Introduction
Cervical cancer is the fourth most common cancer in
women worldwide, with an estimated 527,600 new cases and 265,700
deaths reported in 2012 (1).
Although the association between persistent high-risk human
papillomavirus (HPV) infection and the development of cervical
cancer has been demonstrated by clinical epidemiology, molecular
and functional studies, the specific molecular network mechanisms
underlying the transition from HPV infection to tumorigenesis have
not been fully elucidated. Therefore, there is an urgent clinical
need to explore the potential mechanisms underlying cervical
tumorigenesis and identify novel tumor markers and treatment
targets, in order to ultimately prolong survival and improve the
quality of life of the patients.
Interleukin-17 (IL-17) is a CD4 T-cell-derived
pro-inflammatory cytokine, initially referred to as CTLA-8
(2). IL-17A plays an important
role in adaptive immune response and is a mediator of chronic
inflammation and autoimmune diseases (3,4). The
IL-17 receptor (IL-17R) is widely expressed on various cells,
including macrophages, granulocytes, T cells, fibroblasts and
endothelial cells, among others; thus, IL-17A acts on various cell
types to exert its biological effects.
Recent studies have demonstrated that IL-17A
promotes tumor progression in several types of cancer, such as
colorectal (5), lung (6), breast (7), gastric (8) and hepatocellular carcinoma (9). Thus, IL-17 is considered as an
important mediator of inflammation-related cancers (10); it can also induce the production of
several inflammatory factors, including tumor necrosis factor-α,
IL-6 and IL-1β (11), which have
been implicated in the development of tumors. IL-17A may also
promote vascular endothelial growth factor (VEGF) secretion and
tumor angiogenesis, as well as cell invasion and metastasis
(12,13).
Heparanase (HPSE) is an enzyme that acts both on the
cell surface and within the extracellular matrix (ECM) to degrade
polymeric heparan sulfate molecules into shorter-chain
oligosaccharides, thereby activating macrophages to release
inflammatory factors and chemokines, and ultimately promoting tumor
cell growth (14). It has been
demonstrated that HPSE is expressed in several malignancies
(15-17), is associated with tumor progression
and prognosis, and is involved in the regulation of tumor-related
processes, such as angiogenesis, inflammation, tumor cell invasion
and metastasis (18,19). According to the literature, IL-17A
and HPSE have been found to be associated with tumor and
inflammation, and mediate inflammation-related tumor
development.
Our previous studies confirmed that the genotypes of
IL-17A rs2275913 may play an important role in the development of
cervical cancer, particularly in patients with HPV-16 or HPV-18
infection (20). Moreover, it has
been confirmed that HPSE silencing significantly reduced the
invasiveness of cervical cancer cells (21). Based on this previous research, the
present study further analyzed the association between IL-17 and
microvessel density (MVD) in cervical cancer by
immunohistochemistry. Immunohistochemistry, Transwell and MTT
assays and flow cytometry were performed to study the potential
roles of IL-17A and HPSE in the development of cervical cancer, as
well as the underlying molecular mechanisms, with the aim of
providing a theoretical molecular basis for therapeutic
targeting.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of Zhongnan Hospital of Wuhan University. The human
cervical tissue samples used in this study were obtained from
patients following written informed consent.
Cell lines and samples
A total of 80 pairs of samples were obtained from
patients with primary cervical cancer who had undergone surgery
without chemotherapy or radiotherapy at Zhongnan Hospital of Wuhan
University between March 2015 and March 2017. The diagnosis of
cervical cancer was confirmed by pathological examination, and
adjacent normal cervical tissues (>3 cm away from the tumor)
were used as controls. The human cervical cancer cell lines HeLa
and SiHa were obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and cultured in DMEM (Gibco; Thermo
Fisher Scientific, Shanghai, China) containing 10% FBS (HyClone; GE
Healthcare, Logan, UT, USA).
Immunohistochemistry
Immunohistochemistry was performed as previously
described (22). Briefly, the
cervical tissues were dissected, fixed in 4% paraformaldehyde,
embedded in paraffin, sectioned at a thickness of ~7 µm,
deparaffinized, rehydrated, subjected to antigen retrieval in a
microwave oven and probed with primary rat antibodies against IL17A
(sc-374218), HPSE (sc-515935) and CD31 (sc-71872) (mouse monoclonal
antibodies, 1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). Secondary antibodies combined with streptavidin-horseradish
peroxidase (sc-516102, 1:100; Santa Cruz Biotechnology, Inc.) were
used to detect IL-17A, HPSE and CD31 expression. In negative
controls, primary antibodies were omitted and phosphate-buffered
saline (PBS; pH 7.4) was used instead. To analyze the results, the
sections were examined and images were captured with an Olympus
BX-40 microscope (Olympus Corp., Melville, NY, USA).
Transfection of plasmids
Pre-designed shRNA against HPSE and IL-17A and
negative control shRNA were purchased from GenePharma (GenePharma
Co., Ltd., Shanghai, China). The shRNA were transfected into cells
seeded on 6-well plates at a density of 10,000 cells/well, as
described previously (23).
Briefly, after 24 h of culture in DMEM, the cells were transfected
with the HPSE, IL-17A and negative control shRNA at a final
concentration of 10 nM using Lipofectamine™ 3000 (Invitrogen;
Thermo Fisher Scientific, Carlsbad, CA, USA) according to the
manufacturer's protocol. At 48 h post-transfection, cells were
harvested for further experiments.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from cell lines with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific)
according to the manufacturer's protocol. cDNA was synthesized by
reverse transcription of the total RNA. RT-qPCR was performed using
a SYBR Green Real Time PCR Kit (Toyobo Co., Ltd., Osaka, Japan) in
a 10-µl reaction volume, which contained 1 µl cDNA
template, 0.2 µl of each primer, 3.6 µl
DEPC-H2O, and 5 µl SYBR Green dye, using an ABI
Step One Plus™ Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific). The cycling conditions for the amplification of
genes were as follows: Initial denaturation at 95°C for 30 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec, annealing
at 60°C for 10 sec and elongation at 72°C for 30 sec. The relevant
primers are listed in Table I.
 | Table IThe relative primers of target
genes. |
Table I
The relative primers of target
genes.
| Gene | | Sequence | Size (bp) |
|---|
| β-actin | Forward |
5′-CACGATGGAGGGGCCGGACTCATC-3′ | 240 |
| Reverse |
5′-TAAAGACCTCTATGCCAACACAGT-3′ | |
| VEGF | Forward |
5′-AAGGAGGAGGGCAGAATCAT-3′ | 226 |
| Reverse |
5′-ATCTGCATGGTGATGTTGGA-3′ | |
| CD31 | Forward |
5′-GTGCTGCAATGTGCTGTGAAT-3′ | 180 |
| HPSE | Forward |
5′-ATCAATGGGTCGCAGTTAG-3′ | 128 |
| Reverse |
5′-AGCATCTTAGCCGTCTTTC-3′ | |
| P53 | Forward |
5′-CCACCATCCACTACAACTACAT-3′ | 135 |
| Reverse |
5′-AAACACGCACCTCAAAGC-3′ | |
| IL17 | Forward |
5′-CCACCTCACCTTGGAATCTC-3′ | 220 |
| Reverse |
5′-CCCACGGACACCAGTATCTT-3′ | |
Western blot analysis
Western blot analysis was performed as described
previously (24). Briefly, total
protein was extracted from cells using radio-immunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) supplemented with protease and phosphatase inhibitors
(Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA). Equal amounts of
protein samples were loaded and separated by 10% SDS-PAGE, prior to
being electrophoretically transferred to 0.44-µm PVDF
membranes (Millipore, Bedford, MA, USA) at 250 mA for 60 min on
ice. The non-specific binding sites on the membrane were blocked
with 5% fat-free milk in TBS containing 0.1% Tween-20 at room
temperature for 2 h. Then, the membranes were incubated with a
primary antibody (Santa Cruz Biotechnology, Inc.) at 4°C overnight
and developed with a secondary antibody conjugated to HRP (Santa
Cruz Biotechnology Inc.) at room temperature for 1.5 h. Finally,
the proteins were visualized using enhanced chemiluminescence
luminol reagent (PerkinElmer, Inc., Boston, MA, USA) and band
intensities were quantified using ImageJ software (National
Institutes of Health, Bethesda, MD, USA). GAPDH was used as a
loading control.
Cell cycle distribution analysis
At 48 h after transfection with shRNA or treatment
with recombinant proteins (HPSE, ab232817, ProSpec-Tany; IL-17A,
ab9567, Abcam, Cambridge, UK), the cells were detached and fixed
with 70% ethanol at −20°C overnight. Subsequently, the cells were
collected by centrifugation at 500 × g for 5 min, washed with PBS
and incubated with 25 µg/ml RNase A and 50 µg/ml
propidium iodide (PI) for 30 min in the dark. In a total of
2×104 cells, the cell cycle distribution was mapped by
flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) under
excitation and emission wavelengths of 488 and 525 nm,
respectively.
Cell proliferation
CCK-8 assays were performed to evaluate cell
proliferation. For the CCK-8 assays, 5×103 cells/well
transfected with shRNA or treated with recombinant proteins were
seeded into 96-well plates, cultured in DMEM for 72 h and incubated
for another 2 h after CCK-8 (10 µl; American Type Culture
Collection, Manassas, VA, USA) was added into each well. The cell
proliferation status was determined from the absorbance of culture
plates using ELISA readers (Tecan, Port Melbourne, Australia) at
450 nm after 24 h.
Transwell assays
Transwell assays were used to examine cell invasion.
At 24 h after transfection with shRNA or treatment with recombinant
proteins, 8×104 cells/well were cultured in 200
µl serum-free DMEM and then transferred to an upper
Transwell chamber containing an 8-µm pore size membrane
coated with Matrigel (BD Biosciences), while the lower chamber was
filled with 800 µl DMEM supplemented with 10% FBS. After 48
h, the invaded cells on the lower side of the membrane were fixed
in methanol and stained with 0.1% crystal violet solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were
then counted under a microscope (Olympus Corp., Tokyo, Japan).
Statistical analysis
Statistical analyses were conducted with SPSS v.22.0
software (IBM Corp., Armonk, NY, USA). Data are expressed as the
mean ± standard error of ≥3 independent experiments. The
correlation between variables was determined using Spearman's
correlation. Differences between groups were evaluated using the
Student's t-test or one-way analysis of variance, and P-values
<0.05 were considered to indicate statistically significant
differences.
Results
IL-17A and HPSE are highly expressed in
cervical cancer and may promote tumor angiogenesis
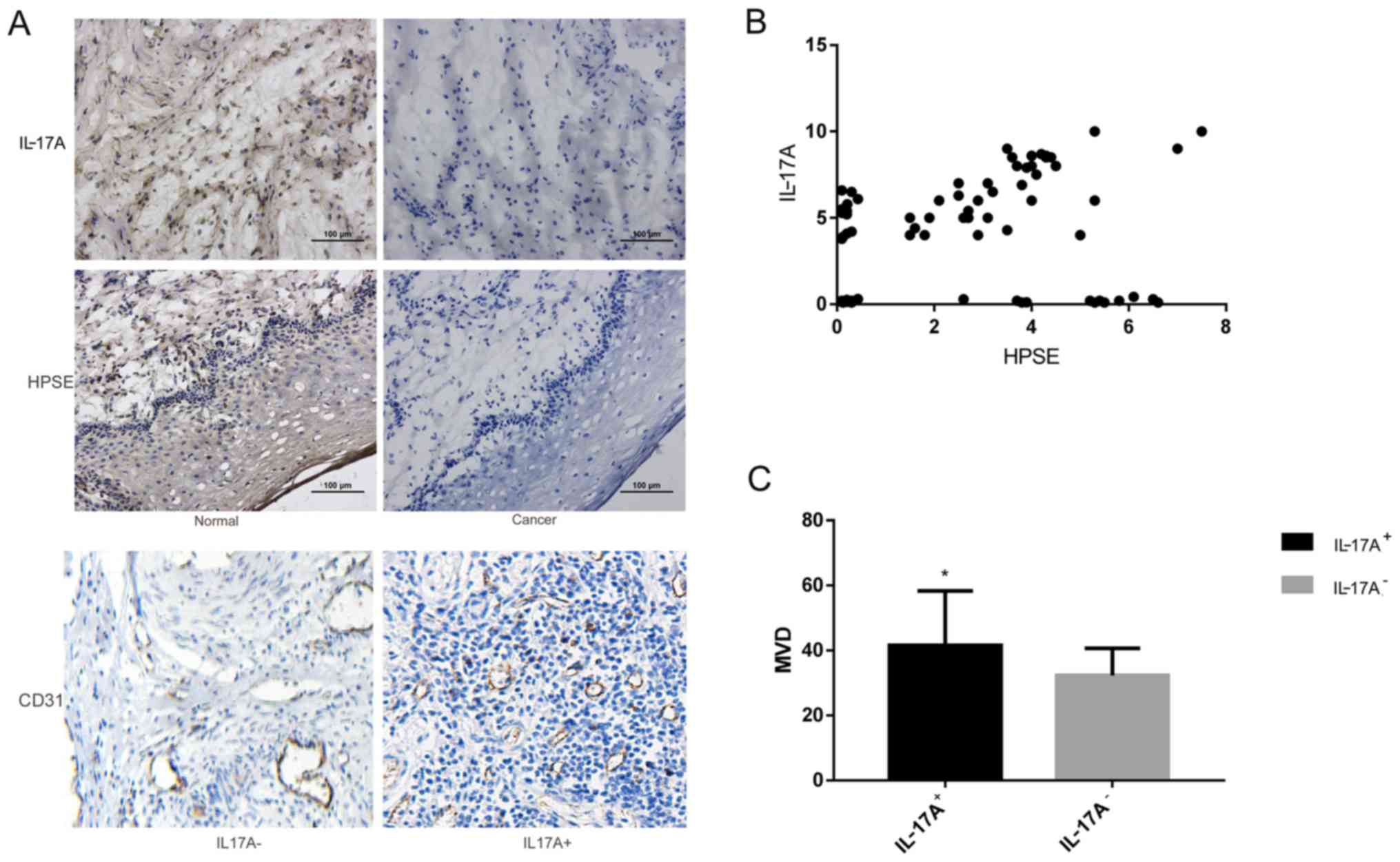
According to the results of immunohistochemistry,
IL-17A was mainly expressed in cancer cells, with the staining
mainly distributed in the cytoplasm; HPSE staining was also
distributed in the cytoplasm, as well as being occasionally visible
in the nucleus (Fig. 1A). The
positive expression rates of IL-17A and HPSE in cervical cancer
tissues were both ~70%, and according to the correlation analysis,
there was a significant positive correlation between the two
(Fig. 1B, Table II). Among patients with cervical
cancer, there were 56 cases with IL-17A-positive expression, in
which the MVD was 41.567±16.72, while in the 24 cases with
IL-17A-negative expression the MVD was 32.332±8.31. The MVD was
significantly higher in the IL-17A-positive group compared with
that in the IL-17A-negative group (P<0.001; Fig. 1C, Table III).
 | Table IIAssociation between IL-17A and
HPSE. |
Table II
Association between IL-17A and
HPSE.
| HPSE
| r | P-value |
|---|
| Positive | Negative |
|---|
| IL-17A | | | | 0.01 |
| Positive | 44 | 12 | 0.286 | |
| Negative | 12 | 12 | | |
 | Table IIIAssociation between IL-17A and
MVD. |
Table III
Association between IL-17A and
MVD.
| Patient no. | MVD
|
|---|
| x̄±s | t | P-value |
|---|
| IL-17A | | | | 0.000 |
| Positive | 56 | 41.567±16.72 | | |
| Negative | 24 | 32.332±8.31 | 6.085 | |
IL-17A and HPSE promote cell
proliferation and invasion
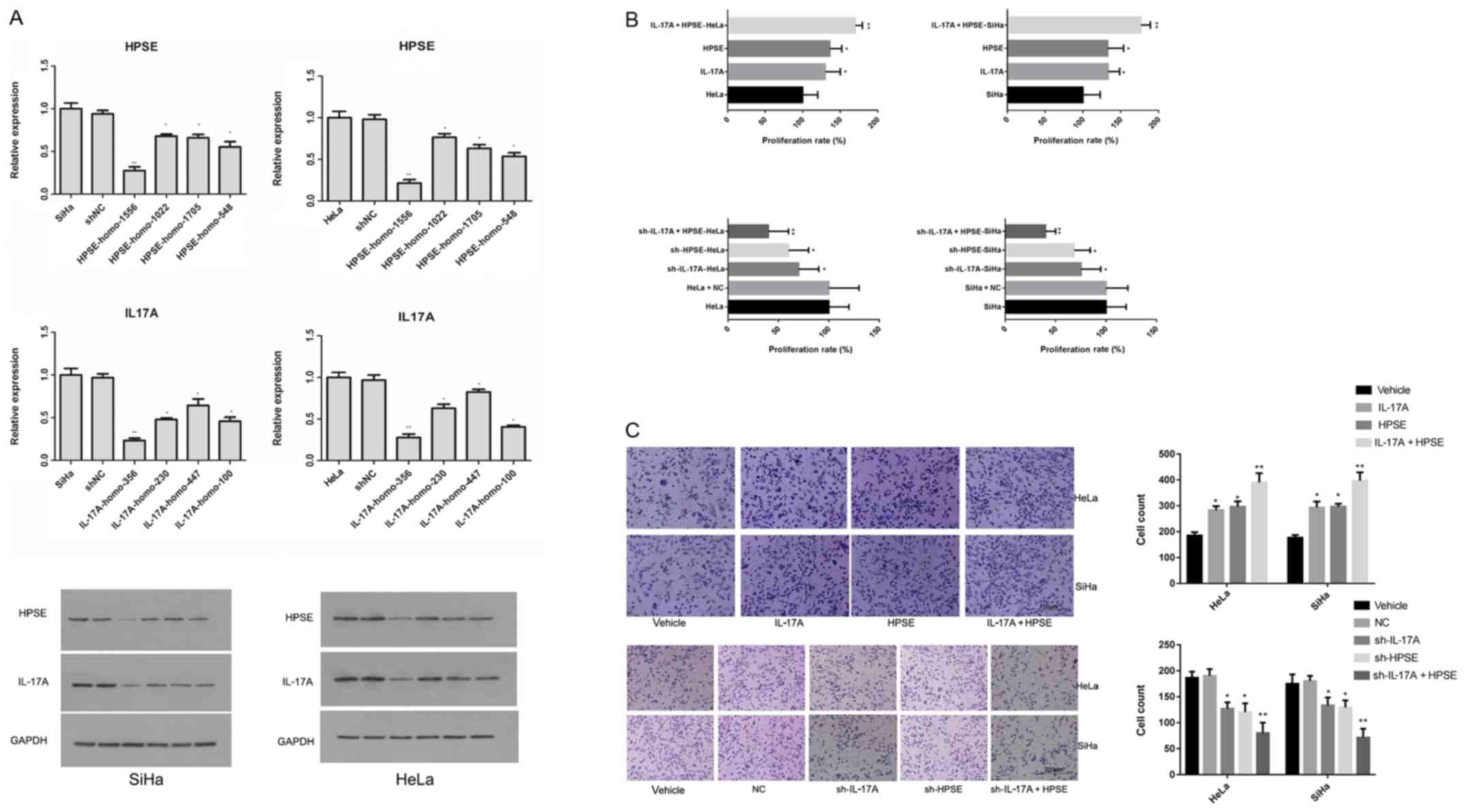
HPSE shRNA experimental groups were established as
follows: HPSE-1556, HPSE-1022, HPSE-1705, HPSE-548, negative
control (NC) and blank control groups. IL-17A shRNA experimental
groups were established as follows: IL-17A-356, IL-17A-230,
IL-17A-447, IL-17A-100, NC and blank control groups. The levels of
IL-17A and HPSE mRNA in both SiHa and HeLa cells in the HPSE-1556
and IL-17A-356 groups were the lowest, indicating that the two
shRNA plasmids had the best silencing effect on the relative genes.
Western blot analysis yielded the same results (Fig. 2A). SiHa and HeLa cells were treated
with recombinant protein (HPSE: 500 ng/ml; IL-17A: 20 ng/ml) or
shRNA. After 24 h of incubation, the proliferation rate of cells
was measured. The IL-17A and HPSE recombinant proteins were found
to promote cell proliferation; moreover, the IL-17A and HPSE
recombinant proteins exerted an additive effect; after the IL-17A
and HPSE genes were silenced, the cell proliferation rate was
significantly decreased, with the inhibition induced by IL-17A and
HPSE shRNA co-transfection being the most notable (Fig. 2B). The invasion of cervical cancer
cells was detected using a Transwell assay following treatment with
recombinant protein or shRNA. It was observed that the numbers of
cells invading to the lower chamber in the IL-17A, HPSE and IL-17A
+ HPSE groups were significantly higher compared with those in the
NC and blank control groups (P<0.01). Moreover, the number of
invaded cells in the sh-IL-17A, sh-HPSE and sh-IL-17A + HPSE groups
was significantly lower compared with that in the NC and blank
control groups (P<0.01) (Fig.
2C).
The proportion of cells in the G2/M phase
was increased by IL-17A and HPSE
Cell cycle distribution was measured by flow
cytometry. The proportion of cells in the G2/M phase in the IL-17A
HeLa, HPSE HeLa and IL-17A + HPSE HeLa cell groups was
significantly higher compared with that in the control HeLa cell
groups (P<0.01). These results demonstrated that IL-17A and HPSE
recombinant proteins significantly increased the rate of HeLa cell
proliferation. The SiHa cell groups exhibited similar changes
(Fig. 3A). Conversely, following
silencing of IL-17A and/or HPSE in HeLa and SiHa cells, the number
of cells in the G2/M phase was significantly reduced compared with
the corresponding control groups (P<0.01). Moreover, IL-17A and
HPSE gene silencing exerted an additive effect in inhibiting cell
proliferation (Fig. 3B).
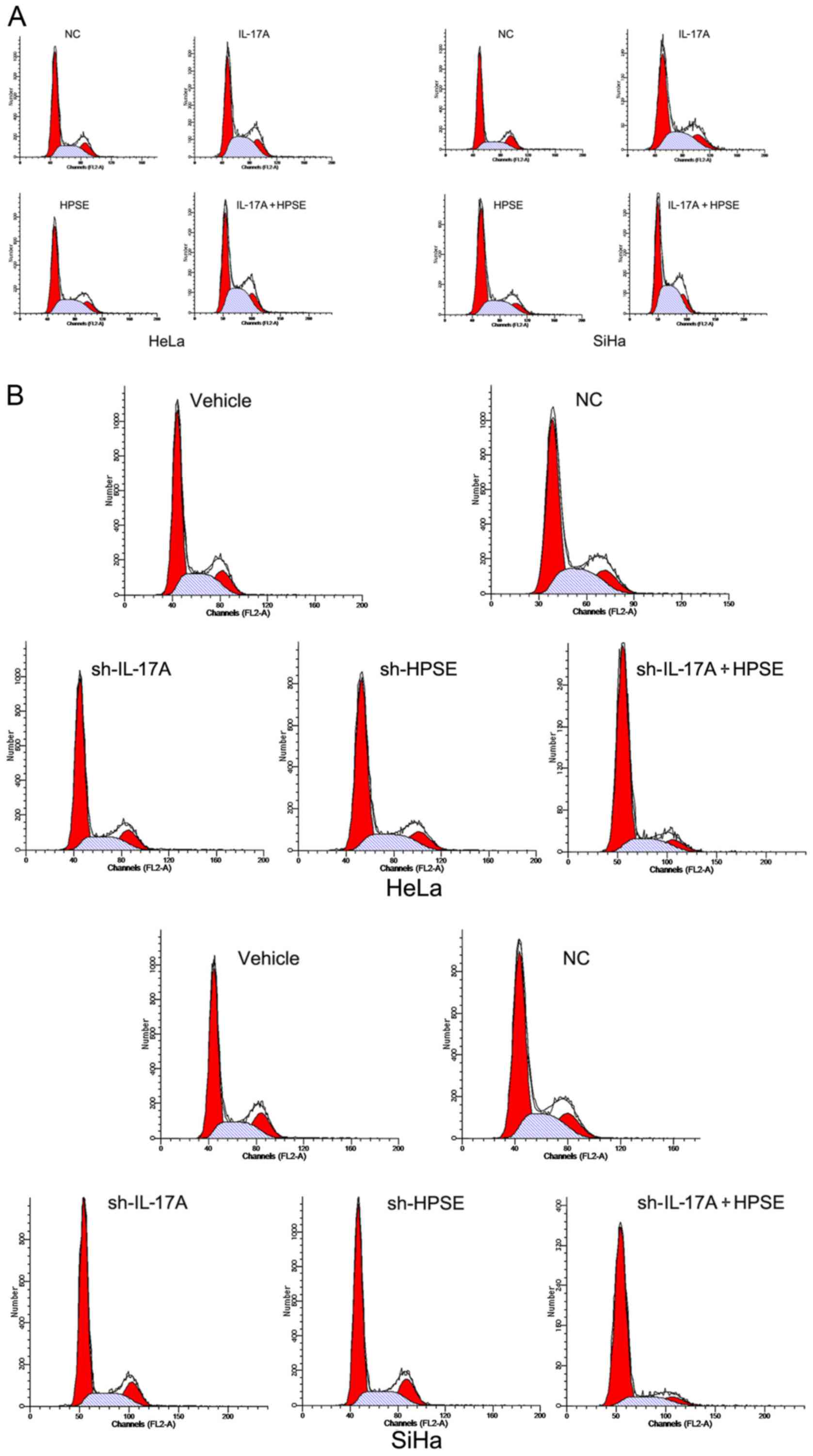 | Figure 3(A) Cell cycle distribution measured
by flow cytometry following target protein upregulation. The left
flow chart shows the proportion of cells in the G2/M phase in the
HeLa, IL-17A-HeLa, HPSE-HeLa and IL-17A + HPSE-HeLa cell groups.
The right flow chart shows the proportion of cells in the G2/M
phase in the SiHa, IL-17A-SiHa, HPSE-SiHa and IL-17A + HPSE-SiHa
cell groups. (B) Cell cycle distribution measured by flow cytometry
following target protein knockdown. The upper flow chart shows the
proportion of cells in the G2/M phase in the HeLa, NC, IL-17A-HeLa,
HPSE-HeLa and IL-17A + HPSE-HeLa cell groups. The lower flow chart
shows the proportion of cells in the G2/M phase in the SiHa, NC,
IL-17A-SiHa, HPSE-SiHa and IL-17A + HPSE-SiHa cell groups. (C) The
relative statistical analysis of parts A and B. IL, interleukin;
HPSE, heparanase; NC, negative control. |
IL-17A and HPSE can increase the mRNA and
protein levels of HPV E6, CD31, VEGF and P53
Compared with NC cells, the expression levels of HPV
E6, CD31, VEGF, HPSE, L17A and P53 in HeLa and SiHa cells were
significantly increased following treatment with IL-17 or HSPE
recombinant protein (P<0.05) (Fig.
4A). However, after silencing of HPSE and IL-17A gene
expression, the mRNA and protein levels of HPV E6, CD31, VEGF, HPSE
and L17A in HeLa and SiHa cells were significantly decreased
(P<0.05; Fig. 4B).
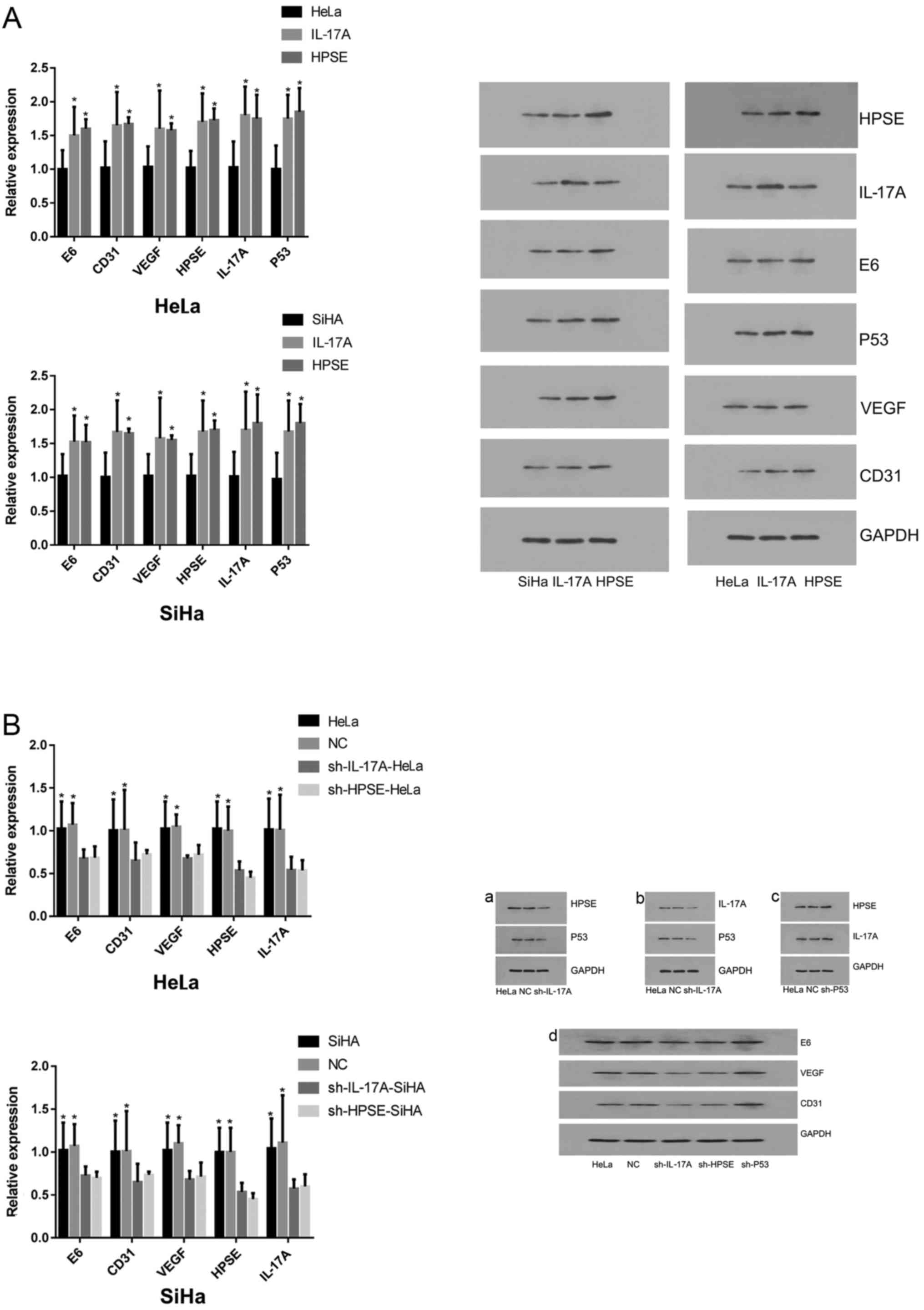 | Figure 4(A) mRNA and protein expression of HPV
E6, CD31, VEGF, HPSE, IL-17A and P53 following target protein
upregulation. Shown are the histogram results of the relative mRNA
levels of HPV E6, CD31, VEGF, HPSE, IL-17A and P53 in HeLa and SiHa
cells following treatment with recombinant proteins of IL-17A and
HPSE for 48 h. The data shown are representative of multiple
experiments; (B) mRNA and protein expression of HPV E6, CD31, VEGF,
HPSE and IL-17A in HeLa and SiHa cells following target protein
knockdown. Shown are the histogram results of the relative mRNA
levels of HPV E6, CD31, VEGF, HPSE and IL-17A in HeLa and SiHa
cells following treatment with shRNAs against IL-17A and HPSE for
24 h. The data shown are representative of multiple experiments
(presented as the mean ± standard deviation, n=10;
*P<0.05, **P<0.01). HPV, human
papillomavirus; VEGF, vascular endothelial growth factor; HPSE,
heparanase; IL, interleukin. |
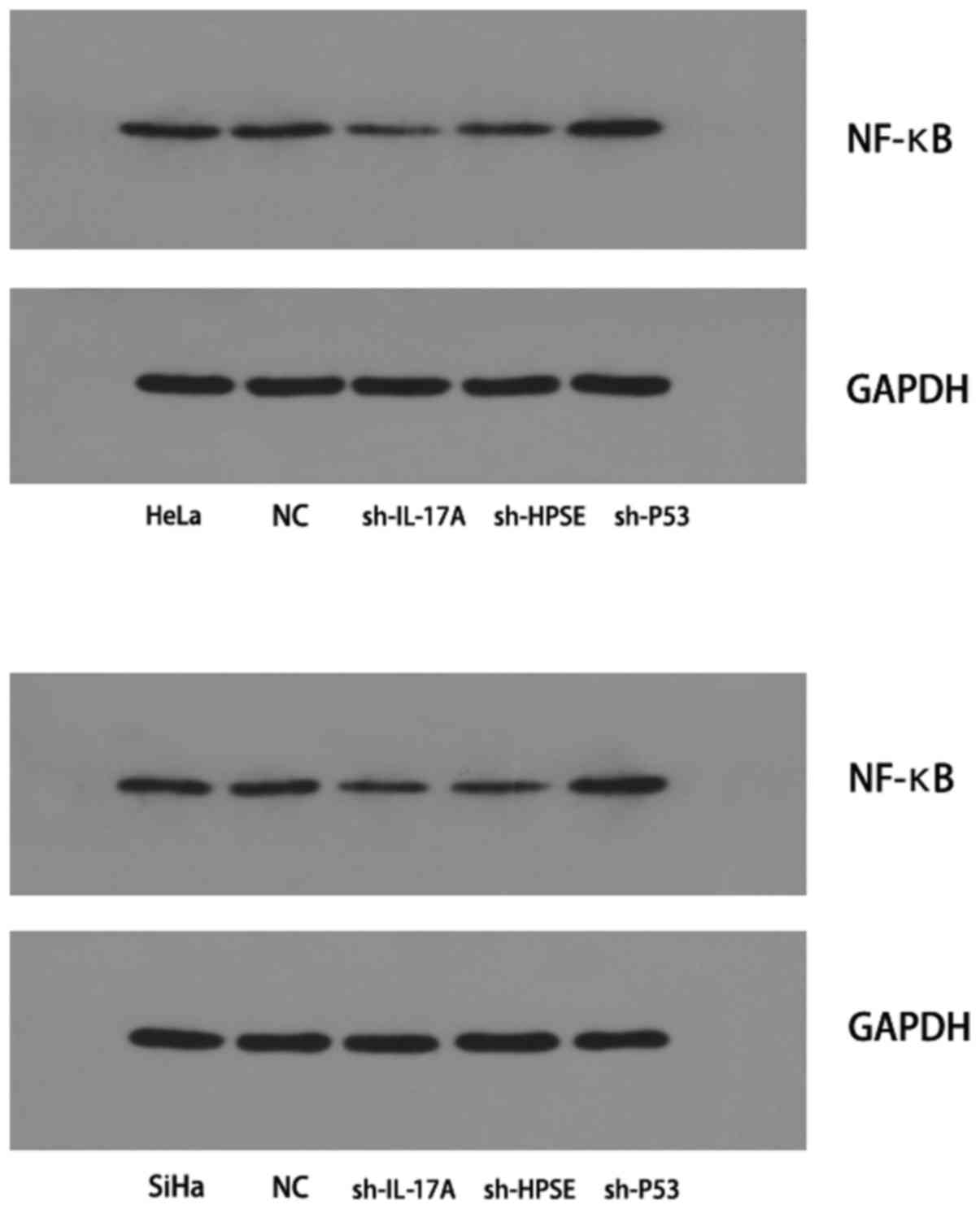
Detection of NF-κB P65 protein expression
by western blotting
The expression of the NF-κB P65 protein in HeLa and
SiHa cells was found to be decreased following inhibition of IL-17A
or HPSE. However, inhibition of P53 gene expression significantly
increased the protein expression of NF-κB P65; compared with the
control group, the difference was statistically significant
(P<0.05; Fig. 5).
Discussion
Since Rudolph Virchow identified inflammatory cells
in tumor tissue and formulated the hypothesis that tumors may
originate from chronic inflammation, the association between tumors
and inflammation has become an important focus in the study of
tumorigenesis. Inflammation is considered to be a defense mechanism
by which the body resists invasion by pathogens. When the
inflammatory response initiates an innate immune response, the
immune response cells release a range of inflammatory cytokines,
such as IL-1, IL-6, IL-17 and IL-23. Furthermore,
inflammatory-related transcription factors, such as NF-κB and
STAT3, are also activated (25,26).
When such an inflammatory microenvironment forms around tumor
tissue, it may promote tumor growth.
IL-17A is a recently identified pro-inflammatory
cytokine that plays an important role in the development of several
chronic inflammatory conditions and cancers by binding to its
receptor, IL-17R. The progression from persistent HPV infection to
cervical cancer has been associated with an inflammatory
microenvironment containing Th17 cells. As a catalyst of heparan
sulfate cleavage, HPSE is associated with the shedding of
biologically active molecules and remodeling of the ECM, which are
involved in the regulation of tumor-related processes, including
angiogenesis, cell invasion, metastasis and inflammation (15). HPSE not only promotes cell-to-cell
transmission, but also promotes the growth of primary tumor blood
vessels and accelerates the migration of tumor cells (27).
Studies to date have demonstrated that IL-17A and
HPSE are involved in inflammation and tumorigenesis, and that both
are associated with the expression of the HPV E6 protein (28,29).
In the present study, recombinant protein stimulation and RNA
interference were used to up- and down-regulate, respectively, the
expression of IL-17A and HPSE in cervical cancer cell lines. The
cell proliferation and invasion abilities were significantly
enhanced following treatment with recombinant protein, particularly
following combined treatment with recombinant IL-17A and HPSE. By
contrast, upon downregulation of the expression of HPSE and IL-17A,
the cell proliferation and invasion rates were significantly
attenuated; this effect was more obvious upon suppression of both
genes, suggesting that HPSE and IL-17A can promote the
proliferation and invasion of cervical cancer cells, and that their
effects are additive.
In order to further investigate the specific
mechanisms of action of IL-17A and HPSE in cervical cancer, the
expression of HPV E6, VEGF, CD31 and P53 were assessed, and it was
observed that they were all upregulated when IL-17A and HPSE were
upregulated, and vice versa; moreover, when P53 was inhibited, the
expression of both IL-17A and HPSE increased. IL-17A and HPSE
regulate the expression of VEGF and CD31, both of which are markers
of angiogenesis, and the results of the immunohistochemical
examination revealed that MVD was significantly higher in tumor
tissues of the IL-17A-positive group compared with the
IL-17A-negative group. Therefore, IL-17A and HPSE may promote
cervical cancer growth, invasion and metastasis by inducing tumor
angiogenesis. The results of the present study are consistent with
previously published results (13,30).
Yan et al recently demonstrated that loss of histone
deacetylase 6 (HDAC6) in mice stimulated the development of
IL-17-producing γδ T cells, indicating that HDAC6 may repress IL-17
production in T cells (31). These
results, combined with those of the present study, indicate that
inhibition of HDAC6 promotes the expression of IL-17, which may be
involved in cervical cancer cell proliferation and invasion, and
tumor angiogenesis. However, Lin et al reported that
inhibition of HDAC activity specifically triggered cervical cancer
cell death through interruption of E6 and E7 signaling (32). Therefore, the precise role of HDAC
in cervical cancer requires further investigation.
NF-κB is a pleiotropic transcription factor that
specifically binds to κB sites on multiple gene promoters to
facilitate transcriptional expression (33). In recent years, the role of NF-κB
in tumors has been a focus of numerous studies. NF-κB is at the
junction of multiple types of stimulating signals that affect
various aspects of the steady state of cells, and it may thereby
serve as a mediator of tumor development. In the present study,
following inhibition of IL-17A or HPSE, the expression of NF-κB in
SiHa and HeLa cells was significantly decreased. As NF-κB is
regulated by stimuli such as oxidative stress, bacterial
lipopolysaccharide and cytokines, among others, and can regulate
the production of pro-inflammatory cytokines, cell surface
receptors, transcription factors and adhesion molecules, it was
hypothesized that IL-17A may affect the development of cervical
cancer through activating the NF-κB signaling pathway.
However, the present study had several limitations.
First, patients were not followed up postoperatively; therefore,
survival analysis results were not provided. Second, in the present
study, IL-17A and HPSE were found to promote angiogenesis and cell
proliferation and invasion in cervical cancer; however, the
specific upstream and downstream mechanisms linking IL-17A and HPSE
molecules were not investigated in detail. Therefore, we plan to
perform cell and animal studies to investigate the exact pathways
associated with both IL-17A and HPSE in cervical cancer.
Large-scale, well-designed studies elucidating issues such as
whether IL-17A can activate the JAK/STAT pathway to regulate
angiogenesis and, thus, contribute to cervical carcinogenesis, as
well as clinical research trials, are required to fully elucidate
the pathogenesis of cervical cancer.
In conclusion, IL-17 appears to promote cell
proliferation and invasion and angiogenesis in cervical cancer,
which may be enhanced by the additive effect of HPSE. The present
and future studies on these molecular aspects are of great value,
as they may provide a novel experimental basis and potential
clinical targets to improve the treatment and prognosis of patients
with cervical cancer.
Acknowledgments
Not applicable.
Funding
The present study was supported by Zhongnan Hospital
of Wuhan University, Science, Technology and Innovation Seed Fund
(grant no. znpy2016040).
Availability of data and materials
All the datasets collected and analyzed in the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
QL and KW performed the experimental work and wrote
the manuscript, FL and WW helped collect the clinical samples, YC
analyzed the data, WZ designed the study and reviewed the
manuscript. All authors discussed the results and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhongnan Hospital of Wuhan University. The human
cervical tissue samples used in this study were obtained from
patients following written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao Z, Painter SL, Fanslow WC, Ulrich D,
Macduff BM, Spriggs MK and Armitage RJ: Human IL-17: A novel
cytokine derived from T cells. J Immunol. 155:5483–5486.
1995.PubMed/NCBI
|
|
3
|
Gaffen SL: An overview of IL-17 function
and signaling. Cytokine. 43:402–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerhardt S, Abbott WM, Hargreaves D,
Pauptit RA, Davies RA, Needham MR, Langham C, Barker W, Aziz A,
Snow MJ, et al: Structure of IL-17A in complex with a potent, fully
human neutralizing antibody. J Mol Biol. 394:905–921. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Omrane I, Medimegh I, Baroudi O, Ayari H,
Bedhiafi W, Stambouli N, Ferchichi M, Kourda N, Bignon YJ,
Uhrhammer N, et al: Involvement of IL17A, IL17F and IL23R
polymorphisms in colorectal cancer therapy. PLoS One.
10:e01289112015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu B, Guenther JF, Pociask DA, Wang Y,
Kolls JK, You Z, Chandrasekar B, Shan B, Sullivan DE and Morris GF:
Promotion of lung tumor growth by interleukin-17. Am J Physiol Lung
Cell Mol Physiol. 307:L497–L508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slattery ML, Herrick JS, Torres-Mejia G,
John EM, Giuliano AR, Hines LM, Stern MC, Baumgartner KB, Presson
AP and Wolff RK: Genetic variants in interleukin genes are
associated with breast cancer risk and survival in a genetically
admixed population: The Breast Cancer Health Disparities Study.
Carcinogenesis. 35:1750–1759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai ZM, Zhang TS, Lin S, Zhang WG, Liu J,
Cao XM, Li HB, Wang M, Liu XH, Liu K, et al: Role of IL-17A
rs2275913 and IL-17F rs763780 polymorphisms in risk of cancer
development: An updated meta-analysis. Sci Rep. 6:204392016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomes AL, Teijeiro A, Burén S, Tummala KS,
Yilmaz M, Waisman A, Theurillat JP, Perna C and Djouder N:
Metabolic inflammation-associated IL-17A causes non-alcoholic
steatohepatitis and hepatocellular carcinoma. Cancer Cell.
30:161–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang SH, Mirabolfathinejad SG, Katta H,
Cumpian AM, Gong L, Caetano MS, Moghaddam SJ and Dong C: T helper
17 cells play a critical pathogenic role in lung cancer. Proc Natl
Acad Sci USA. 111:5664–5669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong Z, Yang XO, Yan H, Liu W, Niu X, Shi
Y, Fang W, Xiong B, Wan Y and Dong C: A protective role by
interleukin-17F in colon tumorigenesis. PLoS One. 7:e349592012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kulig P, Burkhard S, Mikita-Geoffroy J,
Croxford AL, Hövelmeyer N, Gyülvészi G, Gorzelanny C, Waisman A,
Borsig L and Becher B: IL17A-mediated endothelial breach promotes
metastasis formation. Cancer Immunol Res. 4:26–32. 2016. View Article : Google Scholar
|
|
14
|
Meirovitz A, Goldberg R, Binder A,
Rubinstein AM, Hermano E and Elkin M: Heparanase in inflammation
and inflammation-associated cancer. FEBS J. 280:2307–2319. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vlodavsky I, Beckhove P, Lerner I, Pisano
C, Meirovitz A, Ilan N and Elkin M: Significance of heparanase in
cancer and inflammation. Cancer Microenviron. 5:115–132. 2012.
View Article : Google Scholar :
|
|
16
|
Boyango I, Barash U, Naroditsky I, Li JP,
Hammond E, Ilan N and Vlodavsky I: Heparanase cooperates with Ras
to drive breast and skin tumorigenesis. Cancer Res. 74:4504–4514.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandes dos Santos TC, Gomes AM,
Paschoal ME, Stelling MP, Rumjanek VM, Junior AR, Valiante PM, Madi
K, Pereira de Souza HS, Pavão MS, et al: Heparanase expression and
localization in different types of human lung cancer. Biochim
Biophys Acta. 1840:2599–2608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poon IK, Goodall KJ, Phipps S, Chow JD,
Pagler EB, Andrews DM, Conlan CL, Ryan GF, White JA, Wong MK, et
al: Mice deficient in heparanase exhibit impaired dendritic cell
migration and reduced airway inflammation. Eur J Immunol.
44:1016–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shteingauz A, Boyango I, Naroditsky I,
Hammond E, Gruber M, Doweck I, Ilan N and Vlodavsky I: Heparanase
enhances tumor growth and chemoresistance by promoting autophagy.
Cancer Res. 75:3946–3957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv Q, Zhu D, Zhang J, Yi Y, Yang S and
Zhang W: Association between six genetic variants of IL-17A and
IL-17F and cervical cancer risk: A case-control study. Tumour Biol.
36:3979–3984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lü QY, Zhang W, Cheng J, Zhang WT and
Zhong YJ: Effect on invasion ability of cervical cancer cells after
silence heparanase gene expression in HeLa cells. Zhonghua Fu Chan
Ke Za Zhi. 48:532–537. 2013.In Chinese.
|
|
22
|
Chen RJ, Shun CT, Yen ML, Chou CH and Lin
MC: Methyltransferase G9a promotes cervical cancer angiogenesis and
decreases patient survival. Oncotarget. 8:62081–62098.
2017.PubMed/NCBI
|
|
23
|
Li X, Song Y, Liu F, Liu D, Miao H, Ren J,
Xu J, Ding L, Hu Y, Wang Z, et al: Long non-coding RNA MALAT1
promotes proliferation, angiogenesis, and immunosuppressive
properties of mesenchymal stem cells by inducing VEGF and IDO. J
Cell Biochem. 118:2780–2791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y,
Zhu J, Cui F, Zhao W and Shi H: miR-203 suppresses tumor growth and
angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol
Biochem. 32:64–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernandes JV, DE Medeiros Fernandes TA, DE
Azevedo JC, Cobucci RN, DE Carvalho MG, Andrade VS and DE Araújo
JM: Link between chronic inflammation and human
papillomavirus-induced carcinogenesis (Review). Oncol Lett.
9:1015–1026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He G and Karin M: NF-κB and STAT3 - key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar
|
|
27
|
Vreys V and David G: Mammalian heparanase:
What is the message? J Cell Mol Med. 11:427–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang YH, Yu CW, Lai LC, Tsao CH, Ho KT,
Yang SC, Lee H, Cheng YW, Wu TC and Shiau MY: Up-regulation of
interleukin-17 expression by human papillomavirus type 16 E6 in
nonsmall cell lung cancer. Cancer. 116:4800–4809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirshoren N, Bulvik R, Neuman T,
Rubinstein AM, Meirovitz A and Elkin M: Induction of heparanase by
HPV E6 oncogene in head and neck squamous cell carcinoma. J Cell
Mol Med. 18:181–186. 2014. View Article : Google Scholar :
|
|
30
|
Bowers JS, Nelson MH, Kundimi S, Bailey
SR, Huff LW, Schwartz KM, Cole DJ, Rubinstein MP and Paulos CM:
Dendritic cells in irradiated mice trigger the functional
plasticity and antitumor activity of adoptively transferred Tc17
cells via IL12 signaling. Clin Cancer Res. 21:2546–2557. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan B, Liu Y, Bai H, Chen M, Xie S, Li D,
Liu M and Zhou J: HDAC6 regulates IL-17 expression in T
lymphocytes: Implications for HDAC6-targeted therapies.
Theranostics. 7:1002–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Z, Bazzaro M, Wang MC, Chan KC, Peng S
and Roden RB: Combination of proteasome and HDAC inhibitors for
uterine cervical cancer treatment. Clin Cancer Res. 15:570–577.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|