Introduction
Small-cell lung cancer (SCLC) constitutes ~20% of
all lung cancer cases. It is the most aggressive form and a rapidly
metastasizing malignancy, with a high propensity to metastasize to
bone. The metastasis is formed by disseminated tumor cells in
distant organs, and is the primary cause of cancer-associated
mortality in the majority of patients with cancer. Bone is one of
the most common sites of metastasis for several types of tumor,
including breast, prostate and lung cancer, and is accompanied by
severe complications (1). Tumor
metastasis is regulated by a complex molecular signaling network,
which may be a major cause of poor prognosis in lung cancer. The
interaction of tumor cells with the local microenvironment at the
metastatic site serves a critical function in the establishment of
metastases.
Bone morphogenetic proteins (BMPs) are signaling
molecules that belong to the transforming growth factor β (TGFβ)
superfamily and are pluripotent regulators of embryonic development
and organogenesis, and tissue remodeling and repair (2,3). As
their name implies, BMPs were first identified by their ability to
induce ectopic bone in vivo (4,5). To
date, >20 BMP family members have been identified in humans. Of
these BMPs, BMP7 is a high-profile molecule owing to its
pluripotent ability to regulate cellular differentiation,
proliferation and apoptosis (6).
As BMP-7 is a broad-spectrum growth factor, there is increasing
interest in investigating its function in various malignancies,
particularly bone metastasis. For example, BMP7 was identified to
inhibit bone metastasis formation and growth in animal models of
breast and prostate cancer (7,8).
TGFβ family members, including BMPs, exhibit
structural similarity and a cysteine knot (9). BMP-induced gene responses are
transduced by surface serine/threonine kinase receptors and Smad
family transcription factors (10). The membrane receptors contain three
type I receptors [BMP receptor (BMPR)IA, BMPRIB and activin A
receptor type (ACVR)I and three type II receptors (BMPRII, ACVR2A,
AVCR2B) that specifically bind to BMP ligands (11). Upon binding, BMPs activate these
membrane receptors, which in turn phosphorylate the cytosolic Smad
proteins. Through a versatile system of Smad co-regulators,
different responses of BMPs are initiated (12). In addition, BMPs also utilize
Smad-independent pathways, including extracellular-signal-regulated
kinase, c-Jun N-terminal kinase (JNK), and p38 mitogen-activated
protein kinase pathways to transmit signals (13,14).
The cross-talk among these important cellular signaling pathways
contributes to the highly intricate and multifunctional effects of
BMPs in cancer. Signaling of BMPs are intracellularly regulated by
the inhibitory Smads Smad6 and Smad7, extracellularly by the
expression of a pseudoreceptor (BMP and activin membrane-bound
inhibitor homolog) (15), and by
secreted BMP antagonists, including noggin (16) and gremlin (17). Owing to the complexity of the
signaling networks associated with BMPs, research into BMPs by
demonstrating their stimulation may result in diverse, even
contradictory, phenotypes depending on the ligand and the type of
cancer investigated. The research field is currently controversial
and the impact of BMP7 on SCLC as well as its downstream signaling
pathway requires elucidation, which may in turn provide new
insights as a therapeutic target for the clinical treatment of
SCLC.
Materials and methods
Cell culture
The human SCLC cell lines SBC-3 and SBC-5 were gifts
from Professor Saburo Sone and Professor Seiji Yano (Tokushima
University, Tokushima, Japan). Cells were incubated at 37°C with 5%
CO2 in RPMI-1640 medium supplemented with 10%
heated-inactivated fetal bovine serum (FBS) (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell proliferation assay
SBC-3 and SBC-5 cells were plated in 96-well plates
at a concentration of 3,000 cells/well and cultured in RPMI-1640
medium containing recombinant human (rh)BMP7 (EMD Millipore,
Billerica, MA, USA) at sequential concentrations (0, 50, 100, 150
and 200 ng/ml). At the indicated time-points, an MTT assay was
performed to determine the cell proliferation rate (24, 48 and 72
h). Briefly, 20 µl MTT solution (5 mg/ml; Sigma; Merck KGaA,
Darmstadt, Germany) was added to each well. After 3 h of incubation
at 37°C, the MTT solution was removed and 150 µl DMSO was
added to the wells to dissolve the blue formazan crystals. Finally,
the optical density values were determined at a wavelength of 490
nm using a Model 680 microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
To investigate further the effect of rhBMP7 on cell
proliferation, SBC-3 and SBC-5 cells were seeded into 96-well
plates and incubated for 6 days (the day of plating was designated
day 0). Each cell line was divided into three groups: Group A cells
were incubated in RPMI-1640 medium with 150 ng/ml rhBMP7 between
days 1 and 6 (+/+/+); for group B cells, rhBMP7 was supplied only
on days 3 and 4 (−/+/−); and group C cells (control) were
maintained in the RPMI-1640 medium without rhBMP7 (−/−/−). MTT
assays were performed every 24 h. Each test was performed three
times for accuracy.
Migration and invasion assays
Transwell inserts (8.0 µm pore size) were
coated with 70 µl Matrigel (1:8 dilution) (both from Corning
Incorporated, Corning, NY, USA). SBC-3 or SBC-5 cells at a density
of 2×104 were resuspended in 200 µl serum-free
RPMI-1640 medium and were then transferred to the upper chamber. A
500 µl volume of standard cell culture medium with or
without rhBMP7 was added to the lower chamber. The cells were
allowed to pass through the inserts for 24 h at 37°C. The Matrigel
and non-invading cells were removed using cotton swabs, whereas
cells that had migrated through the Matrigel were fixed with 95%
ethyl alcohol and stained with 0.5% crystal violet, and images were
captured under a light microscope at ×200 magnification. The mean
number of migrated cells on one insert was determined in five
high-magnification fields of view selected randomly. Each assay was
performed three times.
For the migration assay, the inserts were not coated
with Matrigel. The other processes were similar to the invasion
assay. Finally, the fields of view for migrated cells were selected
randomly for analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SBC-3 and SBC-5 cells
with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse-transcribed into cDNA using a
PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's protocol. mRNA expression levels of
BMPRIs were determined using qPCR in a Bio-Rad iCycler IQ™ 5
instrument (Bio-Rad Laboratories, Inc.) with SYBR Master mix
(Takara Bio, Inc.) and β-actin was used as a reference. The PCR
procedure was as follows: Pre-denaturation at 95°C for 30 sec; 40
cycles of dena-turation at 95°C for 5 sec and annealing at 60°C for
30 sec. The data were analyzed using the comparative Cq
(2−ΔΔCq) method (18).
The primers used were as follows: BMPRIA forward, 5′-AGA
TGACCAGGGAGAAACCAC-3′ and reverse, 5′-CAACATTCT ATTGTCCGGCGTA-3′;
BMPRIB forward, 5′-CTTTTGCGA AGTGCAGGAAAAT-3′ and reverse,
5′-TGTTGACTGAGT CTTCTGGACAA-3′; ACVRI forward, 5′-GTGAAGGTCTCT
CCTGCGGTA-3′ and reverse, 5′-GCCATCGTTGATGCTCAG TGA-3′; β-actin
forward, 5′-GATCATTGCTCCTCCTGAGC-3′ and reverse,
5′-CACCTTCACCGTTCCAGTTT-3′.
Protein extraction and western blot
analysis
Total protein was extracted using
radioimmunoprecipitation assay buffer, and the membrane and
cytosolic proteins were separated using a Membrane and Cytosol
Protein Extraction kit (Beyotime Institute of Biotechnology,
Haimen, China). Protein concentrations were determined using a
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of protein (30 µg) were
separated by SDS-PAGE (10% gels) and transferred onto
polyvinylidene difluoride membranes. Following blocking with 5%
non-fat dry milk in Tris-buffered saline containing 0.05% Tween-20
for 1 h, membranes were incubated overnight at 4°C with specific
primary antibodies: Anti-BMPRIA and anti-BMPRIB (cat. nos. ABD51
and ABD50, respectively; 1:10,000; EMD Millipore), anti-ACVRI (cat.
no. 4398; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA), anti-BMP7 (cat. no. ab56023; 1:500), anti-Smad2 (cat. no.
ab40855; 1:1,000), anti-Smad4 (cat. no. ab40759; 1:1,000) and
anti-p21 (cat. no. ab109520; 1:1,000) (all from Abcam, Cambridge,
UK), and anti-β-actin (cat. no. A5441; 1:10,000; Sigma; Merck
KGaA). The membranes were incubated with horseradish peroxidase
(HRP)-labeled goat anti-mouse/rabbit secondary antibody (1:2,500;
OriGene Technologies, Inc., Beijing, China) for 1 h at room
temperature. Finally, the relative content of the target proteins
was determined using a chemiluminescence detection system (Gel Doc
XR System) and analyzed by Image Lab software (version 2.0) (both
from Bio-Rad Laboratories, Inc.).
Flow cytometric analysis
Cell cycle and apoptotic analyses were performed
after 48 h of incubation with rhBMP7 in SBC-3 and SBC-5 cells.
Briefly, to determine the cell cycle distribution, 1×105
cells were harvested and washed with PBS. Subsequently, 400
µl Cell Cycle Rapid Detection Solution (Dakewe Biotech Co.,
Ltd., Shenzhen, China) was added and the cellular DNA content was
detected by flow cytometry at an excitation wavelength of 488 nm.
The cell cycle distribution was analyzed using CellQuest (version
5.1) and ModFit software (version 3.0) (both from BD Biosciences,
Franklin Lakes, NJ, USA).
Cell apoptosis was detected using an Annexin
V-fluorescein isothiocyanate (FITC)-propidium iodide (PI) kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Briefly,
1×105 cells were collected, washed and resuspended in
200 µl 1X binding buffer. Subsequently, 10 µl Annexin
V-FITC and 5 µl PI were added and incubated for 15 min at
room temperature in the dark. Subsequently, 300 µl binding
buffer was added to each tube prior to analysis using a FACSCalibur
system (BD Biosciences).
In vivo tumor formation assay
All animal studies were performed in accordance with
the protocol approved by the Laboratory Animal Care of The Fourth
Military Medical University (Xi'an, China). Ten female non-obese
diabetic (NOD)/severe combined immunodeficient (SCID) mice
(weighing between 15 and 17 g) aged between 4 and 5 weeks
(Huafukang Bioscience Co., Inc., Beijing, China) were used for the
tumorigenic ability assay and divided into two groups (each group
consisted of 5 mice). The mice were bred in a special pathogen-free
grade animal facility of the Fourth Military Medical University.
The mice were housed in a 12-h light/12-h dark cycle environment,
with ad libitum access to food and water. SBC-3 cells and
SBC-5 cells in the exponential phase were collected, washed,
counted and resuspended in PBS. In total, 2×106 cells
were injected into the mice via the tail vein. Mice were examined
daily for general health and the progression of masses for 6 weeks.
At the experimental endpoint, mice were sacrificed by
anesthetization (200 mg/kg intraperitoneal pentobarbital sodium;
Sigma; Merck KGaA) and bone metastases were examined by X-ray
(model MX-20; Faxitron Bioptics, LLC, Tucson, AZ, USA). Masses in
the bone were collected, fixed in 10% neutral-buffered formalin,
embedded in paraffin, sectioned at 4 µm, stained with
hematoxylin and eosin (H&E) and then visualized
microscopically.
Immunohistochemistry
Paraffin-embedded sections (4 µm) of tibial
bone were dewaxed with xylene, rehydrated in a descending alcohol
series and then immersed in 3% H2O2 solution
for 10 min to inhibit endogenous peroxidase activity. For antigen
retrieval, slides were boiled in citrate buffer (pH 7.0) for 10 min
in a microwave. Following blocking with 1% bovine serum albumin,
the sections were incubated with polyclonal anti-BMP7 antibody
(cat. no. ab27569; 1:50; Abcam) at 4°C overnight. Following
incubation with HRP-conjugated secondary antibody for 1 h at room
temperature, slides were developed with 3,3′-diaminobenzidine for
40 sec and counter-stained with hematoxylin for 1 min. The primary
antibody was replaced by PBS in negative controls. Two pathologists
who were blinded to the clinical and histopathological outcomes
evaluated the results of the staining independently.
Statistical analysis
Results are presented as the mean - standard
abbreviation. For in vitro experiments, Student's t-test or
one-way analysis of variance was used to determine the differences
in BMP7 treatment on cell biological behaviors. P<0.05 was
considered to indicate a statistically significant difference.
Statistical tests were performed using SPSS software (version
13.0.0; SPSS, Inc., Chicago, IL, USA).
Results
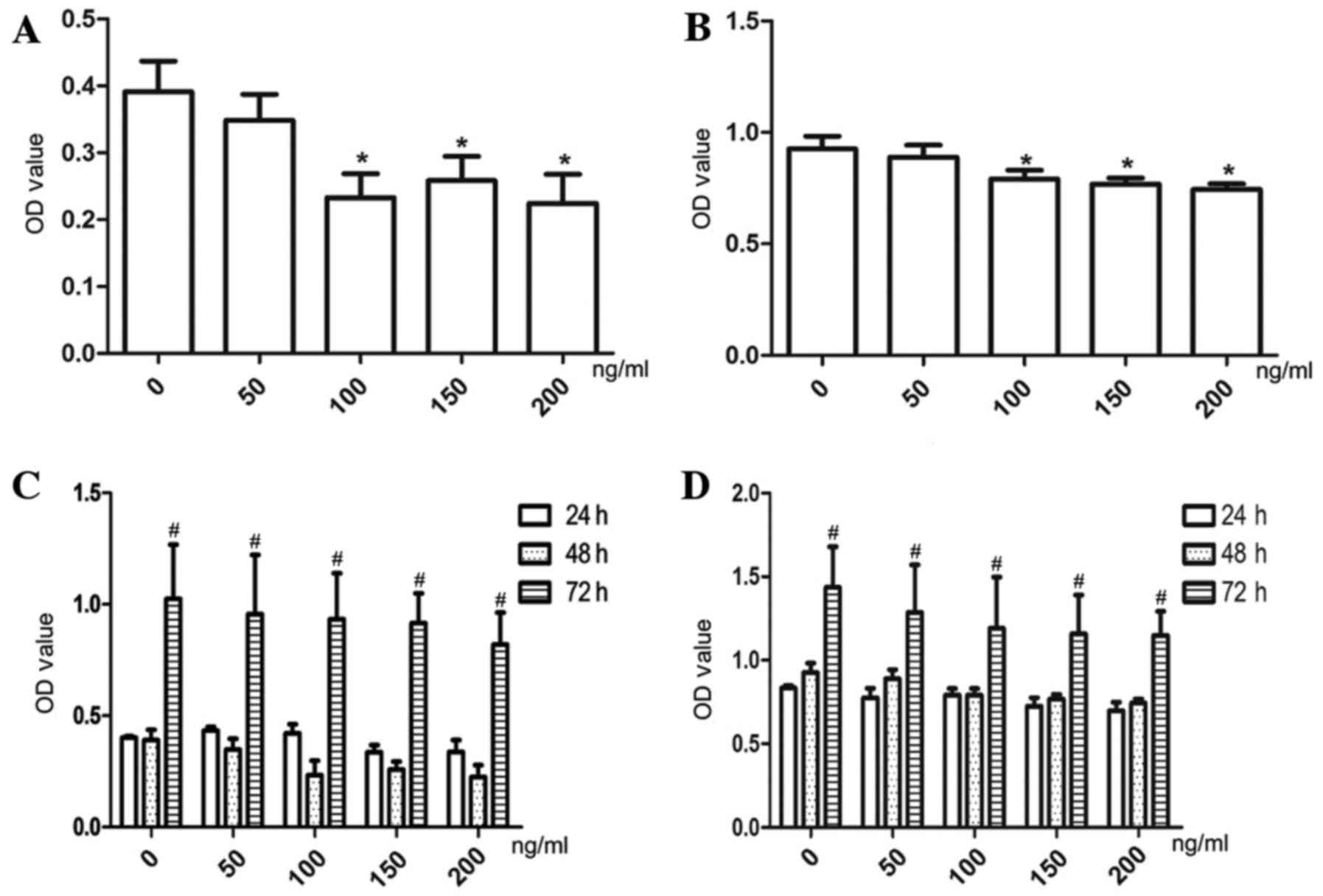
rhBMP7 inhibits the proliferation of SCLC
cells
SBC-3 and SBC-5 cells were treated with various
concentrations of rhBMP7 (0–200 ng/ml) for 24, 48 and 72 h. As
presented in Fig. 1, rhBMP7
inhibited cell proliferation in a dose- and time-dependent manner.
Specifically, rhBMP7 inhibited cell proliferation at a
concentration of ≥150 ng/ml after 24 h (P<0.05), and after 48 h,
100 ng/ml rhBMP7 began to exhibit a clear inhibitory effect
(P<0.01). At 72 h, however, the cells rapidly proliferated at
all concentrations of rhBMP7. Among the three high concentrations
tested (100, 150 and 200 ng/ml), no significant differences were
observed in the inhibition of cell proliferation. On the basis of
these results, a dose of 150 ng/ml was selected for further
analyses.
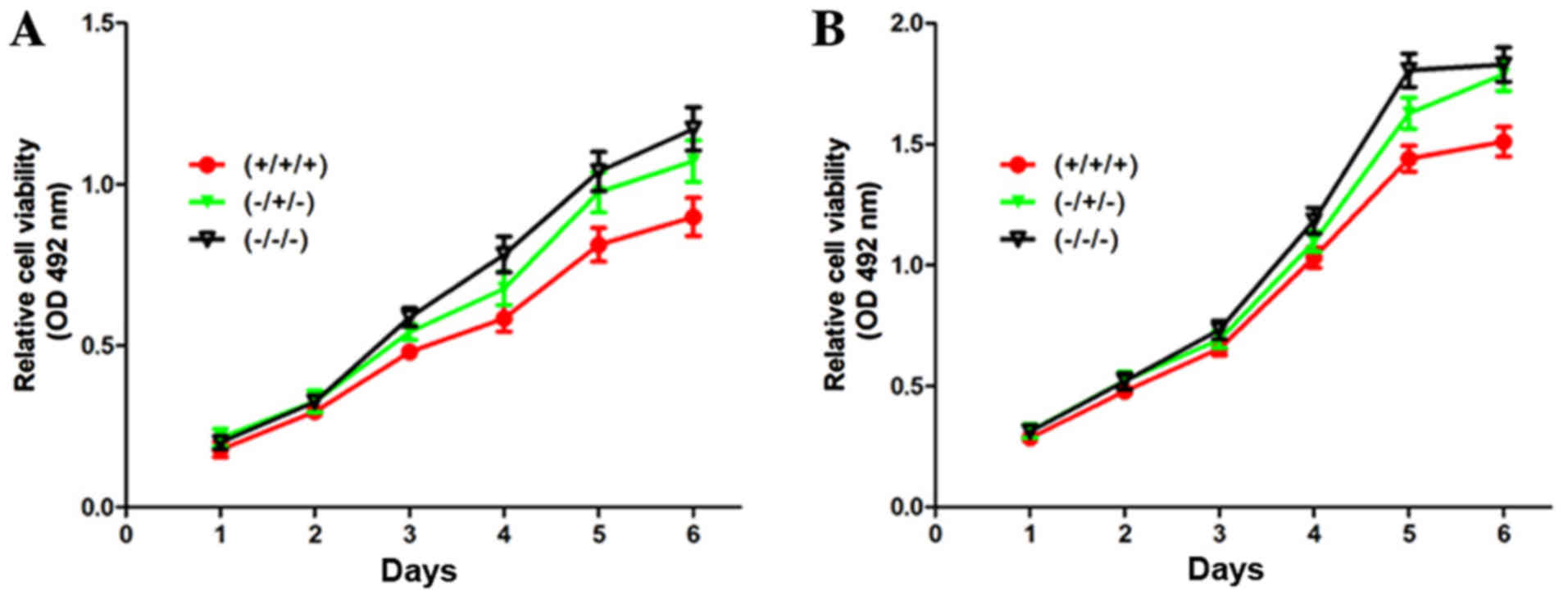
rhBMP7 suppresses the proliferation of
SCLC cells in a reversible manner in vitro
The effect of rhBMP7 on cell proliferation was
investigated further. As presented in Fig. 2, it was identified that rhBMP7
treatment (+/+/+) significantly suppressed the proliferation of
SBC-3 and SBC-5 cells compared with control cells (−/−/−), which
were cultured in the absence of rhBMP7 throughout the period.
Notably, withdrawal of rhBMP7 (−/+/−) rescued the cell
proliferation, suggesting that rhBMP7-induced inhibition was
reversible.
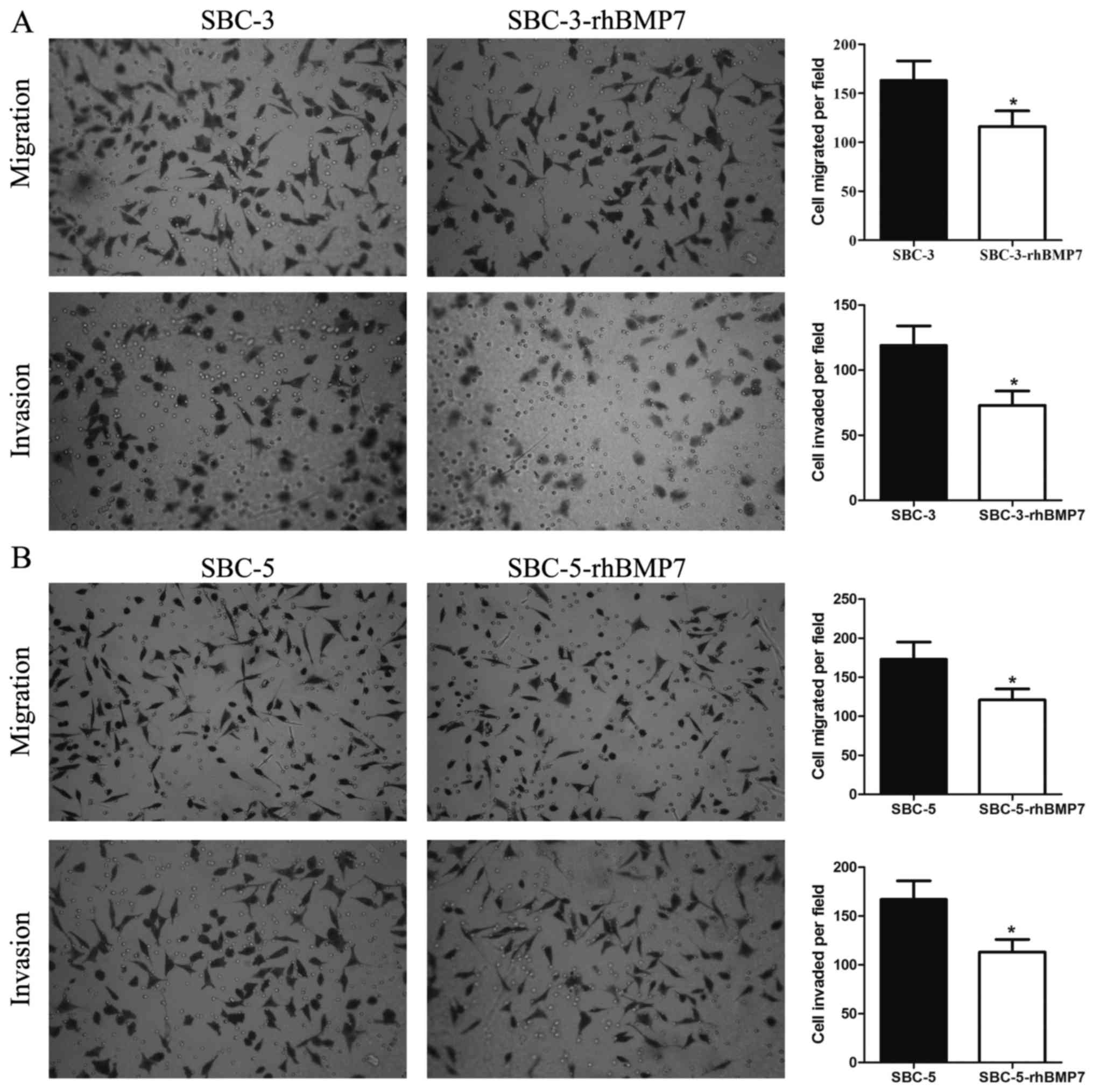
rhBMP7 inhibits the migratory and
invasive potential of SCLC cells
Next, the function of rhBMP7 in cell migration and
invasion of SCLC cells was investigated using in vitro
Transwell assays. The Transwell assays revealed that the number of
migratory SBC-3 cells was decreased from 163-20 without rhBMP7 to
116-16 with rhBMP7, and the number of invasive SBC-3 cells was
decreased from 119-15 to 73-11 (both P<0.05; Fig. 3A). For SBC-5 cells, rhBMP7
decreased the number of migratory cells from 173-22 to 121-14, and
the number of invasive cells from 167-19 to 113-13 (both P<0.05;
Fig. 3B). These results indicated
that rhBMP7 was able to inhibit the migratory and invasive
potential of SBC-3 and SBC-5 cells.
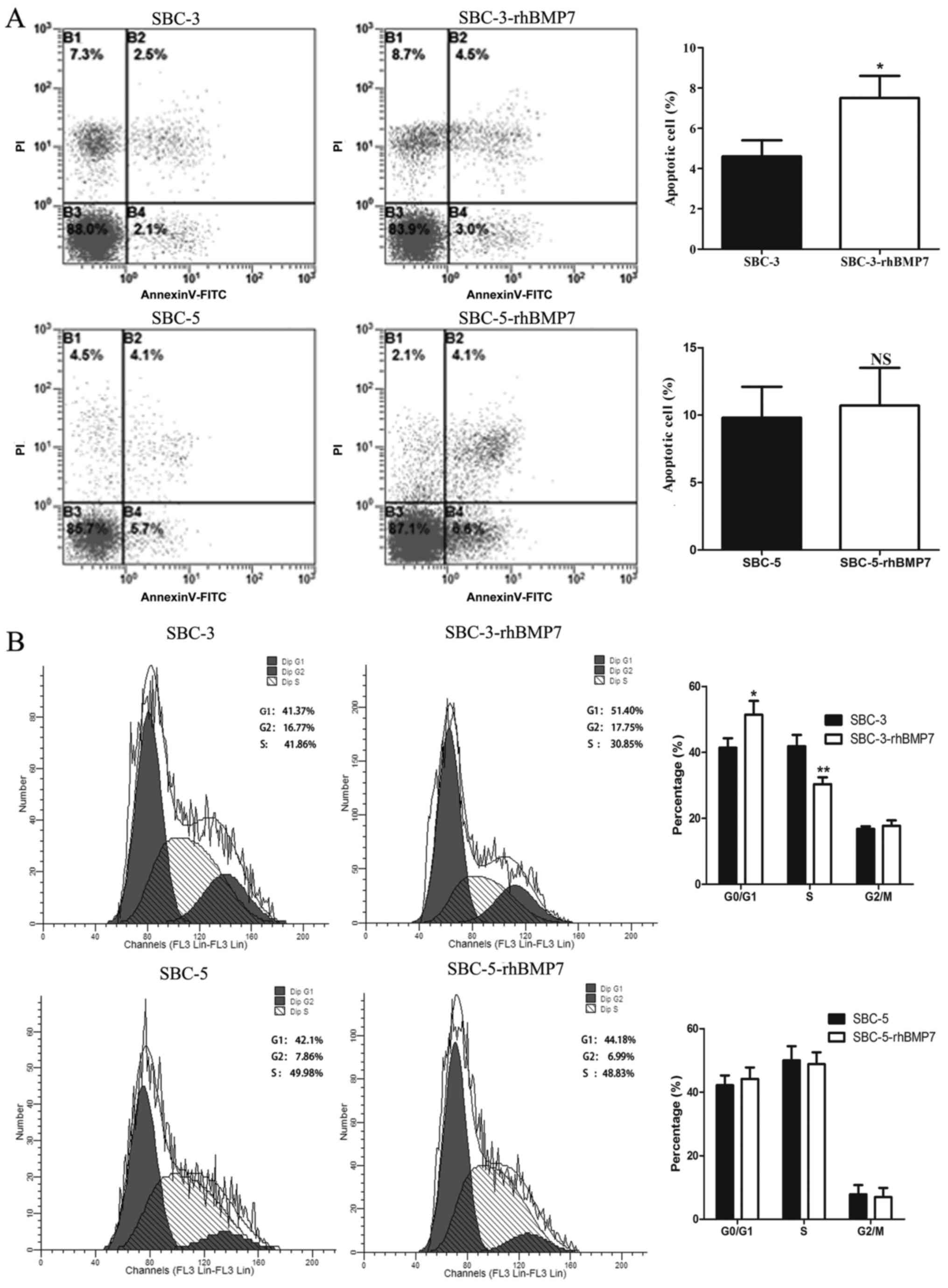
rhBMP7 induces G1 phase arrest
in SCLC cells
To investigate whether the inhibition of
proliferation reflected attenuation of cell apoptosis and cell
cycle, SBC-3 and SBC-5 cells treated with 150 ng/ml rhBMP7 for 48 h
were subjected to flow cytometric analysis. As presented in
Fig. 4A, rhBMP7 significantly
increased the apoptosis of SBC-3 cells (4.6-0.8 vs. 7.5-1.1%;
P<0.05), but did not influence the apoptosis of SBC-5 cells
(9.8-2.3 vs. 10.7-2.8%; P>0.05). There may be distinct
mechanisms of rhBMP7 regulation of the proliferation between SBC-3
and SBC-5 cells.
With regard to the influence on cell cycle, rhBMP7
clearly decreased the proportion of cells in S phase in SBC-3 cells
(from 41.86-3.4 to 30.35-2.1%) and increased the cells in
G1 phase (from 41.37-2.9 to 51.40-4.2%). However, in
SBC-5 cells, this effect was not as evident as it was in SBC-3
cells. The proportion of cells in S phase was decreased from
49.98-4.5 to 48.83-3.7%, whereas the proportion of cells in
G1 phase was increased from 42.16-3.1 to 44.18-3.6%,
suggesting that rhBMP7 induced G1 arrest in SBC-3 cells
(Fig. 4B).
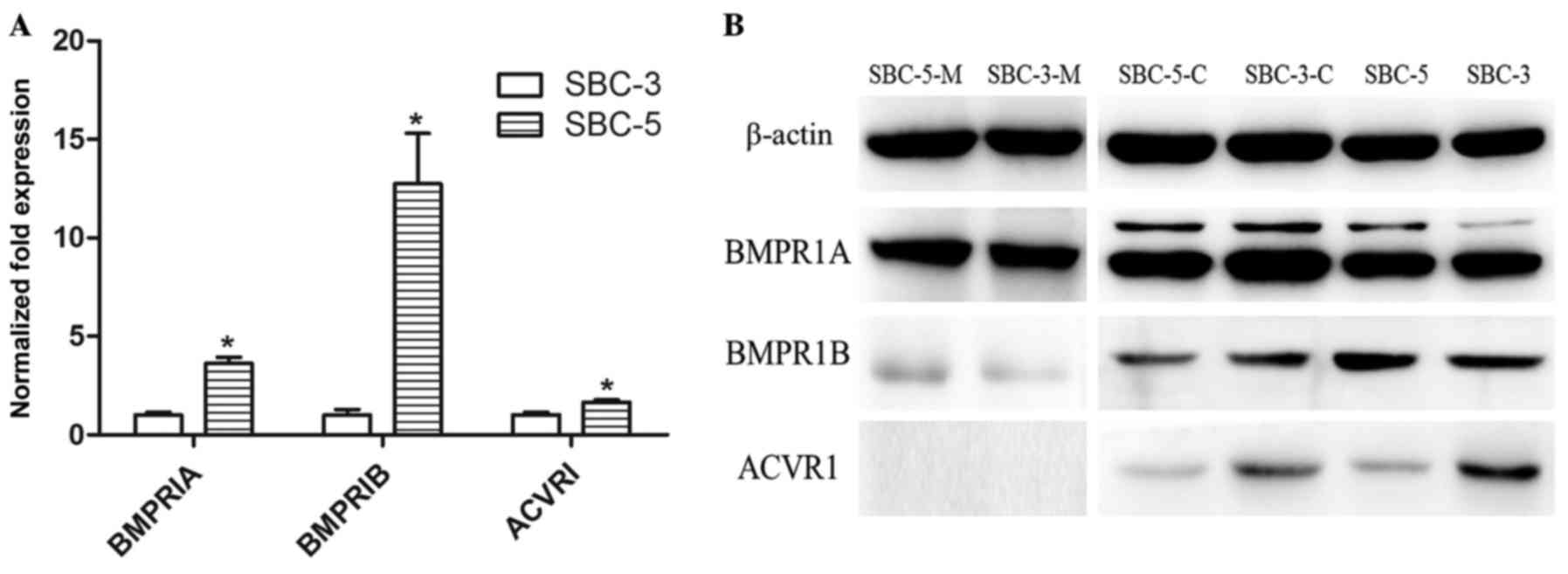
Expression of BMPRIs in SCLC cells
The results of the in vitro experiments
suggested that rhBMP7 inhibited cell proliferation reversibly,
which may serve an important function in tumorigenesis and
formation of metastasis. Therefore, the expression of BMPRIs was
investigated in SBC-3 and SBC-5 cells. Expression of the three type
I receptor subtypes (BMPRIA, BMPRIB and ACVRI) was determined using
RT-qPCR (Fig. 5A) and western blot
analysis (Fig. 5B). The levels of
three type I receptor subtypes (BMPRIs) were higher in SBC-5 cells
compared with in SBC-3 cells at the mRNA level. With regard to
proteins, there were certain notable results. In order to identify
the functional receptors, membrane proteins were extracted
separately. The expression levels of BMPRIA and BMPRIB in total
proteins and membrane proteins were consistent with the results of
qPCR. However, cytoplasmic BMPRIA and BMPRIB were higher in SBC-3
cells. Furthermore, expression of ACVRI was higher in SBC-3 cells
in total and cytoplasmic proteins, although it was only weak. It
was not possible to detect the expression of membrane ACVRI. It may
be hypothesized that distinct expression patterns of BMPRIs in
SBC-3 and SBC-5 cells determined the functions that BMP7 imposed on
them.
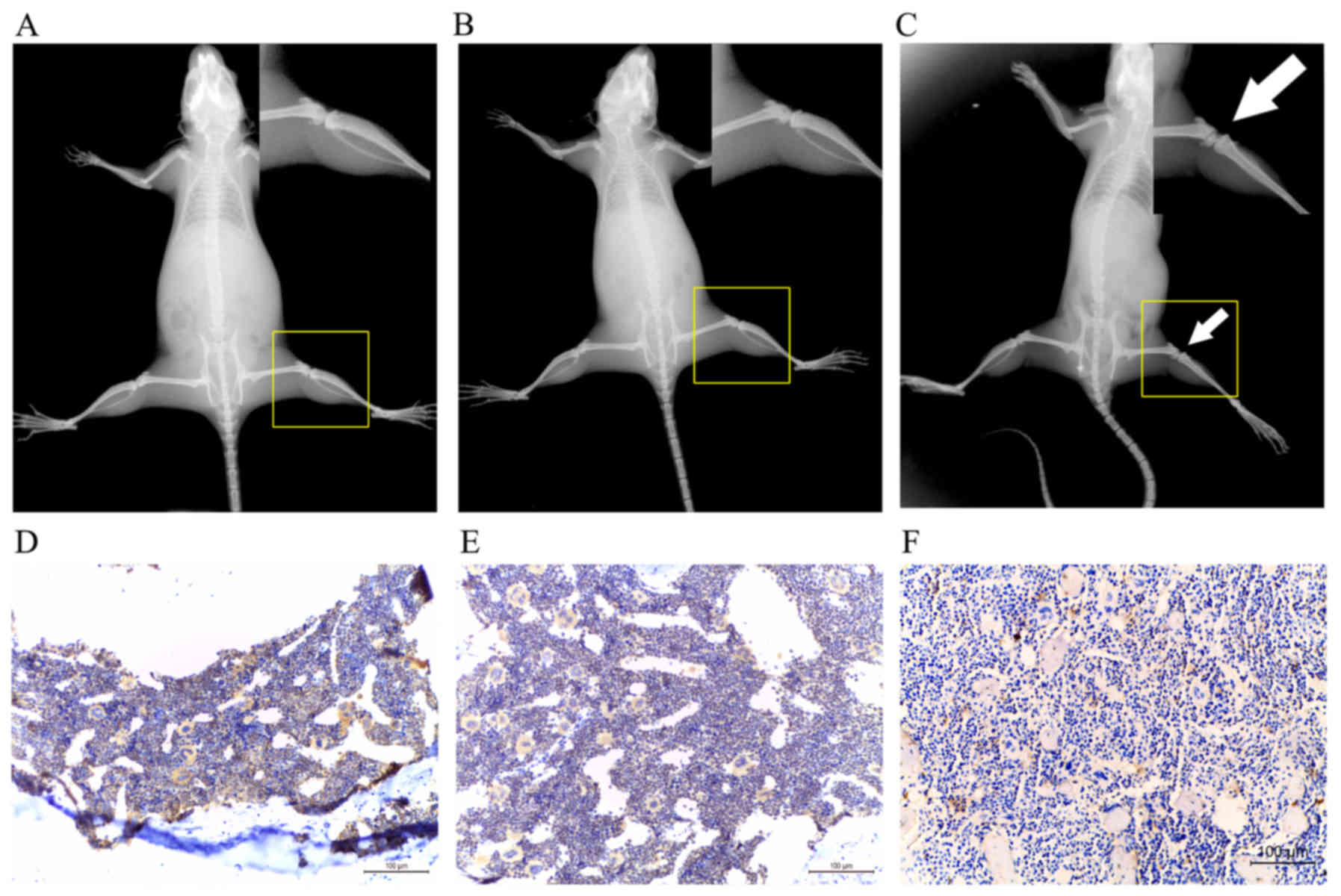
BMP7 is significantly decreased in bone
metastasis
The results of the present study supported the
hypothesis that rhBMP7 served a function in tumor progression by
suppressing the proliferation of SCLCs in vitro. To
determine whether BMP7 was involved in bone metastasis in
vivo, an animal model of bone metastasis was developed.
Finally, bone metastasis was induced only in mice injected with
SBC-5 cells (Fig. 6A–C). The
expression of BMP7 was detected in normal bone, bone metastatic
lesions of mice injected with SBC-5 cells and bone of mice injected
with SBC-3 cells. The results revealed that BMP7 was markedly
decreased in bone metastases (Fig.
6D–F), suggesting that BMP7 may attenuate the survival of
metastatic cancer cells in bone.
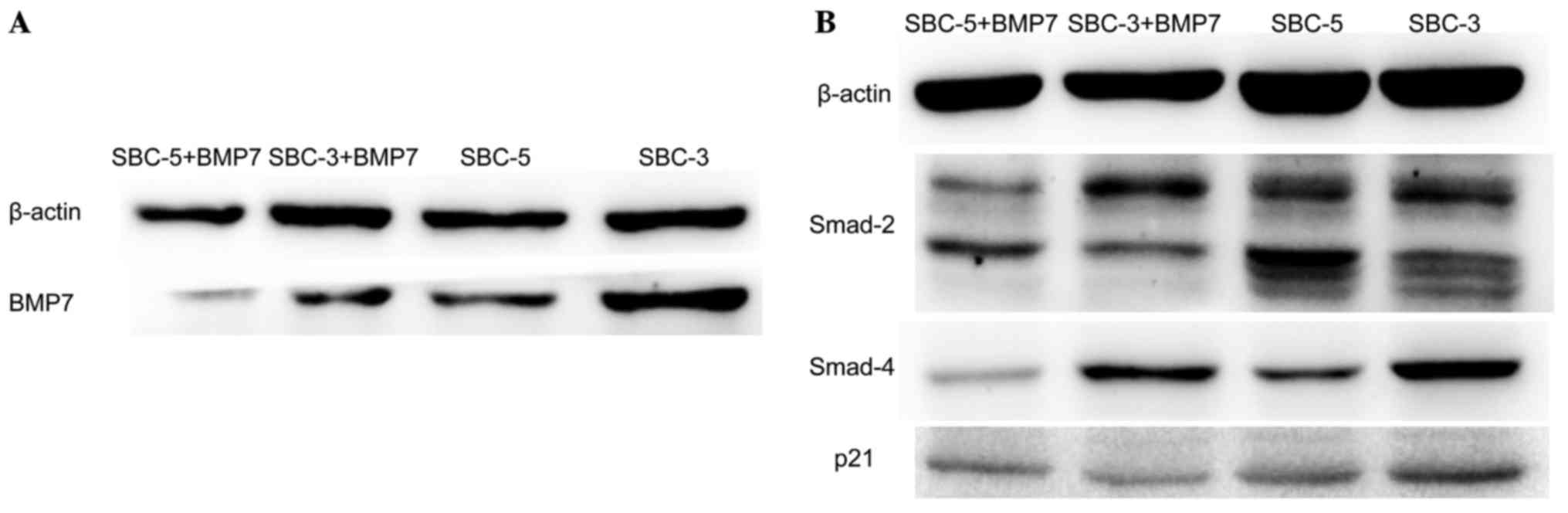
BMP7 signaling pathway is adopted in SCLC
cells
To investigate further the mechanism of BMP7
involved in SCLC cells, associated candidate proteins were detected
by western blot analysis. SBC-3 cells generated more BMP7 compared
with SBC-5 cells (Fig. 7A).
Following treatment with rhBMP7 for 24 h, the self-produced BMP7
was evidently decreased (Fig. 7A).
Furthermore, Smad2 and Smad4 were decreased in SBC-3 and SBC-5
cells upon stimulation with rhBMP7, but p21 was decreased in SBC-3
cells only (Fig. 7B). These
results indicated that BMP7 regulates the SCLC cell cycle through,
at least in part, the Smad signaling pathway.
Discussion
BMP7 is a pleiotropic signaling protein that serves
a major function during physical development. It is a member of the
BMP family, and has been investigated with regard to a possible
contribution to tumorigenesis. This field of research is currently
controversial owing to the marked diversity in the proposed
functions of various BMPs, depending on the BMP ligands and tumor
types investigated.
A previous study conducted on melanoma identified
that BMP7 exhibited metastatic inhibitory ability by inducing MET
(19). In addition, aggressive
melanoma cells escaped from BMP7-mediated autocrine proliferation
inhibition via coordinated upregulation of noggin, a secretory BMP
antagonist (20). Furthermore,
systemic BMP7 treatment of a mouse model significantly inhibited
the growth of glioma (21) and
glioblastoma (22), as well as
micro-metastatic deposits in the bone marrow (7), implying that BMP7 is likely to be a
bone metastatic inhibitor. In the present study, it was
investigated whether BMP7 inhibited proliferation in a dose- and
time-dependent and reversible manner, and its effect on the
invasive ability of SCLC cells. Owing to an increased proliferation
rate and nearly full confluence, SBC-5 cells exhibited a decrease
in proliferation on day 6 in group C, and an inhibitory effect of
rhBMP7 on cell proliferation was evident. In addition, it was
identified that SBC-3 cells have an enhanced secretion ability to
autocrine BMP7 compared with SBC-5 cells. This may be partially
inhibited by exogenous BMP7. Extracellular rhBMP7 may cause
negative feedback, decreasing intrinsic BMP7 autocrine production.
This may be a novel strategy to treat bone metastasis in SCLC. In
the present study, SBC-3 and SBC-5 cells were selected because they
share similar genetic characters, but have different potential in
bone metastasis. As verified by the present in vivo study,
SBC-5 cells have a propensity to metastasize to bone, whereas SBC-3
cells do not. However, BMP7 appears to exert the opposite effects
in certain tumor cells. Sakai et al (23) demonstrated that augmented autocrine
BMP7 signaling induced an increase in the anchorage-independent
cell proliferation and metastatic potential in the bone marrow
metastatic breast cancer model. In esophageal squamous cancer and
colon cancer, BMP7 was identified previously to act as a promoter
in invasion and metastasis (24,25).
Thus, functions of BMP7 are cell-specific and may be either
pro-tumorigenic or anti-tumorigenic (26).
In order to understand how BMP-7 exerts its
anti-proliferative effects in the present study, the impact of
rhBMP7 on the apoptosis and cell cycle in SBC-3 cells and SBC-5
cells was investigated. Results revealed that rhBMP7 significantly
promoted the apoptosis and induced G1 arrest in SBC-3
cells, but exhibited no effect on SBC-5 cells. Thus, the mechanisms
of rhBMP7 regulation of cell proliferation were distinct in SBC-3
and SBC-5 cells. In studies by Yang et al (27,28),
it was also proposed that the apoptotic response to BMP7 was
dependent on the cell type, and, within the same cell type, was
dependent on phenotype, hormone and growth factor status, and
survival conditions. It was also verified whether BMP7 inhibited
the prostate cancer cell proliferation through stabilizing the
levels of survivin and restoring the functions of JNK, which
contributed to the anti-apoptotic activity of BMP7 (27,28).
BMP7 exerted its function by binding to BMPRI. Three type I
receptors were reported. BMPRIB served a negative function for
inducing proliferation and aggressiveness in breast cancer cells by
BMP, but BMPRIA appeared to serve a regulatory function (29). In SBC-3 cells and SBC-5 cells,
different patterns of BMPRI expression meant that BMP7 adopted
different downstream signaling pathways. Total and membrane BMPR-IA
and BMPR-IB were expressed at higher levels in SBC-5 cells, whereas
cytoplasmic BMPR-IA and BMPR-IB were higher in SBC-3 cells.
However, AVCRI was highly expressed in SBC-3 cells, suggesting that
BMP7 inhibited SBC-3 aggressiveness and tumorigenesis mainly by
activating AVCRI. Although the expression of AVCRI was low, it may
serve a critical function. There was a contradictory result about
the expression of AVCRI, which was higher in SBC-5 cells at the
mRNA level, but higher in SBC-3 cells at the protein level. As is
well-known, protein is the final actor of any biomolecule. Thus,
AVCRI was highly expressed in SBC-3 cells compared with in SBC-5
cells. Upon binding to BMPRIs, BMP7 pass down the signal via the
Smad pathway or non-Smad pathway. Smad2 and Smad4 were
downregulated following stimulation with BMP7 in SCLC cells. The
alterations in the sub-bands of Smad2 were notable, with certain
sub-bands enhanced, whereas others were weakened. This may be due
to the alterations in the subtypes of Smad2, which requires further
validation. In addition, as an important cell cycle regulator, p21
levels were also decreased in SBC-3 cells, but there was no change
in p21 levels in SBC-5 cells. Thus, the signaling pathway
associated with BMP7 is complicated. Extrinsic rhBMP7 exerted
different biological effects on SBC-3 and SBC-5 cells. As a
secretory protein, BMP7 employs different receptors to trigger
downstream signaling pathways. A high dose of rhBMP7 was also
linked to cell cycle arrest and apoptotic activation. In addition,
the BMP antagonists noggin and follostatin are also the determining
factors for the cellular response to BMP7. Interestingly, the
expression of these antagonists may be regulated by BMP7 itself
probably via an autocrine or paracrine feedback loop (30). In addition, microRNA (31), gremlin (32) and angiopoietin-like 4 (33) also regulated the expression of BMP7
as an upstream regulator. However, further research to understand
the complexity of BMP7 signaling in SCLC is required.
Clinical data revealed that BMP7 was associated with
the clinicopathological features. For example, in esophageal
squamous cell carcinoma, the expression of BMP7 indicated a poorer
prognosis. This was because the BMP7-positive group demonstrated
deeper progression, more advanced stages and greater venous
invasion compared with the BMP7-negative group (34). Furthermore, in gastric cancer, BMP7
is an independent prognostic factor, and was associated with tumor
size, nodal involvement, lymphatic invasion, venous invasion and
histology, as well as the patient's postoperative outcome (35). Suppression of endogenous BMP7
expression by short interfering RNA in highly metastatic cell lines
led to the upregulation of epithelial cadherin and downregulation
of MMP-9, resulting in the attenuation of cell migration and
invasion in in vitro and in vivo studies (36). However, BMP7 is a double-edged
sword. In clinical prostate cancer specimens and human cancer cell
lines, tumorigenicity and invasive behavior were associated with
decreased expression of BMP7 (8).
BMP7 expression was accompanied with improved surgical outcomes in
renal cell carcinoma (37). In the
present study, the bone metastasis model in NOD/SCID mice was
established and the expression of BMP7 was compared in normal bone
and metastatic bone. It was confirmed that BMP7 was downregulated
in the bone lesions. However, further research is required to
elucidate whether BMP7 is able to inhibit the progression of bone
metastasis in SCLC.
In summary, the results of the present study
suggested that BMP7 served a major function in SCLC progression.
BMP7 is able to inhibit proliferation, migration, and invasion of
SCLC cells. Of note, its inhibitory effect on cell proliferation
was reversible. BMP7 may exert these functions by inducing
apoptosis and/or G1 arrest in the cell cycle.
Furthermore, SBC-3 cells demonstrated a stronger property of
secreting BMP7 and the BMPRI pattern expressed in SBC-3 cells and
SBC-5 cells was distinct, which may be the underlying reasons for
the different responses of SCLC cells to BMP7 exposure. Further
investigation of the mechanisms by which BMP7 expression and
signaling are modulated in tumor cells is required and should
provide novel and deeper insights into the mechanisms underlying
SCLC metastasis as well as strategies for its effective
prevention.
Acknowledgments
The authors are grateful for the interpretation of
immunoassay results provided by the Professor Wei Zhang and Dr Yuan
Ge (Department of Pathology, Tangdu Hospital, Xi'an, China). The
authors thank them for their helpful discussions.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Graff JM: Embryonic patterning: To BMP or
not to BMP, that is the question. Cell. 89:171–174. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wozney JM and Rosen V: Bone morphogenetic
protein and bone morphogenetic protein gene family in bone
formation and repair. Clin Orthop Relat Res. 346:26–37. 1998.
View Article : Google Scholar
|
|
4
|
Urist MR: Bone: Formation by
autoinduction. Science. 150:893–899. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wozney JM, Rosen V, Celeste AJ, Mitsock
LM, Whitters MJ, Kriz RW, Hewick RM and Wang EA: Novel regulators
of bone formation: Molecular clones and activities. Science.
242:1528–1534. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boon MR, van der Horst G, van der Pluijm
G, Tamsma JT, Smit JW and Rensen PC: Bone morphogenetic protein 7:
A broad-spectrum growth factor with multiple target therapeutic
potency. Cytokine Growth Factor Rev. 22:221–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buijs JT, Henriquez NV, van Overveld PG,
van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P,
Cleton-Jansen AM, Driouch K, et al: Bone morphogenetic protein 7 in
the development and treatment of bone metastases from breast
cancer. Cancer Res. 67:8742–8751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buijs JT, Rentsch CA, van der Horst G, van
Overveld PG, Wetterwald A, Schwaninger R, Henriquez NV, Ten Dijke
P, Borovecki F, Markwalder R, et al: BMP7, a putative regulator of
epithelial homeostasis in the human prostate, is a potent inhibitor
of prostate cancer bone metastasis in vivo. Am J Pathol.
171:1047–1057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Botchkarev VA: Bone morphogenetic proteins
and their antagonists in skin and hair follicle biology. J Invest
Dermatol. 120:36–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawabata M, Imamura T and Miyazono K:
Signal transduction by bone morphogenetic proteins. Cytokine Growth
Factor Rev. 9:49–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
ten Dijke P, Korchynskyi O,
Valdimarsdottir G and Goumans MJ: Controlling cell fate by bone
morphogenetic protein receptors. Mol Cell Endocrinol. 211:105–113.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Groppe J, Greenwald J, Wiater E,
Rodriguez-Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale
WW, Izpisua Belmonte JC and Choe S: Structural basis of BMP
signalling inhibition by the cystine knot protein Noggin. Nature.
420:636–642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu DR, Economides AN, Wang X, Eimon PM
and Harland RM: The Xenopus dorsalizing factor Gremlin identifies a
novel family of secreted proteins that antagonize BMP activities.
Mol Cell. 1:673–683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Na YR, Seok SH, Kim DJ, Han JH, Kim TH,
Jung H, Lee BH and Park JH: Bone morphogenetic protein 7 induces
mesenchymal-to-epithelial transition in melanoma cells, leading to
inhibition of metastasis. Cancer Sci. 100:2218–2225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu MY, Rovinsky SA, Lai CY, Qasem S, Liu
X, How J, Engelhardt JF and Murphy GF: Aggressive melanoma cells
escape from BMP7-mediated autocrine growth inhibition through
coordinated Noggin upregulation. Lab Invest. 88:842–855. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klose A, Waerzeggers Y, Monfared P,
Vukicevic S, Kaijzel EL, Winkeler A, Wickenhauser C, Löwik CW and
Jacobs AH: Imaging bone morphogenetic protein 7 induced cell cycle
arrest in experimental gliomas. Neoplasia. 13:276–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
González-Gómez P, Crecente-Campo J,
Zahonero C, de la Fuente M, Hernández-Laín A, Mira H, Sánchez-Gómez
P and Garcia-Fuentes M: Controlled release microspheres loaded with
BMP7 suppress primary tumors from human glioblastoma. Oncotarget.
6:10950–10963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakai H, Furihata M, Matsuda C, Takahashi
M, Miyazaki H, Konakahara T, Imamura T and Okada T: Augmented
autocrine bone morphogenic protein (BMP) 7 signaling increases the
metastatic potential of mouse breast cancer cells. Clin Exp
Metastasis. 29:327–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu M, Cui F, Liu F, Wang J, Wei X and Li
Y: BMP signaling pathways affect differently migration and invasion
of esophageal squamous cancer cells. Int J Oncol. 50:193–202. 2017.
View Article : Google Scholar
|
|
25
|
Zhang T, Fu J, Li Y, Wang Y, Zhang L and
Liu Y: Bone morphogenetic protein 7 is associated with the nodal
invasion of colon cancer. Oncol Lett. 11:1707–1712. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu WH, Thomas TZ, Masumori N, Bhowmick NA,
Gorska AE, Shyr Y, Kasper S, Case T, Roberts RL, Shappell SB, et
al: The loss of TGF-beta signaling promotes prostate cancer
metastasis. Neoplasia. 5:267–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang S, Zhong C, Frenkel B, Reddi AH and
Roy-Burman P: Diverse biological effect and Smad signaling of bone
morphogenetic protein 7 in prostate tumor cells. Cancer Res.
65:5769–5777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang S, Lim M, Pham LK, Kendall SE, Reddi
AH, Altieri DC and Roy-Burman P: Bone morphogenetic protein 7
protects prostate cancer cells from stress-induced apoptosis via
both Smad and c-Jun NH2-terminal kinase pathways. Cancer Res.
66:4285–4290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bokobza SM, Ye L, Kynaston HE, Mansel RE
and Jiang WG: Reduced expression of BMPR-IB correlates with poor
prognosis and increased proliferation of breast cancer cells.
Cancer Genomics Proteomics. 6:101–108. 2009.PubMed/NCBI
|
|
30
|
Ye L, Lewis-Russell JM, Kynaston H and
Jiang WG: Endogenous bone morphogenetic protein-7 controls the
motility of prostate cancer cells through regulation of bone
morphogenetic protein antagonists. J Urol. 178:1086–1091. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ying X, Sun Y and He P: MicroRNA-137
inhibits BMP7 to enhance the epithelial-mesenchymal transition of
breast cancer cells. Oncotarget. 8:18348–18358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin Y, Yang Y, Yang L, Yang Y, Li C, Liu X
and Qu Y: Overexpression of Gremlin promotes non-small cell lung
cancer progression. Tumour Biol. 37:2597–2602. 2016. View Article : Google Scholar
|
|
33
|
Li X, Chen T, Shi Q, Li J, Cai S, Zhou P,
Zhong Y and Yao L: Angiopoietin-like 4 enhances metastasis and
inhibits apoptosis via inducing bone morphogenetic protein 7 in
colorectal cancer cells. Biochem Biophys Res Commun. 467:128–134.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Megumi K, Ishigami S, Uchikado Y, Kita Y,
Okumura H, Matsumoto M, Uenosono Y, Arigami T, Kijima Y, Kitazono
M, et al: Clinicopathological significance of BMP7 expression in
esophageal squamous cell carcinoma. Ann Surg Oncol. 19:2066–2071.
2012. View Article : Google Scholar :
|
|
35
|
Aoki M, Ishigami S, Uenosono Y, Arigami T,
Uchikado Y, Kita Y, Kurahara H, Matsumoto M, Ueno S and Natsugoe S:
Expression of BMP-7 in human gastric cancer and its clinical
significance. Br J Cancer. 104:714–718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu G, Tang S, Yang J, Chen K, Kang J, Zhao
G, Feng F, Yang X, Zhao L, Lu Q, et al: BMP7 expression in
esophageal squamous cell carcinoma and its potential role in
modulating metastasis. Dig Dis Sci. 58:1871–1879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kwak C, Park YH, Kim IY, Moon KC and Ku
JH: Expression of bone morphogenetic proteins, the subfamily of the
transforming growth factor-beta superfamily, in renal cell
carcinoma. J Urol. 178:1062–1067. 2007. View Article : Google Scholar : PubMed/NCBI
|