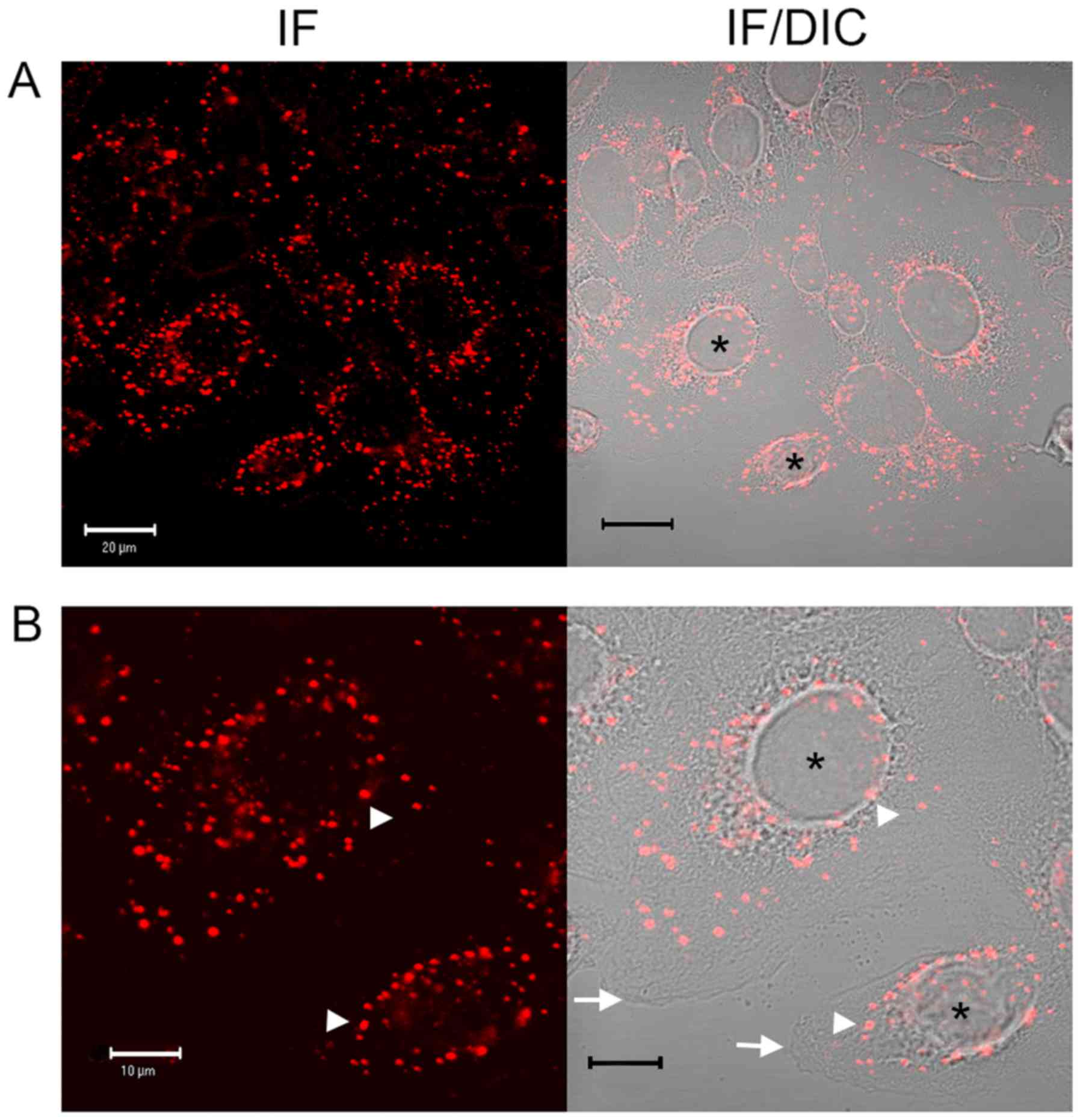
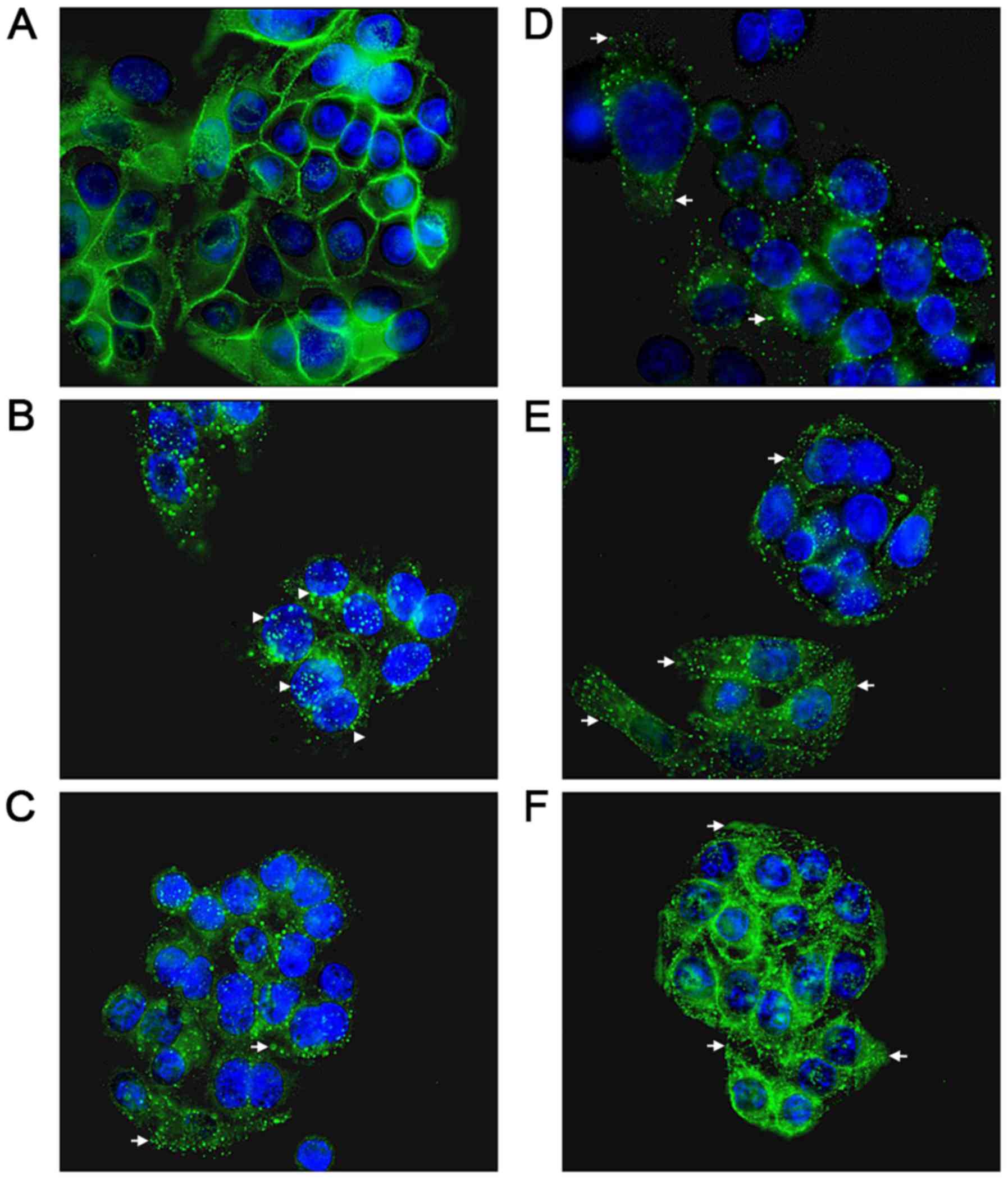
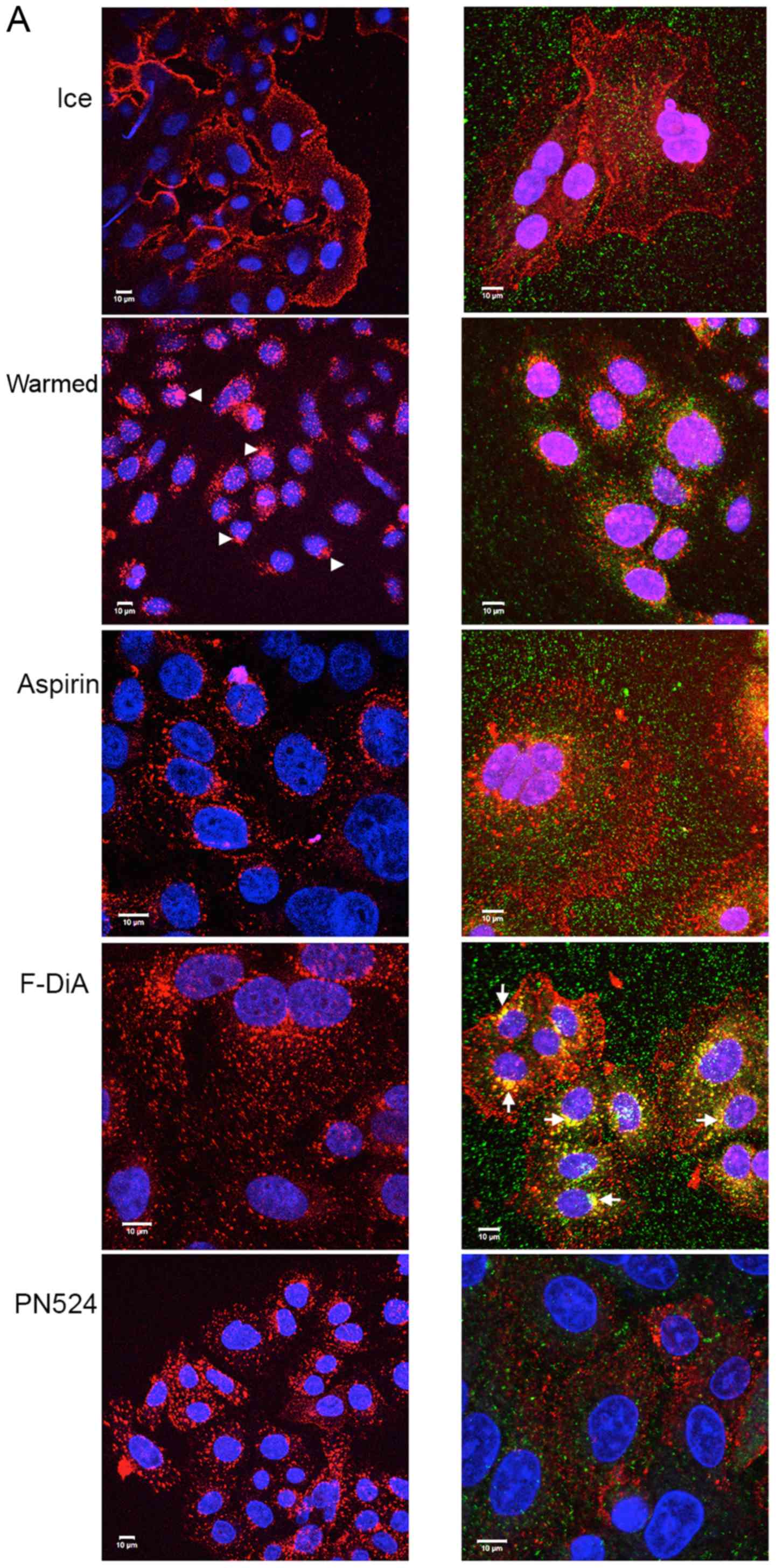
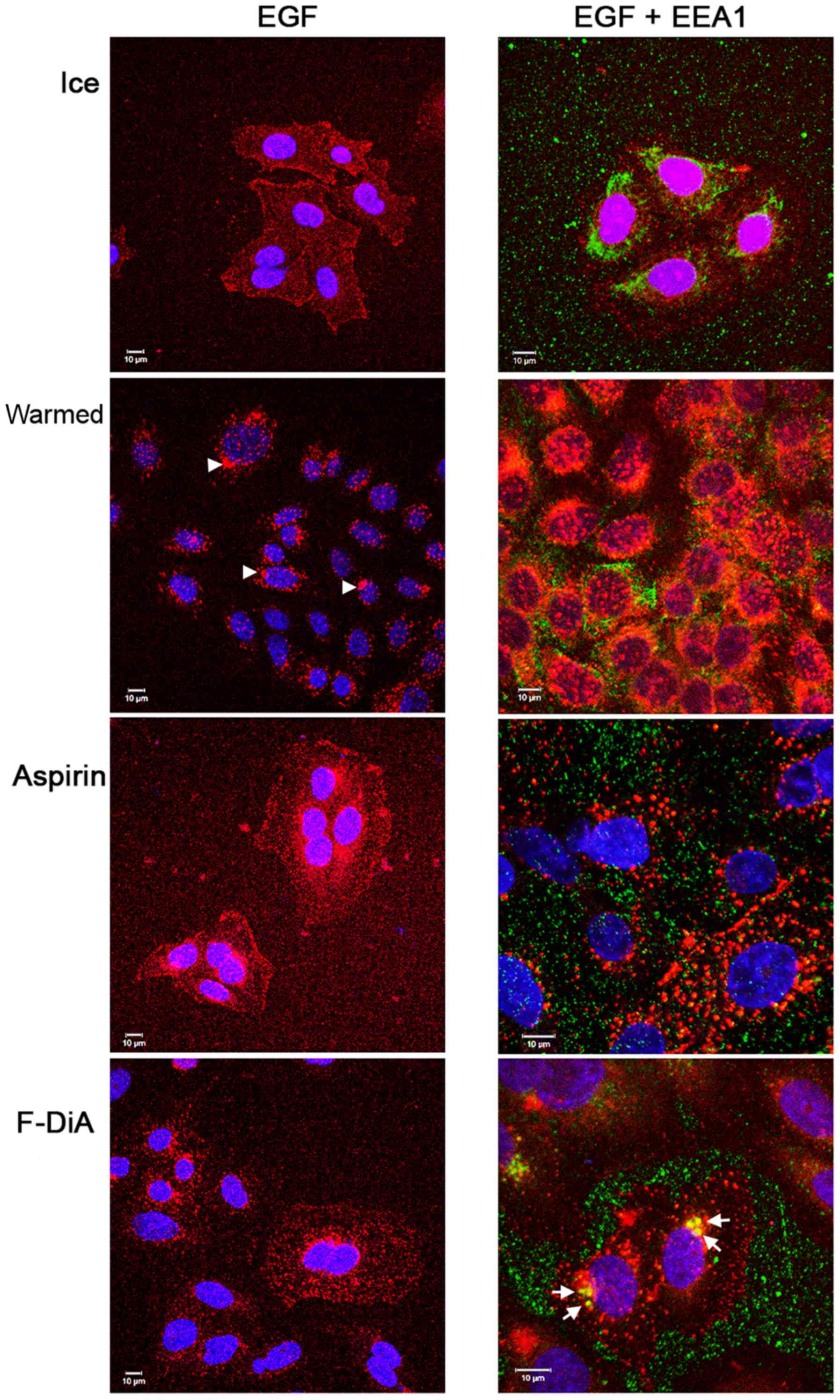
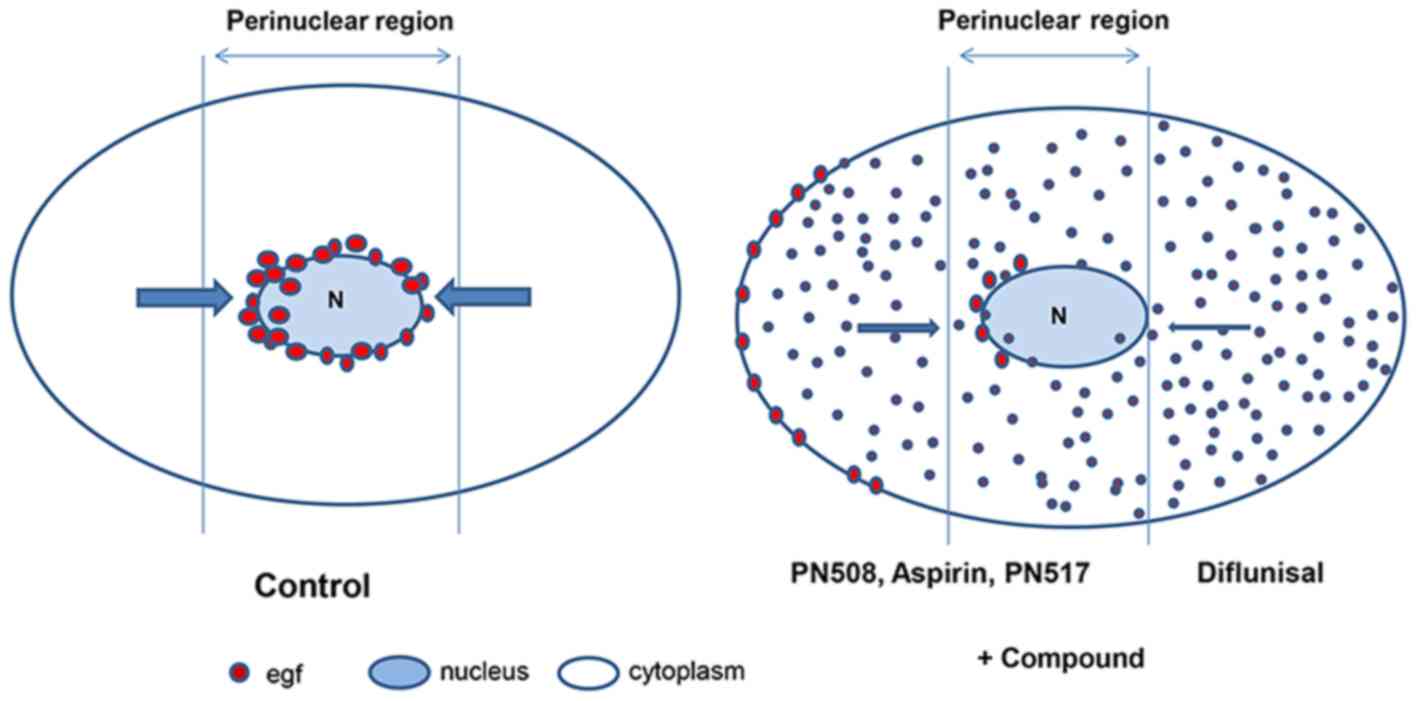
|
1
|
Garcia-Albeniz X and Chan AT: Aspirin for
the prevention of colorectal cancer. Best Pract Res Clin
Gastroenterol. 25:461–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung JJ, Lau JY, Goh KL and Leung WK; Asia
Pacific Working Group on Colorectal Cancer: Increasing incidence of
colorectal cancer in Asia: Implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuriki K and Tajima K: The increasing
incidence of colorectal cancer and the preventive strategy in
Japan. Asian Pac J Cancer Prev. 7:495–501. 2006.PubMed/NCBI
|
|
4
|
Siegel RL, Fedewa SA, Anderson WF, Miller
KD, Ma J, Rosenberg PS and Jemal A: Colorectal Cancer Incidence
Patterns in the United States, 1974–2013 = J Natl Cancer Inst.
109:djw3222017.
|
|
5
|
Shaheen NJ, Straus WL and Sandler RS:
Chemoprevention of gastrointestinal malignancies with nonsteroidal
antiinflam-matory drugs. Cancer. 94:950–963. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandler RS, Halabi S, Baron JA, Budinger
S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi
CL, et al: A randomized trial of aspirin to prevent colorectal
adenomas in patients with previous colorectal cancer. N Engl J Med.
348:883–890. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan AT, Arber N, Burn J, Chia WK, Elwood
P, Hull MA, Logan RF, Rothwell PM, Schrör K and Baron JA: Aspirin
in the chemoprevention of colorectal neoplasia: An overview. Cancer
Prev Res (Phila). 5:164–178. 2012. View Article : Google Scholar
|
|
8
|
Bibbins-Domingo K: Aspirin Use for the
Primary Prevention of Cardiovascular Disease and Colorectal Cancer:
U.S. Preventive Services Task Force Recommendation Statement. Ann
Intern Med. 164:836–845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao X, Lochhead P, Nishihara R, Morikawa
T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et
al: Aspirin use, tumor PIK3CA mutation, and colorectal-cancer
survival. N Engl J Med. 367:1596–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liggett JL, Zhang X, Eling TE and Baek SJ:
Anti-tumor activity of non-steroidal anti-inf lammatory drugs:
Cyclooxygenase-independent targets. Cancer Lett. 346:217–224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal anti-inflammatory drugs as anticancer agents:
Mechanistic, pharmacologic, and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown JR and DuBois RN: COX-2: A molecular
target for colorectal cancer prevention. J Clin Oncol.
23:2840–2855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan AT, Ogino S and Fuchs CS: Aspirin and
the risk of colorectal cancer in relation to the expression of
COX-2. N Engl J Med. 356:2131–2142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bos CL, Kodach LL, van den Brink GR, Diks
SH, van Santen MM, Richel DJ, Peppelenbosch MP and Hardwick JC:
Effect of aspirin on the Wnt/beta-catenin pathway is mediated via
protein phosphatase 2A. Oncogene. 25:6447–6456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kopp E and Ghosh S: Inhibition of NF-kappa
B by sodium salicylate and aspirin. Science. 265:956–959. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu XM, Sansores-Garcia L, Chen XM,
Matijevic-Aleksic N, Du M and Wu KK: Suppression of inducible
cyclooxygenase 2 gene transcription by aspirin and sodium
salicylate. Proc Natl Acad Sci USA. 96:5292–5297. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin MJ, Yamamoto Y and Gaynor RB: The
anti-inflammatory agents aspirin and salicylate inhibit the
activity of I(kappa)B kinase-beta. Nature. 396:77–80. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goel A, Chang DK, Ricciardiello L, Gasche
C and Boland CR: A novel mechanism for aspirin-mediated growth
inhibition of human colon cancer cells. Clin Cancer Res. 9:383–390.
2003.PubMed/NCBI
|
|
19
|
Rüschoff J, Wallinger S, Dietmaier W,
Bocker T, Brockhoff G, Hofstädter F and Fishel R: Aspirin
suppresses the mutator phenotype associated with hereditary
nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad
Sci USA. 95:11301–11306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Din FV, Valanciute A, Houde VP, Zibrova D,
Green KA, Sakamoto K, Alessi DR and Dunlop MG: Aspirin inhibits
mTOR signaling, activates AMP-activated protein kinase, and induces
autophagy in colorectal cancer cells. Gastroenterology.
142:1504–1515.e3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hawley SA, Fullerton MD, Ross FA,
Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green
KA, Mustard KJ, et al: The ancient drug salicylate directly
activates AMP-activated protein kinase. Science. 336:918–922. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirakawa K, Wang L, Man N, Maksimoska J,
Sorum AW, Lim HW, Lee IS, Shimazu T, Newman JC, Schröder S, et al:
Salicylate, diflunisal and their metabolites inhibit CBP/p300 and
exhibit anticancer activity. Elife. 5:pii: e11156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pangburn HA, Kraus H, Ahnen DJ and Rice
PL: Sulindac metabolites inhibit epidermal growth factor receptor
activation and expression. J Carcinog. 4:162005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Selvendiran K, Bratasz A, Tong L, Ignarro
LJ and Kuppusamy P: NCX-4016, a nitro-derivative of aspirin,
inhibits EGFR and STAT3 signaling and modulates Bcl-2 proteins in
cisplatin-resistant human ovarian cancer cells and xenografts. Cell
Cycle. 7:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho M, Kabir SM, Dong Y, Lee E, Rice VM,
Khabele D and Son DS: Aspirin Blocks EGF-stimulated Cell Viability
in a COX-1 Dependent Manner in Ovarian Cancer Cells. J Cancer.
4:671–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deb J, Dibra H, Shan S, Rajan S, Manneh J,
Kankipati CS, Perry CJ and Nicholl ID: Activity of aspirin
analogues and vanillin in a human colorectal cancer cell line.
Oncol Rep. 26:557–565. 2011.PubMed/NCBI
|
|
27
|
Claudius AK, Kankipati CS, Kilari RS,
Hassan S, Guest K, Russell ST, Perry CJ, Stark LA and Nicholl ID:
Identification of aspirin analogues that repress NF-κB signalling
and demonstrate anti-proliferative activity towards colorectal
cancer in vitro and in vivo. Oncol Rep. 32:1670–1680. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed D, Eide PW, Eilertsen IA, Danielsen
SA, Eknæs M, Hektoen M, Lind GE and Lothe RA: Epigenetic and
genetic features of 24 colon cancer cell lines. Oncogenesis.
2:e712013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shigeta K, Hayashida T, Hoshino Y,
Okabayashi K, Endo T, Ishii Y, Hasegawa H and Kitagawa Y:
Expression of epidermal growth factor receptor detected by
cetuximab indicates its efficacy to inhibit in vitro and in vivo
proliferation of colorectal cancer cells. PLoS One. 8:e663022013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richter M, Weiss M, Weinberger I,
Fürstenberger G and Marian B: Growth inhibition and induction of
apoptosis in colorectal tumor cells by cyclooxygenase inhibitors.
Carcinogenesis. 22:17–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin PC, Lin YJ, Lee CT, Liu HS and Lee JC:
Cyclooxygenase-2 expression in the tumor environment is associated
with poor prognosis in colorectal cancer patients. Oncol Lett.
6:733–739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boonstra JJ, van Marion R, Beer DG, Lin L,
Chaves P, Ribeiro C, Pereira AD, Roque L, Darnton SJ, Altorki NK,
et al: Verification and unmasking of widely used human esophageal
adenocar-cinoma cell lines. J Natl Cancer Inst. 102:271–274. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheong E, Ivory K, Doleman J, Parker ML,
Rhodes M and Johnson IT: Synthetic and naturally occurring COX-2
inhibitors suppress proliferation in a human oesophageal
adenocarcinoma cell line (OE33) by inducing apoptosis and cell
cycle arrest. Carcinogenesis. 25:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song S, Honjo S, Jin J, Chang SS, Scott
AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, et
al: The Hippo Coactivator YAP1 Mediates EGFR Overexpression and
Confers Chemoresistance in Esophageal Cancer. Clin Cancer Res.
21:2580–2590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bosetti C, Talamini R, Franceschi S, Negri
E, Garavello W and La Vecchia C: Aspirin use and cancers of the
upper aerodigestive tract. Br J Cancer. 88:672–674. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bosetti C, Gallus S and La Vecchia C:
Aspirin and cancer risk: An updated quantitative review to 2005.
Cancer Causes Control. 17:871–888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: Assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
40
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang X, Huang F, Marusyk A and Sorkin A:
Grb2 regulates internalization of EGF receptors through
clathrin-coated pits. Mol Biol Cell. 14:858–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang F, Khvorova A, Marshall W and Sorkin
A: Analysis of clathrin-mediated endocytosis of epidermal growth
factor receptor by RNA interference. J Biol Chem. 279:16657–16661.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carpenter AE, Jones TR, Lamprecht MR,
Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA,
Moffat J, et al: CellProfiler: Image analysis software for
identifying and quantifying cell phenotypes. Genome Biol.
7:R1002006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bordwell FG and Boutan PJ: Conjugative
Effects in Divalent Sulfur Groupings1. J Am Chem Soc.
78:854–860. 1956. View Article : Google Scholar
|
|
45
|
Nelander L, Johansson G, Toplin I, Melera
A and Nilsson L: The heats of hydrolysis of aspirin, thioaspirin,
and their p-analogues. Acta Chem Scand. 18:973–984. 1964.
View Article : Google Scholar
|
|
46
|
Schneider MR and Wolf E: The epidermal
growth factor receptor ligands at a glance. J Cell Physiol.
218:460–466. 2009. View Article : Google Scholar
|
|
47
|
Yarden Y; The EGFR family and its ligands
in human cancer: signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37(Suppl 4): S3–S8. 2001. View Article : Google Scholar
|
|
48
|
Radinsky R, Risin S, Fan D, Dong Z,
Bielenberg D, Bucana CD and Fidler IJ: Level and function of
epidermal growth factor receptor predict the metastatic potential
of human colon carcinoma cells. Clin Cancer Res. 1:19–31.
1995.PubMed/NCBI
|
|
49
|
Voldborg BR, Damstrup L, Spang-Thomsen M
and Poulsen HS: Epidermal growth factor receptor (EGFR) and EGFR
mutations, function and possible role in clinical trials. Ann
Oncol. 8:1197–1206. 1997. View Article : Google Scholar
|
|
50
|
Citri A and Yarden Y: EGF-ERBB signalling:
Towards the systems level. Nat Rev Mol Cell Biol. 7:505–516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Spano JP, Lagorce C, Atlan D, Milano G,
Domont J, Benamouzig R, Attar A, Benichou J, Martin A, Morere JF,
et al: Impact of EGFR expression on colorectal cancer patient
prognosis and survival. Ann Oncol. 16:102–108. 2005. View Article : Google Scholar
|
|
52
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar
|
|
53
|
Shostak K and Chariot A: EGFR and NF-κB:
Partners in cancer. Trends Mol Med. 21:385–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Habib AA, Chatterjee S, Park SK, Ratan RR,
Lefebvre S and Vartanian T: The epidermal growth factor receptor
engages receptor interacting protein and nuclear factor-kappa B
(NF-kappa B)-inducing kinase to activate NF-kappa B. Identification
of a novel receptor-tyrosine kinase signalosome. J Biol Chem.
276:8865–8874. 2001. View Article : Google Scholar
|
|
55
|
Taub N, Teis D, Ebner HL, Hess MW and
Huber LA: Late endosomal traffic of the epidermal growth factor
receptor ensures spatial and temporal fidelity of mitogen-activated
protein kinase signaling. Mol Biol Cell. 18:4698–4710. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Murphy JE, Padilla BE, Hasdemir B,
Cottrell GS and Bunnett NW: Endosomes: A legitimate platform for
the signaling train. Proc Natl Acad Sci USA. 106:17615–17622. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Din FV, Dunlop MG and Stark LA: Evidence
for colorectal cancer cell specificity of aspirin effects on NF
kappa B signalling and apoptosis. Br J Cancer. 91:381–388. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schwenger P, Bellosta P, Vietor I,
Basilico C, Skolnik EY and Vilcek J: Sodium salicylate induces
apoptosis via p38 mitogen-activated protein kinase but inhibits
tumor necrosis factor-induced c-Jun N-terminal
kinase/stress-activated protein kinase activation. Proc Natl Acad
Sci USA. 94:2869–2873. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Frantz B, O’Neill EA, Ghosh S and Kopp E:
The effect of sodium salicylate and aspirin on NF-kappa B. Science.
270:2017–2019. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Amann R and Peskar BA: Anti-inflammatory
effects of aspirin and sodium salicylate. Eur J Pharmacol. 447:1–9.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nordt SP, Clark RF, Castillo EM and Guss
DA: Comparison of three aspirin formulations in human volunteers.
West J Emerg Med. 12:381–385. 2011. View Article : Google Scholar
|
|
62
|
Borthwick GM, Johnson AS, Partington M,
Burn J, Wilson R and Arthur HM: Therapeutic levels of aspirin and
salicylate directly inhibit a model of angiogenesis through a
Cox-independent mechanism. FASEB J. 20:2009–2016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dawson JP, Berger MB, Lin CC, Schlessinger
J, Lemmon MA and Ferguson KM: Epidermal growth factor receptor
dimerization and activation require ligand-induced conformational
changes in the dimer interface. Mol Cell Biol. 25:7734–7742. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hubbard SR and Miller WT: Receptor
tyrosine kinases: Mechanisms of activation and signaling. Curr Opin
Cell Biol. 19:117–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Arkhipov A, Shan Y, Das R, Endres NF,
Eastwood MP, Wemmer DE, Kuriyan J and Shaw DE: Architecture and
membrane interactions of the EGF receptor. Cell. 152:557–569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang L and Fu L: Mechanisms of resistance
to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 5:390–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sigismund S, Woelk T, Puri C, Maspero E,
Tacchetti C, Transidico P, Di Fiore PP and Polo S:
Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl
Acad Sci USA. 102:2760–2765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sigismund S, Argenzio E, Tosoni D,
Cavallaro E, Polo S and Di Fiore PP: Clathrin-mediated
internalization is essential for sustained EGFR signaling but
dispensable for degradation. Dev Cell. 15:209–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Barbieri E, Di Fiore PP and Sigismund S:
Endocytic control of signaling at the plasma membrane. Curr Opin
Cell Biol. 39:21–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Burke P, Schooler K and Wiley HS:
Regulation of epidermal growth factor receptor signaling by
endocytosis and intracellular trafficking. Mol Biol Cell.
12:1897–1910. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Goh LK, Huang F, Kim W, Gygi S and Sorkin
A: Multiple mechanisms collectively regulate clathrin-mediated
endocytosis of the epidermal growth factor receptor. J Cell Biol.
189:871–883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mellman I and Yarden Y: Endocytosis and
cancer. Cold Spring Harb Perspect Biol. 5:a0169492013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Villaseñor R, Kalaidzidis Y and Zerial M:
Signal processing by the endosomal system. Curr Opin Cell Biol.
39:53–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang YN, Yamaguchi H, Hsu JM and Hung MC:
Nuclear trafficking of the epidermal growth factor receptor family
membrane proteins. Oncogene. 29:3997–4006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang YN and Hung MC: Nuclear functions and
subcellular trafficking mechanisms of the epidermal growth factor
receptor family. Cell Biosci. 2:132012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang YN, Lee HH, Lee HJ, Du Y, Yamaguchi H
and Hung MC: Membrane-bound trafficking regulates nuclear transport
of integral epidermal growth factor receptor (EGFR) and ErbB-2. J
Biol Chem. 287:16869–16879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brand TM, Iida M, Li C and Wheeler DL: The
nuclear epidermal growth factor receptor signaling network and its
role in cancer. Discov Med. 12:419–432. 2011.PubMed/NCBI
|
|
79
|
Jovic M, Sharma M, Rahajeng J and Caplan
S: The early endosome: A busy sorting station for proteins at the
crossroads. Histol Histopathol. 25:99–112. 2010.
|
|
80
|
Pálfy M, Reményi A and Korcsmáros T:
Endosomal crosstalk: Meeting points for signaling pathways. Trends
Cell Biol. 22:447–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Henriksen L, Grandal MV, Knudsen SL, van
Deurs B and Grøvdal LM: Internalization mechanisms of the epidermal
growth factor receptor after activation with different ligands.
PLoS One. 8:e581482013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Capuani F, Conte A, Argenzio E, Marchetti
L, Priami C, Polo S, Di Fiore PP, Sigismund S and Ciliberto A:
Quantitative analysis reveals how EGFR activation and
downregulation are coupled in normal but not in cancer cells. Nat
Commun. 6:79992015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pennock S and Wang Z: A tale of two Cbls:
Interplay of c-Cbl and Cbl-b in epidermal growth factor receptor
downregulation. Mol Cell Biol. 28:3020–3037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rojas M, Yao S and Lin YZ: Controlling
epidermal growth factor (EGF)-stimulated Ras activation in intact
cells by a cell-permeable peptide mimicking phosphorylated EGF
receptor. J Biol Chem. 271:27456–27461. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nyati MK, Morgan MA, Feng FY and Lawrence
TS: Integration of EGFR inhibitors with radiochemotherapy. Nat Rev
Cancer. 6:876–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Downward J, Waterfield MD and Parker PJ:
Autophosphorylation and protein kinase C phosphorylation of the
epidermal growth factor receptor. Effect on tyrosine kinase
activity and ligand binding affinity. J Biol Chem. 260:14538–14546.
1985.PubMed/NCBI
|
|
87
|
Keilhack H, Tenev T, Nyakatura E,
Godovac-Zimmermann J, Nielsen L, Seedorf K and Böhmer FD:
Phosphotyrosine 1173 mediates binding of the protein-tyrosine
phosphatase SHP-1 to the epidermal growth factor receptor and
attenuation of receptor signaling. J Biol Chem. 273:24839–24846.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hsu JM, Chen CT, Chou CK, Kuo HP, Li LY,
Lin CY, Lee HJ, Wang YN, Liu M, Liao HW, et al: Crosstalk between
Arg 1175 methylation and Tyr 1173 p hosphorylation negatively
modulates EGFR-mediated ERK activation. Nat Cell Biol. 13:174–181.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li H, Zhu F, Boardman LA, Wang L, Oi N,
Liu K, Li X, Fu Y, Limburg PJ, Bode AM, et al: Aspirin Prevents
Colorectal Cancer by Normalizing EGFR Expression. EBioMedicine.
2:447–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Murthy U, Basu M, Sen-Majumdar A and Das
M: Perinuclear location and recycling of epidermal growth factor
receptor kinase: Immunofluorescent visualization using antibodies
directed to kinase and extracellular domains. J Cell Biol.
103:333–342. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kesarwala AH, Samrakandi MM and
Piwnica-Worms D: Proteasome inhibition blocks ligand-induced
dynamic processing and internalization of epidermal growth factor
receptor via altered receptor ubiquitination and phosphorylation.
Cancer Res. 69:976–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Farrow DC, Vaughan TL, Hansten PD,
Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne
ST, et al: Use of aspirin and other nonsteroidal anti-inflammatory
drugs and risk of esophageal and gastric cancer. Cancer Epidemiol
Biomarkers Prev. 7:97–102. 1998.PubMed/NCBI
|
|
93
|
Funkhouser EM and Sharp GB: Aspirin and
reduced risk of esophageal carcinoma. Cancer. 76:1116–1119. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kobayashi S, Shimamura T, Monti S, Steidl
U, Hetherington CJ, Lowell AM, Golub T, Meyerson M, Tenen DG,
Shapiro GI, et al: Transcriptional profiling identifies cyclin D1
as a critical downstream effector of mutant epidermal growth factor
receptor signaling. Cancer Res. 66:11389–11398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Thoms HC, Dunlop MG and Stark LA:
p38-mediated inactivation of cyclin D1/cyclin-dependent kinase 4
stimulates nucleolar translocation of RelA and apoptosis in
colorectal cancer cells. Cancer Res. 67:1660–1669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cavalli V, Vilbois F, Corti M, Marcote MJ,
Tamura K, Karin M, Arkinstall S and Gruenberg J: The stress-induced
MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5
complex. Mol Cell. 7:421–432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fratti RA, Chua J and Deretic V: Induction
of p38 mitogen-activated protein kinase reduces early endosome
auto-antigen 1 (EEA1) recruitment to phagosomal membranes. J Biol
Chem. 278:46961–46967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vergarajauregui S, San Miguel A and
Puertollano R: Activation of p38 mitogen-activated protein kinase
promotes epidermal growth factor receptor internalization. Traffic.
7:686–698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Macé G, Miaczynska M, Zerial M and Nebreda
AR: Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid
receptor endocytosis. EMBO J. 24:3235–3246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Higgs GA, Salmon JA, Henderson B and Vane
JR: Pharmacokinetics of aspirin and salicylate in relation to
inhibition of arachidonate cyclooxygenase and antiinflammatory
activity. Proc Natl Acad Sci USA. 84:1417–1420. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hatanpaa KJ, Burma S, Zhao D and Habib AA:
Epidermal growth factor receptor in glioma: Signal transduction,
neuropathology, imaging, and radioresistance. Neoplasia.
12:675–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Moris D, Kontos M, Spartalis E and
Fentiman IS: The Role of NSAIDs in Breast Cancer Prevention and
Relapse: Current Evidence and Future Perspectives. Breast Care
(Basel). 11. pp. 339–344. 2016, View Article : Google Scholar
|
|
103
|
Taub M, Parker R, Mathivanan P, Ariff MA
and Rudra T: Antagonism of the prostaglandin E2 EP1 receptor in
MDCK cells increases growth through activation of Akt and the
epidermal growth factor receptor. Am J Physiol Renal Physiol.
307:F539–F550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Barrett MA, Zheng S, Roshankar G, Alsop
RJ, Belanger RK, Huynh C, Kučerka N and Rheinstädter MC:
Interaction of aspirin (acetylsalicylic acid) with lipid membranes.
PLoS One. 7:e343572012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Alsop RJ, Toppozini L, Marquardt D,
Kučerka N, Harroun TA and Rheinstädter MC: Aspirin inhibits
formation of cholesterol rafts in fluid lipid membranes. Biochim
Biophys Acta. 1848.805–812. 2015.
|
|
106
|
Puri C, Tosoni D, Comai R, Rabellino A,
Segat D, Caneva F, Luzzi P, Di Fiore PP and Tacchetti C:
Relationships between EGFR signaling-competent and
endocytosis-competent membrane microdomains. Mol Biol Cell.
16:2704–2718. 2005. View Article : Google Scholar : PubMed/NCBI
|