Introduction
Ovarian cancer has a high mortality rate compared
with the majority of types of cancer associated with the female
reproductive system (1). The
incidence and mortality rates of ovarian cancer have been
increasing gradually in China, with at least 230,000 new cases and
a mortality rate of >150,000 on an annual basis worldwide
(2). Of all ovarian cancer cases,
~70% are detected at an advanced stage, with 85% of these patients
succumbing to the disease (3,4). The
risk factors for ovarian cancer include nulliparity and refractory
infertility, however, these risks can be mitigated with the use of
oral contraceptives for 5 years (5). Surgical intervention in combination
with chemotherapy is a common treatment method for ovarian cancer,
as ovarian cancer cells are reported to be initially sensitive to
chemotherapeutic drugs (6).
Although there are a number of treatment modalities available for
ovarian cancer, radioresistance is often observed (7,8).
Gene therapy has emerged as a novel treatment to overcome
radioresistance in ovarian cancer (9,10).
Metadherin (MTDH), also known as astrocyte elevated
gene-1 and lysine-rich CEACAM1 co-isolated, is as an important
molecule that regulates multiple biological behaviors in
carcinogenesis (11,12). It has been suggested that MTDH is
associated with chemoresistance and metastasis leading to poor
prognoses for patients with breast cancer (13). Initially considered to be a human
immunodeficiency virus-induced gene in astrocytes, MTDH is a
membrane protein that regulates the homing of tumor cells to the
lung endothelium, and is a lysine-rich protein associated with
tight junctions in prostate epithelial cells (14). It has been demonstrated that MTDH
is upregulated in various types of cancer, including
hepatocellular, breast and lung cancer (11,15,16).
Additionally, a previous study reported that silencing MTDH
markedly improved the radiosensitivity of ovarian cancer and
hindered the repair of radiation-induced DNA double strand breaks
(17). RNA interference-mediated
silencing has been verified to inhibit the expression of
therapeutic genes (18). A
previous study revealed that silencing HOTAIR or mitogen-activated
protein kinase 1 inhibited the proliferation, migration and
invasion of SKOV3 ovarian cancer cells (19). Therefore, the aim of the present
study was to investigate the effects of MTDH silencing on the
radiosensitivity, proliferation and metastasis of SKOV3 ovarian
cancer cells.
Materials and methods
Study subjects
Paraffin-embedded ovarian cancer tissues (n=273)
were obtained from patients with primary ovarian cancer who were
diagnosed at the Second Hospital of Jilin University (Changchun,
China) between January, 2012 and March, 2016. The patients (22-59
years of age with a mean age of 39.9±6.44 years) had no other
medical history or previous history of radio- or chemotherapy.
Tumors were histologically confirmed to be serous cystadenoma (124
cases), mucinous cystadenoma (82 cases), endometriod carcinoma (38
cases) and epithelial carcinoma (29 cases; five with mixed
epithelial carcinoma, five with clear cell carcinoma and 19 with
poorly differentiated carcinoma). All cases were grouped into stage
I (35 cases), stage II (78 cases) and stage III (160 cases)
according to FIGO2000 (20).
Pathological grading was as follows: 48 cases were grade I, 61
cases were grade II and 164 cases were grade III. The histological
grading was as follows: 171 cases were poorly differentiated and
102 cases were medium to well differentiated. Lymph node metastasis
was present as follows: 172 cases with lymph node metastasis and
101 cases without. For comparison, fresh normal ovarian tissues
(n=277) from patients aged 20-56 years old (mean, 36.3±6.1 years),
who had undergone prophylactic oophorectomy for endometrial
carcinoma at the Second Hospital of Jilin University between
January 2012 and March 2016, were collected. There was no
significant difference in baseline characteristics between the two
groups of samples (P>0.05). The present study was approved by
the Ethics Committee of the Second Hospital of Jilin University
(no. 201112006) and informed consent was obtained from patients or
their guardians in accordance with the Declaration of Helsinki.
Cell culture
The SKOV3 ovarian cancer cells were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
The human immortalized ovarian epithelial HOSEpiC cell line (no.
BNCC340096; Beina Biotechnology, Co., Ltd., Shanghai, China) was
routinely cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum in a sterile humidified incubator with 5% CO2 at
37°C.
Cell transfection and grouping
MTDH-short hairpin (sh)RNA (interfering plasmid) and
control-shRNA (negative plasmid) were synthesized by Shanghai
Bioengineering Technology Service Co., Ltd. (Shanghai, China). At
24 h prior to transfection, the SKOV3 ovarian cancer cells were
inoculated into 24-well plates (1.0×105 cells/well). The
cells were divided into blank, control-shRNA and MTDH-shRNA groups,
with three wells in each group, and cultured until cell adherence
and confluence (80-90%) were reached. The transfection reagent kits
and transfection reagents used in the experiment were provided by
Beijing Kangwei Century Biotechnology Co., Ltd. (Beijing, China).
Only Lipofectamine 2000 was added to the blank group. The
appropriate plasmids and transfection reagent were added to the
control-shRNA group and MTDH-shRNA group (0.1 µg; 0.3
µl) and suspensions were incubated in RPMI-1640 with 10%
serum for 10 min. Transfection was performed in accordance with the
manufacturer’s protocol for the transfection reagent.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The ovarian cancer tissues (1 g) and total RNA from
the SKOV3 cells were treated using TRIzol (Thermo Fisher
Scientific, Inc.) according to the manufacturer’s protocol. A
Transgen RT-PCR kit (Beijing Quanshijin Biotechnology Co., Ltd.,
Beijing, China) was used to reverse transcribe RNA to cDNA. cDNA
was amplified using SYBR-Green (Toyobo, Osaka, Japan) with the
specific primers. PCR was initiated with pre-denaturation at 94°C
for 5 min, followed by 30 cycles of denaturation at 94°C for 30
sec, annealing (MTDH, 58°C; β-actin, 54°C) for 30 sec and extension
at 72°C with terminal extension at 72°C for 5 min. The primer
sequences were as follows: MTDH, forward, 5′-AGCAAAGCAGCCACCAGAG-3′
and reverse, 5′-AGGAAATGATGCGGTTGTA-3′; β-actin, forward,
5′-ACCGAGCGCGGCTACAGC-3′ and reverse, 5′-CTCATTGCCAATGGTGAT-3′.
Immunohistochemical staining
An SP kit (Zymed Laboratories, San Francisco, CA,
USA) was used. Surgical specimens with routine immobilization were
paraffin-embedded, cut into 4-m thick sections, dewaxed, heated
with a citric acid buffer (pH 6.0; Beijing Nobleryder Technology
Co., Ltd., Beijing, China) in a microwave for antigen retrieval (20
min) and stored at room temperature. Endogenous peroxidase was
blocked by incubation with 3% H2O2 for 10
min. Subsequently, 10% goat serum (Shanghai Haoran Biotechnology
Co., Ltd., Shanghai, China) was used to block samples for 10 min,
and primary antibody against MTDH (1:200, PB0309; Wuhan Boster
Biological Engineering Co., Ltd., Wuhan, China) was added and
incubated overnight at 4°C. The secondary mouse biotinylated
antibody (1:500, BZKT0379; Shanghai Yijisy Co., Ltd., Shanghai,
China) was then added and incubated at 37°C for 10 min. Horseradish
peroxidase (HRP)-labeled streptavidin (Guangzhou Geruilin
Biotechnology Co., Ltd., Guangzhou, China) was added at 37°C for 10
min and the tissues were stained with diaminobenzidine. The
percentage of positive cells and the staining intensity were
scored. A total of five high-power fields were randomly selected in
each section (magnification, ×400) captured by image analysis
software (NikonH600L microscope and image analysis system; Nikon,
Tokyo, Japan). The percentage of positive cells was scored as
follows: <5%, 0; 6-25%, 1; 26-50%, 2; 51-75%, 3; and ≥76%, 4.
Staining intensity was scored as follows: 0 (no staining), 1
(yellow), 2 (brown-yellow) and 3 (yellow-brown). The positive cell
staining and staining intensity scores were combined and 0-1 was
considered as negative (−), 2 as weak positive (+), 3-4 as positive
(++) and ≥5 as strong positive (+++). +, ++ and +++ were considered
as positive.
Western blot analysis
PMSF cell lysates were added to the SKOV3 cells. The
cells were lysed and centrifuged by 200 W ultrasonic waves to
extract the total protein. Total protein concentration was
determined using a bicinchoninic acid assay. The proteins (100 g/l)
were separated by 12% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes. The membranes were blocked and primary
antibodies, including rabbit anti-human MTDH (1:1,000, ab45338) and
internal reference β-actin (1:200, ab8227) (both from Abcam,
Shanghai, China), were added and incubated overnight at 4°C. The
membranes were then incubated with HRP-labeled IgG secondary
antibody (1:500, ab6721; Abcam) at 3°C for 2 h, followed by three
washes with Tris-buffered saline with Tween-20 (10 min each). All
antibodies used were purchased from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, USA). The samples were developed using a
chemiluminescence system. The gray value was assessed using a
grayscale scanner and the relative protein expression was
calculated using ImageJ software.
MTT assay
A cell suspension was prepared from SKOV3 cells from
the three groups in the logarithmic growth phase, and the density
was adjusted to 3×104 cells/ml. Subsequently, 100
µl cell suspension was seeded into 96-well plates with five
wells for each group. At 24, 48 and 72 h post-transfection, MTT
solution (20 µl per well) was added. Following 4 h of
incubation in the dark, the supernatant was aspirated.
Dimethylsulfoxide (150 µl) was added to each well. The
absorbance values were measured at 570 nm using a microplate
reader. The optical density (OD) values were used to calculate the
in vitro proliferation rate of each group.
Transwell assay
Matrigel was homogenized and added to the upper
chambers of a Transwell chamber (Corning Incorporated, Corning, NY,
USA). Following 6 h of transfection, the SKOV3 cells were digested
and centrifuged (300 × g, 3 min, 37°C) for further use. The cell
suspensions from the blank, control-shRNA, and MTDH-shRNA groups
were seeded into Transwell chambers in 24-well plates with
chemokines and culture medium. The chambers were incubated at 37°C
in an atmosphere containing 5% CO2 for 24 h. A total of
five high-power fields were observed under a microscope (Olympus)
to calculate the number of cells penetrating the membrane.
Wound-healing assay
The SKOV3 cells were seeded into 6-well plates with
three wells for each group. The SKOV3 cells were grown until ~90%
density was reached, following which the culture medium was
aspirated and a pipette tip was used to create a number of parallel
scratches in the middle of culture wells. The cells were washed and
continually cultured. A total of nine fields with scratches were
randomly selected in each group, and images were captured at 0, 24,
48 and 72 h under a microscope (Olympus). Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) was used to measure the
distance between two scratches and calculate the rates of cell
migration. Cell migration = (scratch distance at 0 h − scratch
distance at 24, 48 or 72 h)/scratch distance at 0 h.
Colony-forming assay
According to different doses of irradiation, cells
were inoculated into culture dishes (6 cm). When the cells had
adhered, the culture dishes were irradiated with a single dose of
X-rays at 0, 2, 4, 6 and 8 Gy respectively, and the irradiation was
performed at room temperature at the dose rate of 2 Gy/min.
Following irradiation, three parallel samples were incubated for 2
weeks and stained using Giemsa. The number of colonies (clusters of
≥50 cells) was counted using a microscope and the experiment was
performed in triplicate. Planting efficiency (PE) = number of
clones/number of inoculated cells ×100 (%).
Flow cytometry
When the cells had been centrifuged and trypsinized,
the final concentration of the single cell suspension was adjusted
to 1.25×105 cells/ml. Blank, control-shRNA and
MTDH-shRNA cell suspensions were inoculated into 6-well plates
(1.25×105 cells/well). The cells were exposed to
irradation at 0, 2, 4, 6 and 8 Gy, which was performed at room
temperature at the dose rate of 2 Gy/min. The samples were washed
twice with PBS following irradiation and cell suspensions were
collected in the corresponding flow tubes following digestion with
0.25% trypsin. Binding buffer (500 µl) and Annexin V-FITC (5
µl) were added to each tube and mixed well, followed by the
addition of propidium iodide (5 µl) and further mixing. The
samples were incubated at room temperature for 15 min in the dark.
The apoptotic rate was measured by flow cytometry for 1 h, and the
experiment was performed in triplicate. The above reagents were
purchased from BD Biosciences (San Jose, CA, USA).
Nude mouse xenograft
Nude mouse xenograft models were established using
female BALB/c-nude mice (4-6 weeks old, weighing 16-20 g; n=30;
Beijing Weitong Lihua Co., Beijing, China), which were randomly
divided into three groups (10 in each group; 17-18 g) and
maintained in a specific pathogen-free (SPF) ‘barrier’ facility
with controlled at room temperature and 55-62% humidity, and
alternating 12-h light and dark cycles, food and water is
accessible. The SKOV3 cells in the logarithmic growth phase were
extracted from the blank, control-shRNA and MTDH-shRNA groups and
cell suspensions were produced (4×107 cells/ml).
Subsequently, 0.3 ml of cell suspension was inoculated into the
subcutaneous tissue above the right scapula of mice in each group,
and tumor formation was induced after 10 days. When the tumor size
was 100 mm3, all female BALB/c-nude mice were
anesthetized with 3% pentobarbital sodium (30 mg/kg
intraperitoneally; P3761; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). All mice were placed in a lead box following
anesthesia. The right scapula was fixed outside the lead box and
locally irradiated with X-rays every other day; irradiation was
performed at room temperature at the dose rate of 2 Gy/min each
time (five times), with the total dose of 10 Gy (MultiRad225;
Faxitron X-ray Corporation, Wheeling, IL, USA). A total of 30
female BALB/c-nude mice were sacrificed following X-ray irradiation
for 28 days. Immediately after sacrifice, body weight was detected.
No significant difference was found in body weight of all
BALB/c-nude mice, as all mice weighed approximately 24 g. The gross
morphology and growth of the nude mouse xenografts were examined
and recorded carefully. The stripped tumor was washed with 100
µl sterile water four times. The experiment was approved by
the Animal Ethics Committee of the Second Hospital of Jilin
University (no. 201201003), and the experiment strictly followed
the National Institutes of Health guide for the care and use of
laboratory animals.
Statistical analysis
All data were processed using SPSS 20.0 software
(IBM SPSS, Armonk, NY, USA) and are presented as the mean ±
standard deviation. Student’s t-test was used for comparisons
between two groups and one-way analysis of variance was used to
compare multiple groups. The association between MTDH expression,
ovarian cancer and clinicopathological factors in ovarian cancer
was assessed using the χ2 test. For homogenous data, the
Least Significant Difference test was performed; for non-homogenous
data, the Games-Howell test was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
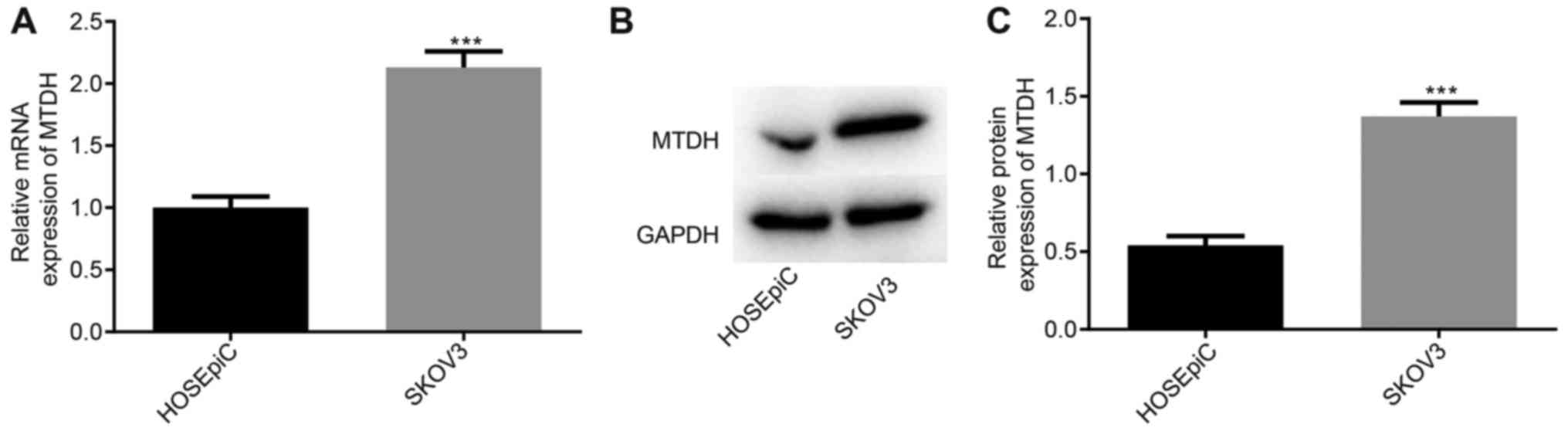
MTDH is expressed at high levels in SKOV3
cells
To measure the expression of MTDH in SKOV3 cells,
RT-qPCR and western blot analyses were performed. The results
demonstrated that the expression of MTDH was increased in SKOV3
cells compared with that in HOSEpiC cells (P<0.05; Fig. 1). These results suggested that MTDH
is associated with ovarian cancer.
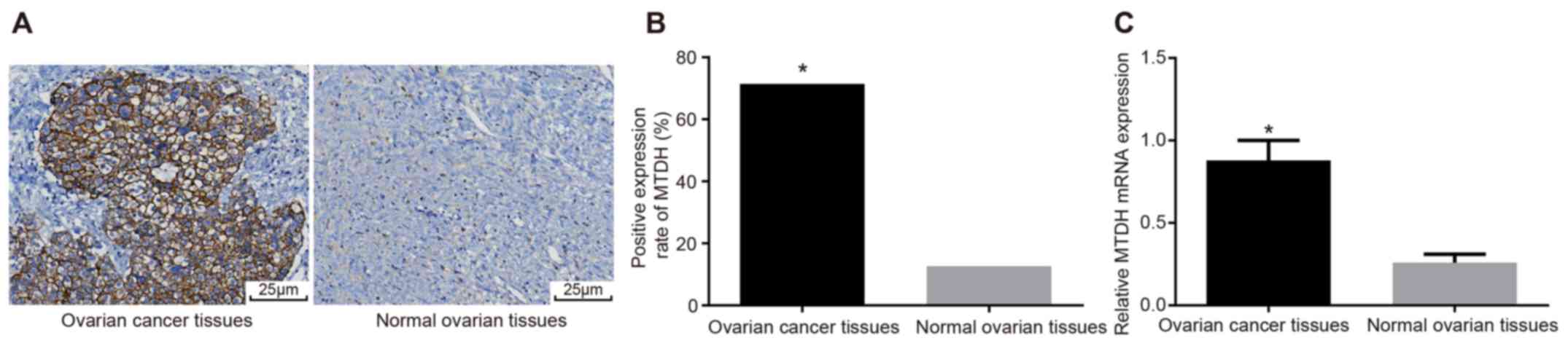
MTDH is expressed at high levels in
ovarian cancer tissues
Subsequently, the expression of MTDH in ovarian
cancer tissues was examined using immunohistochemistry and RT-qPCR
analysis. The cytoplasm or cell membrane was stained brown-yellow
in positive cells, indicating MTDH expression. The positive rate of
MTDH expression in ovarian cancer tissues was 71.43%, compared with
12.64% in normal ovarian tissues (P<0.05; Table I). More positive cells were
expressed in the ovarian cancer tissues than in the normal ovarian
tissues (P<0.05; Fig. 2A and
B). The RT-qPCR results demonstrated that the expression of
MTDH was significantly elevated in ovarian cancer tissues compared
with normal ovarian tissues (P<0.05; Fig. 2C). The association between the
expression of MTDH and clinicopathological features in ovarian
cancer tissues is shown in Table
II; the results suggested that the expression of MTDH was
associated with clinical stage, pathological grade, histological
grade and lymph node metastasis. No significant differences were
observed between histological subtype and age (Table II).
 | Table IExpression of MTDH in ovarian cancer
tissues and normal ovarian tissues. |
Table I
Expression of MTDH in ovarian cancer
tissues and normal ovarian tissues.
| Group | n | MTDH expression
| Positive rate
(%) | P-value |
|---|
| Positive | Negative |
|---|
| Normal ovarian
tissues | 277 | 35 | 242 | 12.64 | 0.001 |
| Ovarian cancer
tissues | 273 | 195 | 78 | 71.43 | |
 | Table IICorrelation between the expression of
MTDH and the clinicopathological features of ovarian cancer. |
Table II
Correlation between the expression of
MTDH and the clinicopathological features of ovarian cancer.
| Clinicopathological
factor | n | MTDH expression
| χ2
value | P-value |
|---|
| Positive | Negative |
|---|
| Histological
classification | | | | | |
| Serous
cystadenocarcinoma | 124 | 90 | 34 | 1.982 | 0.576 |
| Mucinous
cystadenocarcinoma | 82 | 55 | 27 | | |
| Endometrioid
carcinoma | 38 | 30 | 8 | | |
| Epithelial
carcinoma | 29 | 20 | 9 | | |
| FIGO stage | | | | | |
| I, II | 113 | 54 | 59 | 52.800 | <0.001 |
| III | 160 | 141 | 19 | | |
| Grade | | | | | |
| I, II | 109 | 52 | 57 | 50.030 | <0.001 |
| III | 164 | 143 | 21 | | |
| Histology | | | | | |
| Poor
differentiation | 171 | 146 | 25 | 43.650 | <0.001 |
| Middle or high
differentiation | 102 | 49 | 53 | | |
| Age (years) | | | | | |
| ≥40 | 145 | 98 | 47 | 2.237 | 0.135 |
| <40 | 128 | 97 | 31 | | |
| Lymph node
metastasis | | | | | |
| No | 101 | 47 | 54 | 48.680 | <0.001 |
| Yes | 172 | 148 | 24 | | |
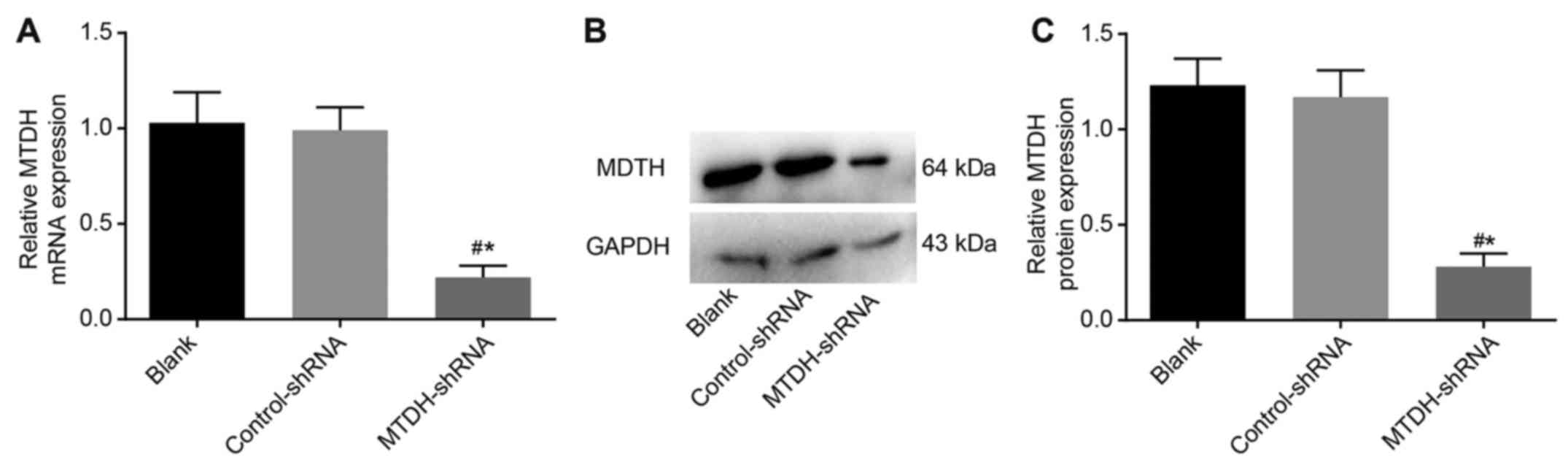
MTDH silencing significantly reduces the
expression of MTDH
RT-qPCR and western blot analyses were performed to
evaluate the mRNA and protein expression of MTDH in SKOV3 cells
following transfection in each group. Compared with the blank and
control-shRNA groups, SKOV3 cells in the MTDH-shRNA group had
decreased mRNA and protein levels of MTDH (P<0.05), whereas no
significant differences in expression were observed in the blank
and control-shRNA groups (P>0.05). This suggested successful
shRNA-mediated inhibition of MTDH in theSKOV3 cells (Fig. 3).
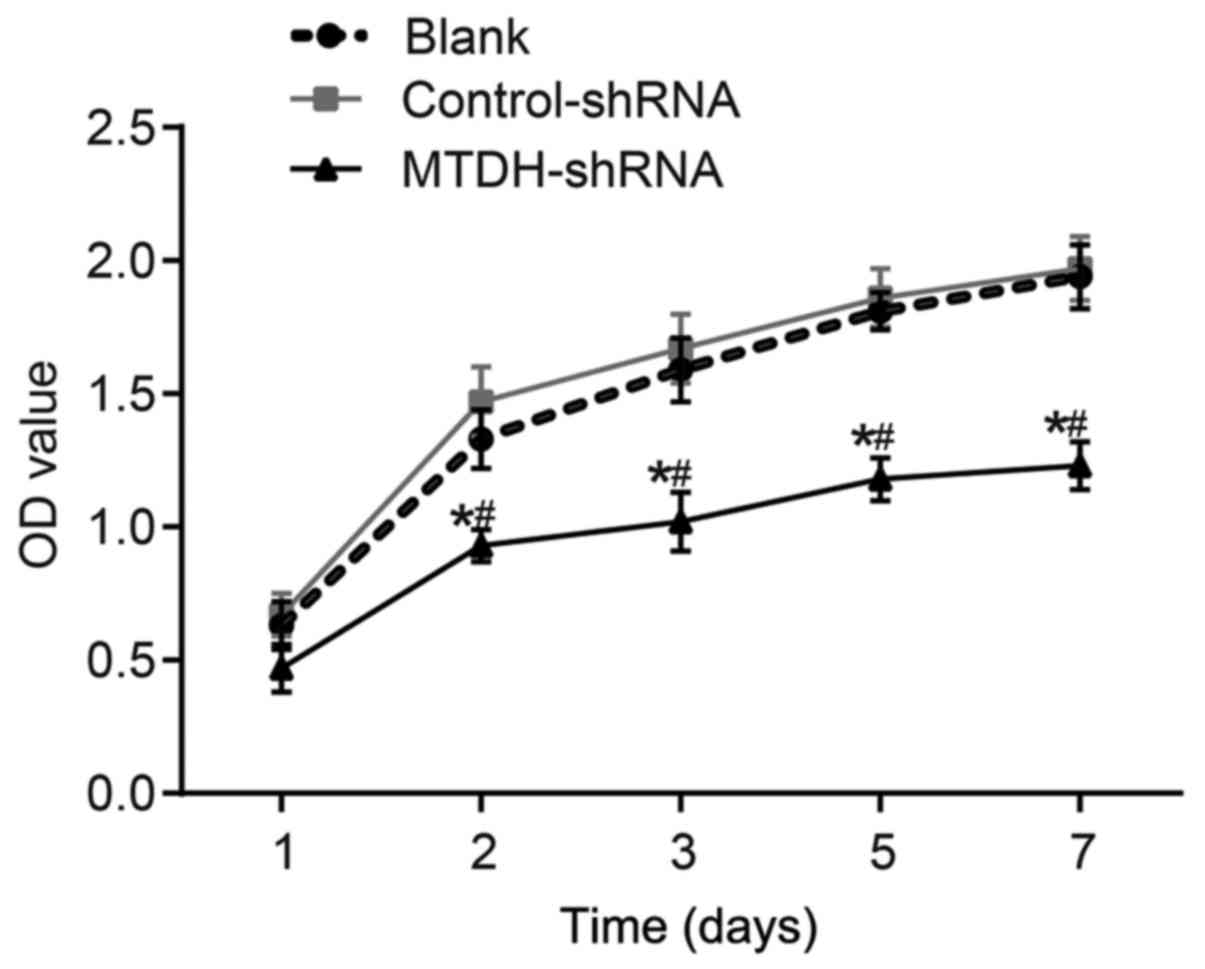
MTDH silencing significantly suppresses
ovarian cancer growth
The viability of SKOV3 cells was assessed using an
MTT assay (Fig. 4). The respective
OD values in the blank, control-shRNA and MTDH-shRNA groups were
0.63±0.09, 0.67±0.08 and 0.40±0.04 at 1 day, 1.33±0.11, 1.47±0.13
and 0.93±0.06 at 2 days, and 1.59±0.12, 1.67±0.13 and 1.02±0.11 at
3 days of tumor growth. The respective OD values in the blank,
control-shRNA and MTDH-shRNA groups were 1.81±0.07, 1.86±0.11 and
1.18±0.08 at 5 days, and 1.94±0.12, 1.97±0.12 and 1.23±0.09 at 7
days of tumor growth. The cell growth in the MTDH-shRNA group was
inhibited compared with that in the blank and control-shRNA groups
at each time point following transfection (P<0.05), whereas no
significant differences were observed in the blank and
control-shRNA groups (P>0.05). These results suggested that the
shRNA-mediated inhibition of MTDH suppressed the proliferation of
SKOV3 cells.
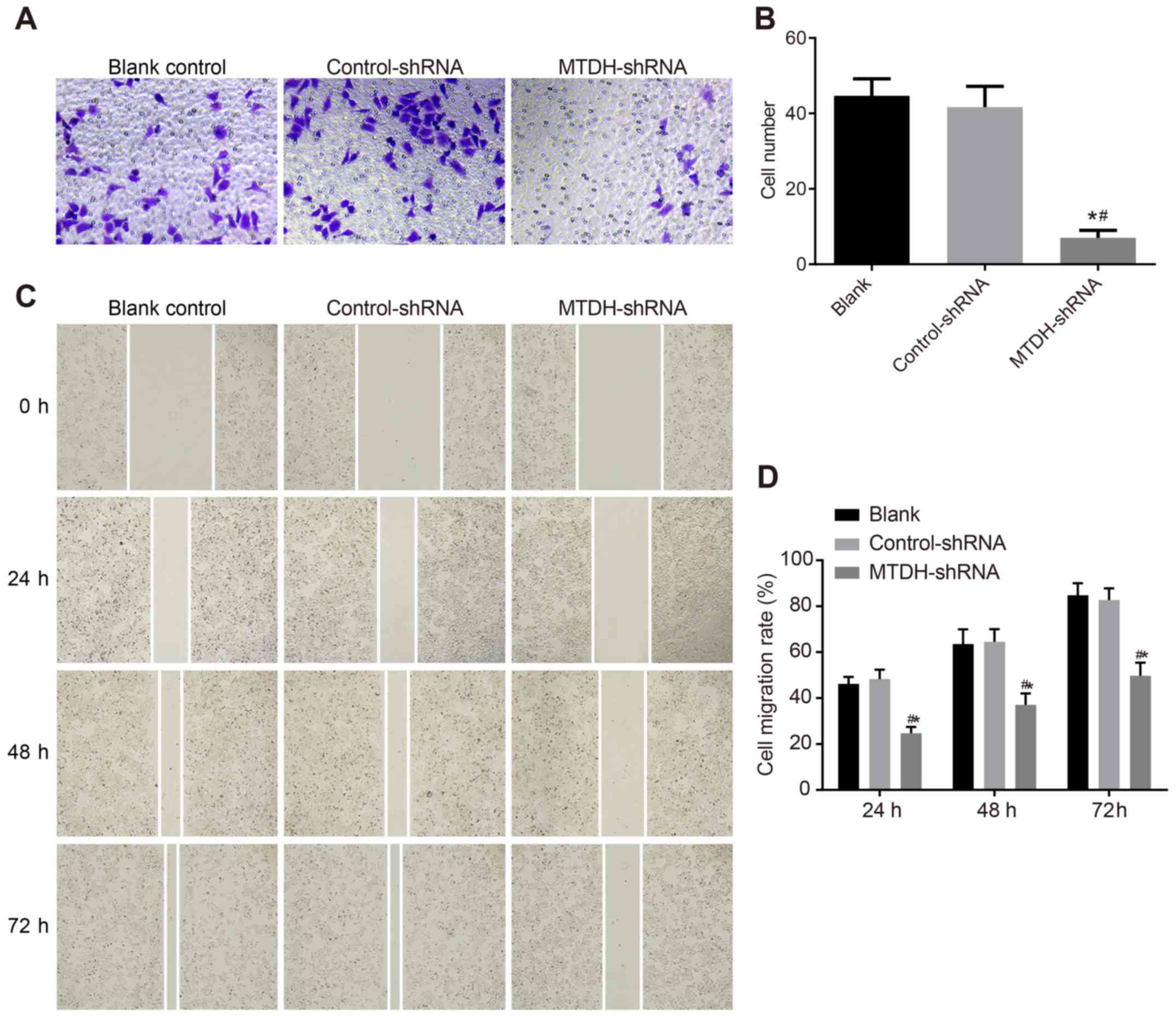
MTDH-shRNA suppresses the migration and
invasion of ovarian cancer SKOV3 cells
The effects of MTDH-shRNA transfection on ovarian
cancer cell migration and invasion were determined using Transwell
and wound-healing assays. The Transwell results revealed no
significant differences in the number of cells penetrating the
membrane between the blank (44.67±4.51) and control-shRNA
(41.67±5.51) groups (P>0.05). Compared with the blank and
control-shRNA groups, a significantly lower number of cells
(7.00±2.00) penetrated the membrane in the MTDH-shRNA group
(P<0.05; Fig. 5A and B). The
wound-healing assay revealed that cell migration was significantly
reduced at each time point in the MTDH-shRNA group compared with
migration in the blank and control-shRNA groups (P<0.05;
Fig. 5C and D). These results
suggested that shRNA-mediated inhibition of MTDH suppressed the
migration and invasion of SKOV3 cells.
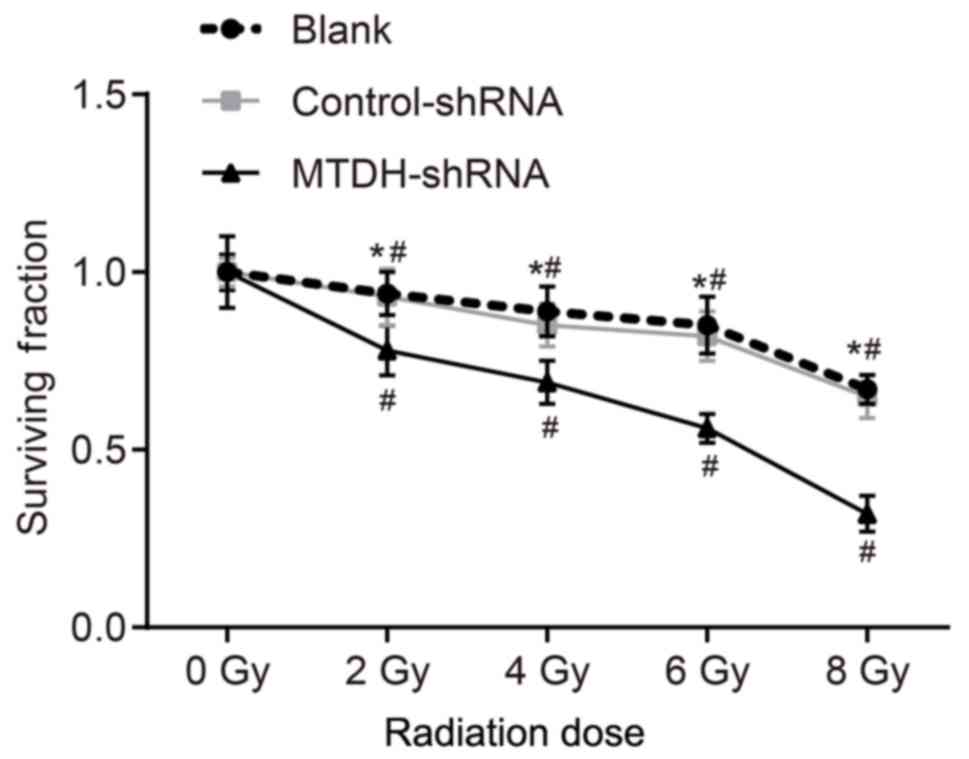
MTDH gene silencing increases the
radiosensitivity of SKOV3 cells
In order to analyze the effect of MTDH gene
silencing on the radiosensitivity of SKOV3 cells, a
colony-formation assay was performed. The colony-formation assay is
regarded as a standard measurement to evaluate the effect of
external radiotherapy on tumors by measuring the reproductive
integrity of tumor cells without proliferation (21). The cell survival fraction was
decreased in the blank, control-shRNA transfection and MTDH-shRNA
groups depending on the radiation dose (P<0.05). The surviving
fraction was lower in the MTDH-shRNA group compared with that in
the blank and control-shRNA groups (all P<0.05), whereas no
significant differences in the cell survival fraction were observed
between the blank and control-shRNA groups (P>0.05; Fig. 6). Taken together, these results
indicated that MTDH silencing increased the sensitivity of the
SKOV3 cells to X-ray radiation.
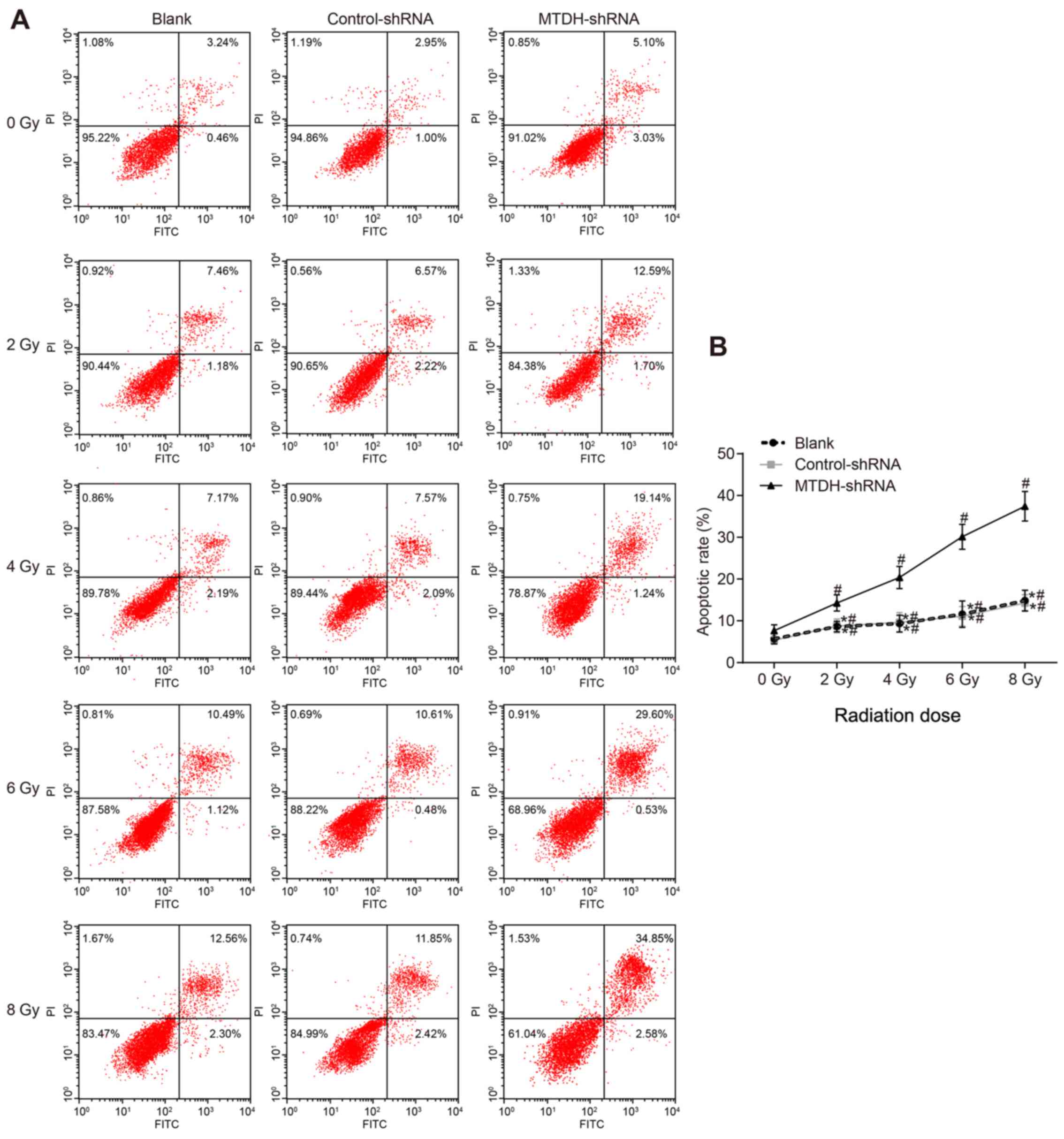
MTDH silencing increases the apoptosis of
SKOV3 cells following radiotherapy
Flow cytometry was used to assess the apoptotic rate
following irradiation (Fig. 7).
The variance analysis indicated that apoptosis was increased with
increasing doses of radiation in the blank, control-shRNA and
MTDH-shRNA groups (P<0.05). The apoptotic rate was significantly
increased in the MTDH-shRNA group compared with that in the blank
and control-shRNA groups (P<0.05), whereas no significant
difference was observed between the blank and control-shRNA groups
(P>0.05). These results suggested that MTDH silencing enhanced
radiotherapy-induced apoptosis of SKOV3 cells.
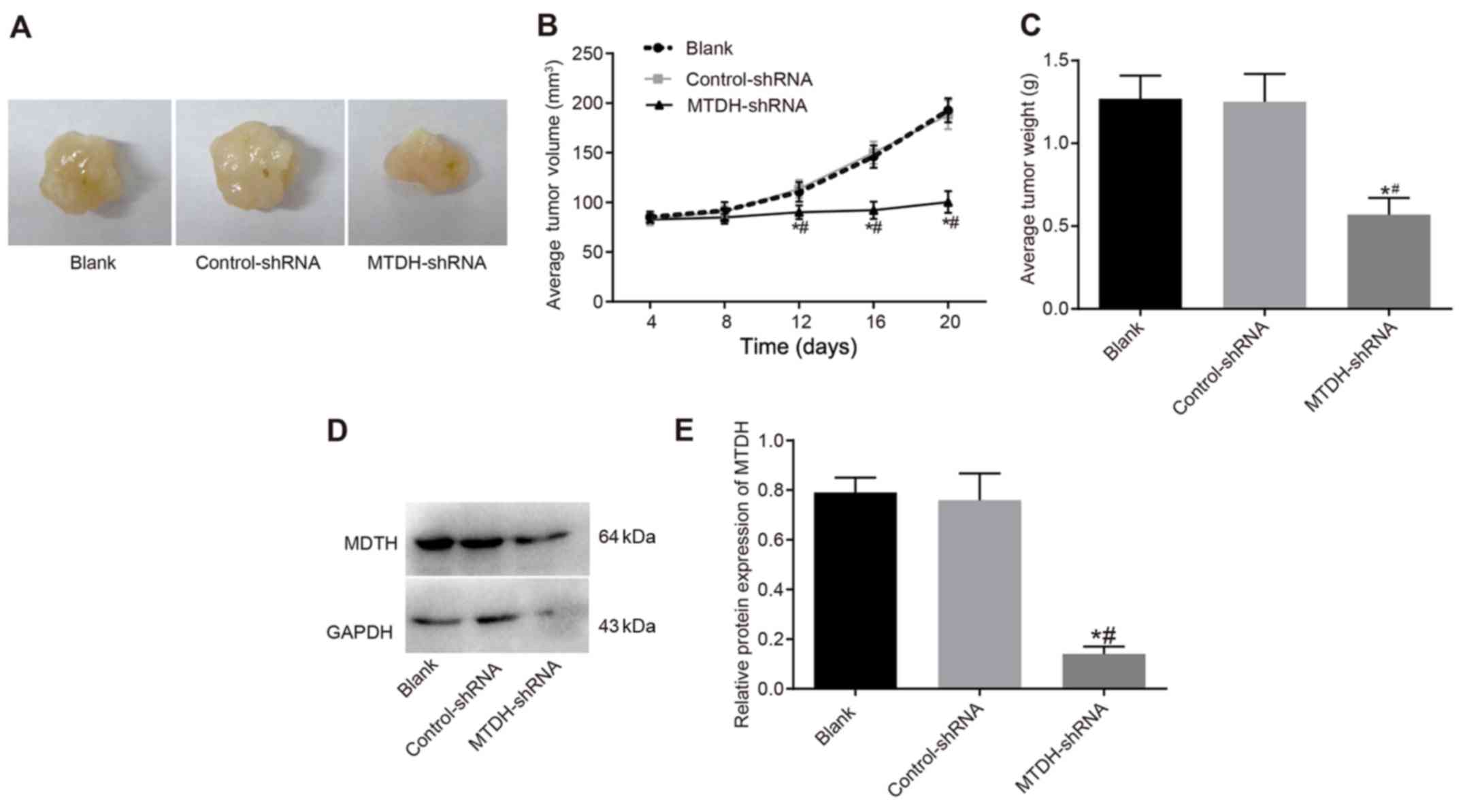
MTDH silencing inhibits tumor growth and
enhances the radiosensitivity of SKOV3 cells
A nude mouse xenograft model was utilized to assess
differences in tumor growth following inoculation with SKOV3 cells.
The growth of xenograft tumors was observed in nude mice as the
efficacy of radiotherapy. Slow tumor growth was observed for 8 days
prior to radiotherapy in the blank, control-shRNA and MTDH-shRNA
groups, and no significant differences in tumor volume were
observed (P>0.05). On day 12 of radiotherapy, the tumor volume
was 110.51±9.73 and 113.72±8.96 mm3 in the blank and
control-shRNA groups, respectively, with no significant differences
observed (P>0.05). In the MTDH-shRNA group, tumor volume was
90.13±6.92 mm3, indicating a significant decrease in
tumor growth rate compared with that in the blank and control-shRNA
groups (P<0.05). Radiotherapy was continued, and the tumor
volume in the MTDH-shRNA group decreased compared with that in the
blank and control-shRNA groups (P<0.05). The mice were
sacrificed at the end of radiotherapy and the tumor tissues were
weighed. The mean tumor weights in the blank, control-shRNA and
MTDH-shRNA groups were 1.27±0.14, 1.25±0.17 and 0.57±0.10 g,
respectively. The mean tumor weight was decreased in the MTDH-shRNA
group compared with that in the blank and control-shRNA groups
(P<0.05), however, no significant differences were observed
between the blank and control-shRNA groups (P>0.05; Fig. 8A–C). These results suggested that
MTDH silencing enhanced the radiosensitivity of the nude mouse
xenograft models and effectively delayed tumor growth. Furthermore,
the protein level of MTDH was reduced in the MTDH-shRNA group
compared with that in the blank and control-shRNA groups
(P<0.05), whereas no such difference was observed between the
blank and control-shRNA groups (P>0.05; Fig. 8D and E).
Discussion
Ovarian cancer is a type of cancer that affects the
female reproductive tract and is the fifth-leading cause of
cancer-associated mortality among women (22). In the last decade, MTDH has been
identified as an important oncogene and is a valuable prognostic
marker in patients with various types of cancer. It has been
reported that MTDH is localized in the cell membrane, cytoplasm,
endoplasmic reticulum, nucleus and nucleolus (23-25).
The application of MTDH gene silencing to overcome radioresistance
in ovarian cancer is of great interest. The present study
investigated the effects of MTDH silencing on the radiosensitivity,
proliferation, migration, invasion and apoptosis of SKOV3 ovarian
cancer cells. The results indicated that MTDH silencing inhibited
cell proliferation, migration and invasion, and promoted cell
apoptosis and radiosensitivity in vitro and in
vivo.
The expression of MTDH was assessed in ovarian
cancer and normal ovarian tissues, and in ovarian cancer and normal
cell lines. The results demonstrated that the expression of MTDH
was high in ovarian cancer tissues compared with that in normal
tissues, and that transfection with MTDH-shRNA reduced the
expression of MTDH in the SKOV3 ovarian cancer cells. The
association between MTDH and oncology has been investigated in
several types of cancer, providing an insight into factors
affecting prognoses (26-28). It has been reported that MTDH is
expressed in human ovarian cancer tissues and is negatively
correlated with the overall survival rate of patients (29). The upregulation of MTDH is
frequently observed in various types of cancer, including liver,
brain and breast cancer, and contributes to poor prognoses
(28,30). Consistent with the results of the
present study, Zhou et al (31) reported that there was minimal or no
MTDH immunore-activity in normal tissues. Similarly, the
overexpression of MTDH is associated with the prognosis of patients
with metastatic ovarian cancer, and MTDH staining was increased in
chemoresistant patients compared with chemosensitive patients
(32,33). Hu et al (27) demonstrated that lung metastasis was
reduced following MTDH knockdown
The results of the MTT, Transwell and wound-healing
assays in the present study revealed that cell growth and migration
were inhibited in the MTDH-shRNA group compared with the blank and
control-shRNA groups. MTDH is potentially a key regulator of tumor
malignancy and is associated with the progression of certain types
of cancer (34). A study by Hu
et al (27) indicated that
the therapeutic targeting of MTDH inhibited tumor growth and
inhibited metastasis, which is consistent with the results of the
present study. The expression of MTDH during developmental and
differentiation processes is mediated by the phosphoinositide
3-kinase/Akt, nuclear factor-κB and Wnt/β-catenin signaling
pathways (26,27). The proliferation and invasion of
cancer cells can be enhanced by activating these pathways (27). Furthermore, the development of
hepatocellular carcinoma can be delayed by the microRNA-375-induced
downregulation of MTDH (30). It
has been reported that MTDH is involved in a number of
physiological and pathological tumors, including brain tumors and
neuroblastomas, whereas MTDH interference suppresses the
proliferation and migration of these cells (35,36).
RNA interference contributes to sequence-specific gene silencing
through double-stranded RNAs, which inhibit gene expression by
degrading a specific mRNA (37).
MTDH silencing in the present study was able to inhibit the
proliferation and metastasis of SKOV3 ovarian cancer cells.
The colony-formation assay and flow cytometry
results revealed that the radiosensitivity and apoptotic rate of
SKOV3 cells were enhanced following MTDH silencing. Radioresistance
is a common issue that results in treatment failure, and it has
been reported that radioresistance is mediated by tumor-related
genes affecting cellular processes (38,39).
A number of factors are associated with cancer risk and
radio-sensitivity, including alterations in DNA repair, cell cycle
or apoptotic pathways (40).
Previous evidence has revealed that the downregulation of MTDH can
reduce the viability, colony formation and invasion of U87 human
glioma cells and 9L rat gliosarcoma cells (41,42).
In addition, MTDH knockdown was shown to reduce radioresistance in
colon cancer cell lines following irradiation (43). Cell viability and apoptosis were
measured following treatment with specific interfering RNA, and it
was reported that the downregulation of MTDH had no significant
effects on cell cycle distribution, rather reducing cell viability
via apoptosis (44). MTDH acts by
interfering with protein translation via mRNA binding in the
cytoplasm or by loading other mRNAs to the polysome (29). A study by Chang et al
(45) revealed that cell apoptosis
was significantly elevated in MTDH-knockdown groups compared with
negative control groups. These results were supported by the in
vivo experiment with nude mouse xenograft models in the present
study, in which MTDH silencing enhanced the therapeutic efficacy of
radiation and effectively inhibited tumor growth.
In conclusion, the results of the present study
suggested that MTDH is expressed at a high level in patients with
ovarian cancer. MTDH silencing inhibited the proliferation and
metastasis of SKOV3 ovarian cancer cells and simultaneously induced
apoptosis. Furthermore, MTDH silencing led to increased
radio-sensitivity of SKOV3 cells. Therefore, MTDH gene silencing
may serve as a novel therapeutic strategy for the management of
ovarian cancer. However, the effects of X-ray radiation on the
expression of p53 and MDTH have not been investigated, and are to
be the focus of future investigations. Gene expression is affected
by various factors, and further investigations are required to
confirm the aforementioned conclusions.
Acknowledgments
The authors are grateful to the reviewers for their
critical comments on this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JC conceived and designed the study. YJ and YMJ
collated the data, designed and developed the database, performed
data analyses and produced the initial draft of the manuscript. ZHJ
obtained the results and validated them, YZ reviewed the results
and discussions, ZHI and YZ revised the figures and tables and
contributed to the revision of the manuscript. All authors have
read and approved the final submitted manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Hospital of Jilin University (grant no.
201112006). Informed consent was obtained from patients or their
guardians in accordance with the Declaration of Helsinki. The
animal experiment was approved by the Animal Ethics Committee of
the Second Hospital of Jilin University (no. 201201003), and the
experiment strictly followed the National Institutes of Health
guide for the care and use of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meng Y, Hu J, Chen Y, Yu T and Hu L:
Silencing MARCH1 suppresses proliferation, migration and invasion
of ovarian cancer SKOV3 cells via downregulation of NF-κB and
Wnt/β-catenin pathways. Oncol Rep. 36:2463–2470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan Y, Feng Q, Sun X, Xue M, Jiang N and
Deng X: Effects of methylseleninic acid on cisplatin-resistant
ovarian cancer cells (SKOV3/DDP) and the mechanisms. Zhong Nan Da
Xue Xue Bao Yi Xue Ban. 41:1305–1311. 2016.Chinese.
|
|
3
|
Kaur A, Sultan SH, Murugaiah V, Pathan AA,
Alhamlan FS, Karteris E and Kishore U: Human C1q induces apoptosis
in an ovarian cancer cell line via tumor necrosis factor pathway.
Front Immunol. 7:5992016. View Article : Google Scholar
|
|
4
|
Musto A, Grassetto G, Marzola MC, Rampin
L, Chondrogiannis S, Maffione AM, Colletti PM, Perkins AC, Fagioli
G and Rubello D: Management of epithelial ovarian cancer from
diagnosis to restaging: An overview of the role of imaging
techniques with particular regard to the contribution of 18F-FDG
PET/CT. Nucl Med Commun. 35:588–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He M, Sun HG, Hao JY, Li YL, Yu JK, Yan
YY, Zhao L, Li N, Wang Y, Bai XF, et al: RNA interference-mediated
FANCF silencing sensitizes OVCAR3 ovarian cancer cells to
adriamycin through increased adriamycin-induced apoptosis dependent
on JNK activation. Oncol Rep. 29:1721–1729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang NN, Zhao LJ, Wu LN, He MF, Qu JW,
Zhao YB, Zhao WZ, Li JS and Wang JH: Mechanistic analysis of
taxol-induced multidrug resistance in an ovarian cancer cell line.
Asian Pac J Cancer Prev. 14:4983–4988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Concin N, Zeillinger C, Stimpfel M,
Schiebel I, Tong D, Wolff U, Reiner A, Leodolter S and Zeillinger
R: p53-dependent radio-resistance in ovarian carcinoma cell lines.
Cancer Lett. 150:191–199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Y, Lu Y and Lu B: MicroRNA and long
non-coding RNA in ovarian carcinoma: Translational insights and
potential clinical applications. Cancer Invest. 34:465–476. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cocco E, Deng Y, Shapiro EM, Bortolomai I,
Lopez S, Lin K, Bellone S, Cui J, Menderes G, Black JD, et al:
Dual-targeting nanoparticles for in vivo delivery of suicide genes
to chemotherapy-resistant ovarian cancer cells. Mol Cancer Ther.
16:323–333. 2017. View Article : Google Scholar :
|
|
11
|
Shi X and Wang X: The role of MTDH/AEG-1
in the progression of cancer. Int J Clin Exp Med. 8:4795–4807.
2015.PubMed/NCBI
|
|
12
|
Wan L, Lu X, Yuan S, Wei Y, Guo F, Shen M,
Yuan M, Chakrabarti R, Hua Y, Smith HA, et al: MTDH-SND1
interaction is crucial for expansion and activity of
tumor-initiating cells in diverse oncogene- and carcinogen-induced
mammary tumors. Cancer Cell. 26:92–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan L and Kang Y: Pleiotropic roles of
AEG-1/MTDH/LYRIC in breast cancer. Adv Cancer Res. 120:113–134.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo F, Wan L, Zheng A, Stanevich V, Wei Y,
Satyshur KA, Shen M, Lee W, Kang Y and Xing Y: Structural insights
into the tumor-promoting function of the MTDH-SND1 complex. Cell
Reports. 8:1704–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan L, Qin H, Piao Y, Liu Z, Han Y, Song F
and Xie X: Expression and clinical significance of MTDH and VEGF in
triple-negative breast cancer. Zhonghua Zhong Liu Za Zhi.
37:827–832. 2015.Chinese.
|
|
16
|
Jia X, Shan C, Xu O and Wang J: Expression
and clinical significance of MTDH, HIF-1α and TKTL1 in laryngeal
carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
29:2133–2138. 2015.Chinese.
|
|
17
|
Zhao Y, Moran MS, Yang Q, Liu Q, Yuan C,
Hong S and Kong B: Metadherin regulates radioresistance in cervical
cancer cells. Oncol Rep. 27:1520–1526. 2012.PubMed/NCBI
|
|
18
|
Gu X, Wang C, Wang X, Ma G, Li Y, Cui L,
Chen Y, Zhao B and Li K: Efficient inhibition of human glioma
development by RNA interference-mediated silencing of PAK5. Int J
Biol Sci. 11:230–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yiwei T, Hua H, Hui G, Mao M and Xiang L:
HOTAIR interacting with MAPK1 regulates ovarian cancer skov3 cell
proliferation, migration, and invasion. Med Sci Monit.
21:1856–1863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanter M, Turan G, Usta C, Usta A, Esen
HH, Tavlı L, Celik C, Demirkol Y and Kanter B: Survivin and cycline
D1 expressions are associated with malignant potential in mucinous
ovarian neoplasms. J Mol Histol. 47:145–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steinbichler TB, Alshaimaa A, Maria MV,
Daniel D, Herbert R, Jozsef D and Ira-Ida S: Epithelial-mesenchymal
crosstalk induces radioresistance in HNSCC cells. Oncotarget.
9:3641–3652. 2017.
|
|
22
|
No authors listed. Summaries for patients.
Screening for ovarian cancer: U.S. Preventive Services Task Force
reaffirmation recommendation statement. Ann Intern Med. 157:I–56.
2012.
|
|
23
|
Lee SG, Kang DC, DeSalle R, Sarkar D and
Fisher PB: AEG-1/MTDH/LYRIC, the beginning: Initial cloning,
structure, expression profile, and regulation of expression. Adv
Cancer Res. 120:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan C, Li X, Yan S, Yang Q, Liu X and
Kong B: The MTDH (−470G>A) polymorphism is associated with
ovarian cancer susceptibility. PLoS One. 7:e515612012. View Article : Google Scholar
|
|
25
|
Emdad L, Hu B, Das SK, Sarkar D and Fisher
PB: AEG-1-AKT2: A novel complex controlling the aggressiveness of
glioblastoma. Mol Cell Oncol. 2:e9950082015. View Article : Google Scholar
|
|
26
|
Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche
H, Sarkar D and Fisher PB: Astrocyte elevated gene-1 (AEG-1)
functions as an oncogene and regulates angiogenesis. Proc Natl Acad
Sci USA. 106:21300–21305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC,
Bruce JN, Volsky DJ and Fisher PB: Astrocyte elevated gene-1:
Recent insights into a novel gene involved in tumor progression,
metastasis and neurodegeneration. Pharmacol Ther. 114:155–170.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haug S, Schnerch D, Halbach S, Mastroianni
J, Dumit VI, Follo M, Hasenburg A, Köhler M, Dierbach H, Herzog S,
et al: Metadherin exon 11 skipping variant enhances metastatic
spread of ovarian cancer. Int J Cancer. 136:2328–2340. 2015.
View Article : Google Scholar
|
|
30
|
He XX, Chang Y, Meng FY, Wang MY, Xie QH,
Tang F, Li PY, Song YH and Lin JS: MicroRNA-375 targets AEG-1 in
hepatocellular carcinoma and suppresses liver cancer cell growth in
vitro and in vivo. Oncogene. 31:3357–3369. 2012. View Article : Google Scholar
|
|
31
|
Zhou B, Yang J, Shu B, Liu K, Xue L, Su N,
Liu J and Xi T: Overexpression of astrocyte-elevated gene-1 is
associated with ovarian cancer development and progression. Mol Med
Rep. 11:2981–2990. 2015. View Article : Google Scholar
|
|
32
|
Li C, Chen K, Cai J, Shi QT, Li Y, Li L,
Song H, Qiu H, Qin Y and Geng JS: Astrocyte elevated gene-1: A
novel independent prognostic biomarker for metastatic ovarian
tumors. Tumour Biol. 35:3079–3085. 2014. View Article : Google Scholar
|
|
33
|
Sarkar D and Fisher PB: AEG-1/MTDH/LYRIC:
Clinical significance. Adv Cancer Res. 120:39–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Y and Li LP: Progress of cancer
research on astrocyte elevated gene-1/Metadherin (Review). Oncol
Lett. 8:493–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Zhang W, Zhu X and Wang Y, Mao X,
Xu X and Wang Y: Upregulation of AEG-1 Involves in Schwann Cell
Proliferation and Migration After Sciatic Nerve Crush. J Mol
Neurosci. 60:248–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao Y, Gu X, Liu H, Wu G, Yuan D, Yang X
and Song Y: Metadherin regulates proliferation and metastasis via
actin cyto-skeletal remodelling in non-small cell lung cancer. Br J
Cancer. 111:355–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park JB, Chang DG, Oh SY and Park EY:
Effect of RNA Interference-Mediated Suppression of p75 on the
Viability of Rat Notochordal Cells. Asian Spine J. 10:985–992.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu R, Li W, Xu Y, Wan J and Zhang Z:
Upregulation of BTG1 enhances the radiation sensitivity of human
breast cancer in vitro and in vivo. Oncol Rep. 34:3017–3024. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huerta S, Gao X, Dineen S, Kapur P, Saha D
and Meyer J: Role of p53, Bax, p21, and DNA-PKcs in radiation
sensitivity of HCT-116 cells and xenografts. Surgery. 154:143–151.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hornhardt S, Rößler U, Sauter W,
Rosenberger A, Illig T, Bickeböller H, Wichmann HE and Gomolka M:
Genetic factors in individual radiation sensitivity. DNA Repair
(Amst). 16:54–65. 2014. View Article : Google Scholar
|
|
41
|
Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK,
Dash R, Yacoub A, Fuller CE, Shah K, Dent P, et al: Astrocyte
elevated gene-1: A novel target for human glioma therapy. Mol
Cancer Ther. 9:79–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Awasthi S, Singhal SS, Awasthi YC, Martin
B, Woo JH, Cunningham CC and Frankel AE: RLIP76 and cancer. Clin
Cancer Res. 14:4372–4377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gnosa S, Zhang H, Brodin VP, Carstensen J,
Adell G and Sun XF: AEG-1 expression is an independent prognostic
factor in rectal cancer patients with preoperative radiotherapy: A
study in a Swedish clinical trial. Br J Cancer. 111:166–173. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nikpour M, Emadi-Baygi M, Fischer U,
Niegisch G, Schulz WA and Nikpour P: MTDH/AEG-1 contributes to
central features of the neoplastic phenotype in bladder cancer.
Urol Oncol. 32:670–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang Y, Li B, Xu X, Shen L, Bai H, Gao F,
Zhang Z and Jonas JB: Lentivirus-mediated knockdown of astrocyte
elevated gene-1 inhibits growth and induces apoptosis through MAPK
pathways in human retinoblastoma cells. PLoS One. 11:e01487632016.
View Article : Google Scholar : PubMed/NCBI
|