Introduction
Prostate cancer (PC) is a common malignant cancer
that often occurs in aged males and is the second leading cause of
cancer-associated mortality (1,2). As
of its notable heterogeneity, PC is difficult to diagnose according
to current diagnostic and prognostic indexes; for example, an
underestimated Gleason score and elevated levels of prostate
specific antigen in the serum of patients with prostatitis and
benign prostatic hyperplasia (3,4).
Given the molecular heterogeneity of PC (5), its diagnosis, treatment and prognosis
may be improved by further investigations into the translation of
several molecular markers in specific stages of disease (6). Recently, the roles of micro
(miRNAs/miRs) in PC have been widely studied, and aberrant miRNAs
expression has been regarded as crucial biomarkers for the
diagnosis, grading and prognosis of PC (7,8).
miRNAs can regulate carcinogenesis of prostate tissue by
interacting with their target genes (9,10).
Therefore, it is necessary to explore the regulatory mechanism of
miRNA, which may provide insight into novel therapeutic strategies
for the treatment of PC.
As small, non-coding and single-stranded RNAs, the
endogenously expressed miRNAs can reversely mediate the expression
of target genes at the post-transcriptional level, and inhibit
translation or induce mRNA degradation to activate various pathways
mainly by binding to the 3′-untranslated region (3′-UTR) (11-13).
It has been demonstrated that miR-335 is an important miRNA
associated with numerous types of cancer (14). For instance, the overexpression of
miR-335 has been reported to suppress the metastatic invasion of
breast cancer (BCa) cells (15).
It was revealed that reduced miR-335 expression in BCa tissues is
associated with the clinicopathological characteristics of BCa
(16). Additionally, miR-335 has
been demonstrated to be related to the chemoresistance of ovarian
cancer cells (17). On the
contrary, in astrocytoma cells, miR-335 can promote tumor growth
and invasion by targeting a potential tumor inhibitor, while
miR-335 inhibition could notably induce growth arrest and apoptosis
in vitro and in vivo (18). Furthermore, miR-335 expression is
dysregulated in several cancers, including hepatocellular
carcinoma, meningioma, PC and colorectal cancer (19-22).
Additionally, miR-335 was reported to possess diverse targets in
the same cancer (23,24). Therefore, exploring the target
genes of miR-335 may reveal novel therapeutic targets.
Early growth response (EGR) transcription factors
can be induced in a variety of cells to respond to stimuli,
including stress, hypoxia, injury, cytokines and growth factors
(25). In the EGR family of
proteins, EGR1 has been reported to be associated with
inflammation, ischemic injury and atherosclerosis (26), in addition to its antitumor effects
by inducing apoptosis in cancer (27-29).
However, to the best of our knowledge, the number of the studies on
EGR3 is limited. A recent study has suggested that high expression
of EGR3 in A549 cells could inhibit cell growth and is associated
with improved prognosis in lung adenocarcinoma (30). Conversely, elevated EGR3 expression
was determined to be associated with poor prognosis in PC (31). Furthermore, EGR3 can regulate the
levels of inflammatory cytokines, including interleukin-6 (IL-6)
and IL-8 in PC cells, and these cytokines can exert critical
effects on the development of PC (32). Considering the roles of miR-335 and
EGR3 in the PC, we aimed to examine whether miR-335 exhibits
anticancer effects by regulating its potential target gene
EGR3.
Materials and methods
Prostate tissue specimens
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Xi'an Jiaotong
University and informed consent was obtained from each patient. A
total of 53 male patients in our hospital from December 2017 to
January 2018 were enrolled in this study: 36 patients with prostate
cancer (mean age, 68.2; age range, 55-82 years) and 18 patients
with metastatic prostate cancer (mean age, 72.4; age range, 58-86
years). Patients with prostate cancer included in the present study
received no preoperative medication and had no history of surgical
castration or radiotherapy. Fifty-three PC tissues and matched
normal tissues from patients were frozen for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis and immunohistochemistry (IHC).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from Promocell (Jiangyin, China) and cultured in
endothelial cell growth medium (Promocell). DU145 and PC-3M cells
(human prostate carcinoma cell line) were obtained from the
American Type Culture Collection (Manassas, VA, USA) and maintained
in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
containing 10% fetal bovine serum (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 2 mM L-glutamine (Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotic mixture (penicillin and
streptomycin; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified incubator with 5% CO2.
Cell transfection
The prostate cancer DU145 Cells (2x105)
were transfected with miR-335 mimics (5′-UCAAGAGCAA
UAACGAAAAAUGU-3′), miR-335 inhibitors (5′-ACAUUU
UUCGUUAUUGCUCUUGA-3′) and corresponding controls (Thermo Fisher
Scientific, Inc.) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at a final concentration of 50 nM
according to the manufacturer's protocols. The PC-3M cells were
only transfected with miR-335 mimics and corresponding controls. To
knockdown EGR3, DU145 cells were transfected with 30 nM small
interfering RNA (siRNA) against EGR3 (siEGR3; #115514; Ambion,
Austin, TX, USA) and negative controls (siNC; #4642; Ambion) using
siPORT NeoFX (Ambion) according to the manufacturer's protocol and
incubated for 24 h. The sequence of siRNAs for EGR3 was
5′-CCAACACAACAGAUAGAAUtt-3′. Puromycin was used to select
transfected cells. In addition, siEGR3 and miRNAs were
co-transfected into DU145 cells at a concentration of 50 nM/well.
At 48 h after transfection, cells were harvested for further
analyses. Non-transfected cells were served as another control.
Cell viability assay
Transfected cells (1x104) were cultivated for 24, 48
and 72 h, respectively. Subsequently, 10 µl Cell Counting Kit-8
solution (Beyotime Institute of Biotechnology, Shanghai, China) was
added into the cells at each time point. After the cells were
incubated for 2 h at 37°C, the optical density at 450 nm was
measured with a FlexStation3 microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Apoptosis analysis
Apoptosis was detected according to the protocols of
the Annexin V-fluorescein isothiocyanate /propidium iodide
apoptosis detection kit (Nanjing KeyGen Biotech, Co., Ltd.,
Nanjing, China). The results were analyzed with a FACSCalibur flow
cytometer (BD Biosciences, San Jose, CA, USA).
In vitro angiogenesis
HUVECs (3x105) were seeded with Matrigel
(BD Biosciences) in a 96-well plate in the respective presence of
supernatants comprising non-transfected DU145 cells, DU145 cells
transfected with miR-NC or miR-335 mimics, DU145 cells transfected
with miR-335 inhibitors, or DU145 cells transfected with siNC or
siEGR3, and then incubated at 37°C for 15 h. Then, the cells were
fixed with 3.7% formaldehyde for 15 min at room temperature. The
images of capillary-like structures from six replicates of each
group were captured using an IX81 Olympus microscope (Olympus
Corporation, Tokyo, Japan; magnification, x100). The overall length
and cell junctions in each frame from five randomly selected fields
were calculated using ImageJ v1.48 software (National Institutes of
Health, Bethesda, MD, USA). Data were normalized to the
controls.
Immunocytochemistry
For caspase-3 expression, the cells were immobilized
in 4% paraformaldehyde for 24-48 h at room temperature and
permeabilized in Tris-buffered saline with 0.1% Triton X-100 (TBST;
pH 7.4; Sigma-Aldrich; Merck KGaA). The cells were treated with
0.1% H2O2 for 30 min at room temperature to
inhibit endogenous peroxidase, followed by incubation with 5%
normal goat serum (Vector Laboratories, Inc., Burlingame, CA, USA)
for 40 min at room temperature to block non-specific binding sites.
The cells were then incubated with anti-caspase-3 (1:50; cat. no.
9661, Cell Signaling Technology, Inc., Danvers, MA, USA) overnight
at 4°C and then with a biotinylated goat anti-rabbit IgG (1:200) as
a secondary antibody (BP-9100, Vector Laboratories, Inc.) for 1 h
at room temperature. Subsequently, the cells were washed in TBST
and incubated with avidin-biotin-peroxidase complex (1:1:100;
Strept ABC complex/HRP; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) for 45 min in dark at room temperature. The results
was visualized with 3′3′-diaminobenzidene (DAB; Vector
Laboratories, Inc.) for 10 min at room temperature and stained with
hematoxylin (Sigma-Aldrich; Merck KGaA) for 30 sec at room
temperature.
Target prediction
To predict the potential targets of miR-335,
TargetScan 7.2 (http://www.targetscan.org) was utilized and the
candidate targets were identified based on the phenotype of
miR-335.
Luciferase assay
DU145 cells were transfected with a firefly
luciferase reporter pGL3 vector (Promega Corporation, Madison, WI,
USA) containing the wild-type or mutant EGR3 3′-UTR, the control
pRL-TK vector containing Renilla luciferase (Promega) with
miR-335 mimics and miR-335 inhibitors using Lipofectamine 2000. The
cells were maintained for 24 h and the activities of firefly and
Renilla luciferase were determined by Dual-Luciferase
Reporter Assay System (Promega Corporation).
RNA extraction, cDNA synthesis and
RT-qPCR
Total RNA of prostate tissues and DU145 cells was
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.). In brief, TRIzol reagent and 200 ml chloroform were added to
samples and mixed for 5 min, followed by centrifugation (12,000 x g
for 15 min at 4°C) to recover the supernatant. Then, the
supernatant was incubated with an equal volume of isopropyl alcohol
for 10 min at room temperature and centrifuged at 12,000 x g for 15
min at 4°C. After dislodging the supernatant, 75% ethanol was added
to for precipitation and the RNA was eluted with nuclease-free
water. For the RT, the concentration and purity were detected by a
NanoDrop 2000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.). cDNA was obtained from 1 µg RNA at 25°C
for 10 min, 37°C for 120 min and 85°C for 5 min using the High
Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The qPCR reaction system was as follows:
2.5 µl dNTPs (2.5 mM); 2.5 µl 10X PCR buffer; 1.5 µl
MgCl2 solution; 1 unit Taq polymerase; 0.25X SYBR Green
I (Sigma-Aldrich; Merck KGaA); 1 µl primers (10 µM each); 1 µl
cDNA; water (to a total volume of 25 µl). The qPCR reactions were
conducted with a LightCycler system (Roche Applied Science,
Indianapolis, IN, USA) using the following parameters: 95°C for 10
min, 40 cycles at 95°C for 10 sec, at 60°C for 20 sec and at 72°C
for 30 sec. The specific primers were listed in Table I. U6 and β-actin served as the
internal reference genes. Expression was calculated using the
2-ΔΔCq method (33).
 | Table ISequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| miR-335 |
GTCGTATCCAGTGCAGGGTCCG |
GTGCAGGGTCCGACCT |
| EGR3 |
TTCGCTTTCGACTCTCC |
CTCCGAGTAGAGATCGC |
| IL-6 |
AAATTCGGTACATCCTCGACGGCA |
AGTGCCTCTTTGCTGCTTTCACAC |
| IL-8 |
AGGACAAGAGCCAGGAAGAAACCA |
AGAGCTGCAGAAATCAGGAAGGCT |
| IL-1β |
AACAGGCTGCTCTGGGATTCTCTT |
AACAGGCTGCTCTGGGATTCTCTT |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| β-actin |
GTGACGTTGACATCCG |
GAGCGTTTGTTGTACCT |
Western blotting
The cells were lysed in lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) and centrifuged at
10,000 x g for 10 min at 4°C, and placed on ice for 30 min to
obtain the supernatant. The protein concentration was determined
using a BCA kit (Beyotime Institute of Biotechnology). After being
separated by 10% SDS-PAGE (40 µg protein was used), the proteins
were transferred onto nitrocellulose membranes (EMD Millipore,
Bedford, MA, USA) and blocked for 1 h. The membranes were incubated
overnight at 4°C with anti-EGR3 (1:500, sc-390967, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-IL-6 (1:1,000, ab9324,
Abcam, Cambridge, USA), anti-IL-8 (1:1,000, ab18672, Abcam),
anti-IL-1β (1:1,000, ab156791, Abcam) and anti-β-actin (1:1,000,
sc-517582, Santa Cruz Biotechnology, Inc.) and incubated with
secondary antibodies (1:1,000, HRP-labeled goat anti-mouse IgG,
A0216, Beyotime Institute of Biotechnology) for 2 h at room
temperature. Proteins were visualized with an ECL kit (GE
Healthcare, Chicago, IL, USA). The Gray value was detected by
ImageJ 1.48 software.
Xenograft experiment of prostate
cancer
Female sever combined immunodeficient mice (20-22 g;
n=6; 6 weeks old) were purchased from the Animal Experimental
Center of Zhejiang Academy of Medical Sciences. Mice were housed in
cages at room temperature (22±3°C) with constant humidity (50±10%)
with a free access to food and water under a light/dark cycle (12
h). The animal experiments were approved by The Second Affiliated
Hospital of Xi'an Jiaotong University Animal Ethics Committee
(approval no. JT201805032M) and according to the Guidelines for the
Care and Use of Laboratory Animals (34).
PC-3M and DU145 cells were transfected with miR-335
mimic and miR-NC as aforementioned. PC model was induced by a
hypodermic injection of 5x106 non-transfected or
transfected cells into lateral abdominal wall of mice. Tumor volume
was measured every 6 days for 42 days. The mice were sacrificed 42
days after the cell transplantation and weighing.
IHC
IHC was performed to detect the EGR3 expression in
53 prostate cancer tissues and matched adjacent tissues and in the
PC xenograft tissues of mice as previously described (35). In brief, tissue sections (4 µm)
were dewaxed in 4% paraformaldehyde for 10 min at room temperature
and rehydrated with gradient ethanol (Sigma-Aldrich; Merck KGaA),
followed by heat-induced antigen retrieval in 10 mM sodium citrate
(pH 6.0) for 20 min. Then the sections were blocked in 10% normal
goat serum (Vector Laboratories, Inc.) at room temperature for 10
min. Thereafter, the sections were incubated in 3%
H2O2 at room temperature for 30 min to
inactivate endogenous peroxidase. The sections were subsequently
incubated with the anti-EGR3 (1:50, sc-390967, Santa Cruz
Technology, Inc.) overnight at 4°C and hybridized with a
biotinylated horse anti-mouse IgG secondary antibody (1:200,
BP-2000, Vector Laboratories, Inc.) for 1 h at room temperature.
The cells were washed in TBST and incubated with
avidin-biotin-peroxidase complex (1:1:100; Strept ABC complex/HRP;
Dako; Agilent Technologies, Inc.) for 45 min in dark at room
temperature. DAB was then added to incubate for 10 min at room
temperature and sections were stained with hematoxylin for 30 sec
at room temperature. The images were diagnosed under an Olympus
microscope (Olympus Corporation; magnification, x400). Image-Pro
Plus version 6.0 software (Media Cybernetics, Inc.) was used to
evaluate the integrated optical density value of the tissue areas
from five randomly selected fields.
Statistical analysis
All data were obtained from independent experiments
and were presented as he mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference. A
Student's t-test and one-way analysis of variance with a Dunnett's
post-test were conducted using SPSS 17.0 software (SPSS, Inc.
Chicago, IL, USA). Comparisons between tumor tissues and matched
adjacent tissues were analyzed with an independent samples t-test.
Correlations between miR-335 expression and EGR3 mRNA were analyzed
by Spearman's correlation analysis.
Results
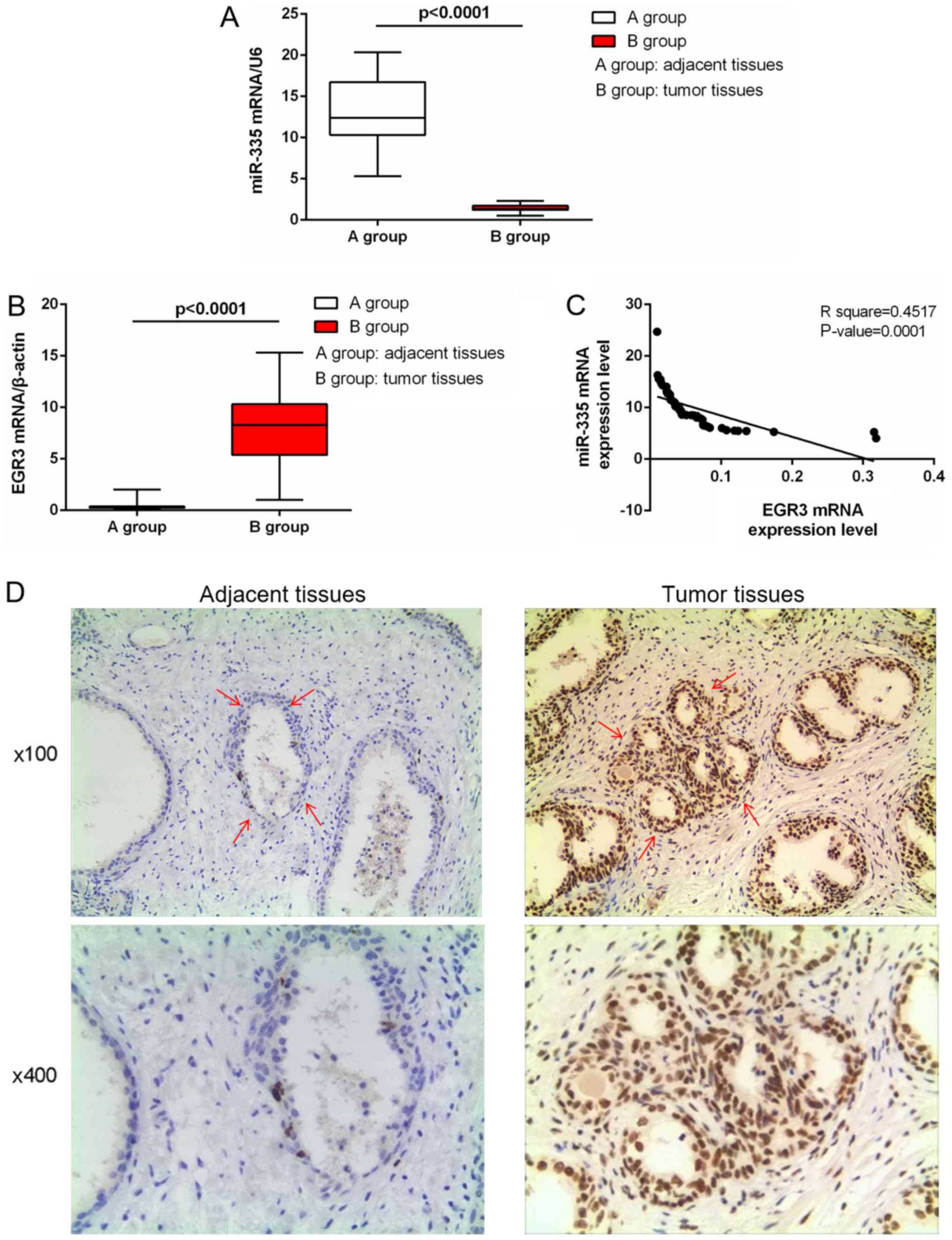
Expression of miR-335 and EGR3 in tissues
of patients with PC
Among the 53 pairs of tissues, the expression levels
of miR-335 in the prostate tumor tissues were significantly reduced
than in normal tissues (P<0.0001; Fig. 1A). On the contrary, EGR3 expression
in tumor tissues was significantly elevated than in normal tissues
(P<0.0001; Fig. 1B). The
expression of miR-335 was negatively correlated with that of EGR3
in patients with PC (P=0.0001; Fig.
1C). Furthermore, upregulated EGR3 expression was observed in
cancer tissues (Fig. 1D).
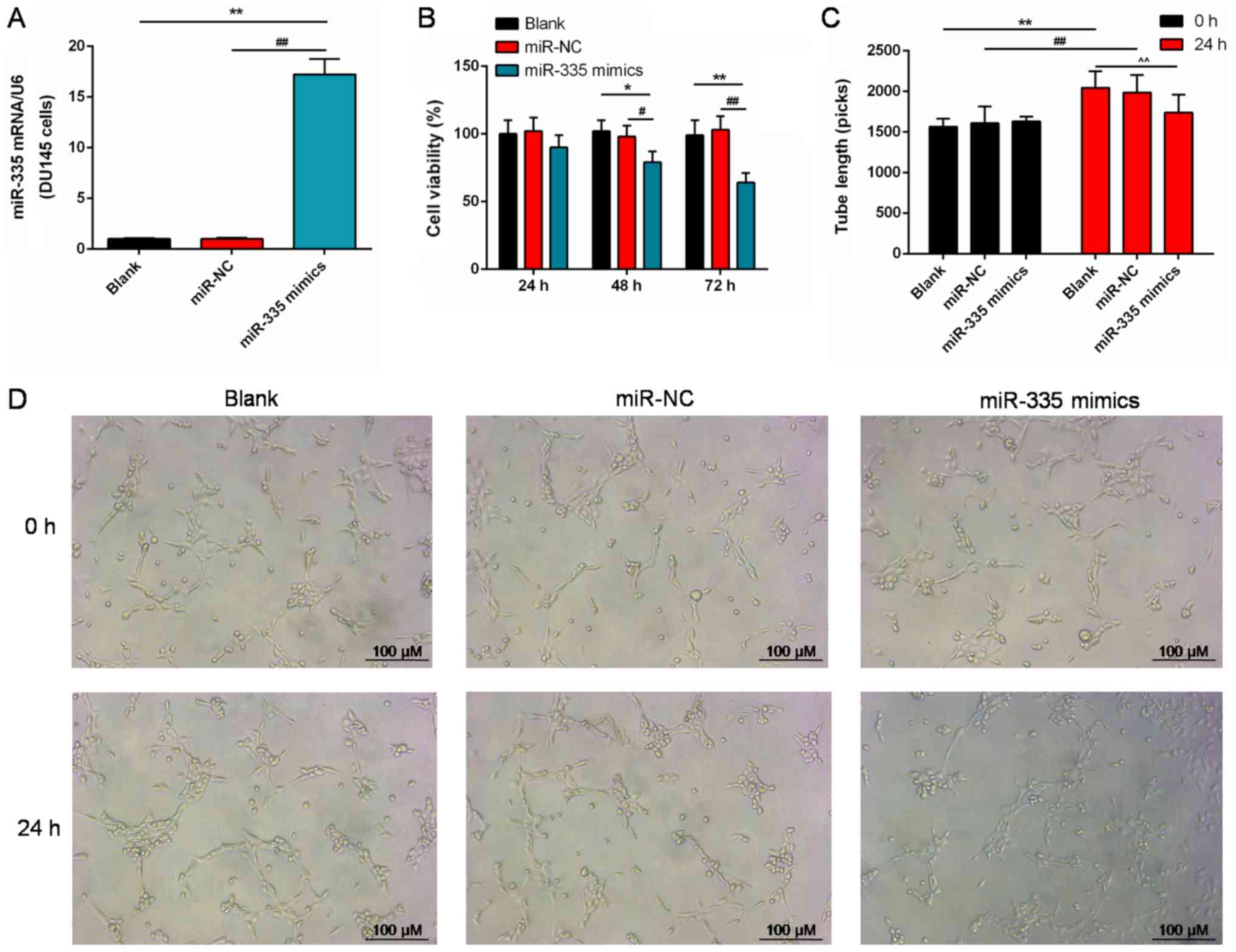
Effects of miR-335 on cell viability and
angiogenesis
The notably reduced expression of miR-335 in DU145
cells was significantly enhanced following transfecting cells with
miR-335 mimics compared with the controls (P<0.01; Fig. 2A). Additionally, miR-335 mimics
significantly inhibited cell viability after 48 h compared with the
controls (P<0.01; Fig. 2B).
After 24 h of culture, the length of neoformative tubes of HUVECs
was significantly suppressed by miR-335 mimics, compared with the
blank and miR-NC groups (P<0.01; Fig. 2C). In addition, the number of tubes
formed in the miR-335 mimics group was markedly lower than in the
blank and miR-NC groups (Fig.
2D).
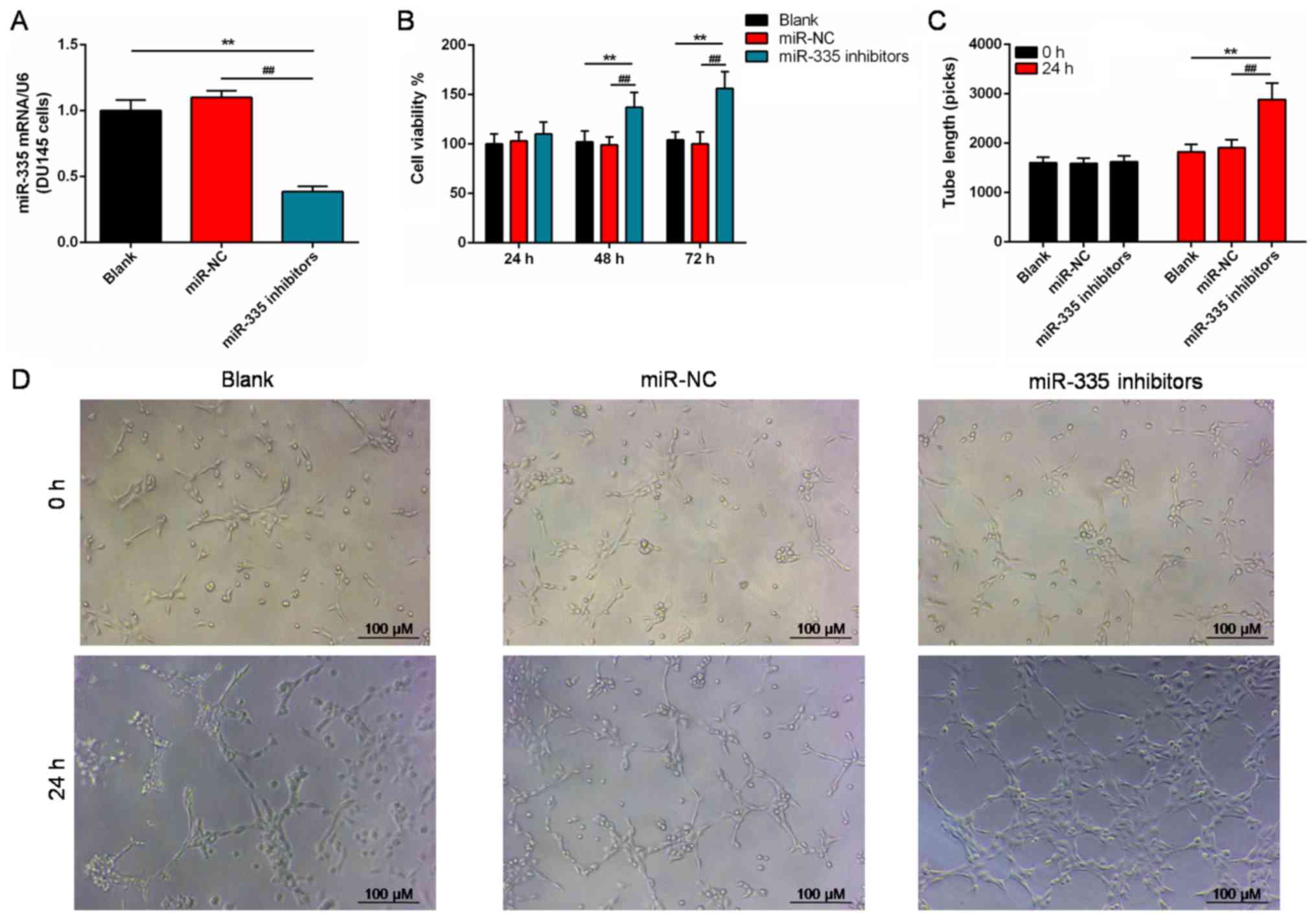
To further explore the role of miR-335 in the PC, we
also successfully silenced the miR-335 expression in DU145 cells
(P<0.01; Fig. 3A). After being
cultured for >48 h, the viability of DU145 cells was
significantly enhanced in the miR-335 inhibitors group compared
with the controls (P<0.01; Fig.
3B). Additionally, the length of the tubes were significantly
increased compared with the controls (P<0.01; Fig. 3C and D) in miR-335 inhibitors
group.
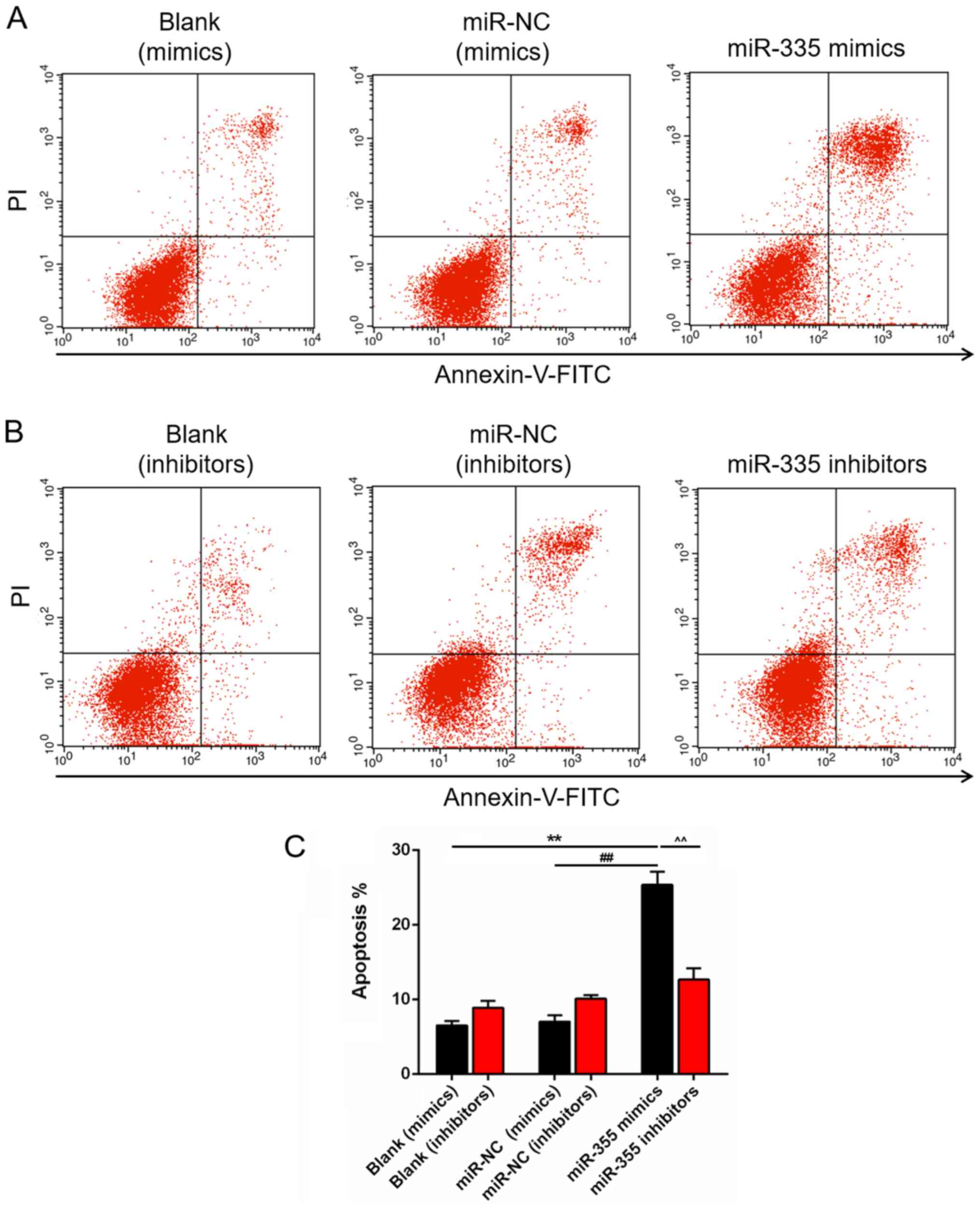
Role of miR-335 in the apoptosis of DU145
cells
miRNAs serve important roles in apoptosis; thus, the
apoptosis of DU145 cells was analyzed in our study. A significant
increase in the number of apoptotic cells was observed in the
miR-335 mimics group, compared with the blank and miR-NC groups
(P<0.01; Fig. 4A). Whereas, the
number of apoptotic cells in miR-335 inhibitors group exhibited no
significant difference compared with the blank and miR-NC groups
(Fig. 4B). The number of apoptotic
DU145 cells was significantly increased in the miR-335 mimics
group, compared with that in cells transfected with miR-335
inhibitors (P<0.01; Fig.
4C).
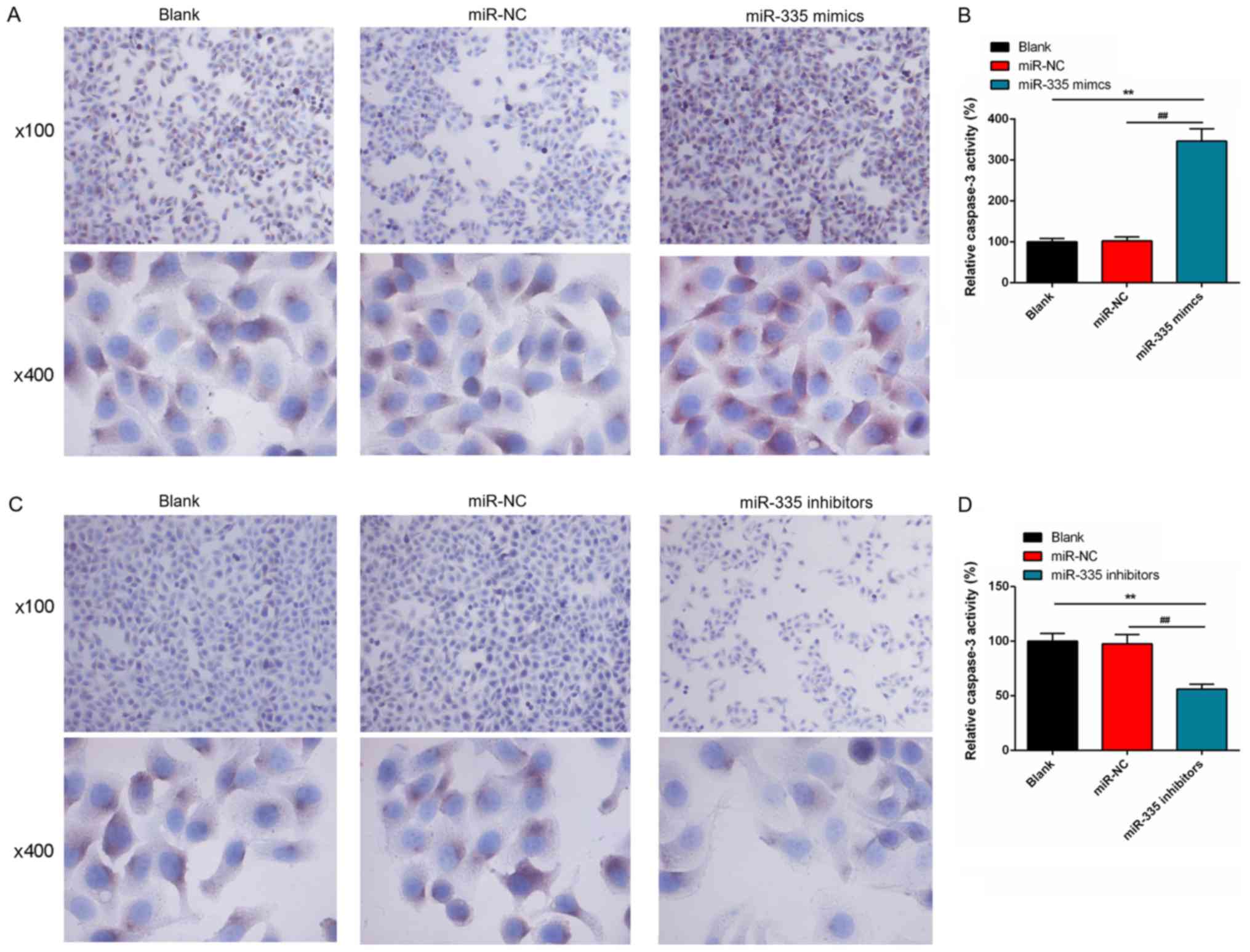
Furthermore, the number of caspase-3-positive cells
markedly increased following iR-355 upregulation; a signifi-cant
increase in activity of caspase-3 was observed in the miR-335
mimics group compared with the controls (P<0.01; Fig. 5A and B). However, the proportion of
caspase-3 positive cells in the miR-335 inhibitors group was
notably less than that in blank group or miR-NC group; caspase-3
activity was significantly reduced following miR-355 knockdown
(P<0.01; Fig. 5C and D).
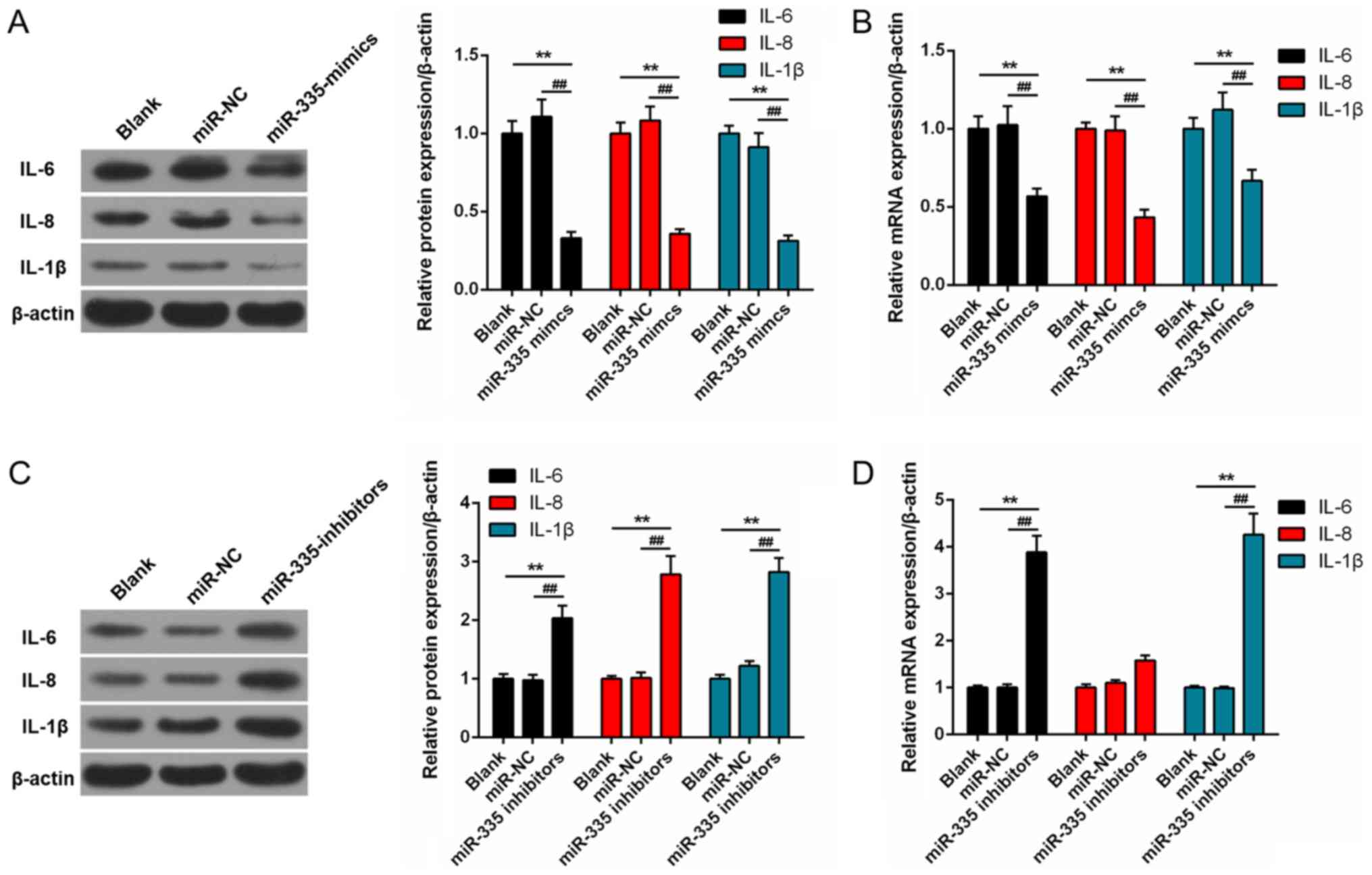
Regulation of miR-335 in the expression
of inflammatory cytokines
The protein expression levels of several
pro-inflammatory factors, including IL-6, IL-8 and IL-1β were
significantly suppressed in the miR-335 mimics group compared with
blank and miR-NC groups (P<0.01; Fig. 6A). Similarly, the mRNA expression
levels of IL-6, IL-8 and IL-1β in the miR-335 mimics group were
significantly down-regulated than in blank and miR-NC groups
(P<0.01; Fig. 6B). The protein
expression levels of IL-6, IL-8 and IL-1β were significantly
enhanced following transfection with miR-335 inhibitors compared
with the control groups (P<0.01; Fig. 6C); a similar trend in mRNA
expression was also for IL-6, IL-8 and IL-1β (P<0.01; Fig. 6D).
EGR3 is a potential target of
miR-335
Based on the bioin-formatic prediction analysis, a
miR-335-binding site was mapped to the 3′-UTR of EGR3 (Fig. 7A). The luciferase activity in cells
transfected with a wild-type EGR3-3′ UTR vector containing miR-335
mimics was significantly reduced compared with the mutated 3′-UTR.
However, the luciferase activity was significantly enhanced in
DU145 cells transfected with wild-type EGR3-3′-UTR vector
containing miR-335 inhibitors (Fig.
7B). EGR3 expression in miR-335 mimics group was significantly
suppressed at the protein and mRNA levels compared with the
controls (P<0.01; Fig. 7C and
D). Conversely, the protein and mRNA expression levels of EGR3
were significantly increased in the cells transfected with miR-335
inhibitors compared with the controls (P<0.01; Fig. 7E and F).
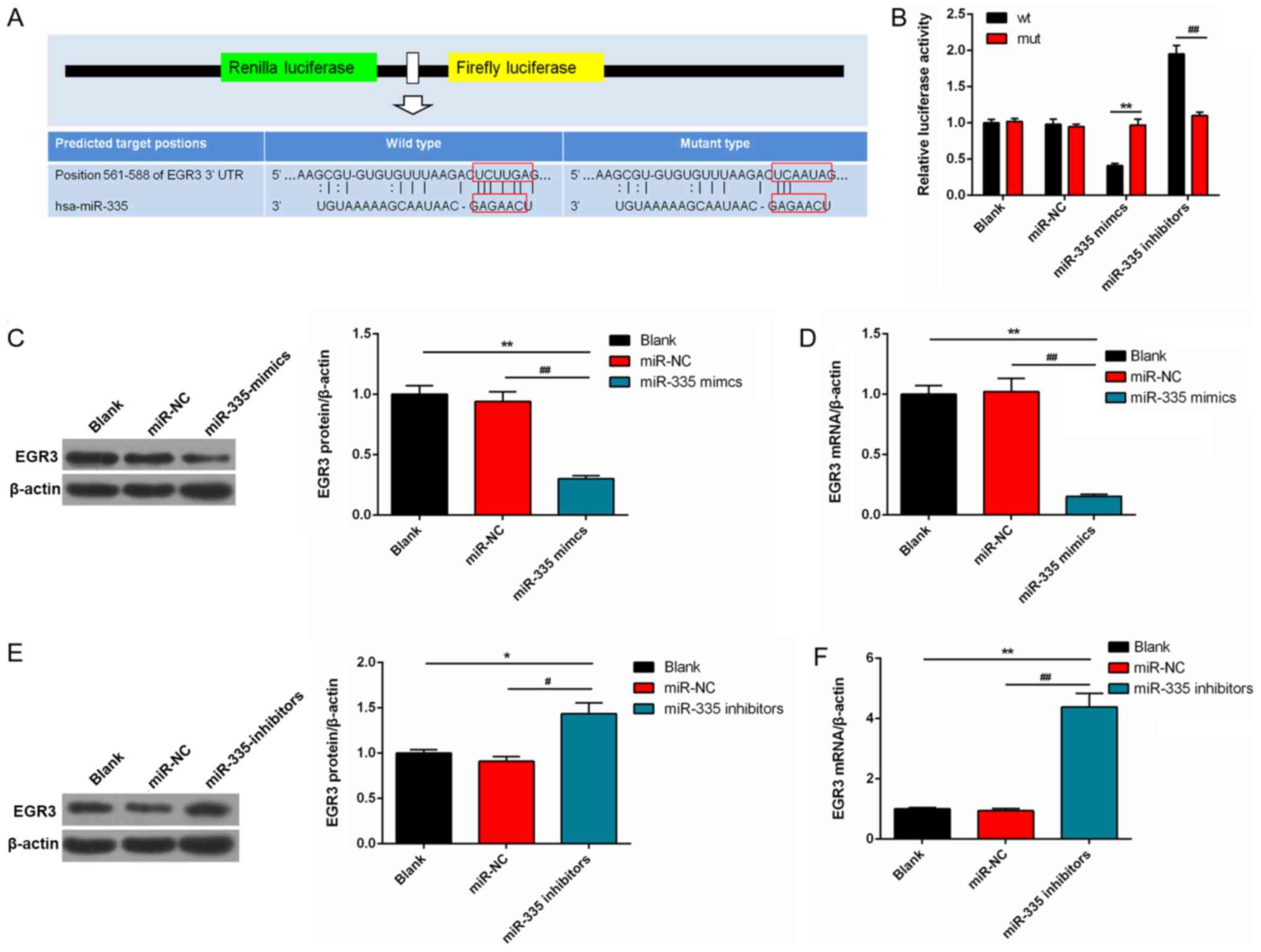 | Figure 7EGR3 is a potential target gene of
miR-335. (A) EGR3 had a miR-335-binding site mapped to its 3′-UTR.
(B) The effects of miR-335 mimics and miR-335 inhibitors on the
luciferase activities of DU145 cells transfected with EGR3-wt 3′-
UTR vector or EGR3-mut 3′-UTR. (C and D) EGR3 expression was
significantly reduced in the miR-335 mimics group at protein and
mRNA levels. (E and F) Protein and mRNA expression levels of EGR3
were significantly enhanced in the miR-335 inhibitors group.
*P<0.05, **P<0.01,
#P<0.05, ##P<0.01, n=3. EGR3, early
growth response 3; miR, microRNA; mut, mutant; NC, negative
control; 3′-UTR, 3′-untranslated region; wt, wild-type. |
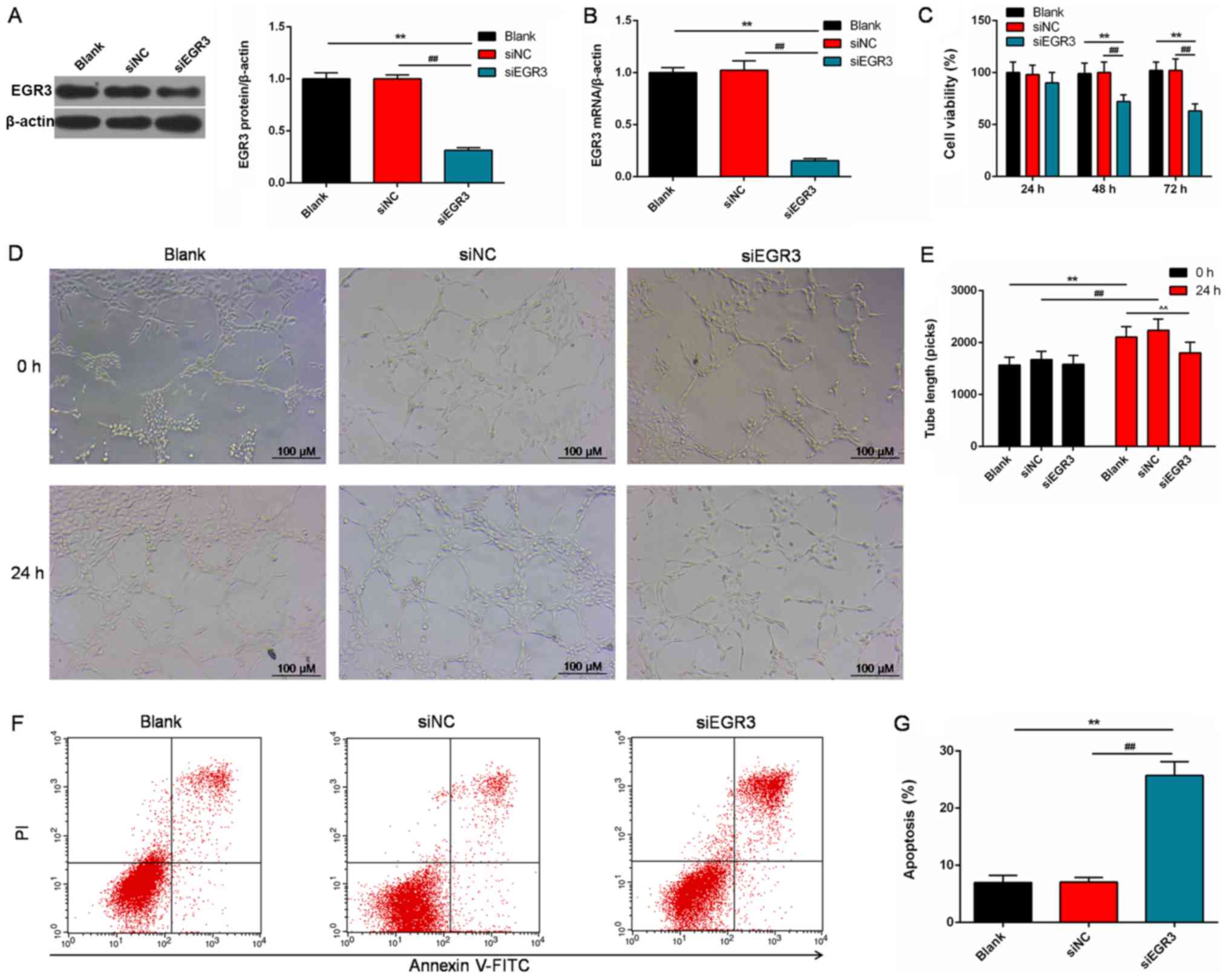
Roles of EGR3 in the PC cells
To understand the effects of EGR3 on prostate
tumors, we detected the angiogenesis and apoptosis of the DU145
cells. EGR3 expression was significantly knocked down at the
transcription and translation levels in cells following
transfection with siEGR3 (P<0.01; Fig. 8A and B). Cell viability was
significantly decreased following EGR3 knockdown in a
time-dependent manner compared with the controls (P<0.01;
Fig. 8C). In addition, the number
of regenerative vessels were notably reduced in siEGR3 group, and
the tube length was significantly decreased compared with the
controls (P<0.01; Fig. 8D and
E). Following transfection with siEGR3, the number of apoptotic
cells was significantly elevated compared with the control cells
(P<0.01; Fig. 8F and G).
Furthermore, the number of caspase-3 positive cells markedly
increased following transfection with siEGR3 (Fig. 9A). Correspondingly, caspase-3
activity was significantly promoted in the siEGR3 group compared
with the controls (P<0.01; Fig.
9B). Additionally, the protein and mRNA expression levels of
IL-6, IL-8 and IL-1β were significantly suppressed in the siEGR3
group compared with the controls (P<0.01; Fig. 9C and D). The viability of cells was
significantly increased after miR-335 knockdown compared with cells
transfected with miR-355 inhibitors and siEGR3, of which the
viability was notably higher than those in non-transfected cells,
and cells transfected with miR-NC or siNC (P<0.05; Fig. 9E).
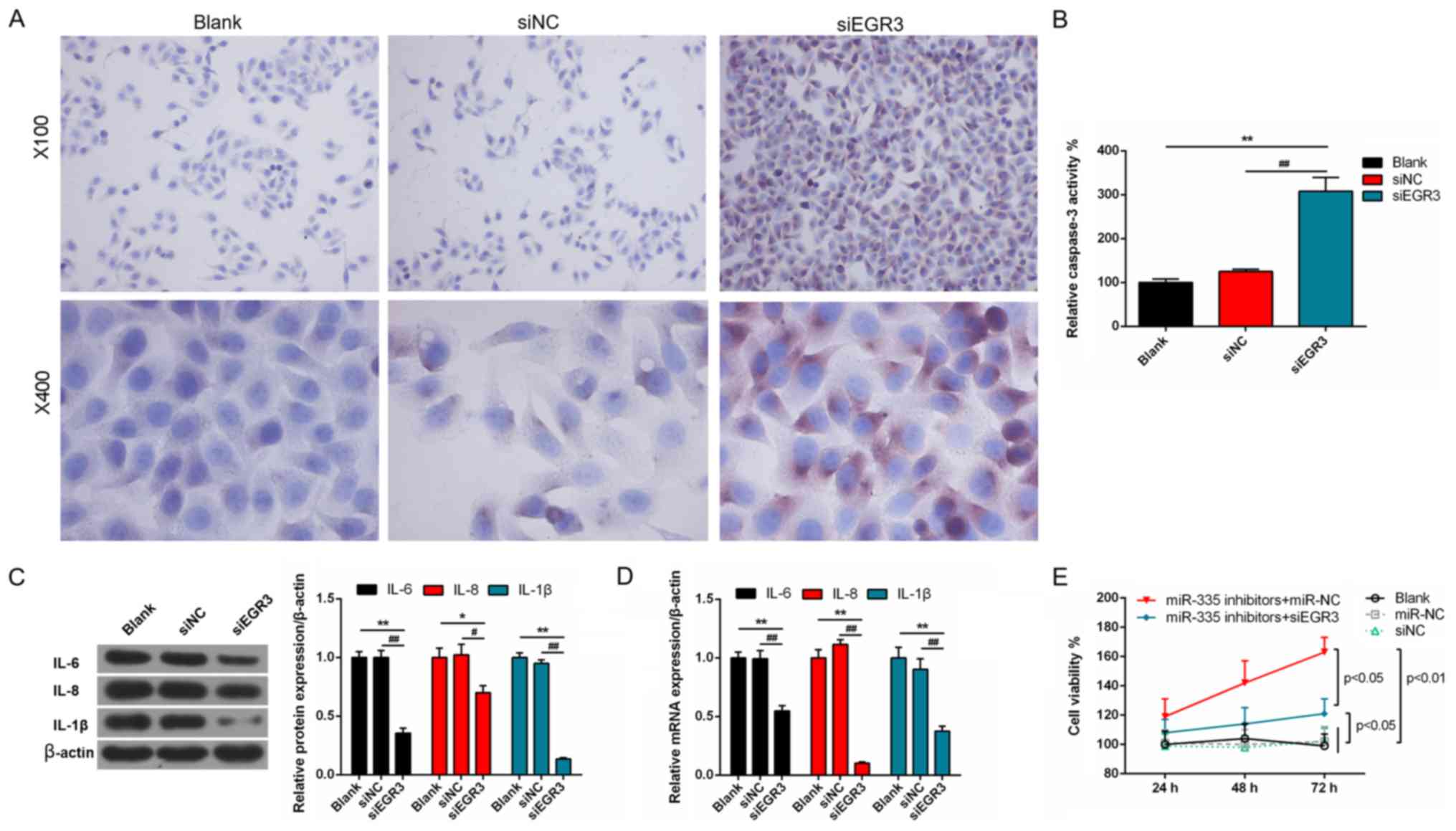 | Figure 9miR-335 exhibits anticancer effects
by regulating EGR3 in DU145 cells. (A and B) Caspase-3 activity was
significantly enhanced by silencing EGR3 expression.
Magnifications, x200 and 400. (C and D) The protein and mRNA
expression levels of IL-6, IL-8 and IL-1β were significantly
inhibited by downregulating EGR3. (E) miR-335 inhibitors suppressed
the viability of cells following the downregulation of EGR3.
*P<0.05, **P<0.01,
#P<0.05, ##P<0.01, n=3. EGR3, early
growth response 3; IL, interleukin; miR, microRNA; NC, negative
control; si, small interfering RNA. |
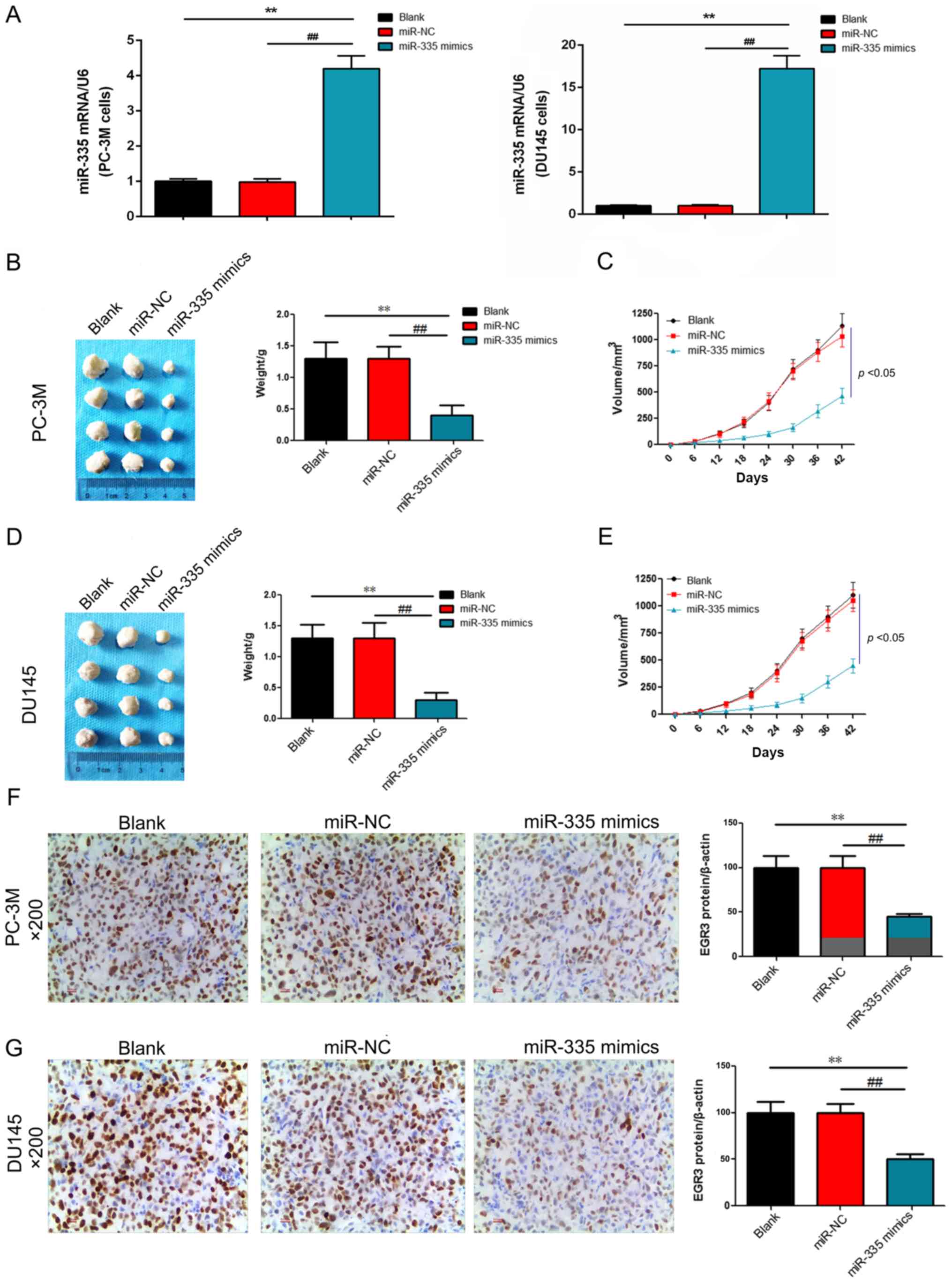
miR-335 inhibits the formation of PC
solid tumor in vivo
In order to explore the effects of miR-335 on the
tumorigenicity of PC, nude mice were transplanted with PC-3M or
DU145 cells to establish PC models in vivo. The results
revealed that the mRNA expression levels of miR-335 were
significantly upregulated in PC-3M and DU145 cells compared with
the controls (P<0.01; Fig.
10A). The tumor weight and volume exhibited no significant
differences between the blank and miR-NC groups; however, when mice
were transplanted with PC-3M + miR-335 mimic or DU145 + miR-335
mimic, the weight and volume of tumors were significantly reduced
(P<0.01 and P<0.05, respectively; Fig. 10B-E). IHC staining demonstrated
that EGR3 was positively expression in the blank and NC groups;
tissues expressing miR-335 mimic exhibited significantly
downregulated EGR3 protein expression (P<0.01; Fig. 10F and G). The results indicated
that miR-335 inhibited the formation of PC solid tumor possibly by
decreasing the expression of EGR3.
Discussion
miRNAs can regulate numerous genes at the
post-transcriptional level, and the aberrant expression of miRNAs
is closely associated with the development of neoplasms in humans
(36). Recently, a substantial
number of cancer-specific miRNAs have been identified as tumor
biomarkers in various types of cancer (37,38).
Several miRNAs, including miR-1, miR-133a, miR-143 and miR-145 were
reported to inhibit tumor development by targeting oncogenic genes
in PC (39-41). EGR3, a member of zinc finger
transcription factors, was demonstrated to be involved in
angiogenesis, inflammation and neurodevelopment (42-44).
Furthermore, it was revealed that EGR3 is involved in the
occurrence and development of numerous types of cancers, and that
EGR3 may be regarded as a tumor suppressor in various cancers
(30,35,45).
In the study, we demonstrated that miR-335 expression was
downregulated, while EGR3 expression was enhanced in PC tissues.
Additionally, miR-335 suppressed cell viability, angiogenesis and
inflammation, and promoted the apoptosis of PC cells. In
vivo miR-355 inhibited the formation of PC solid tumor. We also
reported that EGR3 could be a potential target of miR-335 and
inhibition of EGR3 inhibited the tumorigenesis of a xenograft PC
in vivo.
Downregulated miR-335 expression has been reported
in many types of cancer, such as BCa, acute myeloid leukemia,
gastric cancer and adrenocortical carcinomas (16,46-48).
In the present study, the miR-335 expression was significantly
reduced in the tissues of patients with PC, indicating that miR-335
may serve a vital role in tumorigenesis (49). The malignant transformation of
normal cells is a multilevel and intricate process that is
associated with hereditary and epigenetic changes (50). Oncogenes and tumor-suppressor genes
could be closely related to proliferation and/or apoptosis;
transformed cells may possess anti-apoptosis, invasive, and/or
metastatic properties, thus leading to tumorigenesis (51). In addition, miR-335 can function as
a tumor suppressor by inhibiting proliferation and inducing
apoptosis in various cancer types. For instance, miR-335 expression
is reduced in BCa tissues, blood and cells; and miR-335 regulates
the viability and apoptosis of cells by targeting the upstream
genes of BRCA1 in BCa (23,24).
Furthermore, elevated miR-335 expression inhibits the proliferation
and mammosphere formation of BCa stem-like cells (52). Overexpression of miR-335 can also
reduce cell viability by inhibiting rho-associated,
coiled-coil-containing 1 expression in hepatocellular carcinoma
cells (53), and can promote
apoptosis by targeting specificity protein 1 and BCL-w in lung
cancer (54). Furthermore, miR-335
can also suppress cell viability and induce apoptosis by targeting
members of the inhibitors of apoptosis protein family IAP; miR-335
can regulate the cell cycle and the apoptotic pathways in gastric
cancer (55). miR-335 can initiate
the p53 pathway to inhibit cell proliferation by targeting the
3′-UTR of retinoblastoma 1, and increased proliferation caused by
impaired p53 signaling has been linked to increased miR-335 levels,
suggesting that the regulation of cell viability by miR-335 may be
dependent on p53 (56). Apoptosis
is relevant to various pathological processes, and the cells which
evade apoptosis are involved in tumorigenesis (25). miR-335 was reported as a
proapoptotic miRNA (57); low
miR-335 expression can result in the proliferation and migration of
mesenchymal stem cells (58).
Additionally, inflammation is one of the indicators of cancer and
exerts a crucial effect on tumor development (59,60).
A previous study indicated that miR-335 may be involved in the
IL-6/JNK2/STAT3 pathway (61). We
observed that overexpression of miR-335 could inhibit the viability
and angiogenesis, and promote the apoptosis of PC cells and reduce
the expression of inflammatory cytokines. In addition, we also
silenced the expression of miR-335, and found that the inhibition
of miR-335 could exert opposing effects on than miR-335
overexpression. These results confirmed that miR-335 could function
as a potential tumor suppressor of PC (62).
miR-335 regulates numerous target genes involved in
the progression of cancers (14).
In the present study, EGR3 was identified as a candidate target
gene of miR-335 based on bioinformatics analysis. Furthermore, the
dual-luciferase assay also suggested that EGR3 was a potential
target of miR-335 and was negatively regulated by miR-335 in PC
cells. A previous study reported reduced EGR3 expression in gastric
cancer tissues, which was associated with adverse prognosis
(35); however, EGR3
overexpression increased cell invasion in BCa (63), suggesting that EGR3 may serve
complicated roles in a tumor-specific manner. Of note, we
demonstrated that EGR3 expression was enhanced in PC tissues, which
was inversely correlated with the expression of miR-335. This
suggested that EGR3 may serve a potential role in PC. Furthermore,
EGR3 can mediate multiple biological processes (64). EGR3 was revealed to be involved in
angiogenesis, inflammation and cancer (32,65).
It has been reported that IL-6 can result in enhanced proliferation
and epithelial-mesenchymal transition of PC cells (66). In addition, IL-8 silencing
increased apoptosis, induced cell cycle arrest and improve
chemotherapeutic outcomes of PC cells (67). In the present study, we reduced the
expression of EGR3 in DU145 cells, and observed that the knockdown
of EGR3 inhibited the viability and angiogenesis, promoted
apoptosis and decreased the expression of inflammatory cytokines in
these cells. The results were related to suppressed cell viability,
as reported with miR-335 overexpression. Furthermore, we
synchronously inhibited the expression of miR-335 and EGR3, and
found that the viability of cells was reduced, indicating that
miR-335 inhibited cell viability by targeting the EGR3.
In this study, there were some limitations. For
instance, more cell lines are required to validate the presented
data; more groups could also be established to demonstrate the
targeting of miR-355 to EGR3. We aim to conduct more detailed and
comprehensive experiments in the future, using certain groups:
miR-355 mimics + EGR3 [wild-type (wt)], miR-355 mimics + EGR3
(mutant), miR-355 inhibitors + EGR3 (wt), miR-355 inhibitor + EGR3
(mutant) and another cell line.
In conclusion, miR-335 decreased cell viability and
angiogenesis, suppressed inflammatory-marker expression and
promoted apoptosis by targeting the EGR3 in PC cells. These
findings suggested that miR-335 is closely associated with the
initiation and progression of cancer, and may act as a potential
biomarker in the treatment of patients with PC.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and XY made substantial contributions to
conception and design of the present study. LW and DZ conducted
data acquisition, data analysis and interpretation. PZ drafted the
article and critically revised it for important intellectual
content. All authors approved for the final version to be
published. BW and QL agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of the work are appropriately investigated and resolved.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Xi'an Jiaotong
University
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Acknowledgments
Not applicable.
References
|
1
|
Xiao W, Dai B, Zhu Y and Ye D:
Norcantharidin induces autophagy-related prostate cancer cell death
through Beclin-1 upregulation by miR-129-5p suppression. Tumour
Biol. Dec 5–2015.Epub ahead of print.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Utomo NB, Mochtar CA and Umbas R: Primary
hormonal treatment in localized and locally advanced prostate
cancer: Effectiveness and survival predictive factors. Acta Med
Indones. 44:10–15. 2012.PubMed/NCBI
|
|
4
|
Dasgupta S, Srinidhi S and Vishwanatha JK:
Oncogenic activation in prostate cancer progression and metastasis:
Molecular insights and future challenges. J Carcinog. 11:42012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fraser M, Berlin A, Bristow RG and van der
Kwast T: Genomic, pathological, and clinical heterogeneity as
drivers of person-alized medicine in prostate cancer. Urol Oncol.
33:85–94. 2015. View Article : Google Scholar
|
|
6
|
Fabris L, Ceder Y, Chinnaiyan AM, Jenster
GW, Sorensen KD, Tomlins S, Visakorpi T and Calin GA: The potential
of microRNAs as prostate cancer biomarkers. Eur Urol. 70:312–322.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porkka KP, Pfeiffer MJ, Waltering KK,
Vessella RL, Tammela TL and Visakorpi T: MicroRNA expression
profiling in prostate cancer. Cancer Res. 67:6130–6135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer - the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar :
|
|
9
|
Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu
M, Tepper CG, Evans CP, Kung HJ and deVere White RW: An
androgen-regulated miRNA suppresses Bak1 expression and induces
androgen-independent growth of prostate cancer cells. Proc Natl
Acad Sci USA. 104:19983–19988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu TI, Hsu CH, Lee KH, Lin JT, Chen CS,
Chang KC, Su CY, Hsiao M and Lu PJ: MicroRNA-18a is elevated in
prostate cancer and promotes tumorigenesis through suppressing STK4
in vitro and in vivo. Oncogenesis. 3:e992014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Li B, Li L and Wang T:
MicroRNA-497 suppresses proliferation and induces apoptosis in
prostate cancer cells. Asian Pac J Cancer Prev. 14:3499–3502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nazarov PV, Reinsbach SE, Muller A, Nicot
N, Philippidou D, Vallar L and Kreis S: Interplay of microRNAs,
transcription factors and target genes: Linking dynamic expression
changes to function. Nucleic Acids Res. 41:2817–2831. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cellini F, Morganti AG, Genovesi D,
Silvestris N and Valentini V: Role of microRNA in response to
ionizing radiations: Evidences and potential impact on clinical
practice for radiotherapy. Molecules. 19:5379–5401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo LJ, Wang DD, Wang J, Yang F and Tang
JH: Diverse roles of miR-335 in development and progression of
cancers. Tumour Biol. 37:1–12. 2016. View Article : Google Scholar
|
|
15
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang F, Zheng Z, Guo J and Ding X:
Correlation and quanti-tation of microRNA aberrant expression in
tissues and sera from patients with breast tumor. Gynecol Oncol.
119:586–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shu M, Zheng X, Wu S, Lu H, Leng T, Zhu W,
Zhou Y, Ou Y, Lin X, Lin Y, et al: Targeting oncogenic miR-335
inhibits growth and invasion of malignant astrocytoma cells. Mol
Cancer. 10:592011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dohi O, Yasui K, Gen Y, Takada H, Endo M,
Tsuji K, Konishi C, Yamada N, Mitsuyoshi H, Yagi N, et al:
Epigenetic silencing of miR-335 and its host gene MEST in
hepatocellular carcinoma. Int J Oncol. 42:411–418. 2013. View Article : Google Scholar :
|
|
20
|
Shi L, Jiang D, Sun G, Wan Y, Zhang S,
Zeng Y, Pan T and Wang Z: miR-335 promotes cell proliferation by
directly targeting Rb1 in meningiomas. J Neurooncol. 110:155–162.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu Q, Liu X, Liu Y, Yang J, Lv G and Dong
S: MicroRNA-335 and -543 suppress bone metastasis in prostate
cancer via targeting endothelial nitric oxide synthase. Int J Mol
Med. 36:1417–1425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Z, Zhang Z, Liu Z, Qiu B and Liu K:
MicroRNA-335 inhibits invasion and metastasis of colorectal cancer
by targeting ZEB2. Med Oncol. 31:9822014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heyn H, Engelmann M, Schreek S, Ahrens P,
Lehmann U, Kreipe H, Schlegelberger B and Beger C: MicroRNA miR-335
is crucial for the BRCA1 regulatory cascade in breast cancer
development. Int J Cancer. 129:2797–2806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Y, Zeng F, Wu JY, Li HY, Fan JJ, Mai
L, Zhang J, Ma DM, Li Y and Song FZ: miR-335 inhibits migration of
breast cancer cells through targeting oncoprotein c-Met. Tumour
Biol. 36:2875–2883. 2015. View Article : Google Scholar
|
|
25
|
Zhang S, Xia C, Xu C, Liu J, Zhu H, Yang
Y, Xu F, Zhao J, Chang Y and Zhao Q: Early growth response 3
inhibits growth of hepatocellular carcinoma cells via upregulation
of Fas ligand. Int J Oncol. 50:805–814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhattacharyya S, Fang F, Tourtellotte W
and Varga J: Egr-1: New conductor for the tissue repair orchestra
directs harmony (regeneration) or cacophony (fibrosis). J Pathol.
229:286–297. 2013. View Article : Google Scholar
|
|
27
|
Bolli N, Avet-Loiseau H, Wedge DC, Van Loo
P, Alexandrov LB, Martincorena I, Dawson KJ, Iorio F, Nik-Zainal S,
Bignell GR, et al: Heterogeneity of genomic evolution and
mutational profiles in multiple myeloma. Nat Commun. 5:29972014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boone DN, Qi Y, Li Z and Hann SR: Egr1
mediates p53-independent c-Myc-induced apoptosis via a noncanonical
ARF-dependent transcriptional mechanism. Proc Natl Acad Sci USA.
108:632–637. 2011. View Article : Google Scholar
|
|
29
|
Wirth M, Stojanovic N, Christian J, Paul
MC, Stauber RH, Schmid RM, Häcker G, Krämer OH, Saur D and
Schneider G: MYC and EGR1 synergize to trigger tumor cell death by
controlling NOXA and BIM transcription upon treatment with the
proteasome inhibitor bortezomib. Nucleic Acids Res. 42:10433–10447.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salotti J, Sakchaisri K, Tourtellotte WG
and Johnson PF: An Arf-Egr-C/EBPβ pathway linked to ras-induced
senescence and cancer. Mol Cell Biol. 35:866–883. 2015. View Article : Google Scholar :
|
|
31
|
Pio R, Jia Z and Baron VT: Early growth
response 3 (Egr3) is highly over-expressed in non-relapsing
prostate cancer but not in relapsing prostate cancer. PLoS One.
8:e540962013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baron VT, Pio R, Jia Z and Mercola D:
Early growth response 3 regulates genes of inflammation and
directly activates IL6 and IL8 expression in prostate cancer. Br J
Cancer. 112:755–764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. The National
Academies Press; Washington, DC: 2011
|
|
35
|
Liao F, Ji MY, Shen L, Qiu S, Guo XF and
Dong WG: Decreased EGR3 expression is related to poor prognosis in
patients with gastric cancer. J Mol Histol. 44:463–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang ZD, Qu FY, Chen YY, Ran ZS, Liu HY
and Zhang HD: Involvement of microRNA-718, a new regulator of EGR3,
in regulation of malignant phenotype of HCC cells. J Zhejiang Univ
Sci B. 18:27–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu X, Schwarz JK, Lewis JS Jr, Huettner
PC, Rader JS, Deasy JO, Grigsby PW and Wang X: A microRNA
expression signature for cervical cancer prognosis. Cancer Res.
70:1441–1448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin M, Chen W, Huang J, Gao H, Ye Y, Song
Z and Shen X: MicroRNA expression profiles in human colorectal
cancers with liver metastases. Oncol Rep. 25:739–747. 2011.
|
|
39
|
Fuse M, Nohata N, Kojima S, Sakamoto S,
Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T,
et al: Restoration of miR-145 expression suppresses cell
proliferation, migration and invasion in prostate cancer by
targeting FSCN1. Int J Oncol. 38:1093–1101. 2011.PubMed/NCBI
|
|
40
|
Kojima S, Chiyomaru T, Kawakami K, Yoshino
H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M, et
al: Tumour suppressors miR-1 and miR-133a target the oncogenic
function of purine nucleoside phosphorylase (PNP) in prostate
cancer. Br J Cancer. 106:405–413. 2012. View Article : Google Scholar :
|
|
41
|
Kojima S, Enokida H, Yoshino H, Itesako T,
Chiyomaru T, Kinoshita T, Fuse M, Nishikawa R, Goto Y, Naya Y, et
al: The tumor-suppressive microRNA-143/145 cluster inhibits cell
migration and invasion by targeting GOLM1 in prostate cancer. J Hum
Genet. 59:78–87. 2014. View Article : Google Scholar
|
|
42
|
Nishimura Y, Takizawa R, Koike S,
Kinoshita A, Satomura Y, Kawasaki S, Yamasue H, Tochigi M, Kakiuchi
C, Sasaki T, et al: Association of decreased prefrontal hemodynamic
response during a verbal fluency task with EGR3 gene polymorphism
in patients with schizophrenia and in healthy individuals.
Neuroimage. 85:527–534. 2014. View Article : Google Scholar
|
|
43
|
Li S, Miao T, Sebastian M, Bhullar P,
Ghaffari E, Liu M, Symonds AL and Wang P: The transcription factors
Egr2 and Egr3 are essential for the control of inflammation and
antigen-induced proliferation of B and T cells. Immunity.
37:685–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu D, Evans I, Britton G and Zachary I:
The zinc-finger transcription factor, early growth response 3,
mediates VEGF-induced angiogenesis. Oncogene. 27:2989–2998. 2008.
View Article : Google Scholar
|
|
45
|
Cheng H, Hao S, Liu Y, Pang Y, Ma S, Dong
F, Xu J, Zheng G, Li S, Yuan W, et al: Leukemic marrow infiltration
reveals a novel role for Egr3 as a potent inhibitor of normal
hematopoietic stem cell proliferation. Blood. 126:1302–1313. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Marcucci G, Maharry K, Radmacher MD,
Mrózek K, Vukosavljevic T, Paschka P, Whitman SP, Langer C, Baldus
CD, Liu CG, et al: Prognostic significance of, and gene and
microRNA expression signatures associated with, CEBPA mutations in
cytogenetically normal acute myeloid leukemia with high-risk
molecular features: A Cancer and Leukemia Group B Study. J Clin
Oncol. 26:5078–5087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Soon PS, Tacon LJ, Gill AJ, Bambach CP,
Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson
BG, et al: miR-195 and miR-483-5p identified as predictors of poor
prognosis in adrenocortical cancer. Clin Cancer Res. 15:7684–7692.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2012. View Article : Google Scholar :
|
|
49
|
Vickers MM, Bar J, Gorn-Hondermann I,
Yarom N, Daneshmand M, Hanson JE, Addison CL, Asmis TR, Jonker DJ,
Maroun J, et al: Stage-dependent differential expression of
microRNAs in colorectal cancer: Potential role as markers of
metastatic disease. Clin Exp Metastasis. 29:123–132. 2012.
View Article : Google Scholar
|
|
50
|
Olson P, Lu J, Zhang H, Shai A, Chun MG,
Wang Y, Libutti SK, Nakakura EK, Golub TR and Hanahan D: MicroRNA
dynamics in the stages of tumorigenesis correlate with hallmark
capabilities of cancer. Genes Dev. 23:2152–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang W, Lee DY and Ben-David Y: The roles
of microRNAs in tumorigenesis and angiogenesis. Int J Physiol
Pathophysiol Pharmacol. 3:140–155. 2011.PubMed/NCBI
|
|
52
|
Polytarchou C, Iliopoulos D and Struhl K:
An integrated transcriptional regulatory circuit that reinforces
the breast cancer stem cell state. Proc Natl Acad Sci USA.
109:14470–14475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu H, Li W, Chen C, Pei Y and Long X:
miR-335 acts as a potential tumor suppressor miRNA via
downregulating ROCK1 expression in hepatocellular carcinoma. Tumour
Biol. 36:6313–6319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang H, Li M, Zhang R, Wang Y, Zang W, Ma
Y, Zhao G and Zhang G: Effect of miR-335 upregulation on the
apoptosis and invasion of lung cancer cell A549 and H1299. Tumour
Biol. 34:3101–3109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang B, Huang J, Liu H, Guo W and Li G:
miR-335 directly, while miR-34a indirectly modulate survivin
expression and regulate growth, apoptosis, and invasion of gastric
cancer cells. Tumour Biol. 37:1771–1779. 2016. View Article : Google Scholar
|
|
56
|
Scarola M, Schoeftner S, Schneider C and
Benetti R: miR-335 directly targets Rb1 (pRb/p105) in a proximal
connection to p53-dependent stress response. Cancer Res.
70:6925–6933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qiao J, Lee S, Paul P, Theiss L, Tiao J,
Qiao L, Kong A and Chung DH: miR-335 and miR-363 regulation of
neuroblastoma tumorigenesis and metastasis. Surgery. 154:226–233.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tomé M, López-Romero P, Albo C, Sepúlveda
JC, Fernández-Gutiérrez B, Dopazo A, Bernad A and González MA:
miR-335 orchestrates cell proliferation, migration and
differentiation in human mesenchymal stem cells. Cell Death Differ.
18:985–995. 2011. View Article : Google Scholar :
|
|
59
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Balkwill FR and Mantovani A:
Cancer-related inflammation: Common themes and therapeutic
opportunities. Semin Cancer Biol. 22:33–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shu M, Zhou Y, Zhu W, Zhang H, Wu S, Chen
J and Yan G: MicroRNA 335 is required for differentiation of
malignant glioma cells induced by activation of cAMP/protein kinase
A pathway. Mol Pharmacol. 81:292–298. 2012. View Article : Google Scholar
|
|
62
|
Xiong SW, Lin TX, Xu KW, Dong W, Ling XH,
Jiang FN, Chen G, Zhong WD and Huang J: MicroRNA-335 acts as a
candidate tumor suppressor in prostate cancer. Pathol Oncol Res.
19:529–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Suzuki T, Inoue A, Miki Y, Moriya T,
Akahira J, Ishida T, Hirakawa H, Yamaguchi Y, Hayashi S and Sasano
H: Early growth responsive gene 3 in human breast carcinoma: A
regulator of estrogen-meditated invasion and a potent prognostic
factor. Endocr Relat Cancer. 14:279–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fang F, Shangguan AJ, Kelly K, Wei J,
Gruner K, Ye B, Wang W, Bhattacharyya S, Hinchcliff ME,
Tourtellotte WG, et al: Early growth response 3 (Egr-3) is induced
by transforming growth factor-β and regulates fibrogenic responses.
Am J Pathol. 183:1197–1208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gómez-Martín D, Díaz-Zamudio M,
Galindo-Campos M and Alcocer-Varela J: Early growth response
transcription factors and the modulation of immune response:
Implications towards autoimmunity. Autoimmun Rev. 9:454–458. 2010.
View Article : Google Scholar
|
|
66
|
Rojas A, Liu G, Coleman I, Nelson PS,
Zhang M, Dash R, Fisher PB, Plymate SR and Wu JD: IL-6 promotes
prostate tumorigenesis and progression through autocrine
cross-activation of IGF-IR. Oncogene. 30:2345–2355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Singh RK and Lokeshwar BL: Depletion of
intrinsic expression of Interleukin-8 in prostate cancer cells
causes cell cycle arrest, spontaneous apoptosis and increases the
efficacy of chemotherapeutic drugs. Mol Cancer. 8:572009.
View Article : Google Scholar : PubMed/NCBI
|