Introduction
Ovarian cancer is a gynecological cancer with a high
mortality rate. The disease has an occult onset, and approximately
75% of patients with ovarian cancer have progressed to the advanced
stages of the disease by the time of diagnosis. A common treatment
for ovarian cancer is cytoreductive surgery, which is surgical
removal of the tumor combined with platinum and paclitaxel-based
chemotherapy. Although the sensitivity rate of ovarian cancer to
paclitaxel and platinum reaches 80% and the use of chemotherapy has
significantly improved overall survival, due to drug resistance, up
to 70% of patients will eventually relapse; the 5-year survival
rate remains stagnant at approximately 30% (1,2).
Chemoresistance is a major challenge in the treatment of ovarian
cancer. The exact mechanisms responsible for chemoresistance are
not yet fully understood, despite extensive research in this area
(3,4).
In recent years, the association between
glycoconjugates and drug resistance has attracted increasing
attention. Glycoconjugates are important components of the cell
membrane, and the structure and length of the sugar chain of
complex carbohydrates has been found to be altered in cancer cells
(5). In ovarian cancer, such
changes mainly occur in type II sugar chains, such as Lewis(y).
Lewis(y) antigen is a bi-fucosyl containing an oligosaccharide
chain in which the fucosylation of the end of the sugar chain is
catalyzed by specific α1,2-fucosyltransferase (FUT1). In our
previous studies, we transferred the human FUT1 gene into
the ovarian cancer cell line, RMG-I, to establish the
stably-expressing high-Lewis(y) ovarian cancer cell line, RMG-I-H.
We found that the RMG-I-H cells not only exhibited a stronger
prolifera-tive and invasive ability, but also exhibited reduced
sensitivity to common chemotherapeutic drugs such, as carboplatin,
5-fluorouracil (5-FU) and paclitaxel (6–9). We
previously used immunohistochemical staining to examine ovarian
cancer tissue samples, and found that more sugar chain antigens and
cell membrane receptors, including CD44, CD147, human epididymis
protein 4 (HE4) and integrin α5β1 were expressed in chemoresistant
ovarian cancer tissues (10–13).
These findings suggest that Lewis(y) may be an independent risk
factor for chemotherapeutic resistance in ovarian cancer.
The progression from normal ovarian epithelial cells
to cancer cells and further to chemotherapy-resistant cells is a
complex process, in which cell behaviors, such as proliferation and
apoptosis are extensively altered due to gene mutation and related
changes in the regulatory mechanism. Therefore, the present study
aimed to elucidate the mechanisms responsible for the
chemoresistance of ovarian cancer associated with the Lewis(y)
antigen. We used a gene chip assay to identify differentially
expressed genes (DEGs) between the resistant group and the
sensitive group and predicted involved pathways by bioinformatics
analysis. The findings were validated at both the mRNA and protein
level. This study provides valuable targets for the targeted
therapy and for the molecular diagnosis of ovarian cancer.
Materials and methods
Ethics statement
Samples were fully encoded to protect patient
confidentially. The study and its protocols were approved by the
Research Ethics committees of Shengjing Hospital Affiliated with
China Medical University (2013PS66K). The ethics committee waived
the need for patient consent, as the patient information was
withheld.
Patient specimens
All tissue samples used in this study (including the
colon cancer sample) were obtained from the tissue bank at
Shengjing Hospital of China Medical University. Between May, 2006
and July, 2010, 92 ovarian cancer patients were treated at the
Department of Gynecology of Shengjing Hospital of China Medical
University and were categorized according to the National
Comprehensive Cancer Network (NCCN) guidelines, which uses the
following definitions: Recurrence during the chemotherapy period
(paclitaxel and carboplatin) or within 6 months after the end of
chemotherapy is defined as drug resistance; recurrence between 6–12
months after the end of chemotherapy is defined as partial
sensitivity; and recurrence beyond 12 months after the end of
chemotherapy or no recurrence is defined as sensitivity. Based on
these criteria, we divided all patients into the resistant group
(n=37) and sensitive group (n=55). Lewis(y) expression in the
ovarian cancer tissues was detected by immunohistochemistry and its
association with the chemo-resistance status of the patients was
examined. All the cases are primary, the information and follow-up
data are complete, and chemical treatment was not used in any of
the patients prior to surgery.
Materials
The following reagents were purchased from
commercial sources: Mouse anti-human Lewis(y) monoclonal antibody
(cat. no. F3, ab3359) from Abcam (Cambridge, UK); carboplatin as an
apoptosis-inducing factor from Shandong Qilu Pharmaceutical Co.,
Ltd. (Jinan, China); DMEM and fetal bovine serum from HyClone
(Logan, UT, USA); the streptavidin-biotin-peroxidase (SP) test kit
from Boshide Biotech Co. (Wuhan, China). The protein content in the
cell lysates was measured by BCA assay (Beyotime Institute of
Biotechnology, Jiangsu, China); HRP-labeled secondary antibodies
(goat anti-mouse IgG-HRP, cat. no. sc-2005, 1:2,000; goat
anti-rabbit IgG-HRP, cat. no. sc-2004, 1:2,000) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). For immunoblot
analysis, the following antibodies were used: Annexin A4 (ANXA4)
(cat. no. sc-28827, 1:200), Bax (cat. no. sc-20067, 1:500), p-Bad
(cat. no. sc-166932, 1:500), Bcl-2 (cat. no. sc-509, 1:500), Bcl-xL
(cat. no. sc-8392, 1:500) and β-actin (cat. no. sc-47778, 1:1,000)
from Santa Cruz Biotechnology, Inc.; p-Akt (cat. no. 9271, 1:1,000)
and caspase-3 (cat. no. 9662, 1:500) from Cell Signaling
Technology, Inc. (Danvers, MA, USA). All other reagents were
commercially available in China.
Cell lines and treatment
The RMG-I-H cell line, highly expressing the
FUT1 gene and Lewis(y) antigen, was established by
transfecting the pcDNA3.1(−)-HFUT-H expression vector (containing
the FUT1 gene) into RMG-I cells (a human ovarian clear cell
carcinoma cell line, donated by Professor Iwamori Masao of Tokyo
University, Tokyo, Japan) (6). The
SKOV3, A2780 and COC1 cells, and their cognate cisplatin-resistant
cells SKOV3/DDP, A2780/DDP, COC1/DDP were obtained from the
American Tissue Culture Collection (ATCC, Manassas, VA, USA).
The cells were maintained in DMEM supplemented with
10% fetal bovine serum at 37°C in a humidified 5% CO2
atmosphere. Cells in the exponential growth phase were used in the
subsequent experiments. In the case of carboplatin treatment,
carboplatin was added to the culture medium at 60 mg/l and the
cells were incubated for 48 h. For the inhibition assay, the final
concentration of Lewis(y) antibody was 20 µg/ml. The
duration of treatment was 1 h.
Immunohistochemistry
For SP immunohistochemistry, the following protocol
was used. Tissues were fixed in 4% formaldehyde and embedded in
paraffin. A colon cancer sample served as a positive control for
Lewis(y) antigen. The group treated with phosphate-buffered saline
(PBS) instead of primary antibody was used as a negative control.
The working concentration of the primary antibody against Lewis(y)
was 1:160. The empirical procedure was performed based on the kit
instructions [streptavidin-biotin-peroxidase (SP) test kit].
Immunocytochemistry
The cells cultured in chamber slides were fixed with
4% of paraformaldehyde, then stained according to the SP test kit
instructions. The primary antibody, mouse anti-human Lewis(y)
antibody, was used at a 1:100 dilution. Lewis(y) immunostaining was
performed using an avidin-biotin peroxidase complex kit and then
slides were photographed. The presence of brownish-yellow granules
in the cytoplasm and cell membrane were considered as a positive
result.
Confocal laser scanning microscopy
The protocols strictly followed the instructions of
the reagent suppliers. The ANXA4 antibody and Lewis(y) antibody
were simultaneously added to monolayered cell slides prepared from
the RMG-I-H cells. To these, the following secondary antibodies
were applied: Fluorescein isothiocyanate green fluorescence-labeled
mouse IgM fluorescence (cat. no. sc-2859; 1:8) and
tetramethylrhoda-mine red fluorescence-labeled rabbit IgG (cat. no.
sc-2492; 1:50) (both from Santa Cruz Biotechnology, Inc.). Cell
nuclei were stained with 4′,6-diamidino-2-phenylindole. For
negative controls, PBS replaced the primary antibodies.
Double-labeled immunofluorescence samples were viewed under a
fluorescence confocal microscope (C1-SI; Nikon, Tokyo, Japan).
Western blot analysis
Briefly, after the various treatments, the cells
were washed twice with ice-cold PBS, scraped in lysis buffer [50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% NP-40, 100 mM NaF, 200
µM Na3VO4 and 10 µg/ml each
aprotinin, leupeptin, phenylmethanesulfonyl fluoride and
pepstatin], and incubated for 20 min at 4°C while rocking. Lysates
were cleared by centrifugation (15 min at 13,000 × g, 4°C). For
western blot analysis, 50 µg of total protein were resolved
with 10% SDS-PAGE and transferred onto poly (vinylidene difluoride)
membranes. The membranes were blocked with Tris-buffered saline
Tween [25 mM Tris-HCl, 150 mM NaCl (pH 7.5), and 0.1% Tween-20]
containing 5% non-fat milk and incubated overnight at 4°C with
primary antibody in TBST/1% non-fat milk. The blots were washed in
TTBS and incubated with the appropriate horseradish
peroxidase-linked IgG at room temperature for 1 h, and
immunoreactive proteins were visualized using an ECL detection
system. The protein bands were visualized using the Molecular
Imager system GDS8000b (UVP, Inc., Upland, CA, USA). Total protein
levels were normalized to β-actin expression on the same membrane,
and the bands were quantified using ImageJ software v1.8.0
(National Institutes of Health, Bethesda, MD, USA).
Co-immunoprecipitation
The cold PBS washed monolayer cells were lysed with
200 µl lysis buffer as described above. The protein content
was measured using the protein assay BCA kit (Beyotime
Biotechnology, Jiangsu, China). Following protein determination,
cell lysate containing 500 µg protein was incubated with 5
µg of one of the following antibodies, and incubated at 4°C
overnight. Protein G plus-agarose was added and the samples were
incubated at 4°C for 3 h for immunoprecipitation. ANXA4 was
subjected to 10% SDS-PAGE, then transferred onto a poly (vinylidene
difluoride) membrane and treated with 1:500 diluted anti-Lewis(y)
and anti-ANXA4 sera in Tris-buffered saline with 5% non-fat milk,
followed by HRP-labeled secondary antibody at a 1:1,000 dilution.
Finally, the proteins were visualized with ECL reagent (ECL Prime
Western Blotting Detection Reagent, Amersham, Pittsburgh, PA, USA).
The densitometric analysis of the protein bands was performed using
ImageJ software v1.8.0 (National Institutes of Health, Bethesda,
MD, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the ovarian cancer
cells using TRIzol reagent (Invitrogen, Shanghai, China).
Complementary DNA (cDNA) was synthesized using reverse
transcriptase from a Perfect Real-Time PrimeScript™ RT Reagent kit
(Takara Bio, Inc.). The reaction conditions were as follows: 37°C
for 15 min, 85°C for 5 sec, 4°C for 5 min. Real-time PCR and Ct
value analysis were performed with a Roche LightCycler system. The
primers of target genes were commercially synthesized (Table I). The real-time PCR reaction
conditions were denature at 95°C for 30 sec, 40 cycles of 95°C for
5 sec and 60°C for 30 sec in a 20-µl reaction mixture
containing SYBR@ Premix Ex Taq™ (2X) 10 µl, PCR
Forward Primer (10 µmol/l) 0.4 µl, PCR Reverse Primer
(10 µmol/l) 0.4 µl, cDNA 2 µl, dH2O
7.2 µl. GAPDH was used as an endogenous control.
Relative quantification was performed according to the ΔΔCq method,
and results were expressed in the linear form using the formula
2−ΔΔCq (14). Results
were considered significant when at least a 2-fold difference in
expression levels was detected.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene name | Primer sequence
(5′-3′) |
|---|
| FUT1 | F:
AGGTATAAACACACCCTCTGTGCTT |
| R:
GAGTTCAGGGACAGACAGTGGTT |
| ANXA4 | F:
AGCCTACAAGAGCACCATCG |
| R:
GACAGAGACACCAGCACTCG |
| BIK | F:
GGTTCTTGGCATGACTGA |
| R:
GGCCAATGCGTCACT |
| TM4SF4 | F:
CTTCCACGACGGGGATTAT |
| R:
ATTGTAGTCGTCATGCTGTAGAGTC |
| PHLDA1 | F:
TCATCCTTACTCTCACCCGC |
| R:
CTGGAGTTGGTACGGGTGAG |
| GAPDH | F:
CCTTCATTGACCTCCACTAC |
| R:
GTTGTCATACTTCTCATGGTTC |
Functional and pathway enrichment
analysis
Using human whole genome oligonucleotide microarrays
(Agilent whole human genome oligo microarray; Agilent Technologies,
Santa Clara, CA, USA), gene expression profile alteration in
response to Lewis(y) was investigated and 390 DEGs were identified
and validated in the epithelial ovarian cell lines (RMG-I and COC1)
compared with their sublines (RMG-I-H and COC1/DDP) with greater
resistance to chemotherapy, in which 229 genes were upregulated and
161 genes were down-regulated (15). Gene ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis were performed for the identified DEGs using the Database
for Annotation, Visualization and Integrated Discovery (DAVID)
database. A value of P<0.05 was set as the cut-off
criterion.
Protein-protein interaction (PPI) network
construction and module selection
The functional interactions between proteins can
provide context for the molecular mechanism of cellular processing.
In this study, a PPI network of DEGs was constructed using the
Search Tool for the Retrieval of Interacting Genes (STRING,
http://string.embl.de/) database and subsequently
was visualized using Cytoscape. A confidence score ≥0.4 was set as
the cut-off criterion. Subsequently, Molecular Complex Detection
(MCODE) was performed to screen the modules of the PPI network with
a degree cut-off of 2, a node score cut-off of 0.2, a k-core of 2,
and a max. depth of 100. Finally, the functional enrichment
analysis of genes in each module was performed using the DAVID
database.
Statistical analysis
SPSS 17.0 statistical software (IBM, Armonk, NY,
USA) was applied for statistical analysis. Statistical methods were
varied based on the data type. Quantitative data are presented as
the means ± SD. As for the analysis of the data from RT-qPCR, data
are expressed as the means ± SEM. Positive ratio rates were
evaluated using the Chi-square (χ2) test. A Student's
t-test was employed for comparisons between 2 groups and one-way
ANOVA with the LSD or Bonferroni post hoc test was used for
comparisons between >2 groups. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
Expression of Lewis(y) antigen and the
FUT1 gene in ovarian cancer tissues and cells
Our results revealed that in the 92 samples of
ovarian cancer tissues, Lewis(y) antigen was mainly expressed on
the cell membrane and to a lesser extent in the cytoplasm (Fig. 1A). The positive rate of Lewis(y)
antigen in chemoresistant ovarian cancer tissues was 91.89%, which
was significantly higher than that in the sensitive group (61.82%,
P<0.05) (Table II). The
expression intensity of Lewis(y) antigen in the resistant group was
also significantly higher than that in the sensitive group. In the
resistant group, 8 of the 34 positive cases exhibited a strong
positive expression, while in the sensitive group, there were no
strongly positive cases among the 34 positive cases (Table II).
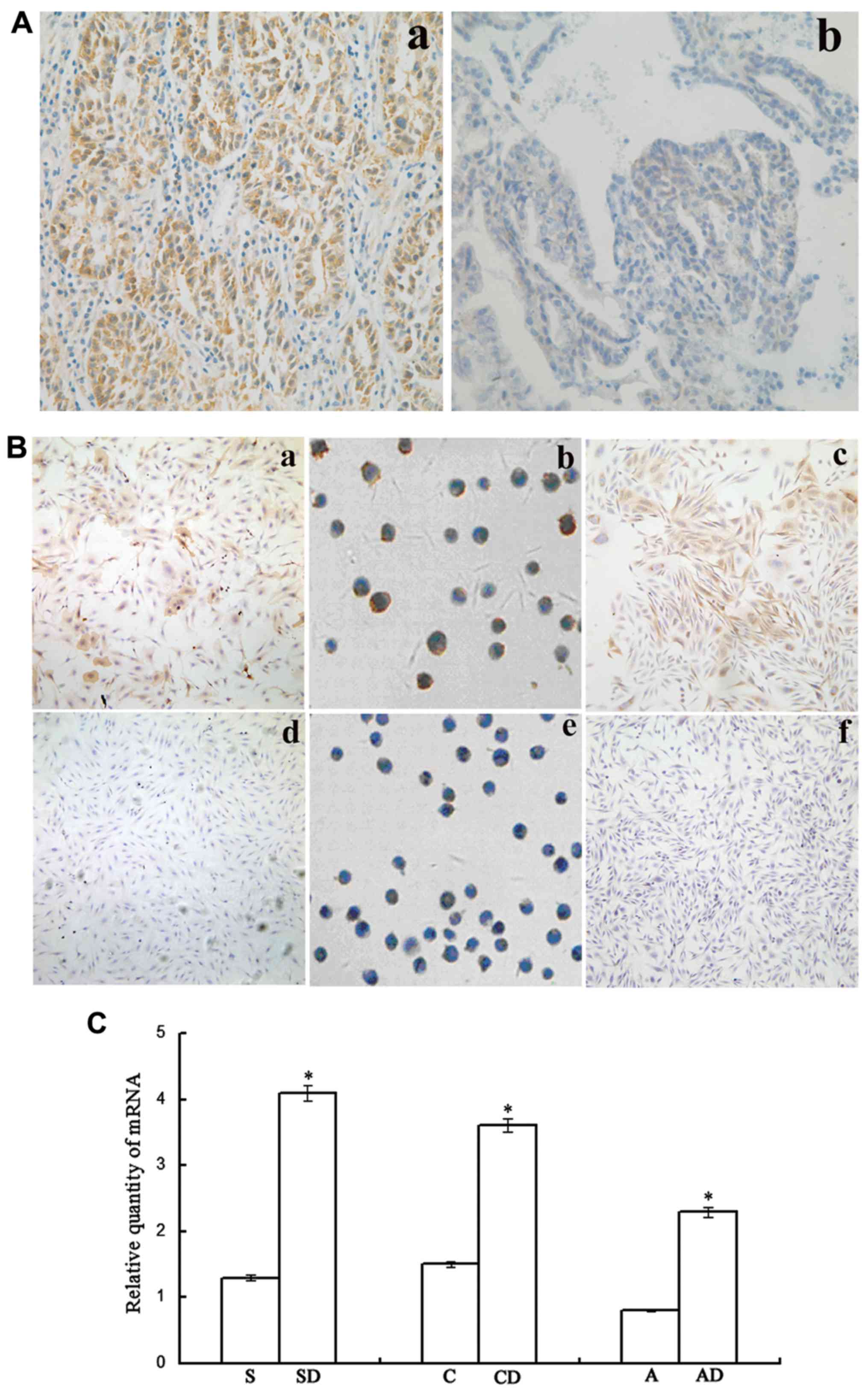 | Figure 1Association between Lewis(y) antigen
and chemotherapy resistance in ovarian cancer. (A)
Immunohistochemical detection of the expression of Lewis(y) antigen
in ovarian cancer tissues (magnification, ×200). (a)
Chemotherapy-resistant group; (b) chemotherapy-sensitive group. (B)
Immunocytochemical detection of Lewis(y) antigen expression in 3
groups of ovarian cancer cells with varying degrees of malignancy.
(a-c) Chemotherapy-resistant cells (SKOV3/DDP, COC1/DDP and
A2780/DDP cells); (d-f) chemotherapy-sensitive cells (SKOV3, COC1
and A2780 cells). (C) RT-qPCR detection of FUT1 expression
in 3 groups of ovarian cancer cells with varying degrees of
malignancy. *P<0.05, SKOV3/DDP compared to SKOV3
cells, COC1/DDP compared to COC1 cells, and A2780/DDP compared to
A2780 cells. Bars are labeled as follows: S, SKOV3 cells; SD,
SKOV3/DDP cells; C, COC1 cells; CD, COC1/DDP cells; A, A2780 cells;
AD, A2780/DDP cells. FUT1, fucosyltransferase 1. |
 | Table IIExpression of Lewis(y) antigen in the
different group. |
Table II
Expression of Lewis(y) antigen in the
different group.
| Group | N | Lewis(y) antigen
| Positive cases | Positive rate
(%) |
|---|
| − | + | ++ | +++ |
|---|
| Resistant
group | 37 | 3 | 8 | 18 | 8 | 34 | 91.89a |
| Sensitive
group | 55 | 21 | 16 | 18 | 0 | 34 | 61.82 |
In previous studies, we introduced the FUT1
gene into human ovarian carcinoma-derived RMG-I cells through gene
transfection and established a cell model overexpressing the
FUT1 gene and Lewis(y) antigen. Further experiments
demonstrated that the FUT1-transfected cells exhibited a
reduced sensitivity to common chemotherapeutic drugs, such as
carboplatin, 5-FU and paclitaxel (6–8). In
this study, to further examine whether the expression of Lewis(y)
antigen was increased when the cells became resistant to
chemotherapy, the expression of Lewis(y) antigen and FUT1 in
ovarian cancer cells was detected by immunocytochemical staining
and RT-qPCR, respectively. The results revealed that after the
cells were challenged by chemotherapeutic drugs, the expression of
Lewis(y) antigen in the cisplatin-resistant cell lines (SKOV3/DDP,
COC1/DDP and A2780/DDP) was significantly higher than the
expression in their parental non-resistant cell lines (SKOV3, COC1
and A2780); the antigen was also widely distributed on the cell
membrane (Fig. 1B). The results of
RT-qPCR confirmed that the mRNA expression level of FUT1 in
the SKOV3/DDP cells increased by 3.17-fold in comparison to that in
the SKOV3 cells, the mRNA expression level of FUT1 in the
COC1/DDP cells increased by 2.42-fold in comparison to that in the
COC1 cells, and the mRNA expression level of FUT1 in the
A2780/DDP cells increased by 2.84-fold in comparison to that in the
A2780 cells (all P<0.05) (Fig.
1C).
Functional and pathway enrichment
analysis
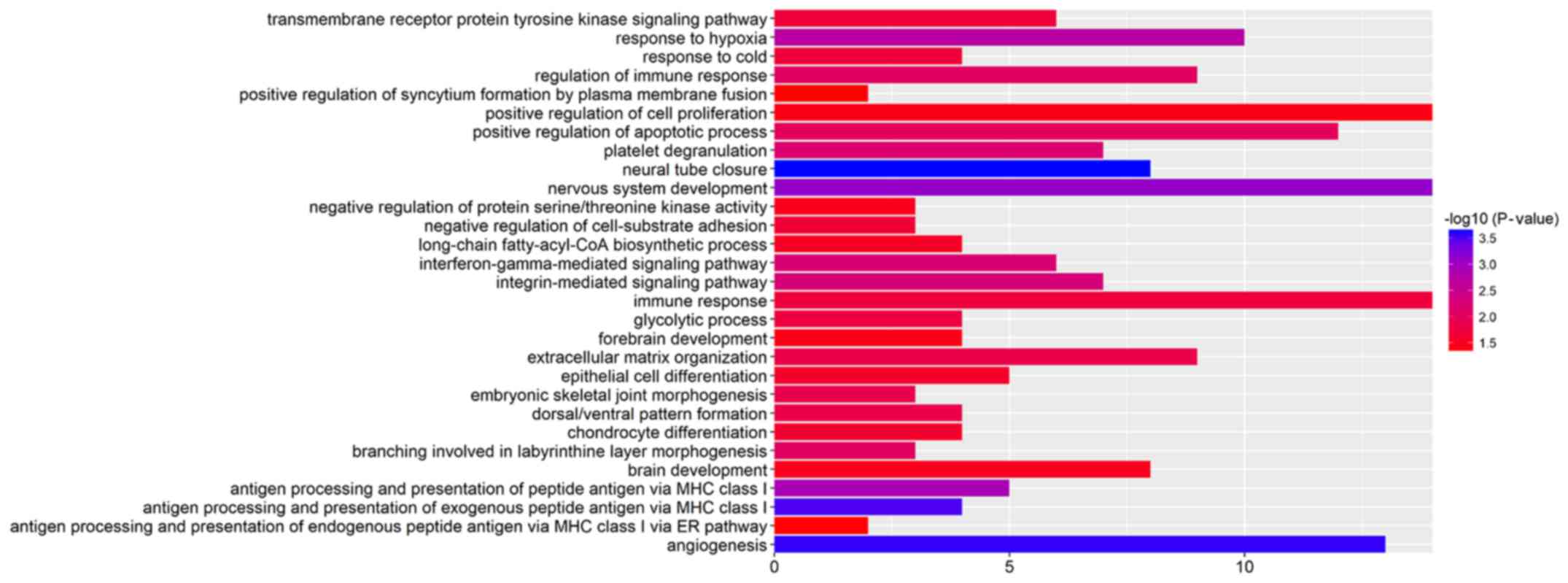
GO enrichment analysis revealed that the DEGs were
mainly involved in the transmembrane receptor protein tyrosine
kinase signaling pathway, the positive regulation of cell
proliferation, the integrin-mediated signaling pathway,
extracellular matrix organization and angiogenesis (Fig. 2 and Table III).
 | Table IIITop 12 significantly differentially
expressed genes determined by enriched analysis in response to
Lewis(y) antigen in ovarian cancer cells. |
Table III
Top 12 significantly differentially
expressed genes determined by enriched analysis in response to
Lewis(y) antigen in ovarian cancer cells.
| Term | Description | Count | P-value |
|---|
| GO:0001525 | Angiogenesis | 13 | 2.72E-04 |
| GO:0007399 | Nervous system
development | 14 | 7.89E-04 |
| GO:0001666 | Response to
hypoxia | 10 | 0.001899323 |
| GO:0007229 | Integrin-mediated
signaling pathway | 7 | 0.005265788 |
| GO:0060333 |
Interferon-gamma-mediated signaling
pathway | 6 | 0.005686303 |
| GO:0050776 | Regulation of
immune response | 9 | 0.008269841 |
| GO:0043065 | Positive regulation
of apoptotic process | 12 | 0.009485865 |
| GO:0030198 | Extracellular
matrix organization | 9 | 0.014169248 |
| GO:0007169 | Transmembrane
receptor protein tyrosine kinase signaling pathway | 6 | 0.019359692 |
| GO:0002062 | Chondrocyte
differentiation | 4 | 0.024541795 |
| GO:0030855 | Epithelial cell
differentiation | 5 | 0.026205904 |
| GO:0008284 | Positive regulation
of cell proliferation | 14 | 0.039256986 |
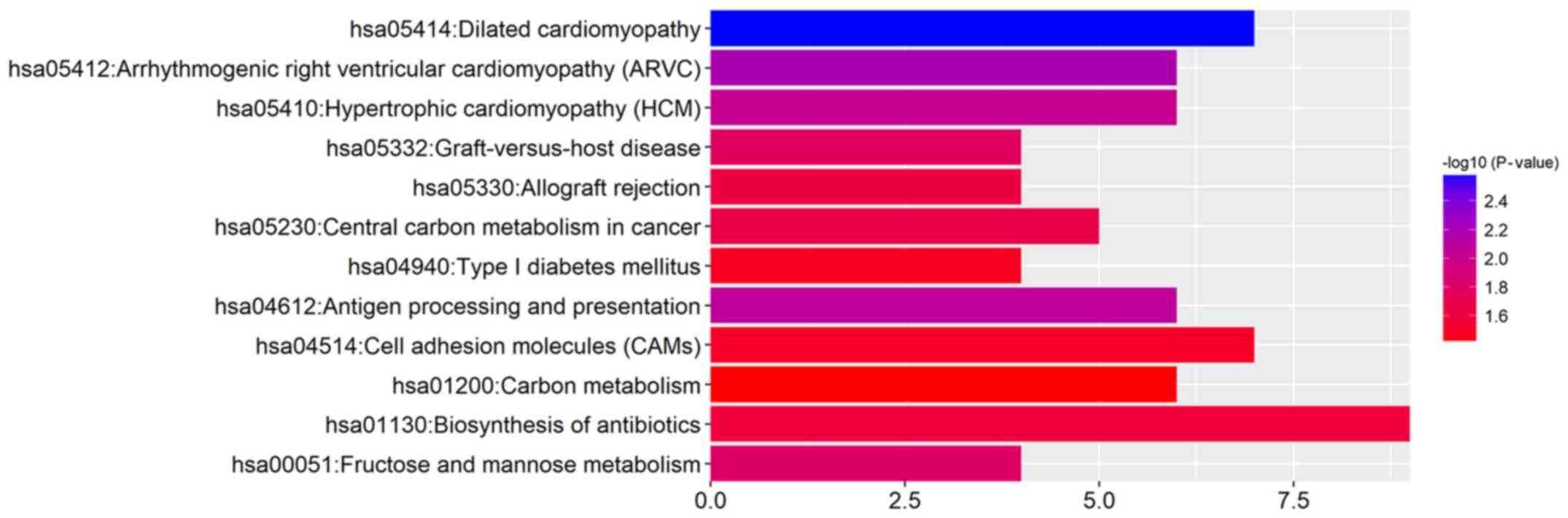
KEGG pathway analysis of the DEGs suggested that
these genes were significantly enriched in antigen processing and
presentation, fructose and mannose metabolism, and central carbon
metabolism in cancer (Fig. 3 and
Table IV).
 | Table IVSignaling pathway enrichment analysis
of differentially expressed genes in response to Lewis(y) antigen
in ovarian cancer cells. |
Table IV
Signaling pathway enrichment analysis
of differentially expressed genes in response to Lewis(y) antigen
in ovarian cancer cells.
| Term | Description | Count | P-value |
|---|
| hsa05414 | Dilated
cardiomyopathy | 7 | 0.002639 |
| hsa05412 | Arrhythmogenic
right ventricular cardiomyopathy (ARVC) | 6 | 0.006362 |
| hsa04612 | Antigen processing
and presentation | 6 | 0.008456 |
| hsa05410 | Hypertrophic
cardiomyopathy (HCM) | 6 | 0.009414 |
| hsa00051 | Fructose and
mannose metabolism | 4 | 0.015682 |
| hsa05332 | Graft-versus-host
disease | 4 | 0.017045 |
| hsa05230 | Central carbon
metabolism in cancer | 5 | 0.021516 |
| hsa05330 | Allograft
rejection | 4 | 0.023141 |
| hsa01130 | Biosynthesis of
antibiotics | 9 | 0.024633 |
| hsa04514 | Cell adhesion
molecules (CAMs) | 7 | 0.030688 |
| hsa04940 | Type I diabetes
mellitus | 4 | 0.032215 |
| hsa01200 | Carbon
metabolism | 6 | 0.039745 |
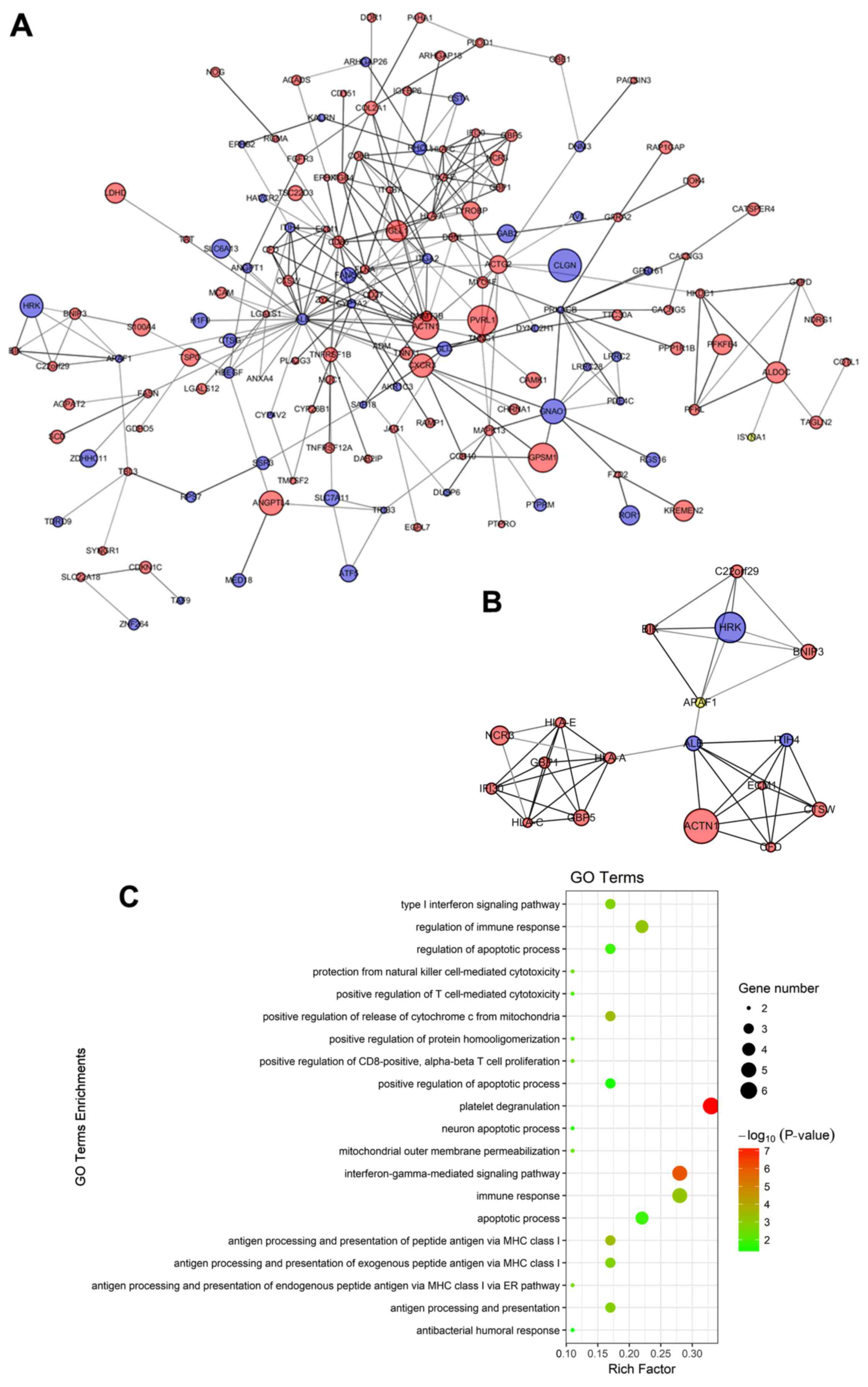
PPI network construction and module
selection
We also constructed a PPI network to examine the
mechanisms through which these genes interact with each other, as
well as to identify the central node of the PPI network. The PPI
network of the DEGs consisted of 323 nodes and 287 edges. A
significant module was obtained from the PPI network of DEGs using
MCODE, including 18 nodes and 45 edges (Fig. 4A and B). Functional enrichment
analysis revealed that genes in this module were mainly associated
with the regulation of the apoptotic process, the positive
regulation of release of the cytochrome c from the
mitochondria, and protection from natural killer cell-mediated
cytotoxicity (Fig. 4C).
Validation of the DEGs
Four DEGs, ANXA4, BCL2 interacting killer
(BIK), transmembrane 4 L six family member 4 (TM4SF4)
and pleckstrin homology like domain family A member 1
(PHLDA1), were selected to validate the results of gene chip
analysis. The results of RT-qPCR revealed that the mRNA levels of
the 4 genes were significantly increased in the Lewis(y)
highly-expressing chemoresistant ovarian cancer cells (RMG-I-H and
COC1/DDP cells), exhibiting 2.82-(ANXA4), 4,21-(BIK),
9.04-(TM4SF4) and 3.38-fold (PHLDA1) increases over
the expression levels in the non-resistant cells (RMG-I and COC1
cells) (P<0.01) (Fig. 5). This
was consistent with the results of gene chip analysis. These
results suggest that Lewis(y) causes cancer chemotherapeutic
resistance may be due to the inhibition of apoptosis.
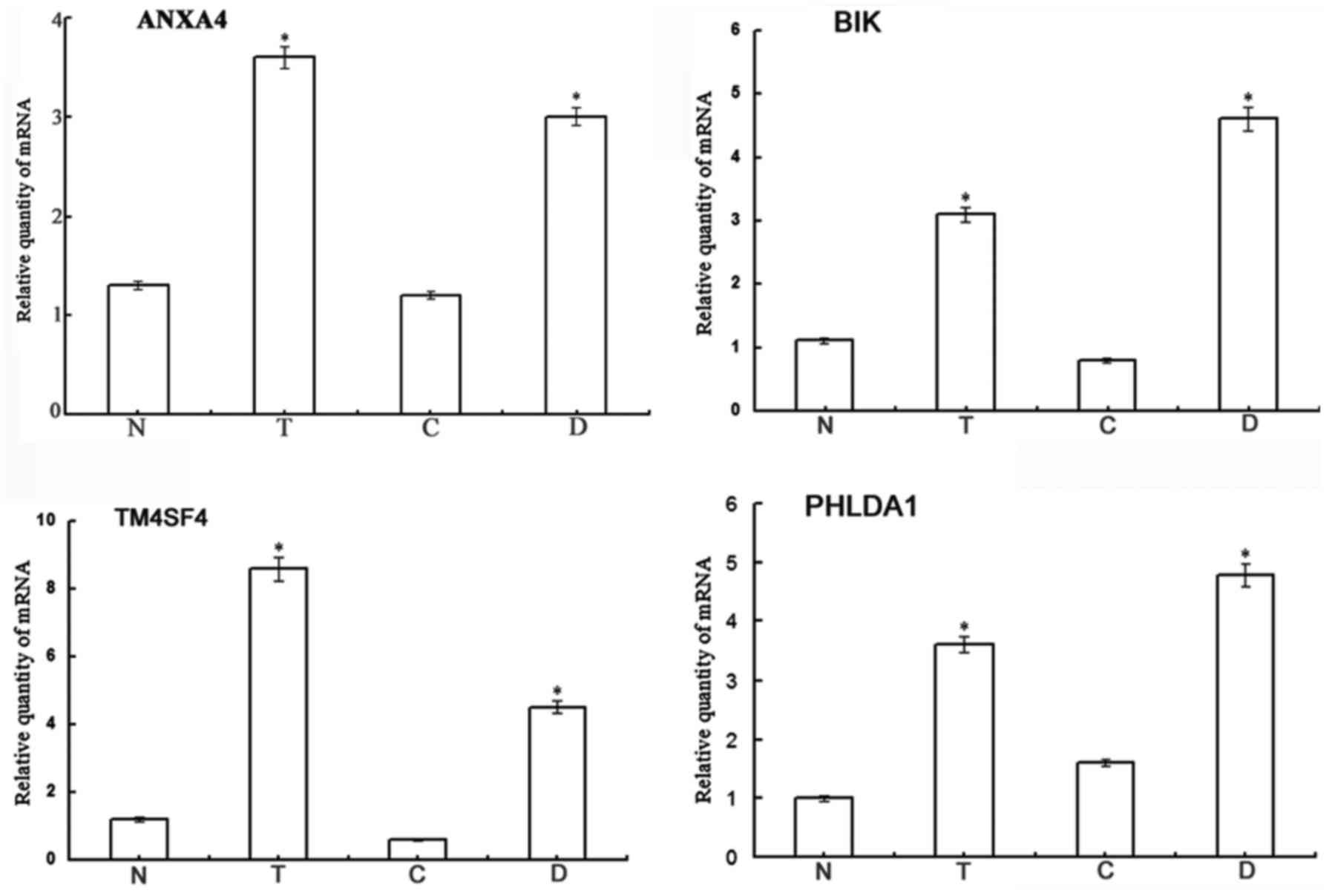 | Figure 5Validation of the differentially
expressed genes. The results of RT-qPCR revealed that the mRNA
expression levels of 4 selected genes (ANXA4, BIK,
TM4SF4 and PHLDA1) exhibited obvious differences
among the 4 ovarian cancer cell lines (RMG-I-H, RMG-I, COC1/DDP and
COC1 cells). Bars are labeled as follows: N, RMG-I cells; T,
RMG-I-H cells; C, COC1cells; D, COC1/DDP cells.
*P<0.01, RMG-I-H compared to RMG-I cells, and
COC1/DDP compared to COC1 cells. ANXA4, Annexin A4;
BIK, BCL2 interacting killer; TM4SF4, transmembrane 4
L six family member 4; PHLDA1, pleckstrin homology-like
domain family A member 1. |
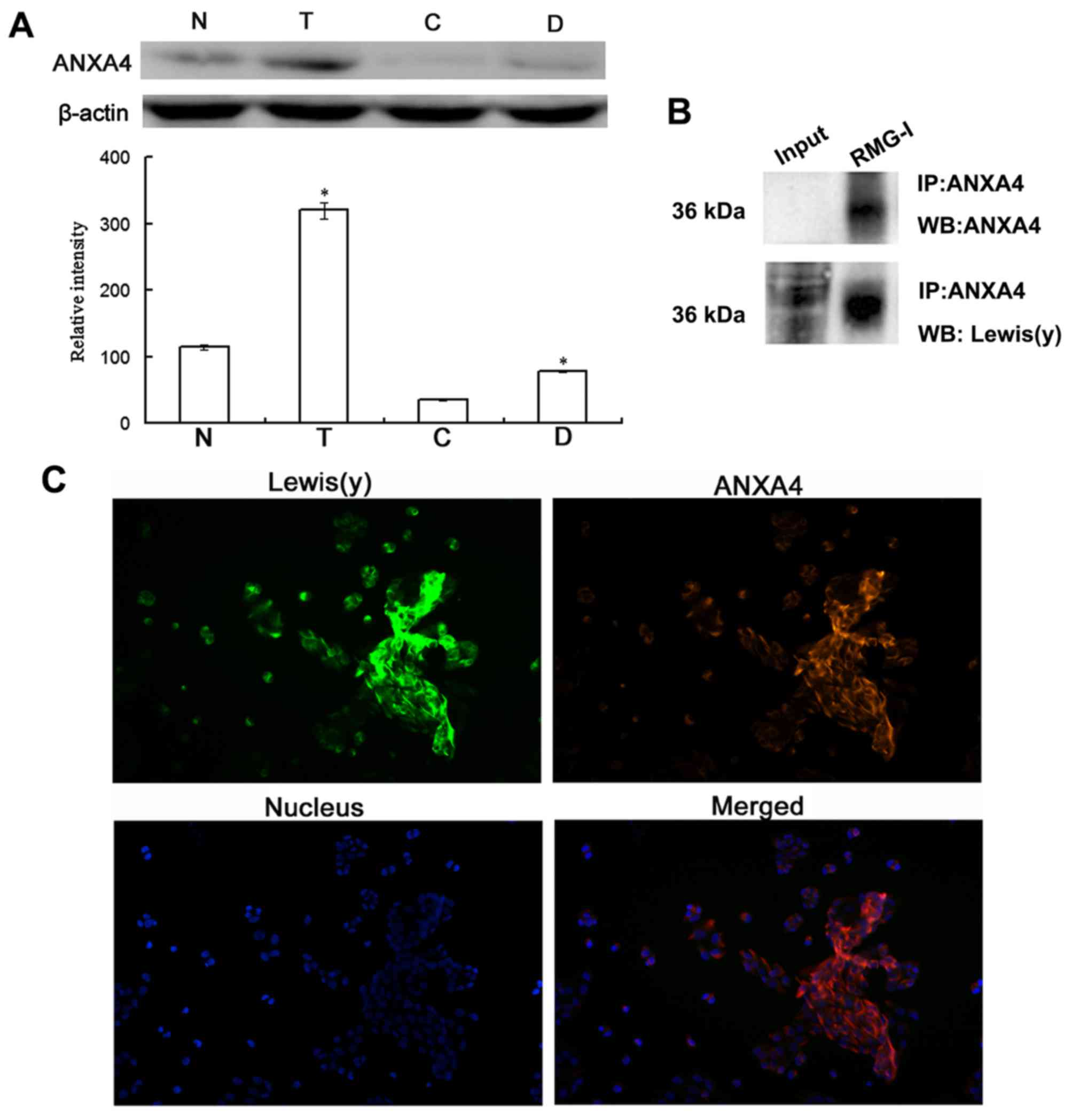
Co-expression of ANXA4 and Lewis(y)
antigen in ovarian cancer cells
As ANXA4 is a membrane phospholipid-binding protein,
we further investigated the protein expression of ANXA4 in ovarian
cancer cells and the presence of Lewis(y) antigen in this protein.
The results of western blot analysis revealed that the protein
expression of ANXA4 was significantly increased in the ovarian
cancer chemotherapy-resistant cells (RMG-I-H and COC1/DDP cells)
expressing high levels of Lewis(y), with an expression level
increase of 2.72-fold and 2.24-fold over the level in RMG-I cells
and COC1 cells, respectively, exhibiting the same trend as the mRNA
expression (Fig. 6A).
Co-immunoprecipitation and laser confocal microscopy
were used to determine whether there was a Lewis(y) structure in
the ANXA4 protein. The results revealed that ANXA4
co-immunoprecipitated with Lewis(y) antigen corresponding to the 36
kDa band (Fig. 6B). Dual-labeling
immunofluorescence revealed red immunofluorescence, indicating that
the Lewis(y) antigen was mainly distributed in the membrane, while
green immunofluorescence indicated ANXA4 was also mainly
distributed in the membrane, although it could also be observed in
the cytoplasm. Moreover, red and green immunofluorescence were
overlaid in the membrane, suggesting the co-localization of
Lewis(y) antigen and ANXA4 (Fig.
6C).
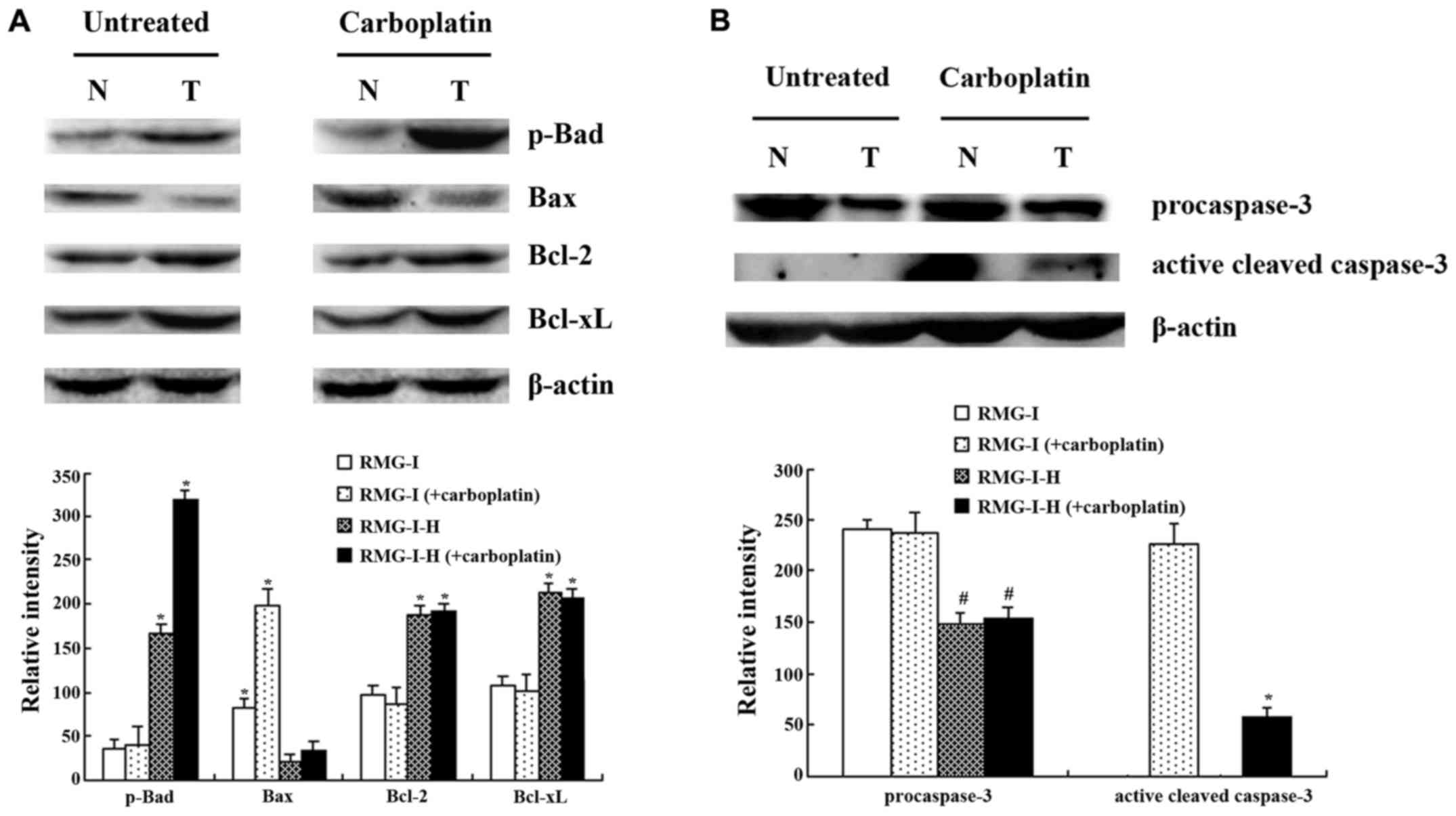
Differences in apoptosis-related protein
expression in ovarian cancer cells
We further examined the expression of
apoptosis-related proteins, such as Bcl-2 in ovarian cancer cells.
Following carboplatin treatment, the RMG-I-H cells exhibited an
upregulated expression of the pro-apoptotic protein, Bax, by 57%,
and the phosphorylation of the anti-apoptotic protein, Bad,
markedly increased by 197%, compared with the pretreatment protein
levels (Fig. 7A) (Bad itself is a
pro-apoptotic protein that becomes anti-apoptotic upon
phosphorylation by Akt.) The expression of other anti-apoptotic
proteins, such as Bcl-2 and Bcl-xL, was not noticeably altered
following carboplatin treatment. Before treatment, the Bax
expression level in the RMG-I cells was higher than that in the
RMG-I-H cells by approximately 3.95-fold. In addition, the
post-treatment expression of Bax in the RMG-I cells increased by
approximately 6.02-fold greater than the increase exhibited by the
RMG-I-H cells. The phosphorylation levels of Bad, and the levels of
Bcl-2 and Bcl-xL in the RMG-I-H cells significantly increased by
4.64-, 1.91- and 2.07-fold as much as in the RMG-I cells,
respectively. Following carboplatin treatment, the phosphorylation
level of Bad in the RMG-I-H cells was approximately 7.78-fold
higher than that in the RMG-I cells. Following carboplatin
treatment, there was no evident change in the levels of
phosphorylated Bad, and the levels of Bcl-2, or Bcl-xL in the RMG-I
cells.
We also examined the expression of caspase-3, and
found that, in the absence of carboplatin, caspase-3 existed in its
inactive, pro-enzyme form in the RMG-I and RMG-I-H cells, and thus
no hydrolysis of caspase-3 was observed (Fig. 7B). The hydrolytic breakdown of
caspase-3 into a small fragment subunit is required for its
activation. The caspase-3 pro-enzyme level in the RMG-I cells was
approximately 1.6-fold higher than that in the RMG-I-H cells.
Treatment with carboplatin markedly increased the level of active
caspase-3 in the RMG-I cells to a level approximately 4.03-fold as
much as that in the RMG-I-H cells. However, the level of the
caspase-3 pro-enzyme exhibited no apparent changes in response to
carboplatin treatment in either cell line.
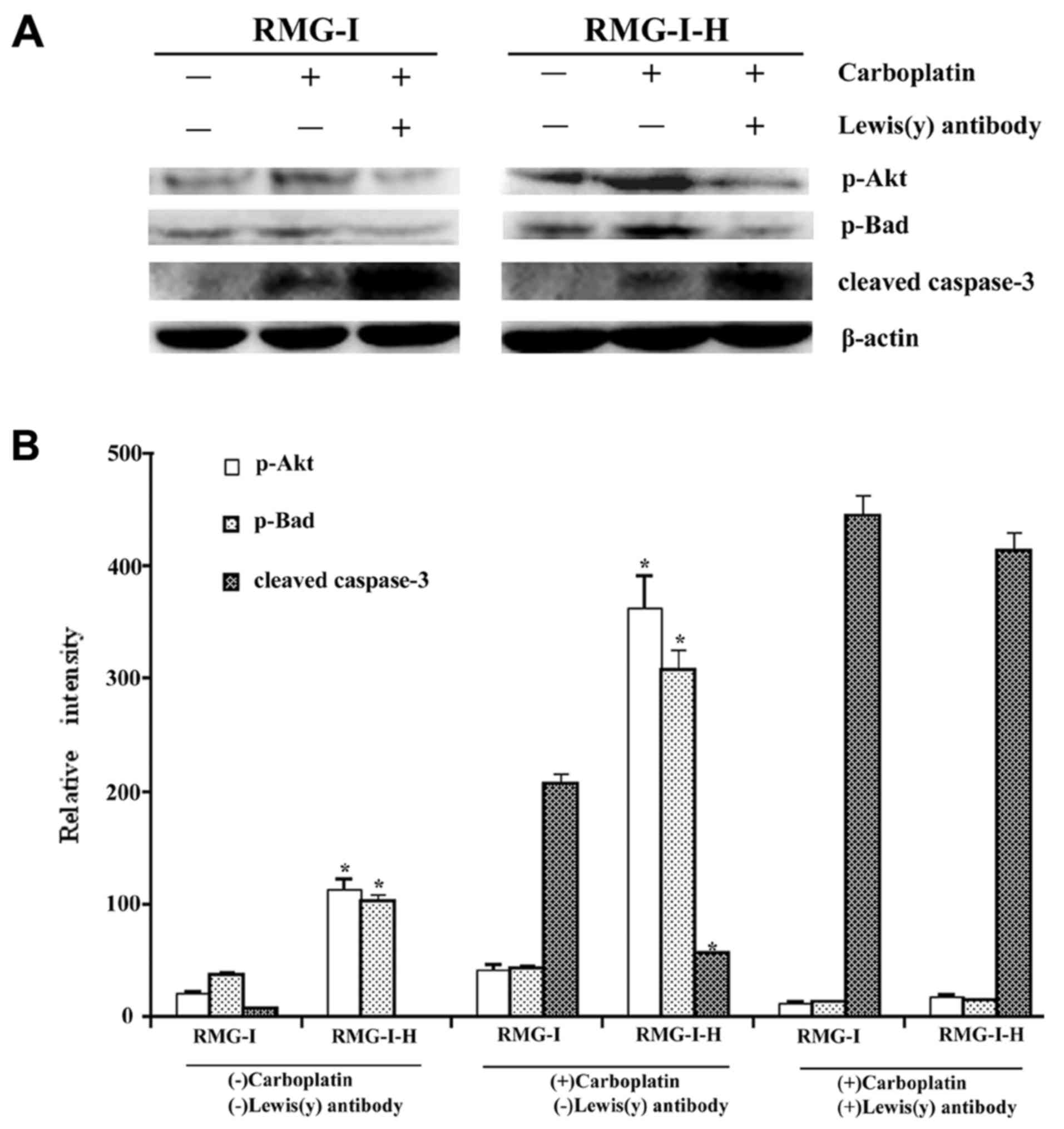
Effect of Lewis(y) antibody on the
expression of apoptosis related proteins in ovarian cancer cells
following carboplatin treatment
To further determine whether the Lewis(y) antigen is
involved in the inhibitory effects on the apoptosis of ovarian
cancer cells, we used Lewis(y) antibody to treat the cells before
carboplatin treatment. The phosphorylation levels of Akt and Bad
were decreased in both cell lines in response to Lewis(y) antibody
pre-treatment, although this decrease was greater in the RMG-I-H
cells, which overexpress Lewis(y) (Fig. 8). The active fragment of caspase-3
was increased in both cell lines, but more predominantly in the
RMG-I-H cells (Fig. 8). High-and
low-level differences between the RMG-I and RMG-I-H cells with
regard to the levels of phosphorylated Akt, phosphorylated Bad and
caspase-3 fragments were also suppressed after Lewis(y) antibody
pre-treatment.
Discussion
Lewis antigens are a group of carbohydrate antigens
present on cell surface glycolipids or glycoproteins. They are also
tumor-associated carbohydrate antigens that are abnormally highly
expressed in malignant tumors derived from epithelial tissues, such
as the breast, ovaries, colon and rectum. A high expression of the
Lewis(y) antigen has been shown to be associated with a poorer
prognosis of cancer (16,17). Our previous studies demonstrated
that approximately 80% of ovarian cancer samples had an increased
expression of Lewis(y). This change in Lewis(y) expression affected
the biological behaviors of ovarian cancer cells. Using an
anti-Lewis(y) monoclonal antibody to block the antigen or
α-L-fucosidase to digest cell surface sugar chain antigens, we
found that the proliferation and adhesion of ovarian cancer cells
decreased, and their sensitivity to chemotherapy increased
(9,18–20).
In the current study, the chemoresistance of ovarian cancer cells
was induced and the expression of Lewis(y) was found to be
significantly increased after the induction of chemoresistance.
Combined with the clinical drug resistance observed in patients
with ovarian cancer, this study suggests a significant association
between Lewis(y) and drug resistance. In the chemoresistant group,
the positive expression rate and expression intensity of Lewis(y)
were both significantly higher than those in the sensitive group.
Our previous study also found that Lewis(y)-associated drug
resistance was not only closely related to the upregulation of
certain drug resistant genes, including multidrug resistance
protein (MDR)-1, multidrug resistance-associated protein
(MRP)-1, MRP-2 and protein kinase C
(PKC)-α (21), but
was also involved in the upregulation of some cell repair genes,
such as topoisomerase (Topo)-I and Topo-IIβ
(22). Lewis(y) may thus be a
novel target for reversing the chemoresistance of ovarian cancer
cells. Moreover, the analysis of the molecular mechanisms
responsible for the Lewis(y)-induced chemoresistance may provide
theoretical support for improving the treatment efficacy of ovarian
cancer.
In this study, we used a gene chip assay to analyze
the gene expression profiles of a cisplatin-resistant ovarian
cancer cell line with high expression of Lewis(y) antigen and its
parental non-resistant cell line. There were 390 genes
differentially expressed between the drug-resistant and the
non-resistant cell lines. These DEGs were mainly associated with
transmembrane receptor protein tyrosine kinase signaling pathway,
the positive regulation of cell proliferation, the
integrin-mediated signaling pathway, extracellular matrix
organization, the regulation of apoptotic process, the positive
regulation of release of cytochrome c from mitochondria and
antigen processing and presentation. We constructed an interaction
network map and predicted a large number of interacting genes,
which may prove to be helpful for understanding the mechanisms of
the chemoresistance of ovarian cancer cells.
The majority of cell surface receptors are
glycoproteins. Changes in the expression of glycosyltransferase can
affect the sugar chain structure of cell surface receptors, which
further affects the abundance and functions of these receptors
(23). In our previous study, we
found that Lewis(y), which is a bi-fucosyl-containing
oligosaccharide chain, was a component of multiple membrane
proteins, including epidermal growth factor receptor (EGFR),
integrin α5β1, transforming growth factor beta receptor (TGF-βR)
and HE4 (13,19,24–26).
Furthermore, Lewis(y) expression increased with the increase in the
malignant degree of ovarian cancer tissues or cells. In the present
study, we found that ANXA4 was abnormally increased at both the
mRNA and protein levels in drug-resistant ovarian cancer cells.
ANXA4 is an important member of the membrane protein family, and we
found that Lewis(y) antigen was also present in ANXA4 protein.
Recent studies suggest that abnormally-high expression of ANXA4
occurs in many cancer types, including ovarian cancer; ANXA4 is
also associated with the proliferation, apoptosis, metastasis,
chemoresistance, and with other biological behaviors of tumor cells
(27,28). A previous study by Choi et
al (29) indicated that ANXA4
was overexpressed in chemoresistant ovarian serous carcinoma
tissues compared with chemosensitive tissues, which was consistent
with our study. Lin et al (30) found that ANXA4 promoted the
proliferation of gastric cancer cells; during this process, Akt was
activated by phosphorylation to regulate the PI3K/Akt signaling
pathway, leading to cell proliferation and resistance to
chemotherapy or other stress conditions. In the present study,
after the RMG-I-H cells were treated with the anti-Lewis(y)
antibody, the expression of phosphorylated Akt was significantly
decreased.
The mechanisms of malignant tumor resistance to
chemotherapeutic drugs are complex and poorly understood; all
chemotherapeutic drugs exert their antitumor effects ultimately by
inducing the apoptosis of tumor cells. If an apoptosis signaling
pathway becomes defective or anti-apoptotic mechanisms are
enhanced, this may result in drug resistance in tumor cells. The
process of apoptosis is tightly controlled in eukaryotes and
consists of two main pathways: The mitochondrial and death receptor
pathways. Bcl-2 family proteins, including the pro-apoptotic
proteins, Bax and Bad, and the apoptosis-inhibitory proteins Bcl-2
and Bcl-xL, play vital roles in the mitochondrial pathway of
apoptosis. In this study, we found that regardless of carboplatin
treatment, the levels of phosphorylated Bad, Bcl-2 and Bcl-xL in
the RMG-I-H cells [which highly express Lewis(y) antigen] were
higher than those in the RMG-I cells. Following carboplatin
treatment, Bax expression in the RMG-I cells and p-Bad levels in
the RMG-I-H cells were markedly upregulated, whereas Bcl-2 and
Bcl-xL expression remained unaltered in both cell lines, compared
with expression levels before carboplatin treatment. These results
suggest that Lewis(y) may be involved in controlling apoptosis by
regulating the expression of Bcl-2 family proteins. Using a breast
cancer cell line, Kelly et al (31) reported that exposure to the
anti-Lewis(y) monoclonal antibody markedly increased the
sensitivity to γ-rays and led to an increased number of cancer
cells undergoing apoptosis. Baldus et al (32) found that the expression of Lewis(y)
increased in colon mucinous cancer cells, and simultaneously, the
number of apoptotic cells declined. Taken together, these results
demonstrate that Lewis(y) is closely related to apoptosis. A
homodimer or a heterodimer can be formed between most members of
the Bcl-2 family, and heterodimers formed between pro-apoptotic and
anti-apoptotic members repress opponent function. Thus, the ratio
of Bcl-2(Bcl-xL)/Bax determines the survival of cells. If Bax is
dominant, Bax homodimers enhance sensitivity of cells to
apoptosis-inducing signals. If Bcl-2 or Bcl-xL is dominant,
Bcl-2(Bcl-xL)/Bax heterodimers exhibit antiapoptotic functions,
which inhibit the release of cytochrome c from the
mitochondria to the cytoplasm and prevent activation of specific
caspase family pro-enzymes (including caspase-3 proenzyme) by
cytochrome c. Caspase-3 is the primary apoptosis-executing
protein that functions by partially or completely hydrolyzing
proteins essential to cell survival, such as DNA repair-associated
proteins. An increase in caspase-3 activity (i.e., the hydrolysis
of the proenzyme to small fragments) indicates that cellular
apoptosis has been initiated. We found that both the caspase-3
pro-enzyme and active fragment were expressed at lower levels in
the RMG-I-H cells than in the RMG-I cells, regardless of
carboplatin treatment. Our results also suggested that the
overexpression of Lewis(y) antigen induced an increased expression
of the apoptosis-inhibitory proteins, Bcl-2 and Bcl-xL, and a
decreased expression of the pro-apoptosic protein, Bax.
Consequently, the ratio of Bcl-2(Bcl-xL)/Bax increased, which
further inhibited the activation of caspase-3 pro-enzyme, decreased
the availability of caspase-3 active fragments, inhibited cell
apoptosis, and promoted the development of tumor drug
resistance.
Azuma et al (33,34)
found that the expression of both FUT4 and its products,
[Lewis(x) and Lewis(y)], increased after an anti-Fas antibody was
used to induce the apoptosis of a human T cell line (Jurkat), and a
caspase-3 inhibitor suppressed the increase in Lewis(y) antigen.
Iwamori et al (16)
reported that the levels of fucosyl antigens [e.g., Lewis(b) and
Lewis(x)] on the cell surface increased markedly when a human
ovarian cancer cell line (KFr13TX) exhibited drug resistance to
paclitaxel. In our previous study, we found that c-Jun/AP-1
expression was increased in highly malignant ovarian cancer tissues
and cells, and its expression was positively associated with the
mRNA expression of FUT1 and Lewis(y) antigen. We also found that
the promoter region of FUT1 contained an AP-1 response
element; c-Jun/AP-1 can specifically bind to the promoter of
FUT1 to promote FUT1 transcription and Lewis(y)
antigen expression (35). In the
present study, we found that after highly-expressing Lewis(y)
ovarian cancer cells were treated with carboplatin, the increases
in the levels of phosphorylated AKT were more significant than
those before treatment.
In conclusion, the expression of Lewis(y) antigen is
elevated in chemoresistant ovarian cancer cells, and ovarian cancer
cells with a high expression of Lewis(y) are more likely to develop
resistance, thus forming a positive feedback loop, which promotes
the progression of ovarian cancer. Based on existing research and
our experimental results, we hypothesized that ovarian cancer cells
activate their own anti-apoptotic mechanism, while the apoptotic
program is activated under the control of apoptosis-inducing
factors, which promote Lewis(y) antigen expression; the increased
expression of Lewis(y) antigen then upregulates the relevant
pathways and ultimately enhances the anti-apoptotic activity of
ovarian cancer cells. The potential mechanisms are as follows:
Lewis(y) is a component of many membrane proteins including ANXA4;
its expression increases with increasing malignancy of ovarian
cancer tissues or cells; the overexpression of Lewis(y) can then
activate downstream signaling pathways to regulate the expression
of multiple factors and mediate drug resistance of cancer cells;
Lewis(y) can also affect the expression and activity of c-Jun/AP-1
to regulate the activity of α1,2-fucosyltransferase and the
transcription of FUT1, which promotes the expression of Lewis(y)
via a positive feedback mechanism. These effects eventually promote
the development and progression of ovarian cancer.
Acknowledgments
Not applicable.
Funding
This study was supported by grants from The National
Natural Science Foundation of China (nos. 81172491, 81101527,
81472437 and 81672590), and Outstanding Scientific Fund of
Shengjing Hospital (no. 201303).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL carried out most parts of the experiment; MZ, YQ
and HW participated in the experiments; BL participated in the
conception and design of the study; ML and QL performed the
statistical analysis. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Samples were fully encoded to protect patient
confidentially and thus patient consent was waived by the Ethics
Committee. The study and its protocols were approved by the
Research Ethics committees of Shengjing Hospital Affiliated with
China Medical University (2013PS66K).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Markman M: Current standards of care for
chemotherapy of optimally cytoreduced advanced epithelial ovarian
cancer. Gynecol Oncol. 131:241–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pliarchopoulou K and Pectasides D:
Epithelial ovarian cancer: Focus on targeted therapy. Crit Rev
Oncol Hematol. 79:17–23. 2011. View Article : Google Scholar
|
|
3
|
He S, Niu G, Shang J, Deng Y, Wan Z, Zhang
C, You Z and Shen H: The oncogenic Golgi phosphoprotein 3 like
overexpression is associated with cisplatin resistance in ovarian
carcinoma and activating the NF-κB signaling pathway. J Exp Clin
Cancer Res. 36:1372017. View Article : Google Scholar
|
|
4
|
Roberts CM, Tran MA, Pitruzzello MC, Wen
W, Loeza J, Dellinger TH, Mor G and Glackin CA: TWIST1 drives
cisplatin resistance and cell survival in an ovarian cancer model,
via upregulation of GAS6, L1CAM, and Akt signalling. Sci Rep.
6:376522016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roseman S: Reflections on glycobiology. J
Biol Chem. 276:41527–41542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwamori M, Tanaka K, Kubushiro K, Lin B,
Kiguchi K, Ishiwata I, Tsukazaki K and Nozawa S: Alterations in the
glycolipid composition and cellular properties of ovarian
carcinoma-derived RMG-1 cells on transfection of the
α1,2-fucosyltransferase gene. Cancer Sci. 96:26–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Lin B, Hao YY, Yan LM, Liu JJ, Zhu
LC and Zhang SL: The effects of Lewis(y) antigen content on drug
resistance to carboplatin in ovarian cancer line RMG-I. Prog
Biochem Biophys. 35:1175–1182. 2008.
|
|
8
|
Zhang F, Liu J, Lin B, Liu Q, Zhao Y, Zhu
L, Hao Y, Zhang S and Iwamori M: Increase in docetaxel-resistance
of ovarian carcinoma-derived RMG-1 cells with enhanced expression
of Lewis Y antigen. Int J Mol Sci. 12:7323–7334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Lin B, Hao Y, Qi Y, Zhu L, Li F,
Liu D, Cong J, Zhang S and Iwamori M: Lewis y antigen promotes the
proliferation of ovarian carcinoma-derived RMG-I cells through the
PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 28:1542009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Z, Gao J, Zhang D, Liu Q, Yan L, Gao L,
Liu J, Liu D, Zhang S and Lin B: High expression of Lewis y antigen
and CD44 is correlated with resistance to chemotherapy in
epithelial ovarian cancers. PLoS One. 8:e572502013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao J, Hu Z, Liu J, Liu D, Wang Y, Cai M,
Zhang D, Tan M and Lin B: Expression of CD147 and Lewis y antigen
in ovarian cancer and their relationship to drug resistance. Med
Oncol. 31:9202014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu LC, Gao J, Hu ZH, Schwab CL, Zhuang
HY, Tan MZ, Yan LM, Liu JJ, Zhang DY and Lin B: Membranous
expressions of Lewis y and CAM-DR-related markers are independent
factors of chemotherapy resistance and poor prognosis in epithelial
ovarian cancer. Am J Cancer Res. 5:830–843. 2015.PubMed/NCBI
|
|
13
|
Hu Z, Gao S, Gao J, Hou R, Liu C, Liu J,
Li B, Liu D, Zhang S and Lin B: Elevated levels of Lewis y and
integrin α5β1 correlate with chemotherapeutic drug resistance in
epithelial ovarian carcinoma. Int J Mol Sci. 13:15588–15600. 2012.
View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
15
|
Zhu L, Hu Z, Liu J, Gao J and Lin B: Gene
expression profile analysis identifies metastasis and
chemoresistance-associated genes in epithelial ovarian carcinoma
cells. Med Oncol. 32:4262015. View Article : Google Scholar
|
|
16
|
Iwamori M, Iwamori Y, Kubushiro K,
Ishiwata I and Kiguchi K: Characteristic expression of
Lewis-antigenic glycolipids in human ovarian carcinoma-derived
cells with anticancer drug-resistance. J Biochem. 141:309–317.
2007. View Article : Google Scholar
|
|
17
|
Madjd Z, Parsons T, Watson NF, Spendlove
I, Ellis I and Durrant LG: High expression of Lewis y/b antigens is
associated with decreased survival in lymph node negative breast
carcinomas. Breast Cancer Res. 7:R780–R787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao L, Yan L, Lin B, Gao J, Liang X, Wang
Y, Liu J, Zhang S and Iwamori M: Enhancive effects of Lewis y
antigen on CD44-mediated adhesion and spreading of human ovarian
cancer cell line RMG-I. J Exp Clin Cancer Res. 30:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan LM, Lin B, Zhu LC, Hao YY, Qi Y, Wang
CZ, Gao S, Liu SC, Zhang SL and Iwamori M: Enhancement of the
adhesive and spreading potentials of ovarian carcinoma RMG-1 cells
due to increased expression of integrin alpha5beta1 with the Lewis
Y-structure on transfection of the alpha1,2-fucosyltrans-ferase
gene. Biochimie. 92:852–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan M, Zhu L, Zhuang H, Hao Y, Gao S, Liu
S, Liu Q, Liu D, Liu J and Lin B: Lewis Y antigen modified CD47 is
an independent risk factor for poor prognosis and promotes early
ovarian cancer metastasis. Am J Cancer Res. 5:2777–2787.
2015.PubMed/NCBI
|
|
21
|
Gao S, Liu Q, Wang X, Lin B and Zhang S:
Effects of Lewis Y antigen on the gene expression of multiple drug
resistance-associated proteins in human ovarian cancer RMG-I-H
cells. Med Oncol. 27:960–967. 2010. View Article : Google Scholar
|
|
22
|
Wang C, Yan L, Wang Y, Lin B, Liu S, Li Q,
Gao L, Zhang S and Iwamori M: Overexpression of Lewis(y) antigen
protects ovarian cancer RMG-1 cells from carboplatin-induced
apoptosis by the upregulation of Topo-I and Topo-II β. Anat Rec
(Hoboken). 294:961–969. 2011. View
Article : Google Scholar
|
|
23
|
Wang XQ, Sun P, O'Gorman M, Tai T and
Paller AS: Epidermal growth factor receptor glycosylation is
required for ganglioside GM3 binding and GM3-mediated suppression
[correction of suppresion] of activation. Glycobiology. 11:515–522.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu JJ, Lin B, Hao YY, Li FF, Liu DW, Qi
Y, Zhu LC, Zhang SL and Iwamori M: Lewis(y) antigen stimulates the
growth of ovarian cancer cells via regulation of the epidermal
growth factor receptor pathway. Oncol Rep. 23:833–841.
2010.PubMed/NCBI
|
|
25
|
Li FF, Liu JJ, Liu DW, Lin B, Hao YY, Cong
JP, Zhu LC, Gao S, Zhang SL and Iwamori M: Lewis Y regulates
signaling molecules of the transforming growth factor β path way in
ovarian carcinoma derived RMG-I cells. Int J Oncol. 40:1196–1202.
2012. View Article : Google Scholar
|
|
26
|
Zhuang H, Gao J, Hu Z, Liu J, Liu D and
Lin B: Co-expression of Lewis y antigen with human epididymis
protein 4 in ovarian epithelial carcinoma. PLoS One. 8:e689942013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee T, Guo K, Li S, Qin S and Liu S:
Effects of ANXA4 on cell adhesive ability and expression of
adhesion-related genes in hepatocellular carcinoma MHCC97H cell
line. J Clin Trials. 31:588–591. 2013.
|
|
28
|
Matsuzaki S, Serada S, Morimoto A, Ueda Y,
Yoshino K, Kimura T and Naka T: Annexin A4 is a promising
therapeutic target for the treatment of platinum-resistant cancers.
Expert Opin Ther Targets. 18:403–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi CH, Sung CO, Kim HJ, Lee YY, Song SY,
Song T, Kim J, Kim TJ, Lee JW, Bae DS, et al: Overexpression of
Annexin A4 is associated with chemoresistance in papillary serous
adenocar-cinoma of the ovary. Hum Pathol. 44:1017–1023. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin LL, Huang HC and Juan HF: Revealing
the molecular mechanism of gastric cancer marker Annexin A4 in
cancer cell proliferation using exon arrays. PLoS One.
7:e446152012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kelly MP, Lee FT, Tahtis K, Smyth FE,
Brechbiel MW and Scott AM: Radioimmunotherapy with alpha-particle
emitting 213Bi-C-functionalized
trans-cyclohexyl-diethylenetriamine-pentaacetic acid-humanized
3S193 is enhanced by combination with paclitaxel chemotherapy. Clin
Cancer Res. 13:5604s–5612s. 2007. View Article : Google Scholar
|
|
32
|
Baldus SE, Mönig SP, Zirbes TK, Thakran J,
Köthe D, Köppel M, Hanisch FG, Thiele J, Schneider PM, Hölscher AH,
et al: Lewis(y) antigen (CD174) and apoptosis in gastric and
colorectal carcinomas: Correlations with clinical and prognostic
parameters. Histol Histopathol. 21:503–510. 2006.PubMed/NCBI
|
|
33
|
Azuma Y, Ito M, Taniguchi A and Matsumoto
K: Expression of cell surface Lewis X and Y antigens and FUT4 mRNA
is increased in Jurkat cells undergoing apoptosis. Biochim Biophys
Acta. 1672:157–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azuma Y, Kurusu Y, Sato H, Higai K and
Matsumoto K: Increased expression of Lewis X and Y antigens on the
cell surface and FUT 4 mRNA during granzyme B-induced Jurkat cell
apoptosis. Biol Pharm Bull. 30:655–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao N, Liu J, Liu D, Hao Y, Yan L, Ma Y,
Zhuang H, Hu Z, Gao J, Yang Z, et al: c-Jun transcriptionally
regulates alpha 1, 2-fucosyltransferase 1 (FUT1) in ovarian cancer.
Biochimie. 107:286–292. 2014. View Article : Google Scholar : PubMed/NCBI
|