Introduction
Programmed cell death ligand 1 (PD-L1) is a
functional ligand of programmed cell death 1 (PD-1) (1). The binding of tumor cell PD-L1 to
immune T-cell PD-1 inhibits T-cell activation and attenuates
T-cell-mediated immunosuppression (2–4).
This results in the evasion of host antitumor immunity, potentially
reducing the efficacy of anticancer therapies and resulting in a
poor clinical outcome (2,5,6). To
counter the escape from the host immune surveillance system by
tumor cells, blockade strategies using monoclonal antibodies (mAbs)
to prevent the binding of PD-L1 to PD-1 have been developed
(7-12). The clinical efficacy of this
approach has been demonstrated in certain cancer types, including
melanoma (13-16), non-small-cell lung cancer (17-19)
and renal carcinoma (20).
Blockade therapy differs from tumoricidal chemotherapy in that its
antitumorigenic effects involve boosting host immunity concomitant
with the modulation of the expression/activity of the repertoire of
cytotoxic T-cell and T-regulatory cells (21–24).
Although success in anti-PD-1/PD-L1 therapy has made it possible to
achieve tumor eradication and disease remission/cure, outstanding
challenges remain. Only 31% of patients with advanced melanoma
treated intravenously with anti-PD-1 drugs (nivolumab) have
exhibited objective tumor regression (14). Likewise, the same therapeutic
regimen has shown response rates ranging from 25% in renal cancer
(20), to 19% in non-squamous
non-small-cell lung cancer (17)
and to 20% in squamous non-small-cell lung cancer (18), respectively. Moreover, a positive
therapeutic response typically occurs in patients with
PD-L1-positive tumors (13,25).
Since the expression of PD-L1 in cancer cells may
affect the patient response to immune blockade therapy, it is of
interest to identify agents capable of modulating the expression of
PD-L1. In this study, we focused on resveratrol, a stilbenoid
present abundantly in red wine, red grape skin and peanuts
(26,27). Interest in resveratrol stems
largely from the report in 1997 by Jang et al, showing that
the molecule prevents the development of pre-neoplastic lesions in
carcinogen-exposed mammary glands, and the inhibition of initiation
and promotion of skin cancer in a mouse model (28). Since then, numerous studies have
demonstrated its broad-spectrum beneficial health effects,
including, anti-inflammatory (29,30)
and anticancer activities (26).
Several mechanisms (31,32) and target proteins for the
biological and pharmacological activities of resveratrol have been
identified and characterized (33–42).
Resveratrol has also been reported to exert immunomodulatory
effects, as illustrated by the induction of interferon (IFN)-γ
expression in CD8+ T-cells both ex vivo and in
vivo (43), and the inhibition
of the proliferation of CD4+ T-cells (43,44).
Craveiro et al (45)
recently demonstrated that low-dose resveratrol (20 μM)
activates human CD4+ cells and induces DNA damage
response, while high-dose resveratrol (100 μM) induces
G1 phase cell cycle arrest, suggesting that resveratrol
may act on host immune cell types in a dose-dependent manner. The
chemopreventive activity of resveratrol was first demonstrated
using skin and breast cancer models (28), and recent clinical trials support
the use of resveratrol in colorectal cancer (46,47).
Thus, in this study, we selected breast and colorectal cancer cell
lines to examine the regulatory effects of resveratrol and its
biotransformed product, piceatannol, on the expression of PD-L1.
The results revealed that both dietary stilbenoids, alone or in
combination, copiously increased the expression level of PD-L1 in
some breast and colorectal cancer cells via HDAC3/p300-mediated
nuclear factor (NF)-κB signaling. In addition, both stilbenoids
exerted cytotoxic effects on the tumor cells.
Materials and methods
Reagents
Resveratrol, piceatannol, resminostat, entinostat,
mocetinostat, vorinostat, curcumin, garcinol, anacardic acid and
Tip60i were purchased from Selleckchem (Houston, TX, USA). MB-3 and
BMS-345541 were purchased from MilliporeSigma (Burlington, MA,
USA). Pterostilbene and myricetin were from LKT Laboratories (St.
Paul, MN, USA) and trimethoxy-resveratrol
(trans-3,5,4′-trimethoxystilbene) was from Cayman Chemical Co. (Ann
Arbor, MI, USA). Stock solutions of the chemicals were prepared
based on the information provided by the manufacturer and
maintained at −20°C. The antibodies for human PD-L1 (E1L3N, 13684),
p38 MAPK (D13E1, 8690), NF-κB p65 (D14E12, 8242), γH2AX (20E3,
9718), cleaved caspase 3 (D3E9, 9579), IRF-1 (D5E4, 8478) and
rabbit IgG isotype monoclonal antibody (DA1E, 5742) conjugated to
PE were obtained from Cell Signaling Technology (Beverly, MA, USA).
c-Myc antibody (9E10, sc-40) was obtained from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). Fetal bovine serum,
RPMI-1640, DMEM, streptomycin and penicillin were from Gibco/Thermo
Fisher Scientific (Waltham, MA, USA). All other chemicals and
solvents were of analytical grade.
Cell culture and treatment
Human BT549 (breast cancer), BT474 (invasive ductal
carcinoma), SKBR3 (breast cancer), HCT116 (colon cancer), SW480
(colon cancer), HT29 (recto-sigmoid adenocarcinoma) and SW620
(colon cancer) cells were obtained from the American Type Culture
Collection (ATCC, Rockville, MD, USA). Human Cal51 (breast cancer)
cells were from the Leibniz Institute DSMZ-German Collection of
Microorganisms and Cell Cultures (Braunschweig, Germany). The cells
were maintained in RPMI-1640 or DMEM culture media supplemented
with penicillin, streptomycin and 10% heat-inactivated fetal bovine
serum according to the manufacturer's instructions. The cells were
split once a week and the media were changed every 3-4 days.
In all experiments described in this study,
parent/parental or DMSO treated cells all refer to untreated,
control cells. For treated cells, the conditions (dose and
treatment duration) and whether any reagents were used together at
specific doses were as indicated in the figure legends.
Immunohistochemistry
Paraffin-embedded SW620 colon cancer cells were
immunohistochemically stained to evaluate the protein expression of
PD-L1, c-Myc, p38 MAPK, γH2AX and cleaved caspase 3. Following
deparaffinization and rehydration, sections of SW620 cells were
prepared. The slides were heated in the Retriever 2000 pressure
cooker (Electron Microscopy Sciences, Hatfield, PA, USA) in Borg
buffer pH 9.5 (Biocare Medical, Concord, CA, USA) and cooled to
room temperature. Endogenous peroxidase activity was inactivated
with Peroxidazed 1 (Biocare Medical) for 10 min. Non-specific
protein interactions were blocked for 10 min with Background
Punisher (Biocare Medical). The sections were incubated with the
primary antibodies, indicated above, at a dilution of 1/200 for 1
h, washed in TBS and incubated with SignalStain Boost IHC Detection
Reagent (Cell Signaling Technology) for 30 min. Following washes in
TBS, immunoreactivity was visualized by development with
3,3′-diaminobenzidine (DAB+, Dako, Carpinteria, CA, USA)
for 5 min. Immunostained sections were briefly counterstained with
CAT hematoxylin, washed in tap water, dehydrated in a graded
alcohol series, cleared in xylene and coverslipped with Permount
mounting medium (Fisher Scientific Co. L.L.C., Pittsburgh, PA,
USA).
Flow cytometric analysis for the surface
expression of PD-L1
The cells were harvested and fixed in 4%
parafor-maldehyde for 10 min. The cells were then rinsed twice with
phosphate-buffered saline (PBS) before a 30-min staining was
performed to prepare the samples for flow cytometry using a rabbit
anti-human PD-L1 monoclonal antibody (E1L3N) conjugated to
phycoerythrin (PE). As controls, cell samples were also stained for
30 min using rabbit IgG isotype monoclonal antibody (DA1E)
conjugated to PE. Following labeling with antibody, the cells were
rinsed twice with PBS and re-suspended with PBS. The data shown as
the geometric means from n=3-4 independent experiments were
acquired on a DB LSR Fortessa X-20, and analyzed with FlowJo
version 10 software (BD Biosciences, San Jose, CA, USA).
PD-L1 mRNA analyses using the Cancer Cell
Line Encyclopedia (CCLE) database
The basal PD-L1 mRNA expression levels shown in
Fig. 2B, presented as transcript
per million (TPM) were obtained from a public Cancer Cell Line
Encyclopedia (CCLE, https://portals.broadinstitute.org/ccle/home) database
for cancer cell lines tested.
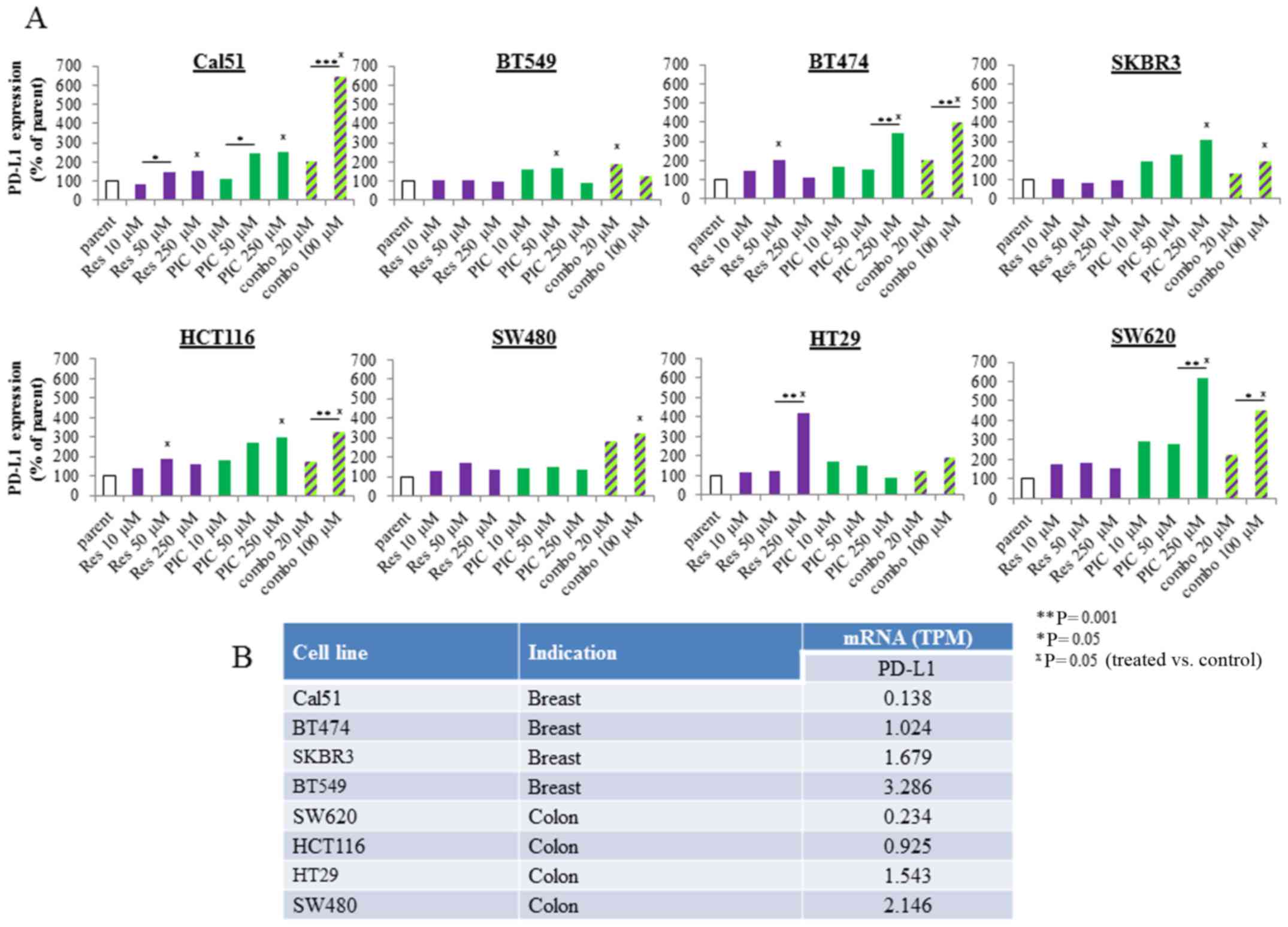 | Figure 2Effects of resveratrol and
piceatannol on programmed cell death ligand 1 (PD-L1) expression in
human breast and colon cancer cells. (A) A panel of 4 breast
(Cal51, BT549, BT474 and SKBR3) and colorectal (HCT116, SW480, HT29
and SW620) cancer cell lines were incubated with increasing
concentrations of resveratrol or piceatannol, as single agents or
in combination for 48 h. Following treatment, the cells were
harvested and stained and quantified for PD-L1-PE surface
expression by flow cytometry. The induction of PD-L1 expression was
based on comparison to a DMSO-treated sample (control used
throughout all experiments), and to isotype control staining. The
geometric mean of MFI of PE area was used as the PD-L1 readout. The
levels of PD-L1 from n=3 experiments were converted to a bar graph
to represent the respective changes in PD-L1 expression following
treatment. The parental condition represents the untreated control.
The statistical difference reflects comparison of treated samples
to parental condition. (B) RNA-seq analysis of constitutive mRNA
expression of PD-L1 in panel of breast and colon cancer cells.
Basal PD-L1 mRNA levels, shown as transcript per million (TPM) were
obtained from a public Cancer Cell Line Encyclopedia (CCLE)
database and ranked from low to high according to the relative TPM
expression by most of the breast and colon cancer cell lines tested
in (A) https://portals.broadinstitute.org/ccle/home. The
asterisks apply to comparisons made among treated samples:
*P<0.05, **P<0.01, ***P<0.001. In
addition, the 'x' symbol refers to comparisons made between the
untreated, control sample with the treated sample: xP<0.05
(treated vs. control). |
NF-κB reporter assay
The A549-Dual cells purchased from InvivoGen (San
Diego, CA, USA) were used. These are derivatives of the A549 human
lung carcinoma cells containing the stable integration of two
inducible reporter constructs. The constructs allow for the
co-expression of a secreted embryonic alkaline phosphatase (SEAP)
reporter gene under the control of the IFN-β minimal promoter fused
5 five NF-κB binding sites, and, a Lucia luciferase gene encoding a
secreted luciferase whose transcription is driven by an ISG54
promoter fused to 5 IFN-stimulated response elements. The cells
were treated for the specified amount of time (8, 24 and 48 h) with
resveratrol or piceatannol, each at 100 μM or combined, each
at 50 μM (referred to as combo-100) to yield a concentration
of 100 μM and the secreted alkaline phosphatase and Lucia
luciferase in the supernatant of the control and treated cells were
detected using the Quanti-Blue reagent from InvivoGen. The results
were scored by the fluorescence intensity on a Perkin Elmer EnSpire
set at a wavelength of 650 nm (PerkinElmer Inc., Shelton, CT, USA).
To determine the role of NF-κB in mediating the induction of PD-L1,
the IKK inhibitor, BMS-345541, was administered for 24 h in
vitro prior to exposure to the combination of piceatannol and
resveratrol, each at 50 μM to yield a concentration of 100
μM, for a further 48 h. BMS-345541 was added at increasing
concentrations (1, 4 and 8 μM), while the dose of the
combination was kept constant.
Cell cycle/apoptosis analysis
The cells were harvested and washed with PBS then
re-suspended in cold 1% formaldehyde in PBS solution for 15 min at
4°C. The cells were washed twice in PBS and re-suspended in
ice-cold 70% ethanol and stored at −20°C for 2 h prior to analysis.
Prior to fluorescence measurement the cells were stained with
4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI). The
intensity of blue fluorescence emission of DAPI stained DNA,
excited with the UV laser (355 nm) was measured, recorded and
analyzed on a MoFlo flow cytometer (Beckman Coulter Life Sciences,
Indianapolis IN, USA) using Kaluza fluorescence intensity analysis
software (48). Experiments were
repeated and representative data are presented.
Statistical analysis
All data are presented as the mean ± the standard
error of the mean. A Student's t-test or two-way ANOVA with
Bonferroni correction were performed to determine statistical
significance between frequencies or mean fluorescence intensities
of assessed cell populations using GraphPad Prism 6 (GraphPad
Software). Statistical significance of results was as indicated in
each figure.
Results
PD-L1 expression is increased by dietary
stilbenoids, resveratrol and/or piceatannol in breast and
colorectal cancer cells
We first assessed any alterations in PD-L1 levels
using the Cal51 breast cancer and HCT116 colon cancer cells treated
with stilbenoids, specifically, resveratrol, piceatannol,
pterostilbene and 3,5,4′-trimethoxystilbene, for comparison with
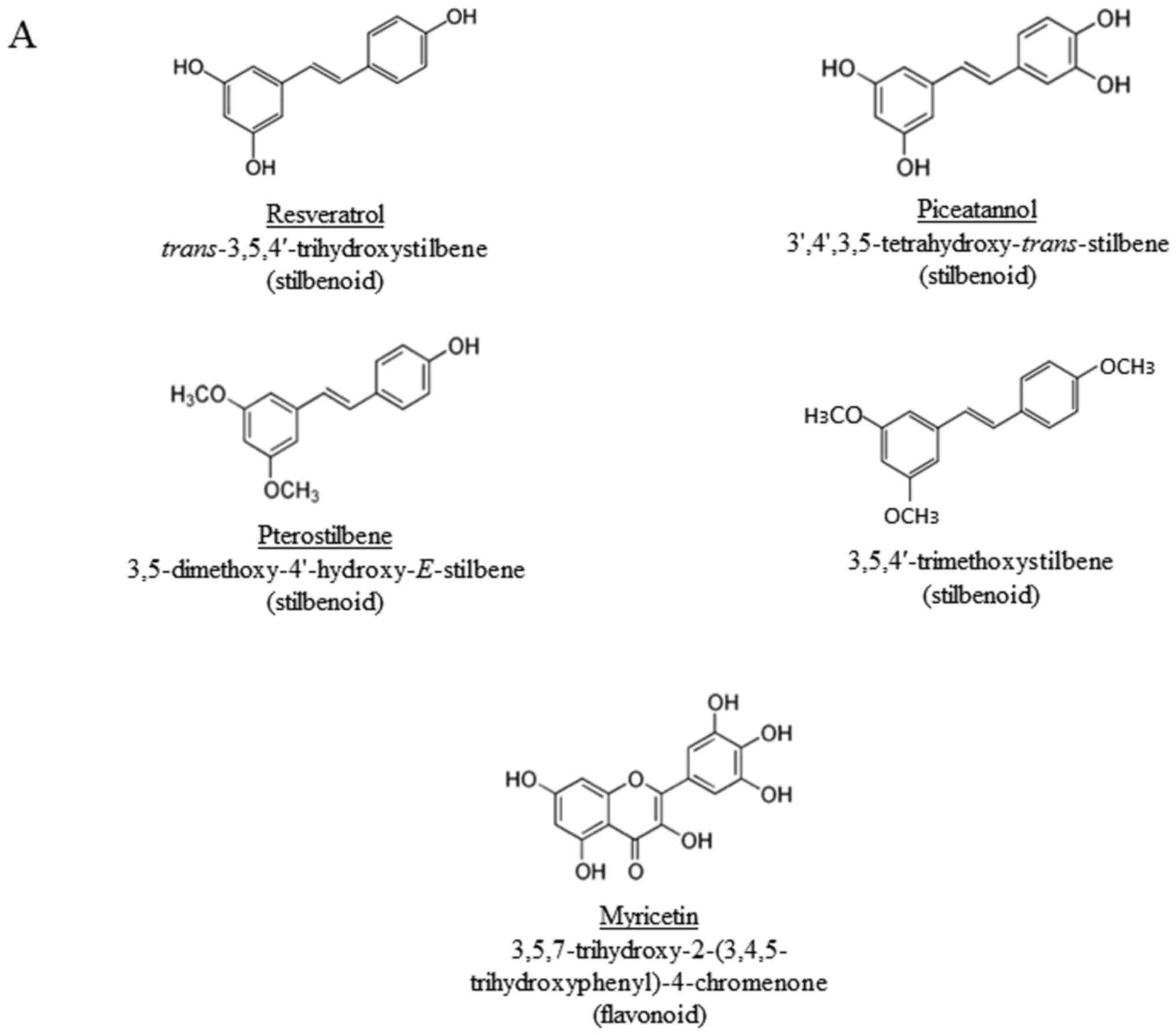
the flavonoid, myricetin (the chemical structures of the compounds
are shown in Fig. 1A). The surface
expression of PD-L1 in the control (DMSO-treated, also referred to
as parent/parental cells) and treated Cal51 and HCT116 cells was
assayed by flow cytometry. Resveratrol significantly increased the
expression of PD-L1 in the Cal51 cells, while treatment with
piceatannol resulted in a marked increase in the PD-L1 level in
HCT116 cells (Fig. 1B).
To determine whether the upregulation of PD-L1 by
resveratrol and piceatannol was broadly or uniquely observed in
specific breast or colon cancer cell lines, we assayed any
alterations in PD-L1 expression using a panel of breast (Cal51,
BT549, BT474 and SKBR3) and colorectal (HCT116, SW480, HT29 and
SW620) cancer cell lines. In addition, we also determined whether
the synergistic upregulation of PD-L1 may result from treatment
with the two stilbenoids. The differential increase in PD-L1
expression induced by resveratrol or piceatannol was observed in
2/4 breast and 3/4 colorectal cancer cell lines treated with either
of the stilbenoids as a single agent (Fig. 2A). The combination of resveratrol
and piceatannol acted synergistically; 50 μM each of
resveratrol and piceatannol combined and referred to as 'combo-100'
resulted in significantly greater induction of PD-L1 expression;
specifically, ≥4.5-fold in the Cal51 and ≥3.5-fold in the SW620
cells than 50 μM of either stilbenoid added alone (Fig. 2A). Gene expression analyses
frequently reveal that the relative abundance of mRNA is only
weakly or even inversely associated with the level of protein
expression (49–51). Thus, in this study, to determine
whether the differential expression level of endogenous PD-L1 mRNA
might contribute to the observed induction of PD-L1 by resveratrol
and piceatannol in these two cell lines, relative to the panel of
the other studied cell lines with the same cancer type grouping,
the basal PD-L1 mRNA expression levels, shown as TPM were obtained
from a public Cancer Cell Line Encyclopedia (CCLE, https://portals.broadinstitute.org/ccle/home) database
for cancer cell lines tested. In the breast cancer cell lines, the
endogenous level of PD-L1 mRNA ranked as follows: Cal51 ≤ BT474
< SKBR3 ≤ BT549 (Fig. 2B);
however, the induction of PD-L1 by co-treatment with resveratrol
and piceatannol yielded the opposite result: Cal51 (~7-fold) ≥
BT474 (~ 4-fold) > SKBR3 (~2-fold) ≥ BT549 (~2-fold) (Fig. 2A). A similar trend was also
observed in the colorectal cancer cells; namely, the endogenous
PD-L1 mRNA ranking was as follows: SW620 ≤ HCT116 < HT29 ≤ SW480
(Fig. 2B), whereas the relative
induction of PD-L1 decreased from high to low as follows: SW620
(~4-fold) > HCT116 (~3-fold) > SW480 (~3-fold) > HT29
(~1.5-fold) (Fig. 2A). These
results suggest that tumors with a low endogenous mRNA level of
PD-L1 are more likely to be affected by resveratrol and/or
piceatannol, alone or in combination.
NF-κB mediates the upregulation of PD-L1
induced by resveratrol and/or piceatannol
IFN-γ is known to induce PD-L1 expression by
upregulating its transcription through the activation of interferon
regulatory transcription factor (IRF-1) (52,53).
Thus, in this study, we investigated whether the same mechanism may
contribute to the resveratrol-and/or piceatannol-mediated induction
of PD-L1 expression. The results of immunohistochemistry revealed
no change in IRF-1 expression in the SW620 cells treated for 48 h
with 'combo-100' compared to the control (data not shown). Since
the NF-κB consensus sequence is also present in the PD-L1 gene
promoter (52,53), and NF-κB plays a major role in the
transcription of PD-L1 by IFN-γ (54-56),
in this study, we examined whether the induction of PD-L1 by
resveratrol and/or piceatannol was due to the activation of NF-κB.
The A549 cells co-expressing the SEAP reporter gene and Lucia
luciferase gene were used to investigate the association between
NF-κB activity and the PD-L1 expression levels following treatment
with the two stilbenoids, alone or combination. In this dual
reporter assay, the secreted SEAP and Lucia luciferase in the
culture supernatant were separately measured to provide a
quantitative readout of the transcriptional impact of NF-κB and the
IFN signaling pathways. The time-dependent (≥24 h) and synergistic
induction of NF-κB expression induced by piceatannol alone and by
combined treatment with resveratrol was observed (Fig. 3A). In response to stimuli, the
inhibitory protein IκB is degraded, which leads to the
release/translocation of heterodimer p65/p50 from the cytoplasm to
the nucleus (57,58). Therefore, the activation of NF-κB
by the combined stilbenoids was examined in the SW620 cells by
immunohistochemistry to analyze changes in the localization of p65;
48 h of exposure to 'combo-100' resulted in an increase in the
translocation and nuclear accumulation of p65, as shown in Fig. 3B (inset).
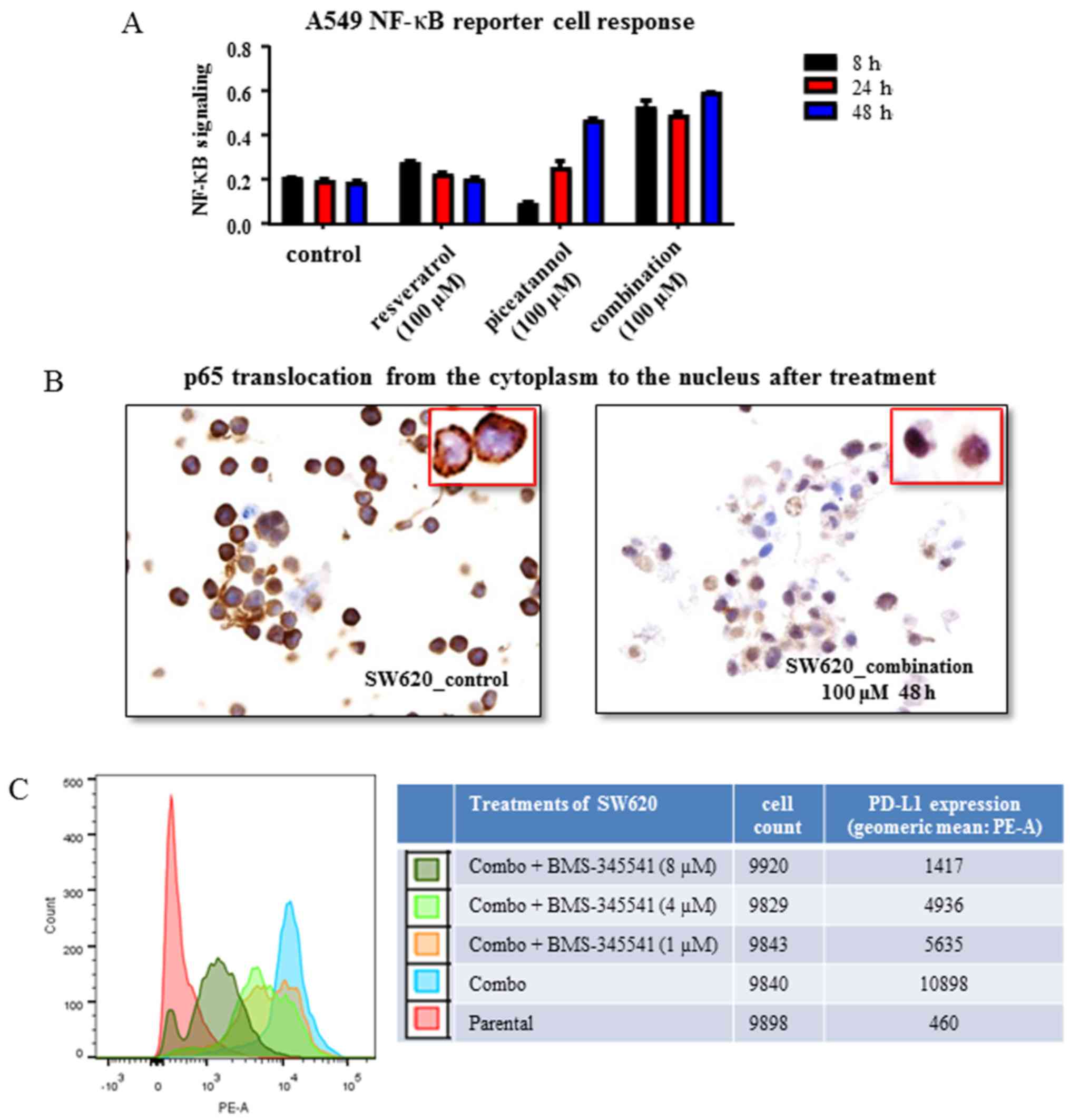 | Figure 3Control of nuclear factor
(NF)-κB-mediated programmed cell death ligand 1 (PD-L1) expression
by resveratrol and piceatannol. (A) A549 dual reporter cells were
used to assay the activation of NF-κB following treatment with
resveratrol or piceatannol, each at 100 μM or combined, each
at 50 μM (referred to as combo-100) to yield a concentration
of 100 μM. Resveratrol as a single agent resulted in an
optimal early time activation of NF-κB, while piceatannol alone
decreased NF-κB signaling at 8 h and followed by a progressive
increases at 24 and 48 h, respectively. When used in combination,
the two stilbenoids led to a marked increase in NF-κB signaling
over the tested duration of 48 h. The parental condition represents
the untreated control. The data shown are from 1 experiment. (B)
Immunohistochemistry of the subcellular localization of NF-κB p65
subunit in SW620 colon cancer cells treated with a combination of
piceatannol and resveratrol, each at 50 μM to yield a
concentration of 100 μM vs. the untreated cells. The
analysis showed that p65 translocated from the cytoplasm to the
nucleus by the combined use of the stilbenoids, indicating the
activation of NF-κB signaling. Images were captured at ×20
magnification and cropped to show the field of cells representative
of the effect of treatment. The results are representative of 5
sections from 1 experiment. We did not quantify the percentage of
cells in which translocation occurred in these samples. The
parental condition represents the control. The image in the inset
represents a high magnification image of the cells in the field of
view. (C) BMS-345541, an inhibitor of IKK, was administered for 24
h in vitro prior to exposure to the combination of
piceatannol and resveratrol, each at 50 μM to yield a
concentration of 100 μM, for a further 48 h. BMS-345541 was
added at increasing concentrations (1, 4 and 8 μM) while the
dose of the combination was kept constant. Following treatment, the
cells were harvested and stained for PD-L1 expression by flow
cytometry. The geometric mean of the mean fluorescent intensity
(MFI) of the phytoerythrin (PE) area was used as the readout for
PD-L1 expression. The parental condition represents the untreated
control. The results are representative of 3 experiments. |
The small molecule, BMS-345541, is an IKK kinase
inhibitor (59) that prevents IκB
phosphorylation, to effectively suppress the translocation of NF-κB
into the nucleus for participation in transcriptional activation of
NF-κB-responsive genes (57,58).
Thus, in this study, we then examined whether the
stilbenoid-induced PD-L1 expression can be blocked by BMS-345541.
In SW620 cells, the induction of PD-L1 by 'combo-100' was inhibited
by 48% with 1 μM BMS-345541 (Fig. 3C). Furthermore, a dose-dependent
inhibition was observed with the 1, 4 and 8 μM of IKK
inhibitor concentration range (Fig.
3C). These results, showing that the inhibition of IKK
significantly decreased PD-L1 expression in SW620 cells suggest
that NF-κB activation is involved in the induction of PD-L1
expression by resveratrol or piceatannol either alone, or in
combination.
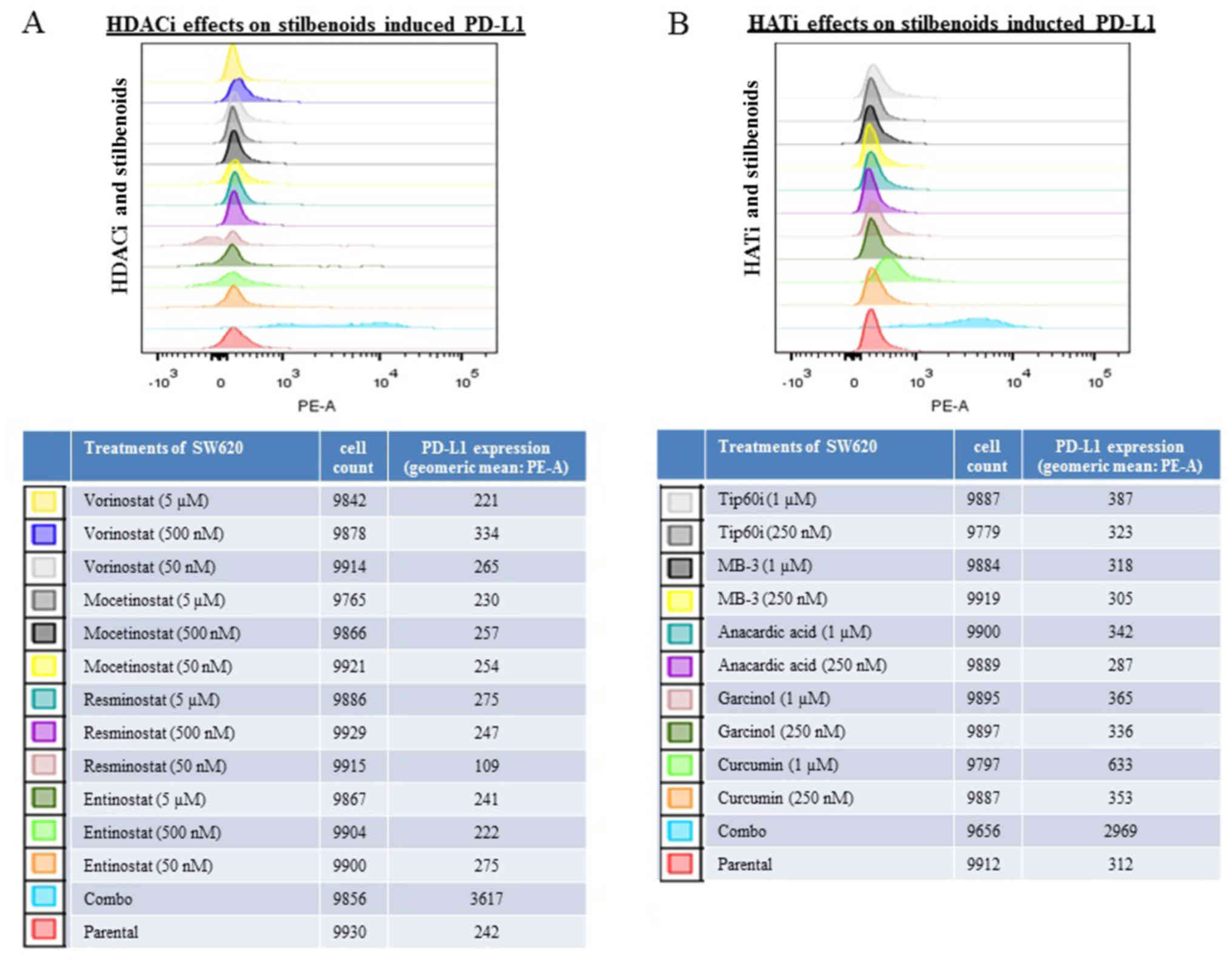
Histone deacetylase inhibitors (HDACis)
and histone acetyltransferase inhibitors (HATis) modulate the
induction of PD-L1 expression induced by the combination of
resveratrol and piceatannol
The expression of PD-L1 can be regulated via histone
acetylation/deacetylation (60,61)
and resveratrol is an activator of HDAC (62). To investigate whether the induction
of PD-L1 by the combination of resveratrol and piceatannol is
blocked by inhibitors of HDAC or HAT, HDACis (vorinostat,
mocetinostat, resminostat and entinostat) and HATis (curcumin,
garcinol, anacardic acid, MB-3 and Tip60i) were used to assess
their effects on the modulation of PD-L1 by the combined use of the
stilbenoids. The cells were pre-treated for 48 h with individual
HDACis/HATis alone or in combination with 60 μM of either of
the stilbenoids, followed by the flow cytometric analysis of PD-L1
expression. The addition of HDACis or HATis alone did not affect
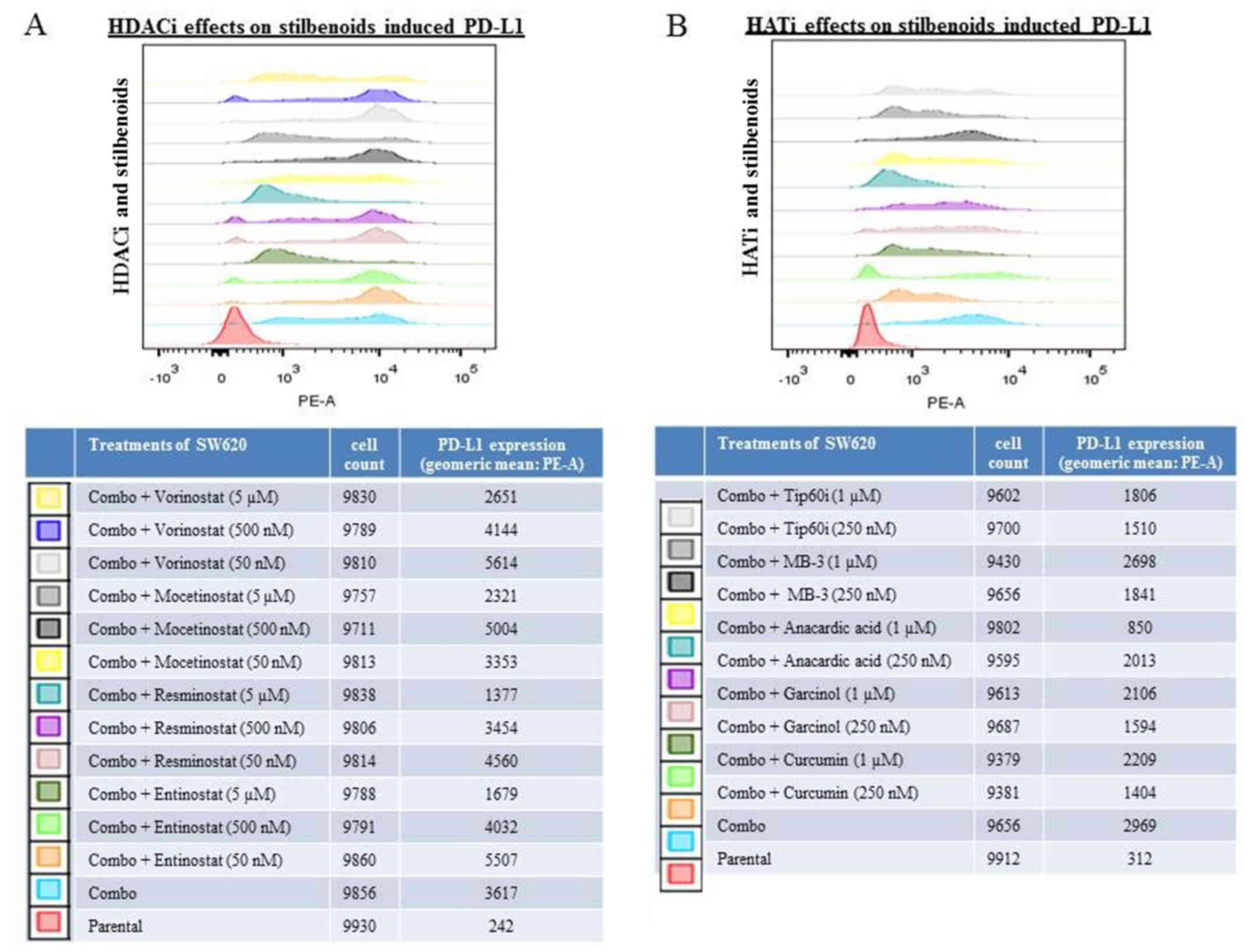
PD-L1 expression compared to the untreated controls (Fig. 4). When the SW620 cells were
pre-treated with histone modification inhibitors, the ability of
the stilbenoids to induce PD-L1 was markedly reduced by two HDACis
(5 μM of resminostat and entinostat) and also by the HATi,
anacardic acid (1 μM) (Fig.
5). These results demonstrated that histone modification
inhibitors can suppress the induction of PD-L1 expression by
stilbenoids. The data are consistent with the interpretation that
the upregulation of PD-L1 by stilbenoids involves transcriptional
control.
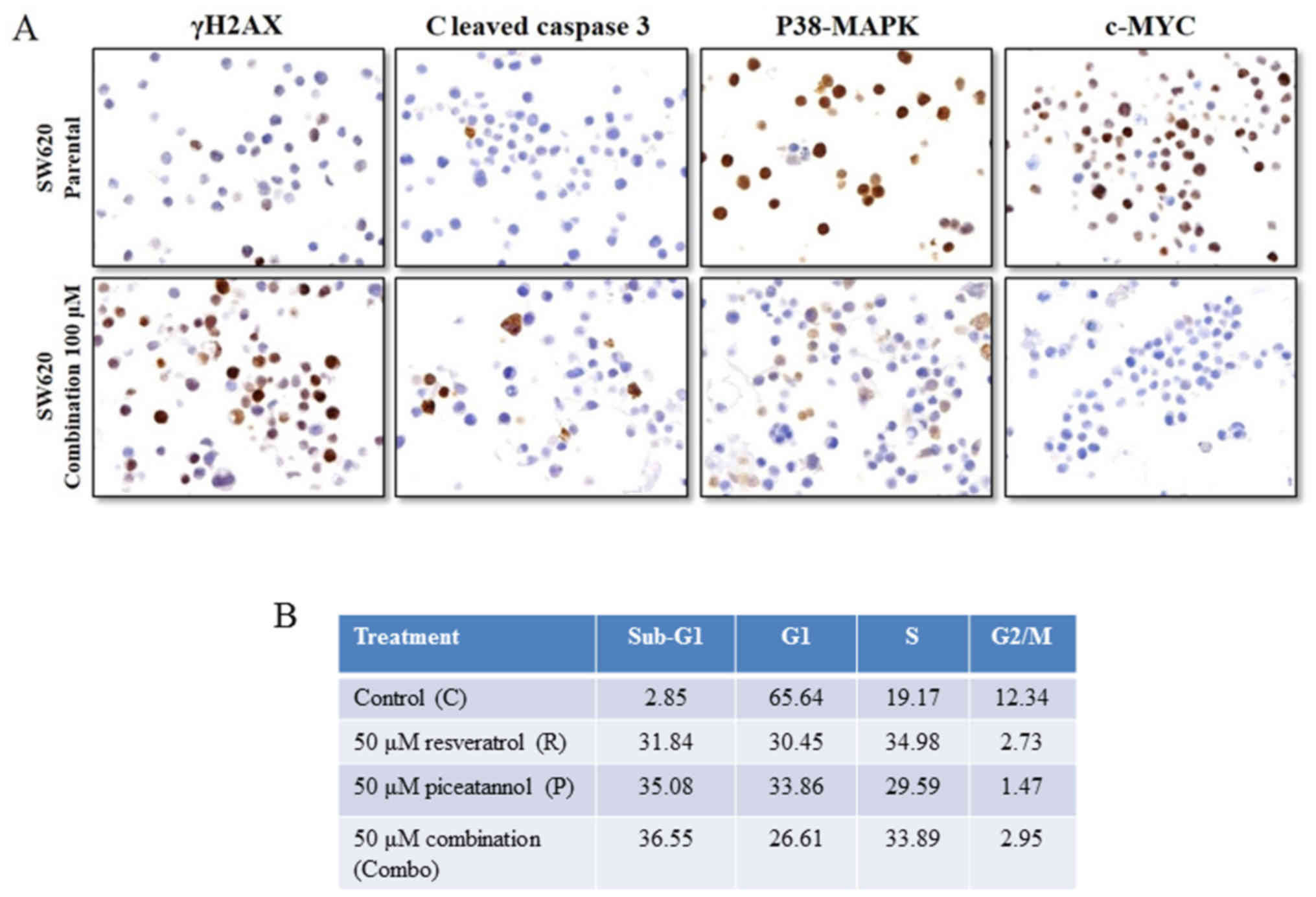
Induction of apoptotic and cell cycle
changes by the combined use of resveratrol and piceatannol
The upregulation of PD-L1 may allow cancers to evade
the host immune system and acquire resistance to anticancer drugs.
Having demonstrated that the upregulation of PD-L1 expression by
stilbenoids in the SW620 colon cancer cells, we then investigated
whether stilbenoids affect the survival status of cells by
analyzing two biomarkers related to apoptosis, namely, the
expression of the DNA damage indicator γH2AX, and that of cleaved
caspase 3. In addition, markers associated with cell survival,
p38-MAPK and c-Myc, were also assessed using immunohistochemistry.
Treatment of the SW620 cells for 48 h with 'combo-100' resveratrol
and piceatannol increased the expression of γH2AX and that of
cleaved caspase 3, and downregulated the p38-MAPK and c-Myc levels
(Fig. 6A). The induction of γH2AX
is characteristic of DNA fragmentation and damage during apoptosis,
and thus supports the interpretation that exposure to resveratrol
and/or piceatannol causes DNA damage and apoptosis via the
activation of caspase 3. We then assessed whether treatment with
the stilbenoids altered cell cycle distribution by flow cytometric
analysis. An increase in the percentage of cells in the S phase of
the cell cycle, from 19 to ~30%, a distinct reduction in the
proportion of G1 phase cells (from 66 to ~30%), and a
marked decrease in the percentage of G2M phase cells,
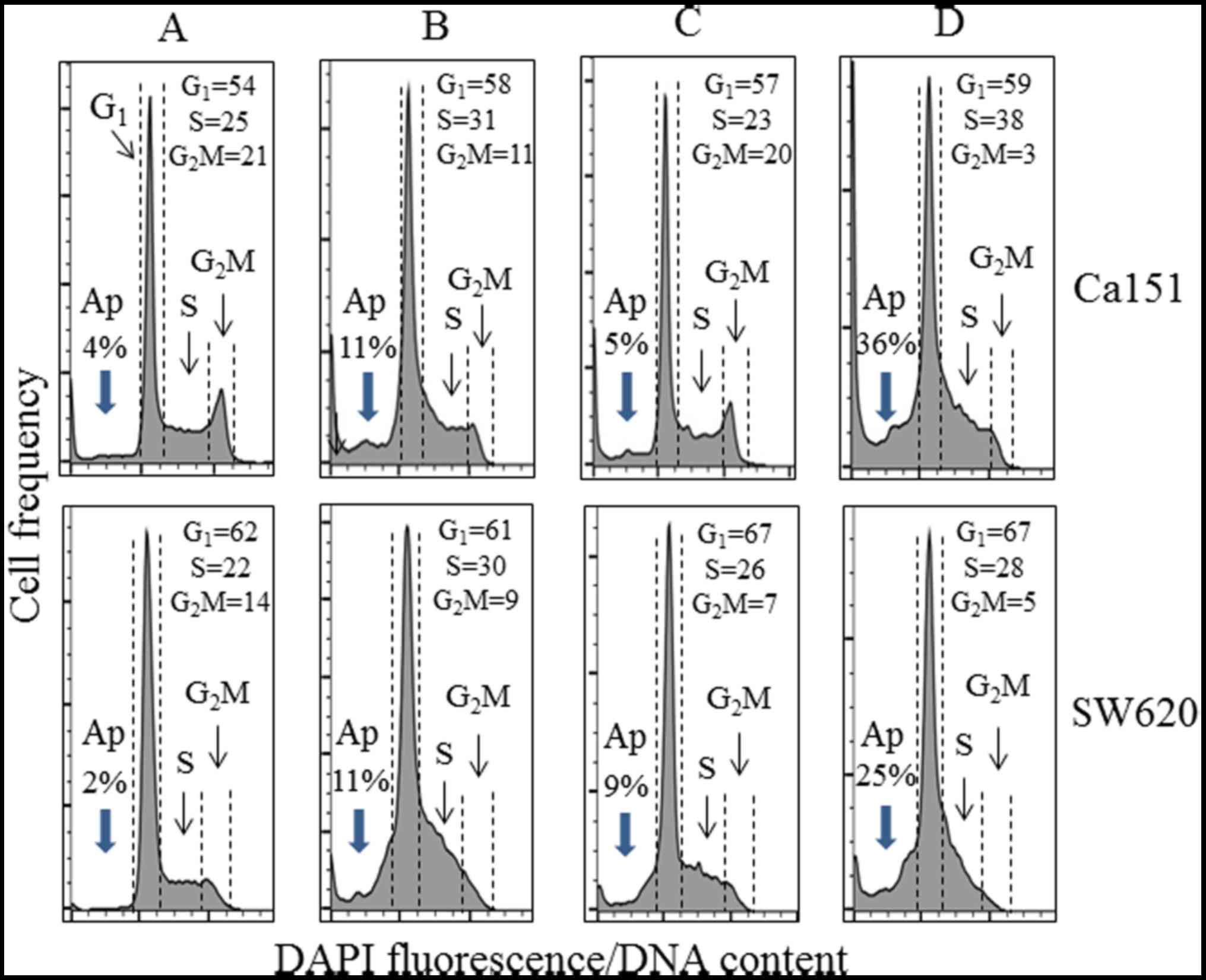
from 12 to ~3% were observed. There was also an increase in the
percentage of cells with fractional DNA content ('sub-G1
cells'), an indication of apoptosis from 2.85% in the control cells
to 31.84, 35.08 and 36.55% in the cells treated with 50 μM
resveratrol, 50 μM piceatannol or 50 μM of these two
compounds for 48 h, respectively (Fig.
6B). We also examined and determined that low-dose (10
μM) resveratrol and/or piceatannol did not induce PD-L1
expression, whereas the analysis of cell cycle phase distribution
changes using Cal51 and SW620 cell cultures treated for 48 h
revealed an apparent increase in the proportion of S-phase cells
concomitant with the reduction of G2M-phase cells in
cultures treated with resveratrol alone, and to an even greater
extent following treatment with both stilbenoids (Fig. 7). In addition, the combination of
resveratrol and piceatannol induced apoptosis in both cell types to
a much greater degree than each of them alone, as demonstrated by
an increase in the percentage of cells with fractional DNA content
('sub-G1 cells'), an indication of apoptosis (compare
Fig. 7A with Fig. 7B–D) (48,63,64).
Taken together, our findings indicate that stilbenoids not only
increase PD-L1 expression, but may also induce DNA damage, leading
to increased cell death in tumor cells, such as SW620 colon cancer
cells.
Discussion
An association has been observed between a decrease
in T-cell proliferation and an increase in apoptosis and tumor
immune evasion, with an increase in the expression of T-cell
inhibitory protein PD-L1 on cancer cells (2,3),
providing the impetus for understanding the control of PD-L1
expression using cancer cells as a model. In this study, we
examined the hypothesis that dietary stilbenoids may act as
modulators of PD-L1 expression in tumor cells. We focused on the
effects of resveratrol, a grape-derived polyphenol that has shown
chemopreventive effects in various cancer types (33–42),
and its hydroxylated derivative, piceatannol. Questions raised and
addressed in this study included whether: i) Structurally-modified
stilbenoids have the same ability to modulate PD-L1 expression; ii)
the effects of stilbenoids on PD-L1 expression can apply to
different cancer types; iii) signaling pathways that control the
stilbenoid-induced PD-L1 expression are effected; and iv) histone
modification inhibitors (HDACis and HATis) block stilbenoid-induced
PD-L1 expression.
Stilbenoids in general exert beneficial effects on
human health (65). It has been
reported that the 4′-hydroxy group of resveratrol is essential for
its bioactivity (66). In this
study, we used breast and colorectal cancer cells to compare the
modulatory effects of stilbenoids on PD-L1 expression, namely,
piceatannol (3′,4′,3,5-trans-trihydroxystilbene),
3,5,4′-trimethoxystilbene, and pterostilbene
(3,5-dimethoxy-4′-hydroxy-E-stilbene), with resveratrol
(3,5,4′-trans-trihydroxystilbene) and myricetin, a naturally
occurring flavonoid present in abundance in edible foods (67). The results presented in Figs. 1 and 2 demonstrate that the hydroxyl, but not
methoxy groups on stilbenoids are important for the induction of
PD-L1. However, no increase in PD-L1 expression was evident in the
cells treated with myricetin
(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone), a
flavonoid that contains 6 hydroxyl groups (Fig. 1). These results suggest that the
3,5-dihydroxy-trans-stilbene structure is required for the
induction of PD-L1 in the cells used.
The expression level PD-L1 of was significantly
increased in the breast and colon cancer cells treated with
resveratrol and piceatannol, both as a single agent and when used
in combination (Figs. 1 and
2). Moreover, PD-L1 expression was
synergistically upregulated 4.5-fold in the Cal51 breast cancer and
≥3.5-fold in the SW620 colon cancer cells by the combined use of
resveratrol and piceatannol (Fig.
2A). Of note, both cancer cells express low basal levels of
PD-L1 mRNA expression (Fig. 2B).
Since, as noted, a positive response and improved clinical outcome
to anti-PD-L1 blockade therapy are best observed in patients with
PD-L1-positive tumors (presumably reflecting a high expression of
PD-L1), we surmise that agents capable of upregulating PD-L1
expression in tumor cells expressing low PD-L1 can sensitize such
cancer cells for an improved response to PD-L1 blockade. As a
corollary, we also hypothesized that the combined use of
resveratrol and piceatannol co-administered with anti-PD-L1
immunotherapy may exhibit clinical benefits in cancer patients with
no-or- low-PD-L1 tumors. Whether the efficacy to PD-L1 blockade may
be enhanced by the combined intake of resveratrol and/or
piceatannol, concomitantly or sequentially, remains to be
verified.
This study also provides evidence showing that the
resvera-trol- and piceatannol-mediated upregulation of PD-L1
requires the activation of NF-κB (Fig.
3). In the SW620 cells, we showed that the induction of PD-L1
expression induced by resveratrol/piceatannol was attenuated by the
IKK inhibitor, BMS 345541 (Fig.
3C). It has been previously demonstrated that the duration and
function of nuclear NF-κB is regulated by reversible
acetylation/deacetylation (68),
and that NF-κB transcriptional response is controlled by the
HDAC3-mediated deacetylation of RelA acting as an intranuclear
molecular switch for turning 'on-off' the NF-κB response (68); we therefore examined whether
histone modification inhibitors affect the resveratrol/
piceatannol-mediated transcriptional control of PD-L1. Our findings
revealed that two HDACis (e.g., resminostat and entinostat) and one
HATi, anacardic acid, effectively blocked the induction of PD-L1
expression by resveratrol/piceatannol (Fig. 5). Both resminostat and entinostat
are HDAC3 inhibitors, thus lending support that the HDAC3-mediated
NF-κB response plays a role in resveratrol/piceatannol-induced
PD-L1 expression. The suppression of PD-L1 induction by resveratrol
using anacardic acid is in accordance with the described effect of
anacardic acid as a HATi for p300 and p300/CBP and data reporting
that resveratrol is a p300 activator (69,70).
These results indicate that the expression of PD-L1 is regulated by
the mechanism of histone acetylation/deacetylation and that
resveratrol/piceatannol induces PD-L1 expression through
HDAC3/p300-mediated NF-κB control.
It should be mentioned that the upregulation of
PD-L1 by resveratrol or piceatannol occur at doses not achievable
physiologically and may exceed pharmacologically relevant
concentrations (26,71). Conceivably, the effective dose
could also be modulated by factors present locally at the site of
responsive cells/tumors (e.g., different hormones, cytokines,
products of cell metabolism or variable oxygen tension) and thus
may additionally affect sensitivity of PD-1/PD-L1 checkpoint to
these compounds, perhaps amplifying their potential anticancer
effect. It should also be noted that the doses used in the present
experiments were based on titration studies (data not shown), and
that the effectiveness of single or combined agents on the
induction of PD-L1 in each cell line is above IC50. It
would be of interest to determine what might account for the
variations observed in dosage dependence in different cell lines.
Since the induction of PD-L1 expression by resveratrol and
piceatannol are mediated through the NF-κB signaling pathway; the
different dose-dependent responses and the upregulation of PD-L1 by
resveratrol and piceatannol may be due to the variation in the
endogenous level of NF-κB components, vis-à-vis, NF-κB1 (p105),
NF-κB2 (p65), CHUK (IKK-α), IKBKB (IKK-β) and IKBKG (IKK-γ), in
each of the cell lines tested. In RNA-seq analyses, we found that
Cal51 and SW620, both with a low endogenous level of PD-L1
expression, expressed high levels NF-κB2 (p65) and CHUK (IKK-α)
compared to cancer cell lines from same anatomical origin showing
high PD-L1 expression (data not shown). Thus, it is tempting to
propose that response in the induction of PD-L1 by stilbenoids in
different cell lines from identical cancer types may be attributed
to the level of expression of NF-κB2 (p65) and CHUK (IKK-α).
Currently, experiments are underway to further test and confirm our
hypothesis.
Another result of note in this study is that the
SW620 cells exposed to a high dose of both stilbenoids were
partially restricted in cell cycle transition in the
G2/M phase and display evidence of apoptosis (Fig. 6). Furthermore, the accumulation of
cells in the S phase of the cell cycle may also be associated with
an increase in their expression of PD-L1. This suggests that
resveratrol and piceatannol affect cancer cells by a dual
mechanism: i) The induction of PD-L1 that sensitizes tumor cells
for recognition by anti-PD-L1 antibodies; an effect that could
diminish cancer cell evasion from immune surveillance; and ii) the
direct induction of cell cycle arrest, increase in DNA damage and
cancer cell destruction via induction of apoptosis.
Since cancer patients expressing tumors positive for
PD-L1, a negative T-cell regulatory molecule, demonstrate efficacy
to anti-PD-L1 blockade therapy with an improved clinical outcome,
one might surmise that low PD-L1-expressing tumors may be
sensitized and may display an improved responsiveness to PD-L1
blockade therapy using dietary agents. The cell culture experiments
used in this study may be considered as a model for testing whether
the sensitivity and responsiveness of tumor cells to PD-L1 targeted
therapy can be augmented by priming with or co-exposure to
stilbenoids, such as resveratrol and/or piceatannol. The hypothesis
raised is as follows: The upregulation of membrane-associated PD-L1
in low PD-L1-expressing tumor cells is a 'find-me' approach for
targeting by immune checkpoint inhibitors to potentially improve
the effi-cacy of anti-PD-L1 blockade therapy via stilbenoids.
Indeed, we believe that the elevation of PD-L1 expression, as we
have demonstrated in this study using pharmacological doses of the
stilbenoids, resveratrol and piceatannol, may underlie the
unresolved challenge in that the positive response to immune
checkpoint blockade therapy in 19-31% of treated individuals is a
limited to number of clinical indications, typically in patients
whose tumors express elevated-PD-L1, which we stated explicitly in
the Introduction. Thus, while on teleological grounds, the
upregulation of PD-L1 by polyphenols in cancer could promote
disease progression, we offer the consideration that the observed
stilbenoid-induced PD-L1 increase be viewed from the hypothesis
that agents capable of upregulating PD-L1 expression in tumor cells
could sensitize cancer cells for an improved clinical response to
PD-L1 immune checkpoint blockade therapy. Testing these aspects
would constitute a novel approach to confirm our hypothesis. These
possibilities are under further investigative considerations in our
laboratory. Studies are also planned to explore whether stilbenoids
may impact host immune response, for example, by affecting PD-1
expression in PD-1-expressing Jurkat T-cells.
Funding
This study was supported in part by the Robert A.
Welke Cancer Research Foundation (to HDH and ZD).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL performed experiments and analyzed the data (cell
culture analysis, immunohistochemistry, flow cytometric analysis,
NF-κB reporter assay and statistical analysis) and edited the
manuscript. TCH interpreted the data and wrote the manuscript. HDH
collected and analyzed/interpreted the data (cell cycle and
apoptosis analyses). ZD interpreted the data and wrote the section
of cell cycle and apoptosis analyses and edited the manuscript. JMW
interpreted the data, and wrote and edited the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors acknowledge that the RNA-seq data
presented in Fig. 2B were obtained
from the Cancer Cell Line Encyclopedia (CCLE) database (https://portals.broadinstitute.org/ccle/home).
References
|
1
|
Okazaki T and Honjo T: PD-1 and PD-1
ligands: From discovery to clinical application. Int Immunol.
19:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamazaki T, Akiba H, Iwai H, Matsuda H,
Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al:
Expression of programmed death 1 ligands by murine T cells and APC.
J Immunol. 169:5538–5545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hino R, Kabashima K, Kato Y, Yagi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 ligand 1 is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Hamrouni A, Wolowiec D, Coiteux V,
Kuliczkowski K, Hetuin D, Saudemont A and Quesnel B: Plasma cells
from multiple myeloma patients express B7-H1 (PD-L1) and increase
expression after stimulation with IFN-{gamma} and TLR ligands via a
MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 110:296–304.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lyford-Pike S, Peng S, Young GD, Taube JM,
Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et
al: Evidence for a role of the PD-1:PD-L1 pathway in immune
resistance of HPV-associated head and neck squamous cell carcinoma.
Cancer Res. 73:1733–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases–elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He J, Hu Y, Hu M and Li B: Development of
PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment
for Non-Small Cell Lung Cancer. Sci Rep. 5:131102015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noh H, Hu J, Wang X, Xia X, Satelli A and
Li S: Immune checkpoint regulator PD-L1 expression on tumor cells
by contacting CD11b positive bone marrow derived stromal cells.
Cel. Commun Signal. 13:142015. View Article : Google Scholar
|
|
12
|
Kim JM and Chen DS: Immune escape to
PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann
Oncol. 27:1492–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Topalian SL, Sznol M, McDermott DF, Kluger
HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB,
Powderly JD, et al: Survival, durable tumor remission, and
long-term safety in patients with advanced melanoma receiving
nivolumab. J Clin Oncol. 32:1020–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gadiot J, Hooijkaas AI, Kaiser AD, van
Tinteren H, van Boven H and Blank C: Overall survival and PD-L1
expression in metastasized malignant melanoma. Cancer.
117:2192–2201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous von-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar
|
|
20
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: CheckMate 025 investigators: Nivolumab versus
everolimus in advanced renal-cell carcinoma. N Engl J Med.
373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L and Han X: Anti-PD-1/PD-L1 therapy
of human cancer: Past, present, and future. J Clin Invest.
125:3384–3391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv42016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar :
|
|
26
|
Hsieh TC and Wu JM: Resveratrol:
Biological and pharmaceutical properties as anticancer molecule.
Biofactors. 36:360–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saud SM, Li W, Morris NL, Matter MS,
Colburn NH, Kim YS and Young MR: Resveratrol prevents tumorigenesis
in mouse model of Kras activated sporadic colorectal cancer by
suppressing oncogenic Kras expression. Carcinogenesis.
35:2778–2786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rahman I, Biswas SK and Kirkham PA:
Regulation of inflammation and redox signaling by dietary
polyphenols. Biochem Pharmacol. 72:1439–1452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dvorakova M and Landa P: Anti-inflammatory
activity of natural stilbenoids: A review. Pharmacol Res.
124:126–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park EJ and Pezzuto JM: The pharmacology
of resveratrol in animals and humans. Biochim Biophys Acta.
1852:1071–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Britton RG, Kovoor C and Brown K: Direct
molecular targets of resveratrol: Identifying key interactions to
unlock complex mechanisms. Ann N Y Acad Sci. 1348:124–133. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harikumar KB and Aggarwal BB: Resveratrol:
A multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tennen RI, Michishita-Kioi E and Chua KF:
Finding a target for resveratrol. Cell. 148:387–389. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova
SP, Lee KW, Bode AM and Dong Z: Resveratrol directly targets COX-2
to inhibit carcinogenesis. Mol Carcinog. 47:797–805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ito Y, Mitani T, Harada N, Isayama A,
Tanimori S, Takenaka S, Nakano Y, Inui H and Yamaji R:
Identification of carbonyl reductase 1 as a resveratrol-binding
protein by affinity chromatography using
4′-amino-3,5-dihydroxy-trans-stilbene. J Nutr Sci Vitaminol
(Tokyo). 59:358–364. 2013. View Article : Google Scholar
|
|
37
|
Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh
TC, Wu JM and Zhang Z: Crystal structure of quinone reductase 2 in
complex with resveratrol. Biochemistry. 43:11417–11426. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsieh TC, Wang Z, Deng H and Wu JM:
Identification of glutathione sulfotransferase-pi (GSTP1) as a new
resveratrol targeting protein (RTP) and studies of
resveratrol-responsive protein changes by resveratrol affinity
chromatography. Anticancer Res. 28A:29–36. 2008.
|
|
39
|
Wang Z, Hsieh TC, Zhang Z, Ma Y and Wu JM:
Identification and purification of resveratrol targeting proteins
using immobilized resveratrol affinity chromatography. Biochem
Biophys Res Commun. 323:743–749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Athar M, Back JH, Kopelovich L, Bickers DR
and Kim AL: Multiple molecular targets of resveratrol:
Anti-carcinogenic mechanisms. Arch Biochem Biophys. 486:95–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsieh TC, Lin CY, Bennett DJ, Wu E and Wu
JM: Biochemical and cellular evidence demonstrating AKT-1 as a
binding partner for resveratrol targeting protein NQO2. PLoS One.
9:e1010702014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Calleri E, Pochetti G, Dossou KSS,
Laghezza A, Montanari R, Capelli D, Prada E, Loiodice F, Massolini
G, Bernier M, et al: Resveratrol and its metabolites bind to PPARs.
ChemBioChem. 15:1154–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Y, Paik JH, Cho D, Cho JA and Kim CW:
Resveratrol induces the suppression of tumor-derived
CD4+CD25+ regulatory T cells. Int
Immunopharmacol. 8:542–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zou T, Yang Y, Xia F, Huang A, Gao X, Fang
D, Xiong S and Zhang J: Resveratrol inhibits CD4+ T cell
activation by enhancing the expression and activity of Sirt1. PLoS
One. 8:e751392013. View Article : Google Scholar
|
|
45
|
Craveiro M, Cretenet G, Mongellaz C,
Matias MI, Caron O, de Lima MCP, Zimmermann VS, Solary E, Dardalhon
V, Dulić V, et al: Resveratrol stimulates the metabolic
reprogramming of human CD4+ T cells to enhance effector
function. Sci Signal. 10:102017. View Article : Google Scholar
|
|
46
|
Patel KR, Scott E, Brown VA, Gescher AJ,
Steward WP and Brown K: Clinical trials of resveratrol. Ann N Y
Acad Sci. 1215:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Patel KR, Brown VA, Jones DJ, Britton RG,
Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA,
et al: Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Darzynkiewicz Z, Bruno S, Del Bino G,
Gorczyca W, Hotz MA, Lassota P and Traganos F: Features of
apoptotic cells measured by flow cytometry. Cytometry. 13:795–808.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maier T, Güell M and Serrano L:
Correlation of mRNA and protein in complex biological samples. FEBS
Lett. 583:3966–3973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vogel C and Marcotte EM: Insights into the
regulation of protein abundance from proteomic and transcriptomic
analyses. Nat Rev Genet. 13:227–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Y, Beyer A and Aebersold R: On the
dependency of cellular protein levels on mRNA abundance. Cell.
165:535–550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2015. View Article : Google Scholar
|
|
53
|
Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI,
Park YM, Oh S, Shin JG, Yao S, Chen L, et al: Interferon regulatory
factor-1 is prerequisite to the constitutive expression and
IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett.
580:755–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y
and Zhang P: Interferon-γ-induced PD-L1 surface expression on human
oral squamous carcinoma via PKD2 signal pathway. Immunobiology.
217:385–393. 2012. View Article : Google Scholar
|
|
55
|
Abiko K, Matsumura N, Hamanishi J,
Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I
and Mandai M: IFN-γ from lymphocytes induces PD-L1 expression and
promotes progression of ovarian cancer. Br J Cancer. 112:1501–1509.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mandai M, Hamanishi J, Abiko K, Matsumura
N, Baba T and Konishi I: Dual faces of IFNγ in cancer progression:
A role of PD-L1 induction in the determination of pro- and
antitumor immunity. Clin Cancer Res. 22:2329–2334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Goto K, Chiba Y and Misawa M: IL-13
induces translocation of NF-kappaB in cultured human bronchial
smooth muscle cells. Cytokine. 46:96–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hideshima H, Yoshida Y, Ikeda H, Hide M,
Iwasaki A, Anderson KC and Hideshima T: IKKβ inhibitor in
combination with bortezomib induces cytotoxicity in breast cancer
cells. Int J Oncol. 44:1171–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Burke JR, Pattoli MA, Gregor KR, Brassil
PJ, MacMaster JF, McIntyre KW, Yang X, Iotzova VS, Clarke W, Strnad
J, et al: BMS-345541 is a highly selective inhibitor of I kappa B
kinase that binds at an allosteric site of the enzyme and blocks
NF-kappa B-dependent transcription in mice. J Biol Chem.
278:1450–1456. 2003. View Article : Google Scholar
|
|
60
|
Kroesen M, Gielen P, Brok IC, Armandari I,
Hoogerbrugge PM and Adema GJ: HDAC inhibitors and immunotherapy; a
double edged sword. Oncotarget. 5:6558–6572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Woods DM, Sodré AL, Villagra A, Sarnaik A,
Sotomayor EM and Weber J: HDAC inhibition upregulates PD-1 ligands
in melanoma and augments immunotherapy with PD-1 blockade. Cancer
Immunol Res. 3:1375–1385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kajstura M, Halicka HD, Pryjma J and
Darzynkiewicz Z: Discontinuous fragmentation of nuclear DNA during
apoptosis revealed by discrete 'sub-G1' peaks on DNA content
histograms. Cytometry A. 71:125–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Darzynkiewicz Z, Halicka HD and Zhao H:
Analysis of cellular DNA content by flow and laser scanning
cytometry. Adv Exp Med Biol. 676:137–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Akinwumi BC, Bordun KM and Anderson HD:
Biological activities of stilbenoids. Int J Mol Sci. 19:192018.
View Article : Google Scholar
|
|
66
|
Matsuoka A, Takeshita K, Furuta A, Ozaki
M, Fukuhara K and Miyata N: The 4′-hydroxy group is responsible for
the in vitro cytogenetic activity of resveratrol. Mutat Res.
521:29–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Semwal DK, Semwal RB, Combrinck S and
Viljoen A: Myricetin: A dietary molecule with diverse biological
activities. Nutrients. 8:902016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen Lf, Fischle W, Verdin E and Greene
WC: Duration of nuclear NF-kappaB action regulated by reversible
acetylation. Science. 293:1653–1657. 2001. View Article : Google Scholar
|
|
69
|
Shakibaei M, Buhrmann C and Mobasheri A:
Resveratrol-mediated SIRT-1 interactions with p300 modulate
receptor activator of NF-kappaB ligand (RANKL) activation of
NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived
cells. J Biol Chem. 286:11492–11505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zeng Z, Cheng S, Chen H, Li Q, Hu Y, Wang
Q, Zhu X and Wang J: Activation and overexpression of Sirt1
attenuates lung fibrosis via P300. Biochem Biophys Res Commun.
486:1021–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gescher AJ and Steward WP: Relationship
between mechanisms, bioavailibility, and preclinical
chemopreventive efficacy of resveratrol: A conundrum. Cancer
Epidemiol Biomarkers Prev. 12:953–957. 2003.PubMed/NCBI
|