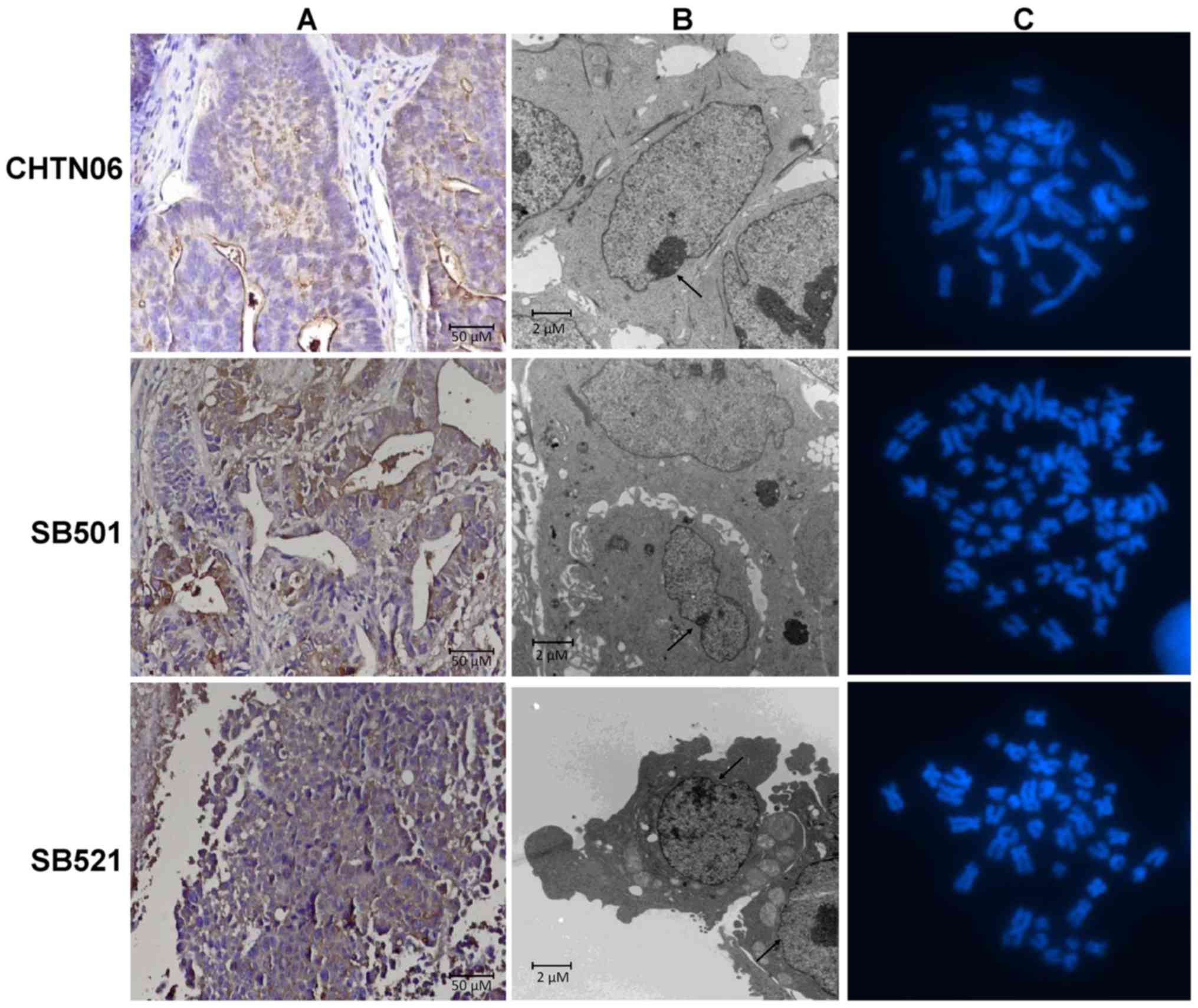
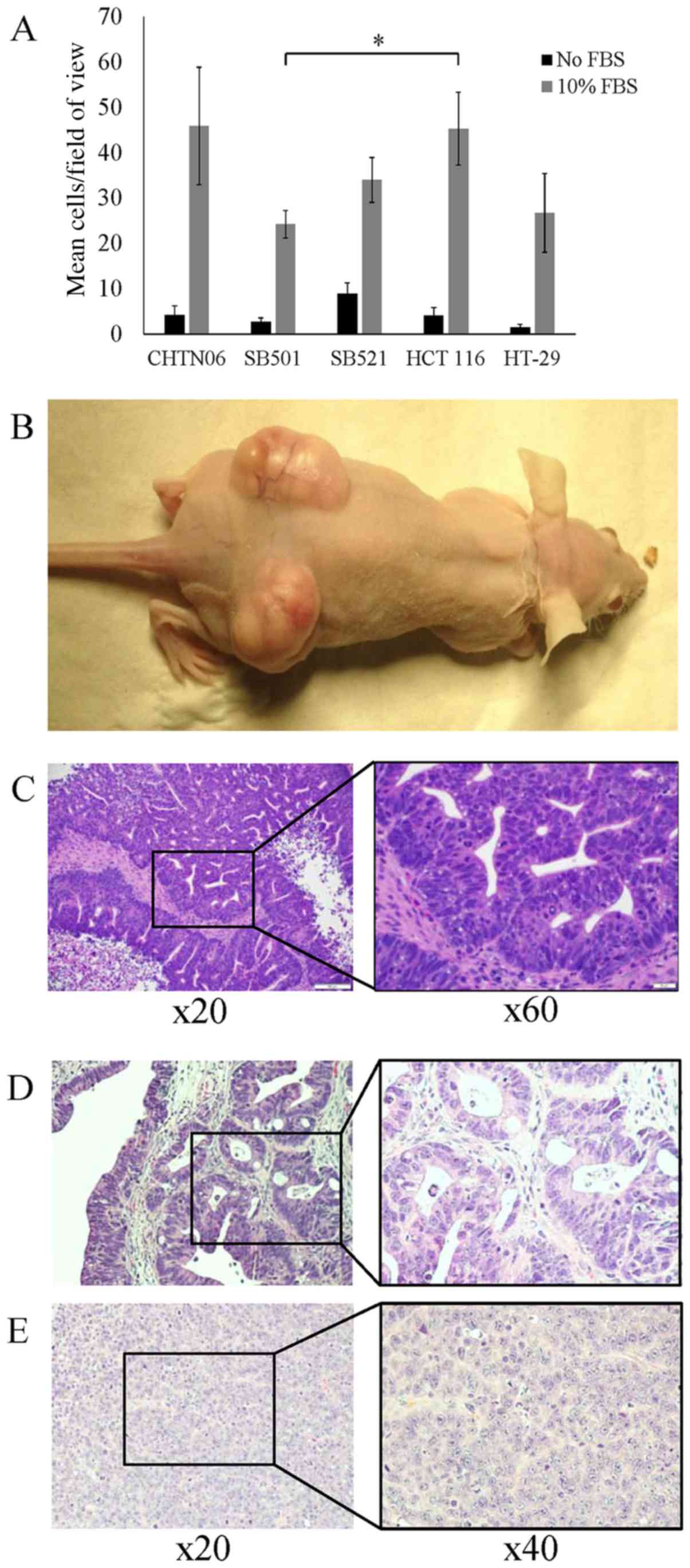
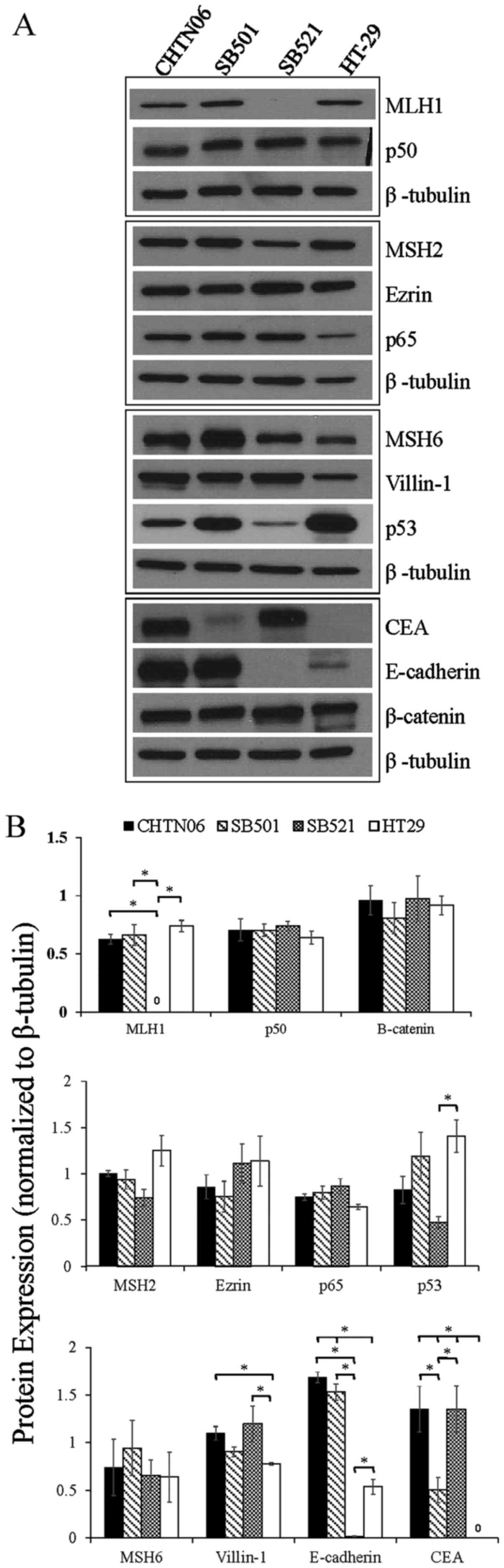
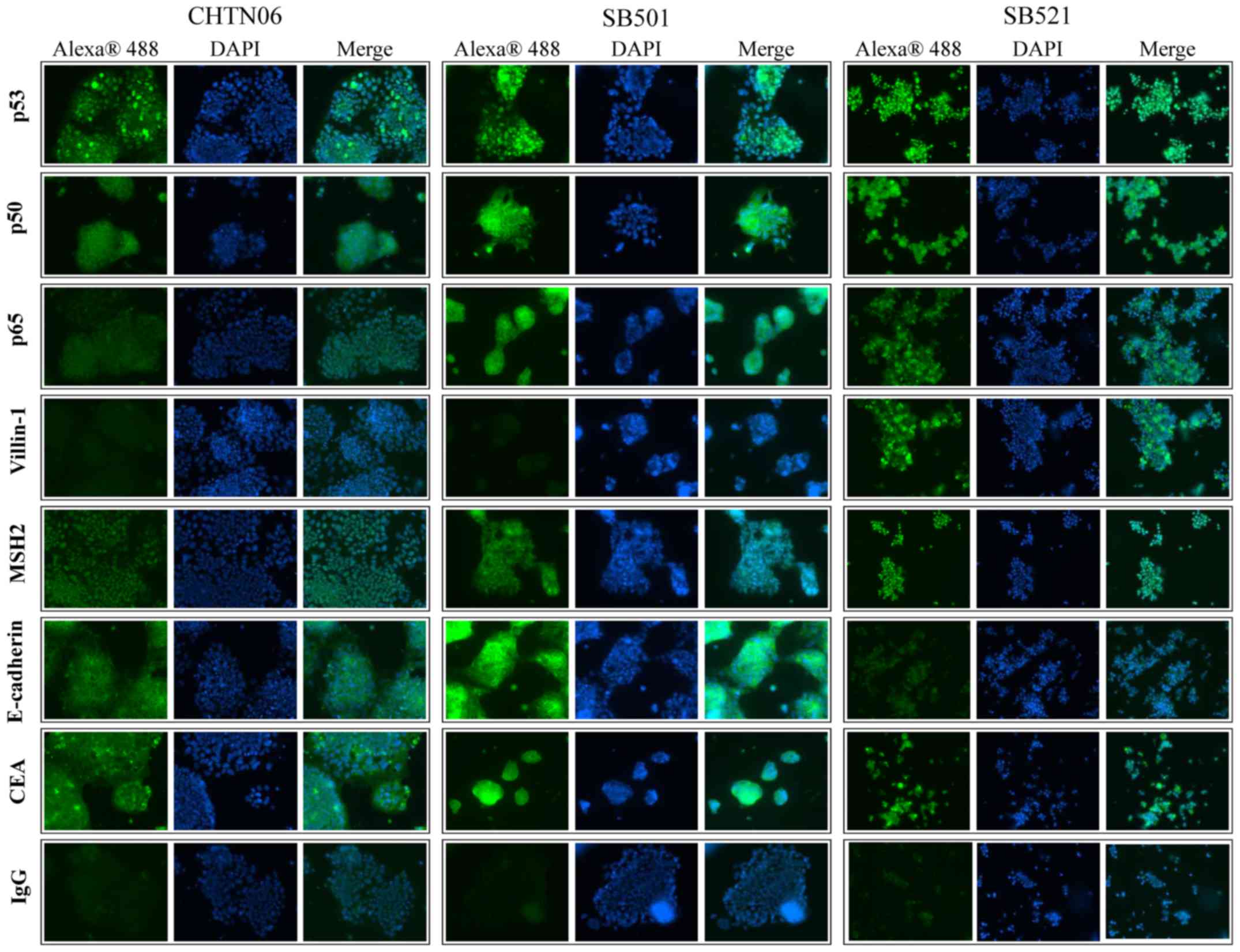
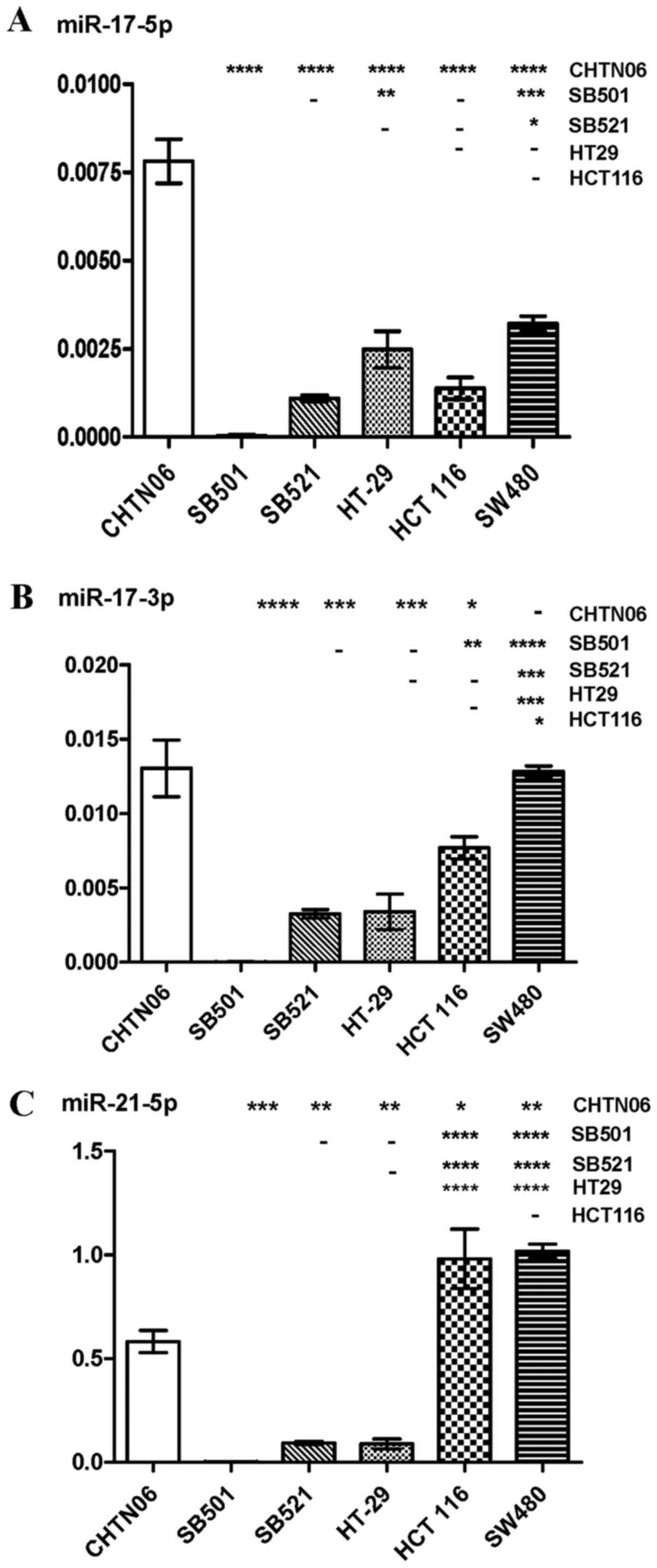
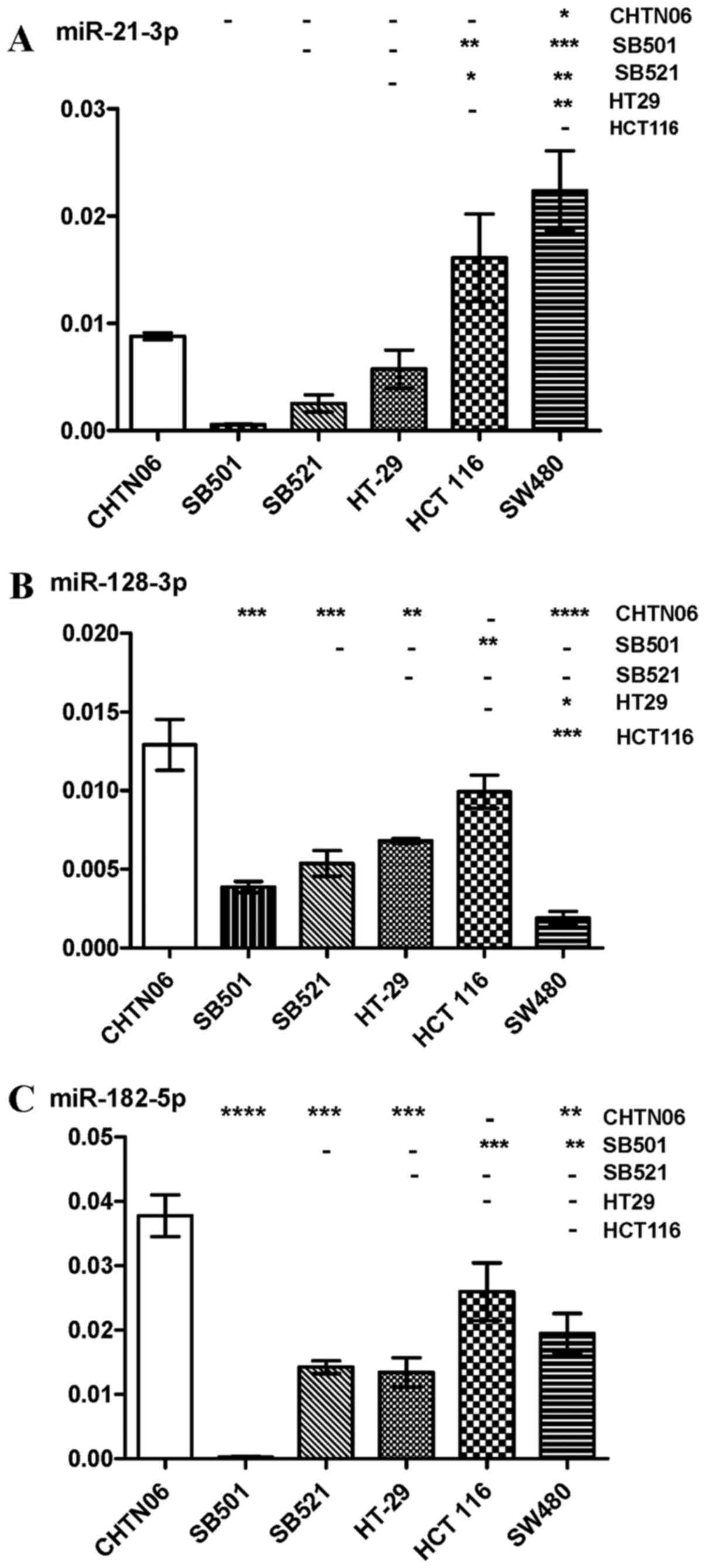
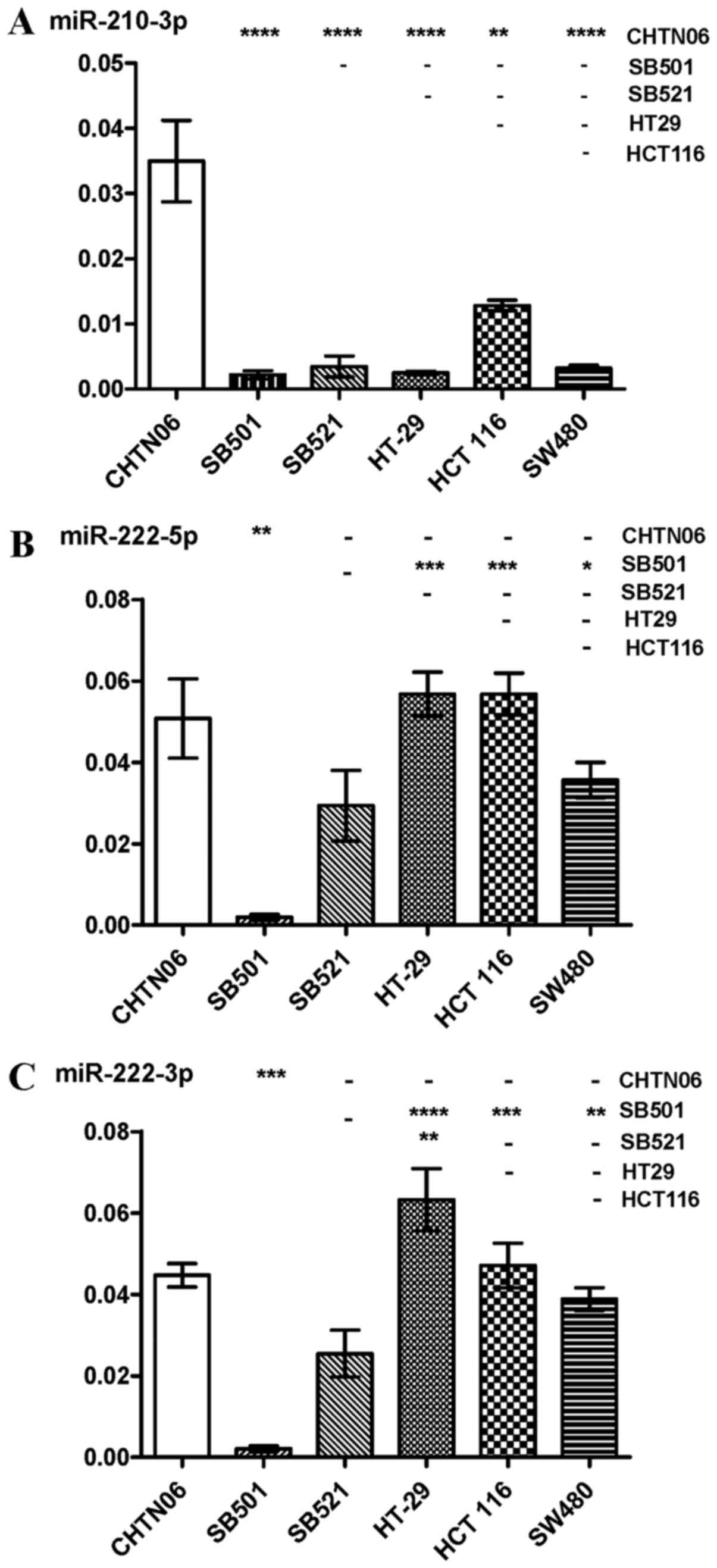
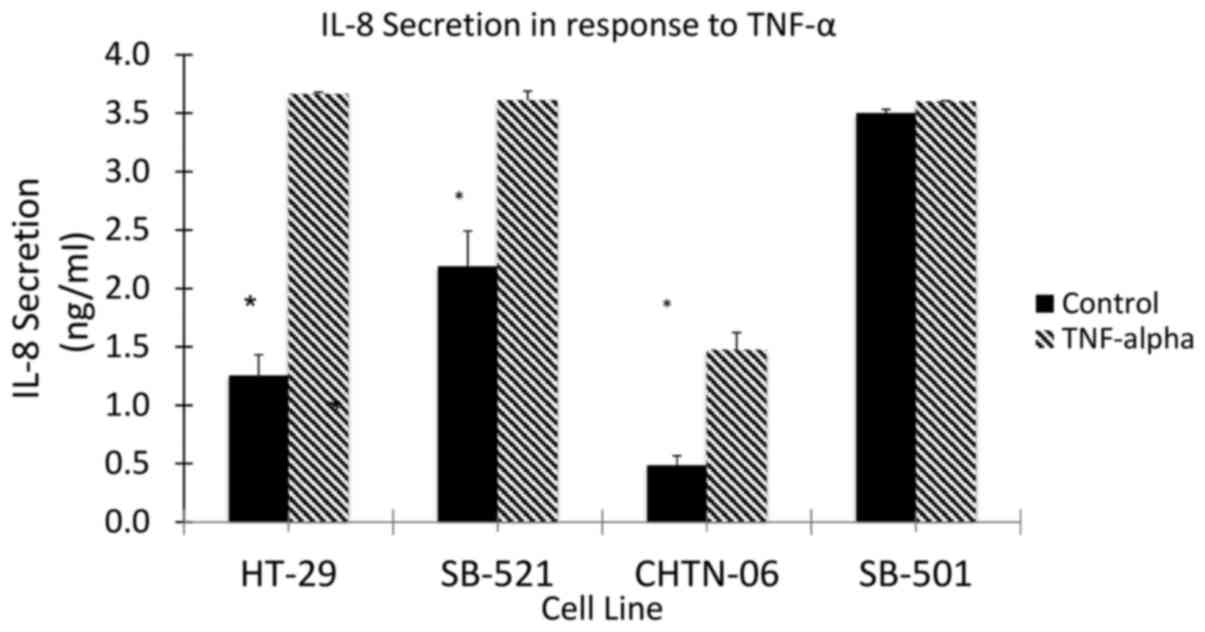
|
1
|
Lieberman DA, Holub JL, Moravec MD, Eisen
GM, Peters D and Morris CD: Prevalence of colon polyps detected by
colonoscopy screening in asymptomatic black and white patients.
JAMA. 300:1417–1422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alexander D, Jhala N, Chatla C, Steinhauer
J, Funkhouser E, Coffey CS, Grizzle WE and Manne U: High-grade
tumor differentiation is an indicator of poor prognosis in African
Americans with colonic adenocarcinomas. Cancer. 103:2163–2170.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chien C, Morimoto LM, Tom J and Li CI:
Differences in colorectal carcinoma stage and survival by race and
ethnicity. Cancer. 104:629–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clegg LX, Li FP, Hankey BF, Chu K and
Edwards BK: Cancer survival among US whites and minorities: A SEER
(Surveillance, Epidemiology, and End Results) Program
population-based study. Arch Intern Med. 162:1985–1993. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper GS, Yuan Z and Rimm AA: Racial
disparity in the incidence and case-fatality of colorectal cancer:
Analysis of 329 United States counties. Cancer Epidemiol Biomarkers
Prev. 6:283–285. 1997.PubMed/NCBI
|
|
6
|
Hodgson DC, Fuchs CS and Ayanian JZ:
Impact of patient and provider characteristics on the treatment and
outcomes of colorectal cancer. J Natl Cancer Inst. 93:501–515.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hodgson DC, Zhang W, Zaslavsky AM, Fuchs
CS, Wright WE and Ayanian JZ: Relation of hospital volume to
colostomy rates and survival for patients with rectal cancer. J
Natl Cancer Inst. 95:708–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mayberry RM, Coates RJ, Hill HA, Click LA,
Chen VW, Austin DF, Redmond CK, Fenoglio-Preiser CM, Hunter CP,
Haynes MA, et al: Determinants of black/white differences in colon
cancer survival. J Natl Cancer Inst. 87:1686–1693. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ries LAG, Kosary CL, Hankey BF, Miller BA,
Harras A and Edwards BK: SEER Cancer Statistics Review. 1973–1994.
1997.
|
|
10
|
Weber TK, Chin HM, Rodriguez-Bigas M,
Keitz B, Gilligan R, O'Malley L, Urf E, Diba N, Pazik J and
Petrelli NJ: Novel hMLH1 and hMSH2 germline mutations in African
Americans with colorectal cancer. JAMA. 281:2316–2320. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carethers JM: Racial and ethnic factors in
the genetic pathogenesis of colorectal cancer. J Assoc Acad Minor
Phys. 10:59–67. 1999.
|
|
12
|
Burn J, Gerdes AM, Macrae F, Mecklin JP,
Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L,
et al: CAPP2 Investigators: Long-term effect of aspirin on cancer
risk in carriers of hereditary colorectal cancer: An analysis from
the CAPP2 randomised controlled trial. Lancet. 378:2081–2087. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tougeron D, Sha D, Manthravadi S and
Sinicrope FA: Aspirin and colorectal cancer: Back to the future.
Clin Cancer Res. 20:1087–1094. 2014. View Article : Google Scholar :
|
|
14
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-kB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015. View Article : Google Scholar
|
|
16
|
Goodman JE, Bowman ED, Chanock SJ, Alberg
AJ and Harris CC: Arachidonate lipoxygenase (ALOX) and
cyclo-oxygenase (COX) polymorphisms and colon cancer risk.
Carcinogenesis. 25:2467–2472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sansbury LB, Millikan RC, Schroeder JC,
Moorman PG, North KE and Sandler RS: Use of nonsteroidal
antiinflammatory drugs and risk of colon cancer in a
population-based, case-control study of African Americans and
Whites. Am J Epidemiol. 162:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mackenzie GG, Sun Y, Huang L, Xie G,
Ouyang N, Gupta RC, Johnson F, Komninou D, Kopelovich L and Rigas
B: Phosphosulindac (OXT-328), a novel sulindac derivative, is safe
and effective in colon cancer prevention in mice. Gastroenterology.
139:1320–1332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ouyang N, Williams JL and Rigas B:
NO-donating aspirin isomers downregulate peroxisome
proliferator-activated receptor (PPAR)delta expression in
APC(min/+) mice proportionally to their tumor inhibitory effect:
Implications for the role of PPARdelta in carcinogenesis.
Carcinogenesis. 27:232–239. 2006. View Article : Google Scholar
|
|
20
|
Ouyang N, Ji P and Williams JL: A novel
NSAID derivative, phosphoibuprofen, prevents AOM-induced colon
cancer in rats. Int J Oncol. 42:643–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams JL, Ji P, Ouyang N, Kopelovich L
and Rigas B: Protein nitration and nitrosylation by NO-donating
aspirin in colon cancer cells: Relevance to its mechanism of
action. Exp Cell Res. 317:1359–1367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puvvada SD, Funkhouser WK, Greene K, Deal
A, Chu H, Baldwin AS, Tepper JE and O'Neil BH: NF-κB and Bcl-3
activation are prognostic in metastatic colorectal cancer.
Oncology. 78:181–188. 2010. View Article : Google Scholar :
|
|
23
|
Al-Maghrabi J, Gomaa W, Buhmeida A,
Al-Qahtani M and Al-Ahwal M: Loss of villin immunoexpression in
colorectal carcinoma is associated with poor differentiation and
survival. ISRN Gastroenterol. 2013:6797242013.PubMed/NCBI
|
|
24
|
Arango D, Al-Obaidi S, Williams DS, Dopeso
H, Mazzolini R, Corner G, Byun DS, Carr AA, Murone C, Tögel L, et
al: Villin expression is frequently lost in poorly differentiated
colon cancer. Am J Pathol. 180:1509–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brat DJ, Bellail AC and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro Oncol. 7:122–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alfaro C, Suárez N, Martínez-Forero I,
Palazón A, Rouzaut A, Solano S, Feijoo E, Gúrpide A, Bolaños E,
Erro L, et al: Carcinoma-derived interleukin-8 disorients dendritic
cell migration without impairing T-cell stimulation. PLoS One.
6:e179222011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frouws MA, Reimers MS, Swets M,
Bastiaannet E, Prinse B, van Eijk R, Lemmens VE, van Herk-Sukel MP,
van Wezel T, Kuppen PJ, et al: The influence of BRAF and KRAS
mutation status on the association between aspirin use and survival
after colon cancer diagnosis. PLoS One. 12:e01707752017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao S, Wu D, Wu P, Wang Z and Huang J:
Serum IL-10 Predicts worse outcome in cancer patients: A
meta-analysis. PLoS One. 10:e01395982015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peddareddigari VG, Wang D and Dubois RN:
The tumor microenvironment in colorectal carcinogenesis. Cancer
Microenviron. 3:149–166. 2010. View Article : Google Scholar
|
|
30
|
Rodrigues NR, Rowan A, Smith ME, Kerr IB,
Bodmer WF, Gannon JV and Lane DP: p53 mutations in colorectal
cancer. Proc Natl Acad Sci USA. 87:7555–7559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bosari S, Viale G, Bossi P, Maggioni M,
Coggi G, Murray JJ and Lee AK: Cytoplasmic accumulation of p53
protein: An independent prognostic indicator in colorectal
adenocarcinomas. J Natl Cancer Inst. 86:681–687. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bosari S, Viale G, Roncalli M, Graziani D,
Borsani G, Lee AK and Coggi G: p53 gene mutations, p53 protein
accumulation and compartmentalization in colorectal adenocarcinoma.
Am J Pathol. 147:790–798. 1995.PubMed/NCBI
|
|
33
|
Iorio MV and Croce CM: Causes and
consequences of microRNA dysregulation. Cancer J. 18:215–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li E, Ji P, Ouyang N, Zhang Y, Wang XY,
Rubin DC, Davidson NO, Bergamaschi R, Shroyer KR, Burke S, et al:
Differential expression of miRNAs in colon cancer between African
and Caucasian Americans: Implications for cancer racial health
disparities. Int J Oncol. 45:587–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Sarver AL, Khatri R, Hajeri PB,
Kamenev I, French AJ, Thibodeau SN, Steer CJ and Subramanian S:
Sequential expression of miR-182 and miR-503 cooperatively targets
FBXW7, contributing to the malignant transformation of colon
adenoma to adenocarcinoma. J Pathol. 234:488–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang MH, Yu J, Jiang DM, Li WL, Wang S and
Ding YQ: microRNA-182 targets special AT-rich sequence-binding
protein 2 to promote colorectal cancer proliferation and
metastasis. J Transl Med. 12:1092014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qu A, Du L, Yang Y, Liu H, Li J, Wang L,
Liu Y, Dong Z, Zhang X, Jiang X, et al: Hypoxia-inducible MiR-210
is an independent prognostic factor and contributes to metastasis
in colorectal cancer. PLoS One. 9:e909522014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
Implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brew R, Erikson JS, West DC, Kinsella AR,
Slavin J and Christmas SE: Interleukin-8 as an autocrine growth
factor for human colon carcinoma cells in vitro. Cytokine.
12:78–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carethers JM, Koi M and Tseng-Rogenski SS:
EMAST is a form of microsatellite instability that is initiated by
inflammation and modulates colorectal cancer progression. Genes
(Basel). 6:185–205. 2015. View Article : Google Scholar :
|
|
41
|
Jin WJ, Xu JM, Xu WL, Gu DH and Li PW:
Diagnostic value of interleukin-8 in colorectal cancer: A
case-control study and meta-analysis. World J Gastroenterol.
20:16334–16342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Ji P, Zhang Y, LaComb JF, Tian X,
Li E and Williams JL: Aberrant DNA Methylation: Implications in
Racial Health Disparity. PLoS One. 11:e01531252016. View Article : Google Scholar : PubMed/NCBI
|