Introduction
Melanoma is a type of skin cancer caused by
excessive hyperplasia of abnormal melanocytes. It is prone to
relapse and metastasis, which makes it one of the leading causes of
mortality among skin cancers (1).
Recently, molecular targeted therapy has provided potential for
intervention at a molecular level by modulating certain signaling
pathways as a form of cancer treatment and offers the promise of
novel treatments for melanoma through the associated in-depth
analyses of signal transduction pathways. Several antagonistic
drugs directed against melanoma have been put into clinical use
(2–4). However, melanoma has developed into
one of the fastest growing cancers in terms of incidence rate in
recent years, with an annual rate of growth of 3–5% (5,6). As
a result, identifying novel targets for the treatment of melanoma
remains an urgent need at this stage.
Matrine is a major alkaloid extracted from
Sophora flavescens (7) and
is also naturally present in subprostrate Sophora (8) and Sophora alopecuroides
(9). Matrine exhibits several
pharmacological effects, including anti-arrhythmia (10), anti-inflammation (11), and antitumor activities (12–15).
The wide spread use of matrine is attributed to its low toxicity.
The anticancer properties of matrine are associated with its
ability to inhibit proliferation and invasion and induce apoptosis
in tumor cell lines through a vast number of pathways (13,16,17).
However, in the case of melanoma, the matrine mechanism of action
has not been clarified.
MicroRNAs (miRNAs/miRs) are considered to have
important roles in tumors. It has recently been reported that
matrine alters miRNA expression profiles in SGC-7901 human gastric
cancer cells, providing a novel and promising approach to identify
the mechanisms of action of matrine (18). In this study, an miRNA microarray
was used to screen relative miRNA levels of SGC-7901 human gastric
cancer cells following matrine treatment (18). Among the results, miR-19b was of
particular interest. Compared with the untreated cells, miR-19b was
significantly downregulated in SGC-7901 cells following matrine
treatment. Another study reported that miR-19b promoted breast
cancer metastasis by targeting myosin regulatory light chain
interacting protein and associated cell adhesion molecules
(19). Additionally, it has been
reported that miR-19b-3p may promote colon cancer proliferation and
oxaliplatin-based chemoresistance by targeting SMAD4 (20). Notably, in melanoma, miR-19b was
reported to be a novel upstream effector of telomerase reverse
transcriptase transcription via direct targeting of mRNA encoding
pituitary homeobox 1 (21).
Additionally, miR-19b was downregulated following matrine treatment
in gastric cancer cell lines (18). Thus, in the present study, it was
aimed to determine whether matrine regulates miR-19b in melanoma
via certain pathways to alter the proliferation, invasion and
apoptosis of melanoma cells.
Materials and methods
Cell lines and cell culture
A375 and SK-MEL-2 cells have been widely used in
melanoma research in vitro (22,23).
The two cell lines were purchased from the cell bank of the Chinese
Academy of Sciences (Shanghai, China). Normal human epidermal
melanocytes (NHEMs) and three melanoma cell lines (SK-MEL-1, A875
and M21) were purchased from Zhong Qiao Xin Zhou Biotechnology Co.,
Ltd. (Shanghai, China). The cell lines were cultured in a
humidified atmosphere of 5% CO2, with the temperature
set to 37°C. Cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin.
Reagents
Matrine (>98% purity) was purchased from Rochen
Pharma Co., Ltd. (Shanghai, China) and dissolved in DMEM medium to
make a 10 mg/ml stock solution and stored at −20°C in the dark.
Cell treatment
A375 and SK-MEL-2 cells were exposed to different
treatments and divided into several groups according to the
experimental design. A375 and SK-MEL-2 cells were directly treated
with 0, 250 and 500 µg/ml matrine; additionally, the two
cells were transfected with anti-miR-19b-3p (cat. no. B03001;
Shanghai GenePharma Co., Ltd., Shanghai, China), of miR-19b-3p
mimics (cat. no. B01001) or small interfering RNA (si) targeting
phosphatase and tensin homolog (PTEN; cat. no. A01001; Shanghai
GenePharma Co., Ltd.) or co-transfected with si-PTEN and the first
two miRNAs. In the experiments, anti-miR-control (cat. no. B04003)
was used as a control for anti-miR-19b-3p mimics, miR-control (cat.
no. B04001) (both from Shanghai GenePharma Co., Ltd.) was used as a
control for miR-19b-3p mimics, and the blank group and scramble
si-PTEN group was used as a control for si-PTEN. The blank group
was untransfected cells. For transfection, A375 and SK-MEL-2 cells
were cultured in the complete medium without antibiotics for >24
h and washed with 1X PBS (pH 7.4) and then transiently transfected
with the appropriate constructs (20 pmol for 24-well plate or 100
pmol for 6-well plate) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturers' instructions. The sequences were as follows:
Anti-miR-19b-3p, 5′-UCAGUUUUGCAUGGAUUUGCACA-3′; miR-19b-3p mimic,
5′-UGUGCAAAUCCAUGCAAAACUGA-3′ and 5′-AGUUUUGCAUGGAUUUGCACAAG-3′;
si-PTEN, 5′-AGAUGUUAGUGACAAUGAACC-3′ and
5′-GGUUCAUUGUCACUAACAUCU-3′.
Cell counting kit-8 (CCK-8) assay
The CCK-8 assay is a method for evaluating the
proliferation of A375 and SK-MEL-2 cells in vitro. The cells
into several groups as described above. Following treatment or
transfection for 24–72 h, 10 µl CCK-8 reagent was added to
the medium with 90 µl DMEM and FBS. The cells were incubated
for 4 h. The absorbance at 450 nm [optical density (OD)450] was
measured using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) to estimate viable cell numbers.
Transwell assay
A Transwell assay was performed to analyze cell
invasion. For this assay, 5×105 cells/ml A375 and
SK-MEL-2 cells were cultured in the upper chamber of a 24-well
Transwell Permeable Support with 100 µl serum-free DMEM;
whereas, the lower chamber was filled with 400 µl medium
containing 10% FBS. Subsequently, the plate was incubated for 48 h.
Subsequently, cells in the upper chamber were then removed with a
clean cotton swab, and cells that had migrated to the lower chamber
through Matrigel were fixed with 99.5% methanol at room temperature
for 30 min. To determine the number of cells via microscopy, 0.1%
crystal violet staining for 10 min at room temperature was
performed. Cells were counted in three randomly selected fields
under an inverted light microscope and each experiment was repeated
in triplicate independently.
Flow cytometry
In normal cells, phosphatidylserine (PS) is only
distributed inside of the membrane lipid bilayer. When the earliest
steps of apoptosis occur, PS translocates to the outside of the
membrane, and can bind with the calcium-dependent
phospholipid-binding protein Annexin V. Thus, Annexin V binding is
a sensitive marker for detecting early cell apoptosis. Propidium
iodide (PI) is a nucleic acid dye. It is unable to penetrate the
whole cell membrane of intact cells, while it is able to penetrate
and stain late apoptotic and dead cells due to the increased
permeability of the cell membrane. In the current study, Annexin
V-fluorescein isothiocyanate (FITC)/PI was applied to detect the
apoptosis of A375 and SK-MEL-2 cells via flow cytometry according
to the instructions of the FITC Annexin V Apoptosis Detection Kit I
(BestBio Science, Shanghai, China). For this assay,
5×105 cells/ml were cultured following treatment of
matrine for 48 h or transfection with miRs and siRNA for 24 h. A
total of 1×104 cells were harvested during the flow
cytometry and each experiment was performed in triplicate.
Caspase-3/7 activity detection
Caspase-3/7 activity detection is another method to
evaluate apoptosis. According to the instructions of the
Caspase-Glo® 3/7 assay (Promega Corporation, Madison,
WI, USA), 4×104 of cells were seeded in 96-well plates
and cultured with matrine (0, 250 and 500 µg/ml) for 24 h.
Caspase-Glo® 3/7 buffer and substrate were thoroughly
dissolved to form the Caspase-Glo® 3/7 reagent. The
wells containing matrine, cell culture medium and
Caspase-Glo® 3/7 reagent without cells were used as a
blank group. The wells containing Caspase-Glo® 3/7
reagent and cells without matrine treatment in culture medium were
used as negative control group. The wells containing
Caspase-Glo® 3/7 reagent and cells under treatment of
matrine in cell culture medium were used as the experimental
groups. After incubation for 3 h at 37°C, the luminescence of each
group was measured in a luminometer plate reader at an excitation
wavelength of 490 nm and an emission wavelength of 530 nm.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA exaction from A375 and SK-MEL-2 cells
following transfection or treatment for 24 h was performed with an
E.Z.N.A. Total RNA Kit I (Omega Bio-Tek, Inc., Norcross, GA, USA).
RNA was reverse transcribed to cDNA using a First Strand cDNA
Synthesis Kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. For the detection of miR-19b-3p,
RT-qPCR was performed with a high-specificity miR-19b-3p RT-qPCR
Detection Kit and ABI Power SYBR Green PCR Master Mix (both from
Applied Biosystems; Thermo Fisher Scientific, Inc.), with U6 small
nuclear RNA used as the endogenous control for normalization. For
detection of PTEN mRNA, qPCR was performed with an ABI 7500 Fast
System (Applied Biosystems; Thermo Fisher Scientific, Inc.), with
GAPDH for PTEN normalization used as the endogenous control. The
relative expression of miR-19b-3p and PTEN mRNA were calculated
using the 2−ΔΔCq method (21).
The primers for miR-19b-3p were as follows: RT
primer, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACACGTT-3′;
forward, 5′-TCCGAAGTCAAAACGTACCTA-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′. The primers for U6 were as follows: RT
primer, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATA-3′;
forward, 5′-TCCGATCGTGAAGCGTTC-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′. The primers for PTEN mRNA were as follows:
forward, 5-CAAGATGATGTTTGAAACTATTCCAATG-3 and reverse,
5′-5-CCTTTAGCTGGCAGACCACAA-3′. The primers for GAPDH were as
follows: forward, 5′-ACAACAGCCTCAAGATCATCAGC-3′ and reverse,
5′-CACGCCACAGTTTCCCGGAG-3′. The RT-PCR reaction conditions were
16°C for 10 min, 42°C for 60 min, 85°C for 5 min, and paused at
16°C. The qPCR reaction conditions were 95°C for 3 min, 40 cycles
at 95°C for 30 sec and 60°C for 40 sec, melting curve (60°C for 60
sec; 95°C for 15 sec).
Western blotting
Total protein from A375 and SK-MEL-2 cells
(5×106 cells) after transfection or treatment for 24 h
was homogenized in lysis buffer and was quantified. The lysis
buffer was a mix of phenylmethylsulfonyl fluoride and
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) according to the ratio of 1:99. A
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) was used to quantify the total protein. In the
process of western blotting, the total protein (30 µg) was
separated by 10% SDS-PAGE and transferred onto a nitrocellulose
membrane at 90 V for 80 min. Tris-buffered saline (TBS; 1X) and 20%
Tween-20 (5 ml) were mixed to produce TBST. Following washing in 1X
TBST for 3 min, the membrane was blocked in 3% FBS for 1 h at room
temperature and probed with the primary antibody (mouse anti-PTEN
monoclonal antibody; cat. no. BM4114; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) overnight at 4°C at a 1:500
dilution. Following rinsing with 1X TBST 5 times for 5 min, the
membrane was probed with a secondary antibody (goat anti-mouse IgG;
1:10,000 dilution; cat. no. SA00001-1; ProteinTech Group, Inc.,
Chicago, IL, USA) for 2 h at room temperature. Following five
washes in TBST, the films of immunoreactive products were scanned
using an enhanced chemiluminescence western blot detection system
(FluorChem E; ProteinSimple, San Jose, CA, USA) with the ECL
Western Blotting Substrate (Thermo Fisher Scientific, Inc.).
Quantified data were analyzed using IPP 6.0 image analysis software
(Media Cybernetics, Inc., Rockville, MD, USA). GAPDH (cat. no.
BM3876; Wuhan Boster Biological Technology, Ltd.) served as an
endogenous reference and antibody was incubated overnight at 4°C in
a 1:400 dilution.
Luciferase reporter assay
A luciferase reporter assay was used to examine the
association between miR-19b-3p and PTEN, following identification
of two putative binding sites in the PTEN 3′ untranslated region
(UTR) using bioinformatics analysis (TargetScan; targetscan.org/vert_72/). PCR primers were
designed (forward, 5′-GTACTCGAGAGGATTAATAAAGATGGCACT-3′ and
reverse, 5′-ACGTCTAGAATCAATAAAGCACATGTAGGAC-3′) to amplify
wild-type (Wt) and mutant (Mt) PTEN 3′UTR sequences by PCR
amplification, and the PUC57-Wt-PTEN 3′UTR and PUC57-Mt-PTEN 3′UTR
(Sangon Biotech Co., Ltd., Shanghai, China) were used as the
templates for amplification, respectively. A pmirGLO reporter
vector was purchased from Promega Corporation, and the fragments
that had been amplified beforehand were cloned into the multiple
cloning site region of the vector to produce the recombinant
vectors pmirGLO-3′UTR PTEN-Wt and pmirGLO-3′UTR PTEN-Mt.
To perform out the luciferase reporter assay, A375
cells were divided into 8 groups and cultured in two 6-well plates.
pmirGLO-3′UTR PTEN-Wt or pmirGLO-3′UTR PTEN-Mt vectors (2
µg) were co-transfected with miR-control, miR-19b-3p mimic
(cat. no. B01001), anti-miR-control or anti-miR-19b-3p (cat. no.
B03001) into A375 cells as described above. Luciferase activity was
then determined at 24 h post-transfection with a Dual-Luciferase
Assay Kit (Promega Corporation).
Statistical analyses
The software SPSS v.21.0 (IBMCorp., Armonk, NY, USA)
was used for data analysis. The Student's t-test was used to
compare between two groups. One-way analysis of variance was
performed to compare data among three or more groups, and further
analysis was achieved using Bonferroni or Least Significant
Difference test. All values are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Matrine inhibits proliferation and
invasiveness, and promotes apoptosis in human A375 and SK-MEL-2
melanoma cell lines
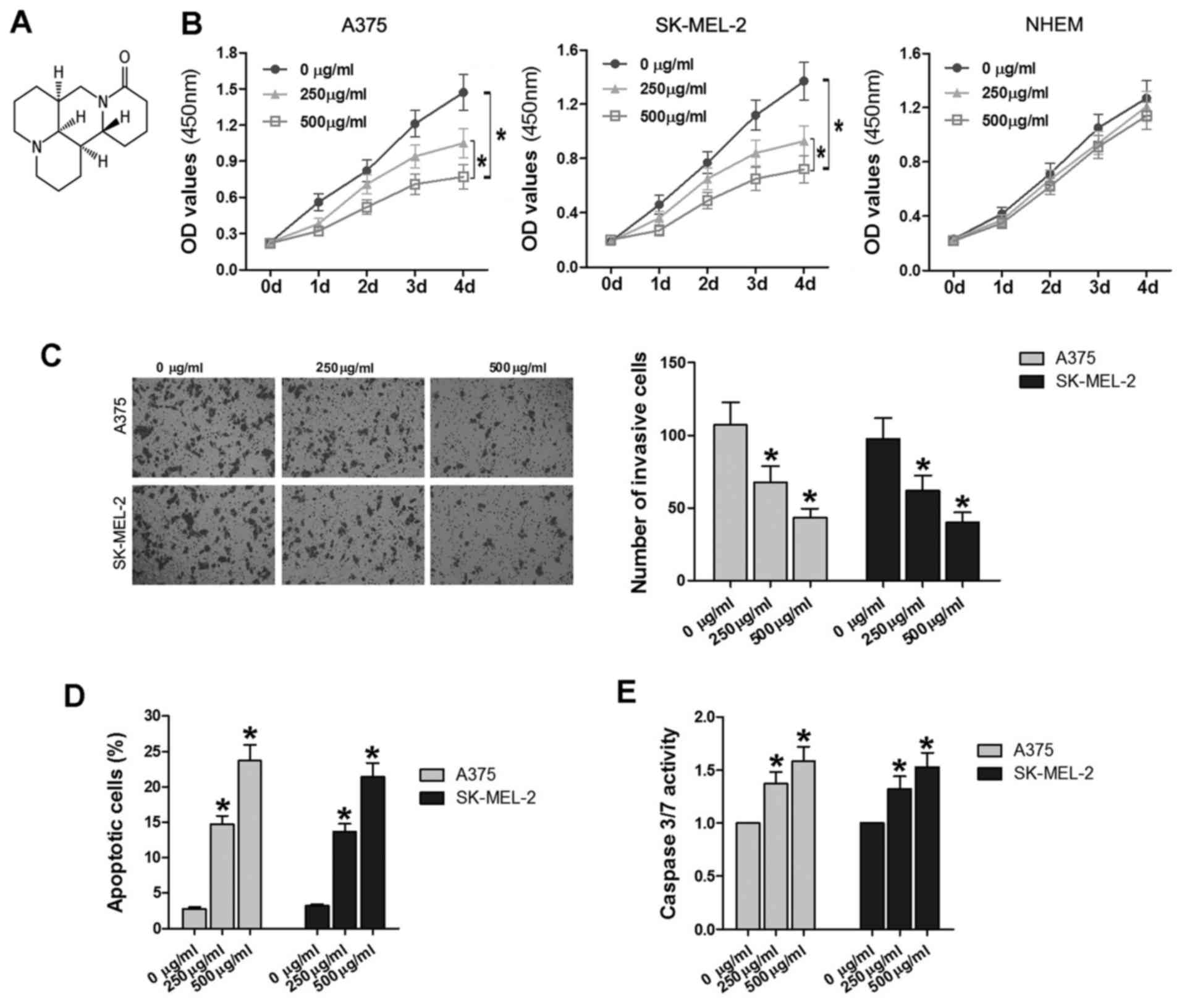
Matrine, one of the main alkaloid components
extracted from a traditional Chinese herb, Sophora
flavescens Ait, has wide-spread pharmacological effects. The
chemical formula of matrine is
C15H24N2O (Fig. 1A) (24). In the current study, NHEMs, A375
and SK-MEL-2 cells were treated with various concentrations of
matrine (0, 250 and 500 µg/ml) for 0–4 days. The CCK-8 assay
was used to compare the proliferation rates of cells at different
matrine concentrations. The OD450 values were measured over time
and the growth curves were plotted (Fig. 1B). NHEM cells treated with the
increasing concentrations of matrine shared similar OD values
(P>0.05); while A375 cells treated with 500 µg/ml matrine
exhibited significantly lower OD values than the cells treated with
250 µg/ml matrine and 0 µg/ml matrine (P<0.05).
Furthermore, the OD values in the 250 µg/ml group were also
significantly lower than those in the 0 µg/ml group
(P<0.05). A similar phenomenon was also observed in the SK-MEL-2
cell line.
Additionally, Transwell assays were performed to
determine the invasiveness of following matrine treatment. After
incubation for 48 h, the number of invaded A375 cells in the 250
µg/ml group (62.4±9.8) and 500 µg/ml group (43.6±6.1)
was significantly decreased compared with the control group
(105.3±15.7; P<0.05; Fig. 1C).
Compared with the cell numbers obtained from CCK-8 assay (Fig. 1B), the relative rate of invaded
cells treated in the same condition were relatively decreased,
revealing that matrine could inhibit the invasiveness of A375 cells
in some degree. Similarly, the number of SK-MEL-2 cells in the 250
µg/ml group (62.4±9.8) and 500 µg/ml group (60.8±7.1)
was also significantly decreased compared with the control group
(95.5±14.6; P<0.05; Fig.
1C).
In addition to proliferation and invasion, abnormal
apoptosis is another important feature of tumor pathology (25). As demonstrated in Fig. 1D, the flow cytometry analysis
revealed that when treated with 250–500 µg/ml matrine, A375
and SK-MEL-2 cells exhibited significantly increased proportions of
apoptotic cells (P<0.05); furthermore, the apoptotic rates were
increased with elevated matrine concentration, suggesting that
matrine induces cell apoptosis in a dose-dependent manner in
vitro. To confirm the results, a caspase-3/7 assay was also
used to detect cell apoptosis. The results demonstrated that
caspase-3/7 activity was significantly increased in the 250–500
µg/ml of matrine treatment groups compared with 0
µg/ml (P<0.05; Fig. 1E),
which was consistent with the findings of flow cytometry.
These findings indicated that matrine did not induce
marked cytotoxicity in normal cells; and that following treatment
with 250 and 500 µg/ml matrine, melanoma cells proliferation
and invasion were significantly suppressed, while apoptosis was
promoted, and the effects were dose-dependent.
Matrine decreases the expression of
miR-19b-3p and increases the expression of PTEN in A375 and
SK-MEL-2 cells
The aforementioned experiments demonstrated that
matrine inhibited cell proliferation and invasion, and induced cell
apoptosis. Subsequently, the effect of matrine on the expression of
miR-19b-3p and PTEN was detected using RT-qPCR and western blot
assays. miR-19b is part of the miR-17-92 and miR-106-363 clusters.
The two miRNA clusters are in the chromosomal region 13q31.3 and
Xq26.2, respectively; with miRNAs at these locations often
overexpressed in cancer cells (26–28).
In the current study, the expression of miR-19b-3p was measured in
five types of melanoma cells, with NHEMs used as a negative
control. miR-19b-3p expression in the five melanoma cell lines was
upregulated compared with that in the NHEMs (P<0.05; Fig. 2A). Additionally, following
treatment with various concentrations of matrine for 48 h, the
expression of miR-19b-3p in A375 and SK-MEL-2 cells significantly
reduced in the 250–500 µg/ml matrine groups (P<0.05,
Fig. 2B); while there was no
significant difference in the expression of miR-19b-3p in NHEMs
(P>0.05; Fig. 2B).
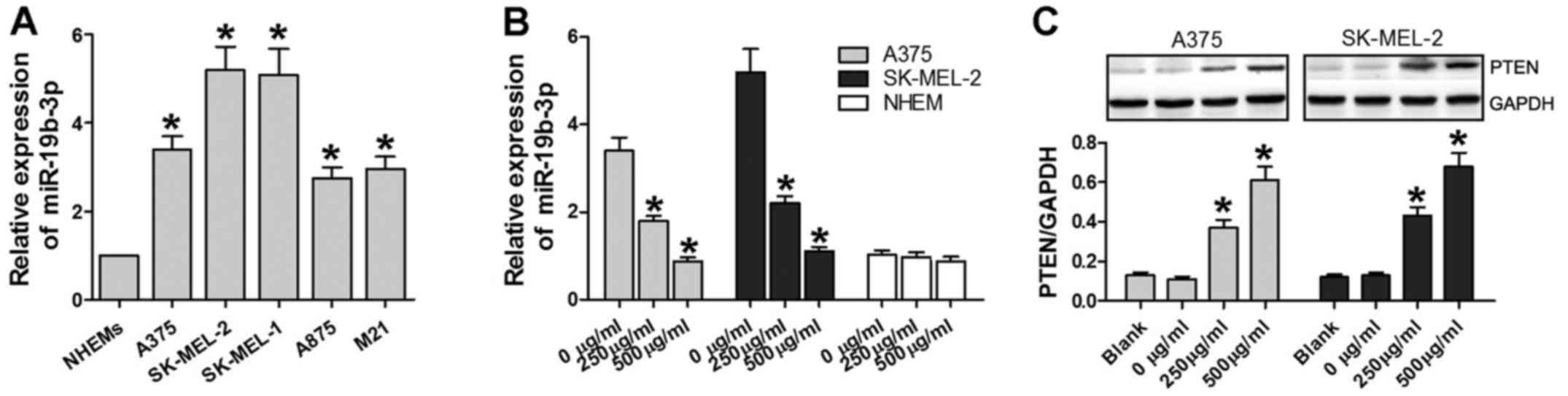 | Figure 2Matrine increases the expression of
PTEN, and decreases the expression of miR-19b-3p. (A) miR-19b-3p
expression levels as determined by RT-qPCR in NHEMs, A375,
SK-MEL-2, SK-MEL-1, A875 and M21 cells. *P<0.05 vs. NHEMs. (B)
miR-19b-3p expression levels as detected by RT-qPCR in NHEMs, A375
and SK-MEL-2 cells treated with 0, 250 and 500 µg/ml of
matrine for 24 h. (C) PTEN protein expression levels as determined
by western blot analysis in A375 and SK-MEL-2 cells treated with 0,
250 and 500 µg/ml of matrine for 24 h. *P<0.05
vs. 0 µg/ml or blank. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; NHEM, normal
human epidermal melanocytes; miR, microRNA; PTEN, phosphatase and
tensin homolog. |
PTEN has been reported to be activated by matrine to
induce growth inhibition and apoptosis in
V600EBRAF-harboring melanoma cells (29). In the current study, PTEN protein
expression was significantly increased in A375 and SK-MEL-2 cells
treated with 250 µg/ml matrine groups and 500 µg/ml
compared with the blank and 0 µg/ml groups (P<0.05;
Fig. 2C). Overall, matrine
decreased the expression of miR-19b-3p and increased the expression
of PTEN in A375 and SK-MEL-2 cells.
miR-19b-3p targets PTEN in A375 and
SK-MEL-2 cells
The findings of the current study indicated that the
expression of miR-19b-3p and PTEN was altered in matrine-treated
A375 and SK-MEL-2 cells; subsequently, it was aimed to determine
whether there is an association between miR-19b-3p and PTEN in
melanoma cells. The bioinformatics analysis provided two
interactive binding regions between miR-19b-3p and PTEN mRNA
(Fig. 3A). Vast amounts of
published data indicate that miRNAs can target one or more mRNA
species to regulate their expression. To determine whether PTEN
expression is regulated by miR-19b-3p, a dual-luciferase reporter
assay was performed. During this experiment, A375 cells were
divided into eight groups for the different treatments. miR-19b-3p
significantly suppressed the luciferase activity in cells
transfected with the pmirGLO-3′UTR PTEN-Wt; however, miR-19b-3p
failed to inhibit this activity in cells containing the
pmirGLO-3′UTR PTEN-Mt (Fig. 3B).
Additionally, the cells were also trans-fected with anti-miR-19b-3p
and pmirGLO-3′UTR PTEN-Wt or pmirGLO-3′UTR PTEN-Mt groups, with an
anti-miR-control group as a negative control. The results revealed
that luciferase activity in cells transfected with anti-miR-19b-3p
and the pmirGLO-3′UTR PTEN-Wt was at its highest level compared
with the other groups (P<0.05; Fig.
3B). Combined with the fact that the protein expression of PTEN
was inhibited by miR-19b-3p overexpression and increased by
miR-19b-3p downregulation (Fig.
3C), it was concluded that miR-19b-3p targeted PTEN in A375 and
SK-MEL-2 cells.
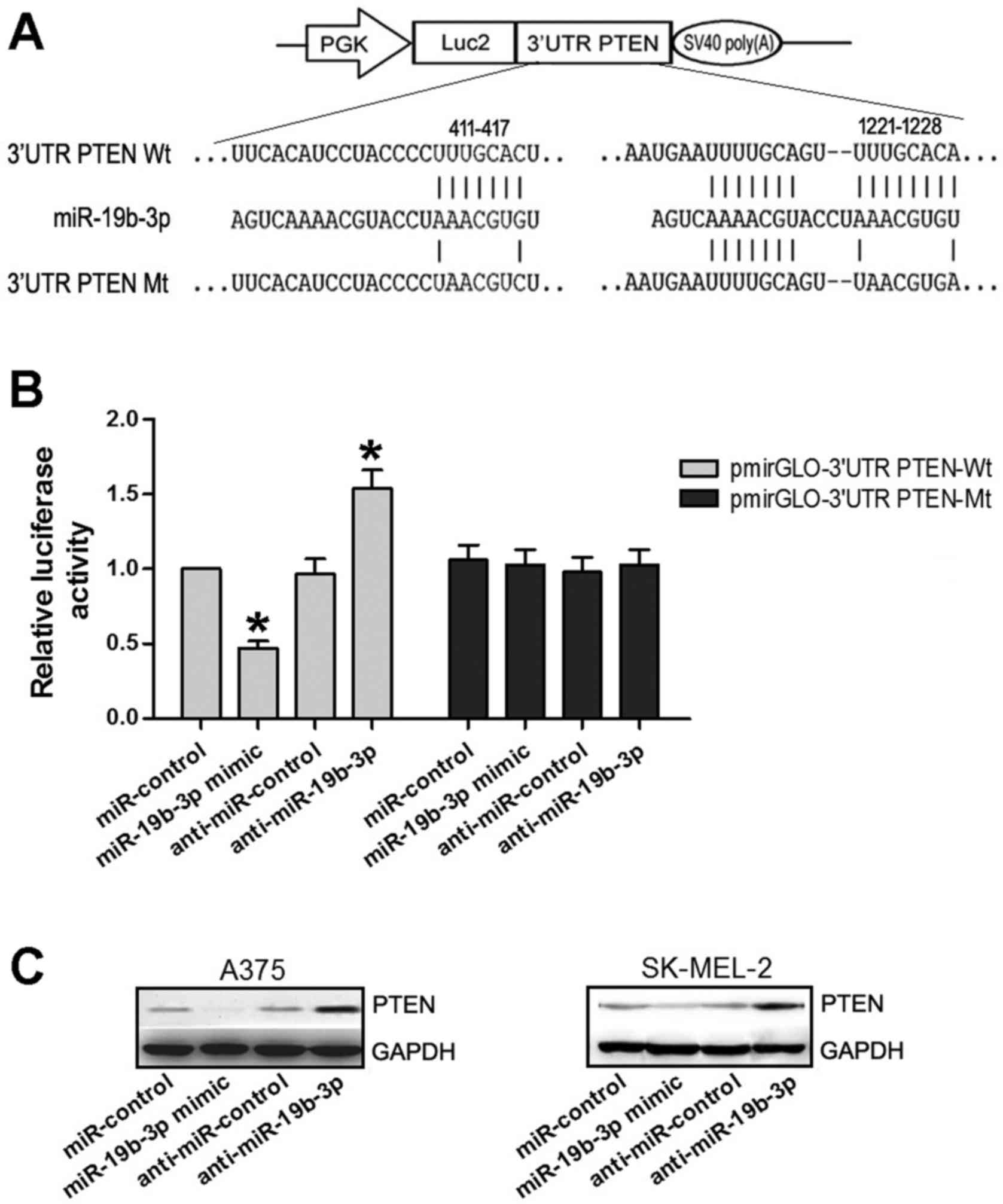 | Figure 3miR-19b-3p targeted PTEN in A375 and
SK-MEL-2 cells. (A) Putative binding sites between miR-19b-3p and
PTEN 3′UTR identified through bioinformatics analysis. (B)
Luciferase activity of Wt and Mt PTEN 3′UTR reporter constructs,
relative to miR-control and anti-control groups, in the presence of
miR-19b-3p mimic and anti-miR-19b-3p. *P<0.05 vs.
respective control. (C) Expression of PTEN as detected through
western blot assay in A375 and SK-MEL-2 cells transfected with
miR-control, miR-19b-3p mimic, anti-miR-19b-3p or anti-miR-control.
PGK, phospho-glycerate kinase promoter; Luc2, firefly luciferase;
UTR, untranslated region; PTEN, phosphatase and tensin homolog; Wt,
wild-type; miR, microRNA; Mt, mutant. |
Matrine shares the similar effects on
cell proliferation, invasiveness and apoptosis with miR-19b-3p in
A375 and SK-MEL-2 cells
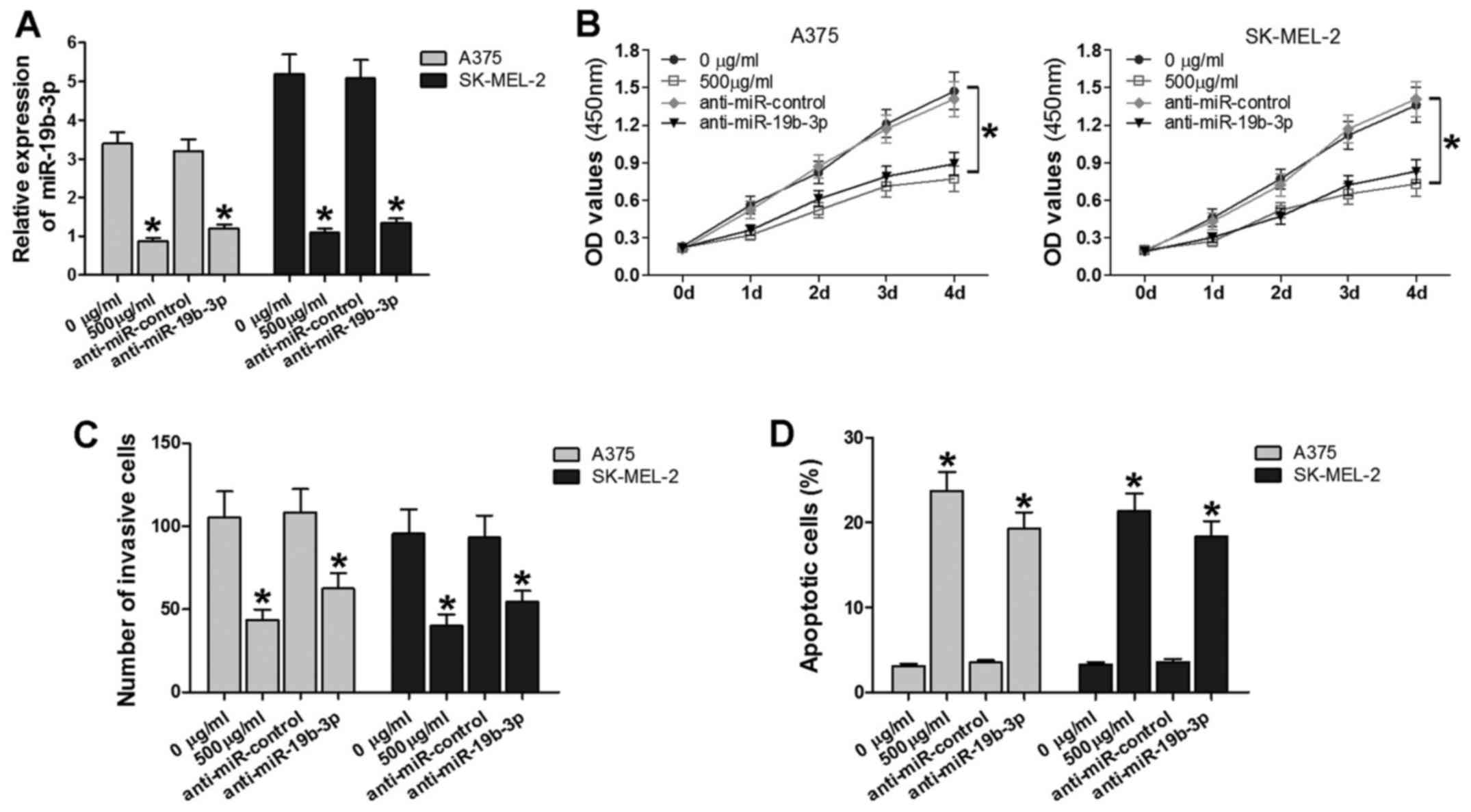
miR-19b-3p was verified to be downregulated by
matrine. To analyze the biological effects of matrine-mediated
regulation of miR-19b-3p in melanoma, the A375 and SK-MEL-2 cell
lines were treated with 0 or 500 µg/ml matrine, or
transfected with anti-miR-19b-3p or anti-miR-control. As
demonstrated in Fig. 4A, the
expression of miR-19b-3p was suppressed in the 500 µg/ml
matrine groups and anti-miR-19b-3p groups. Subsequently, CCK-8,
Transwell assay and flow cytometry assays were performed to assess
cell proliferation, invasiveness and apoptosis. Cell growth and
invasion were significantly inhibited, and the number of apoptotic
cells was increased in the 500 µg/ml matrine and
anti-miR-19b-3p groups compared with the control groups (Fig. 4B–D). These results indicated that
matrine and downregulated miR-19b-3p suppressed cell proliferation
and invasiveness, and promoted cell apoptosis. Thus, the two
approaches exert similar effects on melanoma cells.
Silencing of PTEN expression reverses the
effects of matrine and miR-19b-3p in A375 and SK-MEL-2 cells
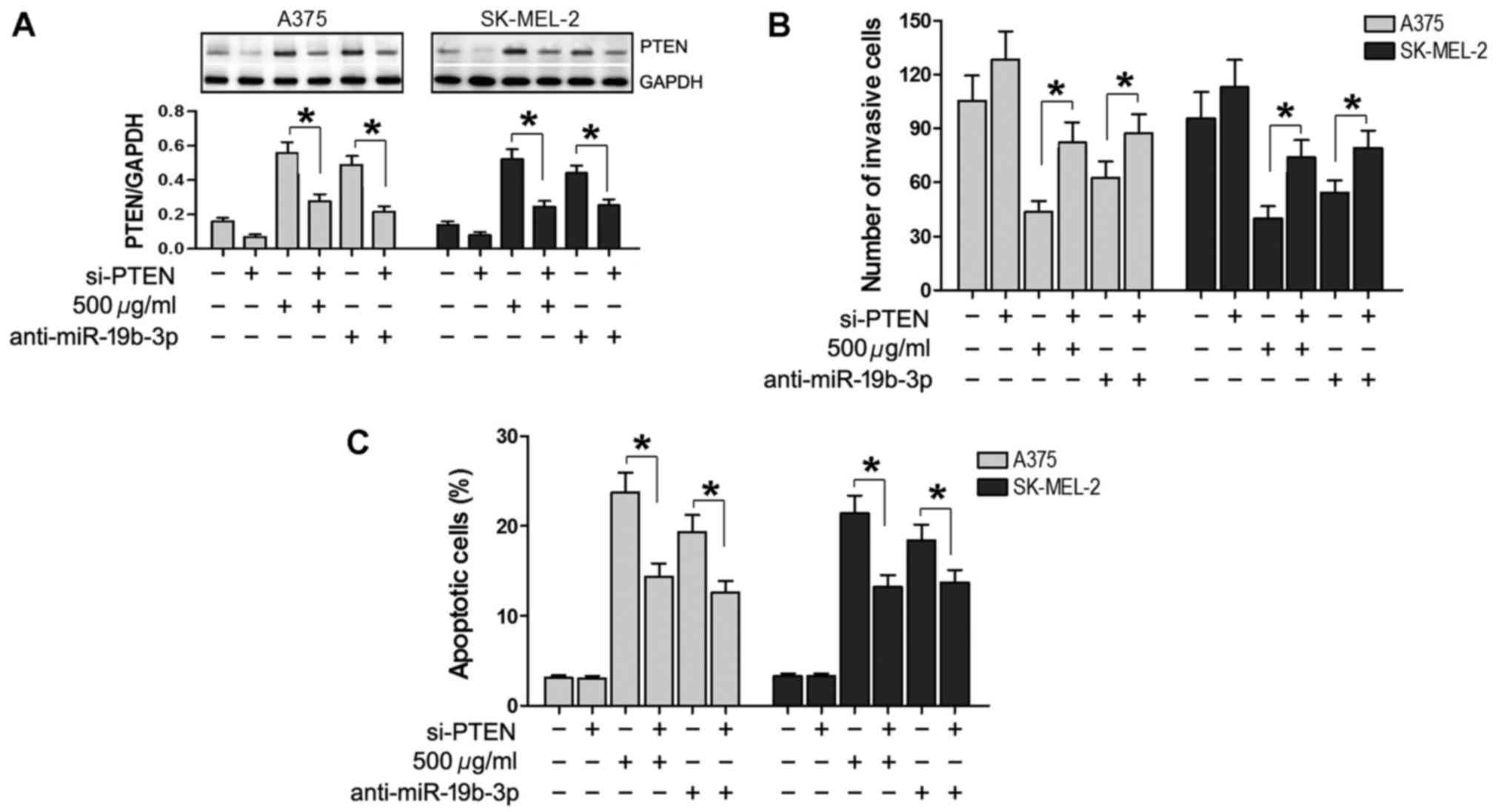
It was attempted to determine the advanced molecular
mechanisms underlying the effects of matrine and miR-19b-3p. In the
current study, si-PTEN was used to silence the expression of PTEN
in A375 and SK-MEL-2 cells. PTEN expression was then evaluated by
western blot analysis, which demonstrated that PTEN was markedly
increased following transfection with anti-miR-19b-3p or treatment
with 500 µg/ml matrine, and decreased by transfection with
si-PTEN. Additionally, PTEN was reduced in cells co-transfected
with si-PTEN and anti-miR-19b-3p, and in cells treated with matrine
and si-PTEN, compared with those in the anti-miR-19b-3p and matrine
treatment groups, respectively (P<0.05; Fig. 5A). Transwell assay results
demonstrated that compared with cells transfected with
anti-miR-19b-3p or treated with 500 µg/ml matrine alone, the
number of invasive cells was significantly increased in the cells
transfected with si-PTEN, co-transfected with si-PTEN and
anti-miR-19b-3p, or simultaneously treated with matrine and si-PTEN
(P<0.05; Fig. 5B). Flow
cytometry revealed that compared with cells transfected with
anti-miR-19b-3p or treated with 500 µg/ml matrine alone, the
number of apoptotic cells was significantly reduced in cells
transfected with si-PTEN, co-transfected with si-PTEN and
anti-miR-19b-3p, or simultaneously treated with matrine and si-PTEN
(P<0.05; Fig. 5C). In these
experiments, a scramble si-PTEN was used; when compared with the
blank group, the scramble si-PTEN group shared a similar effect.
Thus, only the blank group is shown as a negative control in
Fig. 5. These results further
suggested that silencing of PTEN expression reverses the effects of
matrine and miR-19b-3p downregulation in A375 and SK-MEL-2
cells.
Discussion
Due to its rapidly increasing incidence rate and
poor prognosis, melanoma is one of the most lethal skin diseases.
Several high-throughput studies have indicated that matrine is
associated with antitumor activity in melanoma. For example,
matrine has a significant inhibitory effect on the adhesion and
invasiveness of malignant human A375 melanoma cell line by
downregulating the expression of heparanase mRNA (30); and matrine inhibits the
invasiveness and metastasis of A375 cells in vitro (31). However, the antitumor potential and
underlying mechanisms of matrine remain largely unknown.
The mechanisms may be associated with the inhibition
of cellular proliferation, induction of apoptosis and autophagy,
arrest of cell cycle, inhibition of angiogenesis and downregulation
of target mRNA and protein expression. As reported, matrine induces
cell cycle arrest and apoptosis with recovery of the expression of
miR-126 in the A549 non-small cell lung cancer cell line (32). Matrine inhibited the invasion and
metastasis of lung cancer cells by elevating expression of
miR-133a, which further suppressed activation of epidermal growth
factor receptor/protein kinase B (Akt)/matrix metalloproteinase-9
pathway (33). Furthermore,
matrine can inhibit breast cancer growth via an miR-21/PTEN/Akt
pathway in MCF-7 cells (34).
These studies reinforce the notion that miRNAs can act as mediators
of the therapeutic efficacy of natural medicines.
The current study initially confirmed the effects of
matrine on cell proliferation, invasion and apoptosis in A375 and
SK-MEL-2 melanoma cell lines. The results of CCK-8 assay
demonstrated that the number of A375 and SK-MEL-2 cells was
significantly decreased following treatment with matrine. Cell
apoptosis or other factors all influence the number of living
cells. In the current study, in the different groups of A375 and
SK-MEL-2 cells treated with various concentrations of matrine for
48 h, live cells in the 250 µg/ml group accounted for ~75%
of the 0 µg/ml group, live cells in 500 µg/ml group
accounted for ~60% of the 0 µg/ml group. The results of
tran-swell assay demonstrated that in the different groups of A375
and SK-MEL-2 cells treated with various concentrations of matrine
for 48 h, invaded cells in 250 µg/ml group accounted for
~60% of the 0 µg/ml group, which was lower than the 75%
observed in the CCK-8 assay, and invaded cells in the 500
µg/ml group accounted for ~40% of the 0 µg/ml group,
which was lower than the 60% observed in the CCK-8 assay. The
results above verified that the decrease of cells in the invasion
assay was not caused solely by reduced cell viability, but also
caused by the reduced invasion capacity. Matrine has an inhibitory
effect on cell invasion to a certain degree in melanoma cells. It
is concluded that matrine inhibited the proliferation and
invasiveness of A375 and SK-MEL-2 cells, and induced cell apoptosis
with dose-dependence in vitro. The concentrations used in
the current study were 0, 250 and 500 µg/ml, which were
similar to the concentration used in an in vivo experiment
by Liou et al (35) and far
beyond the effectual dose in normal cells in vitro (29). Next, based on the fact that miR-19b
was found to be overexpressed through our previous miRNA microarray
(18), miR-19b was verified to be
higher in five types of melanoma cells compared with in NHEMs
(36,37). Moreover, when treated with matrine,
miR-19b expression was significantly downregulated in A375 and
SK-MEL-2 cells, revealing that matrine could inhibit miR-19b
expression in vitro. To explore the advanced molecular
mechanisms, bioinformatics analysis was performed, which indicated
the presence of two interactive binding regions between miR-19b-3p
and PTEN mRNA.
The tumor inhibitor gene PTEN is a 47-kDa protein
first identified as a candidate tumor suppressor gene in 1997
(38,39). Thus far, vast amounts of data
published indicate that PTEN has antitumor activity. Cordes et
al (40) reported that PTEN is
associated with an aggressive tumor phenotype and with unfa-vorable
outcome in early bladder cancer. Additionally, a high level of PTEN
expression has been associated with low-grade liver metastasis and
satisfactory patient survival in pancreatic cancer (41). PTEN was also reported to be
activated by matrine to induce growth inhibition and apoptosis in
V600EBRAF-harboring melanoma cells (29). In the current study, treatment with
matrine increased the PTEN protein expression in A375 and SK-MEL-2
cells in vitro. Based on the results of bioinformatics
analysis, a dual-luciferase reporter assay was performed to explore
the association between miR-19b-3p and PTEN. The results
demonstrated that PTEN was a target of miR-19b-3p. A subsequent
western blot assay demonstrated that miR-19b-3p regulated the
protein level of PTEN in A375 and SK-MEL-2 cells. Numerous studies
(42–48) have also reported that miR-19
(miR-19a or miR-19b) regulates the expression of PTEN, and as a
result modulates cancer biology, in processes including cancer cell
apoptosis, proliferation, invasion, metastasis and cell cycling
(Table I). The findings of the
current study demonstrated that miR-19b targets PTEN in melanoma
cells; matrine also decreased the expression of miR-19b-3p and
increased the expression of PTEN in A375 and SK-MEL-2 cells, while
the association between matrine, miR-19b and PTEN in melanoma
remained unclear. To address the biological functions of matrine
and miR-19b-3p, the effects of the two components on cell
proliferation, invasion and apop-tosis were compared. Matrine and
anit-miR-19b-3p induced similar effects in the melanoma cell lines.
Furthermore, when the expression of PTEN was silenced, the
inhibitory effects on proliferation and invasion and the promotion
of apoptosis by matrine or downregulated miR-19b-3p were
reversed.
 | Table IAssociation between PTEN gene and
miR-19 in different type of cancer. |
Table I
Association between PTEN gene and
miR-19 in different type of cancer.
| Author, year | Cancer type | miR-19
expression | PTEN
expression | Function | Ref. |
|---|
| Liu et al,
2017 | Wilms' tumor | Up | Down | Inhibition of
miR-19b suppresses the progression of Wilms' tumor by modulating
the PTEN/PI3K/Akt signaling pathway | (42) |
| Li et al,
2017 | Breast cancer | Up | Down | Enhanced PTEN
pseudogene 1 could inhibit breast cancer cell growth, metastasis
and tumorigenicity by inhibiting miR-19b and facilitating PTEN in
breast cancer | (43) |
| Chen et al,
2016 | Human
neuroblastoma | No description | No description | Antagomir of
miR-19b decreases cell viability and phospho-Akt expression and
increases PTEN expression in neuroblastoma cells | (44) |
| Li et al,
2015 | Lung cancer | No description | No description | PTEN is involved in
miR-19-induced epithelial-mesenchymal transition, migration and
invasion in lung cancer cells | (45) |
| Li et al,
2014 | Breast cancer | Up | Down | Curcumin modulates
miR-19/PTEN/Akt/p53 axis to exhibit its protective effects against
bisphenol A-associated breast cancer promotion | (46) |
| Tian et al,
2013 | Gliomas | Up | Down | miR-19a and miR-19b
may have an oncogenic role in gliomagenesis at least partially via
the negative regulation of PTEN | (47) |
| Jia et al,
2013 | Prostate
cancer | Up | Down | miR-19b could
promote prostate cancer cell proliferation by coregulating the
expression of PTEN, PI3K/Akt pathway and cyclin D1 in
vitro | (48) |
In summary, the findings of the current study
suggested that matrine suppresses cell proliferation, invasion and
induce cell apoptosis partially through miR-19b-3p targeting PTEN,
thus enriching the understanding of the molecular mechanisms of
matrine in reducing melanoma progression and metastasis. However,
the current study did not explore whether overexpression of
miR-19b-3p inhibited the effects of matrine, which requires future
investigation. Additionally, determining whether matrine has
potential for use in vivo or in a clinical setting will
require thorough investigation. Various approaches, including the
use of liposomes, nanoparticles, micelles and phospholipid
complexes, may improve the pharmacokinetic profile of matrine,
which may promote the future application of matrine in the
clinic.
Acknowledgments
Thanks are given to the People's Hospital of Jiaozuo
City (Jiaozuo, China) for providing the experimental platform.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YPW and LDZ designed the study and performed part of
the statistical analysis of data. YPW, XHW, GL and JFZ performed
the cell-based experiments including CCK-8, Transwell, flow
cytometry, caspase-3/7 activity detection and luciferase reporter
assay. YXY and JZ were responsible for consulting the literature,
involved in experimental design, bioinformatics analysis, writing
the manuscript and participating in revising it critically for the
comments. XLS and ZGL performed the RT-qPCR and western blot assay
and performed the statistical analysis. All authors have approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Reference
|
1
|
Flaherty KT, Robert C, Hersey P, Nathan P,
Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et
al METRIC Study Group: Improved survival with MEK inhibition in
BRAF-mutated melanoma. N Engl J Med. 367:107–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo J, Si L, Kong Y, Flaherty KT, Xu X,
Zhu Y, Corless CL, Li L, Li H, Sheng X, et al: Phase II,
open-label, single-arm trial of imatinib mesylate in patients with
metastatic melanoma harboring c-Kit mutation or amplification. J
Clin Oncol. 29:2904–2909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hauschild A, Grob JJ, Demidov LV, Jouary
T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr,
Kaempgen E, et al: Dabrafenib in BRAF-mutated metastatic melanoma:
A multicentre, open-label, phase 3 randomised controlled trial.
Lancet. 380:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lanoy E: Epidemiology, risk factor and
screening for melanoma and other skin cancers. Rev Prat. 64:31–36.
2014.In French. PubMed/NCBI
|
|
6
|
Haiducu ML, Hinek A, Astanehe A, Lee TK
and Kalia S: Extracutaneous melanoma epidemiology in British
Columbia. Melanoma Res. 24:377–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu Q, Fang Q, Feng B, Sun S, Du W, Amut E,
Xiao A and Chang C: Matrine-imprinted monolithic stationary phase
for extraction and purification of matrine from Sophorae
flavescentis Ait. J Chromatogr B Analyt Technol Biomed Life Sci.
879:894–900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo Z, Zhang L, Song C and Zhang X:
Molecularly imprinted solid-phase extraction of matrine from radix
Sophorae tonkinensis. Analyst (Lond). 136:3016–3022. 2011.
View Article : Google Scholar
|
|
9
|
Kucukboyaci N, Ozkan S, Adiguzel N and
Tosun F: Characterisation and antimicrobial activity of Sophora
alopecuroides L. var. alopecuroides alkaloid extracts. Turk J Biol.
35:379–385. 2011.
|
|
10
|
Zhang JP, Zhang M, Zhou JP, Liu FT, Zhou
B, Xie WF and Guo C: Antifibrotic effects of matrine on in vitro
and in vivo models of liver fibrosis in rats. Acta Pharmacol Sin.
22:183–186. 2001.PubMed/NCBI
|
|
11
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG, et al: Antiinflammatory
effects of matrine in LPS-induced acute lung injury in mice. Eur J
Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang C, Liu SP, Fang CH, He RS, Wang Z,
Zhu YQ and Jiang SW: Effects of matrine on the proliferation of
HT29 human colon cancer cells and its antitumor mechanism. Oncol
Lett. 6:699–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YQ, Li Y, Qin J, Wang Q, She YL, Luo
YL, He JX, Li JY and Xie XD: Matrine reduces proliferation of human
lung cancer cells by inducing apoptosis and changing miRNA
expression profiles. Asian Pac J Cancer Prev. 15:2169–2177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao H, Yang B, Hu R and Wang Y: Matrine
effectively inhibits the proliferation of breast cancer cells
through a mechanism related to the NF-κB signaling pathway. Oncol
Lett. 6:517–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang P, Wang Z, Chong T and Ji Z: Matrine
inhibits proliferation and induces apoptosis of the androgen
independent prostate cancer cell line PC-3. Mol Med Rep. 5:783–787.
2012.
|
|
16
|
Li Y, Zhang ZN, Zhao HM, Tong ZC, Yang J,
Wang H and Liang XJ: Matrine inhibits the invasive properties of
human osteosarcoma cells by downregulating the ERK-NF-κB pathway.
Anticancer Drugs. 25:1035–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Antitumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Xie S, Liu X, Wu H, Lin X, Gu J,
Wang H and Duan Y: Matrine alters microRNA expression profiles in
SGC-7901 human gastric cancer cells. Oncol Rep. 32:2118–2126. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao L, Zhao Y, He Y and Mao Y: miR-19b
promotes breast cancer metastasis through targeting MYLIP and its
related cell adhesion molecules. Oncotarget. 8:64330–64343.
2017.PubMed/NCBI
|
|
20
|
Jiang T, Ye L, Han Z, Liu Y, Yang Y, Peng
Z and Fan J: miR-19b-3p promotes colon cancer proliferation and
oxaliplatin-based chemoresistance by targeting SMAD4: Validation by
bioinformatics and experimental analyses. J Exp Clin Cancer Res.
36:1312017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohira T, Naohiro S, Nakayama Y, Osaki M,
Okada F, Oshimura M and Kugoh H: miR-19b regulates hTERT mRNA
expression through targeting PITX1 mRNA in melanoma cells. Sci Rep.
5:82012015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Chen Q, Deng G, Kuang S, Lian J,
Wang M and Zhu H: AMPK activation by GSK621 inhibits human melanoma
cells in vitro and in vivo. Biochem Biophys Res Commun.
480:515–521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilking MJ, Singh C, Nihal M, Zhong W and
Ahmad N: SIRT1 deacetylase is overexpressed in human melanoma and
its small molecule inhibition imparts anti-proliferative response
via p53 activation. Arch Biochem Biophys. 563:94–100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai JP, He XW, Jiang Y and Chen F:
Preparative separation and determination of matrine from the
Chinese medicinal plant Sophora flavescens Ait by molecularly
imprinted solid-phase extraction. Anal Bioanal Chem. 375:264–269.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu S: Development of anticancer drugs
mediated by apoptosis and autophagy. Nihon Rinsho. 73:1302–1307.
2015.PubMed/NCBI
|
|
26
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He L, Thomson JM, Hemann MT,
Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe
SW, Hannon GJ, et al: A microRNA polycistron as a potential human
oncogene. Nature. 435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ota A, Tagawa H, Karnan S, Tsuzuki S,
Karpas A, Kira S, Yoshida Y and Seto M: Identification and
characterization of a novel gene, C13orf25, as a target for
13q31-q32 amplification in malignant lymphoma. Cancer Res.
64:3087–3095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin H, Sun Y, Wang S and Cheng X: Matrine
activates PTEN to induce growth inhibition and apoptosis in
V600EBRAF harboring melanoma cells. Int J Mol Sci.
14:16040–16057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu XY, Fang H, Yang ZG, Teng LS, Ruan LM,
Fang DR and Jiang XL: Inhibition of invasiveness and expression of
heparanase-mRNA in human malignant melanoma cell line A375 by
matrine. Zhong Yao Cai. 29:253–256. 2006.PubMed/NCBI
|
|
31
|
Liu XY, Fang H, Yang ZG, Wang XY, Ruan LM,
Fang DR, Ding YG, Wang YN, Zhang Y, Jiang XL, et al: Matrine
inhibits invasiveness and metastasis of human malignant melanoma
cell line A375 in vitro. Int J Dermatol. 47:448–456. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An Q, Han C, Zhou Y, Li F, Li D, Zhang X,
Yu Z, Duan Z and Kan Q: Matrine induces cell cycle arrest and
apoptosis with recovery of the expression of miR-126 in the A549
non-small cell lung cancer cell line. Mol Med Rep. 14:4042–4048.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao H, Zhao X, Qu J, Zhang J and Cai H:
Matrine suppresses invasion and metastasis of NCI-H1299 cells by
enhancing microRNA-133a expression. Int J Clin Exp Med.
8:10714–10722. 2015.PubMed/NCBI
|
|
34
|
Li LQ, Li XL, Wang L, et al: Matrine
inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7
cells. Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liou CJ, Lai YR, Chen YL, Chang YH, Li ZY
and Huang WC: Matrine attenuates COX-2 and ICAM-1 expressions in
human lung epithelial cells and prevents acute lung injury in
LPS-induced mice. Mediators Inflamm. 2016:36304852016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhatt KV, Spofford LS, Aram G, McMullen M,
Pumiglia K and Aplin AE: Adhesion control of cyclin D1 and p27Kip1
levels is deregulated in melanoma cells through BRAF-MEK-ERK
signaling. Oncogene. 24:3459–3471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Satoh R, Hagihara K, Matsuura K, Manse Y,
Kita A, Kunoh T, Masuko T, Moriyama M, Moriyama H, Tanabe G, et al:
Identification of ACA-28, a 1′-acetoxychavicol acetate analogue
compound, as a novel modulator of ERK MAPK signaling, which
preferentially kills human melanoma cells. Genes Cells. 22:608–618.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cordes I, Kluth M, Zygis D, Rink M, Chun
F, Eichelberg C, Dahlem R, Fisch M, Höppner W, Wagner W, et al:
PTEN deletions are related to disease progression and unfavourable
prognosis in early bladder cancer. Histopathology. 63:670–677.
2013.PubMed/NCBI
|
|
41
|
Feng C, Yao R, Huang F, Liu X and Nie W:
High level of PTEN expression is associated with low-grade liver
metastasis and satisfactory patient survival in pancreatic cancer.
Arch Med Res. 42:584–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu GL, Yang HJ, Liu B and Liu T: Effects
of microRNA-19b on the proliferation, apoptosis, and migration of
Wilms' tumor cells via the PTEN/PI3K/AKT signaling pathway. J Cell
Biochem. 118:3424–3434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li RK, Gao J, Guo LH, Huang GQ and Luo WH:
PTENP1 acts as a ceRNA to regulate PTEN by sponging miR-19b and
explores the biological role of PTENP1 in breast cancer. Cancer
Gene Ther. 24:309–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Y, Tsai YH, Tseng BJ, Pan HY and
Tseng SH: Suppression of miR-19b enhanced the cytotoxic effects of
mTOR inhibitors in human neuroblastoma cells. J Pediatr Surg.
51:1818–1825. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li J, Yang S, Yan W, Yang J, Qin YJ, Lin
XL, Xie RY, Wang SC, Jin W, Gao F, et al: MicroRNA-19 triggers
epithelial-mesenchymal transition of lung cancer cells accompanied
by growth inhibition. Lab Invest. 95:1056–1070. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, Xie W, Xie C, Huang C, Zhu J, Liang
Z, Deng F, Zhu M, Zhu W, Wu R, et al: Curcumin modulates
miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7
breast cancer cell proliferation. Phytother Res. 28:1553–1560.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tian L, Fang YX, Xue JL and Chen JZ: Four
microRNAs promote prostate cell proliferation with regulation of
PTEN and its downstream signals in vitro. PLoS One. 8:e758852013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jia Z, Wang K, Zhang A, Wang G, Kang C,
Han L and Pu P: miR-19a and miR-19b overexpression in gliomas.
Pathol Oncol Res. 19:847–853. 2013. View Article : Google Scholar : PubMed/NCBI
|