Introduction
Gastric cancer is one of the dangerous malignant
tumor types, due to its threat to the health of people globally,
with it being the second most common cancer type and a leading
cause of mortality in China in 2015 (1). A significant decline in incidence has
occurred since 20th century due to the efficient prevention and
treatment of H. pylori infection, which commonly results in
non-cardia gastric cancer; however, the morbidity of cardia gastric
cancer is rising in Europe and North America in 2012, due to the
increasingly high-fat diets (2,3). No
significant improvements have been produced in advancing the
diagnosis and treatment of gastric cancer, and it frequently
metastasizes prior to identification (4). In a number of metastatic models of
gastric cancer, lymph node metastasis is not only a major type of
metastasis but also the early route (4,5).
Additionally, gastric cancer with lymphatic metastasis will be
refractory, due to the difficulties in full elimination with
surgical resection or chemoradiotherapy (6). Therefore, it is of vital importance
to develop novel therapies for the early intervention of lymph node
metastasis in gastric cancer.
Obesity is a well-recognized risk factor for gastric
cancer in the USA and China (7,8).
However, it remains controversial whether the body mass index
(BMI), which is currently the standard measure of obesity, has a
positive association with the incidence and mortality of gastric
cancer (9,10). A recent review reported that BMI
was predominantly an indicator for cardia gastric cancer, rather
than non-cardia (9); additionally,
a meta-analysis indicated high fat-intake was positively associated
with gastric cancer incidence (10). Furthermore, increasing evidence
indicates that hyperlipidemia is positively associated with
lymphatic metastasis in a number of cancer types, including
esophageal and gastric cancer (11,12).
Thus, it is indicated that abnormal lipid level may have a negative
effect on patients with gastric cancer through promoting lymphatic
metastasis. Nevertheless, it remains unclear which variety of
dyslipidemia stimulates gastric cancer lymphatic metastasis, and by
which mechanisms.
Hypercholesterolemia is a type of dyslipidemia,
characterized by increased low-density lipoprotein (LDL)
cholesterol in the plasma. LDL is susceptible to oxidation by
reactive oxygen species (ROS), therefore increasing lipid
peroxidation in the tissue or plasma (13). Additionally, there is increasing
evidence indicating that oxidized LDL (oxLDL) is a common
pathogenic factor in cardiovascular diseases, and breast and
ovarian cancer (14,15). For example, a previous study
indicated that increasing oxLDL is positively associated with
cancer development, through binding to the lectin-like oxLDL
receptor (LOX) and stimulating inflammation, proliferation and
migration pathways (16). However,
it has not been reported whether oxLDL could have an initial
adverse effect on gastric cancer lymphatic metastasis, and the
regulatory mechanisms remain unidentified.
Thus, the purpose of the present study was to
investigate the association between the plasma oxLDL levels and the
lymph node metastasis in gastric cancer, and then to elucidate the
underlying mechanisms.
Materials and methods
Cell lines and cell culture
Gastric cancer cell lines HGC-27, MGC-803 and AGS
were purchased from the American Type Culture Collection (Manassas,
VA, USA), and SGC-7901 cells were purchased from the Chinese
Academy of Sciences Cell Bank (Shanghai, China). All cell lines
were maintained in Dulbecco’s modified Eagle’s medium (DMEM;
Corning Life Sciences, New York, NY, USA) supplemented with 10%
(v/v) fetal bovine serum, 100 U/ml penicillin and 100 mg/ml
streptomycin, and cultivated in a humidified 37°C incubator
containing 5% CO2.
Tissue specimens, clinical and
pathological data collection
A total of 17 cases of gastric cancer specimens
(mean age, 64.5 years; age range, 50-75 years; 9 male and 8 female
patients), including cancerous and paracancerous tissues used as
control, as well as plasma, were collected from the Department of
Gastrointestinal Surgery, Traditional Chinese Medicine Hospital of
Guangdong Province (Guangzhou, China) from November 2015 to June
2016. Another 11 cases of plasma from patients with gastric cancer
(mean age, 67.7 years; age range, 53-76 years; 5 male and 6 emale
patients) were collected from the Guangzhou First People’s Hospital
(Guangzhou, China) from April to June 2016. All cases were
histopathologically confirmed as gastric cancer by three
pathologists blinded to the present study. All patients provided
written informed consent prior to surgery, and the use of medical
records and biospecimens was approved by the Institutional Research
Ethics Committee of Traditional Chinese Medicine Hospital of
Guangdong Province and Guangzhou First People’s Hospital.
Native LDL (nLDL) and oxLDL
preparation
The density of the human plasma sample was adjusted
to 1.200 mg/ml with NaBr previously. Subsequently, nLDL, with
density between 1.020-1.063 mg/ml, was extracted from human plasma
samples by density gradient centrifugation (4°C at 6,000 × g for 6
h) with 1.020 mg/ml NaBr solution layer (upper layer) and 1.063
mg/ml NaBr solution layer (lower layer) in the centrifugation tube.
Prior to centrifugation, the solutions were added from bottom to
the top in the order: 1.063 mg/ml NaBr solution, 1.020 mg/ml NaBr
solution and 1.200 mg/ml adjusted human plasma. Following the
density gradient centrifugation, the extracted nLDL was located
between the 1.063 mg/ml NaBr solution and 1.020 mg/ml NaBr
solution, and then it was collected with an injector. oxLDL was
obtained by oxidizing nLDL with 5 µM CuSO4
solution at 37°C for 18 h. Subsequently, nLDL and oxLDL were
purified with a dialysis bag in PBS solution with 200 µM
EDTA (4°C for 24 h). The concentrations were measured with a
Lowry’s assay (17). Finally, the
oxidative activity of oxLDL was determined by Oil Red O
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) staining (room
temperature for 8 min) of foaming macrophages RAW264.7 (American
Type Culture Collection) treated with oxLDL (37°C for 24 h), which
was measured by light microscope at ×200 magnification. As the
endocytosis of oxidized LDL occurred in macrophages, the
macrophages became foaming cells and were stained by Oil Red O.
Animal experiments
Animal experiments using female BALB/c nude mice
(n=18, 5-6 weeks old, 18-22 g body weight) were purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd.,
(Beijing, China). The mice were provided access to food and water
ad libitum, and housed under specific pathogen-free
conditions with a 12/12 h light/dark cycle, and controlled humidity
(40-70%) and temperature (22±3°C). Mice would be removed from the
group if they exhibited tumor-associated complications, including
signs of emaciation, bleeding, skin ulceration or necrosis;
additionally, sick mice were removed from the experiment and
sacrificed by cervical dislocation. The mice sacrificed were all
mature, and no mice were sacrificed prior to grouping. The
restrictions placed on the tumor sizes in the mice specified that
they must be <10% of the weight of the mice (<2,500
mm3). Nude mice were treated with oxLDL for 28 days, and
then the volume and weight of tumors were determined. Following the
experiment, all the mice were sacrificed by cervical dislocation.
The mice were anaesthetized by intraperitoneal injection with 10%
chloral hydrate (3 ml/kg body weight) prior to cervical
dislocation. All procedures associated with animal handling,
experimentation and welfare were conducted in compliance with the
Institutional Animal Care and Use Committee of Sun Yat-sen
University (approval no. 20151011007; Guangzhou, China).
Popliteal lymph node metastasis
model
HGC-27 cells (2×106) previously infected
with pLenti-CMV-EGFP-linker-Luc-P GK-Puro virus (Obio Biotech
Corp., Ltd., Shanghai, China) were inoculated subcutaneously into
the footpads of the nude mice. Following the tumor volume reaching
~100 mm3, fluorescein luciferin (Promega Corporation,
Madison, WI, USA) was injected intraperitoneally, and then the
footpad tumors were imaged with a spectroscopic IVIS (PerkinElmer,
Inc., Waltham, MA, USA). Subsequently, the mice were randomized
into three groups, the control, nLDL and oxLDL groups. The latter
two treatment groups were injected in the tail vein with 5
µg/g body weight nLDL (5 mg/ml) or oxLDL (5 mg/ml),
respectively, every two days while the control group was treated
with the same volume of PBS. At 21 days after the first
administration, in vivo imaging of the footpad transplanted
tumors and the popliteal lymph nodes metastatic tumors was
conducted with a spectroscopic IVIS. The mice were then sacrificed
at day 28, and the tumors were excised, dissected and weighed.
Tumor volume was calculated with the following formula: Volume
(mm3) = (length × width2) / 2.
VEGF-C expression levels in HGC-27, AGS, MGC-803 and
SGC-7901 cell lines are sensitive to oxLDL. Thus, all of these cell
lines are suitable for in vitro signaling experiments.
According to the outcomes of our preliminary animal experiments,
the tumor formation rate of HGC-27 cells was highest amongst all of
the cell lines tested. Considering this, HGC-27 was selected to
start the animal experiments, and for later in vivo and
in vitro experiments.
Tube formation assay
A human lymphatic endothelial cells (hLECs) in
vitro tube formation assay was performed by first pipetting 200
µl Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) into
each well of a 24-well plate, which was then polymerized for 30 min
at 37°C. hLECs (2×104 cells; Procell Life Technology
Co., Ltd., Wuhan, China) in 200 µl conditioned medium (the
culture medium from HGC-27 cells treated with oxLDL) were added to
each well and incubated at 37°C in an atmosphere containing 5%
CO2 for 12 h. Images were captured using a bright-field
with ZEISS Axio Observer Z1 (Carl Zeiss AG, Oberkochen,
Germany).
ELISA
A collection of clinical samples, including fresh
plasma, cancerous and paracancerous tissues all obtained from the
same patient with gastric cancer were preserved in liquid nitrogen
(−196°C); ELISAs were performed according to the manufacturer’s
protocols of the ELISA test kits. Firstly, for the tissue ELISA
experiment, the tissues were broken up by a homogenizer and with
ultrasonication, and the concentration of total protein in each
sample was determined with the bicinchoninic acid (BCA) method.
Subsequently, the concentration of VEGF-C was normalized to the
concentration of total protein. Secondly, for the collection of
cell culture supernatants, the gastric cancer cell lines (SGC-7901
and HGC-27) seeded (5×104 cells/500 µl) in 6-well
culture plates were cultured in growth medium until they reached
70-80% confluency, and were then treated with 50 µg/ml nLDL
or oxLDL for 24 h as aforementioned, while PBS was used in place of
this for the control group, and then the supernatants were
collected. Finally, the supernatants were centrifuged (4°C at 300 ×
g for 10 min), and the concentration of oxLDL and VEGF-C was
measured using an ELISA kit specific for VEGF-C (oxLDL kit; cat.
no. STA-388; Cell Biolabs, Inc., San Diego, CA, USA; and VEGF-C
kit; cat. no. DVEC00; R&D Systems, Inc., Minneapolis, MN, USA).
The procedure steps were conducted according to the manufacturer’s
protocols.
Nucleoprotein extraction
HGC-27 cells were treated with 50 µg/ml nLDL,
oxLDL or PBS for 12 h at 37°C, prior to extraction of total
protein, following which a Nucleoprotein Extraction kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used according to
the manufacturer’s protocols. The procedural steps were conducted
according to the manufacturer’s protocols. Subsequently, the
content of P65, histones (internal reference for nucleoprotein) and
β-actin (internal reference for cytoplasmic protein) among the
total nucleoprotein was detected by western blot analysis,
according to the subsequent protocol.
Western blotting
The antibodies used for western blotting were:
Rabbit polyclonal to human LOX-1 antibody (dilution, 1:500; cat.
no. sc-20753; Santa Cruz Biotechnology, Inc., Dallas, TX, USA);
rabbit monoclonal to human phospho-IκB kinase α (p-IKKα)
(Ser176)/IKKβ (Ser177) antibody (dilution, 1:1,000; cat. no.
CST#2078); rabbit polyclonal to human IKKα antibody (dilution,
1:1,000; cat. no. CST#2862); rabbit monoclonal to human p-inhibitor
of κB α (IκBα) (Ser32) antibody (dilution, 1:1,000; cat. no.
CST#2859); rabbit monoclonal to human IκBα antibody (dilution,
1:1,000; cat. no. CST#4812); rabbit monoclonal to human p-P65
(Ser536) antibody (dilution, 1:1,000; cat. no. CST#3033); rabbit
monoclonal to human P65 antibody (dilution, 1:1,000; cat. no.
CST#8242); rabbit polyclonal to human VEGF-C antibody (dilution,
1:1,000; cat. no. CST#2445); rabbit polyclonal to human β-actin
antibody (dilution, 1:5,000; cat. no. CST#3700); rabbit monoclonal
to human Histone H3 (D1H2) antibody (dilution, 1:5,000; cat. no.
CST#3700); and mouse monoclonal to human β-tubulin (D3U1W) antibody
(dilution, 1:2,000; cat. no. CST# 86298) (all from Cell Signaling
Technology, Inc., Danvers, MA, USA). All antibodies were incubated
for 12 h at 4°C. The secondary antibody goat anti-rabbit [1:2,000,
horseradish peroxidase (HRP) conjugate; cat. no. 31460] and goat
anti-mouse (1:2,000, HRP conjugate; cat. no. 31430) (both from
Thermo Fisher Scientific, Inc.) were incubated for 4 h at 4°C.
Additionally, 10% non-fat milk (cat. no. 100-04504-SDS; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was applied as a blocking
reagent (1 h at room temperature). A total of 20 µM
pyrrolidine dithiocarbamic acid, ammonium salt (PDTC; cat. no.
#P8765; Sigma-Aldrich; Merck KGaA) was used as an inhibitor of
nuclear factor (NF)-κB activation and 250 µg/ml polyinosinic
acid (Poly I; cat. no. P4154; Sigma-Aldrich; Merck KGaA) as a LOX-1
inhibitor.
The cells (AGS, SGC-7901, MGC-803 and HGC-27) were
lysed with 1X SDS and the total protein was extracted. The protein
concentration was determined using a BCA protein quantitation kit
(Nanjing KGI Biological Technology Development Co., Ltd., Nanjing,
China), according to the manufacturer’s protocol. Aliquots of equal
amounts of protein (30 µg) [containing loading buffer:
sodium lauryl sulfate, glycerol, bromophenol blue and tris
(hydroxymethyl) aminomethane] from the lysates were loaded onto a
10% SDS-PAGE gel, and then electrophoresis was conducted at 80 V
(constant voltage) until the markers had separated fully. A piece
of polyvinylidene fluoride membrane was cut according to the size
of the gel, and it was incubated in methanol for ~10 sec on a
rocker at room temperature prior to use. Subsequently, a
gel-membrane sandwich was arranged and the electrodes were
attached, and then the power supply was set to 300 mA (constant
current) for 3 h at 4°C. Following this, 10% milk in TBS with 0.1%
Tween-20 (TBST) buffer was prepared, the membranes were rocked in
TBST gently for 1 h at room temperature, and then the membranes
were washed with TBST buffer three times, for 10 min each time.
Additionally, the primary antibodies (anti-LOX-1, anti-IKK,
anti-p-IKK, anti-IκB, anti-p-IκB, anti-P65, anti-p-P65, anti-VEGF-C
and anti-β-actin) was added at the corresponding dilution in TBST
buffer (containing 5% bovine serum albumin; cat. no. SW3015;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). The membrane was incubated at 4°C for ~12 h, and then the
first antibody solution was poured out and the membrane was washed
three times with TBST buffer for 10 min each. Subsequently, the
secondary antibodies (goat anti-mouse IgG and goat ant-rabbit IgG)
was added at the corresponding dilution in TBST buffer (containing
2% bovine serum albumin). This was incubated at 4°C for ~4 h,
following which the secondary antibody solution was poured out and
the membrane was washed three times with TBST buffer for 10 min
each. Following this, the TBST buffer from the membranes was poured
out, and an enhanced chemiluminescent solution (Applygen
Technologies, Inc., Beijing, China) was added, with exposure to
X-ray film and imaging with an ImageQuant LAS 4000 mini being
performed. Gray level scanning was performed using ImageJ software
(Version 1.48; National Institutes of Health, Bethesda, MD, USA),
and the results were normalized to β-actin in order to analyze the
changes in target protein expression.
RNA interference
LOX-1 siRNA and a non-specific control siRNA
(NCsiRNA) were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). siLOX-1-1, sense, 5′-AGGACG
GUUCUCCUUUGAUdTdT-3′, and anti-sense, 3′-dTdTUCCU
GCCAAGAGGAAACUA-5′; siLOX-1-2, sense, 5′-CAGGUA
CCUGUGCAUAUAUdTdT3′, and antisense, 3′-dTdTGUCCA UGGACACGUAUAUA-5′;
and siLOX-1-3, sense, 5′-CGAACU CAAGGAAAUGAUAdTdT-3′, and
antisense, 3′-dTdTGCUU GAGUUCCUUUACUAU-5′.
Transfection was performed according to the
manufacturer’s protocols of the interference kit (Guangzhou RiboBio
Co., Ltd.). When HGC-27 gastric cancer cells were grown to ~60%
confluency, riboFECT (Guangzhou RiboBio Co., Ltd.) was used for
transfection. For each transfection reaction, transfection
complexes were prepared at room temperature using 20 nM siLOX-1 or
NCsiRNA, incubated at 37°C for 10 min and then added to the
serum-free DMEM. RNA and proteins were collected 24 h after
transfection and the effect of interference was determined by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) or western blot analysis, as aforementioned. Cells were
used for other experiments 24-72 h after transfection.
RT-qPCR
Total RNA was extracted from HGC-27 and SGC-7901
gastric cancer cell lines using a total RNA extraction kit (Qiagen
China Co., Ltd., Shanghai, China) according to the manufacturer’s
protocols. Subsequently, 500 ng RNA was reverse transcribed using a
PrimeScript Reverse Transcription kit (Takara Bio, Inc., Otsu,
Japan), and then qPCR was configured using SYBR® II
Premix Ex Taq™ (Takara Bio, Inc.). Finally, a
LightCycler® 2.0 system (Roche Diagnostics, Basel,
Switzerland) was used for RT-qPCR analysis. Primer sequences were
as follows: human LOX-1, sense, 5′-CCCTTGCTCGGAAGCTGAAT-3′, and
antisense, 5′-GCT TGCTGGATGAAGTCCTGAA-3′; human VEGF-C, sense
5′-AGAGCCGAGGGCAAAAGT-3′, and antisense 5′-GCTGA
GGTCCTCTCCTGGTC-3′; and human β-actin, sense, 5′-ACT
CTTCCAGCCTTCCTTC-3′, and antisense, 5′-ATCTCCTTCT GCATCCTGTC-3′.
These primers were all synthesized by GeneRay Biotech Co., Ltd.
(Shanghai, China). β-actin was used as an internal control and all
samples were assayed in triplicate.
Cycling conditions were as follows: Preheat for 95°C
for 30 sec for 1 cycle, and amplification at 95°C for 10 sec and
60°C for 45 sec for 40 cycles. Data were analyzed using the
2-∆∆Cq method (18),
and β-actin was used as the internal reference gene.
Immunofluorescence
Gastric cancer cells (HGC-27) were seeded
(1×104 cells/ml DMEM medium) in petri dishes and treated
with 50 µg/ml nLDL or oxLDL for 12 h at 37°C. Cells were
washed three times with PBS and fixed in 4% paraformaldehyde for 15
min at room temperature. The non-specific binding sites of the
cells were then blocked with normal non-immunized goat serum (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) for 1 h at 37°C,
and then the cells were washed three times with PBS. A rabbit
polyclonal to human NF-κB p65 antibody (dilution, 1:200; cat. no.
ab16502; Abcam, Cambridge, MA, USA) was added and the membranes
were incubated overnight at 4°C, following which the secondary
antibody (anti-rabbit; Alexa Fluor 488 conjugate; dilution,
1:1,000; cat. no. A-11029; Thermo Fisher Scientific, Inc.) was
added for 1 h at room temperature. Finally, cell nuclei were
stained with DAPI (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature. The coverslips were then added, and the cells were
imaged at ×200 magnification using a laser confocal microscope
(Carl Zeiss).
Immunohistochemistry
Tissues were surgically resected, fixed in formalin
(37%; overnight at room temperature) and embedded in paraffin.
Subsequently, 5-µm thick histological sections were
prepared. The sections were treated with the blocking solution (3%
H2O2) and normal goat serum (Boster
Biological Technology, Pleasanton, CA, USA) to block non-specific
background binding for 1 h at 37°C. Sections were then incubated
with a mouse monoclonal antibody specific to D2-40 (1:200,
dilution; cat. no. ab77854; Abcam). Following an overnight
incubation at 4°C, sections were incubated with the secondary
antibody from the EnVision™ Detection Kit
(peroxidase/DAB-conjugated, anti-rabbit/mouse; cat. no. K500705;
Dako; Agilent Technologies, Inc.) at room temperature for 20 min,
and then incubated with enzyme conjugate (horseradish
peroxidase-streptavidin) under the same conditions (all steps were
performed according to the manufacturer’s protocols). The vessels
were revealed with the streptavidin-peroxidase complex followed by
the chromogenic substrate 3,3′-diaminobenzidine, and the tissue
sections were counterstained with hematoxylin for 20 sec at 37°C.
Lymphatic vessel density (LVD) was determined by calculating the
tube number per ×100 field of view. Sections were imaged at ×100
magnification with a light microscope (Leica Microsystems, GmbH,
Wetzlar, Germany).
Statistical analysis
All data are presented as the means ± standard
deviations. The data were analyzed using SPSS 21.0 software (IBM
Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) was
used to analyze the differences between groups, followed by
Fisher’s least significant difference test. Following confirming
the data obeyed bivariate normal distribution, Pearson’s
correlation analysis was used to determine the correlation between
LVD and plasma oxLDL, LVD and plasma VEGF-C, and plasma VEGF-C and
plasma oxLDL. Pearson’s correlation analysis was performed with
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA) to determine the correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Level of oxLDL in the plasma of patients
with gastric cancer is positively correlated with the lymphatic
metastasis of gastric cancer cells
Clinical studies demonstrated that there is an
association between hyperlipidemia and gastric cancer metastasis
(2,3). Furthermore, patients with gastric
cancer and hyperlipidemia have an increased risk of lymphatic
metastasis, compared with patients without hyperlipidemia (19). OxLDL was reported to be associated
with the development of various tumor types, including breast,
cervical and ovarian cancer (14,15).
However, whether oxLDL in patients with hyperlipidemia would
promote the lymph node metastasis of gastric cancer remains
unknown. To verify this scientific hypothesis, plasma samples from
28 patients with gastric cancer were collected and the
concentration of oxLDL was detected via ELISAs. Subsequently, the
association between oxLDL levels and lymph node metastasis in
gastric cancer was analyzed. As depicted in Fig. 1A, the concentration of oxLDL in
plasma was significantly increased at higher stages of lymph node
metastasis.
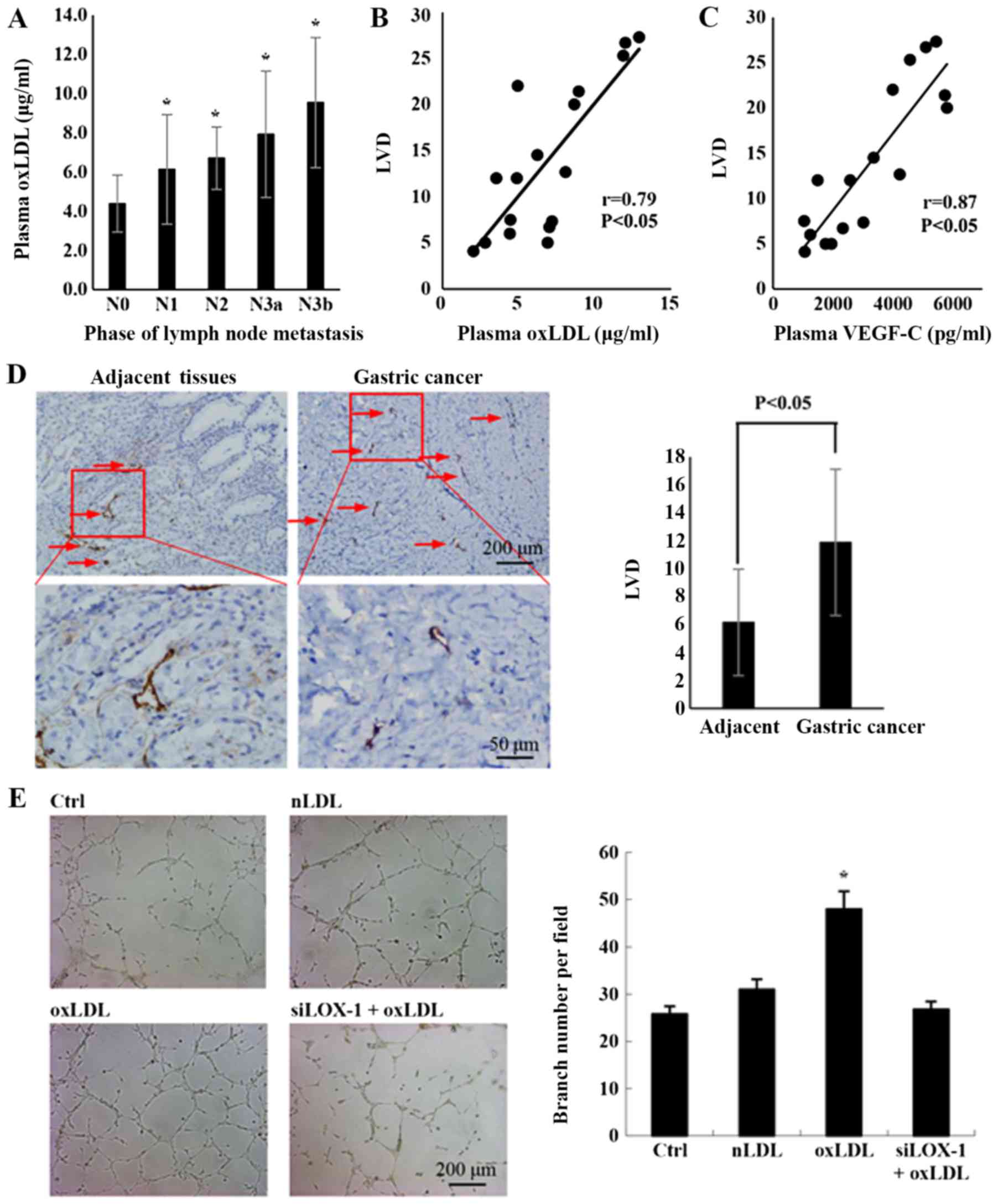 | Figure 1Plasma oxLDL in patients with gastric
cancer is positively correlated with lymph node metastasis. (A) The
association between plasma oxLDL and lymph node metastasis in
gastric cancer (case numbers: N0=8, N1=4, N2=4, N3a=5 and N3b=7;
*P<0.05, compared with N0). (B) The correlation
between the plasma oxLDL and the LVD was determined by Pearson’s
correlation analysis (n=17; r=0.79 and P<0.05). Plasma oxLDL in
patients with gastric cancer was detected via ELISAs. Lymphatic
vessels in gastric cancer tissues were stained with D2-40. LVD was
calculated using the number of vessels per field. (C) The
correlation between the plasma VEGF-C and the LVD. Plasma VEGF-C in
patients with gastric cancer was detected by ELISAs (n=17, r=0.87
and P<0.05). (D) Lymphatic vessel in adjacent tissues and
gastric cancer tissues were stained with D2-40 by
immunohistochemistry. Histograms represent the number of lymphatic
tubes per field (for adjacent and gastric cancer tissues n=17). (E)
Tube formation of LEC incubated with the condition medium from the
culture medium of HGC-27 treated by nLDL, oxLDL or siLOX-1+oxLDL
for 24 h (*P<0.05 vs, Ctrl). A total of
2×104 human lymphatic endothelial cells were seeded with
200 µl conditioned medium in each well and incubated for 12
h, and then the tube formation was observed with microscope.
Results are presented as the means ± standard deviations. N0, no
regional lymph node metastasis; N1, 1-2 regional lymph node
metastases; N2, 3-6 regional lymph node metastases; N3, 7 or more
regional lymph node metastases; N3a, 7-15 regional lymph nodes
metastases; N3b, >16 regional lymph nodes with metastasis; LVD,
lymphatic vessel density; VEGF-C, vascular endothelia growth
factor-C; oxLDL, oxidized low-density lipoprotein; Ctrl, control;
nLDL, native LDL, siLOX-1, small interfering lectin-like oxLDL. |
Correlation analysis indicated that there was a
positive correlation between the oxLDL concentration and LVD in
cancer tissues (Fig. 1B; r=0.79;
P<0.05). VEGF-C is a predominant factor for lymphangiogenesis
(20), and the present data
verified its consistent effect in gastric cancer cells (Fig. 1C), highlighting the importance of
VEGF-C in the lymphatic metastasis process of gastric cancer.
Lymphangiogenesis is an important step in the process of lymphatic
metastasis (21). To confirm if
gastric cancer lymph node metastasis is associated with
lymphangiogenesis stimulated by oxLDL, the LEC-specific marker
D2-40 was used to stain lymphatic vessels in gastric cancer samples
and matched adjacent tissues. Subsequently, the LVD, which can
reflect the degree of lymphangiogenesis (20), was calculated. The results
demonstrated that LVD was significantly increased in gastric cancer
tissues, compared with adjacent tissues (Fig. 1D). In vitro experiments also
demonstrated that oxLDL treatment significantly promoted
lymphangiogenesis in the cancer cell microenvironment, compared
with the control group (Fig. 1E).
These results indicate that oxLDL may be a notable risk factor for
lymph node metastasis in gastric cancer, due to stimulating
lymphangiogenesis.
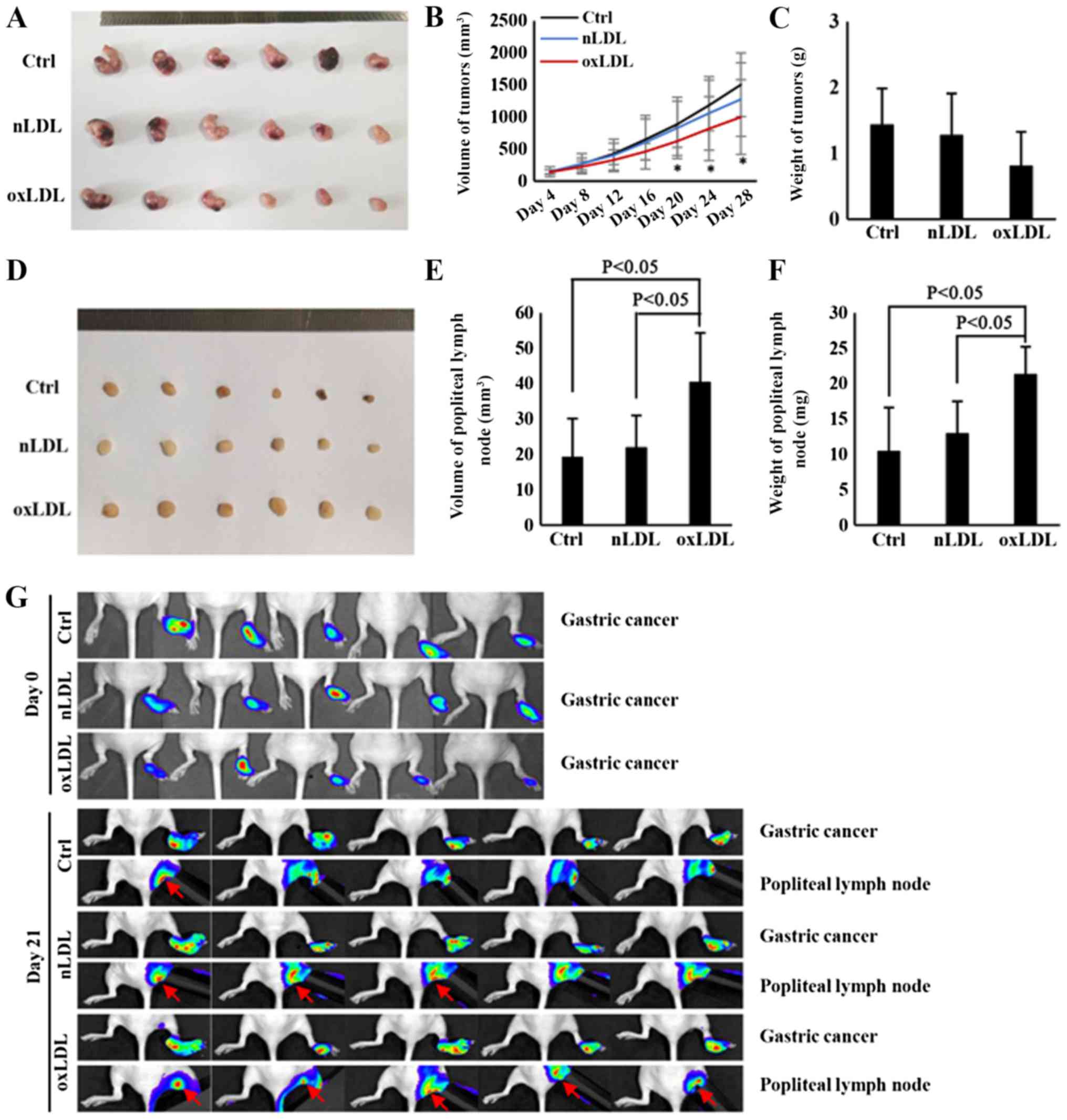
OxLDL promotes the lymph node metastasis
of gastric cancer in vivo
To investigate the direct effect of oxLDL on the
lymph node metastasis of gastric cancer in vivo, a
footpad-popliteal lymph node metastasis model was constructed.
Following nude mice being treated with oxLDL for 28 days, it was
determined that the volume of tumors from the ox-LDL-treated group
was decreased, compared with the control group (Fig. 2A and B). Additionally, although the
weight of tumors was also decreased in the ox-LDL-treated group,
there was no significant statistical differences among the three
groups (Fig. 2C). However, the
volume and weight of popliteal lymph nodes in oxLDL-treated nude
mice were significantly increased, compared with the control nude
mice (Fig. 2D-F). Therefore, these
results indicate that oxLDL could significantly promote the lymph
node metastasis of gastric cancer, an effect that was independent
of increasing tumor cell proliferation. Previous studies
demonstrated that tumor-induced inflammation could also result in
enlarged lymph nodes (19,22,23).
To confirm that the enlargement of popliteal lymph nodes in nude
mice was due to metastasized gastric cancer cells rather than
tumor-induced inflammation, the metastasized gastric cancer cells
were traced via live imaging with IVIS system. Prior to footpad
injection, the HGC-27 cells were labeled with luciferase and then
observed with the IVIS system as described previously (24). Finally, the results demonstrated
that there were increased popliteal lymph node metastatic cancer
cells migrating from footpad xenografts in the nLDL and oxLDL
groups, compared with the control group, whilst oxLDL promoted
lymphatic metastasis, primarily from orthotopic implantation
(Fig. 2G). These results indicate
that oxLDL has a significant role in promoting the lymph node
metastasis of gastric cancer.
Concentration of plasma oxLDL in patients
with gastric cancer is positively correlated with the plasma
VEGF-C
The previous data confirmed the role of oxLDL in
promoting the lymph node metastasis of gastric cancer, but the
molecular mechanism remains unclear. On the basis of previous
studies, including our own (24,25),
VEGF-C is a verified major VEGF that promotes lymphangiogenesis and
lymph node metastasis. To investigate the mechanism of oxLDL in
promoting lymph node metastasis, the effect of oxLDL on VEGF-C
expression in and secretion from gastric cancer cells was examined,
and the association between the concentration of plasma oxLDL and
VEGF-C in patients with gastric cancer was analyzed. Finally, it
was determined that they were positively correlated (Fig. 3A; r=0.64; P<0.05).
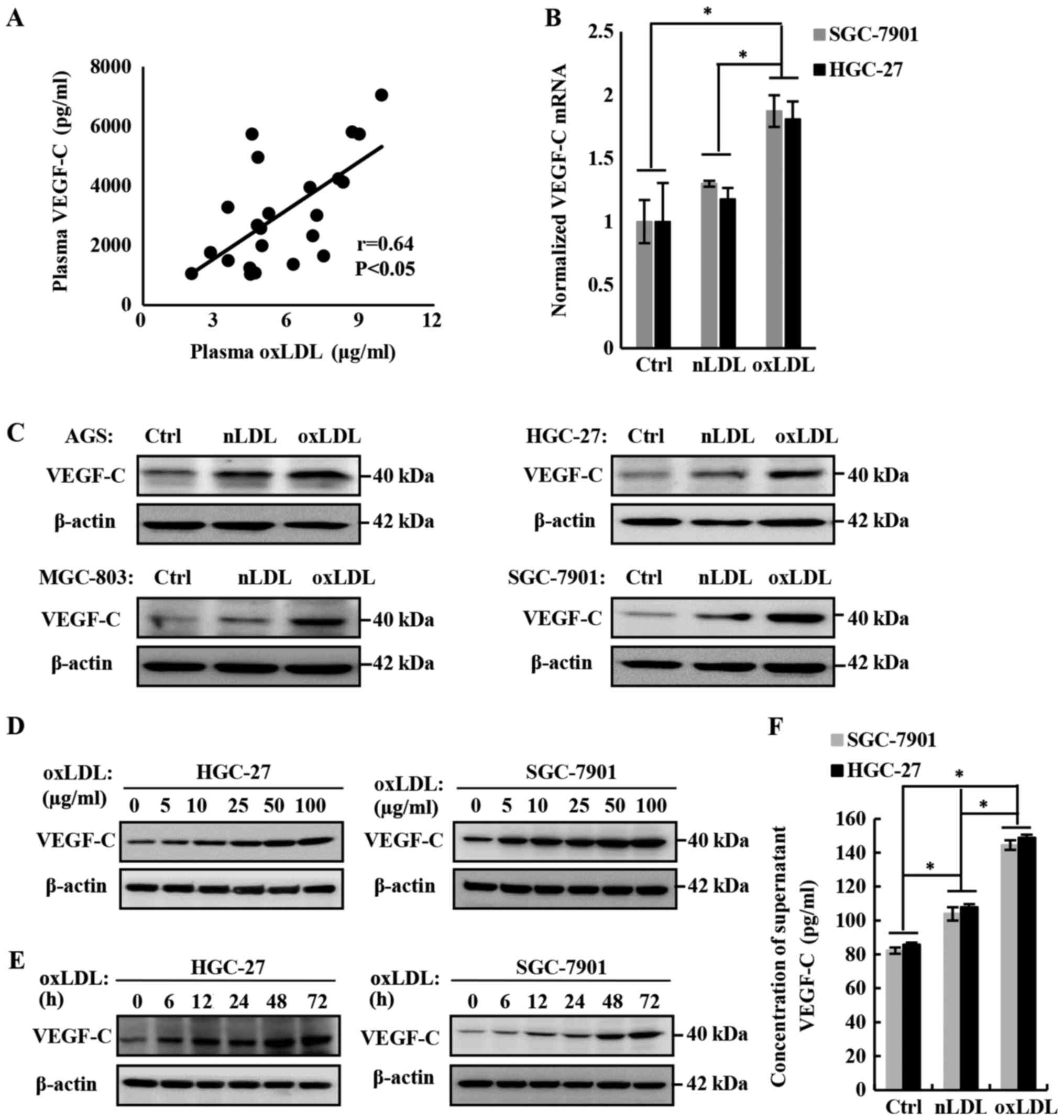 | Figure 3OxLDL promotes the expression and
secretion of VEGF-C. (A) Plasma oxLDL was correlated with plasma
VEGF-C in patients with gastric cancer. Plasma oxLDL and VEGF-C
levels in patients with gastric cancer were detected using ELISA
kits. The number of patients was 23 (r=0.64 and P<0.05). (B)
VEGF-C mRNA levels in HGC-27 cells treated with 50 µg/ml
nLDL or oxLDL for 24 h, as measured by reverse
transcription-quantitative polymerase chain reaction. A total of
three independent experiments were performed. (C) VEGF-C expression
levels were upregulated by nLDL and oxLDL in AGS, HGC-27, MGC-803
and SGC-7901 gastric cancer cell lines. (D) OxLDL dose-dependently
downregulated VEGF-C expression in gastric cancer cells. (E) OxLDL
time-dependently downregulated VEGF-C expression in gastric cancer
cells. VEGF-C protein levels in cell lysates were measured by
western blotting, with β-actin as the control. (F) To measure the
effect of oxLDL on the secretion of VEGF-C by HGC-27 and SGC-7901
gastric cancer cells, VEGF-C was detected in the supernatants using
an ELISA kit. *P<0.05, results are presented as the
means ± standard deviations. OxLDL, oxidized low-density
lipoprotein; Ctrl, control; nLDL, native LDL; VEGF-C, vascular
endothelia growth factor-C. |
OxLDL promotes the expression and
secretion of VEGF-C in gastric cancer cells
To verify the role of oxLDL in promoting VEGF-C
expression and secretion in gastric cancer cells, a series of in
vitro studies were also conducted. Firstly, the mRNA level of
VEGF-C in HGC-27 and SGC-7901 gastric cancer cell lines was
detected by RT-qPCR. The results demonstrated that the
transcription level of VEGF-C was significantly upregulated
following treatment with oxLDL, compared with the nLDL or blank
control groups (Fig. 3B).
Subsequently, the expression of VEGF-C in oxLDL-treated gastric
cancer cells was detected with western blotting and it was
determined that oxLDL promoted VEGF-C expression in a dose- and
time-dependent manner (Fig. 3C-E).
Finally, the level of VEGF-C secretion was measured using an ELISA
kit and it was determined that oxLDL also significantly increased
the secretion of VEGF-C, compared with the nLDL treatment or blank
control groups (Fig. 3F). In
summary, oxLDL promotes the expression and secretion of VEGF-C in
gastric cancer cells.
OxLDL upregulates VEGF-C expression and
secretion in gastric cancer cells by activating the NF-κB signaling
pathway
Previous studies, including our own, have revealed
that NF-κB signaling can regulate the expression of VEGF-C
(24,26), and that oxLDL can promote the
activation of the NF-κB signaling pathway (27,28).
Therefore, the present study investigated whether oxLDL regulates
the expression and secretion of VEGF-C through the NF-κB signaling
pathway in gastric cancer cells. To investigate this hypothesis,
the following experiments were conducted. Firstly, the HGC-27
gastric cancer cell line was treated with oxLDL, and then
activation of the NF-κB signaling pathway was examined. Western
blot analyses (Fig. 4A) revealed
that HGC-27 cells incubated with 50 µg/ml oxLDL for various
time intervals could sequentially induce increased phosphorylation
of IκB and p65. Previous studies confirmed that when the NF-κB
signaling pathway is activated, the transcription factor P65 would
be released into the nucleus to regulate the expression of its
downstream target genes (29,30).
The results of immunofluorescence staining and immunoblotting for
nucleoproteins in HGC-27 cells (Fig.
4B and C) demonstrated that oxLDL upregulated the nuclear
translocation of P65. Subsequently, the NF-κB signaling inhibitor
PDTC was used to antagonize the activating effect of oxLDL, and it
was determined that VEGF-C expression and secretion were restored
to their original level, to an extent (Fig. 4D and E). As our previous study
verified, IKK-dependent phosphorylation of IκB and P65 was
responsible for the positive transcriptional regulation of VEGF-C,
and demonstrated that P65 was directly involved in the
transcription of VEGF-C on the promoter region of VEGF-C using a
chromatin immunoprecipitation assay (24). Thus, it is necessary to clarify
whether oxLDL mediates the upregulation of VEGF-C by P65.
Therefore, P65 was knocked down with siRNA, and it was observed
that the upregulation of VEGF-C was inhibited (Fig. 4F and G). These data indicate that
oxLDL upregulates VEGF-C expression by promoting NF-κB signaling,
which could directly regulate VEGF-C transcription.
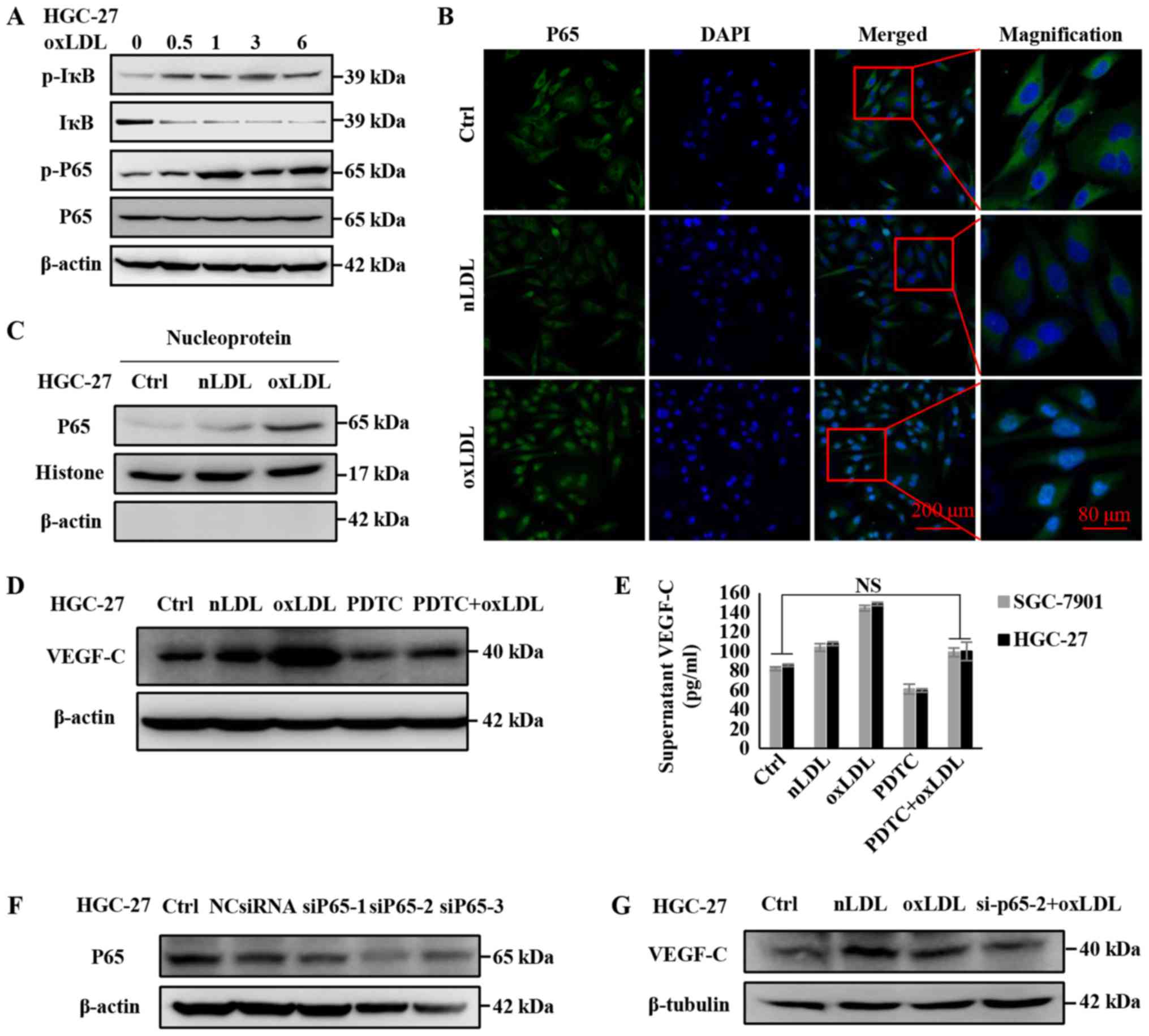 | Figure 4NF-κB signaling is involved in the
regulation of VEGF-C expression by oxLDL. (A) Activation of the
NF-κB signaling pathway in HGC-27 cells following oxLDL treatment.
IκB/p-IκB, P65/p-P65 and β-actin in gastric cancer cell lysates
were measured by western blot analysis. (B) Translocation of NF-κB
and P65 following oxLDL treatment; HGC-27 cells were incubated with
50 µg/ml oxLDL for 6 h. (C) The amount of P65 that was
translocated into the nucleus following oxLDL treatment was
measured by western blotting. HGC-27 cells were incubated with 50
µg/ml nLDL and oxLDL for 12 h. (D) The upregulated VEGF-C
level was rescued by the NF-κB inhibitor PDTC. The protein levels
were measured via western blotting. (E) VEGF-C levels in the
supernatants were detected using an ELISA kit. (F) The knockdown
effect of P65 siRNA was verified by western blotting. (G) The
upregulated VEGF-C level was rescued by P65 siRNA. P65/p-P65 and
VEGF-C protein levels in gastric cancer cell lysates were measured
via western blotting. *P<0.05, results are presented
as the means ± standard deviations. OxLDL, oxidized low-density
lipoprotein; Ctrl, control; nLDL, native LDL; VEGF-C, vascular
endothelia growth factor-C; NF-κB, nuclear factor-κB; p-, phospho-;
si, small interfering; PDTC, pyrrolidine dithiocarbamic acid,
ammonium salt; IκB, inhibitor of κB α; NS, non-significant. |
OxLDL promotes the activation of the
NF-κB signaling pathway and the expression of VEGF-C in gastric
cancer cells, mediated by the LOX-1 receptor
Previous studies confirmed that oxLDL could
specifically bind to LOX-1, while others reported that LOX-1
mediated the atherogenesis and tumorigenesis of oxLDL (31,32).
Thus, it is necessary to examine whether LOX-1 mediates the
function of oxLDL to promote VEGF-C expression in gastric cancer
cells. Therefore, LOX-1 was knocked down using siRNA (Fig. 5A) and the expression of VEGF-C was
assessed. As depicted in Fig. 5B and
C, the expression and secretion of VEGF-C in SGC7901 or HGC-27
cells treated with oxLDL were rescued by siLOX-1. Additionally, the
gastric cell lines were pre-treated with 250 µg/ml Poly I,
which is a recognized LOX-1 receptor antagonist (33), and it was determined that the
expression and secretion of VEGF-C was downregulated, even
following oxLDL treatment (Fig. 5D and
E). Furthermore, the activation of the NF-κB signaling pathway
was investigated, and it was determined that the phosphorylation of
P65, promoted by oxLDL, was rescued via oxLDL/LOX-1 interaction
inhibition by Poly I (Fig. 5D).
These outcomes indicate that LOX-1 mediates the activation of the
NF-κB signaling pathway induced by oxLDL, and upregulates the
expression and secretion of VEGF-C in gastric cancer cells.
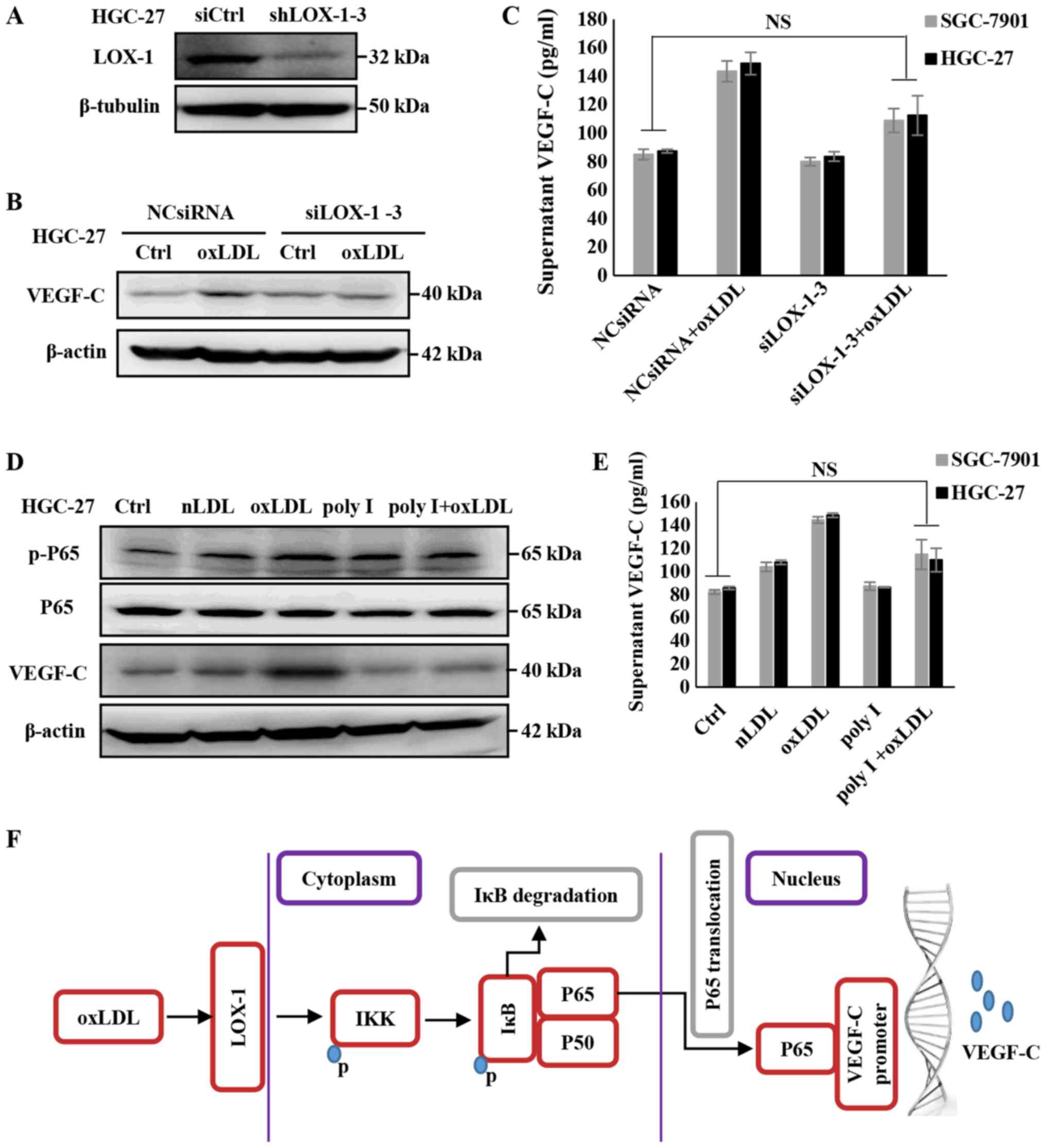 | Figure 5OxLDL promotes the activation of the
NF-κB signaling pathway, as well as the expression and secretion of
VEGF-C, through LOX-1. (A) Validation of siRNA efficacy in HGC-27
cells. (B) The effects of oxLDL on NF-κB activity and VEGF-C
expression were blocked following the knockdown of LOX-1. VEGF-C
protein levels in the cell lysates were measured via western
blotting, (C) supernatant VEGF-C levels were detected using an
ELISA kit. (D) The regulatory effects of oxLDL on NF-κB activity
and VEGF-C expression were blocked following LOX-1 being inhibited
by 250 µg/ml Poly I. VEGF-C protein levels in the cell
lysates were measured via western blotting, (E) supernatant VEGF-C
levels were detected using an ELISA kit. (F) Schematic depicting
the mechanism by which oxLDL promotes lymph node metastasis in
gastric cancer. Results are presented as the means ± standard
deviations. NS, non-significant; IKK, IBκ kinase; OxLDL, oxidized
low-density lipoprotein; Ctrl, control; nLDL, native LDL; VEGF-C,
vascular endothelia growth factor-C; NF-κB; nuclear factor-κB; p-,
phospho-; si, small interfering; LOX, lectin-like oxLDL; IκB,
inhibitor of κB α; NCsiRNA, non-specific control siRNA; poly I,
polyinosinic acid. |
Discussion
There has been a decline in the incidence of gastric
cancer globally, which is currently being attributed to the
efficient prevention and treatment of H. pylori infection
(1); however, it remains the
second most common malignancy diagnosed, and a leading cause of
cancer-associated mortalities in China in 2012 (1). The reason may lie in the increasing
widespread high-fat diet, along with improvements in people’s
living standards since the 1990s. Over recent years, increasing
evidence demonstrated that there is an association between obesity
and the malignant progression of gastric cancer (7,9).
However, BMI, the standard diagnostic indicator for obesity, cannot
definitively predict the incidence and mortality of gastric cancer,
while increasing evidence has indicated that hyperlipidemia is more
relevant to the progress of gastric cancer than BMI (10-12).
The present study indicated that excessive oxLDL, one of the
abnormal lipid forma in obesity, has disadvantages with respect to
the lymph node metastasis of gastric cancer, which is recognized as
an initial and lethal process of cancer spreading.
There is sufficient evidence (34,35),
including our previous data (24),
to verify that lymphangiogenesis is a primary step for lymphatic
metastasis and an important indicator for the aggressiveness of
malignancies. Furthermore, studies also demonstrated that various
malignancies, including breast, ovarian and cervical cancer, could
induce the abnormal growth of peripheral lymphatic vessels and
increase their permeability by secreting VEGF-C in order to
facilitate the lymphatic metastasis of tumor cells (36,37).
Thus, LVD was calculated and the expression and secretion of VEGF-C
was detected, and it was determined that oxLDL is positively
associated with increased lymph node metastasis, in addition to
VEGF-C upregulation and lymphangiogenesis stimulation. However, due
to the weak correlation (r=0.64) determined between plasma oxLDL
and VEGF-C levels and the undetectable oxLDL in tissue sections
using immunochemical methods of detection, further in vitro
and in vivo experiments were conducted to define whether
oxLDL can regulate VEGF-C expression and stimulate
lymphangiogenesis. An in vitro study revealed that oxLDL
upregulated the expression of VEGF-C in gastric cell lines. Similar
results were obtained from the popliteal lymph node metastasis
animal experiment. These results demonstrate that the level of
oxLDL is associated with VEGF-C expression levels and
lymphangiogenesis in gastric cancer, but further clinical studies
are required to determine whether oxLDL is an independent marker
for the early prediction of gastric cancer lymphatic metastasis. It
is also necessary to identify if oxLDL promotes gastric cancer cell
spread through lymph vessels or if it enhances the proliferation of
cancer cells in lymph nodes. As other studies have demonstrated,
elevated oxLDL, or the upregulation of LOX-1 expression, could
promote tumor proliferation via ROS generation and cell cycle
protein induction (31,38). However, similar phenomenon was not
observed in the present gastric cancer implantation models
following treatment with oxLDL, and it was tentatively interpreted
as the space limitation in the footpad or the loss of gastric
cancer cells caused by oxLDL toxicity. Furthermore, to address this
point, gastric cancer cells pre-labeled with lucif-erase were
traced using a live imaging system IVIS, and as we had
hypothesized, there were increased labeled cells observed in the
oxLDL treatment group, compared with the nLDL treatment or blank
control groups. In conclusion, oxLDL promoted the lymph node
metastasis of gastric cancer independently of increasing cancer
cell proliferation.
Lymphangiogenesis is mediated by the balance between
stimulating and inhibiting factors, including the regulatory
mechanisms in angiogenesis (39,40).
Our previous study indicated that kallistatin, one of the
angiogenesis inhibitors, could impede the NF-κB signaling pathway
and downregulate the expression of VEGF-C to impair the lymph node
metastasis of gastric cancer cells (24). NF-κB is an important inducible
transcription factor that serves an important role in cell growth,
proliferation, differentiation, apoptosis and carcinogenesis
(29,30). Primarily, IκB, which covers the
nuclear localization sequence of NF-κB, prevents P65 from
translocating to the nucleus and regulating gene transcription
(29); when cells are stimulated
by various endogenous, including DNA damage, ROS and lysosomal
proteases, or exogenous factors, including interleukin (IL)-1, IL-6
and tumor necrosis factor-α, the NF-κB signaling pathway is
activated and IκB will be phosphorylated and degraded, resulting in
increased nuclear translocation of P65, which then binds to the
promoter region of the corresponding gene to regulate its
transcription and expression (30). The continuous activation of the
NF-κB signaling pathway will result in the continuous expression of
its target genes, which may result in an increased number of
diseases, including cancer (29,30).
It has been reported that the NF-κB signaling pathway is involved
in the regulation of VEGF-C expression (41). Previous studies reported that oxLDL
can effectively promote the activation of NF-κB signaling pathway
proteins (27,28). The present data indicated that
oxLDL promoted IκB and P65 phosphorylation to increase the
degradation of IκB. Subsequently, the nuclear translocation of P65
increased and the expression of VEGF-C was upregulated.
Additionally, VEGF-C is primarily expressed in and
secreted from cancer tissues, and acts as a strong stimulator able
to promote lymphangiogenesis and lymph node metastasis through
binding to VEGF receptor-3 (42).
It was demonstrated that oxLDL promotes VEGF-C expression and
secretion in gastric cancer cells by activating the NF-κB signaling
pathway through its receptor, LOX, and there was no evidence
indicating that oxLDL can act on VEGF-C directly. It has been
reported that LOX-1 can activate tumor-associated signaling
pathways to promote the development of tumors (43). Simultaneously, it can maintain the
growth of numerous tumors and is an important regulator of
angiogenesis (42). This indicates
that oxLDL may serve a role in the NF-κB signaling pathway through
the mediation of LOX-1. The present results demonstrated the
knockdown of LOX-1 on HGC-27 gastric cancer cells using interfering
fragments and specific inhibitors can inhibit the aforementioned
function of oxLDL to a certain extent. This indicates that LOX-1
mediates oxLDL activation of the NF-κB signaling pathway and
upregulation of VEGF-C. Combined with the aforementioned data, the
present study may preliminarily outline the specific molecular
mechanism by which oxLDL aggravates the lymph node metastasis of
gastric cancer, providing a notable theoretical basis for the
future prevention and treatment of gastric cancer lymphatic
metastasis.
Li et al (44) demonstrated that ROS elimination
could induce resistance to anoikis and promote nasopharyngeal
carcinoma metastasis through blood vessels. As aforementioned,
oxLDL or LOX-1 enhance ROS levels in cancer cells. The present data
cannot exclude the possibility that oxLDL may promote the lymph
node metastasis of gastric cancer cells by inducing resistance to
anoikis, or even metastasis through blood vessels to lymphatic
vessel. Considering that lymphatic is an important metastatic route
of gastric cancer cells (19), it
can be proposed that the lymph node metastasis enhancing mechanism
of oxLDL is due to the upregulation of VEGF-C caused by activating
the NF-κB signaling pathway. Furthermore, as a recent study
elucidated, metastasis-initiating cells rely on dietary lipid
uptake via cluster of differentiation 36 (CD36) to promote
lymphatic metastasis (45). Hence,
it is considered that if oxLDL/LOX-1 could interact with or
regulate the expression or function of CD36, so far as to increase
the quantity of metastasis-initiating cells, oxLDL will promote the
lymph node metastasis of gastric cancer multi-dimensionally.
In conclusion, to the best of our knowledge, this
was the first report that oxLDL promotes lymph node metastasis in
gastric cancer, and to indicate the elucidated molecular mechanisms
of oxLDL, including the upregulation of VEGF-C expression in and
increased secretion from gastric cancer cells through the
LOX-1/NF-κB signaling pathway (Fig.
5E). These data support oxLDL elimination as a significant
therapeutic target for the prevention and intervention of early
lymph node metastasis in gastric cancer. However, whether oxLDL can
be an independent marker for the early prediction of gastric cancer
lymphatic metastasis remains to be determined. On account of our
limited clinical data, a larger study population, further
stratified and analyzed in terms of age, sex and BMI, should be
used to resolve this problem in future studies.
An animal model of in situ induction would
have been an improved choice for the present study. However, when
the tracing of lymphatic metastatic gastric cancer cells was
performed with an IVIS system, it was determined that it was
difficult to distinguish the metastatic gastric cancer cells from
the insitu ones. To solve this problem, a popliteal lymph
node metastasis model was constructed that was successfully used in
tracing the lymph node metastasis of cancer cells. BALB/C nude mice
are a strain with a genetic mutation that causes the absence of a
thymus, resulting in an inhibited immune system due to a notably
reduced number of T cells. To avoid the negative influence of the
inhibited immune system, an improved animal model such as a
traceable orthotopic implantation model should be used to
investigate this in future studies.
Lymphangiogenesis is a complicated process
influenced with numerous factors, including VEGF-C, VEGF-D and
VEGF-A, among others (42). In the
preliminary experiment, the transcription levels of VEGF-C, VEGF-D
and VEGF-A were detected in HGC-27 gastric cancer cells following
treatment with oxLDL, and it was determined that VEGF-D and VEGF-A
were upregulated, although only slightly. This indicates that the
mechanism by which oxLDL regulates lymph node metastasis is
complicated, and that it is worth further investigation in the
future.
In summary, the present data preliminarily verified
the correlation between oxLDL and lymph node metastasis of gastric
cancer, and clarified the molecular mechanism of oxLDL promoting
the lymph node metastasis in gastric cancer. These data indicate
that oxLDL elimination could be a novel choice for the prevention
and intervention of early lymph node metastasis in gastric
cancer.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81572342, 81770808,
81471033, 81600641, 81370945, 81400639, 81570871, 81570764,
81872165 and 81702879), the National Key Sci-Tech Special Project
of China (grant nos. 2013ZX09102-053 and 2015GKS-355), the Key
Project of Nature Science Foundation of Guangdong Province, China
(grant nos. 2015A030311043, 2016A030311035 and 2016A020214001), the
Guangdong Natural Science Fund (grant nos. 2014A020212023,
2014A030313073, 2015A030313029 and 2015A030313103), the Guangdong
Science Technology Project (grant no. 2017A020215075), the Initiate
Research Funds for the Central Universities of China (Youth
Program) (grant nos. 13ykpy06, 14ykpy05 and 16ykpy24), the Key
Sci-tech Research Project of Guangzhou Municipality, China (grant
nos. 201508020033, 201510010052, 201707010084 and 201803010017) the
Pearl River Nova Program of Guangzhou Municipality, China (grant
no. 201610010186) and the Fundamental Research Funds for the
Central Universities (grant no. 2017BQ112).
Availability of data and materials
The datasets and materials used and/or analyzed
during the current study are available from the corresponding
author on reasonable request.
Authors’ contributions
XY and GG designed the experiments and revised the
manuscript. CM, JX and CL conducted the majority of the experiments
and analyzed the data. CM and JX organized the figures and wrote
the manuscript. HY, RL, XW, WX, TZha and PJ participated in the
experiments. WQ, TZho, ZY, WW and JM participated in the
experiments and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All patient’s provided written informed consent
prior to surgery, and the use of medical records and biospecimens
was approved by the Institutional Research Ethics Committee. All
procedures relating to animal handling, experimentation and welfare
were conducted in compliance with the Institutional Animal Care and
Use Committee of Sun Yat-sen University (approval no.
20151011007).
Patient consent for publication
All of the patients recruited to the present study
provided their consent for their data to be published in this
paper.
Competing interests
The authors confirmed that they have no conflicts of
interest.
Acknowledgments
The authors would like to thank the assistance of Dr
Wei Wang and Dr Wenjun Xiong from the Department of
Gastrointestinal Surgery, Traditional Chinese Medicine Hospital of
Guangdong Province (Guangzhou, China), and Dr Ting Zhang from the
Guangzhou First People’s Hospital (Guangzhou, China) for providing
the clinical samples.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends - an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar
|
|
3
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okholm C, Svendsen LB and Achiam MP:
Status and prognosis of lymph node metastasis in patients with
cardia cancer - a systematic review. Surg Oncol. 23:140–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colquhoun A, Arnold M, Ferlay J, Goodman
KJ, Forman D and Soerjomataram I: Global patterns of cardia and
non-cardia gastric cancer incidence in 2012. Gut. 64:1881–1888.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Witte MH, Bernas MJ, Martin CP and Witte
CL: Lymphangiogenesis and lymphangiodysplasia: From molecular to
clinical lymphology. Microsc Res Tech. 55:122–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey
SM, Dong ZW, Mark SD, Qiao YL and Taylor PR: Prospective study of
risk factors for esophageal and gastric cancers in the Linxian
general population trial cohort in China. Int J Cancer.
113:456–463. 2005. View Article : Google Scholar
|
|
9
|
De Pergola G and Silvestris F: Obesity as
a major risk factor for cancer. J Obes. 2013.291546:2013.
|
|
10
|
Han J, Jiang Y, Liu X, Meng Q, Xi Q,
Zhuang Q, Han Y, Gao Y, Ding Q and Wu G: Dietary fat intake and
risk of gastric cancer: A meta-analysis of observational studies.
PLoS One. 10:e01385802015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sako A, Kitayama J, Kaisaki S and Nagawa
H: Hyperlipidemia is a risk factor for lymphatic metastasis in
superficial esophageal carcinoma. Cancer Lett. 208:43–49. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung JI, Cho HJ, Jung YJ, Kwon SH, Her S,
Choi SS, Shin SH, Lee KW and Park JH: High-fat diet-induced obesity
increases lymphangiogenesis and lymph node metastasis in the B16F10
melanoma allograft model: Roles of adipocytes and M2-macrophages.
Int J Cancer. 136:258–270. 2015. View Article : Google Scholar
|
|
13
|
Levitan I, Volkov S and Subbaiah PV:
Oxidized LDL: Diversity, patterns of recognition, and
pathophysiology. Antioxid Redox Signal. 13:39–75. 2010. View Article : Google Scholar :
|
|
14
|
Chen KC, Liao YC, Wang JY, Lin YC, Chen CH
and Juo SHH: Oxidized low-density lipoprotein is a common risk
factor for cardiovascular diseases and gastroenterological cancers
via epigenomical regulation of microRNA-210. Oncotarget.
6:24105–24118. 2015.PubMed/NCBI
|
|
15
|
Delimaris I, Faviou E, Antonakos G,
Stathopoulou E, Zachari A and Dionyssiou-Asteriou A: Oxidized LDL,
serum oxidizability and serum lipid levels in patients with breast
or ovarian cancer. Clin Biochem. 40:1129–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khaidakov M and Mehta JL: Do
atherosclerosis and obesity-associated susceptibility to cancer
share causative link to oxLDL and LOX-1. Cardiovasc Drugs Ther.
25:477–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koskinen S, Enockson C, Lopes-Virella MF
and Virella G: Preparation of a human standard for determination of
the levels of antibodies to oxidatively modified low-density
lipoproteins. Clin Diagn Lab Immunol. 5:817–822. 1998.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)). Method Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
19
|
Kitayama J, Hatano K, Kaisaki S, Suzuki H,
Fujii S and Nagawa H: Hyperlipidaemia is positively correlated with
lymph node metastasis in men with early gastric cancer. Br J Surg.
91:191–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skobe M, Hawighorst T, Jackson DG, Prevo
R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K and Detmar
M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swartz MA and Skobe M: Lymphatic function,
lymphangiogenesis, and cancer metastasis. Microsc Res Tech.
55:92–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pereira ER, Jones D, Jung K and Padera TP:
The lymph node microenvironment and its role in the progression of
metastatic cancer. Semin Cell Dev Biol. 38:98–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mumprecht V, Honer M, Vigl B, Proulx ST,
Trachsel E, Kaspar M, Banziger-Tobler NE, Schibli R, Neri D and
Detmar M: In vivo imaging of inflammation- and tumor-induced lymph
node lymphangiogenesis by immuno-positron emission tomography.
Cancer Res. 70:8842–8851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma C, Luo C, Yin H, Zhang Y, Xiong W,
Zhang T, Gao T, Wang X, Che D, Fang Z, et al: Kallistatin inhibits
lymphangiogenesis and lymphatic metastasis of gastric cancer by
downregulating VEGF-C expression and secretion. Gastric Cancer.
21:617–631. 2018. View Article : Google Scholar
|
|
25
|
Mäkinen T, Veikkola T, Mustjoki S,
Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H,
Kerjaschki D, et al: Isolated lymphatic endothelial cells transduce
growth, survival and migratory signals via the VEGF-C/D receptor
VEGFR-3. EMBO J. 20:4762–4773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu G, Huang Q, Huang Y, Zheng W, Hua J,
Yang S, Zhuang J, Wang J and Ye J: Lipopolysaccharide increases the
release of VEGF-C that enhances cell motility and promotes
lymphangiogenesis and lymphatic metastasis through the TLR4-
NF-κB/JNK pathways in colorectal cancer. Oncotarget. 7:73711–73724.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimura S, Akagi M, Yoshida K, Hayakawa
S, Sawamura T, Munakata H and Hamanishi C: Oxidized low-density
lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1
(LOX-1) in cultured bovine articular chondrocytes increases
production of intracellular reactive oxygen species (ROS) resulting
in the activation of NF-kappaB. Osteoarthritis Cartilage.
12:568–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Guo Y, Zhu P and Yang T: Role of
Ox-LDL/LOX-1/NF-κB signaling pathway in regulation of
atherosclerotic plaque growth by testosterone in male rabbits.
Vascul Pharmacol. 59:131–137. 2013. View Article : Google Scholar
|
|
29
|
Ray A and Prefontaine KE: Physical
association and functional antagonism between the p65 subunit of
transcription factor NF-kappa B and the glucocorticoid receptor.
Proc Natl Acad Sci USA. 91:752–756. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Christian F, Smith EL and Carmody RJ: The
regulation of NF-κB subunits by phosphorylation. Cells. 5:52016.
View Article : Google Scholar
|
|
31
|
Lu J, Mitra S, Wang X, Khaidakov M and
Mehta JL: Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in
atherogenesis and tumorigenesis. Antioxid Redox Signal.
15:2301–2333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akhmedov A, Rozenberg I, Paneni F, Camici
GG, Shi Y, Doerries C, Sledzinska A, Mocharla P, Breitenstein A,
Lohmann C, et al: Endothelial overexpression of LOX-1 increases
plaque formation and promotes atherosclerosis in vivo. Eur Heart J.
35:2839–2848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Falconi M, Ciccone S, D’Arrigo P, Viani F,
Sorge R, Novelli G, Patrizi P, Desideri A and Biocca S: Design of a
novel LOX-1 receptor antagonist mimicking the natural substrate.
Biochem Biophys Res Commun. 438:340–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beasley NJ, Prevo R, Banerji S, Leek RD,
Moore J, van Trappen P, Cox G, Harris AL and Jackson DG:
Intratumoral lymphangiogenesis and lymph node metastasis in head
and neck cancer. Cancer Res. 62:1315–1320. 2002.PubMed/NCBI
|
|
35
|
Coşkun U, Akyürek N, Dursun A and Yamaç D:
Peritumoral lymphatic microvessel density associated with tumor
progression and poor prognosis in gastric carcinoma. J Surg Res.
164:110–115. 2010. View Article : Google Scholar
|
|
36
|
Alitalo K and Carmeliet P: Molecular
mechanisms of lymphangiogenesis in health and disease. Cancer Cell.
1:219–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su JL, Yang PC, Shih JY, Yang CY, Wei LH,
Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zettler ME, Prociuk MA, Austria JA,
Massaeli H, Zhong G and Pierce GN: OxLDL stimulates cell
proliferation through a general induction of cell cycle proteins.
Am J Physiol Heart Circ Physiol. 284:H644–H653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng R and Ma JX: Angiogenesis in
diabetes and obesity. Rev Endocr Metab Disord. 16:67–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao G, Li Y, Zhang D, Gee S, Crosson C and
Ma J: Unbalanced expression of VEGF and PEDF in ischemia-induced
retinal neovascularization. FEBS Lett. 489:270–276. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsai PW, Shiah SG, Lin MT, Wu CW and Kuo
ML: Up-regulation of vascular endothelial growth factor C in breast
cancer cells by heregulin-beta 1. A critical role of p38/nuclear
factor-kappa B signaling pathway. J Biol Chem. 278:5750–5759. 2003.
View Article : Google Scholar
|
|
42
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li C, Zhang J, Wu H, Li L, Yang C, Song S,
Peng P, Shao M, Zhang M, Zhao J, et al: Lectin-like oxidized
low-density lipo-protein receptor-1 facilitates metastasis of
gastric cancer through driving epithelial-mesenchymal transition
and PI3K/Akt/GSK3 beta activation. Sci Rep. 7:452752017. View Article : Google Scholar
|
|
44
|
Li S, Mao Y, Zhou T, Luo C, Xie J, Qi W,
Yang Z, Ma J, Gao G and Yang X: Manganese superoxide dismutase
mediates anoikis resistance and tumor metastasis in nasopharyngeal
carcinoma. Oncotarget. 7:32408–32420. 2016.PubMed/NCBI
|
|
45
|
Pascual G, Avgustinova A, Mejetta S,
Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:41–45. 2017. View Article : Google Scholar
|