|
1
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bassi P, Ferrante GD, Piazza N, Spinadin
R, Carando R, Pappagallo G and Pagano F: Prognostic factors of
outcome after radical cystectomy for bladder cancer: A
retrospective study of a homogeneous patient cohort. J Urol.
161:1494–1497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madersbacher S, Hochreiter W, Burkhard F,
Thalmann GN, Danuser H, Markwalder R and Studer UE: Radical
cystectomy for bladder cancer today - a homogeneous series without
neoadjuvant therapy. J Clin Oncol. 21:690–696. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shariat SF, Karakiewicz PI, Palapattu GS,
Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ,
Sagalowsky AI, et al: Outcomes of radical cystectomy for
transitional cell carcinoma of the bladder: A contemporary series
from the Bladder Cancer Research Consortium. J Urol. 176:2414–2422.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dhar NB, Klein EA, Reuther AM, Thalmann
GN, Madersbacher S and Studer UE: Outcome after radical cystectomy
with limited or extended pelvic lymph node dissection. J Urol.
179:873–878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konety BR, Joslyn SA and O'Donnell MA:
Extent of pelvic lymph-adenectomy and its impact on outcome in
patients diagnosed with bladder cancer: Analysis of data from the
Surveillance, Epidemiology and End Results Program data base. J
Urol. 169:946–950. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leissner J, Ghoneim MA, Abol-Enein H,
Thüroff JW, Franzaring L, Fisch M, Schulze H, Managadze G, Allhoff
EP, el-Baz MA, et al: Extended radical lymphadenectomy in patients
with urothelial bladder cancer: Results of a prospective
multi-center study. J Urol. 171:139–144. 2004. View Article : Google Scholar
|
|
8
|
Koppie TM, Vickers AJ, Vora K, Dalbagni G
and Bochner BH: Standardization of pelvic lymphadenectomy performed
at radical cystectomy: Can we establish a minimum number of lymph
nodes that should be removed? Cancer. 107:2368–2374. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Azuma H, Inamoto T, Takahara K, Nomi H,
Hirano H, Ibuki N, Uehara H, Komura K, Minami K, Uchimoto T, et al:
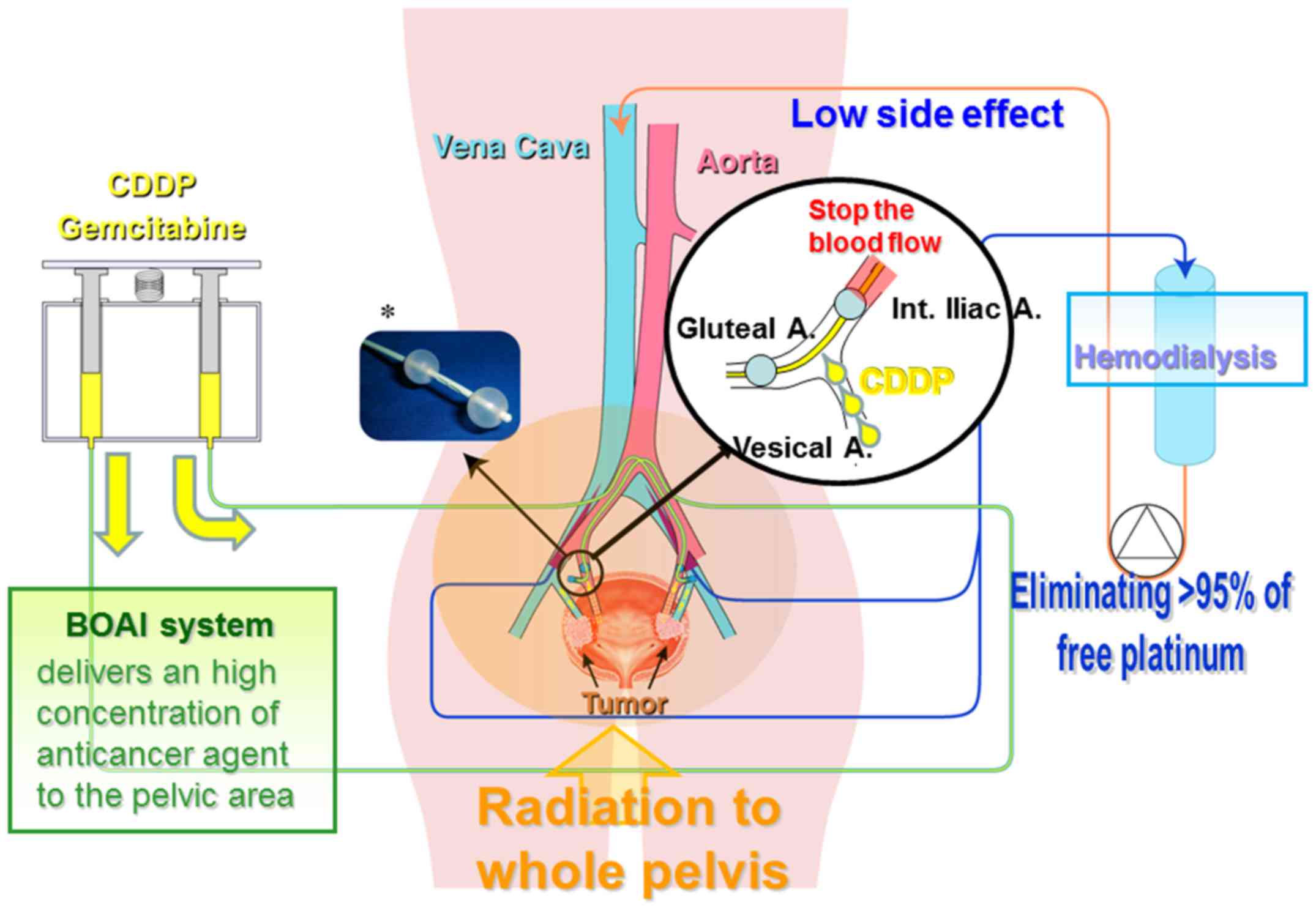
Novel bladder preservation therapy, OMC-regimen: Combined therapy
using balloon-occluded arterial infusion of anticancer agent and
hemodialysis with concurrent radiation. J Urol. 193:443–450. 2015.
View Article : Google Scholar
|
|
10
|
Sun M and Trinh QD: Diagnosis and staging
of bladder cancer. Hematol Oncol Clin North Am. 29:205–218.
vii2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Kock I, Mirhosseini M, Lau F, Thai V,
Downing M, Quan H, Lesperance M and Yang J: Conversion of Karnofsky
Performance Status (KPS) and Eastern Cooperative Oncology Group
Performance Status (ECOG) to Palliative Performance Scale (PPS),
and the interchangeability of PPS and KPS in prognostic tools. J
Palliat Care. 29:163–169. 2013.
|
|
12
|
Basch E, Iasonos A, McDonough T, Barz A,
Culkin A, Kris MG, Scher HI and Schrag D: Patient versus clinician
symptom reporting using the National Cancer Institute Common
Terminology Criteria for Adverse Events: Results of a
questionnaire-based study. Lancet Oncol. 7:903–909. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Azuma H, Inamoto T, Takahara K, Nomi H,
Uehara H, Komura K, Minami K, Kouno J, Kotake Y, Abe H, et al:
Effect of a novel bladder preservation therapy, BOAI-CDDP-radiation
(OMC-regimen). Int J Oncol. 43:79–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azuma H, Inamoto T, Takahara K, Nomi H,
Hirano H, Ibuki N, Uehara H, Komura K, Minami K, Uchimoto T, et al:
The novel bladder preservation therapy BOAI-CDDP-radiation
(OMC-regimen): A new treatment option for invasive bladder cancer
patients with lymph node metastasis. Int J Oncol. 44:1895–1903.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abol-Enein H, Tilki D, Mosbah A, El-Baz M,
Shokeir A, Nabeeh A and Ghoneim MA: Does the extent of
lymphadenectomy in radical cystectomy for bladder cancer influence
disease-free survival? A prospective single-center study. Eur Urol.
60:572–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zehnder P, Studer UE, Skinner EC, Dorin
RP, Cai J, Roth B, Miranda G, Birkhäuser F, Stein J, Burkhard FC,
et al: Super extended versus extended pelvic lymph node dissection
in patients undergoing radical cystectomy for bladder cancer: A
comparative study. J Urol. 186:1261–1268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karakiewicz PI, Shariat SF, Palapattu GS,
Perrotte P, Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A,
Bastian PJ, et al: Precystectomy nomogram for prediction of
advanced bladder cancer stage. Eur Urol. 50:1254–1262. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shariat SF, Karakiewicz PI, Palapattu GS,
Amiel GE, Lotan Y, Rogers CG, Vazina A, Bastian PJ, Gupta A,
Sagalowsky AI, et al: Nomograms provide improved accuracy for
predicting survival after radical cystectomy. Clin Cancer Res.
12:6663–6676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kassouf W, Agarwal PK, Herr HW, Munsell
MF, Spiess PE, Brown GA, Pisters L, Grossman HB, Dinney CP and
Kamat AM: Lymph node density is superior to TNM nodal status in
predicting disease-specific survival after radical cystectomy for
bladder cancer: Analysis of pooled data from MDACC and MSKCC. J
Clin Oncol. 26:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruins HM, Veskimae E, Hernandez V,
Imamura M, Neuberger MM, Dahm P, Stewart F, Lam TB, N'Dow J, van
der Heijden AG, et al: The impact of the extent of lymphadenectomy
on oncologic outcomes in patients undergoing radical cystectomy for
bladder cancer: A systematic review. Eur Urol. 66:1065–1077. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitamura H, Masumori N and Tsukamoto T:
Role of lymph node dissection in management of bladder cancer. Int
J Clin Oncol. 16:179–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jensen JB, Ulhøi BP and Jensen KM: Lymph
node mapping in patients with bladder cancer undergoing radical
cystectomy and lymph node dissection to the level of the inferior
mesenteric artery. BJU Int. 106:199–205. 2010. View Article : Google Scholar
|