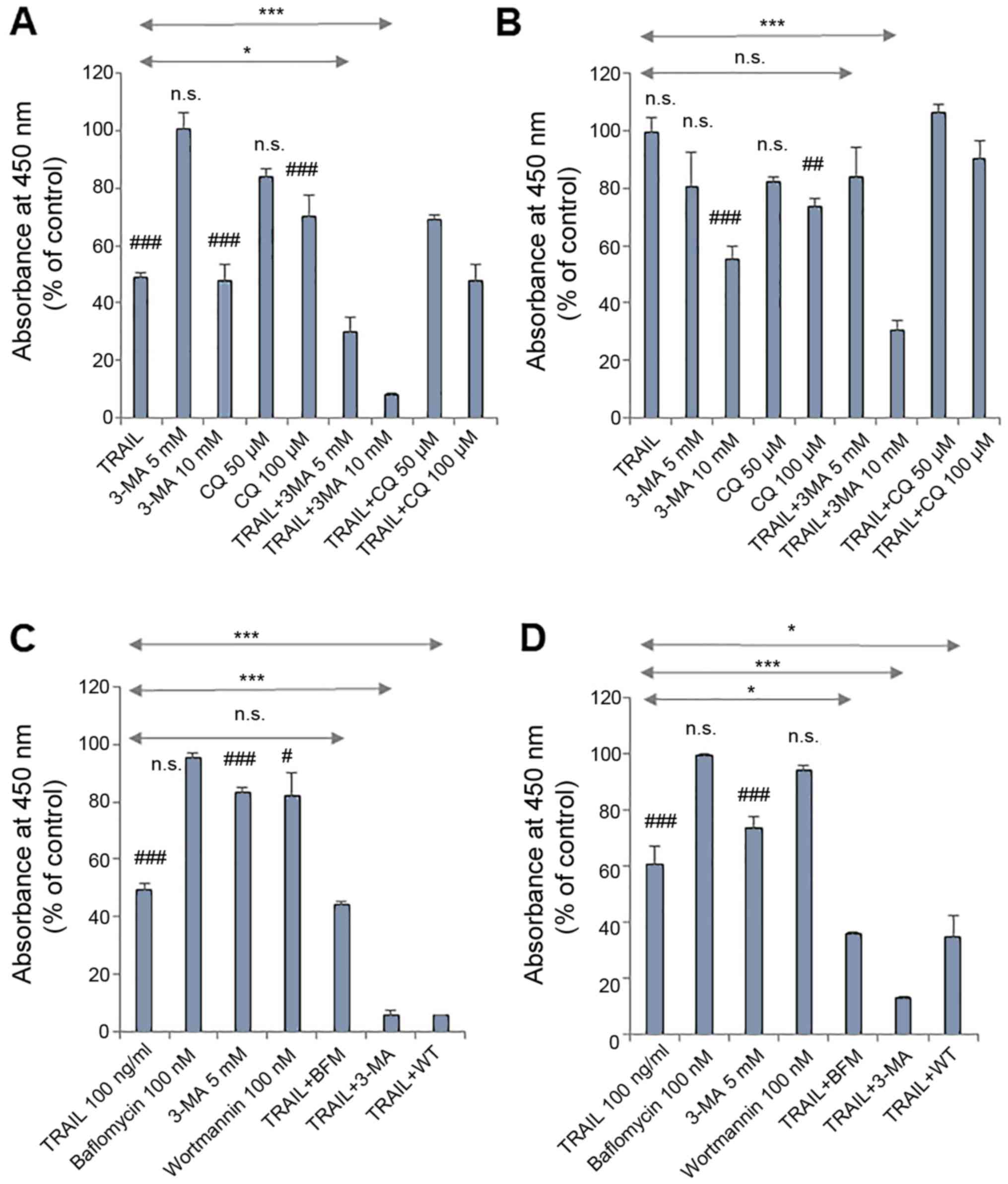
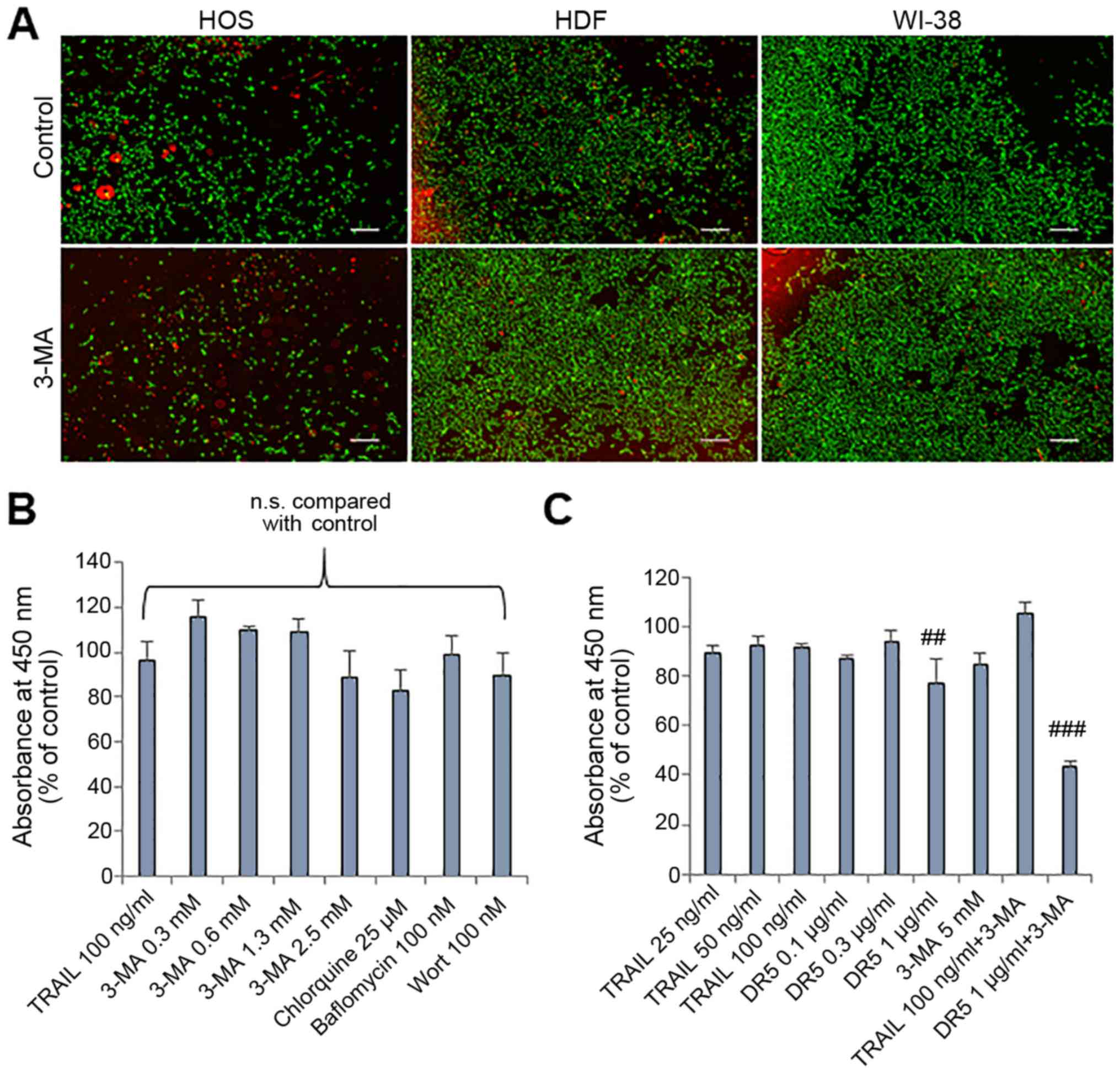
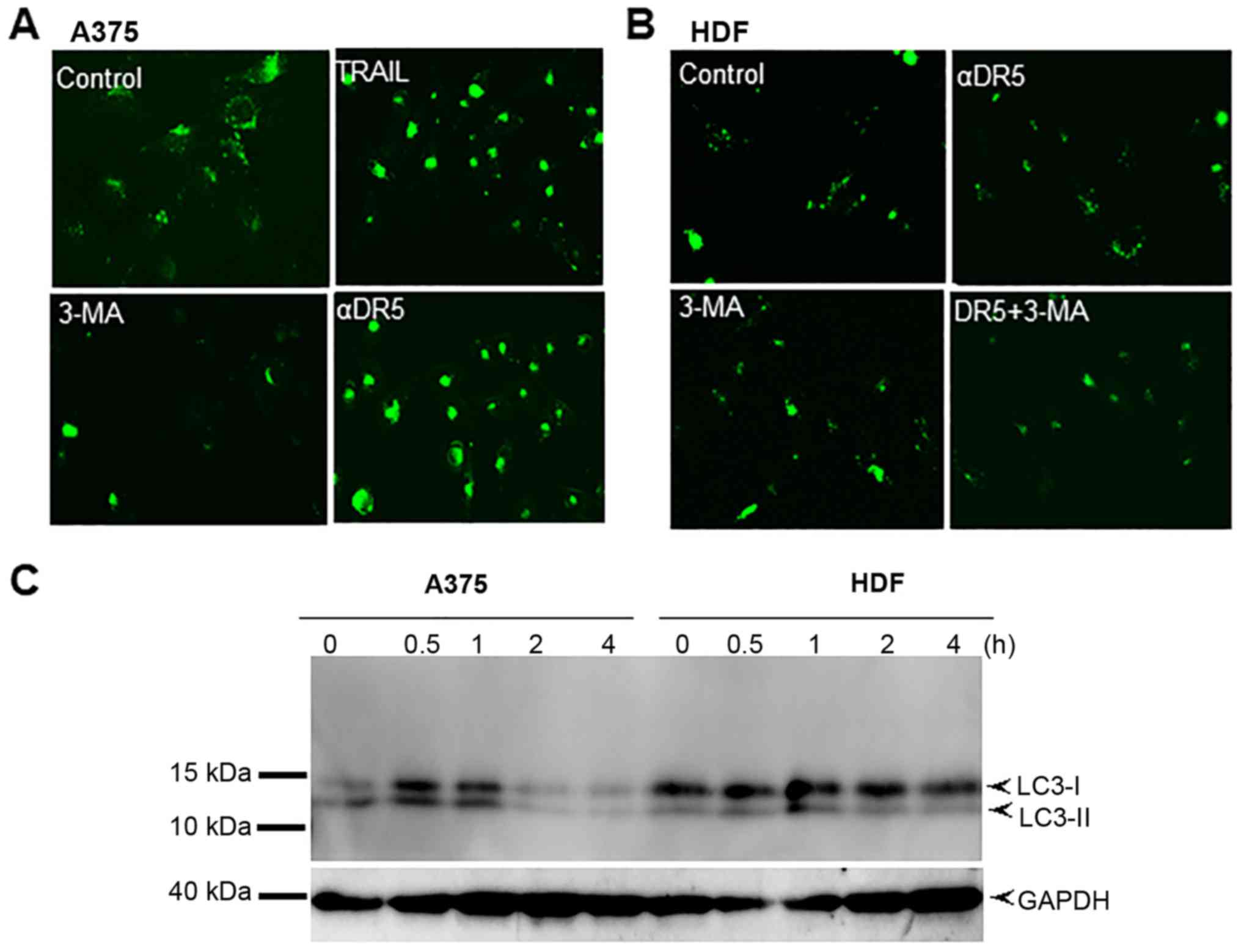
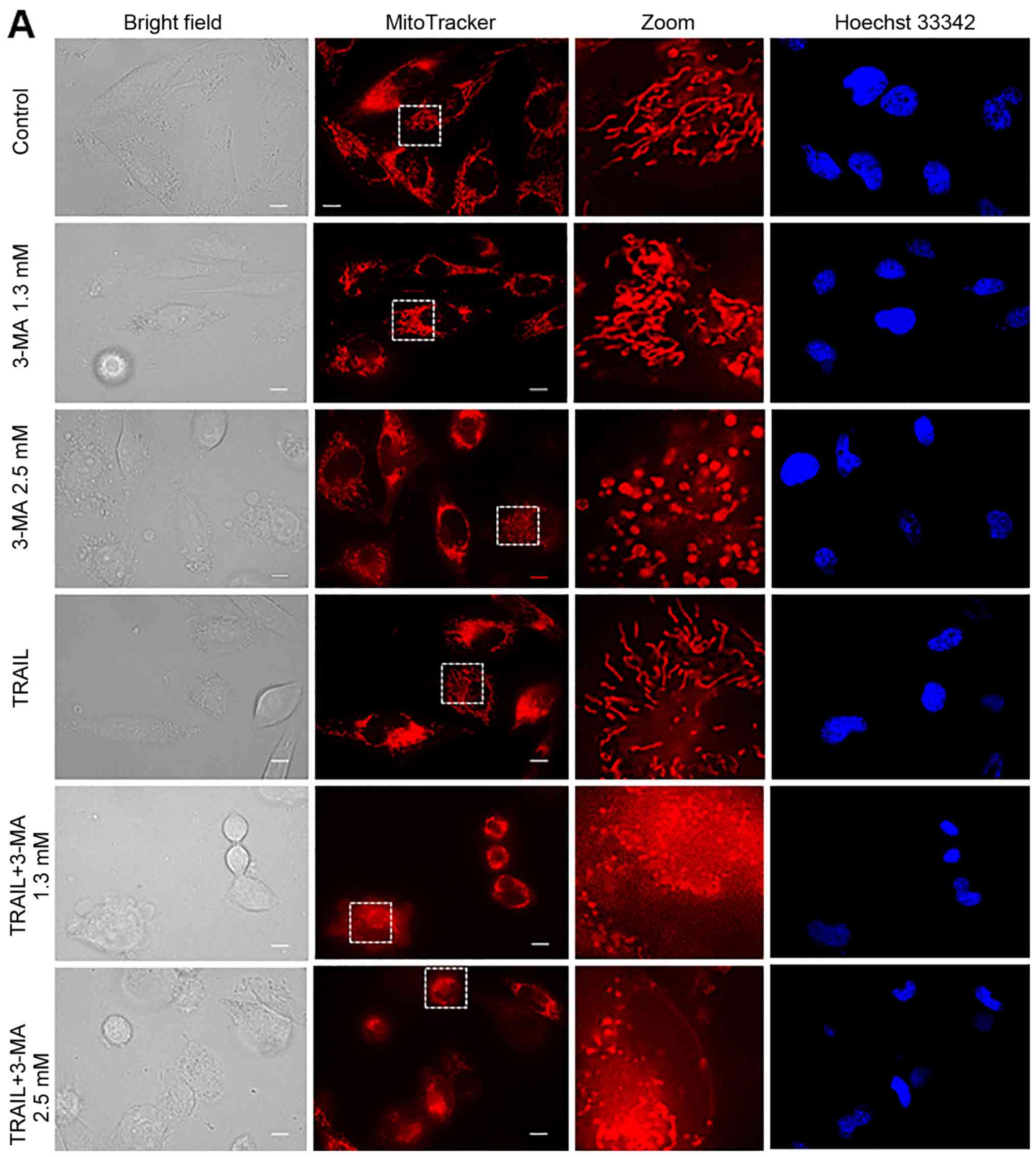
|
1
|
Almasan A and Ashkenazi A: Apo2L/TRAIL:
Apoptosis signaling, biology, and potential for cancer therapy.
Cytokine Growth Factor Rev. 14:337–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang S: The promise of cancer therapeutics
targeting the TNF-related apoptosis-inducing ligand and TRAIL
receptor pathway. Oncogene. 27:6207–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonzalvez F and Ashkenazi A: New insights
into apoptosis signaling by Apo2L/TRAIL. Oncogene. 29:4752–4765.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kischkel FC, Lawrence DA, Chuntharapai A,
Schow P, Kim KJ and Ashkenazi A: Apo2L/TRAIL-dependent recruitment
of endogenous FADD and caspase-8 to death receptors 4 and 5.
Immunity. 12:611–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ivanov VN, Bhoumik A and Ronai Z: Death
receptors and melanoma resistance to apoptosis. Oncogene.
22:3152–3161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dyer MJ, MacFarlane M and Cohen GM:
Barriers to effective TRAIL-targeted therapy of malignancy. J Clin
Oncol. 25:4505–4506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dimberg LY, Anderson CK, Camidge R,
Behbakht K, Thorburn A and Ford HL: On the TRAIL to successful
cancer therapy? Predicting and counteracting resistance against
TRAIL-based therapeutics. Oncogene. 32:1341–1350. 2013. View Article : Google Scholar
|
|
10
|
Guiho R, Biteau K, Heymann D and Redini F:
TRAIL-based therapy in pediatric bone tumors: How to overcome
resistance. Future Oncol. 11:535–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Miguel D, Lemke J, Anel A, Walczak H
and Martinez-Lostao L: Onto better TRAILs for cancer treatment.
Cell Death Differ. 23:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Codogno P and Meijer AJ: Autophagy and
signaling: Their role in cell survival and cell death. Cell Death
Differ. 12(Suppl 2): 1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Díaz-Troya S, Pérez-Pérez ME, Florencio FJ
and Crespo JL: The role of TOR in autophagy regulation from yeast
to plants and mammals. Autophagy. 4:851–865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dennis MD, Baum JI, Kimball SR and
Jefferson LS: Mechanisms involved in the coordinate regulation of
mTORC1 by insulin and amino acids. J Biol Chem. 286:8287–8296.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhutia SK, Mukhopadhyay S, Sinha N, Das
DN, Panda PK, Patra SK, Maiti TK, Mandal M, Dent P, Wang XY, et al:
Autophagy: Cancer's friend or foe? Adv Cancer Res. 118:61–95. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fulda S and Kögel D: Cell death by
autophagy: Emerging molecular mechanisms and implications for
cancer therapy. Oncogene. 34:5105–5113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fulda S: Autophagy in cancer therapy.
Front Oncol. 7:1282017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knoll G, Bittner S, Kurz M, Jantsch J and
Ehrenschwender M: Hypoxia regulates TRAIL sensitivity of colorectal
cancer cells through mitochondrial autophagy. Oncotarget.
7:41488–41504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He W, Wang Q, Xu J, Xu X, Padilla MT, Ren
G, Gou X and Lin Y: Attenuation of TNFSF10/TRAIL-induced apoptosis
by an autophagic survival pathway involving TRAF2- and
RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy. 8:1811–1821.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim SC, Jeon HJ, Kee KH, Lee MJ, Hong R
and Han SI: Involvement of DR4/JNK pathway-mediated autophagy in
acquired TRAIL resistance in HepG2 cells. Int J Oncol.
49:1983–1990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Knizhnik AV, Roos WP, Nikolova T, Quiros
S, Tomaszowski KH, Christmann M and Kaina B: Survival and death
strategies in glioma cells: Autophagy, senescence and apoptosis
triggered by a single type of temozolomide-induced DNA damage. PLoS
One. 8:e556652013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo W, Wang Y, Wang Z, Wang YP and Zheng
H: Inhibiting autophagy increases epirubicin's cytotoxicity in
breast cancer cells. Cancer Sci. 107:1610–1621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prieto-Domínguez N, Ordóñez R, Fernández
A, García-Palomo A, Muntané J, González-Gallego J and Mauriz JL:
Modulation of Autophagy by Sorafenib: Effects on Treatment
Response. Front Pharmacol. 7:1512016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito T, Ando T, Suzuki-Karasaki M, Tokunaga
T, Yoshida Y, Ochiai T, Tokuhashi Y and Suzuki-Karasaki Y: Cold
PSM, but not TRAIL, triggers autophagic cell death: A therapeutic
advantage of PSM over TRAIL. Int J Oncol. 53:503–514.
2018.PubMed/NCBI
|
|
30
|
Landes T and Martinou JC: Mitochondrial
outer membrane permeabilization during apoptosis: The role of
mitochondrial fission. Biochim Biophys Acta. 1813:540–545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Elgass K, Pakay J, Ryan MT and Palmer CS:
Recent advances into the understanding of mitochondrial fission.
Biochim Biophys Acta. 1833:150–161. 2013. View Article : Google Scholar
|
|
32
|
Twig G and Shirihai OS: The interplay
between mitochondrial dynamics and mitophagy. Antioxid Redox
Signal. 14:1939–1951. 2011. View Article : Google Scholar :
|
|
33
|
Chen H, Chomyn A and Chan DC: Disruption
of fusion results in mitochondrial heterogeneity and dysfunction. J
Biol Chem. 280:26185–26192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoppins S, Lackner L and Nunnari J: The
machines that divide and fuse mitochondria. Annu Rev Biochem.
76:751–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pendin D, Filadi R and Pizzo P: The
concerted action of mitochondrial dynamics and positioning: New
characters in cancer onset and progression. Front Oncol. 7:1022017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kingnate C, Charoenkwan K, Kumfu S,
Chattipakorn N and Chattipakorn SC: Possible roles of mitochondrial
dynamics and the effects of pharmacological interventions in
chemoresistant ovarian cancer. EBioMedicine. 34:256–266. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akita M, Suzuki-Karasaki M, Fujiwara K,
Nakagawa C, Soma M, Yoshida Y, Ochiai T, Tokuhashi Y and
Suzuki-Karasaki Y: Mitochondrial division inhibitor-1 induces
mitochondrial hyper-fusion and sensitizes human cancer cells to
TRAIL-induced apoptosis. Int J Oncol. 45:1901–1912. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suzuki-Karasaki Y, Fujiwara K, Saito K,
Suzuki-Karasaki M, Ochiai T and Soma M: Distinct effects of TRAIL
on the mitochondrial network in human cancer cells and normal
cells: Role of plasma membrane depolarization. Oncotarget.
6:21572–21588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takata N, Ohshima Y, Suzuki-Karasaki M,
Yoshida Y, Tokuhashi Y and Suzuki-Karasaki Y: Mitochondrial
Ca2+ removal amplifies TRAIL cytotoxicity toward
apoptosis-resistant tumor cells via promotion of multiple cell
death modalities. Int J Oncol. 51:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ohshima Y, Takata N, Suzuki-Karasaki M,
Yoshida Y, Tokuhashi Y and Suzuki-Karasaki Y: Disrupting
mitochondrial Ca2+ homeostasis causes tumor-selective
TRAIL sensitization through mitochondrial network abnormalities.
Int J Oncol. 51:1146–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suzuki Y, Inoue T, Murai M,
Suzuki-Karasaki M, Ochiai T and Ra C: Depolarization potentiates
TRAIL-induced apoptosis in human melanoma cells: Role for
ATP-sensitive K+ channels and endoplasmic reticulum
stress. Int J Oncol. 41:465–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lockshin RA and Zakeri Z: Apoptosis,
autophagy, and more. Int J Biochem Cell Biol. 36:2405–2419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mukhopadhyay S, Panda PK, Sinha N, Das DN
and Bhutia SK: Autophagy and apoptosis: Where do they meet?
Apoptosis. 19:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kulikov AV, Luchkina EA, Gogvadze V and
Zhivotovsky B: Mitophagy: Link to cancer development and therapy.
Biochem Biophys Res Commun. 482:432–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Narendra DP, Jin SM, Tanaka A, Suen DF,
Gautier CA, Shen J, Cookson MR and Youle RJ: PINK1 is selectively
stabilized on impaired mitochondria to activate Parkin. PLoS Biol.
8:e10002982010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jangamreddy JR, Ghavami S, Grabarek J,
Kratz G, Wiechec E, Fredriksson BA, Rao Pariti RK, Cieślar-Pobuda
A, Panigrahi S and Łos MJ: Salinomycin induces activation of
autophagy, mitophagy and affects mitochondrial polarity:
Differences between primary and cancer cells. Biochim Biophys Acta.
1833:2057–2069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Marchi S and Pinton P: Alterations of
calcium homeostasis in cancer cells. Curr Opin Pharmacol. 29:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Monteith GR, Prevarskaya N and
Roberts-Thomson SJ: The calcium-cancer signalling nexus. Nat Rev
Cancer. 17:367–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jardin I and Rosado JA: STIM and calcium
channel complexes in cancer. Biochim Biophys Acta. 1863:1418–1426.
2016. View Article : Google Scholar
|
|
51
|
Izzo V, Bravo-San Pedro JM, Sica V,
Kroemer G and Galluzzi L: Mitochondrial permeability transition:
New findings and persisting uncertainties. Trends Cell Biol.
26:655–667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Galluzzi L, Bravo-San Pedro JM, Kepp O and
Kroemer G: Regulated cell death and adaptive stress responses. Cell
Mol Life Sci. 73:2405–2410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Calcium and mitochondria in the regulation of cell death. Biochem
Biophys Res Commun. 460:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Danese A, Patergnani S, Bonora M,
Wieckowski MR, Previati M, Giorgi C and Pinton P: Calcium regulates
cell death in cancer: Roles of the mitochondria and
mitochondria-associated membranes (MAMs). Biochim Biophys Acta
Bioenerg. 1858:615–627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Elustondo PA, Nichols M, Robertson GS and
Pavlov EV: Mitochondrial Ca2+ uptake pathways. J
Bioenerg Biomembr. 49:113–119. 2017. View Article : Google Scholar
|
|
56
|
Deak AT, Blass S, Khan MJ, Groschner LN,
Waldeck-Weiermair M, Hallström S, Graier WF and Malli R:
IP3-mediated STIM1 oligomerization requires intact mitochondrial
Ca2+ uptake. J Cell Sci. 127:2944–2955. 2014. View Article : Google Scholar : PubMed/NCBI
|