Introduction
Lung cancer has the highest morbidity and mortality
rates among malignant tumors globally (1). Although a number of advanced
technologies and high-precision testing methods have been applied
in the detection and treatment of lung cancer in recent decades,
the 5-year survival rate among patients with invasive and
metastatic lung cancer remains low (2-6).
Hypoxia, a hallmark of cancer development that occurs mainly in
fast-growing solid tumors, is characterized as closely associated
with cell metastasis (7-9) and may be a major contributor to tumor
progression and poor clinical outcomes (10-12).
Hypoxia is known to trigger epithelial-to-mesenchymal transition
(EMT) in lung cancer cells, but a clearer understanding of the
underlying mechanism is required for the development of treatments
targeting this process.
Netrin-1, the best-characterized ligand of the
netrin family, is a highly conserved cell-secreted soluble protein.
Accumulating evidence has confirmed that netrin-1 is not only
involved in the development of the central nervous system (13-17),
but also plays a completely different role regulating the adhesion
and proliferation of multiple cancer cell types (18-20).
In recent studies, the overexpression of netrin-1 has been
considered as a selective advantage for tumor progression in
several human cancers, such as colorectal cancer, metastatic breast
cancer and neuroblastoma (18,21,22).
Moreover, Gibert and Mehlen proposed that monoclonal antibodies to
netrin-1 can block ligand-receptor interaction with dependence
receptors, thereby inducing tumor cell apoptosis (23). It has been demonstrated that FAK is
an important downstream molecule of netrin-1 that interacts within
the phosphoinositide 3 kinase (PI3K)/AKT and mitogen-activated
protein kinase (MAPK) pathways to induce EMT (24-26).
Moreover, netrin-1 was linked to the p38 MAPK and PI3K/AKT
signaling pathways in experiments of netrin-1-mediated induction of
Schwann cell migration in peripheral nervous system injury
(19). However, additional
research is required to determine whether netrin-1 activation is
directly associated with hypoxia and EMT.
The aim of present study was to investigate whether
netrin-1 is involved in the hypoxia-induced EMT of non-small cell
lung cancer (NSCLC) cells. The concentration of netrin-1 in the
serum of NSCLC patients was compared with that in the healthy
control group in order to determine whether netrin-1 is associated
with lung cancer progression.
Materials and methods
Cell culture and simulation of hypoxic
conditions
The human NSCLC cell lines A549, NCI-H1299,
NCI-H1975, SPC-A1, PC9 and NCI-H522, along with the normal human
bronchial epithelial cell line 16-HBE, were obtained from the Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
(Gibco, Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA,
USA) containing 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2. To simulate a hypoxic microenvironment in
vitro, the cells were treated with 100 µmol/l
CoCl2 (Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA)
for 48 h.
Cell transfection with small interfering
(si)RNA
A549 and PC9 lung cancer cells were seeded into
6-well plates (2×105/well) and incubated overnight. For
transfection beginning on the next day, the cells were treated with
50 nmol/l siRNA or only a siRNA-Mate transfection reagent
(GenePharma, Jiangsu, China) as a negative control (NC) for 48 h.
The target sequences for si-netrin-1 #1 and
si-netrin-1 #2 were 5′-GCAAGAAGUUCGAAGUGACTT-3′ and
5′-UCCAGCAGCGUGAGAAGAATT-3′, respectively.
Western blot analysis
Total protein was extracted from the cells using
radioimmunoprecipitation assay buffer (Cell Signaling Technology,
Danvers, MA, USA), and a BCA protein assay kit (Abcam, Cambridge,
UK) was used to determine the concentration of total protein.
Proteins were then separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene fluoride membrane. After blocking with a 5% milk
solution in 1X Tris-buffered saline containing 0.1% Tween-20 (TBST)
for 1 h at room temperature, the membranes were incubated with
primary rabbit monoclonal antibodies to netrin-1 (dilution 1:100
cat. no. BA1671-1 ProteinTech Group, Inc., Chicago, IL, USA),
E-cadherin (dilution 1:500 cat. no. 28874-1-AP ProteinTech Group,
Inc.), vimentin (dilution 1:4,000 cat. no. 10366-1-AP ProteinTech
Group, Inc.), AKT (dilution 1:400 cat. no. ab81238 Abcam), or
β-actin (dilution 1:5,000 cat. no. 20536-1-AP ProteinTech Group,
Inc.) overnight at 4°C. After three washes with TBST buffer, the
membranes were incubated with the corresponding secondary
antibodies (anti-rabbit IgG, dilution 1:10,000 cat. no. SA00001-2,
ProteinTech Group, Inc.) for 1 h at room temperature.
Antibody binding signals were visualized using the
Super Signal West Pico kit (Thermo Fisher Scientific Inc., Anthem,
AZ, USA), and quantitative densitometric analysis was then
performed using Eagle Eye II soſtware (Eagle Eye Technology Ltd.,
London, UK). Quantification of the intensities of the
immunoreactive bands was performed by the Gel Doc™ XR imaging
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
QuantityOne (Bio-Rad Laboratories, Inc.). Experiments were repeated
three times, and the expression level of each target protein was
normalized to the corresponding expression level of β-actin.
Microfluidic chip assay
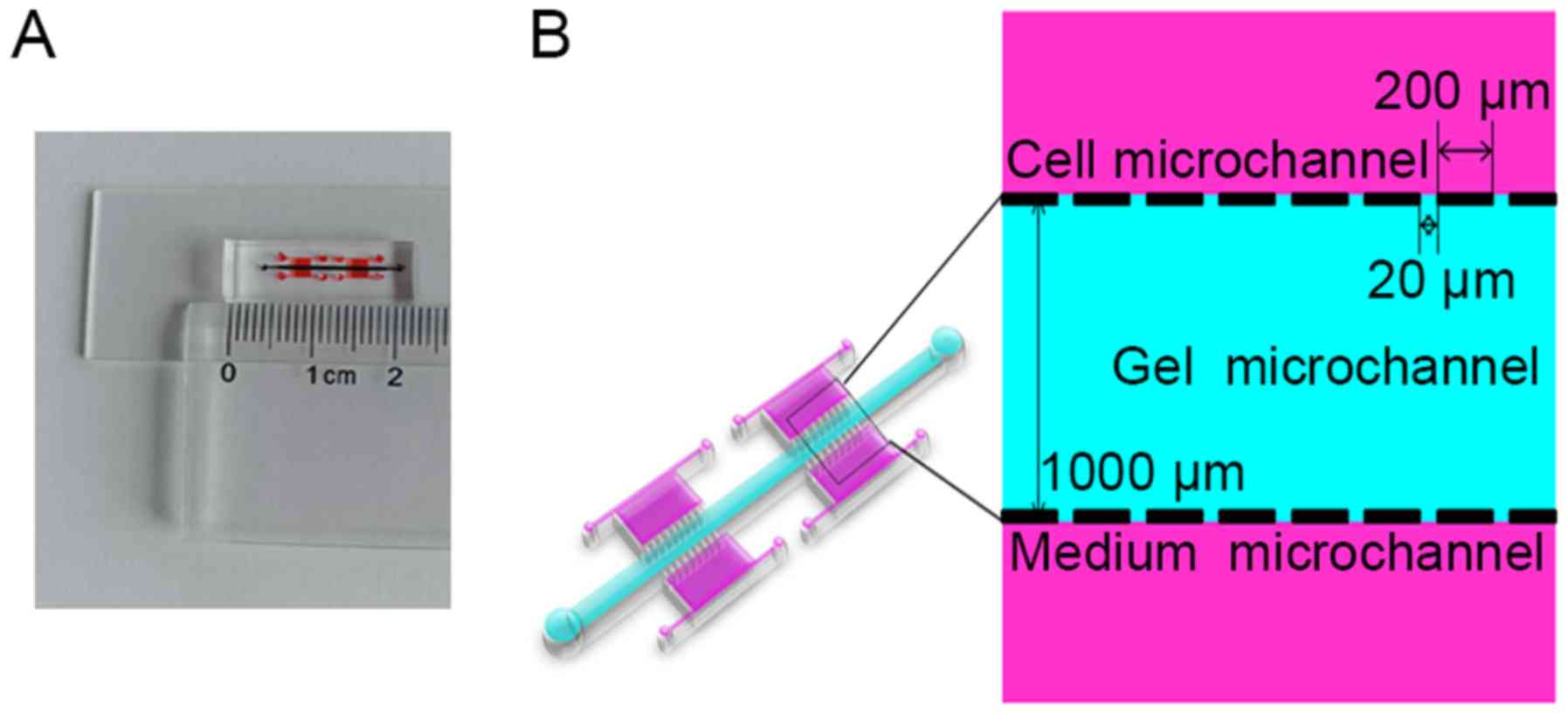
A three-dimensional (3D) microfluidic chip (Fig. 1A and B) was constructed for use in
in vitro assays to investigate the invasive behavior of A549
and PC9 cells under hypoxic conditions (27). The microchip was fabricated with
poly-dimethylsiloxane (Sylgard 184; Dow Chemical, Midland, MI,
USA). This device contains four units composed of three parts each,
including two lateral media micro-channels and one central gel
micro-channel. Cancer cells were cultured in suspension within the
two media channels, which are separated from the central gel
channel by micro-columns with micro-gaps that allow diffusion of
chemoattractants from the Matrigel. Cell invasion was evaluated
after culture of the cells within the assay system for 48 h at 37°C
in 5% CO2. For each condition, three independent
experiments were performed.
Transwell assay
Cell culture inserts (8 µm; BD-Falcon,
Franklin Lakes, NJ, USA) were used in a Transwell assay to
investigate the invasive behavior of A549 and PC9 cells under
hypoxic conditions. The upper chambers were coated with 100
µl Matrigel (Becton, Dickinson and Company, Franklin, Lakes,
NJ, USA; 1:1 diluted in phosphate-buffered saline). Cells exposed
to the indicated hypoxic conditions were seeded into the upper
chamber, and ~0.5 ml of culture medium containing 10% FBS was added
to the lower chamber. After 24 h, cells still present on the upper
membrane were gently removed with a cotton swab. Cells that had
migrated to the lower surface of the membrane were fixed in 4%
paraformaldehyde and then stained with 0.1% crystal violet solution
(Abcam) according to the manufacturer’s instructions. The mean
numbers of migrating cells were calculated from five random fields
under the microscope.
Wound healing assay
To analyze cell migration under various conditions,
A549 and PC9 cells (5×105/well) were grown in 6-well
Petri dishes under conditions of hypoxia or normoxia. After 6 h of
serum starvation, a scratch was made in each well using a sterile
100-µl pipette tip. After 24 h, a digital camera connected
to an inverted microscope (Nikon TE200; Nikon Corp., Tokyo, Japan)
was used to capture images. The experiments were performed in
triplicate, and representative images are shown.
Patient sample collection
Serum samples were collected from NSCLC patients and
healthy donors at the Second Affiliated Hospital of Dalian Medical
University (Dalian, China) between January 2016 and May 2017. The
present study was approved by the Institutional Review Boards of
the Second Affiliated Hospital of Dalian Medical University and all
procedures were performed according to the principles outlined in
the Declaration of Helsinki. All participants provided written
informed consent to the use of their tissues for the purposes of
the present study. Prior to any surgical or drug treatment,
peripheral blood samples were collected. These samples were then
centrifuged to obtain serum samples, which were stored at −80°C
until analysis. The clinical characteristics of all participants,
including age, sex, smoking history and pathological results, were
recorded.
ELISA
Netrin-1 concentrations in the conditioned media of
cells treated under various conditions, as well as in human serum
samples, were detected using a netrin-1 ELISA kit (Cusabio,
Houston, TX, USA) following the manufacturer’s instructions.
Statistical analysis
Data are expressed as mean ± standard deviation. The
SPSS v. 20 statistical software package (IBM Corp., Armonk, NY,
USA) was used to perform Student’s t-test, analysis of variance
with least significant difference test or χ2 test to
assess differences among data. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
A549 and PC9 cells express high levels of
netrin-1
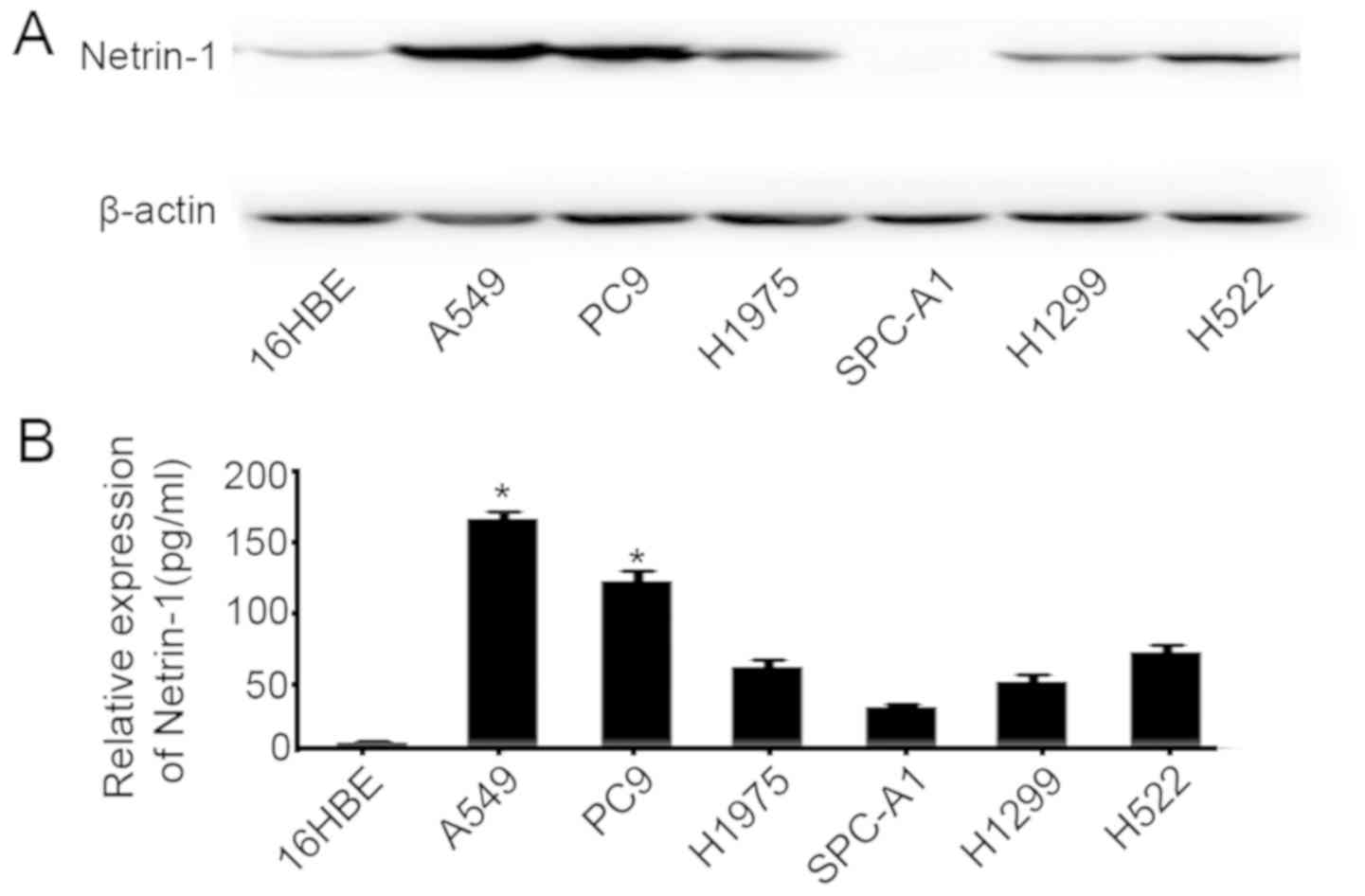
Netrin-1 expression in the 16HBE, A549, NCI-H1299,
NCI-H1975, SPC-A1, PC9 and NCI-H522 cell lines was examined by
western blotting (Fig. 2A) and
ELISA, using medium collected after culture for 24 h (Fig. 2B). The results demonstrated that
netrin-1 was expressed in most of these lung cancer cell lines, and
its expression was the highest in A549 and PC9 cells.
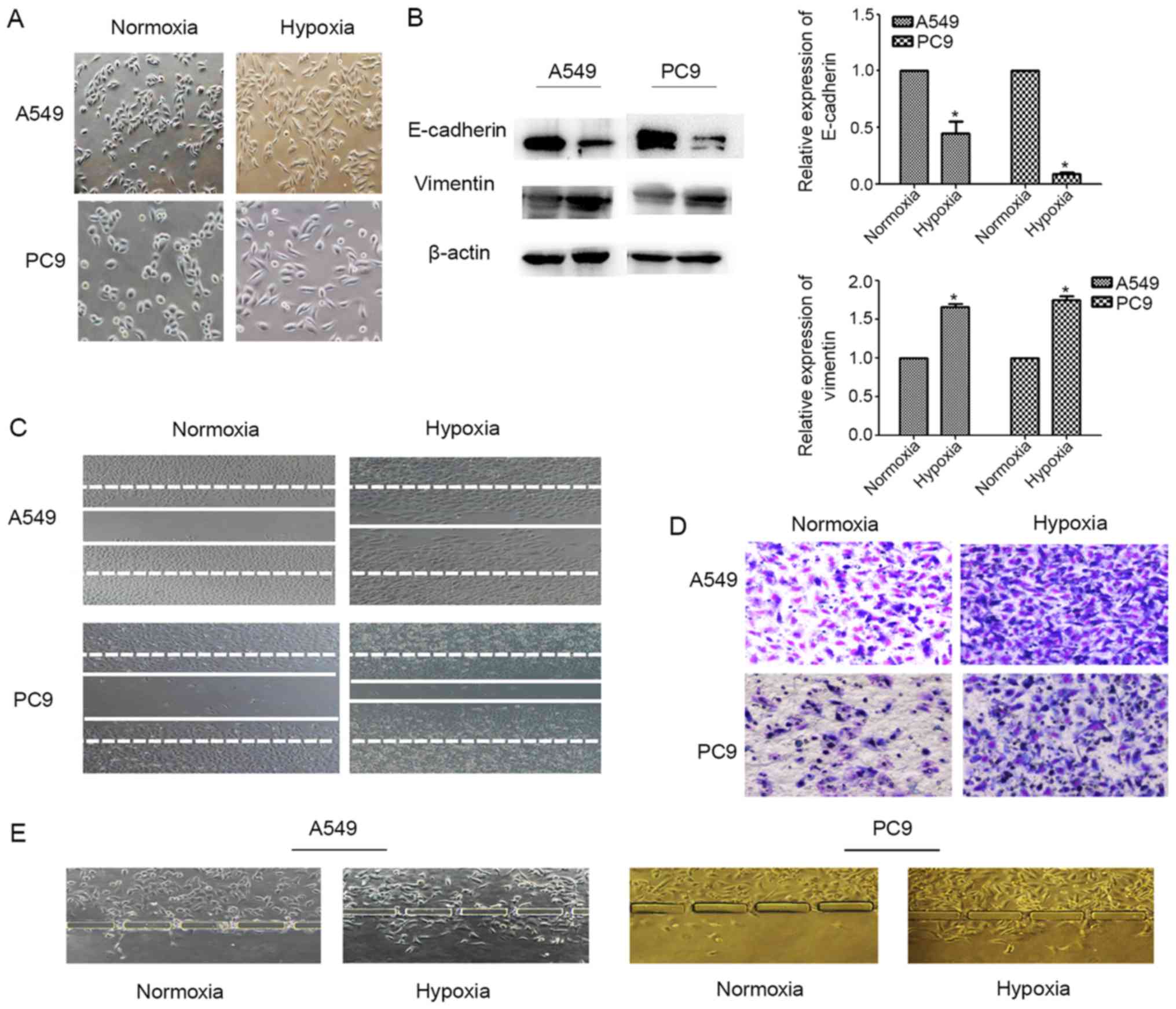
A549 and PC9 cells undergo EMT upon
exposure to hypoxic conditions
Following exposure to 100 µM CoCl2
for 24-48 h to create hypoxic culture conditions, the morphology of
A549 and PC9 cells was characterized by marked stretching and
elongation (Fig. 3A). The
epithelial biomarker E-cadherin was downregulated in these cells
after exposure to hypoxia, whereas expression of the mesenchymal
biomarker vimentin was increased (P<0.05; Fig. 3B). In both the Transwell and wound
healing assays, we found that the invasion (Fig. 3D and E) and migration (Fig. 3C) capacities of A549 and PC9 cells
were increased in the hypoxic microenvironment. These findings
represent typical events that occur during EMT of lung
adenocarcinoma cells under hypoxia.
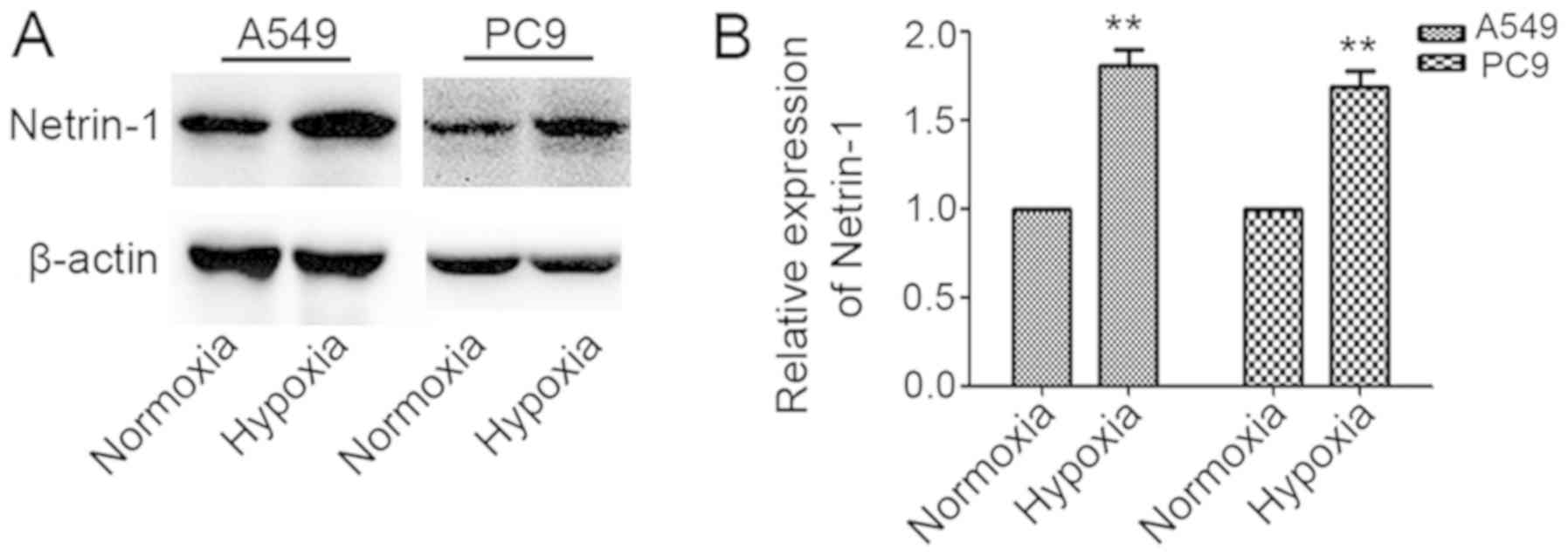
Netrin-1 expression increases in A549 and
PC9 cells under hypoxic conditions
Netrin-1 expression in A549 and PC9 cells was
measured following exposure of the cells to normoxia or hypoxia for
48 h. Netrin-1 expression was significantly higher in A549 and PC9
cells cultured under hypoxic conditions compared with its levels in
cells cultured under normoxic conditions (P<0.05; Fig. 4A and B).
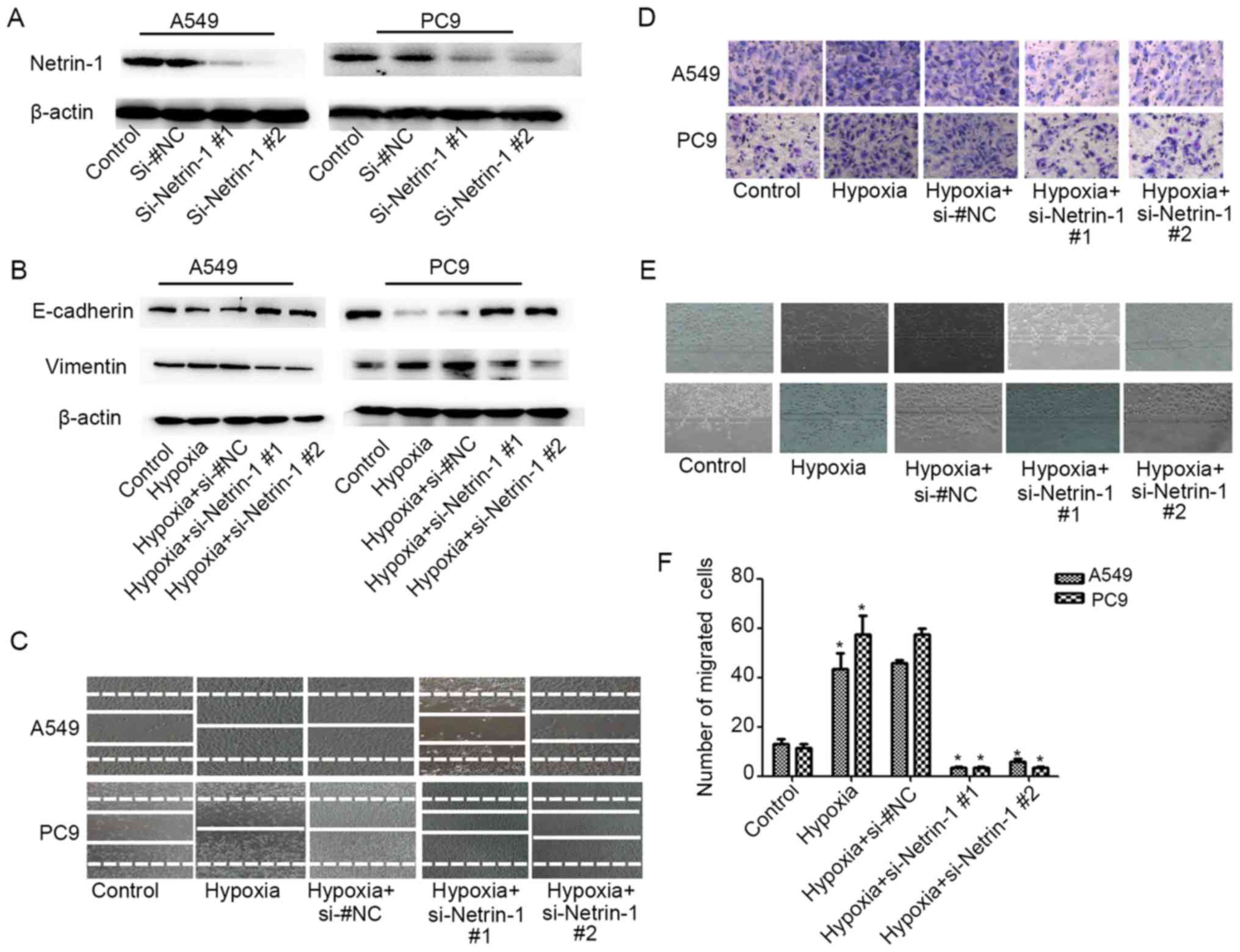
Netrin-1 silencing inhibits
hypoxia-induced EMT of A549 and PC9 cells
To investigate the role of Netrin-1 in
hypoxia-induced EMT, A549 and PC9 cells were transfected with two
netrin-1 siRNAs to effectively knock down netrin-1 expression
(P<0.05; Fig. 5A). With
knockdown of netrin-1, the hypoxia-triggered EMT of A549 and PC9
cells was attenuated, with restoration of E-cadherin and vimentin
expression (Fig. 5B) upon exposure
of the cells to hypoxia. The Transwell (Fig. 5D) and microfluidic chip assays
(Fig. 5E and F) revealed that the
hypoxia-induced invasive behavior of A549 and PC9 cells was notably
suppressed with knockdown of netrin-1 expression. In addition,
netrin-1 silencing effectively abrogated the effects of hypoxia on
the migratory behavior of these cells (Fig. 5C). Taken together, these results
indicate that netrin-1 plays an important role in hypoxia-induced
EMT and subsequent invasion and migration of lung cancer cells.
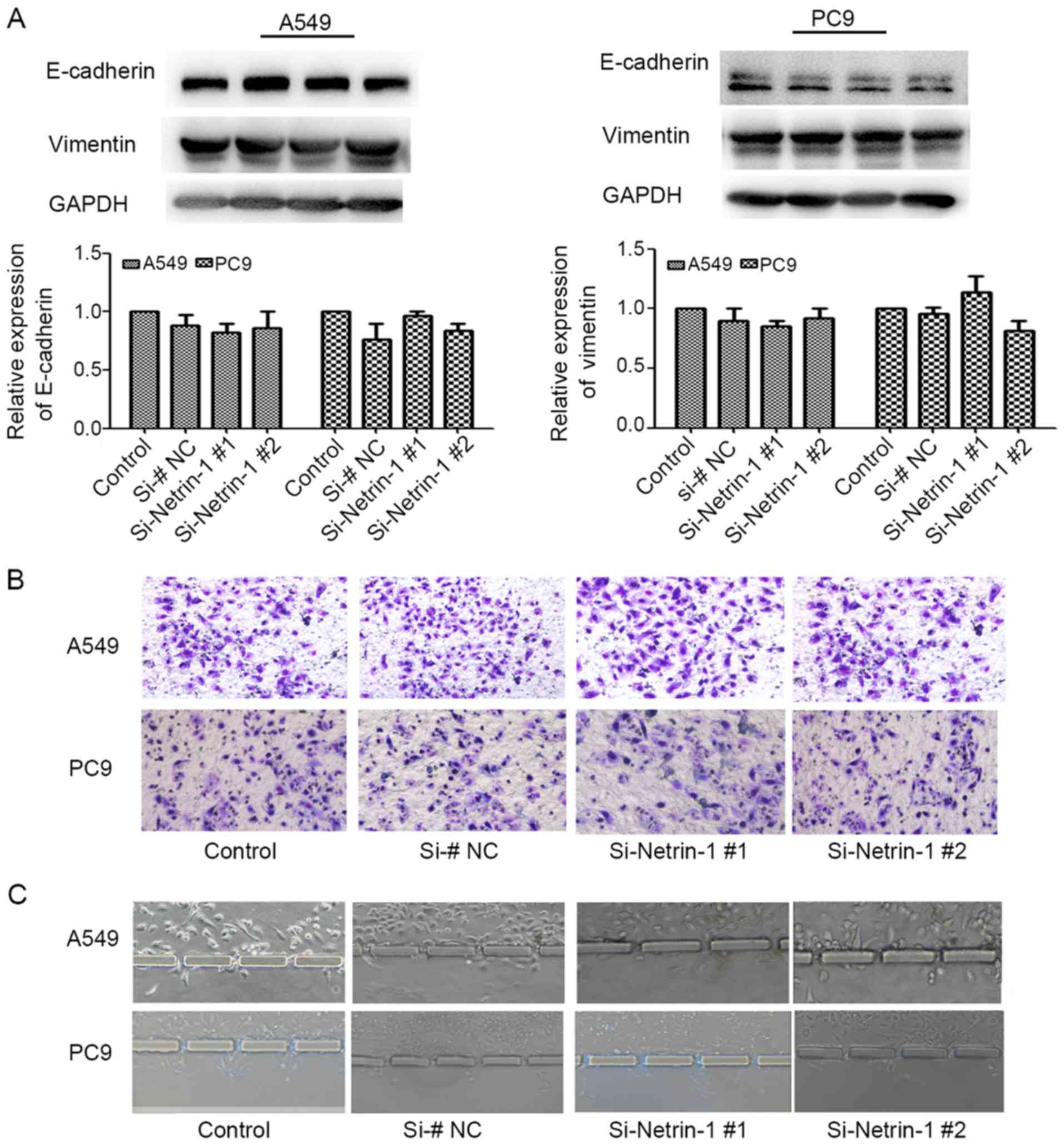
By comparison, under normoxic conditions, E-cadherin
and vimentin expression in A549 and PC9 cells was not obviously
altered by silencing of netrin-1 expression (P>0.05; Fig. 6A). Moreover, only slight
differences in the invasive behavior of A549 and PC9 cells were
observed with netrin-1 knockdown and culture under normoxic
conditions (P>0.05; Fig. 6B and
C).
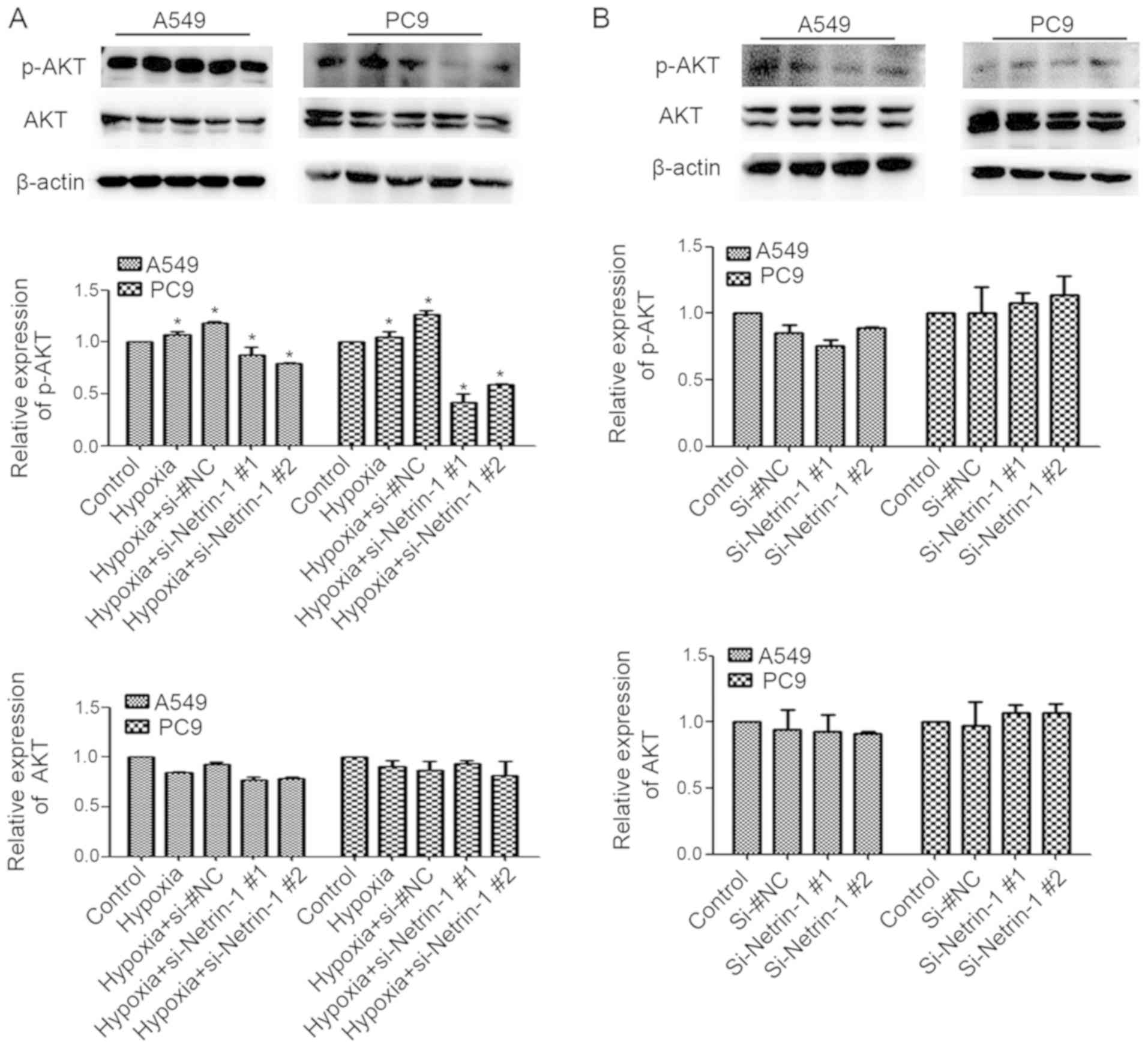
Netrin-1 mediates hypoxia-induced EMT by
activating the PI3K/AKT pathway
To investigate whether the role of netrin-1 in
hypoxia-induced EMT of lung adenocarcinoma cells involves PI3K/AKT
signaling, the expression of p-AKT was measured in A549 and PC9
cells following netrin-1 knockdown. Western blotting confirmed that
knockdown of netrin-1 resulted in reduced hypoxia-induced p-AKT
expression in A549 and PC9 cells (P<0.05; Fig. 7A); however, under normoxic
conditions, silencing of netrin-1 did not affect the accumulation
of p-AKT (P>0.05; Fig. 7B).
Taken together, these data suggest that netrin-1 is involved in
hypoxia-triggered activation of p-AKT.
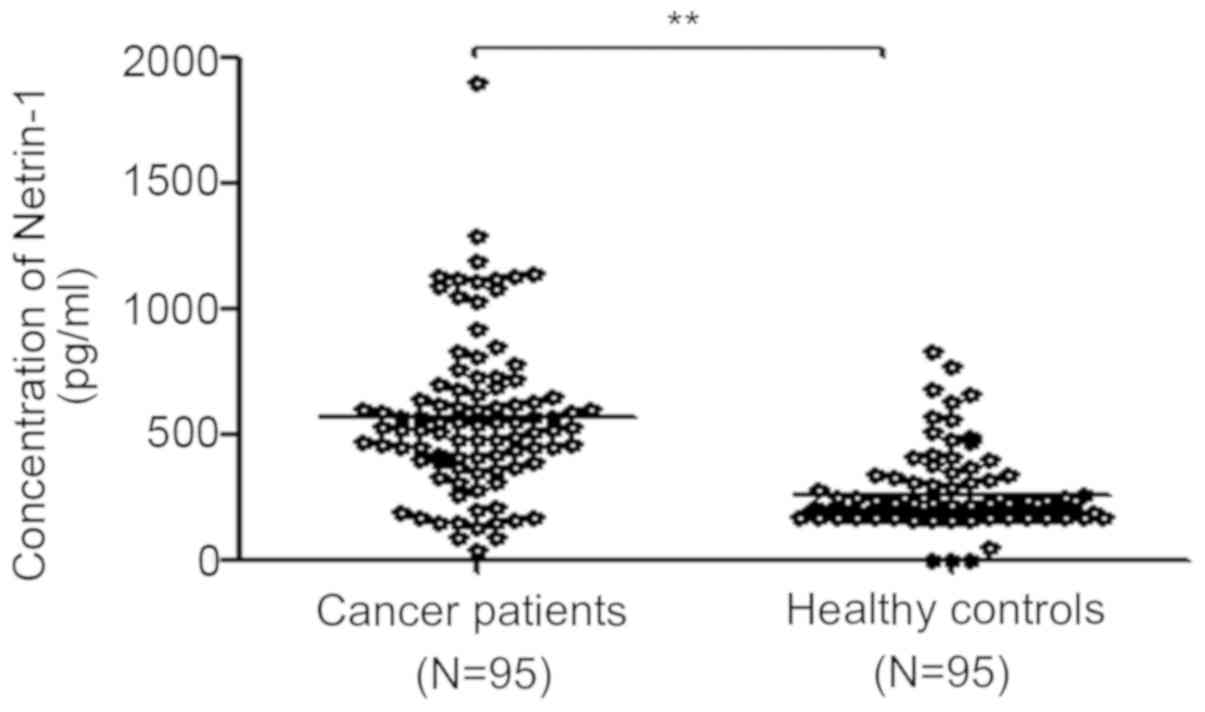
Netrin-1 concentration is elevated in the
serum of NSCLC patients
The concentration of netrin-1 in serum samples from
95 NSCLC patients and 95 control donors was first analyzed by
ELISA. The patients’ clinical data are summarized in Table I. There was no significant
difference in age or sex between cancer patients and healthy
control participants (P>0.05). Moreover, no correlations were
found between the basal netrin-1 level and patient age, sex or
histopathological subtype (P>0.05; Table II), apart from TNM stage
(P<0.05; Table II). The serum
concentration of netrin-1 was higher in NSCLC patients compared
with that in control participants (570.29±314.46 vs. 259.96±152.37
pg/ml, respectively, P<0.05; Fig.
8 and Table II).
 | Table IClinical characteristics of 95
patients with non-small cell lung cancer and 95 healthy
controls. |
Table I
Clinical characteristics of 95
patients with non-small cell lung cancer and 95 healthy
controls.
|
Characteristics | Patients
(n=95) | Controls
(n=95) | P-valuea |
|---|
| Age, years | | | 0.146 |
| ≤60 | 55 | 45 | |
| >60 | 40 | 50 | |
| Sex | | | 0.374 |
| Male | 54 | 60 | |
| Female | 41 | 35 | |
 | Table IICorrelations between serum levels of
netrin-1 and clinicopathological characteristics of patients with
non-small cell lung cancer (n=95). |
Table II
Correlations between serum levels of
netrin-1 and clinicopathological characteristics of patients with
non-small cell lung cancer (n=95).
|
Characteristics | Patients
(n=95) | Netrin-1 (mean ±
SD) | P-valuea |
|---|
| Cancer
patients | 55 | 570.39±314.46 | 0.00 |
| Healthy
subjects | 40 | 258.66±153.74 | |
| Age, years | | | 0.574 |
| ≤60 | 54 | 548.84±261.75 | |
| >60 | 41 | 585.88±349.38 | |
| Sex | | | 0.253 |
| Male | 72 | 545.03±291.08 | |
| Female | 23 | 603.56±343.69 | |
| Stage | | | 0.03 |
| I+II | 69 | 628.98±319.06 | |
| III+IV | 26 | 422.46±251.95 | |
|
Differentiation | | | 0.143 |
| Low | 17 | 671.79±401.71 | |
| Moderate +
high | 78 | 548.16±290.55 | |
| Histological
type | | | 0.686 |
|
Adenocarcinoma | 80 | 575.97±304.50 | |
| Squamous cell
carcinoma | 15 | 539.95±373.58 | |
Discussion
With >1.61 million new cases of lung cancer
diagnosed each year and an estimated 1.59 million deaths annually
worldwide (28), lung cancer has a
high mortality rate that is likely due to early metastasis
(29). However, the mechanisms
underlying lung cancer metastasis remain to be fully elucidated.
Over the past decade, extensive research on lung cancer has focused
on cancer cells, while overlooking the unique but complex tumor
microenvironment (7,30). Interestingly, both experimental and
clinical studies have recently reported that hypoxia within tumors
may contribute to the invasive potential of tumor cells and, thus,
promote metastasis (11). In the
present study, lung cancer cells were treated with CoCl2
to mimic hypoxia and the hypoxic response was then investigated
(6,30). A simple but effective 3D
microfluidic chip based on an in vitro assay system was used
to investigate the role of netrin-1 in the hypoxia-induced EMT of
A549 and PC9 cells. The use of the microfluidic chip overcomes some
of the limitations associated with previous 2D platforms, including
subjectivity in the quantification and the inability to reflect
real-time changes. Furthermore, the 3D chip is used to culture the
cells, thereby mimicking cell growth in vivo, and the
accuracy of the device is increased by enabling multiple
repetitions of the same conditions (27). The results of the present study
demonstrated that CoCl2-induced hypoxia caused changes
in the morphology of A549 and PC9 cells, as well as in the
expression of E-cadherin and vimentin. These findings suggest that
exposure to hypoxia led to EMT in these cells, and that netrin-1
plays a key role in this process and is thus potentially implicated
in the progression of NSCLC.
In recent years, netrin-1 has been shown to regulate
cancer progression in a number of cancer types. For example,
netrin-1 was identified as a novel candidate biomarker for ovarian
carcinoma, and also as a prognostic factor for brain metastases
(31,32). In the present study, the expression
of netrin-1 was found to be increased in response to hypoxia in
A549 and PC9 cells. Moreover, netrin-1 expression was strongly
upregulated in the serum of NSCLC patients compared with control
donors. Interestingly, An et al reported that netrin-1 is
markedly underexpressed and suppresses the growth of cancer cells
in stage I/II pancreatic ductal adenocarcinoma (33). These conflicting activities of
netrin-1 may be attributed to differences in the test methods and
heterogeneities between these tumor types. Moreover, upregulation
of netrin-1 was shown to promote cancer cell migration and invasion
in human hepatocellular carcinoma (34). We observed that knockdown of
netrin-1 using si-RNA significantly reduced the invasiveness and
EMT of lung cancer cells in a simulated hypoxic environment. These
data indicate that netrin-1 was activated in hypoxia-induced EMT.
Furthermore, NP137, the first humanized netrin-1 antibody
generated, was found to have antitumor activity both in solid
tumors and hematological malignancies in a preclinical trial
(35). Those findings indicate a
direct association of netrin-1 activation with hypoxia-induced EMT,
and suggest its potential as a therapeutic target for lung cancer
treatment.
Several classical signaling pathways, including the
Notch, Wnt and PI3K/AKT pathways, are involved in hypoxia-induced
EMT (36-38), but the underlying molecular
mechanisms remain unclear. Netrin-1 was shown to promote cell
migration and tube formation mediated by activation of the
FAK/PI3K/AKT signaling pathway in hypopharyngeal cancer (24). Zhang et al also suggested
that netrin-1 may act as a pro-metastatic factor in NSCLC by
enhancing cell invasion and migration via PI3K/AKT-mediated EMT
(39). The aim of the present
study was to determine whether netrin-1 promotes hypoxia-induced
EMT through activation of the PI3K/AKT pathway. We observed that
p-AKT, a critical protein in the PI3K/AKT pathway, was upregulated
during hypoxia-induced EMT of A549 and PC9 cells (40). Moreover, knockdown of netrin-1 led
to reduced expression of p-AKT under hypoxic conditions and
indirectly inhibited tumor cell migration and invasion.
Furthermore, these effects of netrin-1 silencing were not observed
in A549 and PC9 cells cultured under normoxic conditions. A
limitation of the present study is that the mechanism through which
netrin-1 promotes EMT via the PI3K/AKT pathway has yet to be
verified by upregulating netrin-1 expression in lung cancer
cells.
In conclusion, the results of the present study
provide evidence that netrin-1 promotes hypoxia-induced EMT in lung
cancer cells via the PI3K/AKT pathway in vitro. The analysis
of the patients’ serum samples also revealed that netrin-1 may
serve as a useful clinical biomarker for predicting lung cancer
progression. Therefore, netrin-1 may represent a novel therapeutic
target for preventing lung cancer progression and metastasis.
Funding
This study was supported by a grant from the Natural
Science Foundation of Liaoning Province (no. 20170540242).
Availability of data and materials
All the datasets generated/analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
XYJ designed the study, completed all experiments,
and wrote and revised the manuscript. HQL appraised relevant
studies and assisted with drafting and revising the manuscript. HC
performed data analysis and figure preparation. LNY and JZ
appraised relevant studies. QW participated in conceiving and
designing the study, LHC conceived and designed the study, and
assisted with drafting and revising the manuscript. All the authors
have read and approved the final version of this manuscript for
publication.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Boards of the Second Affiliated Hospital of Dalian Medical
University and all procedures were performed according to the
principles outlined in the Declaration of Helsinki. All
participants provided written informed consent regarding the use of
their sera for research purposes.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests to disclose.
Acknowledgments
The authors would like to thank the staff of the
laboratory for their help in carrying out the present study.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shaikh D, Zhou Q, Chen T, Ibe JC, Raj JU
and Zhou G: cAMP-dependent protein kinase is essential for
hypoxia-mediated epithelial-mesenchymal transition, migration, and
invasion in lung cancer cells. Cell Signal. 24:2396–2406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunther S, Ostheimer C, Stangl S, Specht
HM, Mozes P, Jesinghaus M, Vordermark D, Combs SE, Peltz F, Jung
MP, et al: Correlation of Hsp70 Serum Levels with Gross Tumor
Volume and Composition of Lymphocyte Subpopulations in Patients
with Squamous Cell and Adeno Non-Small Cell Lung Cancer. Front
Immunol. 6:5562015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foss KM, Sima C, Ugolini D, Neri M, Allen
KE and Weiss GJ: miR-1254 and miR-574-5p: serum-based microRNA
biomarkers for early-stage non-small cell lung cancer. J Thorac
Oncol. 6:482–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Li E, Gao Y, Wang Y, Guo Z, He J,
Zhang J, Gao Z and Wang Q: Study on invadopodia formation for lung
carcinoma invasion with a microfluidic 3D culture device. PLoS One.
8:e564482013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao M, Zhang Y, Zhang H, Wang S, Zhang M,
Chen X, Wang H, Zeng G, Chen X, Liu G, et al: Hypoxia-induced cell
stemness leads to drug resistance and poor prognosis in lung
adenocar-cinoma. Lung Cancer. 87:98–106. 2015. View Article : Google Scholar
|
|
7
|
Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia
XF, Sun X, Li GG, Hu QD, Fu QH, et al: Hypoxia-Induced
Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma
Induces an Immunosuppressive Tumor Microenvironment to Promote
Metastasis. Cancer Res. 76:818–830. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Huang G, Li X, Zhang Y, Jiang Y,
Shen J, Liu J, Wang Q, Zhu J, Feng X, et al: Hypoxia induces
epithelial-mesenchymal transition via activation of SNAI1 by
hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC
Cancer. 13:1082013. View Article : Google Scholar
|
|
9
|
Chen S, Chen JZ, Zhang JQ, Chen HX, Yan
ML, Huang L, Tian YF, Chen YL and Wang YD: Hypoxia induces
TWIST-activated epithelial-mesenchymal transition and proliferation
of pancreatic cancer cells in vitro and in nude mice. Cancer Lett.
383:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaupel P and Multhoff G: Accomplices of
the Hypoxic Tumor Microenvironment Compromising Antitumor Immunity:
Adenosine, Lactate, Acidosis, Vascular Endothelial Growth Factor,
Potassium Ions, and Phosphatidylserine. Front Immunol. 8:18872017.
View Article : Google Scholar
|
|
12
|
Nakamura H, Ichikawa T, Nakasone S,
Miyoshi T, Sugano M, Kojima M, Fujii S, Ochiai A, Kuwata T, Aokage
K, et al: Abundant tumor promoting stromal cells in lung
adenocarcinoma with hypoxic regions. Lung Cancer. 115:56–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dominici C, Moreno-Bravo JA, Puiggros SR,
Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P and Chédotal A:
Floor-plate-derived netrin-1 is dispensable for commissural axon
guidance. Nature. 545:350–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu K, Wu Z, Renier N, Antipenko A,
Tzvetkova-Robev D, Xu Y, Minchenko M, Nardi-Dei V, Rajashankar KR,
Himanen J, et al: Neural migration. Structures of netrin-1 bound to
two receptors provide insight into its axon guidance mechanism.
Science. 344:1275–1279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dun XP and Parkinson DB: Role of Netrin-1
Signaling in Nerve Regeneration. Int J Mol Sci. 18:182017.
View Article : Google Scholar
|
|
16
|
Chen J, Du H, Zhang Y, Chen H, Zheng M,
Lin P, Lan Q, Yuan Q, Lai Y, Pan X, et al: Netrin-1 Prevents Rat
Primary Cortical Neurons from Apoptosis via the DCC/ERK Pathway.
Front Cell Neurosci. 11:3872017. View Article : Google Scholar
|
|
17
|
Bai L, Mei X, Wang Y, Yuan Y, Bi Y, Li G,
Wang H, Yan P and Lv G: The Role of Netrin-1 in Improving
Functional Recovery through Autophagy Stimulation Following Spinal
Cord Injury in Rats. Front Cell Neurosci. 11:3502017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ko SY, Blatch GL and Dass CR: Netrin-1 as
a potential target for metastatic cancer: Focus on colorectal
cancer. Cancer Metastasis Rev. 33:101–113. 2014. View Article : Google Scholar
|
|
19
|
Lv J, Sun X, Ma J, Ma X, Zhang Y, Li F, Li
Y and Zhao Z: Netrin-1 induces the migration of Schwann cells via
p38 MAPK and PI3K-Akt signaling pathway mediated by the UNC5B
receptor. Biochem Biophys Res Commun. 464:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akino T, Han X, Nakayama H, McNeish B,
Zurakowski D, Mammoto A, Klagsbrun M and Smith E: Netrin-1 promotes
medulloblastoma cell invasiveness and angiogenesis, and
demonstrates elevated expression in tumor tissue and urine of
patients with pediatric medulloblastoma. Cancer Res. 74:3716–3726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grandin M, Mathot P, Devailly G, Bidet Y,
Ghantous A, Favrot C, Gibert B, Gadot N, Puisieux I, Herceg Z,
Delcros JG, Bernet A and Mehlen P: Inhibition of DNA methylation
promotes breast tumor sensitivity to netrin-1 interference.
8:863–877. 2016.
|
|
22
|
Wang H, Zhang B, Gu M, Li S, Chi Z and Hao
L: Overexpression of the dependence receptor UNC5H4 inhibits cell
migration and invasion, and triggers apoptosis in neuroblastoma
cell. Tumour Biol. 35:5417–5425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibert B and Mehlen P: Dependence
Receptors and Cancer: Addiction to Trophic Ligands. Cancer Res.
75:5171–5175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Wang B, Chen X, Li W and Dong P:
AGO2 involves the malignant phenotypes and FAK/PI3K/AKT signaling
pathway in hypopharyngeal-derived FaDu cells. Oncotarget.
8:54735–54746. 2017.PubMed/NCBI
|
|
25
|
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR,
Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al: Galectin-1
induces hepato-cellular carcinoma EMT and sorafenib resistance by
activating FAK/PI3K/AKT signaling. Cell Death Dis. 7:e22012016.
View Article : Google Scholar
|
|
26
|
Yang X, Li S, Zhong J, Zhang W, Hua X, Li
B and Sun H: CD151 mediates netrin-1-induced angiogenesis through
the Src-FAK-Paxillin pathway. J Cell Mol Med. 21:72–80. 2017.
View Article : Google Scholar
|
|
27
|
Song J, Zhang Y, Zhang C, Du X, Guo Z,
Kuang Y, Wang Y, Wu P, Zou K, Zou L, et al: A microfluidic device
for studying chemotaxis mechanism of bacterial cancer targeting.
Sci Rep. 8:63942018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Torre LA, Siegel RL and Jemal A: Lung
Cancer Statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar
|
|
29
|
Quéré G, Descourt R, Robinet G, Autret S,
Raguenes O, Fercot B, Alemany P, Uguen A, Férec C, Quintin-Roué I,
et al: Mutational status of synchronous and metachronous tumor
samples in patients with metastatic non-small-cell lung cancer. BMC
Cancer. 16:2102016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chu CY, Jin YT, Zhang W, Yu J, Yang HP,
Wang HY, Zhang ZJ, Liu XP and Zou Q: CA IX is upregulated in
CoCl2-induced hypoxia and associated with cell invasive potential
and a poor prognosis of breast cancer. Int J Oncol. 48:271–280.
2016. View Article : Google Scholar
|
|
31
|
Papanastasiou AD, Pampalakis G, Katsaros D
and Sotiropoulou G: Netrin-1 overexpression is predictive of
ovarian malignancies. Oncotarget. 2:363–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harter PN, Zinke J, Scholz A, Tichy J,
Zachskorn C, Kvasnicka HM, Goeppert B, Delloye-Bourgeois C,
Hattingen E, Senft C, et al: Netrin-1 expression is an independent
prognostic factor for poor patient survival in brain metastases.
PLoS One. 9:e923112014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
An XZ, Zhao ZG, Luo YX, Zhang R, Tang XQ,
Hao D, Zhao X, Lv X and Liu D: Netrin-1 suppresses the MEK/ERK
pathway and ITGB4 in pancreatic cancer. Oncotarget. 7:24719–24733.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han P, Fu Y, Liu J, Wang Y, He J, Gong J,
Li M, Tan Q, Li D, Luo Y, et al: Netrin-1 promotes cell migration
and invasion by down-regulation of BVES expression in human
hepatocellular carcinoma. Am J Cancer Res. 5:1396–1409.
2015.PubMed/NCBI
|
|
35
|
Ducarouge B, Delcros JG, Abès R,
Goldschneider D, Gibert B, Blachier J, Neves D, Mehlen P, Bernet A
and Depil S: Abstract 2921: Preclinical characteristics of NP137, a
first-in-class monoclonal antibody directed against netrin-1 and
inducing dependence receptors-mediated cell death. Cancer Res.
75(Suppl): 2921. 2015. View Article : Google Scholar
|
|
36
|
Fujiki K, Inamura H, Miyayama T and
Matsuoka M: Involvement of Notch1 signaling in malignant
progression of A549 cells subjected to prolonged cadmium exposure.
J Biol Chem. 292:7942–7953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hong CF, Chen WY and Wu CW: Upregulation
of Wnt signaling under hypoxia promotes lung cancer progression.
Oncol Rep. 38:1706–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Z, Wang S, Song C and Hu Z:
Paeoniflorin prevents hypoxia-induced epithelial-mesenchymal
transition in human breast cancer cells. OncoTargets Ther.
9:2511–2518. 2016. View Article : Google Scholar
|
|
39
|
Zhang X, Cui P, Ding B, Guo Y, Han K, Li
J, Chen H and Zhang W: Netrin-1 elicits metastatic potential of
non-small cell lung carcinoma cell by enhancing cell invasion,
migration and vasculogenic mimicry via EMT induction. Cancer Gene
Ther. 25:18–26. 2018. View Article : Google Scholar
|
|
40
|
Wang H, Zhang C, Xu L, Zang K, Ning Z,
Jiang F, Chi H, Zhu X and Meng Z: Bufalin suppresses hepatocellular
carcinoma invasion and metastasis by targeting HIF-1α via the
PI3K/AKT/mTOR pathway. Oncotarget. 7:20193–20208. 2016.PubMed/NCBI
|