|
1
|
Tew WP and Fleming GF: Treatment of
ovarian cancer in the older woman. Gynecol Oncol. 136:136–142.
2015. View Article : Google Scholar
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H, Su J, Xu Y, Kang J, Li H, Zhang L,
Yi H, Xiang X, Liu F and Sun L: p62/SQSTM1 involved in cisplatin
resistance in human ovarian cancer cells by clearing ubiquitinated
proteins. Eur J Cancer. 47:1585–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Xu Y, Su J, Yu H, Kang J, Li H, Li
X, Xie Q, Yu C, Sun L, et al: Autophagic flux promotes cisplatin
resistance in human ovarian carcinoma cells through ATP-mediated
lysosomal function. Int J Oncol. 47:1890–1900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie Q, Su J, Jiao B, Shen L, Ma L, Qu X,
Yu C, Jiang X, Xu Y and Sun L: ABT737 reverses cisplatin resistance
by regulating ER-mitochondria Ca2+ signal transduction
in human ovarian cancer cells. Int J Oncol. 49:2507–2519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lagadinou ED, Sach A, Callahan K, Rossi
RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer
KM, et al: BCL-2 inhibition targets oxidative phosphorylation and
selectively eradicates quiescent human leukemia stem cells. Cell
Stem Cell. 12:329–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weiler M, Bähr O, Hohlweg U, Naumann U,
Rieger J, Huang H, Tabatabai G, Krell HW, Ohgaki H, Weller M, et
al: BCL-xL: Time-dependent dissociation between modulation of
apoptosis and invasiveness in human malignant glioma cells. Cell
Death Differ. 13:1156–1169. 2006. View Article : Google Scholar
|
|
11
|
Bae IH, Yoon SH, Lee SB, Park JK, Ho JN
and Um HD: Signaling components involved in Bcl-w-induced migration
of gastric cancer cells. Cancer Lett. 277:22–28. 2009. View Article : Google Scholar
|
|
12
|
Kelekar A and Thompson CB: Bcl-2-family
proteins: The role of the BH3 domain in apoptosis. Trends Cell
Biol. 8:324–330. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manfredi G, Kwong JQ, Oca-Cossio JA,
Woischnik M, Gajewski CD, Martushova K, D'Aurelio M, Friedlich AL
and Moraes CT: BCL-2 improves oxidative phosphorylation and
modulates adenine nucleotide translocation in mitochondria of cells
harboring mutant mtDNA. J Biol Chem. 278:5639–5645. 2003.
View Article : Google Scholar
|
|
14
|
Dey R and Moraes CT: Lack of oxidative
phosphorylation and low mitochondrial membrane potential decrease
susceptibility to apoptosis and do not modulate the protective
effect of Bcl-x(L) in osteosarcoma cells. J Biol Chem.
275:7087–7094. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warburg O: Iron, the oxygen-carrier of
respiration-ferment. Science. 61:575–582. 1925. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandel NS: Mitochondria and cancer.
Cancer Metab. 2:82014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matassa DS, Amoroso MR, Lu H, Avolio R,
Arzeni D, Procaccini C, Faicchia D, Maddalena F, Simeon V,
Agliarulo I, et al: Oxidative metabolism drives
inflammation-induced platinum resistance in human ovarian cancer.
Cell Death Differ. 23:1542–1554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ippolito L, Marini A, Cavallini L, Morandi
A, Pietrovito L, Pintus G, Giannoni E, Schrader T, Puhr M, Chiarugi
P, et al: Metabolic shift toward oxidative phosphorylation in
docetaxel resistant prostate cancer cells. Oncotarget.
7:61890–61904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Denise C, Paoli P, Calvani M, Taddei ML,
Giannoni E, Kopetz S, Kazmi SM, Pia MM, Pettazzoni P, Sacco E, et
al: 5-fluorouracil resistant colon cancer cells are addicted to
OXPHOS to survive and enhance stem-like traits. Oncotarget.
6:41706–41721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montopoli M, Bellanda M, Lonardoni F,
Ragazzi E, Dorigo P, Froldi G, Mammi S and Caparrotta L: 'Metabolic
reprogramming' in ovarian cancer cells resistant to cisplatin. Curr
Cancer Drug Targets. 11:226–235. 2011. View Article : Google Scholar
|
|
22
|
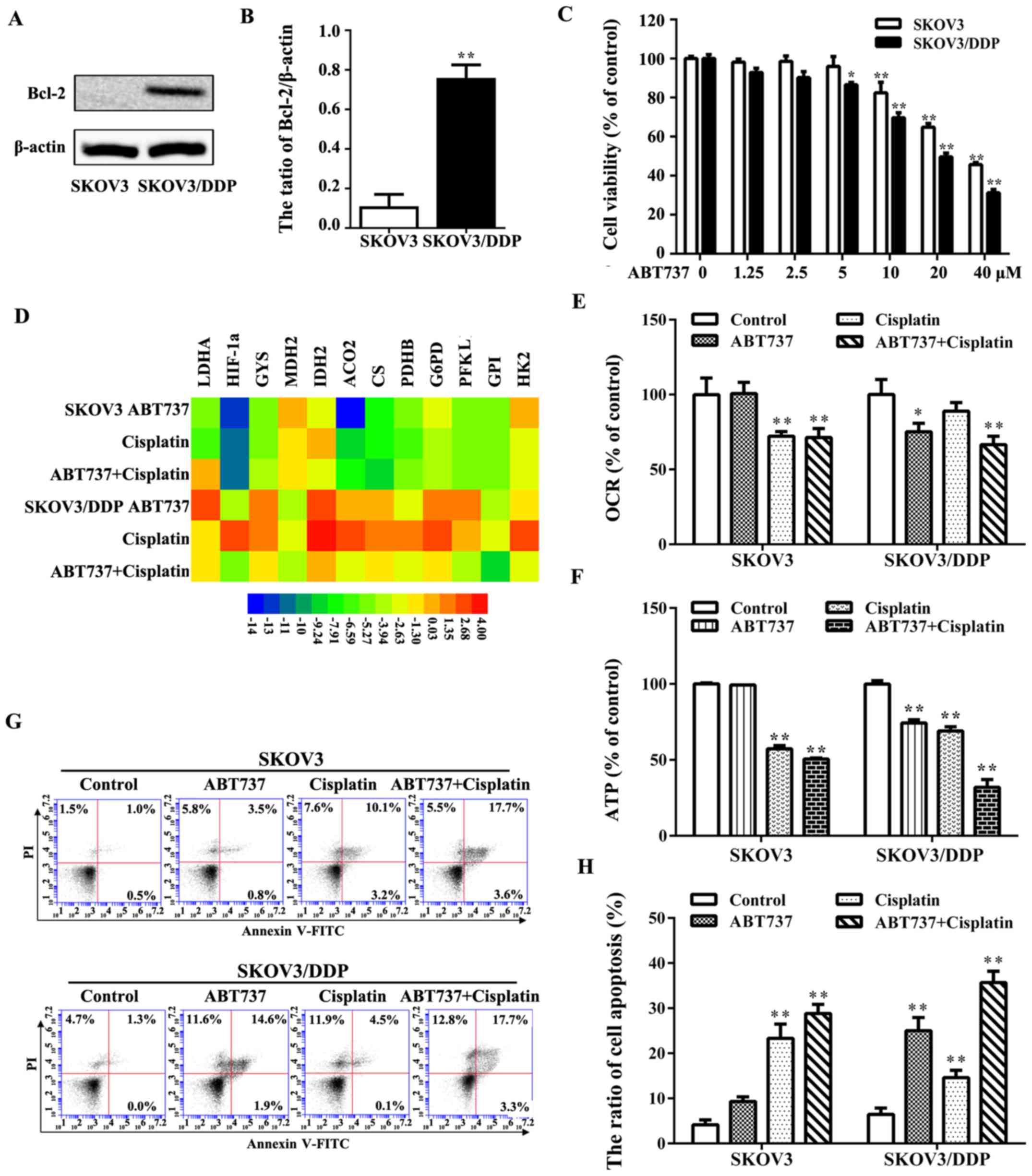
Fan Z, Yu H, Cui N, Kong X, Liu X, Chang
Y, Wu Y, Sun L and Wang G: ABT737 enhances cholangiocarcinoma
sensitivity to cisplatin through regulation of mitochondrial
dynamics. Exp Cell Res. 335:68–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Bol V, Bol A, Bouzin C, Labar D, Lee JA,
Janssens G, Porporato PE, Sonveaux P, Feron O and Grégoire V:
Reprogramming of tumor metabolism by targeting mitochondria
improves tumor response to irradiation. Acta Oncol. 54:266–274.
2015. View Article : Google Scholar
|
|
25
|
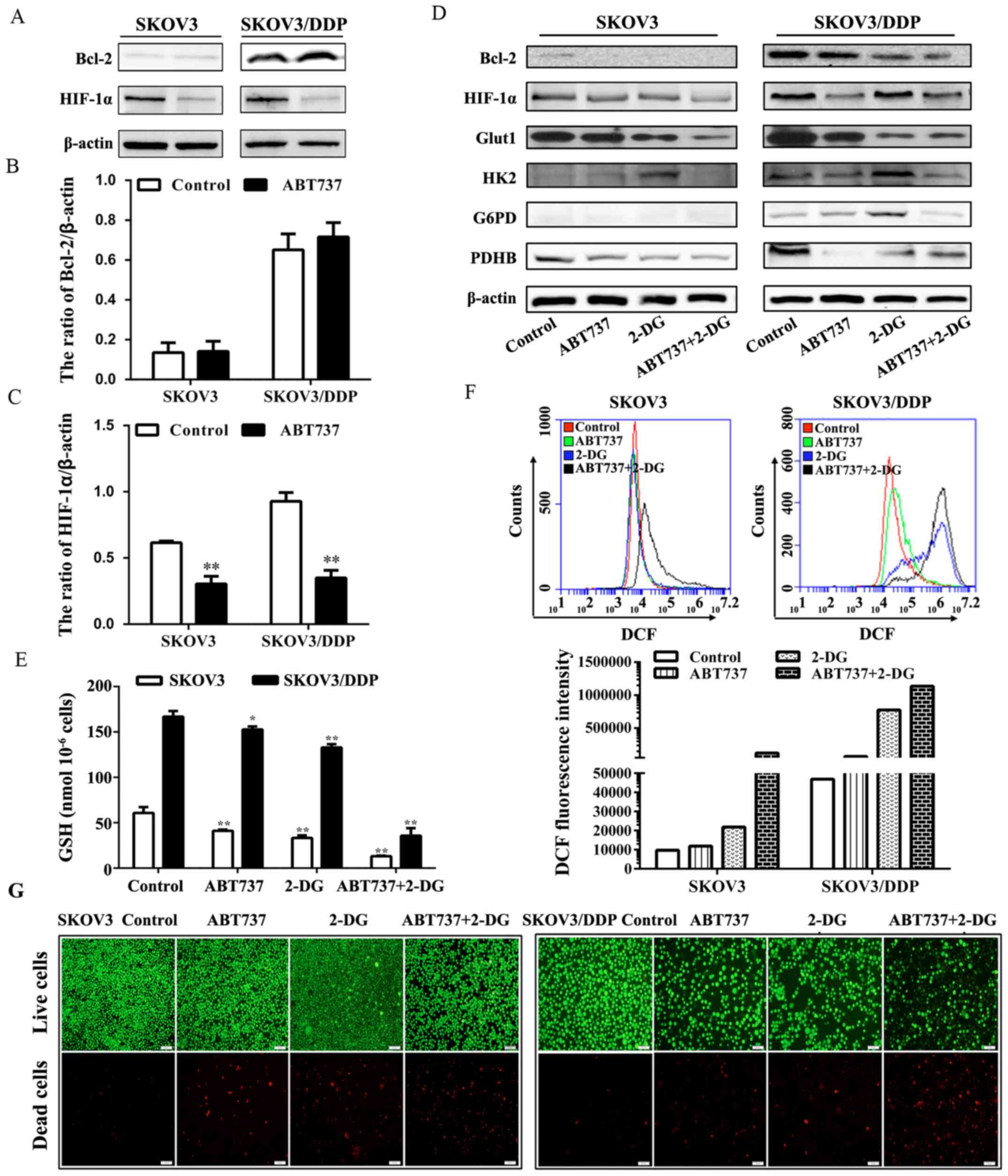
Xiang XY, Kang JS, Yang XC, Su J, Wu Y,
Yan XY, Xue YN, Xu Y, Liu YH, Yu CY, et al: SIRT3 participates in
glucose metabolism interruption and apoptosis induced by BH3
mimetic S1 in ovarian cancer cells. Int J Oncol. 49:773–784. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Floreani M, Petrone M, Debetto P and
Palatini P: A comparison between different methods for the
determination of reduced and oxidized glutathione in mammalian
tissues. Free Radic Res. 26:449–455. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deberardinis RJ, Sayed N, Ditsworth D and
Thompson CB: Brick by brick: Metabolism and tumor cell growth. Curr
Opin Genet Dev. 18:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caneba CA, Yang L, Baddour J, Curtis R,
Win J, Hartig S, Marini J and Nagrath D: Nitric oxide is a positive
regulator of the Warburg effect in ovarian cancer cells. Cell Death
Dis. 5:e13022014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
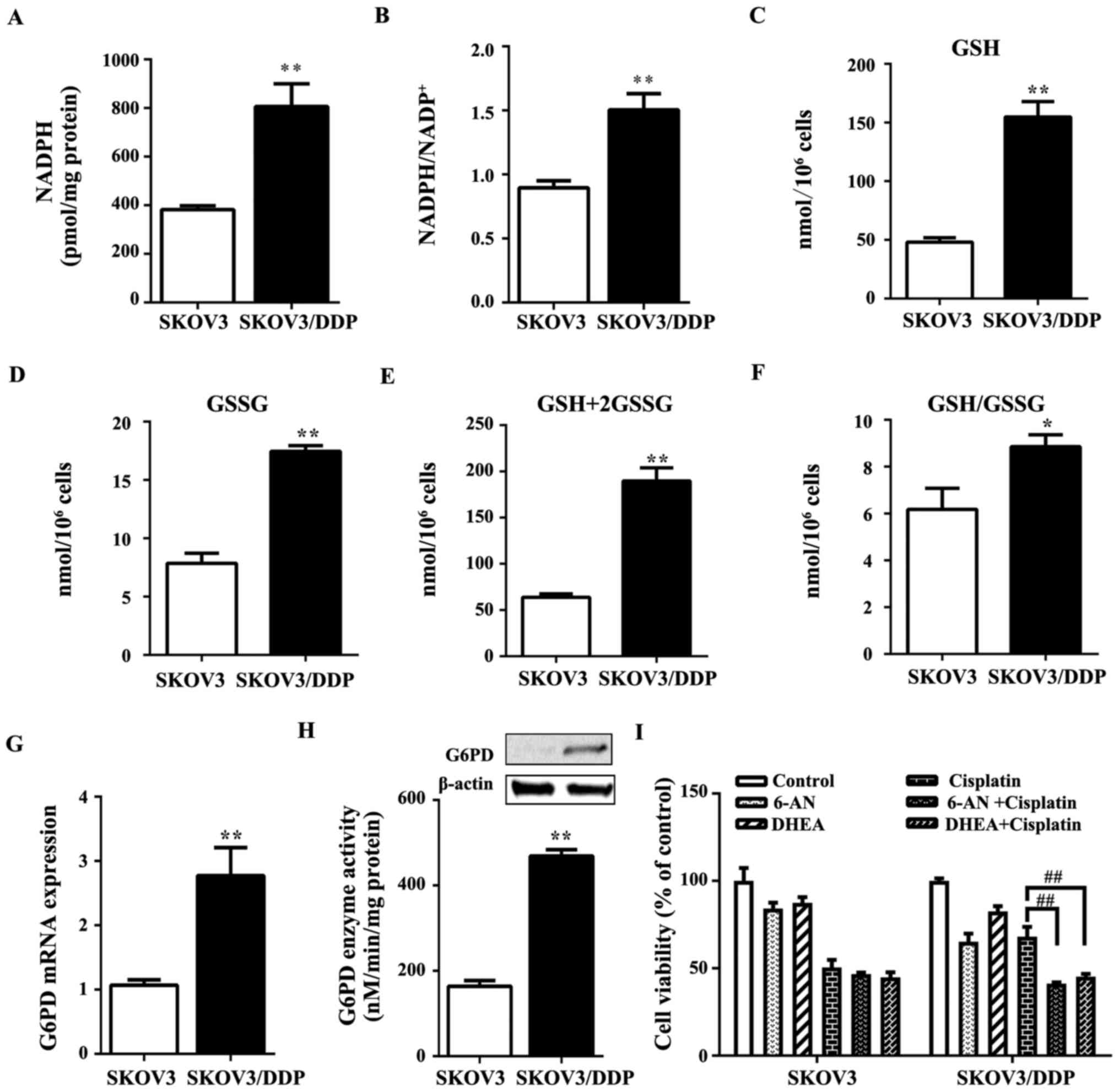
Stanton RC: Glucose-6-phosphate
dehydrogenase, NADPH, and cell survival. IUBMB Life. 64:362–369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borst P, Evers R, Kool M and Wijnholds J:
A family of drug transporters: The multidrug resistance-associated
proteins. J Natl Cancer Inst. 92:1295–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Köhler E, Barrach H and Neubert D:
Inhibition of NADP dependent oxidoreductases by the
6-aminonicotinamide analogue of NADP. FEBS Lett. 6:225–228. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raineri R and Levy HR: On the specificity
of steroid interaction with mammary glucose 6-phosphate
dehydrogenase. Biochemistry. 9:2233–2243. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gross A: BCL-2 family proteins as
regulators of mitochondria metabolism. Biochim Biophys Acta.
1857.1243–1246. 2016.
|
|
35
|
Caro P, Kishan AU, Norberg E, Stanley IA,
Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, et
al: Metabolic signatures uncover distinct targets in molecular
subsets of diffuse large B cell lymphoma. Cancer Cell. 22:547–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weinberg SE and Chandel NS: Targeting
mitochondria metabolism for cancer therapy. Nat Chem Biol. 11:9–15.
2015. View Article : Google Scholar :
|
|
37
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berridge MV, Herst PM and Tan AS:
Metabolic flexibility and cell hierarchy in metastatic cancer.
Mitochondrion. 10:584–588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jose C, Hébert-Chatelain E, Bellance N,
Larendra A, Su M, Nouette-Gaulain K and Rossignol R: AICAR inhibits
cancer cell growth and triggers cell-type distinct effects on
OXPHOS biogenesis, oxidative stress and Akt activation. Biochim
Biophys Acta. 1807.707–718. 2011.
|
|
40
|
Roesch A, Vultur A, Bogeski I, Wang H,
Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA,
Philipp SE, et al: Overcoming intrinsic multidrug resistance in
melanoma by blocking the mitochondrial respiratory chain of
slow-cycling JARID1B(high) cells. Cancer Cell. 23:811–825. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vander Heiden MG, Locasale JW, Swanson KD,
Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G,
Rabinowitz JD, Asara JM, et al: Evidence for an alternative
glycolytic pathway in rapidly proliferating cells. Science.
329:1492–1499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahn CS and Metallo CM: Mitochondria as
biosynthetic factories for cancer proliferation. Cancer Metab.
3:12015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vander Heiden MG: Targeting cancer
metabolism: A therapeutic window opens. Nat Rev Drug Discov.
10:671–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee YJ, Galoforo SS, Berns CM, Tong WP,
Kim HR and Corry PM: Glucose deprivation-induced cytotoxicity in
drug resistant human breast carcinoma MCF-7/ADR cells: Role of
c-myc and bcl-2 in apoptotic cell death. J Cell Sci. 110:681–686.
1997.PubMed/NCBI
|
|
45
|
Catanzaro D, Gaude E, Orso G, Giordano C,
Guzzo G, Rasola A, Ragazzi E, Caparrotta L, Frezza C and Montopoli
M: Inhibition of glucose-6-phosphate dehydrogenase sensitizes
cisplatin-resistant cells to death. Oncotarget. 6:30102–30114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Z, Fukushima H, Gao D, Inuzuka H, Wan
L, Lau AW, Liu P and Wei W: The two faces of FBW7 in cancer drug
resistance. BioEssays. 33:851–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sengupta N, Rose ST and Morgan JA:
Metabolic flux analysis of CHO cell metabolism in the late
non-growth phase. Biotechnol Bioeng. 108:82–92. 2011. View Article : Google Scholar
|
|
49
|
Polimeni M, Voena C, Kopecka J, Riganti C,
Pescarmona G, Bosia A and Ghigo D: Modulation of doxorubicin
resistance by the glucose-6-phosphate dehydrogenase activity.
Biochem J. 439:141–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang J, Yuan W and Chen Z, Wu S, Chen J,
Ge J, Hou F and Chen Z: Overexpression of G6PD is associated with
poor clinical outcome in gastric cancer. Tumour Biol. 33:95–101.
2012. View Article : Google Scholar
|
|
51
|
Clément MV, Hirpara JL and Pervaiz S:
Decrease in intracellular superoxide sensitizes
Bcl-2-overexpressing tumor cells to receptor and drug-induced
apoptosis independent of the mitochondria. Cell Death Differ.
10:1273–1285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen ZX and Pervaiz S: Bcl-2 induces
pro-oxidant state by engaging mitochondrial respiration in tumor
cells. Cell Death Differ. 14:1617–1627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen ZX and Pervaiz S: Involvement of
cytochrome c oxidase subunits Va and Vb in the regulation of cancer
cell metabolism by Bcl-2. Cell Death Differ. 17:408–420. 2010.
View Article : Google Scholar
|
|
54
|
Chen L, Willis SN, Wei A, Smith BJ,
Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM and Huang DC:
Differential targeting of prosurvival Bcl-2 proteins by their
BH3-only ligands allows complementary apoptotic function. Mol Cell.
17:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dai Y, Jin S, Li X and Wang D: The
involvement of Bcl-2 family proteins in AKT-regulated cell survival
in cisplatin resistant epithelial ovarian cancer. Oncotarget.
8:1354–1368. 2017.
|
|
56
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar :
|
|
57
|
Wang GL and Semenza GL: General
involvement of hypoxia-inducible factor 1 in transcriptional
response to hypoxia. Proc Natl Acad Sci USA. 90:4304–4308. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
59
|
Ai Z, Lu Y, Qiu S and Fan Z: Overcoming
cisplatin resistance of ovarian cancer cells by targeting
HIF-1-regulated cancer metabolism. Cancer Lett. 373:36–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|