Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor among children and adolescents between
10-25-years-old, and the second leading cause of cancer-mortality
in pediatric age between 10-14-years-old (1). Despite great advances in therapeutic
strategies for OS, including surgical resection combined with
adjuvant chemotherapy or radiotherapy, the clinical prognosis and
5-year survival rate of patients with OS (65%) remains
unsatisfactory due to high metastasis and recurrence (2). The pathogenesis of OS is an extremely
compli-cated process, which has been associated with multifarious
molecular changes (3). Therefore,
an improved understanding of the pathogenesis of OS may aid the
identification of novel diagnostic biomarkers and the development
of effective therapeutic strategies for the treatment of OS.
Long non-coding RNAs (lncRNAs) are defined as
evolutionarily conserved non-coding RNAs (ncRNAs) that are >200
nucleotides in length with no or little protein-coding capacity
(4). An increasing number of
studies have revealed that lncRNAs serve important regulatory roles
in a variety of pathophysiological processes, including cell
proliferation, cell cycle progression, apoptosis and carcinogenesis
(5-7). It is well reported that lncRNAs are
frequently dysregulated in numerous types of tumors, and act as
oncogenes or tumor suppressors in the development of various
cancers, such as OS (8,9). As one of the first reported
cancer-associated lncRNAs (10),
metastasis associated lung adenocarcinoma transcript 1 (MALAT1),
located on chromosome 11q13, was highly expressed in metastasizing
non-small cell lung cancer (11).
Subsequently, research has revealed that MALAT1 was widely
expressed in human tissues, including the heart, kidney, spleen and
brain (12). Previously,
accumulating evidence has demonstrated that MALAT1 serves diverse
roles in the carcinogenesis of numerous types of tumors, including
breast cancer, hepatocellular carcinoma and cervical cancer
(13-15). In addition, several studies have
reported that MALAT1 expression was increased in OS tissues and
cells, and correlated with the poor prognosis of patients with OS.
Furthermore, enhanced expression of MALAT1 promoted the progression
of OS (16-18); however, the underlying molecular
mechanism by which MALAT1 promotes OS progression requires further
investigation.
MicroRNAs (miRNAs/miRs) are another class of highly
conserved ncRNAs with a length of 18-25 nucleotides, and regulate
gene expression by inducing the degradation or post-transcriptional
inhibition of mRNAs (19).
Specifically, dysregulated miRNAs have been closely associated with
the development and progression of human cancers by affecting
biological processes, including differentiation, proliferation,
invasion and migration (20).
Recently, increasing evidence has demonstrated that lncRNAs could
function as competing endogenous RNAs (ceRNAs) to suppress the
expression and activities of miRNAs, thus regulating the expression
of target mRNA (21-23). As a well-studied miRNA, miR-34a, a
member of the miR-34 family, has been reported as downregulated in
OS cells and functioned as a tumor suppressor in the pathogenesis
of OS (24,25). Considering the importance of MALAT1
and miR-34a in the pathogenesis of OS, the present study
investigated whether MALAT1 functions as a molecular sponge of
miR-34a in OS.
In the present study, it was reported that MALAT1
was upregulated in OS tissues and cells. Additionally, the
expression of MALAT1 was associated with tumor size, clinical stage
and distant metastasis in patients with OS; however, MALAT1
silencing suppressed the viability, invasion and migration in OS
cells. Mechanistically, it was demonstrated that MALAT1
depletion-mediated suppression on OS progression was attributed to
its function as a ceRNA of miR-34a and thus the regulation of
cyclin D1 (CCND1) expression. The findings of the present study may
contribute to the development of lncRNA-directed diagnosis and
treatment of patients with OS.
Materials and methods
Human OS tissue collection
A total of 30 pairs of OS and adjacent normal tissue
samples were surgically resected from patients at the Shangqiu
First People’s Hospital (Shangqiu, China) between 2016 and 2017.
All specimens were confirmed as OS samples via histological
analysis following surgery. None of the patients with OS had
received any preoperative treatments, including radiotherapy or
chemotherapy prior to surgery. All tissues were immediately
snap-frozen in liquid nitrogen following surgical removal and
stored at -80°C until RNA extraction. The present study was
approved by the Research Ethics Committee of Shangqiu First
People’s Hospital and written informed consent was obtained from
each patient. Patients with OS were classified into high (n=16) and
low (n=14) MALAT1 expression groups according to the median
expression levels of MALAT1 and the details of clinical
characteristics of patients are presented in Table I.
 | Table IAssociation of MALAT1 expression with
clinicopathological factors in osteosarcoma. |
Table I
Association of MALAT1 expression with
clinicopathological factors in osteosarcoma.
| Clinicopathological
feature | n | MALAT1 expression
| P-value |
|---|
| Low [(n) %)] | High [(n) %] |
|---|
| Age | | | | |
| <20 years | 16 | 7 (43.8) | 9 (56.2) | >0.05 |
| ≥20 years | 14 | 7 (50.0) | 7 (50.0) | |
| Sex | | | | |
| Female | 13 | 5 (38.5) | 8 (61.5) | >0.05 |
| Male | 17 | 9 (52.9) | 8 (47.1) | |
| Tumor size | | | | |
| >8 cm | 18 | 5 (27.8) | 13 (72.2) | <0.05 |
| ≤8 cm | 12 | 9 (75.0) | 3 (25.0) | |
| Clinical stage | | | | |
| IIA | 8 | 6 (75.0) | 2 (25.0) | <0.05 |
| IIB/III | 22 | 8 (36.4) | 14 (63.6) | |
| Distant
metastasis | | | | |
| Absent | 20 | 12 (60.0) | 8 (40.0) | <0.05 |
| Present | 10 | 2 (20.0) | 8 (80.0) | |
Cell culture and transfection
The OS cell lines (Saos-2, MG63 and SOSP-9607) and
the normal human osteoblastic cell line, hFOB were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA). All
cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) and 1% penicillin/streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.), and incubated at 37°C in a humidified
atmosphere containing 5% CO2.
PcDNA-MALAT1 (MALAT1) with sites of restriction
enzymes NheI and BamHI, pcDNA vector (Vector), small
interfering (si)RNAs against MALAT1 (si-MALAT1) and pcDNA-CCND1
(CCND1) with sites of restriction enzymes NheI and
BamHI, CCND1 (si-CCND1), an siRNA scrambled control (si-NC),
miR-34a mimics (miR-34a), miRNA scram-bled control (miR-NC),
miR-34a inhibitor (anti-miR-34a) and inhibitor control
(anti-miR-NC) were obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The sequences were as follows: si-MALAT1, sense
5′-GATCCATAATCGGTTTCAAGG-3′, antisense,
5′-TTGAAACCGATTATGGATCAT-3′; si-CCND1, sense
5′-GGAGCAUUUUGAUACCAGATT-3′, antisense,
5′-UCUGGUAUCAAAAUGCUCCGG-3′; si-NC, sense
5′-UUCUCCGAACGUGUCACGUTT-3′, antisense,
5′-ACGUGACACGUUCGGAGAATT-3′; miR-34a mimics, sense
5′-UGGCAGUGUCUUAGCUGGUUGU-3′, antisense,
5′-AACCAGCUAAGACACUGCCAUU-3′; miR-NC, sense
5′-UUCUCCGAACGUGUCACGTT-3′, antisense 5′-ACGUGACACGUUCGGAGAATT-3′;
and anti-miR-34a 5′-ACAACCAGCUAAGACACUGCCA-3′. SOSP-9607 and Saos-2
cells exhibited greater variations in MALAT1 expression due to
transfection, hence subsequent experiments were conducted in
SOSP-9607 and Saos-2 cells. Transient transfection with the 40 nM
aforementioned oligonucleotides or 1 µg plasmids into
SOSP-9607 and Saos-2 cells was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were collected for further analyses at 48
h post-transfection and transfection efficacy was investigated by
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR) as described below.
Construction of MALAT1 lentiviral
expression vector
The MALAT1 lentiviral vector was produced by
Shanghai GenePharma Co., Ltd. The full-length of MALAT1 or miR-34a
cDNA was synthesized and subcloned into pGLV/H1/green fluorescent
protein lentiviral frame plasmids using the restriction enzymes
XhoI and KpnI (Addgene, Inc., Cambridge, MA, USA).
Following DNA sequencing, lentivirus containing MALAT1 or miR-34a
and the packaging vectors (pCMV-VSVG, pMDLg/pRRE and pRSV-REV) were
transduced into 293T cells (ATCC, multiplicity of infection=50) to
generate the recombinant lentiviral vectors MALAT1 (lenti-MALAT1)
and miR-34a (lenti-miR-34a). At 48 h following cotransduction, the
lentivirus-containing supernatant was collected and purified with a
0.45-µm filter. Lentivirus was collected via
ultracentrifugation for 60 min at 50,000 × g (4°C) and stored at
−80°C until further use. Empty lentiviral vectors (lenti-NC) were
used as the control. SOSP-9607 cells were inoculated in a 24-well
plate and infected with the aforementioned recombinant
vector-containing lentiviruses at a multiplicity of infection of 20
until 60% confluence was attained. The stably transfected cells
were used for in vivo analysis.
RT-qPCR analysis
Total RNA from the tissue samples and cells was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The first strand cDNA was synthesized from 1
µg of total RNA using a PrimeScript RT Reagent kit (Takara
Bio, Inc., Otsu, Japan) according to the manufacturer’s protocols.
The expression levels of MALAT1 were determined using SYBR Green
Real-Time PCR Master Mix (Roche Diagnostics, Basel, Switzerland) on
an ABI 7500 PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.), with GAPDH as an internal control. qPCR was
performed to detect miR-34a expression using the TaqMan microRNA
Assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) on
an ABI 7500 PCR system and U6 small nuclear RNA was used as an
endogenous control. Three replicates were involved in this
experiment. RT-qPCR was conducted with the following amplification
protocol: 95°C for 1 min, 40 cycles of 95°C for 15 sec and 60°C for
1 min. The relative gene expression was calculated using the
2−ΔΔCq quantification method (26). The primer sequences used were as
follows: MALAT1 forward, 5′-AAAGCAAGGTCTCCCCACAAG-3′, reverse,
5′-GGTCTGTGCTAGATCAAAAGGCA-3′; GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′;
miR-34a forward, 5′-GTGCAGGGTCCGAGGT-3′, reverse,
5′-GCCGCTGGCAGTGTCTTAGCTG-3′ and U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
MTT assay
Cell viability was assessed using an MTT assay.
Following transfection, SOSP-9607 and Saos-2 cells were seeded into
96-well plates at a density of 3,000 cells/well and incubated at
37°C for 0, 24, 48 and 72 h. Then, 20 µl of 0.5 mg/ml MTT
(Beyotime Institute of Biotechnology, Haimen, China) was added into
each well and incubated for another 4 h at 37°C. Following removal
of the supernatant, 150 µl dimethyl sulfoxide was
administrated to cells to dissolve formazan. The relative
absorbance at wavelength of 490 nm was measured with a microplate
reader (Model 680; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Transwell invasion and migration
assays
Cell invasion assay was performed using a Transwell
chamber (8-µm; Corning Incorporated, Corning, NY, USA)
containing a Matrigel-coated membrane (BD Bioscience, San Jose, CA,
USA) and cell migration assay was conducted in a similar fashion
without Matrigel. The transfected SOSP-9607 and Saos-2 cells
(5×105 cells) in serum-free DMEM medium were seeded in
the upper chambers, and the DMEM containing 10% FBS was added to
the lower chambers as a chemoat-tractant. Following incubation at
37°C for 24h, the cells that did not migrate or invade on the upper
chamber were removed with a cotton swab, while cells that invaded
or migrated to the lower chamber were fixed with 100% methanol for
20 min and stained with 0.5% crystal violet at room temperature for
5 min. The number of invaded or migrated cells was counted under a
light microscope (Olympus Corporation, Tokyo, Japan).
Luciferase reporter assay
The potential binding sites of miR-34a and
3′-untranslated region (UTR) of MALAT1 or CCND1 were predicted by
bioinformatics software miRcode 11 (http://www.mircode.org/) or TargetScan Human Release
7.1 (http://www.targetscan.org/vert_71/). For the
luciferase reporter assay, SOSP-9607 cells were seeded into 24-well
plates at 3×104 cells/well and cotransfected with 50 nM
miR-34a or miR-NC, 50 ng of recombinant luciferase pGL3 vectors
(Promega Corporation, Madison, WI, USA) with sites of restriction
enzymes NheI and XhoI containing the wild-type or
mutant MALAT1 or 3′-UTR of CCND1 fragments and 2 ng pRL-TK (Promega
Corporation) using Lipofectamine 2000. Cells were harvested at 48 h
post-transfection and then the luciferase activity was measured
using the Dual-Luciferase Reporter Assay System (Promega
Corporation). Renilla lucif-erase activity was used for
normalization.
RNA immunoprecipitation (RIP)
RIP was conducted using a Magna RNA-binding protein
immunoprecipitation kit (EMD Millipore, Billerica, MA, USA).
Briefly, after the centrifugation at 2,000 × g for 10 min at 4°C,
the cell pellets of SOSP-9607 transfected with miR-34a mimics were
collected and resuspended in NP-40 lysis buffer (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) supplemented with 1 mM PMSF
(Sigma-Aldrich; Merck KGaA), 1 mM dithiothreitol (Invitrogen;
Thermo Fisher Scientific., Inc.), 1% protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA), as well as 200 U/ml RNase inhibitor
(Invitrogen; Thermo Fisher Scientific., Inc.). The supernatant from
whole cell lysate was incubated with RIP buffer containing 0.5 M
EDTA, 0.1% RNase inhibitor, A + G magnetic beads conjugated with
human anti-Argonaute2 (Ago2) antibody (ab32381, 1:200; Abcam,
Cambridge, MA, USA) and IgG (PP6421-K, 1:200; EMD Millipore), as
well as a positive control (input). Following incubation overnight
at 4°C, the magnetic beads were rinsed with cold NT2 buffer to
remove the non-specific binding and then incubated with 10 mg/ml
proteinase K (Sigma-Aldrich; Merck KGaA) at 55°C for 30 min. The
co-precipitated RNAs were isolated and detected by RT-qPCR as
aforementioned to demonstrate MALAT1 and miR-34a in the
precipitates.
Western blotting
Total cell lysates were obtained from tissues and
cells with radioimmunoprecipitation assay buffer solution (Beyotime
institute of Biotechnology) containing 1% proteinase and
phosphatase inhibitors (Sangon Biotech Co., Ltd., Shanghai, China).
The protein concentration was determined using a Bicinchoninic Acid
protein assay kit (Sigma-Aldrich; Merck KGaA). The supernatant (20
µg) from cell lysates was separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.). Following blocking with 5% non-fat milk for 1
h in Tris-buffer saline with 0.05% Tween-20 buffer at room
temperature, the membranes were incubated overnight with primary
antibody against CCND1 (ab134175, 1:10,000; Abcam) and β-actin
(ab20272, 1:5,000; Abcam) at 4°C. Following washing, the membranes
were incubated for 2 h with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The protein bands were detected and visualized
using an electrochemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.) and analyzed by Image Lab software 5.2 (Bio-Rad
Laboratories, Inc.). Three replicates were involved in this
experiment.
Xenograft tumor growth assay
The animal study was approved by the Institutional
Animal Care Committee of Shangqiu First People’s Hospital. A total
of 24 female nude BALB/c mice (5-weeks-old, 15-18 g) were purchased
from Henan Experimental Animals Centre (Zhengzhou, China) and
maintained in an air content (10-15 air change/h), temperature
(20-25°C) and humidity (50-60%) controlled environment with 12-h
light/dark cycle and free access to food and water under specific
pathogen-free conditions. SOSP-9607 cells (5×106) stably
transduced with lenti-NC, lenti-MALAT1 or lenti-MALAT1 +
lenti-miR-34a were subcutaneously inocu-lated into the posterior
flank of nude mice. Tumor size was measured every 5 days starting
from 5 days post-inoculation; tumor volumes were calculated by
using the equation: Volume (mm3) = Length ×
width2/2. After 25 days, the mice were sacrificed, and
the tumors were imaged and weighted. The xenograft tumors were
excised and subjected to RT-qPCR analysis of MALAT1 and miR-34a
expression and western blot analysis of CCND1 expression as
aforementioned.
Statistical analysis
Data are presented as the mean ± standard deviation
from three independent experiments. All statistical analyses were
performed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA).
The differences between two groups were evaluated by using a
Student’s t-test or one-way analysis of variance followed by a
Bonferroni post hoc test. Spearman’s correlation analysis was
conducted on the abundances of lncRNA and miRNA or mRNA. P<0.05
was considered to indicate a statistically significant
difference.
Results
MALAT1 is highly expressed in OS and
correlates with clinical features
To address the function of MALAT1 in OS, the
expression of MALAT1 in 30 pairs of OS and adjacent normal tissues
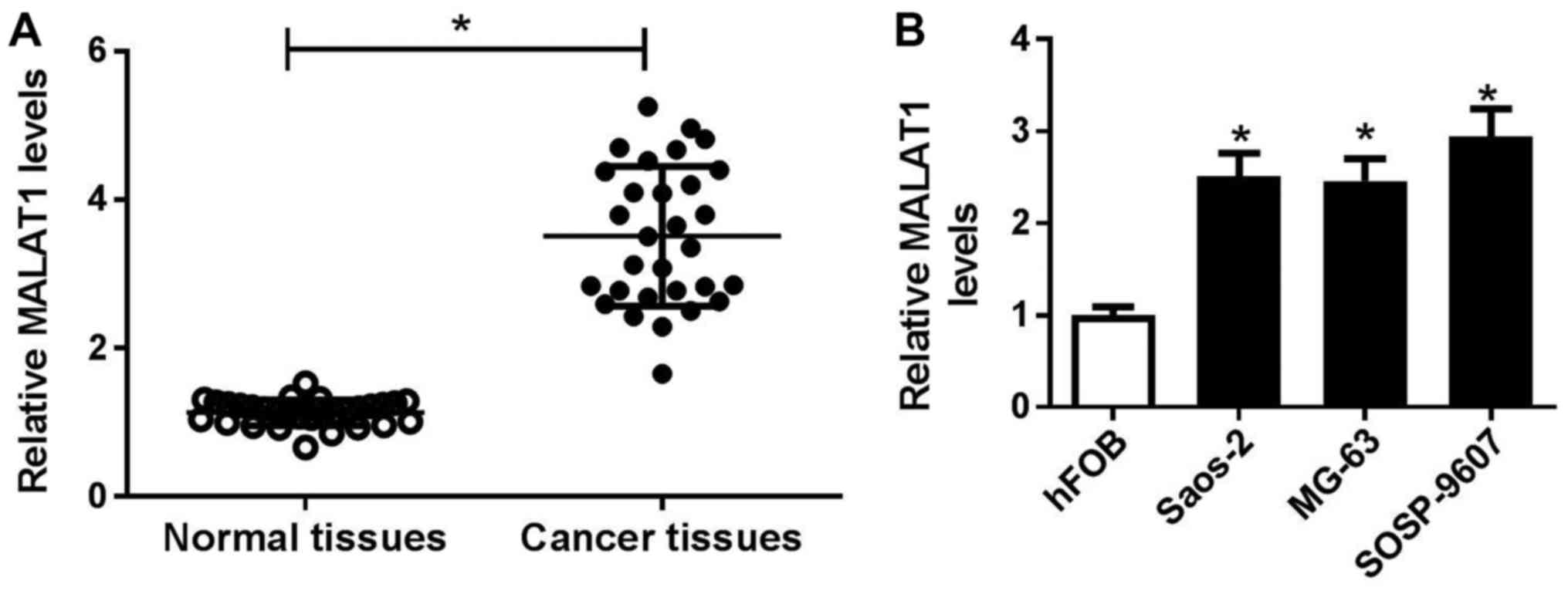
were detected by RT-qPCR. As presented in Fig. 1A, MALAT1 expression was
significantly higher in OS tissues than in adjacent normal tissues.
To determine the association of MALAT1 expression with
clinicopathologic features, including age, sex, tumor size,
clinical stage and distant metastasis were analyzed in high (n=16)
and low (n=14) MALAT1 expression groups. The results revealed that
high MALAT1 expression levels were associated with tumor size,
clinical stage and distant metastasis, respectively (P<0.05;
Table I); however, the expression
of MALAT1 was independent of age and sex (P>0.05). Additionally,
the expression levels of MALAT1 were detected in OS cell lines
(Saos-2, MG63 and SOSP-9607) and the normal human osteoblastic cell
line hFOB. RT-qPCR analysis demonstrated that MALAT1 expression was
significantly upregulated in OS cell lines compared with in hFOB
cells (Fig. 1B), particularly in
SOSP-9607 and Saos-2 cells. Therefore, SOSP-9607 and Saos-2 cells
were selected for further experiments.
MALAT1 knockdown suppresses cell
viability, migration and invasion in OS cells
To confirm the association between MALAT1 and the
development of OS, gain-of-function and loss-of-function approaches
were performed in the present study. MALAT1 was stably
overexpressed in SOSP-9607 cells via transfection of MALAT1 and
downregulated in Saos-2 cells via transfection of si-MALAT1, as
demonstrated by RT-qPCR (Fig. 2A).
An MTT assay revealed that, compared with in the control, ectopic
expression of MALAT1 significantly promoted the viability of
SOSP-9607 cells, whereas MALAT1 downregulation significantly
reduced the viability of Saos-2 cells (Fig. 2B). In addition, Transwell migration
and invasion assays demonstrated that MALAT1 overexpression led to
significant enhancements of the migration and invasive abilities of
SOSP-9607 cells compared with the control. On the contrary,
downregulation of MALAT1 resulted in a significant reduction in the
migration and invasive abilities of Saos-2 cells compared with the
control (Fig. 2C and D).
Collectively, these findings indicated that MALAT1 knockdown
delayed the progression of OS.
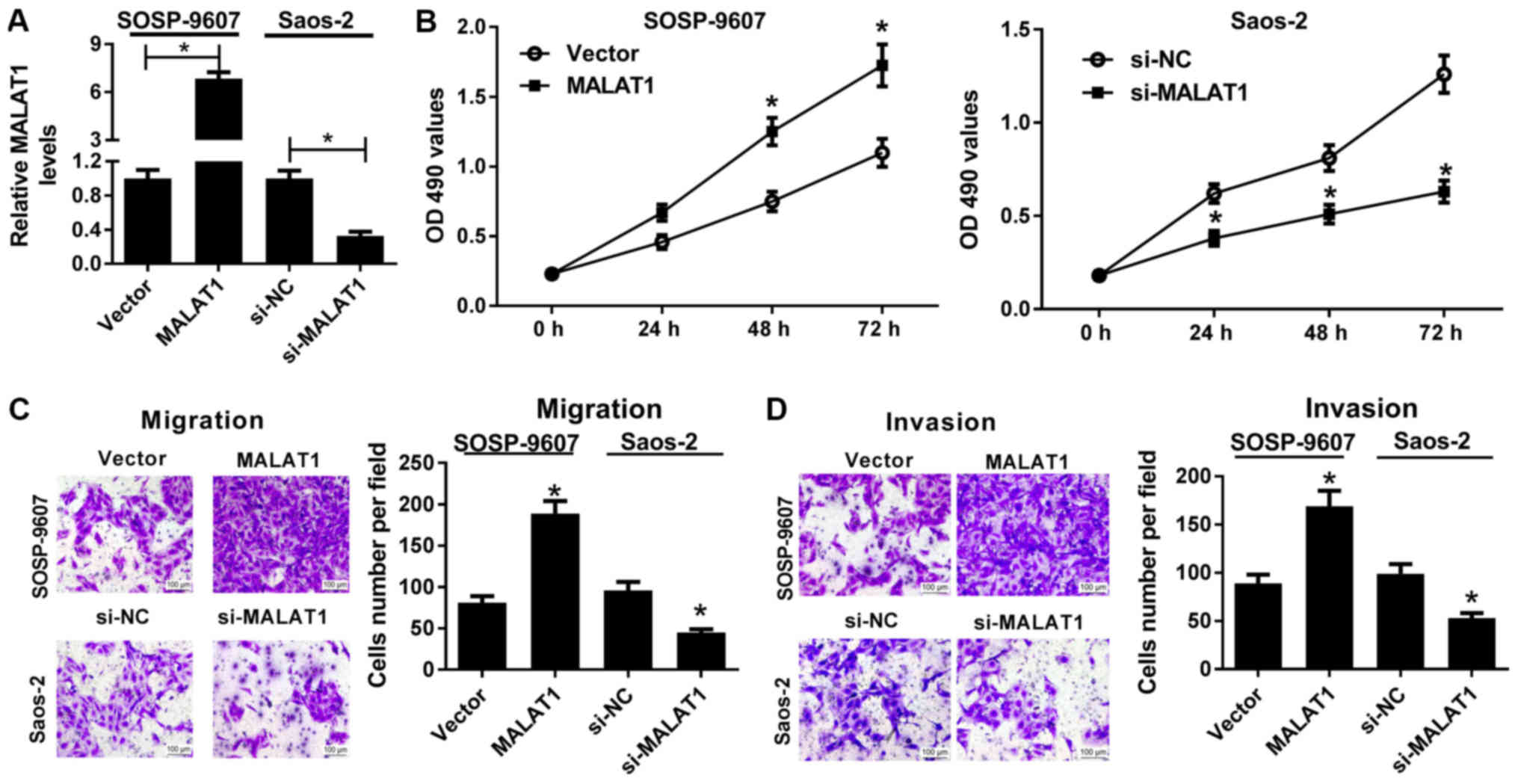 | Figure 2Effects of MALAT1 on the viability,
migration and invasion of osteosarcoma cells. SOSP-9607 cells were
transfected with MALAT1 or Vector, and Saos-2 cells were
transfected with si-MALAT1 or si-NC. (A) Reverse
transcription-quantitative polymerase chain reaction was conducted
to detect the expression of MALAT1 in the transfected SOSP-9607 and
Saos-2 cells. (B) MTT assay was employed to assess cell viability
at 0, 24, 48 and 72 h of the transfected SOSP-9607 and Saos-2
cells. (C and D) Transwell migration and invasion assays were
conducted to evaluate the migration and invasive abilities in the
transfected SOSP-9607 and Saos-2 cells. Scale bar, 100 µm.
*P<0.05 vs. Vector or si-NC. MALAT1, metastasis
associated lung adenocarcinoma transcript 1; NC, negative control;
NS, not significant; si, small interfering RNA; Vector, empty
vector control. |
MALAT1 suppresses miR-34a expression via
functioning as a ceRNA
Bioinformatics software miRcode 11 was employed to
predict the potential miRNA candidates and the results revealed
that MALAT1 contained the binding sites of miR-34a (Fig. 3A). To confirm the interaction
between MALAT1 and miR-34a, luciferase reporter and RIP assays were
performed. The results of the luciferase reporter assay
demonstrated that miR-34a mimics significantly reduced the
luciferase activity of cells transfected with wild-type MALAT1
compared with in the control, but not within cells containing
mutant MALAT1 (Fig. 3B). The RIP
assay demonstrated that MALAT1 and miR-34a were preferentially
enriched with Ago2-targeting beads compared with the IgG-treated
group (Fig. 3C). miR-34a was
observed to be significantly downregulated in OS cell lines
(Saos-2, MG63 and SOSP-9607) compared with in hFOB cells (Fig. 3D). In addition, a significant
decrease in miR-34a expression levels within OS tissues was
observed compared with in corresponding adjacent normal tissues
(Fig. 3E). Furthermore, Spearman’s
correlation analysis suggested a negative correlation between
MALAT1 and miR-34a in OS tissues (Fig.
3F). To further assess the potential association between MALAT1
and miR-34a, SOSP-9607 and Saos-2 cells were transfected with
MALAT1, si-MALAT1 or corresponding controls, respectively. RT-qPCR
analysis demonstrated that miR-34a expression levels were
significantly decreased in MALAT1-overexpressed SOSP-9607 cells and
significantly increased in si-MALAT1-transfected Saos-2 cells
compared with in the corresponding controls (Fig. 3G). The results of the present study
suggested that MALAT1 served as a molecular sponge of miR-34a to
inhibit miR-34a expression in OS cells.
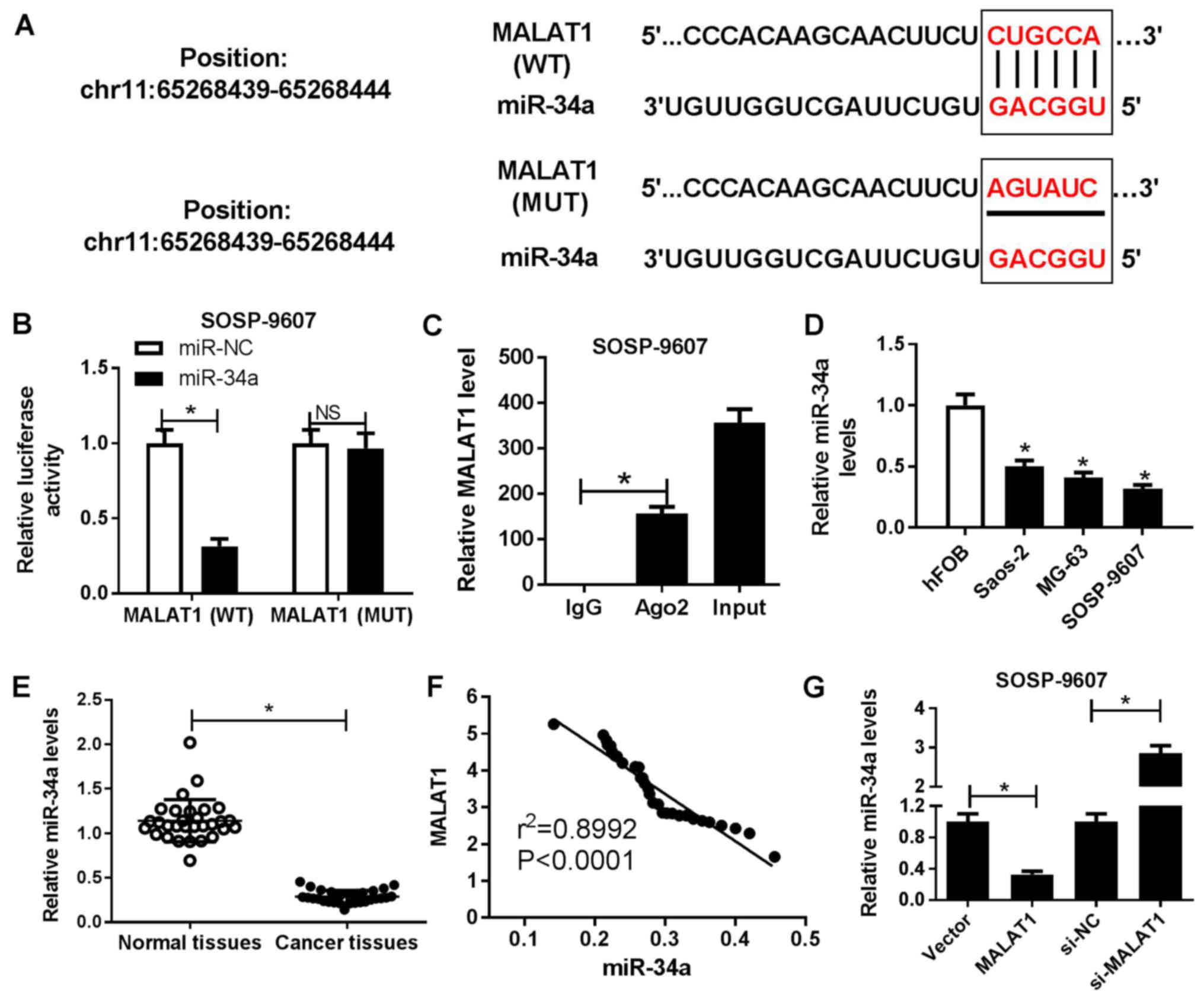 | Figure 3Regulatory association between MALAT1
and miR-34a in OS cells. (A) Predicted binding sites between
miR-34a and MALAT1, and the mutations in the MALAT1 sequence that
disrupts the interaction between miR-34a and MALAT1. (B) Luciferase
reporter assay was performed to detect the luciferase activity in
SOSP-9607 cells following cotransfection with miR-34a or miR-NC,
and the luciferase reporter plasmids containing the WT or MUT
MALAT1 sequence. (C) Association between MALAT1 and miR-34a with
Ago2 was determined by an RNA immunoprecipitation assay. MALAT1 and
miR-34a expression levels in the immunoprecipitates were examined
by RT-qPCR. (D) miR-34a expression OS cell lines (Saos-2, MG63 and
SOSP-9607) and hFOB cells was estimated by RT-qPCR. (E) miR-34a
expression levels in OS tissues and adjacent normal tissues were
detected by RT-qPCR. (F) Correlation between MALAT1 and miR-34a
expression in OS tissues. (G) Expression of miR-34a in SOSP-9607 or
saos-2 cells transfected with MALAT1, si-MALAT1 or corresponding
controls was measured by RT-qPCR. *P<0.05 vs. miR-NC,
IgG, hFOB, normal tissues, Vector or si-NC. Ago2, Argonaute2; IgG,
immunoglobulin G, MALAT1, metastasis associated lung adenocarcinoma
transcript 1; miR, microRNA; MUT, mutant; NC, negative control; OS,
osteosarcoma; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; si, small interfering RNA; Vector, empty
vector control; WT, wild-type. |
MALAT1 knockdown partially reverses
anti-miR-34a-mediated promotion of OS cell viability, migration and
invasion
To gain insight into the mechanism by which MALAT1
regulates the progression of OS, SOSP-9607 cells were transfected
with miR-34a or cotransfected with MALAT1, and Saos-2 cells
transfected with anti-miR-34a or cotransfected with si-MALAT1 were
employed to investigate the MALAT1-mediated effects of miR-34a on
cell viability, migration and invasion. RT-qPCR demonstrated that
miR-34a expression levels were significantly elevated following
transfection with miR-34a mimics in SOSP-9607 cells, whereas
overexpression of MALAT1 significantly suppressed the increase of
miR-34a expression compared with in the corresponding control
(Fig. 4A). On the contrary,
anti-miR-34a significantly reduced miR-34a expression in Saos-2
cells compared with in the control, which was significantly
reversed by MALAT1 knockdown (Fig.
4A). MTT assay demonstrated that miR-34a mimics significantly
suppressed the viability of SOSP-9607 cells compared with in the
control; overexpression of MALAT1 significantly reversed
miR-34a-induced suppres-sion of cell viability. Conversely,
anti-miR-34a significantly promoted the viability of Saos-2 cells
compared with in the control, while MALAT1 silencing significantly
abrogated the effects of anti-miR-34a on inducing cell viability
(Fig. 4B). Furthermore, Transwell
migration and invasion assays suggested that miR-34a overexpression
significantly inhibited the migration and invasion of SOSP-9607
cells compared with in the control; however, exogenous MALAT1
significantly ameliorated the inhibitory effects of miR-34a on cell
migration and invasion (Fig. 4C and
D). Furthermore, MALAT1 down-regulation significantly
attenuated anti-miR-34a-mediated promotion of migration and
invasion of Saos-2 cells (Fig. 4C and
D). These findings indicated that MALAT1 knockdown could
reverse anti-miR-34a-mediated promotion of OS cell viability,
migration and invasion.
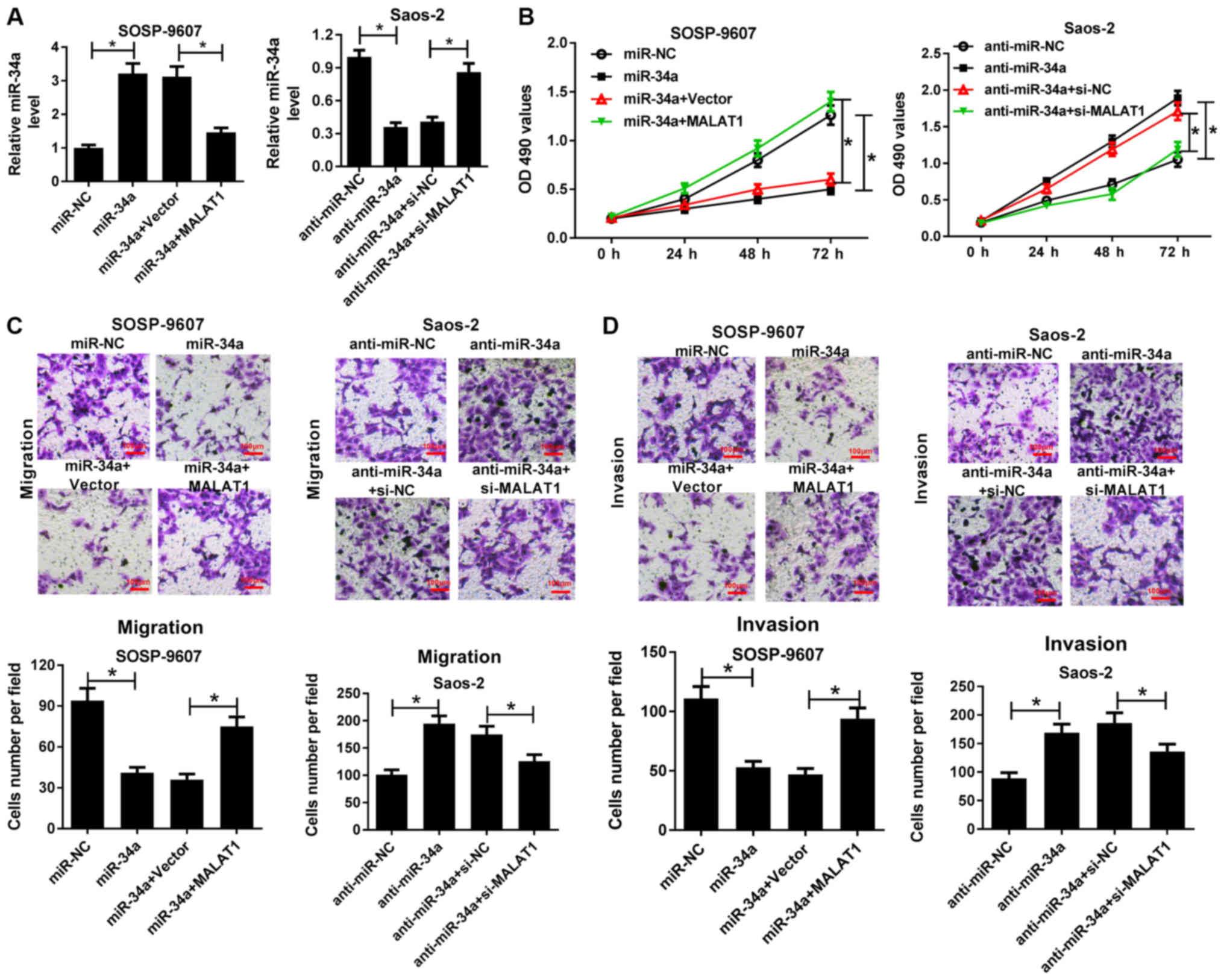 | Figure 4MALAT1 knockdown reversed
anti-miR-34a-mediated promotion of OS cell viability, migration and
invasion. SOSP-9607 cells were transfected with miR-34a or miR-NC,
or cotransfected with MALAT1 or Vector, and Saos-2 cells were
transfected with anti-miR-34a, anti-miR-NC, or cotransfected with
si-MALAT1 or si-NC. (A) Expression of miR-34a in transfected
SOSP-9607 and Saos-2 cells was determined by reverse
transcription-quantitative polymerase chain reaction. (B) Cell
viability at 0, 24, 48 or 72 h in transfected SOSP-9607 and Saos-2
cells was assessed via an MTT assay. (C and D) Migration and
invasion of the transfected SOSP-9607 and Saos-2 cells were
evaluated by Transwell migration and invasion assays. Scale bar,
100 µm. *P<0.05 vs. miR-NC, miR-34a + Vector,
anti-miR-NC or anti-miR-34a + si-NC. Anti-miR, inhibitor; MALAT1,
metastasis associated lung adenocarcinoma transcript 1; miR,
microRNA; NC, negative control, si, small interfering RNA; Vector,
empty vector control. anti-miR-NC, CCND1-WT, CCND1-WT + miR-34a +
Vector, Vector, MALAT1 + miR-NC, si-NC or anti-miR-NC + si-MALAT1.
Anti-cyclin D1; MALAT1, metastasis associated lung adenocarcinoma
transcript 1; miR, microRNA; NC, negative control; si, small
interfering RNA; UTR, region; Vector, empty vector control; WT,
wild-type. |
MALAT1 modulates CCND1 expression by
targeting miR-34a
CCND1, a regulator of the cell cycle, which is
involved in tumor progression, has been identified as a target of
miR-34a in recent studies (27,28).
Accordingly, to investigate whether miR-34a targets CCND1 in OS
cells, the putative miR-34a recognition sequence in the 3′UTR of
CCND1 was determined using TargetScan Human Release 7.1 (Fig. 5A). The subsequent luciferase
reporter assay demonstrated that miR-34a significantly suppressed
the luciferase activity of the wild-type reporter compared with in
the control, while miR-34a did not notably affect the activity of
the reporter containing the mutant sequence of the potential
binding sites (Fig. 5B). miR-34a
transfection led to a significant decrease in CCND1 expression
levels, whereas transfection of anti-miR-34a induced a substantial
increase in the expression levels of CCND1 in SOSP-9607 cells
compared with in the control (Fig.
5C); MALAT1 overexpression significantly reversed the
suppressive effects of miR-34a on the luciferase activity of
wild-type reporter compared with in the corresponding control
(Fig. 5D). Furthermore,
overexpression of MALAT1 significantly enhanced the protein
expression levels of CCND1 in SOSP-9607 cells, while restoration of
miR-34a expression inhibited the increase in CCND1 expression
levels mediated by MALAT1 overexpression (Fig. 5E). Conversely,
si-MALAT1-transfected SOSP-9607 cells exhibited a significant
decrease in CCND1 expression levels compared with in the control,
while cotransfection with anti-miR-34a significantly abrogated this
effect (Fig. 5F). Therefore, these
results suggested that MALAT1 acts as an endogenous sponge of
miR-34a to regulate CCND1 expression in OS cells.
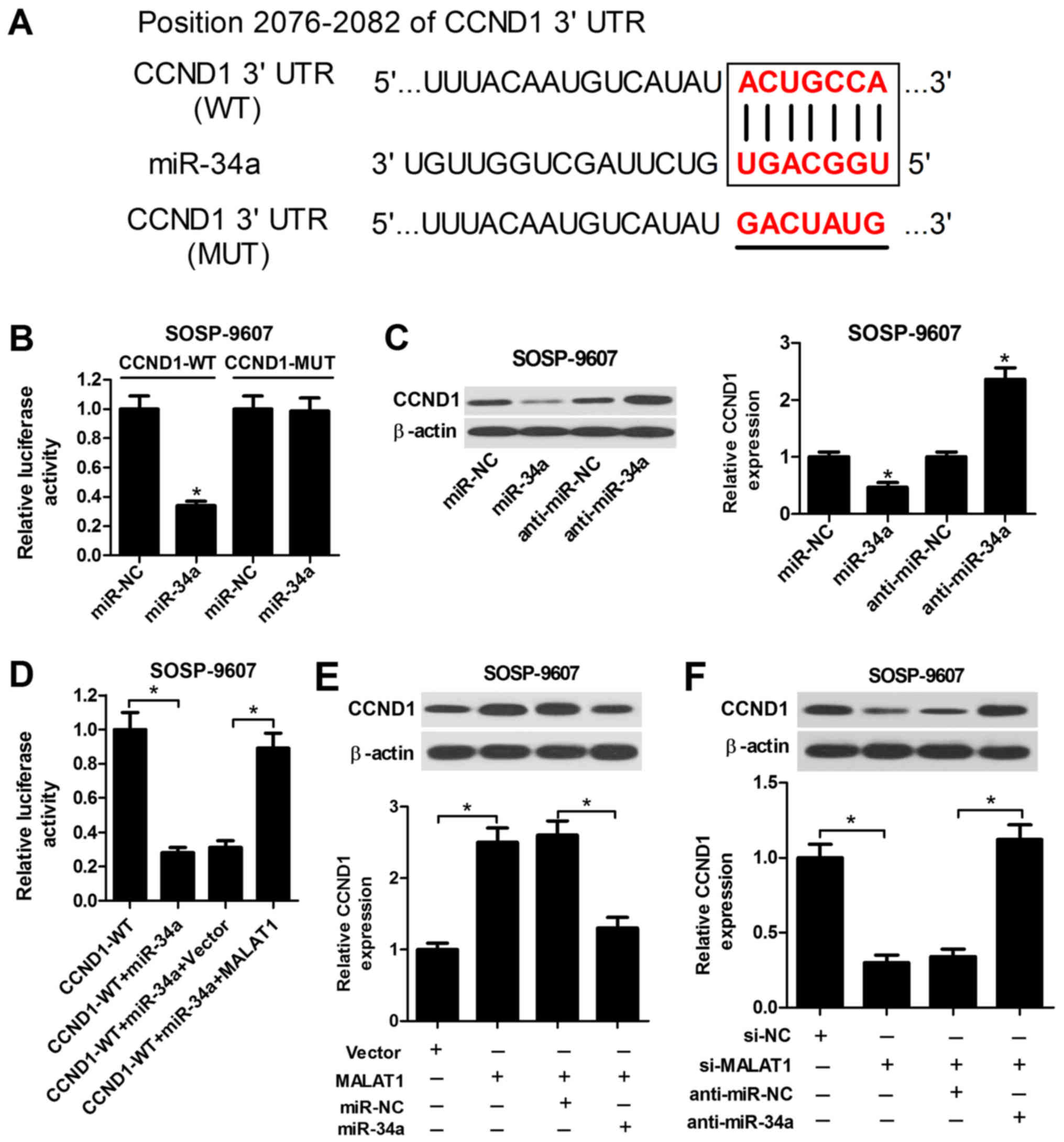 | Figure 5MALAT1 suppresses CCND1 expression by
acting as a sponge of miR‐34a. (A) Predicted miR‐34a binding sites
in the 3′UTR region of CCND1 and the corresponding mutant sequence.
(B) Luciferase activity was measured by a luciferase reporter assay
in SOSP‐9607 cells following cotransfection with CCND1 (WT) or
CCND1 (MUT), and miR‐34a or miR‐NC. (C) Western blot analysis of
CCND1 expression levels in SOSP‐9607 cells transfected with
miR‐34a, anti‐miR‐34a or corresponding controls. (D) Luciferase
activity was determined via a luciferase reporter assay in
SOSP‐9607 cells following transfection with CCND1 (WT) and miR‐34a,
miR‐34a + Vector, or miR‐34a + MALAT1. (E) Western blotting was
performed to examine the protein expression levels of CCND1 in
SOSP‐9607 cells transfected with MALAT1 or Vector, or combined with
miR‐34a or miR‐NC. (F) Western blotting was conducted to detect the
protein expression levels of CCND1 in SOSP‐9607 cells transfected
with si‐MALAT1 or si‐NC, or with anti‐miR‐34a or anti‐miR‐NC.
*P<0.05 vs. miR‐NC, anti‐miR‐NC, CCND1‐WT, CCND1‐WT + miR‐34a +
Vector, Vector, MALAT1 + miR‐NC, si‐NC or anti‐miR‐NC + si‐MALAT1.
Anti‐miR, inhibitor; CCND1, cyclin D1; MALAT1, metastasis
associated lung adenocarcinoma transcript 1; miR, microRNA; NC,
negative control; si, small interfering RNA; UTR, untranslated
region; Vector, empty vector control; WT, wild‐type. |
MALAT1 knockdown suppresses the
viability, migration and invasion of OS cells by downregulating
CCND1
RT-qPCR analysis demonstrated that CCND1 mRNA
expression was significantly higher in OS tissues than in normal
tissues (Fig. 6A) and positively
correlated with MALAT1 expression in OS tissues (Fig. 6B). In addition, the present study
reported that CCND1 mRNA expression was significantly increased
within OS cells (Saos-2, MG63 and SOSP-9607) compared with in hFOB
cells (Fig. 6C). To further
investigate the combined effects of MALAT1 and CCND1 on the
progression of OS, rescue experiments with MALAT1-transfected
SOSP-9607 cells and si-MALAT1-transfected Saos-2 cells were
conducted via CCND1 knockdown or overexpression, respectively.
Western blotting demonstrated that overexpression of MALAT1
significantly promoted CCND1 expression in SOSP-9607 cells, while
CCND1 silencing significantly reduced MALAT1
overexpression-mediated increased CCND1 expression compared with in
the corresponding control (Fig.
6D). Conversely, MALAT1 silencing significantly suppressed
CCND1 expression in Saos-2 cells compared with in the control,
which was reversed by exogenous CCND1 expression (Fig. 6D). Subsequently, an MTT assay
revealed that CCND1 downregulation significantly reduced the
promotion of viability induced by MALAT1 overexpression in
SOSP-9607 cells; however, increased CCND1 expression significantly
reversed the inhibition of cell viability mediated by MALAT1
downregulation compared with in the corresponding control (Fig. 6E). Furthermore, Transwell migration
and invasion assays indicated that MALAT1 overexpression-induced
cell migration and invasion were significantly mitigated following
CCND1 silencing in SOSP-9607 cells. On the contrary, MALAT1
silencing-induced inhibition of migration and invasion were
significantly ameliorated by CCND1 overexpression in Saos-2 cells
compared with in the control (Fig. 6F
and G). Therefore, these results indicated that MALAT1
knockdown suppressed cell viability, migration and invasion in OS
cells by downregulation of CCND1 expression.
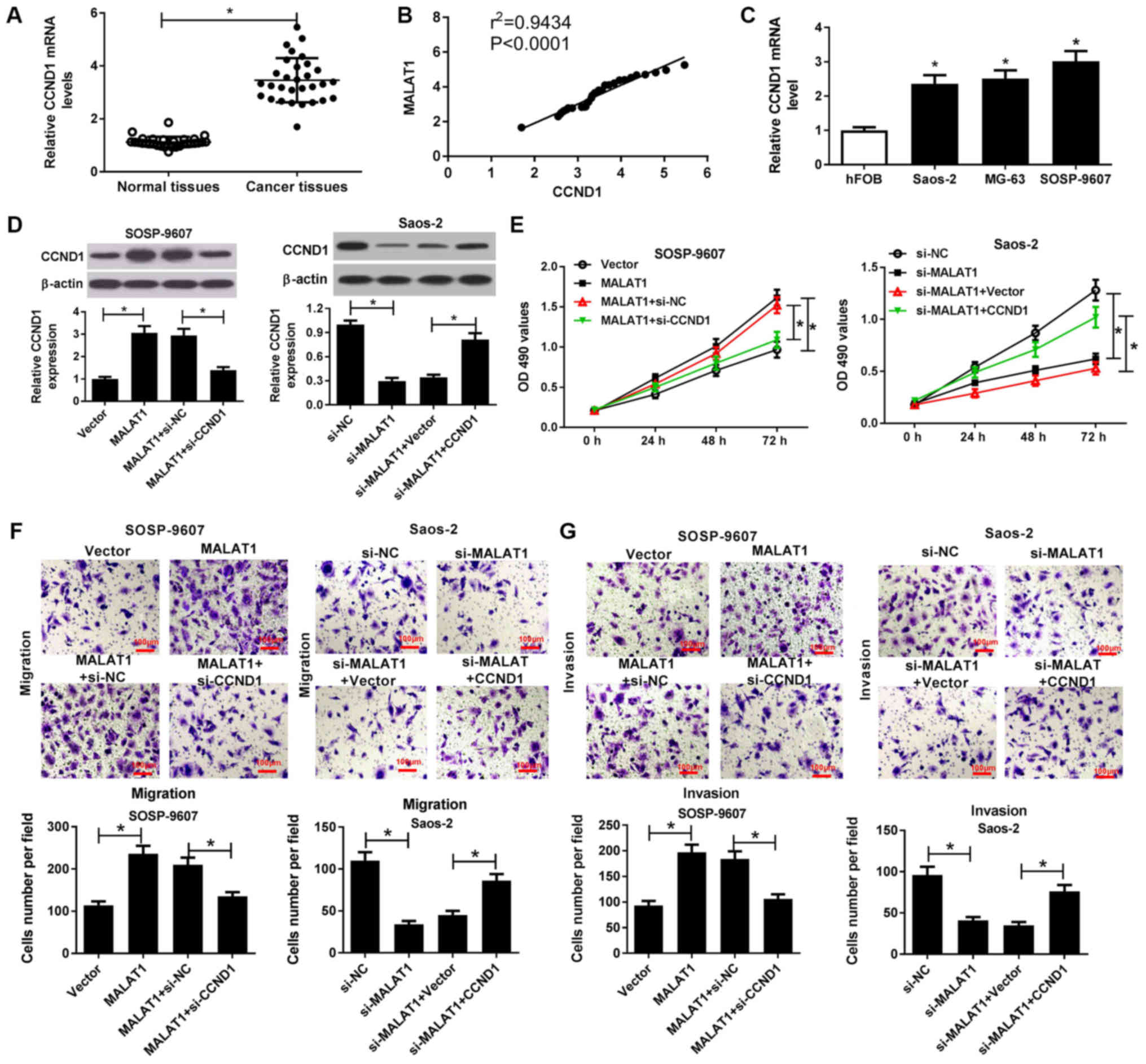 | Figure 6MALAT1 knockdown suppresses the
viability, migration and invasion of OS cells by downregulation
CCND1. (A) CCND1 mRNA expression in OS tissues and normal tissues
was detected by RT-qPCR. (B) Correlation between MALAT1 and CCND1
expression in OS tissues. (C) CCND1 mRNA expression in OS cells
(Saos-2, MG63 and SOSP-9607) and hFOB cells was evaluated by
RT-qPCR. SOSP-9607 cells were transfected with MALAT1, Vector,
MALAT1 + si-NC, or MALAT1 + si-CCND1; Saos-2 cells were transfected
with si-MALAT1, si-NC, si-MALAT1 + CCND1 or si-MALAT1 + Vector, and
subjected to analysis at 48 h post-transfection. (D) Protein
expression levels of CCND1 in transfected SOSP-9607 and Saos-2
cells were detected by western blotting. (E) Viability of the
transfected SOSP-9607 and Saos-2 cells was assessed by an MTT
assay. (F and G) Transwell migration and invasion assays were
conducted to evaluate the migration and invasive ability of
transfected SOSP-9607 and Saos-2 cells. Scale bar, 100 µm.
*P<0.05 vs. normal tissues, hFOB, Vector, si-NC,
MALAT1 + si-NC, si-MALAT1 + Vector. CCND1, cyclin D1; MALAT1,
metastasis associated lung adenocarcinoma transcript 1; miR,
microRNA; NC, negative control; OS, osteosarcoma; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; si,small
interfering RNA; Vector, empty vector control. |
MALAT1 promotes OS tumor growth in vivo
by inhibiting miR-34a and upregulating CCND1
To validate whether MALAT1 affects OS tumorigenesis
in vivo, SOSP-9607 cells stably transfected with lenti-NC,
lenti-MALAT1, or lenti-MALAT1 + lenti-miR-34a were subcutaneously
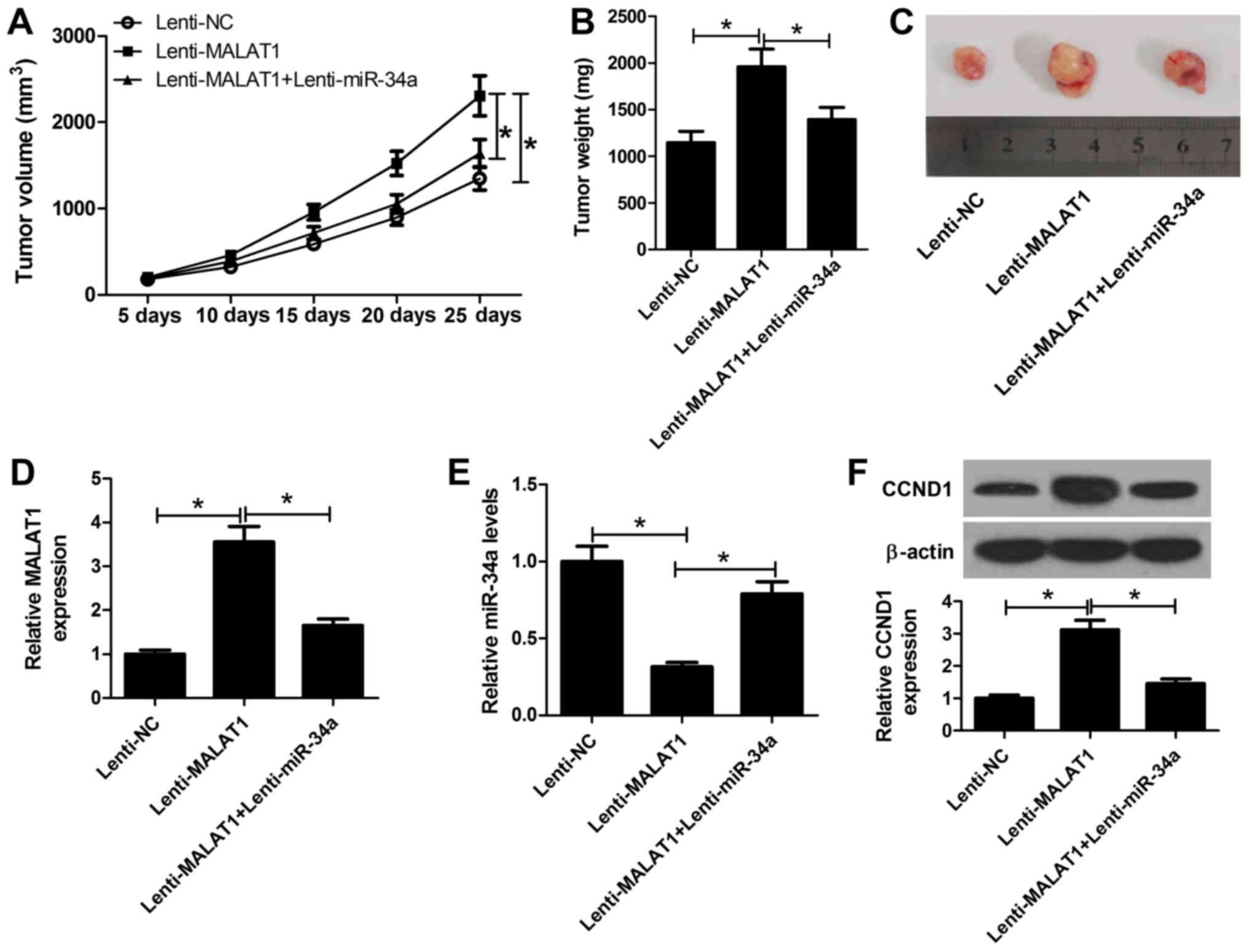
inoculated into nude mice. As presented in Fig. 7A, tumor growth was significantly
promoted via exogenous overexpression of MALAT1 compared with in
the control; however, overexpression of miR-34a significantly
suppressed the effects of MALAT1 on tumor growth. Additionally,
tumor weight in the MALAT1-overexpressing group was significantly
higher compared with in the control group, which was significantly
reduced in the MALAT1 and miR-34a overexpression group (Fig. 7B and C). Then, RT-qPCR analyses of
MALAT1 and miR-34a expression in the excised tumors were performed.
The results indicated that MALAT1 expression was significantly
upregulated in the lenti-MALAT1 group, but decreased following the
transduction of lenti-miR-34a (Fig.
7D). In addition, ectopic expression of MALAT1 led to a
significant decrease in miR-34a expression within xenograft tumor
tissues, which was reversed via the overexpression of miR-34a
(Fig. 7E). Additionally, western
blot analysis revealed that the expression levels of CCND1 in the
lenti-MALAT1 group were significantly increased compared with in
the control; however, a significant reduction in the lenti-MALAT1 +
enti-miR-34a group was also observed (Fig. 7F). Collectively, the results of the
present study indicated that MALAT1 promoted OS tumor growth in
vivo via the miR-34a/CCND1 axis.
Discussion
Numerous studies have suggested that alterations in
the expression of certain lncRNAs serve crucial roles the
tumorigenesis and progression of several cancer types, such as OS
(6,7,29).
For instance, upregulated lncRNA Hox transcript antisense
intergenic RNA promoted the viability, invasion and migration of OS
cells via the activation of the protein kinase B (Akt)/mechanistic
target of rapamycin pathway (30).
LncRNA colorectal neoplasia differentially exerted its oncogenic
role in OS cells via enhancing the activity of Notch1 signaling and
promoting epithelial-mesenchymal transition (31). LncRNA forkhead box F1 adjacent
non-coding developmental regulatory RNA reversed doxorubicin
resistance and suppressed the progression of OS cells by
downregulating ATP binding cassette subfamily B member 1 and ATP
binding cassette subfamily C member 1 (32); the present study investigated the
role of MALAT1.
In recent years, accumulating evidence has suggested
a positive association between MALAT1 and the progres-sion of OS
(33). For instance, MALAT1
silencing delayed the progression of OS by altering the expression
and localization of β-catenin (34). Additionally, high serum levels of
MALAT1 predicted poor survival in OS patients; overexpression of
MALAT1 promoted cell metastasis and decreased E-cadherin levels by
associating with enhancer of zeste homolog 2 (35). Furthermore, MALAT1 was proposed as
an oncogenic lncRNA that promoted tumor growth and metastasis of OS
via activation of the phosphoinositide 3-kinase/Akt signaling
pathway (36). Therefore, MALAT1
may be a promising therapeutic target for the treatment of patients
with OS (37). In the present
study, the oncogenic role of MALAT1 was also reported in OS as
MALAT1 was highly expressed in OS tissues and cells. In addition,
the correlation between MALAT1 expression and the
clinicopathological features of patients with OS were reported.
This was in accordance with recent findings, which suggested MALAT1
as a predictor of poor survival, and was associated with tumor
size, stage and metastasis in patients with OS (16). Additionally, ectopic expression of
MALAT1 enhanced the progression of OS by promoting cell viability,
migration and invasion, while MALAT1 silencing elicited opposing
effects, which was consistent with previous studies (17).
In recent years, compelling evidence has suggested
that lncRNAs act as ceRNAs or a molecular sponge to antagonize the
expression and functions of miRNAs, modulating the derepression of
these miRNAs targets via post-transcriptional regulation (38). The crosstalk between lncRNAs and
miRNAs has been associated with the pathogenesis of human diseases,
such as cancer (39). For
instance, enhanced MALAT1 expression levels served as a predictor
of unfavorable outcomes in OS and promoted OS progression via
miR-205 suppression and activating mothers against decapentaplegic
homolog 4 function (16). LncRNA
X-inactive specific transcript inhibited OS cell growth and
mobility by competitively binding to miR-21-5p and upregulating
programmed cell death 4 (40). To
determine the underlying mechanism by which MALAT1 promotes the
progression of OS, a functional role of MALAT1 as a miRNA decoy for
miR-34a was determined; MALAT1 suppressed miR-34a expression in OS
cells in the present study. Conversely, a previous study revealed
that miR-34a may be independent of MALAT1 by an RNA pulldown assay
(41). This may be due to the
presence of surfactant and the dilution of miR-34a by addition of
cell lysate buffer, which may induce false negative results during
immunoprecipitation. miR-34a has been proposed as a direct
transcriptional target of the tumor suppressor p53 (42). The inactivating mutations of p53
often induce reductions in the expression of miR-34a within tumors
(43). miR-34a has been frequently
reported as a tumor suppressor with lost or reduced expression in
various types of tumors (44). In
addition, miR-34a was regarded as a novel prognostic biomarker and
correlated with tumor size and stage in OS (45). The present study reported that
miR-34a was downregulated in OS tissues and cells, in accordance
with previous studies (24,25).
Additionally, MALAT1 was negatively correlated with miR-34a
expression in OS cells. Rescue experiments further revealed that
MALAT1 knockdown partially reversed anti-miR-34a-mediated increases
in OS cell viability, migration and invasion, suggesting that
MALAT1 exerted its oncogenic role in OS by functioning as a sponge
of miR-34a.
CCND1, one of the highly conserved members of the
cyclin family, is well-known for its role in the response to the
mitogenic signals that regulate of the G1 phase of the cell cycle
(46). CCND1 has been notably
associated with tumorigenesis and metastasis in clinical studies
and in vivo experiments (46-48).
The overexpression of CCND1 is frequently observed in a variety of
tumors and is thus regarded as an oncogene in numerous human
tumors, including breast, colon and prostate cancers (49,50).
CCND1 was also reported to be upregulated in OS and contributed to
the progression of OS (51).
Interestingly, previous studies have demonstrated that miR-34a
could directly target the 3′UTR of CCND3 in laryngeal carcinoma
cells (27), breast cancer
(28), and lung cancer (52). Similarly, it was demonstrated that
CCND1 was identified as a target of miR-34a and that miR-34a
suppressed CCND1 expression in OS cells in the present study.
Furthermore, MALAT1 was proposed to function as a sponge of miR-34a
to positively regulate CCND1 expression in OS cells. Mechanistic
analyses demonstrated that overexpression of CCND1 partially
reversed the effects of MALAT1 silencing on OS cell viability,
invasion and migration. In addition, in vivo experiments
demonstrated that MALAT1 promoted OS tumor growth via miR-34a
inhibition. Therefore, the present study proposed that MALAT1
exerted its oncogenic role in OS in vitro and in vivo
by regulating the miR-34a/CCND1 axis.
In conclusion, it was demonstrated that MALAT1 was
upregulated in OS and associated with the clinicopathological
features of OS. Knockdown of MALAT1 suppressed the progression of
OS by stimulating miR-34a expression and in turn inhibiting CCND1
expression. To the best of our knowl-edge, the present study is the
first to report of the crosstalk between MALAT1, miR-34a and CCND1,
providing novel insight into the diagnosis and therapy of OS.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors’ contributions
GD contributed to the experimental design, most
experiments and analysis. GD also drafted the manuscript and
collected clinical samples. CZ, ChX and CX conducted the
experiments and analyzed the data. LZ and YZ were conducted in
vivo experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Shangqiu First People’s Hospital and written
informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cortini M, Avnet S and Baldini N:
Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett.
405:90–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao X, Wang W and Wang Z: The role of
chemotherapy for metastatic, relapsed and refractory osteosarcoma.
Paediatr Drugs. 16:503–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maire G, Martin JW, Yoshimoto M,
Chilton-MacNeill S, Zielenska M and Squire JA: Analysis of
miRNA-gene expression-genomic profiles reveals complex mechanisms
of microRNA deregulation in osteosarcoma. Cancer Genet.
204:138–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu X, Sood AK, Dang CV and Zhang L: The
role of long noncoding RNAs in cancer: The dark matter matters.
Curr Opin Genet Dev. 48:8–15. 2018. View Article : Google Scholar
|
|
7
|
Sanchez Calle A, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li CH and Chen Y: Targeting long
non-coding RNAs in cancers: Progress and prospects. Int J Biochem
Cell Biol. 45:1895–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheetham SW, Gruhl F, Mattick JS and
Dinger ME: Long noncoding RNAs and the genetics of cancer. Br J
Cancer. 108:2419–2425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gutschner T, Hämmerle M and Diederichs S:
MALAT1 - a paradigm for long noncoding RNA function in cancer. J
Mol Med (Berl). 91:791–801. 2013. View Article : Google Scholar
|
|
11
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brauze D, Mikstacka R and Pelkonen O:
Monoclonal antibody characterization of NADH- and NADPH-dependent
hydroxylation of benzo(a)pyrene in liver microsomes from
5,6-benzoflavone-induced C57Bl/6 mice. Acta Biochim Pol.
37:219–225. 1990.PubMed/NCBI
|
|
13
|
Huang NS, Chi YY, Xue JY, Liu MY, Huang S,
Mo M, Zhou SL and Wu J: Long non-coding RNA metastasis associated
in lung adenocarcinoma transcript 1 (MALAT1) interacts with
estrogen receptor and predicted poor survival in breast cancer.
Oncotarget. 7:37957–37965. 2016.PubMed/NCBI
|
|
14
|
Konishi H, Ichikawa D, Yamamoto Y, Arita
T, Shoda K, Hiramoto H, Hamada J, Itoh H, Fujita Y, Komatsu S, et
al: Plasma level of metastasis-associated lung adenocarcinoma
transcript 1 is associated with liver damage and predicts
development of hepatocellular carcinoma. Cancer Sci. 107:149–154.
2016. View Article : Google Scholar :
|
|
15
|
Guo F, Li Y, Liu Y, Wang J, Li Y and Li G:
Inhibition of metas-tasis-associated lung adenocarcinoma transcript
1 in CaSki human cervical cancer cells suppresses cell
proliferation and invasion. Acta Biochim Biophys Sin (Shanghai).
42:224–229. 2010. View Article : Google Scholar
|
|
16
|
Li Q, Pan X, Wang X, Jiao X, Zheng J, Li Z
and Huo Y: Long noncoding RNA MALAT1 promotes cell proliferation
through suppressing miR-205 and promoting SMAD4 expression in
osteosarcoma. Oncotarget. 8:106648–106660. 2017.
|
|
17
|
Wang Y, Zhang Y, Yang T, Zhao W, Wang N,
Li P, Zeng X and Zhang W: Long non-coding RNA MALAT1 for promoting
metastasis and proliferation by acting as a ceRNA of miR-144-3p in
osteosarcoma cells. Oncotarget. 8:59417–59434. 2017.PubMed/NCBI
|
|
18
|
Luo W, He H, Xiao W, Liu Q, Deng Z, Lu Y,
Wang Q, Zheng Q and Li Y: MALAT1 promotes osteosarcoma development
by targeting TGFA via MIR376A. Oncotarget. 7:54733–54743. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang S, Sun Z, Zhou Q, Wang W, Wang G,
Song J, Li Z, Zhang Z, Chang Y, Xia K, et al: MicroRNAs, long
noncoding RNAs, and circular RNAs: Potential tumor biomarkers and
targets for colorectal cancer. Cancer Manag Res. 10:2249–2257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dang Y, Wei X, Xue L, Wen F, Gu J and
Zheng H: Long non-coding RNA in glioma: Target miRNA and signaling
pathways. Clin Lab. 64:887–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gang L, Qun L, Liu WD, Li YS, Xu YZ and
Yuan DT: MicroRNA-34a promotes cell cycle arrest and apoptosis and
suppresses cell adhesion by targeting DUSP1 in osteosarcoma. Am J
Transl Res. 9:5388–5399. 2017.
|
|
25
|
Wen J, Zhao YK, Liu Y and Zhao JF:
MicroRNA-34a inhibits tumor invasion and metastasis in osteosarcoma
partly by effecting C-IAP2 and Bcl-2. Tumour Biol.
39:10104283177057612017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Ye J, Li L, Feng P, Wan J and Li J:
Downregulation of miR-34a contributes to the proliferation and
migration of laryngeal carcinoma cells by targeting cyclin D1.
Oncol Rep. 36:390–398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li ZH, Weng X, Xiong QY, Tu JH, Xiao A,
Qiu W, Gong Y, Hu EW, Huang S and Cao YL: miR-34a expression in
human breast cancer is associated with drug resistance. Oncotarget.
8:106270–106282. 2017.
|
|
29
|
Li Z, Dou P, Liu T and He S: Application
of long noncoding RNAs in osteosarcoma: Biomarkers and therapeutic
targets. Cell Physiol Biochem. 42:1407–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li E, Zhao Z, Ma B and Zhang J: Long
noncoding RNA HOTAIR promotes the proliferation and metastasis of
osteosarcoma cells through the AKT/mTOR signaling pathway. Exp Ther
Med. 14:5321–5328. 2017.PubMed/NCBI
|
|
31
|
Li Z, Tang Y, Xing W, Dong W and Wang Z:
LncRNA, CRNDE promotes osteosarcoma cell proliferation, invasion
and migration by regulating Notch1 signaling and
epithelial-mesenchymal transition. Exp Mol Pathol. 104:19–25. 2018.
View Article : Google Scholar
|
|
32
|
Kun-Peng Z, Xiao-Long M and Chun-Lin Z:
LncRNA FENDRR sensitizes doxorubicin-resistance of osteosarcoma
cells through downregulating ABCB1 and ABCC1. Oncotarget.
8:71881–71893. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao KT and Lian D: Long non-coding RNA
MALAT1 is an independent prognostic factor of osteosarcoma. Eur Rev
Med Pharmacol Sci. 20:3561–3565. 2016.PubMed/NCBI
|
|
34
|
Zhang ZC, Tang C, Dong Y, Zhang J, Yuan T
and Li XL: Targeting LncRNA-MALAT1 suppresses the progression of
osteosarcoma by altering the expression and localization of
β-catenin. J Cancer. 9:71–80. 2018. View Article : Google Scholar :
|
|
35
|
Huo Y, Li Q, Wang X, Jiao X, Zheng J, Li Z
and Pan X: MALAT1 predicts poor survival in osteosarcoma patients
and promotes cell metastasis through associating with EZH2.
Oncotarget. 8:46993–47006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar
|
|
37
|
Cai X, Liu Y, Yang W, Xia Y, Yang C, Yang
S and Liu X: Long noncoding RNA MALAT1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 34:932–941. 2016. View Article : Google Scholar
|
|
38
|
Sen R, Ghosal S, Das S, Balti S and
Chakrabarti J: Competing endogenous RNA: The key to
posttranscriptional regulation. ScientificWorldJournal.
2014.896206:2014.
|
|
39
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859.169–176. 2016.
|
|
40
|
Zhang R and Xia T: Long non-coding RNA
XIST regulates PDCD4 expression by interacting with miR-21-5p and
inhibits osteosarcoma cell growth and metastasis. Int J Oncol.
51:1460–1470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leucci E, Patella F, Waage J, Holmstrøm K,
Lindow M, Porse B, Kauppinen S and Lund AH: microRNA-9 targets the
long non-coding RNA MALAT1 for degradation in the nucleus. Sci Rep.
3:25352013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bommer GT, Gerin I, Feng Y, Kaczorowski
AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Misso G, Di Martino MT, De Rosa G, Farooqi
AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P,
Tassone P, et al: Mir-34: A new weapon against cancer? Mol Ther
Nucleic Acids. 3:e1942014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Jia LS, Yuan W, Wu Z, Wang HB, Xu
T, Sun JC, Cheng KF and Shi JG: Low miR-34a and miR-192 are
associated with unfavorable prognosis in patients suffering from
osteosarcoma. Am J Transl Res. 7:111–119. 2015.PubMed/NCBI
|
|
46
|
Li Z, Wang C, Prendergast GC and Pestell
RG: Cyclin D1 functions in cell migration. Cell Cycle. 5:2440–2442.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ramos-García P, González-Moles MA,
González-Ruiz L, Ruiz-Ávila I, Ayén Á and Gil-Montoya JA:
Prognostic and clinicopathological significance of cyclin D1
expression in oral squamous cell carcinoma: A systematic review and
meta-analysis. Oral Oncol. 83:96–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo J, Xu LN, Zhang SJ, Jiang YG, Zhuo DX,
Wu LH, Jiang X and Huang Y: Downregulation of LncRNA-RP11-317J10.2
promotes cell proliferation and invasion and predicts poor
prognosis in colorectal cancer. Scand J Gastroenterol. 53:38–45.
2018. View Article : Google Scholar
|
|
49
|
Inoue K and Fry EA: Aberrant expression of
cyclin D1 in cancer. Signal Transduct Insights. 4:1–13. 2015.
View Article : Google Scholar
|
|
50
|
Ju X, Casimiro MC, Gormley M, Meng H, Jiao
X, Katiyar S, Crosariol M, Chen K, Wang M, Quong AA, et al:
Identification of a cyclin D1 network in prostate cancer that
antagonizes epithelial-mesenchymal restraint. Cancer Res.
74:508–519. 2014. View Article : Google Scholar :
|
|
51
|
Cai CK, Zhao GY, Tian LY, Liu L, Yan K, Ma
YL, Ji ZW, Li XX, Han K, Gao J, et al: miR-15a and miR-16-1
downregulate CCND1 and induce apoptosis and cell cycle arrest in
osteosarcoma. Oncol Rep. 28:1764–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R,
Sun Z and Zheng X: Downregulation of CCND1 and CDK6 by miR-34a
induces cell cycle arrest. FEBS Lett. 582:1564–1568. 2008.
View Article : Google Scholar : PubMed/NCBI
|