Introduction
Breast cancer is the most frequently diagnosed
malignancy in women (1). Despite
improved methods of early detection and diagnosis, as well as
usually effective clinical management, breast cancer is the second
leading cause of cancer-related mortality due to recurrent
metastatic disease (2). Of note,
90% of breast cancer-related deaths are not due to the primary
tumor, but due to metastatic disease. Current models of metastasis
support the hypothesis that cells can detach from the primary tumor
and move to distant sites via the blood vessels and lymphatic
system (3). For this reason, new
methods are necessary for the detection and treatment of the
residual tumor cells in order to prevent metastasis (4). A large number of studies have
documented disseminated tumor cells in the bone marrow or
circulating tumor cells in the peripheral blood from patients with
most types of epithelial cancer (5–10).
The detection and characterization of tumor cells in the peripheral
blood have various potential applications in oncology (11). In the early stage of the disease,
the enumeration and characterization of circulating tumor cells can
potentially help to monitor the effect of systemic therapy, detect
an early relapse of malignancy, predict the risk for metastatic
disease and, thus, help to improve prognosis (12,13).
In the more advanced stages of the disease,
circulating tumor cells may provide prognostic information and aid
the physician in monitoring the response to treatment (14,15).
Moreover, circulating tumor cells may represent characteristics of
the residual tumor, inform about the sensitivity to anticancer
drugs and, thus, can be used for a personalized anticancer therapy
(16). The phenotypic
characterization of tumor cells circulating in the blood has the
potential to improve the current understanding of metastasis
formation and immune modulation. They can be used as a real-time
liquid biopsy in a variety of human cancers and may play a major
role in helping to administer a targeted therapy.
B7-H3 is a surface antigen against which a targeted
therapy can be envisioned. It is a type I transmembrane protein and
an important immune checkpoint member of the B7 ligand family, for
which the receptor(s) have not yet been identified. Its expression
is induced on immune cells, particularly antigen-presenting cells
(17). It is assumed that B7-H3 is
involved in the inhibition of T-cells. On the other hand, it has
been found that B7-H3 has also stimulatory immunological functions
(18,19). B7-H3 protein has been detected in
several cell lines (18,20) and numerous studies have described
B7-H3 expression in human malignancies (17,21–27).
Apart from immune evasion, B7-H3 plays a role in cancer
progression, including invasion and migration, angiogenesis and
gene regulation (19). The
proportion of the expression is associated with both a negative
prognosis and a poor clinical outcome in patients (17). The blocking immune checkpoints,
such as CTLA-4, programmed cell death protein 1 (PD-1) and its
ligand, PD-L1, has shown clinical benefit in patients with
different tumor entities. Due to its comparable role in immune
evasion, B7-H3 has become an interesting target for novel
immunotherapeutic treatments (17,28–30).
Ki-67 (also known as MKI67) is a cellular marker
that is tightly linked to the cell cycle. The fact that Ki-67 is
universally expressed among proliferating cells and is absent in
quiescent cells has led to the further evaluation of Ki-67 as a
marker of proliferation (31).
Although little is known about the exact function of the protein in
dividing cells, Ki-67 is expressed during the G1, S and G2 phases
of the cell cycle with a peak during mitosis and it is absent in
the G0 phase (32,33). There is a strong association
between the proportion of Ki-67-positive cells and tumor size,
aggressiveness, the level of angiogenesis and the survival of
patients. Patients with breast cancer and a Ki-67 index >15%
have a poor prognosis associated with a shortened disease-free and
overall survival (34). On the
other hand numerous studies indicate a positive correlation between
the percentage of proliferating cells and the response to
preoperative treatment with chemotherapy. The higher the level of
Ki-67, the more pronounced the sensitivity of breast cancer to
neoadjuvant therapy (35). The
detection of Ki-67 on circulating epithelial tumor cells (CETCs)
may be clinically more informative than the examination of total
CETC numbers as it allows for the quantification of proliferative
and non-proliferative subpopulations among the CETCs.
The aim of this study was to evaluate B7-H3 and
Ki-67 expression on CETCs in breast cancer patients which may
contribute to a better understanding of the immune escape
mechanisms of these cells. Identifying the proliferative
subpopulation of CETCs may serve as a useful tool with which to
predict the aggressiveness of cancer.
Patients and methods
Peripheral blood (7.5 ml) from 50 breast cancer
patients in different stages of disease was drawn into blood count
tubes with ethylenediamine-tetra-acetic acid (EDTA) as an
anticoagulant and processed within 48 h of collection. Medical
records were reviewed for determination of ER/PR and HER2 status in
the primary tumor or metastatic biopsy upon diagnosis. The primary
tissue was processed in the corresponding hospitals according to
the ASCO-CAP guidelines. In parallel, healthy control blood samples
were collected from 12 male and 8 female donors aged between 20–40
years. The sampling of peripheral blood was carried out 6–12 weeks
after the end of standard therapy (tumor resection, adjuvant
chemotherapy and adjuvant radiotherapy). In patients with local or
distant recurrence, blood was collected prior to the treatment for
recurrent disease. All patients and healthy volunteers gave their
informed consent to participate in the study, which was approved by
the Ethics and Scientific Committees of the University of Jena
(Jena, Germany).
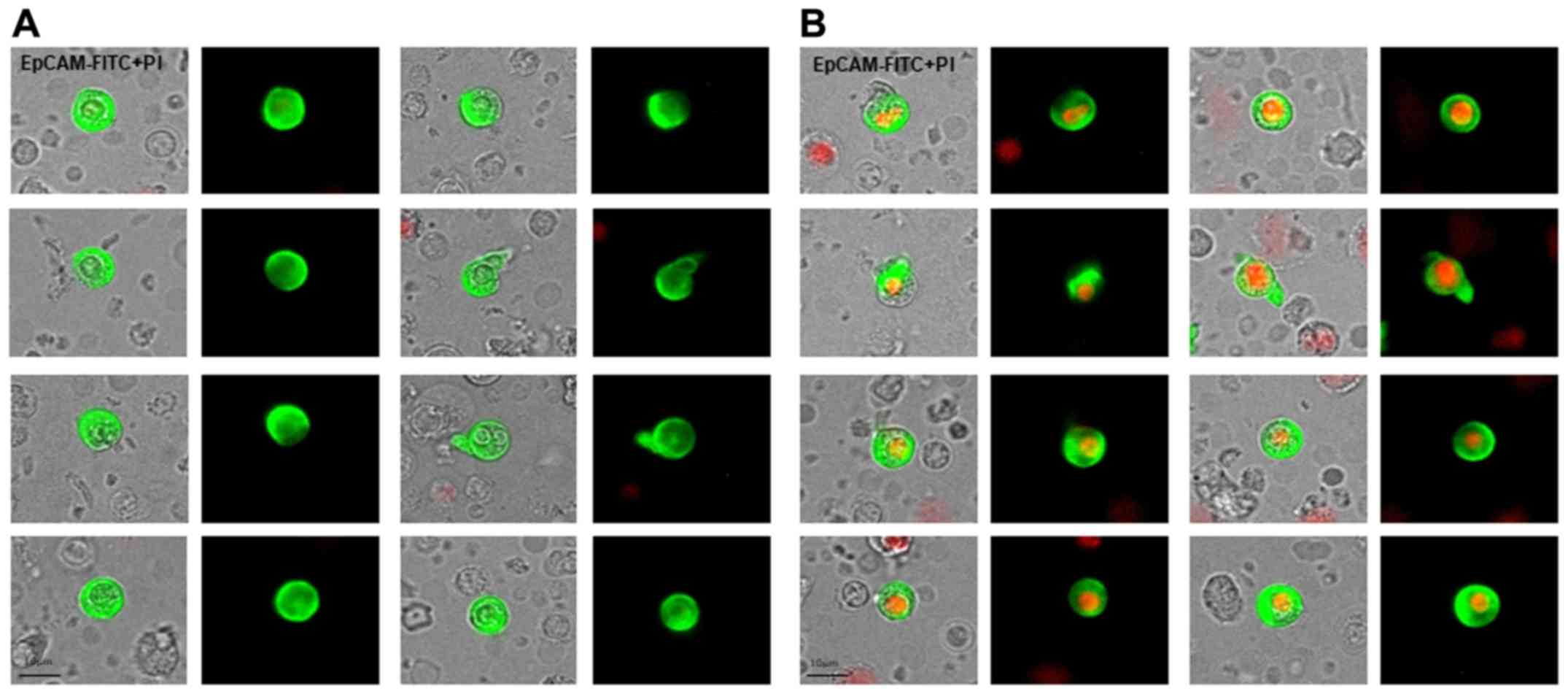
For CETC enumeration and further characterization,
the maintrac® approach was used, as reported previously
(36). Briefly, 1 ml blood was
subjected to red blood cell lysis using 15 ml of erythrocyte lysis
solution (Qiagen, Hilden, Germany) for 15 min at 4°C spun down at
700 × g and re-diluted in 500 ml of PBS-EDTA. Subsequently, 5
µl of fluoresceinisothiocyanate (FITC)-conjugated anti-human
epithelial cell adhesion molecule antibody (EpCAM, dilution 1:4,
clone HEA-125, cat. no. 130-113-203, Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany) at a final concentration of up to 107
cells/100 µl cell suspension were added and incubated for 15
min at 4°C. The corresponding isotypic control for EpCAM (Mouse
IgG1K FITC, Miltenyi Biotec GmbH) was used at the same final
concentration. The samples were subsequently diluted with 430
µl PBS-EDTA. A defined volume of the cell suspension and
propidium iodide (PI; Sigma-Aldrich, St. Louis, MO, USA) was
transferred to wells of ELISA plates (Greiner Bio-One, Monroe, NC,
USA). The analysis of red and green fluorescence of the cells was
performed using a Fluorescence Scanning Microscope, ScanR,
(Olympus, Tokyo, Japan), enabling the detection and relocation of
cells for the visual examination of EpCAM-positive cells. For data
analysis, we used the ScanR Analysis software (Olympus). Vital
CETCs were defined as EpCAM-positive cells, lacking in nuclear PI
staining and with intact morphology, and only these cells were
counted (Fig. 1). We used
fluorospheres (Flow-Check 770, Beckman Coulter, Brea, CA, USA) for
the daily verification of optical components and detectors of the
microscope, which are required to ensure the consistent analysis of
samples.
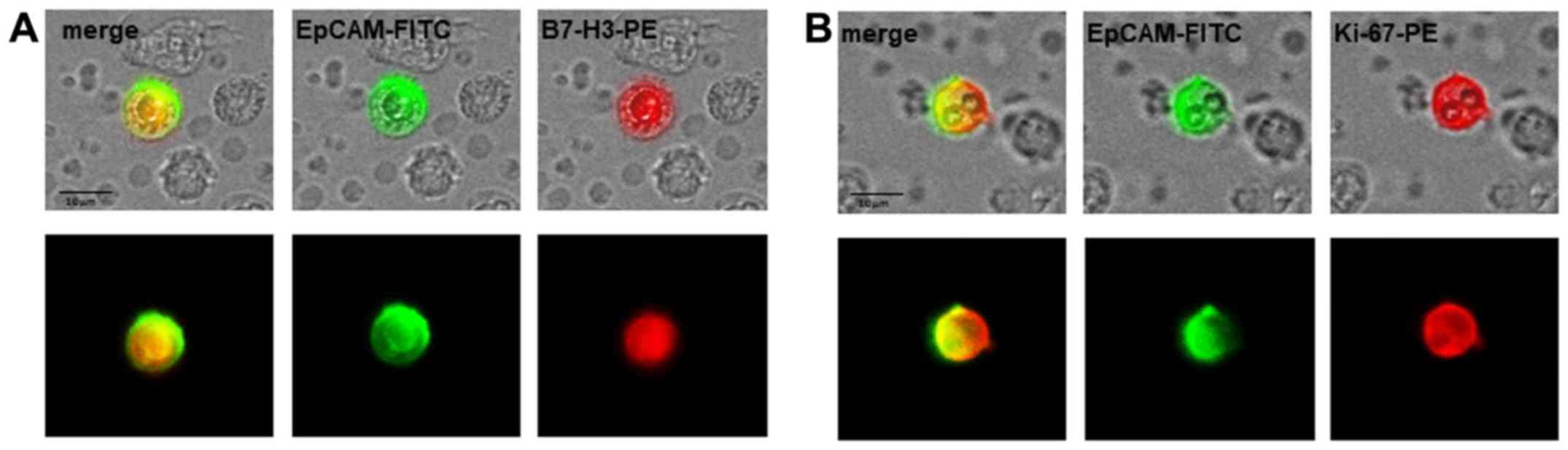
The analyses of B7-H3 and Ki-67 expression on the
CETCs were performed with an extended maintrac®
approach. For B7-H3 expression analysis, we used an anti-human
B7-H3 phycoerythrin (PE)-conjugated antibody (dilution 1:10, clone
MIH42, cat. no. 351002, BioLegend, San Diego, CA, USA) at a final
concentration of 0.9 µg/ml and for Ki-67 we used an
anti-human Ki-67 phycoerythrin (PE)-conjugated antibody (dilution
1:10, clone Ki-67, cat. no. 350503, BioLegend) at a final
concentration of 0.1 µg/ml. The corresponding isotype
controls for B7-H3 (Mouse IgG1 PE, cat. no. 400101, BioLegend) and
Ki-67 (Mouse IgG1 PE, cat. no. 400111, BioLegend) were used at the
same final concentration. Finally, the cells were visually
inspected looking for a green and red surface staining, but also a
well-preserved nucleus (Fig. 2).
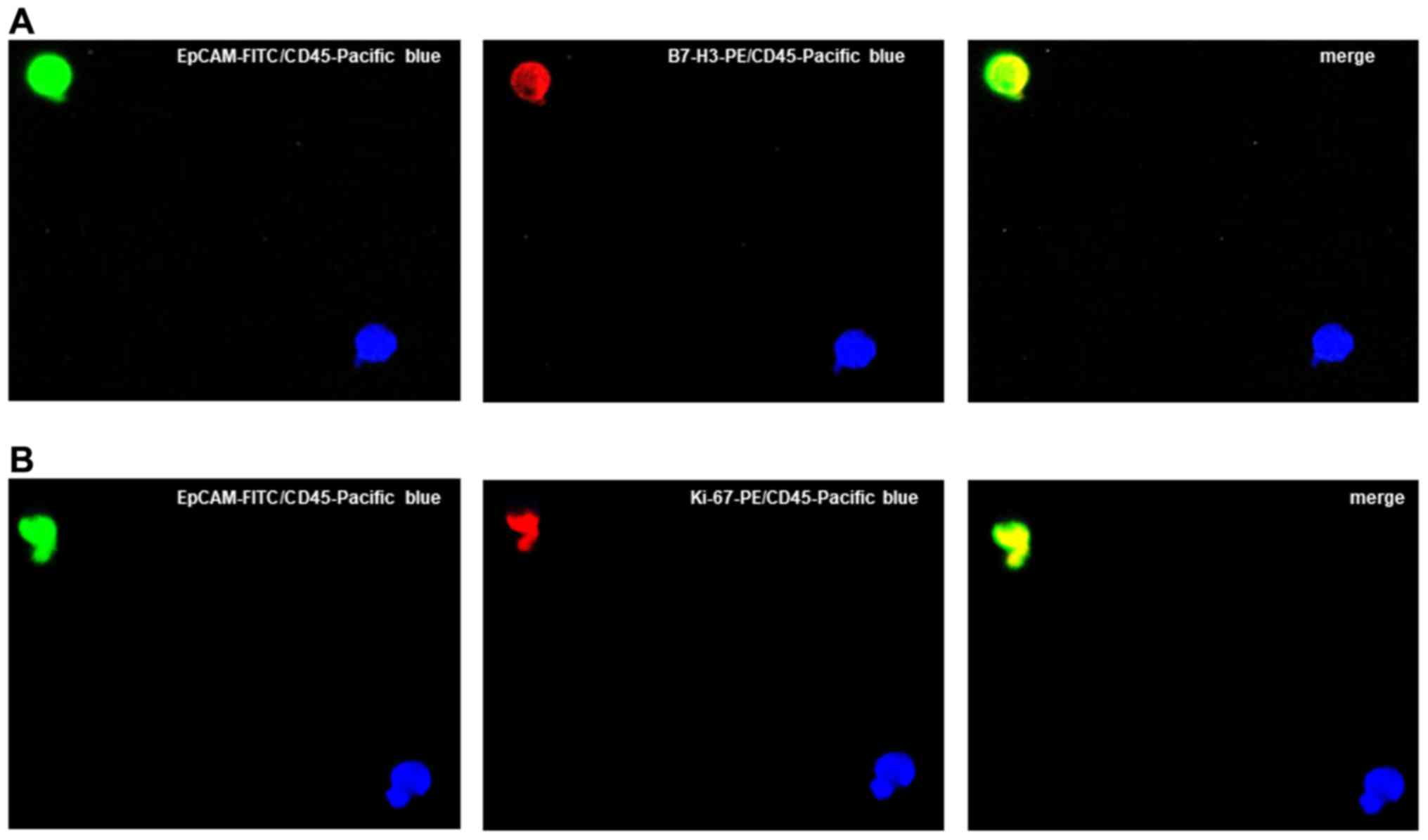
For excluding the expression of B7-H3/Ki-67 on hematopoetic cells,
we additionally performed staining with EpCAM-FITC,
B7-H3-PE/Ki-67-PE and CD45-Pacific blue (clone J.33, dilution 1:1,
cat. no. A74763, Beckman Coulter, Krefeld, Germany) antibodies
(Fig. 3). The results for B7-H3
and Ki-67 were calculated as a percentage of the total number of
CETCs.
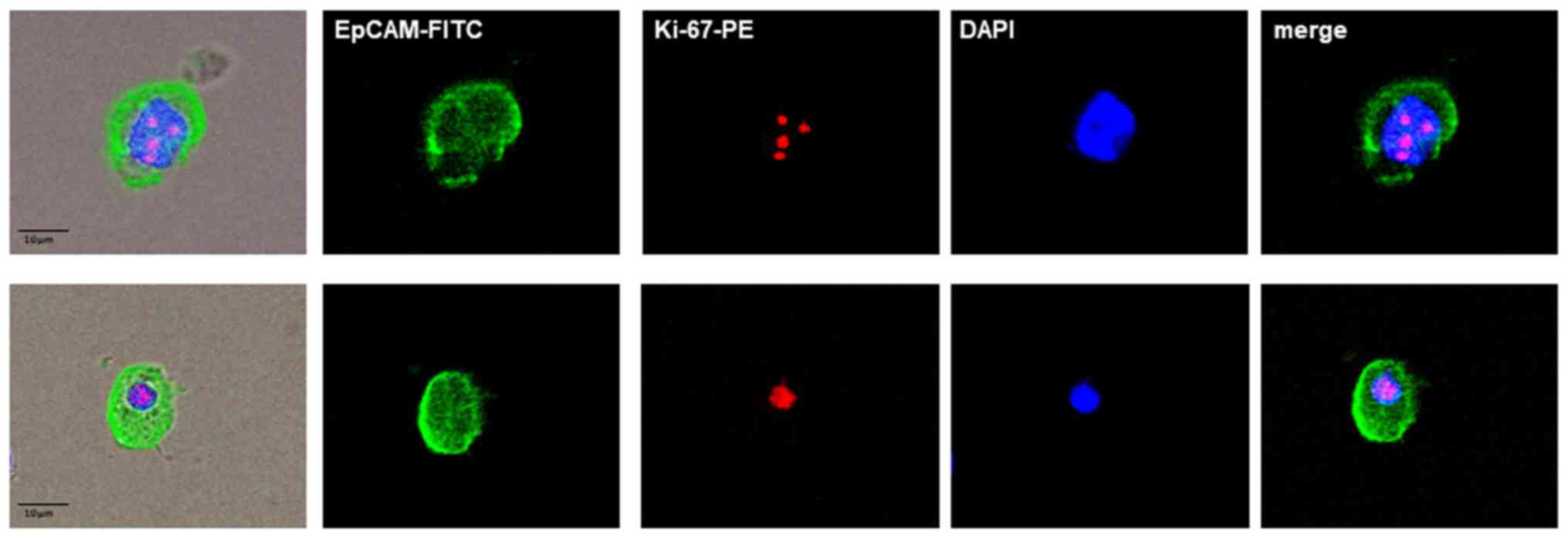
MCF-7 and Sk-Br-3 (data not shown) breast cancer
cells, which were used as positive controls for Ki-67 analysis,
were obtained from the CLS Cell Lines Service (Eppenheim, Germany).
The Sk-Br-3 cell line was grown in Dulbecco’s modified Eagle’s
medium with 4,5 g/l glucose, 2 mM L-glutamine (Gibco/Thermo Fisher
Scientific, Waltham, MA, USA) and 10% FBS. The cells were
maintained at 37°C in 5% CO2. The MCF-7 cells were grown
in minimum essential medium Eagle ready-to-use medium (CLS Cell
Lines Service). For immuno-fluorescence analysis, the cells were
detached from the cell culture flasks using StemPro®
Accutase® Cell Dissociation Reagent (Gibco/Thermo Fisher
Scientific) washed and stained for Ki-67 with the same protocol as
the patient samples (Fig. 4).
Statistical analysis
Statistical analysis was performed using the
software programs SigmaPlot version 13.0 (Systat Software Inc.,
Chicago, IL, USA) for Windows. Comparisons between variables were
performed using a Student t-test for normal distributed variables
or Mann-Whitney Rank Sum Test for not normally distributed
variables. Correlation analysis was carried out was calculated with
Pearson’s correlation coefficient. The significance level was set
at P<0.05.
Results
A total of 50 patients with histologically confirmed
breast cancer were enrolled in this study. Out of these, 25 (50%)
patients had T1; 8 (16%) patients had T2 and 11 (22%) patients had
T3/4 tumor size. The primary tumors were histologically positive
for estrogen receptor (ER) and progesterone receptor (PR) in 30
patients (60%) and positive for human epidermal growth factor
receptor 2 (HER2) in 11 patients (22%). In total, 15 (30%) patients
were in stage I; 8 (16%) were in stage II; 15 (30%) and 6 (12%)
were in stage III and IV, respectively. The age of the patients
ranged from 32 to 78 years (median, 59 years). The median number of
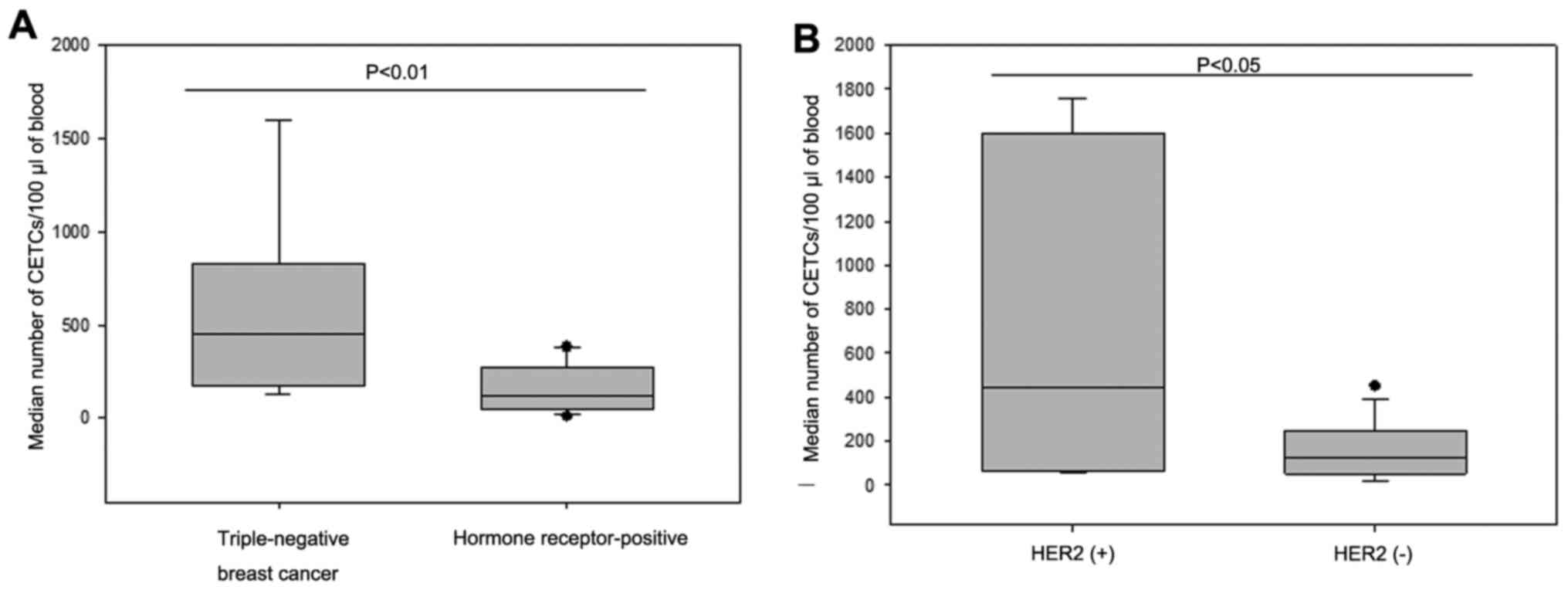
CETCs was 145 per 100 µl of blood (range, 10–1,760).
Patients with triple-negative breast cancer (TNBC; n=7) had
significantly more CETCs as compared to hormone receptor-positive
patients (n=30; median 450 vs. 125; P<0.01) (Fig. 5A). Additionally, patients with
primary HER2-positive tumors (n=11) had significantly more CETCs as
compared to patients with HER2-negative tumors (n=33; median 445
vs. 125; P<0.05) (Fig. 5B). No
statistically significant differences in CETC numbers were observed
according to tumor size and lymph node/ distant-metastasis
(Table I). As a negative control,
we tested blood samples from 20 healthy controls and confirmed that
none of the samples were positive for CETCs (data not shown).
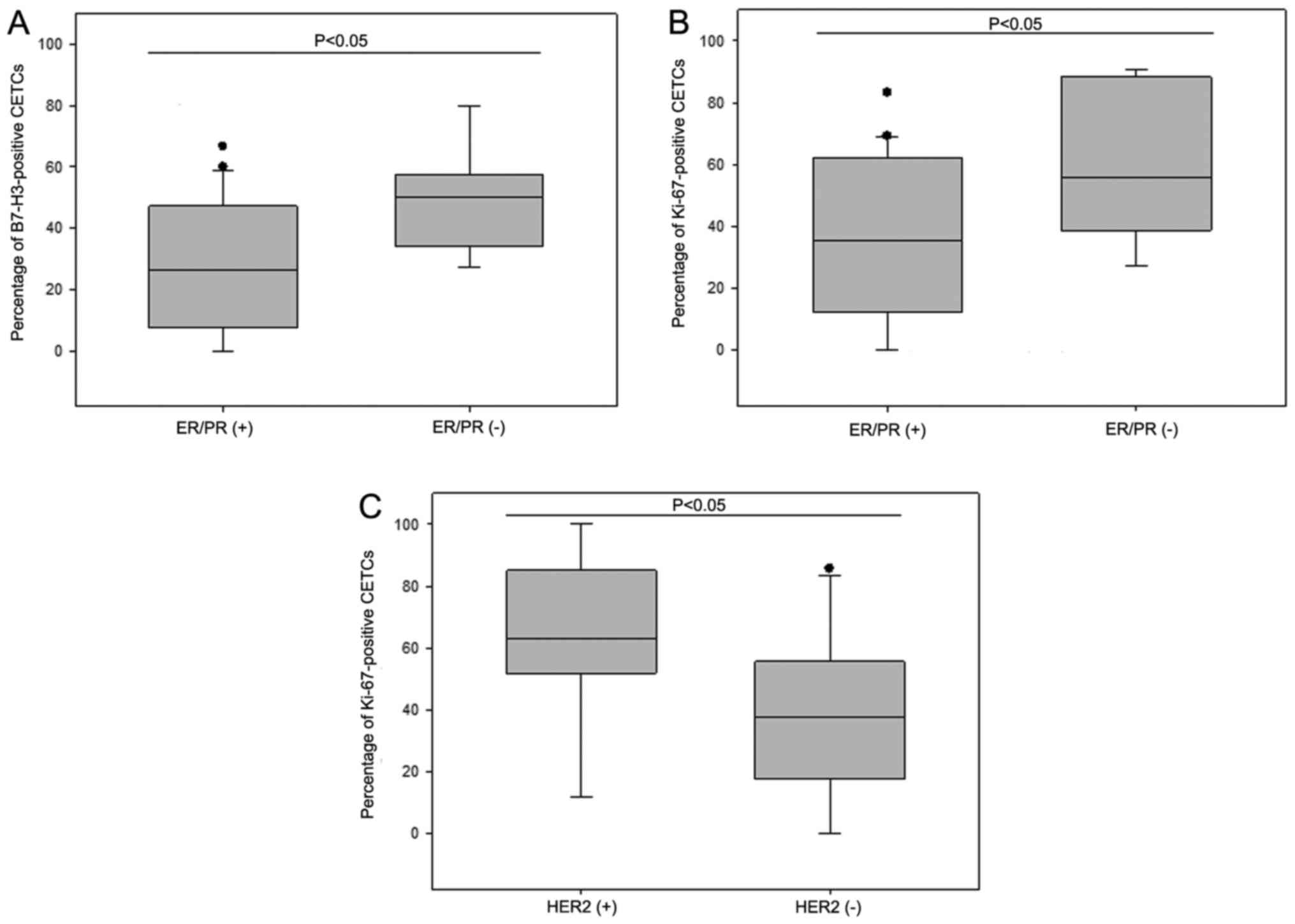
B7-H3-positive CETCs were observed in 43 patients (86%). The
percentage of B7-H3-positive CETCs ranged from 0 to 80% (median,
35%). An association between hormone receptor-status in primary
tumors and the percentage of B7-H3-positive CETCs was observed.
Patients (n=14) with tumor tissue negative for ER/PR had a
significantly greater number of B7-H3-positive CETCs as compared to
patients (n=30) with a positive ER/PR status (median 50% vs. 26%;
P<0.05) (Fig. 6A).
 | Table ICharacteristics of cancer patients
according to B7-H3 and Ki-67 expression. |
Table I
Characteristics of cancer patients
according to B7-H3 and Ki-67 expression.
| Clinicopathological
characteristics | Number of patients
(%) with CETCs | P-value | Number of patients
(%) with CETCs positive for B7-H3 | P-value | Number of patients
(%) with CETCs positive for Ki-67 | P-value |
|---|
| Age (years) | | P=0.164 | | P=0.745 | | P=0.583 |
| ≤50 | 10 (20) | | 10 (23) | | 9 (20) | |
| >50 | 40 (80) | | 33 (77) | | 36 (80) | |
| Tumor size | | P=0.876 | | P=0.168 | | P=0.315 |
| T1 | 25 (50) | | 20 (47) | | 21 (47) | |
| T2 | 8 (16) | | 7 (16) | | 8 (18) | |
| T3/4 | 11 (22) | | 10 (23) | | 10 (22) | |
| n.a. | 6 (12) | | 6 (14) | | 6 (13) | |
| Lymph node
status | | P=0.424 | | P=0.189 | |
P<0.05 |
| Positive | 23 (46) | | 21 (49) | | 22 (49) | |
| Negative | 21 (42) | | 16 (37) | | 17 (38) | |
| n.a. | 6 (12) | | 6 (14) | | 6 (13) | |
| Metastasis | | P=0.253 | | P=0.616 | | P=0.568 |
| Positive | 6 (12) | | 6 (14) | | 6 (13) | |
| Negative | 38 (76) | | 31 (72) | | 33 (74) | |
| n.a. | 6 (12) | | 6 (14) | | 6 (13) | |
| ER status | |
P<0.05 | | P=0.178 | |
P<0.05 |
| Positive | 30 (60) | | 23 (53) | | 25 (56) | |
| Negative | 14 (28) | | 14 (33) | | 14 (31) | |
| n.a. | 6 (12) | | 6 (14) | | 6 (13) | |
| PR status | |
P<0.05 | | P=0.178 | |
P<0.05 |
| Positive | 30 (60) | | 23 (53) | | 25 (56) | |
| Negative | 14 (28) | | 14 (33) | | 14 (31) | |
| n.a. | 6 (12) | | 6 (14) | | 6 (13) | |
| HER2 status | |
P<0.05 | | P=0.412 | |
P<0.05 |
| Positive
(2+/3+) | 11 (22) | | 10 (23) | | 11 (25) | |
| Negative
(0/1+) | 33 (66) | | 27 (63) | | 28 (62) | |
| n.a. | 6 (12) | | 6 (14) | | 6 (13) | |
| Stage | | P=0.181 | | P=0.404 | | P=0.453 |
| I | 15 (30) | | 10 (23) | | 11 (25) | |
| II | 8 (16) | | 8 (19) | | 8 (18) | |
| III | 15 (30) | | 13 (30) | | 14 (31) | |
| IV | 6 (12) | | 6 (14) | | 6 (13) | |
| n.a. | 6 (12) | | 6 (14) | | 6 (13) | |
| Radiotherapy | | P=0.523 | |
P<0.05 | |
P<0.05 |
| Yes | 22 (44) | | 22 (51) | | 22 (49) | |
| No | 26 (52) | | 19 (44) | | 21 (47) | |
| n.a. | 2 (4) | | 2 (5) | | 2 (4) | |
Ki-67-positive CETCs were detected in 45 patients
(90%) and the percentage ranged from 0–100 (median, 45%).
Furthermore, the percentage of Ki-67-positive CETCs was
significantly associated with the ER/PR and HER2 status in the
primary tumor. Patients (n=14) with hormone receptor-negative
tumors had a greater number of Ki-67-positive CETCs than patients
with hormone receptor-positive tumors (n=30) (median, 56% vs. 35%;
P<0.05) (Fig. 6B). In addition,
patients (n=11) with a HER2-positive primary tumor had a greater
number of Ki-67-positive CETCs than patients (n=33) with a
HER2-negative primary tumor (median, 63% vs. 38%; P<0.05)
(Fig. 6C). An association was
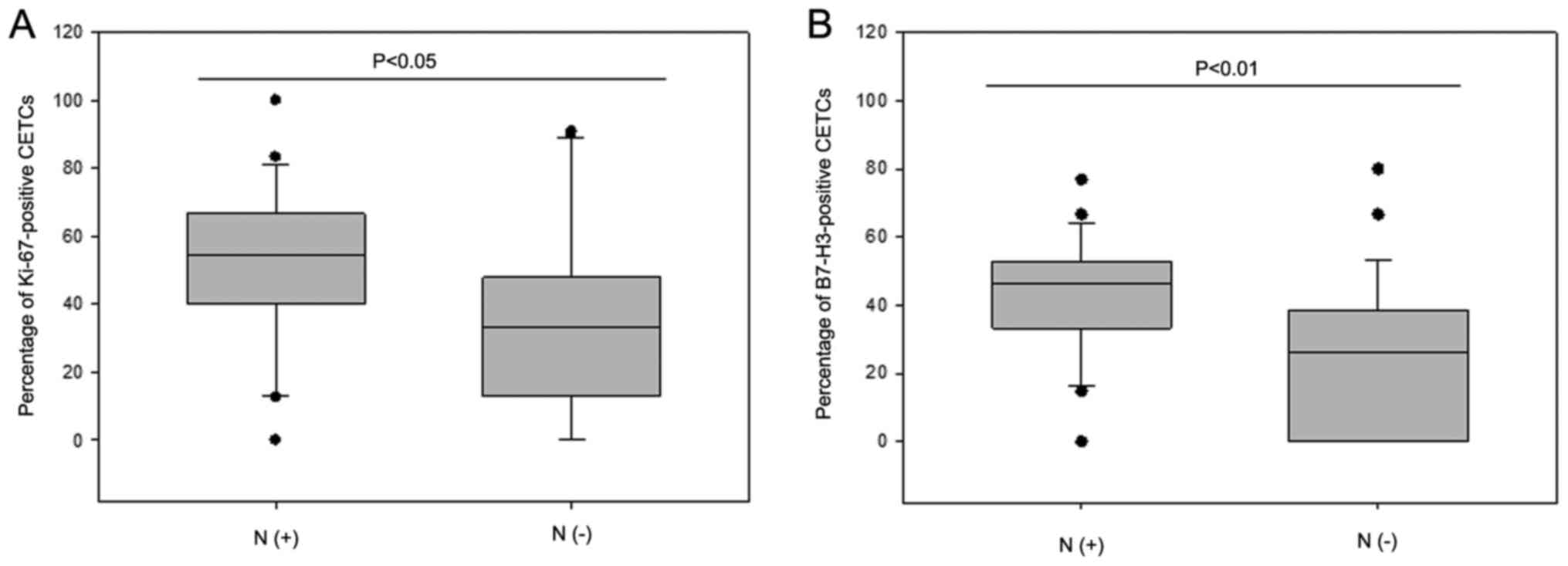
observed between the percentage of Ki-67 positive CETCs and the
lymph node status. Patients (n=23) with positive lymph nodes had a
significantly greater number of Ki-67-positive CETCs than patients
(n=21) with negative lymph nodes (median, 55% vs. 33%; P<0.05)
(Fig. 7A). Additionally an
association was observed between the percentage of B7-H3-positive
CETCs and the lymph node status (median, 46% vs. 26%; P<0.01)
(Fig. 7B).
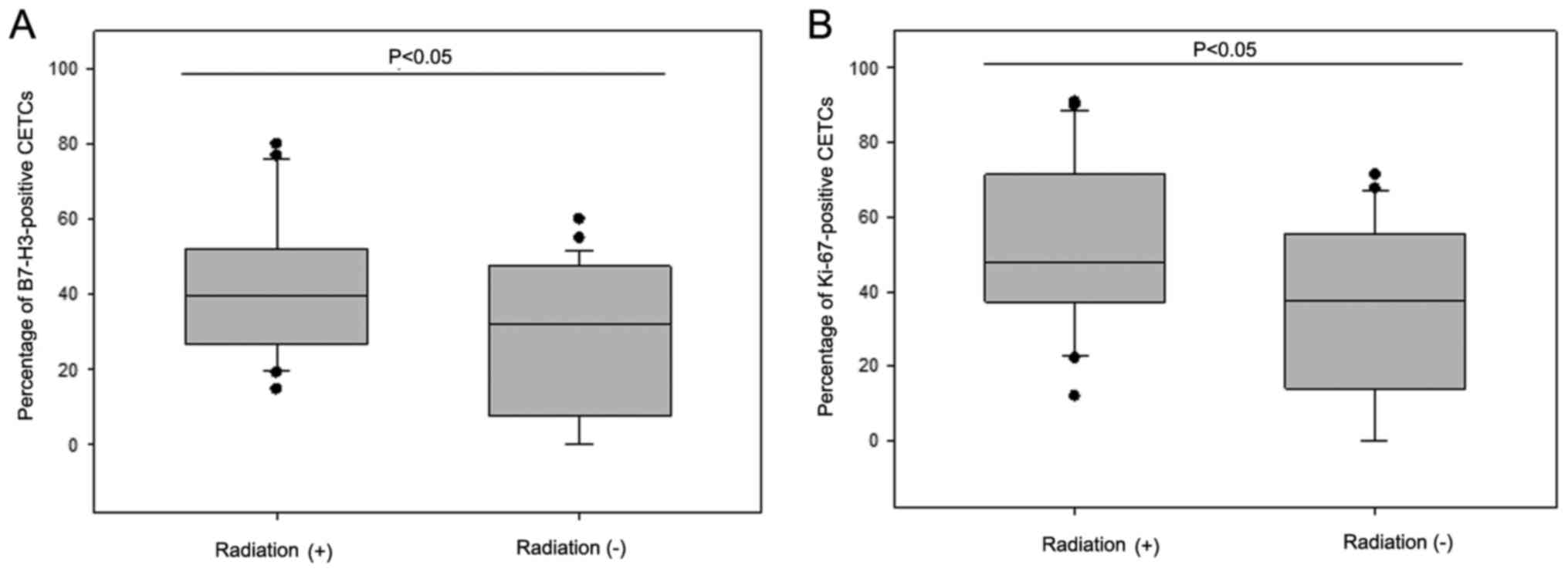
Patients (n=22) who had received radiotherapy had a
higher fraction of B7-H3- and Ki-67-positive CETCs as compared to
patients (n=26) without radiotherapy (median B7-H3, 40% vs. 23%,
P<0.05; median Ki-67, 48% vs. 38%, P<0.05) regardless of the
radiation regimen (Fig. 8).
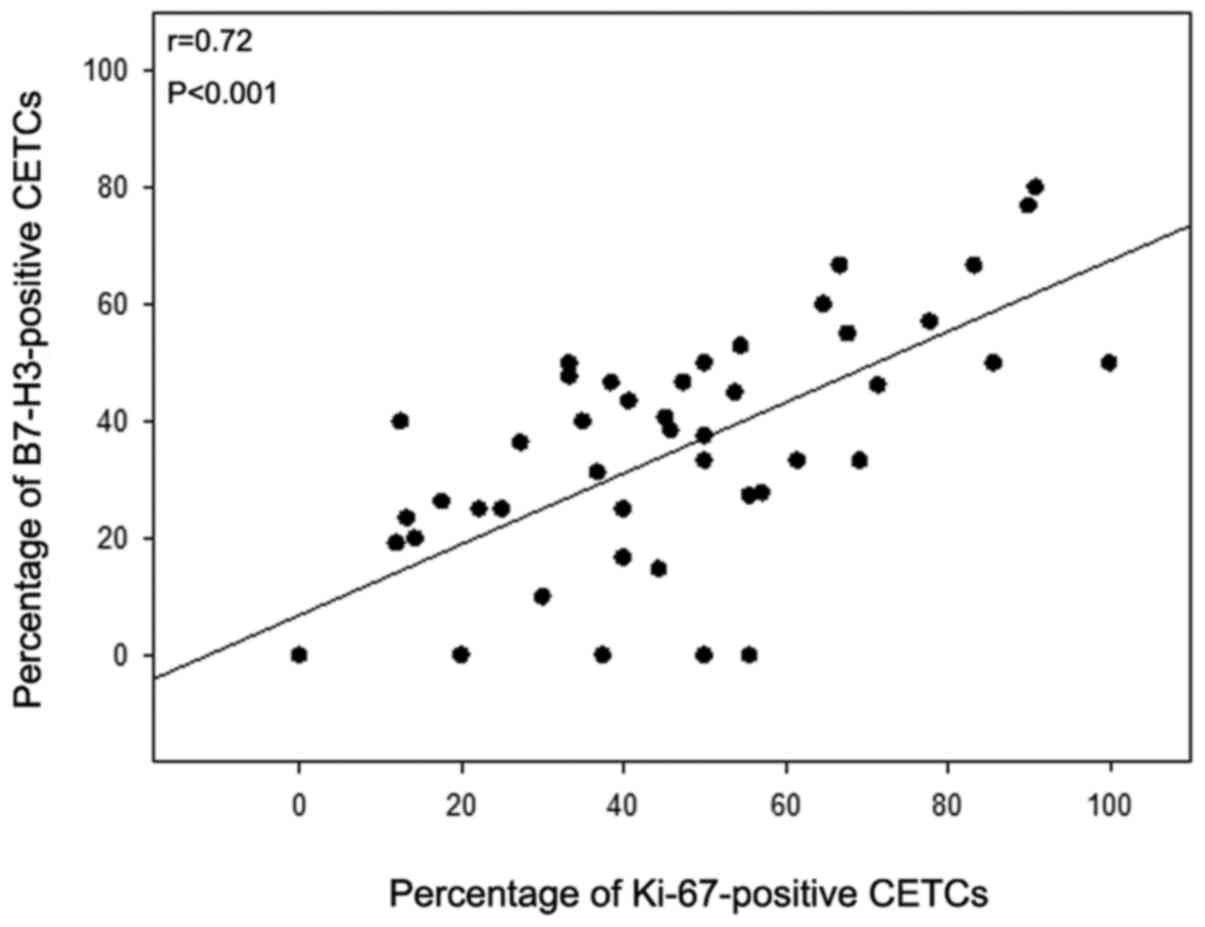
Comparing the percentage of B7-H3-positive CETCs with the
percentage of Ki-67-positive CETCs, both biomarkers significantly
correlated with each other (r=0.721; P=0.001) (Fig. 9). Statistical analysis revealed
that the highest association was observed between the percentage of
B7-H3- and Ki-67-positive CETCs (P<0.001) (Fig. 9), followed by lymph node positivity
(P=0.013) (Fig. 7) and the
administration of radiation (P=0.038) (Fig. 8A). All other parameters were not
significant.
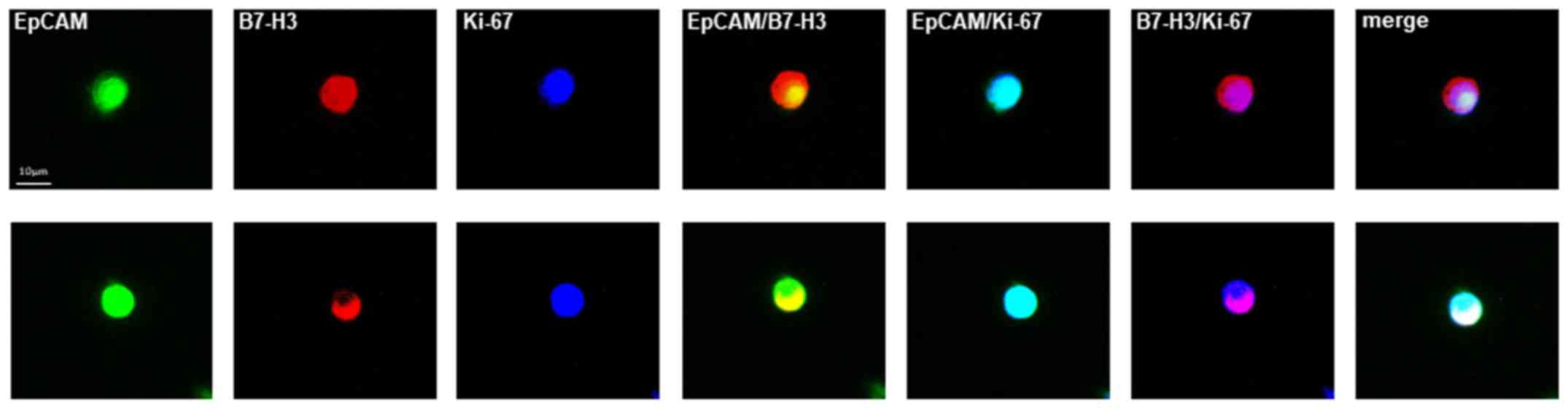
Subsequently, we evaluated and compared the
percentage of B7-H3- and Ki-67-positive CETCs in 20 breast cancer
patients by performing co-expression analysis. The co-expression of
B7-H3 and Ki-67 was confirmed in 90% of patients. Fig. 10 shows two typical cell galleries
of CETCs from one patient which show that these cells have a
parallel expression of both, B7-H3 and Ki-67 on their surface. The
percentage of B7-H3-and Ki-67-positive CETCs ranged from 23–75%
with a median of 35% (data not shown).
Discussion
The analysis of circulating tumor cells is essential
for understanding the vascular spread of cancer to distant sites
and for being able to make use of these cells for real-time and
non-invasive tumor monitoring. A number of techniques have been
developed over the past 20 years to detect, isolate and
characterize circulating tumor cells (37). Circulating tumor cell analysis may
play an important role as a ‘liquid biopsy’, which will allow
physicians to follow changes of the systemic part of the disease
over time, enabling adjustment of treatment and thus a promising
new diagnostic tool for patients suffering from e.g., breast cancer
(36). The majority of the
procedures have, however, been hampered by the paucity of these
cells recovered by the different approaches particularly those
using magnetic bead enrichment after a fixation step (38), which impedes statistical analysis
of the circulating tumor cells (39). Apart from mere quantitative
analysis, current research on circulating tumor cells is focusing
on the identification of novel diagnostic and therapeutic
biomarkers expressed by these cells, such as B7-H3 and Ki-67 on
tumor cells circulating in the blood, which allows for the
determination of the aggressiveness of the residual tumor load, and
can thus contribute to the determination of prognosis. They reflect
the biological properties of the remnant tumor left in the body
after previous interventions, which are crucial for the further
development of the disease and can provide starting points for
targeted treatment.
The association between the total number of
circulating tumor cells and immunohistochemistry in breast cancer
is controversial. Punnoose et al reported lower numbers of
circulating tumor cells in TNBC as compared to luminal subtypes
(40). By contrast, Peeters et
al found no significant association between
immunohistochemically defined subtypes and circulating tumor cell
numbers, but very high cell counts of >80 circulating tumor
cells in 7.5 ml blood were found more frequently in patients with
Luminal A and TNBC metastatic tumors (41). In this study, using a
non-enrichment approach, the number of CETCs was significantly
higher in patients with TNBC consistent with the clinical findings
that this type of tumor is more aggressive and has an increased
potential to be invasive, to migrate and to metastasize.
Additionally, we observed a significant difference in the absolute
CETC count in patients with HER2-positive tumors who had higher
CETC counts as compared to patients with HER2-negative tumors. Our
results are contradictory to the results by Liu et al
(42) and Giordano et al
(43) who reported a lower
circulating tumor cell count in HER2-positive subgroups. These
discrepancies may be explained by the different methods used for
the enumeration of circulating tumor cells.
Due to the growing interest in immunotherapy, the
analysis of B7-H3 expression in the primary breast tumor and on the
circulating tumor cells may be crucial. B7-H1, known as PD-L1, is
one of the most studied targets in present clinical trials. There
is a high homology between B7-H3 and B7-H1; therefore, the blockade
of both these molecules is highly feasible (17,18).
Different antibodies which inhibit B7-H3 are currently being
investigated in clinical trials [e.g., 8H9: NCT01099644,
NCT01502917, and NCT00089245; enoblituzumab (MGA271): NCT01391143]
(19). The present study was
designed to demonstrate for the first time, at least to the best of
our knowledge, B7-H3 expression in connection with Ki-67 on CETCs
in patients with breast cancer. We were able to show that B7-H3 was
expressed on a fraction of CETCs in the majority (82%) of breast
cancer patients. Comparably, Maeda et al (18) detected B7-H3 expression in 92% of
breast cancer tissues at various levels and Sun et al found
that B7-H3 expression was present in 80.55% of breast cancer
tissues compared to 16.48% in normal breast tissues (28). Arigami et al demonstrated a
strong expression of B7-H3 in 39% of breast cancers and the
expression was related to the progression of primary breast cancer
to axillary lymph nodes (30). In
gastric cancer, Arigami et al found that B7-H3 expression
can be a useful blood marker for predicting tumor progression
(44).
On the single cell level, on average of 30% of the
CETCs expressed B7-H3 in our patient population. There is little
data with respect to the expression of B7-H3 on circulating tumor
cells in breast cancer patients and none regarding the extent of
expression on these cells, at least to the best of our knowledge.
Arigami et al (30) only
reported on mRNA expression for B7-H3, but this did not allow for
the calculatation at the single tumor cell level.
This study suggests that B7-H3 expression in breast
cancer is significantly higher in hormone receptor-negative
patients, whereas Liu et al and Sun et al found no
association between B7-H3 positivity in breast tumor tissue and ER
or PR status, histological subtype or differentiation (23,45).
The association between B7-H3 on CETCs and hormone receptor status
may contribute to explain the worse prognosis of patients with
hormone-negative tumors.
The proliferation biomarker, Ki-67, is established
as a prognostic factor for breast cancer. The proportion of
dividing cells reflects the proliferative potential of the tumor.
The expression of Ki-67 in healthy breast tissue is very low
(<3%). By contrast, in breast cancer tissues, Ki-67 is often
overexpressed (33,46). Müller et al using the
CellSearch approach reported no expression of Ki-67 in the
circulating tumor cells detected in the 47 examined patients
(47). This may be due to the low
frequency and low number of circulating tumor cells detected by
their approach. By contrast, Spiliotaki et al (48), using a different approach, found
Ki-67 positive cells in 27.5% out of 40 circulating tumor cell
positive patients. Kallergi et al, using the same approach
as Spiliotaki et al, observed Ki-67 positive circulating
tumor cells in 51.7 and 44% of patients with early and metastatic
breast cancer, respectively (49).
In this study, we found positive staining for Ki-67 on the CETCs in
45 of 50 patients (90%). This difference may be explained by the
different technologies used to detect circulating tumor cells.
Furthermore, we also found an association between a
higher frequency of Ki-67-positive CETCs and a negative ER/PR and
positive HER2 status. Similar results were observed in breast
cancer tissue by Nishimura et al, where a higher Ki-67 index
was significantly associated with a larger tumor, younger age,
positive lymph nodes, a higher nuclear grade, a negative ER/PR
status, p53 overexpression and a positive HER2 status (50). In addition, they reported that a
high Ki-67 index in breast cancer tissue was significantly
associated with positive lymph nodes (50). In this study, the expression of
Ki-67 on CETCs was statistically significantly associated with a
positive lymph node status. Furthermore, comparable to the findings
of Arigami et al, the percentage of B7-H3-positive CETCs was
significantly associated with a positive lymph node status
(30). Liu et al found an
association between B7-H3 expression in tumor tissue and a positive
lymph node metastasis (45). B7-H3
overexpression in CETCs may thus be important for tumor progression
and invasiveness.
Post-surgery adjuvant therapies, such as chemo- and
radiotherapy are essential for patients with breast cancer;
however, many patients suffer from local recurrence or metastasis.
In this study, we observed that patients who have received
radiotherapy had an upregulated expression of B7-H3 on CETCs. This
may be due to inflammatory processes occurring during radiotherapy.
Maeda et al demonstrated that B7-H3 was induced by
inflammatory cytokines in dendritic cells and monocytes (18). Additionally, Sun et al
demonstrated that the stimulation of hepatocellular carcinoma cells
with interferon-γ in vitro led to a significant upregulation
of B7-H3 expression (51).
Subsequent radiotherapy resistance observed in breast cancer
patients may be due to the upregulation of B7-H3 in CETCs.
Combination therapies with B7-H3 blockade may in the future be able
to overcome radio-resistance. Of note, in this study, we found that
not only B7-H3, but also Ki-67 were more highly expressed in the
CETCs in patients who had received radiotherapy. Although there are
data available about changes in Ki-67 index during radiotherapy in
breast tumor tissue (52), a
comparison between tumor tissue and circulating tumor cells has not
been made to date, at least to the best of our knowledge. It is
well known that a high Ki-67 index in tumor tissue is associated
with lower disease-free and overall survival rates in breast cancer
(50).
In samples with a >50% fraction of B7-H3
expression and Ki-67 expression, a correlation between B7-H3 and
Ki-67 can be assumed in individual CETCs. The exact physiological
and pathological function of B7-H3 and particularly its role in the
development and progression of human cancers remains unclear, as
both stimulatory and inhibitory properties have been described
(53). B7-H3 may serve as an
inhibitor of anti-tumor immunity. Thus, proliferation may induce
B7-H3 and at the same time protect CETCs from destruction by
self-reactive T lymphocytes. Understanding the mechanisms through
which B7-H3 can be induced in and is association with the
proliferation of CETCs may, in the future, contribute the designing
of suitable drugs for breast cancer therapy.
The limitations of the study include the small
patient sample size and the preponderance of patients in early
stage of breast cancer. On the other hand, the possibility of
detection of circulating tumor cells also in this patient
population may be of advantage as they are still in a situation
where a cure is possible.
In conclusion, the analysis of the properties of
CETCs offers a low invasive, easy-to-repeat, real-time ‘liquid
biopsy’ approach, which reflects the actual aggressiveness of the
tumor. Furthermore, we demonstrate for the first time, at least to
the best of our knowledge, that B7-H3 and Ki-67 are expressed on
the CETCs in patients with breast cancer. The B7-H3 pathway
regulates the innate and adaptive immunity and promotes cancer cell
aggressiveness through various immunological functions. Therefore
it could become a unique and interesting target for future cancer
immunotherapies.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
MP and KP designed the study. MP and DSS performed
the experiments. MP, DSS and UP analyzed the data. MP and DSS wrote
the paper in consultation with KP and UP. All authors discussed the
results and contributed to the final manuscript.
Ethics approval and consent to
participate
All patients and healthy volunteers gave their
informed consent to participate in the study, which was approved by
the Ethics and Scientific Committees of the University of Jena
(Jena, Germany).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davison Z, de Blacquière GE, Westley BR
and May FE: Insulin-like growth factor-dependent proliferation and
survival of triple-negative breast cancer cells: Implications for
therapy. Neoplasia. 13:504–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar
|
|
4
|
Swaby RF and Cristofanilli M: Circulating
tumor cells in breast cancer: A tool whose time has come of age.
BMC Med. 9:432011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathiesen RR, Borgen E, Renolen A,
Løkkevik E, Nesland JM, Anker G, Ostenstad B, Lundgren S, Risberg
T, Mjaaland I, et al: Persistence of disseminated tumor cells after
neoadjuvant treatment for locally advanced breast cancer predicts
poor survival. Breast Cancer Res. 14:R1172012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hartkopf AD, Taran F-A, Wallwiener M,
Hagenbeck C, Melcher C, Krawczyk N, Hahn M, Wallwiener D and Fehm
T: The presence and prognostic impact of apoptotic and nonapoptotic
disseminated tumor cells in the bone marrow of primary breast
cancer patients after neoadjuvant chemotherapy. Breast Cancer Res.
15:R942013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berg A, Berner A, Lilleby W, Bruland ØS,
Fosså SD, Nesland JM and Kvalheim G: Impact of disseminated tumor
cells in bone marrow at diagnosis in patients with nonmetastatic
prostate cancer treated by definitive radiotherapy. Int J Cancer.
120:1603–1609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LWMM, et al: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma XL, Xiao ZL, Liu L, Liu XX, Nie W, Li
P, Chen NY and Wei YQ: Meta-analysis of circulating tumor cells as
a prognostic marker in lung cancer. Asian Pac J Cancer Prev.
13:1137–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahbari NN, Aigner M, Thorlund K, Mollberg
N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz
J: Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dotan E, Cohen SJ, Alpaugh KR and Meropol
NJ: Circulating tumor cells: Evolving evidence and future
challenges. Oncologist. 14:1070–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pachmann K, Camara O, Kavallaris A,
Krauspe S, Malarski N, Gajda M, Kroll T, Jörke C, Hammer U,
Altendorf-Hofmann A, et al: Monitoring the response of circulating
epithelial tumor cells to adjuvant chemotherapy in breast cancer
allows detection of patients at risk of early relapse. J Clin
Oncol. 26:1208–1215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pierga JY, Bidard FC, Mathiot C, Brain E,
Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A
and Marty M: Circulating tumor cell detection predicts early
metastatic relapse after neoadjuvant chemotherapy in large operable
and locally advanced breast cancer in a phase II randomized trial.
Clin Cancer Res. 14:7004–7010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Budd GT, Cristofanilli M, Ellis MJ,
Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV,
Terstappen LW, et al: Circulating tumor cells versus imaging -
predicting overall survival in metastatic breast cancer. Clin
Cancer Res. 12:6403–6409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dawood S, Broglio K, Valero V, Reuben J,
Handy B, Islam R, Jackson S, Hortobagyi GN, Fritsche H and
Cristofanilli M: Circulating tumor cells in metastatic breast
cancer: From prognostic stratification to modification of the
staging system? Cancer. 113:2422–2430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paterlini-Brechot P and Benali NL:
Circulating tumor cells (CTC) detection: Clinical impact and future
directions. Cancer Lett. 253:180–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Picarda E, Ohaegbulam KC and Zang X:
Molecular pathways: Targeting B7-H3 (CD276) for human cancer
immunotherapy. Clin Cancer Res. 22:3425–3431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maeda N, Yoshimura K, Yamamoto S, Kuramasu
A, Inoue M, Suzuki N, Watanabe Y, Maeda Y, Kamei R, Tsunedomi R, et
al: Expression of B7-H3, a potential factor of tumor immune evasion
in combination with the number of regulatory T cells, affects
against recurrence-free survival in breast cancer patients. Ann
Surg Oncol. 21 (Suppl 4): S546–S554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castellanos JR, Purvis IJ, Labak CM, Guda
MR, Tsung AJ, Velpula KK and Asuthkar S: B7-H3 role in the immune
landscape of cancer. Am J Clin Exp Immunol. 6:66–75.
2017.PubMed/NCBI
|
|
20
|
Wang Z, Yang J, Zhu Y, Zhu Y, Zhang B and
Zhou Y: Differential expression of 2IgB7-H3 and 4IgB7-H3 in cancer
cell lines and glioma tissues. Oncol Lett. 10:2204–2208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Chong KK, Nakamura Y, Nguyen L,
Huang SK, Kuo C, Zhang W, Yu H, Morton DL and Hoon DS: B7-H3
associated with tumor progression and epigenetic regulatory
activity in cutaneous melanoma. J Invest Dermatol. 133:2050–2058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Lv X, Wu Y, Xu J, Wang L, Chen W,
Zhang W, Li J, Zhang S and Qiu H: Expression of costimulatory
molecule B7-H3 and its prognostic implications in human acute
leukemia. Hematology. 20:187–195. 2015. View Article : Google Scholar
|
|
23
|
Sun J, Guo Y-D, Li X-N, Zhang Y-Q, Gu L,
Wu P-P, Bai GH and Xiao Y: B7-H3 expression in breast cancer and
upregulation of VEGF through gene silence. OncoTargets Ther.
7:1979–1986. 2014. View Article : Google Scholar
|
|
24
|
Zang X, Thompson RH, Al-Ahmadie HA, Serio
AM, Reuter VE, Eastham JA, Scardino PT, Sharma P and Allison JP:
B7-H3 and B7x are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad Sci
USA. 104:19458–19463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zang X, Sullivan PS, Soslow RA, Waitz R,
Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, et al:
Tumor associated endothelial expression of B7-H3 predicts survival
in ovarian carcinomas. Mod Pathol. 23:1104–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Sun J, Zhao H, Zhu D, Zhi Q, Song
S, Zhang L, He S, Kuang Y, Zhang Z, et al: The coexpression and
clinical significance of costimulatory molecules B7-H1, B7-H3, and
B7-H4 in human pancreatic cancer. OncoTargets Ther. 7:1465–1472.
2014. View Article : Google Scholar
|
|
27
|
Ingebrigtsen VA, Boye K, Nesland JM,
Nesbakken A, Flatmark K and Fodstad Ø: B7-H3 expression in
colorectal cancer: Associations with clinicopathological parameters
and patient outcome. BMC Cancer. 14:6022014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M,
Tan Y, Wang HT, Lu BF and Zhang XG: Clinical significance and
regulation of the costimulatory molecule B7-H3 in human colorectal
carcinoma. Cancer Immunol Immunother. 59:1163–1171. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamato I, Sho M, Nomi T, Akahori T,
Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H and Nakajima Y:
Clinical importance of B7-H3 expression in human pancreatic cancer.
Br J Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arigami T, Narita N, Mizuno R, Nguyen L,
Ye X, Chung A, Giuliano AE and Hoon DS: B7-h3 ligand expression by
primary breast cancer and associated with regional nodal
metastasis. Ann Surg. 252:1044–1051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li FY, Wu SG, Zhou J, Sun JY, Lin Q, Lin
HX, Guan XX and He ZY: Prognostic value of Ki-67 in breast cancer
patients with positive axillary lymph nodes: A retrospective cohort
study. PLoS One. 9:e872642014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dowsett M, Nielsen TO, A’Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al International Ki-67 in Breast Cancer Working Group: Assessment
of Ki67 in breast cancer: Recommendations from the International
Ki67 in Breast Cancer working group. J Natl Cancer Inst.
103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan PH, Bay BH, Yip G, Selvarajan S, Tan
P, Wu J, Lee CH and Li KB: Immunohistochemical detection of Ki67 in
breast cancer correlates with transcriptional regulation of genes
related to apoptosis and cell death. Mod Pathol. 18:374–381. 2005.
View Article : Google Scholar
|
|
34
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: Panel members: Thresholds for
therapies: Highlights of the St Gallen International Expert
Consensus on the primary therapy of early breast cancer 2009. Ann
Oncol. 20:1319–1329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yerushalmi R, Woods R, Ravdin PM, Hayes MM
and Gelmon KA: Ki67 in breast cancer: Prognostic and predictive
potential. Lancet Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pachmann K, Clement JH, Schneider CP,
Willen B, Camara O, Pachmann U and Höffken K: Standardized
quantification of circulating peripheral tumor cells from lung and
breast cancer. Clin Chem Lab Med. 43:617–627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tao M, Ma D, Li Y, Zhou C, Li Y, Zhang Y,
Duan W, Xu X, Wang R, Wu L, et al: Clinical significance of
circulating tumor cells in breast cancer patients. Breast Cancer
Res Treat. 129:247–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pachmann U, Hekimian K, Carl S, Ruediger
N, Rabenstein C and Pachmann K: Comparing sequential steps for
detection of circulating tumor cells: More specific or just less
sensitive? . Webmedcentral. 2:WMC0014902011.
|
|
39
|
Lorente D, Ravi P, Mehra N, Pezaro C,
Omlin A, Gilman A, Miranda M, Rescigno P, Kolinsky M, Porta N, et
al: Interrogating metastatic prostate cancer treatment switch
decisions: A multi-institutional survey. Eur Urol Focus:.
S2405–4569(16): pp. 30142-02016, doi.org/10.1016/j.euf.2016.09.005urisimpledoi.org/10.1016/j.euf.2016.09.005.
|
|
40
|
Punnoose EA, Atwal SK, Spoerke JM, Savage
H, Pandita A, Yeh RF, Pirzkall A, Fine BM, Amler LC, Chen DS, et
al: Molecular biomarker analyses using circulating tumor cells.
PLoS One. 5:e125172010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peeters DJ, van Dam PJ, Van den Eynden GG,
Rutten A, Wuyts H, Pouillon L, Peeters M, Pauwels P, Van Laere SJ,
van Dam PA, et al: Detection and prognostic significance of
circulating tumour cells in patients with metastatic breast cancer
according to immunohistochemical subtypes. Br J Cancer.
110:375–383. 2014. View Article : Google Scholar :
|
|
42
|
Liu Y, Liu Q, Wang T, Bian L, Zhang S, Hu
H, Li S, Hu Z, Wu S, Liu B, et al: Circulating tumor cells in
HER2-positive metastatic breast cancer patients: A valuable
prognostic and predictive biomarker. BMC Cancer. 13:2022013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Giordano A, Giuliano M, De Laurentiis M,
Arpino G, Jackson S, Handy BC, Ueno NT, Andreopoulou E, Alvarez RH,
Valero V, et al: Circulating tumor cells in immunohistochemical
subtypes of metastatic breast cancer: Lack of prediction in
HER2-positive disease treated with targeted therapy. Ann Oncol.
23:1144–1150. 2012. View Article : Google Scholar
|
|
44
|
Arigami T, Uenosono Y, Hirata M, Yanagita
S, Ishigami S and Natsugoe S: B7-H3 expression in gastric cancer: A
novel molecular blood marker for detecting circulating tumor cells.
Cancer Sci. 102:1019–1024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu C, Liu J, Wang J, Liu Y, Zhang F, Lin
W, Gao A, Sun M, Wang Y and Sun Y: B7-H3 expression in ductal and
lobular breast cancer and its association with IL-10. Mol Med Rep.
7:134–138. 2013. View Article : Google Scholar
|
|
46
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Müller V, Stahmann N, Riethdorf S, Rau T,
Zabel T, Goetz A, Jänicke F and Pantel K: Circulating tumor cells
in breast cancer: Correlation to bone marrow micrometastases,
heterogeneous response to systemic therapy and low proliferative
activity. Clin Cancer Res. 11:3678–3685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Spiliotaki M, Mavroudis D, Kapranou K,
Markomanolaki H, Kallergi G, Koinis F, Kalbakis K, Georgoulias V
and Agelaki S: Evaluation of proliferation and apoptosis markers in
circulating tumor cells of women with early breast cancer who are
candidates for tumor dormancy. Breast Cancer Res. 16:4852014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kallergi G, Konstantinidis G,
Markomanolaki H, Papadaki MA, Mavroudis D, Stournaras C,
Georgoulias V and Agelaki S: Apoptotic circulating tumor cells in
early and metastatic breast cancer patients. Mol Cancer Ther.
12:1886–1895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nishimura R, Osako T, Okumura Y, Hayashi
M, Toyozumi Y and Arima N: Ki-67 as a prognostic marker according
to breast cancer subtype and a predictor of recurrence time in
primary breast cancer. Exp Ther Med. 1:747–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi
Y, Shi JY, Xu YF, Shi YH, Song K, et al: B7-H3 is expressed in
human hepatocellular carcinoma and is associated with tumor
aggressiveness and postoperative recurrence. Cancer Immunol
Immunother. 61:2171–2182. 2012. View Article : Google Scholar
|
|
52
|
Kovarík J, Skry GD, Mikel J and Svoboda
VH: Changes of Ki67 index of various tumors during radiation
therapy. Neoplasma. 43:89–92. 1996.PubMed/NCBI
|
|
53
|
Madu CO and Lu Y: Novel diagnostic
biomarkers for prostate cancer. J Cancer. 1:150–177. 2010.
View Article : Google Scholar : PubMed/NCBI
|