Introduction
Natural products represent a valuable source for
developing therapeutic agents against cancer (1-5).
However, the variations in pharmacological responses observed in
cancer therapy, along with the development of drug resistance,
represent major factors that inhibit the development of therapeutic
agents with improved efficacy and safety profiles. To address these
issues and achieve major clinical benefits, it is crucial to
improve our understanding of the underlying molecular mechanisms of
the pharmacological effects of natural products in cancer cells
prior to further developing them as novel treatments (6,7).
The clinical application of naturally derived
sesquiterpene compounds as potential anticancer agents remains
challenging with respect to their total chemical synthesis and
limited understanding of their molecular behavior within cancer
cells. Sesquiterpenes and their subclass, sesquiterpene lactones
(SLs), represent secondary metabolites of plant-derived products;
the pharmacological effects of these compounds have been found to
be involved in the regulation of several complex molecular
signaling pathways, the most common being the nuclear factor-κB
(NF-κB) signaling pathway (8).
Diverse oxidized carbocycle members of the SLs include the
germacranolides, guaianolides and eudesmanolides, which have
recently attracted a great deal of attention due to their potential
anticancer properties. Certain SL members were found to selectively
target tumor and cancer stem cells, while avoiding normal cells
(9,10). This property has led to the
application of SLs, including artemisin, thapsigargin and
parthenolide, in clinical trials as therapeutic anticancer agents
(11-17).
Recently, an array of arglabin analogues of the
guaianolide subclass have been reported to be selective inhibitors
of stem and progenitor cells related to acute myelogenous leukemia;
however, the molecular mechanisms underlying their potential
therapeutic properties remain unknown (17). In the development of novel
innovative sesquiterpene derivatives with improved pharmacological
profiles, eight structurally diverse sesquiterpenes and SLs,
classified as three major subclasses (elemanes, germacranes and
guaianes) were synthesized and their cytotoxicity profiles were
investigated in the glioblastoma U-87 MG cell line in the present
study. Among them, the compound VDS58 showed the most promising
effects in a panel of human cancer cell lines (glioblastoma U-87
MG, breast MCF-7 and erythroleukemia K562), in mouse
erythroleukemia (MEL-745) cells and in normal cells (human lung
fibroblast MRC-5). The time-dependent assessment of VDS58-treated
cultures suggested that mainly U-87 MG cells are able to recover
their proliferation rates after 48 h. Gene expression analysis
indicated a transient induction of cyclin-dependent kinase
inhibitor 1A (CDKN1) expression within the first 24 h of
exposure to VDS58. Notably, during this analysis, no notable
alterations in tumor protein 53 (TP53) expression were
observed. In addition, subsequent application of VDS58 following an
initial 48 h of exposure revealed that cells exhibited increased
expression levels of caspase-3 (CASP3), CASP9,
BCL2-associated agonist of cell death (BAD),
cyclin-dependent kinase (CDK6), CDK inhibitor 1A
(CDKN1), MYC proto-oncogene bHLH transcription factor
(MYC) and TP53; however, this upregulation persisted
for only 24 h. Following this, only the expression levels of
MYC were maintained at high levels. This indicates that
upregulated MYC expression may facilitate cancer cell
proliferation, which coincides with the proliferation kinetics
reported upon 48-72 h of exposure of cells to VDS58.
Materials and methods
Synthesis of natural sesquiterpenoids and
analogs
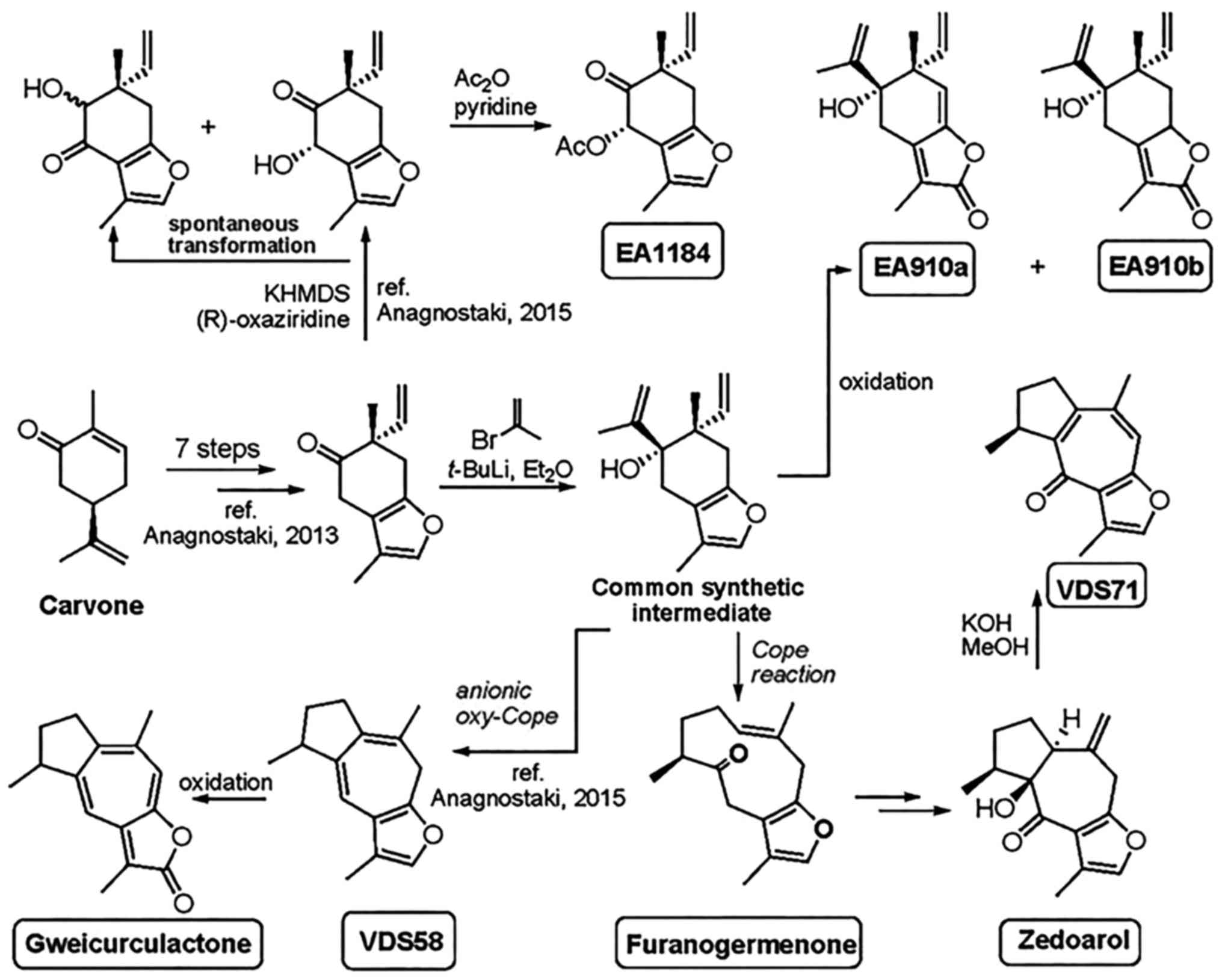
The chemical structures and the applied method of
synthesis for the production of sesquiterpenes (EA910a, EA910b,
zedoarol, gweicurculactone, VDS71, VDS58, furanogermenone and
EA1184) are shown in Fig. 1. The
total chemical synthesis of natural substances furanogermenone,
zedoarol and gweicurculactone was based on the unified synthetic
protocol and methods in our previously published studies (18-21).
All natural and synthetic substances were purified by flash column
chromatography with Merck silica gel 60 (particle size, 0.040-0.063
mm; EMD Millipore, Billerica, MA, USA). The purity of all compounds
was established by nuclear magnetic resonance on a Bruker 300 AM
(Bruker Corporation, Billerica, MA, USA) and Agilent 500 MHz
spectrometer and a high-resolution mass spectra recorder with an
Agilent Electrospray ionisation time-of-flight mass spectrometer
(Agilent Technologies, Inc., Santa Clara, CA, USA). All tested
compounds had purity levels of >95%.
Cell cultures
The previously established cancer U-87 MG, MCF-7,
murine erythroleukemia FLC clone 745 (MEL-745) and K562 cell lines,
and a normal MRC-5 cell line, were obtained, stored and used in a
routine manner (22,23) in the Laboratory of Pharmacology,
School of Pharmacy, Aristotle University of Thessaloniki (Greece)
(<25 passages were applied). The murine erythroleukemia MEL-745
cells were obtained from Dr C. Friend (Division of Cytology, The
Sloan-Kettering Institute for Cancer Research, New York, NY, USA)
(24) and were cultured as
described by our previous study (25). Regarding the dispute on the
misidentification of U87 MG (26),
the U87 MG cell line used in this study is a clone originating from
that established at the University of Uppsala (Uppsala, Sweden).
Malignant U-87 MG (human epithelial glioblastoma grade IV
astrocytoma), MCF-7 (human breast cancer) and MEL-745 cells, along
with the normal MRC-5 (human fetal lung fibroblast) cells, were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% v/v fetal bovine serum (FBS) and 100 µg/ml penicillin and
streptomycin. K562 (human leukemic) cells were cultured in Roswell
Park Memorial Institute (RPMI)-1640 medium. Cells were maintained
in culture (37°C in 5% v/v CO2), and were passaged every
2-3 days when 75-80% confluence was achieved using trypsin-EDTA
(0.25% w/v), for the attached cultures. Trypsin-EDTA, DMEM, RPMI
and FBS were purchased from Thermo Fisher Scientific, Inc. (Waltham
MA, USA).
Cytotoxicity assessment
In all experiments, the new synthesized
sesquiterpene compounds were tested by dissolving them in DMSO
prior to application to the various cell cultures. The final
concentration of DMSO was ≤0.1%, which had no effect on cell
proliferation (data not shown). The malignant (U-87 MG, MCF-7, K562
and MEL-745) and normal (MRC-5) cell lines were seeded in 24-well
plates at an initial concentration of 1×105 cells/ml.
For the attached cultures (U-87 MG, MCF-7 and MRC-5), cells were
allowed to attach for 3-4 h (at 37°C in 5% v/v CO2)
prior to the addition of the sesquiterpenoids. The specified
concentrations used in the cultures were between 1×10−7
and 1×10−4 M. Cells were grown in the presence of the
molecules for 48 h prior to the cell proliferation being measured
with Neubauer counting chambers under an optical microscope (x10
magnification). Subsequently, the calculation of the half-maximal
inhibitory concentration (IC50) values of each compound
for a specific cell line was estimated. Moreover, cell death within
cell cultures was also determined using the Trypan blue
dye-exclusion method, as previously described (23).
Cell pretreatment and washout
experiments
Cytotoxicity was further assessed through the
re-application of pretreatment and washout experiments using human
malignant U-87 MG and normal MRC-5 cell cultures, respectively. The
cells were pretreated for 24 and 48 h with the previously
calculated IC50 concentrations of VDS58. VDS58 is a
guaiane sesquiterpene that was synthesized by our group in the
Laboratory of Organic Chemistry of the Aristotle University of
Thessaloniki, starting from the commercially available (R)-carvone
and following an 12-step route (20,21).
The re-application of VDS58 to U-87 MG cells was achieved by adding
fresh DMEM containing the IC50 concentration of VDS58 to
the cells. The replenishment of the MRC-5 cell population and the
removal of VDS58 from the cultures were achieved by washing out the
cells twice with PBS and then incubating them with fresh DMEM in
the absence of drug treatment. MRC-5 and U-87 MG cells were then
cultured for 96 and 120 h, respectively. The assessment of cell
proliferation and death in the cultures was performed every 24 h
using the aforementioned methods.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total cytoplasmic RNA isolation from U-87 MG and
K562 cells, and the two-step RT-qPCR analysis (kit KK4602; Kapa
Biosystems, Wilmington, MA, USA) were performed as previously
described (22). The following
primer sequences were used to amplify the indicated genes: β-actin
(ACTB) forward, 5′-ttgctgacaggatgcagaag-3′ and reverse,
5′-tgatccacatctgctggaag-3′; BCL2 associated X apoptosis regulator
(BAX) forward, 5′-tctgacggcaacttcaactg-3′ and reverse,
5′-gaggaagtccaatgtccagc-3′; BCL2 apoptosis regulator (BCL2)
forward, 5′-acttcgccgagatgtcca-3′ and reverse, 5′-caaagaaggcc
acaatcctc-3′; CDKN1 forward, 5′-gagcgatggaacttcgactt-3′ and
reverse, 5′-gtgggaaggtagagcttggg-3′; CASP9 forward,
5′-tcgaagc caaccctagaaaa-3′ and reverse,
5′-cctccagaaccaatgtccac-3′; BAD forward,
5′-cagatcccagagtttgagcc-3′ and reverse, 5′-ctgctcctgctg gtgactg-3′;
CASP3 forward, 5′-ggttcatccagtcgctttgt-3′ and reverse,
5′-aattctgttgccacctttcg-3′; CDK4 forward, 5′-accagatggcactta
caccc-3′ and reverse, 5′-ccacagaagagaggctttcg-3′; CDK2
forward, 5′-ttgtcaagctgctggatgtc-3′ and reverse,
5′-tgatgaggggaagagga atg-3′; CDK6 forward,
5′-tgcacagtgtcacgaacaga-3′ and reverse,
5′-acctcg-gagaagctgaaaca-3′; cyclin D1 (CCND1) forward,
5′-ctgc gaagtggaaaccatc-3′ and reverse, 5′-ttgaagtaggacaccgaggg-3′;
CASP8 forward, 5′-gatgacatgaacctgctgga-3′ and reverse,
5′-caggctcttgttgatttggg-3′; MYC forward,
5′-aggagaatgtcaagaggcga-3′ and reverse, 5′-ggccttttcattgttttcca-3′;
catenin β1 (CTNNB1) forward, 5′-gctgggaccttgcataacctt-3′ and
reverse, 5′-attttcac cagggcaggaatg-3′; RB transcriptional
corepressor 1 (RB1) forward, 5′-tgtcagagagagagcttggt-3′ and
reverse, 5′-ctcatctaggtcaactgctgc-3′; and transforming growth
factor β1 (TGFB1) forward, 5′-actgcg gatctctgtgtcattg-3′ and
reverse, 5′-acagtagtgttccccactggtc-3′. The ΔΔCq values of gene
expression corresponding to the amplified mRNAs from each sample
were calculated by normalizing the values to the internal control
(ACTB). The fold-change in expression levels was calculated
using the 2−ΔΔCq method (27). All experiments were performed in
triplicate.
Bioinformatic analysis of gene expression
data
Bioinformatic analysis was performed to estimate the
levels of gene expression for the U-87 MG and K562 cell lines
employed throughout this study to assess the cytotoxicity behavior
of VDS58. In detail, processed gene expression values [transcript
per million reads (TPM)] resulting from the analysis of RNA-Seq
data obtained from the ENCODE project (28) were downloaded from the Gene
Expression Omnibus repository for untreated samples of U-87
(GSE90176) and K562 (GSE78561) cell lines. Spike-in controls and
genes with TPM values equal to zero were removed from the data, and
the means of the TPM expression values for each gene were then
calculated. Next, for each cell line, the mean TPM values were
processed via logarithm (log2) transformation and normalized
to z-scores. The density plots of the z-score gene expression
values obtained for each cell line exhibited a great degree of
similarity (data not shown). Using the z-scores for gene expression
in U-87 MG and K562 cell lines and the Pathview R package (29), visualizations were created for: i)
The Homo sapiens pathway of the cell cycle (hsa04110) using
information from the Kyoto Encyclopedia of Genes and Genomes
database (30-32); and ii) the expression of the genes
that constitute this pathway.
Statistical analysis
All data represent at least 2 independent biological
experiments and the data are expressed as the mean ± standard
deviation. Comparisons were made using two-way repeated measures
analysis of variance, followed by Dunnett’s test. All statistical
analyses were performed using GraphPad Prism 6.0 (GraphPad
Software, Inc. (La Jolla, CA, USA). P<0.05 was used to indicate
a statistically significant difference.
Results
Preliminary pharmacological evaluation of
natural sesquiterpenoids and analogues
The preliminary pharmacological evaluation of the
obtained structural sesquiterpene derivatives shown in Fig. 1, including EA910a, EA910b,
zedoarol, gweicurculactone, VDS71, VDS58, furanogermenone and
EA1184, was performed in human glioblastoma U-87 MG cells by
assessing their cytotoxicity profiles. The cultures were exposed to
different concentrations (0.0001-0.1 mM;
10−7-10−4 M) of these compounds; cell
proliferation and death were evaluated after 48 h of treatment. A
small proportion of dead cells was observed in culture following
treatment with all analogs (data not shown). Moreover, the majority
of the synthetic sesquiterpene derivatives exhibited no substantial
cytotoxicity based on their concentration-dependent inhibitory
effects on the proliferation of U-87 MG cells; their
IC50 values were >10−4 M (data not shown).
Notably, however, sesquiterpene VDS58 exhibited increased
cytotoxicity in a concentration-dependent manner. The proliferation
of U-87 MG cells was decreased ~70% following 48 h of exposure to
VDS58 at 1×10−4 M, compared with untreated cells
(Fig. 2A and B). The latter
prompted further analysis of the behavior of VDS58 in cell
cultures.
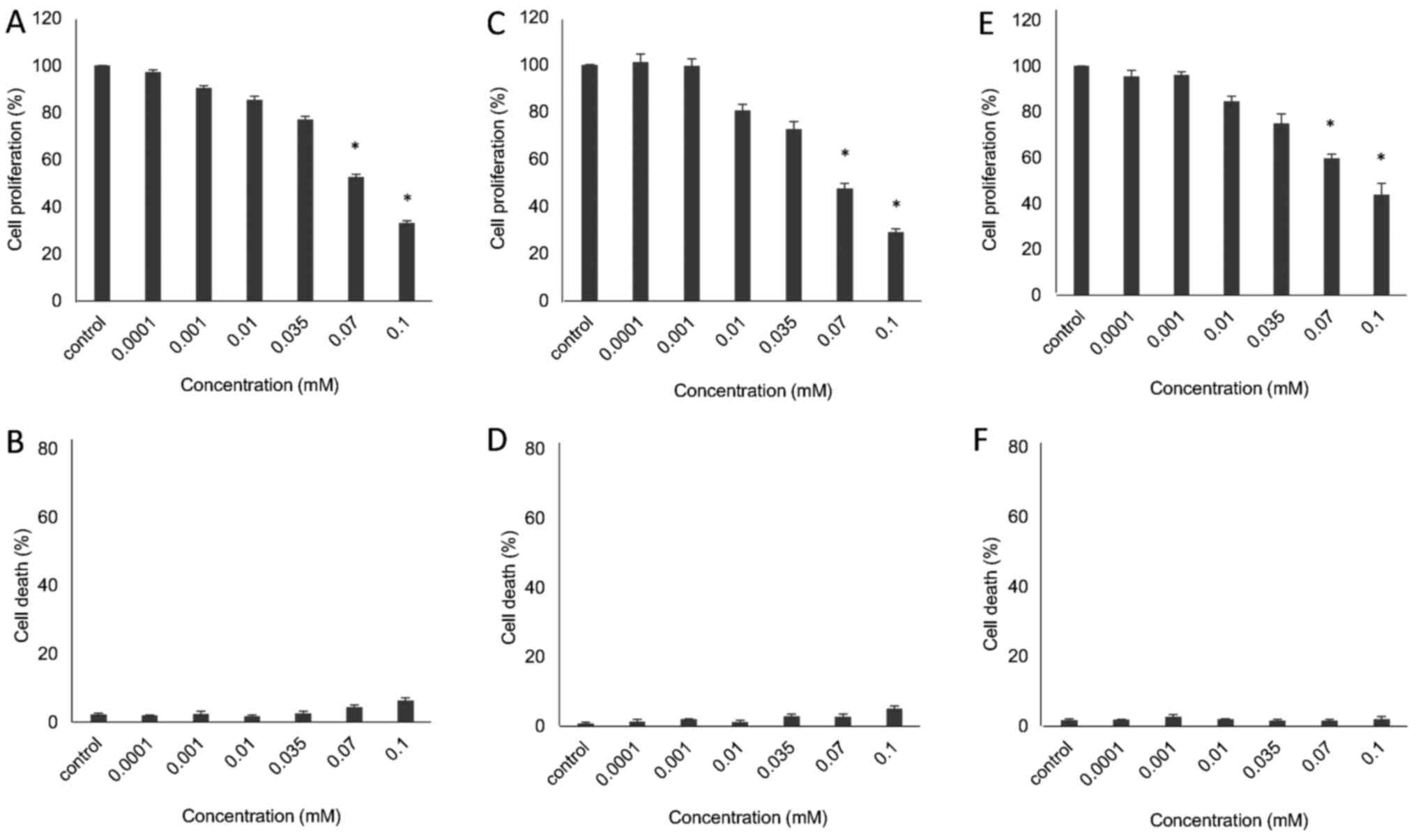
Assessment of VDS58 cytotoxicity profile
in various human monolayer and suspension cell cultures
The initial VDS58-induced anti-proliferative
activity exhibited by U-87 MG cells prompted the further
investigation of its cytotoxicity profile in various cell lines
(MCF-7, MRC-5, K562 and MEL-745). These cultures were exposed to
increasing concentrations of VDS58 (10−7-10−4
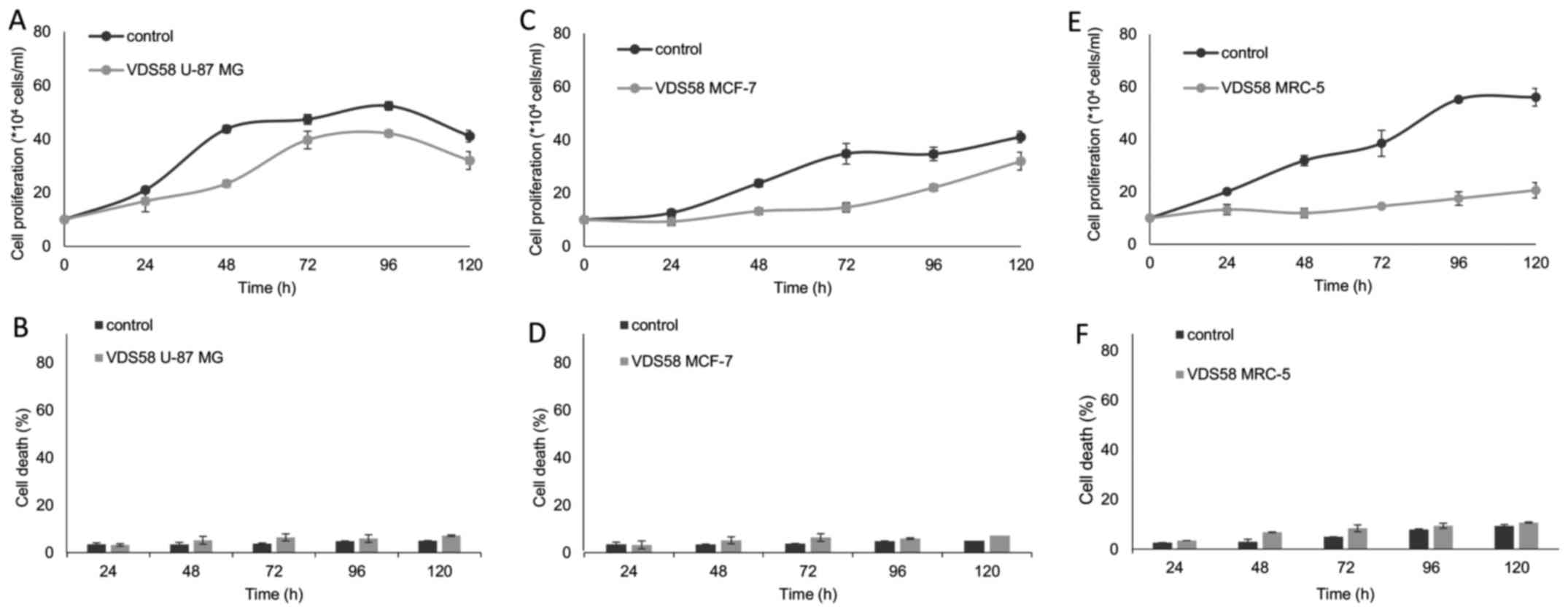
M) for 48 h. As shown in Figs. 2
and 3, VDS58 reduced cellular
proliferation of all cell lines in a concentration-dependent
manner; and cells were grown either as attached monolayers (U-87
MG, MCF-7 and MRC-5) or as suspension (K562 and MEL-745) cultures.
The estimated IC50 values of VDS58 varied between the
cell lines: 7×10−5 M for U-87 MG; 6.9×10−5 M
for MCF-7; 9.2×10−5 M for MRC-5; 4.2×10−5 M
for K562; and 2.9×10−5 M for MEL-745 (Table I). Notably, however, only in
suspension, erythroleukemia cultures (K562 and MEL-745 cells)
showed an increase in the proportion of dead cells, reaching ~80%
at higher concentrations (1×10−4 M for K562, Fig. 3B; >7×10−5 M for
MEL-745, Fig. 3D). Moreover,
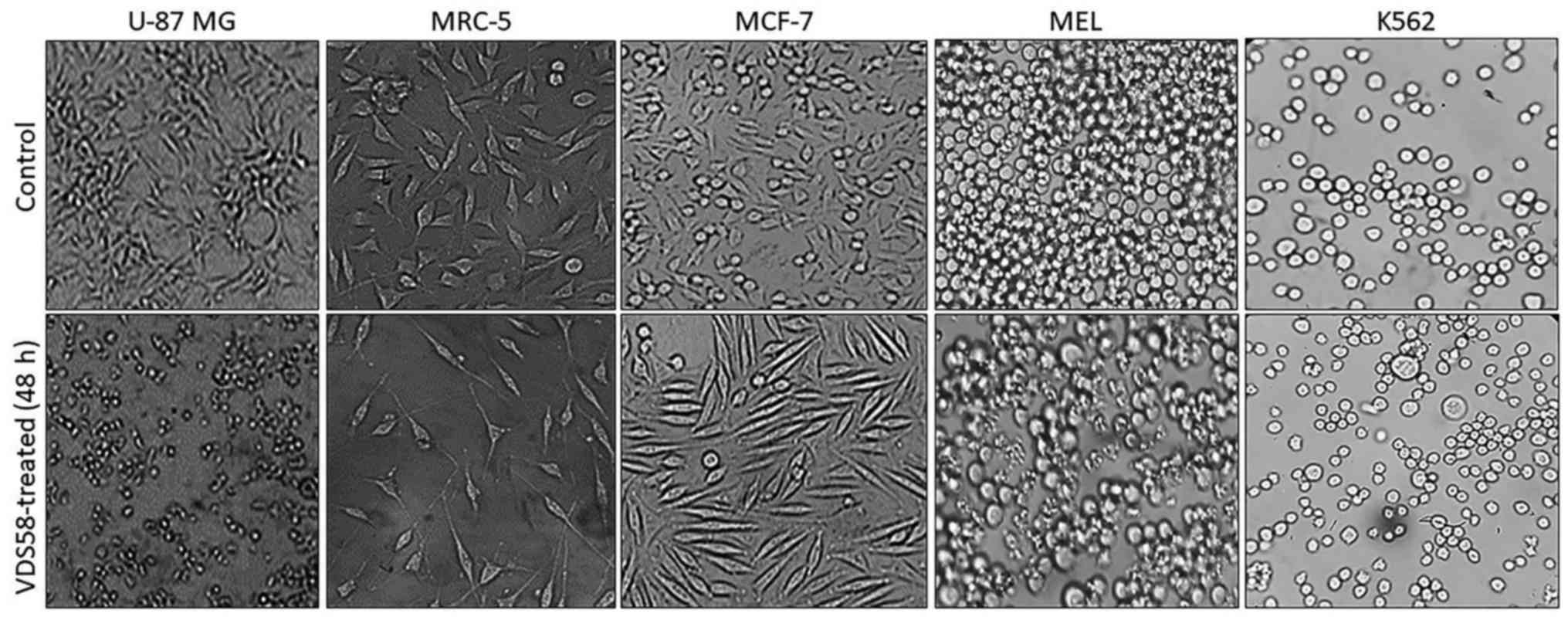
specific morphological changes have been recorded in the
VDS58-treated cell cultures. As shown in Fig. 4, the treated U-87 MG cells lost
their epithelial morphology and adherence properties. MRC-5
cultures exhibited a spindle-like phenotype and MCF-7 cells
obtained a fibroblastic-mesenchymal type shape, whereas in
suspension, K562 and MEL-745 cultures possessed large cells, an
indicator of cytotoxicity. Thus, contrary to the limited effects
observed for the other sesquiterpene molecules (EA910a, EA910b,
zedoarol, gweicurculactone, VDS71, furanogermenone and EA1184), the
VDS58 analog exhibited a notable concentration-dependent
cytotoxicity profile, which was accompanied by cellular
morphological changes in the malignant and normal (MRC-5)
cells.
 | Table IIC50 values of VDS58 in
various cell lines. |
Table I
IC50 values of VDS58 in
various cell lines.
| Cell line | IC50
(M) |
|---|
| U-87 MG |
7×10−5 |
| MCF-7 |
6.9×10−5 |
| MRC-5 |
9.2×10−5 |
| K562 |
4.2×10−5 |
| MEL |
2.9×10−5 |
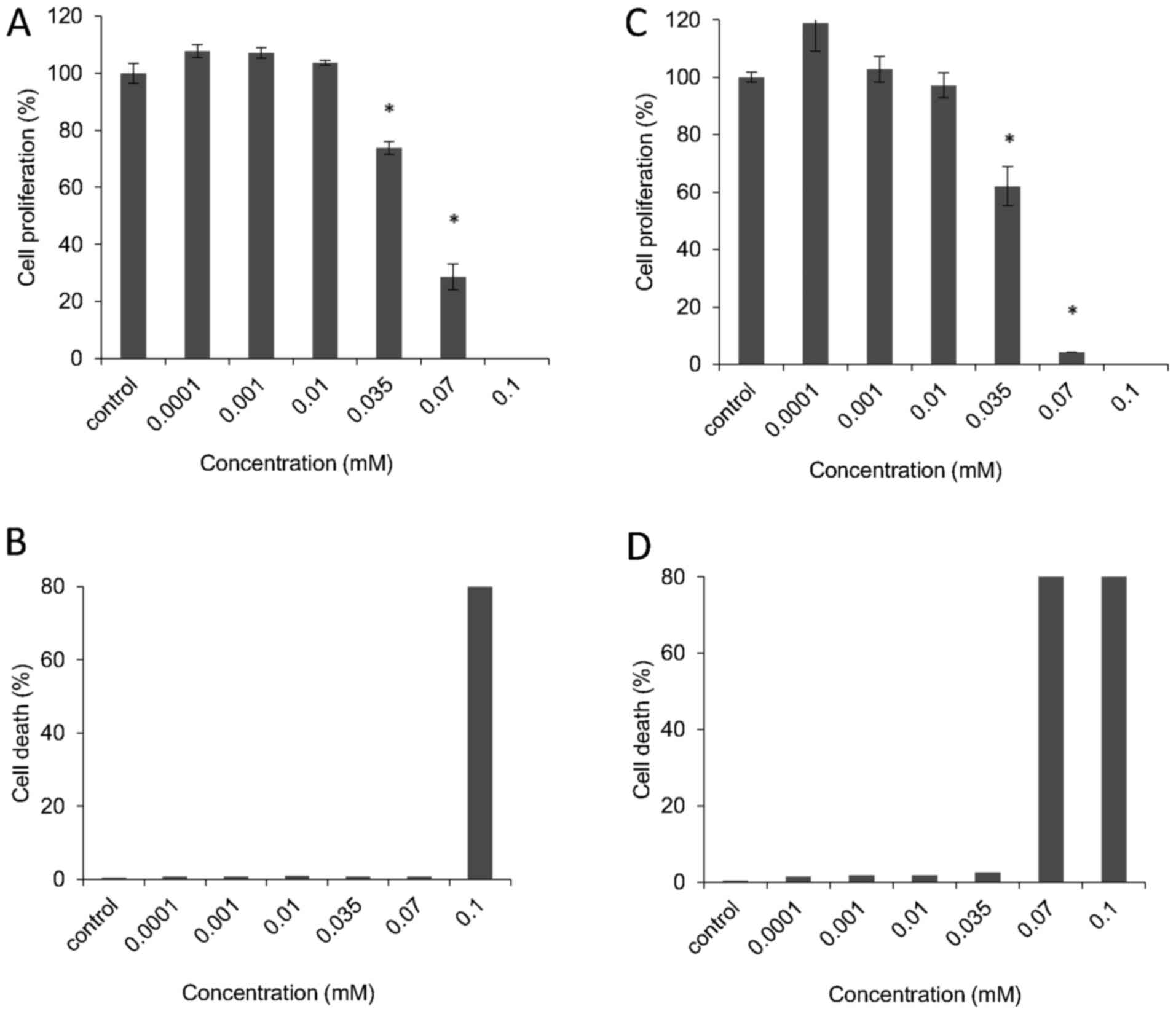
Kinetic analysis of cell proliferation of
human cell lines exposed to VDS58
To better characterize the cytotoxic effects of
VDS58, the cell cultures were treated continuously with the
corresponding IC50 of VDS58 for 4-5 days. As shown in
Fig. 5, U-87 MG cells exposed to
the IC50 of VDS58 grew at slower rates in culture;
however, after 48-72 h, the cell numbers were comparable to those
of the control cells, with no notable changes in the proportion of
dead cells (Fig. 5A and B).
Moreover, MCF-7 cells treated with the IC50 of VDS58
showed similar behaviors, although a treatment time of 96-120 h was
required to obtain cell numbers comparable with those of the
untreated cells (Fig. 5C and D).
Notably, normal MRC-5 cells incubated with the IC50 of
VDS58 did not proliferate notably, even after 120 h of treatment
(Fig. 5E and F). The
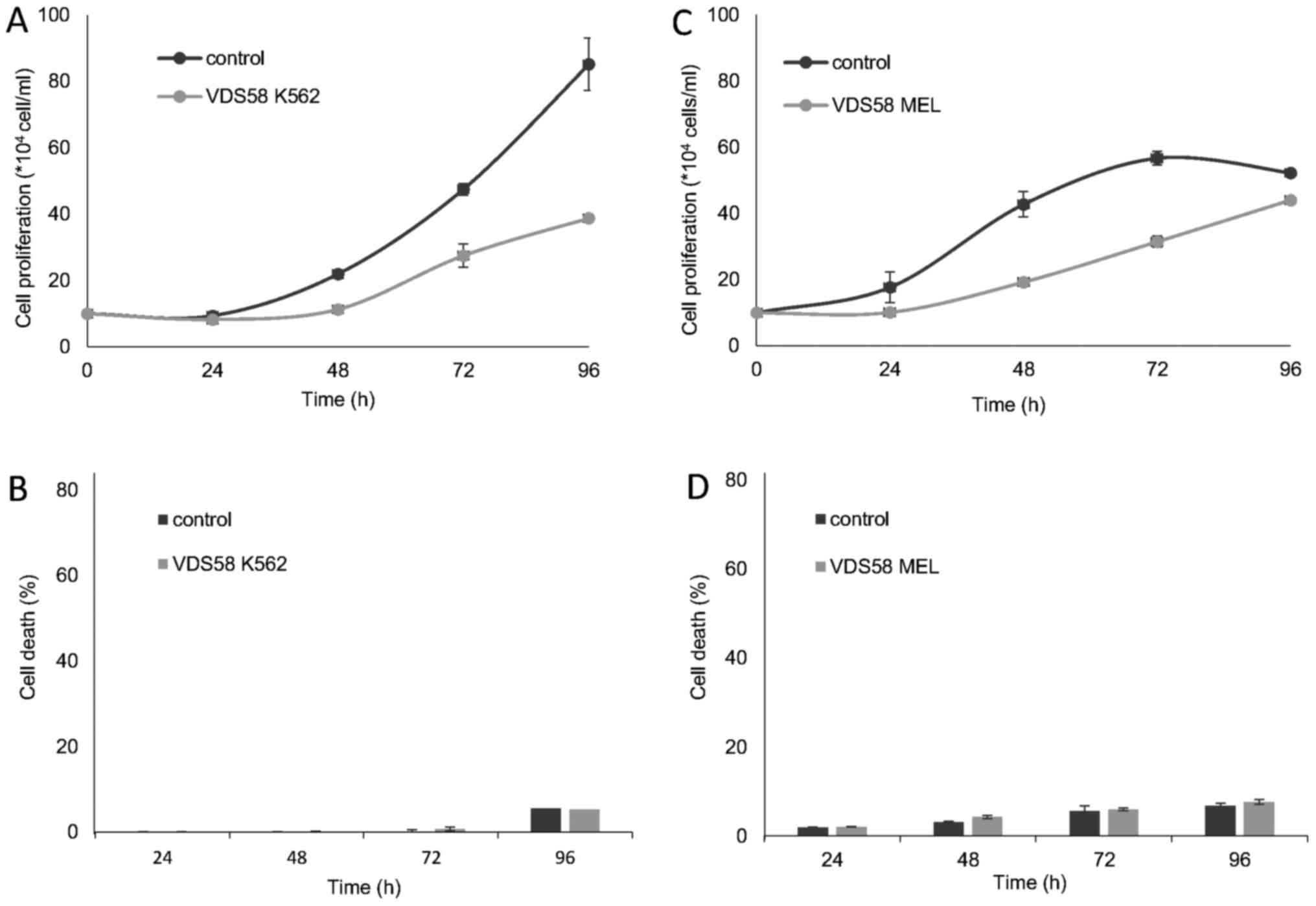
erythroleukemia K562 and MEL-745 cell lines, which were exposed to
the corresponding IC50 of VDS58, exhibited slow
proliferation rates compared with those of the controls (Fig. 6).
Notably, in the attached VDS58-treated cultures,
particularly in the malignant U-87 MG and MCF-7 cells, the cells
survived and exhibited high proliferation rates; an effect that was
more prominent in the U-87 MG cells compared with that observed in
the normal attached MRC-5 cells (Fig.
5). Thus, to gain more insight into the cellular effects of
VDS58, particularly in glioblastoma U-87 MG and normal MRC-5 cells,
a complementary set-up of kinetic experiments was designed. For
U-87 MG cells, the question to be addressed in these experiments
was whether the cells were able to avoid the effects exerted by
VDS58 conveyed by its intracellular metabolism leading to its
cellular inactivity. For MRC-5, the current study investigated
whether the cells regained their proliferation capacity following
the removal of VDS58 from the culture. To this end, these cultures
were initially exposed for 48 h to the corresponding
IC50 of VDS58 for each culture (U-87 MG,
7×10−5 M; MRC-5, 9.2×10−5 M). A washout step
was subsequently introduced, (referred to as time-point ‘0’),
followed by the addition of fresh medium containing VDS58
(7×10−5 M) for U-87 MG cells, whereas for MRC-5 cells,
the culture medium was free of VDS58. In all cases, the control
cultures were included in the experimental design to facilitate the
interpretation of the results. The results of the current study
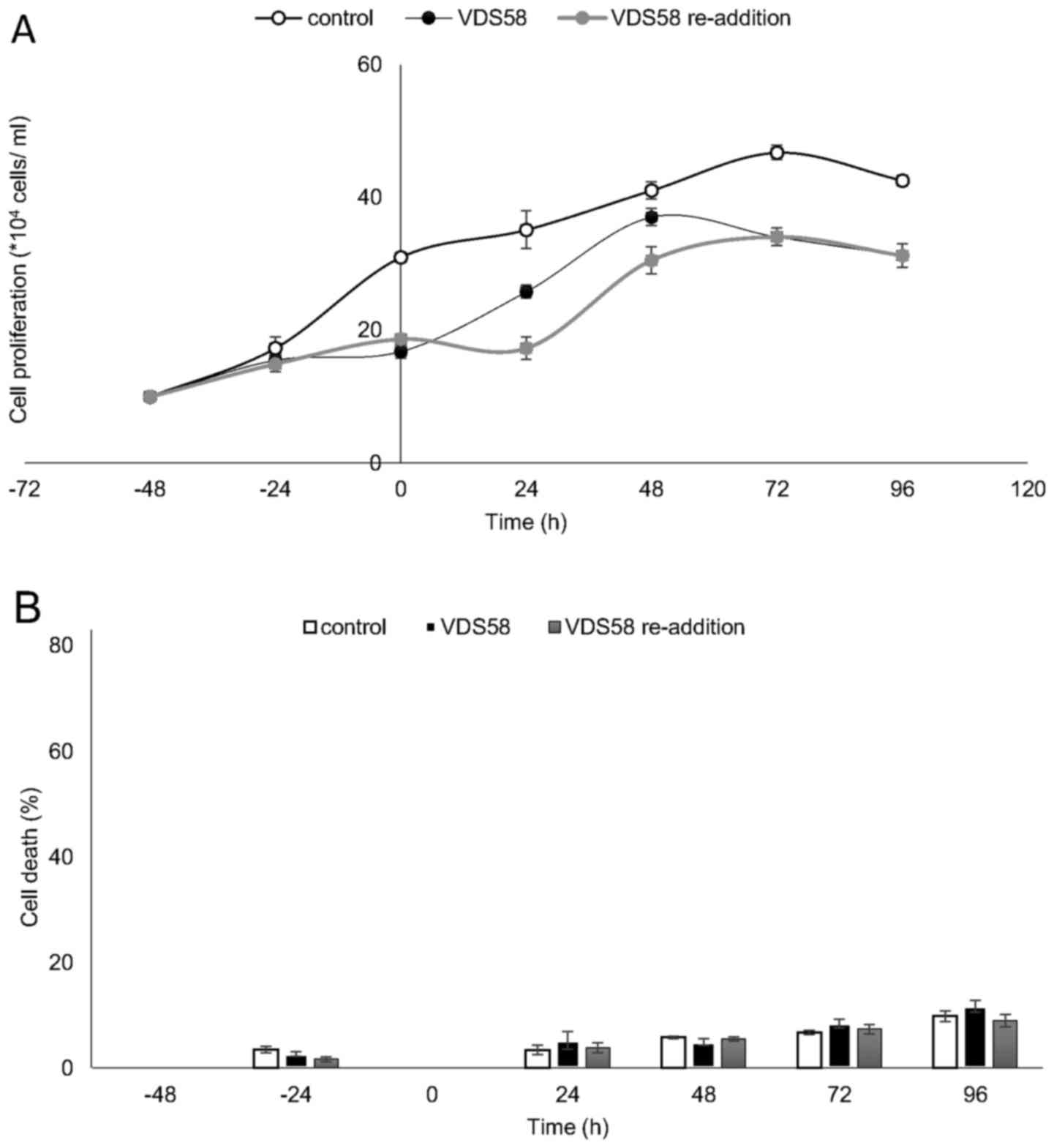
showed that malignant U-87 MG cells have the ability to regain
their proliferative potential subsequent to the initial
pretreatment (Fig. 7A). At 48-72 h
after the re-addition of VDS58, the cell number was similar to that
of the culture continuously treated with VDS58, while the untreated
control exhibited a higher cell accumulation rate (Fig. 7). These data suggest that the
observed behavior of U-87 MG cells to escape and overcome the
proliferation inhibitory effects of VDS58 may be attributed to an
intrinsic capacity of U-87 MG cells rather than the intracellular
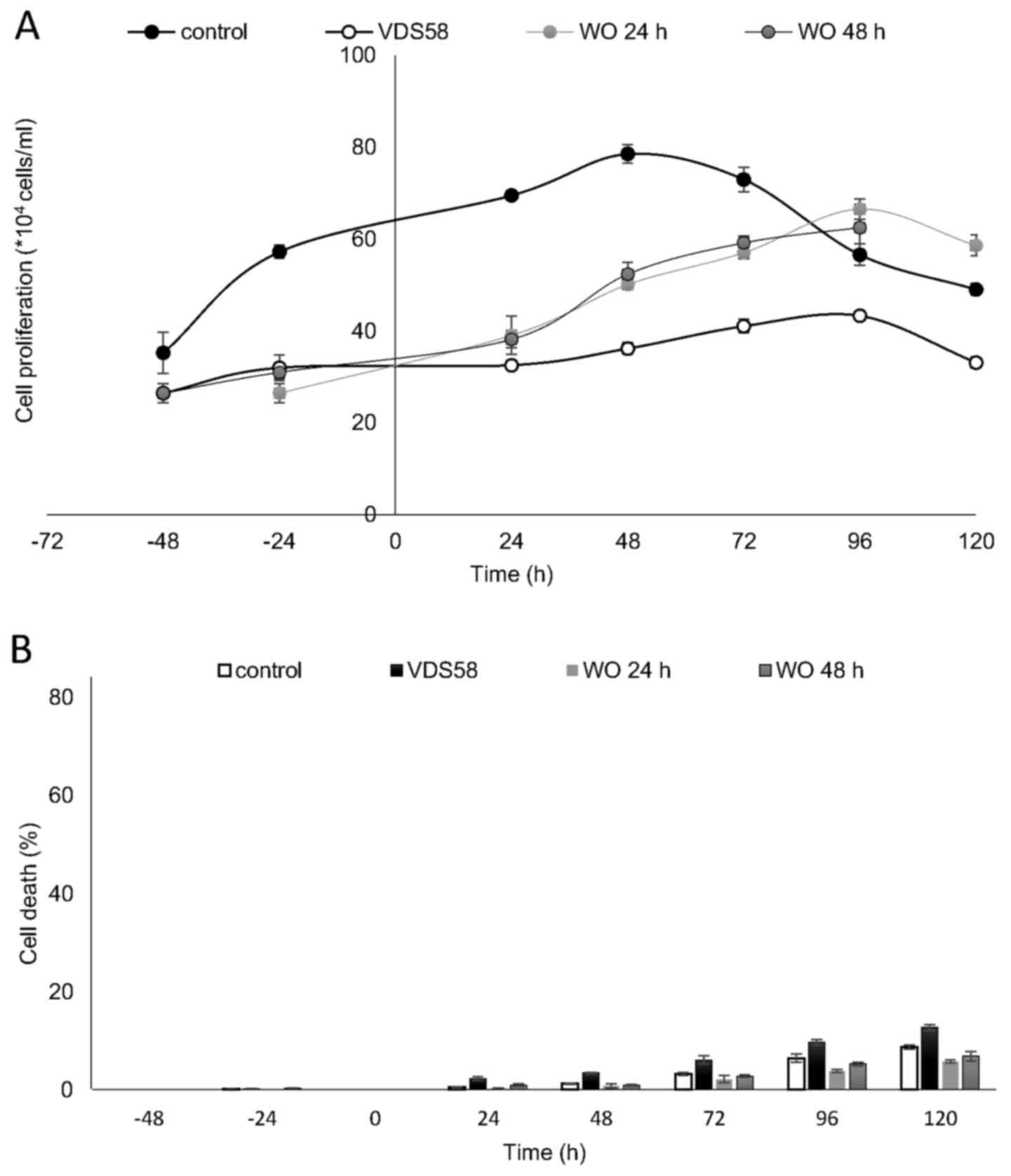
metabolism of VDS58. The results of a typical washout experiment
for MRC-5 cells pretreated for either 24 or 48 h with VDS58 are
shown in Fig. 8. The initial
inhibition of cell proliferation, as expected with exposure to
VDS58 (Fig. 5E), was followed by a
proliferative phase after the replenishment of cultures and
transfer of cells into VDS58-free fresh medium (Fig. 8A). This implies that the
intracellular actions of VDS58 may not cause any permanent harmful
effects on the proliferation of normal MRC-5 cells; thus, cells are
able to grow again, soon after the removal of VDS58 from the
culture. Such behavior suggests the capability of MRC-5 cells to
regain their potential to proliferate even subsequent to exposure
to VDS58. Alternatively, it indicates the presence of inherent
molecular machinery within MRC-5 cells, enabling them to regain
their full proliferation potential, as VDS58-pretreated cells grow
after the removal of the agent and attain cell numbers comparable
with that of the control culture.
Gene expression profiling of human cell
lines incubated with VDS58
Numerous compounds exert their cytotoxic effects by
deregulating the cell cycle or by inducing apoptotic signaling
pathways. The kinetic analysis of cell proliferation prompted the
further elucidation of the molecular mechanisms underlying the
cellular responses in VDS58-treated cultures via RT-qPCR gene
expression analysis in the present study. The assessment focused on
16 genes associated with the cell cycle, apoptosis and/or
senescence (Fig. 9). U-87 MG cells
continuously treated with the IC50 concentration of
VDS58 exhibited a substantial increase in gene expression levels of
CDKN1 (~12-fold), CASP9 (~7-fold), BCL2
(~4-fold), TGFB1 (~3.5-fold) and CTNNB1 (~2-fold)
(P<0.05) (Fig. 9A). However,
the significantly upregulated expression of these genes returned to
basal levels after 48 h of treatment, excluding that of
TGFB1 and CTNNB1, in which upregulated levels were
maintained even after 72 h. Such data indicate the transient
induction of the CDKN1 signaling pathway and the ability of
U-87 MG cultures to overcome the effects of VDS58. Notably, the
re-addition of VDS58 to the U-87 MG cultures after an initial 48 h
exposure caused a more profound activation of all genes, excluding
BAX, CASP8 and RB1 (Fig. 9B). Highly increased expression
levels of BAD (~13-fold), CASP9 (~12-fold),
CASP3 (~9-fold), TP53 (~8-fold), CDK6
(~6-fold) and CDKN1 (~5-fold), as well as MYC,
CCND1, CDK2 and CDK4 (~3-fold), and
TGFB1 (~2.5-fold), were observed (P<0.05). In the latter
case, the expression levels of all genes returned to their initial
levels, excluding those for MYC and TGFB1 following
48 h of cell re-exposure to VDS58. By contrast, the gene expression
profiles of VDS58-treated K562 cells revealed no notable alteration
in gene expression levels, since only TGFB1 (~3-fold),
CTNNB1 (~2-fold), CASP3 (~2-fold) and BAX
(~1.5-fold) showed a time-dependent increase for at least 48 h
(P<0.05) (Fig. 10). Similarly,
RT-qPCR analysis was performed with MCF-7 and MRC-5 cultures
exposed to VDS58; however, no alterations in gene expression levels
were recorded between treated and untreated cells (data not shown).
Notably, within the panel of genes analyzed in MRC-5 cells, only
CDK2, CDK6, BAD, BAX and BCL2
appeared to have been amplified by RT-qPCR, as also reported in our
recent study (22).
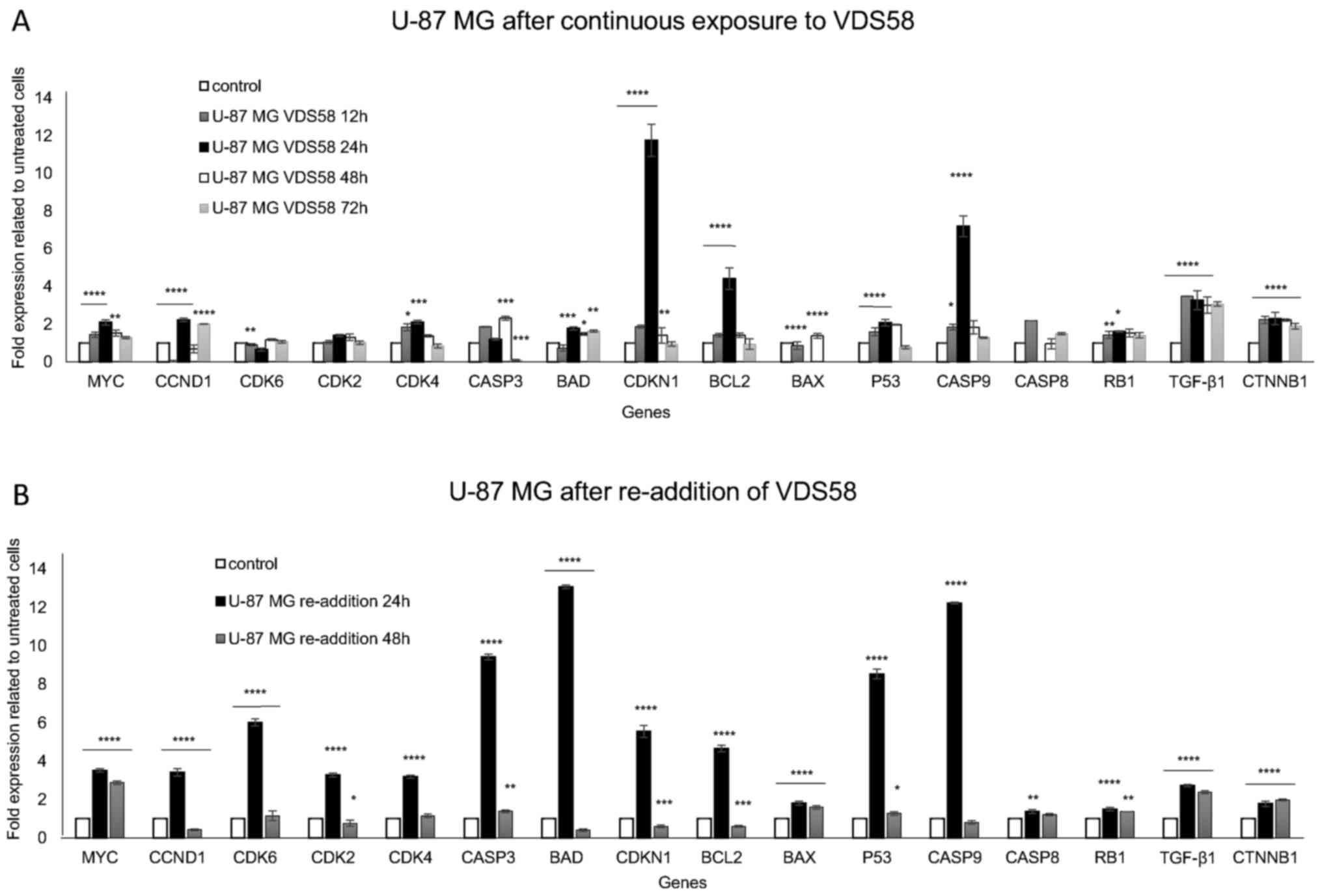 | Figure 9Gene expression profiling by reverse
transcription-quantitative polymerase chain reaction analysis of
U-87 MG cells exposed to VDS58. (A) Expression profiles of
proliferation- and apoptosis-related genes of cytoplasmic RNA
isolated from U-87 MG cells continuously treated with VDS58
(7×10−5 M; IC50 concentration) for 12, 24, 48
and 72 h. (B) Gene expression profiles of U-87 MG cells initially
pretreated with VDS58 for 48 h, prior to the replenishment of cells
and the addition of fresh medium in culture containing VDS58
(7×10−5 M; IC50 concentration), as shown in
Fig. 7. The data shown indicate a
representative experiment where three independent measurements were
used to calculate the mean ± standard deviation (n=4).
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 compared
with the control. A biological replication of this experiment was
performed twice with similar results. IC50, half maximal
inhibitory concentration; MYC, MYC proto-oncogene bHLH
transcription factor; CDK, cyclin-dependent kinase; CASP, caspase;
BAD, BCL2-associated agonist of cell death; CDKN1, cyclin-dependent
kinase inhibitor 1A; BCL2, BCL2-associated agonist of cell death;
BAX, BCL2-associated X apoptosis regulator; TP53, tumor protein 53;
RB1, RB transcriptional corepressor 1; TGF-β1, transforming growth
factor-β1; CTNNB1, catenin β1. |
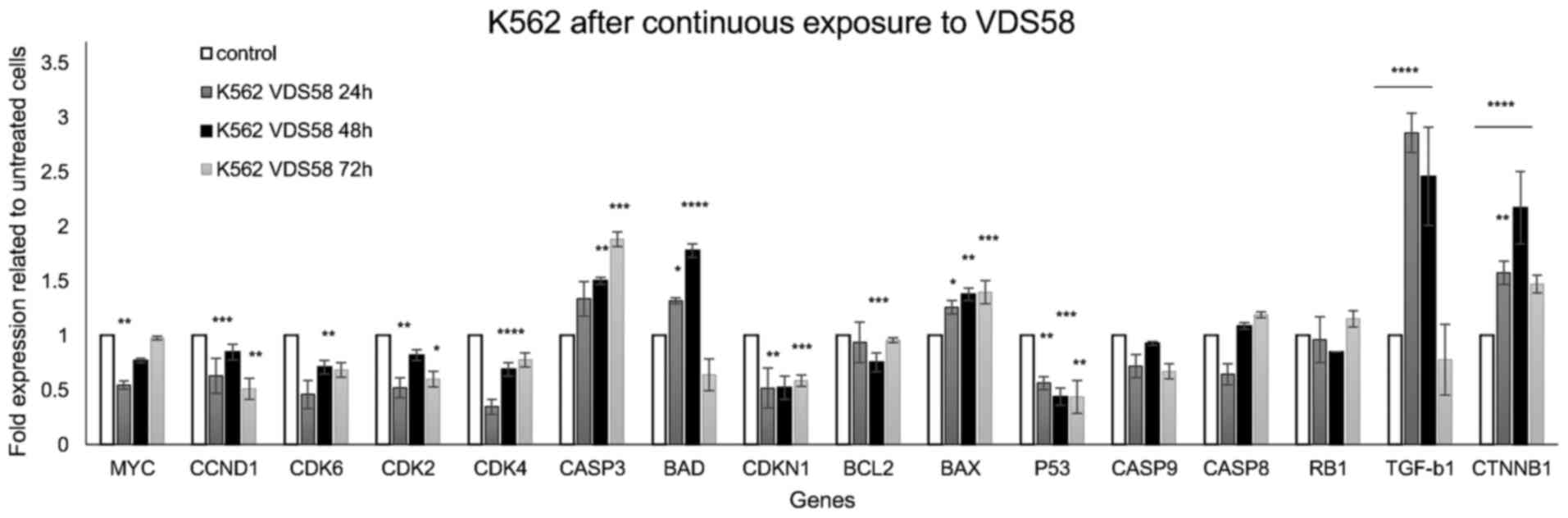 | Figure 10Gene expression profiling by RT-qPCR.
analysis of K562 cells exposed to VDS58. K562 cells were
continuously treated with VDS58 (4.2×10−5 M;
IC50 concentration; Table
I) for 24, 48 and 72 h. The RT-qPCR analysis of isolated
cytoplasmic RNA was performed as shown in Fig. 9. The data shown indicate a
representative experiment where three independent measurements were
used to calculate the mean ± standard deviation (n=4).
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 compared
with the control. A biological replication of this experiment was
performed twice with similar results. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; MYC, MYC
proto-oncogene bHLH transcription factor; CDK, cyclin-dependent
kinase; CASP, caspase; BAD, BCL2-associated agonist of cell death;
CDKN1, cyclin-dependent kinase inhibitor 1A; BCL2, BCL2-associated
agonist of cell death; BAX, BCL2-associated X apoptosis regulator;
TP53, tumor protein 53; RB1, RB transcriptional corepressor 1;
TGF-β1, transforming growth factor-β1; CTNNB1, catenin β1. |
Bioinformatic analysis of gene expression
data to assess the molecular heterogeneity underlying the response
of U-87 MG and K562 cells to VDS58 treatment
The diverse cytotoxic responses observed upon the
exposure of U-87 MG and K562 cells to VDS58 were accompanied by a
differential gene expression profile in the same cultures (Figs. 9 and 10). To improve our understanding of such
cellular behavior, a bioinformatic pathway analysis of whole genome
RNA-Seq expression data from the ENCODE project available for
parental U-87 MG and K562 cells was performed, focusing on genes
involved in the cell cycle, senescence and/or apoptosis signaling
pathways (Fig. 11). Analysis of
the associations between gene expression levels associated with
these aforementioned signaling pathways in the two parental cell
lines unveiled substantial differences (Fig. 11A and D). Notably, the comparison
between the two cell lines showed that crucial genes involved in
the cell cycle pathway, including TGFB1, MYC,
TP53, RB1, CDKN2A, CDKN1B,
CDKN1, CDK2 and CCND1, exhibited greater
variation in expression levels. Consequently, this existing
variability in cell cycle gene expression profiles could contribute
toward the notable diversity in the cytotoxic response of cultures
to VDS58 treatment. To further analyze the gene expression profile
and detect the molecular profiles in VDS58-treated cell cultures
over time, heatmaps were created from the generated RT-qPCR data
(Figs. 9 and 10), and are presented in Fig. 11B, C and E. Evidently, the
existing variability in gene expression levels of parental U-87 MG
and K562 cells from the RNA-Seq data of the ENCODE project was
further confirmed in the RT-qPCR data by comparing control cultures
(Fig. 11B vs. E). Moreover, the
kinetic analysis of the gene expression levels analyzed following
VDS58 exposure exhibited a different pattern during the 24-72 h
course of investigation. This may contribute toward the observed
differential pharmacological response observed in the two cell
lines. The re-addition of VDS58 to U-87 MG cultures, after initial
exposure to the same agent, allowed cells to present a clear
molecular profile, in which two main groups of genes could be
identified. In the first group, the expression of the BAX, RB1,
CTNNB1, TGFB1 and MYC genes was increased after 24 h following the
re-addition of VDS58 and maintained at this level afterwards. In
the second group representing the rest of the genes under
investigation, a transient 24-h activation of expression was
observed, which after 48 h returned to the initial level reaching
that of control untreated cultures (Fig. 11C).
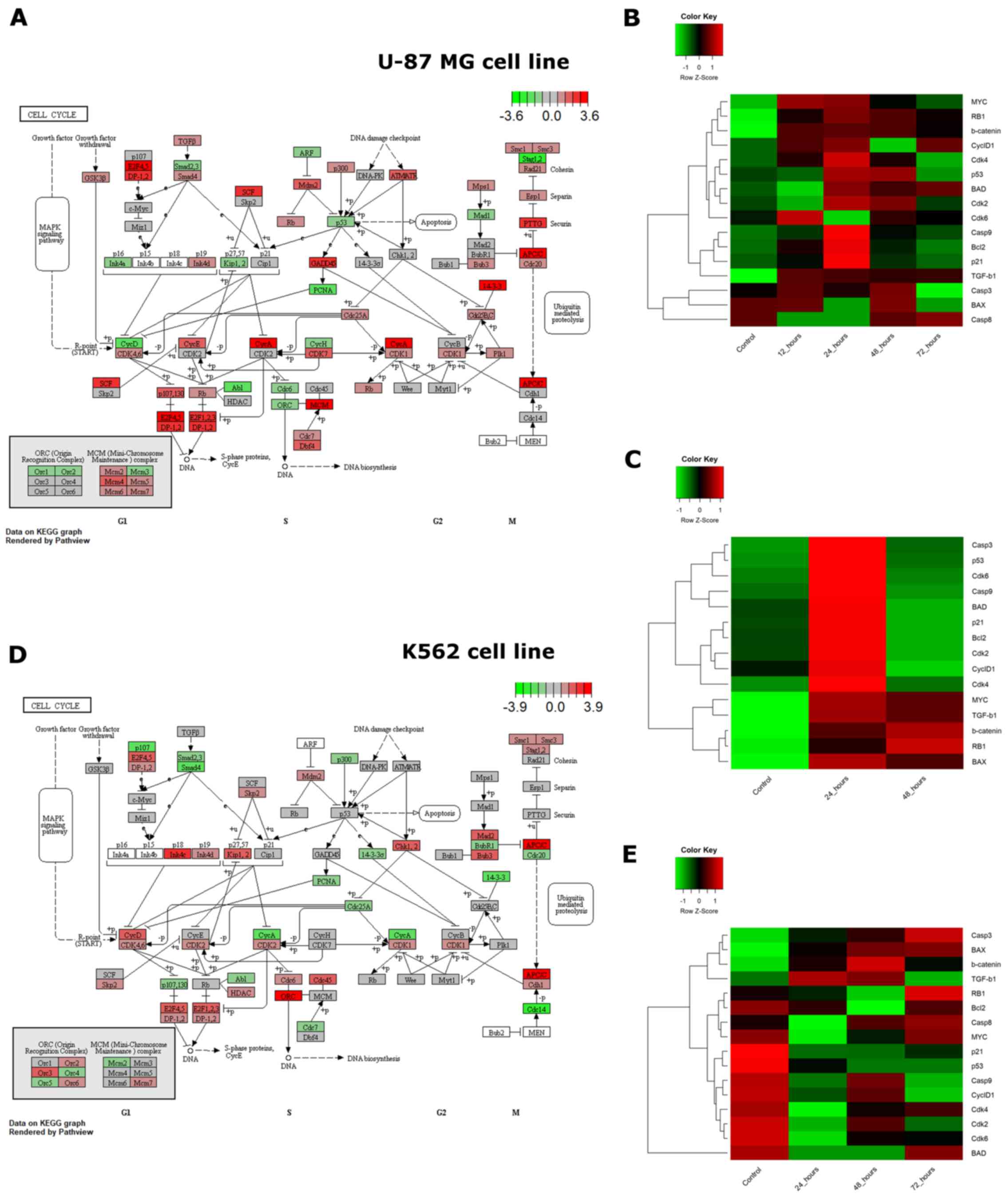 | Figure 11Cell cycle pathway visualization
using RNA-Seq ENCODE data and heatmaps of expression for selected
genes of U-87 MG and K562 cells exposed to VDS58 as analyzed by
RT-qPCR. (A and D) Expression of genes that participate in the cell
cycle pathway, as profiled by RNA-sequencing for untreated (A) U-87
MG and (D) K562 cell lines (data from the ENCODE project).
Transcript per million reads values for all genes expressed in each
cell line were normalized to z-score, and genes associated with the
cell cycle were extracted from the Kyoto Encyclopedia of Genes and
Genomes database (29-31) and rendered in graphs using Pathview
(28). (B and C) The heatmaps show
the expression of selected genes participating in cellular
senescence, apoptosis and the cell cycle in the U-87 MG cell line.
U-87 MG cells were (B) continuously exposed to the substance for 0
(control), 12, 24, 48 and 72 h, and (C) continuously exposed to the
substance for 48 h (control) following re-addition at 24 and 48 h.
(E) The heatmaps show the expression of selected genes involved in
cellular senescence, apoptosis and the cell cycle in K562 cells.
K562 cells were continuously exposed to VDS58 for 0 (control), 24,
48 and 72 h. Gene expression values for all heatmaps were measured
using SYBR RT-qPCR and normalized to z-score values for each gene.
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
Discussion
Tumor cell heterogeneity and genomic instability are
factors that contribute toward the poor outcomes of cancer therapy
in clinical practice. Moreover, the development of drug resistance
represents a hallmark of malignant cells to escape the effects of
anticancer agents. An improved understanding of the molecular
mechanisms underlying the variable response of cancer cells to
environmental stimuli and therapeutic agents is of high demand in
the development of novel anticancer drugs (6,33).
To this end, the selective cytotoxicity observed for the SL
analogs, including artemisin, thapsigargin and parthenolide, has
led to further pharmacological investigation through their
evaluation in clinical trials; however, specific pharmacokinetic
issues have been raised (15-17).
For example, parthenolide presents poor bioavailability and thus
efforts have been made to produce molecules with enhanced
solubility and membrane permeability (9,34-36).
Moreover, the potent ability of parthenolide to inhibit NF-κB is
recognized as one of the potential factors for its selective action
for targeting tumor and cancer stem cells. Parthenolide was found
to directly modify the p65 subunit of NF-κβ and suppress the
activity of the upstream IκB kinase complex leading to
stabilization of the NF-κB inhibitors, IκBα and IκBβ (37-39).
Notably, the total synthesis of sesquiterpenes and
SLs has led to intense research efforts for decades; however, only
a few unified synthetic protocols have been published to analyze
the diverse carbocyclic complexity presented by the sesquiterpenoid
family (20,21,40,41).
Furthermore, despite extensive studies on the chemical synthesis
and biology of 6,12-SLs, the isomeric 8,12-SLs, comprising almost
half of the natural substances, have received relatively limited
attention in the pharmacological field, restricting the
identification of the pharmacological potential of this family of
compounds (42). Thus, efforts to
improve our understanding of the molecular mechanisms underlying
the effects of SLs in cancer cells are urgently required to ensure
the clinical exploitation and application of these compounds as
potential anticancer therapeutic agents.
The CDK inhibitor CDKN1 promotes cell cycle arrest
in response to a number of stimuli, including activation of
TP53 and TGFB1, and suppression of MYC
(43). The knowledge accumulated
thus far regarding the dysregulation of CDKN1 has revealed
that several important tumor suppressor and oncogenic signaling
pathways may alter CDKN1 expression to exert their effects
on cell cycle progression, survival, senescence and apoptosis
(44). Additionally, the
co-operation of CDKN1 with tumor suppressor molecules or
conversely, the antagonism with oncogene products, leads to tumor
cell regression (45). It is
notable that tumor cell senescence is induced by CDKN1
subsequent to restoring TP53 function or inactivating
MYC in tumors with functional TP53 (46,47).
Moreover, the anti-proliferation activity of TGFB1 on tumor
cells is mediated through the activity of a repressive
transcription factor complex constituting SMAD4, p107 and E2F4/5,
which is mediated by the suppression of MYC (48). Consequently, CDKN1
expression can promote and inhibit tumorigenic processes, depending
on the existing molecular heterogeneity within various tumor cell
types and microenvironments (43).
Alternatively, variations in cellular response and the complexity
of the molecular events that are associated with the dysregulation
of CDKN1 imply that further understanding of these molecular
mechanisms is required prior to the potential therapeutic roles of
CDKN1 being investigated for the development of novel drugs
for the treatment of cancer.
The data obtained in the present study presents a
solid foundation of evidence as to how the sesquiterpene derivative
VDS58 exerts its differential cytotoxic effects in cancer and
normal cell lines of various histopathological origins and
heterogeneous gene expression profiles. Normal MRC-5 cells exhibit
higher IC50 to VDS58 than U87 MG and recover their
proliferation capacity in a manner dependent to the exposure period
to VDS58. Moreover, the differential cytotoxicity profile observed
in the cell cultures has prompted us to focus on elucidating the
molecular events underlying the pharmacological response. However
at this time, the high IC50 of VDS58 restricts any
further consideration of in vivo studies. The unique ability
of U-87 MG cells to transiently induce the expression of genes
involved in the cell cycle, senescence and apoptosis imply
heterogeneity at the molecular level and in the underlying
signaling pathways. Indeed, this was verified by conducting a
pathway bioinformatic analysis of whole genome RNA-Seq data of the
ENCODE project, in which the human parental U-87 MG glioblastoma
and K562 erythroleukemia cells presented a substantially marked
difference in the expression levels of crucial genes governing cell
cycle decisions, including MYC, TP53, TGFB1,
CCND1 and RB1. Notably, in K562 cells exposed to
VDS58, the expression of certain genes, mainly TGFB1 and, to a
lesser extent, CTNNB1, BAD, BAX and
CASP3, was induced, and activation was maintained at this
level for 48-72 h. These results further support the hypothesis
that variations in the cytotoxicity observed in different cell
cultures reflect the heterogeneous gene expression profiles that
exist within these parental cell lines. Notably, genes such as
MYC and TGFB1 in U-87 MG cells exposed to VDS58
exhibited a persistent increase in expression, while the remaining
genes, including CDKN1, CASP9, BCL2 and
TP53, were only induced for 24 h. This observation suggests
that the related signaling pathways are differentially affected by
the homeo-static genomic mechanisms that govern the cellular
behavior following exposure to VDS58. The current study observed
that U-87 MG cells were capable of recovering their proliferation
rates soon after 24-48 h following exposure to VDS58, which
coincides with the gene expression profiles. Moreover, the
persistence of induced expression levels for TGFB1 and
MYC only could provide support for cell proliferation after
the initial cell cycle arrest of cells, due to the transient
increase in the expression level of CDKN1. Additionally,
U-87 MG and K562 cells treated with VDS58 exhibited increased
TGFB1 expression levels; however, variations in their
cellular proliferation responses were noticed.
The analysis of gene expression profiles by
creating the corresponding heatmaps permitted the identification of
heterogeneity in the expression of crucial genes and signaling
pathways within the two parental cell lines. Consequently, such an
approach has permitted variations in expression to be revealed
within the molecular signatures of the genes analyzed. This
observation could be important in understanding the molecular
mechanisms that aid cancer cells in evading therapeutic
interventions by overcoming proliferation restriction signals, or
by developing drug resistance. The results of the current study
provide new knowledge on the anticancer properties of sesquiterpene
and present evidence toward understanding the mechanism of action
exhibited by sesquiterpene analogs as potential antitumor drug
targets. Additionally, this study further contributes to improving
methodologies to develop potential candidate molecules as
anticancer agents to target cell cycle signaling pathways. However,
the IC50 values of the tested SLs analogs are high
enough to determine a clearer picture for their cytotoxicity
profile. Also, it is necessary to further clarify the precise
molecular mechanisms underlying the effects of VDS58 against tumor
cells prior to this agent being considered for further
pharmacological evaluation and development. To this end, by
synthesizing more cytotoxic SLs and applying genomics and
proteomics approaches, such therapeutic possibilities will be
effectively addressed.
Acknowledgments
Not applicable.
Funding
This study was funded by interdepartmental public
funds of Aristotle University of Thessaloniki.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors’ contributions
ALZ and VPD synthesized the sesquiterpene
compounds; MGA and NFT performed cell and molecular experimental
work; ISV supervised the whole study; MGA performed the statistical
analysis; KAK performed the bioinformatic analysis; FMC and NG
provided resources; ALZ and ISV designed the experimental
methodology and analyzed the data. MGA, VPD, NFT, FMC, KAK, NG, ALZ
and ISV contributed to the interpretation of the experimental data
and to the writing of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah NP, Nicoll JM, Nagar B, Gorre ME,
Paquette RL, Kuriyan J and Sawyers CL: Multiple BCR-ABL kinase
domain mutations confer polyclonal resistance to the tyrosine
kinase inhibitor imatinib (STI571) in chronic phase and blast
crisis chronic myeloid leukemia. Cancer Cell. 2:117–125. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wall ME, Wani MC, Cook CE, Palmer KH,
McPhail AT and Sim GA: Plant antitumor agents. I. The isolation and
structure of camptothecin, a novel alkaloidal leukemia and tumor
inhibitor from Camptotheca acuminata. J Am Chem Soc. 88:3888–3890.
1966. View Article : Google Scholar
|
|
4
|
Cragg GM and Newman DJ: Nature: A vital
source of leads for anticancer drug development. Phytochem Rev.
8:313–331. 2009. View Article : Google Scholar
|
|
5
|
Song Y, Sun H, Zhang A, Yan G, Han Y and
Wang X: Plant-derived natural products as leads to anti-cancer
drugs. J Med Plant Herb Ther Res. pp. 6–15. 2014
|
|
6
|
Vizirianakis IS, Chatzopoulou M,
Bonovolias ID, Nicolaou I, Demopoulos VJ and Tsiftsoglou AS: Toward
the development of innovative bifunctional agents to induce
differentiation and to promote apoptosis in leukemia: Clinical
candidates and perspectives. J Med Chem. 53:6779–6810. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tao W, Li B, Gao S, Bai Y, Shar PA, Zhang
W, Guo Z, Sun K, Fu Y, Huang C, et al: CancerHSP: Anticancer herbs
database of systems pharmacology. Sci Rep. 5:114812015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kreuger MRO, Grootjans S, Biavatti MW,
Vandenabeele P and D’Herde K: Sesquiterpene lactones as drugs with
multiple targets in cancer treatment: Focus on parthenolide.
Anticancer Drugs. 23:883–896. 2012.PubMed/NCBI
|
|
9
|
Ren Y, Yu J and Kinghorn AD: Development
of anticancer agents from plant-derived sesquiterpene lactones.
Curr Med Chem. 23:2397–2420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosco A and Golsteyn RM: Emerging
anti-mitotic activities and other bioactivities of sesquiterpene
compounds upon human cells. Molecules. 22:4592017. View Article : Google Scholar
|
|
11
|
Zhou J and Zhang Y: Cancer stem cells:
Models, mechanisms and implications for improved treatment. Cell
Cycle. 7:1360–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jordan CT: Searching for leukemia stem
cells - not yet the end of the road? Cancer Cell. 10:253–254. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawasaki BT, Hurt EM, Kalathur M, Duhagon
MA, Milner JA, Kim YS and Farrar WL: Effects of the sesquiterpene
lactone parthenolide on prostate tumor-initiating cells: An
integrated molecular profiling approach. Prostate. 69:827–837.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Efferth T, Sauerbrey A, Olbrich A, Gebhart
E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, et
al: Molecular modes of action of artesunate in tumor cell lines.
Mol Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji Y, Zhang YC, Pei LB, Shi LL, Yan JL and
Ma XH: Anti-tumor effects of dihydroartemisinin on human
osteosarcoma. Mol Cell Biochem. 351:99–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gravett AM, Liu WM, Krishna S, Chan WC,
Haynes RK, Wilson NL and Dalgleish AG: In vitro study of the
anti-cancer effects of artemisone alone or in combination with
other chemotherapeutic agents. Cancer Chemother Pharmacol.
67:569–577. 2011. View Article : Google Scholar
|
|
17
|
Zhang CZ, Zhang H, Yun J, Chen GG and Lai
PB: Dihydroartemisinin exhibits antitumor activity toward
hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol.
83:1278–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zografos AL and Anagnostaki EE:
Sesquiterpenes. From Biosynthesis to Total Synthesis: Strategies
and Tactics for Natural Products. John Wiley & Sons, Inc; pp.
254–278. 2016
|
|
19
|
Anagnostaki EE and Zografos AL: ‘Common
synthetic scaffolds’ in the synthesis of structurally diverse
natural products. Chem Soc Rev. 41:5613–5625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anagnostaki EE and Zografos AL:
Non-natural elemane as the ‘stepping stone’ for the synthesis of
germacrane and guaiane sesquiterpenes. Org Lett. 15:152–155. 2013.
View Article : Google Scholar
|
|
21
|
Anagnostaki EE, Demertzidou VP and
Zografos AL: Divergent pathways to furosesquiterpenes: First total
syntheses of (+)-zedoarol and (Rac)-gweicurculactone. Chem Commun
(Camb). 51:2364–2367. 2015. View Article : Google Scholar
|
|
22
|
Tseligka ED, Rova A, Amanatiadou EP,
Calabrese G, Tsibouklis J, Fatouros DG and Vizirianakis IS:
Pharmacological development of target-specific delocalized
lipophilic cation-functionalized carboranes for cancer therapy.
Pharm Res. 33:1945–1958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vizirianakis IS and Tsiftsoglou AS:
Blockade of murine erythroleukemia cell differentiation by
hypomethylating agents causes accumulation of discrete small
poly(A)-RNAs hybridized to 3′-end flanking sequences of beta(major)
globin gene. Biochim Biophys Acta. 1743:101–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Friend C, Scher W, Holland JG and Sato T:
Hemoglobin synthesis in murine virus-induced leukemic cells in
vitro: Stimulation of erythroid differentiation by dimethyl
sulfoxide. Proc Natl Acad Sci USA. 68:378–382. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vizirianakis IS, Wong W and Tsiftsoglou
AS: Analysis of the inhibition of commitment of murine
erythroleukemia (MEL) cells to terminal maturation by
N6-methyladenosine. Biochem Pharmacol. 44:927–936. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:pp. 354re32016, View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
28
|
Dunham I, Kundaje A, Aldred SF, Collins
PJ, Davis CA, Doyle F, Epstein CB, Frietze S, Harrow J, Kaul R, et
al ENCODE Project Consortium: An integrated encyclopedia of DNA
elements in the human genome. Nature. 489:57–74. 2012. View Article : Google Scholar
|
|
29
|
Luo W and Brouwer C: Pathview: An
R/Bioconductor package for pathway-based data integration and
visualization. Bioinformatics. 29:1830–1831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
31
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar :
|
|
32
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar :
|
|
33
|
Gottesman MM, Lavi O, Hall MD and Gillet
JP: Toward a better understanding of the complexity of cancer drug
resistance. Annu Rev Pharmacol Toxicol. 56:85–102. 2016. View Article : Google Scholar
|
|
34
|
Hartwell JL and Abbott BJ: Antineoplastic
principles in plants: Recent developments in the field. Adv
Pharmacol Chemother. 7:117–209. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Merfort I: Perspectives on sesquiterpene
lactones in inflammation and cancer. Curr Drug Targets.
12:1560–1573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lytton J, Westlin M and Hanley MR:
Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum
Ca-ATPase family of calcium pumps. J Biol Chem. 266:17067–17071.
1991.PubMed/NCBI
|
|
38
|
Hehner SP, Heinrich M, Bork PM, Vogt M,
Ratter F, Lehmann V, Schulze-Osthoff K, Dröge W and Schmitz ML:
Sesquiterpene lactones specifically inhibit activation of NF-kappa
B by preventing the degradation of I kappa B-alpha and I kappa
B-beta. J Biol Chem. 273:1288–1297. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghantous A, Sinjab A, Herceg Z and
Darwiche N: Parthenolide: From plant shoots to cancer roots. Drug
Discov Today. 18:894–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Demertzidou VP and Zografos AL:
Platinum-catalyzed cycloisomerizations of a common enyne: A
divergent entry to cyclopropane sesquiterpenoids. Formal synthesis
of sarcandral-actone A. Org Biomol Chem. 14:6942–6946. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Foo K, Usui I, Götz DCG, Werner EW, Holte
D and Baran PS: Scalable, enantioselective synthesis of germacrenes
and related sesquiterpenes inspired by terpene cyclase phase logic.
Angew Chem Int Ed Engl. 51:11491–11495. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu X, Xu S and Maimone TJ: A double
allylation strategy for gram-scale guaianolide production: Total
synthesis of (+)-mika-nokryptin. Angew Chem Int Ed Engl.
56:1624–1628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Warfel NA and El-Deiry WS: p21WAF1 and
tumourigenesis: 20 years after. Curr Opin Oncol. 25:52–58. 2013.
View Article : Google Scholar
|
|
44
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ventura A, Kirsch DG, McLaughlin ME,
Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R
and Jacks T: Restoration of p53 function leads to tumour regression
in vivo. Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu CH, van Riggelen J, Yetil A, Fan AC,
Bachireddy P and Felsher DW: Cellular senescence is an important
mechanism of tumor regression upon c-Myc inactivation. Proc Natl
Acad Sci USA. 104:13028–13033. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bretones G, Delgado MD and León J: Myc and
cell cycle control. Biochim Biophys Acta. 1849:506–516. 2015.
View Article : Google Scholar
|