the pro-migratory effects of
CSF-1 in this cell line (Fig.
3F).
Expression levels of VEGFA and FLT1 in
cancerous tissues were positively associated with CSF-1 and CSF-1R
expression and are associated with a poor prognosis of patients
with GC
Finally, to clarify the prognostic impact of
angiogenetic factors and its correlation with CSF-1 and CSF1R
expression in GC tissues, we quantified VEGFA and its receptor
(FLT1) expression in GC tissues. A high expression of VEGFA and
FLT1 was significantly associated with a poor prognosis, compared
with the low expression groups, for OS [VEGFA, P=0.0052; FLT1,
P=0.0097; log-rank test (Fig. 4A,
upper panels)]. A high expression of VEGFA and FLT1 was also found
to be associated with a poor prognosis, compared with the low
expression groups, for DFS [VEGFA, P=0.062; FLT1, P=0.11; log-rank
test (Fig. 4A, lower panels)].
Furthermore, the CSF-1 or CSF-1R expression levels positively
correlated with the VEGFA or FLT1 expression levels in GC tissues
(CSF-1 and VEGFA: P<0.0001, r=0.99; CSF-1 and FLT1: P<0.0001,
r=0.99; CSF-1R and VEGFA: P<0.0001, r=0.98; CSF-1R and FLT1:
P<0.0001, r=0.99, respectively) (Fig. 4B).
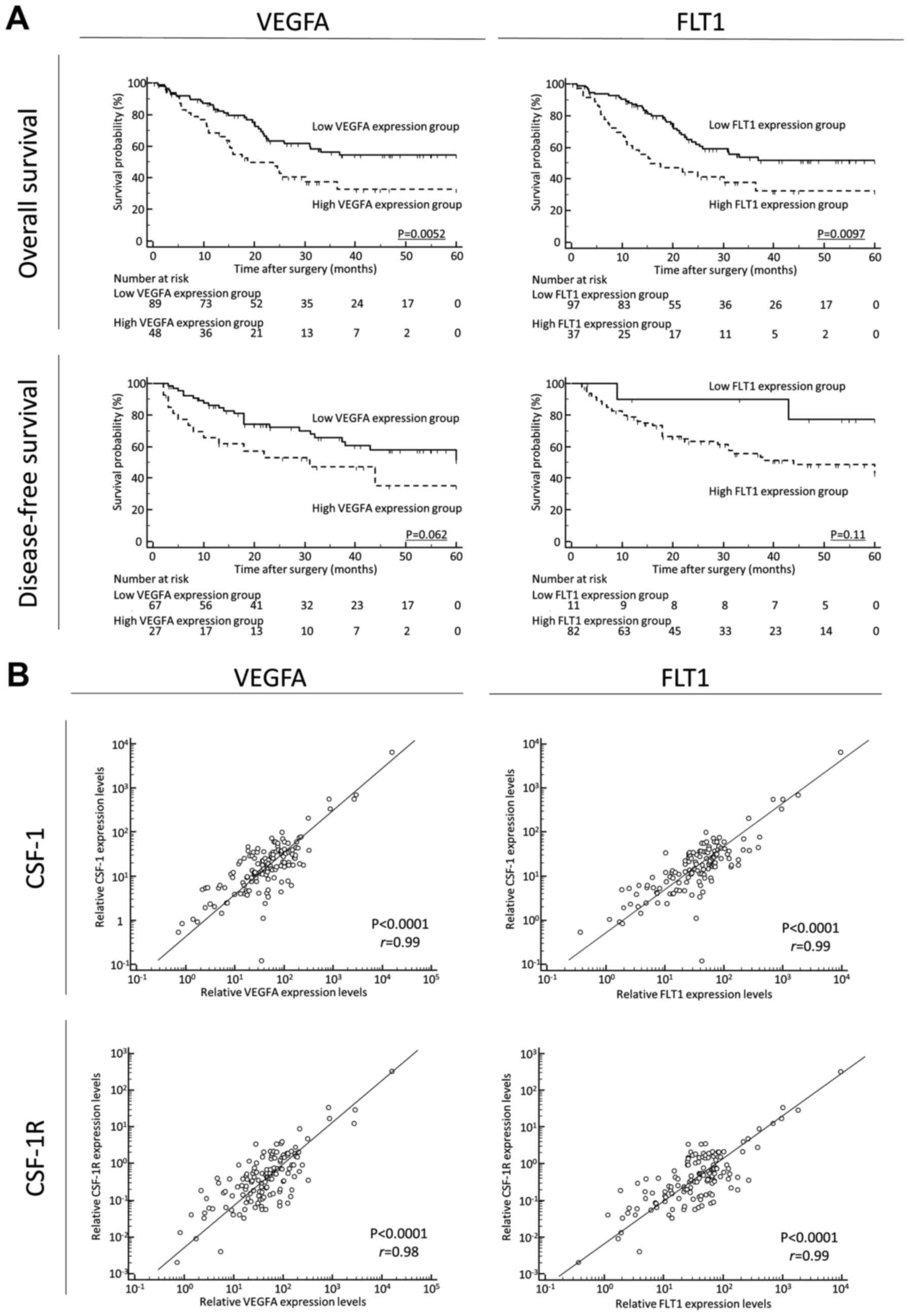 | Figure 4(A) (Upper panels) A high expression
of vascular endothelial growth factor A (VEGFA) and Fms related
tyrosine kinase 1 (FLT1) was significantly associated with a poor
prognosis, compared with the low expression groups, for overall
survival (OS; (VEGFA, P=0.0052; FLT1, P=0.0097; log-rank test).
(Lower panels) A high expression of VEGFA and FLT1 also tended to
be associated with a poor prognosis, compared with the low
expression groups, for disease-free survival (DFS; VEGFA, P=0.062;
FLT1, P=0.11l; log-rank test). It should be noted here that for
disease-free survival, patients with non-curative intent (stage IV)
were not included; thus, the patient numbers differ from those for
OS. In addition, several samples were not sufficient for
amplification and were thus excluded. (B)
Colony-stimulating-factor-1 (CSF-1) or CSF receptor (CSF-1R)
expression levels positively correlated with VEGFA or FLT1
expression levels in gastric cancer tissues (CSF-1 and VEGFA:
P<0.0001, r=0.99; CSF-1 and FLT1: P<0.0001, r=0.99; CSF-1R
and VEGFA: P<0.0001, r=0.98; CSF-1R and FLT1: P<0.0001,
r=0.99, respectively). |
Collectively, these data, including the clinical
significance of CSF-1/CSF-1R expression, multiple in vitro
functional assays, and the positive correlation with angiogenetic
factors, highlight the possibility that the CSF-1/CSF-1R axis may
be an attractive target for the development of novel treatment
strategies for patients with GC.
Discussion
Accumulating evidence suggests the importance of the
CSF-1/CSF-1R axis in various human cancers; however, the
association between the expression of these proteins and their
mechanistic role in driving GC has been unclear. In this study, to
the best of our knowledge, we provide the first evidence supporting
the clinical significance and functional importance of the
CSF-1/CSF-1R axis in GC. First, the elevated expression of CSF-1
and CSF-1R in primary GC tissue was found to be significantly
associated with the presence of lymph node and peritoneal
metastasis, and an advanced TNM stage classification in patients
with GC. Second, not only was a high co-expression of CSF-1/CSF-1R
significantly associated with a poor survival, multivariate Cox
regression analysis revealed that it was also an independent
prognostic factor for OS and DFS in patients with GC. Third, a high
CSF-1/CSF-1R co-expression was an independent risk factor for lymph
node and peritoneal metastasis in patients with GC, suggesting that
the CSF-1/CSF-1R axis may be involved in the progression of lymph
node and peritoneal metastasis. Finally, a series of in
vitro experiments demonstrated that the CSF-1/CSF-1R axis
enhanced, not only the proliferation of GC cells, but also their
migratory capacity and ability to resist anoikis.
Several studies have demonstrated associations
between the overexpression of CSF-1 or CSF-1R and a poor
oncological outcome. Richardsen et al performed tissue IHC
analysis and found that the expression of CSF-1 and CSF-1R was
higher in the prostate tumor cells and tumor stromal areas in
patients with metastatic prostate cancer compared with patients
with non-metastatic prostate cancer (28). Of note, another study analyzed the
expression of CSF-1 and CSF-1R in tumors from 149 patients with
non-gynecological leiomyosarcoma and found that the co-expression
of CSF-1/CSF-1R in the primary tissues was a feasible prognostic
biomarker for patients with this tumor (29). One of the major findings of this
study was that the high expression of CSF-1 and CSF-1R in primary
tissues was significantly associated with disease progression,
recurrence, and a poor survival outcome in patients with GC.
Furthermore, a high CSF-1/CSF-1R expression was an independent
prognostic factor for OS and DFS in patients with GC. Our results
are highly consistent with previous observations in various other
types of cancer (5,6). Collectively, these data suggest that
the CSF-1/CSF-1R axis is intimately involved in GC disease
progression and may be a surrogate parameter in predicting the
prognosis of patients with GC.
Another key finding of this study was the intimate
connection between the CSF-1/CSF-1R expression status and lymph
node and/or peritoneal metastasis in patients with GC. Our results
clearly demonstrate that the co-expression of CSF-1/CSF-1R is a
potential predictor of recurrence and poor prognosis, and is an
independent risk factor for lymph node and peritoneal metastasis in
patients with GC. In current practice, there is a great need to
discover feasible biomarkers that can identify patients with GC
with lymph node or peritoneal metastasis. Metastasis to these areas
is generally recognized to be one of the most important risk
factors for disease recurrence and a poor prognosis of patients
with GC. The accurate detection of lymph node metastasis can assist
in the selection of minimally invasive treatments, such as
endoscopic resection or laparoscopic-assisted gastrectomy, for
patients with early-stage GC. Furthermore, the precise prediction
of peritoneal metastasis may aid the oncologist with
decision-making regarding chemotherapeutic regimens and
peri-operative intraperitoneal chemotherapy for patients with GC.
Therefore, the identification of patients with GC with lymph node
or peritoneal metastasis using molecular biomarkers, such as
CSF-1/CSF-1R may assist the oncologist or surgeon in the
decision-making process as to the proper treatment course to
improve the prognosis of patients with GC.
This study uncovered a novel functional role for the
CSF-1/CSF-1R axis in GC development. CSF-1 is a cytokine secreted
by various cell types, and it regulates the survival, proliferation
and differentiation of monocytes, osteoclasts and macrophages
(4,30,31).
CSF-1 exerts its biological effects by binding to CSF-1R, which is
a 165-kDa glycoprotein encoded by the c-fms proto-oncogene
(32). The CSF-1/CSF-1R axis has
been shown to play a pivotal role in macrophage and osteoclast
function in patients with inflammatory disease (31), and CSF-1 and CSF-1R expressed in
tumor-associated macrophages enhances tumor progression and
metastasis in various types of cancer (33–35).
These findings suggest that the CSF-1/CSF-1R axis functions as a
paracrine loop regulating cancer cells and blood-derived
macrophages in the cancer microenvironment. However, emerging
evidence also supports an autocrine-loop function for the
CSF-1/CSF-1R axis in cancer cells. For example, CSF-1/CSF-1R
signaling activates signal transducer and activator of
transcription-3 (Stat3), which promotes cell survival and
proliferation in renal cell carcinoma (11). The same observation has been
reported in other types of cancer, such as breast, lung and ovarian
cancer (7,12–14).
The IHC analysis in our study revealed that CSF-1 and CSF-1R are
mainly expressed in cancer cells, not in the cancer stroma, in GC
tissues. Based on the combination of previous findings and our
data, we hypothesized that the CSF-1/CSF-1R axis may be involved in
GC development, and we investigated this in vitro using
human GC cells treated with rhCSF-1 and a CSF-1R inhibitor.
Consistent with previous data (7,11–14),
we found that rhCSF-1 treatment enhanced the cell proliferative and
migratory ability and CSF-1R inhibitor treatment suppressed these
effects of rhCSF-1 in cultured GC cells. We demonstrated that the
CSF-1/CSF-1R axis mediates a distinct oncogenic function, namely,
anoikis resistance, in the GC cell line. Metastasis formation is
currently recognized to consist of multiple steps (36,37),
and anoikis resistance plays a pivotal role in cancer cell survival
during dissemination and metastasis (27). Of note, our study successfully
demonstrated that the CSF-1 or CSF-1R expression levels positively
correlated with the VEGFA or FLT1 expression levels in GC tissues.
Although it remains unclear as to the direct evidence of CSF-1
upregulation via VEGF stimulation, several lines of evidence
indicate that the CSF-1/CSF-1R axis can induce the production of
VEGFA in various type of cells (38–41).
Eubank et al demonstrated that recombinant human M-CSF
induces freshly isolated normal human monocytes to produce and
release the growth factor, VEGF, in a dose-dependent manner, and
suggested an important role for M-CSF and monocytes in VEGF
production and angiogenesis (39).
Okazaki et al demonstrated that M-CSF stimulation increased
VEGF protein vis Akt phosphorylation in culture medium of skeletal
muscle cell, which was significantly inhibited by the addition of
CSF-1R-neutralizing antibody (40). Furthermore, another research group
demonstrated that CSF-1 induced VEGF-A overexpression in
tumor-infiltrative macrophages and promoted tumor development via
angiogenesis in colon cancer (41). These previous data combined with
our novel findings suggested that the CSF1/CSF1R axis may promote
metastatic spread via anoikis resistant with tumor angiogenesis,
and highlighted the possibility that therapies targeting the
CSF-1/CSF-1R axis may prove to be attractive for the development of
novel treatment strategies for patients with GC.
Recently, Aharinejad et al assessed
circulating CSF-1 concentration in pre-operative serum specimens
from 1,260 patients with early-stage breast cancer (572 pts) and
benign breast lesion (688 pts) and demonstrated that the serum
CSF-1 concentration level was significantly increased in patients
with early-stage breast cancer compared to those with benign breast
lesion (42). A high serum CSF-1
concentration was significantly associated with nodal involvement
and a poor survival in the patients with breast cancer (41). Although we could not evaluate the
clinical impact of circulating CSF-1 concentration in GC due to the
lack of matched specimens in the current study cohort, the
above-mentioned evidence, together with our findings using tissue
specimens, suggests the potential use of serum CSF-1 as a
non-invasive prognostic biomarker in patients with GC.
In conclusion, this study provides novel evidence
supporting the clinical significance and functional importance of
the CSF-1/CSF-1R axis in GC. Our results demonstrate the clinical
feasibility of CSF-1 and CSF-1R as prognostic and predictive
biomarkers for lymph node and peritoneal metastasis in patients
with GC. Our in vitro analysis also revealed a functional
role for the CSF-1/CSF-1R axis in GC development. It can thus be
concluded that the CSF-1/CSF-1R axis may have clinical utility as a
prognostic and predictive biomarker and, potentially, as a
therapeutic target in GC.
Acknowledgments
The authors would like to thank Mrs. Yuki Orito,
Mrs. Amphone Okada and Ms. Aya Narumi (Department of
Gastrointestinal and Pediatric Surgery, Mie University, Tsu, Japan)
for providing excellent technical assistance.
Funding
YO was supported in part by a Grant-in-Aid for
Scientific Research from the Takeda Science Foundation, Japan. This
study was also supported in part by a Grant-in-Aid for Scientific
Research (no. 25462018) from the Ministry of Education, Culture,
Sports, Science and Technology, Japan to MO.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Study concept and design (YO, YT, CM and MKu);
provision of samples (TI, MKa, HY, HF, SS, MO, KT, YI, MT and TA);
acquisition of data (YO, YT, TI, MKa, HY, HF, SS, MO, KT, YI and
CM); analysis and interpretation of data (YO, YT, MKa, HY, HF, SS,
MO, KT, YI, MT and TA); statistical analysis (YO and YT); drafting
of the manuscript (YO, YT, CM and MKu). All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
For the use of patient samples, written informed
consent was obtained from each patient, and the study was approved
by the Institutional Review Boards of Mie University (no.
2215).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill K, Kirma N, Gunna VS, Santanam N,
Parthasarathy S and Tekmal RR: Regulation of colony stimulating
factor-1 (CSF-1) in endometrial cells: Glucocorticoids and
oxidative stress regulate the expression of CSF-1 and its receptor
c-fms in endometrial cells. Fertil Steril. 76:1005–1011. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pixley FJ and Stanley ER: CSF-1 regulation
of the wandering macrophage: Complexity in action. Trends Cell
Biol. 14:628–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang L, Wu Q, Xu L, Zhang W, Zhu Y, Liu H,
Xu J and Gu J: Increased expression of colony stimulating factor-1
is a predictor of poor prognosis in patients with clear-cell renal
cell carcinoma. BMC Cancer. 15:672015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Komohara Y, Ohnishi K, Kuratsu J and
Takeya M: Possible involvement of the M2 anti-inflammatory
macrophage phenotype in growth of human gliomas. J Pathol.
216:15–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patsialou A, Wyckoff J, Wang Y, Goswami S,
Stanley ER and Condeelis JS: Invasion of human breast cancer cells
in vivo requires both paracrine and autocrine loops involving the
colony-stimulating factor-1 receptor. Cancer Res. 69:9498–9506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soares MJ, Pinto M, Henrique R, Vieira J,
Cerveira N, Peixoto A, Martins AT, Oliveira J, Jerónimo C and
Teixeira MR: CSF1R copy number changes, point mutations, and RNA
and protein overexpression in renal cell carcinomas. Mod Pathol.
22:744–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ide H, Seligson DB, Memarzadeh S, Xin L,
Horvath S, Dubey P, Flick MB, Kacinski BM, Palotie A and Witte ON:
Expression of colony-stimulating factor 1 receptor during prostate
development and prostate cancer progression. Proc Natl Acad Sci
USA. 99:14404–14409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chambers SK, Kacinski BM, Ivins CM and
Carcangiu ML: Overexpression of epithelial macrophage
colony-stimulating factor (CSF-1) and CSF-1 receptor: A poor
prognostic factor in epithelial ovarian cancer, contrasted with a
protective effect of stromal CSF-1. Clin Cancer Res. 3:999–1007.
1997.PubMed/NCBI
|
|
11
|
Komohara Y, Hasita H, Ohnishi K, Fujiwara
Y, Suzu S, Eto M and Takeya M: Macrophage infiltration and its
prognostic relevance in clear cell renal cell carcinoma. Cancer
Sci. 102:1424–1431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hung JY, Horn D, Woodruff K, Prihoda T,
LeSaux C, Peters J, Tio F and Abboud-Werner SL: Colony-stimulating
factor 1 potentiates lung cancer bone metastasis. Lab Invest.
94:371–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goswami S, Sahai E, Wyckoff JB, Cammer M,
Cox D, Pixley FJ, Stanley ER, Segall JE and Condeelis JS:
Macrophages promote the invasion of breast carcinoma cells via a
colony-stimulating factor-1/epidermal growth factor paracrine loop.
Cancer Res. 65:5278–5283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menke J, Kriegsmann J, Schimanski CC,
Schwartz MM, Schwarting A and Kelley VR: Autocrine CSF-1 and CSF-1
receptor coexpression promotes renal cell carcinoma growth. Cancer
Res. 72:187–200. 2012. View Article : Google Scholar
|
|
15
|
Okugawa Y, Toiyama Y, Hur K, Toden S,
Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, et
al: Metastasis-associated long non-coding RNA drives gastric cancer
development and promotes peritoneal metastasis. Carcinogenesis.
35:2731–2739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okugawa Y, Tanaka K, Inoue Y, Kawamura M,
Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, et al:
Brain-derived neurotrophic factor/tropomyosin-related kinase B
pathway in gastric cancer. Br J Cancer. 108:121–130. 2013.
View Article : Google Scholar :
|
|
17
|
Okugawa Y, Inoue Y, Tanaka K, Kawamura M,
Saigusa S, Toiyama Y, Ohi M, Uchida K, Mohri Y and Kusunoki M: Smad
interacting protein 1 (SIP1) is associated with peritoneal
carcinomatosis in intestinal type gastric cancer. Clin Exp
Metastasis. 30:417–429. 2013. View Article : Google Scholar
|
|
18
|
Okugawa Y, Toiyama Y, Tanaka K, Matsusita
K, Fujikawa H, Saigusa S, Ohi M, Inoue Y, Mohri Y, Uchida K, et al:
Clinical significance of Zinc finger E-box Binding homeobox 1
(ZEB1) in human gastric cancer. J Surg Oncol. 106:280–285. 2012.
View Article : Google Scholar
|
|
19
|
Toiyama Y, Tanaka K, Kitajima T, Shimura
T, Imaoka H, Mori K, Okigami M, Yasuda H, Okugawa Y, Saigusa S, et
al: Serum angiopoietin-like protein 2 as a potential biomarker for
diagnosis, early recurrence and prognosis in gastric cancer
patients. Carcinogenesis. 36:1474–1483. 2015.PubMed/NCBI
|
|
20
|
Toiyama Y, Yasuda H, Saigusa S, Matushita
K, Fujikawa H, Tanaka K, Mohri Y, Inoue Y, Goel A and Kusunoki M:
Co-expression of hepatocyte growth factor and c-Met predicts
peritoneal dissemination established by autocrine hepatocyte growth
factor/c-Met signaling in gastric cancer. Int J Cancer.
130:2912–2921. 2012. View Article : Google Scholar
|
|
21
|
Japanese-Gastric-Cancer-Association: Jpn
Classificacion Gastric Carcinoma. 14:10–25. 2010.
|
|
22
|
Tanaka K, Mohri Y, Nishioka J, Kobayashi
M, Ohi M, Miki C, Tonouchi H, Nobori T and Kusunoki M: Neurotrophic
receptor, tropomyosin-related kinase B as an independent prognostic
marker in gastric cancer patients. J Surg Oncol. 99:307–310. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conway JG, McDonald B, Parham J, Keith B,
Rusnak DW, Shaw E, Jansen M, Lin P, Payne A, Crosby RM, et al:
Inhibition of colony-stimulating-factor-1 signaling in vivo with
the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl
Acad Sci USA. 102:16078–16083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conway JG, Pink H, Bergquist ML, Han B,
Depee S, Tadepalli S, Lin P, Crumrine RC, Binz J, Clark RL, et al:
Effects of the cFMS kinase inhibitor
5-(3-methoxy-4-((4-methoxybenzyl)oxy)benzyl) pyrimidine-2,4-diamine
(GW2580) in normal and arthritic rats. J Pharmacol Exp Ther.
326:41–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okugawa Y, Toiyama Y, Toden S, Mitoma H,
Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR and Goel A:
Clinical significance of SNORA42 as an oncogene and a prognostic
biomarker in colorectal cancer. Gut. 66:107–117. 2017. View Article : Google Scholar
|
|
26
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richardsen E, Uglehus RD, Due J, Busch C
and Busund LT: The prognostic impact of M-CSF, CSF-1 receptor, CD68
and CD3 in prostatic carcinoma. Histopathology. 53:30–38. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Espinosa I, Beck AH, Lee CH, Zhu S,
Montgomery KD, Marinelli RJ, Ganjoo KN, Nielsen TO, Gilks CB, West
RB, et al: Coordinate expression of colony-stimulating factor-1 and
colony-stimulating factor-1-related proteins is associated with
poor prognosis in gynecological and nongynecological
leiomyosarcoma. Am J Pathol. 174:2347–2356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu W, Chen J, Xiong Y, Pixley FJ, Yeung YG
and Stanley ER: Macrophage proliferation is regulated through CSF-1
receptor tyrosines 544, 559, and 807. J Biol Chem. 287:13694–13704.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chitu V and Stanley ER: Colony-stimulating
factor-1 in immunity and inflammation. Curr Opin Immunol. 18:39–48.
2006. View Article : Google Scholar
|
|
32
|
Sherr CJ, Rettenmier CW, Sacca R, Roussel
MF, Look AT and Stanley ER: The c-fms proto-oncogene product is
related to the receptor for the mononuclear phagocyte growth
factor, CSF-1. Cell. 41:665–676. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony-stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aharinejad S, Abraham D, Paulus P, Abri H,
Hofmann M, Grossschmidt K, Schäfer R, Stanley ER and Hofbauer R:
Colony-stimulating factor-1 antisense treatment suppresses growth
of human tumor xenografts in mice. Cancer Res. 62:5317–5324.
2002.PubMed/NCBI
|
|
35
|
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley
F, Stanley ER, Graf T, Pollard JW, Segall J and Condeelis J: A
paracrine loop between tumor cells and macrophages is required for
tumor cell migration in mammary tumors. Cancer Res. 64:7022–7029.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan DA and Giaccia AJ: Hypoxia, gene
expression, and metastasis. Cancer Metastasis Rev. 26:333–339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Curry JM, Eubank TD, Roberts RD, Wang Y,
Pore N, Maity A and Marsh CB: M-CSF signals through the MAPK/ERK
pathway via Sp1 to induce VEGF production and induces angiogenesis
in vivo. PLoS One. 3:e34052008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eubank TD, Galloway M, Montague CM,
Waldman WJ and Marsh CB: M-CSF induces vascular endothelial growth
factor production and angiogenic activity from human monocytes. J
Immunol. 171:2637–2643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okazaki T, Ebihara S, Takahashi H, Asada
M, Kanda A and Sasaki H: Macrophage colony-stimulating factor
induces vascular endothelial growth factor production in skeletal
muscle and promotes tumor angiogenesis. J Immunol. 174:7531–7538.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zins K, Abraham D, Sioud M and Aharinejad
S: Colon cancer cell-derived tumor necrosis factor-alpha mediates
the tumor growth-promoting response in macrophages by up-regulating
the colony-stimulating factor-1 pathway. Cancer Res. 67:1038–1045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aharinejad S, Salama M, Paulus P, Zins K,
Berger A and Singer CF: Elevated CSF1 serum concentration predicts
poor overall survival in women with early breast cancer. Endocr
Relat Cancer. 20:777–783. 2013. View Article : Google Scholar : PubMed/NCBI
|


















