Introduction
Osteosarcoma has a double-peak age distribution
occurring in children and young adults <20 years of age (75%)
and those >50-60 years of age (25%), known as secondary
osteosarcoma (1). Osteosarcoma is
the most common pediatric malignant bone tumor, accounting for ~5%
of all pediatric tumors (2,3). The
possible risk factors for secondary osteosarcoma are radiation,
Paget's disease and Li-Fraumeni syndrome (4). Early pulmonary metastasis leads to
the low survival rate of osteosarcoma (5). At present, surgery combined with
chemotherapy is the most common treatment method for osteosarcoma.
In 1979, Rosen et al proposed the concept of neoadjuvant
chemotherapy, and after more than 40 years of development and
improvement, this has become the preferred treatment plan for
osteosarcoma (6). However, current
chemotherapeutic drugs not only have strong cytotoxic effects, but
also have toxic side effects and can induce therapeutic resistance
(7). Therefore, novel treatment
strategies are required.
Rhodiola rosea L., a perennial herbaceous
plant, is the most type of common Chinese medicine and is widely
used in the medical field (8).
Among all of the effective components extracted from Rhodiola
rosea L., salidroside exhibits powerful properties and has
received notable attention. Recent studies have reported that
salidroside has anti-fatigue, anti-aging, anti-oxidant,
anti-inflammatory, neuroprotective and cardiovascular protective
effects (9-12). A literature review revealed that
salidroside exhibits antitumor effects in various tumors, including
fibrosarcoma (13), bladder
carcinoma (14), lung carcinoma
(15), breast carcinoma (16) and renal cell carcinoma (17) in vitro; however, the
association between salidroside and osteosarcoma requires further
investigation. In the present study, we investigated the potential
antitumor effects of salidroside in vitro and the underlying
molecular mechanism.
Materials and methods
Cell culture and treatment
Human osteosarcoma cell lines MG63 and U2OS
(ZQXZBIO, Shanghai, China), were selected to assess the antitumor
effects of salidroside. Cells were cultured in Dulbecco's modified
Eagle's medium combined with high-glucose medium (DMEM-HG)
containing 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and were maintained in a 37°C humidified
incubator with 5% CO2. Cells were harvested with a 0.25%
trypsin-0.02% EDTA solution and passaged when the cells attained
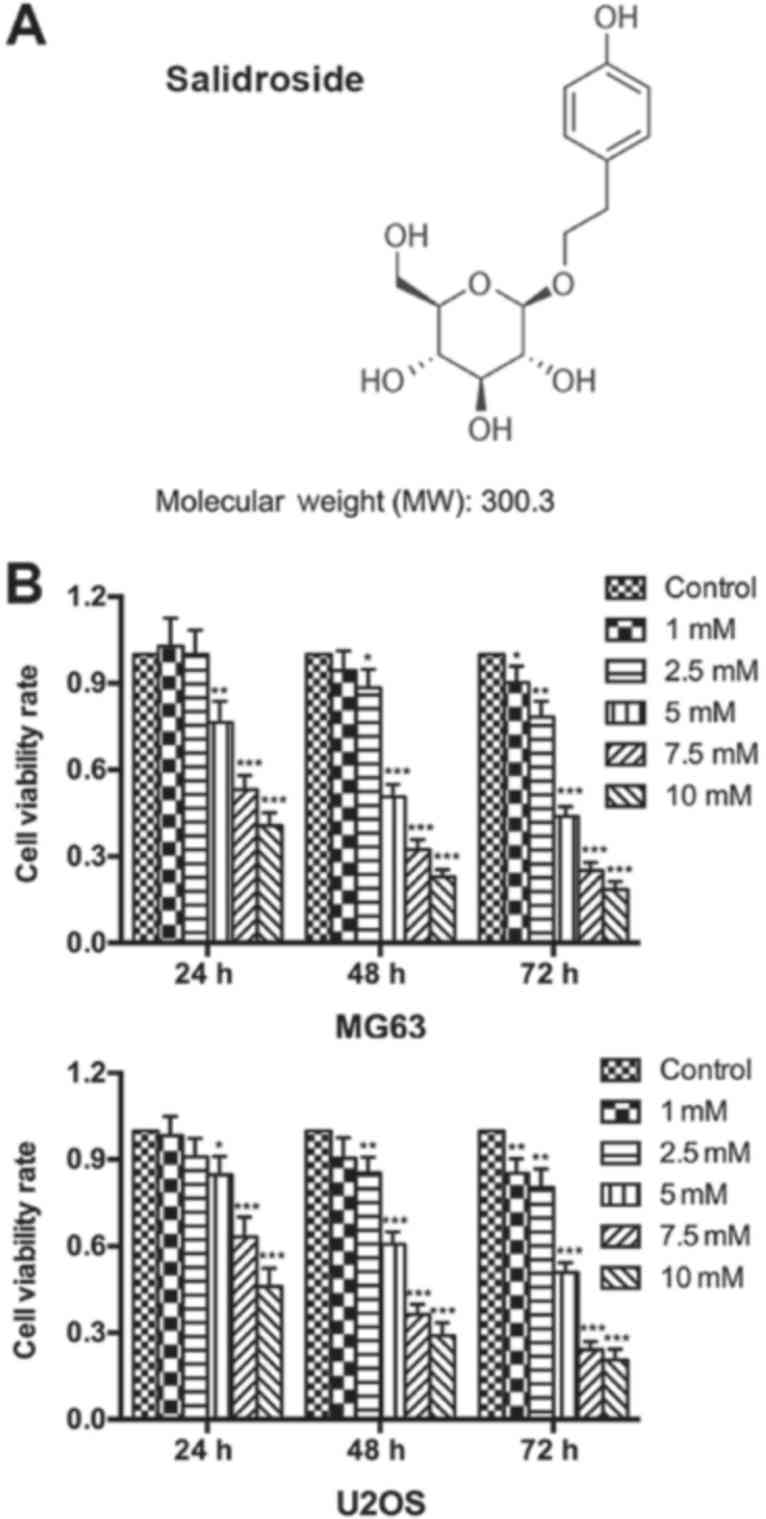
~80% confluence. Salidroside (Fig.
1A; purity >99%, MedChem Express, Monmouth Junction, NJ,
USA) was dissolved in PBS at room temperature and filtered through
a 0.22-μm filter (Merck KGaA, Darmstadt, Germany) prior to
use. To determine the potential role of salidroside in osteosarcoma
cells, the cells were pretreated with different concentrations (0,
1, 2.5, 5, 7.5 and 10 mM) of salidroside at 37°C for 24, 48 and 72
h, respectively. Cells cultured without pretreatment were used as a
control. Z-LEHD-FMK (50 μM at 37°C for 2 h, Selleck
Chemicals, Houston, TX, USA), a caspase-9 specific inhibitor, was
used to explore whether salidroside induced osteosarcoma cell
apoptosis via the caspase-9-dependent apoptotic pathway.
Furthermore, FLLL32 (5 μM at 37°C for 2 h), a specific
inhibitor of JAK2/STAT3 phosphorylation, was used to further
confirm whether salidroside induced osteosarcoma cell apoptosis via
the JAK2/STAT3 signaling pathway.
Cell viability assay
Cell viability was assessed using an MTT assay
(18). Briefly, the two cell lines
were seeded in 96-well plates (6×103 cells/well) for 24
h and when they reached ~80% confluence, they were treated with
different concentrations of salidroside (0, 1, 2.5, 5, 7.5 and 10
mM) at 37°C for 24, 48, and 72 h, respectively. After treatment,
cells were cultured in DMEM-HG medium containing MTT solution (10
μl/well, 5 mg/ml in PBS) in a 37°C humidified incubator for
4 h. Then, dimethyl sulfoxide (150 μl/well) was added to
dissolve the formazan crystals. The absorbance value was measured
at a wavelength of 490 nm using a SpectraMax Plus 384 microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA). Cell viability
rate = (salidroside treatment group absorbance value - blank
control group absorbance value) / (no salidroside treatment group
absorbance value - blank control group absorbance value).
Morphology of apoptotic cells
An inverted phase contrast fluorescence microscope
(Carl Zeiss, Heidenheimer, Germany) was used to directly observe
morphological changes in the two cell lines treated with
salidroside. Cells were separately seeded into 6-well
(12×104 cells/well) and 24-well (3×104
cells/well) plates, cultured to confluence, and treated with (0, 1,
5 and 10 mM) salidroside at 37°C for 48 h. Firstly, we directly
observed the morphology of apoptotic cells seeded in the 6-well
plate (magnification, ×100). Cells were counted from five random
fields for each group, and the average was expressed as the number
of apoptotic cells. Then, we observed the nuclear morphology of
apoptotic cells seeded in the 24-well plate using Hoechst 33258
staining. In brief, cells were washed three times with PBS, fixed
in 4% paraformaldehyde at 4°C for 20 min, stained with Hoechst
33258 staining solution (125 μl/well, Beyotime Institute of
Biotechnology, Haimen, China) for 5 min, rewashed three times with
PBS, covered with anti-fading solution, and then observed using the
aforementioned fluorescence microscope (magnification, ×400). Cells
were counted from five random fields for each group and the number
of apoptotic cells (Hoechst-positive cells) was expressed as a
percentage (%) of the total number of counted cells.
Flow cytometric analysis of cell
apoptosis
To further verify the effect of salidroside on the
apoptosis of osteosarcoma cells, an Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) was used.
Briefly, following treatment with (0, 1, 5 and 10 mM) salidroside
for 48 h, MG63 cells (1×105 cells/sample) were digested
with trypsin (0.25%, room temperature, 1-3 min), centrifuged (300 ×
g, 4°C, 5 min), washed twice with PBS, and resuspended in binding
buffer (500 μl/sample, Nanjing KeyGen Biotech Co., Ltd.).
Each sample was stained with Annexin V-FITC (5 μl) and
propidium iodide (PI; 5 μl) in the dark at 4°C for 15 min
and then immediately analyzed with a flow cytometer equipped with
FACSComp software (BD Biosciences, Franklin Lakes, NJ, USA).
Annexin V-FITC-/PI- cells were identified as
viable cells, Annexin V-FITC+/PI- cells were
identified as early apoptotic cells, and Annexin
V-FITC+/PI+ cells were identified as the sum
of late apoptotic cells and necrotic cells.
Flow cytometric analysis of the cell
cycle
A cell cycle detection kit (Nanjing KeyGen Biotech
Co., Ltd.) was used to evaluate the cell cycle distribution of
cells. In brief, after treatment with (0, 1, 5, 10 mM) salidroside
for 24 h, MG63 cells (1×105 cells/sample) were digested
with trypsin (without EDTA, 0.25%, room temperature, 1-3 min),
centrifuged (300 × g, 4°C, 5 min), washed three times with PBS, and
fixed in 70% ethanol at 4°C overnight. Then, each sample was
centrifuged (300 × g, 4°C, 5 min), washed three times with PBS,
incubated with RNase A (100 μl) at 37°C for 30 min, stained
with PI (400 μl) in the dark at 4°C for 30 min, and then
immediately analyzed with a flow cytometer equipped with CellQuest
software (BD Biosciences).
Cell invasion assay
Cell invasion was analyzed using a Transwell
invasion assay. Matrigel (20 μl/chamber, BD Biosciences) was
evenly applied to the upper surface of the chamber (8.0 μm,
cat. no. 3422; Costar; Corning, Inc., Corning, NY, USA) and
incubated in a 37°C humidified incubator for 30 min. The two cell
lines (1×105 cells/chamber) were suspended in
preprocessed DMEM-HG medium (200 μl/chamber, serum-free
medium alone, or supplemented with 0, 1, 5 or 10 mM salidroside)
and then seeded into the upper surface of the chamber. DMEM-HG
medium (500 μl/well, containing 10% FBS) was added to the
24-well plate. Following incubation in a 37°C humidified incubator
for 24 h, the upper-chamber cells were wiped out with a cotton
swab, and the lower-chamber cells were washed twice with PBS, fixed
in 4% paraformaldehyde at 4°C for 20 min, stained with 0.1% crystal
violet (Beyotime Institute of Biotechnology) at room temperature
for 5 min, rewashed twice with PBS, then observed under an inverted
phase contrast microscope (magnification, ×400, Zeiss AG,
Oberkochen, Germany). Cells were counted in five random fields for
each group, and the number of invasive cells (crystal
violet-positive cells) was expressed as a percentage (%) of the
total number of counted cells.
Western blot analysis
After treatment with (0, 1, 5 and 10 mM) salidroside
for 48 h, MG63 cells were digested with trypsin and lysed by
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The lysates were centrifuged at 12,000 × g at 4°C
for 30 min, then the supernatants were collected and quantified by
a bincinchoninic acid assay (CW Biotechnology, Beijing, China).
Total protein (30 μg) was separated using 10 or 12% SDS-PAGE
with a 5% stacking gel, and then transferred to polyvinylidene
difluoride membranes (0.22 μm; Merck KGaA). The membranes
were subsequently blocked in tris-buffered saline with 10% Tween-20
(TBS-T) containing 5% nonfat milk at room temperature for 2 h,
incubated with primary antibodies: rabbit anti-B-cell lymphoma 2
(Bcl-2; cat. no. 4223; 1:1,000), Bcl-2-associated X protein (Bax;
cat. no. 5023; 1:1,000), caspase-3 (cat. no. 9665; 1:1,000),
caspase-7 (cat. no. 9491; 1:1,000), caspase-9 (cat. no. 9502;
1:1,000), cyclin D1 (cat. no. 2978; 1:1,000), p21 (cat. no. 2947;
1:1,000), matrix metalloproteinase 2 (MMP-2; cat. no.40994;
1:1,000), MMP-9 (cat. no. 13667; 1:1,000), signal transducer and
activator of transcription 3 (STAT3; cat. no. 12640; 1:1,000),
phosphorylated (p)-STAT3 (cat. no. 9145; 1:2,000), JAK2 (cat. no.
3230; 1:1,000) and p-Janus kinase 2 (JAK2; cat. no. 3776; 1:1,000),
all from Cell Signaling Technology, Inc., Danvers, MA, USA; mouse
anti-β-actin (cat. no. CW0264; 1:5,000) and rabbit anti-GAPDH
(CW0101, 1:5,000) were obtained from CWBIO (Beijing, China) in
TBS-T containing 5% bovine serum albumin at 4°C overnight and
washed three times with TBS-T. Then, the membranes were incubated
with secondary antibodies (horseradish peroxidase-conjugated goat,
anti-mouse (cat. no. CW0102; 1:10,000) or rabbit (cat. no. CW0156;
1:10000; CWBIO) in TBS-T for 2 h. An enhanced chemiluminescence
western blot detection kit (Thermo Fisher Scientific, Inc.),
ChemiDoc™ XRS+ System and Image Lab™ 2.1 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) were used to observe the
expression of all proteins.
Statistical analysis
Significant differences between groups of data were
analyzed by one-way analysis of variance and a Student-Newman-Keuls
test using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA).
Graphs were created using GraphPad Prism 6 software (GraphPad
Software, Inc., La Jolla, CA, USA). All data were presented as the
mean ± standard error of the mean and three independent experiments
were conducted. P<0.05 was considered to indicate a
statistically significant difference.
Results
Salidroside inhibits osteosarcoma cell
growth
An MTT assay was performed to determine the growth
inhibitory effects of salidroside on osteosarcoma cells. The
results showed that salidroside significantly inhibited the
viability of osteosarcoma cells in a time- and
concentration-dependent manner. Furthermore, a significant
inhibitory effect was detected with 5 mM salidroside at 24 h, which
peaked at 10 mM salidroside at 72 h compared with the control
(Fig. 1A). The half the maximal
inhibitory concentration (IC50) values were: MG63, 5.09
mM; U2OS, 9.79 mM; MG63, 4.72 mM; U2OS, 5.294 mM; MG63, 4.67 mM;
and U2OS, 4.96 mM following treatment with salidroside for 24, 48,
and 72 h, respectively. Based on the aforementioned results, we
reported that salidroside inhibited osteosarcoma cell growth in a
concentration-dependent manner. Thus, we selected 0, 1, 5 (the
approximate IC50) and 10 mM salidroside as effective
drug concentrations for subsequent analysis.
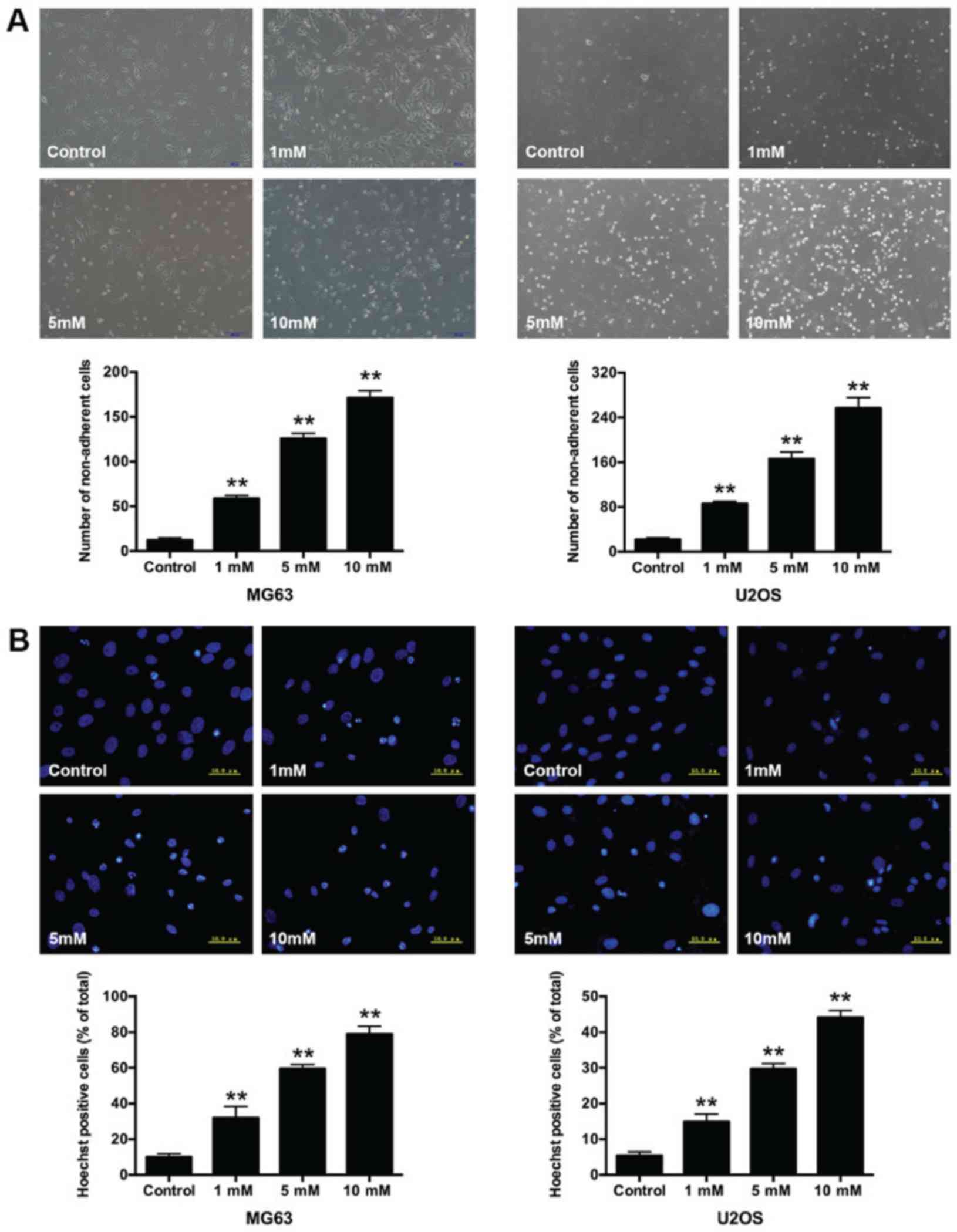
Morphological changes in
salidroside-treated osteosarcoma cells
In addition, we compared the growth inhibitory
effect after treatment with 1, 5, and 10 mM salidroside for 48 h.
Morphological changes in osteosarcoma cells were observed via
microscopy. Cells in the control group presented a long spindle or
polygon appearance and attached uniformly to the culture dish.
Whereas, some cells became small and round, and were suspended in
the medium in the salidroside-treated groups. The number of
non-adherent cells significantly increased (MG63, 1 mM, 58.67±3.51;
5 mM, 125.67±6.03 and 10 mM, 171.00±8.19; U2OS, 1 mM, 85.67±4.04; 5
mM, 166.33±12.22 and 10 mM, 257.00±19.00) compared with the control
group (MG63, 12.33±2.52; U2OS, 12.67±3.51) (Fig. 2A). Hoechst 33258 staining was
performed to further observe the nuclear morphological changes in
osteosarcoma cells. The non-apoptotic nuclei presented weaker blue
fluorescence, while the apoptotic nuclei exhibited brighter
fluorescence and the structure became condensed, fragmented and
crescent-shaped. The percentage of apoptotic nuclei significantly
increased (MG63, 1 mM, 32.03±6.38%; 5 mM, 59.53±2.47% and 10 mM,
78.94±4.44%; U2OS, 1 mM, 14.91±2.16%; 5 mM, 29.72±1.47% and 10 mM
44.13±1.91%); compared with the control groups (MG63, 10.00±1.90%;
U2OS, 5.39±1.06%) (Fig. 2B). The
results indicated that salidroside induced the apoptosis of
osteosarcoma cells in a concentration-dependent manner.
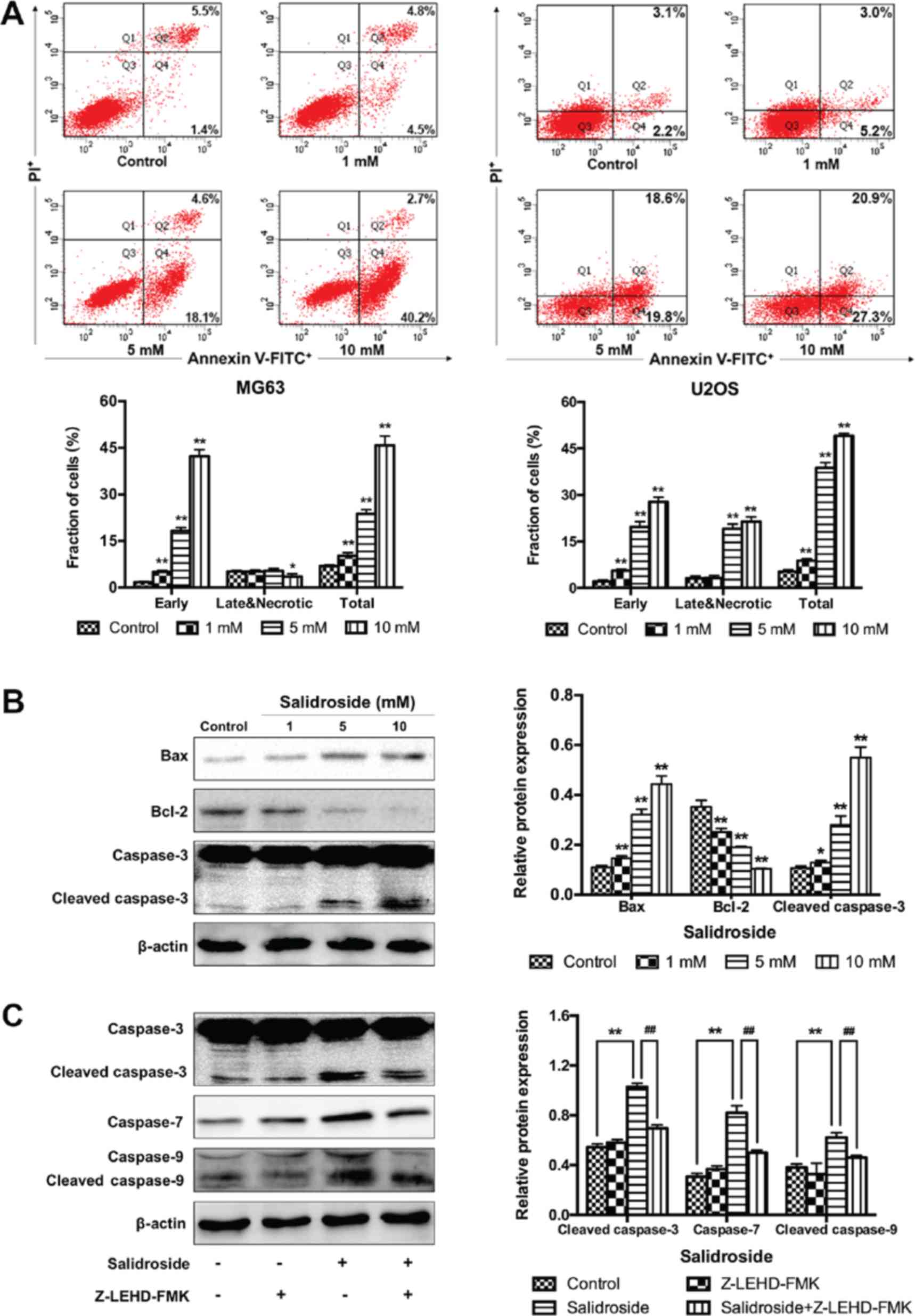
Salidroside induces osteosarcoma cell
apoptosis via the caspase-9-dependent apoptotic pathway
In addition, flow cytometry was performed to further
verify the growth inhibitory effects of salidroside in osteosarcoma
cells associated with apoptosis. The percentage of apoptotic cells
was significantly increased in the presence of salidroside (MG63, 1
mM, 10.20±1.00%; 5 mM, 23.73±1.38% and 10 mM, 45.77±3.07%; U2OS, 1
mM, 8.83±0.55%; 5 mM, 38.80±1.69% and 10 mM, 49.10±0.78%), compared
with the control groups (MG63, 6.90±0.40%; U2OS, 5.23±0.58%)
(Fig. 3A). Then, the Bcl-2 and
caspase family of proteins (Bax, Bcl-2, and caspase-3) were
investigated to explore the potential molecular mechanism in
osteosarcoma cells (MG63 cells were selected). Western blot
analysis demonstrated that the expression of pro-apoptotic protein
was significantly increased and that of anti-apoptotic proteins was
significantly decreased in the salidroside groups in a
concentration-dependent manner, compared with the control group
(Fig. 3B). Additionally, to
evaluate whether the caspase-9-dependent apoptotic pathway was
involved in salidroside-induced osteosarcoma cell apoptosis,
Z-LEHD-FMK, a caspase-9 specific inhibitor, was used and the
molecular mechanism was explored using western blot analysis. Cells
were pretreated with or without Z-LEHD-FMK at 50 μM for 2 h,
then 5 mM salidroside was added to the experimental group and
cultured for another 48 h. The expression of cleaved caspase-3,
caspase-7, and cleaved caspase-9 was significantly increased in the
salidroside group, compared with the control group; however, the
expression of these proteins was significantly reduced in the
presence of Z-LEHD-FMK, compared with the salidroside group
(Fig. 3C). These data not only
confirmed that salidroside induced the apoptosis of osteosarcoma
cells in a concentration-dependent manner, but also indicated that
salidroside induced MG63 cell apoptosis via the caspase-9-dependent
apoptotic pathway.
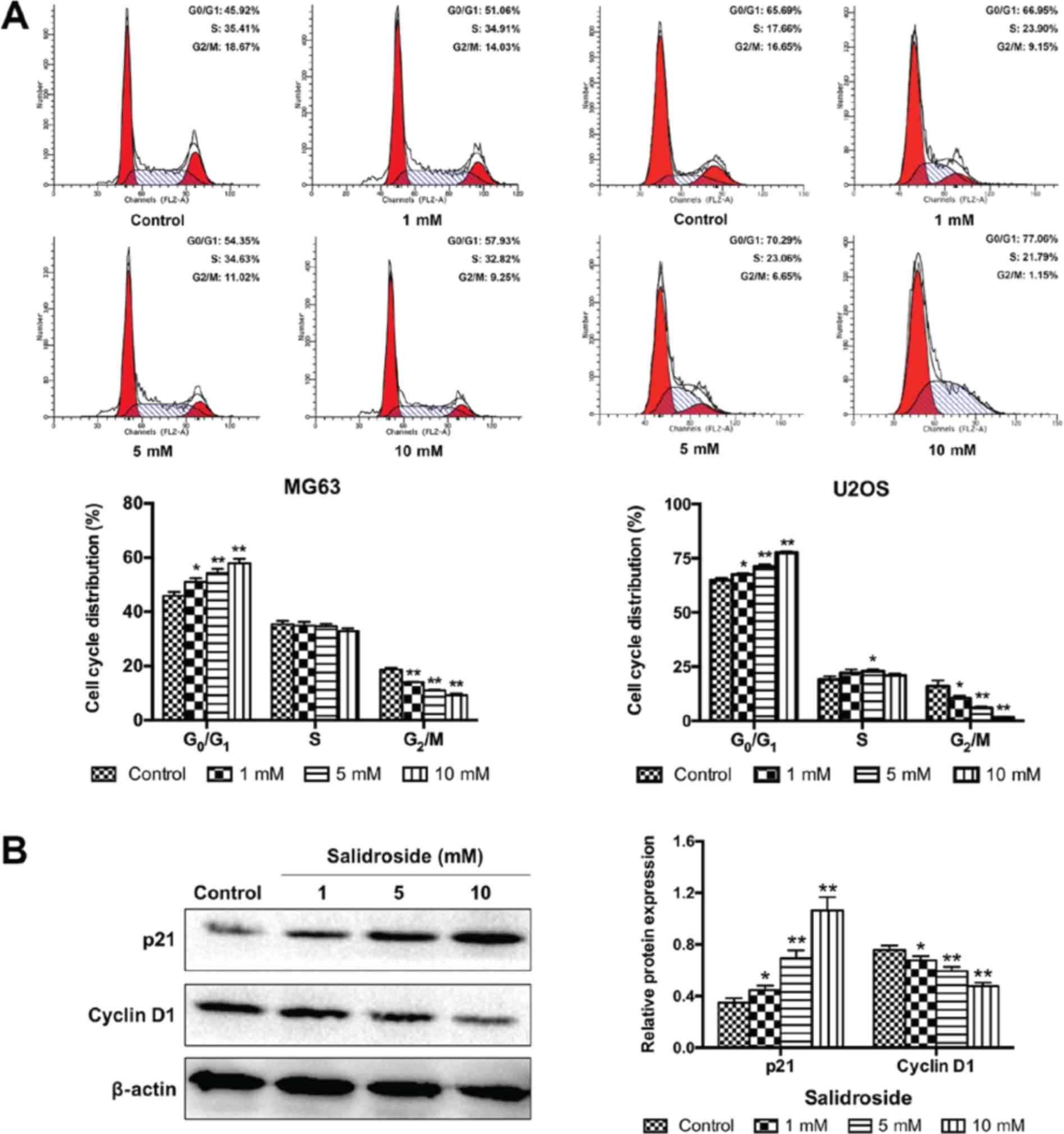
Salidroside induces
G0/G1 arrest in osteosarcoma cells
To determine whether cell-cycle arrest was involved
in the growth inhibitory effects of salidroside, we assessed the
role of salidroside in the progression of the cell cycle in
osteosarcoma cells using flow cytometry. As presented in Fig. 4A, salidroside significantly
increased the percentage of cells in G0/G1
phase (MG63, 1 mM, 51.06±1.38%; 5 mM, 54.35±1.50% and 10 mM,
57.93±1.10%; U2OS, 1 mM 67.51±0.67%; 5 mM 71.22±0.88% and 10 mM
77.57±0.66%), but significantly decreased the percentage of cells
in G2/M phase (MG63, 1 mM, 14.02±0.24%; 5 mM,
11.01±0.21% and 10 mM, 9.25±0.56%; U2OS, 1 mM 10.42±1.11%; 5 mM,
5.93±0.63% and 10 mM, 1.47±0.29%), compared with the control group
(MG63, G0/G1 45.92±1.36% and G2/M
18.68±0.65%; U2OS, G0/G1 64.98±0.96% and
G2/M 15.99±2.63%). Then, the expression of cell
cycle-associated proteins were analyzed (cyclin D1 and p21) to
elucidate the potential molecular mechanism. The expression of p21
was significantly increased and the expression of cyclin D1 was
significantly decreased in the salidroside groups in a
concentration-dependent manner, compared with the control group
(Fig. 4B). These results
demonstrated that salidroside induced G0/G1
phase arrest of osteosarcoma cells in a concentration-dependent
manner.
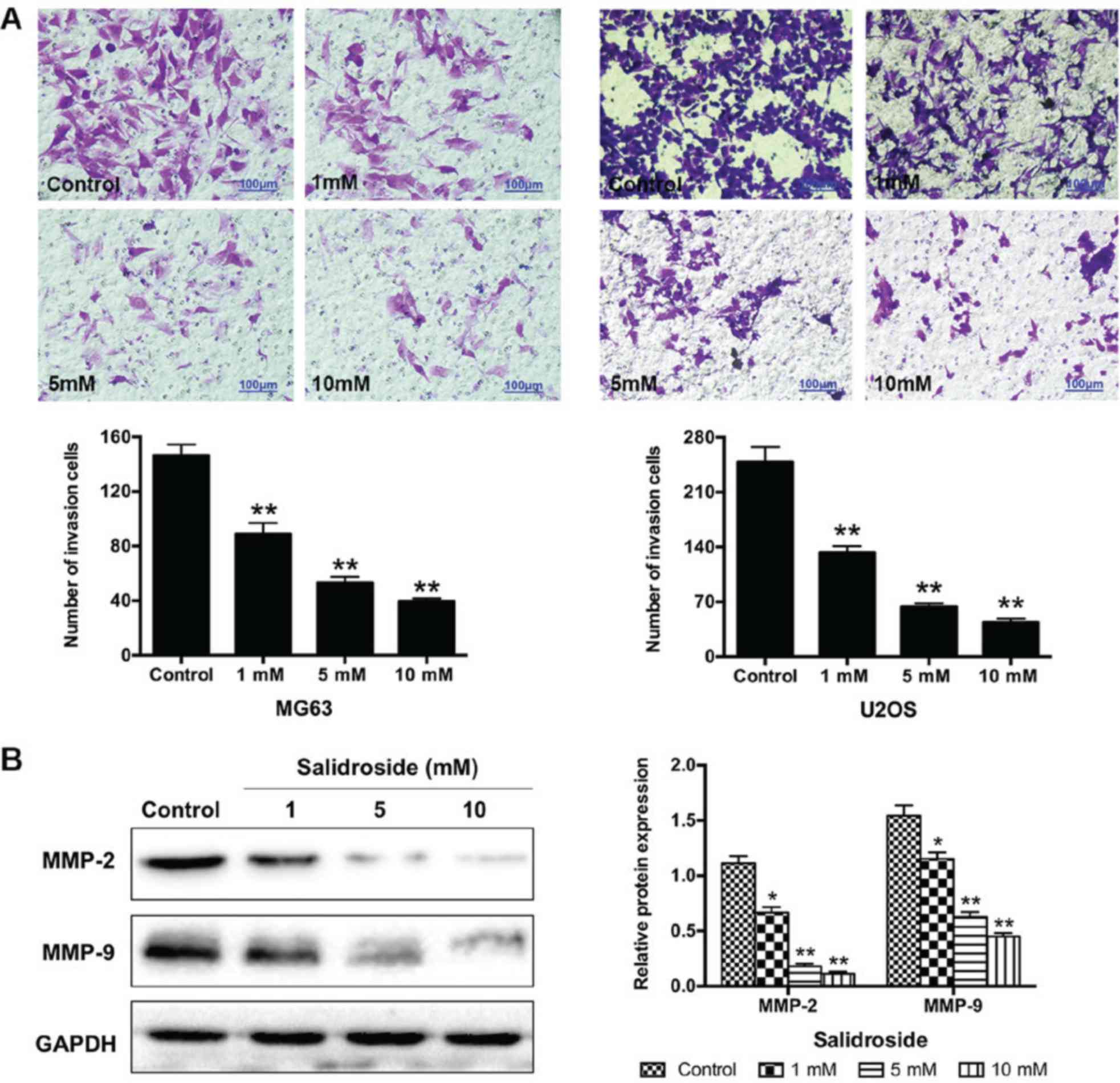
Salidroside inhibits the invasion of
osteosarcoma cells
We performed a Transwell assay to understand the
effects of salidroside on the invasion of osteosarcoma cells. The
results showed that the number of invasive cells significantly
decreased (MG63, 1 mM, 89.00±7.94; 5 mM, 53.33±4.16 and 10 mM,
39.67±2.08; U2OS, 1 mM, 133.00±8.00; 5 mM, U2OS, 63.67±4.16 and 10
mM, 44.00±4.58) compared with the control groups (MG63,
146.33±8.08; U2OS, 248.67±19.04) (Fig.
5A). Additionally, we further investigated the potential
molecular mechanism in MG63 cells using western blot analysis.
Salidroside significantly decreased the expression of
invasion-associated proteins (MMP-2 and MMP-9) in a
concentration-dependent manner, compared with the control groups
(Fig. 5B). In summary, these
findings suggested that salidroside inhibited the invasion of
osteosarcoma cells in a concentration-dependent manner.
Salidroside inhibits the JAK2/STAT3
signaling pathway in osteosarcoma cells
To further investigate the potential mechanism by
which salidroside suppressed the growth of osteosarcoma (MG63)
cells, the JAK2/STAT3 signaling pathway was analyzed. The results
of western blot analysis demonstrated that salidroside
significantly decreased the expression of p-JAK2 and p-STAT3 in a
concentration-dependent manner, compared with the control groups
(Fig. 6A). Furthermore, FLLL32 (5
μM), a specific inhibitor of JAK2/STAT3 phosphorylation, was
used to further confirm these findings. As presented in Fig. 6B, FLLL32 significantly decreased
the expression of p-JAK2 and p-STAT3, compared with the salidroside
group. Similarly, apoptosis-, cell cycle- and invasion-related
proteins were accordingly altered in the salidroside + FLLL32
group, compared with the salidroside group (Fig. 6C and D). Collectively, the
aforementioned results indicated that the JAK2)/STAT3 signaling
pathway was involved in salidroside-induced apoptosis and cell
cycle arrest in MG63 cells. Collectively, the aforementioned
results indicated that salidroside induced apoptosis and cell cycle
arrest, and suppressed the invasion of osteosarcoma cells by
inhibiting the JAK2/STAT3 signaling pathway.
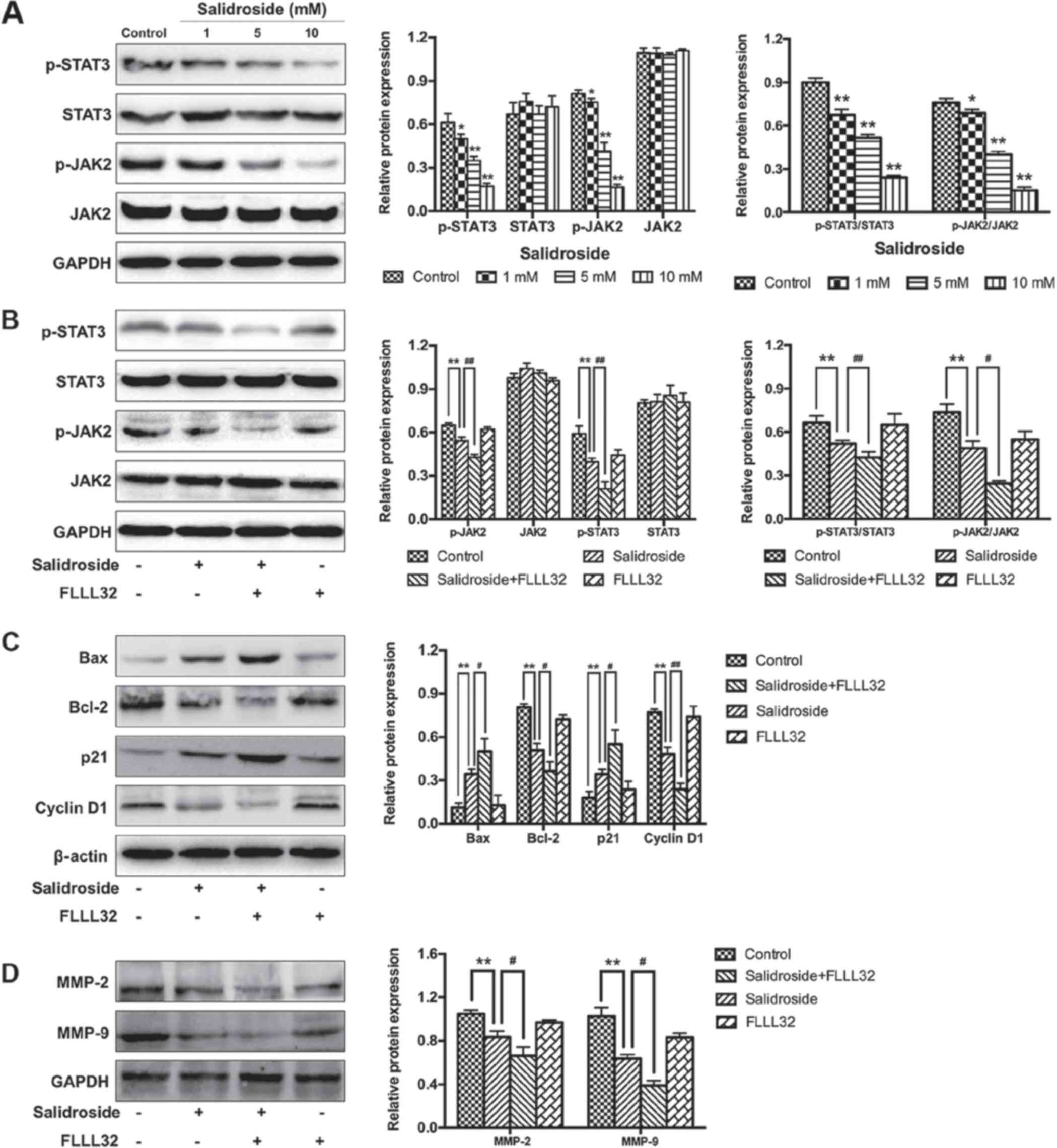 | Figure 6Salidroside inhibits the JAK2/STAT3
signaling pathway. (A) Western blot analysis was used to
investigate whether the JAK2/STAT3 signaling pathway was associated
with the growth inhibitory effect induced by salidroside. (B)
FLLL32 (a specific JAK2/STAT3 inhibitor) was used to confirm the
involvement of the JAK2/STAT3 signaling pathway. (C) Western blot
analysis was used to verify the role of the JAK2/STAT3 pathway in
the regulation of apoptosis- and cell cycle-associated proteins in
salidroside-treated osteosarcoma cells. (D) Western blot analysis
was used to verify the role of the JAK2/STAT3 pathway in the
regulation of invasion-associated proteins in salidroside-treated
osteosarcoma cells. *P<0.05, **P<0.01,
vs. control group. #P<0.05, ##P<0.01,
vs. salidroside group. Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; JAK2, Janus kinase 2; MMP, matrix
metalloproteinase; p, phosphorylated; STAT3, signal transducer and
activator of transcription 3. |
Discussion
Osteosarcoma is a common aggressive malignant bone
tumor. Despite considerable developments in the treatment of
osteosarcoma, current treatments for osteosarcoma still have major
limitations; the long-term survival (5-year survival rate is ~60%)
and mortality rates remained unchanged (19). Therefore, novel therapeutic
strategies are urgently required that can effectively act via
various anticancer mechanisms. The positive effects of
phytochemical drugs on antitumorigenic activity have been
demonstrated, as well as their protective effects against the side
effects of traditional chemotherapeutic drugs, indicating that they
are safer compounds for use in normal cells (20,21).
However, the complex mechanism of action of phytochemical drugs
complicates their application in treating human malignancies
(22). Salidroside, a glucoside of
tyrosol, was considered an effective ingredient of Rhodiola,
which has been demonstrated to have anti-proliferative and
pro-apoptotic effects in previous reports (8-12).
Interestingly, research has reported that salidroside also has
antitumor effects in several human tumor cells, such as
neuroblastoma cells, bladder cancer cells, glioma cells and lung
cancer cells (14,15,20,23).
Furthermore, Li et al (24)
reported that salidroside combined with antitumor agents exerted
excellent antitumor effects in colorectal cancer. Qi et al
(25) revealed that salidroside
had a direct inhibitory effect on the proliferation, migration and
invasion of gastric cancer cells. In the present study, we first
assessed the antitumor effects of salidroside in the treatment of
osteosarcoma. We demonstrated that salidroside induced the growth
and invasion of osteosarcoma cells, which indicated its therapeutic
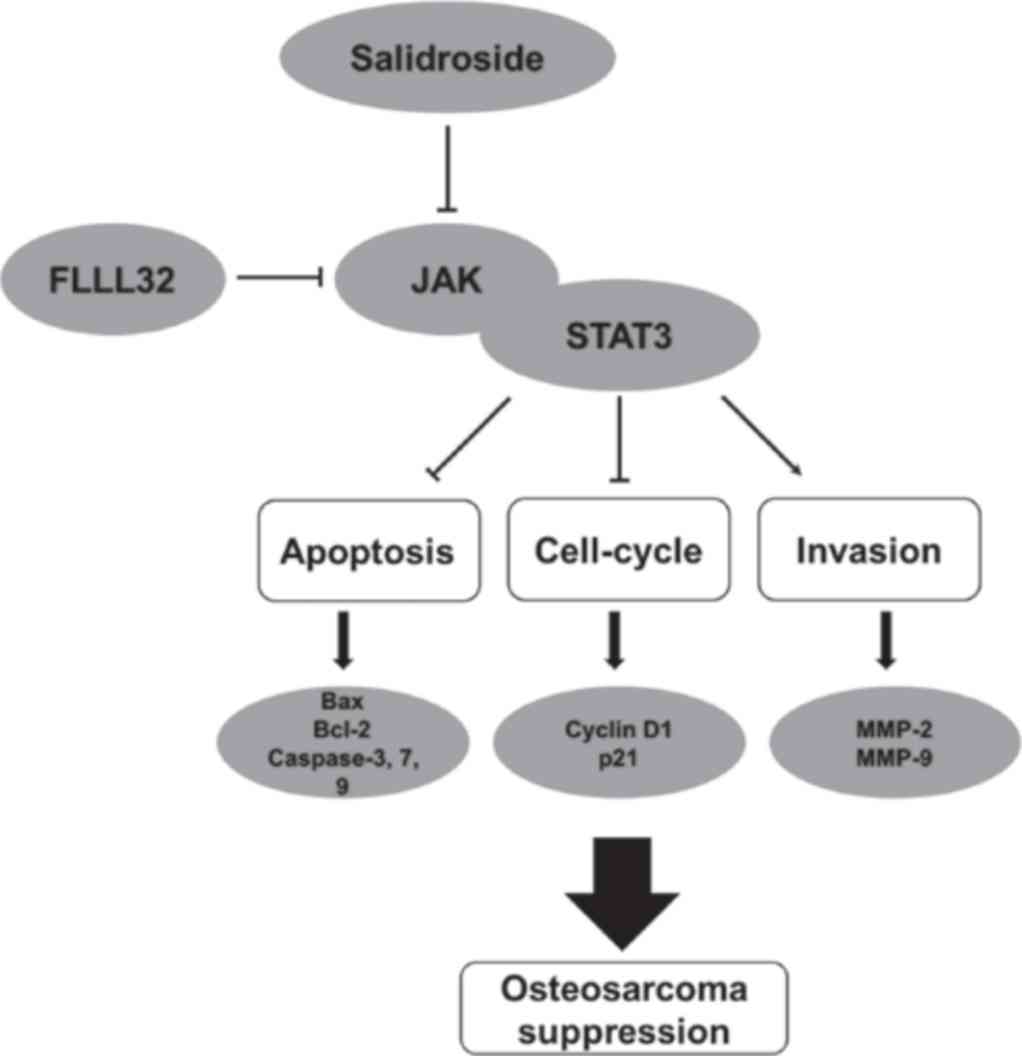
potential. The pharmacological mechanism of salidroside may be
related to the JAK2/STAT3 signaling pathway (Fig. 7).
Cell proliferation is an important marker for tumor
development. Therefore, inhibiting tumor growth (by promoting tumor
cell apoptosis) is the most important objective in preventing tumor
progression (26). The MTT assay
is widely used in bioactive factor activity assays, large-scale
antitumor drug screening and cytotoxicity assays (27). In the present study, the results of
the MTT assay revealed that salidroside significantly inhibited the
viability of osteosarcoma cells in a time- and
concentration-dependent manner. The results of cell morphological
observations and flow cytometric apoptosis detection further
indicated that the decrease in cell viability induced by
salidroside was associated with cell apoptosis. We investigated
whether the expression of apoptotic-related proteins via western
blot analysis, and the expression of the Bcl-2 and caspase
families, critical apoptosis-related proteins, were regulated by
salidroside. The Bcl-2 and caspase families are specific regulatory
proteins of the mitochondrial apoptosis pathway, which is one of
the main pathways of apoptosis (28). Our results indicated that the
mitochondrial apoptosis pathway is involved in salidroside-mediated
apoptosis of osteosarcoma cells. In addition, dysregulated cell
cycle distribution is another feature of tumor development, and the
induction of cell apoptosis is accompanied with cell cycle arrest
(29). Flow cytometric cell cycle
analysis is widely used for evaluating changes in cell cycle
distribution (30). We reported
that salidroside triggered G0/G1 phase arrest
in osteosarcoma cells, which was consistent with previous reports
(16,31). Then, the present study investigated
the expression of cell cycle-related proteins using western blot
analysis; the expression of cyclin D1 and p21 were revealed to be
regulated by salidroside. Therefore, we concluded that salidroside
induced the apoptosis of osteosarcoma cells by inducing
G0/G1 phase arrest. We suggested that
salidroside may function as an agonist to induce the apoptosis and
G0/G1 phase arrest of osteosarcoma cells, and
may represent as an alternative therapeutic strategy for the
treatment of osteosarcoma.
Invasion, which generally leads to metastasis, can
be used to predict tumor malignancy (32). Previous research reported that
early metastasis (particularly pulmonary metastasis) remains as the
main cause of mortality in ~40% of patients with osteosarcoma
(33). The results of Transwell
assays demonstrated that salidroside significantly inhibited the
invasive ability of osteosarcoma cells in a concentration-dependent
manner. We further investigated the molecular mechanism using
western blot analyses and reported that salidroside significantly
decreased the expression of MMP-2 and MMP-9 in a
concentration-dependent manner. MMP-2 and MMP-9, the two most
important proteins in the MMP family, are involved in extracellular
matrix degradation by effectively decomposing collagen IV and
laminin (34). The MMP family of
proteins facilitates tumor metastasis by degrading the basement
membrane of the extracellular matrix, and is an effective marker
for predicting tumor malignancy (35). Thus, our findings indicated that
salidroside reduced the metastatic capabilities of osteosarcoma
cells by suppressing the expression of MMPs.
Increasing evidence has suggested that the
inhibitory effect of salidroside on different tumor cells is
associated with different signal pathways. Sun et al
(13) showed that salidroside
inhibited the metastasis of human fibrosarcoma cells by
downregulating the reactive oxygen species (ROS)/protein kinase
C/extracellular signal-regulated kinase 1/2 pathway. Zhao et
al (16) revealed that
salidroside reduced oxidative stress and suppressed breast cancer
growth by inhibiting the formation of ROS and activating the
mitogen-activated protein kinase pathway. Fan et al
(36) found that salidroside
induced the apoptosis and autophagy of human colorectal cancer
cells via inhibiting the PI3K/Akt/mammalian target of rapamycin
pathway. Lv et al (17)
reported that salidroside suppressed proliferation in renal cell
carcinoma by modulating the JAK2/STAT3 pathway. Kang et al
(37) demonstrated that
salidroside inhibited the mobility and angiogenesis of breast
cancer cells by regulating epidermal growth factor
receptor/Jak2/STAT3 signaling via MMP2. To investigate the
potential signaling pathway involved in salidroside-mediated cell
apoptosis of osteosarcoma cells, western blot analysis was
performed. We showed that the JAK2/STAT3 signaling pathway
participated in salidroside-mediated cell apoptosis of osteosarcoma
cells, which was consistent with previous reports of signaling in
other tumors, including human renal carcinoma cells, human melanoma
cells and colon cancer cells (38,39).
JAK family proteins have been implicated in different cellular
processes, such as cell proliferation, differentiation, apoptosis,
invasion and angiogenesis (40).
JAKs are activated by cytokines to phosphorylate Y residues and
subsequently phosphorylate the downstream molecule STAT3 (40). STAT3 translocates to the nucleus
and binds to DNA, regulating thee expression of apoptosis-related
genes, such as Bcl-2 and other members of this family (41). STAT3 is therefore considered an
essential anti-apoptotic factor. The JAK2/STAT3 pathway is an
evolutionarily conserved pathway that induces tissue homoeostasis
modulation and decreases the extent of damage during cellular
stress; it is therefore considered a critical signaling pathway in
cancer formation and progression (27,42).
Our results revealed that salidroside reduced the phosphorylation
of JAK2 and STAT3. In addition, we reported that FLLL32 (a specific
JAK2/STAT3 inhibitor) significantly enhanced the inhibitory effects
of salidroside on JAK2 and STAT3 phosphorylation in osteosarcoma
cells. Taken together, these results demonstrated that salidroside
induces cell apoptosis, cell cycle arrest and suppresses invasion
of osteosarcoma cells via inhibiting the JAK2/STAT3 signaling
pathway.
ROS can activate multiple pathways involved in
metastasis, invasion, and apoptosis of tumor cells. It has been
reported that ROS and several oxidative substances produced by
tumor cells can promote tumor growth (43). Salidroside, an antioxidant, had
been reported to exert anti-cancer effects in breast cancer
treatment (16); however, whether
autophagy and ROS are involved in salidroside-induced apoptosis and
cell cycle arrest in osteosarcoma cells is yet to be determined and
further studies are required to investigate the mechanism of action
of salidroside. In addition, a limitation of our study is that only
an MTT assay was used to determine the growth inhibition by
salidroside; further methods are required to verify our findings in
the future. By consulting the literature, we found that low
concentrations of salidroside could suppress the growth and
invasion of several human tumor cells (15,17,20,31).
Our preliminary research was based on the concentration at the
micromolar level; however, the results showed that the activity of
osteosarcoma cells was not affected. This could be due to the
relatively mild efficacy of salidroside; thus, investigations were
conducted with salidroside at the millimolar level in the present
study. Additionally, several studies also used high concentrations
(mM) of salidroside (36,44); we used similar concentrations of
salidroside for analysis. Furthermore, the normal osteoblast cell
line hFOB1.19 was employed to explore whether salidroside induced
osteoblast apoptosis with the same concentrations. We reported that
the concentration of salidroside significantly induced the
apoptosis of normal osteoblasts (hFOB1.19) from ≥8.2 mM at 24 h
(data not shown). Therefore, we considered that the sensitivity of
cells to salidroside may differ between cell lines. Our future
study aims to further narrow the concentration gradient of
salidroside.
In the present study, it was salidroside was
demonstrated to be a critical inhibitor of osteosarcoma growth and
invasion, and was associated with the induction of cell apoptosis,
cell cycle arrest and suppressed invasion. Investigations into the
molecular mechanism of action of salidroside suggested that
apoptosis may be induced via the mitochondrial apoptosis pathway,
and the JAK2/STAT3 pathway was reported to be involved in
salidroside-mediated inhibition of osteosarcoma growth and
invasion. In conclusion, the findings of the present study may
provide insight into the molecular mechanism underlying the effects
of salidroside and may be considered as a potential therapeutic
agent for the treatment of osteosarcoma.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CL made substantial contributions to the design of
the study for the analysis of the role of salidroside in modulating
osteosarcoma cell growth and invasion. LH, ZH and LW performed
experiments including the MTT assay, and cell apoptosis and cell
cycle analysis. WL, XC and XZ performed the experiments to analyze
molecular mechanisms, such as western blot analysis. SY conducted
statistical analysis of the experimental data. CL and LH were major
contributors in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Lewis VO: What's new in musculoskeletal
oncology. J Bone Joint Surg Am. 91:1546–1556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dominkus M, Darwish E and Funovics P:
Reconstruction of the pelvis after resection of malignant bone
tumours in children and adolescents. Recent Results Cancer Res.
179:85–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuchs B and Pritchard DJ: Etiology of
osteosarcoma. Clin Orthop Relat Res. 397:40–52. 2002. View Article : Google Scholar
|
|
5
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosen G, Marcove RC, Caparros B, Nirenberg
A, Kosloff C and Huvos AG: Primary osteogenic sarcoma: The
rationale for preoperative chemotherapy and delayed surgery.
Cancer. 43:2163–2177. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kulchitsky VA, Potkin VI, Zubenko YS,
Chernov AN, Talabaev MV, Demidchik YE, Petkevich SK, Kazbanov VV,
Gurinovich TA, Roeva MO, et al: Cytotoxic effects of
chemotherapeutic drugs and heterocyclic compounds at application on
the cells of primary culture of neuroepithelium tumors. Med Chem.
8:22–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tolonen A, Pakonen M, Hohtola A and
Jalonen J: Phenylpropanoid glycosides from Rhodiola rosea. Chem
Pharm Bull (Tokyo). 51:467–470. 2003. View Article : Google Scholar
|
|
9
|
Zhu Y, Zhang YJ, Liu WW, Shi AW and Gu N:
Salidroside suppresses HUVECs cell injury induced by oxidative
stress through activating the Nrf2 signaling pathway. Molecules.
21:212016. View Article : Google Scholar
|
|
10
|
Yang DW, Kang OH, Lee YS, Han SH, Lee SW,
Cha SW, Seo YS, Mun SH, Gong R, Shin DW, et al: Anti-inflammatory
effect of salidroside on phorbol-12-myristate-13-acetate plus
A23187-mediated inflammation in HMC-1 cells. Int J Mol Med.
38:1864–1870. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G
and Wang Z: Neuroprotective effects of salidroside against
beta-amyloid-induced oxidative stress in SH-SY5Y human
neuroblastoma cells. Neurochem Int. 57:547–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Shen WS, Gao CH, Deng LC and Shen
D: Protective effects of salidroside on epirubicin-induced early
left ventricular regional systolic dysfunction in patients with
breast cancer. Drugs R D. 12:101–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun C, Wang Z, Zheng Q and Zhang H:
Salidroside inhibits migration and invasion of human fibrosarcoma
HT1080 cells. Phytomedicine : international journal of phytotherapy
and phytopharmacology. 19:355–363. 2012. View Article : Google Scholar
|
|
14
|
Liu Z, Li X, Simoneau AR, Jafari M and Zi
X: Rhodiola rosea extracts and salidroside decrease the growth of
bladder cancer cell lines via inhibition of the mTOR pathway and
induction of autophagy. Mol Carcinog. 51:257–267. 2012. View Article : Google Scholar
|
|
15
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao G, Shi A, Fan Z and Du Y: Salidroside
inhibits the growth of human breast cancer in vitro and in vivo.
Oncol Rep. 33:2553–2560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv C, Huang Y, Liu ZX, Yu D and Bai ZM:
Salidroside reduces renal cell carcinoma proliferation by
inhibiting JAK2/STAT3 signaling. Cancer Biomark. 17:41–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faisham WI, Mat Saad AZ, Alsaigh LN, Nor
Azman MZ, Kamarul Imran M, Biswal BM, Bhavaraju VM, Salzihan MS,
Hasnan J, Ezane AM, et al: Prognostic factors and survival rate of
osteosarcoma: A single-institution study. Asia Pac J Clin Oncol.
13:e104-e1102017. View Article : Google Scholar
|
|
20
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: The anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Y, Zhao Y, Zheng C, Meng Y and Yang Y:
Synthesis, biological activity of salidroside and its analogues.
Chem Pharm Bull (Tokyo). 58:1627–1629. 2010. View Article : Google Scholar
|
|
22
|
Farzaei MH, Bahramsoltani R and Rahimi R:
Phytochemicals as adjunctive with conventional anticancer
therapies. Curr Pharm Des. 22:4201–4218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan
C, Cao G and Wang Z: Protective effects of salidroside on hydrogen
peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells.
Eur J Pharmacol. 564:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H and Chen C: Inhibition of autophagy
enhances synergistic effects of Salidroside and anti-tumor agents
against colorectal cancer. BMC Complement Altern Med. 17:5382017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi Z, Tang T, Sheng L, Ma Y, Liu Y, Yan L,
Qi S, Ling L and Zhang Y: Salidroside inhibits the proliferation
and migration of gastric cancer cells via suppression of
Src-associated signaling pathway activation and heat shock protein
70 expression. Mol Med Rep. 18:147–156. 2018.PubMed/NCBI
|
|
26
|
Normile D: Cell proliferation. Common
control for cancer, stem cells. Science. 298:18692002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu X, Ma J, Vikash V, Li J, Wu D, Liu Y,
Zhang J and Dong W: Thymoquinone augments cisplatin-induced
apoptosis on esophageal carcinoma through mitigating the activation
of JAK2/STAT3 pathway. Dig Dis Sci. 63:126–134. 2018. View Article : Google Scholar
|
|
28
|
Lv C, Hao Y, Han Y, Zhang W, Cong L, Shi Y
and Tu G: Role and mechanism of microRNA-21 in
H2O2-induced apoptosis in bone marrow
mesenchymal stem cells. J Clin Neurosci. 27:154–160. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin W, Zhu X, Yang S, Chen X, Wang L,
Huang Z, Ding Y, Huang L and Lv C: MicroRNA-203 inhibits
proliferation and invasion, and promotes apoptosis of osteosarcoma
cells by targeting Runt-related transcription factor 2. Biomed
Pharmacother. 91:1075–1084. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu X, Zhang X, Qiu S, Yu D and Lin S:
Salidroside induces cell-cycle arrest and apoptosis in human breast
cancer cells. Biochem Biophys Res Commun. 398:62–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tesser-Gamba F, Lopes LJ, Petrilli AS and
Toledo SR: MAPK7 gene controls proliferation, migration and cell
invasion in osteo-sarcoma. Mol Carcinog. 55:1700–1713. 2016.
View Article : Google Scholar
|
|
33
|
Yu C and Wang W: Relationship between P15
gene mutation and formation and metastasis of malignant
osteosarcoma. Med Sci Monit. 22:656–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui Y, Zhu JJ, Ma CB, Cui K, Wang F, Ni SH
and Zhang ZY: Genetic polymorphisms in MMP 2, 3 and 9 genes and the
susceptibility of osteosarcoma in a Chinese Han population.
Biomarkers. 21:160–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lv C, Yang S, Chen X, Zhu X, Lin W, Wang
L, Huang Z, Wang M and Tu G: MicroRNA-21 promotes bone mesenchymal
stem cells migration in vitro by activating PI3K/Akt/MMPs pathway.
J Clin Neurosci. 46:156–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang DY, Sp N, Kim DH, Joung YH, Lee HG,
Park YM and Yang YM: Salidroside inhibits migration, invasion and
angiogenesis of MDA-MB 231 TNBC cells by regulating EGFR/Jak2/STAT3
signaling via MMP2. Int J Oncol. 53:877–885. 2018.PubMed/NCBI
|
|
38
|
Bill MA, Nicholas C, Mace TA, Etter JP, Li
C, Schwartz EB, Fuchs JR, Young GS, Lin L, Lin J, et al:
Structurally modified curcumin analogs inhibit STAT3
phosphorylation and promote apoptosis of human renal cell carcinoma
and melanoma cell lines. PLoS One. 7:e407242012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun KX, Xia HW and Xia RL: Anticancer
effect of salidroside on colon cancer through inhibiting JAK2/STAT3
signaling pathway. Int J Clin Exp Pathol. 8:615–621.
2015.PubMed/NCBI
|
|
40
|
Mahmoud AM and Abd El-Twab SM: Caffeic
acid phenethyl ester protects the brain against hexavalent chromium
toxicity by enhancing endogenous antioxidants and modulating the
JAK/STAT signaling pathway. Biomed Pharmacother. 91:303–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tian Y, Zhang W, Xia D, Modi P, Liang D
and Wei M: Postconditioning inhibits myocardial apoptosis during
prolonged reperfusion via a JAK2 STAT3-Bcl-2 pathway. J Biomed Sci.
18:532011. View Article : Google Scholar
|
|
42
|
Wu KJ, Huang JM, Zhong HJ, Dong ZZ,
Vellaisamy K, Lu JJ, Chen XP, Chiu P, Kwong DWJ, Han QB, et al: A
natural product-like JAK2/STAT3 inhibitor induces apoptosis of
malignant melanoma cells. PLoS One. 12:e01771232017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shokoohinia Y, Jafari F, Mohammadi Z,
Bazvandi L, Hosseinzadeh L, Chow N, Bhattacharyya P, Farzaei MH,
Farooqi AA, Nabavi SM, et al: Potential anticancer properties of
osthol: A comprehensive mechanistic review. Nutrients. 10:102018.
View Article : Google Scholar
|
|
44
|
Shi X, Zhao W, Yang Y, Wu S and Lv B:
Salidroside could enhance the cytotoxic effect of L-OHP on
colorectal cancer cells. Mol Med Rep. 17:51–58. 2018.
|