Introduction
Hereditary multiple exostoses (HME), also termed
hereditary multiple osteochondroma, is an autosomal dominant
inherited disease characterized by the development of multiple
exostoses, predominantly located on the limbs, shoulder blades,
ribs, and pelvis (1).
Osteochondromas are frequently adjacent to the growth plates of
bones, and can increase in size and number until growth plates
close as the child stops developing. The exostoses can result in
numerous health problems, including skeletal bowing and
deformities, growth restriction, and nerve and blood vessel
compression (1,2). As a common benign bone tumor, HME is
estimated to occur at a rate of 1/50,000 cases; however, HME
progresses into chondrosarcomas or osteosarcomas in ~2% of patients
(3-6).
Heterozygous germline mutations in the exostosin
glycosyltransferase 1 (EXT1) and EXT2 genes are exhibited in
>90% of HME cases (7-9). Although no EXT1 or EXT2 germline
mutations are detected in certain cases, somatic mosaic mutations
were identified in one HME case (10). In patients with HME, 10% of
mutations are spontaneous and 90% of affected individuals have a
family history of HME (11).
Additionally, 80% of mutations in patients with HME are truncation
mutations, including nonsense, frameshift and splice site
mutations, which commonly introduce premature stop codons during
translation, or result in partial or entire loss of gene function
(9). EXT1 and EXT2 are tumor
suppressor genes that encode glycosyltransferases (7,12),
EXT1 and EXT2 form a hetero-oligomeric complex in the Golgi body
that catalyzes chain elongation during the biosynthesis of heparan
sulfate (HS) (13). HS has a key
role in chondrocyte proliferation and endochondral ossification
(14). Therefore, heterozygous
mutations in the EXT1 or EXT2 gene theoretically result in a
reduction in systemic HS levels by ~50% in HME individuals.
However, it has been reported that haploinsufficiency may not
always result in osteochondroma formation. When HS levels are
significantly decreased, but not lost completely, a second event,
such as loss-of-heterozygosity or compound heterozygous mutations,
appears to be the major cause of HME development, which has been
confirmed in animal models and also reported in a number of
patients with HME (15-19).
Recently, 436 mutations in EXT1 and 223 mutations in
EXT2 have been reported in the Multiple Osteochondroma Mutation
Database (http://medgen.ua.ac.be/LOVDv.2.0/home.php), including
various splicing mutations (11).
Alternative splicing is ubiquitous in mammals, and is a major
contributor to molecular diversity and complexity, and gene
regulation; additionally, alternative splicing is required for
numerous critical biological processes in development and disease,
including regulation of cell growth, hormone responsiveness and
cancer (20,21). However, once mutations exist in
splicing elements or splicing signal sequences, particularly at 3'
and 5' splice sites, normal splicing of mRNA and translation will
be disrupted, which can cause exon skipping or aberrant splicing,
where new splicing sites are created, resulting in truncated
proteins with potentially reduced expression and function (21). For example, dysregulation of
alternative splicing has been demonstrated to be associated with
various human diseases, including cancer, muscular dystrophies,
neurodegenerative diseases and obesity (21).
A number of splicing mutations have been detected in
patients with HME, and the molecular mechanisms are reported to
involve the creation of new splice sites or exon skipping due to
splicing mutations, resulting in early termination of translation
and the degradation of truncated peptides via nonsense-mediated
mRNA decay (NMD) (22,23). In the present paper, a splice
mutation in EXT1 (c.1284+2del) was identified in a three-generation
Chinese family with HME. Skipping of EXT1 exon 4 was verified by TA
cloning and sequencing of EXT1 mRNA from the patients with HME. No
premature stop codon was produced by the skipping of exon 4;
however, the expression levels of EXT1/EXT2 mRNA were notably
reduced in the patients, as indicated by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
In vitro, the truncated mutant protein was detected in the
cytoplasm when expressed in Cos-7 cells. Thus, whether mutant EXT1
or EXT2 proteins are biologically functional requires further
research, but the decrease in the expression of wild-type EXT1/EXT2
proteins will hinder the process of HS polymerization and chain
elongation.
Materials and methods
Study participants, cell culture and
reagents
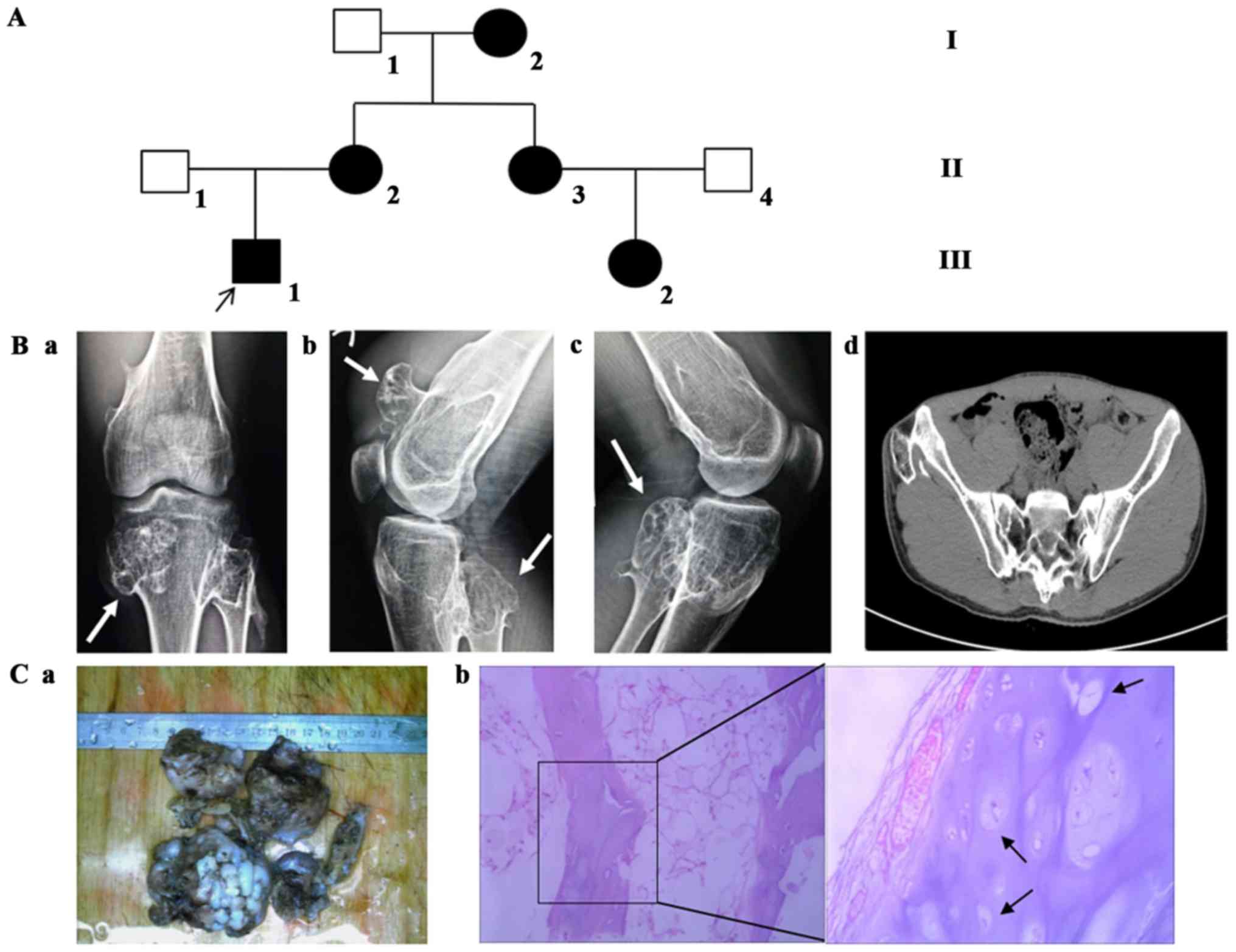
Peripheral blood samples were collected from a
Chinese family with HME in three generations from May 2013 to March
2015 (Fig. 1A). The HME diagnosis
was produced according to their clinical manifestations and
physical examinations, including X-ray, computed tomography and
pathological sections (24).
Osteochondromas tissue was stained with hematoxylin and eosin for 5
min at room temperature. In the present study, two patients with
HME (III 1, the proband, male, 31 years; II 2, the mother of the
proband, female, 62 years), one normal family member (II 1, the
father of the proband, male, 65 years) and one healthy individual
(normal physical examination, male, 31 years) were enrolled in
mutation analysis of EXT1 and EXT2 genes. The proband (III 1) was
the first person who required surgical intervention in the family
with HME, and was an inpatient of the Department of Bone Tumors of
Fuzhou Second Hospital (Fuzhou, China), the healthy individual was
an inpatient at the Medical Examination Center of Fuzhou Second
Hospital. The samples were collected together when the proband was
in hospital for surgery. Written informed consent was obtained from
all participants, and the study was approved by the Ethics
Committee of Fuzhou Second Hospital [approval no. (2014) 63].
293-T and Cos-7 (originating from African green
monkey kidney fibroblasts) cell lines (Cell Bank of the Chinese
Academy of Sciences, Shanghai, China) were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), and incubated at 37°C incubator in an atmosphere
containing 5% CO2. Cells were passaged at 80% confluency
by digestion with trypsin (Gibco; Thermo Fisher Scientific,
Inc.).
Mutation screening for EXT1 and EXT2
genes
Genomic DNA of the participants was extracted from
peripheral blood according to the procedures of the SE Blood DNA
kit (Omega Bio-Tek, Inc., Norcross, GA, USA). DNA samples were used
for mutation screening of the coding exons and the adjacent introns
of EXT1 (GenBank NG_007455.2) (https://www.ncbi.nlm.nih.gov/nuccore/NG_007455.2)
and EXT2 (GenBank NG_007560.1) (https://www.ncbi.nlm.nih.gov/nuccore/NG_007560.1)
genes using previously reported primer sequences (22,25).
The products of the amplified sequences were observed on a 2%
agarose gel and purified with HiBind® columns using an
E.Z.N.A.® Cycle-Pure kit (Omega Bio-Tek, Inc.).
Bidirectional sequencing was performed on purified products using
an ABI 3730 XL genetic analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The possible pathogenic mutation screened from
the sequencing would be proved to be a novel variant or a reported
one in the ExAc database (http://exac.broadinstitute.org/gene/ENSG00000182197)
Bioinformatics analysis and
prediction
Several web-based programs with different algorithms
were used to analyze the potential effect of mutations on exon
splicing. Mutation Taster and Protein Variation Effect Analyzer
(PROVEAN) were selected for pathogenicity prediction. Mutation
Taster uses a Bayes classifier to predict the disease potential of
an alteration (mutationtaster.org/) and PROVEAN is a software tool
that predicts whether an amino acid substitution or indel has an
impact on the biological function of a protein
(provean.jcvi.org/index.php). The CRYP-SKIP algorithm (http://cryp-skip.img.cas.cz/) uses multiple logistic
regression to predict the two aberrant transcripts from the primary
sequence, and was applied in the present study to estimate the
probability of cryptic splice-site activation (P) and exon
skipping (1-P) due to a splicing mutation (26). The Berkeley Drosophila
Genome Project (BDGP) (http://www.fruitfly.org/about/index.html) algorithm
accurately distinguishes between donor and acceptor sites using a
generalized hidden Markov model. As a splice site prediction
program, it predicts cryptic splice sites and highlights changes in
splice sites following input of a mutant sequence. The Human
Splicing Finder (HSF) (http://www.umd.be/HSF3/) is an online tool that uses
various algorithms to predict the effects of mutations on splicing
signals or to identify splicing motifs in any human sequence. It
has been previously used to predict the effects caused by splicing
mutations (27).
Analysis of EXT1 and EXT2 mRNA
Total RNA was obtained from the venous blood of two
patients with HME and normal controls according to the instructions
of a QIAamp RNA Blood Mini kit (Qiagen GmbH, Hilden, Germany).
Total RNA (5 µg) was reverse transcribed into cDNA using random
primers based of a PrimeScript™ 1st Strand cDNA Synthesis kit
(Takara Biotechnology Co., Ltd., Dalian, China), and the
synthesized cDNA was used as a template for PCR amplification of
EXT1 with the following primers: 5′-atgcaggccaaaaaacgctatt-3′
(forward); and 5′-tcaaagtcgctcaatgtctcg-3′ (reverse). LA Taq
(Takara Biotechnology Co., Ltd.) was used as the DNA polymerase
under the conditions: 94°C for 5 min, 94°C for 30 sec, annealing
for 30 sec (53°C), 72°C for 45 sec for a total of 30 cycles; and
the last cycle was extended at 72°C for 10 min (ABI 2720 Thermal
Cycler; Applied Biosystems; Thermo Fisher Scientific, Inc.). The
PCR products were separated by 1% agarose gel electrophoresis and
visualized using ethidium bromide staining (Sangon Biotech Co.,
Ltd., Shanghai, China) for ~1 h at room temperature. The products
were purified using HiBind® columns and then cloned into
the PGEM-T Easy vector (Promega Corporation, Madison, WI, USA).
Ligation products were transformed into XL1-blue bacteria and
cultured in ampicillin/X-gal/IPTG plates. Following transformation,
~50 positive clones were selected randomly from the proband, the
mother and normal control, then cultured separately in a shaker
overnight at 37°C. Following extraction, the plasmids of the three
groups were sequenced to search for the potential abnormal
alternative transcripts in the individuals with HME, and the
percentage of the mutated transcripts was evaluated with respect to
the normal control.
cDNA from peripheral blood lymphocytes of the
proband, the mother and normal control was amplified by two-step
RT-qPCR according to the protocol of a SYBR® Ex Taq
Reagent kit (Takara Biotechnology Co., Ltd.) using the
LightCycler480 Real-Time PCR system (Roche Diagnostics,
Indianapolis, IN, USA). qPCR was performed using the following
primers: EXT1 (GenBank NM_000127.2), forward
5′-CATAGGCGATGAGAGATTGT-3′, and reverse 5′-CAA GAATTGTGTCTGCTGTC-3′
(fragment size, 97 bp); abnormally spliced EXT1, forward
5′-GTGATGCTCAGCAAT GGATG-3′, and reverse 5′-TCTGTCCTGAATAATCTGTA-3′
(fragment size, 108 bp); and EXT2 (GenBank NM_207122.1), forward
5′-GGCTACGATGTCAGCATTCCTG-3′, and reverse
5′-GGCTTCTAGGTCCTCTCTGTAC-3′ (fragment size, 141 bp). 18s rRNA was
amplified simultaneously as an internal control (forward
5′-CGACGACCCATTCGAACGTCT-3′, and reverse
5′-CTCTCCGGAATCGAACCCTGA-3′; fragment size, 102 bp). The conditions
of RT-qPCR were as follows: 95°C for 3 min, 95°C for 15 sec, 60°C
annealing for 15 sec, 72°C for 20 sec for a total of 39 cycles,
95°C for 5 sec, melt curve 60-95°C at increments for 0.5°C every 5
sec (CFX96™ Real-Time System; Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The 2−∆∆Cq method was used to calculate
relative gene expression (28).
Lentiviral transduction in vitro and
observation of growth condition
The full-length coding sequence of EXT1 gene
(EXT1-FL) was amplified using cDNA from the peripheral blood of a
normal participant, and the abnormal mutant transcript of EXT1
(EXT1-DEL) was amplified using the aforementioned recombinant
plasmids from TA cloning and sequencing as the template, which
matched the NCBI reference sequence (GenBank NM_000127.2), except
for exon 4. The products of the amplified transcripts were
confirmed by sequencing. GV358-EXT1-FL and GV358-EXT1-DEL
lentiviral vectors were constructed, and were amplified and
titrated based on the manufacturer's instructions (Shanghai
GeneChem Co., Ltd., Shanghai, China) (29). A GV358-GFP vector was used as a
negative control (NC). 293-T and Cos-7 cells were seeded in 6-well
plates at a density of 5×105 cells/ml of DMEM and then
co-infected with GV358-EXT11-FL, GV358-EXT1-DEL and empty vector
(GV358-GFP vector), which were mixed with Polybrene (Shanghai
GeneChem Co., Ltd.) at a multiplicity of infection of 10. Cell
infection efficiency and growth state were assessed by observation
of green fluorescent protein (EXT1-GFP fusion protein) and cell
morphological characteristics at 24 h after infection using a
fluorescence microscope (x200 magnification; Olympus Corporation,
Tokyo, Japan).
RNA extraction and RT-qPCR
Based on the TRIzol® (Shanghai Pufei
Biotechnology Co., Ltd., Shanghai, China) method, total RNA was
extracted from three groups of 293-T cells infected with lentivirus
after 48 h. Subsequently, RNA was reverse transcribed into cDNA
using Promega M-MLV kits (Promega Corporation). GV358-EXT1-FL,
GV358-EXT1-DEL and NC were simultaneously detected by two-step
RT-qPCR under the same conditions aforementioned. The primer
sequences used were as follows: Forward 5′-TTTTCTGCCCTACGACAA
CAT-3′, and reverse 5′-ACACTGTGAAGGCGAAATCCA-3′, fragment size, 98
bp. As an internal control, GAPDH was also amplified using the
following primers: Forward 5′-TGACTT CAACAGCGACACCCA-3′ and reverse
5′-CACCCTGTT GCTGTAGCCAAA-3′; fragment size, 121 bp.
Protein extraction and western
blotting
Total protein was isolated from cultured 293-T cells
and Cos-7 cells at 72 h after infection. The protein concentration
was measured using a Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology, Shanghai, China) and 30 µg protein from
each group was separated via SDS-PAGE on 10% gels, then blotted
onto polyvinylidene difluoride membranes (cat. no. IPVH00010; EMD
Millipore, Billerica, MA, USA). Following electrophoresis,
membranes were blocked with 5% bovine serum albumin (BSA; Thermo
Fisher Scientific, Inc.) for 1 h at room temperature. Membranes
were probed with mouse anti-Flag monoclonal antibody (cat. no.
RLM3001; Suzhou Ruiying Biotechnology Co., Ltd., Suzhou, China;
1:3,000 dilution) and rabbit anti-human EXT1 (cat. no. ab126305;
Abcam, Cambridge, UK; 1:3,000 dilution) to detect EXT1 and the
novel truncated peptide, which targeted the 205-335 amino acid
sequences of human EXT1, a region upstream of exon 4 (overnight at
4°C). Peroxidase-conjugated goat anti-mouse IgG (cat. no. A0216)
and goat anti-rabbit IgG (cat. no. A0208) secondary antibodies were
incubated for 1 h at room temperature (both from Beyotime Institute
of Biotechnology; both 1:10,000 dilution). GAPDH was also detected
using mouse anti-GAPDH monoclonal antibody (cat. no. RLM3029;
Suzhou Ruiying Biotechnology Co., Ltd.; 1:3,000 dilution) as an
endogenous control for the western blot analysis under the same
conditions aforementioned. Finally, the membranes were developed
using enhanced chemiluminescence (ECL-Plus) reagent (Thermo Fisher
Scientific, Inc.) and exposed to x-ray film (Carestream Health,
Inc., Rochester, NY, USA).
Immunofluorescence analysis and
subcellular localization detection using laser scanning confocal
microscopy
Following infection for 48 h, the monolayer of Cos-7
cells was washed with PBS (3 times), fixed using 4%
paraformaldehyde under room temperature for 30 min, permeabilized
(0.02% Triton X-100), blocked (5% BSA) for 30 min at room
temperature and then incubated at 4°C overnight with rabbit
anti-human EXT1 (cat. no. 126305; Abcam; 1:200 dilution). Following
being washed with PBS (3 times), cells were incubated with Alexa
Fluor® 555-conjugated donkey anti-rabbit antibody (cat.
no. A0453; Beyotime Institute of Biotechnology; 1:500 dilution)
avoiding light for 2 h at room temperature. Finally, stained
monolayer cells were washed with PBS three times and observed under
a laser scanning confocal microscope (x630 magnification; Leica
Microsystems GmbH, Wetzlar, Germany). The nucleus of the cells was
stained with DAPI (cat. no. C1002; Beyotime Institute of
Biotechnology).
Statistical analysis
χ2 test was used to evaluate the
statistical significance of the result of TA cloning sequence
(proportion). One-way analysis of variance with Least Significant
Difference test was used to evaluate the statistical significance
of the results of RT-qPCR, and they were expressed as the mean ±
standard error of the mean [SPSS 19.0 (IBM Corp., Armonk, NY,
USA)]. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical data of the family with HME
According to information provided by the proband, at
least five individuals of the family were suspected to have
multiple exostoses. However, only three members (the proband and
his parents) participated in the present study and agreed to
publication (Fig. 1A). The proband
(III 1) had exhibited exostoses around the joints of the hips,
knees, wrists, and ankles for >20 years. Furthermore, imaging
(X-ray and computed tomography) and pathological sections confirmed
the diagnosis (Fig. 1B and C). The
mother had relatively minor symptoms according to the examination
conducted.
Mutation screening and identification of
a novel mutation (c.1284+2del) in EXT1
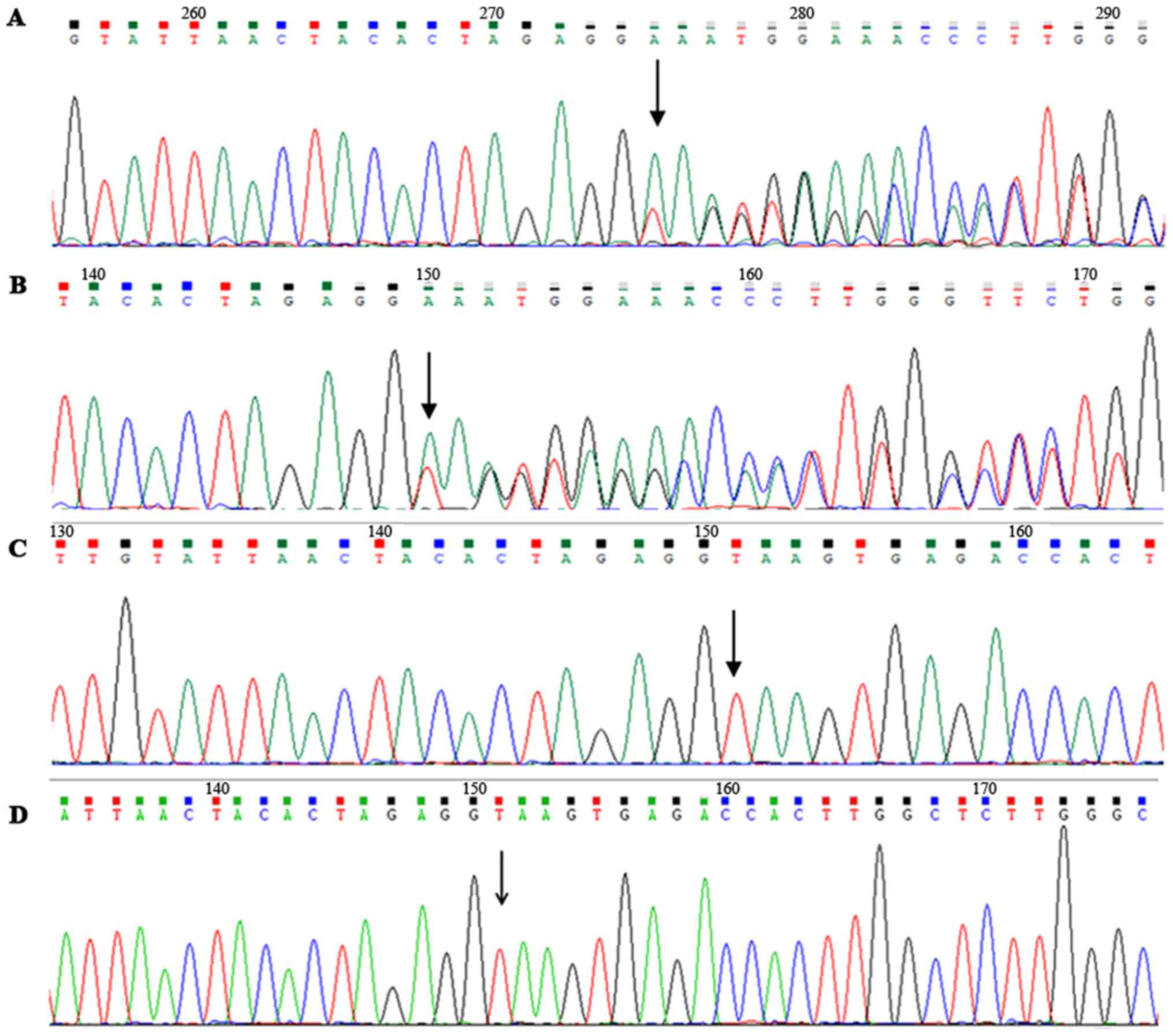
Sequencing results of the coding region and adjacent
intronic sequences in EXT1/EXT2 genes of the proband revealed that
there was a heterozygous deletion (c.1284+2del) in intron 4 of the
EXT1 gene, but no mutation was determined in the EXT2 gene
(Fig. 2A). Furthermore, DNA
sequencing identified the same alteration in the mother (II 2)
(Fig. 2B); however, the mutation
was not exhibited in the normal father or the healthy participants
(Fig. 2C and D), and it was not
reported in the ExAc database. The mutation spectrum indicated that
the delete mutation was co-segregated with HME in this family.
Sequence analysis indicated that c.1284+2del is an intron variation
at the 5' splice site (AGgt) of exon 4.
Abnormal splicing and exon skipping in
EXT1 gene
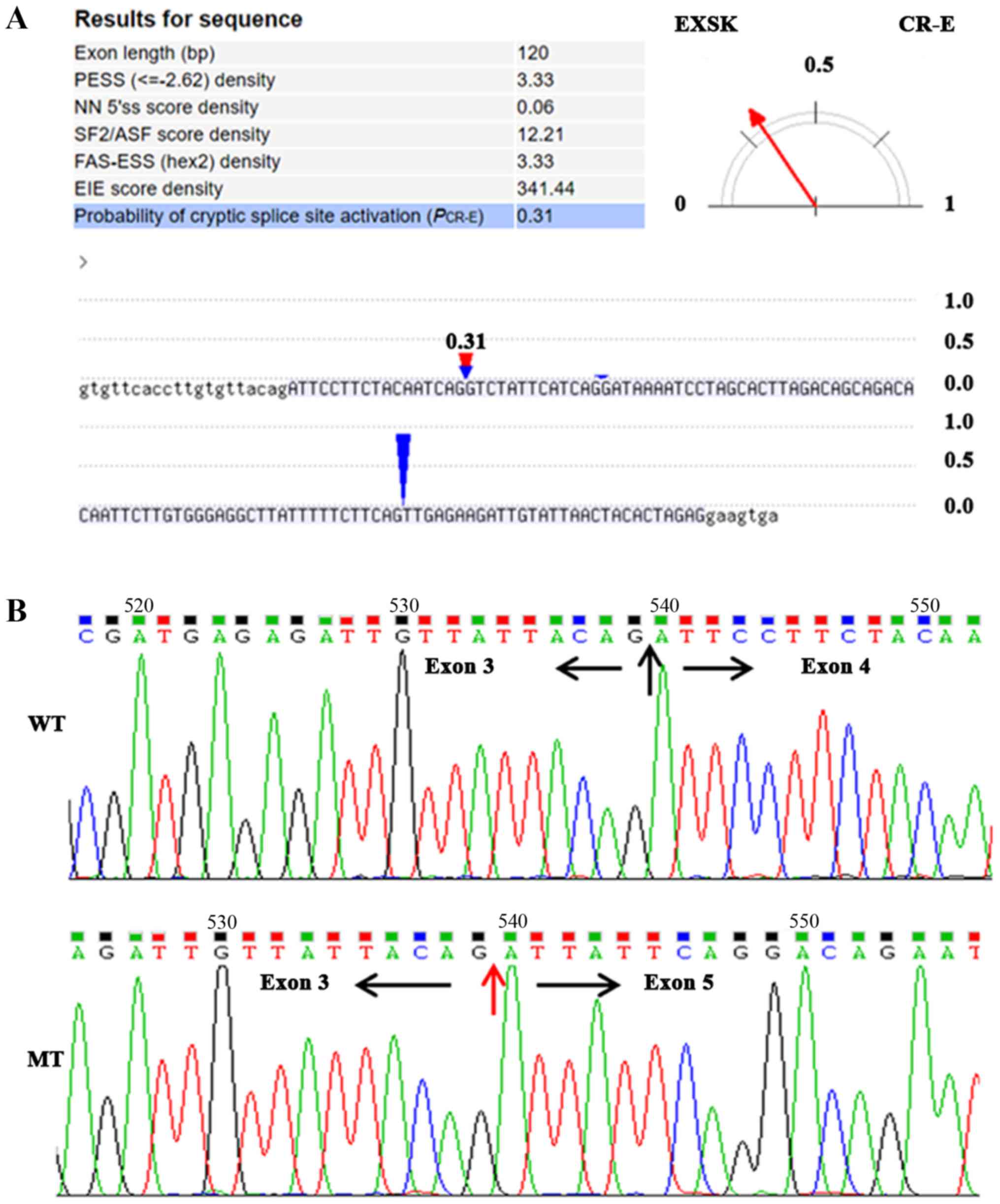
Predictions from multiple bioinformatics databases
revealed that the splicing mutation may cause two potential effects
in the mRNA: Skipping of exon 4, or loss of the primary 5 splice
site (AGGT) and activation of an adjacent cryptic splice site. The
CRYP-AGgt analysis revealed that the probability of exon 4 skipping
was 0.69, whereas the probability of new cryptic splice site
activation was 0.31 (Fig. 3A).
BDGP predicted that the mutation caused the splice site to
disappear at the mutational site. The HSF tool also indicated that
AGgt was absent due to the mutation; however, it also suggested
that a novel splice site (AAgt) emerged 3 bp downstream of AGgt.
Mutation Taster predicted that c.1284+2del may disrupt normal
splicing and that it was a disease-causing mutation that may affect
the protein function. The novel polypeptide lacking amino acids
from 389 to 428 of exostosin-1 protein (I389_E428del) created by
exon 4 skipping was also indicated to be deleterious by PROVEAN
analysis (data not shown).
TA cloning and sequencing of the targeted fragments
of the two patients identified a notable number of abnormal
transcripts with exon 4 skipping in EXT1 mRNA (proband 66.7%,
mother, 58.5%, P<0.05; Fig. 3B;
Table I); however, no aberrantly
spliced transcripts with cryptic splice site were identified in the
patients. Furthermore, although the ratio of transcripts with exon
4 jumping in EXT1 was increased in the proband, compared with the
mother, there was no significant statistical difference between
them (P>0.05; Table I). The
results from TA cloning and sequencing were almost consistent with
the bioinformatics predications, and the corresponding amino acids
coded by the missing exon 4 were amino acids 389-428 of exostosin-1
protein, which form part of the conserved domain of exostosin
(amino acids 110-396; Fig. 4).
 | Table ITA clone and sequencing results of
the proband, mother of HME and normal control. |
Table I
TA clone and sequencing results of
the proband, mother of HME and normal control.
| Subjects | The clones with
skipped exon 4 | The clones without
skipped exon 4 | Total number of
clones | Clones with skipped
exon 4/total number of clones |
|---|
| The probandc | 26 | 13 | 39 | 0.667 |
| The mothera,b | 24 | 17 | 41 | 0.585 |
| Normal control | 2 | 38 | 40 | 0.050 |
Aberrantly reduced expression of
EXT1/EXT2 genes
To investigate the potential effect of the splice
mutation on the gene expression of EXT1/EXT2, the mRNA of EXT1/EXT2
and the abnormally spliced transcript of EXT1 gene was assessed in
the patients with HME and normal controls. To distinguish the
normal EXT1 transcript from the abnormally spliced transcript (with
exon 4 skipping), the primers were designed with the downstream
primer located in exon 4 of EXT1 for detecting the normal
transcript, and the downstream primer spanned exons 3 and 5 of EXT1
to detect the abnormally spliced transcript. As depicted in
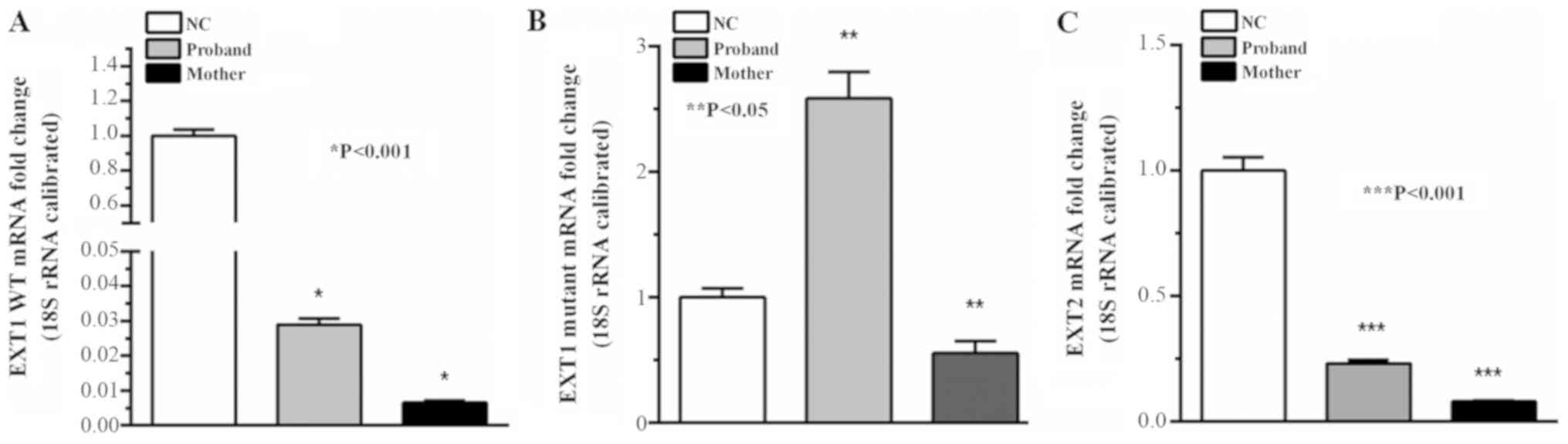
Fig. 5, the levels of wild-type
EXT1/EXT2 mRNA in patients with HME were significantly reduced,
compared with the normal control (P<0.05). The level of the
mutant EXT1 transcript was significantly increased in the proband,
compared with the normal control and the mother (P<0.05).
Overexpression of EXT1-GFP fusion protein
and aberrantly spliced RNA in vitro
Recombinant plasmids were successfully constructed
and then packaged with lentivirus vectors, as confirmed by PCR and
sequencing. After 48 h of infection with lentivirus, GV358-EXT1-FL
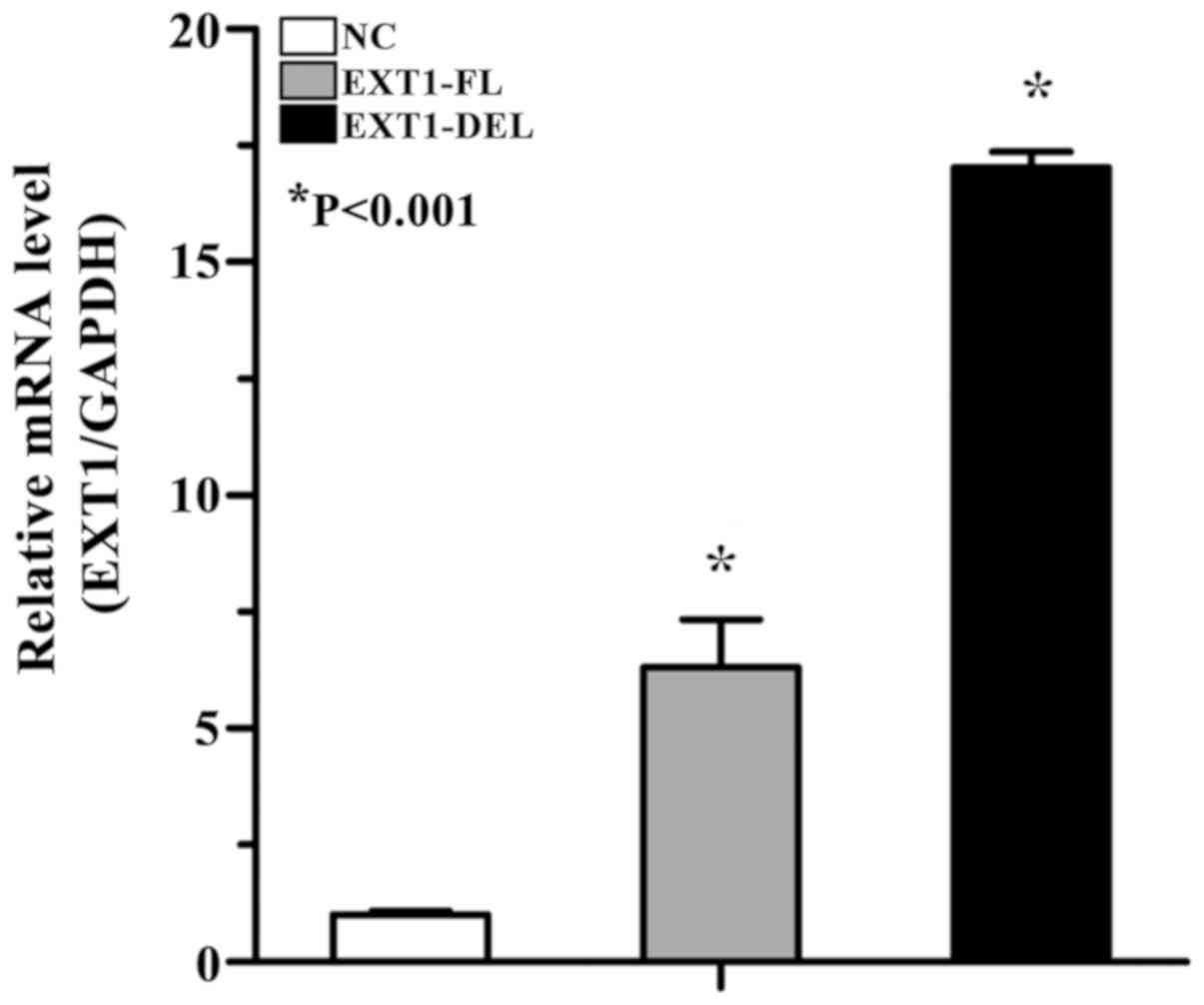
and GV358-EXT1-DEL were overexpressed in the cells. RT-qPCR
indicated that the expression levels of full-length transcript and
aberrantly spliced transcript were significantly increased,
compared with the empty vector infected with lentivirus (17.032-
and 6.309-folds, respectively), and the level of aberrantly spliced
transcript was significantly increased, compared with the
full-length transcript (3.073-folds; P<0.05; Fig. 6).
Increased expression of the truncated
polypeptide, with no notable changes in subcellular
localization
To investigate the cellular functionality of the
aberrant polypeptide, and whether its expression level and
subcellular location are different from EXT1-FL, western blot
analysis was performed and the subcellular location was determined
using laser scanning confocal microscope in cells expressing the
lentiviral constructs. Western blotting revealed that the aberrant
polypeptide was expressed by the vector, and at an increased level,
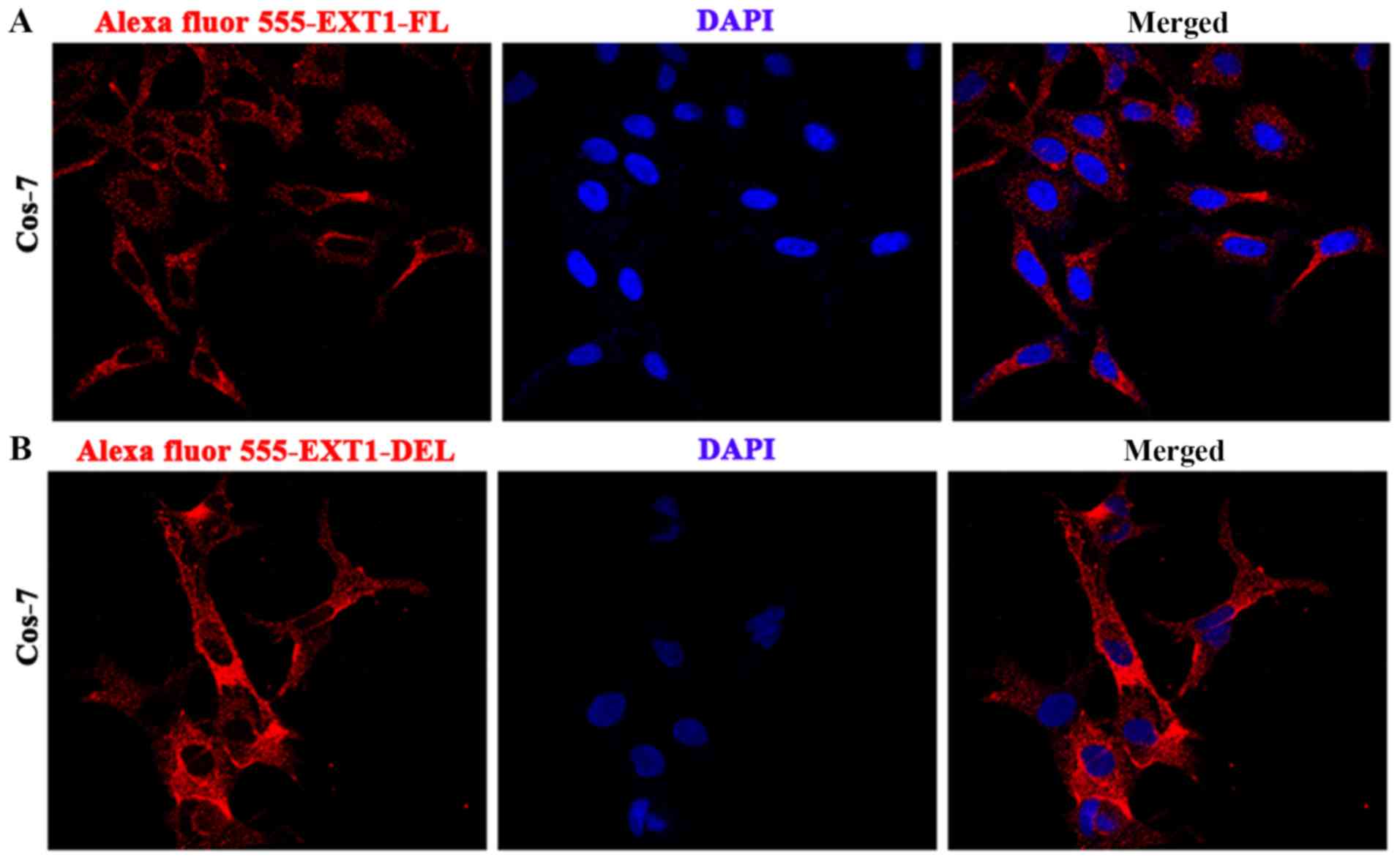
compared with the wild-type protein (Fig. 7). However, the subcellular
localization of the mutated polypeptide exhibited no alteration,
compared with the full-length protein, with the majority of the
EXT1-DEL and EXT1-FL protein located in the cytoplasm of Cos-7
cells (Fig. 8). However,
endogenous EXT1 is generally considered to be located in the Golgi
apparatus of the cytoplasm (30).
Discussion
The present study reported a novel heterozygous
splice mutation (c.1284+2del) in intron 4 of the EXT1 gene
identified in a three-generation family with HME. Mutation Taster
and PROVEAN predicted that c.1284+2del was a disease-causing
mutation. The result of TA cloning and sequencing indicated that
the mutation resulted in skipping of EXT1 exon 4 during mRNA
splicing in the proband and his affected mother with no premature
stop codon. RT-qPCR of the two patients revealed that expression
levels of EXT1/EXT2 mRNA were reduced, compared with normal
controls, and the levels of the abnormal EXT1 transcript (without
exon 4) were increased in the proband, compared with his mother and
normal control. Furthermore, the truncated peptide produced from
the abnormally spliced transcript is potentially translated and
expressed in cells without degradation via NMD. Additionally, the
subcellular localization of the truncated peptide may be the same
as protein produced from the wild-type EXT1 gene, and both proteins
were observed to be localized to the cytoplasm in vitro.
Although the molecular mechanisms associated with
HME are not fully understood, it is clear that HME is predominantly
provoked by mutations in either/both of the EXT1 or EXT2 tumor
suppressor genes. EXT1 accounts for 56-78%, and EXT2 for 21-44% of
HME-causing mutations (11).
Splicing mutations have been investigated to determine the
molecular mechanism of HME (22,23).
Alternative splicing is one of the important mechanisms in
regulating gene expression and protein diversity, splice sites (5'
and 3'), the branch site and the polypyrimidine sequence are the
key splicing signals that have major roles in the splicing of
pre-mRNA. If mutations occur in these sequences, the effective
splicing of exons may be affected, which may interfere with
subsequent transcription and translation (20,21).
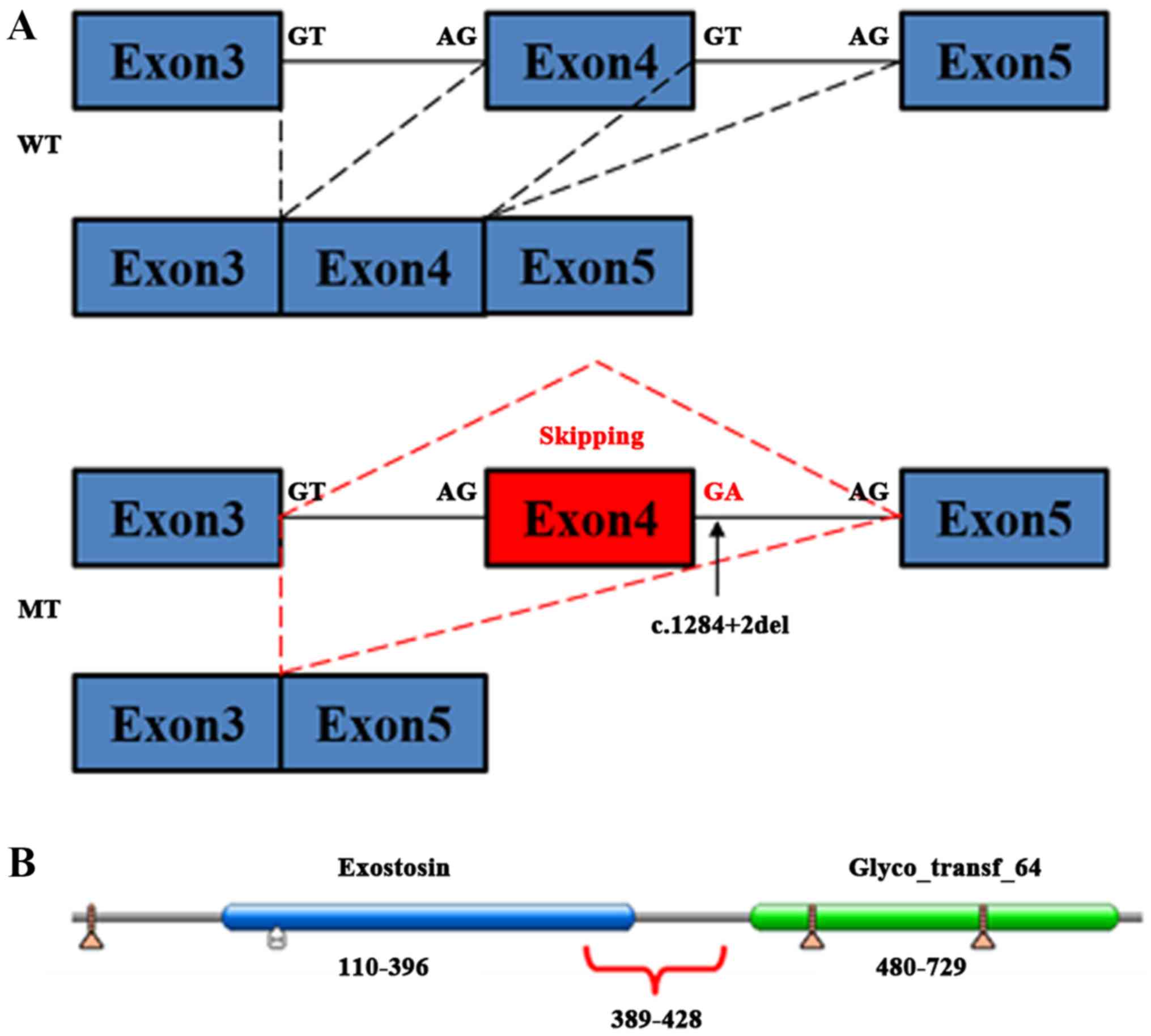
The mutation identified in the present study was located at the 5'
splice site of EXT1 exon 4, and was predicted and verified to cause
exon 4 skipping. As the sequence length of exon 4 is 120 bp and is
a multiple of 3, and the upstream coding region of exon 4 is also a
multiple of 3 (1,164 bp), the skipping of exon 4 does not disturb
the triplicate coding order of the downstream amino acids in EXT1
and premature stop code will not be created, which is different
from other mutations reported in previous studies (22,23).
TA cloning and sequencing results identified
abnormal transcripts with skipping of exon 4 in the proband and his
affected mother (66.7 and 58.5%, respectively) at significantly
increased levels, compared with the normal control participants
(P<0.05); however, a few transcripts with exon 4 skipping were
identified in the normal control (5%; Table I), which may be explained by the
phenomenon that alternative splicing frequently occurs in human
genes with multiple exons (31).
Furthermore, the number of abnormal transcripts in the proband was
increased, compared with his mother, with no statistical
significance between the two patients, potentially due to an
insufficient number of clones detected. However, the results from
RT-qPCR of EXT1/EXT2 mRNA and the abnormal spliced transcript of
EXT1, which lacks exon 4 due to the splice mutation, in the two
patients with HME were different from that of TA cloning and
sequencing. The expression levels of wild-type EXT1/EXT2 mRNA in
both patients were reduced, compared with the normal control (EXT1:
proband, 0.02890; mother, 0.00654; and normal, 1.0; and EXT2:
proband, 0.23216; mother, 0.08038; and normal, 1.0), particularly
for EXT1 mRNA. By contrast, the level of the abnormal spliced EXT1
transcript with exon 4 skipping was significantly increased in the
proband, compared with his mother and the normal control (proband,
2.58735; mother, 0.55260; and normal 1.0; P<0.05). The
difference between the TA cloning data and RT-qPCR analysis may be
due to error in the TA cloning or the numbers of positive colonies
analyzed may have been less than estimated. Additionally, although
the levels of wild-type EXT1/EXT2 mRNA were also deceased in the
mother, the level of the abnormally spliced EXT1 transcript with
exon 4 skipping was significantly reduced, compared with the
proband (Fig. 5; P<0.05). This
may be associated with the evidence that the clinical symptoms of
male patients are prone to be more severe, compared with female
patients, and it may also indicate that the severity increases with
successive generations (32,33).
Previous studies indicated that the mutated EXT1 and
EXT2 were localized to the Golgi apparatus in vitro, which
were similar to the wild-type genes (30,34);
however, the type of the mutations in these studies were truncated
mutation (EXT2-Y419X) and missense mutations (EXT1-R340C and
EXT2-D227N), and the produced protein was a truncated peptide that
lacked the entire domain of Glyco_transf_64 (480-725aa)
(EXT2-Y419X), or a single amino acid was changed (EXT1-R340C and
EXT2-D227N), which were notably different from the identified
splice mutation in the present study. As the splice mutation
(c.1284+2del) in the present study just results in the deletion of
partial amino acid sequences (389-428 aa), which is resident in the
tail of the exostosin domain (110-396 aa) and the junction of the
two domains of EXT1, whether the special peptide decays through NMD
or not deserves intensive investigation. Additionally, if it is not
decayed, where it anchors, and whether it is different from
previous reports is also worth investigation.
Lentiviruses are effective and frequently-used tools
that allow exogenous genes or exogenous short hairpin RNAs to be
integrated into the host genome to achieve stable expression of the
target sequence. 293-T cells and Cos-7 cells are common tool cells
for efficiently expressing exogenous genes (35,36).
In the present study, a lentivirus was used to express the mutant
EXT1 transcript lacking exon 4 in vitro. Overexpression of
the abnormal transcript was confirmed by RT-qPCR in 293-T cells
infected with the EXT1-DEL lentivirus; however, there were also
small amounts of the abnormal transcript detected in cells infected
with NC (empty vector) lentivirus (Fig. 6), indicating there may be some
endogenous expression of this transcript in 293-T cells. Western
blot analysis confirmed the expression of the truncated peptide.
Other bands that were distinctly visible in the empty vector and
EXT1-FL lanes were possibly caused by the relatively low
specificity of the polyclonal EXT1 antibody used (Fig. 7B), while there was single band in
either lane incubated with monoclonal anti-Flag antibody (Fig. 7A). In order to observe the
subcellular localization of the abnormal peptide in the same host
cells as a previous study (30),
Cos-7 cells were also used as host cells and transfected with
lentiviruses. Immunofluorescence demonstrated that the abnormal
peptide was localized in the cytoplasm of Cos-7 cells, which was
similar to the localization of EXT1-FL. Notably, the levels of the
abnormal peptide were increased, compared with EXT1-FL (Fig. 8), which was consistent with the
results of RT-qPCR and western blot analysis.
In conclusion, a novel splice mutation was
identified in two patients from a family with HME in the present
study. However, more family members did not enroll for
co-segregation analysis of the mutation with disease status.
Expression levels of wild-type EXT1/EXT2 mRNA, which possess
glycosyltransferase activity, were notably reduced due to the
mutation in both patients, yet the level of an abnormally spliced
transcript without the full functional domain was increased in the
proband, compared with the normal control. The decrease in the copy
numbers of the wild-type genes and increase in the abnormal
transcript may be the pathogenic mechanism of HME in the family
that participated in the present study. The abnormal transcript was
detected in the patients with HME, and expression and localization
of the protein product were assessed in vitro, revealing
that the abnormal peptide was expressed and located in the
cytoplasm of Cos-7 cells, which is in accordance with previous
studies (30,34). However, it was regrettable that
there was no homologous structure in EXT1, except the C-terminal of
Exostosin-1 (the domain of Glyco_transf_64), so computational
biological analysis of the structure and function was lacking in
the present study, in order to distinguish Exostosin-1 from the
mutant protein; however, although the two proteins were detected in
the cytoplasm of Cos-7 cells, it was not confirmed in vivo
experiment. Furthermore, the observations in the present study was
only derived from one cell line (Cos-7 cells). In conclusion, the
biological function of wild-type EXT1/EXT2 proteins may not be
affected by the emergence of increased levels of mutant EXT1/EXT2,
but by the decrease in the levels of wild-type EXT1/EXT2 proteins,
which will disrupt HS polymerization and chain elongation.
Funding
This study was supported by grants from the Science
and Technology Project of Fuzhou (grant no. 2015-S-141-14), Youth
Research Project of Health and Family Planning in Fujian Province
(grant no. 2016-2-41), Natural Science Funding Project in Fujian
Province (grant no. 2016J01643) and the National Natural Science
Foundation of China (grant no. 81772287).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG designed the study concept and drafted the
manuscript. ML performed data acquisition and analysis. ML, WC and
GH revised the manuscript. WY was responsible for case collection
and diagnosis. All authors reviewed the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants, and the study was approved by the Ethics Committee of
Fuzhou Second Hospital [Fuzhou, China; approval no. (2014) 63].
Patient consent for publication
The patients participated in the study agreed with
the publication of the paper regarding their family research.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
HME
|
hereditary multiple exostoses
|
|
HS
|
heparan sulfate
|
|
NMD
|
nonsense-mediated mRNA decay
|
|
HSF
|
Human Splicing Finder
|
|
BDGP
|
Berkeley Drosophila Genome Project
|
References
|
1
|
Stieber JR and Dormans JP: Manifestations
of hereditary multiple exostoses. J Am Acad Orthop Surg.
13:110–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones KB: Glycobiology and the growth
plate: Current concepts in multiple hereditary exostoses. J Pediatr
Orthop. 31:577–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wicklund CL, Pauli RM, Johnston D and
Hecht JT: Natural history study of hereditary multiple exostoses.
Am J Med Genet. 55:43–46. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmale GA, Conrad EU III and Raskind WH:
The natural history of hereditary multiple exostoses. J Bone Joint
Surg Am. 76:986–992. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porter DE, Lonie L, Fraser M, Dobson-Stone
C, Porter JR, Monaco AP and Simpson AH: Severity of disease and
risk of malignant change in hereditary multiple exostoses. A
genotype-phenotype study. J Bone Joint Surg Br. 86:1041–1046. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Porter DE and Simpson AH: The neoplastic
pathogenesis of solitary and multiple osteochondromas. J Pathol.
188:119–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahn J, Lüdecke HJ, Lindow S, Horton WA,
Lee B, Wagner MJ, Horsthemke B and Wells DE: Cloning of the
putative tumour suppressor gene for hereditary multiple exostoses
(EXT1). Nat Genet. 11:137–143. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hecht JT, Hogue D, Strong LC, Hansen MF,
Blanton SH and Wagner M: Hereditary multiple exostosis and
chondrosarcoma: Linkage to chromosome II and loss of heterozygosity
for EXT-linked markers on chromosomes II and 8. Am J Hum Genet.
56:1125–1131. 1995.PubMed/NCBI
|
|
9
|
Wuyts W and Van Hul W: Molecular basis of
multiple exostoses: Mutations in the EXT1 and EXT2 genes. Hum
Mutat. 15:220–227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szuhai K, Jennes I, de Jong D, Bovée JV,
Wiweger M, Wuyts W and Hogendoorn PC: Tiling resolution array-CGH
shows that somatic mosaic deletion of the EXT gene is causative in
EXT gene mutation negative multiple osteochondromas patients. Hum
Mutat. 32:E2036–E2049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jennes I, Pedrini E, Zuntini M, Mordenti
M, Balkassmi S, Asteggiano CG, Casey B, Bakker B, Sangiorgi L and
Wuyts W: Multiple osteochondromas: Mutation update and description
of the multiple osteochondromas mutation database (MOdb). Hum
Mutat. 30:1620–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stickens D, Clines G, Burbee D, Ramos P,
Thomas S, Hogue D, Hecht JT, Lovett M and Evans GA: The EXT2
multiple exostoses gene defines a family of putative tumour
suppressor genes. Nat Genet. 14:25–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCormick C, Duncan G, Goutsos KT and
Tufaro F: The putative tumor suppressors EXT1 and EXT2 form a
stable complex that accumulates in the Golgi apparatus and
catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci USA.
97:668–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takei Y, Ozawa Y, Sato M, Watanabe A and
Tabata T: Three Drosophila EXT genes shape morphogen gradients
through synthesis of heparan sulfate proteoglycans. Development.
131:73–82. 2004. View Article : Google Scholar
|
|
15
|
Zak BM, Schuksz M, Koyama E, Mundy C,
Wells DE, Yamaguchi Y, Pacifici M and Esko JD: Compound
heterozygous loss of Ext1 and Ext2 is sufficient for formation of
multiple exostoses in mouse ribs and long bones. Bone. 48:979–987.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones KB, Piombo V, Searby C, Kurriger G,
Yang B, Grabellus F, Roughley PJ, Morcuende JA, Buckwalter JA,
Capecchi MR, et al: A mouse model of osteochondromagenesis from
clonal inactivation of Ext1 in chondrocytes. Proc Natl Acad Sci
USA. 107:2054–2059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsumoto K, Irie F, Mackem S and
Yamaguchi Y: A mouse model of chondrocyte-specific somatic mutation
reveals a role for Ext1 loss of heterozygosity in multiple
hereditary exostoses. Proc Natl Acad Sci USA. 107:10932–10937.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuntini M, Pedrini E, Parra A, Sgariglia
F, Gentile FV, Pandolfi M, Alberghini M and Sangiorgi L: Genetic
models of osteochondroma onset and neoplastic progression: Evidence
for mechanisms alternative to EXT genes inactivation. Oncogene.
29:3827–3834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reijnders CM, Waaijer CJ, Hamilton A,
Buddingh EP, Dijkstra SP, Ham J, Bakker E, Szuhai K, Karperien M,
Hogendoorn PC, et al: No haploinsufficiency but loss of
heterozygosity for EXT in multiple osteochondromas. Am J Pathol.
177:1946–1957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barash Y, Calarco JA, Gao W, Pan Q, Wang
X, Shai O, Blencowe BJ and Frey BJ: Deciphering the splicing code.
Nature. 465:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang GS and Cooper TA: Splicing in
disease: Disruption of the splicing code and the decoding
machinery. Nat Rev Genet. 8:749–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian C, Yan R, Wen S, Li X, Li T, Cai Z,
Li X, Du H and Chen H: A splice mutation and mRNA decay of EXT2
provoke hereditary multiple exostoses. PLoS One. 9:e948482014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen XJ, Zhang H, Tan ZP, Hu W and Yang
YF: Novel mutation of EXT2 identified in a large family with
multiple osteochondromas. Mol Med Rep. 14:4687–4691. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bovée JV: Multiple osteochondromas.
Orphanet J Rare Dis. 3:32008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wuyts W, Radersma R, Storm K and Vits L:
An optimized DHPLC protocol for molecular testing of the EXT1 and
EXT2 genes in hereditary multiple osteochondromas. Clin Genet.
68:542–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Divina P, Kvitkovicova A, Buratti E and
Vorechovsky I: Ab initio prediction of mutation-induced cryptic
splice-site activation and exon skipping. Eur J Hum Genet.
17:759–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desmet FO, Hamroun D, Lalande M,
Collod-Béroud G, Claustres M and Béroud C: Human Splicing Finder:
An online bioinformatics tool to predict splicing signals. Nucleic
Acids Res. 37:e672009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Lizée G, Aerts JL, Gonzales MI, Chinnasamy
N, Morgan RA and Topalian SL: Real-time quantitative reverse
transcriptase-polymerase chain reaction as a method for determining
lentiviral vector titers and measuring transgene expression. Hum
Gene Ther. 14:497–507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kobayashi S, Morimoto K, Shimizu T,
Takahashi M, Kurosawa H and Shirasawa T: Association of EXT1 and
EXT2, hereditary multiple exostoses gene products, in Golgi
apparatus. Biochem Biophys Res Commun. 268:860–867. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie BB, Li D, Shi WL, Qin QL, Wang XW,
Rong JC, Sun CY, Huang F, Zhang XY, Dong XW, et al: Deep RNA
sequencing reveals a high frequency of alternative splicing events
in the fungus Trichoderma longibrachiatum. BMC Genomics. 16:542015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clement ND and Porter DE: Hereditary
multiple exostoses: Anatomical distribution and burden of exostoses
is dependent upon genotype and gender. Scott Med J. 59:35–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pedrini E, Jennes I, Tremosini M, Milanesi
A, Mordenti M, Parra A, Sgariglia F, Zuntini M, Campanacci L,
Fabbri N, et al: Genotype-phenotype correlation study in 529
patients with multiple hereditary exostoses: Identification of
'protective' and 'risk' factors. J Bone Joint Surg Am.
93:2294–2302. 2011. View Article : Google Scholar
|
|
34
|
Busse M, Feta A, Presto J, Wilén M,
Grønning M, Kjellén L and Kusche-Gullberg M: Contribution of EXT1,
EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem.
282:32802–32810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bobadilla S, Sunseri N and Landau NR:
Efficient transduction of myeloid cells by an HIV-1-derived
lentiviral vector that packages the Vpx accessory protein. Gene
Ther. 20:514–520. 2013. View Article : Google Scholar
|
|
36
|
Nukuzuma S, Nakamichi K, Kameoka M,
Sugiura S, Nukuzuma C, Miyoshi I and Takegami T: Efficient
propagation of progressive multifocal leukoencephalopathy-type JC
virus in COS-7-derived cell lines stably expressing Tat protein of
human immunodeficiency virus type 1. Microbiol Immunol. 54:758–762.
2010. View Article : Google Scholar
|