Introduction
Lung cancer is the most common malignant carcinoma
worldwide (1). Due to its complex
etiological mechanisms, rapid disease progression and lack of
effective therapeutic drugs, it has become the main cause of
cancer-associated mortality. Therefore, finding methods with which
to effectively delay the progression of lung cancer, or treat the
disease, is of great clinical concern.
Lung adenocarcinoma is the most common type of lung
cancer (2,3). The proliferation and metastasis of
lung adenocarcinoma is key for disease progression (4). The excessive activation of multiple
cell factors, oxidative stress factors and signaling pathways is
known to contribute to tumor cell proliferation and metastasis
(5,6). However, the precise reason for the
proliferation and metastasis of lung adenocarcinoma remains
unclear.
Elevated epidermal growth factor receptor
(EGFR)-mediated proliferative signaling is an independent factor
leading to tumor progression (6),
although anti-EGFR therapies fail to exhibit satisfactory efficacy
in some patients with lung cancer (7). In addition to EGFR mutations, recent
studies have found that a mutation in the RNA-binding protein 10
(RBM10) gene is also an important event in tumor progression in
certain types of cancer (2,8-12).
RBM10 is involved in the tissue damage repair and various
cell processes (13,14). RBM10 mutations can result in
the continuous proliferation and infiltration of tissues and cells
(14,15), and therefore accelerate disease
progression. It was thus hypothesized that RBM10 protein
overexpression may be essential, not only for causing tumor cell
proliferation and metastasis, but also for the proliferation of
stromal cells in the tumor microenvironment and epimatrix
deposition.
Based on previous studies, we hypothesized that
apoptosis would be inhibited and multiple proliferative signaling
pathways would be excessively activated (likely due to inflammation
or oxidative stress) in lung adenocarcinomas. However, whether
RBM10 represents a common target for controlling the activity of
such pathways, or for promoting apoptosis, is unclear.
In this study, we examined the effects of RBM10
overexpression and downregulation on lung tumor proliferation and
apoptosis in vitro and in vivo. We also determined
whether RBM10 expression is associated with the prognosis of lung
cancer. This study provides important insight into the effects
caused by RBM10 gene mutations in lung adenocarcinomas.
Materials and methods
Cells, cell culture and passage
Human lung adenocarcinoma cell lines (A549 and
H1299) and human lung fibroblast cells (HLF), were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA) and
maintained in the Central Laboratory of Dalian Medical University
(Dalian, China). The H1299 and A549 cells were grown in RPMI-1640
medium, which contained 10% fetal bovine serum and the HLF cells
were cultured in DMED/F12 medium. All the cells were maintained in
a 37˚C incubator under an atmosphere of 5% CO2. The
medium was changed every day and passaged when the cells grew to
80% confluence following trypsinization.
Lung tissue specimens
Lung tissue specimens were collected from patients
with lung adenocarcinoma (n=6) and adjacent non-cancerous tissues
(NCTs) (n=6) were also collected for use in immunohistochemistry
and western blot analysis (all samples were collected from April,
2015 to October, 2015). These patients were operated and diagnosed
as having lung adenocarcinoma at the First Affiliated Hospital of
Dalian Medical University. None of the patients had received
chemotherapy or radiotherapy prior to surgery. The samples were
stored at −80°C until use in the experiments. Informed consent was
obtained from all patients. This study was approved by the Human
Research Ethics Committee of the First Affiliated Hospital of
Dalian Medical University.
Lung adenocarcinoma tissue
microarray
Survival data from a lung adenocarcinoma tissue
microarray (90 lung adenocarcinoma tissues and 90 NCTs) were
provided by Shanghai Outdo Biotech (Shanghai, China). None of the
patients received chemotherapy or radiotherapy prior to surgery and
had completed 5 years of follow-up.
Construction and transfection of high and
low RBM10 expression cell strains
The pcDNA3.1RBM10 plasmid vector and RBM10 siRNA
were constructed by ShangHai GenePharma Co. (Shanghai, China). The
sequences of RBM10 siRNA were as follows: RBM10 siRNA-1,
5'-CCGAGAGAAGUGCUUCAAATT-3'; RBM10 siRNA-2,
5'-CCACACAGCACCAUGGAUUTT-3'; RBM10 siRNA-3,
5'-GGACAUGGCCUCCAAUGAATT-3'. The sequence of the negative control
(mock) siRNA was as follows: 5'-UUCUCCGAACGUGUCACGUTT-3'. RBM10
shRNAs were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
sequences of RBM10 shRNA were as follows: RBM10 shRNA-D1,
5'-CCGGCAAGGGTTCTAAGAGGGACATCTCGAGATGTCCCTCTTAGAACCCTTGTTTTTG-3';
RBM10 shRNA-D2,
5'-CCGGCAAGACCATCAATGTTGAGTTCTCGAGAACTCAACATTGATGGTCTTGTTTTTG-3';
RBM10 shRNA-D3,
5'-CCGGGACATGGACTACCGTTCATATCTCGAGATATGAACGGTAGTCCATGTCTTTTTG-3';
RBM10 shRNA-D4,
5'-CCGGGCCCGCAGTCTCAACAAACAACTCGAGTTGTTTGTTGAGACTGCGGGCTTTTTG-3'.
The A549 and H1299 cells in the third generation
logarithmic growth phase were selected. A total of 2×103
cells per well were inoculated in a sterile 24-well culture plate
for 12 h. For the overexpression of RBM10, the cells were
transfected with the pcDNA3.1RBM10 plasmid with liposome particles.
To inhibit RBM10 expression, the cells were transfected with
RBM10siRNA or RBM10 shRNA, using Lipofectamine 2000
(Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA). A mock
transfection was also performed using an empty vector. At 48 h
following transfection, protein expression was examined by western
blot analysis.
Western blot analysis
The cells were harvested following trypsinization
and washed 3 times using PBS. A total of 1×107 cells
were incubated in RIPA lysis buffer for 60 min at 4°C. The cells
were pipetted every 10 min during incubation and centrifuged at
20,800 × g for 2 min at 4°C. The supernatant was harvested and the
protein concentration was measured using the BCA kit (GenePharma
Co.) according to manufacturer's instructions, prior to storage at
−20°C.
A 5% stacking gel and 12.5% acrylamide separating
gel were used in the procedure. A 10 µl sample was then mixed with
5X loading buffer at a 4:1 ratio. Following denaturation by
boiling, the samples were loaded and resolved via 10% SDS-PAGE
followed by transfer onto a nitrocellulose membrane. The membrane
was blocked in 5% non-fat milk in PBS for 1 h at room temperature
and incubated with the primary antibody overnight at 4°C, followed
by the addition of the secondary antibody for 2 h at room
temperature. The membrane was washed for 5 min in TBST 3 times, and
then visualized using the cECL kit (Zhong Shan-Golden Bridge
Biological Technology, Beijing, China). Quantification of the bands
was carried out using the Molecular Imager Chemi Doc XRS + Imaging
System (Bio-Rad Laboratories, Hercules, CA, USA).Western blot
analysis was repeated 3 times. The antibodies used were as follows:
Rabbit anti-RBM10 (ab72423, 1:2,000 dilution), rabbit anti-p53
(ab131442, 1:500 dilution), rabbit anti-EGFR (ab131498, 1:500
dilution), rabbit anti-Bax (ab182733, 1:1,000 dilution), rabbit
anti-Bcl-2 (ab32124, 1:1,000 dilution), rabbit anti-caspase-8
(ab25901, 1:1,000 dilution) and rabbit anti-GAPDH (ab37168, 1:1,000
dilution) antibodies were obtained from Abcam (Cambridge, MA, USA).
Rabbit anti-α-tubulin (2144, 1:1,000 dilution), rabbit anti-AKT
(4691, 1:1,000 dilution), rabbit anti-phospho-AKT (4060, 1:1,000
dilution), rabbit anti-MEK1 (9146, 1:1,000 dilution), rabbit
anti-phospho-MEK1 (98195, 1:1,000 dilution), rabbit anti-ERK1/2
(4695, 1:1,000 dilution), rabbit anti-phospho-ERK1/2 (4370, 1:1,000
dilution), rabbit anti-c-Raf (9422, 1:1,000 dilution) and rabbit
anti-phospho-c-Raf (9421, 1:1,000 dilution) antibodies were
purchased from Cell Signaling Technology (Danvers, MA, USA). Goat
anti-rabbit IgG (H+L), HRP antibody (31460, 1:10,000 dilution) was
obtained from Thermo Fisher Scientific.
Immunofluorescence assay
RBM10 antibody (1:200), anti-p53 antibody (1:200),
or anti-EGFR antibody (1:200) (these antibodies were the same as
the ones mentioned above) were added to the cells, followed by
incubation with FITC-goat anti-rabbit antibody (1:200; ZF-0311,
Zhongshan Biotechnology, Zhongshan, China) for 40 min at 23°C (away
from light). After washing the cells in 0.1 M PBS, the expression
levels of RBM10, EGFR and p53 were observed under a fluorescence
confocal microscope (Olympus BX63; Olympus, Tokyo, Japan).
Immunohistochemistry
The samples from the tissue microarray (the
thickness of each section was 3 µm) were deparaffinized, endogenous
peroxidase activity was quenched in 3% H2O2
for 10 min, and the sections were washed in PBS. They were then
incubated with RBM10 antibody (1:300 dilution) for 1 h, followed by
incubation with the appropriate biotinylated secondary antibodies
(Vector Laboratories, Burlingame, CA, USA) and treatment with
avidin-biotin-coupling (ABC) reagent (Vector Laboratories), as
recommended by the manufacturer. Color development was achieved
with the substrate diaminobenzidine. Two independent pathologists
reviewed and scored the degree of immunohistochemical staining
according to the criteria below using a light microscope (Carl
Zeiss, Thornwood, NY, USA).
Scoring method of immunohistochemical
staining
For the 90 lung adenocarcinoma tissue samples, the
protein expression level of RBM10 was evaluated as follows: for
RBM10 staining in the nucleus and cytoplasm, the staining intensity
was scored as 0/1+/2+/3+ (0, no; 1+, light yellow; 2+, yellow; 3+,
brown-yellow) and the staining positive rate scores (0, negative;
1, 1-25%; 2, 26-50%; 3, 51-75% and 4, 76-100%) were calculated
(i.e., cancer tissues compared to the NCTs). ImageJ software
(National Institutes of Health, Bethesda, MD, USA) was used here.
The ‘staining intensity scores’ were multiplied by the ‘staining
positive rate scores’ to yield a total score, by which patients
were then grouped; that is, a sample with a score of ≤1 was placed
in the low expression group and a score of >1 was placed in the
high expression group.
Cell wound scratch assay
Lines were drawn on the back of a 6-well plate with
a marker pen, and the transverse lines were drawn uniformly at
distances of 1 cm between the lines and 3 lines in each well. A
total of 2×105 cells (H1299) were seeded into the plate
and cultured at 37°C in an atmosphere of 5% CO2. When
the cells grew to 80% confluence, the medium was discarded and the
cells were washed with PBS 3 times. Subsequently, a scratch was
placed in the middle of the well with a sterile 200 µl pipette tip,
and the cells were then washed with PBS 3 times. The cells were
then cultured in RPMI-1640 medium containing 2% FBS and 100 IU/ml
penicillin/gentamycin at 37°C. Images were captured using a DSC-HX1
digital camera (Sony Corp., Tokyo, Japan). Experiments were carried
out in triplicate and repeated 3 times.
Cell colony formation assay
A total of 800 cells (A549 and H1299) were seeded
into each well of 6-well plates and grown overnight, and were then
transfected with the shRNAs. The cells were allowed to form
colonies at the 4 day and then fixed with 4% paraformaldehyde for
15 min, stained with crystal violet for 15 min at room temperature,
and then washed 3 times with dH2O. The cells were
photographed with a digital camera (Leica, Wetzlar, Germany) , and
experiments were performed in triplicate and repeated 3 times.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD) values. A Student's t-test was applied to compare 2 groups of
independent data. One-way ANOVA with Tukey's post hoc test was used
for multiple comparisons. The Chi-square test was used to evaluate
the differences in the categorical variables. Survival curves were
carried out using the Kaplan-Meier method and were compared between
groups using the log-rank test. Statistical significance was set at
P<0.05. All statistical analyses were performed using the SPSS
17.0 software package (SPSS lnc., Chicago, IL, USA).
Results
RBM10 is overexpressed in lung
adenocarcinoma cell lines and tissue samples
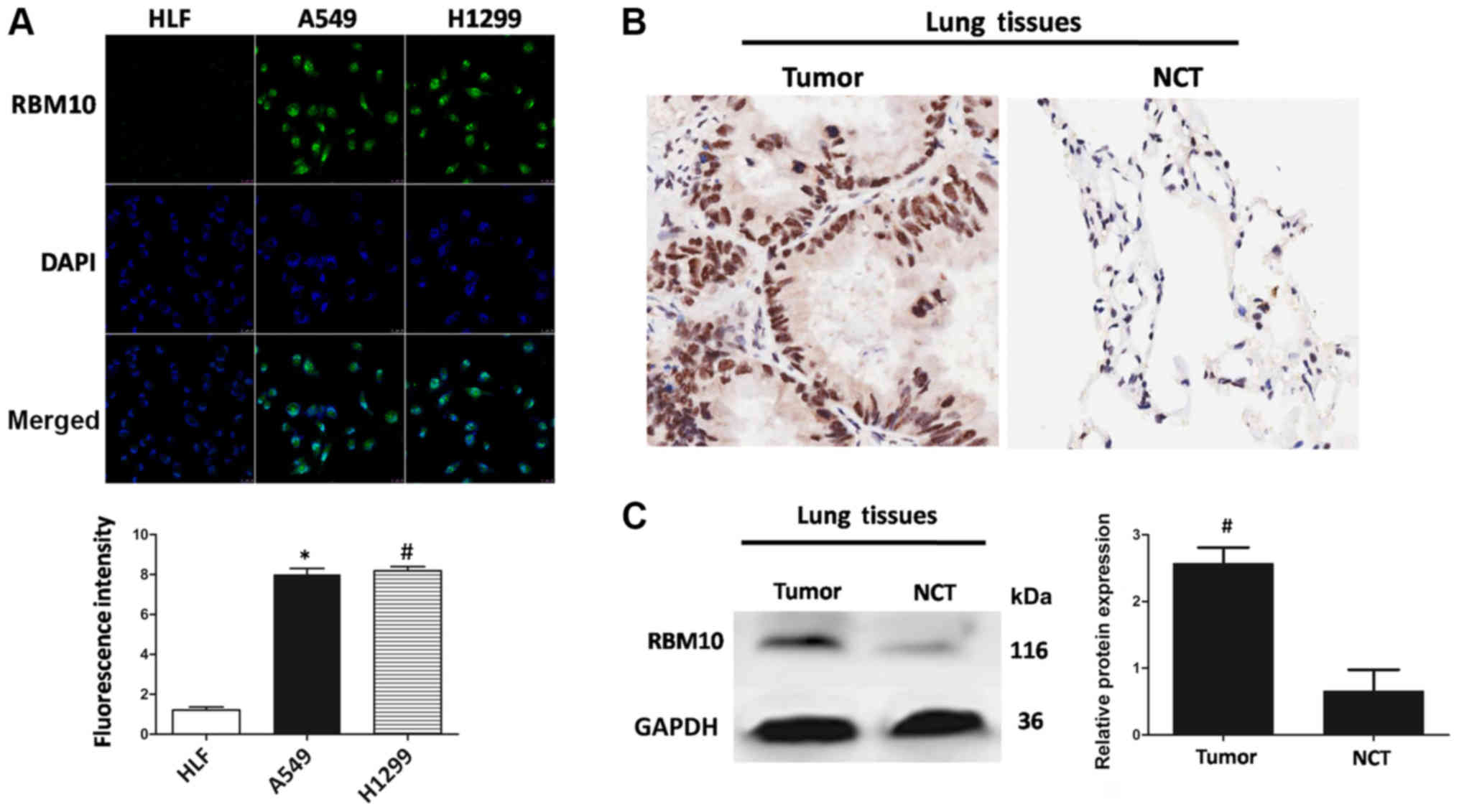
First we examined whether RBM10 protein was
expressed in lung adenocarcinoma cell lines (A549 and H1299) using
the green fluorescent protein RBM10. As shown in Fig. 1A, RBM10 was overexpressed in the
lung adenocarcinoma cells near the cell nucleus compared to the
HLFs (P<0.01); this result indicated that RBM10 may be
overexpressed in the cell nucleus of lung adenocarcinoma cells.
To verify the expression of RBM10 in vitro,
we further examined 6 pairs of lung adenocarcinoma tissues and the
corresponding NCTs. Immunohistochemical staining of RBM10 protein
revealed that the protein expression level of RBM10 in the lung
adenocarcinoma tissues was significantly higher than that in the
NCTs, and was mainly located in the nucleus (Fig. 1B). These results revealed that the
protein expression of RBM10 in human lung adenocarcinoma tissues
was markedly upregulated compared with that in the NCTs.
We also used western blot analysis to detect the
protein expression of RBM10 in the 6 pairs of lung adenocarcinoma
tissues and NCTs. The results revealed that the protein expression
of RBM10 in the tumor tissues was significantly higher than that in
NCTs (Fig. 1C), and the results of
quantitative analysis revealed statistically significant
differences between the 2 groups (P<0.05). It was further
verified by quantitative analysis that the protein expression level
of RBM10 in the lung adenocarcinoma tissues was higher than that in
the normal tissues adjacent to the carcinoma.
Upregulation of RBM10 expression inhibits
the apoptosis of lung adenocarcinoma cells
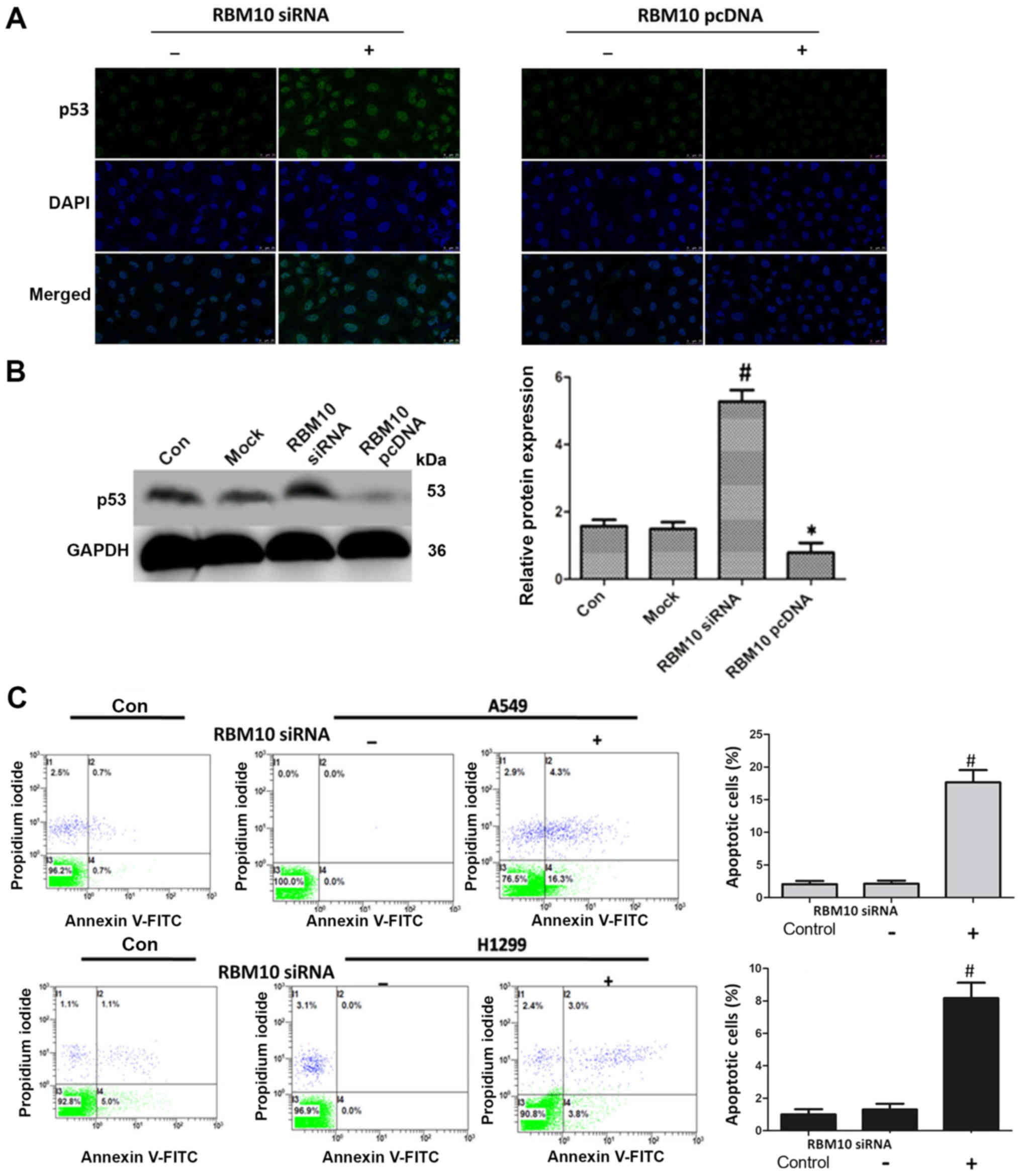
We then examined the effects of the upregulation
(using a pcDNA3.1 plasmid) and downregulation (using an siRNA
technique) of RBM10 expression on the apoptosis of A549 cells. At
48 h following transfection, the cells were stained using
immunofluorescence. First, we examined the effect of RBM10 on the
key tumor suppressor protein, p53; when RBM10 expression was
upregulated in the A549 cells, the protein expression of p53 was
decreased. However, when RBM10 protein expression was silenced, p53
protein expression was increased (Fig.
2A). The same results were obtained by western blot analysis,
and exhibited statistical significance (P<0.05; Fig. 2B).
Subsequently, we examined the effect of RBM10 on
cell apoptosis by flow cytometry. At 48 h following transfection
with RBM10 siRNA, the apoptotic rate of the A549 and H1299 cells
markedly increased [P<0.05 vs. control or RBM10 siRNA (−)
groups] (Fig. 2C). This result
indicated that the upregulation of RBM10 protein may inhibit the
apoptosis of the lung cancer cells.
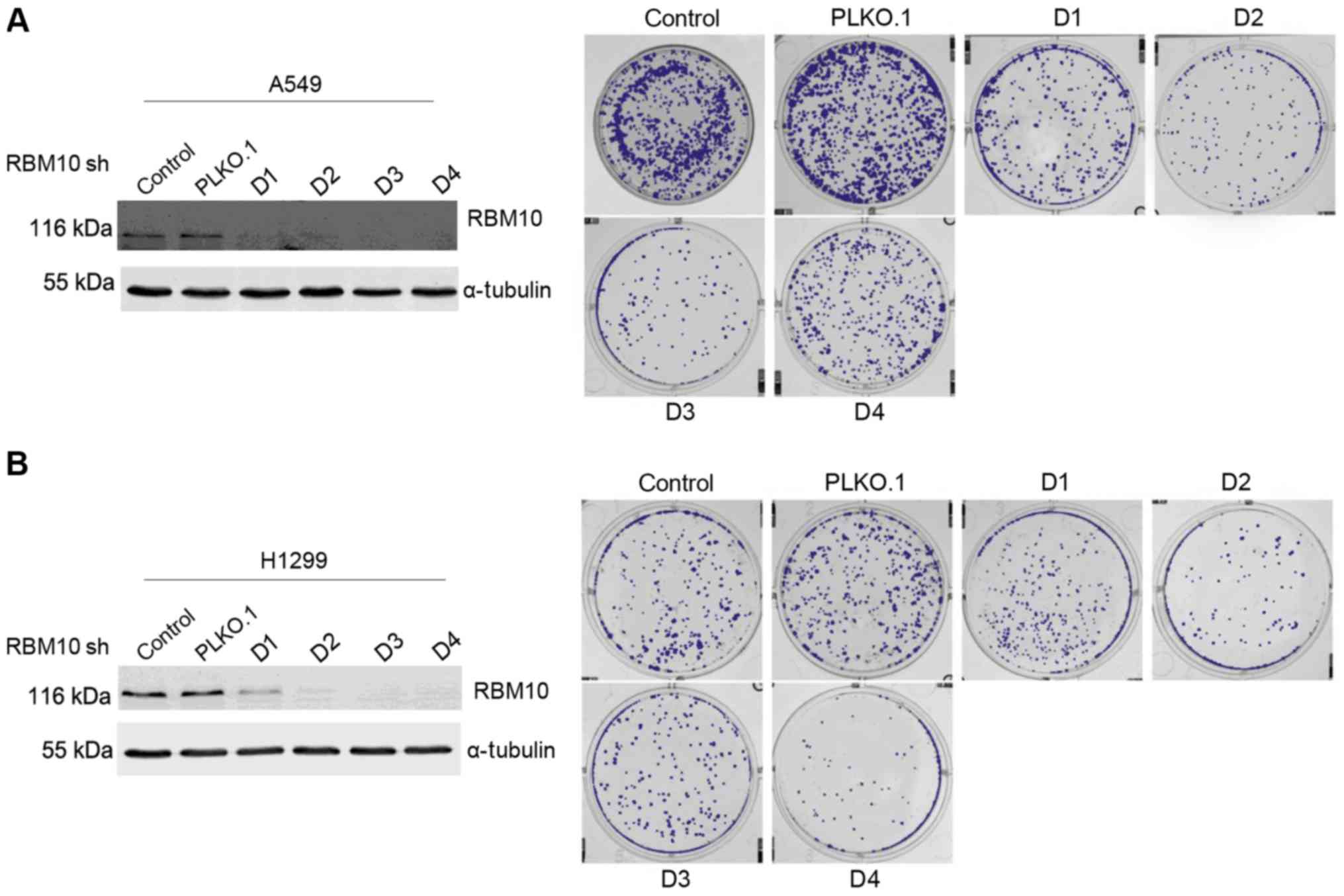
Downregulation of RBM10 expression
inhibits the proliferation of lung adenocarcinoma cells
We then investigated the association between RBM10
expression and the proliferation of lung adenocarcinoma cells. We
transfected the A549 and H1299 cells with either RBM10 shRNA (with
varying DNA sequences) or with a PLKO.1 carrier for 48 h and
verified the transfection by western blot analysis. We found that
the untransfected A549 and H1299 cells (control group), along with
the PLKO.1 carrier-infected A549 and H1299 cells, grew to a medium
extent in soft agar. Conversely, after the silencing of RBM10
expression with different DNA sequences (D1, D2, D3 and D4), the
growth of the clones in soft agar culture medium and their ability
to form cell colonies had weakened compared with that of the
control group or the PLKO.1 carrier group (Fig. 3).
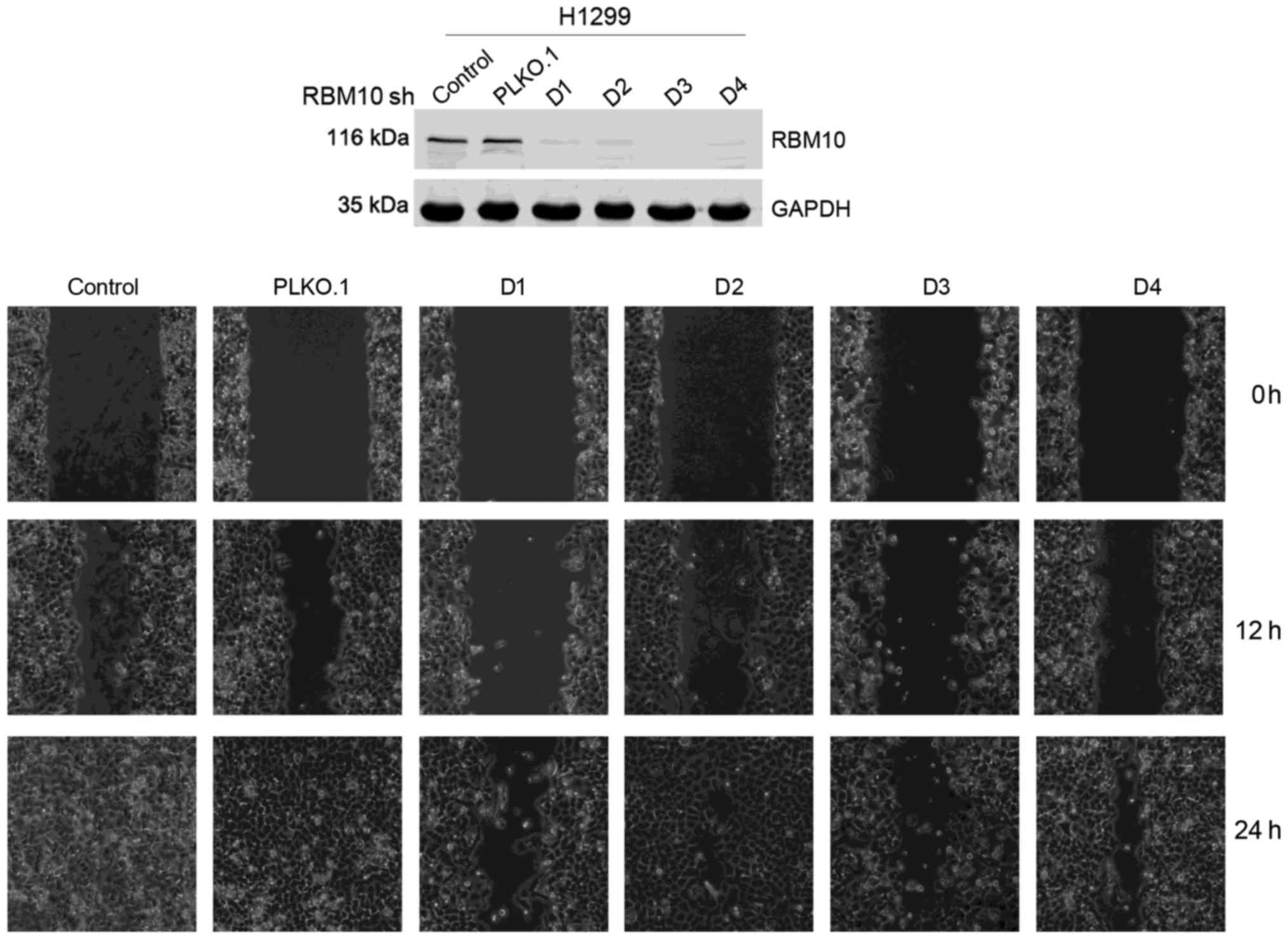
Downregulation of RBM10 expression
inhibits the migration of lung adenocarcinoma cells
Tumor cell migration is important in the metastasis
of lung adenocarcinomas. Therefore, we examined the effects of
RBM10 on cell migration using a cell wound scratch assay. We found
that at 24 h following transfection of the H1299 cells with RBM10
shRNA, the migration of the tumor cells was weaker compared with
that of the control group and PLKO.1 vector control group (Fig. 4). This indicates that the
downregulation of RBM10 protein expression in the lung
adenocarcinoma cells inhibits cellular migration, whereas a high
expression of RBM10 protein can promote tumor cell migration.
Upregulation of RBM10 expression
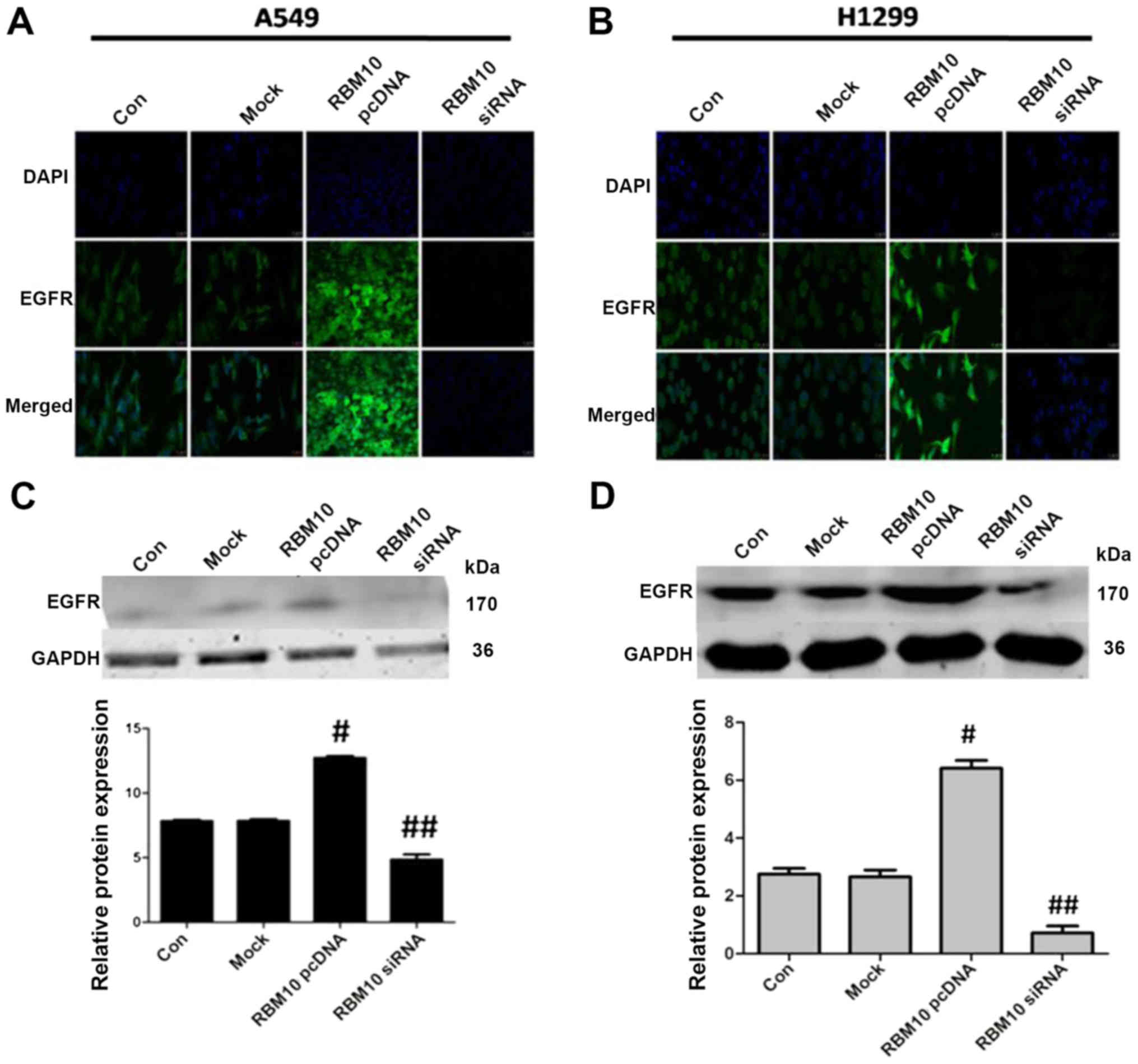
increases EGFR expression in lung adenocarcinoma cells
EGFR is the key protein in the tumor proliferative
signaling pathways. In this study, we examined the effect of RBM10
on the expression of EGFR by immunofluorescence staining. We found
that the upregulation of RBM10 expression (via pcDNA3.1) increased
EGFR expression in the cytosolic membrane of the A549 and H1299
cells, while the downregulation of RBM10 expression (via siRNA)
decreased EGFR expression (Fig. 5A and
B).
To further verify the results of immunofluorescence
staining, we performed western blot analysis. We found that
transfection with RBM10 siRNA downregulated the expression of EGFR
in the lung adenocarcinoma cells compared with the control or mock
groups (P<0.05) and transfection with RBM10 pcDNA3.1 upregulated
the expression of EGFR (P<0.05; Fig. 5C and D).
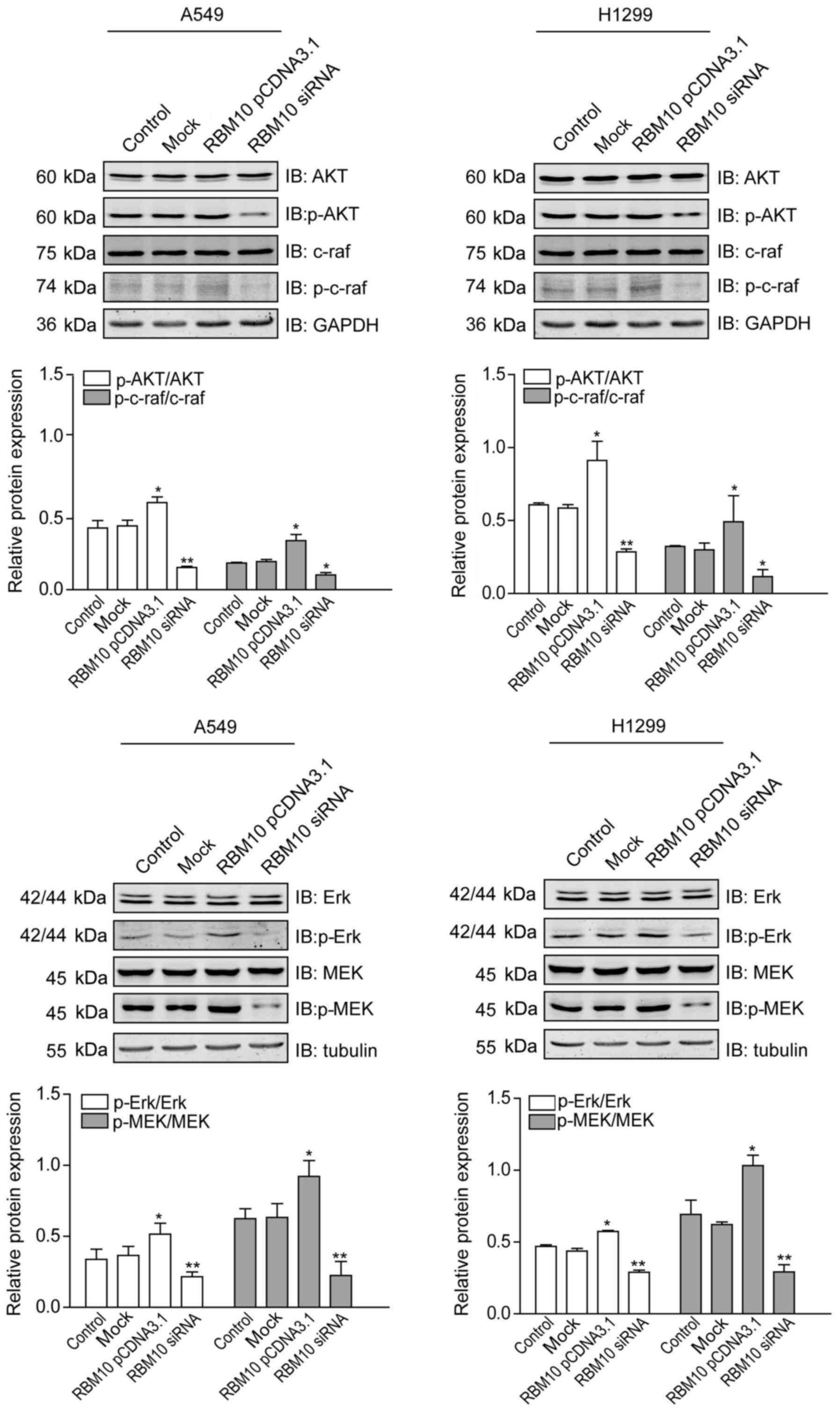
Upregulation of RBM10 expression
stimulates proliferative signaling pathways in lung adenocarcinoma
cells
The EGFR-mediated activation of key signaling
pathways is important for the proliferation of lung
adenocarcinomas. Therefore, we then examined the effects of RBM10
expression on proteins within key proliferative signaling pathways
(MAPK and PI3K) by western blot analysis. We found that p-Akt,
p-c-Raf, p-Erk, and p-MEK1 proteins were expressed at similar
levels in the A549 and H1299 cells in the control or mock groups.
RBM10 overexpression upregulated the expression levels of these
proteins (P<0.05), while the downregulation of RBM10 expression
by RBM10 siRNA downregulated the expression levels of these
proteins, particularly those of p-Akt, p-Erk and p-MEK1
(P<0.01). No significant differences were observed in the total
levels of Akt, c-Raf, Erk and MEK1 between the different groups in
the two cell lines (Fig. 6). These
findings indicate that the protein expression of RBM10 in lung
adenocarcinoma cells can activate key proliferative signaling
pathways.
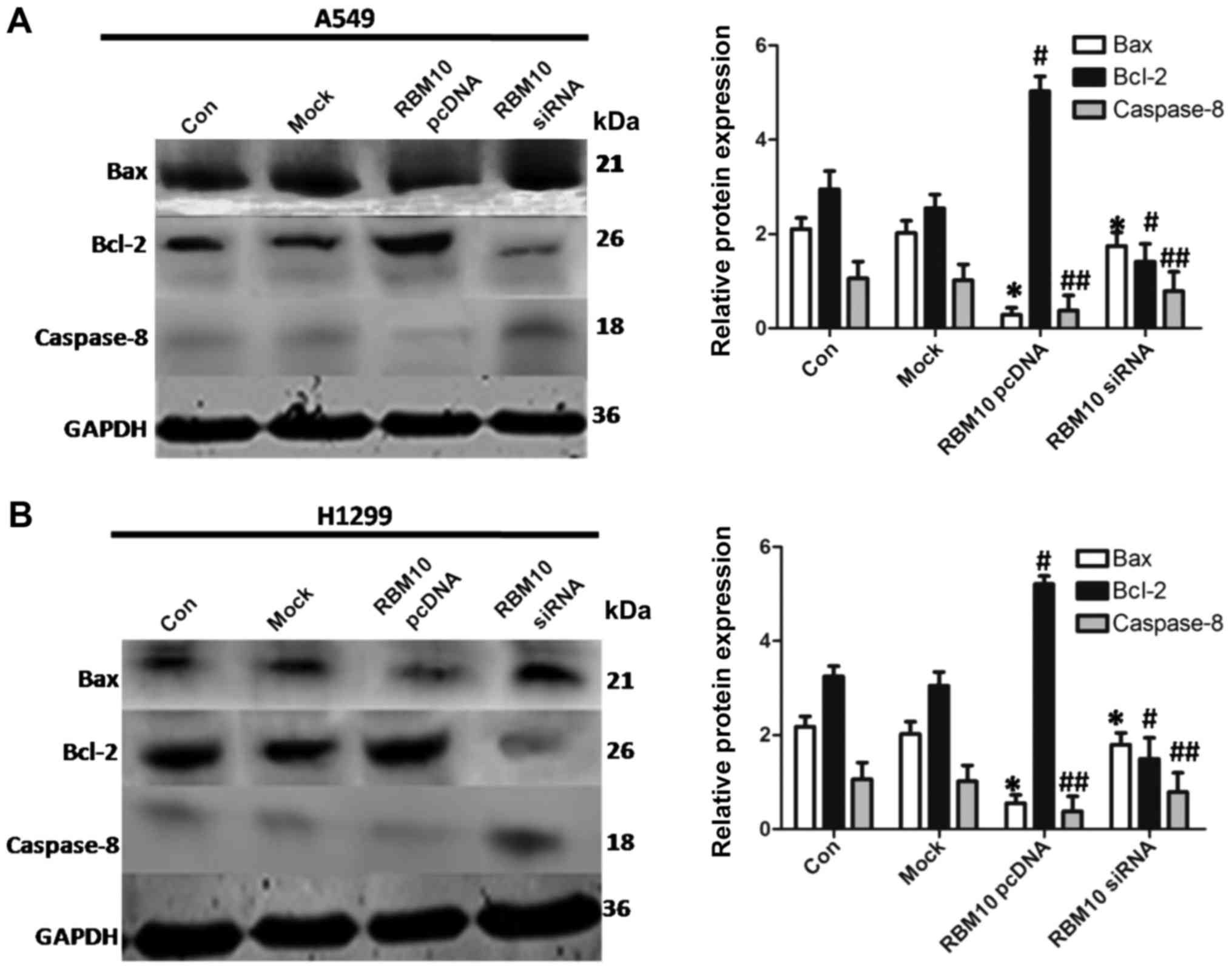
Upregulation of RBM10 expression inhibits
the stimulation of the apoptotic signaling pathways in lung
adenocarcinoma cells
Studies have shown that the inhibition of classical
apoptotic signaling pathways plays an important role in the
pathogenesis of lung adenocarcinoma (16-18).
In this study, we used western blot analysis to detect the
expression levels of key proteins of apoptotic signaling pathways
(including Bax, Bcl-2 and caspase-8) following the upregulation or
downregulation of RBM10 expression. The overexpression of RBM10
decreased the expression levels of the pro-apoptotic proteins, Bax
and caspase-8, whereas it increased the expression of the
anti-apoptotic protein, Bcl-2 (P<0.05). However, RBM10 silencing
increased the protein expression of Bax and caspase-8, and
decreased the protein expression of Bcl-2 (P<0.05; Fig. 7). These results indicate that a
high expression of RBM10 protein may reduce the stimulation of the
apoptotic signaling pathways in lung adenocarcinoma cells.
Associations of the levels of RBM10
protein expression with the clinicopathological characteristics of
patients with lung adenocarcinomas
In order to better evaluate the associations of
RBM10 protein expression levels and clinicopathological parameters
in lung adenocarcinomas, we analyzed the case numbers of high
expression and low expression of RBM10 protein in the nucleus and
cytoplasm of lung adenocarcinomas, and the association analysis was
performed on the TNM stage according to the 7th edition of lung
cancer (19) (Table I). The results revealed that the
level of RBM10 protein expression was not related to sex and age,
respectively (P>0.05, χ2 test), but was associated
with T stage and N stage (P=0.012 and 0.046, respectively;
P<0.05, χ2 test).
 | Table IAssociations of the protein
expression levels of RBM10 with the clinicopathological
characteristics of patients with lung adenocarcinoma. |
Table I
Associations of the protein
expression levels of RBM10 with the clinicopathological
characteristics of patients with lung adenocarcinoma.
| Total (n=69) high
expression | RBM10 low
expression (n=55) | RBM10 (n=14) | P-value |
|---|
| Sex | | | | |
| Male | 39 | 29 | 10 | 0.566 |
| Female | 30 | 26 | 4 | |
| Age (years) | | | | |
| ≥60 | 36 | 32 | 4 | 0.434 |
| <60 | 33 | 23 | 10 | |
| N stage | | | | |
| N0 | 44 | 32 | 12 | 0.046 |
| N1 + N2 | 25 | 23 | 2 | |
| T stage | | | | |
| T1 + T2 | 50 | 42 | 8 | 0.012 |
| T3 + T4 | 19 | 13 | 6 | |
Association between RBM10 expression in
lung adenocarcinomas and clinical prognosis
Immunohistochemical experiments were carried out in
the tissue chip containing 90 cases of lung adenocarcinoma tissues
and NCTs. We found that in the lung adenocarcinoma tissues, both in
the nucleus and in the cytoplasm, the protein expression levels of
RBM10 were higher than those in NCTs (P<0.05; Table II); the high expression levels of
RBM10 protein were more than those in NCTs.
 | Table IIExpression levels of RBM10 in tumors
and NCTs. |
Table II
Expression levels of RBM10 in tumors
and NCTs.
| Patient no. (90)
|
|---|
| Tumors | NCTs |
|---|
| Nucleus | | |
| RBM10 (high) | 60 | 28 |
| RBM10 (low) | 30 | 62 |
| P-value | 0.037 | |
| Cytoplasm | | |
| RBM10 (high) | 72 | 2 |
| RBM10 (low) | 18 | 88 |
| -value | 0.042 | |
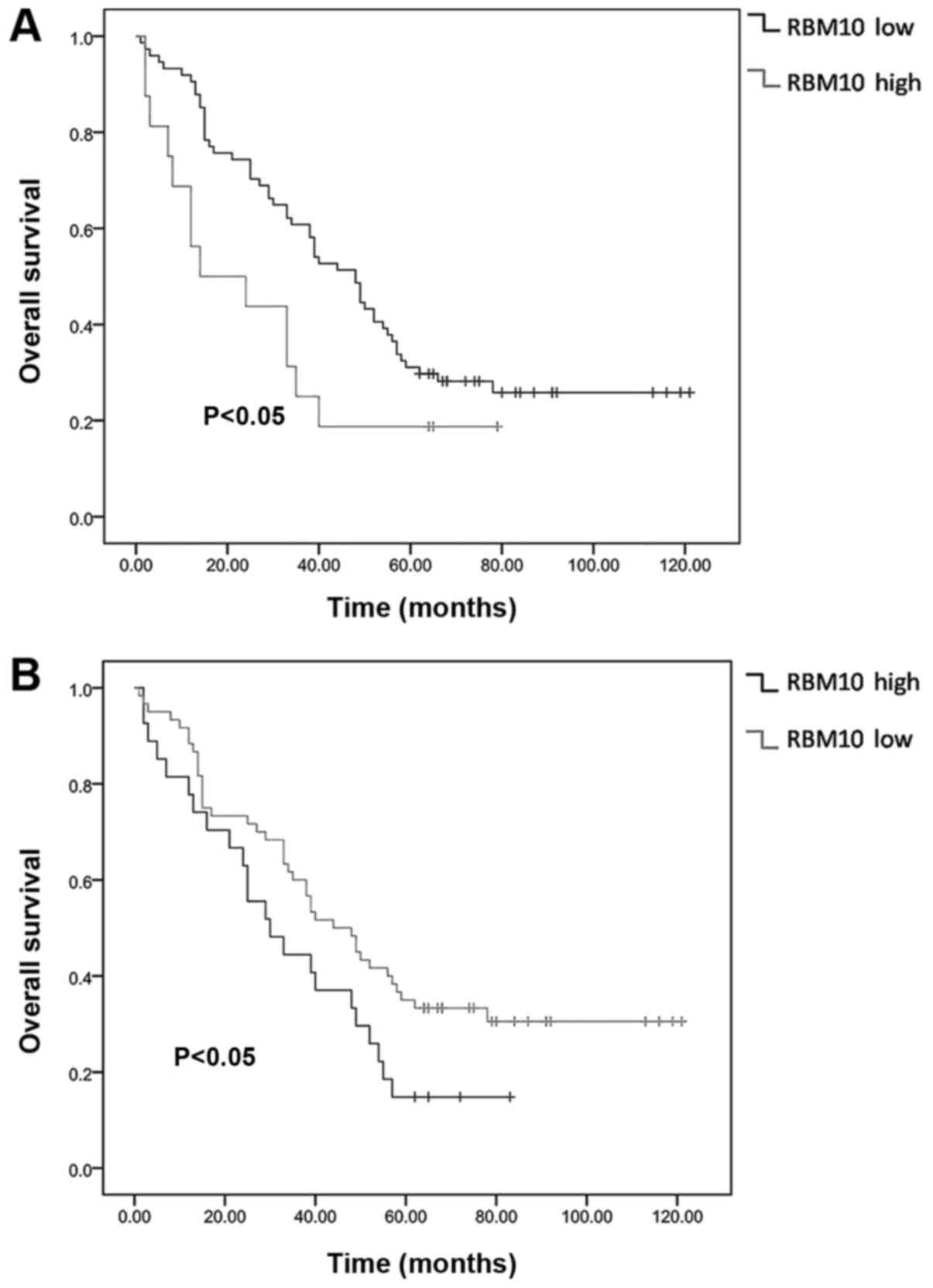
We also obtained follow-up data of the 90 patients 5
years post-operatively and analyzed the survival rates in the
RBM10-high and RBM10-low expression groups. We found that the
protein expression of RBM10 in the tumor tissues, both in the
nucleus and cytoplasm, was significantly associated with the
patient survival time. Patients with a high expression level of
RBM10 had a shorter overall survival time and a poor prognosis
compared to those with a low expression level of RBM10 (P<0.05;
Fig. 8).
Discussion
Recent studies have indicated that RBM10 gene
is mutated in certain types of tumor, including pancreatic cancer,
breast cancer, colon cancer and melanoma (10-12,20).
It is also mutated in up to 21% of invasive lung adenocarcinomas
(9) and 7% of lung adenocarcinomas
(21). In a study on pancreatic
cancer (10) remarkably,
RBM10 gene mutations were associated with an enhanced
survival, although all RBM10-mutated cases were of high
grade. Of note, as they observed (10) in the RBM10-mutated tumors,
cases harbouring mutations at codon 61 paradoxically exhibited
histological and clinical features indicative of poor survival. It
can be speculated from the above that the effect of RBM10
mutations on the opposite prognosis may be related to the
differential expression of mutations, some of which are highly
expressed and some are low expression in tumors.
As RBM10 can influence cellular proliferation and
apoptosis (11,14,22),
some studies have suggested that it functions as a tumor suppressor
gene (11,22-24),
while others consider it to be an oncogene (15,25).
To clarify the role of RBM10 in lung adenocarcinomas, we carried
out related research.
In this study, we confirmed the association between
RBM10 and lung adenocarcinomas both in vitro and in
vivo. RBM10 was upregulated in lung adenocarcinoma cells and
lung adenocarcinoma tissues, compared with normal lung cells and
normal lung tissues adjacent to the tumor. Our findings are
consistent with other findings (21,22,25,26).
We also demonstrated that RBM10 was mainly expressed in the cell
nucleus, and to the best of our knowledge, this is the first time
this has been reported.
We then examined the role of RBM10 in lung
adenocarcinomas. By immunofluorescence assay and western blot
analysis, we found that the overexpression of RBM10 reduced the
expression of p53, which mainly affects the cell cycle and inhibits
tumor cell invasion and proliferation by promoting apoptosis
(27). Our flow cytometry
experiments further verified that the downregulation of RBM10
expression led to the increased apoptosis of lung adenocarcinoma
cells. Therefore, we hypothesized that RBM10 promotes the
proliferation of lung adenocarcinomas by inhibiting apoptosis and
may be a oncogene, which was also confirmed by other studies
(15,22).
The clone formation assay and cell wound scratch
assay are experimental methods used to visually evaluate cell
biological abilities. Our findings indicated that the abilities of
cell proliferation and growth, as well as invasiveness and
metastasis, were reduced following the downregulation of RBM10,
which further indicates that, a high expression of RBM10 can
promote the proliferation and metastasis of lung adenocarcinoma
cells. Thus, we hypothesized that RBM10 is an oncogene in lung
adenocarcinomas. Some researchers have also come to this conclusion
(15,20).
EGFR protein is at the head of one of the most
important signaling pathways in mammalian cell physiology and
oncogenesis (6). First, we
demonstrated that RBM10 expression is positively associated with
EGFR expression. We then detected several key proteins (p-AKT,
p-c-RAF, p-ERK and p-MEK) in the MAPK and PI3K signaling pathways
activated by EGFR, which play key roles in tumor proliferation
(6). Likewise, we found that the
level of RBM10 expression was positively related to these key
proteins, which indicates that RBM10 plays a functional role in
stimulating proliferation in lung adenocarcinoma. This finding is
in line with some previous findings (25), although others have found opposite
results (28).
In this study, there may be such a question: RBM10
is mainly expressed in the nucleus, and EGFR is mainly expressed on
the cell membrane. The mechanism through which RBM10 affect EGFR
should be considered. We consider the following: i) There may be
some type of crosstalk between RBM10 and EGFR in the lung
adenocarcinoma cells that express RBM10 and EGFR; ii) research has
indicated that in specific cases, EGFR can be translocated to the
nucleus to play a role, although the specific mechanisms are not
clear (29,30), and iii) in some cases, RBM10 can
sometimes appear in the cytoplasm (31).
Subsequently, we detected the effects of RBM10 on
tumor apoptosis. We found that the overexpression of RBM10
decreased the expression levels of pro-apoptotic proteins, such as
Bax and caspase-8, whereas it increased that of the anti-apoptotic
protein, Bcl-2. However, the silencing of RBM10 increased the
protein expression levels of Bax and caspase-8, and decreased the
protein expression of Bcl-2. This finding is supported by previous
findings (25). Thus, it can be
hypothesized that RBM10 plays a role in promoting apoptosis in lung
adenocarcinoma. Some scholars have come to the opposite conclusion
(23,28,32);
that is, the anti-apoptotic effect of RBM10. At the same time,
there are also studies showing that RBM10 does not affect the
selective splicing of caspase-9, caspase-3, caspase-2 and c-FLIP,
which does not affect their expression levels (33).
Finally, when examining the clinical samples of 90
cases from a tissue microarray, we found that the expression level
of RBM10 in the nucleus and cytoplasm of lung cancer tissues was
higher than that of normal tissue adjacent to the tumor. This is in
line with our cytological experiments. In addition, the expression
of RBM10 in NCTs was also consistent with the low expression of our
cytological experiments in HLFs. As RBM10 can affect the metabolism
of DNA molecules and a variety of cellular processes, it is
necessary for the development (34,35),
growth and metabolism of normal cells (14,36).
In this study, we also examined the associations
between the expression of RBM10 and the clinicopathological
indicators of lung adenocarcinoma, such as sex, age, T stage and N
stage. RBM10 was highly expressed in males and in patients >60
years of age, although there was no statistical association. The
high expression level in males was consistent with the results of
gene sequencing of 230 cases of lung adenocarcinoma with the cancer
genome research network, that is, the RBM10 mutation is greater
among males (2). The association
between RBM10 and age has not been reported, at least to the best
of our knowledge. We demonstrated that the expression of RBM10 was
higher in older aged patients, and it may also be associated with
the increase in age and the increased incidence of lung
adenocarcinoma. In addition, the deletion of RBM5 and RBM6 in other
family members can be observed in smokers (37); however, to the best of our
knowledge, there is no study available on RBM10 and smoking. Due to
the incomplete information on smoking in this study, it is
impossible to carry out the related analysis. In combination with
the similarity between RBM10 and the structure of RBM5 and RBM6,
the above-mentioned results can not exclude the effect of smoking
in some patients. Of course, the experimental results that we
obtained may also be related to our small sample size.
The high expression of RBM10 was associated with the
T stage and N stage of lung adenocarcinoma, indicating that RBM10
can promote tumor growth, proliferation and lymph node metastasis,
and increase the malignant biological behavior of the tumor, that
is to play the role of an oncogene, which is consistent with the
results of our cytological experiments. Similar studies have been
conducted on breast cancer. Elevated levels of RBM6 and RBM10v2 RNA
are associated with the progression of tumor grade, increased tumor
size, progression of intraductal carcinoma in situ carcinoma and
the loss of progesterone receptor expression. It is suggested that
a coordinated expression of specific RBM6 and RBM10v2 variables is
important for the occurrence of breast cancer (11). It is also suggested that the
expression and function of RBM10 in tumors are not isolated, but
also influenced by other RNA splicing proteins in the family.
The most important method for evaluating the
clinical efficacy of an index is the survival curve. Therefore, in
this study, through the analysis of the high and low expression of
RBM10 in the tissue microarray of lung adenocarcinoma, we found
that patients with high levels of RBM10 expression (in the nucleus
and cytoplasm) had a shorter overall survival and a poorer
prognosis. This is consistent with the clinicopathological
findings, that is, the higher the T stage level is, the higher the
lymph node metastasis is in patients with RBM10 high expression of
lung adenocarcinoma. Therefore, it is suggested that the high
expression of RBM10 is a poor prognostic indicator. To the best of
our knowledge, we are the first to analyze the association between
RBM10 expression and the survival of patients with lung
adenocarcinoma. This result is in line with the findings of a
previous study on breast cancer (11), and opposite to the findings of
another study on pancreatic cancer (10).
At present, there are different opinions about
whether RBM10 is a cancer-suppressor gene or an oncogene. In
previous studies, opposing results have been found (22,26,38);
however, the current study can partly explain the contradictory
findings. RBM10 has three variants (RBM10v1, RBM10v2 and RBM10v3),
with RBM10v1 and RBM10v2 playing the leading roles (11,23,39).
In addition, RBM10v1 can promote proliferation, while RBM10v2
increases apoptosis (25). Thus,
we hypothesized that the reason why RBM10 plays differential roles
in the progression of lung adenocarcinoma may be associated with
the composition ratio between the two RBM10 variants. As the
composition ratio of RBM10v1 and RBM10v2 is diverse among different
mutated lung adenocarcinomas, and even among other tumor types, the
resulting effects of RBM10 will differ. In future experiments, we
aim to investigate the composition ratio of these two variants in
lung adenocarcinomas.
In conclusion, this study confirms that RBM10 plays
an important role in the progression of lung adenocarcinomas by
regulating both proliferative and apoptotic signaling pathways. We
propose that RBM10 may be an oncogene, and may represent a novel
therapeutic target for the treatment of lung adenocarcinomas.
Funding
This study was supported by National Natural Science
Foundation of China (NSFC) (grant no. 81774078).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XS, MJ and WS performed the research. TW, CG and XS
conceived and designed the study. XS, WS and LF analyzed the data,
TW, CG and XS wrote the manuscript.
Ethics approval and consent to
participate
For the use of patient samples, informed consent was
obtained from all patients. This study was approved by the Human
Research Ethics Committee of the First Affiliated Hospital of
Dalian Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Collisson EA, Campbell JD, Brooks AN,
Berger AH, Lee W, Chmielecki J, Beer DG, Cope L, Creighton CJ,
Danilova L, et al: Cancer Genome Atlas Research Network:
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar
|
|
3
|
Ferlay J, Shin H-R, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
4
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar
|
|
5
|
Milkovic L, Siems W, Siems R and Zarkovic
N: Oxidative stress and antioxidants in carcinogenesis and
integrative therapy of cancer. Curr Pharm Des. 20:6529–6542. 2014.
View Article : Google Scholar
|
|
6
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:3–45. 2017.
|
|
7
|
Milik SN, Lasheen DS, Serya RAT and
Abouzid KAM: How to train your inhibitor: Design strategies to
overcome resistance to Epidermal Growth Factor Receptor inhibitors.
Eur J Med Chem. 7:1–21. 2017.
|
|
8
|
Zhao J, Sun Y, Huang Y, Song F, Huang Z,
Bao Y, Zuo J, Saffen D, Shao Z, Liu W, et al: Functional analysis
reveals that RBM10 mutations contribute to lung adenocarcinoma
pathogenesis by deregulating splicing. Sci Rep. 7:404882017.
View Article : Google Scholar
|
|
9
|
Vinayanuwattikun C, Le Calvez-Kelm F,
Abedi-Ardekani B, Zaridze D, Mukeria A, Voegele C, Vallée M,
Purnomosari D, Forey N, Durand G, et al: Elucidating genomic
characteristics of lung cancer progression from in situ to invasive
adenocarcinoma. Sci Rep. 6:316282016. View Article : Google Scholar
|
|
10
|
Witkiewicz AK, McMillan EA, Balaji U, Baek
G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, et
al: Whole-exome sequencing of pancreatic cancer defines genetic
diversity and therapeutic targets. Nat Commun. 6:67442015.
View Article : Google Scholar
|
|
11
|
Rintala-Maki ND, Goard CA, Langdon CE,
Wall VE, Traulsen KE, Morin CD, Bonin M and Sutherland LC:
Expression of RBM5-related factors in primary breast tissue. J Cell
Biochem. 100:1440–1458. 2007. View Article : Google Scholar
|
|
12
|
Giannakis M, Mu XJ, Shukla SA, Qian ZR,
Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, et
al: Genomic correlates of immune-cell infiltrates in colorectal
carcinoma. Cell Reports. 15:857–865. 2016. View Article : Google Scholar
|
|
13
|
Jackson TC, Du L, Janesko-Feldman K, Vagni
VA, Dezfulian C, Poloyac SM, Jackson EK, Clark RS and Kochanek PM:
The nuclear splicing factor RNA binding motif 5 promotes caspase
activation in human neuronal cells, and increases after traumatic
brain injury in mice. J Cereb Blood Flow Metab. 35:655–666. 2015.
View Article : Google Scholar :
|
|
14
|
Loiselle JJ and Sutherland LC:
Differential downregulation of Rbm5 and Rbm10 during skeletal and
cardiac differentiation. In Vitro Cell Dev Biol Anim. 50:331–339.
2014. View Article : Google Scholar
|
|
15
|
Rodor J, FitzPatrick DR, Eyras E and
Cáceres JF: The RNA-binding landscape of RBM10 and its role in
alternative splicing regulation in models of mouse early
development. RNA Biol. 14:45–57. 2017. View Article : Google Scholar
|
|
16
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fulda S: Evasion of apoptosis as a
cellular stress response in cell. Int J Cell Biol. 2:1–6. 2010.
|
|
18
|
Plati J, Bucur O and Khosravi-Far R:
Dysregulation of apoptotic signaling in cancer: Molecular
mechanisms and therapeutic opportunities. J Cell Biochem.
104:1124–1149. 2008. View Article : Google Scholar
|
|
19
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar
|
|
20
|
Garrisi VM, Strippoli S, De Summa S, Pinto
R, Perrone A, Guida G, Azzariti A, Guida M and Tommasi S: Proteomic
profile and in silico analysis in metastatic melanoma with and
without BRAF mutation. PLoS One. 9:e1120252014. View Article : Google Scholar
|
|
21
|
Imielinski M, Berger AH, Hammerman PS,
Hernandez B, Pugh TJ, Hodis E, Cho J, Suh J, Capelletti M,
Sivachenko A, et al: Mapping the hallmarks of lung adenocarcinoma
with massively parallel sequencing. Cell. 150:1107–1120. 2012.
View Article : Google Scholar
|
|
22
|
Bechara EG, Sebestyén E, Bernardis I,
Eyras E and Valcárcel J: RBM5, 6, and 10 differentially regulate
NUMB alternative splicing to control cancer cell proliferation. Mol
Cell. 52:720–733. 2013. View Article : Google Scholar
|
|
23
|
Wang K, Bacon ML, Tessier JJ, Rintala-Maki
ND, Tang V and Sutherland LC: RBM10 modulates apoptosis and
influences TNF-α gene expression. J Cell Death. 5:1–19. 2012.
View Article : Google Scholar
|
|
24
|
Hernández J, Bechara E, Schlesinger D,
Delgado J, Serrano L and Valcárcel J: Tumor suppressor properties
of the splicing regulatory factor RBM10. RNA Biol. 13:466–472.
2016. View Article : Google Scholar
|
|
25
|
Julie J, Loiselle 1, Justin Roy G and
Leslie C: Sutherland: RBM10 promotes transformation-associated
processes in small cell lung cancer and is directly regulated by
RBM5. PLoS One. 12:1–23. 2017.
|
|
26
|
Tessier SJ, Loiselle JJ, McBain A, Pullen
C, Koenderink BW, Roy JG and Sutherland LC: Insight into the role
of alternative splicing within the RBM10v1 exon 10 tandem donor
site. BMC Res Notes. 8:462015. View Article : Google Scholar
|
|
27
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar
|
|
28
|
Martín-Garabato E, Martínez-Arribas F,
Pollán M, Lucas AR, Sánchez J and Schneider J: The small variant of
the apoptosis-associated X-chromosome RBM10 gene is co-expressed
with caspase-3 in breast cancer. Cancer Genomics Proteomics.
5:169–173. 2008.
|
|
29
|
Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF
and Hung MC: Novel prognostic value of nuclear epidermal growth
factor receptor in breast cancer. Cancer Res. 65:338–348. 2005.
|
|
30
|
Psyrri A, Yu Z, Weinberger PM, Sasaki C,
Haffty B, Camp R, Rimm D and Burtness BA: Quantitative
determination of nuclear and cytoplasmic epidermal growth factor
receptor expression in oropharyngeal squamous cell cancer by using
automated quantitative analysis. Clin Cancer Res. 11:5856–5862.
2005. View Article : Google Scholar
|
|
31
|
Xiao SJ, Wang LY, Kimura M, Kojima H,
Kunimoto H, Nishiumi F, Yamamoto N, Nishio K, Fujimoto S, Kato T,
et al: S11/RBM10: Multiplicity and cooperativity of nuclear
localisation domains. Biol Cell. 105:162–174. 2013. View Article : Google Scholar
|
|
32
|
Dvinge H, Kim E, Abdel-Wahab O and Bradley
RK: RNA splicing factors as oncoproteins and tumour suppressors.
Nat Rev Cancer. 16:413–430. 2016. View Article : Google Scholar :
|
|
33
|
Inoue A, Yamamoto N, Kimura M, Nishio K,
Yamane H and Nakajima K: RBM10 regulates alternative splicing. FEBS
Lett. 588:942–947. 2014. View Article : Google Scholar
|
|
34
|
Lukong KE, Chang KW, Khandjian EW and
Richard S: RNA-binding proteins in human genetic disease. Trends
Genet. 24:416–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ozuemba B, Masilamani TJ, Loiselle JJ,
Koenderink B, Vanderbeck KA, Knee J, Larivière C and Sutherland LC:
Co- and post-transcriptional regulation of Rbm5 and Rbm10 in mouse
cells as evidenced by tissue-specific, developmental and
disease-associated variation of splice variant and protein
expression levels. Gene. 580:26–36. 2016. View Article : Google Scholar
|
|
36
|
Johnston JJ, Sapp JC, Curry C, Horton M,
Leon E, Cusmano-Ozog K, Dobyns WB, Hudgins L, Zackai E and
Biesecker LG: Expansion of the TARP syndrome phenotype associated
with de novo mutations and mosaicism. Am J Med Genet A.
164A:120–128. 2014. View Article : Google Scholar
|
|
37
|
Angeloni D: Molecular analysis of
deletions in human chromosome 3p21 and the role of resident cancer
genes in disease. Brief Funct Genomics Proteomics. 6:19–39. 2007.
View Article : Google Scholar
|
|
38
|
Martínez-Arribas F, Agudo D, Pollán M,
Gómez-Esquer F, Díaz-Gil G, Lucas R and Schneider J: Positive
correlation between the expression of X-chromosome RBM genes (RBMX,
RBM3, RBM10) and the proapoptotic Bax gene in human breast cancer.
J Cell Biochem. 97:1275–1282. 2006. View Article : Google Scholar
|
|
39
|
Sutherland LC: RNA binding motif (RBM)
proteins: A novel family of apoptosis modulators? J Cell Biochem.
94:5–24. 2005. View Article : Google Scholar
|