Human papillomaviruses (HPVs) are a family of
non-enveloped viruses with cutaneous and mucosal tropism, causing
the most common sexually transmitted disease (1). The association of HPV infections,
particularly persistent infections, with a series of malignancies
has been well-established, exemplified by anogenital (cervical,
vulvar, vaginal, penile and anal) cancer, head and neck cancer
(oropharyngeal squamous cell carcinoma affecting the tonsils,
tonsillar fossa, tongue, base of the tongue and soft palate),
non-melanoma skin cancer in patients with epidermodysplasia
verruciformis (EV), and malignant progression of recurrent
respiratory papillomatosis (2).
These malignancies generally account for ~4.5% of all cancers
(3), among which cervical cancer
is a major concern. It is estimated that ~530,000 new cases and
275,000 deaths from cervical cancer occur annually worldwide,
causing a major global disease burden and loss of life years,
particularly in developing countries (4-6).
Over the past decades, with the elucidation of the
natural history of HPV and HPV-associated diseases, as well as
technical progress, effective screening strategies and robust
prophylactic vaccines have been developed. As the most
groundbreaking scientific discovery in the fight against cervical
cancer, prophylactic vaccines have an excellent safety and efficacy
profile, conferring type-specific immunity against HPV infection
(7). Prophylactic vaccines are
virus-like particles (VLP) self-assembled by L1 capsid without
viral genome, which trigger neutralizing antibody production, thus
blocking the adherence and internalization of HPV by basal cells in
the epithelium. These vaccines appear to be a promising approach to
decreasing the morbidity and mortality of HPV-associated benign and
malignant diseases.
However, despite the prophylactic effect of
currently available vaccines, they are not effective in eradicating
pre-existing HPV infection and associated lesions. In addition,
these vaccines merely induce immunity specific to certain HPV
types, but are unable to fend off other types of the virus;
furthermore, their immunization longevity, which is presumably not
lifelong, has yet to be evaluated. Finally, the inaccessibility to
vaccines and screening programs in resource-poor regions exposes
local populations to a high risk of HPV-associated malignancies,
which have already been proven to be responsible for a substantial
proportion of the worldwide cancer burden. These unresolved issues
necessitate screening programs and further exploration of
therapeutic modalities for persistent HPV infection and associated
lesions.
However, given the fact that most HPV infections
that are accompanied by simultaneous epithelial dysplasia undergo
spontaneous clearance under immunological surveillance within 1-2
years (8), not all HPV infections
require treatment. Therefore, it is advisable to differentiate
persistent HPV infection from transient infection through
biomarkers or lesion characteristics, which, unfortunately, have
not yet been fully elucidated. What is currently known is that
higher-grade lesions have a lower probability of spontaneous
regression, and the process of oncogenesis, from low-grade squamous
intraepithelial lesion (LSIL) through high-grade squamous
intraepithelial lesion (HSIL) to invasive cervical cancer (ICC), is
consecutive. Hence, a wait-and-watch approach is usually adopted
for patients with LSIL to determine whether there is spontaneous
regression or progression, while HSIL is mostly treated by physical
ablative or surgical modalities (9). Such strategies are practicable, but
cannot address the anxiety of patients with LSIL during the long
wait, or exclude the possibility of LSIL progression. Furthermore,
the currently available therapeutic modalities, primarily surgical
treatment, are somewhat destructive and costly, and are
characterized by a high recurrence rate, several side effects and
complications, restricting their applicability in LSIL management.
Therefore, there is a need for non-invasive interventions, such as
medications, that are appropriate for both LSIL and HSIL, or even
ICC, as well as transformation of the overall concept from treating
cancer to treating infection.
The aim of this review article was to discuss the
extensive previous and ongoing investigations into antiviral
agents, therapeutic vaccines and immunomodulators, along with their
respective advantages and drawbacks.
A certain group of diseases were demonstrated to be
associated with HPV infection; these may be divided into benign and
malignant lesions, according to their prognosis, or into mucosal
and cutaneous lesions, according to their primary location.
Specifically, mucosal and cutaneous lesions in anogenital sites
resulting from HPV infection are classified together into one
category due to their similar natural history and etiological
relevance. Hence, HPV-associated diseases may be classified as
anogenital, aerodigestive and non-genital cutaneous infections.
All HPV-associated diseases share dysplasia of the
epithelium as the common pathological characteristic. In
particular, dysplasia of the stratified squamous epithelium in
anogenital sites is further classified into grade 1, 2 and 3
intraepithelial neoplasia, corresponding to mild, moderate and
severe dysplasia, respectively, with grade 3 intraepithelial
neoplasia also representing carcinoma in situ. The term LISL
in cytopathology is equivalent to grade 1 intraepithelial neoplasia
and HSIL refers to grade 2 and 3 intraepithelial neoplasia.
Although most HPV infections in anogenital sites,
regardless of the HPV type, result in low-grade dysplasia, which
may take the form of a benign condylomatous lesion highly likely to
regress spontaneously within 2 years (10), persistent infection with high-risk
HPV types has been recognized as a strong carcinogenic factor.
The role of high-risk HPV infection as a
prerequisite for cervical cancer development has been well
established due to the work of Boshart et al (11,12).
It is believed that almost all cervical cancer cases are caused by
HPV, and that HPV-negative cases were misclassified due to the
limitation of testing methods (false-negative) (13). HPV-16 is the most frequent type
found in cervical cancer, followed by HPV-18, -45, -31, -33 and
other high-risk types (14).
HPV-18 is more common in adenocarcinoma compared with squamous cell
carcinoma, while adenocarcinoma accounts for ~10% of all cervical
cancer cases (15). As regards
low-risk HPV types, such as HPV-6 and -11, they are mostly found in
low-grade lesions, such as cervical intraepithelial neoplasia
(CIN)1, but are rarely found in high-grade lesions (CIN 2, 3 and
ICC).
Anal cancer ranks second in terms of correlation
with HPV infection. A study in France reported that 97% of the
cases of anal cancer are HPV-positive, most of which are
HPV-16-positive (16). Similarly,
it is estimated that 70% cases of vaginal cancer, 45% cases of
penile cancer and 40% cases of vulvar cancer are attributed to HPV,
particularly HPV-16 (17). Anal
intraepithelial neoplasia (AIN), vaginal intraepithelial neoplasia
(VAIN), penile intraepithelial neoplasia (PIN) and vulvar
intraepithelial neoplasia (VIN) are deemed as precursors of the
respective carcinomas, with a certain risk of progression (18,19).
Low-risk HPVs, mainly HPV-6 and -11, are more common
in the aerodigestive tract; therefore, the majority of the
HPV-related aerodigestive tract lesions are benign, such as
papilloma of the oral cavity and recurrent respiratory
papillomatosis (RRP) of the larynx (20). However, regardless of the low risk,
RRP has the potential of spread and progression. Therefore, even
'low-risk' HPVs may progress to cancer.
HPV-16 is the most common high-risk type affecting
the aerodigestive tract, and is considered to be associated with a
small proportion of oropharyngeal cancers, such as those
originating from the tonsils, tonsillar fossa, base of the tongue
and soft palate. Of note, the prevalence of HPV-positive
oropharyngeal cancers has markedly increased over the past decades
(21).
There has always been controversy on the association
between HPV infection and esophageal squamous cell carcinoma
(ESCC). Numerous studies have attempted to investigate the
association between HPV infection and ESCC, but contradictory
results were reported. As regards studies detecting HPV DNA in ESCC
samples, both negative and positive results have been reported
(22-26). However, the mere presence of HPV
DNA in ESCC tissues cannot confirm its etiological role in
tumorigenesis; thus, a large international study (interSCOPE) was
designed to determine whether there were anti-L1 or anti-E6/E7
antibodies in the serum of ESCC patients, with only 4 samples found
positive for HPV-16 E6 and E7 (27). Further evidence demonstrated no
detectable level of HPV DNA integration in ESCC samples (28,29),
and the status of HPV infection did not affect the prognosis of
ESCC (30). These results indicate
that HPV may play a less important role in the development of ESCC,
but a hit-and-run mechanism may be utilized by HPV to induce ESCC.
Large prospective cohort studies with long follow-up are required
to draw definitive conclusions on the involvement of HPVs in
esophageal carcinogenesis.
The HPV types involved in cutaneous infection,
including HPV-1, -2, -3, -4, -10, -27, -28 and -41, among others,
are quite different from those involved in mucosal infection,
usually causing various types of warts, such as common, flat and
plantar warts (31). While
cutaneous HPV infection does not ordinarily cause skin cancer, it
may become complicated when there is a genetic background of EV. EV
patients are susceptible to HPV infection, particularly HPV-5 and
-8, and a certain proportion of EV patients eventually develop skin
cancer at the location of primary lesion (32). Therefore, HPV-5 and -8 are
considered as possible carcinogens. However, the role of HPVs in
non-melanoma skin cancer in the normal population is yet to be
fully elucidated.
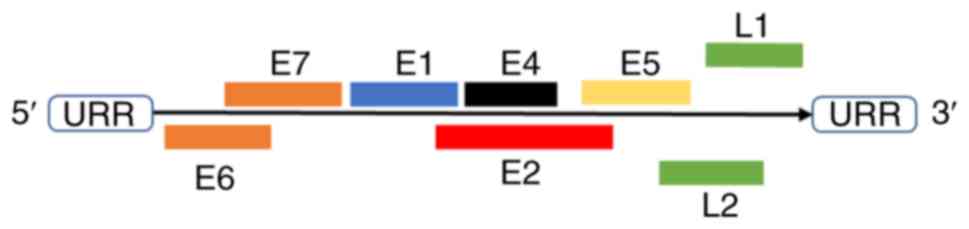
HPVs are non-enveloped, double-stranded circular DNA
viruses with a genome ~8 kb in size, which consist of three parts:
Long control region (LCR), open reading frame (ORF) of six early
genes (E1, E2, E4, E5, E6 and E7) and ORF of two late genes (major
capsid protein L1 and minor capsid protein L2) (Fig. 1) (33). The viral capsid is an icosahedron
composed of 72 pentamers of L1 (360 in total) with variable numbers
of L2 buried inside the capsid surface. To date, >170 types of
HPV have been identified and they may be roughly divided into
cutaneotropic and mucotropic types, while certain types of HPV may
be found in both cutaneous and mucosal lesions. Those mucosal HPVs
are further subdivided into low-risk and high-risk groups,
according to their carcinogenic potency. HPVs only infect the basal
keratinocytes of human stratified squamous epithelia, such as skin
and mucosae. A microwound of the epithelium is a prerequisite for
the transmission procedure, which enables HPVs to reach the
basement membrane (BM) and basal keratinocytes (34). Additionally, active cell division
stimulated by wound healing response is also considered to be
necessary for the infection process (35,36).
It has been demonstrated that HPVs first bind to heparan sulfate
proteoglycans (HSPGs) (37) on the
BM through the L1 capsid protein, which induces subsequent
conformation of L2 minor capsid protein to expose its N-terminal,
where a furin cleavage site is located (38). Upon furin cleavage, viruses shed
from the BM are transferred to the cell surface for secondary
binding events mediated by allosteric L1 (39-41),
and the RG-1 epitope on L2, which is required for L2-mediated
endosomal escape from the late endosomes (42), is exposed. In addition, BM also
acts as a guidance for HPVs to identify permissive cells, i.e.,
basal keratinocytes (mitotically active epithelial cells) rather
than non-permissive (non-dividing) cells (40). Internalization of the virions
follows the secondary binding events, through α6β4 integrins
(43-46), tetraspanins CD63 and CD151
(47-49) and other unidentified receptors.
As regards HPVs adhering to the cell surface through
syndecans (HSPGs located on the cell membrane), it is also possible
that additional components, such as epidermal growth factor (EGF)
and keratinocyte growth factor (KGF), are incorporated after
initial binding occurs, forming large-molecular-weight complexes.
After cleavage by matrix metalloprotease, these complexes are
released from the cell membrane and subsequently bind with
EGFR/KGFR, which mediates the uptake of the complexes (50,51).
The endocytosed virions are transported by
retrograde trafficking sequentially through the endosomal system,
where the capsid disassembles and L1 is retained in a degraded
form, while L2 remains associated with viral DNA (vDNA), trans
Golgi network, endoplasmic reticulum and, finally, into the nucleus
during the nuclear envelope breakdown of mitosis (52).
Following the initial infection by high-risk HPVs,
the viral genome tethers the cellular genome as episomes undergo
transient amplification to extend to ~200 copies per cell,
maintaining the viral episome at a low copy number and forming the
reservoir of infection (53-55).
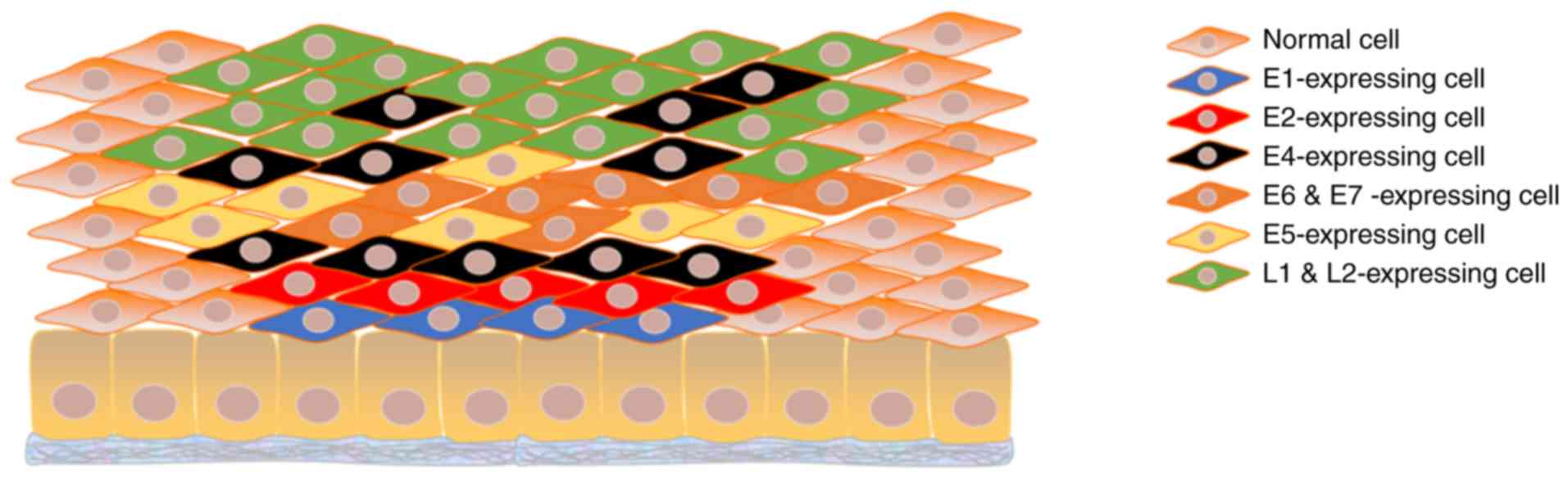
The life cycle of intracellular viruses is closely associated with
the proliferation, differentiation and maturation of keratinocytes,
and the expression of viral proteins is likewise highly ordered. In
the lower layers of the epithelium, where basal and parabasal cells
reside, E6 and E7, referred to as the oncogenic proteins, are
expressed to stimulate cell division. E6, targeting p53, mediates
its ubiquitination through recruitment of E6AP and
proteasome-dependent degradation (56). E7 binds retinoblastoma family
proteins and, therefore, releases E2F to activate gene
transcription necessary for DNA replication. Thus, coordination of
E6 and E7 drives cells to re-enter the cell cycle (35). In addition, E1 helicase is required
for viral genome replication, and E2, which is required for
transcription activation and repression, recruits E1 at the
beginning of replication. Therefore, E1 may be transiently
expressed for the aforementioned initial genome amplification, but
not for genome maintenance (57);
by contrast, E2 is considered to be constitutively expressed for
its role in transcription activation. In the middle layers, with
the advent of genome amplification, the necessary proteins E1, E2,
E4, E5, E6 and E7 increase in abundance. E6 and E7 are still
needed, as they allow cells to re-enter the S-phase, which provides
the conditions for viral genome replication (58). E5 plays a role similar to those of
E6 and E7, but through stabilization of EGFR and enhancement of EGF
signaling and mitogen-activated protein kinase activity (59-61).
In the upper layers, E4, L1 and L2 are predominantly expressed
where packaging of vDNA and assembly of intact virions occur
following genome amplification (Fig.
2). The virus is finally released in the superficial layers of
stratified epithelium along with the shedding of senescent cells.
Apart from virus release, another role of E4 is disintegration of
the stratum corneum by formation of amyloid fibers, enabling
repetitive infection of HPVs (62-64).
On the contrary, this mode is completely changed when lesions
progress (to HSIL or ICC), and the definition 'abortive infection'
is often used to describe the status where most or all layers of
the stratified epithelium are occupied by basal-like cells
overexpressing E6 and E7. Viral genome integration is a late event,
which deregulates E6 and E7 expression by loss of E2 and is highly
associated with invasive lesions (58).
HPVs have long been known to employ multiple tactics
to escape recognition and elimination by the human immune system,
underlying persistent infection.
The unique life cycle of HPV beyond the dermis keeps
it away from immunocompetent cells. The factors contributing to the
immune invisibility of HPV-infected keratinocytes include the
maintenance of low profile of the viral genome in basal cells,
non-secretory proteins, low profile of viral proteins via E2 as
transcription repressor and suboptimal codon usage (65,66),
and the absence of viremia and cell lysis.
HPVs also interfere with normal immune function
through the following mechanisms. A dampened type I interferon
(IFN) signaling cascade results from inhibition of TYK2 kinase
activity (67) and IFN regulatory
factor 3 (IRF3) transactivation (68) by E6, as well as inhibition of IRF1
(69) and IRF9 (70) by E7. An impaired antigen-presenting
process via the major histocompatibility complex-I (MHC-I), also
referred to as human leukocyte antigen (HLA), results from
decreased expression of low-molecular-weight polypeptide (LMP)2,
LMP7, transporter associated with antigen processing (TAP)1, TAP2
and MHC-I (71). Depletion of
Langerhans cells (LC) in the infected epithelium results from
downregulation of E-cadherin on the cell membrane of infected
keratinocytes (72,73). Blocked maturation of LCs results
from activation of the phosphoinositide 3-kinase (PI3K)-Akt pathway
in LCs by L2 capsid protein (74,75).
A shift from Th1- to Th2-response caused by HPV stimulates
interleukin (IL)-10 secretion at the expense of IFN-γ (76,77).
Furthermore, IL-10 is considered to downregulate the expression of
classic HLA-I molecules (76) and
upregulate the expression of non-classic HLA-G molecules (78), which suppress the functions of
cytotoxic T lymphocytes (CTLs) (79), natural killer (NK) cells (80) and dendritic cells (DCs) (81).
Although the infected cells suffer an immune attack,
the apoptosis resistance conferred by E5, which inhibits TRAIL- and
CD95L-mediated apoptosis (82-84),
as well as E6, which accelerates proteasome degradation of p53,
FADD, procaspase-8 and c-Myc (85-87),
enable their survival (88).
Chemical antivirals are crucial for the treatment of
several viral infectious diseases, such as viral hepatitis B and
acquired immunodeficiency syndrome (AIDS), but little is known on
the role of antivirals in HPV infections. This may be partially
attributed to the fact that the targets of classical antivirals are
enzymes encoded by the viral genome, while HPVs hijack the cellular
replication system for their reproduction, except for E1 helicase,
which provides few targets for drug design. However, several
studies and clinical trials have identified and demonstrated the
robust anti-HPV potential of certain acyclic nucleoside
phosphonates (ANPs), among which cidofovir is the most extensively
investigated.
Cidofovir,
(S)-1-(3-hydroxy-2-(phosphonomethoxy)-propyl) cytosine, was
initially designed to inhibit the DNA polymerase and become
incorporated into the daughter DNA, slowing down DNA replication
and viral genome instability. Further studies have demonstrated its
antiviral potential against herpes simplex virus (HSV), which
encodes its own DNA polymerase, and against HPV, in which case no
HPV-specific DNA polymerase is generated. The underlying mechanisms
may involve the fact that cidofovir is more likely to be converted
to its active form as triphosphorylated cidofovir in HPV-infected
cells compared with uninfected cells (89), or that the single replication
origin is the viral episome, in contrast to multiple replication
origins in human genome, which is more susceptible to
chain-terminating factors, with no substitutive origins or
compensatory effects from other origins (90). A phase II clinical trial that
adopted topical cidofovir in the treatment of CIN2 and CIN3
reported a 60.8% response rate in the cidofovir group vs. 20% in
the control group (91). Although
conization may outperform cidofovir in terms of therapeutic
efficacy, these findings have identified an alternative treatment
for patients with concerns regarding postoperative complications.
Similar studies have been performed on women with high-grade vulval
intraepithelial neoplasia, where 4 of 10 had complete regression
and 3 had a partial response (92). Another study evaluated the safety
and efficacy of topical cidofovir in the treatment of PAIN and VIN
in HIV-positive patients, demonstrating 15% complete response, 36%
partial response, 21% stable disease and 6% progressive disease
(93).
However, the two hydroxyls in the phosphonic moiety
of cidofovir decrease its transmembrane activity, and it may be
hypothesized that lipophilic modification of the hydroxyls will
enhance its anti-HPV activity. This hypothesis has already been
confirmed by adefovir and tenafovir, both resulting in
significantly higher efficacy compared with their parent compounds,
but exhibiting no specificity for HPV-infected cells (94), whereas GS-9191 exhibited
selectivity towards HPV-infected cells with enhanced activity,
which was further verified in an animal model (95). More recently, another derivative,
octadecyloxyethyl benzyl 9-((2-Phosphonomethoxy) ethyl)guanine
(ODE-Bn-PMEG), was designed and demonstrated to be effective in
blocking HPV-11, -16 and -18 replication (90). These ANPs appear to be promising,
but further studies are required to evaluate their safety and
efficacy in vivo.
In contrast to ANPs, antivirals targeting proteins
encoded by HPV are characterized by higher specificity. With the
exception of E1 helicase inhibitors, the majority of these
antivirals are novel chemicals hindering protein-DNA or
protein-protein interaction.
As previously mentioned, E1 is recruited to the
origin site of HPV genome with the help of E2, followed by assembly
into double hexamers to start replication, thus hindering the
binding between E1 and E2, or E1/E2 and DNA, which appears to be
very promising in lowering viral load. Both hypotheses have been
evidenced by indandiones for the former (96-98)
and polyamides for the latter (99), respectively. Indandiones were found
to be more effective against HPV-6 and -11, rather than high-risk
HPV types (97). Further
modifications may confer anti-high-risk-HPV activities to these
chemicals. In view of the inability of earlier-synthesized
polyamides to penetrate the cell membrane, previous studies focused
on binding modes between polyamides and DNA, while recent research
has resolved this issue through the synthesis of PA1 and PA25,
which have been proven effective in reducing viral load in cell
experiments (100,101).
The fact that E1 is the only protein encoded by HPV
that has enzymatic activity (102,103), together with the indispensability
of E1 in genome replication, makes E1 the most promising target for
inhibiting viral amplification. Screened out as a small molecule
inhibitor of HPV6 E1 (104),
biphenysulphonacetic acid affects ATP binding of E1 through
allosterism involving Tyr486 (105). Therefore, the activity of
biphenysulphonacetic acid appears to be dependent on the amino acid
sequence (tyrosine residue) and three-dimensional structure of E1,
which is somewhat type-specific. Moreover, it lacks activity in
cell-based assays due to the high intracellular concentration of
ATP (104), which further
prevents the currently available compounds from therapeutic
application.
The well-known interaction between E6 and E6AP,
which mediates the proteasome degradation of p53, provides another
therapeutic target for HPV infection. The recognition of the
E6-binding motif on E6AP, defined as an α-helix with three leucines
on one side and two negatively charged residues on the opposite
side, enabled researchers to screen out small molecular inhibitors
among therapeutic agents (106,107). Further medicinal development
based on this finding may prove to be useful.
Apart from the cellular replication system, several
other host mechanisms usurped by HPV to facilitate its survival and
reproduction may serve as targets, and corresponding agents are
referred to as host-dependent viral inhibitors.
The oncoprotein E7 was also demonstrated to be
associated with class I histone deacetylases (HDAC)1 and 2
(108) under the mediation of
Mi2β (109), responsible for
proliferation-promotion and long-term viral episome maintenance
(108). HDACs decrease the
acetylation state of histones, thereby inhibiting target gene
transcription. The relocation of HDACs induced by E7 from
proliferation-promoting genes to cell cycle-arresting or apoptosis
genes leads to upregulation of the former and downregulation of the
latter. Therefore, HDAC inhibitors may be able to interrupt the
multiple pathogenic processes. Current HDAC inhibitors are mostly
Zn2+-chelating agent binding to the Zn-binding catalytic
domain of HDAC, including short-chain fatty acids (110), hydroxamic acids (111,112), benzamide derivatives (113), epoxyketones and cyclic peptides
(114). Although these were
effective in arresting the proliferation of cervical cancer cells
(115-117), they may need further optimization
prior to clinical application due to their broad spectrum of
cellular targets.
Cyclin-dependent kinase (Cdk) 2, activated by cyclin
A or E, is crucial for driving the cell cycle as well as for the
pathogenesis of HPV. Cdk2 is stimulated by E7 via multiple
mechanisms (118) and
subsequently promotes cellular proliferation. Cdk2 also accelerates
viral genome amplification by phosphorylating E1 at specific sites
in its N-terminal domain (119-121) and causes abnormal copy numbers of
centrosome with genome instability (122-124). Phosphorylation of E1 induces its
nuclear retention and, thus, facilitates the formation of hexamers
that are necessary for replication initiation (125,126). Inhibitors of Cdk2, therefore, are
considered to halt the proliferation of cervical carcinoma cells
and restore normal centrosome replication. Cell-based assays using
roscovine (127,128) and indirubin-3′-oxime (IO)
(129) have already confirmed
this hypothesis. Novel IO derivatives with higher specificity and
potency towards Cdk2 have already been discovered (130) and, together with other, more
potent Cdk2 inhibitors, such as flavopiridol, should be further
evaluated to establish their role in HPV infection treatment.
The cellular transcription factor Sp1 can also bind
to LCR in the viral genome of both low- and high-risk HPV types,
and is involved in the transcription of HPV genes (mainly E6 and
E7) independently of E2 (131,132). Inhibiting this process with
derivatives of nordihydroguaiaretic acid (NDGA), i.e.,
tetra-O-methyl NDGA and tetra-acetyl NDGA (133), resulted in cell growth arrest
(134), apoptosis and tumor size
reduction in tumor-bearing mice (134).
The etiology of cervical carcinoma as a viral
infectious disease established over the last ~50 years has enabled
its prevention through prophylactic vaccination (Fig. 3). Current prophylactic vaccination,
however, will not achieve a significant reduction in the morbidity
and mortality of cervical carcinoma until successful world coverage
by vaccination, which is an elusive goal due to the high cost of
HPV vaccines. In addition, it usually takes 10-30 years (median,
23.5 years) for CIN 2/3 to progress to ICC (135); therefore, considering the size of
the population with existing HPV infections and the natural history
of HPV-associated precancerous diseases, tens of years may pass for
the vaccines to exert their protective effects against cervical
cancer. In summary, a significant decrease in the incidence of
cervical cancer will not be achieved until vaccinated women enter
the peak age range of cervical cancer.
The demand for clearance of established HPV
infection and regression of precancerous/cancerous lesions has
prompted the design of therapeutic vaccines. Apart from the humoral
immunity triggered by prophylactic vaccines, therapeutic vaccines
trigger cell-mediated immune responses. Among the proteins encoded
by HPV, E6 and E7 are the best-characterized and the most
extensively investigated due to their carcinogenic role and
constitutive expression in infected cells (58). Live vector vaccines or DNA vaccines
including wild-type E6 and E7 with the potential to transform cells
are usually inactivated at certain sites into detox forms. Other
targets include E1, E2 and E5, according to their expression mode
during the life cycle of HPV. However, it must be mentioned that
once viral genome is integrated, most genes are lost, except E6 and
E7, which are expressed at even higher levels without repression of
E2 (136-139). Several strategies for the
development of therapeutic vaccines have been studied, including
live vector, nucleic acid, peptide-based, protein and cellular
vaccines, with several vaccine candidates currently in clinical
trials (Fig. 4).
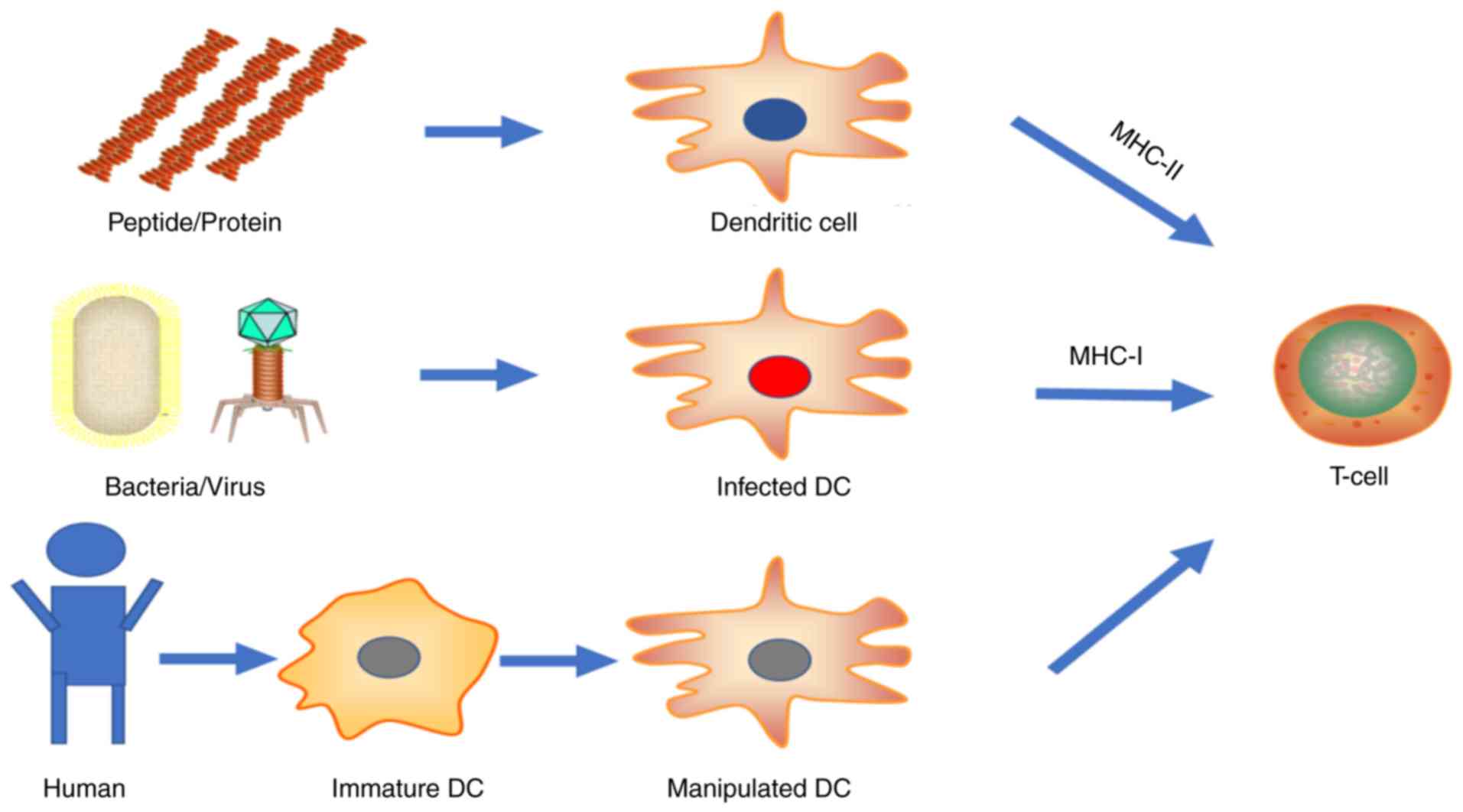
Live vector vaccines utilize attenuated bacteria or
viruses to transport genes of interest into cells. These
microorganisms infect host cells, proliferate intracellularly and
spread to surrounding cells in a restricted manner prior to immune
elimination. The gene of interest is then expressed by the host
protein expression system, leaving the protein at its most natural
state. These allow class I MHC antigen presentation by infected
cells, but inefficiently. Another more high-efficiency antigen
presentation pathway is achieved by dendritic cell (DC) ingestion
of free antigen released by infected cells through exosomes,
secretion or apoptosis. Thereafter, DCs process antigen and present
it on the cell surface for T-cell recognition and activation
through both class II and class I (by cross-presentation) MHC
pathways. More directly, DCs residing in the vaccination sites
(e.g., Langerhans cells in the dermis) may be infected by the live
vectors, which simplifies the antigen presentation process. In
addition, the vector itself acts as an adjuvant to enhance the
immunogenicity of the vaccine due to its pathological nature, thus
promoting an even stronger immune response. Unfortunately, live
vector vaccines carry the risk of overwhelming infection in
immunocompromised patients, and the live vector itself can induce
neutralizing antibody production, thus abrogating the boost effect
of repeated vaccination. In rare cases, the host may have
pre-existing immunity against the live vector, leading to
vaccination failure.
Viral vectors include adenoviruses, adeno-associated
viruses, alphaviruses, lentiviruses and vaccinia viruses.
Alphaviruses, including Semliki forest viruses, Sindbis viruses and
Venezuelan equine encephalitis viruses are RNA viruses that are
transformed into RNA replicon form by substitution of their
3′-terminal structural genes with the genes of interest. These RNA
replicons are capable of autonomous amplification but fail to
assemble into intact virions due to lack of capsomers. In view of
their similarity to nucleic acid vaccines, they will be discussed
in the respective section.
TA-HPV is a recombinant vaccinia virus expressing
HPV-16/18 E6/E7 with 3 completed clinical trials. A phase I/II
study in patients with advanced cervical cancer reported that an
HPV-specific CTL response was detected in one of three evaluable
patients (152). Another phase I
study conducted in patients with International Federation of
Gynecology and Obstetrics stage Ib or IIa cervical cancer found
that 4 of 29 patients developed an HPV-specific CTL response after
a single vaccination (153). A
phase II study in patients with HPV-positive high-grade VIN or VAIN
with a duration of up to 15 years observed a lesion reduction of at
least 50% in 5 of 12 (42%) patients, with 1 patient exhibiting
complete regression (154).
TG4001 is a recombinant modified vaccinia Ankara
(MVA) expressing HPV-16 E6, E7, and IL-2. A phase I study including
21 cases of HPV16+ CIN2/3 patients revealed that 48%
experienced disease regression, whereas 38% exhibited HPV DNA
clearance (155).
MVA E2 is a recombinant MVA expressing BPV E2. In a
phase III study in patients with HPV-induced anogenital
intraepithelial neoplasia, a 90% clearance in female patients and
100% clearance in male patients was reported (156).
Also designed to express the fusion protein of
calreticulin and HPV16 E7, adenovirus vector was demonstrated to
eradicate established tumors in mice (157). Clinical trials of this vaccine,
however, have yet to be conducted.
Antigens delivered in the form of peptides or whole
proteins directly are referred to as subunit vaccines. As the most
classical type of vaccines, they are considered to be safer
compared with live vector vaccines for lack of infectivity and
persistent existence.
Peptide vaccines, with an excellent safety profile
and good stability, are easy to produce and more cost-effective.
However, peptides are truncated from the whole protein and, thus,
may not contain the necessary epitopes for DC processing and
presentation through the MHC pathway. Furthermore, the fact that
each individual has his own HLA type means that epitopes recognized
by MHC may differ among different individuals. Therefore, for valid
immunization, the epitopes have to be identified so as to match the
MHC-specificity of each individual, which limits the mass
production of peptide-based vaccines (158). This was addressed by the
synthesis of long overlapping peptides covering the entire sequence
of the protein. Low immunogenicity is another drawback of
peptide-based vaccines, which may be addressed by co-administration
of adjuvants, co-expression of cytokines and fusion protein with
Toll-like receptor (TLR) ligands.
HPV16-SLP (ISA101) is a peptide-based vaccine
consisting of nine HPV16 E6 and four HPV16 E7 synthetic long
overlapping peptides with adjuvant Montanide ISA51. In a phase II
clinical trial in patients with HPV16+ VIN3, 15 of the
19 patients exhibited a clinical response (79%), with a complete
response in 9 patients (47%). Moreover, all patients developed a
vaccine-induced T-cell response, but patients with stronger
IFN-γ-associated CD4+ and CD8+ T-cell
response were more likely to achieve complete response (159). Other studies have also
demonstrated the therapeutic potential of ISA101 (160-163).
PepCan, a vaccine consisting of four HPV16 E6
synthetic peptides and Candin as an adjuvant, has completed the
dose-escalation phase of a phase I clinical study in patients with
HSIL, with 50 µg reported as the most effective dose, and
histological regression of disease in 45% of all patients (164).
Protein-based vaccines utilize the full-length E6
and/or E7 protein to immunize humans. Compared with peptide-based
vaccines, they contain all the epitopes and exclude MHC
restriction, but due to their exogenous nature mostly presented by
the MHC II pathway (165), they
tend to mount humoral immunity and have low immunogenicity. These
problems may be overcome by fusion protein targeting them to DCs
and giving them access to the MHC I antigen presentation
pathway.
TA-CIN is a fusion protein of HPV16 L2, E6 and E7.
As the first vaccine that combines therapeutic and prophylactic
effects, it was tested on healthy subjects, demonstrating a
TA-CIN-specific IgG in 24 of the 32 vaccinated patients and
cell-mediated immunity in 25 of the 32 patients (166). A phase II clinical trial
conducted in patients with VIN 2/3 combined topical imiquimod and
TA-CIN, reporting a 63% lesion response 1 year after vaccination
(167).
DNA vaccines are plasmid DNAs carrying genes of
interest and transfecting host cells for sustained antigen
expression. DNA vaccines usually do not increase neutralizing
antibody production, allowing repeated vaccinations (172). However, they raise concerns
regarding the risks of exogenous DNA integration, albeit without
supportive evidence. Unlike viral vaccines, DNA plasmids cannot
autonomously amplify or spread intercellularly, resulting in the
main drawback of DNA vaccines, namely poor immunogenicity (173,174).
VGX-3100, a DNA vaccine encoding HPV-16/18 E6/E7,
which is administered intramuscularly with electroporation, has
finished its phase IIb clinical trial in HPV16/18+
CIN2/3 patients. A total of 53/107 (49.5%) patients with VGX-3100
treatment in contrast to 11/36 (30.6%) placebo subjects exhibited
histopathological regression in the per-protocol analysis. In
addition, 55/114 (48.2%) patients with VGX-3100 treatment in
contrast to 12/40 (30.0%) placebo subjects had histopathological
regression in the modified intention-to-treat analysis (195).
RNA replicon-based vaccines are derived from
alphaviruses. They replicate intracellularly and express genes of
interest with no risk of integration. However, the instability of
RNA limits their application and puts forward a more stable form,
namely suicidal DNA vaccines, also referred to as DNA-launched RNA
replicons. In contrast to RNA replicon-based vaccines, suicidal DNA
vaccines have an extra step of transcription into RNA replicons
after transfection. Compared with DNA vaccines, the
self-replication of these vaccines increases antigen expression,
and the final apoptosis of transfected cells resulting from
extensive double-stranded RNA production avoids the possibility of
genomic integration. However, early apoptosis of host cells causes
inadequate stimulation towards T lymphocytes and insufficient
T-cell response. Co-transfection of genes encoding anti-apoptotic
proteins in the vector (199) and
use of flavivirus Kunjin (KUN) (200,201) have been introduced to address
this issue. These vaccines appear to be highly promising for the
treatment of HPV infections, but require further investigation.
Cell-based vaccines include extracting and
isolating cells (such as DCs or T lymphocytes) from the peripheral
blood or excised tumors of patients, manipulating and expanding
them ex vivo, and finally transferring the selected and
modified cells back to the patients.
As the most robust antigen-presenting cells (APCs),
DCs are mostly studied in the context of immune system activation,
circumventing the necessity to access antigens to APCs in
vivo and to use adjuvants. Antigen-loaded DCs are produced
ex vivo through transfection by viral vectors (202,203), transduction by DNA or RNA vectors
(204,205), pulsation of antigenic peptides,
proteins or tumor cell lysates (205-209). Inevitably, DC-based vaccines have
certain drawbacks: First, the production of DC-based vaccines is
resource-intensive and individualized, so that large-scale
production and widespread use appear to be impractical; second, it
is difficult to unify the culturing techniques, which leads to
spotty vaccine quality and lack of standard evaluation criteria;
third, in order to prime immunity against antigens, DCs have to
migrate to lymphoid tissues, and this poses the question of
determining the most efficient administration route among
intramuscular, subcutaneous, intravenous and intranodal injection,
or other options; fourth, the limited longevity of DCs caused by
T-cell-mediated apoptosis weakens the magnitude of immune response,
which has been partially addressed by transfecting DCs with siRNA
silencing pro-apoptotic proteins (207,208,210).
In a phase I clinical study, DCs were pulsed with
HPV16/18 E7 and then co-administered with IL-2 back to the
patients. An E7-specific CD8+ response was observed in
all patients (211). Another
phase I clinical trial was conducted in patients with stage Ib or
IIa cervical cancer and DCs were pulsed with HPV16/18 E7 as well as
keyhole limpet hemocyanin, promoting DC maturation. As a result, 8
of 10 patients exhibited an increase in E7-specific CD8+
T lymphocytes (212).
ACT selects tumor antigen-specific CTLs, engineers
or activates them and expands them ex vivo, and they are
finally re-administered to the patients. A pilot study using HPV16
E6/E7-specific T cells in patients with metastatic cervical cancer
reported complete regression in 2 of 9 patients (218). TCR gene-engineered T cells were
also introduced to target HPV+ epithelial cancer cells
in cell-based assays and exhibited killing avidity (219).
Immunomodulators are agents stimulating innate
and/or adaptive immunity for pathogen elimination. As regards
treatment of persistent HPV infection, imiquimod is the most
extensively studied and widely used immunomodulator.
Imiquimod, an agonist for TLR7, can trigger
expression of cytokines and induce a local immune response. The
raised levels of cytokines activate local immune cells and initiate
immune clearance of HPV-infected cells. Adverse events may include
itching, erythema, burning, irritation, tenderness, ulceration and
pain. The antiviral as well as antitumor properties of imiquimod
have been demonstrated in basal cell carcinoma (220), VAIN (221), VIN (222) and AIN (223). A more popular method is topically
applying imiquimod in combination with therapeutic vaccines.
However, neither of these treatments have been licensed.
IFN is widely used in the treatment of low-risk
HPV-associated anogenital warts, but its role in high-risk
HPV-associated pre-cancerous lesions and cancers remains a subject
of debate; therefore, more large scale, double-blind, randomized
controlled trials are required.
Candidate therapies for HPV infection mainly
include chemical antivirals, therapeutic vaccines and
immunomodulators. Therapeutic vaccines appear to be the most
promising approach to eliminating HPV in terms of effectiveness,
while each type of vaccine comes with its own advantages and
disadvantages. Generally, most vaccines must be injected into
certain sites, except for Lactobacillus-based vaccines, which are
administered orally. Mucosal immunity primed by Lactobacillus-based
vaccines satisfies the needs for anti-HPV immunity, as the life
cycle of HPV expands beyond the BM. These synergistic factors make
Lactobacillus-based vaccines a promising candidate. For all
therapeutic vaccines, enhancement of immunogenicity is the common
requirement for clinical application.
Antivirals robustly inhibit the proliferation of
HPVs, but are unable to eradicate infection, particularly by
integrated viruses. The safety profiles of HDAC, Cdk2 and Sp1
inhibitors must be further investigated, as they have numerous
downstream targets. It would be preferable to verify the
therapeutic effects of these inhibitors at doses not interfering
with normal cell functions.
In summary, the coordinated use of various
strategies may act synergistically against HPV infection. The
combination of prophylactic with therapeutic vaccines, or of
different types of therapeutic vaccines as in prime-booster
strategy, or of therapeutic vaccines with immunomodulators,
antivirals or checkpoint inhibitors, and other similar
combinations, may have a profound impact on the treatment of HPV
infection.
The present study was supported by the Key Research
and Development Program of Sichuan Province (grant no. 2017SZ0002)
and the State Key Laboratories Development Program of China (grant
no. 2018ZX09201018).
Not applicable.
YL and HL wrote the initial manuscript. RP created
the figures and YY contributed writing material and new ideas. XZ
and XQ revised the manuscript and approved the final version. All
authors have read and approved the final version of the manuscript
for publication.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests to disclose.
The authors would like to thank Professor Xia Zhao
for her guidance and insightful suggestions.
|
1
|
Dunne EF, Unger ER, Sternberg M, McQuillan
G, Swan DC, Patel SS and Markowitz LE: Prevalence of HPV infection
among females in the United States. JAMA. 297:813–819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M and Franceschi S: Global burden of human
papil-lomavirus and related diseases. Vaccine. 30(Suppl 5):
F12–F23. 2012. View Article : Google Scholar
|
|
3
|
de Martel C, Plummer M, Vignat J and
Franceschi S: Worldwide burden of cancer attributable to HPV by
site, country and HPV type. Int J Cancer. 141:664–670. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plummer M, de Martel C, Vignat J, Ferlay
J, Bray F and Franceschi S: Global burden of cancers attributable
to infections in 2012: A synthetic analysis. Lancet Glob Health.
4:e609–e616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
6
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kash N, Lee MA, Kollipara R, Downing C,
Guidry J and Tyring SK: Safety and efficacy data on vaccines and
immunization to human papillomavirus. J Clin Med. 4:614–633. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: Unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View Article : Google Scholar
|
|
9
|
Massad LS, Einstein MH, Huh WK, Katki HA,
Kinney WK, Schiffman M, Solomon D, Wentzensen N and Lawson HW: 2012
ASCCP Consensus Guidelines Conference: 2012 updated consensus
guidelines for the management of abnormal cervical cancer screening
tests and cancer precursors. Obstet Gynecol. 121:829–846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giuliano AR, Harris R, Sedjo RL, Baldwin
S, Roe D, Papenfuss MR, Abrahamsen M, Inserra P, Olvera S and Hatch
K: Incidence, prevalence, and clearance of type-specific human
papillomavirus infections: The Young Women's Health Study. J Infect
Dis. 186:462–469. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boshart M, Gissmann L, Ikenberg H,
Kleinheinz A, Scheurlen W and Zur Hausen H: A new type of
papillomavirus DNA, its presence in genital cancer biopsies and in
cell lines derived from cervical cancer. EMBO J. 3:1151–1157. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dürst M, Gissmann L, Ikenberg H and Zur
Hausen H: A papil-lomavirus DNA from a cervical carcinoma and its
prevalence in cancer biopsy samples from different geographic
regions. Proc Natl Acad Sci USA. 80:3812–3815. 1983. View Article : Google Scholar
|
|
13
|
Tjalma W: HPV negative cervical cancers
and primary HPV screening. Facts Views Vis Obgyn. 10:107–113.
2018.
|
|
14
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abramowitz L, Jacquard AC, Jaroud F,
Haesebaert J, Siproudhis L, Pradat P, Aynaud O, Leocmach Y,
Soubeyrand B, Dachez R, et al: Human papillomavirus genotype
distribution in anal cancer in France: The EDiTH V study. Int J
Cancer. 129:433–439. 2011. View Article : Google Scholar
|
|
17
|
De Vuyst H, Clifford GM, Nascimento MC,
Madeleine MM and Franceschi S: Prevalence and type distribution of
human papillomavirus in carcinoma and intraepithelial neoplasia of
the vulva, vagina and anus: A meta-analysis. Int J Cancer.
124:1626–1636. 2009. View Article : Google Scholar
|
|
18
|
Stanley MA, Winder DM, Sterling JC and
Goon PK: HPV infection, anal intra-epithelial neoplasia (AIN) and
anal cancer: Current issues. BMC Cancer. 12:3982012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Seters M, van Beurden M and de Craen
AJ: Is the assumed natural history of vulvar intraepithelial
neoplasia III based on enough evidence? A systematic review of 3322
published patients. Gynecol Oncol. 97:645–651. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gillison ML, Broutian T, Pickard RK, Tong
ZY, Xiao W, Kahle L, Graubard BI and Chaturvedi AK: Prevalence of
oral HPV infection in the United States, 2009-2010. JAMA.
307:693–703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaturvedi AK, Engels EA, Pfeiffer RM,
Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M,
Cozen W, et al: Human papillomavirus and rising oropharyngeal
cancer incidence in the United States. J Clin Oncol. 29:4294–4301.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koh JS, Lee SS, Baek HJ and Kim YI: No
association of high-risk human papillomavirus with esophageal
squamous cell carcinomas among Koreans, as determined by polymerase
chain reaction. Dis Esophagus. 21:114–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang SK, Guo LW, Chen Q, Zhang M, Liu SZ,
Quan PL, Lu JB and Sun XB: Prevalence of human papillomavirus 16 in
esophageal cancer among the Chinese population: A systematic review
and meta-analysis. Asian Pac J Cancer Prev. 15:10143–10149. 2014.
View Article : Google Scholar
|
|
24
|
Guo F, Liu Y, Wang X, He Z, Weiss NS,
Madeleine MM, Liu F, Tian X, Song Y, Pan Y, et al: Human
papillomavirus infection and esophageal squamous cell carcinoma: A
case-control study. Cancer Epidemiol Biomarkers Prev. 21:780–785.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yong F, Xudong N and Lijie T: Human
papillomavirus types 16 and 18 in esophagus squamous cell
carcinoma: A meta-analysis. Ann Epidemiol. 23:726–734. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liyanage SS, Rahman B, Ridda I, Newall AT,
Tabrizi SN, Garland SM, Segelov E, Seale H, Crowe PJ, Moa A and
Macintyre CR: The aetiological role of human papillomavirus in
oesophageal squamous cell carcinoma: A meta-analysis. PLoS One.
8:e692382013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sitas F, Egger S, Urban MI, Taylor PR,
Abnet CC, Boffetta P, O'Connell DL, Whiteman DC, Brennan P,
Malekzadeh R, et al: InterSCOPE study: Associations between
esophageal squamous cell carcinoma and human papillomavirus
serological markers. J Natl Cancer Inst. 104:147–158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cancer Genome Atlas Research Network;
Analysis Working Group; Asan University; BC Cancer Agency; Brigham
and Women's Hospital; Broad Institute; Brown University; Case
Western Reserve University; Dana-Farber Cancer Institute; Duke
University; et al: Integrated genomic characterization of
oesophageal carcinoma. Nature. 541:169–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu W, Snell JM, Jeck WR, Hoadley KA,
Wilkerson MD, Parker JS, Patel N, Mlombe YB, Mulima G, Liomba NG,
et al: Subtyping sub-Saharan esophageal squamous cell carcinoma by
comprehensive molecular analysis. JCI Insight. 1:e887552016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo L, Liu S, Zhang S, Chen Q, Zhang M,
Quan P and Sun XB: Human papillomavirus-related esophageal cancer
survival: A systematic review and meta-analysis. Medicine
(Baltimore). 95:e53182016. View Article : Google Scholar
|
|
31
|
Kilkenny M, Merlin K, Young R and Marks R:
The prevalence of common skin conditions in Australian school
students: 1. Common, plane and plantar viral warts. Br J Dermatol.
138:840–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orth G: Host defenses against human
papillomaviruses: Lessons from epidermodysplasia verruciformis.
Curr Top Microbiol Immunol. 321:59–83. 2008.PubMed/NCBI
|
|
33
|
Shanmugasundaram S and You J: Targeting
persistent human papillomavirus infection. Viruses. 9:pii.
E2292017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roberts JN, Buck CB, Thompson CD, Kines R,
Bernardo M, Choyke PL, Lowy DR and Schiller JT: Genital
transmission of HPV in a mouse model is potentiated by nonoxynol-9
and inhibited by carrageenan. Nat Med. 13:857–861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doorbar J: Molecular biology of human
papillomavirus infection and cervical cancer. Clin Sci (Lond).
110:525–541. 2006. View Article : Google Scholar
|
|
36
|
Schiller JT, Day PM and Kines RC: Current
understanding of the mechanism of HPV infection. Gynecol Oncol.
118(1 Suppl): S12–S17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giroglou T, Florin L, Schäfer F, Streeck
RE and Sapp M: Human papillomavirus infection requires cell surface
heparan sulfate. J Virol. 75:1565–1570. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Selinka HC, Giroglou T, Nowak T,
Christensen ND and Sapp M: Further evidence that papillomavirus
capsids exist in two distinct conformations. J Virol.
77:12961–12967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Day PM, Gambhira R, Roden RB, Lowy DR and
Schiller JT: Mechanisms of human papillomavirus type 16
neutralization by l2 cross-neutralizing and l1 type-specific
antibodies. J Virol. 82:4638–4646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kines RC, Thompson CD, Lowy DR, Schiller
JT and Day PM: The initial steps leading to papillomavirus
infection occur on the basement membrane prior to cell surface
binding. Proc Natl Acad Sci USA. 106:20458–20463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Richards KF, Bienkowska-Haba M, Dasgupta
J, Chen XS and Sapp M: Multiple heparan sulfate binding site
engagements are required for the infectious entry of human
papillomavirus type 16. J Virol. 87:11426–11437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Richards RM, Lowy DR, Schiller JT and Day
PM: Cleavage of the papillomavirus minor capsid protein, L2, at a
furin consensus site is necessary for infection. Proc Natl Acad Sci
USA. 103:1522–1527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Evander M, Frazer IH, Payne E, Qi YM,
Hengst K and McMillan NA: Identification of the alpha6 integrin as
a candidate receptor for papillomaviruses. J Virol. 71:2449–2456.
1997.PubMed/NCBI
|
|
44
|
Abban CY and Meneses PI: Usage of heparan
sulfate, integrins, and FAK in HPV16 infection. Virology. 403:1–16.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang HS and Lambert PF: Use of an in vivo
animal model for assessing the role of integrin a(6)β(4) and
syndecan-1 in early steps in papillomavirus infection. Virology.
433:395–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aksoy P, Abban CY, Kiyashka E, Qiang W and
Meneses PI: HPV16 infection of HaCaTs is dependent on β4 integrin,
and a6 integrin processing. Virology. 449:45–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scheffer KD, Berditchevski F and Florin L:
The tetraspanin CD151 in papillomavirus infection. Viruses.
6:893–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Spoden G, Freitag K, Husmann M, Boller K,
Sapp M, Lambert C and Florin L: Clathrin- and caveolin-independent
entry of human papillomavirus type 16-involvement of
tetraspanin-enriched microdomains (TEMs). PLoS One. 3:e33132008.
View Article : Google Scholar
|
|
49
|
Scheffer KD, Gawlitza A, Spoden GA, Zhang
XA, Lambert C, Berditchevski F and Florin L: Tetraspanin CD151
mediates papillomavirus type 16 endocytosis. J Virol. 87:3435–3446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Surviladze Z, Dziduszko A and Ozbun MA:
Essential roles for soluble virion-associated heparan sulfonated
proteoglycans and growth factors in human papillomavirus
infections. PLoS Pathog. 8:e10025192012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cerqueira C, Samperio Ventayol P, Vogeley
C and Schelhaas M: Kallikrein-8p roteolytically processes human
papillomaviruses in the extracellular space to facilitate entry
into host cells. J Virol. 89:7038–7052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Aksoy P, Gottschalk EY and Meneses PI: HPV
entry into cells. Mutat Res Rev Mutat Res. 772:13–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pyeon D, Pearce SM, Lank SM, Ahlquist P
and Lambert PF: Establishment of human papillomavirus infection
requires cell cycle progression. PLoS Pathog. 5:e10003182009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Parish JL, Bean AM, Park RB and Androphy
EJ: ChlR1 is required for loading papillomavirus E2 onto mitotic
chromosomes and viral genome maintenance. Mol Cell. 24:867–876.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
McBride AA: Replication and partitioning
of papillomavirus genomes. Adv Virus Res. 72:155–205. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tomaić V, Pim D and Banks L: The stability
of the human papillomavirus E6 oncoprotein is E6AP dependent.
Virology. 393:7–10. 2009. View Article : Google Scholar
|
|
57
|
Egawa N, Nakahara T, Ohno S,
Narisawa-Saito M, Yugawa T, Fujita M, Yamato K, Natori Y and Kiyono
T: The E1 protein of human papillomavirus type 16 is dispensable
for maintenance replication of the viral genome. J Virol.
86:3276–3283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Doorbar J, Quint W, Banks L, Bravo IG,
Stoler M, Broker TR and Stanley MA: The biology and life-cycle of
human papillomavi-ruses. Vaccine. 30(Suppl 5): F55–F70. 2012.
View Article : Google Scholar
|
|
59
|
Genther SM, Sterling S, Duensing S, Münger
K, Sattler C and Lambert PF: Quantitative role of the human
papillomavirus type 16 E5 gene during the productive stage of the
viral life cycle. J Virol. 77:2832–2842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fehrmann F, Klumpp DJ and Laimins LA:
Human papilloma-virus type 31 E5 protein supports cell cycle
progression and activates late viral functions upon epithelial
differentiation. J Virol. 77:2819–2831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pim D, Collins M and Banks L: Human
papillomavirus type 16 E5 gene stimulates the transforming activity
of the epidermal growth factor receptor. Oncogene. 7:27–32.
1992.PubMed/NCBI
|
|
62
|
Wang Q, Griffin H, Southern S, Jackson D,
Martin A, McIntosh P, Davy C, Masterson PJ, Walker PA, Laskey P, et
al: Functional analysis of the human papillomavirus type 16 E1=E4
protein provides a mechanism for in vivo and in vitro keratin
filament reorganization. J Virol. 78:821–833. 2004. View Article : Google Scholar :
|
|
63
|
McIntosh PB, Martin SR, Jackson DJ, Khan
J, Isaacson ER, Calder L, Raj K, Griffin HM, Wang Q, Laskey P, et
al: Structural analysis reveals an amyloid form of the human
papillomavirus type 16 E1-E4 protein and provides a molecular basis
for its accumulation. J Virol. 82:8196–8203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Brown DR, Kitchin D, Qadadri B, Neptune N,
Batteiger T and Ermel A: The human papillomavirus type 11 E1-E4
protein is a transglutaminase 3 substrate and induces abnormalities
of the cornified cell envelope. Virology. 345:290–298. 2006.
View Article : Google Scholar
|
|
65
|
Zhao KN and Chen J: Codon usage roles in
human papilloma-virus. Rev Med Virol. 21:397–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou J, Liu WJ, Peng SW, Sun XY and Frazer
I: Papillomavirus capsid protein expression level depends on the
match between codon usage and tRNA availability. J Virol.
73:4972–4982. 1999.PubMed/NCBI
|
|
67
|
Li S, Labrecque S, Gauzzi MC, Cuddihy AR,
Wong AH, Pellegrini S, Matlashewski GJ and Koromilas AE: The human
papilloma virus (HPV)-18 E6 oncoprotein physically associates with
Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene.
18:5727–5737. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ronco LV, Karpova AY, Vidal M and Howley
PM: Human papillomavirus 16 E6 oncoprotein binds to interferon
regulatory factor-3 and inhibits its transcriptional activity.
Genes Dev. 12:2061–2072. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Um SJ, Rhyu JW, Kim EJ, Jeon KC, Hwang ES
and Park JS: Abrogation of IRF-1 response by high-risk HPV E7
protein in vivo. Cancer Lett. 179:205–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
The human papilloma-virus E7 protein is
able to inhibit the antiviral and anti-growth functions of
interferon-alpha. Virology. 277:411–419. 2000. View Article : Google Scholar
|
|
71
|
Evans M, Borysiewicz LK, Evans AS, Rowe M,
Jones M, Gileadi U, Cerundolo V and Man S: Antigen processing
defects in cervical carcinomas limit the presentation of a CTL
epitope from human papillomavirus 16 E6. J Immunol. 167:5420–5428.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Matthews K, Leong CM, Baxter L, Inglis E,
Yun K, Bäckström BT, Doorbar J and Hibma M: Depletion of Langerhans
cells in human papillomavirus type 16-infected skin is associated
with E6-mediated down regulation of E-cadherin. J Virol.
77:8378–8385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
D'Costa ZJ, Leong CM, Shields J, Matthews
C and Hibma MH: Screening of drugs to counteract human
papillomavirus 16 E6 repression of E-cadherin expression. Invest
New Drugs. 30:2236–2251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fausch SC, Fahey LM, Da Silva DM and Kast
WM: Human papillomavirus can escape immune recognition through
Langerhans cell phosphoinositide 3-kinase activation. J Immunol.
174:7172–7178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fahey LM, Raff AB, Da Silva DM and Kast
WM: A major role for the minor capsid protein of human
papillomavirus type 16 in immune escape. J Immunol. 183:6151–6156.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mota F, Rayment N, Chong S, Singer A and
Chain B: The antigen-presenting environment in normal and human
papillomavirus (HPV)-related premalignant cervical epithelium. Clin
Exp Immunol. 116:33–40. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
de Jong A, van Poelgeest MI, van der Hulst
JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R and
van der Burg SH: Human papillomavirus type 16-positive cervical
cancer is associated with impaired CD4+ T-cell immunity against
early antigens E2 and E6. Cancer Res. 64:5449–5455. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rodriguez JA, Galeano L, Palacios DM,
Gómez C, Serrano ML, Bravo MM and Combita AL: Altered HLA class I
and HLA-G expression is associated with IL-10 expression in
patients with cervical cancer. Pathobiology. 79:72–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Le Gal FA, Riteau B, Sedlik C,
Khalil-Daher I, Menier C, Dausset J, Guillet JG, Carosella ED and
Rouas-Freiss N: HLA-G-mediated inhibition of antigen-specific
cytotoxic T lymphocytes. Int Immunol. 11:1351–1356. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Marchal-Bras-Goncalves R, Rouas-Freiss N,
Connan F, Choppin J, Dausset J, Carosella ED, Kirszenbaum M and
Guillet J: A soluble HLA-G protein that inhibits natural killer
cell-mediated cytotoxicity. Transplant Proc. 33:2355–2359. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gros F, Cabillic F, Toutirais O, Maux AL,
Sebti Y and Amiot L: Soluble HLA-G molecules impair natural
killer/dendritic cell crosstalk via inhibition of dendritic cells.
Eur J Immunol. 38:742–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kabsch K, Mossadegh N, Kohl A, Komposch G,
Schenkel J, Alonso A and Tomakidi P: The HPV-16 E5 protein inhibits
TRAIL- and FasL-mediated apoptosis in human keratinocyte raft
cultures. Intervirology. 47:48–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Venuti A, Paolini F, Nasir L, Corteggio A,
Roperto S, Campo MS and Borzacchiello G: Papillomavirus E5: The
smallest oncopro-tein with many functions. Mol Cancer. 10:1402011.
View Article : Google Scholar
|
|
84
|
Lagunas-Martínez A, Madrid-Marina V and
Gariglio P: Modulation of apoptosis by early human papillomavirus
proteins in cervical cancer. Biochim Biophys Acta. 1805:6–16.
2010.
|
|
85
|
Gross-Mesilaty S, Reinstein E, Bercovich
B, Tobias KE, Schwartz AL, Kahana C and Ciechanover A: Basal and
human papillomavirus E6 oncoprotein-induced degradation of Myc
proteins by the ubiquitin pathway. Proc Natl Acad Sci USA.
95:8058–8063. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Filippova M, Parkhurst L and
Duerksen-Hughes PJ: The human papillomavirus 16 E6 protein binds to
Fas-associated death domain and protects cells from Fas-triggered
apoptosis. J Biol Chem. 279:25729–25744. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Garnett TO, Filippova M and
Duerksen-Hughes PJ: Accelerated degradation of FADD and procaspase
8 in cells expressing human papilloma virus 16 E6 impairs
TRAIL-mediated apoptosis. Cell Death Differ. 13:1915–1926. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Garnett TO and Duerksen-Hughes PJ:
Modulation of apoptosis by human papillomavirus (HPV) oncoproteins.
Arch Virol. 151:2321–2335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Johnson JA and Gangemi JD: Selective
inhibition of human papillomavirus-induced cell proliferation by
(S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine. Antimicrob
Agents Chemother. 43:1198–1205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Beadle JR, Valiaeva N, Yang G, Yu JH,
Broker TR, Aldern KA, Harden EA, Keith KA, Prichard MN, Hartman T,
et al: Synthesis and antiviral evaluation of octadecyloxyethyl
Benzyl 9-[(2-Phosphonomethoxy)ethyl]guanine (ODE-Bn-PMEG), a potent
inhibitor of transient HPV DNA amplification. J Med Chem.
59:10470–10478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Van Pachterbeke C, Bucella D, Rozenberg S,
Manigart Y, Gilles C, Larsimont D, Vanden Houte K, Reynders M,
Snoeck R, Bossens M, et al: Topical treatment of CIN 2+ by
cidofovir: Results of a phase II, double-blind, prospective,
placebo-controlled study. Gynecol Oncol. 115:69–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tristram A and Fiander A: Clinical
responses to Cidofovir applied topically to women with high grade
vulval intraepithe-lial neoplasia. Gynecol Oncol. 99:652–655. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Stier EA, Goldstone SE, Einstein MH, Jay
N, Berry JM, Wilkin T, Lee JY, Darragh TM, Da Costa M, Panther L,
et al: Safety and efficacy of topical cidofovir to treat high-grade
perianal and vulvar intraepithelial neoplasia in HIV-positive men
and women. AIDS. 27:545–551. 2013. View Article : Google Scholar :
|
|
94
|
Pertusati F, Hinsinger K, Flynn ÁS, Powell
N, Tristram A, Balzarini J and McGuigan C: PMPA and PMEA prodrugs
for the treatment of HIV infections and human papillomavirus (HPV)
associated neoplasia and cancer. Eur J Med Chem. 78:259–268. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wolfgang GH, Shibata R, Wang J, Ray AS, Wu
S, Doerrfler E, Reiser H, Lee WA, Birkus G, Christensen ND, et al:
GS-9191 is a novel topical prodrug of the nucleotide analog
9-(2-phosphonylmethoxyethyl)guanine with antiproliferative activity
and possible utility in the treatment of human papilloma-virus
lesions. Antimicrob Agents Chemother. 53:2777–2784. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yoakim C, Ogilvie WW, Goudreau N, Naud J,
Haché B, O'Meara JA, Cordingley MG, Archambault J and White PW:
Discovery of the first series of inhibitors of human
papilloma-virus type 11: Inhibition of the assembly of the
E1-E2-Origin DNA complex. Bioorg Med Chem Lett. 13:2539–2541. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
White PW, Titolo S, Brault K, Thauvette L,
Pelletier A, Welchner E, Bourgon L, Doyon L, Ogilvie WW, Yoakim C,
et al: Inhibition of human papillomavirus DNA replication by small
molecule antagonists of the E1-E2 protein interaction. J Biol Chem.
278:26765–26772. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang Y, Coulombe R, Cameron DR, Thauvette
L, Massariol MJ, Amon LM, Fink D, Titolo S, Welchner E, Yoakim C,
et al: Crystal structure of the E2 transactivation domain of human
papillomavirus type 11 bound to a protein interaction inhibitor. J
Biol Chem. 279:6976–6985. 2004. View Article : Google Scholar
|
|
99
|
Schaal TD, Mallet WG, McMinn DL, Nguyen
NV, Sopko MM, John S and Parekh BS: Inhibition of human papilloma
virus E2 DNA binding protein by covalently linked polyamides.
Nucleic Acids Res. 31:1282–1291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Edwards TG, Koeller KJ, Slomczynska U, Fok
K, Helmus M, Bashkin JK and Fisher C: HPV episome levels are
potently decreased by pyrrole-imidazole polyamides. Antiviral Res.
91:177–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Vasilieva E, Niederschulte J, Song Y,
Harris GD Jr, Koeller KJ, Liao P, Bashkin JK and Dupureur CM:
Interactions of two large antiviral polyamides with the long
control region of HPV16. Biochimie. 127:103–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wilson VG, West M, Woytek K and Rangasamy
D: Papillomavirus E1 proteins: Form, function, and features. Virus
Genes. 24:275–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Stenlund A: Initiation of DNA replication:
Lessons from viral initiator proteins. Nat Rev Mol Cell Biol.
4:777–785. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Faucher AM, White PW, Brochu C,
Grand-Maître C, Rancourt J and Fazal G: Discovery of small-molecule
inhibitors of the ATPase activity of human papillomavirus E1
helicase. J Med Chem. 47:18–21. 2004. View Article : Google Scholar
|
|
105
|
White PW, Faucher AM, Massariol MJ,
Welchner E, Rancourt J, Cartier M and Archambault J:
Biphenylsulfonacetic acid inhibitors of the human papillomavirus
type 6 E1 helicase inhibit ATP hydrolysis by an allosteric
mechanism involving tyrosine 486. Antimicrob Agents Chemother.
49:4834–4842. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Be X, Hong Y, Wei J, Androphy EJ, Chen JJ
and Baleja JD: Solution structure determination and mutational
analysis of the papillomavirus E6 interacting peptide of E6AP.
Biochemistry. 40:1293–1299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Baleja JD, Cherry JJ, Liu Z, Gao H,
Nicklaus MC, Voigt JH, Chen JJ and Androphy EJ: Identification of
inhibitors to papillomavirus type 16 E6 protein based on
three-dimensional structures of interacting proteins. Antiviral
Res. 72:49–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Longworth MS and Laimins LA: The binding
of histone deacetylases and the integrity of zinc finger-like
motifs of the E7 protein are essential for the life cycle of human
papillomavirus type 31. J Virol. 78:3533–3541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Brehm A, Nielsen SJ, Miska EA, McCance DJ,
Reid JL, Bannister AJ and Kouzarides T: The E7 oncoprotein
associates with Mi2 and histone deacetylase activity to promote
cell growth. Embo J. 18:2449–2458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lu Q, Yang YT and Chen CS, Davis M, Byrd
JC, Etherton MR, Umar A and Chen CS: Zn2+-chelating motif-tethered
short-chain fatty acids as a novel class of histone deacetylase
inhibitors. J Med Chem. 47:467–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Suzuki T, Matsuura A, Kouketsu A, Nakagawa
H and Miyata N: Identification of a potent non-hydroxamate histone
deacetylase inhibitor by mechanism-based drug design. Bioorg Med
Chem Lett. 15:331–335. 2005. View Article : Google Scholar
|
|
112
|
Suzuki T, Nagano Y, Kouketsu A, Matsuura
A, Maruyama S, Kurotaki M, Nakagawa H and Miyata N: Novel
inhibitors of human histone deacetylases: Design, synthesis, enzyme
inhibition, and cancer cell growth inhibition of SAHA-based
non-hydroxamates. J Med Chem. 48:1019–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Suzuki T, Ando T, Tsuchiya K, Fukazawa N,
Saito A, Mariko Y, Yamashita T and Nakanishi O: Synthesis and
histone deacety-lase inhibitory activity of new benzamide
derivatives. J Med Chem. 42:3001–3003. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Nakajima H, Kim YB, Terano H, Yoshida M
and Horinouchi S: FR901228, a potent antitumor antibiotic, is a
novel histone deacetylase inhibitor. Exp Cell Res. 241:126–133.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Finzer P, Ventz R, Kuntzen C, Seibert N,
Soto U and Rösl F: Growth arrest of HPV-positive cells after
histone deacetylase inhibition is independent of E6/E7 oncogene
expression. Virology. 304:265–273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Finzer P, Kuntzen C, Soto U, zur Hausen H
and Rösl F: Inhibitors of histone deacetylase arrest cell cycle and
induce apoptosis in cervical carcinoma cells circumventing human
papillomavirus oncogene expression. Oncogene. 20:4768–4776. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chavez-Blanco A, Perez-Plasencia C,
Perez-Cardenas E, Carrasco-Legleu C, Rangel-Lopez E, Segura-Pacheco
B, Taja-Chayeb L, Trejo-Becerril C, Gonzalez-Fierro A, Candelaria
M, et al: Antineoplastic effects of the DNA methylation inhibitor
hydralazine and the histone deacetylase inhibitor valproic acid in
cancer cell lines. Cancer Cell Int. 6:22006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hebner CM and Laimins LA: Human
papillomaviruses: Basic mechanisms of pathogenesis and
oncogenicity. Rev Med Virol. 16:83–97. 2006. View Article : Google Scholar
|
|
119
|
Lin BY, Ma T, Liu JS, Kuo SR, Jin G,
Broker TR, Harper JW and Chow LT: HeLa cells are phenotypically
limiting in cyclin E/CDK2 for efficient human papillomavirus DNA
replication. J Biol Chem. 275:6167–6174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ma T, Zou N, Lin BY, Chow LT and Harper
JW: Interaction between cyclin-dependent kinases and human
papillomavirus replication-initiation protein E1 is required for
efficient viral replication. Proc Natl Acad Sci USA. 96:382–387.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cueille N, Nougarede R, Mechali F,
Philippe M and Bonne-Andrea C: Functional interaction between the
bovine papillomavirus virus type 1 replicative helicase E1 and
cyclin E-Cdk2. J Virol. 72:7255–7262. 1998.PubMed/NCBI
|
|
122
|
Duensing S and Münger K: Human
papillomaviruses and centrosome duplication errors: Modeling the
origins of genomic instability. Oncogene. 21:6241–6248. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Duensing S and Münger K: Centrosome
abnormalities and genomic instability induced by human
papillomavirus oncoproteins. Prog Cell Cycle Res. 5:383–391.
2003.PubMed/NCBI
|
|
124
|
Duensing S, Duensing A, Flores ER, Do A,
Lambert PF and Münger K: Centrosome abnormalities and genomic
instability by episomal expression of human papillomavirus type 16
in raft cultures of human keratinocytes. J Virol. 75:7712–7716.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hsu CY, Mechali F and Bonne-Andrea C:
Nucleocytoplasmic shuttling of bovine papillomavirus E1 helicase
downregulates viral DNA replication in S phase. J Virol.
81:384–394. 2007. View Article : Google Scholar :
|
|
126
|
Deng W, Lin BY, Jin G, Wheeler CG, Ma T,
Harper JW, Broker TR and Chow LT: Cyclin/CDK regulates the
nucleocy-toplasmic localization of the human papillomavirus E1 DNA
helicase. J Virol. 78:13954–13965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Schang LM: Cyclin-dependent kinases as
cellular targets for antiviral drugs. J Antimicrob Chemother.
50:779–792. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Vitali L, Yakisich JS, Vita MF, Fernandez
A, Settembrini L, Siden A, Cruz M, Carminatti H, Casas O and
Idoyaga Vargas V: Roscovitine inhibits ongoing DNA synthesis in
human cervical cancer. Cancer Lett. 180:7–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Duensing S, Duensing A, Lee DC, Edwards
KM, Piboonniyom SO, Manuel E, Skaltsounis L, Meijer L and Münger K:
Cyclin-dependent kinase inhibitor indirubin-3′-oxime selectively
inhibits human papillomavirus type 16 E7-induced numerical
centrosome anomalies. Oncogene. 23:8206–8215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yan L, Lai F, Chen X and Xiao Z: Discovery
of novel indirubin-3′-monoxime derivatives as potent inhibitors
against CDK2 and CDK9. Bioorg Med Chem Lett. 25:2447–2451. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Gloss B and Bernard HU: The E6/E7 promoter
of human papillomavirus type 16 is activated in the absence of E2
proteins by a sequence-aberrant Sp1 distal element. J Virol.
64:5577–5584. 1990.PubMed/NCBI
|
|
132
|
Butz K and Hoppe-Seyler F: Transcriptional
control of human papillomavirus (HPV) oncogene expression:
Composition of the HPV type 18 upstream regulatory region. J Virol.
67:6476–6486. 1993.PubMed/NCBI
|
|
133
|
Craigo J, Callahan M, Huang RC and DeLucia
AL: Inhibition of human papillomavirus type 16 gene expression by
nordihydroguaiaretic acid plant lignan derivatives. Antiviral Res.
47:19–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Heller JD, Kuo J, Wu TC, Kast WM and Huang
RC: Tetra-O-methyl nordihydroguaiaretic acid induces G2 arrest in
mammalian cells and exhibits tumoricidal activity in vivo. Cancer
Res. 61:5499–5504. 2001.PubMed/NCBI
|
|
135
|
Vink MA, Bogaards JA, van Kemenade FJ, de
Melker HE, Meijer CJ and Berkhof J: Clinical progression of
high-grade cervical intraepithelial neoplasia: Estimating the time
to preclinical cervical cancer from doubly censored national
registry data. Am J Epidemiol. 178:1161–1169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Schwarz E, Freese UK, Gissmann L, Mayer W,
Roggenbuck B, Stremlau A and Zur Hausen H: Structure and
transcription of human papillomavirus sequences in cervical
carcinoma cells. Nature. 314:111–114. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yu T, Ferber MJ, Cheung TH, Chung TK, Wong
YF and Smith DI: The role of viral integration in the development
of cervical cancer. Cancer Genet Cytogenet. 158:27–34. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Jeon S, Allen-Hoffmann BL and Lambert PF:
Integration of human papillomavirus type 16 into the human genome
correlates with a selective growth advantage of cells. J Virol.
69:2989–2997. 1995.PubMed/NCBI
|
|
139
|
Jeon S and Lambert PF: Integration of
human papillomavirus type 16 DNA into the human genome leads to
increased stability of E6 and E7 mRNAs: Implications for cervical
carcinogenesis. Proc Natl Acad Sci USA. 92:1654–1658. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Souders NC, Sewell DA, Pan ZK, Hussain SF,
Rodriguez A, Wallecha A and Paterson Y: Listeria-based vaccines can
overcome tolerance by expanding low avidity CD8+ T cells capable of
eradicating a solid tumor in a transgenic mouse model of cancer.
Cancer Immun. 7:22007.PubMed/NCBI
|
|
141
|
Sewell DA, Pan ZK and Paterson Y:
Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth
in a transgenic mouse model for HPV-16 transformed tumors. Vaccine.
26:5315–5320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Adachi K, Kawana K, Yokoyama T, Fujii T,
Tomio A, Miura S, Tomio K, Kojima S, Oda K, Sewaki T, et al: Oral
immunization with a Lactobacillus casei vaccine expressing human
papil-lomavirus (HPV) type 16 E7 is an effective strategy to induce
mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine.
28:2810–2817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bermudez-Humaran LG, Langella P, Miyoshi
A, Gruss A, Guerra RT, Montes de Oca-Luna R and Le Loir Y:
Production of human papillomavirus type 16 E7 protein in
Lactococcus lactis. Appl Environ Microbiol. 68:917–922. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Bermudez-Humaran LG, Cortes-Perez NG, Le
Loir Y, Alcocer-González JM, Tamez-Guerra RS, de Oca-Luna RM and
Langella P: An inducible surface presentation system improves
cellular immunity against human papillomavirus type 16 E7 antigen
in mice after nasal administration with recombinant lactococci. J
Med Microbiol. 53:427–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cortes-Perez NG, Azevedo V,
Alcocer-González JM, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier
G, Gruss A, Langella P and Bermúdez-Humarán LG: Cell-surface
display of E7 antigen from human papillomavirus type-16 in
Lactococcus lactis and in Lactobacillus plantarum using a new
cell-wall anchor from lactobacilli. J Drug Target. 13:89–98. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Krul MR, Tijhaar EJ, Kleijne JA, Van Loon
AM, Nievers MG, Schipper H, Geerse L, Van der Kolk M, Steerenberg
PA, Mooi FR and Den Otter W: Induction of an antibody response in
mice against human papillomavirus (HPV) type 16 after immunization
with HPV recombinant Salmonella strains. Cancer Immunol Immunother.
43:44–48. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Kawana K, Adachi K, Kojima S, Taguchi A,
Tomio K, Yamashita A, Nishida H, Nagasaka K, Arimoto T, Yokoyama T,
et al: Oral vaccination against HPV E7 for treatment of cervical
intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific
mucosal immunity in the cervix of CIN3 patients. Vaccine.
32:6233–6239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Schnupf P and Portnoy DA: Listeriolysin O:
A phagosome-specific lysin. Microbes Infect. 9:1176–1187. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chen Z, Ozbun L, Chong N, Wallecha A,
Berzofsky JA and Khleif SN: Episomal expression of truncated
listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy
by preferentially inducing expansions of CD4+FoxP3- and CD8+ T
cells. Cancer Immunol Res. 2:911–922. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Peters C and Paterson Y: Enhancing the
immunogenicity of bioengineered Listeria monocytogenes by passaging
through live animal hosts. Vaccine. 21:1187–1194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Maciag PC, Radulovic S and Rothman J: The
first clinical use of a live-attenuated Listeria monocytogenes
vaccine: A Phase I safety study of Lm-LLO-E7 in patients with
advanced carcinoma of the cervix. Vaccine. 27:3975–3983. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Borysiewicz LK, Fiander A, Nimako M, Man
S, Wilkinson GW, Westmoreland D, Evans AS, Adams M, Stacey SN,
Boursnell ME, et al: A recombinant vaccinia virus encoding human
papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy
for cervical cancer. Lancet. 347:1523–1527. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kaufmann AM, Stern PL, Rankin EM, Sommer
H, Nuessler V, Schneider A, Adams M, Onon TS, Bauknecht T, Wagner
U, et al: Safety and immunogenicity of TA-HPV, a recombinant
vaccinia virus expressing modified human papillomavirus (HPV)-16
and HPV-18 E6 and E7 genes, in women with progressive cervical
cancer. Clin Cancer Res. 8:3676–3685. 2002.PubMed/NCBI
|
|
154
|
Baldwin PJ, van der Burg SH, Boswell CM,
Offringa R, Hickling JK, Dobson J, Roberts JS, Latimer JA, Moseley
RP, Coleman N, et al: Vaccinia-expressed human papillomavirus 16
and 18 e6 and e7 as a therapeutic vaccination for vulval and
vaginal intraepithelial neoplasia. Clin Cancer Res. 9:5205–5213.
2003.PubMed/NCBI
|
|
155
|
Brun JL, Dalstein V, Leveque J, Mathevet
P, Raulic P, Baldauf JJ, Scholl S, Huynh B, Douvier S, Riethmuller
D, et al: Regression of high-grade cervical intraepithelial
neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol.
204:169.e1–e8. 2011. View Article : Google Scholar
|
|
156
|
Rosales R, López-Contreras M, Rosales C,
Magallanes-Molina JR, Gonzalez-Vergara R, Arroyo-Cazarez JM,
Ricardez-Arenas A, Del Follo-Valencia A, Padilla-Arriaga S,
Guerrero MV, et al: Regression of human papillomavirus
intraepithelial lesions is induced by MVA E2 therapeutic vaccine.
Hum Gene Ther. 25:1035–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Gomez-Gutierrez JG, Elpek KG, Montes de
Oca-Luna R, Shirwan H, Sam Zhou H and McMasters KM: Vaccination
with an adenoviral vector expressing calreticulin-human
papilloma-virus 16 E7 fusion protein eradicates E7 expressing
established tumors in mice. Cancer Immunol Immunother. 56:997–1007.
2007. View Article : Google Scholar
|
|
158
|
Kast WM, Brandt RM, Sidney J, Drijfhout
JW, Kubo RT, Grey HM, Melief CJ and Sette A: Role of HLA-A motifs
in identification of potential CTL epitopes in human papillomavirus
type 16 E6 and E7 proteins. J Immunol. 152:3904–3912.
1994.PubMed/NCBI
|
|
159
|
Kenter GG, Welters MJ, Valentijn AR, Lowik
MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM,
Offringa R, Drijfhout JW, et al: Vaccination against HPV-16
oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med.
361:1838–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
de Vos van Steenwijk PJ, Ramwadhdoebe TH,
Löwik MJ, van der Minne CE, Berends-van der Meer DM, Fathers LM,
Valentijn AR, Oostendorp J, Fleuren GJ, Hellebrekers BW, et al: A
placebo-controlled randomized HPV16 synthetic long-peptide
vaccination study in women with high-grade cervical squamous
intraepithelial lesions. Cancer Immunol Immunother. 61:1485–1492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
van Poelgeest MI, Welters MJ, van Esch EM,
Stynenbosch LF, Kerpershoek G, van Persijn van Meerten EL, van den
Hende M, Löwik MJ, Berends-van der Meer DM, Fathers LM, et al:
HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of
patients with advanced or recurrent HPV16-induced gynecological
carcinoma, a phase II trial. J Transl Med. 11:882013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
de Vos van Steenwijk PJ, van Poelgeest MI,
Ramwadhdoebe TH, Löwik MJ, Berends-van der Meer DM, van der Minne
CE, Loof NM, Stynenbosch LF, Fathers LM, Valentijn AR, et al: The
long-term immune response after HPV16 peptide vaccination in women
with low-grade pre-malignant disorders of the uterine cervix: A
placebo-controlled phase II study. Cancer Immunol Immunother.
63:147–160. 2014. View Article : Google Scholar
|
|
163
|
Welters MJ, van der Sluis TC, van Meir H,
Loof NM, van Ham VJ, van Duikeren S, Santegoets SJ, Arens R, de Kam
ML, Cohen AF, et al: Vaccination during myeloid cell depletion by
cancer chemotherapy fosters robust T cell responses. Sci Transl
Med. 8:334ra522016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Coleman HN, Greenfield WW, Stratton SL,
Vaughn R, Kieber A, Moerman-Herzog AM, Spencer HJ, Hitt WC, Quick
CM, Hutchins LF, et al: Human papillomavirus type 16 viral load is
decreased following a therapeutic vaccination. Cancer Immunol
Immunother. 65:563–573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Su JH, Wu A, Scotney E, Ma B, Monie A,
Hung CF and Wu TC: Immunotherapy for cervical cancer: Research
status and clinical potential. BioDrugs. 24:109–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
de Jong A, O'Neill T, Khan AY, Kwappenberg
KM, Chisholm SE, Whittle NR, Dobson JA, Jack LC, St Clair Roberts
JA, Offringa R, et al: Enhancement of human papillomavirus (HPV)
type 16 E6 and E7-specific T-cell immunity in healthy volunteers
through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein
vaccine. Vaccine. 20:3456–3464. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Daayana S, Elkord E, Winters U, Pawlita M,
Roden R, Stern PL and Kitchener HC: Phase II trial of imiquimod and
HPV therapeutic vaccination in patients with vulval intraepithelial
neoplasia. Br J Cancer. 102:1129–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Van Damme P, Bouillette-Marussig M, Hens
A, De Coster I, Depuydt C, Goubier A, Van Tendeloo V, Cools N,
Goossens H, Hercend T, et al: GTL001, A therapeutic vaccine for
women infected with human papillomavirus 16 or 18 and normal
cervical cytology: Results of a phase I clinical trial. Clin Cancer
Res. 22:3238–3248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Granadillo M, Vallespi MG, Batte A,
Mendoza O, Soria Y, Lugo VM and Torrens I: A novel fusion
protein-based vaccine comprising a cell penetrating and
immunostimulatory peptide linked to human papillomavirus (HPV) type
16 E7 antigen generates potent immunologic and anti-tumor responses
in mice. Vaccine. 29:920–930. 2011. View Article : Google Scholar
|
|
170
|
Vallespi MG, Glaria LA, Reyes O, Garay HE,
Ferrero J and Araña MJ: A Limulus antilipopolysaccharide
factor-derived peptide exhibits a new immunological activity with
potential applicability in infectious diseases. Clin Diagn Lab
Immunol. 7:669–675. 2000.PubMed/NCBI
|
|
171
|
Lin KH, Chang LS, Tian CY, Yeh YC, Chen
YJ, Chuang TH, Liu SJ and Leng CH: Carboxyl-terminal fusion of E7
into Flagellin shifts TLR5 activation to NLRC4/NAIP5 activation and
induces TLR5-independent anti-tumor immunity. Sci Rep. 6:241992016.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Lee SJ, Yang A, Wu TC and Hung CF:
Immunotherapy for human papillomavirus-associated disease and
cervical cancer: Review of clinical and translational research. J
Gynecol Oncol. 27:e512016. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Vici P, Pizzuti L, Mariani L, Zampa G,
Santini D, Di Lauro L, Gamucci T, Natoli C, Marchetti P, Barba M,
et al: Targeting immune response with therapeutic vaccines in
premalignant lesions and cervical cancer: Hope or reality from
clinical studies. Expert Rev Vaccines. 15:1327–1336. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yang A, Farmer E, Wu TC and Hung CF:
Perspectives for therapeutic HPV vaccine development. J Biomed Sci.
23:752016. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Trimble C, Lin CT, Hung CF, Pai S, Juang
J, He L, Gillison M, Pardoll D, Wu L and Wu TC: Comparison of the
CD8+ T cell responses and antitumor effects generated by DNA
vaccine administered through gene gun, biojector, and syringe.
Vaccine. 21:4036–4042. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Best SR, Peng S, Juang CM, Hung CF,
Hannaman D, Saunders JR, Wu TC and Pai SI: Administration of HPV
DNA vaccine via electroporation elicits the strongest CD8+ T cell
immune responses compared to intramuscular injection and
intradermal gene gun delivery. Vaccine. 27:5450–5459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Tsen SW, Wu CY, Meneshian A, Pai SI, Hung
CF and Wu TC: Femtosecond laser treatment enhances DNA transfection
efficiency in vivo. J Biomed Sci. 16:362009. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Klencke B, Matijevic M, Urban RG, Lathey
JL, Hedley ML, Berry M, Thatcher J, Weinberg V, Wilson J, Darragh
T, et al: Encapsulated plasmid DNA treatment for human
papilloma-virus 16-associated anal dysplasia: A Phase I study of
ZYC101. Clin Cancer Res. 8:1028–1037. 2002.PubMed/NCBI
|
|
179
|
Hung CF, Hsu KF, Cheng WF, Chai CY, He L,
Ling M and Wu TC: Enhancement of DNA vaccine potency by linkage of
antigen gene to a gene encoding the extracellular domain of
Fms-like tyrosine kinase 3-ligand. Cancer Res. 61:1080–1088.
2001.PubMed/NCBI
|
|
180
|
Hauser H and Chen SY: Augmentation of DNA
vaccine potency through secretory heat shock protein-mediated
antigen targeting. Methods. 31:225–231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Cheung YK, Cheng SC, Sin FW and Xie Y:
Plasmid encoding papillomavirus Type 16 (HPV16) DNA constructed
with codon optimization improved the immunogenicity against HPV
infection. Vaccine. 23:629–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Liu W, Gao F, Zhao KN, Zhao W, Fernando
GJ, Thomas R and Frazer IH: Codon modified human papillomavirus
type 16 E7 DNA vaccine enhances cytotoxic T-lymphocyte induction
and anti-tumour activity. Virology. 301:43–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Lin CT, Tsai YC, He L, Calizo R, Chou HH,
Chang TC, Soong YK, Hung CF and Lai CH: A DNA vaccine encoding a
codon-optimized human papillomavirus type 16 E6 gene enhances CTL
response and anti-tumor activity. J Biomed Sci. 13:481–488. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Smahel M, Poláková I, Pokorná D, Ludvíková
V, Dusková M and Vlasák J: Enhancement of T cell-mediated and
humoral immunity of beta-glucuronidase-based DNA vaccines against
HPV16 E7 oncoprotein. Int J Oncol. 33:93–101. 2008.PubMed/NCBI
|
|
185
|
Massa S, Simeone P, Muller A, Benvenuto E,
Venuti A and Franconi R: Antitumor activity of DNA vaccines based
on the human papillomavirus-16 E7 protein genetically fused to a
plant virus coat protein. Hum Gene Ther. 19:354–364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Chen CH, Wang TL, Hung CF, Yang Y, Young
RA, Pardoll DM and Wu TC: Enhancement of DNA vaccine potency by
linkage of antigen gene to an HSP70 gene. Cancer Res. 60:1035–1042.
2000.PubMed/NCBI
|
|
187
|
Cheng WF, Hung CF, Chai CY, Hsu KF, He L,
Ling M and Wu TC: Tumor-specific immunity and antiangiogenesis
generated by a DNA vaccine encoding calreticulin linked to a tumor
antigen. J Clin Invest. 108:669–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Bolhassani A, Zahedifard F, Taghikhani M
and Rafati S: Enhanced immunogenicity of HPV16E7 accompanied by
Gp96 as an adjuvant in two vaccination strategies. Vaccine.
26:3362–3370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Hung CF, Cheng WF, Hsu KF, Chai CY, He L,
Ling M and Wu TC: Cancer immunotherapy using a DNA vaccine encoding
the translocation domain of a bacterial toxin linked to a tumor
antigen. Cancer Res. 61:3698–3703. 2001.PubMed/NCBI
|
|
190
|
Hung CF, Cheng WF, He L, Ling M, Juang J,
Lin CT and Wu TC: Enhancing major histocompatibility complex class
I antigen presentation by targeting antigen to centrosomes. Cancer
Res. 63:2393–2398. 2003.PubMed/NCBI
|
|
191
|
Huang CH, Peng S, He L, Tsai YC, Boyd DA,
Hansen TH, Wu TC and Hung CF: Cancer immunotherapy using a DNA
vaccine encoding a single-chain trimer of MHC class I linked to an
HPV-16 E6 immunodominant CTL epitope. Gene Ther. 12:1180–1186.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Kim TW, Hung CF, Boyd D, Juang J, He L,
Kim JW, Hardwick JM and Wu TC: Enhancing DNA vaccine potency by
combining a strategy to prolong dendritic cell life with
intracellular targeting strategies. J Immunol. 171:2970–2976. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Kim TW, Lee JH, He L, Boyd DA, Hardwick
JM, Hung CF and Wu TC: Modification of professional
antigen-presenting cells with small interfering RNA in vivo to
enhance cancer vaccine potency. Cancer Res. 65:309–316.
2005.PubMed/NCBI
|
|
194
|
Huang B, Mao CP, Peng S, Hung CF and Wu
TC: RNA interference-mediated in vivo silencing of fas ligand as a
strategy for the enhancement of DNA vaccine potency. Hum Gene Ther.
19:763–773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Trimble CL, Morrow MP, Kraynyak KA, Shen
X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, et
al: Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic
synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6
and E7 proteins for cervical intraepithelial neoplasia 2/3: A
randomised, double-blind, placebo-controlled phase 2b trial.
Lancet. 386:2078–2088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB,
Hong SR, Lee CW, Kim S, Woo JW, Park KS, et al: Clearance of
persistent HPV infection and cervical lesion by therapeutic DNA
vaccine in CIN3 patients. Nat Commun. 5:53172014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Maldonado L, Teague JE, Morrow MP, Jotova
I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, et al:
Intramuscular therapeutic vaccination targeting HPV16 induces T
cell responses that localize in mucosal lesions. Sci Transl Med.
6:221ra132014. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Alvarez RD, Huh WK, Bae S, Lamb LS Jr,
Conner MG, Boyer J, Wang C, Hung CF, Sauter E, Paradis M, et al: A
pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients
with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3).
Gynecol Oncol. 140:245–252. 2016. View Article : Google Scholar :
|
|
199
|
Kim TW, Hung CF, Juang J, He L, Hardwick
JM and Wu TC: Enhancement of suicidal DNA vaccine potency by
delaying suicidal DNA-induced cell death. Gene Ther. 11:336–342.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Varnavski AN, Young PR and Khromykh AA:
Stable high-level expression of heterologous genes in vitro and in
vivo by noncy-topathic DNA-based Kunjin virus replicon vectors. J
Virol. 74:4394–4403. 2000. View Article : Google Scholar
|
|
201
|
Herd KA, Harvey T, Khromykh AA and Tindle
RW: Recombinant Kunjin virus replicon vaccines induce protective
T-cell immunity against human papillomavirus 16 E7-expressing
tumour. Virology. 319:237–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Tillman BW, Hayes TL, DeGruijl TD, Douglas
JT and Curiel DT: Adenoviral vectors targeted to CD40 enhance the
efficacy of dendritic cell-based vaccination against human
papillomavirus 16-induced tumor cells in a murine model. Cancer
Res. 60:5456–5463. 2000.PubMed/NCBI
|
|
203
|
Mackova J, Kutinova L, Hainz P, Krystofova
J, Sroller V, Otahal P, Gabriel P and Nemeckova S: Adjuvant effect
of dendritic cells transduced with recombinant vaccinia virus
expressing HPV16-E7 is inhibited by co-expression of IL12. Int J
Oncol. 24:1581–1588. 2004.PubMed/NCBI
|
|
204
|
Wang TL, Ling M, Shih IM, Pham T, Pai SI,
Lu Z, Kurman RJ, Pardoll DM and Wu TC: Intramuscular administration
of E7-transfected dendritic cells generates the most potent
E7-specific anti-tumor immunity. Gene Ther. 7:726–733. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Benencia F, Courreges MC and Coukos G:
Whole tumor antigen vaccination using dendritic cells: Comparison
of RNA electroporation and pulsing with UV-irradiated tumor cells.
J Transl Med. 6:212008. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Murakami M, Gurski KJ, Marincola FM,
Ackland J and Steller MA: Induction of specific CD8+ T-lymphocyte
responses using a human papillomavirus-16 E6/E7 fusion protein and
autologous dendritic cells. Cancer Res. 59:1184–1187.
1999.PubMed/NCBI
|
|
207
|
Peng S, Kim TW, Lee JH, Yang M, He L, Hung
CF and Wu TC: Vaccination with dendritic cells transfected with BAK
and BAX siRNA enhances antigen-specific immune responses by
prolonging dendritic cell life. Hum Gene Ther. 16:584–593. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Kim JH, Kang TH, Noh KH, Bae HC, Kim SH,
Yoo YD, Seong SY and Kim TW: Enhancement of dendritic cell-based
vaccine potency by anti-apoptotic siRNAs targeting key
pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell
death. Immunol Lett. 122:58–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Adams M, Navabi H, Jasani B, Man S,
Fiander A, Evans AS, Donninger C and Mason M: Dendritic cell (DC)
based therapy for cervical cancer: Use of DC pulsed with tumour
lysate and matured with a novel synthetic clinically non-toxic
double stranded RNA analogue poly (I):poly (C(12)U) (Ampligen R).
Vaccine. 21:787–790. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Ahn YH, Hong SO, Kim JH, Noh KH, Song KH,
Lee YH, Jeon JH, Kim DW, Seo JH and Kim TW: The siRNA cocktail
targeting interleukin 10 receptor and transforming growth factor-β
receptor on dendritic cells potentiates tumour antigen-specific
CD8(+) T cell immunity. Clin Exp Immunol. 181:164–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Santin AD, Bellone S, Palmieri M, Ravaggi
A, Romani C, Tassi R, Roman JJ, Burnett A, Pecorelli S and Cannon
MJ: HPV16/18 E7-pulsed dendritic cell vaccination in cervical
cancer patients with recurrent disease refractory to standard
treatment modalities. Gynecol Oncol. 100:469–478. 2006. View Article : Google Scholar
|
|
212
|
Santin AD, Bellone S, Palmieri M, Zanolini
A, Ravaggi A, Siegel ER, Roman JJ, Pecorelli S and Cannon MJ: Human
papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination
of stage IB or IIA cervical cancer patients: A phase I
escalating-dose trial. J Virol. 82:1968–1979. 2008. View Article : Google Scholar :
|
|
213
|
Bubenik J, Símová J, Hájková R, Sobota V,
Jandlová T, Smahel M, Sobotková E and Vonka V: Interleukin 2 gene
therapy of residual disease in mice carrying tumours induced by HPV
16. Int J Oncol. 14:593–597. 1999.PubMed/NCBI
|
|
214
|
Mikyskova R, Indrová M, Símová J, Jandlová
T, Bieblová J, Jinoch P, Bubeník J and Vonka V: Treatment of
minimal residual disease after surgery or chemotherapy in mice
carrying HPV16-associated tumours: Cytokine and gene therapy with
IL-2 and GM-CSF. Int J Oncol. 24:161–167. 2004.
|
|
215
|
Hallez S, Detremmerie O, Giannouli C,
Thielemans K, Gajewski TF, Burny A and Leo O:
Interleukin-12-secreting human papillomavirus type 16-transformed
cells provide a potent cancer vaccine that generates E7-directed
immunity. Int J Cancer. 81:428–437. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Chang EY, Chen CH, Ji H, Wang TL, Hung K,
Lee BP, Huang AY, Kurman RJ, Pardoll DM and Wu T: Antigen-specific
cancer immunotherapy using a GM-CSF secreting allogeneic tumor
cell-based vaccine. Int J Cancer. 86:725–730. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Thompson PL and Dessureault S: Tumor cell
vaccines. Adv Exp Med Biol. 601:345–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Stevanović S, Draper LM, Langhan MM,
Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry
RM, Kammula US, et al: Complete regression of metastatic cervical
cancer after treatment with human papillomavirus-targeted
tumor-infiltrating T cells. J Clin Oncol. 33:1543–1550. 2015.
View Article : Google Scholar
|
|
219
|
Draper LM, Kwong ML, Gros A, Stevanović S,
Tran E, Kerkar S, Raffeld M, Rosenberg SA and Hinrichs CS:
Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered
T cells directed against E6. Clin Cancer Res. 21:4431–4439. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Beutner KR, Geisse JK, Helman D, Fox TL,
Ginkel A and Owens ML: Therapeutic response of basal cell carcinoma
to the immune response modifier imiquimod 5% cream. J Am Acad
Dermatol. 41:1002–1007. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Buck HW and Guth KJ: Treatment of vaginal
intraepithelial neoplasia (primarily low grade) with imiquimod 5%
cream. J Low Genit Tract Dis. 7:290–293. 2003. View Article : Google Scholar
|
|
222
|
Terlou A, van Seters M, Ewing PC, Aaronson
NK, Gundy CM, Heijmans-Antonissen C, Quint WG, Blok LJ, van Beurden
M and Helmerhorst TJ: Treatment of vulvar intraepithelial neoplasia
with topical imiquimod: Seven years median follow-up of a
randomized clinical trial. Gynecol Oncol. 121:157–162. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Fox PA, Nathan M, Francis N, Singh N, Weir
J, Dixon G, Barton SE and Bower M: A double-blind, randomized
controlled trial of the use of imiquimod cream for the treatment of
anal canal high-grade anal intraepithelial neoplasia in
HIV-positive MSM on HAART, with long-term follow-up data including
the use of open-label imiquimod. Aids. 24:2331–2335.
2010.PubMed/NCBI
|