Introduction
Epithelial ovarian cancer (EOC) remains the most
lethal gynecological cancer threatening women worldwide, due to its
late presentation, metastasis, recurrence and chemo-resistance.
Although various forms of therapy have been created, >70% of
patients relapse within 18 months (1). Ovarian cancer stem cells are a
specific population of tumor cells with tumor initiation and
self-renewal ability (2), which
are potentially responsible for chemo-resistance,
invasion/metastasis, tumor immune privilege and poor prognosis
(3).
Cancer multicellular spheroids cultured in
suspension system or three-dimensional culture are widely used
approaches to investigate cancer stem cells. Numerous researchers
have demonstrated that culturing cancer cells in suspension to form
spheroids is a more accurate reflection and imitation of clinical
cancer behavior in vitro than traditional adherent culture
system (4-7). Cancer spheroids exhibit resistance to
apoptosis-inducing drugs (4), and
have notably different metabolic profiles (5) and signaling pathways compared with
individual cancer cells (6). 3D
culture systems have gained increasing attention and recognition as
tools for researching antitumor drugs, molecular regulation
mechanisms in cancer stem cells and establishment of personalized
cancer therapy (7).
The standard front-line therapy for EOC is a
combination of debulking surgery and platinum-based chemotherapy.
Certain preclinical and clinical studies have reported the benefits
of adoptive immunotherapy in EOC, including human leukocyte
antigen-restricted tumor infiltrating lymphocytes (8) and MHC-independent immune effectors
such as natural killer (NK) cells (9), lymphokine-activated killer (10) and cytokine-induced killer (CIK)
cells (11,12).
CIK cells are a heterogeneous population of
CD3+CD56+ NK T cells that were first
discovered in the 1990s (13). CIK
cells are generated from human peripheral blood mononuclear cell
(PBMCs) and can be easily expanded in vitro. Numerous basic
research investigations and clinical studies have demonstrated the
safety and efficiency of CIK-based therapies in treating malignant
tumors in vitro and in vivo, including the role of
CIK in killing cancer stem cells (14).
In this study, it was aimed to identify the effects
of CIK cells on ovarian cancer spheroid cells to determine the
mechanism involved in resistance to CIK cells.
Materials and methods
Collection of PBMCs
This study was approved by the Institutional Ethics
Committee of the International Peace Maternity and Child Health
Hospital and Shanghai Red Cross Blood Center (Shanghai, China).
Written informed consent was obtained from all participants.
Criteria for donors were no history of chronic diseases (including
diabetes and hypertension), viral infections (such as hepatitis),
autoimmune diseases (including systemic lupus erythematous,
rheumatoid arthritis and nephritis) and cancer.
Isolation of PBMC, culture and
characterization of CIK cells
Blood samples (n=20) were collected at the Shanghai
Blood Center between January 2016 and December 2016. The male to
female ratio of donors was 1:1. Age range of donors was 28-42 years
old. Peripheral blood (20 ml) was collected with EDTA anticoagulant
from each donor and centrifuged at 400 × g for 10 min to remove
plasma at 4°C. The blood cell pellet was resuspended in 20 ml
phosphate-buffered saline (PBS) and centrifuged at 800 × g for 15
min in Ficoll centrifuge tube at 4°C. PBMCs at the interface were
collected and resuspended in 40 ml PBS and centrifuged at 400 × g
for 10 min at 4°C. The cell pellet was resuspended in 40 ml PBS and
centrifuged at 400 × g for 10 min at 4°C for the second time. PBMCs
were adjusted to 1×106 cells/ml and cultured in 10 ml
GT-T551 culture medium (Takara Bio, Inc., Otsu, Japan) supplemented
with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 1,000 U/ml interferon-γ (Shanghai Chemo Wanbang
Biopharma Co., Ltd., Shanghai, China) in T25 flask for 24 h at 37°C
with 5% CO2. Then cells were stimulated with 30 ng/ml
anti-CD3 antibody (cat. no. TL-101, T&L Biological Technology,
Beijing, China) and 1,000 IU/ml interlukin-2 (IL-2; Shanghai Huaxin
High Biotechnology, Inc., Shanghai, China) to induce CIK cell
proliferation. The cell culture was supplemented with 1,000 IU/ml
IL-2 every 2 days.
To characterize CIK cells induced from PBMCs, cells
were harvested and suspended in ice cold PBS with 10% FBS. Then
cells were stained with labeled primary antibodies according to the
technical data sheet as follows: CD3-fluorescein isothiocyanate
(cat. no. 300305; 1:20; BioLegend, Inc., San Diego, CA, USA), and
CD56-allophycocyanin (cat. no. 362503; 1:20; BioLegend, Inc.) for
30 min at 4°C. Then cells were washed in cold PBS and analyzed
using a flow cytometer (Accuri C6; BD Biosciences, Franklin, Lakes,
NJ, USA).
Cancer cell lines
A2780 epithelial ovarian cancer cell line was
obtained from the Shanghai Cell Bank of Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium (DMEM)/high glucose (Thermo Fisher Scientific, Inc.) medium
supplemented with 10% FBS, streptomycin (100 U/ml) and penicillin
(100 U/ml). Cancer spheroid cells were cultured in DMEM/F12 (Thermo
Fisher Scientific, Inc.) medium supplemented with 10% knock-out
serum (Thermo Fisher Scientific, Inc.), streptomycin (100 U/ml;
Thermo Fisher Scientific, Inc.), penicillin (100 U/ml; Thermo
Fisher Scientific), 1X non-essential amino acids (Thermo Fisher
Scientific, Inc.), 1 mM sodium pyruvate (Thermo Fisher Scientific,
Inc.), 20 ng/ml human recombinant epidermal growth factor, and 10
ng/ml basic fibroblast growth factor. Cobalt chloride
(CoCl2; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was used to induce chemical hypoxia. Recombinant human transforming
growth factor-β1 protein (rhTGF-β1; Abcam, Cambridge, UK) was used
to treat cancer cells for 24 h and then used for subsequent
experiments. All cells were incubated at 37°C in an incubator
containing 5% CO2.
Immunofluorescence (IF) staining
Aldehyde dehydrogenase 1 family member A1 (ALDH1A1)
and Nanog were identified by using the indirect immunofluorescent
labeling technique. Cells (80% confluence) were fixed with 4%
paraformaldehyde for 15 min at room temperature. After washing in
PBS with 0.1% Tween-20 three times, samples were permeabilized
using PBS containing 0.25% Triton X-100 for 10 min at room
temperature. After washing in PBS with 0.1% Tween-20 three times,
samples were blocked by PBS containing 1% bovine serum albumin
(Yeasen, Shanghai, China), 22.52 mg/ml glycine and 0.1% Tween-20
for 30 min at room temperature, and then incubated with the
following primary antibodies at 4°C overnight: ALDH1A1 (cat. no.
ab52492; 1:200; Abcam) and Nanog (cat. no. ab109250; 1:200; Abcam).
Subsequently, cells were incubated with secondary antibody
conjugated to Alexa Fluor® 488 (cat. no. A-11008; 1:200; Thermo
Fisher Scientific, Inc.). Cells were counterstained with DAPI
(Thermo Fisher Scientific, Inc.) and examined under the
fluorescence microscope (three fields of each sample were
evaluated; Leica Microsystems GmbH, Wetzlar, Germany).
Flow cytometry-based cytotoxic activity
assay
Target cells (adherent cancer cells and cancer
spheroid cells of 80% confluence) in 2 ml PBS were labeled with 5
μM fluorescent dye 5- or 6-(N-succinimidyloxycarbonyl)
fluorescein 3′,6′-diacetate (CFSE; Beyotime Institute of
Biotechnology, Haimen, China) at 37°C for 10 min and terminated by
addition of 1 ml FBS. The fluorescence-labeled cells were washed in
PBS three times, resuspended in complete culture medium, and
adjusted cell density to 1×106 cells/ml. CIK cells were
collected, resuspended in complete culture medium and adjusted cell
density to 1×107 cells/ml. To evaluate cellular lysis
effects of CIK cells on cancer cells in different E:T and at
different time, different number of CIK cells were mixed with
100,000 target cells (adherent cells and cancer spheroids) to set
the ratio of effector cells (CIK cells) to target cells (cancer
cells) (E:T) at 5: 1, 10:1 or 20:1. The death rate of target cells
untreated with CIK cells was considered as the ratio of the
spontaneous cell death. The mixture of target cells and CIK cells
were centrifuged at 400 × g for 5 min and cultured at 37°C for 4,
24 and 48 h. To evaluate the death of cancer cells, propidium
iodide (PI; 100 mg/ml, 20 μl/test; Sigma-Aldrich; Merck
KGaA) was used. Following addition of PI, cells were incubated at
4°C for 15 min, washed in PBS, resuspended in 100 μl PBS,
and then analyzed by flow cytometry (Accuri C6; BD Biosciences).
Specific cytotoxicity (%) was calculated according to the formula:
Specific killing efficiency = (target cells from
PI+/CFSE+ quadrant - spontaneous death)/(100
- spontaneous death) x 100 (12).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol (Thermo Fisher Scientific, Inc.). RT to produce cDNA was
performed using PrimeScript™ RT reagent kit with gDNA Eraser
(Takara Bio, Inc.) according to the manufacturer's instructions.
qPCR reactions were performed using the SYBR Green Real-time PCR
Master Mix (Takara Bio, Inc.). PCR primers were designed according
to cDNA sequences in the NCBI database. The following primers were
used: 18s RNA, forward 5′-CGTTGATTAAGTCCCTGC CCTT-3′ and reverse
5′-TCAAGT TCGACCGTCTTCTCAG-3′; intercellular adhesion molecule
(ICAM)-1, forward 5′-GAACC TTACCCTACGCTGCC-3′ and reverse
5′-CAGTGCGGCACGAGAAATTG-3′; ICAM-2, forward
5′-GGTACACGTGAGGCCAAAGA-3′ and reverse 5′-TTTCCACTGAGCCTGTTCGT-3′;
ICAM-3, forward 5′-CCCAAAATTGACCGAGCCAC-3′ and reverse
5′-ACGTTGACGAAGAACGGGAT-3′; HIF1A, forward
5′-GCACAGGCCACATTCACGTA-3′ and reverse 5′-GGCTGTGTCGACTGAGGAAA-3′.
Cycling conditions for the PCR machine were as follows: 95°C for 5
sec, 60°C for 30 sec and 72°C for 30 sec for 40 cycles. All
reactions were performed in a 10 μl volume. Gene expression
levels were evaluated using the ∆∆Cq method (15), standardized to levels of 18s RNA
amplification.
Western blot analysis
For western blotting, cells were lysed using RIPA
(Yeasen) and protein concentration was determined by BCA Protein
Assay Kit (Thermo Fisher Scientific, Inc.). Lysates (10-20
μg) were separated on by 10% SDS-PAGE gel and transferred to
a polyvinyldifluoridine membrane (EMD Millipore, Billerica, MA,
USA). Membranes were blocked with 5% non-fat milk in Tris-HCl
buffer solution containing 0.1% Tween-20 (TBST) and were separately
incubated in the following primary antibodies at 4°C overnight:
ALDH1A1 (cat. no. ab52492, 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), Nanog (cat. no. ab109250, 1:1,000; Cell
Signaling Technology, Inc.), β-actin (cat. no. 66009-1-Ig,
1:20,000; ProteinTech Group, Inc., Chicago, IL, USA), ICAM-1 (cat.
no. ab53013, 1:1,000; Abcam), GAPDH (cat. no. 30203ES10, 1:5,000;
Yeasen), HIF1A (cat. no. WL01607, 1:500; Wanleibio Co., Ltd.,
Shanghai, China), phosphor-mothers against decapentaplegic homolog
3 (phosphor-SMAD3; cat. no. ab52903, 1:1,000; Abcam), SMAD3 (cat.
no. 9523, 1:1000; Cell Signaling Technology, Inc.), phosphor-SMAD2
(cat. no. ab188334, 1:1,000; Abcam), SMAD2 (cat. no. 5339, 1:1,000;
Cell Signaling Technology, Inc.), TGF-β1 (cat. no. ab64715,
1:1,000; Abcam), phospho-IκB kinase (Ikk)-α/β (cat. no. 2697,
1:1000; Cell Signaling Technology, Inc.), phosphor-NF-κB inhibitor
(phosphor-IκB; cat. no. 2859, 1:1,000; Cell Signaling Technology,
Inc.), IκB (cat. no. 4812, 1:1,000; Cell Signaling Technology,
Inc.), phosphor-p65 (cat. no. 3031, 1:1,000; Cell Signaling
Technology, Inc.), p65 (cat. no. 8242, 1:1,000; Cell Signaling
Technology, Inc.), and vascular endothelial growth factor A (VEGFA;
cat. no. ab52917, 1:1000; Abcam). Following washing with TBST,
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit IgG (cat. no. 33101ES60, 1:2,500; Yeasen).
Visualization of blots was performed using a standard protocol for
electro-chemiluminescence (cat. no. P10100, New Cell &
Molecular Biotech Co., Suzhou, China). Levels of GAPDH or β-actin
were used as internal standards.
Flow cytometry-based method for
evaluating cell cycle distribution and anoikis
To assess the cell cycle progression of adherent
cells and cancer spheroids of A2780 (80% confluence), cells were
harvested and fixed with 70% ethanol at -20°C. After 24 h, cells
were stained with 0.1 mg/ml PI (Sigma-Aldrich; Merck KGaA) and cell
cycle distribution was evaluated by flow cytometry (Accuri C6; BD
Biosciences.
To assess the anoikis-resistance of adherent cells
and cancer spheroids of A2780 (80% confluence), cells were
harvested and cultured in sterile flow cytometry tube at 37°C.
After 24 or 48 h, cells were stained with 0.1 mg/ml PI and the
positive rates of PI were evaluated by flow cytometry (Accuri C6;
BD Biosciences).
Blocking CIK-mediated lysis by using
antibody against ICAM-1 on cancer cells
To block the function of ICAM-1 of cancer cells,
cells were incubated with 10 μg/μl ICAM-1 antibody
(cat. no. ab53013; Abcam) or GFP antibody (as a negative control;
cat. no. ab183734; Abcam) in MACS buffer at 4°C for 30 min. Then
cells were used for subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was detected with CCK-8 (Yeasen)
according to the manufacturer's protocol. Cancer cells (5,000 cells
per well) were seeded in 96-well plate with complete culture medium
for 24 h. Then different number of CIK cells were added at
different E:T ratios as follows: 25,000 CIK for 5:1, 50,000 CIK
cells for 10:1 and 100,000 CIK cells for 20:1. After 24 h, cell
viability was measured using Synergy microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Construction of HIF1A knockdown plasmid
and stable transfection
The pGPU6/short hairpin RNA (shRNA) construction for
HIF1A and negative control (NC) were designed and purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). The shRNA
sequences are as follows: HIF1A-KD-1, sense
CACCGGGATTAACTCAGTTTGAACTTTCAAGAGAAGTTCAAACTGAGTTAATCCCTTTTTTG,
anti-sense
GATCCAAAAAAGGGATTAACTCAGTTTGAACTTCTCTTGAAAGTTCAAACTGAGTTAATCCC;
HIF1A-KD-2, sense
CACCGGAAATGAGAGAAATGCTTACTTCAAGAGAGTAAGCATTTCTCTCATTTCCTTTTTTG,
anti-sense
GATCCAAAAAAGGAAATGAGAGAAATGCTTACTCTATTGAAGTAAGCATTTCTCTCATTTCC; NC,
sense CACCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTG,
anti-sense
GATCCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAC. To
establish shRNA cell lines, 1×105 A2780 cells were
transfected with 1 μg pGPU6/shRNA constructs by
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) for 24 h and
selected in complete medium containing 100 μg/ml neomycin
for 14 days. The colonies stably expressing shRNAs were expanded
and used for subsequent experiments.
ELISA
An ELISA kit was used to test whether A2780 spheroid
cells secret TGF-β1. A2780 cells (2×105) were cultured
in suspension conditions. After 48 h, culture supernatant was
collected and tested using the kit according to the instructions of
the manufacturer (cat. no. DB100B; R&D Systems, Inc.,
Minneapolis, MN, USA).
Statistical analysis
Data are presented as the mean ± standard error.
Student's t-test, one-way analysis of variance (ANOVA) or two-way
ANOVA were used to evaluate statistical differences via GraphPad
Prism version 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Tukey's multiple comparisons test was used to compare specific
groups following ANOVAs. P<0.05 was considered to indicate a
statistically significant difference.
Results
A2780 cancer spheroid cells exhibit
cancer stem cell characteristics
In our previous research, cancer stem-like cells
were isolated with drug selection in SK-OV-3 ovarian cancer cell
lines (16) and could be enriched
in ovarian cancer spheroids (17).
Other studies revealed that certain cancer stem cell-associated
markers were upregulated in ovarian cancer spheroids, including
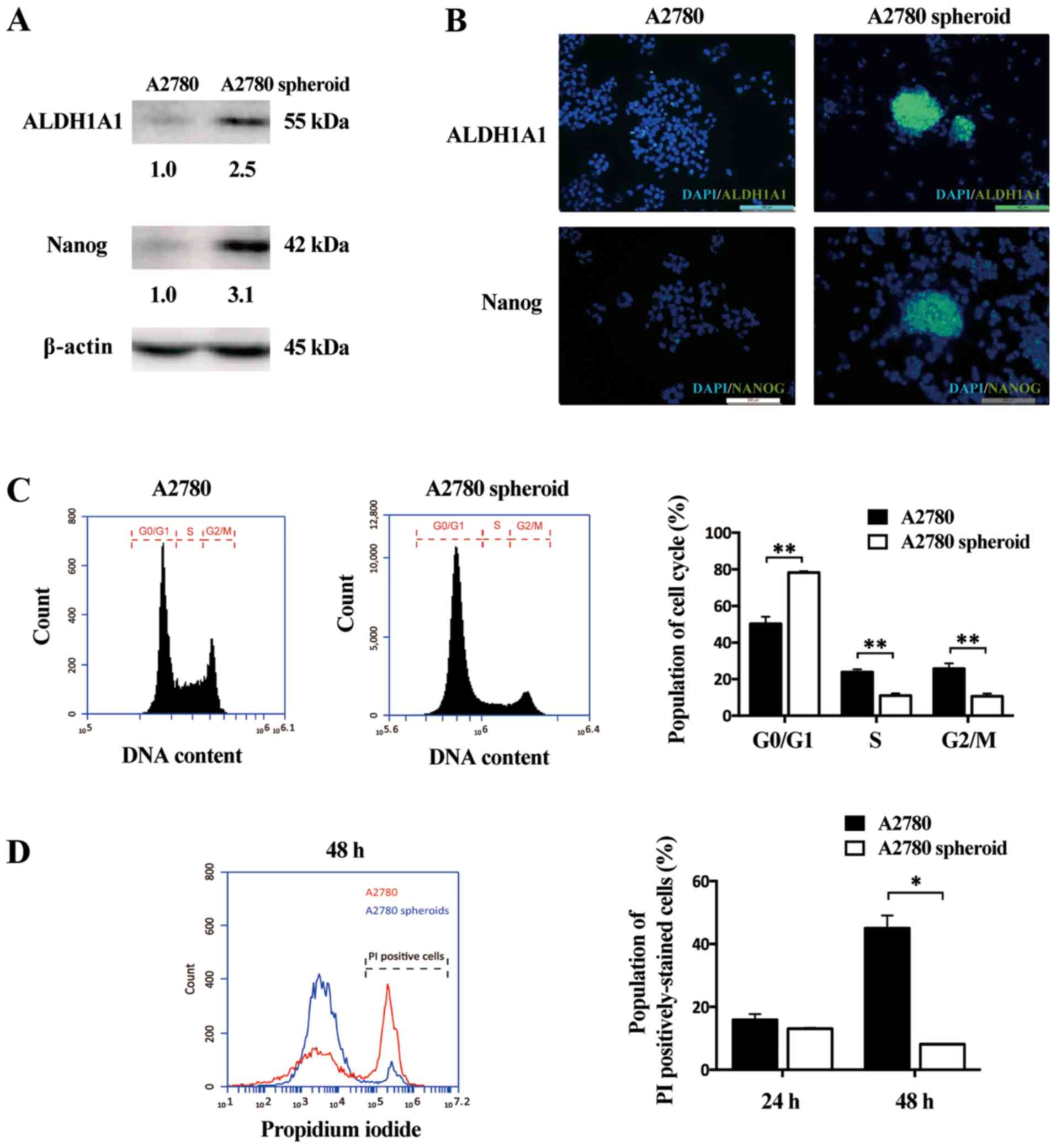
ALDH1A1 (18) and Nanog (19). In the current study, western blot
analysis demonstrated that the protein expressions of ALDH1A1 and
Nanog were strongly upregulated in A2780 spheroids compared with in
adherent cells (Fig. 1A).
Furthermore, IF was performed to examine the protein expression of
ALDH1A and Nanog between A2780 adherent cells and spheroid cells.
These two stemness-related markers (green) were highly expressed in
A2780 spheroid cells than adherent cells, indicating cancer stem
cell-like cells were enriched in spheroid cells (Fig. 1B). The cell cycle distribution of
A2780 adherent cells and A2780 spheroids was also determined. The
population of G0/G1 phases increased and the population of S/G2/M
phases decreased in ovarian cancer spheroids compared with adherent
cells (Fig. 1C). To further
evaluate the difference of proliferative ability between adherent
cells and cancer spheroids, a flow cytometry-based method to detect
anoikis was conducted. Following culture in tubes for 24 or 48 h,
adherent cells and cancer spheroids were stained with PI to label
dead cells. The rate of PI-positive stained cells was lower for
A2780 spheroids than A2780 adherent cells (Fig. 1D). This result indicated that A2780
cancer spheroid exhibited a greater ability of anoikis-resistance
in the anchorage-independent stage than adherent cells. Taken
together, these data indicated that ovarian epithelial cancer
spheroid cells have properties of cancer stem cells.
A2780 ovarian cancer spheroid cells
resist CIK-mediated cellular lysis
CIK cells were generated from PBMCs donated from
healthy donors. The efficiency of inducing
CD3+CD56+ CIK cells was identified by flow
cytometry. The positive rates of CD3, CD56 and CD3/CD56 cells were
95.20±3.96, 46.40±1.84, and 44.50±5.94% (Fig. 2A). To evaluate the cytotoxic
effects of CIK cells against adherent cells and spheroid cells, a
flow cytometry-based method was used. CIK cells induced cell death
of A2780 adherent cells in vitro time-dependently and
dose-dependently (Fig. 2B). After
24 h, although CIK cells could induce death of A2780 spheroid cells
dose-dependently, the cytotoxic was significantly lower compared
with the adherent cells (Fig. 2C).
The effects of CIK cells against A2780 spheroid cells or A2780
adherent cells were as follows: 2.8±0.5 vs. 22.9±1.1% at E:T 5:1;
4.2±3.1 vs. 47.5±0.4% at E:T 10:1; 9.3±1.4 vs. 55.8±5.7% at E:T
20:1 (Fig. 2B). All these results
indicate that A2780 epithelial ovarian cancer spheroid cells
exhibited the ability to resist the cytotoxic effects mediated by
CIK cells.
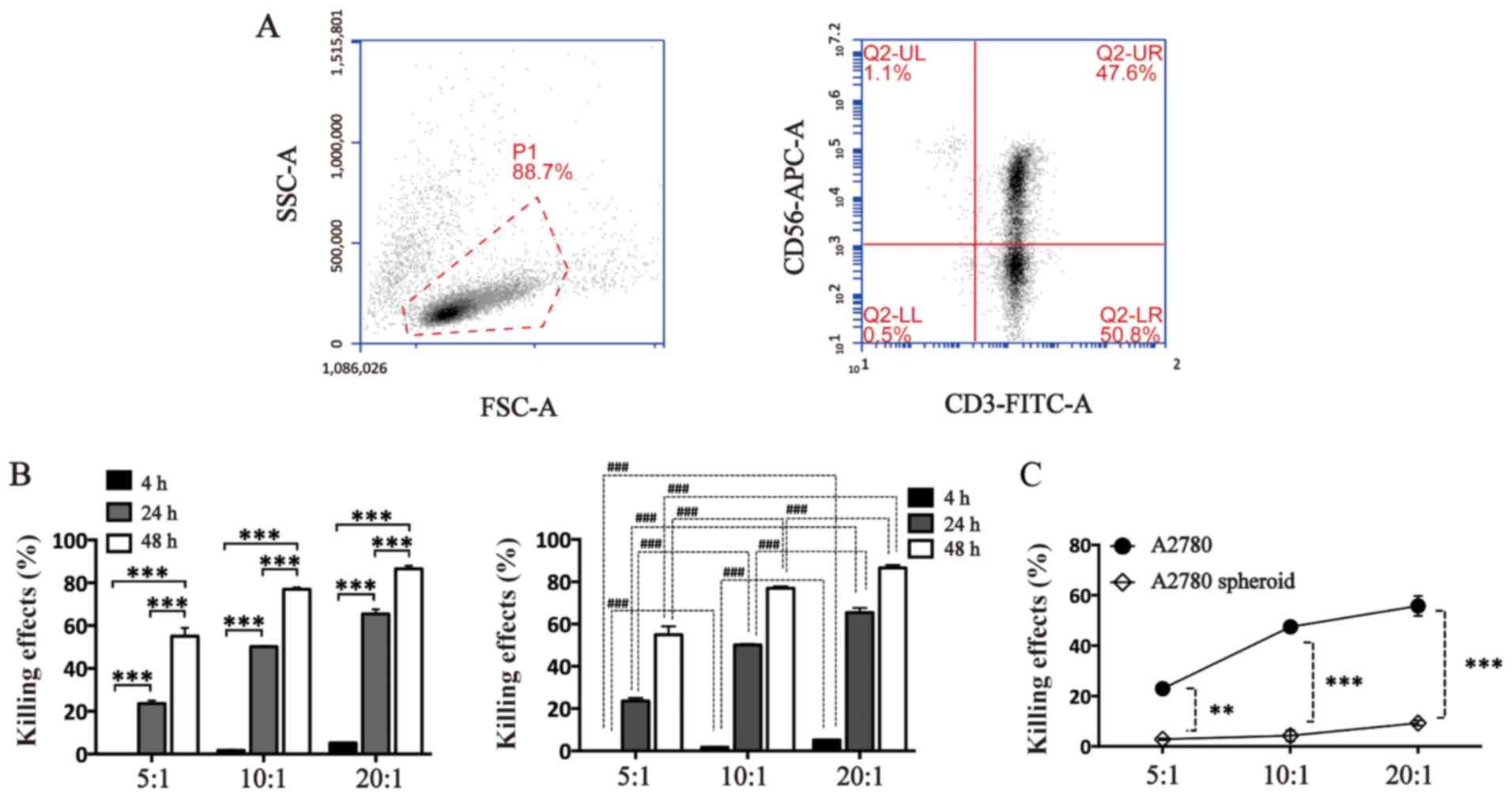 | Figure 2A2780 ovarian cancer spheroid cells
are resistant to CIK-mediated cellular lysis. (A) Efficiency of
inducing CIK cells evaluated by analyzing the positive rate of CD3
and CD56 of CIK cells using flow cytometry. The positive rate of
CD3/CD56 was 44.50±5.94%. (B) Cell death effects of CIK cells
against A2780 adherent cells at different E:T ratios and times (4,
24 and 48 h). CFSE was used to label cancer cells and PI was used
to stain dead cells. Both CFSE-positive and PI-positive cells
presented dead A2780 cells. (C) A2780 adherent cells and spheroid
cells treated with CIK cells at different E:Ts for 24 h. Data are
presented as the mean ± standard error (n=3).
**P<0.01 and *** or
###P<0.001. Two-way analysis of variance was used in
B. CIK, cytokine-induced killer cells; E:T, effector cells to
target cells; SSC, side scatter; FSC, forward scatter; APC,
allophycocyanin; FITC, isothiocyanate; CFSE, 5- or
6-(N-succinimidyloxycarbonyl) fluorescein 3′,6′-diacetate; PI,
propidium iodide. |
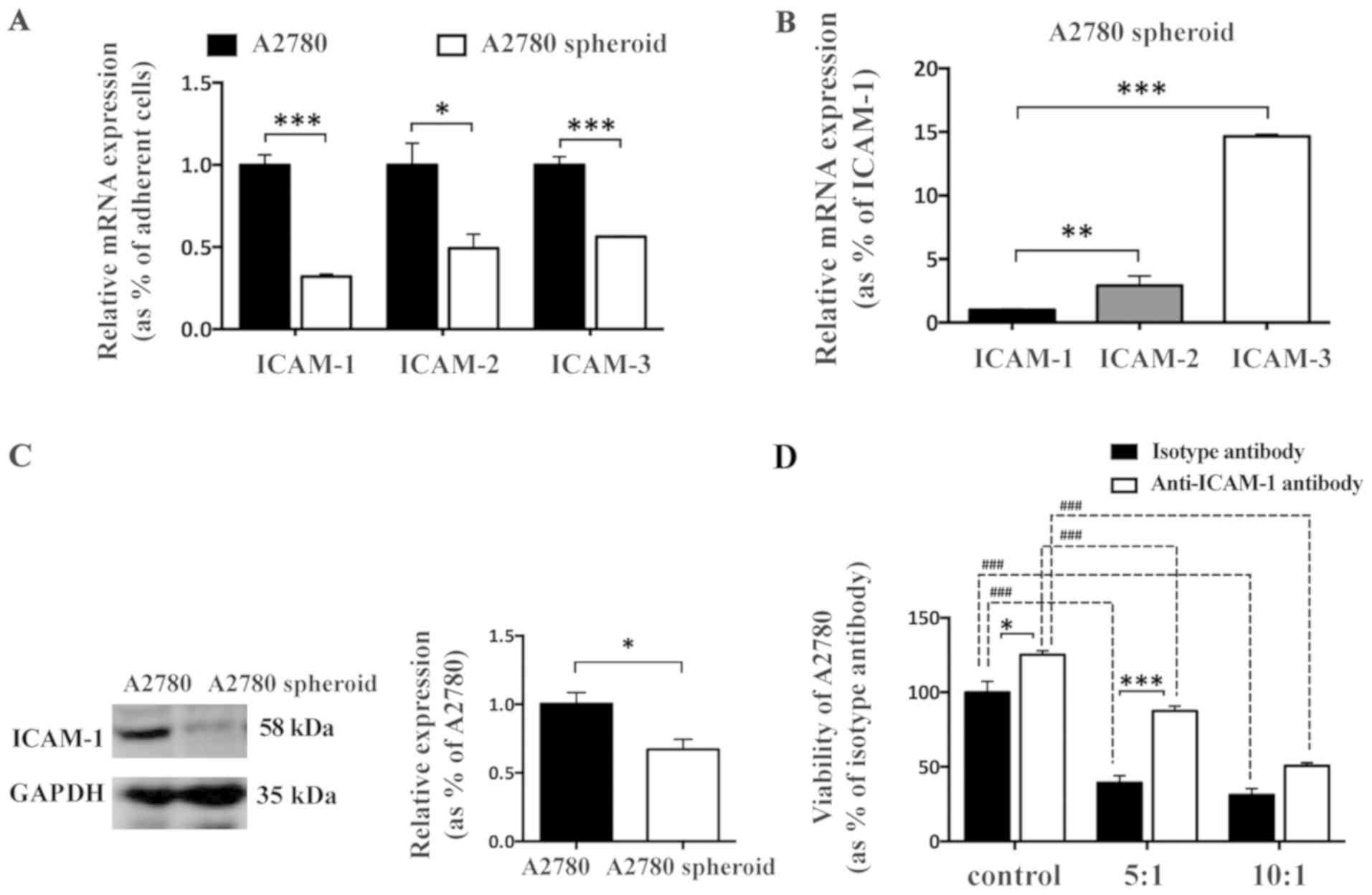
ICAM-1 is downregulated in A2780 spheroid
cells
ICAMs are reported to be important for CIK-mediated
cellular lysis via a role in recognizing tumor target cells
(20). To determine whether
downregulation of ICAMs in EOC spheroid cells contributes to
evasion from CIK-mediated cytotoxic effects, the transcriptional
expression of ICAM-1, -2 and -3 between A2780 adherent cells and
spheroid cells was analyzed by RT-qPCR. The mRNA expression levels
of ICAM-1, -2 and -3 were all downregulated in A2780 spheroid cells
compared with in A2780 adherent cells (Fig. 3A). Additionally, the most
significantly downregulated gene among ICAMs in A2780 spheroid
cells was ICAM-1 (Fig. 3B). The
mRNA expression levels of ICAM-2 and ICAM-3 were 3-fold and 15-fold
higher than the expression level of ICAM-1 in A2780 spheroid cells,
respectively. These results indicated the importance of ICAMs in
resistance to CIK-mediated lysis of A2780 spheroid cells. To
further evaluate the protein expression level of ICAM-1 between
adherent and spheroid A2780 cells, western blot analysis was
performed. ICAM-1 was significantly reduced in A2780 spheroid cells
compared with adherent A2780 cells (Fig. 3C), which was consistent with the
results of RT-qPCR (Fig. 3A). To
further explore whether ICAM-1 was involved in CIK-induced cellular
lysis against A2780 adherent cells, anti-ICAM-1 antibody was used
to directly block the function of ICAM-1 in A2780 adherent cells.
The CCK-8 cellular viability assay demonstrated that adding
antibody against ICAM-1 inhibited CIK-mediated cellular lysis and
increased the viability of A2780 adherent cells dose-dependently
in vitro (Fig. 3D),
indicating that cell-cell interaction is required to mediate the
cytotoxic effect of CIK cells, and ICAM-1 may be involved in cancer
cell target recognition and cellular lysis by CIK cells.
Additionally, anti-ICAM-1 antibody increased the viability of A2780
cells.
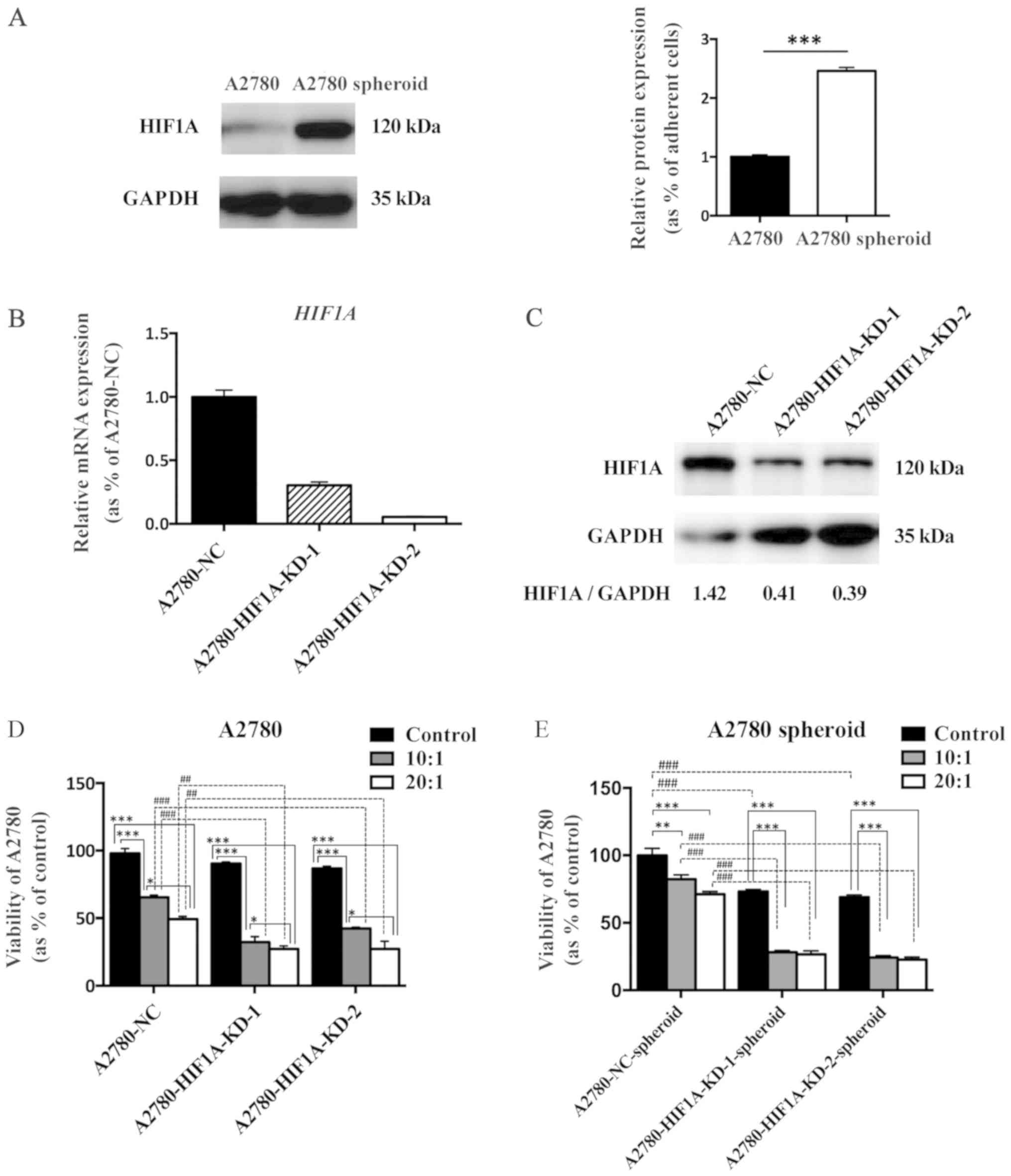
Knockdown of HIF1A in A2780 cells
promotes CIK-mediated cellular lysis
Hypoxia occurs during tumor development. Hypoxia can
be easily established in a 3D culture system in vitro. HIF1A
is upregulated and expression is maintained in the core of cancer
spheroids. HIF1A is also one of the most important hypoxia-related
transcription factors that control the production of pro-angiogenic
molecules and cancer stem cell-like traits in EOC cells (21). Western blot analysis demonstrated
that HIF1A was significantly upregulated in A2780 spheroid cells
compared with adherent cells (Fig.
4A). To explore whether HIF1A contributes to the resistance to
CIK-mediated cellular lysis of spheroid cells, HIF1A knockdown
(A2780-HIF1A-KD-1 and -2) and negative control (A2780-NC) A2780
cell lines were established. RT-qPCR and western blot analysis
demonstrated that expression of HIF1A was significantly
downregulated at the mRNA and protein levels in the knockdown cells
compared with the negative control group (Fig. 4B). A CCK-8 cell viability assay
revealed that knockdown of HIF1A promoted the cytotoxic effects of
CIK cells and decreased the viability of both adherent cancer cells
and cancer spheroids at different E:Ts (Fig. 4C). Additionally, knockdown of HIF1A
in A2780 cells slightly decreased cellular proliferation in
vitro (Fig. 4D and E).
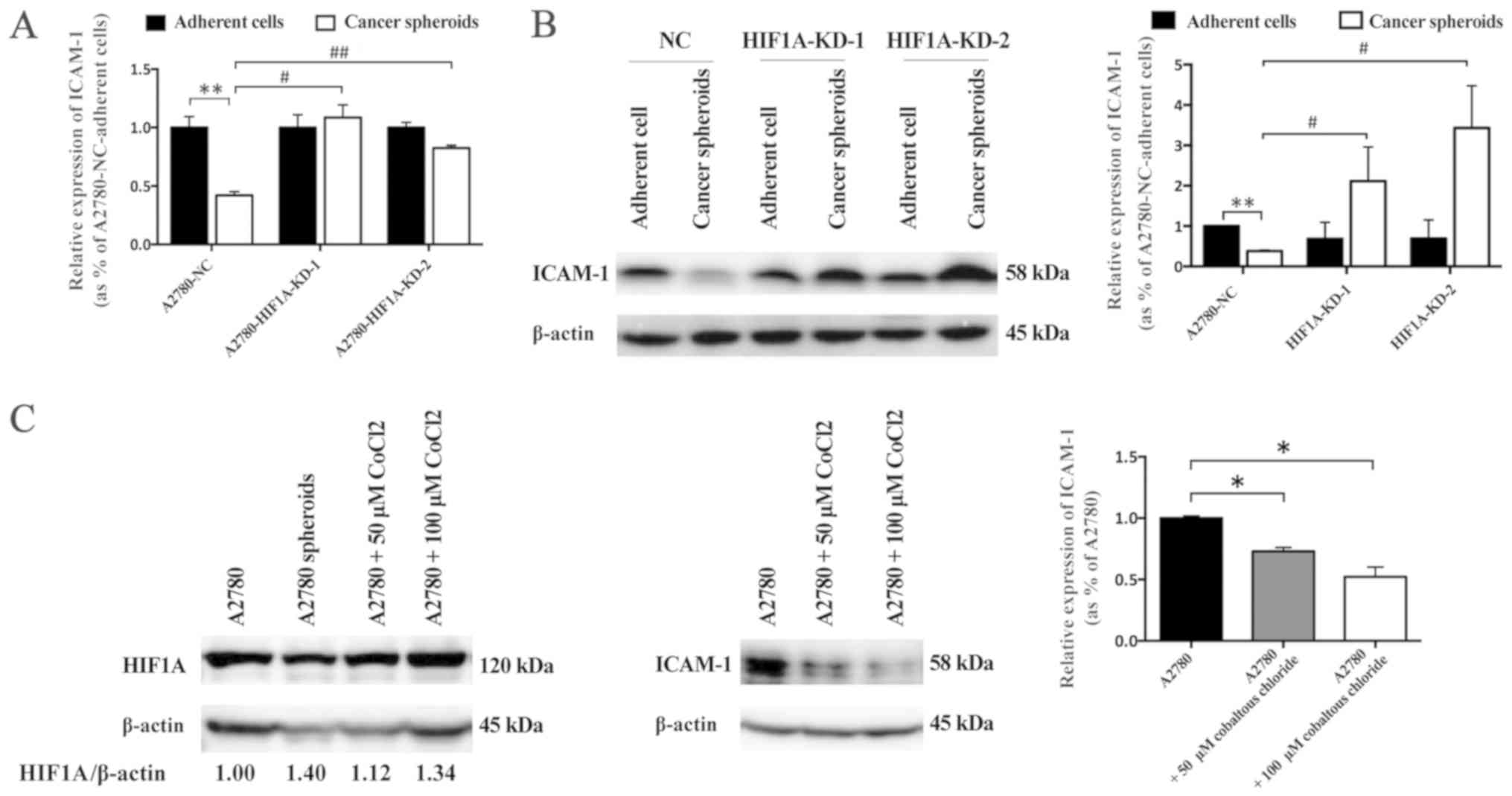
HIF1A regulates expression of ICAM-1 in
A2780 spheroid cells
Previous studies reported that hypoxia promoted
ICAM-1 expression in endothelial cells via activation of
HIF-independent nuclear factor-κB (NF-κB) pathway (22) and C-C motif chemokine ligand
15/Janus kinase 2/signal transducer and activator of transcription
3 pathway (23). However, whether
HIF1A regulates ICAM-1 expression in EOC spheroid cells has not
been reported before. In the current study, the association between
HIF1A and ICAM-1 in A2780 spheroid cells was evaluated. RT-qPCR
demonstrated that the mRNA expression of ICAM-1 was lower in
A2780-NC spheroid cells compared with A2780-NC adherent cells.
However, in the comparison with A2780-HIF1A-KD adherent cells, the
mRNA expression level of ICAM-1 was only slightly affected in the
two HIF1A knockdown spheroid cells. In addition, the mRNA
expression level of ICAM-1 in A2780-HIF1A-KD spheroid cells was
higher than that in A2780-NC spheroid cells (Fig. 5A). Western blot analysis
demonstrated that the protein expression level of ICAM-1 in
A2780-NC spheroid cells was lower than that in A2780-NC adherent
cells (Fig. 5B). Notably,
knockdown of HIF1A did not have an effect on protein expression of
ICAM-1 in adherent cells, but the protein expression of ICAM-1
significantly upregulated in HIF1A knockdown spheroid cells
compared with NC spheroid cells (Fig.
5B). To further elucidate the association between hypoxia and
ICAM-1 in EOC cells, CoCl2 was used to induce chemical
hypoxia and determine the effects of hypoxia/HIF1A in vitro.
Controversial results have been reported regarding the effects of
CoCl2-induced hypoxia on the expression of ICAM-1 in
different cell lines in vitro. It was reported that
CoCl2 upregulated ICAM-1 expression in endothelial cells
(24), but downregulated ICAM-1 in
colorectal cancer cells (25).
Western blot analysis demonstrated that CoCl2
dose-dependently decreased the protein expression level of ICAM-1
in A2780 adherent cells (Fig. 5C).
These observations indicated that HIF1A/hypoxia may regulate the
expression of ICAM-1 in the formation of A2780 spheroids, and may
be partially involved in the resistance of A2780 spheroid cells to
CIK cells.
HIF1A-associated signaling pathways are
activated in EOC cells
Various hypoxia-associated signaling pathways have
been reported to be involved in the regulation of ICAM-1
expression, including TGF-β1/SMADs and NF-κB signaling pathway. The
therapeutic potential of targeting TGF-β1/SMADs (26) and NF-κB signaling pathways
(27) in ovarian cancer were also
investigated. However, it is not clear whether TGF-β1/SMADs and
NF-κB signaling pathways are active in EOC spheroids.
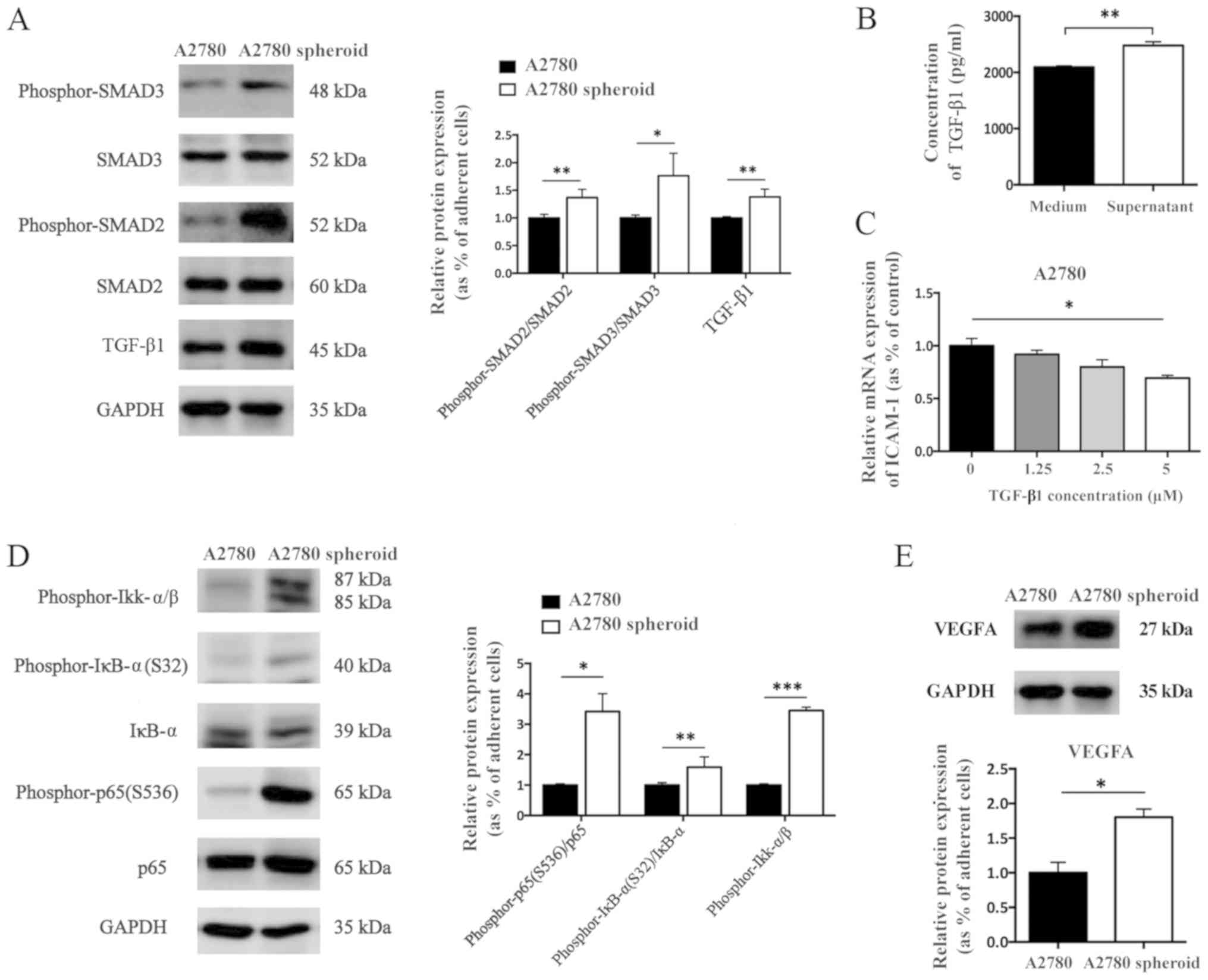
Western blot analysis demonstrated that the ratio of
phosphor-SMAD3 to SMAD3 and phosphor-SMAD2 to SMAD2 were
significantly increased in A2780 spheroid cells compared with in
adherent cells. In addition, the protein expression level of TGF-β1
was upregulated in A2780 spheroid cells compared with in A2780
adherent cells (Fig. 6A). To
evaluate whether A2780 spheroid cells secret TGF-β1 into the
culture medium, an ELISA measuring TGF-β1 was used. The amount of
TGF-β1 in the supernatant of A2780 spheroids was higher than that
in fresh culture medium, which indicated that A2780 spheroids could
secrete TGF-β1 into culture medium (Fig. 6B). To further investigate the
influence of TGF-β1 on the transcriptional regulation of ICAM-1 in
EOC cells, rhTGF-β1 was added to treat A2780 adherent cells for 24
h. rhTGF-β1 significantly decreased the mRNA expression level of
ICAM-1 in A2780 cells (Fig.
6C).
Further results demonstrated that the
phosphorylation level of Ikk-α/β, ratio of phosphor-IκB to IκB, and
ratio of phosphor-p65 to p65 were all upregulated in A2780 spheroid
cells compared with in adherent cells (Fig. 6D). In addition, the protein
expression level of VEGFA was upregulated in A2780 spheroid cells
compared with in adherent cells (Fig.
6E). These results indicated the increased activation of NF-κB
and TGF-β1/SMADs signaling pathway in A2780 spheroid cells compared
with adherent A2780 cells.
Discussion
Numerous studies have reported that epithelial
cancer spheroid cells are more tumorigenic than their parental
tumor cells, adherent cancer cells or differentiated cancer cells,
including primary cells and EOC cell lines (7). The tumor-promoting trait is
associated with the enrichment of ovarian cancer stem cells and
activation of tumorigenic and stemness-associated signaling
pathways in EOC spheroids (18).
Condello et al (18) found
that β-catenin-regulated ALDH1A1 was a target in ovarian cancer
spheroids. Xu et al (19)
observed that microRNA-214 regulates ovarian cancer cell stemness
by targeting p53/Nanog. In this study, ALDH1A1 and Nanog were
significantly upregulated in A2780 cancer spheroids, as determined
via western blot analysis and immunofluorescence. A2780 spheroid
cells exhibited higher capacity for self-renewal compared with
adherent cells in a soft agar colony formation assay in
vitro. Additionally, autophagy-mediated transition from G0
phase to G1 phase also contributes to the expression of OCT-4 and
NOTCH1, two stemness-associated markers, in cancer spheroid cells
(2). Determining the cell cycle
distribution of A2780 and A2780 spheroid can provide the rate of
G0/G1 phase, which is associated with cancer stem cells traits.
Carduner et al (28)
reported that ovarian cancer spheroid cells exhibited
anoikis-resistance, which was important for survival of cancer
cells; the present results are consistent with that. It is
essential to confirm this trait as A2780 spheroid cells exhibited
anoikis-resistance. It may be concluded that the death of cancer
cells is associated with CIK-mediated cellular lysis, but not
anoikis. These results lead to the examination of
stemness-associated markers (ALDH1A1 and Nanog) by western blot
analysis and immunofluorescence, cell cycle distribution and
anoikis-resistance via flow cytometry to investigated the
differences, including in stemness, between adherent A2780cancer
cells and A2780 spheroids.
Cancer cells employ many mechanisms of immune
escape, including downregulation of major histocompatibility
complex (MHC) I expression (29),
secretion of immune suppressive molecules (30), disrupting functions of immune cells
and creating a tumor-favoring microenvironment (31). CIK cells have MHC-unrestricted
activity against cancer stem cells, including in melanoma (32) and hepatocellular carcinoma
(33). However, the effectiveness
of CIK against EOC spheroids and whether spheroid cells endow the
ability to evade immune attack has rarely been discussed
previously.
ICAM-1 is well known for its anti-cancer function by
recruiting immune cells (34).
ICAM-1 is also important for recognition of tumor target cells of
CIK cells. Some studies have reported the tumor-suppressive effects
of ICAM-1 on ovarian cancer. Arnold et al (35) reported that ICAM-1 expressed at low
levels in cancer cells and had a reverse association with patient
survival. de Groote et al (36) showed an immune-independent cellular
role of ICAM-1 in ovarian cancer, whereby upregulation of
endogenous ICAM-1 reduced cancer cell growth in the absence of
immune cells. Lymphocyte function-associated antigen 1/ICAM-1 are
involved in target recognition leading to target cell lysis by CIK
cells, which can be blocked anit-ICAM-1 antibody. In the present
study, the expression level of ICAM-1 was lower in ovarian cancer
spheroids compared with in adherent cancer cells. Furthermore,
blockage of ICAM-1 inhibited CIK-mediated cellular lysis in
adherent cancer cells. These results suggest that ICAM-1 may have a
role in the resistance to immune attack of ovarian cancer
cells.
Tumor hypoxia and HIF1A are known to have important
roles in ovarian cancer metastasis, angiogenesis and resistance to
chemotherapy. In addition, the hypoxic tumor microenvironment and
HIF1A contribute to immune escape in various cancer types,
including prostate cancer, breast cancer (37). Terry et al (38) reported that HIF1A-dependent
induction of epithelial-mesenchymal transition-associated
transcription factors and secretion of immunosuppressive TGF-β
enabled cancer cells to resist cytotoxic T cell and NK
cell-mediated lysis. Furthermore, HIF1A-induced activation of
PI3K/Akt, VEGFA/ERK, nitric oxide/cyclic GMP/protein kinase G
signaling pathways promote immune evasion of cancer cells (39-41).
MHC class I chain-related molecule A (MICA) is an NKG2D ligand that
is also important for recognition of cancer cells and cancer stem
cells by CIK cells in hepatocellular carcinoma (35), nasopharyngeal carcinoma (42). Barsoum et al (34) reported that hypoxia/HIF1A increased
the expression of ADAM10 and decreased surface MICA levels on
cancer cells, and contributed to resistance to lysis mediated by
innate immune effectors, which was attenuated by using a nitric
oxide mimetic. In the present study, EOC spheroid cells evaded CIK
cells-mediated lysis, which was associated with HIF1A-mediated
downregulation of ICAM-1. However, one cell line is not enough to
avoid the significant variations among cancer cell lines due to
their different genetic backgrounds. In future studies more
suitable cell lines, animal models and clinical ovarian cancer
samples will be used to further investigate the association among
HIF1A, ICAM1 and immune reaction.
Various hypoxia-related signaling pathways are
reported to be involved in the regulation of ICAM-1. Sawada et
al (43) reported that TGF-β1
decreased ICAM-1 expression in pancreatic cancer cells, inhibited
lymphocyte-mediated cytotoxic effects and enhanced liver metastatic
potential. Furthermore, Bessa et al (44) reported that immunoblockade of
TGF-β1 partially restored impaired leukocyte functions, indicating
the immunosuppressive role of TGF-β1. In the current study, A2780
spheroid cells could secrete TGF-β1 and the TGF-β1/SMADs signaling
pathway was activated in A2780 spheroid cells. This observation
indicates that TGF-β1/SMADs signaling pathway may have important
role in evasion from CIK cells in A2780 spheroid cells.
Additionally, NF-κB signaling was activated in A2780
cancer spheroids, which is reported to facilitate upregulation of
ICAM-1 in cardiovascular disorders (45). It was also observed that
HIF1A-regulated VEGFA was upregulated in A2780 spheroid cells,
which may have a role in inhibiting the expression of ICAM-1.
Thichanpiang et al (46)
reported that VEGF-A(165) b inhibited NF-κB/tumor necrosis factor-α
(TNF-α)-mediated upregulation of ICAM-1 expression in human retinal
pigment epithelium cells. Li et al (47) also reported that NF-κB promoted
programmed cell death 1 ligand 1 (PD-L1) expression and fostered an
immunosuppressive tumor microenvironment in ovarian cancer, and the
upregulation of PD-L1 promoted immune escape of cancer cells from
CIK cells (48). Furthermore,
combination of PD-L1/PD-1 blockade and CIK cells brought more
benefits to patients with ovarian cancer (33). These results indicate that
HIF1A-mediated upregulation of VEGFA may reduce the effects of
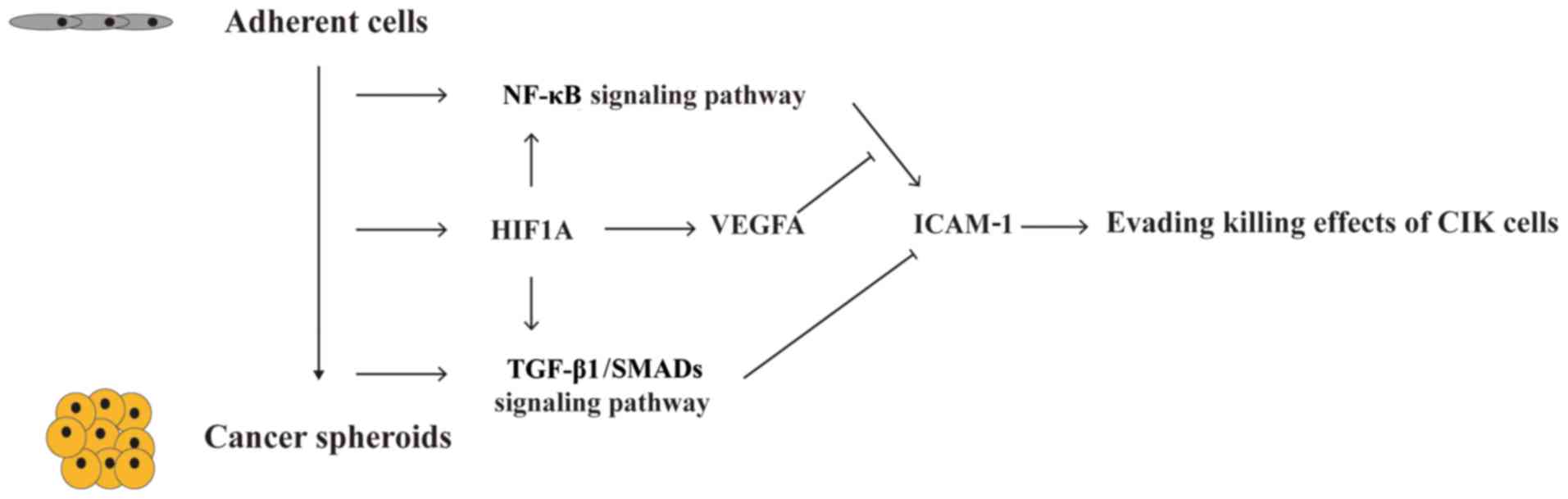
NF-κB/TNF-α on ICAM-1 in A2780 spheroid cells. Thus, the results of
the present study suggest that hypoxia/HIF1A-induced downregulation
of ICAM-1 is responsible for the resistance of A2780 spheroids to
CIK-mediated cellular lysis. HIF1A-associated TGF-β1/SMADs and
VEGFA signaling pathways may mediate the downregulation of ICAM-1
in A2780 spheroid cells (Fig.
7).
CIK cell-based adoptive cell therapy (ACT) has
exhibited activity in several pre-clinical models, suggesting that
ACT may provide benefits in settings of low tumor burden, minimal
residual disease or maintenance therapy. Liu et al (12) reported the clinical feasibility and
efficacy of CIK cells in maintenance therapy against ovarian
cancer, observing a significant increase in median progression free
survival and no severe CIK cell infusion-associated toxicity.
However, clinical research is still at early stage and few studies
of CIK-based therapy in EOC have been conducted. Well-organized,
individualized and combined cell therapy may promote its clinical
effectiveness and application. Additionally, modifying CIK cells
using chemical hetero-conjugation (11) and chimeric antigen receptors
(49) may provide strategies to
optimize and enhance CIK-based cancer therapy.
In conclusion, this work highlighted the
HIF1A-mediated immune evasion of EOC spheroids from CIK
cell-mediated lysis via downregulation of ICAM-1. Although CIK
cells exhibit promising properties against many tumor and cancer
stem cells, paying attention to the mechanisms of immune escape by
cancer cells and identifying solutions to reduce evasion may
generate more effective therapeutic strategies in ovarian cancer
treatment.
Funding
This work was supported by grants from Shanghai
Municipal Council for Science and Technology (grant no.
14411961500), Shanghai Municipal Education Commission-Gaofeng
Clinical Medicine (grant no. 20152236), Shanghai Jiao Tong
University Medicine-Engineering Fund (grant no. YG2015ZD11), The
Interdisciplinary Program of Shanghai Jiao Tong University (grant
no. YG2016MS43) and The National Natural Science Foundation of
China under Grants (grant no. 81741013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL and SB participated in the design of the study.
SB conducted the experiments. BL, QW, TG, QD and XM provided
technical expertise and analyzed the data. SB prepared the
manuscript and DL critically reviewed the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of the International Peace Maternity and Child Health
Hospital and Shanghai Red Cross Blood Center (Shanghai, China).
Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
EOC
|
epithelial ovarian cancer
|
|
CIK
|
cytokine-induced killer
|
|
HIF1A
|
hypoxia inducible factor-1α
|
|
ICAM
|
intercellular adhesion molecule
|
|
CoCl2
|
cobalt chloride
|
|
rhTGF-β1
|
recombinant human transforming growth
factor-β1
|
|
SMADs
|
mothers against decapentaplegic
homologs
|
|
NK
|
natural killer
|
|
PBMC
|
peripheral blood mononuclear cells
|
|
PBS
|
phosphate-buffered saline
|
|
FBS
|
fetal bovine serum
|
|
E:T
|
effector cells to target cells
ratio
|
|
PCR
|
polymerase chain reaction
|
|
CFSE
|
5- or 6-(N-succinimidyloxycarbonyl)
fluorescein 3′,6′-diacetate
|
Acknowledgments
The authors thank Chunhui Wang (Shanghai iCELL
Biotechnology Co., Ltd.), Yan Zhang, Jing Gu and Jing Zhou for
their technical advices. Thanks for Professor Hong Xu (Med-X
Research Institute) and Dr Dingshengzi Zhang (Med-X Research
Institute) for their assistance with flow cytometry.
References
|
1
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Q, Bu S, Xin D, Li B, Wang L and Lai
D: Autophagy is indispensable for the self-renewal and quiescence
of ovarian cancer spheroid cells with stem cell-like properties.
Oxid Med Cell Longev. 2018:70104722018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
You Y, Li Y, Li M, Lei M, Wu M, Qu Y, Yuan
Y, Chen T and Jiang H: Ovarian cancer stem cells promote tumour
immune privilege and invasion via CCL5 and regulatory T cells. Clin
Exp Immunol. 191:60–73. 2018. View Article : Google Scholar
|
|
4
|
Jeong JY, Kang H, Kim TH, Kim G, Heo JH,
Kwon AY, Kim S, Jung SG and An HJ: MicroRNA-136 inhibits cancer
stem cell activity and enhances the anti-tumor effect of paclitaxel
against chemoresistant ovarian cancer cells by targeting Notch3.
Cancer Lett. 386:168–178. 2017. View Article : Google Scholar
|
|
5
|
Liao J, Qian F, Tchabo N,
Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB,
Morrison CD, et al: Ovarian cancer spheroid cells with stem
cell-like properties contribute to tumor generation, metastasis and
chemotherapy resistance through hypoxia-resistant metabolism. PLoS
One. 9:e849412014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Mezencev R, Bowen NJ, Matyunina LV
and McDonald JF: Isolation and characterization of stem-like cells
from a human ovarian cancer cell line. Mol Cell Biochem.
363:257–268. 2012. View Article : Google Scholar
|
|
7
|
Chen MW, Yang ST, Chien MH, Hua KT, Wu CJ,
Hsiao SM, Lin H, Hsiao M, Su JL and Wei LH: The STAT3-miRNA-92-Wnt
signaling pathway regulates spheroid formation and malignant
progression in ovarian cancer. Cancer Res. 77:1955–1967. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiriva-Internati M, Weidanz JA, Yu Y,
Frezza EE, Jenkins MR, Kennedy RC, Cobos E and Kast WM: Sperm
protein 17 is a suitable target for adoptive T-cell-based
immunotherapy in human ovarian cancer. J Immunother. 31:693–703.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carlsten M, Björkström NK, Norell H,
Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K,
Kiessling R, Ljunggren HG, et al: DNAX accessory molecule-1
mediated recognition of freshly isolated ovarian carcinoma by
resting natural killer cells. Cancer Res. 67:1317–1325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Law KS, Chen HC and Liao SK: Non-cytotoxic
and sublethal paclitaxel treatment potentiates the sensitivity of
cultured ovarian tumor SKOV-3 cells to lysis by
lymphokine-activated killer cells. Anticancer Res. 27:841–850.
2007.PubMed/NCBI
|
|
11
|
Chan JK, Hamilton CA, Cheung MK, Karimi M,
Baker J, Gall JM, Schulz S, Thorne SH, Teng NN, Contag CH, et al:
Enhanced killing of primary ovarian cancer by retargeting
autologous cytokine-induced killer cells with bispecific
antibodies: A preclinical study. Clin Cancer Res. 12:1859–1867.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Li H, Cao S, Zhang X, Yu J, Qi J,
An X, Yu W, Ren X and Hao X: Maintenance therapy with autologous
cytokine-induced killer cells in patients with advanced epithelial
ovarian cancer after first-line treatment. J Immunother.
37:115–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rong X, Wei F, Li A, Xiao D and Luo R:
Effective activity of cytokine induced killer cells against
hepatocellular carcinoma including tumor-initiating cells. Med
Hypotheses. 84:159–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Ma L, Lai D, Liu T, Cheng W and Guo L:
Cancer stem-like cells can be isolated with drug selection in human
ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin
(Shanghai). 42:593–602. 2010. View Article : Google Scholar
|
|
17
|
Luo X, Dong Z, Chen Y, Yang L and Lai D:
Enrichment of ovarian cancer stem-like cells is associated with
epithelial to mesenchymal transition through an miRNA-activated AKT
pathway. Cell Prolif. 46:436–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Condello S, Morgan CA, Nagdas S, Cao L,
Turek J, Hurley TD and Matei D: β-catenin-regulated ALDH1A1 is a
target in ovarian cancer spheroids. Oncogene. 34:2297–2308. 2015.
View Article : Google Scholar
|
|
19
|
Xu CX, Xu M, Tan L, Yang H, Permuth-Wey J,
Kruk PA, Wenham RM, Nicosia SV, Lancaster JM, Sellers TA, et al:
MicroRNA MiR-214 regulates ovarian cancer cell stemness by
targeting p53/Nanog. J Biol Chem. 291:228512016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt-Wolf IG, Lefterova P, Mehta BA,
Fernandez LP, Huhn D, Blume KG, Weissman IL and Negrin RS:
Phenotypic characterization and identification of effector cells
involved in tumor cell recognition of cytokine-induced killer
cells. Exp Hematol. 21:1673–1679. 1993.PubMed/NCBI
|
|
21
|
Qin J, Liu Y, Lu Y, Liu M, Li M, Li J and
Wu L: Hypoxia-inducible factor 1 alpha promotes cancer stem
cells-like properties in human ovarian cancer cells by upregulating
SIRT1 expression. Sci Rep. 7:105922017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Winning S, Splettstoesser F, Fandrey J and
Frede S: Acute hypoxia induces HIF-independent monocyte adhesion to
endothelial cells through increased intercellular adhesion
molecule-1 expression: The role of hypoxic inhibition of prolyl
hydroxylase activity for the induction of NF-kappa B. J Immunol.
185:1786–1793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park KH, Lee TH, Kim CW and Kim J:
Enhancement of CCL15 expression and monocyte adhesion to
endothelial cells (ECs) after hypoxia/reoxygenation and induction
of ICAM-1 expression by CCL15 via the JAK2/STAT3 pathway in ECs. J
Immunol. 190:6550–6558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goebeler M, Meinardus-Hager G, Roth J,
Goerdt S and Sorg C: Nickel chloride and cobalt chloride, two
common contact sensitizers, directly induce expression of
intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion
molecule-1 (VCAM-1), and endothelial leukocyte adhesion molecule
(ELAM-1) by endothelial cells. J Invest Dermatol. 100:759–765.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawczyk-Krupka A, Czuba ZP, Kwiatek B,
Kwiatek S, Krupka M and Sieroń K: The effect of ALA-PDT under
normoxia and cobalt chloride (CoCl2)-induced hypoxia on
adhesion molecules (ICAM-1, VCAM-1) secretion by colorectal cancer
cells. Photodiagn Photodyn Ther. 19:103–115. 2017. View Article : Google Scholar
|
|
26
|
Rynne-Vidal A, Au-Yeung CL,
Jiménez-Heffernan JA, Pérez-Lozano ML, Cremades-Jimeno L, Bárcena
C, Cristóbal-García I, Fernández-Chacón C, Yeung TL, Mok SC, et al:
Mesothelial-to-mesenchymal transition as a possible therapeutic
target in peritoneal metastasis of ovarian cancer. J Pathol.
242:140–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan XY, Zhang Y, Zhang JJ, Zhang LC, Liu
YN, Wu Y, Xue YN, Lu SY, Su J and Sun LK: p62/SQSTM1 as an
oncotarget mediates cisplatin resistance through activating
RIP1-NF-κB pathway in human ovarian cancer cells. Cancer Sci.
108:1405–1413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carduner L, Picot CR, Leroy-Dudal J, Blay
L, Kellouche S and Carreiras F: Cell cycle arrest or survival
signaling through αv integrins, activation of PKC and ERK1/2 lead
to anoikis resistance of ovarian cancer spheroids. Exp Cell Res.
320:329–342. 2014. View Article : Google Scholar
|
|
29
|
Aust S, Felix S, Auer K, Bachmayr-Heyda A,
Kenner L, Dekan S, Meier SM, Gerner C, Grimm C and Pils D: Absence
of PD-L1 on tumor cells is associated with reduced MHC I expression
and PD-L1 expression increases in recurrent serous ovarian cancer.
Sci Rep. 7:429292017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo EY, Chu CS, Goletz TJ, Schlienger K,
Yeh H, Coukos G, Rubin SC, Kaiser LR and June CH: Regulatory
CD4(+)CD25(+) T cells in tumors from patients with early-stage
non-small cell lung cancer and late-stage ovarian cancer. Cancer
Res. 61:4766–4772. 2001.PubMed/NCBI
|
|
31
|
Peng J, Hamanishi J, Matsumura N, Abiko K,
Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, et
al: Chemotherapy induces programmed cell death-ligand 1
overexpression via the nuclear factor-κB to foster an
immunosuppressive tumor microenvironment in ovarian cancer. Cancer
Res. 75:5034–5045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gammaitoni L, Giraudo L, Macagno M, Leuci
V, Mesiano G, Rotolo R, Sassi F, Sanlorenzo M, Zaccagna A, Pisacane
A, et al: Cytokine-induced killer cells kill chemo-surviving
melanoma cancer stem cells. Clin Cancer Res. 23:2277–2288. 2017.
View Article : Google Scholar
|
|
33
|
Rong XX, Wei F, Lin XL, Qin YJ, Chen L,
Wang HY, Shen HF, Jia LT, Xie RY, Lin TY, et al: Recognition and
killing of cancer stem-like cell population in hepatocellular
carcinoma cells by cytokine-induced killer cells via NKG2d-ligands
recognition. OncoImmunology. 5:e10860602015. View Article : Google Scholar
|
|
34
|
Long EO: ICAM-1: Getting a grip on
leukocyte adhesion. J Immunol. 186:5021–5023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arnold JM, Cummings M, Purdie D and
Chenevix-Trench G: Reduced expression of intercellular adhesion
molecule-1 in ovarian adenocarcinomas. Br J Cancer. 85:1351–1358.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Groote ML, Kazemier HG, Huisman C, van
der Gun BT, Faas MM and Rots MG: Upregulation of endogenous ICAM-1
reduces ovarian cancer cell growth in the absence of immune cells.
Int J Cancer. 134:280–290. 2014. View Article : Google Scholar
|
|
37
|
Barsoum IB, Hamilton TK, Li X, Cotechini
T, Miles EA, Siemens DR and Graham CH: Hypoxia induces escape from
innate immunity in cancer cells via increased expression of ADAM10:
Role of nitric oxide. Cancer Res. 71:7433–7441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Terry S, Buart S, Tan TZ, Gros G, Noman
MZ, Lorens JB, Mami-Chouaib F, Thiery JP and Chouaib S: Acquisition
of tumor cell phenotypic diversity along the EMT spectrum under
hypoxic pressure: Consequences on susceptibility to cell-mediated
cytotoxicity. OncoImmunology. 6:e12718582017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wan J, Wu W, Che Y, Kang N and Zhang R:
Low dose photodynamic-therapy induce immune escape of tumor cells
in a HIF-1α dependent manner through PI3K/Akt pathway. Int
Immunopharmacol. 28:44–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee YH, Bae HC, Noh KH, Song KH, Ye SK,
Mao CP, Lee KM, Wu TC and Kim TW: Gain of HIF-1α under normoxia in
cancer mediates immune adaptation through the AKT/ERK and VEGFA
axes. Clin Cancer Res. 21:1438–1446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu Y, Hu J, Sun W, Duan X and Chen X:
Hypoxia-mediated immune evasion of pancreatic carcinoma cells. Mol
Med Rep. 11:3666–3672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei F, Rong XX, Xie RY, Jia LT, Wang HY,
Qin YJ, Chen L, Shen HF, Lin XL, Yang J, et al: Cytokine-induced
killer cells efficiently kill stem-like cancer cells of
nasopharyngeal carcinoma via the NKG2D-ligands recognition.
Oncotarget. 6:35023–35039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sawada T1, Kimura K, Nishihara T, Onoda N,
Teraoka H, Yamashita Y, Yamada N, Yashiro M, Ohira M and Hirakawa
K: TGF-beta1 down-regulates ICAM-1 expression and enhances liver
metastasis of pancreatic cancer. Adv Med Sci. 51:60–65. 2006.
|
|
44
|
Bessa X, Elizalde JI, Mitjans F, Piñol V,
Miquel R, Panés J, Piulats J, Piqué JM and Castells A: Leukocyte
recruitment in colon cancer: Role of cell adhesion molecules,
nitric oxide, and transforming growth factor beta1.
Gastroenterology. 122:1122–1132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ohga E and Matsuse T: The relationship
between adhesion molecules and hypoxia. Nihon Rinsho. 58:1587–1591.
2000.In Japanese. PubMed/NCBI
|
|
46
|
Thichanpiang P, Harper SJ, Wongprasert K
and Bates DO: TNF-α-induced ICAM-1 expression and monocyte adhesion
in human RPE cells is mediated in part through autocrine VEGF
stimulation. Mol Vis. 20:781–789. 2014.
|
|
47
|
Li J, Chen L, Xiong Y, Zheng X, Xie Q,
Zhou Q, Shi L, Wu C, Jiang J and Wang H: Knockdown of PD-L1 in
human gastric cancer cells inhibits tumor progression and improves
the cytotoxic sensitivity to CIK therapy. Cell Physiol Biochem.
41:907–920. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen CL, Pan QZ, Zhao JJ, Wang Y, Li YQ,
Wang QJ, Pan K, Weng DS, Jiang SS, Tang Y, et al: PD-L1 expression
as a predictive biomarker for cytokine-induced killer cell
immunotherapy in patients with hepatocellular carcinoma.
OncoImmunology. 5:e11766532016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ren X, Ma W, Lu H, Yuan L, An L, Wang X,
Cheng G and Zuo S: Modification of cytokine-induced killer cells
with chimeric antigen receptors (CARs) enhances antitumor immunity
to epidermal growth factor receptor (EGFR)-positive malignancies.
Cancer Immunol Immunother. 64:1517–1529. 2015. View Article : Google Scholar : PubMed/NCBI
|