Introduction
Oxysterols are oxygenated derivatives of cholesterol
formed in the human body or ingested in the diet. Oxysterols are
associated with various cardiovascular, metabolic,
neurodegenerative and cancerous pathologies (1). Oxysterols have been shown to play
pro-cancerous and pro-proliferative roles through the modulation of
inflammation or oxysterol-binding proteins in various types of
cancer, including breast cancer and lung cancer (2-4).
Gastric cancer (GC) is the third leading cause of cancer-related
mortality worldwide (5). In China,
GC is considered as the second leading cause of cancer-related
mortality (6). Dietary patterns,
obesity, smoking and chronic infections contribute to the
development of GC (7).
Hyperlipidemia is associated with lymph node metastasis in patients
with GC (8,9). Metformin, a drug used in the
treatment of type 2 diabetes for the regulation of glucose and
fatty acid metabolism, has been found to inhibit the proliferation
and epithelial-mesenchymal transition of AGS GC cells (10,11).
In addition, use of statins can reduce the risk of developing GC
(12). However, the role of
oxysterols in GC and the related mechanisms remain largely
unknown.
25-Hydroxycholesterol (25-HC) is a type of oxysterol
which is synthesized from cholesterol (13). Johnson et al found that
25-HC was upregulated in serum following the ingestion of a meal
rich in oxysterols and following a dietary cholesterol challenge
(14). In addition, the levels of
25-HC have been shown to be higher in hypercholesterolemic serum
compared to those in normocholesterolemic serum (15). 25-HC has also been found to be
involved in the progression of breast and ovarian tumors by
activating the estrogen receptor (ER) α-mediated signaling pathway
(16) and promoting resistance to
anti-hormone treatment in ER-positive breast cancer (17). More recently, 25-HC has been
reported to promote the migration and invasion of lung
adenocarcinoma cells (18).
Increased cholesterol levels are often associated with obesity
(19), which has been found to be
a risk factor for the development of GC (20). Thus, we hypothesized that 25-HC may
play a role in the development of GC. To date, at least to the best
of our knowledge, the mechanisms of oxysterol-induced GC
progression remain largely unknown.
Therefore, in the present study, we evaluated the
role of 25-HC in GC both in vitro and in vivo. Our
data revealed that 25-HC had no effects on GC cell proliferation
and apoptosis, whereas it decreased the sensitivity of the cells to
5-fluorouracil (5-FU). Moreover, 25-HC promoted GC cell invasion
and the underlying mechanisms partly involved the upregulation of
Toll-like receptor 2 (TLR2)/nuclear factor (NF)-κB
signaling-dependent matrix metalloproteinase (MMP) expression.
Materials and methods
Cells and reagents
The human GC cell lines, AGS and MGC-803, were
obtained from the Cell Bank of the Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China). The AGS cells were
cultured in F-12K medium and the MGC-803 cells were cultured in
RPMI-1640 medium complemented with 10% heat-inactivated fetal
bovine serum (FBS) and maintained at 37˚C with 5% CO2 in
a humidified tissue culture incubator. All cell culture reagents
were purchased from Invitrogen (Shanghai, China).
25-HC was purchased from Sigma-Aldrich (Shanghai,
China) and dissolved in ethanol as a stock solution. Rabbit
anti-MMP1 polyclonal antibody (pAb, #10371-2-AP), rabbit anti-MMP2
pAb (#10373-2-AP), rabbit anti-MMP3 pAb (#17873-1-AP), rabbit
anti-MMP9 pAb (#10375-2-AP) and rabbit anti-MMP13 pAb (#18165-1-AP)
were obtained from Proteintech (Wuhan, China). Rabbit anti-Bcl-2
monoclonal antibody (mAb, #4223), rabbit anti-Bax mAb (#5023),
rabbit anti-phospho-PI3K p85/ p55 mAb (#4228), rabbit anti-PI3K p85
mAb (#4292), rabbit anti-phospho-AKT mAb (#4060), rabbit anti-AKT
mAb (#4691), rabbit anti-phosphosignal transducer and activator of
transcription 3 (STAT3) mAb (#9145), rabbit anti-STAT3 mAb (#4904),
rabbit anti-phospho-p38 mAb (#9211), rabbit anti-p38 mAb (#9212),
rabbit anti-phospho-extracellular signal-regulated protein kinase
(Erk)1/2 mAb (#4370), rabbit anti-Erk1/2 mAb (#4695), rabbit
anti-phospho-stress-activated protein kinase (SAPK)/c-Jun
NH2-terminal kinase (JNK) mAb (#4668), rabbit anti-SAPK/JNK pAb
(#9252), rabbit anti-phospho-NF-κB p65 mAb (#3039), rabbit
anti-NF-κB p65 mAb (#8242) and rabbit anti-β-actin mAb (#8457) were
purchased from Cell Signaling Technologies (CST; Danvers, MA, USA).
Rabbit anti-TLR2 pAb (#DF7002), rabbit anti-TLR3 pAb (#DF6415),
rabbit anti-TLR4 pAb (#AF7017), rabbit anti-TLR9 pAb (#DF2970) were
purchased from Affinity Biosciences (Jiangsu, China). HRP-linked
anti-rabbit IgG Ab (#70-GAR007) was obtained from MultiSciences
(Lianke) Biotech Co., Ltd. (Hangzhou, China). FITC anti-human CD44
antibody (#103021) was purchased from BioLegend (San Diego, CA,
USA).
Measurement of cell viability
Cell viability was detected by Cell Counting kit-8
assay (Beyotime, Jiangsu, China) according to the manufacturer's
instructions. The cells were seeded in 96-well plate at a density
of 1×103 cells/well and allowed to grow for 24 h before
the medium was replaced with either ethanol as the vehicle or
various concentrations of 25-HC (2.5, 5 and 10 µM) in a
total volume of 100 µl. Following culture for 24, 48 and 72
h, 10 µl of CCK-8 solution reagent were added to each well
and cultured for a further 1 h. The absorbance at 450 nm was
measured using an ultra-microplate reader (Emax, Molecular Devices,
CA, USA).
To investigate the role of 25-HC in the
chemosensitivity to 5-FU, the AGS or MGC-803 cells seeded in
96-well plates were treated with various concentrations of 25-HC
with or with 5-FU (5 µM) for 24 or 48 h before cell
viability was determined by CCK-8 assay as described above.
Annexin V cell apoptosis assay
Cells in a 6-well plate were treated with various
concentrations of 25-HC with or without 5-FU (5 uM) for 48 h before
the cells were trypsinized, washed with ice-cold PBS and
resuspended. Cell apoptosis was determined with the Annexin V
apoptosis kit (Lianke Biotech, Co., Ltd.) according to the
manufacturer's instructions. The stained cells were then analyzed
by flow cytometry (BD FACScan; BD Biosciences, San Jose, CA,
USA).
Western blot analysis
Cells or tissues from the lungs of mice were washed
with ice-cold PBS 3 times and lysed with RIPA buffer (CST, Danvers,
MA) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) (CST) and
protease inhibitor cocktail. Equal amounts of proteins were
separated by 10% SDS-polyacrylamide gel electrophoresis and blotted
onto PVDF membranes. The membranes were blocked with PBS containing
5% non-fat milk for 1.5 h at room temperature, followed by
incubation with primary antibodies against MMP1, MMP2, MMP3, MMP9,
MMP13, Bcl-2, Bax, p-PI3K p85/p55, PI3K p85, p-AKT, AKT p-STAT3,
STAT3, p-p38, p38, p-Erk1/2, Erk1/2, p-SAPK/JNK, SAPK/JNK p-NF-κB
p65, NF-κB p65, TLR2, TLR3, TLR4, TLR9 and β-actin (all diluted
1:1,000) overnight at 4°C. Following incubation with the HRP-linked
goat anti-rabbit secondary antibody (dilution 1:5,000) for 1 h at
room temperature, immunoreactive proteins were detected with
FluorChem E System (ProteinSimple, Santa Clara, CA, USA).
For the inhibition assay, cells in 6-well plates
were pre-treated with the NF-κB-specific inhibitor, Bay 11-7082 at
2 µg/ml (Beyotime Institute of Biotechnology, Shanghai,
China) for 1 h and medium were replaced with the complete medium
supplemented with 25-HC.
Animal experiments
All the experiments using animals were approved by
the Animal Care and Use Committee of Zhejiang University. Five to
six-week-old female BALB/c nude mice (90 in total), weighing
between 18-20 g were purchased from Shanghai Laboratory Animal
Company (SLAC; Shanghai, China) and maintained in the animal
facility at Zhejiang University. Mice were provided with water and
food (SLAC) ad libitum and kept under standard conditions
(temperature 24±2°C, humidity, 50-70%, 12-h light/dark cycle).
For tumor growth assays, 5×106 AGS cells
were subcutaneously injected into the right flanks of the nude
mice. When the volumes of the xenograft tumors reached an average
of 100 mm3, the mice were randomly divided into 4 groups
as follows: The PBS and 25-HC groups (with 5 mice in each group),
and the PBS + 5-FU and 25-HC + 5-FU groups (with 10 mice in each
group). The mice in the PBS + 5-FU and 25-HC + 5-FU groups received
5-FU or/and 25-HC via intraperitoneal injection with 5-FU (25
mg/kg) or/and 25-HC (10 mg/kg) every 3 days for 3 weeks. After 3
weeks, the mice were sacrificed, and the tumors were harvested and
weighed, and embedded in paraffin for use in further analyses.
Tumor volume was calculated using the following formulae: V = ½×
(length × width2). This experiment was repeated under
the same setting 3 times (once with 10 mice in total, and another 2
times with 20 mice each time).
For lung metastasis assay, the mice were injected
with 1×106 of AGS cells through the tail vein and
randomly divided into 2 groups (PBS and 25-HC group) with 8 mice in
each group. Mice in the 25-HC group were intraperitoneally injected
with 25-HC (10 mg/kg) every 3 days for 3 weeks. This experiment was
repeated twice (with 20 mice being prepared each time). After 3
weeks, the mice were sacrificed, and the lungs were removed and
weighted. The lung metastatic tumors on the surface were calculated
and H&E staining was performed on the lung tissues or part of
the lung tissues were extracted for protein extraction for use in
western blot analysis. H&E staining was performed by Google
Biotechnology Co., Ltd. (Wuhan, China).
Cell cycle assay
The cell cycle was analyzed with the Cell Cycle
Staining kit (Lianke Biotech, Co., Ltd.) according to the
manufacturer's instructions. Cells in a 6-well plate were treated
with various concentrations of 25-HC with or without 5-FU (5
µM) for 48 h before the cells were trypsinized, washed with
ice-cold PBS and fixed with 75% ethanol at −20°C overnight. The
cells were then stained with PI solution for 30 min in the dark.
Cell cycles were analyzed by flow cytometry (BD FACScan; BD
Biosciences).
Wound healing assay
The AGS cells were seeded and allowed to grow to 80%
confluence in complete medium before cells were wounded by a
200-µl pipette. The cells were then washed with PBS twice
and replenished with fresh culture medium with 5% FBS containing
the indicated concentrations of 25-HC. Images of the wound
morphology were acquired under a light microscope (CKX41; Olympus,
Shanghai, China).
Cell invasion assay
Cell invasion assay was carried out using 24-well
cell culture chambers with an 8-µm pore filter (Corning
Costar Corp., Cambridge, MA, USA). Before the cells were seeded,
the upper surfaces of the membranes were coated with 50 µl
Matrigel (BD Biosciences) for 6 h at 37°C. The cells were then
seeded at a density of 1×105 cells/well in 100 µl
serum-free F-12K medium added onto the top chamber of each
Transwell with various concentrations of 25-HC. The cells were
allowed to invade for 36 h at 37°C. After removing the cells that
had remained in the upper side of the membrane with a cotton swab,
cells in the lower side of the membrane were fixed with ice-cold
methanol, stained with crystal violet (Hushi, Shanghai, China) in
20% ethanol overnight and then counted in 5 pre-determined fields
under a light microscope (CKX41; Olympus).
For the inhibition assay, cells in the upper chamber
were pre-treated with NF-κB inhibitor Bay 11-7082 at 2 µg/ml
(Beyotime) for 1 h and the medium was replaced with FBS-free medium
supplemented with 25-HC or not.
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qPCR)
The AGS cells were exposed to 25-HC at various
concentrations for 24 h and total RNA was extracted using the
Ultrapure RNA kit (Cwbiotech, Beijing, China). Reverse
transcriptase PCR was performed with the HiFiScript cDNA Synthesis
kit (Cwbiotech). Subsequently, quantitative PCR (qPCR) was
performed using the iTaq Universal SYBR-Green Supermix on a CFX96
Touch Real-Time PCR Detection System (both from Bio-Rad, Hercules,
CA, USA). The primer sequences used are listed in Table I. The results of qPCR were analyzed
with the relative quantification Delta delta CT (2−ΔΔCq)
strategy (21). The calculated
threshold cycle was normalized to the value of internal β-actin
amplified from the same cDNA and the fold-change was calculated as
referenced to control.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Forward | Reverse |
|---|
| β-actin |
GTATCCTGACCCTGAAGTACC |
TGAAGGTCTCAAACATGATCT |
| TLR2 |
CTCTTCAGCAAACGCTGTTCT |
GGCGTCTCCCTCTATTGTATTG |
| TLR3 |
GTGAGATACAACGTAGCTGACTG |
TCCTGCATCCAAGATAGCAAGT |
| TLR4 |
GCCTTTCAGGGAATTAAGCTCC |
GATCAACCGATGGACGTGTAAA |
| TLR9 |
ACAACTCTGACTTCGTCCACC |
TCTGGGCTCAATGGTCATGTG |
Luciferase reporter assay
The AGS cells were seeded in 96-well plate at a
density 1×104 per well 1 day prior to transfection. The
cells were transiently transfected with NF-κB luciferase reporter
plasmid with Lipofectamine 2000 (Invitrogen/Thermo Fisher
Scientific, Waltham, MA, USA) following the manufacturer's
instructions. At 24 h post-transfection, the cells were stimulated
with the indicated concentrations of 25-HC. After 24 h, the cells
were collected, and the luciferase activities were determined by
using the Bright-Glo luciferase assay system (Promega Corp.,
Madison, WI, USA).
siRNA transfection
siRNA targeting human TLR2 (5'-GGA
GUCUCUGUCAUGUGAUdTdT-3') and scramble siRNA (siNC)
(5'-UUCUCCGAACGUGUCACGUTTdTdT-3') were purchased from GenePharma
Technologies (Shanghai, China). The AGS cells were transfected with
the siRNAs using Lipofectamine 2000 (Invitrogen/Thermo Fisher
Scientific) following the manufacturer's instructions. Following
transfection for 24 h, the cells were prepared for use in further
experiments.
Flow cytometry for the determination of
CD44 expression
The AGS cells in 6-well plate were treated with
various concentrations of 25-HC for 48 h before the cells were
trypsinized, washed with ice-cold PBS, resuspended and counted
prior to staining for flow cytometric analysis. In 100 µl of
buffer, 1×106 cells were incubated with the
anti-CD44-FITC on ice for 30 min. The cells were then washed with
buffer and fixed in 1% paraformaldehyde for 30 min on ice.
Following fixation, the cells were washed, and the fluorescence
intensity was assessed by flow cytometry (BD FACScan; BD
Biosciences, San Jose, CA, USA).
Statistical analysis
Data are presented as the means ± SEM. Statistical
significance was determined by a Student's t-test or one-way
analysis of variance (ANOVA) followed by Dunnett's test or Tukey's
test. A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
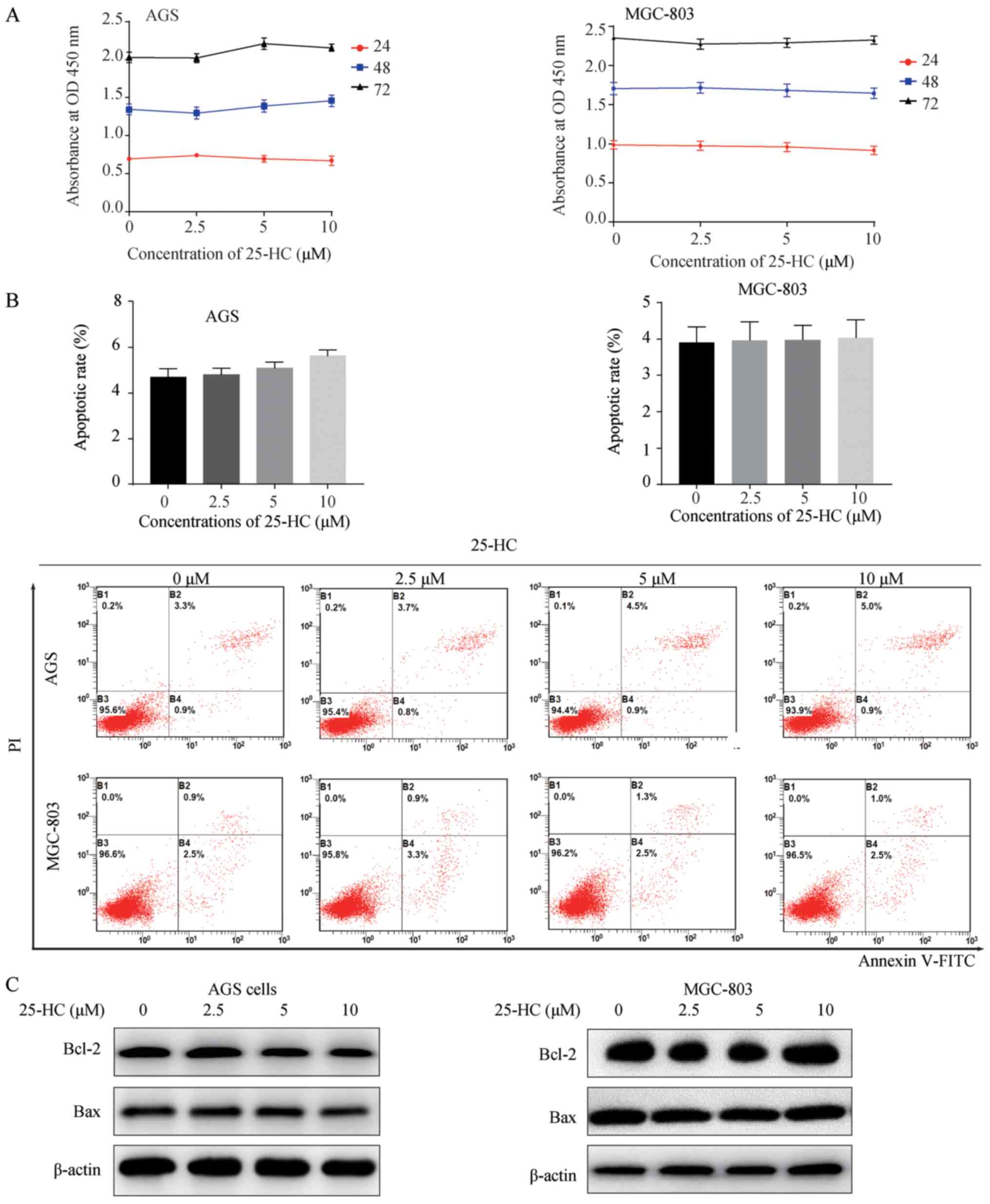
25-HC has no effects on GC cell
proliferation and apoptosis in vitro
To explore the biological effects of 25-HC on GC
cell proliferation, we first evaluated cell proliferation by CCK-8
assay. The AGS or MGC-803 cells were exposed to the indicated
concentrations of 25-HC for 24, 48 and 72 h. As shown in Fig. 1A, 25-HC had no effect on GC cell
proliferation. The regulation of fatty acid metabolism can decrease
GC cell viability and induce cell apoptosis (10). Thus, in this study, we also
detected cell apoptosis by flow cytometry, as well as the
expression levels of the apoptotic regulators, Bcl-2 and Bax by
western blot analysis. Consistent with the results of cell
proliferation assay, 25-HC had no marked effects on cell apoptosis
(Fig. 1B) or on the expression
levels of Bcl-2 and Bax (Fig. 1C).
These results demonstrate that 25-HC (2.5-10 µM) has no
effects on GC cell proliferation and apoptosis.
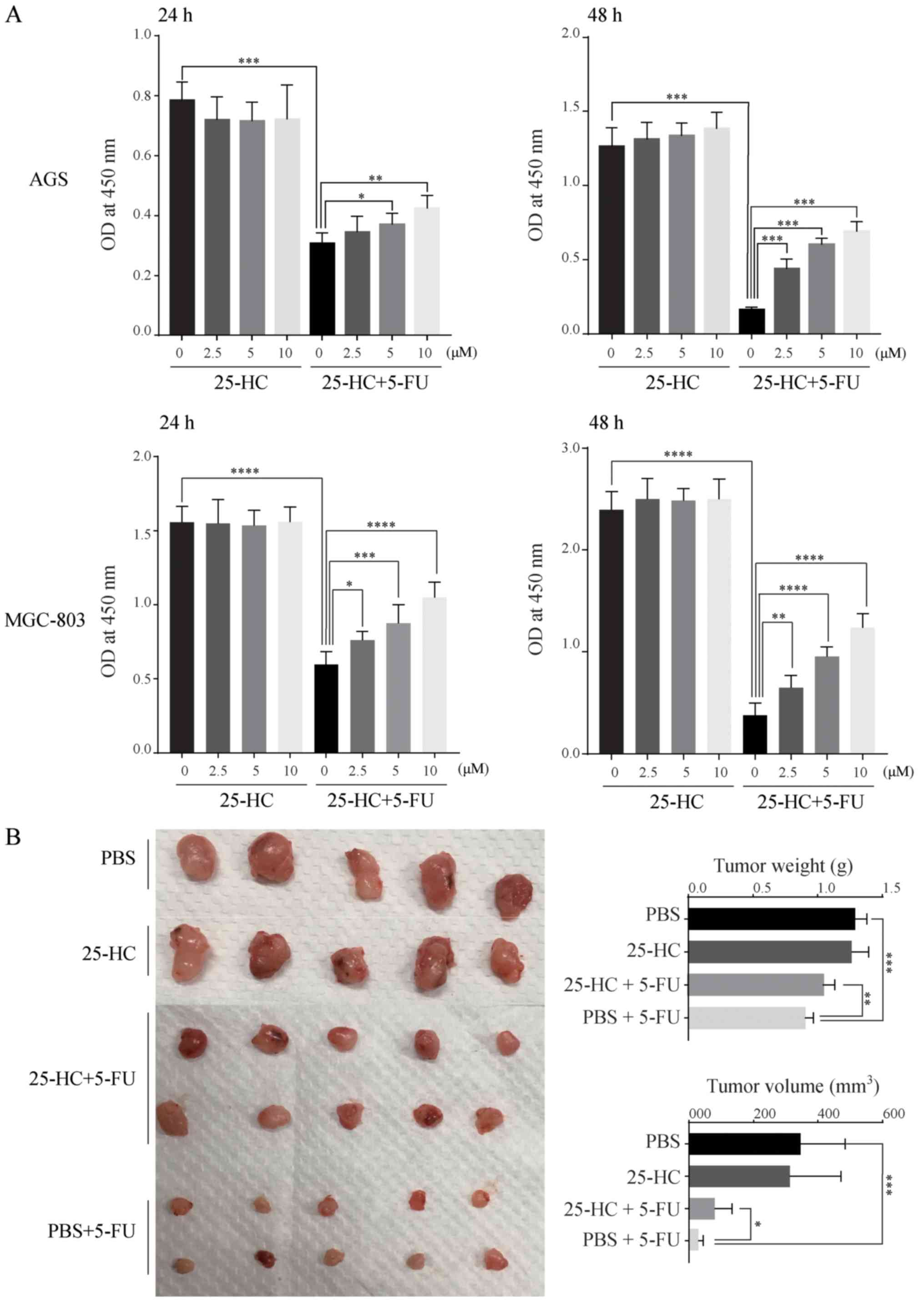
25-HC decreases the sensitivity of GC
cells to 5-FU
To examine the effects of 25-HC on the
chemoresistance of GC cells, the AGS and MGC-803 cells were treated
with 25-HC with or without 5-FU and cell proliferation were
evaluated. As shown in Fig. 2A,
although 25-HC had no effect on cell proliferation directly, it
decreased the sensitivity of the GC cells to 5-FU with a
significant increase in absorbance at 450 nm compared to 5-FU
stimulation alone in a dose-dependent manner. In mouse tumor
xenograft experiments, the tumor volume and weight in the 25-HC
group exhibited no marked difference compared with the PBS group.
However, following 5-FU treatment, tumor growth was enhanced by
25-HC, as evidenced by the greater tumor volume and increased tumor
weight compared to the PBS + 5-FU group (Fig. 2B). Thus, these results indicate
that 25-HC decreases the sensitivity to 5-FU in AGS cells both
in vitro and in vivo.
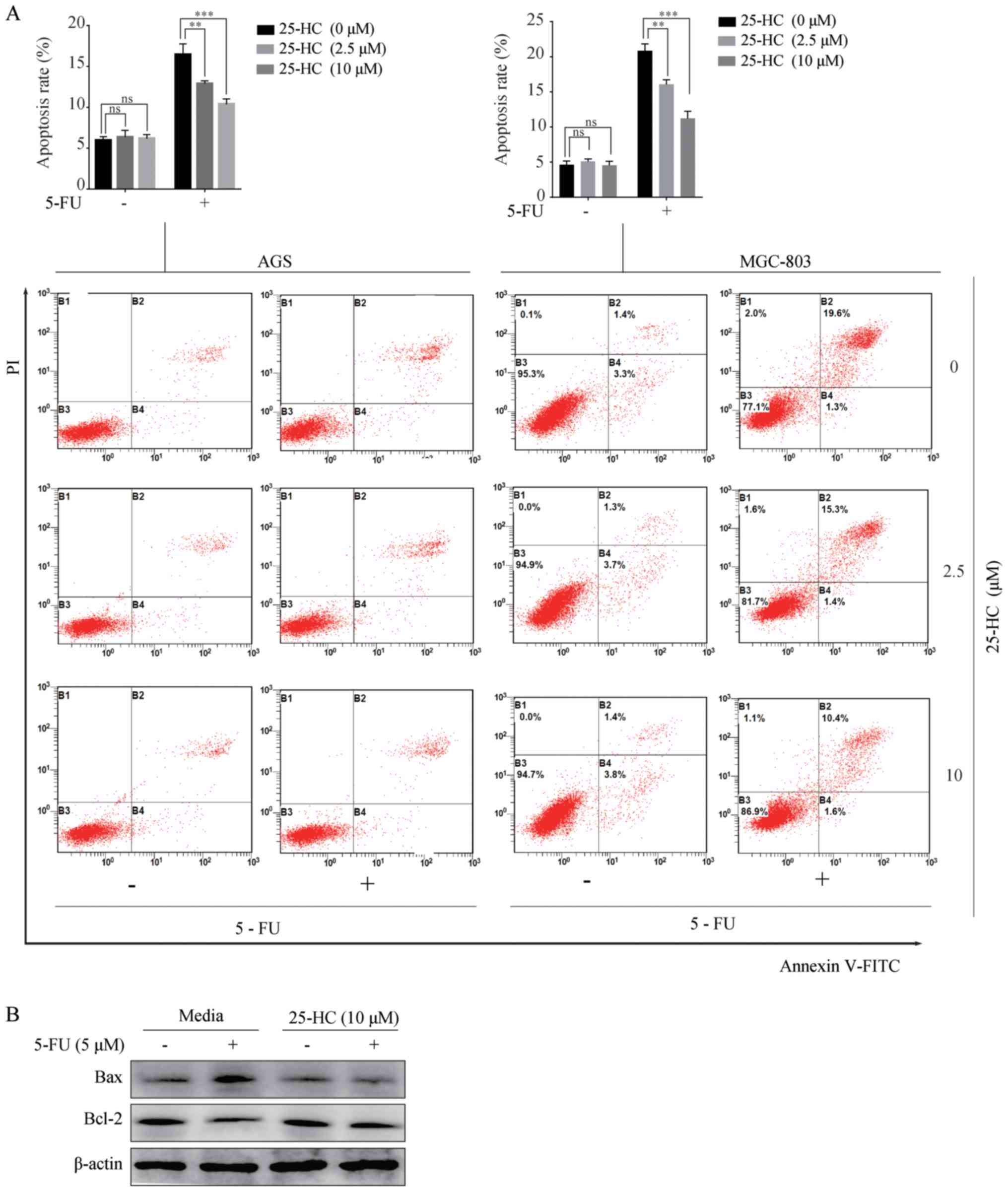
25-HC decreases the pro-apoptotic effects
of 5-FU on GC cells
We then investigated the effects of 25-HC on cell
apoptosis induced by 5-FU. The cells were treated with 25-HC with
or without 5 µM 5-FU for 48 h and the apoptotic cells were
determined by Annexin V/PI staining. As shown in Fig. 3A, upon 5-FU treatment, the
apoptotic rates of the AGS cells (16.7±0.75%) decreased
significantly following 25-HC stimulation (12.9±0.19% at 2.5
µM and 10.4±0.37% at 10 µM; P<0.05). Similar
results were obtained with the MGC-803 cells. Moreover, the
increased expression of Bcl-2 and the decreased expression of Bax
in the AGS cells following treatment with 5-FU were detected
(Fig. 3B). Overall, these results
indicate that 25-HC decreases the pro-apoptotic effects of 5-FU on
GC cells.
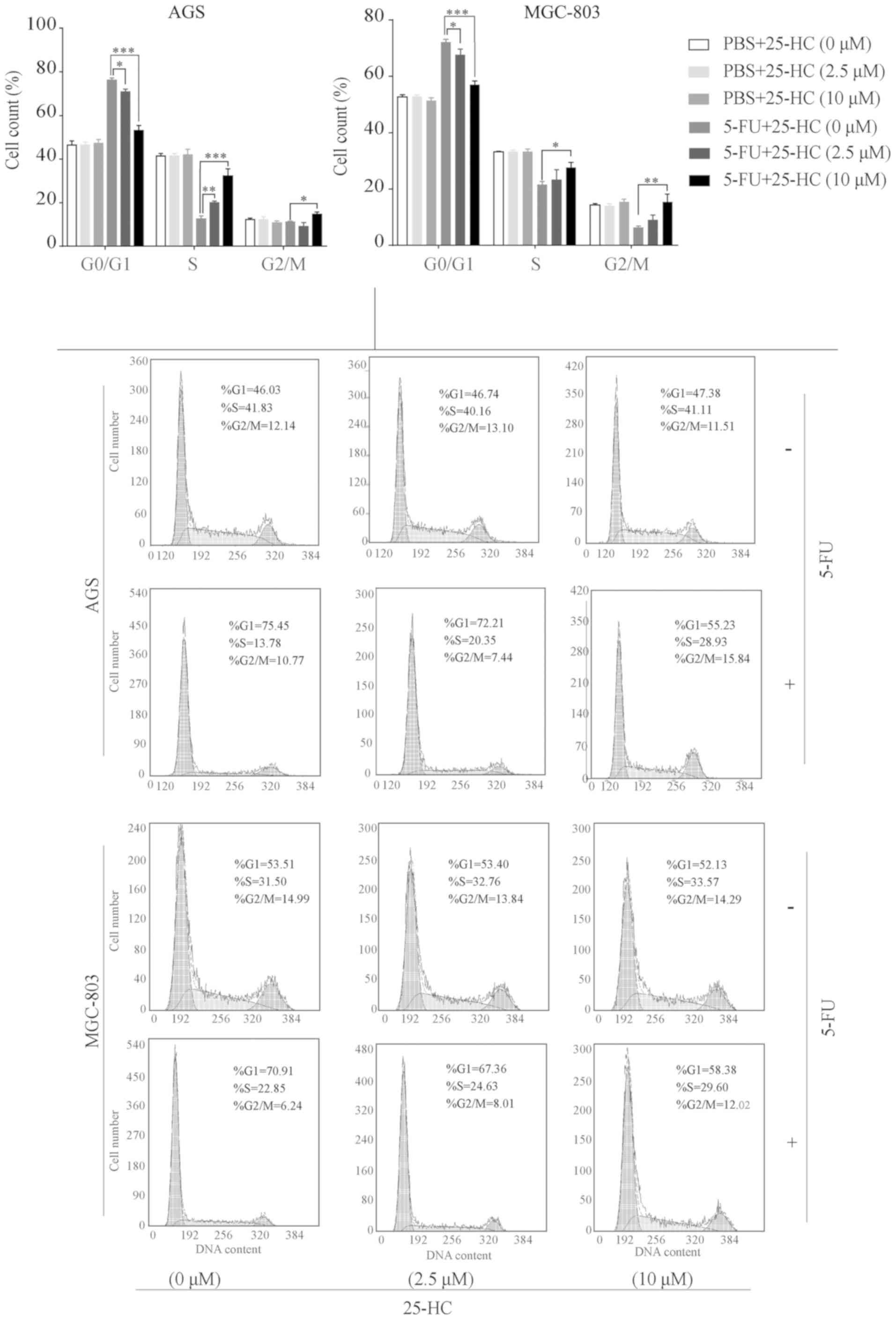
25-HC arrests the cell cycle of the GC
cells at the S-phase following 5-FU treatment
The cell cycle distribution of the 25-HC-exposed GC
cells treated with or without 5-FU was analyzed and quantified by
flow cytometry. As shown in Fig.
4, 25-HC treatment alone had minimal effects on cell cycle
arrest in the AGS and MGC-803 cells, which was consistent with the
results of proliferation assay. However, an increase in the
population of cells in the S-phase following 5-FU treatment was
observed in a dose-dependent manner, ranging from 12.62±0.8% to
20.1±0.4% to 32.24±1.9% in the AGS cells and 21.53±0.7% to
23.33±2.0% to 27.67±0.9% in the MGC-803 cells (25-HC at 0, 2.5 and
10 µM, respectively, P<0.05). Additionally, the numbers
of cells in the G0/G1 phase were significantly decreased with the
increasing concentrations of 25-HC (P<0.05).
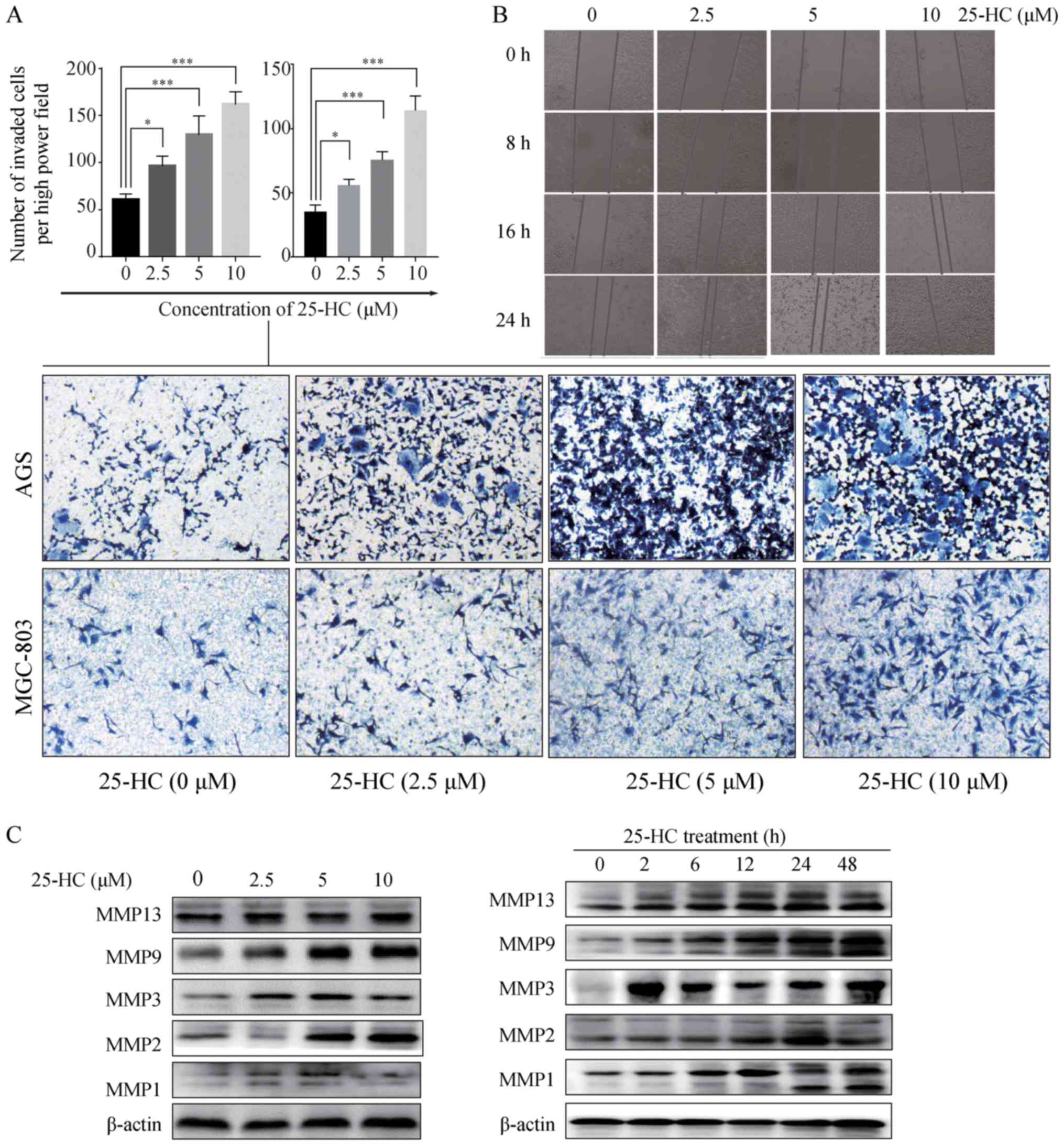
25-HC promotes GC cell migration and
invasion in vitro
We then investigated the effects of 25-HC on GC cell
migration and invasion in vitro. First, we performed a
Transwell invasion assay. Compared to the control group, 25-HC
treatment significantly enhanced the AGS cell and MGC-803 invasive
ability (P<0.05; Fig. 5A). A
wound healing assay was then carried out on the AGS cells. The
results revealed that 25-HC treatment induced the more rapid
closing of the scratch wounds in the AGS cells (Fig. 5B), indicating that 25-HC
efficiently promoted the motility of the AGS cells.
Metastasis involves tumor cell adhesion to the
extracellular matrix (ECM), proteolytic cleavage or destruction of
the ECM, and leads to cell migration through the resultant defect
(22,23). MMPs are a group of enzymes that can
degrade proteins in the ECM by the endopeptidase activity (24). Thus, in this study, we detected the
MMP1, MMP2, MMP3, MMP9 and MMP13 expression levels which have been
found to be overexpressed in human GC (25,26).
As shown in Fig. 5C, 25-HC
increased the expression levels of the detected MMPs in a dose- and
time-dependent manner.
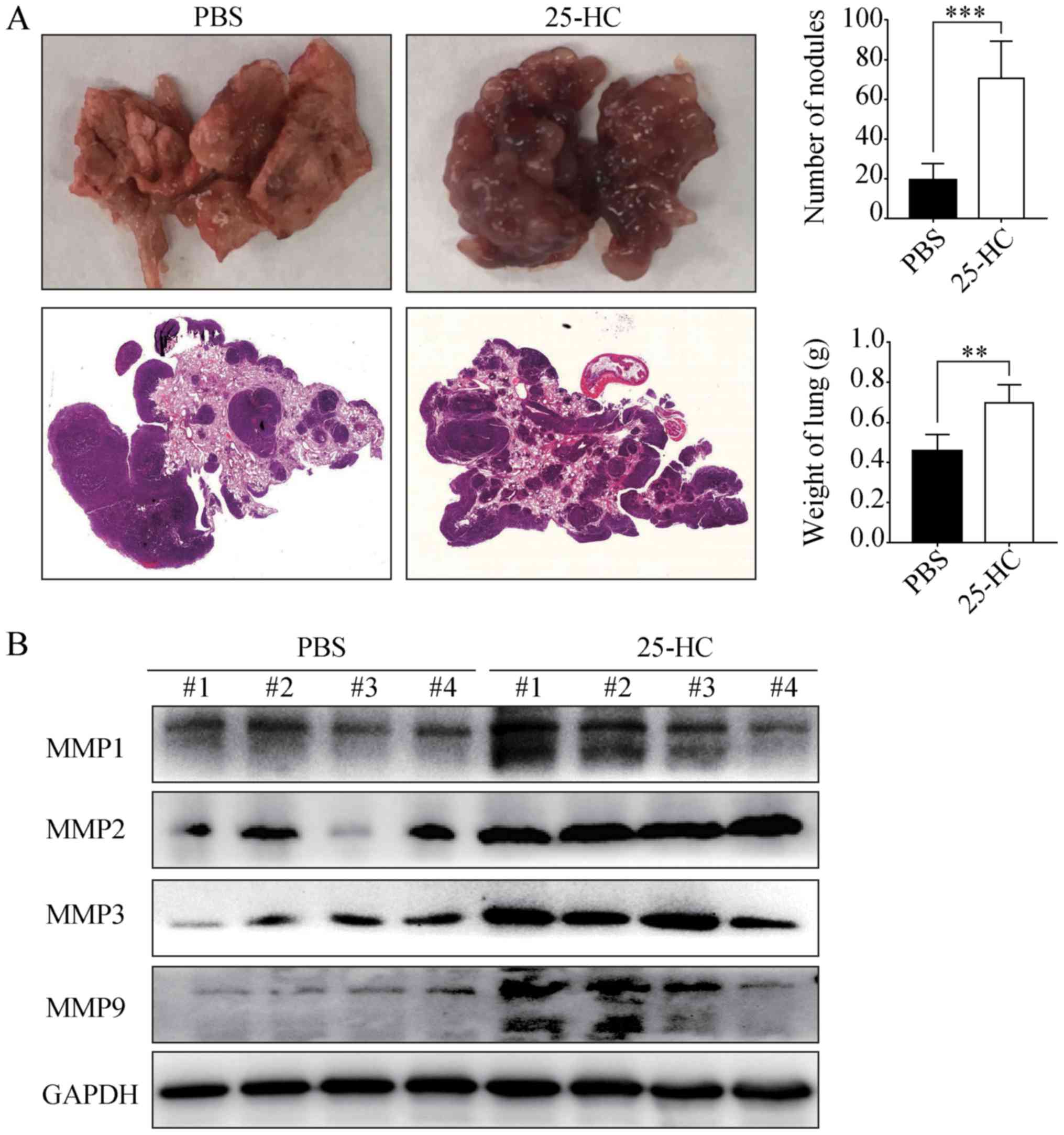
25-HC promotes the distant lung
metastasis of AGS cells in vivo
To determine whether the promoting effects of 25-HC
on GC cell invasion in vitro can be reproduced in
vivo, the AGS cells were injected into the tail veins of BALB/c
nude mice pre-treated with PBS or 25-HC. As shown in Fig. 6A, following 25-HC treatment, the
number of metastatic nodules in lungs was significantly increased
and the lung weight was also increased (P<0.01). In addition,
the protein expression levels of MMP1, MMP2, MMP3 and MMP9 in the
lung tissues were determined by western blot analysis. As shown in
Fig. 6B, the expression levels of
all the detected MMPs were much higher in the mice treated with
25-HC. Taken together, these data demonstrate that 25-HC strongly
promotes the in vivo lung metastatic potential of GC
cells.
25-HC regulates multiple signaling
pathways
To identify the 25-HC-associated signaling pathways,
western blot analysis was performed. The mitogen-activated protein
kinase (MAPKs, including JNK, Erk and p38), NF-κB, PI3K/ AKT and
STAT3 signaling pathways have been associated with GC cell
migration and invasion (27,28).
The suppression of these signaling pathways can inhibit the
migration and invasion of AGS cells, and the activation of these
pathways promotes cell invasion and metastasis (29-31).
The results of this study demonstrated that 25-HC upregulated the
phosphorylation of NF-κB p65 in a dose- and time-dependent manner,
while it decreased the phosphorylation levels of all the other
proteins detected (Fig. 7A). We
also assessed NF-κB activation using a luciferase reporter assay.
Following exposure to increasing concentrations of 25-HC, NF-κB
activity was significantly induced in a dose-dependent manner
(Fig. 7B). These results indicate
that NF-κB may be responsible for the induction of the migration
and invasion of AGS cells.
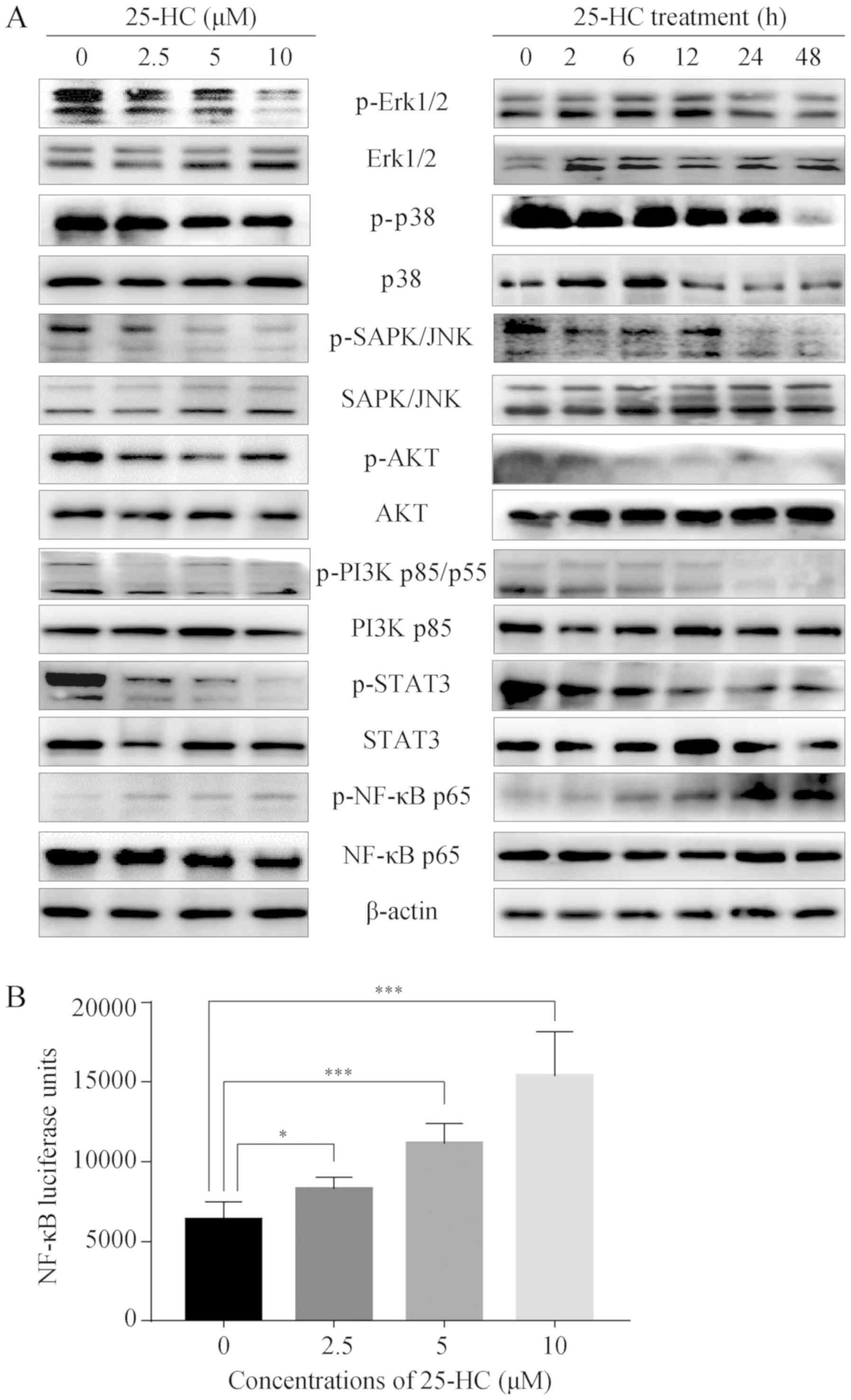 | Figure 725-HC regulates multiple signaling
pathways. (A) AGS cells were treated with the indicated
concentrations of 25-HC for 24 h or treated with 5 µM 25-HC
for the indicated times before total cellular proteins were
extracted for western blot analysis to detect the p-Erk1/2, Erk1/2,
p-p38, p38, p-SAPK/JNK, SAPK/JNK, p-AKT, AKT, p-PI3K p85/p55, PI3K
p85/p55, p-STAT3, STAT3, p-NF-κB p65, NF-κB p65 expression levels.
Results were representative of 3 independent experiments. (B) AGS
cells were transfected with NF-κB luciferase reporter plasmid
before the cells were treated with 25-HC. After 24 h, the
luciferase activities were determined by using the Bright-Glo
luciferase assay system. Results were obtained from 3 independent
experiments and are expressed as the means ± SEM. Statistical
significance was determined by one-way ANOVA followed by Dunnett's
test. Representative images are presented. *P<0.05
and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC,
gastric cancer. |
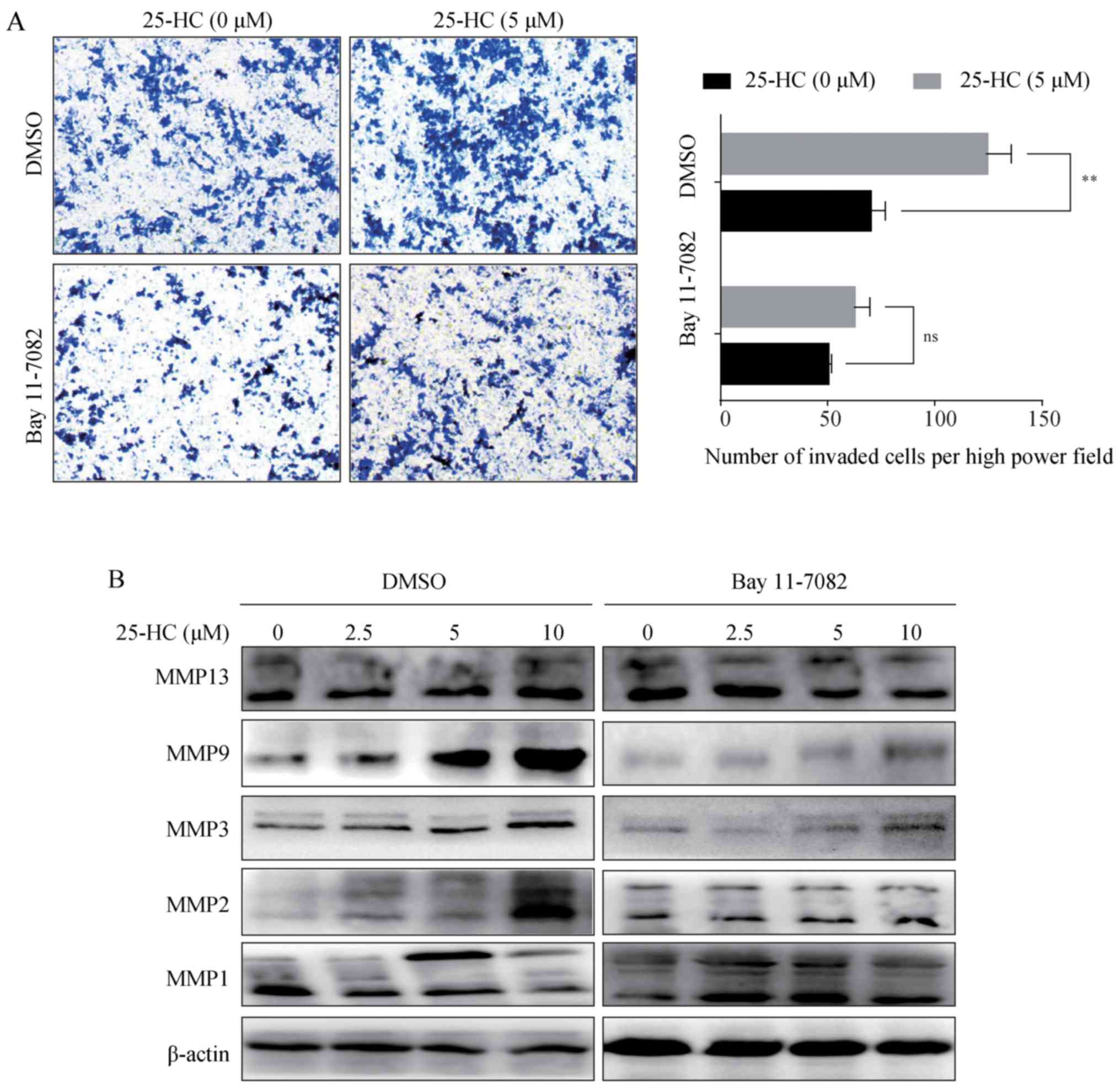
25-HC promotes AGS cell invasion and MMP
expression levels through the upregulation of the NF-κB
pathway
Subsequently, the AGS cells were treated with the
NF-κB specific inhibitor, Bay 11-7082, prior to 25-HC treatment,
and the invasive ability and MMP expression levels were analyzed.
The results demonstrated that Bay 11-7082 treatment reversed the
invasion induced by 25-HC (Fig.
8A) and abolished the upregulation of MMP expression levels
(Fig. 8B). Taken together, these
results demonstrated that 25-HC-promoted the invasion of and MMP
expression levels in AGS cells by upregulating the NF-κB signaling
pathway.
The promoting effects of 25-HC on AGS
cell invasion and MMP expression levels are partly dependent on
TLR2
In this study, to investigate whether TLRs are
involved in the promoting effects of 25-HC on AGS cell invasion, we
first detected the expression levels of TLRs by RT-qPCR (Fig. 9A) and western blot analysis
(Fig. 9B). The results revealed
that 25-HC significantly induced TLR2 expression in the AGS cells
in a time- and dose-dependent manner, while it had no effects on
the TLR3, 4 and 9 expression levels. We then knocked down TLR2 in
the AGS cells by siRNA transfection and the knock down efficiency
was shown in Fig. 9D. As shown in
Fig. 9C, the knockdown of TLR2
partly abolished the 25-HC-induced cell invasion. In addition, the
expression levels of MMPs were also decreased by the knockdown of
TLR2 (Fig. 9D). We also noted that
the knockdown of TLR2 only partly decreased the 25-HC-induced AGS
cell migration and MMP expressionlevels. Thus, we speculated that
TLR2 is not the only signaling pathway responsible for
25-HC-promoted AGS cells invasion and MMPs expressions.
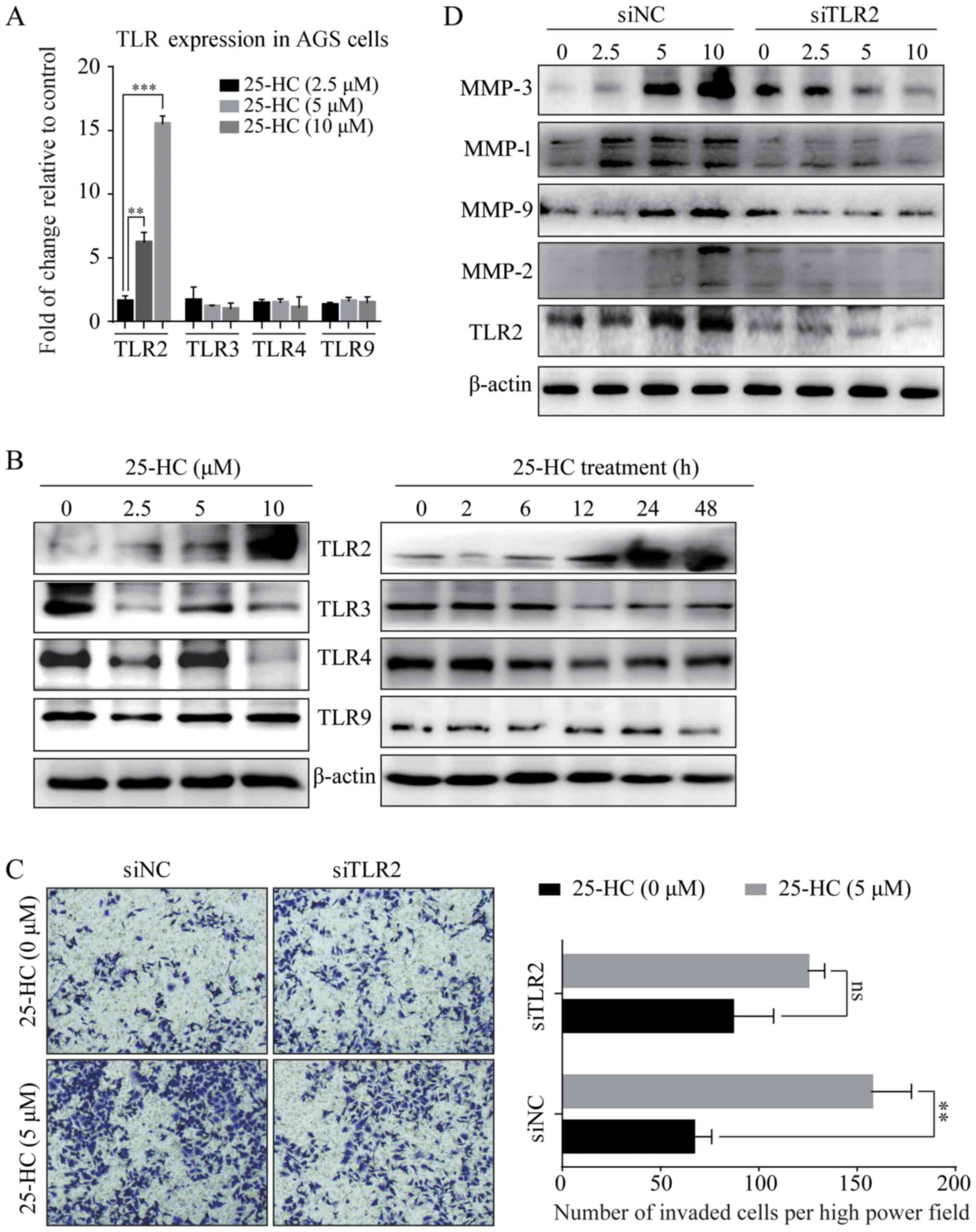 | Figure 925-HC-promotes AGS cell invasion and
MMP expression and these effects are partly dependent on TLR2. (A)
AGS cells were treated with the indicated concentrations of 25-HC
for 24 h before the cells were collected, RNA was extracted for
RT-qPCR to determine the mRNA expression levels of TLR2, TLR3, TLR4
and TLR9. Statistical significance was determined by one-way ANOVA
followed by Dunnett's test. (B) AGS cells were treated with the
indicated concentrations of 25-HC for 24 h or treated with 5
µM 25-HC for the indicated times before total cellular
proteins were extracted for western blot analysis to detect the
TLR2, TLR3, TLR4 and TLR9 expression levels. AGS cells were
transfected with siNC or siTLR2 for 24 h. (C) Invasion assay was
carried out following stimulation with 5 µM 25-HC.
Statistical significance was determined by a Student's t-test. (D)
Cells were treated with various concentrations of 25-HC for 24 h
before total cellular proteins were extracted for western blot
analysis to detect the MMP and TLR2 expression levels. Results were
obtained from 3 independent experiments and are expressed as the
means ± SEM. Representative images are presented; ns, no
significance; **P<0.01 and ***P<0.001.
25-HC, 25-hydroxycholesterol; GC, gastric cancer; MMP, matrix
metalloproteinase; TLR, Toll-like receptor. |
Discussion
25-HC is one of the major cytotoxins in oxidized
low-density lipoprotein (oxLDL) and has been demonstrated to exert
dose-dependent effects on cell proliferation (32) and the induction of cell apoptosis
(33-35). However, little is known about the
role of 25-HC in GC. In this study, we demonstrated that 25-HC
significantly decreased the sensitivity of AGS and MGC-803 cells to
5-FU, whereas it had no direct effects on cell proliferation and
apoptosis. Moreover, 25-HC treatment led to the enhanced invasion
of GC cells, accompanied by the increased expression levels of
MMPs. Further investigations revealed that 25-HC promoted cells
invasion via the TLR2-mediated activation of the NF-κB signaling
pathway. These results provide new insight into the roles of 25-HC
in GC progression.
The results of the present study are inconsistent
with prior studies, since we reported that 25-HC had no direct
effects on cell proliferation and apoptosis. We speculate that
there are two reasons for this. One reason is that the effector
cells are different since other studies were performed on non-tumor
cells, including macrophages, vascular cells and differentiated
PG12 cells (33-35) and perhaps these cells are more
sensitive to 25-HC treatment. The other reason may be the different
concentrations of 25-HC used in this study. Chen et al also
reported 25-HC promoted A549 and NCL-H1975 lung adenocarcinoma and
cell migration and invasion at the concentration of 0.1 µM
without affecting cell proliferation, while displaying the
inhibitory effect on cell proliferation from 1.0-25 µM
(18), indicating that 25-HC plays
differential roles at various concentrations. In this study, we
reported that 25-HC promoted the migration and invasion of GC cells
at the concentrations from 2.5-10 µM without affecting cell
proliferation.
NF-κB is constitutively activated in many types of
cancer and exerts a variety of pro-tumorigenic effects (36,37).
A previous study reported that 25-HC amplified inflammatory
signaling by mediating the recruitment of the AP-1 components FBJ
osteosarcoma oncogene (FOS) and jun proto-oncogene (JUN) to the
promoters of a subset of Toll-like receptor-responsive genes,
resulting in the alteration of the inflammatory response (38). 25-HC can enhance the NF-κB DNA
binding activity and the translocation of phosphorylated c-Jun into
the nucleus (39). The activation
of NF-κB is involved in the induction of the MMPs which are
associated with the invasion and metastasis of tumor cells. Thus,
the activated NF-κB signaling and the upregulated MMP expression
levels following treatment with 25-HC in this study could be
expected. It has been proven that the activation of NF-κB promotes
GC cell proliferation (40). As
shown in Figs. 7 and 8, the promotion of the invasion of GC
cells by 25-HC was dependent on the NF-κB signaling pathway. It is
noteworthy that 25-HC had no effects on cell proliferation, as
shown in Fig. 1. Thus, we detected
stem cell marker expression in AGS cells treated with 25-HC
(Fig. S1), and the results
revealed that 25-HC had no effects on the expression levels of
CD44, ALDH1, Sox2 and KLF4. We assumed that the probable reason for
this was that the effects of 25-HC are complex. Reboldi et
al found that 25-HC decreased inflammasome activation in
macrophages and consequently decreased the expression of IL-1β and
caspase-1 activation (41) and
Tricarico et al reported that 25-HC reduced inflammation,
but was ineffective in restoring the autophagic flux and decreasing
the apoptotic levels (42). All
these controversial findings suggest that the effects of 25-HC are
complex. Thus, we have reasons to assume that 25-HC may exert
inhibitory effects on the activation of other signaling pathways,
such as the Wnt or Hedgehog pathways (43) which could affect cell proliferation
and apoptosis.
Oxysterols, including 7β-hydroxycholesterol (7β-OHC)
has been reported to enhance the sensitivity of tumor cell lines,
such as HepG2, U937 and K562 to adriamycin, VP-16, 5-FU and
bleomycin (44). In this study,
having determined that 25-HC had no direct effects on AGS and
MGC-803 cell proliferation (Fig.
1), we thus detected whether 25-HC affects the sensitivity of
GC cells to 5-FU. As shown in Fig.
3, 25-HC decreased the sensitivity of GC cells to 5-FU. To the
best of our knowledge, this is a novel finding of this study.
To the best of our knowledge, there is limited
research available on the association between 25-HC and TLRs.
Erridge et al reported that in THP-1 cells, 25-HC stimulated
inflammatory signaling via the lipid-recognizing TLRs 1, 2, 4 and
6, while independent of TLR signaling (45). In addition, TLR4 agonist can induce
the synthesis of 25-HC in macrophages (46). In this study, we detected the
expression levels of TLR2, TLR3, TLR4 and TLR9, which have been
reported to be expressed in GC cells (47-50).
The results of this study revealed that 25-HC upregulated TLR2
expression; however, the exact mechanisms involved are not yet
clear and want further investigation. To the best of our knowledge,
this is the first study to report the regulation of TLRs by
25-HC.
In conclusion, in this study, we identified a novel
role of 25-HC in GC, in that 25-HC promotes GC cell migration and
invasion by upregulating TLR2-NF-κB-mediated MMP expression.
Therefore, the targeting of 25-HC may prove to be a potential
therapeutic strategy for the treatment of GC.
Supplementary Data
Funding
The present study was supported by a grant from the
Key Project of Natural Science Foundation of Zhejiang Province
(LZ16H160003 to WC).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
SW and WC participated in the conception and design
of the study. SW, YY, CR and GZ performed the statistical analysis
and were involved in the preparation of the figures. SW and YY
reviewed the results and participated in the discussion of the
data. SW, GZ and WC prepared the manuscript and revised it. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All the experiments using animals were approved by
the Animal Care and Use Committee of Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Kloudova A, Guengerich FP and Soucek P:
The role of oxysterols in human cancer. Trends Endocrinol Metab.
28:485–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nelson ER, Chang CY and McDonnell DP:
Cholesterol and breast cancer pathophysiology. Trends Endocrinol
Metab. 25:649–655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang KA, Chae S, Lee KH, Park MT, Lee SJ,
Lee YS and Hyun JW: Cytotoxic effect of 7beta-hydroxycholesterol on
human NCI-H460 lung cancer cells. Biol Pharm Bull. 28:1377–1380.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagano K, Imai S, Zhao X, Yamashita T,
Yoshioka Y, Abe Y, Mukai Y, Kamada H, Nakagawa S, Tsutsumi Y, et
al: Identification and evaluation of metastasis-related proteins,
oxysterol binding protein-like 5 and calumenin, in lung tumors. Int
J Oncol. 47:195–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitayama J, Hatano K, Kaisaki S, Suzuki H,
Fujii S and Nagawa H: Hyperlipidaemia is positively correlated with
lymph node metastasis in men with early gastric cancer. Br J Surg.
91:191–198. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo E, Chen L, Xie Q, Chen J, Tang Z and
Wu Y: Serum HDL-C as a potential biomarker for nodal stages in
gastric cancer. Ann Surg Oncol. 14:2528–2534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen G, Feng W, Zhang S, Bian K, Yang Y,
Fang C, Chen M, Yang J and Zou X: Metformin inhibits gastric cancer
via the inhibition of HIF1α/PKM2 signaling. Am J Cancer Res.
5:1423–1434. 2015.
|
|
11
|
Valaee S, Yaghoobi MM and Shamsara M:
Metformin inhibits gastric cancer cells metastatic traits through
suppression of epithelial-mesenchymal transition in a
glucose-independent manner. PLoS One. 12:e01744862017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh PP and Singh S: Statins are
associated with reduced risk of gastric cancer: A systematic review
and meta-analysis. Ann Oncol. 24:1721–1730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russell DW: The enzymes, regulation, and
genetics of bile acid synthesis. Annu Rev Biochem. 72:137–174.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson KA, Morrow CJ, Knight GD and
Scallen TJ: In vivo formation of 25-hydroxycholesterol from
endogenous cholesterol after a single meal, dietary cholesterol
challenge. J Lipid Res. 35:2241–2253. 1994.PubMed/NCBI
|
|
15
|
Hodis HN, Chauhan A, Hashimoto S, Crawford
DW and Sevanian A: Probucol reduces plasma and aortic wall
oxysterol levels in cholesterol fed rabbits independently of its
plasma cholesterol lowering effect. Atherosclerosis. 96:125–134.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lappano R, Recchia AG, De Francesco EM,
Angelone T, Cerra MC, Picard D and Maggiolini M: The cholesterol
metabolite 25-hydroxycholesterol activates estrogen receptor
α-mediated signaling in cancer cells and in cardiomyocytes. PLoS
One. 6:e166312011. View Article : Google Scholar
|
|
17
|
Simigdala N, Gao Q, Pancholi S,
Roberg-Larsen H, Zvelebil M, Ribas R, Folkerd E, Thompson A, Bhamra
A, Dowsett M, et al: Cholesterol biosynthesis pathway as a novel
mechanism of resistance to estrogen deprivation in estrogen
receptor-positive breast cancer. Breast Cancer Res. 18:582016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Zhang L, Xian G, Lv Y, Lin Y and
Wang Y: 25-Hydroxycholesterol promotes migration and invasion of
lung adenocarcinoma cells. Biochem Biophys Res Commun. 484:857–863.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burkard I, von Eckardstein A, Waeber G,
Vollenweider P and Rentsch KM: Lipoprotein distribution and
biological variation of 24S- and 27-hydroxycholesterol in healthy
volunteers. Atherosclerosis. 194:71–78. 2007. View Article : Google Scholar
|
|
20
|
Yang P, Zhou Y, Chen B, Wan HW, Jia GQ,
Bai HL and Wu XT: Overweight, obesity and gastric cancer risk:
Results from a meta-analysis of cohort studies. Eur J Cancer.
45:2867–2873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Gilkes DM, Semenza GL and Wirtz D: Hypoxia
and the extracellular matrix: Drivers of tumour metastasis. Nat Rev
Cancer. 14:430–439. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Høye AM and Erler JT: Structural ECM
components in the premetastatic and metastatic niche. Am J Physiol
Cell Physiol. 310:C955–C967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yadav L, Puri N, Rastogi V, Satpute P,
Ahmad R and Kaur G: Matrix metalloproteinases and cancer - roles in
threat and therapy. Asian Pac J Cancer Prev. 15:1085–1091. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murray GI, Duncan ME, Arbuckle E, Melvin
WT and Fothergill JE: Matrix metalloproteinases and their
inhibitors in gastric cancer. Gut. 43:791–797. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elnemr A, Yonemura Y, Bandou E, Kinoshita
K, Kawamura T, Takahashi S, Tochiori S, Endou Y and Sasaki T:
Expression of collagenase-3 (matrix metalloproteinase-13) in human
gastric cancer. Gastric Cancer. 6:30–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang M and Huang CZ: Mitogen-activated
protein kinase signaling pathway and invasion and metastasis of
gastric cancer. World J Gastroenterol. 21:11673–11679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang MD, Lai KC, Lai TY, Hsu SC, Kuo CL,
Yu CS, Lin ML, Yang JS, Kuo HM, Wu SH, et al: Phenethyl
isothiocyanate inhibits migration and invasion of human gastric
cancer AGS cells through suppressing MAPK and NF-kappaB signal
pathways. Anticancer Res. 30:2135–2143. 2010.PubMed/NCBI
|
|
30
|
Yakata Y, Nakayama T, Yoshizaki A, Kusaba
T, Inoue K and Sekine I: Expression of p-STAT3 in human gastric
carcinoma: Significant correlation in tumour invasion and
prognosis. Int J Oncol. 30:437–442. 2007.PubMed/NCBI
|
|
31
|
Duan H, Qu L and Shou C: Activation of
EGFR-PI3K-AKT signaling is required for Mycoplasma
hyorhinis-promoted gastric cancer cell migration. Cancer Cell Int.
14:1352014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Velázquez E, Santos A, Montes A, Blázquez
E and Ruiz-Albusac JM: 25-Hydroxycholesterol has a dual effect on
the proliferation of cultured rat astrocytes. Neuropharmacology.
51:229–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Travert C, Carreau S and Le Goff D:
Induction of apoptosis by 25-hydroxycholesterol in adult rat Leydig
cells: Protective effect of 17beta-estradiol. Reprod Toxicol.
22:564–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi YK, Kim YS, Choi IY, Kim SW and Kim
WK: 25-hydroxy-cholesterol induces mitochondria-dependent apoptosis
via activation of glycogen synthase kinase-3beta in PC12 cells.
Free Radic Res. 42:544–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang L and Sinensky MS:
25-Hydroxycholesterol activates a cytochrome c release-mediated
caspase cascade. Biochem Biophys Res Commun. 278:557–563. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
37
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gold ES, Diercks AH, Podolsky I,
Podyminogin RL, Askovich PS, Treuting PM and Aderem A:
25-Hydroxycholesterol acts as an amplifier of inflammatory
signaling. Proc Natl Acad Sci USA. 111:10666–10671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koarai A, Yanagisawa S, Sugiura H,
Ichikawa T, Kikuchi T, Furukawa K, Akamatsu K, Hirano T, Nakanishi
M, Matsunaga K, et al: 25-Hydroxycholesterol enhances cytokine
release and Toll-like receptor 3 response in airway epithelial
cells. Respir Res. 13:632012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha
TK, Byun DS, Chae KS, Lee BH, Chun HS, et al: NF-kappaB activates
transcription of the RNA-binding factor HuR, via PI3K-AKT
signaling, to promote gastric tumorigenesis. Gastroenterology.
135:2030–2042. 2042.e1–3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reboldi A, Dang EV, McDonald JG, Liang G,
Russell DW and Cyster JG: Inflammation. 25-Hydroxycholesterol
suppresses interleukin-1-driven inflammation downstream of type I
interferon. Science. 345:679–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tricarico PM, Gratton R, Braga L, Celsi F
and Crovella S: 25-Hydroxycholesterol and inflammation in
Lovastatin-deregulated mevalonate pathway. Int J Biochem Cell Biol.
92:26–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Weille J, Fabre C and Bakalara N:
Oxysterols in cancer cell proliferation and death. Biochem
Pharmacol. 86:154–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hyun JW, Holl V, Weltin D, Dufour P, Luu B
and Bischoff P: Effects of combinations of 7beta-hydroxycholesterol
and anticancer drugs or ionizing radiation on the proliferation of
cultured tumor cells. Anticancer Res. 22:943–948. 2002.PubMed/NCBI
|
|
45
|
Erridge C, Webb DJ and Spickett CM:
25-Hydroxycholesterol, 7beta-hydroxycholesterol and
7-ketocholesterol upregulate interleukin-8 expression independently
of Toll-like receptor 1, 2, 4 or 6 signalling in human macrophages.
Free Radic Res. 41:260–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bauman DR, Bitmansour AD, McDonald JG,
Thompson BM, Liang G and Russell DW: 25-Hydroxycholesterol secreted
by macrophages in response to Toll-like receptor activation
suppresses immunoglobulin A production. Proc Natl Acad Sci USA.
106:16764–16769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Li Y, Li Y, Li R, Ma Y, Wang H
and Wang Y: Chloroquine inhibits MGC803 gastric cancer cell
migration via the Toll-like receptor 9/nuclear factor kappa B
signaling pathway. Mol Med Rep. 11:1366–1371. 2015. View Article : Google Scholar
|
|
48
|
Yue Y, Zhou T, Gao Y, Zhang Z, Li L, Liu
L, Shi W, Su L and Cheng B: High mobility group box 1/toll-like
receptor 4/myeloid differentiation factor 88 signaling promotes
progression of gastric cancer. Tumour Biol. Mar 28–2017.Epub ahead
of print. View Article : Google Scholar
|
|
49
|
Fernandez-Garcia B, Eiró N, González-Reyes
S, González L, Aguirre A, González LO, Del Casar JM, García-Muñiz
JL and Vizoso FJ: Clinical significance of toll-like receptor 3, 4,
and 9 in gastric cancer. J Immunother. 37:77–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
West AC, Tang K, Tye H, Yu L, Deng N,
Najdovska M, Lin SJ, Balic JJ, Okochi-Takada E, McGuirk P, et al:
Identification of a TLR2-regulated gene signature associated with
tumor cell growth in gastric cancer. Oncogene. 36:5134–5144. 2017.
View Article : Google Scholar : PubMed/NCBI
|