Introduction
Neuroblastoma (NB) is the most frequent
extra-cranial childhood type of cancer and is responsible for
approximately 15% of all paediatric deaths due to cancer (1,2). NB
is substantially heterogeneous (3,4).
Several critical genetic aberrations have been associated with NB;
however, few molecular markers have been associated with the
prognosis of the disease. Among these, the V-myc avian
myelocytomatosis viral oncogene neuroblastoma derived homolog
(MYCN) locus amplification is the most important, being
present in approximately 20-30% of all NB cases, and is strongly
associated with a poor prognosis (5). Deletions of part of 1p, of 11q23
(6,7) and the gain of 17q chromosomes
(8) has also been shown to be
associated with to a negative outcome. However, NB-related tumour
suppressors or oncogenes have not been identified at these
chromosomal regions. Functional guidelines for the prognosis of
patients with NB were drawn as risk grouping. The Children's
Oncology Group (COG) (9)
incorporated the tumour stage as assessed by the International NB
Staging System (INSS), MYCN amplification level, and
histology according to Shimada et al (10). These guidelines identify high-risk
patients, with disseminated disease, frequent MYCN
amplification and poorly differentiated tumour cells. The survival
probability is <40%, regardless of the cancer treatment being
administered. In recent years, gene expression profiles and NB
outcome have been studied with different technical platforms and
analytical methods in order to distinguish high-risk from low-risk
tumours; these methods however, result in poor prognostic relevance
when they are used to discriminate patients with low-and
intermediate-stage disease (11,12),
an issue that has now become a major clinical challenge in the
field of NB. Oxidative stress plays an important role in switching
from cell proliferation to cell death (13), and the metabolic pathways
responsible for the polyamine (PA) inter-conversion and degradation
are responsible for oxidant by-products (14,15).
Over the past years, spermine (SPM) metabolism has been found to be
closely related to DNA oxidation in NB cell lines; however, the
ectopic expression of spermine oxidase (SMOX), that specifically
oxidizes SPM to produce spermidine (SPD), 3-aminopropanal and
hydrogen-peroxide (16,17), induces DNA damage without any
increase in cell mortality (18).
SMOX over-activity provokes sub-lethal chronic DNA damage and
repair, visualized as an induction of apurinic/apyrimidinic
endonuclease protein (APE1) and the hyper-phosphorylation of the
H2AX histone, a well characterized marker of DNA damage and repair
(19). In the present study, we
further characterized the effects of SMOX, stably transfected into
NB cells. In parallel, we investigated the effects of SMOX in a
mouse model (Total-SMOX mice) overexpressing SMOX in all tissues
(20,21), and also examined the expression of
the cytoprotective gene, apoptosis-antagonizing transcription
factor (AATF) (otherwise known as Che-1) (22). AATF has been characterized to be
stress-activated and to inhibit apoptosis, and it is induced and
stabilized upon DNA damage (22,23).
In this study, it was demonstrated that a high level of AATF
expression is associated with SMOX gene overexpression in
both NB cells and in Total-SMOX mice. Of note, the anti-apoptotic
effect due to AATF has recently been described as a transcriptional
competitor in the promoter regions of the p53-driven expression of
the pro-apoptotic genes BAX, BAK and PUMA
(24,25). The results presented in this study
also confirm the antagonizing role of AATF towards BAX, BAK and
PUMA in the in vitro and in vivo SMOX-overexpressing
model systems investigated.
Materials and methods
Cells, cell culture and reagents
All reagents were purchased from Sigma-Aldrich,
unless otherwise specified. The inhibitor of FAD-dependent SMOX
activity, N,N'-Bis(2,3-butadienyl)-1,4-butanediamine
dihydrochloride (MDL-72527, herein referred to as MDL), was a gift
from Hoechst Marion Roussel Inc. Plastic-wares were purchased from
Nunc (Nunc A/S). The transfections and growth conditions of the
parental N18TG2 murine NB cell line (Sigma-Aldrich),
pcDNA3-transfected (NP), pcDNA3-SMOX-transfected (NS) and
pcDNA3-γSMO-transfected (NG) cell lines were as previously
described in the study by Cervelli et al (26), Amendola et al (18) and Bianchi et al (19). The γSMO isoform is a splicing
variant of SMOX lacking the FAD domain (26). The cells were pre-treated with MDL
at a final concentration of 50 µM for 24 h. For ROS
counteraction, the cells were pre-treated with
N-acetyl-cysteine (NAC) at a final concentration of 10 mM of
24 h. The control cells were cells treated in the absence of MDL
and NAC.
Animals
A total of 9 syngeneic (SG) male mice and 9
Total-SMOX transgenic (TG) male mice (3 months old, with a mean
body weight of 30 g), were used as the experimental subjects. In
the experiments, only male mice were used in order to exclude all
biochemical and behavioural changes due to female cycle hormones.
They were born and raised at the ‘Stazione per la Tecnologia
Animale' at the University of Tor Vergata and they were transferred
to the animal house of the University of Roma Tre for the
experiments. The animals were housed in an environment with a
controlled temperature (20±1°C), humidity (55±10%) and a 12-h
light/dark cycle. The housing conditions were in accordance with
standard laboratory settings including cage enrichment with free
access to food and water.
Transgenic GFP-SMOX (former JoSMOX) mice described
in the study by Cervelli et al (27) were crossed with Total-CRE mice to
obtain the Total-SMOX genetic line described in the study by Ceci
et al (20). In order to
stabilize the Total-SMOX transgenic line, mice were further
genetically stabilised back-crossing 10 times with C57BL/6 mice
(20). The genotyping for the
Total-SMOX mice was carried out using the β-gal staining of the
tail biopsies from the mouse pups and confirmed by PCR methods
according to Ceci et al (20). In the experiments, as control mice,
the syngeneic littermates were used. All experiments were performed
on independent groups of mice. The organs (brain, intestine and
heart) were obtained from sacrificed SG and TG mice by cervical
dislocation. The experiments were carried out in accordance with
the ethical guidelines for the conduct of animal research of the
European Community's Council Directive 77/499/EEC e 81/309/EEC.
Formal approval of these experiments was obtained from the Italian
Ministry of Health (Official Italian Regulation D.L.vo 26/2014,
‘Authorization from Ministero della Salute no. 964/2015-PR'). All
experiments were performed on independent groups of mice. All
efforts were made to minimize the number of animals used and their
suffering.
RNA isolation, reverse
transcription-quantitative PCR (RT-qPCR) and semi-quantitative
RT-PCR
Total RNA from the NG, NP, NS cell lines, and from
the SG and TG mouse brains, intestines and hearts was isolated
using the GeneElute system, and reverse transcribed into cDNA using
the SuperScript First-Strand Synthesis System (Invitrogen; Thermo
Fisher Scientific), according to the manufacturer's instructions.
For quantitative PCR (qPCR), cDNA was amplified by SYBR Premix Ex
Taq (Takara Bio Inc.) according to the manufacturer's instructions.
Specific primers pairs were designed using Primer Express Software
(Applied Biosystems). The thermo-cycling conditions were as
follows: 95°/30 sec, followed by 40 cycles of [95°/5 sec; 60-63°
(according to Table SI)/30 sec].
Three experimental replicates were performed, and
the results were analysed using the relative expression software
tool (REST) (28). The sequence
and position of the specific primers for AATF, BAK,
BAX and PUMA are listed in Table SI. The sequence and
position of the specific primers for SMOX, β-actin
and RPS7 as the housekeeping reference genes have been
described elsewhere (18,19,27).
For semi-quantitative RT-PCR, the Takara PCR Amplification kit was
used (Takara Bio Inc.). The thermocycling conditions were as
follows: 94°/4 min, followed by 30 cycles of [94°/1 min; 60-63°
(according to Table SI)/30 sec; 72°/30 sec] and then 72°/7 min. The
amplified products were run on a 1.5% agarose gel. Gel images using
ethidium bromide were obtained with automatic exposure by Diana III
dedicated device (Raytest Italia).
Analysis of ROS levels, stress and
apoptosis
The levels of intracellular hydrogen peroxide were
determined by flow cytometric (FCM) analysis of the fluorescence
intensity of 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA)
(Invitrogen; Thermo Fisher Scientific). Briefly, the cells were
treated with H2DCFDA for 30 min with/without pre-treatment for 24 h
with 10 mM NAC. At least 2×104 cells were analyzed by a
FACSCalibur flow cytometer (BD Biosciences), with laser excitation
set at 495 and a 525 nm emission filter to detect green
fluorescence. Oxidative stress, on the basis of number of 8-oxo-dG
residues (29), was analysed using
the OxyDNA kit, following the manufacturer's instructions (Merck
KGaA). Negative control was obtained by omitting the fluorescence
probe-mix from the reaction. The levels of γH2AX accumulation were
determined by FCM analysis of the green fluorescence intensity,
only inside the G1 sub-cohort of cells evidenced by DNA red
fluorescence using 1 µg/ml of propidium iodide (PI), with
laser excitation set at 488 and 630 nm band pass emission filter to
detect both red and green fluorescence (30). The cells were stained with a
primary γH2AX, rabbit polyclonal antibody (4418-APC-020; Trevigen)
at a dilution of 1:100, overnight a 4°C, and then with a secondary
monoclonal anti-rabbit FITC-conjugated antibody (sc-2359; Santa
Cruz Biotechnology) at a dilution of 1:200, for 2 h at room
temperature. The level of 3′-terminal deoxy-transferase (TdT) by
the TUNEL method was used to detect apoptosis, following the
manufacturer's instructions (in situ Cell Death Fluorescent
kit; Roche Diagnostic S.p.A.) and was performed as previously
described (31). AATF was detected
and quantified by both semi-quantitative RT-PCR (see above) and
western blot analysis. Protein extraction and western blot analysis
were carried out as previously described by Ceci et al
(20). The Briefly, tissues were
homogenized in 5 vol (w/v) of ice-cold lysis buffer (50 mM Tris-HCl
pH 7.5, 10% glycerol, 320 mM sucrose, 1% Triton, 1 mM PMSF, 1X
complete protease inhibitor cocktail) using a Potter Elvehjem
Tissue Homogenizer for 45 sec. The samples were kept on ice for 30
min and then centrifuged at 16,100 × g for 15 min at 4°C. The
supernatant was collected and protein determination was carried out
as previously described by Ceci et al (20). Primary rabbit polyclonal antibody
developed against AATF (dilution 1:1,000, not commercially
available; provided by Professor Fanciulli, Università degli Studi
di Milano-Bicocca) (22) was used
for western blot analysis (incubated overnight at 4°C). Equal
proteins amounts (20 µg/sample) were loaded on
electrophoresis in a 10% poly-acrylamide gel and transferred onto
nitrocellulose membranes. The membranes were blocked with 5%
non-fat dry milk for 1 h and incubated overnight at 4°C with a
polyclonal anti-β-actin (dilution 1:2,000, cat. no. A5060;
Sigma-Aldrich S.r.l) diluted in 2.5% non-fat dry milk. β-actin was
taken as the reference. Secondary peroxidase-labelled anti-rabbit
IgG antibody (dilution 1:5,000, cat. no. SA00001-2, in 1.25%
non-fat dry milk) was from Proteintech. The secondary antibody was
incubated for 1 h at room temperature. Detection was performed
using ECL Western blotting detection reagents (GE Healthcare).
Densitometric measurements were obtained using ImageJ 1.52a
software (Wayne Rasband National Institutes of Health).
Analysis of enzymatic activities of PA
metabolism
PA oxidases [SMOX and the peroxisomal FAD-dependent
enzyme N1-acetyltransferase oxidase (PAOX)] activity was
assayed as previously described (19,32,33).
Ornithine decarboxylase (ODC) and spermidine/spermine
N1-acetyltransferase (SSAT) activities were determined
using 14C-labelled substrate and scintillation counting
of end metabolized products, as described elsewhere (18,19).
Cell pellets were sonicated on ice and frozen in aliquots. The
protein concentration was determined using the Bradford method and
at least 3 determination replicas were carried out for the
enzymatic activity of SMOX, PAOX, ODC and SSAT.
Statistical analysis
Significant differences at P<0.05 were evaluated
by one-way ANOVA, followed by the multiple comparison Tukey
post-hoc test (SPSS-11 statistical dedicated software; SPSS Inc.).
All experiments were repeated 3 times unless otherwise
indicated.
Results
SMOX induces cellular stress via an
imbalance in ROS generation, but not apoptosis
In previous studies, SMOX activity has been
described to induce a chronic sub-lethal DNA damage, with a 3-fold
increase in 8-oxo-dG residues, but with no increase in cell
mortality being observed (18,19,31).
Treatments with inhibitors of PA oxidases have been shown to
abolish the higher amount of oxidative stress in different cell
line models (34,35). Similar results were obtained in the
present study, since treatment with NAC was able to counteract ROS
overproduction (36). ROS
(Fig. 1A, left panel) and 8-oxo-dG
(Fig. 1B, left panel) were induced
by SMOX in the NS cells, as shown by the FCM histograms of H2DCFDA
and 8-oxo-dG residues, when compared with both NP and NG cells. A
final concentration of 10 mM NAC treatment scavenged ROS
overproduction (Fig. 1A, right
panel) and hampered 8-oxo-dG residues (Fig. 1B, right panel) in NS cells. The
γH2AX phosphorylated content was also evaluated to monitor the
onset of cellular response to DNA damage (Fig. 1C, left panel). SMOX induced γH2AX
phosphorylation in the NS cells and NAC subverted this event
(Fig. 1C, right panel). These data
confirm the ability of SMOX to produce oxidative cellular stress,
driving cells to a preliminary phase of repair. Notably, we confirm
previous data on NB cells (18,19)
and in human colon adenocarcinoma cell (31), whereby SMOX over-expression does
not induce cell mortality and apoptosis per se when evaluated by
the ipo-G0/G1 sub-population and TUNEL assay, as shown in Fig. S1.
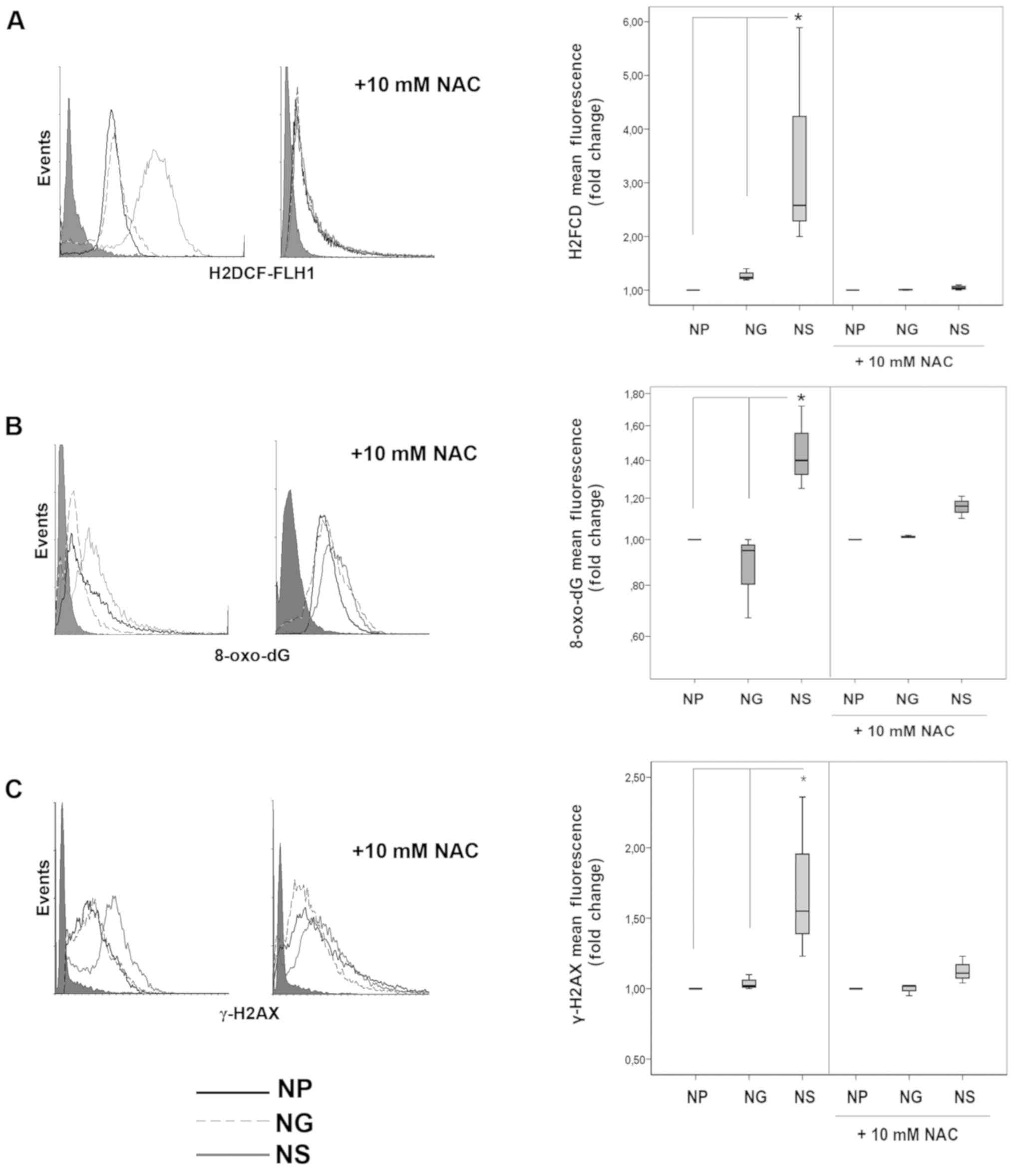 | Figure 1SMOX induces oxidative stress.
Mono-parametric FCM analysis of (A) H2DCF, (B) 8-oxo-dG, and (C)
γH2AX. Auto-fluorescence is closed to the y-axes (dark grey-filled
histograms). NP, N18TG2 cells transfected with pcDNA3 (black line);
NG, N18TG2 cells transfected with pcDNA3/γSMO (dashed line); NS,
N18TG2 cells transfected with pcDNA3/SMOX. In the right panels,
analyses were performed following pre-treatment of the cells for 24
h with 10 mM of NAC as a ROS scavenger. H2DCF and 8-oxo-dG are
gathered as FLH-1 green fluorescence emission. γH2AX is quantified
within the Go/G1 cellular sub-population (PI counter-stain) to
avoid the S-phase γH2AX-positive cells as a confounding factor. In
the left panels, histograms are representative of at least 20,000
events gated. In the right panels, the Whisker plots represent the
statistical analysis of the fold change of the mean and the
dispersion of the fluorescence in the cell population, relative to
NP cells. Data were analysed by one-way ANOVA, followed by Tukey's
post hoc test. *P<0.05 vs. NP cells and NG cells.
SMOX, spermine oxidase; NAC, N-acetyl-cysteine. |
SMOX induces AATF gene expression via an
imbalance in ROS generation
The cellular ROS disequilibrium can drive cells to
enter a complex mechanistic molecular pathway to arrest the cell
cycle and promote DNA repair or apoptosis. From this point of view,
SMOX represents an apparently self-contradictory response, since it
has the ability to induce preliminary stress without driving the
onset of apoptosis. Thus, we wished to examine the level of the
AATF gene (22). Upon
genotoxic stress, AATF has been described to become phos-phorylated
and to subsequently translocate to the nucleus to bind the
PUMA, BAX and BAK promoter regions (23). PUMA, BAX and
BAK are well known pro-apoptotic genes driven by p53, and NB
cells are almost exclusively p53-proficient (23).
AATF mRNA expression was evaluated by
semi-quantitative RT-PCR in the NP, NG and NS cells, under normal
growth conditions and following treatment with NAC (Fig. 2A). At 25 cycles, AATF mRNA
expression was higher in the NS cells. With the increasing PCR
cycles, amplification rises to saturate the signals. The exhaustive
ROS deprivation due to treatment with 10 mM NAC (Fig. 2A), enforced AATF expression in all
the cell lines. AATF protein accumulation detected by western blot
analysis was in full accordance with the RNA expression data
(Fig. 2B, left panel), while NAC
treatment induced the over-accumulation of AATF protein in all cell
lines (Fig. 2B, right panel). As a
first step, a semi-quantitative RT-PCR indicated the diminished
amount of mRNA for the pro-apoptotic genes, BAK, BAX
and PUMA, in the NS cells (Fig. S2A). MDL treatment induced an equal
expression of these pro-apoptotic genes in the NP, NG and NS cell
lines (Fig. S2A). The expression
levels of BAK, BAX and PUMA were then quantified by qPCR, as a
ratio with the RPS7 housekeeping reference gene, without analysing
the NAC-treated cells, since they exhibited an equal amount of
AATF gene transcripts. The results of qPCR confirmed the
lower mRNA expression levels of BAK, BAX and PUMA in the NS vs. the
NP and NG cell lines, and the normalization effect due to MDL
(Fig. 3A). Subsequently, we
analysed these effects of SMOX overexpres-sion in tissues from the
genetic-engineered conditional mouse model, Total-SMOX (20). When comparing the level of AATF in
different organs between the transgenic and syngeneic animals, we
observed a higher level of this anti-apoptotic factor in the
intestinal tract and heart tissues than in the brain, according to
SMOX expression in these organs (Fig.
3B). Consistent with the results obtained in vitro, the
AATF levels were associated with SMOX overexpression, as
demonstrated by semi-quantitative RT-PCR (Fig. S2B) and quantified by qPCR
(Fig. 3B), where the expression of
genes are reported as ratio between syngeneic vs. transgenic mice.
AATF activation was associated with the transcriptional inhibition
of BAK, BAX and PUMA, thus representing
indirect evidence in vivo of the anti-apoptotic activity of
SMOX. Taken together, these results strongly indicate that SMOX may
act as an indirect inhibitor of apoptosis, via an imbalance in ROS
generation, and may be considered a tumorigenic gene.
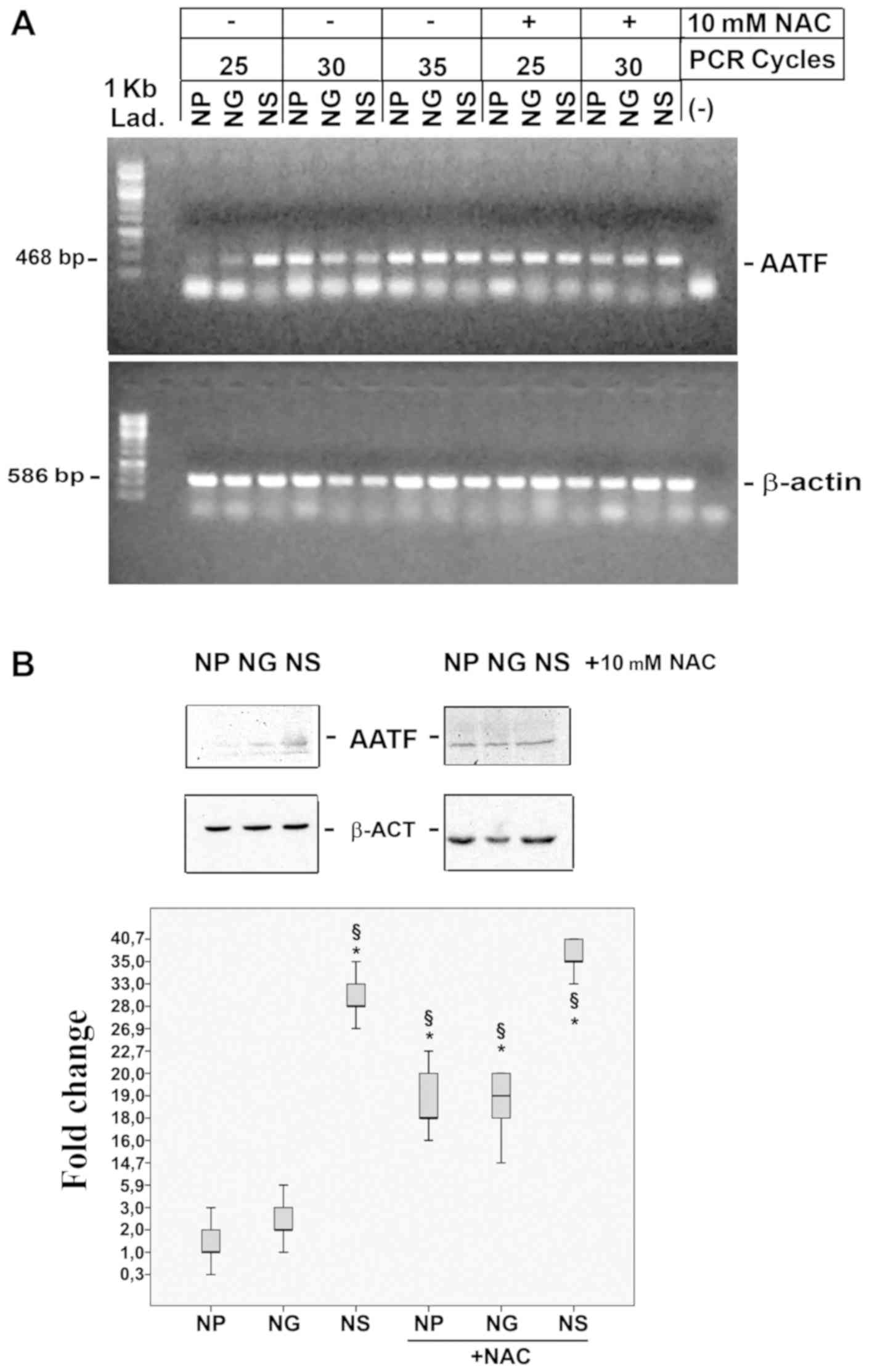 | Figure 2SMOX induces AATF expression. (A)
Semi-quantitative RT-PCR quantification of the antagonizing
apoptosis transcription factor (AATF) and β-actin expression levels
in NP, NS, NG cells, in the presence 10 mM NAC as a ROS scavenger
at different PCR cell cycles (as indicated). The symbol ‘-'
represents the negative control of the PCR reaction. (B) Western
blot analysis of AATF in NP, NG and NS cells in the presence of 10
mM NAC as a ROS scavenger. The Whisker plot represents the fold
changes in AATF protein content vs. NP, calculated as arbitrary
densitometric units normalized vs. actin signals of 3 independent
experiments. Data were analysed by one-way ANOVA, followed by
Tukey's post hoc test. *P<0.05 vs. NP cells;
§P<0.05 vs. NG cells. SMOX, spermine oxidase; AATF,
apoptosis antagonizing-factor; β-ACT, β-actin; NAC,
N-acetyl-cysteine; NP cells. NP, N18TG2 cells transfected
with pcDNA3; NS, N18TG2 cells transfected with pcDNA3/SMOX; NG,
N18TG2 cells transfected with pcDNA3/γSMO. |
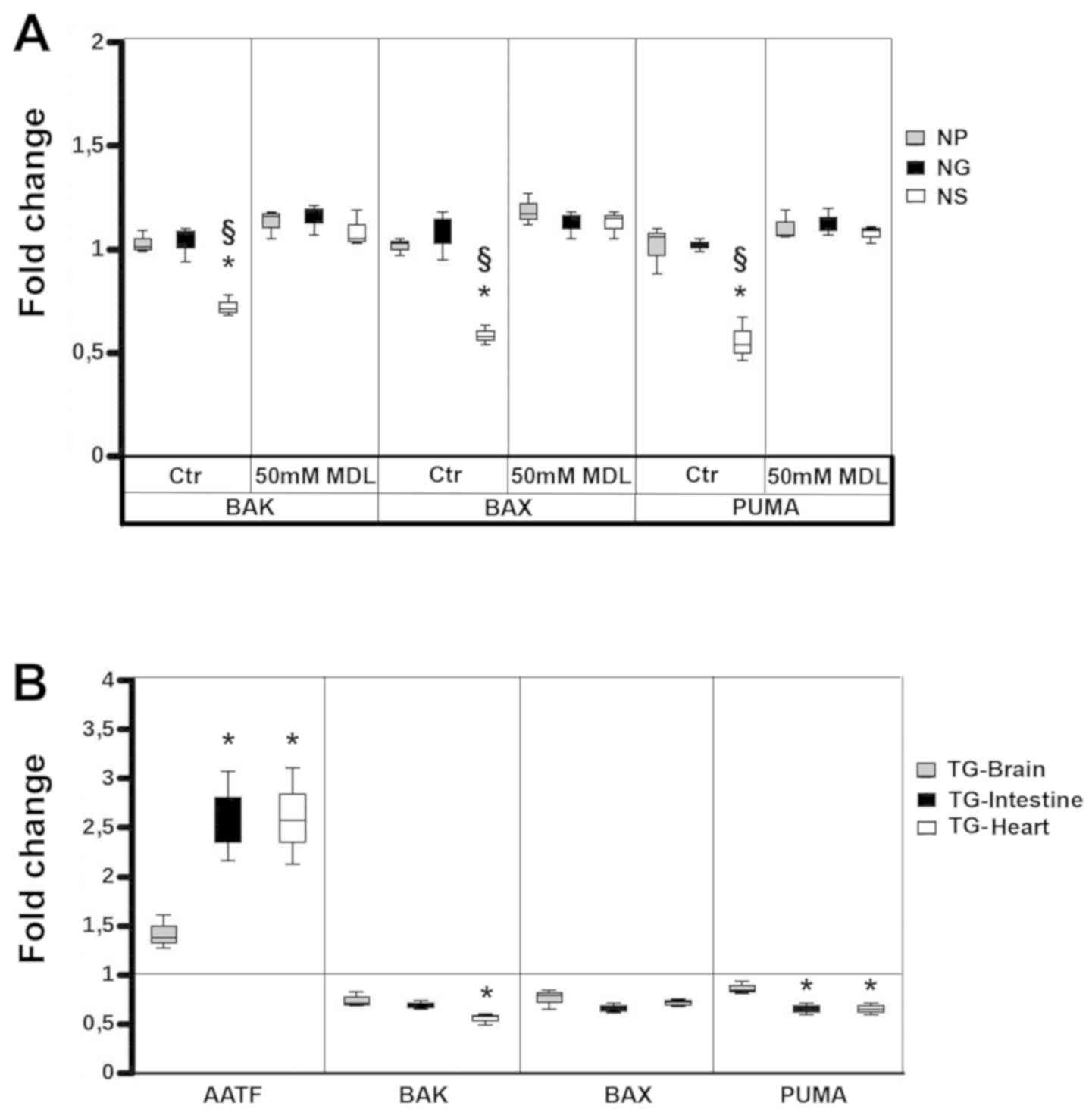 | Figure 3SMOX indirectly inhibits the
expression of the pro-apoptotic genes, BAK, BAX and PUMA. (A) Fold
change of BAK, BAX and PUMA expression between NG (black bars) and
NS (white bars) cell lines vs. NP (black bars). Whisker-plot graph
of qPCR normalized vs.RPS7 as the control housekeeping gene, in the
absence or presence of 50 µM MDL as a SMOX inhibitor. (B)
Fold change of AATF, BAK, BAX and PUMA expression between tissues
from Total-SMOX transgenic mice (TG) vs. syngeneic mice.
Whisker-plot graph of qPCR normalized vs. RPS7 as the control
housekeeping gene. TG-Brain (grey bars), TG-Intestine (black bars),
and TG-Heart (white bars). Data were analysed by one-way ANOVA,
followed by Tukey's post hoc test. *P>0.05 vs. NP
cells in (A) and TG-Brain in (B); §P<0.05 vs. NG
cells in (A). SMOX, spermine oxidase, AATF, apoptosis
antagonizing-factor, BAK, Bcl-2 antagonist/killer, BAX,
Bcl-2-associated X protein, PUMA, p53 upregulated modulator of
apoptosis; MDL-72527, N,N′-Bis(2,3-butadienyl)-1,4-butanediamine
dihydrochloride; NP cells. NP, N18TG2 cells transfected with
pcDNA3; NS, N18TG2 cells transfected with pcDNA3/SMOX; NG, N18TG2
cells transfected with pcDNA3/γSMO. |
SMOX and PA metabolism in NB cells
We also found that PA metabolism in the NS cells was
consistent with what was expected (19,37).
Namely, a low ODC activity corresponds to a higher SSAT activity
and to a 3- or 4-fold enhancement in SMOX activity (19,31)
(Fig. 4A). The intracellular PA
concentrations followed the enzymatic variation consistently, with
less putrescine (PUT) and more SPD, mainly due to SMOX
overactivity, although the amount of SPM was consistently buffered
by the interconversion of the PA metabolism (Fig. 4B, whisker box-plot). Of note, NAC
induced an increase in the level of intracellular SPD in both NP
and NS cell lines.
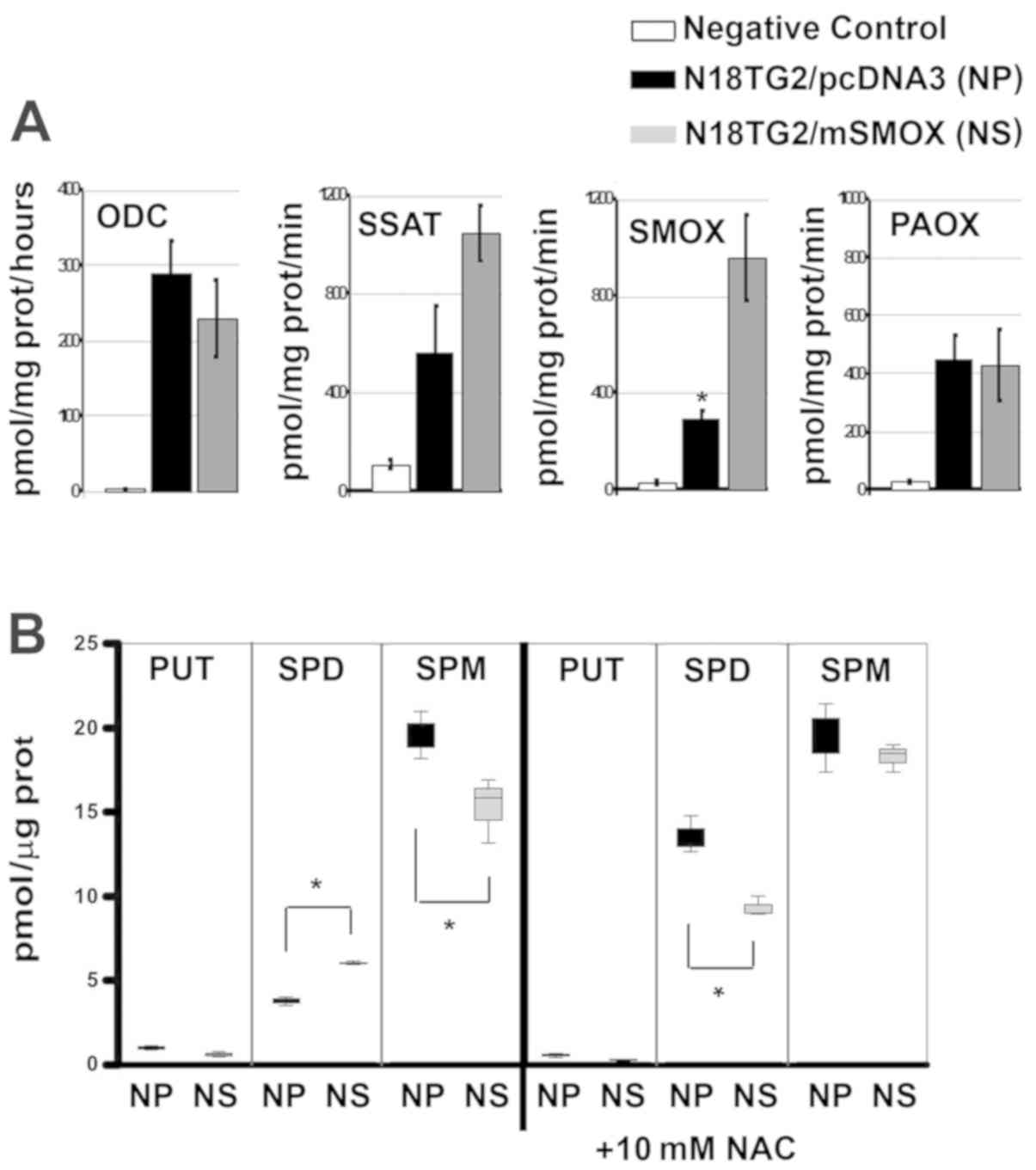 | Figure 4PA metabolism. (A) Column bar graphs
of ODC, SSAT, SMOX and PAOX activities in expressed as pmol/mg
proteins/h in NP and NS cells. White columns represent the negative
controls of enzymatic reactions, with no cellular extracts.
*P>0.05, difference in SMOX activity between NP and
NS cell lines. (B) Column bar graphs of PUT, SPD and SPM
concentrations expressed as pmoles/µg of protein, in NP and
NS cells and in the presence of 10 mM NAC (as indicated). PUT, SPD,
and SPM concentrations obtained from NG control cells were
identical to NP cells, and then omitted for the sake of clarity.
Data were analysed by one-way ANOVA, followed by Tukey's post hoc
test. *P>0.05 vs. NP cells. NP, N18TG2 cells
transfected with pcDNA3 (black bars); NS, N18TG2 cells transfected
with pcDNA3/SMOX (grey bars). PA, polyamine; ODC, ornithine
decarboxylase; SSAT, spermidine/spermine
N1-acetyltransferase; SMOX, spermine oxidase; APAO,
N1-acetylpolyamine oxidase; PUT, putrescine; SPD,
spermidine; SPM, spermine. |
Discussion
Previous studies have demonstrated that SMOX
activity is involved in the delivery of oxidative stress (18,19,35).
At such a level, ROS may act as signalling molecules in various
intracellular processes (38,39),
one of these being apoptosis. However, without any further
induction of damage, SMOX did not induce the onset of apoptosis
(18,19,35 and this study). Recently, the AATF gene has been
characterized to be stress-activated and to inhibit apoptosis
(22,23). AATF is induced and stabilized upon
DNA damage (22,23) and, in this study, its expression
was found to be associated with SMOX overexpression in NB cells and
tissues. In our in vitro and in vivo experimental
models, the expression levels of BAK, BAX and PUMA were
downregulated in the presence of SMOX overexpression and the
SMOX-induced increase in the AATF levels. However, the chromosomal
gains on the AATF locus (17q12) with the consistent
augmented mRNA expression level of AATF are associated per se with
a negative outcome in NB patients (23), thus justifying that SMOX activity
can preserve cell survival under stress conditions, playing an
indirect anti-apoptotic role, inducing AATF expression. This could
have detrimental effects on cancer prognosis (40,41).
The PA bio-synthesis rates are associated with a negative prognosis
(37), and each biosyn-thetic and
catabolic step of PA metabolism is a putative target for cancer
diagnosis. The findings of this study confirm that dysfunctions in
PA metabolism are strictly related to cancer. In conclusion, ROS
overproduction due to SMOX activity may activate AATF and may
indirectly inhibit the apoptosis of NB cells.
Supplementary Data
Funding
This study was funded by the generous support of ‘La
Sapienza' University of Rome and the Italian MIUR (Ministero
dell'Istruzione, dell'Università e della Ricerca). This study was
also supported by the Italian Ministry of Education, University and
Research (MIUR), Grant to Department of Science, University ‘Roma
Tre'-‘Dipartimenti di Eccellenza', (Legge 232/2016, Articolo 1,
Comma 314–337) and by the ‘Roma Tre' University of Rome
contribution to the laboratories (CAL/2017 and CAL/2018) to MC. PM
and EA would also like to thank Wakunaga Pharmaceutical Co., Ltd.
for the scholarship given to YK for supporting his PhD
research.
Availability of data and materials
All data generated or analysed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
EF, MC, PM, YK, EA and RA conceived this study and
coordinated the collab oration among the authors. EF, RA and MC
performed all the experiments. EF, MC, EA and RA optimized the
protocols for the analyses. All authors wrote the manuscript and
all authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments were carried out in accordance with
the ethical guidelines for the conduct of animal research of the
European Community's Council Directive 77/499/EEC e 81/309/EEC.
Formal approval of these experiments was obtained from the Italian
Ministry of Health (Official Italian Regulation D.L.vo 26/2014,
“Authorization from Ministero della Salute no. 964/2015-PR”). All
experiments were performed on independent groups of mice. All
efforts were made to minimize the number of animals used and their
suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the ‘International
Polyamine Foundation - ONLUS' for the availability to look up the
poly-amines documentation. The authors are indebted to Professor
Roberto Fanciulli (Università degli Studi di Milano-Bicocca) for
providing the antibodies against AATF.
Abbreviations:
|
AATF
|
apoptosis-antagonizing-factor
|
|
APE1
|
apurinic/apyrimidinic endonuclease
1
|
|
H2DCFDA
|
2′,7′-dichloro-dihydrofluorescein
diacetate
|
|
FCM
|
flow cytometry
|
|
PAOX
|
peroxisomal
N1-acetyl-spermine/spermidine oxidase
|
|
NAC
|
N-acetyl-cysteine
|
|
MDL-72527
|
N,N1-Bis(2,3-butadienyl)-1,4-butanediamine
|
|
NB
|
neuroblastoma
|
|
ODC
|
ornithine decarboxylase
|
|
PA
|
polyamines
|
|
PI
|
propidium iodide
|
|
PUT
|
putrescine
|
|
SPD
|
spermidine
|
|
SSAT
|
spermidine/spermine
N1-acetyltransferase
|
|
SMOX
|
spermine oxidase
|
|
SPM
|
spermine
|
|
MYCN
|
V-myc avian myelocytomatosis viral
oncogene, neuroblastoma-derived homolog
|
|
TdT
|
3′-terminal deoxy-transferase
|
References
|
1
|
Brodeur GM and Maris JM: Neuroblastoma In:
Principles and Practice of Pediatric Oncology. Pizzo PA and Poplack
DG: JB Lippincott; Philadelphia: pp. 933–970. 2006
|
|
2
|
Steliarova-Foucher E, Colombet M, Ries
LAG, Moreno F, Dolya A, Bray F, Hesseling P, Shin HY, Stiller CA,
Bouzbid S, et al IICC-3 contributors: International incidence of
childhood cancer, 2001-10: A population-based registry study.
Lancet Oncol. 18:719–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brodeur GM: Neuroblastoma: Biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ngan ES: Heterogeneity of neuroblastoma.
Oncoscience. 2:837–838. 2015.PubMed/NCBI
|
|
5
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Attiyeh EF, London WB, Mossé YP, Wang Q,
Winter C, Khazi D, McGrady PW, Seeger RC, Look AT, Shimada H, et al
Children's Oncology Group: Chromosome 1p and 11q deletions and
outcome in neuroblastoma. N Engl J Med. 353:2243–2253. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mlakar V, Jurkovic Mlakar S, Lopez G,
Maris JM, Ansari M and Gumy-Pause F: 11q deletion in neuroblastoma:
A review of biological and clinical implications. Mol Cancer.
16:1142017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bown N, Cotterill S, Lastowska M, O'Neill
S, Pearson AD, Plantaz D, Meddeb M, Danglot G, Brinkschmidt C,
Christiansen H, et al: Gain of chromosome arm 17q and adverse
outcome in patients with neuroblastoma. N Engl J Med.
340:1954–1961. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Leary M, Krailo M, Anderson JR and
Reaman GH; Children's Oncology Group: Progress in childhood cancer:
50 years of research collaboration, a report from the Children's
Oncology Group. Semin Oncol. 35:484–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimada H, Chatten J, Newton WA Jr, Sachs
N, Hamoudi AB, Chiba T, Marsden HB and Misugi K: Histopathologic
prognostic factors in neuroblastic tumors: Definition of subtypes
of ganglioneuroblastoma and an age-linked classification of
neuro-blastomas. J Natl Cancer Inst. 73:405–416. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oberthuer A, Berthold F, Warnat P, Hero B,
Kahlert Y, Spitz R, Ernestus K, König R, Haas S, Eils R, et al:
Customized oligo-nucleotide microarray gene expression-based
classification of neuroblastoma patients outperforms current
clinical risk stratification. J Clin Oncol. 24:5070–5078. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Diskin S, Rappaport E, Attiyeh E,
Mosse Y, Shue D, Seiser E, Jagannathan J, Shusterman S, Bansal M,
et al: Integrative genomics identifies distinct molecular classes
of neuroblastoma and shows that multiple genes are targeted by
regional alterations in DNA copy number. Cancer Res. 66:6050–6062.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casero RA Jr and Marton LJ: Targeting
polyamine metabolism and function in cancer and other
hyperproliferative diseases. Nat Rev Drug Discov. 6:373–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cervelli M, Angelucci E, Germani F,
Amendola R and Mariottini P: Inflammation, carcinogenesis and
neurodegeneration studies in transgenic animal models for polyamine
research. Amino Acids. 46:521–530. 2014. View Article : Google Scholar
|
|
16
|
Cervelli M, Amendola R, Polticelli F and
Mariottini P: Spermine oxidase: Ten years after. Amino Acids.
42:441–450. 2012. View Article : Google Scholar
|
|
17
|
Polticelli F, Salvi D, Mariottini P,
Amendola R and Cervelli M: Molecular evolution of the polyamine
oxidase gene family in Metazoa. BMC Evol Biol. 12:902012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amendola R, Bellini A, Cervelli M, Degan
P, Marcocci L, Martini F and Mariottini P: Direct oxidative DNA
damage, apoptosis and radio sensitivity by spermine oxidase
activities in mouse neuro-blastoma cells. Biochim Biophys Acta.
1755:15–24. 2005.PubMed/NCBI
|
|
19
|
Bianchi M, Bellini A, Cervelli M, Degan P,
Marcocci L, Martini F, Scatteia M, Mariottini P and Amendola R:
Chronic sub-lethal oxidative stress by spermine oxidase
overactivity induces continuous DNA repair and hypersensitivity to
radiation exposure. Biochim Biophys Acta. 1773:774–783. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ceci R, Duranti G, Leonetti A, Pietropaoli
S, Spinozzi F, Marcocci L, Amendola R, Cecconi F, Sabatini S,
Mariottini P, et al: Adaptive responses of heart and skeletal
muscle to spermine oxidase overexpression: Evaluation of a new
transgenic mouse model. Free Radic Biol Med. 103:216–225. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cervelli M, Leonetti A, Duranti G,
Sabatini S, Ceci R and Mariottini P: Skeletal muscle
pathophysiology: The emerging role of spermine oxidase and
spermidine. Med Sci (Basel). 6:62018.
|
|
22
|
Bruno T, De Nicola F, Iezzi S, Lecis D,
D'Angelo C, Di Padova M, Corbi N, Dimiziani L, Zannini L, Jekimovs
C, et al: Che-1 phosphorylation by ATM/ATR and Chk2 kinases
activates p53 transcription and the G2/M checkpoint. Cancer Cell.
10:473–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Cai S, Zhang C, Liu Z, Luo J, Xing
B and Du X: Deacetylation of NAT10 by Sirt1 promotes the transition
from rRNA biogenesis to autophagy upon energy stress. Nucleic Acids
Res. 46:9601–9616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Höpker K, Hagmann H, Khurshid S, Chen S,
Hasskamp P, Seeger-Nukpezah T, Schilberg K, Heukamp L, Lamkemeyer
T, Sos ML, et al: AATF/Che-1 acts as a phosphorylation-dependent
molecular modulator to repress p53-driven apoptosis. EMBO J.
31:3961–3975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sang Q, Liu X, Wang L, Qi L, Sun W, Wang
W, Sun Y and Zhang H: Sun Y andZhang H: CircSNCA downregulation by
pramipexole treatment mediates cell apoptosis and autophagy in
Parkinson's disease by targeting miR-7. Aging (Albany NY).
10:62018.
|
|
26
|
Cervelli M, Bellini A, Bianchi M, Marcocci
L, Nocera S, Polticelli F, Federico R, Amendola R and Mariottini P:
Mouse spermine oxidase gene splice variants. Nuclear subcellular
localization of a novel active isoform. Eur J Biochem. 271:760–770.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cervelli M, Bellavia G, D'Amelio M,
Cavallucci V, Moreno S, Berger J, Nardacci R, Marcoli M, Maura G,
Piacentini M, et al: A new transgenic mouse model for studying the
neurotoxicity of spermine oxidase dosage in the response to
excitotoxic injury. PLoS One. 8:e648102013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cadet J, Douki T, Gasparutto D and Ravanat
JL: Oxidative damage to DNA: Formation, measurement and biochemical
features. Mutat Res. 531:5–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang X, Okafuji M, Traganos F, Luther E,
Holden E and Darzynkiewicz Z: Assessment of histone H2AX
phosphorylation induced by DNA topoisomerase I and II inhibitors
topotecan and mitoxantrone and by the DNA cross-linking agent
cisplatin. Cytometry A. 58:99–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amendola R, Cervelli M, Fratini E,
Sallustio DE, Tempera G, Ueshima T, Mariottini P and Agostinelli E:
Reactive oxygen species spermine metabolites generated from amine
oxidases and radiation represent a therapeutic gain in cancer
treatments. Int J Oncol. 43:813–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cervelli M, Fratini E, Amendola R, Bianchi
M, Signori E, Ferraro E, Lisi A, Federico R, Marcocci L and
Mariottini P: Increased spermine oxidase (SMO) activity as a novel
differentiation marker of myogenic C2C12 cells. Int J Biochem Cell
Biol 4. 1:934–944. 2009. View Article : Google Scholar
|
|
33
|
Cervelli M, Bellavia G, Fratini E,
Amendola R, Polticelli F, Barba M, Federico R, Signore F, Gucciardo
G, Grillo R, et al: Spermine oxidase (SMO) activity in breast tumor
tissues and biochemical analysis of the anticancer spermine
analogues BENSpm and CPENSpm. BMC Cancer. 10:5552010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pledgie A, Huang Y, Hacker A, Zhang Z,
Woster PM, Davidson NE and Casero RA Jr: Spermine oxidase
SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the
primary source of cytotoxic H2 O2 in
polyamine analogue-treated human breast cancer cell lines. J Biol
Chem. 280:39843–39851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Capone C, Cervelli M, Angelucci E,
Colasanti M, Macone A, Mariottini P and Persichini T: A role for
spermine oxidase as a mediator of reactive oxygen species
production in HIV-Tat-induced neuronal toxicity. Free Radic Biol
Med. 63:99–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun SY: N-acetylcysteine, reactive oxygen
species and beyond. Cancer Biol Ther. 9:109–110. 2010. View Article : Google Scholar :
|
|
37
|
Fogel-Petrovic M, Vujcic S, Miller J and
Porter CW: Differential post-transcriptional control of ornithine
decarboxylase and spermidine-spermine
N1-acetyltransferase by polyamines. FEBS Lett.
391:89–94. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mastrantonio R, Cervelli M, Pietropaoli S,
Mariottini P, Colasanti M and Persichini T: HIV-Tat induces the
Nrf2/ARE pathway through NMDA receptor-elicited spermine oxidase
activation in human neuroblastoma cells. PLoS One. 11:e01498022016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pietropaoli S, Leonetti A, Cervetto C,
Venturini A, Mastrantonio R, Baroli G, Persichini T, Colasanti M,
Maura G, Marcoli M, et al: Glutamate Excitotoxicity Linked to
Spermine Oxidase Overexpression. Mol Neurobiol. 55:7259–7270. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caforio M, Sorino C, Iacovelli S,
Fanciulli M, Locatelli F and Folgiero V: Recent advances in
searching c-Myc transcriptional cofactors during tumorigenesis. J
Exp Clin Cancer Res. 37:2392018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jing P, Zou J, Weng K and Peng P: The
PI3K/AKT axis modulates AATF activity in Wilms' tumor cells. FEBS
Open Bio. 8:1615–1623. 2018. View Article : Google Scholar : PubMed/NCBI
|


















