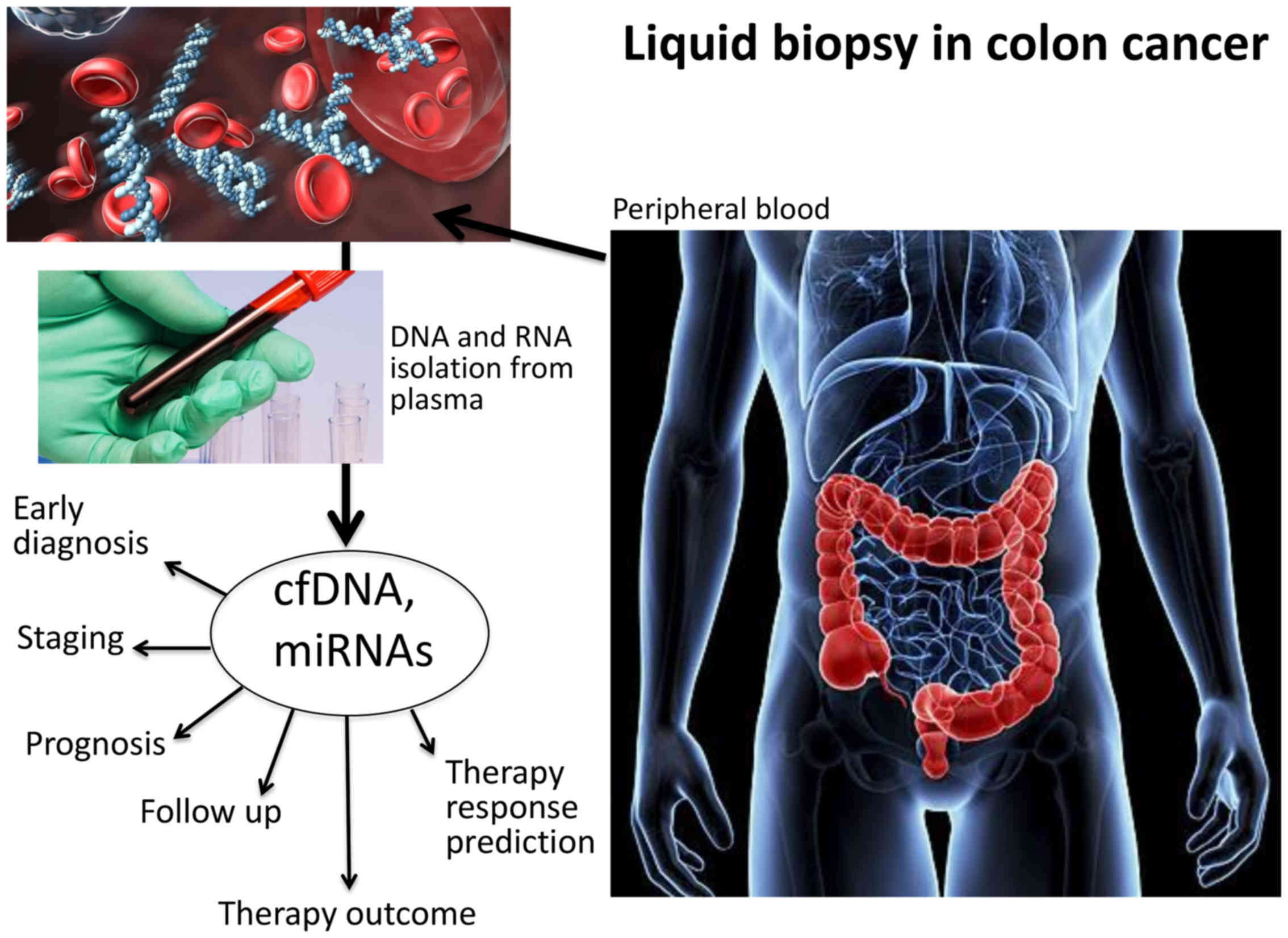
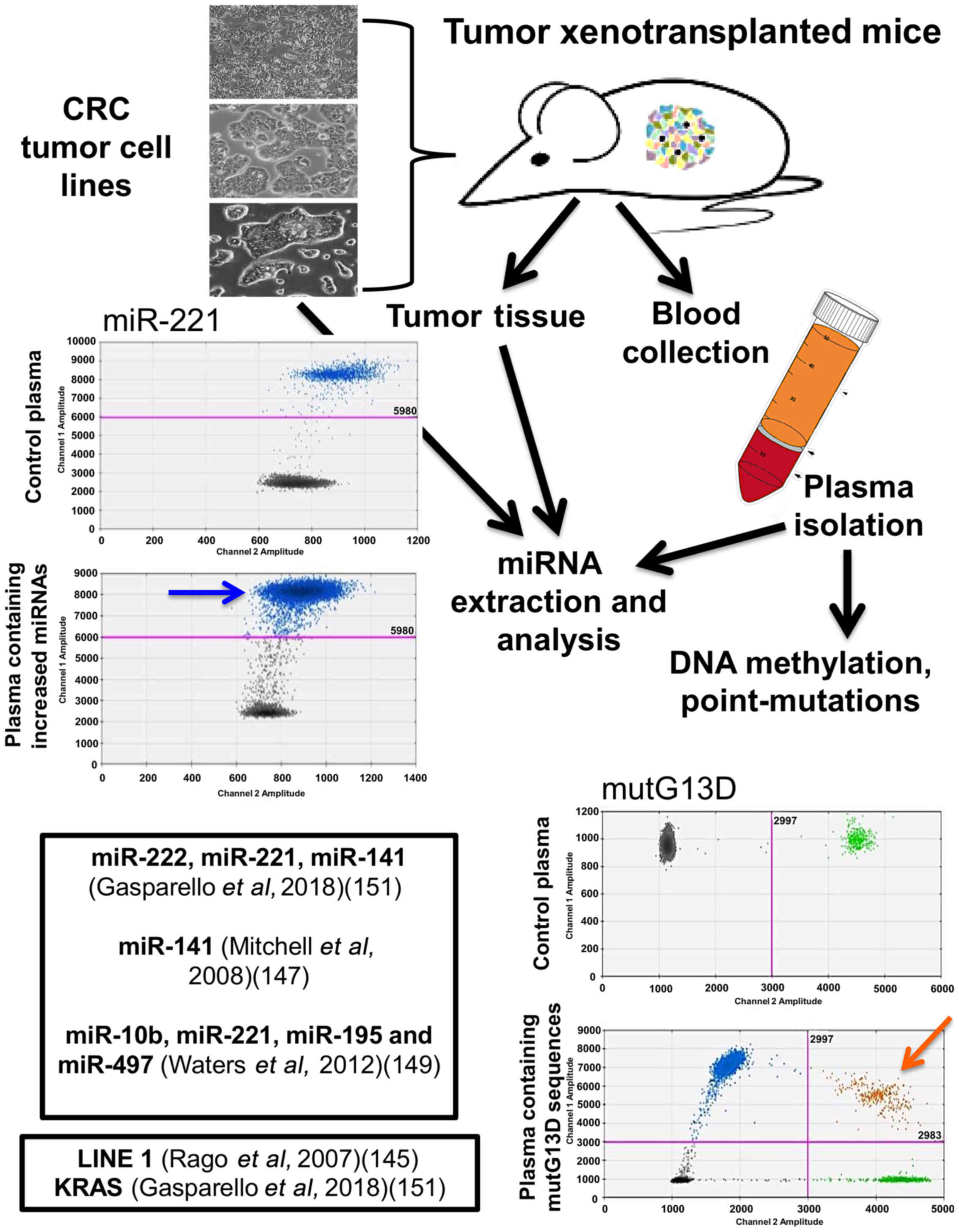
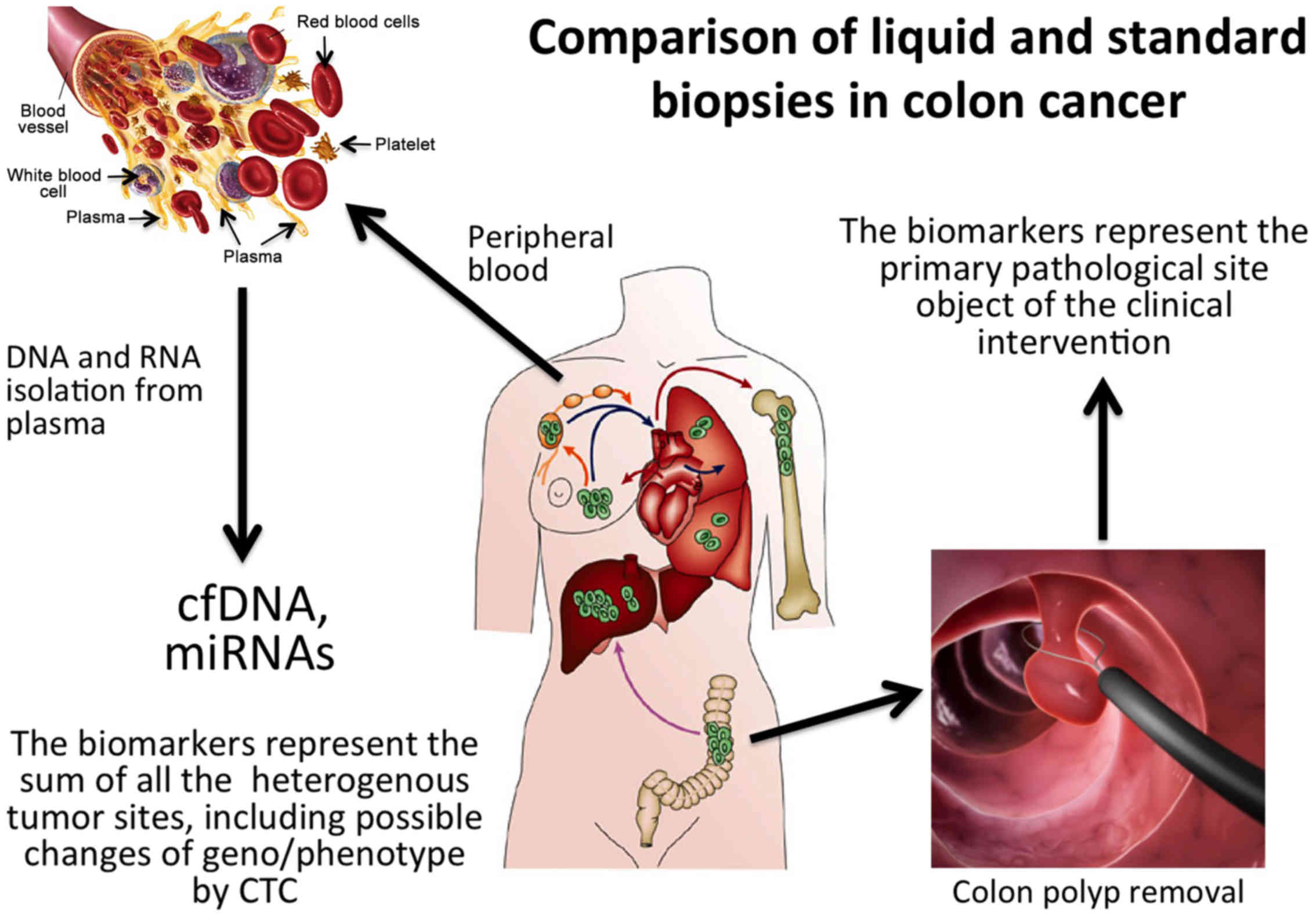
|
1
|
Heitzer E, Auer M, Ulz P, Geigl JB and
Speicher MR: Circulating tumor cells and DNA as liquid biopsies.
Genome Med. 5:732013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra242014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domínguez-Vigil IG, Moreno-Martínez AK,
Wang JY, Roehrl MHA and Barrera-Saldaña HA: The dawn of the liquid
biopsy in the fight against cancer. Oncotarget. 9:2912–2922.
2017.
|
|
4
|
Breitbach S, Tug S, Helmig S, Zahn D,
Kubiak T, Michal M, Gori T, Ehlert T, Beiter T and Simon P: Direct
quantification of cell-free, circulating DNA from unpurified
plasma. PloS One. 9:e878382014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heitzer E, Ulz P and Geigl JB: Circulating
tumor DNA as a liquid biopsy for cancer. Clin Chem. 61:112–123.
2015. View Article : Google Scholar
|
|
6
|
Yang M, Forbes ME, Bitting RL, O'Neill SS,
Chou PC, Topaloglu U, Miller LD, Hawkins GA, Grant SC, DeYoung BR,
et al: Incorporating blood-based liquid biopsy information into
cancer staging: Time for a TNMB system. Ann Oncol. 29:311–323.
2018. View Article : Google Scholar
|
|
7
|
Kleppe M and Levine RL: Tumor
heterogeneity confounds and illuminates: Assessing the
implications. Nat Med. 20:342–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leon SA, Shapiro B, Sklaroff DM and Yaros
MJ: Free DNA in the serum of cancer patients and the effect of
therapy. Cancer Res. 37:646–650. 1977.PubMed/NCBI
|
|
10
|
Burz C, Pop VV, Buiga R, Daniel S, Samasca
G, Aldea C and Lupan I: Circulating tumor cells in clinical
research and monitoring patients with colorectal cancer.
Oncotarget. 9:24561–24571. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sozzi G, Conte D, Mariani L, Lo Vullo S,
Roz L, Lombardo C, Pierotti MA and Tavecchio L: Analysis of
circulating tumor DNA in plasma at diagnosis and during follow-up
of lung cancer patients. Cancer Res. 61:4675–4678. 2001.PubMed/NCBI
|
|
12
|
Spindler KL, Pallisgaard N, Vogelius I and
Jakobsen A: Quantitative cell-free DNA, KRAS, and BRAF mutations in
plasma from patients with metastatic colorectal cancer during
treatment with cetuximab and irinotecan. Clin Cancer Res.
18:1177–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perkins G, Yap TA, Pope L, Cassidy AM,
Dukes JP, Riisnaes R, Massard C, Cassier PA, Miranda S, Clark J, et
al: Multi-purpose utility of circulating plasma DNA testing in
patients with advanced cancers. PloS One. 7:e470202012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ignatiadis M, Lee M and Jeffrey SS:
Circulating tumor cells and circulating tumor DNA: Challenges and
opportunities on the path to clinical utility. Clin Cancer Res.
21:4786–4800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krebs MG, Hou JM, Ward TH, Blackhall FH
and Dive C: Circulating tumour cells: Their utility in cancer
management and predicting outcomes. Ther Adv Med Oncol. 2:351–365.
2010. View Article : Google Scholar
|
|
16
|
Millner LM, Linder MW and Valdes R Jr:
Circulating tumor cells: a review of present methods and the need
to identify heterogeneous phenotypes. Ann Clin Lab Sci. 43:295–304.
2013.PubMed/NCBI
|
|
17
|
Kuipers EJ and Spaander MC: Personalized
screening for colorectal cancer. Nat Rev Gastroenterol Hepatol.
15:391–392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kloten V, Rüchel N, Brüchle NO, Gasthaus
J, Freudenmacher N, Steib F, Mijnes J, Eschenbruch J, Binnebösel M,
Knüchel R, et al: Liquid biopsy in colon cancer: Comparison of
different circulating DNA extraction systems following absolute
quantification of KRAS mutations using Intplex allele-specific PCR.
Oncotarget. 8:86253–86263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomsen CEB, Appelt AL, Andersen RF,
Lindebjerg J, Jensen LH and Jakobsen A: The prognostic value of
simultaneous tumor and serum RAS/RAF mutations in localized colon
cancer. Cancer Med. 6:928–936. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spindler KL, Pallisgaard N, Andersen RF,
Brandslund I and Jakobsen A: Circulating free DNA as biomarker and
source for mutation detection in metastatic colorectal cancer. PLoS
One. 10:e01082472015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hardingham JE, Grover P, Winter M, Hewett
PJ, Price TJ and Thierry B: Detection and clinical significance of
circulating tumor cells in colorectal cancer-20 years of progress.
Mol Med. 21:S25–S31. 2015. View Article : Google Scholar
|
|
22
|
Veldore VH, Choughule A, Routhu T, Mandloi
N, Noronha V, Joshi A, Dutt A, Gupta R, Vedam R and Prabhash K:
Validation of liquid biopsy: Plasma cell-free DNA testing in
clinical management of advanced non-small cell lung cancer. Lung
Cancer (Auckl). 9:1–11. 2018.
|
|
23
|
Anfossi S, Babayan A, Pantel K and Calin
GA: Clinical utility of circulating non-coding RNAs - an update.
Nat Rev Clin Oncol. May 21–2018.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Izzotti A, Carozzo S, Pulliero A,
Zhabayeva D, Ravetti JL and Bersimbaev R: Extracellular MicroRNA in
liquid biopsy: Applicability in cancer diagnosis and prevention. Am
J Cancer Res. 6:1461–1493. 2016.PubMed/NCBI
|
|
25
|
Coombs CC, Zehir A, Devlin SM, Kishtagari
A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, et
al: Therapy-related clonal hematopoiesis in patients with
non-hematologic cancers is common and associated with adverse
clinical outcomes. Cell Stem Cell. 21:374–382e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taly V, Pekin D, Benhaim L, Kotsopoulos
SK, Le Corre D, Li X, Atochin I, Link DR, Griffiths AD, Pallier K,
et al: Multiplex picodroplet digital PCR to detect KRAS mutations
in circulating DNA from the plasma of colorectal cancer patients.
Clin Chem. 59:1722–1731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang JY, Hsieh JS, Chang MY, Huang TJ,
Chen FM, Cheng TL, Alexandersen K, Huang YS, Tzou WS and Lin SR:
Molecular detection of APC, K-ras, and p53 mutations in the serum
of colorectal cancer patients as circulating biomarkers. World J
Surg. 28:721–726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohan S, Heitzer E, Ulz P, Lafer I, Lax S,
Auer M, Pichler M, Gerger A, Eisner F, Hoefler G, et al: Changes in
colorectal carcinoma genomes under anti-EGFR therapy identified by
whole-genome plasma DNA sequencing. PLoS Genet. 10:e10042712014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng WN, Gu WQ, Zhao N, Pan YM, Luo W,
Zhang H, Liang JM, Yang J and Deng YM: Comparison of the SuperARMS
and Droplet Digital PCR for detecting EGFR mutation in ctDNA from
NSCLC patients. Transl Oncol. 11:542–545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Newman AM, Bratman SV, To J, Wynne JF,
Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE, et
al: An ultrasensitive method for quantitating circulating tumor DNA
with broad patient coverage. Nat Med. 20:548–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujiwara K, Fujimoto N, Tabata M, Nishii
K, Matsuo K, Hotta K, Kozuki T, Aoe M, Kiura K, Ueoka H, et al:
Identification of epigenetic aberrant promoter methylation in serum
DNA is useful for early detection of lung cancer. Clin Cancer Res.
11:1219–1225. 2005.PubMed/NCBI
|
|
32
|
Szpechcinski A, Chorostowska-Wynimko J,
Struniawski R, Kupis W, Rudzinski P, Langfort R, Puscinska E,
Bielen P, Sliwinski P and Orlowski T: Cell-free DNA levels in
plasma of patients with non-small-cell lung cancer and inflammatory
lung disease. Br J Cancer. 113:476–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pisanic TR 2nd, Athamanolap P, Poh W, Chen
C, Hulbert A, Brock MV, Herman JG and Wang TH: DREAMing: A simple
and ultrasensitive method for assessing intratumor epigenetic
heterogeneity directly from liquid biopsies. Nucleic Acids Res.
43:e1542015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dawson SJ, Tsui DW, Murtaza M, Biggs H,
Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B,
et al: Analysis of circulating tumor DNA to monitor metastatic
breast cancer. N Engl J Med. 368:1199–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murtaza M, Dawson SJ, Tsui DW, Gale D,
Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS,
et al: Non-invasive analysis of acquired resistance to cancer
therapy by sequencing of plasma DNA. Nature. 497:108–112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yanagawa T, Kagara N, Miyake T, Tanei T,
Naoi Y, Shimoda M, Shimazu K, Kim SJ and Noguchi S: Detection of
ESR1 mutations in plasma and tumors from metastatic breast cancer
patients using next-generation sequencing. Breast Cancer Res Treat.
163:231–240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mastoraki S, Strati A, Tzanikou E,
Chimonidou M, Politaki E, Voutsina A, Psyrri A, Georgoulias V and
Lianidou E: ESR1 Methylation: A liquid biopsy-based epigenetic
assay for the follow-up of patients with metastatic breast cancer
receiving endocrine treatment. Clin Cancer Res. 24:1500–1510. 2018.
View Article : Google Scholar
|
|
38
|
Gray ES, Rizos H, Reid AL, Boyd SC,
Pereira MR, Lo J, Tembe V, Freeman J, Lee JH, Scolyer RA, et al:
Circulating tumor DNA to monitor treatment response and detect
acquired resistance in patients with metastatic melanoma.
Oncotarget. 6:42008–42018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schreuer M, Meersseman G, Van Den
Herrewegen S, Jansen Y, Chevolet I, Bott A, Wilgenhof S, Seremet T,
Jacobs B, Buyl R, et al: Quantitative assessment of BRAF V600
mutant circulating cell-free tumor DNA as a tool for therapeutic
monitoring in metastatic melanoma patients treated with BRAF/MEK
inhibitors. J Transl Med. 14:952016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Madic J, Piperno-Neumann S, Servois V,
Rampanou A, Milder M, Trouiller B, Gentien D, Saada S, Assayag F,
Thuleau A, et al: Pyrophosphorolysis-activated polymerization
detects circulating tumor DNA in metastatic uveal melanoma. Clin
Cancer Res. 18:3934–3941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pereira E, Camacho-Vanegas O, Anand S,
Sebra R, Catalina Camacho S, Garnar-Wortzel L, Nair N, Moshier E,
Wooten M, Uzilov A, et al: Personalized Circulating Tumor DNA
Biomarkers dynamically predict treatment response and survival in
gynecologic cancers. PLoS One. 10:e01457542015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Forshew T, Murtaza M, Parkinson C, Gale D,
Tsui DW, Kaper F, Dawson SJ, Piskorz AM, Jimenez-Linan M, Bentley
D, et al: Noninvasive identification and monitoring of cancer
mutations by targeted deep sequencing of plasma DNA. Sci Transl
Med. 4:136ra682012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
No JH, Kim K, Park KH and Kim YB:
Cell-free DNA level as a prognostic biomarker for epithelial
ovarian cancer. Anticancer Res. 32:3467–3471. 2012.PubMed/NCBI
|
|
44
|
Giannopoulou L, Chebouti I, Pavlakis K,
Kasimir-Bauer S and Lianidou ES: RASSF1A promoter methylation in
high-grade serous ovarian cancer: A direct comparison study in
primary tumors, adjacent morphologically tumor cell-free tissues
and paired circulating tumor DNA. Oncotarget. 8:21429–21443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chan KC, Jiang P, Zheng YW, Liao GJ, Sun
H, Wong J, Siu SS, Chan WC, Chan SL, Chan AT, et al: Cancer genome
scanning in plasma: Detection of tumor-associated copy number
aberrations, single-nucleotide variants, and tumoral heterogeneity
by massively parallel sequencing. Clin Chem. 59:211–224. 2013.
View Article : Google Scholar
|
|
46
|
Zhang P, Wen X, Gu F, Deng X, Li J, Dong
J, Jiao J and Tian Y: Methylation profiling of serum DNA from
hepatocellular carcinoma patients using an Infinium Human
Methylation 450 BeadChip. Hepatol Int. 7:893–900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ren N, Qin LX, Tu H, Liu YK, Zhang BH and
Tang ZY: The prognostic value of circulating plasma DNA level and
its allelic imbalance on chromosome 8p in patients with
hepatocellular carcinoma. J Cancer Res Clin Oncol. 132:399–407.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang A, Zhang X, Zhou SL, Cao Y, Huang
XW, Fan J, Yang XR and Zhou J: Detecting circulating tumor DNA in
hepatocellular carcinoma patients using Droplet Digital PCR is
feasible and reflects intratumoral heterogeneity. J Cancer.
7:1907–1914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Heitzer E, Ulz P, Belic J, Gutschi S,
Quehenberger F, Fischereder K, Benezeder T, Auer M, Pischler C,
Mannweiler S, et al: Tumor-associated copy number changes in the
circulation of patients with prostate cancer identified through
whole-genome sequencing. Genome Med. 5:302013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Annala M, Vandekerkhove G, Khalaf D,
Taavitsainen S, Beja K, Warner EW, Sunderland K, Kollmannsberger C,
Eigl BJ, Finch D, et al: Circulating tumor DNA genomics correlate
with resistance to Abiraterone and Enzalutamide in prostate cancer.
Cancer Discov. 8:444–457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Delgado PO, Alves BC, Gehrke Fde S,
Kuniyoshi RK, Wroclavski ML, Del Giglio A and Fonseca FL:
Characterization of cell-free circulating DNA in plasma in patients
with prostate cancer. Tumour Biol. 34:983–986. 2013. View Article : Google Scholar
|
|
52
|
Buelens S, Claeys T, Dhondt B, Poelaert F,
Vynck M, Yigit N, Thas O, Ost P, Vandesompele J, Lumen N, et al:
Prognostic and therapeutic implications of circulating androgen
receptor gene copy number in prostate cancer patients using Droplet
Digital polymerase chain reaction. Clin Genitourin Cancer.
16:197–205. 2017. View Article : Google Scholar
|
|
53
|
Pu XX, Huang GL, Guo HQ, Guo CC, Li H, Ye
S, Ling S, Jiang L, Tian Y and Lin TY: Circulating miR-221 directly
amplified from plasma is a potential diagnostic and prognostic
marker of colorectal cancer and is correlated with p53 expression.
J Gastroenterol Hepatol. 25:1674–1680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu GH, Zhou ZG, Chen R, Wang MJ, Zhou B,
Li Y and Sun XF: Serum miR-21 and miR-92a as biomarkers in the
diagnosis and prognosis of colorectal cancer. Tumour Biol.
34:2175–2181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma miR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lv ZC, Fan YS, Chen HB and Zhao DW:
Investigation of microRNA-155 as a serum diagnostic and prognostic
biomarker for colorectal cancer. Tumour Biol. 36:1619–1625. 2015.
View Article : Google Scholar
|
|
57
|
Krawczyk P, Powrózek T, Olesiński T,
Dmitruk A, Dziwota J, Kowalski D and Milanowski J: Evaluation of
miR-506 and miR-4316 expression in early and non-invasive diagnosis
of colorectal cancer. Int J Colorectal Dis. 32:1057–1060. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar
|
|
59
|
Liu C, Eng C, Shen J, Lu Y, Takata Y,
Mehdizadeh A, Chang GJ, Rodriguez-Bigas MA, Li Y, Chang P, et al:
Serum exosomal miR-4772-3p is a predictor of tumor recurrence in
stage II and III colon cancer. Oncotarget. 7:76250–76260.
2016.PubMed/NCBI
|
|
60
|
Ogata-Kawata H, Izumiya M, Kurioka D,
Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H,
et al: Circulating exosomal microRNAs as biomarkers of colon
cancer. PLoS One. 9:e929212014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zheng G, Du L, Yang X, Zhang X, Wang L,
Yang Y, Li J and Wang C: Serum microRNA panel as biomarkers for
early diagnosis of colorectal adenocarcinoma. Br J Cancer.
111:1985–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dou H, Wang Y, Su G and Zhao S: Decreased
plasma let-7c and miR-152 as noninvasive biomarker for
non-small-cell lung cancer. Int J Clin Exp Med. 8:9291–9298.
2015.PubMed/NCBI
|
|
64
|
Yu H, Jiang L, Sun C, Li Guo L, Lin M,
Huang J and Zhu L: Decreased circulating miR-375: A potential
biomarker for patients with non-small-cell lung cancer. Gene.
534:60–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li N, Ma J, Guarnera MA, Fang H, Cai L and
Jiang F: Digital PCR quantification of miRNAs in sputum for
diagnosis of lung cancer. J Cancer Res Clin Oncol. 140:145–150.
2014. View Article : Google Scholar :
|
|
66
|
Geng Q, Fan T, Zhang B, Wang W, Xu Y and
Hu H: Five microRNAs in plasma as novel biomarkers for screening of
early-stage non-small cell lung cancer. Respir Res. 15:1492014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu
Y, Chen Y, Xu L, Zen K, Zhang C, et al: Serum microRNA signatures
identified in a genome-wide serum microRNA expression profiling
predict survival of non-small-cell lung cancer. J Clin Oncol.
28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yu L, Todd NW, Xing L, Xie Y, Zhang H, Liu
Z, Fang H, Zhang J, Katz RL and Jiang F: Early detection of lung
adenocarcinoma in sputum by a panel of microRNA markers. Int J
Cancer. 127:2870–2878. 2010. View Article : Google Scholar
|
|
69
|
Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ,
Wang YK, Zeng F, Zhou JH and Zhang YK: High expression of serum
miR-21 and tumor miR-200c associated with poor prognosis in
patients with lung cancer. Med Oncol. 29:618–626. 2012. View Article : Google Scholar
|
|
70
|
Leng Q, Lin Y and Jiang F, Lee CJ, Zhan M,
Fang H, Wang Y and Jiang F: A plasma miRNA signature for lung
cancer early detection. Oncotarget. 8:111902–111911. 2017.
View Article : Google Scholar
|
|
71
|
Asaga S, Kuo C, Nguyen T, Terpenning M,
Giuliano AE and Hoon DS: Direct serum assay for microRNA-21
concentrations in early and advanced breast cancer. Clin Chem.
57:84–91. 2011. View Article : Google Scholar
|
|
72
|
Zhu W, Qin W, Atasoy U and Sauter ER:
Circulating microRNAs in breast cancer and healthy subjects. BMC
Res Notes. 2:892009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Heneghan HM, Miller N, Lowery AJ, Sweeney
KJ, Newell J and Kerin MJ: Circulating microRNAs as novel minimally
invasive biomarkers for breast cancer. Ann Surg. 251:499–505. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Stückrath I, Rack B, Janni W, Jäger B,
Pantel K and Schwarzenbach H: Aberrant plasma levels of circulating
miR-16, miR-107, miR-130a and miR-146a are associated with lymph
node metastasis and receptor status of breast cancer patients.
Oncotarget. 6:13387–13401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mangolini A, Ferracin M, Zanzi MV,
Saccenti E, Ebnaof SO, Poma VV, Sanz JM, Passaro A, Pedriali M,
Frassoldati A, et al: Diagnostic and prognostic microRNAs in the
serum of breast cancer patients measured by droplet digital PCR.
Biomark Res. 3:122015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kleivi Sahlberg K, Bottai G, Naume B,
Burwinkel B, Calin GA, Børresen-Dale AL and Santarpia L: A serum
microRNA signature predicts tumor relapse and survival in
triple-negative breast cancer patients. Clin Cancer Res.
21:1207–1214. 2015. View Article : Google Scholar
|
|
77
|
Margue C, Reinsbach S, Philippidou D,
Beaume N, Walters C, Schneider JG, Nashan D, Behrmann I and Kreis
S: Comparison of a healthy miRNome with melanoma patient miRNomes:
are microRNAs suitable serum biomarkers for cancer. Oncotarget.
6:12110–121127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fleming NH, Zhong J, da Silva IP,
Vega-Saenz de Miera E, Brady B, Han SW, Hanniford D, Wang J,
Shapiro RL, Hernando E, et al: Serum-based miRNAs in the prediction
and detection of recurrence in melanoma patients. Cancer.
121:51–59. 2015. View Article : Google Scholar
|
|
79
|
Ono S, Oyama T, Lam S, Chong K, Foshag LJ
and Hoon DS: A direct plasma assay of circulating microRNA-210 of
hypoxia can identify early systemic metastasis recurrence in
melanoma patients. Oncotarget. 6:7053–7064. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stark MS, Klein K, Weide B, Haydu LE,
Pflugfelder A, Tang YH, Palmer JM, Whiteman DC, Scolyer RA, Mann
GJ, et al: The prognostic and predictive value of melanoma-related
microRNAs using tissue and serum: A microRNA expression analysis.
EBioMedicine. 2:671–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Friedman EB, Shang S, de Miera EV, Fog JU,
Teilum MW, Ma MW, Berman RS, Shapiro RL, Pavlick AC, Hernando E, et
al: Serum microRNAs as biomarkers for recurrence in melanoma. J
Transl Med. 10:1552012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kanemaru H, Fukushima S, Yamashita J,
Honda N, Oyama R, Kakimoto A, Masuguchi S, Ishihara T, Inoue Y,
Jinnin M, et al: The circulating microRNA-221 level in patients
with malignant melanoma as a new tumor marker. J Dermatol Sci.
61:187–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fogli S, Polini B, Carpi S, Pardini B,
Naccarati A, Dubbini N, Lanza M, Breschi MC, Romanini A and Nieri
P: Identification of plasma microRNAs as new potential biomarkers
with high diagnostic power in human cutaneous melanoma. Tumour
Biol. 39:10104283177016462017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yokoi A, Yoshioka Y, Hirakawa A, Yamamoto
Y, Ishikawa M, Ikeda S, Kato T, Niimi K, Kajiyama H, Kikkawa F, et
al: A combination of circulating miRNAs for the early detection of
ovarian cancer. Oncotarget. 8:89811–89823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Todeschini P, Salviato E, Paracchini L,
Ferracin M, Petrillo M, Zanotti L, Tognon G, Gambino A, Calura E,
Caratti G, et al: Circulating miRNA landscape identifies miR-1246
as promising diagnostic biomarker in high-grade serous ovarian
carcinoma: A validation across two independent cohorts. Cancer
Lett. 388:320–327. 2017. View Article : Google Scholar
|
|
86
|
Zuberi M, Mir R, Das J, Ahmad I, Javid J,
Yadav P, Masroor M, Ahmad S, Ray PC and Saxena A: Expression of
serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in
epithelial ovarian cancer and their association with
clinicopathological features. Clin Transl Oncol. 17:779–787. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liang H, Jiang Z, Xie G and Lu Y: Serum
microRNA-145 as a novel biomarker in human ovarian cancer. Tumour
Biol. 36:5305–5313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gao YC and Wu J: MicroRNA-200c and
microRNA-141 as potential diagnostic and prognostic biomarkers for
ovarian cancer. Tumour Biol. 36:4843–4850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhou J, Gong G, Tan H, Dai F, Zhu X, Chen
Y, Wang J, Liu Y, Chen P, Wu X, et al: Urinary microRNA-30a-5p is a
potential biomarker for ovarian serous adenocarcinoma. Oncol Rep.
33:2915–2923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shah JS, Gard GB, Yang J, Maidens J,
Valmadre S, Soon PS and Marsh DJ: Combining serum microRNA and
CA-125 as prognostic indicators of preoperative surgical outcome in
women with high-grade serous ovarian cancer. Gynecol Oncol.
148:181–188. 2018. View Article : Google Scholar
|
|
91
|
Gui J, Tian Y, Wen X, Zhang W, Zhang P,
Gao J, Run W, Tian L, Jia X and Gao Y: Serum microRNA
characterization identifies miR-885-5p as a potential marker for
detecting liver pathologies. Clin Sci (Lond). 120:183–193. 2011.
View Article : Google Scholar
|
|
92
|
Yamamoto Y, Kosaka N, Tanaka M, Koizumi F,
Kanai Y, Mizutani T, Murakami Y, Kuroda M, Miyajima A, Kato T, et
al: MicroRNA-500 as a potential diagnostic marker for
hepatocellular carcinoma. Biomarkers. 14:529–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu J, Wu C, Che X, Wang L, Yu D, Zhang T,
Huang L, Li H, Tan W, Wang C, et al: Circulating microRNAs, miR-21,
miR-122, and miR-223, in patients with hepatocellular carcinoma or
chronic hepatitis. Mol Carcinog. 50:136–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z,
Wang JF, Zhang Z, Lu S, Huang X, et al: Plasma microRNA panel to
diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin
Oncol. 29:4781–4788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY,
Zhang JF, Shen HB, Zhang CY and Zen K: Serum microRNA profiles
serve as novel biomarkers for HBV infection and diagnosis of
HBV-positive hepatocarcinoma. Cancer Res. 70:9798–9807. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Moshiri F, Salvi A, Gramantieri L,
Sangiovanni A, Guerriero P, De Petro G, Bassi C, Lupini L, Sattari
A, Cheung D, et al: Circulating miR-106b-3p, miR-101-3p and
miR-1246 as diagnostic biomarkers of hepatocellular carcinoma.
Oncotarget. 9:15350–15364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kachakova D, Mitkova A, Popov E, Popov I,
Vlahova A, Dikov T, Christova S, Mitev V, Slavov C and Kaneva R:
Combinations of serum prostate-specific antigen and plasma
expression levels of let-7c, miR-30c, miR-141, and miR-375 as
potential better diagnostic biomarkers for prostate cancer. DNA
Cell Biol. 34:189–200. 2015. View Article : Google Scholar :
|
|
98
|
Bryant RJ, Pawlowski T, Catto JW, Marsden
G, Vessella RL, Rhees B, Kuslich C, Visakorpi T and Hamdy FC:
Changes in circulating microRNA levels associated with prostate
cancer. Br J Cancer. 106:768–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lin HM, Castillo L, Mahon KL, Chiam K, Lee
BY, Nguyen Q, Boyer MJ, Stockler MR, Pavlakis N, Marx G, et al:
Circulating microRNAs are associated with docetaxel chemotherapy
outcome in castration-resistant prostate cancer. Br J Cancer.
110:2462–2471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kotb S, Mosharafa A, Essawi M, Hassan H,
Meshref A and Morsy A: Circulating miRNAs 21 and 221 as biomarkers
for early diagnosis of prostate cancer. Tumour Biol.
35:12613–12617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer-the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar
|
|
102
|
Singh PK, Preus L, Hu Q, Yan L, Long MD,
Morrison CD, Nesline M, Johnson CS, Koochekpour S, Kohli M, et al:
Serum microRNA expression patterns that predict early treatment
failure in prostate cancer patients. Oncotarget. 5:824–840. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Farran B, Dyson G, Craig D, Dombkowski A,
Beebe-Dimmer JL, Powell IJ, Podgorski I, Heilbrun L, Bolton S and
Bock CH: A study of circulating microRNAs identifies a new
potential biomarker panel to distinguish aggressive prostate
cancer. Carcinogenesis. 39:556–561. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cochetti G, Poli G, Guelfi G, Boni A,
Egidi MG and Mearini E: Different levels of serum microRNAs in
prostate cancer and benign prostatic hyperplasia: Evaluation of
potential diagnostic and prognostic role. Onco Targets Ther.
9:7545–7553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brychta N, Krahn T and von Ahsen O:
Detection of KRAS mutations in circulating tumor DNA by digital PCR
in early stages of pancreatic cancer. Clin Chem. 62:1482–1491.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kinugasa H, Nouso K, Miyahara K, Morimoto
Y, Dohi C, Tsutsumi K, Kato H, Matsubara T, Okada H and Yamamoto K:
Detection of K-ras gene mutation by liquid biopsy in patients with
pancreatic cancer. Cancer. 121:2271–2280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Couraud S, Zalcman G, Milleron B, Morin F
and Souquet PJ: Lung cancer in never smokers-a review. Eur J
Cancer. 48:1299–1311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Perez-Carbonell L, Sinicrope FA, Alberts
SR, Oberg AL, Balaguer F, Castells A, Boland CR and Goel A:
miR-320e is a novel prognostic biomarker in colorectal cancer. Br J
Cancer. 113:83–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Case M, Matheson E, Minto L, Hassan R,
Harrison CJ, Bown N, Bailey S, Vormoor J, Hall AG and Irving JA:
Mutation of genes affecting the RAS pathway is common in childhood
acute lymphoblastic leukemia. Cancer Res. 68:6803–6809. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lin CC, Huang WL, Wei F, Su WC and Wong
DT: Emerging platforms using liquid biopsy to detect EGFR mutations
in lung cancer. Expert Rev Mol Diagn. 15:1427–1440. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Warton K, Mahon KL and Samimi G:
Methylated circulating tumor DNA in blood: Power in cancer
prognosis and response. Endocr Relat Cancer. 23:R157–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mitchell SM, Ho T, Brown GS, Baker RT,
Thomas ML, McEvoy A, Xu ZZ, Ross JP, Lockett TJ, Young GP, et al:
Evaluation of methylation biomarkers for detection of crculating
tumor DNA and application to colorectal cancer. Genes (Basel).
7:E1252016. View Article : Google Scholar
|
|
113
|
Ghelani HS, Rachchh MA and Gokani RH:
MicroRNAs as newer therapeutic targets: A big hope from a tiny
player. J Pharmacol Pharmacother. 3:217–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chekulaeva M and Filipowicz W: Mechanisms
of miRNAmediated post-transcriptional regulation in animal cells.
Curr Opin Cell Biol. 21:452–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cammaerts S, Strazisar M, De Rijk P and
Del Favero J: Genetic variants in microRNA genes: Impact on
microRNA expression, function, and disease. Front Genet. 6:1862015.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Friedländer MR, Lizano E, Houben AJS,
Bezdan D, Báñez-Coronel M and Kudla G: Evidence for the biogenesis
of more than 1,000 novel human microRNAs. Genome Biol. 15:R572014.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cheng WC, Chung IF, Tsai CF, Huang TS,
Chen CY and Wang SC: YM500v2: A small RNA sequencing (smRNA-seq)
database for human cancer miRNome research. Nucleic Acids Res.
43:D862–D867. 2015. View Article : Google Scholar :
|
|
121
|
Londin E, Loher P, Telonis AG, Quann K,
Clark P and Jing Y: Analysis of 13 cell types reveals evidence for
the expression of numerous novel primate-and tissue-specific
microRNAs. Proc Natl Acad Sci USA. 112:E1106–E1115. 2015.
View Article : Google Scholar
|
|
122
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: MicroRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144. 2006.
View Article : Google Scholar :
|
|
123
|
Gambari R, Fabbri E, Borgatti M, Lampronti
I, Finotti A, Brognara E, Bianchi N, Manicardi A, Marchelli R and
Corradini R: Targeting microRNAs involved in human diseases: A
novel approach for modification of gene expression and drug
development. Biochem Pharmacol. 82:1416–1429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Piva R, Spandidos DA and Gambari R: From
microRNA functions to microRNA therapeutics: Novel targets and
novel drugs in breast cancer research and treatment. Int J Oncol.
43:985–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: Νew trends in the development of miRNA therapeutic
strategies in oncology. Int J Oncol. 49:5–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Bertucci A, Prasetyanto EA, Septiadi D,
Manicardi A, Brognara E, Gambari R, Corradini R and De Cola L:
Combined Delivery of temozolomide and anti-miR221 PNA using
mesoporous silica nanoparticles induces apoptosis in resistant
glioma cells. Small. 11:5687–5695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Gheinani AH, Vögeli M, Baumgartner U,
Vassella E, Draege RA, Burkhard FC and Monastyrskaya K: Improved
isolation strategies to increase the yield and purity of human
urinary exosomes for biomarker discovery. Sci Rep. 8:39452018.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
da Silveira JC, Andrade GM, Del Collado M,
Sampaio RV, Sangalli JR, Silva LA, Pinaffi FVL, Jardim IB, Cesar
MC, Nogueira MFG, et al: Supplementation with small-extracellular
vesicles from ovarian follicular fluid during in vitro production
modulates bovine embryo development. PLoS One. 12:e01794512017.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Halvorsen AR, Helland Å, Gromov P,
Wielenga VT, Talman MM, Brunner N, Sandhu V, Børresen-Dale AL,
Gromova I and Haakensen VD: Profiling of microRNAs in tumor
interstitial fluid of breast tumors - a novel resource to identify
biomarkers for prognostic classification and detection of cancer.
Mol Oncol. 11:220–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Valentino A, Reclusa P, Sirera R,
Giallombardo M, Camps C, Pauwels P, Crispi S and Rolfo C: Exosomal
microRNAs in liquid biopsies: Future biomarkers for prostate
cancer. Clin Transl Oncol. 19:651–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wecker T, Hoffmeier K, Plötner A, Grüning
BA, Horres R, Backofen R, Reinhard T and Schlunck G: MicroRNA
profiling in aqueous humor of individual human eyes by
next-generation sequencing. Invest Ophthalmol Vis Sci.
57:1706–1713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Nishida-Aoki N and Ochiya T: Interactions
between cancer cells and normal cells via miRNAs in extracellular
vesicles. Cell Mol Life Sci. 72:1849–1861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Schetter AJ, Okayama H and Harris CC: The
role of microRNAs in colorectal cancer. Cancer J. 18:244–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bellassai N and Spoto G: Biosensors for
liquid biopsy: Circulating nucleic acids to diagnose and treat
cancer. Anal Bioanal Chem. 408:7255–7264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Das J, Ivanov I, Montermini L, Rak J,
Sargent EH and Kelley SO: An electrochemical clamp assay for
direct, rapid analysis of circulating nucleic acids in serum. Nat
Chem. 7:569–575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Pinheiro LB, Coleman VA, Hindson CM,
Herrmann J, Hindson BJ, Bhat S and Emslie KR: Evaluation of a
Droplet Digital polymerase chain reaction format for DNA copy
number quantification. Anal Chem. 84:1003–1011. 2012. View Article : Google Scholar :
|
|
138
|
Podlesniy P and Trullas R: Biomarkers in
cerebrospinal fluid: Analysis of cell-free circulating
mitochondrial DNA by digital PCR. Methods Mol Biol. 1768:111–126.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Macagno N, Fina F, Penel N, Bouvier C,
Nanni I, Duffaud F, Rouah R, Lacarelle B, Ouafik L, Bonvalot S, et
al: Proof of concept: Prognostic value of the plasmatic
concentration of circulating cell free DNA in desmoid tumors using
ddPCR. Oncotarget. 9:18296–18308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
DiNardo CD, Routbort MJ, Bannon SA, Benton
CB, Takahashi K, Kornblau SM, Luthra R, Kanagal-Shamanna R,
Medeiros LJ, Garcia-Manero G, et al: Improving the detection of
patients with inherited predispositions to hematologic malignancies
using next-generation sequencing-based leukemia prognostication
panels. Cancer. 124:2704–2713. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Müllauer L: Next generation sequencing:
Clinical applications in solid tumours. Memo. 10:244–247. 2017.
View Article : Google Scholar
|
|
142
|
Giuffrida MC and Spoto G: Integration of
isothermal amplification methods in microfluidic devices: Recent
advances. Biosens Bioelectron. 90:174–186. 2017. View Article : Google Scholar
|
|
143
|
Giuffrida MC, Zanoli LM, D'Agata R,
Finotti A, Gambari R and Spoto G: Isothermal
circular-strand-displacement polymerization of DNA and microRNA in
digital microfluidic devices. Anal Bioanal Chem. 407:1533–1543.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Garcia-Olmo DC, Gutierrez-Gonzalez L,
Ruiz-Piqueras R, Picazo MG and Garcia-Olmo D: Detection of
circulating tumor cells and of tumor DNA in plasma during tumor
progression in rats. Cancer Lett. 217:115–123. 2005. View Article : Google Scholar
|
|
145
|
Rago C, Huso DL, Diehl F, Karim B, Liu G,
Papadopoulos N, Samuels Y, Velculescu VE, Vogelstein B, Kinzler KW,
et al: Serial assessment of human tumor burdens in mice by the
analysis of circulating DNA. Cancer Res. 67:9364–9370. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Thierry AR, Mouliere F, Gongora C, Ollier
J, Robert B, Ychou M, Del Rio M and Molina F: Origin and
quantification of circulating DNA in mice with human colorectal
cancer xenografts. Nucleic Acids Res. 38:6159–6175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci US A.
105:10513–10518. 2008. View Article : Google Scholar
|
|
148
|
Selth LA, Townley S, Gillis JL, Ochnik AM,
Murti K, Macfarlane RJ, Chi KN, Marshall VR, Tilley WD and Butler
LM: Discovery of circulating microRNAs associated with human
prostate cancer using a mouse model of disease. Int J Cancer.
131:652–661. 2012. View Article : Google Scholar
|
|
149
|
Waters PS, McDermott AM, Wall D, Heneghan
HM, Miller N, Newell J, Kerin MJ and Dwyer RM: Relationship between
circulating and tissue microRNAs in a murine model of breast
cancer. PLoS One. 7:e504592012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Greystoke A, Ayub M, Rothwell DG, Morris
D, Burt D, Hodgkinson CL, Morrow CJ, Smith N, Aung K, Valle J, et
al: Development of a circulating miRNA assay to monitor tumor
burden: From mouse to man. Mol Oncol. 10:282–291. 2016. View Article : Google Scholar :
|
|
151
|
Gasparello J, Allegretti M, Tremante E,
Fabbri E, Amoreo CA, Romania P, Melucci E, Messana K, Borgatti M,
Giacomini P, et al: Liquid biopsy in mice bearing colorectal
carcinoma xenografts: Gateways regulating the levels of circulating
tumor DNA (ctDNA) and miRNA (ctmiRNA). J Exp Clin Cancer Res.
37:1242018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Hofman P: Liquid biopsy for early
detection of lung cancer. Curr Opin Oncol. 29:73–78. 2017.
View Article : Google Scholar
|
|
153
|
Pérez-Ramírez C, Cañadas-Garre M, Robles
AI, Molina MÁ, Faus-Dáder MJ and Calleja-Hernández MÁ: Liquid
biopsy in early stage lung cancer. Transl Lung Cancer Res.
5:517–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Bedin C, Enzo MV, Del Bianco P,
Pucciarelli S, Nitti D and Agostini M: Diagnostic and prognostic
role of cell-free DNA testing for colorectal cancer patients. Int J
Cancer. 140:1888–1898. 2017. View Article : Google Scholar
|
|
155
|
Allenson K, Castillo J, San Lucas FA,
Scelo G, Kim DU, Bernard V, Davis G, Kumar T, Katz M, Overman MJ,
et al: High prevalence of mutant KRAS in circulating
exosome-derived DNA from early-stage pancreatic cancer patients.
Ann Oncol. 28:741–747. 2017.PubMed/NCBI
|
|
156
|
Shimomura A, Shiino S, Kawauchi J,
Takizawa S, Sakamoto H, Matsuzaki J, Ono M, Takeshita F, Niida S,
Shimizu C, et al: Novel combination of serum microRNA for detecting
breast cancer in the early stage. Cancer Sci. 107:326–334. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Schröck A, Leisse A, de Vos L, Gevensleben
H, Dröge F, Franzen A, Wachendörfer M, Schröck F, Ellinger J,
Teschke M, et al: Free-circulating methylated DNA in blood for
diagnosis, staging, prognosis, and monitoring of head and neck
squamous cell carcinoma patients: An observational prospective
cohort study. Clin Chem. 63:1288–1296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Quandt D, Dieter Zucht H, Amann A,
Wulf-Goldenberg A, Borrebaeck C, Cannarile M, Lambrechts D,
Oberacher H, Garrett J, Nayak T, et al: Implementing liquid
biopsies into clinical decision making for cancer immunotherapy.
Oncotarget. 8:48507–48520. 2018.
|
|
159
|
Goodall J, Mateo J, Yuan W, Mossop H,
Porta N, Miranda S, Perez-Lopez R, Dolling D, Robinson DR, Sandhu
S, et al: Circulating cell-free DNA to quide prostate cancer
treatment with PARP inhibition. Cancer Discov. 7:1006–1017. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
He J, Tan W and Ma J: Circulating tumor
cells and DNA for real-time EGFR detection and monitoring of
non-small-cell lung cancer. Future Oncol. 13:787–797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Craw P and Balachandran W: Isothermal
nucleic acid amplification technologies for point-of-care
diagnostics: A critical review. Lab Chip. 12:2469–2486. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kim J and Easley CJ: Isothermal DNA
amplification in bioanalysis: strategies and applications.
Bioanalysis. 3:227–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Guo Q, Yang X, Wang K, Tan W, Li W, Tang H
and Li H: Sensitive fluorescence detection of nucleic acids based
on isothermal circular strand-displacement polymerization reaction.
Nucleic Acids Res. 37:e202009. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
D'Agata R, Breveglieri G, Zanoli LM,
Borgatti M, Spoto G and Gambari R: Direct detection of point
mutations in nonamplified human genomic DNA. Anal Chem.
83:8711–8717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Albitar M: Hematopoietic cell phenotyping
using circulating cell-free markers. US Patent 9,255,926 B2. Filed
August 17, 2006, issued February 9, 2016.
|
|
166
|
Thierry A and Molina F: Analytical methods
for cell free nucleic acids and applications. European Patent
2,426,217A1. Filed September 9, 2010; issued March 7, 2012.
|
|
167
|
Platica O: Method of mutation detection in
blood cell-free DNA using primer extension (PE) and PCR. US Patent
9,062,350 B2. Filed March 11, 2012; issued June 23, 2015.
|
|
168
|
Hoon DSB and Taback B: DNA markers for
management of cancer. US Patent 7,718,364 B2. Filed March 25, 2004;
issued May 18, 2010.
|
|
169
|
Cortese Rand Petronis A: Method for
analysis of DNA methylation profiles of cell-free circulating DNA
in bodily fluids. European Patent 2,483,426 A4. Filed October 1,
2010; issued April 4, 2013.
|
|
170
|
Schutz E, Beck J and Urnovitz H:
Colorectal cancer associated circulating nucleic acid biomarkers.
US Patent 2014/0303008A1. Filed October 19, 2012; issued October 9,
2014.
|
|
171
|
Murtaza M and Contente-Cuomo T: Quality
assessment of circulating cell-free DNA using multiplexed droplet
digital PCR. WO Patent 2016/168844A1. Filed April 17, 2015; issued
October 10, 2016.
|
|
172
|
Hoon DSB, Umetani N and Sunami E: Use of
free circulating DNA for diagnosis, prognosis, and treatment of
cancer. WO Patent 2006/128192 A2. Filed May 27, 2005; issued
November 30, 2006.
|
|
173
|
Raymond CK, Lim LP and Armour CD: Methods
for quantitative genetic analysis of cell free DNA. US Patent
2016/0053301 A1. Filed August 22, 2014; issued February 25,
2016.
|
|
174
|
Ambros V, Lee R and Fusco AP: Isolating
Circulating microRNA (miRNA). US Patent 9,896,683 B2. Filed July
29, 2015; issued February 20, 2018.
|
|
175
|
Taylor DD and Gercel-Taylor C:
Cancer-derived microvesicle-associated microrna as a diagnostic
marker. US Patent 8,216,784 B2. Filed July 25, 2008; issued July
10, 2012.
|
|
176
|
Taylor DD and Gercel-Taylor C:
Exosome-associated microRNA as a diagnostic marker. European Patent
2,806,273 B1. Filed August 12, 2013; issued December 5, 2013.
|
|
177
|
Croce CM, Calin GA and Volinia S: Methods
for Diagnosing Pancreatic Cancer Using MicroRNAs. US Patent
2013/0324589 A1. Filed August 12, 2013; issued December 5,
2013.
|
|
178
|
Ditzel H and Kodahl AR: Circulating
microRNA based cancer biomarkers. European Patent 3,011,058 A1.
Filed December 24, 2014; issued April 27, 2016.
|
|
179
|
Croce CM: MicroRNA signatures in human
ovarian cancer. European Patent 3,138,926 A3. Filed September 8,
2008; issued April 5, 2017.
|
|
180
|
Plasma microRNAs for the detection of
early colorectal cancer. European Patent 2,944,700 B1. Filed
October 10, 2012; issued October 18, 2017.
|
|
181
|
Zhang C, Zeng K, Zhang J, Ba Y, Chen X and
Li H: Serum or plasma microRNA as biomarkers for non-small cell
lung cancer. US Patent 9,388,470 B2. Filed December 14, 2009;
issued July 12, 2016.
|
|
182
|
Croce CM, Calin GA and Volinia S: Methods
for diagnosing breast cancer using MicroRNAs. US Patent. 8,603,744
B2. Filed February 28, 2012; issued December 10, 2013.
|
|
183
|
NCT02639832, A Pilot Surveillance
Study to Monitor Natural Killer Cells and Circulating Tumor Cells
in Women With Previously Treated Non-metastatic Triple Negative
Breast Cancer and Women With Previously Treated Non-metastatic
Breast Cancer With a Confirmed BRCA Mutation, 2015
|
|
184
|
NCT02626039, Characterization &
Comparison of Drugable Mutations in Primary and Metastatic Tumors,
CTCs and cfDNA in MBCpatients, 2015
|
|
185
|
NCT02186236, Detection of Oncogenic
Tumor Mutations in the Urine and Blood of Lung and Colorectal
Cancer Patients, 2014
|
|
186
|
NCT02788084, Development of a
Tissue-Based & Cell Free DNA Next-Generation Sequencing
Workflow, 2016
|
|
187
|
NCT02883517, Cell-free Circulating
DNA in Primary Cutaneous Lymphomas, 2016
|
|
188
|
NCT02887612, ctDNA for Prediction of
Relapse in Gastric Cancer, 2016
|
|
189
|
NCT02738593, Detection Cell Free DNA
in Lung Cancer Patients, 2016
|
|
190
|
NCT02610218, Liquid Biopsy in
Monitoring the Therapeutic Efficacy of Targeted Therapy in
Advanced/Metastatic Gastric Cancer, 2015
|
|
191
|
NCT02872779, Correlation Between
Circulating Tumour Markers Early Variations and Clinical Response
in First Line Treatment of Metastatic Colorectal Cancer
(COCA-MACS), 2016
|
|
192
|
NCT02443948, Circulating Cell-free
Tumor DNA in the Plasma of Patients With Gastrointestinal Stromal
Tumors (GIST), 2015
|
|
193
|
NCT02133222, Mutational Analyses in
Consecutive Measurement Before and After Chemotherapy (AMMAM),
2014
|
|
194
|
NCT02934984, Circulating Cell-free
Tumor DNA (ctDNA) in Pancreatic Cancer, 2016
|
|
195
|
NCT02784639, Comparison of KRAS/BRAF
Mutational Status With Conventional Techniques and Plasma Samples
Analysis, 2016
|
|
196
|
NCT02036216, Circulating Cell-free
DNA as a Predictive Biomarker for Hepatocelluar Carcinoma, 2014
|
|
197
|
NCT02791217, Identification of
Hematological Malignancies and Therapy Predication Using microRNAs
as a Diagnostic Tool, 2016
|
|
198
|
NCT02928627, Clinical Significance of
Hepatic and Circulating microRNAs miR-221 and miR-222 in
Hepatocellular Carcinoma, 2016
|
|
199
|
NCT02964351, microRNA Profiles
Identification in Adeno Carcinoma Prostate Cancer, 2016
|
|
200
|
NCT01541800, Circulating microRNAs as
Disease Markers in Pediatric Cancers, 2012
|
|
201
|
NCT02065908, Circulating MicroRNA as
Biomarker of Cardiotoxicity in Breast Cancer, 2014
|
|
202
|
NCT02812680, The Utility of
Circulating Tumour Cells and Plasma microRNA in Esophageal
Adenocarcinoma, 2016
|
|
203
|
NCT01612871, Circulating miRNAs as
Biomarkers of Hormone Sensitivity in Breast Cancer (MIRHO),
2012
|
|
204
|
NCT01722851. Circulating miRNAs ICORG.
10(11): V22012.
|
|
205
|
D'Agata R, Corradini R, Ferretti C, Zanoli
L, Gatti M, Marchelli R and Spoto G: Ultrasensitive detection of
non-amplified genomic DNA by nanoparticle-enhanced surface-plasmon
resonance imaging. Biosens Bioelectron. 25:2095–2100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
D'Agata R and Spoto G: Surface plasmon
resonance imaging for nucleic acid detection. Anal Bioanal Chem.
405:573–584. 2013. View Article : Google Scholar
|