Cancer is the second leading cause of global
mortality, with ~20 million cases and 9.7 million deaths reported
in 2022 (1). While therapeutic
strategies such as surgical resection, chemotherapy, radiotherapy,
targeted therapy and immunotherapy have advanced, drug resistance
and adverse reactions undermine clinical outcomes (2,3).
Drug resistance not only drives tumor recurrence but also
perpetuates cancer as a key public health challenge (4).
Current investigations into therapy resistance
mechanisms extend beyond ATP-binding cassette (ABC)
transporter-mediated drug efflux to encompass metabolic
reprogramming, exemplified by the Warburg effect, alongside dynamic
mitochondrial interactions (5,6).
Cancer cells exhibit a range of metabolic adaptations, enabling
them to secure energy despite limited nutrient and oxygen
availability (7). In the 1920s,
Otto Warburg demonstrated that cancer cells preferentially generate
ATP through glycolysis, even in the presence of sufficient oxygen
(8). Research on cancer cell
metabolism primarily focuses on glycolysis, glutamine metabolism
and mitochondrial protective mechanisms (9,10).
Moreover, abnormal lipid metabolism is a hallmark of metabolic
reprogramming (11). These
adaptations create interconnected networks that sustain
tumorigenesis and therapeutic evasion.
Targeting cancer metabolism gained momentum in the
20th century with nucleotide metabolism inhibitors such as
methotrexate (12). However,
clinical setbacks with glycolysis inhibitors such as 2-deoxyglucose
(13) shifted focus toward
oncogene-targeting therapies due to the limited efficacy and
undesirable side effects. Metabolic intervention strategies have
been facilitated by discoveries linking non-coding RNAs (ncRNAs) to
cancer molecular biology. ncRNAs are a class of RNAs that lack
protein-coding potential and were once considered non-essential
components of the genome. Functionally diverse ncRNAs, including
long ncRNAs (lncRNAs), circular RNAs (circRNAs) and microRNAs
(miRNAs or miRs) (14), regulate
tumor progression through nuclear chromatin remodeling, cytoplasmic
mRNA interactions and competing endogenous RNA (ceRNA) network
formation (15,16). In addition to their roles in cell
proliferation, invasion, migration, and apoptosis, ncRNAs
orchestrate metabolic reprogramming across glycolysis,
mitochondrial function and glutamine and lipid metabolism (17), thereby driving drug resistance in
multiple types of cancer, such as prostate and breast cancer (BC)
(18,19). ncRNAs have thus become an
increasingly recognized target for cancer treatment, as well as
potential biomarkers (14,19).
The present review aimed to outline the mechanisms through which
metabolic changes influence drug resistance in tumor cells and how
ncRNAs mediate drug resistance via metabolic regulation.
Metabolism-regulating ncRNAs hold promise as both biomarkers and
therapeutic targets for overcoming drug resistance in cancer.
Chromosomal variability and immune escape predispose
tumors to both intrinsic and acquired resistance to treatment.
Intrinsic drug resistance stems from cancer cell genetic and
epigenetic traits, making certain types of tumor hard to treat with
conventional therapy (20). For
example, in colorectal cancer (CRC), the activation of the
Wnt/β-catenin signaling pathway enhances stem cell characteristics,
thereby rendering the cells resistant to cisplatin (20). Acquired drug resistance develops
gradually under therapeutic pressure, driven by dynamic changes in
cancer cell behavior and signaling pathways (21). In parallel with tumor metabolism
research, drug resistance is frequently driven by metabolic
reprogramming (10,17) (Fig.
1). As a hallmark of cancer, metabolic reprogramming supplies
the essential nutrients and energy required for tumor progression,
while ncRNAs exert a notable impact. ncRNAs modulate tumor
metabolism by activating metabolism-associated signaling pathways
or targeting key metabolic enzymes (22,23). In metabolically dysregulated tumor
cells, ncRNAs display aberrant expression, influenced by factors
such as DNA methylation (24),
transcription factors (25), and
hypoxic or oncogenic stimuli (26,27). Additionally, ncRNAs can be
transferred between cells via exosomes. For example, temozolomide
(TMZ)-resistant glioma cells transfer circ-0072083 to TMZ-sensitive
cells through exosomes (28). The
deregulation of ncRNAs disrupts metabolic pathways in tumor cells,
thereby driving metabolic reprogramming and altering tumor
sensitivity to therapeutic intervention (Table I).
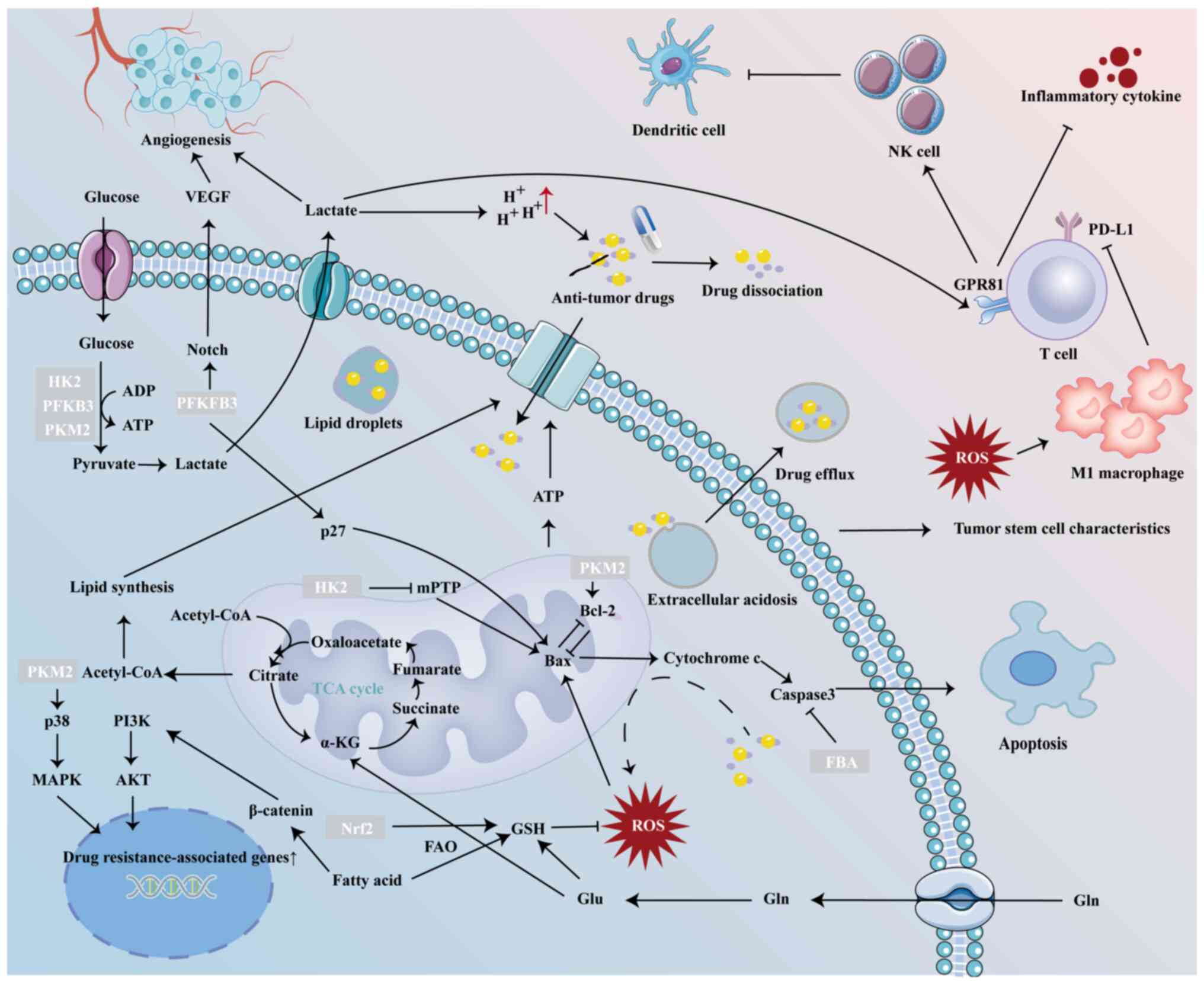
Aerobic glycolysis serves as a prominent example of
a reprogrammed metabolic pathway in cancer cells. Tumor cells
uptake glucose in large quantities, which is converted to pyruvate
by enzymes such as hexokinase (HK), phosphofructokinase (PFK) and
pyruvate kinase (PK). Pyruvate is subsequently converted to lactic
acid by lactate dehydrogenase A (LDHA) or transported to the
mitochondria, where it is catalyzed by pyruvate dehydrogenase (PDH)
to form acetyl-CoA (9).
Glycolysis contributes to drug resistance through
multiple mechanism. Glycolysis provides cancer cells with
sufficient energy to maintain malignancy (6). 3-bromopyruvate is a glycolysis
inhibitor that markedly decreases glycolytic activity by inhibiting
the enzyme HK2. This inhibition impairs the energy supply derived
from glycolysis, leading to a decrease in ATP and glutathione (GSH)
levels in tumor cells, thereby enhancing their sensitivity to
chloroethylnitrosoureas, a bifunctional anti-tumor alkylating agent
(29). In drug-resistant cancer
cells, the PI3K/AKT signaling pathway is activated, which regulates
glycolysis (30), causing higher
ATP levels than in drug-sensitive cells (31,32). This process provides the necessary
energy for ABC transporters to facilitate drug efflux (33). Chondroitin sulfate disrupts
mitochondrial electron transport and glycolysis to deplete ATP.
This energy depletion impairs P-glycoprotein (P-gp) activity,
thereby decreasing doxorubicin efflux (34).
Numerous glycolysis enzymes are associated with cell
death. For example, HK2 translocates to mitochondria where it
inhibits mitochondrial permeability transition pores to block
cytochrome c/Bax release, enhancing tumor cell survival (35). Mitochondrial PKM2 binds BCL2 to
suppress reactive oxygen species (ROS)-mediated apoptosis (36). Additionally,
fructose-1,6-diphosphate aldolase delays drug-induced apoptosis by
decreasing caspase-3 activity through catalytic product
accumulation (37). Furthermore,
PDH kinase 3 (PDK3) overexpression promotes lycorine hydrochloride
resistance in glioblastoma cells via apoptosis inhibition (38).
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3)
activates cyclin-dependent kinases (CDKs), leading to the
phosphorylation of p27, a potent inhibitor of G1/S transformation
and activator of apoptosis (39).
PFKFB3 also regulates angiogenesis through vascular endothelial
growth factor (VEGF)-mediated endothelial morphogenesis and Notch
signaling suppression (40). The
morphology of tumor blood vessels is irregular. This leads to
localized nutritional and oxygen deficiency, which in turn promote
tumor metastasis (41). Combining
anti-angiogenic therapies that target PFKFB3 reverses the abnormal
changes in tumor blood vessels, improving the delivery of
chemotherapy drugs (41).
The Warburg effect results in the accumulation of
lactic acid, which dilates blood vessels and stimulates
angiogenesis to enhance local energy supply through an increase in
oxygen and nutrients (42).
Excessive lactic acid also decreases the pH around tumor cells,
creating a pH gradient both inside and outside the cells (43). Weakly basic drugs dissociate in
the acidic extracellular environment, hindering their passage
through the cell membrane or sequestering them in acidic lysosomal
vesicles (43,44), rendering them ineffective.
Conversely, weakly acidic drugs enter cells but are often
inactivated before reaching their target due to the alkaline
intracellular conditions (45).
Furthermore, extracellular acidosis promotes drug efflux via P-gp
(46), multidrug
resistance-related protein 1 (MRP1) (47) and intracellular acidic vesicles
(48), further contributing to
chemotherapy resistance. Elevated lactate levels serve as agonists
for the G protein-coupled receptor GPR81, which is expressed on
immune cells. Activation of GPR81 decreases the release of
pro-inflammatory cytokines from T cells and may impair the function
of natural killer cells, partially through recruitment of
monocyte-derived dendritic cells (49). Monocarboxylate
transporter-1-mediated lactate transport is essential for
maintaining intracellular pH in cancer cells, a process that
supports the stem cell properties of pancreatic adenocarcinoma and
glioblastoma stem cells (50,51). Additionally, when several key
enzymes, such as HK2, PFKFB3 and PKM2, are abnormally elevated, the
Wnt/β-catenin, Erk1/2 and YAP/Hippo signaling pathways become
activated (52-54). These pathways are recognized as
key regulators that promote resistance to anti-cancer drugs.
In solid tumors, cancer cells frequently exist in a
hypoxic environment, where they tend to derive energy primarily
through glycolysis. HIF-1 serves as a crucial regulator of cancer
cell responses to hypoxia and facilitates metabolic switching,
while HIF-1α is an important subtype (55). The enhancement of glycolysis by
HIF-1 is associated with its ability to induce the expression of
various glycolytic enzymes at the transcriptional level (56). These enzymes differ from the
subtypes of glycolytic enzymes present in non-malignant cells,
including HK2, PKM and LDHA, playing a key role in the metabolic
shift of cancer cells from oxidative phosphorylation to glycolysis
(57). Besides, HIF-1α also
enhances the expression of Bcl-2/adenovirus E1B 19 kDa interacting
protein 3, which triggers mitochondrial selective autophagy,
thereby decreasing oxidative metabolism in cancer cells (58).
A number of ncRNAs regulate HIF-1α to promote
glycolysis. For example, circHIF1A upregulates HIF1α by
competitively binding miR-361-5p, leading to the overexpression of
enzymes such as glucose transporter 1 (GLUT1) and LDHA. In a
xenograft model, silencing circHIF1A enhances sensitivity to
cetuximab treatment (59).
Similarly, circNRIP1 acts as a miR-138-5p sponge, promoting
HIF-1α-dependent glycolysis and contributing to 5-fluorouracil (FU)
resistance in gastric cancer (GC) (60). Li et al (61) demonstrated that lncRNA
RP11-536K7.3 recruits sex-determining region Y-box2 (SOX2) to
activate Ubiquitin-specific protease 7 (USP7) transcription, which
stabilizes HIF-1α by deubiquitination, thus enhancing glycolysis
and conferring resistance to oxaliplatin in CRC. In BC,
tumor-associated macrophages (TAMs) transfer HIF1-stabilizing
lncRNA HISLA to cancer cells via extracellular vesicles. HISLA
facilitates glycolysis and enhances resistance to
chemotherapy-induced apoptosis by preventing the interaction
between prolyl-4-hydroxylase domain protein 2 and HIF-1.
Additionally, elevated HIF-1 induces lactic acid release from BC
cells, which upregulates HISLA expression in TAMs, creating a
positive feedback loop (62).
Such positive feedback mechanisms of HIF-1α on ncRNAs are common.
For example, lncRNA HIF1α-AS1 activates AKT, promoting the
phosphorylation of Y-box binding protein 1 (YB1), which recruits
phosphorylated-YB1 to HIF-1α mRNA, enhancing its translation. HIF1α
directly binds the HIF1α-AS1 promoter, driving its transcription
(63). HIF1α also regulates the
transcription of circZNF91, notably increasing its presence in
exosomes derived from hypoxic pancreatic cancer (PC) cells.
circZNF91 is subsequently transferred to normoxic PC cells, where
it competitively binds miR-23b-3p, thereby alleviating miR-23b-3p's
inhibition of sirtuin1 (SIRT1). Elevated SIRT1 then promotes
glycolysis and confers resistance to gemcitabine (GEM) in PC cells
(64).
GLUT is a high-affinity membrane protein responsible
for glucose uptake into the cytoplasm. In cancer cells, GLUT1 is
often overexpressed, facilitating enhanced glucose uptake to meet
the energy demands required for aerobic glycolysis (62). A recent study (65) indicated that elevated GLUT1
expression in endometrial cancer (EC) is associated with increased
cell proliferation and invasion and glycolysis. Moreover, GLUT1
overexpression promotes the expression of MMP1, MMP14 and Cyclin D1
in EC cells (66).
Dual-luciferase assays confirmed GLUT1 as a direct target of
miR-140 and miR-143, which suppress EC cell proliferation and
glycolysis by inhibiting GLUT1 expression, thereby enhancing
sensitivity to Paclitaxel (PTX) (66). Additionally, circRNA-0002130,
secreted in serum exosomes of patients with non-small cell lung
cancer (NSCLC), promotes glycolysis and exacerbates resistance to
osimertinib. Mechanistically, circRNA-0002130 serves as a sponge
for miR-498, which targets GLUT1, thereby indirectly upregulating
GLUT1 expression in NSCLC cells (67). Furthermore, miR-1291-5p and
miR-218 serve as direct regulators of GLUT1, counteracting
cisplatin resistance in pancreatic and bladder cancer cells
(68,69).
HKs, located in the cytoplasm, are the primary
rate-limiting enzymes in glycolysis. HKs catalyzes the
phosphorylation of glucose to form glucose-6-phosphate,
representing the initial step in the glycolytic pathway (70). HK2, in particular, is
overexpressed in numerous types of cancer and is associated with
resistance to chemotherapy (34).
A study demonstrated that HK2 can enhance cisplatin-induced
phosphorylation of ERK1/2, thereby promoting cellular autophagy
induced by cisplatin (52).
miRNAs can directly target the 3′ untranslated region (UTR) of HK2
to suppress its expression, including miR-708-5p, miR-202,
miR-125b-5p and miR-216b-5p (71-74). These miRNAs inhibit glycolysis by
downregulating HK2 expression. However, in cancer cells, these
miRNAs may be suppressed by upstream lncRNAs, contributing to drug
resistance (71-74), along with increased glucose uptake
and lactate secretion (74,75). For example, lncRNA-UCA1,
functioning as an oncogene in both solid and hematological tumors,
is associated with decreased drug sensitivity. Overexpression of
UCA1 promotes glycolysis by upregulating HK2, and UCA1 can
indirectly regulate HK2 by sponging miR-125a, thereby promoting
drug resistance (76). Inhibiting
UCA1 enhances the sensitivity of cancer cells to chemotherapy drugs
such as PTX and doxorubicin (76,77).
PFK, located in the cytoplasm, is divided into two
subtypes: PFK1, which catalyzes the conversion of fructose
6-phosphate to fructose-1,6-bisphosphate, marking the second
rate-limiting step in glycolysis, and PFKFB, which converts
fructose-6-phosphate to fructose-2,6-bisphosphate (F2,6P2)
(70). PFKFB3, the most active
isoform of PFKFB, is upregulated in numerous types of cancer in
response to activation of hypoxic signals, RAS signaling, estrogen
receptor and P53 mutations, driving glycolytic metabolism in tumors
(40). PFKFB3 generates F2,6P2,
which activates CDKs. This triggers CDK-mediated phosphorylation of
p27, leading to its ubiquitination and proteasomal degradation via
CDK1, thereby reducing p27 levels (40). PFKFB3 also enhances cancer cell
stemness and promotes resistance to numerous chemotherapeutic
agents by upregulating the YAP/Hippo signaling pathway in small
cell lung carcinoma (53). In
CRC, miR-488 has been shown to target and inhibit PFKFB3 expression
(78). High levels of PFKFB3
promote the proliferation, invasion and migration of CRC cells,
while also contributing to resistance to chemotherapy agents such
as 5-FU and oxaliplatin (78).
Similarly, the circ-SAMD4A is highly expressed in CRC. SAMD4A can
indirectly upregulate PFKFB3 by sponging miR-545-3p, thereby
inhibiting apoptosis and decreasing sensitivity to 5-FU in CRC
cells through the miR-545-3p/PFKFB3 axis (79).
PKM2 catalyzes the final rate-limiting step of
glycolysis in cancer cells, producing pyruvate and ATP (80). The upregulation of PKM2 isoforms
in cancer is associated with drug resistance (81). In the cytoplasm, PKM2 facilitates
the process of glycolysis. Following post-translational
modification, PKM2 is translocated to the nucleus, where it serves
as a transcriptional co-activator for STAT3, β-catenin and NF-κB.
This activates drug resistance-related genes, which enhance cancer
drug resistance (54). TGF-β and
STAT3 synergistically promote the expression of PKM2, which
upregulates PD-L1 expression in cancer cells, further enhancing
cancer immune escape (82).
Recent studies (83-86) have revealed that ncRNAs mediate
PKM2-associated drug resistance through various mechanisms. For
example, miR-122 directly binds the 3′UTR of PKM2, inhibiting its
expression and reversing resistance to oxaliplatin, docetaxel and
doxorubicin by attenuating glycolysis (87-89). The glycolysis-related lncRNA SNHG3
promotes resistance to enzalutamide in prostate cancer via the
miR-139-5p/PKM2 axis (83). In
tumor cells, the ratio of PKM1 to PKM2 serves a critical role in
regulating both glycolysis and drug resistance. PKM1 and PKM2 are
isoforms generated by alternative splicing of PKM pre-mRNA, which
is regulated by the PKM gene. In cisplatin-resistant NSCLC cells,
the highly expressed lncRNA PCIF1 competes with miR-326, which can
directly interact with PKM to downregulate its expression. This
decreases the production of PKM2 by influencing PKM splicing.
Knockdown of lncRNA PCIF1 restores miR-326 expression, leading to
decreased glycolysis and PKM2 levels, which enhances cellular
sensitivity to cisplatin (84).
Additionally, RNA-binding proteins, such as Heterogeneous Nuclear
Ribonucleoprotein A1 (HNRNPA1), Polypyrimidine tract binding
protein 1 (PTBP1) and Sam68, serve critical roles in the specific
splicing of PKM (90). miR-326
also regulates PKM2 production by inhibiting HNRNPA1, HNRNPA2 and
PTBP1 (85). In hepatocellular
carcinoma (HCC), miR-374b directly binds to HNRNPA1, reducing
selective splicing of PKM into PKM2 and increasing cellular
sensitivity to sorafenib (91).
In CRC, the lncRNA LINC01852l interacts with the RNA-binding
protein SR-like splicing factors5 (SRSF5), which promotes PKM
splicing to generate PKM2. Overexpression of LINC01852l leads to
upregulated PKM2 levels and increased glycolysis, thereby
contributing to resistance to 5-FU (86). Furthermore, in
doxorubicin-resistant BC, lncRNA TBX15 is downregulated. TBX15
serves as a sponge for miR-152, which targets Kinesin family member
2C (KIF2C) to inhibit PKM2-mediated glycolysis. KIF2C binds PKM2
and promotes the ubiquitination of PKM2 domain 2, enhancing PKM2
stability. The TBX15/miR-152 axis reverses doxorubicin resistance
in BC by targeting KIF2C and blocking cellular glycolysis and
autophagy (92). In ovarian
cancer (OC), the lncRNA CTSLP8 directly binds to PKM2, forming a
dimer. This PKM2-CTSLP8 dimer transcriptionally regulates c-Myc,
promoting glycolysis and cisplatin resistance in OC cells (93).
LDH, located in the cytoplasm, catalyzes the
conversion of pyruvate to lactic acid, the final product of
glycolysis. LDHA, a prominent isoform of LDH that is upregulated in
cancer, stimulates cellular lactate production, which contributes
to the acidic tumor microenvironment and enhances cancer cell
resistance to chemotherapy drugs (94). In various cancers, miRNAs such as
miR-34a, miR-101-3p and miR-329-3p target and inhibit LDHA,
suppressing lactate production and enhancing cancer cell
sensitivity to cisplatin. miR-34a and miR-101-3p are sponged by
upstream lncRNAs such as SNHG7 and XIST, respectively, leading to
their downregulation. Overexpression of these miRNAs can reverse
glycolytic metabolism and lactate-mediated cisplatin resistance
(95-97). In GC cells, the lncRNA HAGLR
sponges miR-338-3p to promote 5-FU resistance by targeting LDHA
(98). Similarly, in cervical
cancer (CC), knocking down lncRNA-NEAT1 restores miR-34a
expression, reversing LDHA-induced 5-FU resistance (99). Besides these ncRNAs that directly
bind to and inhibit LDHA expression, Shi et al (100) recently discovered that lncRNA
GLTC is overexpressed in thyroid cancer and negatively associated
with clinical prognosis. GLTC enhances LDHA succinylation at the
K155 site by disrupting the interaction between SIRT5 and LDHA.
This modification increases lactate content and the
NAD+/NADH ratio in papillary thyroid carcinoma cells,
promoting resistance to 131I therapy (100). c-Myc, a transcription factor
highly expressed in cancers, is closely linked to the tumor
microenvironment, metabolic reprogramming and the activation of
various oncogenic signaling pathways (101). c-Myc promotes glycolysis in
cancer cells by upregulating key enzymes of glycolytic metabolism.
Several ncRNAs have been shown to promote glycolysis and drug
resistance by stabilizing c-Myc mRNA. For example, circARHGAP12,
through m6A modification, binds c-Myc, promoting doxorubicin
resistance. Inhibition of circARHGAP29 decreases its interaction
with Insulin-like Growth Factor 2 mRNA-Binding Protein 1 (IGF2BP2),
enhancing c-Myc stability and increasing tumor cell sensitivity to
docetaxel. Similarly, miR-3679-5p stabilizes c-Myc by inhibiting
NEDD4-like E3 ubiquitin protein ligase (NEDD4L). Mechanistically,
c-Myc enhances the transcription of LDHA, promoting glycolysis, ATP
production and lactate generation in tumor cells, thereby mediating
cellular drug resistance (24,102,103).
PDH is an enzyme in the pyruvate dehydrogenase
complex (PDC), which catalyzes the irreversible decarboxylation of
pyruvate to acetyl-CoA in mitochondria. However, cancer cells
exhibit a preference for aerobic glycolysis rather than the
mitochondrial oxidation of pyruvate (104). miR-21-5p contributes to
cisplatin resistance in OC cells by targeting PDH E1 subunit α1
(PDHA1) (105). Additionally,
PDK1 regulates the activity of the PDC by inhibiting PDH (104). The upregulation of PDK1 inhibits
the tricarboxylic acid cycle (TCA), leading to enhanced ATP
synthesis and decreased production of ROS under cellular hypoxic
conditions (106). Previous
studies have identified upstream regulators of PDK1, including
miR-148a and miR-4290, which suppress cellular glycolysis and
promote sensitivity to cisplatin and doxorubicin by inhibiting PDK1
expression (107,108). Overexpression of PDK1 can
reverse the effects of these miRNAs, promoting cell proliferation
and drug resistance (107,108). PTBP1 is a nuclear regulator of
alternative splicing. miR-134 and miR-506-3p directly bind to
PTBP1, reducing its expression and inhibiting glucose uptake and
lactate production in cells. The inhibition of miR-134 and
miR-506-3p induces resistance to doxorubicin and 5-FU, respectively
(25,109). PTBP1 can bind to the 3′ UTR of
LDHA, enhancing its stability. In aromatase inhibitor
(AI)-resistant BC cells, the lncRNA DIO3OS interacts with PTBP1 in
the nucleus, promoting BC cell proliferation and glycolysis
(110). Phosphoglycerate mutase
1 (PGAM1) is a key enzyme in glycolysis, catalyzing the conversion
of 2-phosphoglycerate to 3-phosphoglycerate (111). The m6A-modified circQSOX1
indirectly upregulates PGAM1 expression by sponging miR-326 and
miR-330-5p, thereby activating glycolysis in CRC cells. This
decreases the response of CRC to anti-CTLA-4 therapy and promotes
immune evasion in CRC (112).
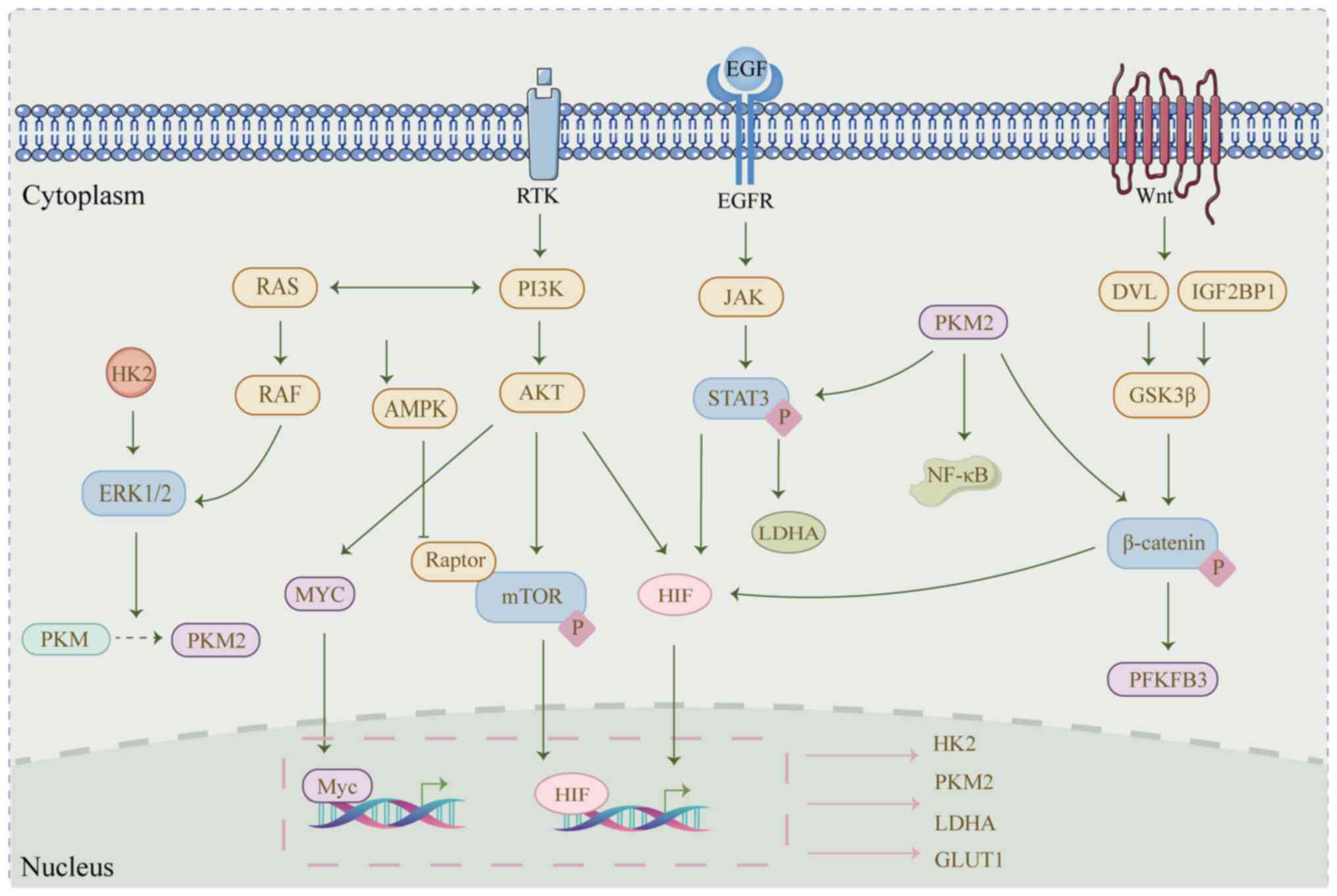
Previous studies have highlighted the activation of
signaling pathways such as PI3K/AKT, MAPK and EGFR as key drivers
of glycolysis in cancer cells through the upregulation of
glycolysis-associated genes (113,114). For example, the PI3K/AKT
signaling pathway can directly promote the phosphorylation of
glycolytic enzymes HK2 and PFKFB. Additionally, PI3K/AKT regulates
the expression of the transcription factor MYC, which further
enhances the expression of HK2, PFK-1, PDK1, PKM2 and LDHA
(115). In FMS-like tyrosine
kinase 3 (FLT3)-resistant acute myeloid leukemia (AML) cells,
elevated expression of miR-155 activates the PI3K/AKT pathway by
directly binding to the 3′UTR of PIK3R1, a PI3K inhibitor. This
process enhances glycolysis, which is a key feature of resistance
to FLT3 tyrosine kinase inhibitors (116). By contrast, the inhibitory
effect of miR-149-3p on AKT1 decreases the expression of HK2, LDHA
and GLUT1 in AML cells, thereby enhancing the sensitivity of AML to
chemotherapy drugs (117).
EGFR stimulation can enhance the activation of HK2
and PKM2, thereby increasing lactate excretion to support the
acidic tumor microenvironment (118). This phenomenon may be associated
with the upregulation of HIF-1α and LDHA expression (119). Targeting EGFR has been shown to
improve glycolysis-mediated chemotherapy (120-122). Gao et al (120) found that EGFR positively
regulates lncRNA FGD5-AS1 expression in CRC. FGD5-AS1 indirectly
upregulates HK2 by sponging miR-330-3p, a negative regulator of
HK2. The EGFR-targeted inhibitor erlotinib suppresses HK2
expression and glycolysis in CRC, enhancing sensitivity to 5-FU
(119). miR-143 can target and
inhibit HK2, restoring sensitivity to cisplatin and 5-FU by
decreasing HK2-dependent glycolysis. Notably, EGFR negatively
regulates miR-143, and its downregulation in drug-resistant cancer
cells results in excess HK2 (121,122). Epiregulin (EREG) is an EGFR
ligand, which upregulates HK2, GLUT3 and PDK1 by activating EGFR
(123). miR-186-3p suppresses
the expression of EREG. However, tamoxifen decreases miR-186-3p
levels in ER-positive BC cells. The miR-186-3p/EREG axis activates
EGFR signaling, promoting glycolysis and mediating tamoxifen
resistance in BC cells (124).
The interaction between ncRNAs and the MAPK signal
transduction pathways plays a key role in tumor cell proliferation,
survival and metabolic reprogramming (125). ERK1/2 serves as the downstream
and final effector of the MAPK pathway (125) and promotes glycolysis by
facilitating the nuclear translocation of PKM2 or upregulating
HIF-1α (126,127). In apatinib-resistant GC cells,
both LINC00665 and ERK2 are upregulated. LINC00665 enhances ERK2
expression by sequestering miR-665, which stimulates the expression
of GLUT1, LDHB and HK2, thereby promoting glycolysis and inducing
apatinib resistance (128).
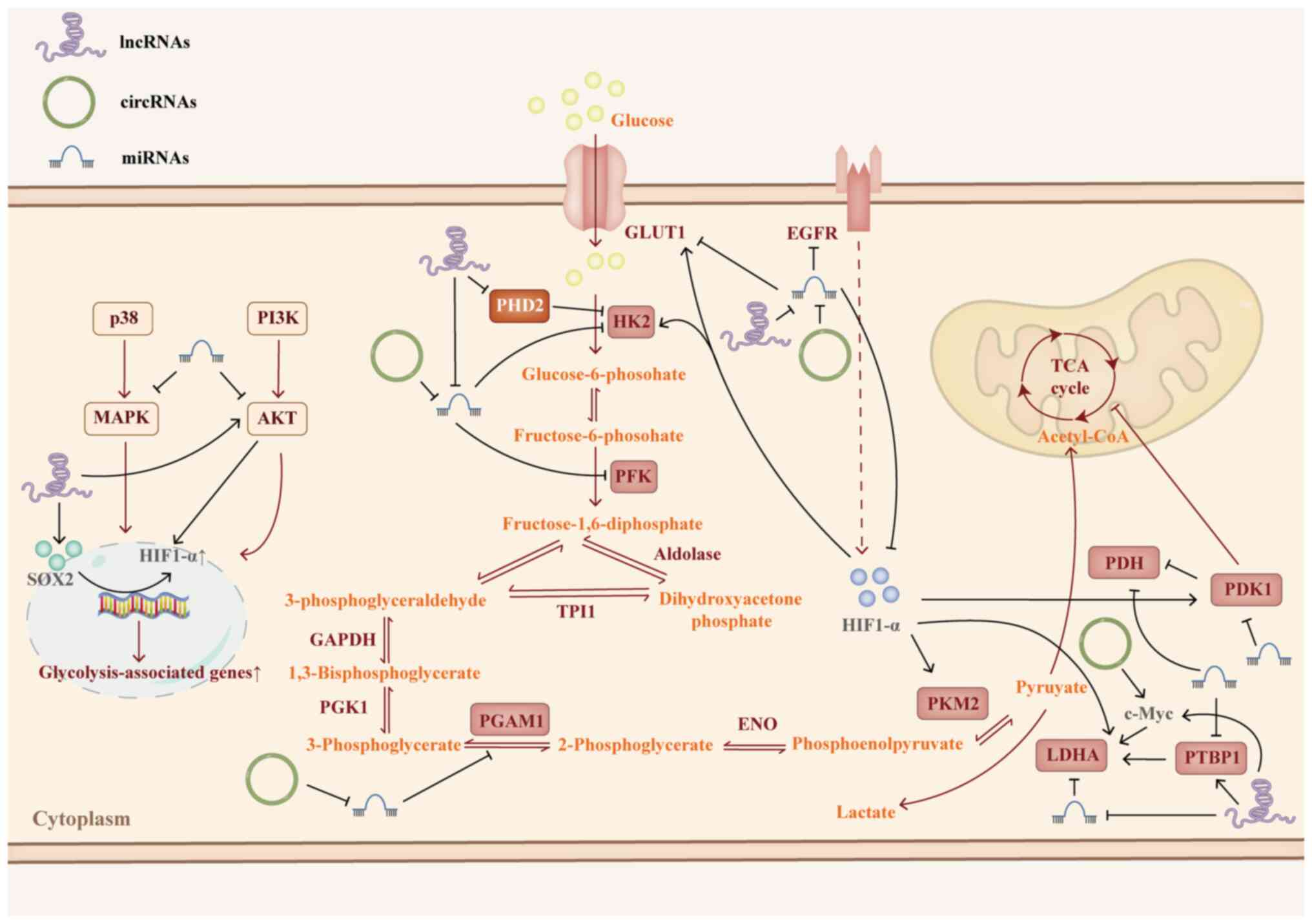
In summary, ncRNAs target key glycolytic enzymes and
associated signaling pathways (Fig.
2; Table II). These crucial
glycolytic enzymes not only facilitate glycolysis in cancer cells
but also activate downstream signaling pathways associated with
drug resistance (Fig. 3). While
some studies suggest ncRNAs contribute to chemotherapy resistance
through glycolysis enhancement (135,136), the precise mechanisms by which
downstream targets promote glycolysis remain unclear, with only
phenotypical changes in glycolysis observed.
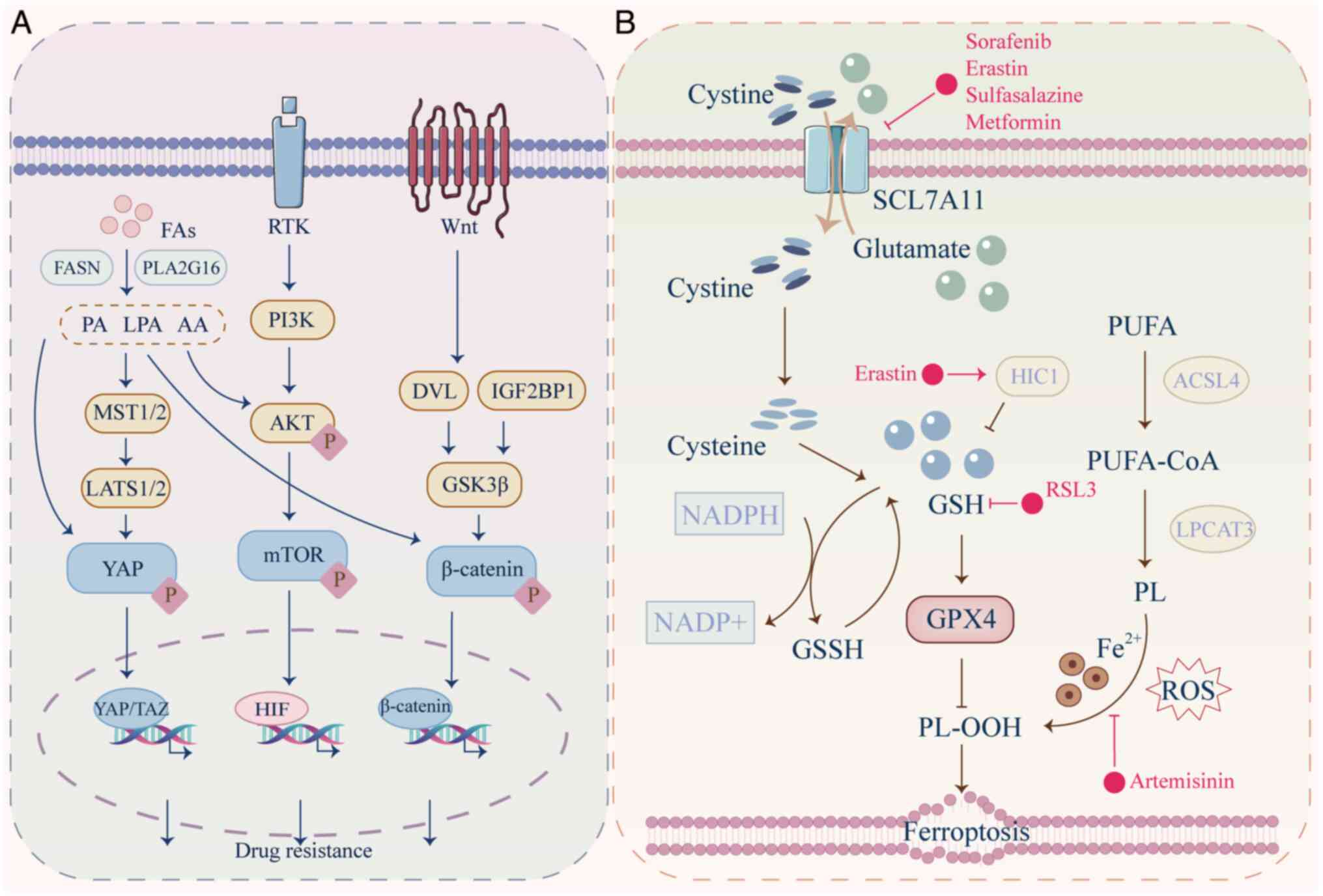
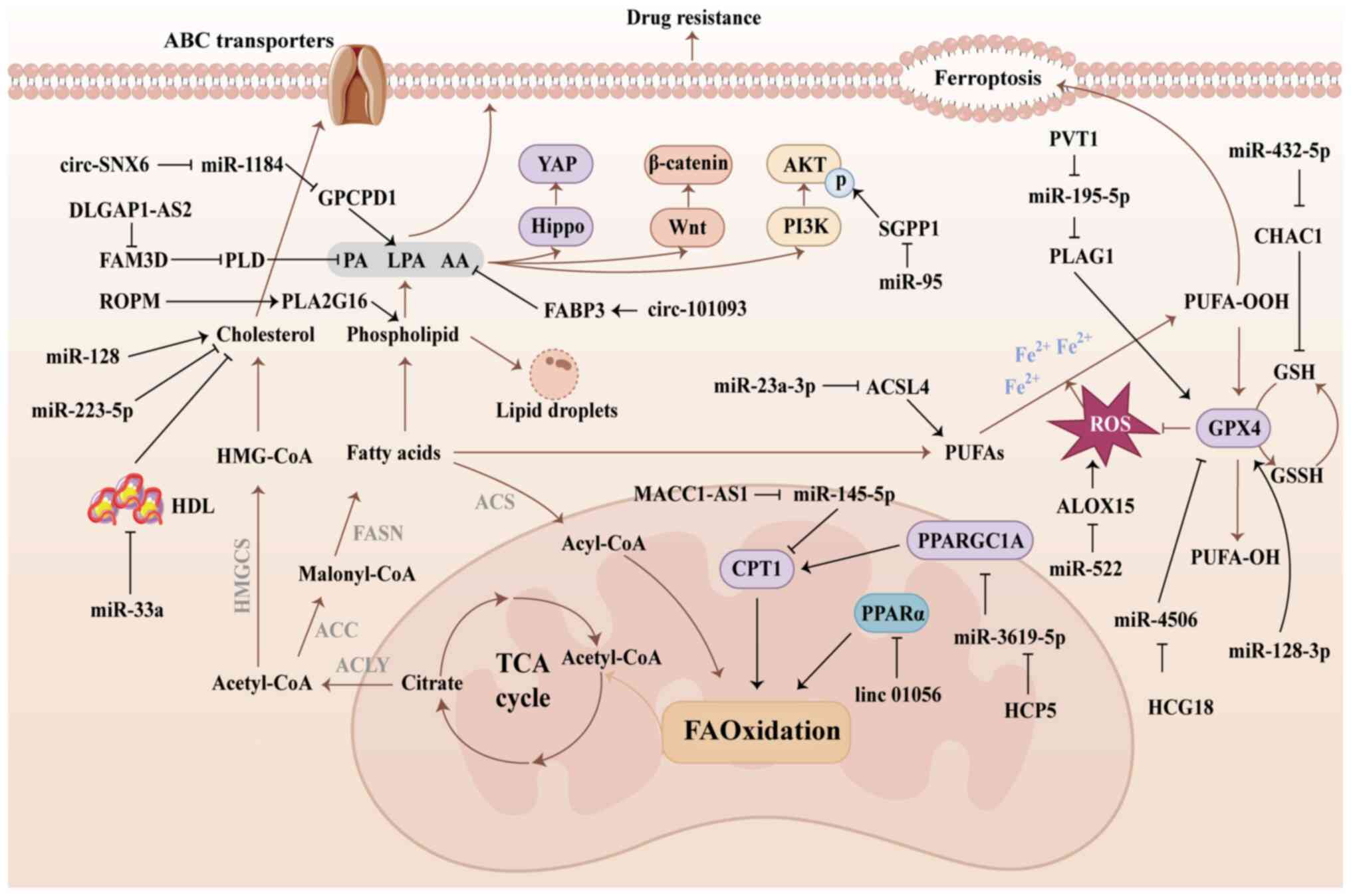
By contrast with normal cells, tumor cells require
dysregulated lipid metabolism to support proliferation, metastasis,
membrane synthesis and signaling (137). Fatty acids (FAs) accumulate in
tumor cells through both direct external uptake and de novo
synthesis (138). These
up-regulated FAs participate in lipid synthesis, leading to the
production of phospholipids, cholesterol and other lipid
metabolites, thereby fueling processes such as FA oxidation (FAO)
(138). This provides essential
ATP, lipid products and signaling molecules for tumor cell
proliferation, metastasis and drug resistance.
In drug-resistant cancer cells, the uptake and
synthesis of FAs are notably enhanced. This leads to the
accumulation of cholesterol and lipid droplets within the cells,
which decreases ROS levels and promote stemness, thereby
contributing to drug resistance (138). Furthermore, FAs and synthesized
lipid products regulate the activation of the PI3K/AKT,
Wnt/β-catenin and Hippo/YAP signaling pathways, thereby promoting
survival and drug resistance in cancer cells (Fig. 4A) (139-141).
As numerous chemotherapeutic agents require cellular
entry to target DNA and induce cell death, lipid metabolism also
mitigate toxic effects by altering drug uptake in cancer cells
(142). Drugs typically enter
cells through non-specific lipophilic interactions with the cell
membrane. Abnormal lipid metabolism alters the membrane
composition, decreasing its permeability to anti-cancer drugs
(143). Although the exact
changes in membrane composition are not fully understood, the low
permeability of certain drugs is associated with poor membrane
fluidity (144). Additionally,
lipid metabolism affects the expression of transporters on the cell
membrane. Free FAs activate the G protein-coupled receptor GPR120,
which in turn upregulates ABC transporter expression via the
AKT/NF-κB signaling pathway, leading to decreased epirubicin
accumulation in BC cells (145).
By contrast, long-chain PUFAs decrease multidrug resistance 1 gene
(MDR1) expression on the cell membrane, thereby decreasing PTX
efflux and enhancing the chemotherapy sensitivity of tumor cells
(146). Furthermore, lipid
metabolism promotes adipocyte-like transformation in cancer cells,
leading to the production of lipid droplets, which act as storage
sites for fat-soluble drugs. This results in diminished
concentrations of chemotherapeutic agents reaching intracellular
targets (147).
Cholesterol serves a pivotal role in cell membrane
protein function, receptor transport, proliferation, membrane
biogenesis and signal transduction. Increased cholesterol synthesis
is associated with poorer survival rates and cancer progression
(152). High cholesterol levels
are observed in BC cells, where miR-128 and miR-223 regulate
cholesterol metabolism by targeting genes such as
3-Hydroxy-3-methylglutaryl-CoA Synthase 1 (HMGCS1), Low-density
lipoprotein receptor (LDLR) and ATP-Binding Cassette Transporter A1
(ABCA1), modulating cholesterol biosynthesis, uptake and efflux.
Cholesterol accumulation enhances lipid raft formation and membrane
rigidity, decreasing membrane permeability and contributing to drug
resistance. These miRNAs indirectly influence cell sensitivity to
tamoxifen via the regulation of cholesterol (153). Additionally, miR-33a mitigates
drug resistance in BC by inhibiting high-density lipoprotein (HDL)
expression and preventing HDL-mediated cholesterol extraction
(154).
FAs that accumulate in cancer cells are converted
into fatty acyl-CoA through the action of acyl-CoA synthetase
(ACS). Acyl-CoA is transported to the mitochondrial matrix, where
it undergoes β-oxidation to generate acetyl-CoA and ATP (43). Long-term exposure of cancer cells
to elevated ROS levels induced by chemotherapy may enhance
resistance to further treatment. This phenomenon is due to the
activation of several factors, including Nrf2, c-Jun and HIF-1α,
which enhance the antioxidant capacity of cancer cells (155). To neutralize ROS, tumor cells
produce large amounts of antioxidants such as GSH, which is reduced
from its oxidized form via NADPH (156). Elevated ROS levels can promote
FAO, enabling cancer cells to generate sufficient NADPH and reduced
GSH (157). Increased FAO has
been observed in drug-resistant GC and AML (158,159). This increase not only meets the
energy demands of tumor cells but also supports their survival in
oxidative stress environments (160). Inhibition of FAO reduces NADPH
and GSH production in tumor cells while simultaneously increasing
ROS production (161). In
addition, cancer cells counteract ferroptosis by enhancing FAO and
phospholipid synthesis, which elevates the levels of saturated FAs
and restores GSH levels, thereby improving resistance to ROS
(162).
In sorafenib-resistant HCC cells, the lncRNA
LINC01056 is notably downregulated and inversely associated with
sorafenib resistance. Mechanistically, LINC01056 binds specifically
to peroxisome proliferator-activated receptor α (PPARα), inhibiting
its nuclear translocation and transcriptional activity, thus
decreasing the expression of FAO-related genes. Overexpression of
LINC01056 suppresses PPARα, attenuating FAO in HCC cells and
restoring their sensitivity to sorafenib in vitro (163). Previous studies (26,164) have highlighted the key role of
mesenchymal stem cells (MSCs) in mediating drug resistance in GC
cells. MSCs stimulate the expression of lncRNA MACC1-AS1 in GC
through TGF-β secretion. As a sponge for miR-145-5p, MACC1-AS1
enhances the expression of FAO-associated metabolic enzymes such as
octamer-binding transcription factor 4 and carnitine
palmitoyltransferase 1 (CPT1), thereby promoting FAO in GC cells
and contributing to resistance to the 5-FU+leucovorin + oxaliplatin
(FOLFOX) regimen (26).
Furthermore, exosomes derived from MSCs elevate the expression of
lncRNA HCP5 in GC cells, which specifically inhibits miR-3619-5p.
By preventing miR-3619-5p from binding PPARG coactivator 1α, HCP5
enhances the transcriptional complex of PPARγ coactivator
1-α/CEBPB, leading to the induction of CPT1 transcription. This
process promotes stemness and chemoresistance in GC cancer cells by
driving FAO (164).
Ferroptosis is a form of programmed cell death
characterized by the accumulation of lipid peroxidation products
that serves a key role in cancer occurrence, treatment resistance
and tumor suppression (165).
Polyunsaturated FAs (PUFAs) are catalyzed by acyl-CoA synthetase
long-chain family member 4 (ACSL4) and lysophosphatidylcholine
acyltransferases to produce phospholipids (PLs). Catalyzed by
lipoxygenase and P450 oxidoreductase, PL interacts with ROS and
iron ions to form PL hydroperoxides (PLOOH). The significant
production of PLOOH results in the instability and increased
permeability of cell membranes, leading to cell death (166). The occurrence of ferroptosis is
influenced by two pathways. The crucial pathway is the inactivation
of GSH peroxidase 4 (GPX4). GPX4 serves a pivotal role in the
reduction of PLOOH through the utilization of GSH. The other
pathway involves inhibition of the cystine/glutamate antiporter
system (xCT), mediated by solute carrier family 7A member 11
(SLC7A11). SLC7A11 facilitates the uptake of cysteine, which is key
for the synthesis of GSH, a prerequisite for the functional
activity of GPX4 (167).
Ferroptosis is induced by anti-tumor drugs that
suppress GPX4 or promote lipid peroxidation, as summarized by Li
et al (168). For
example, the combined treatment of albumin-bound paclitaxel and TMZ
enhances ferroptosis in glioblastoma by inhibiting GPX4 expression
(169). Sorafenib induces lipid
peroxidation in HCC by inhibiting solute carrier family 3A member 2
(SLC3A2) and SLC7A11, thereby suppressing GSH production and GPX4
expression, which upregulates ferroptosis in HCC cells (170). However, in drug-resistant tumor
cells, numerous types of lipid metabolic enzyme and endogenous
antioxidant mitigate the occurrence of ferroptosis by decreasing
lipid peroxidation (171). The
SLC7A11-GPX4 system is dysregulated in numerous types of
drug-resistant cancer, contributing to resistance against
oxaliplatin (172).
HER2-positive BC cells activate the β-catenin signaling pathway via
fibroblast growth factor receptor 4, leading to an increased
synthesis of GSH via the transcription factor 4/SLC7A11 axis
(173). Nrf2 is also implicated
in cancer drug resistance and ferroptosis. Excessive ROS inactivate
Keap1 via oxidation of its cysteine residues, releasing the
transcription factor Nrf2 into the nucleus, where it activates the
transcription of antioxidant genes, such as GPX4 (174). Furthermore, the activation of
Nrf2 induces Metallothionein-1G, which reduces GSH depletion and
lipid peroxidation, thereby inhibiting sorafenib-induced
ferroptosis (175).
The lncRNA PVT1 enhances pleiomorphic adenoma gene
1 (PLAG1) expression at the transcriptional level by targeting
miR-195-5p. PLAG1 interacts with GPX4, facilitating its role in
stabilizing lipid peroxidation and protecting HCC cells from
sorafenib-induced ferroptosis (187). Both miR-128-3p and miR-450b-5p
promote ferroptosis and lipid peroxidation by inhibiting the
enzymatic activity of GSH and GPX4 (188,189). Additionally, sorafenib
upregulates miR-23a-3p expression in HCC via ETS proto-oncogene 1
induction, which targets ACSL4. Sorafenib-mediated overexpression
of miR-23a-3p decreases lipid peroxidation and ferroptosis in HCC
cells by inhibiting ACSL4, thereby contributing to HCC cell
resistance to sorafenib (170).
Cancer-associated fibroblasts secrete miR-522 and miR-432-5p to
inhibit lipoxygenase15 (ALOX15) and cyclotransferase1 (CHAC1).
ALOX15 degrades ROS generated by lipid peroxidation, whereas CHAC1
enhances the production of GSH, thus inducing drug resistance
(190,191).
In summary, ncRNAs modulate the sensitivity of
tumor cells to drugs by influencing lipid metabolism (Fig. 5; Table III). These mechanisms involve
lipid synthesis, FAO and the regulation of ferroptosis. While most
of the aforementioned studies have focused on how ncRNAs affect
lipid oxidation balance within cancer cells, future investigations
should explore how ncRNA-mediated lipid metabolism alters cell
membrane components and intracellular lipid droplets to promote
drug resistance.
Mitochondria are key in metabolic reprogramming,
supporting the catabolism of amino acids, nucleotides and lipids,
as well as the production of NADH and NADPH (192). In tumor cells, mitochondrial DNA
(mtDNA) mutations or alterations in content lead to mitochondrial
dysfunction. For example, the deletion of mtDNA in PC cells results
in mitochondrial dysfunction, driving a shift to the Warburg effect
and promoting stem cell-like characteristics. This reprogramming
helps the cells resist docetaxel treatment (193). In HCC, mtDNA loss contributes to
chemotherapy resistance by activating the NRF-2 signaling pathway
and upregulating multidrug resistance genes such as MDR1 and MRP1/2
(194). Further evidence from
colon cancer cells with mtDNA deletions supports the involvement of
MDR1 in resistance mechanisms (195). On the contrary,
chemotherapy-induced mitochondrial dysfunction in cancer cells is
often accompanied by mutations in mtDNA, which increase ROS
production (196).
Resistance to apoptosis is a key mechanism
underlying drug resistance in cancer. Under physiological and
pathological conditions, cells maintain a balance in redox
homeostasis. However, certain chemotherapeutic agents, such as
5-FU, epirubicin and gemcitabine, induce oxidative stress by
inhibiting the antioxidant system or mitochondrial function,
leading to excessive production of ROS (197). Elevated ROS levels disrupt
mitochondrial membrane potential, resulting in the release of
cytochrome c and activation of caspases-3, -6 and -7, thereby
triggering apoptosis in cancer cells (198). Additionally, tumors often
release excess ROS into the microenvironment, which can induce
macrophages to adopt a pro-cancer phenotype (199). This phenotype reduces T cell
infiltration into tumors and decreases the expression of programmed
death-ligand 1 (PD-L1), thereby hindering the effectiveness of
anti-PD-L1 immunotherapy (200).
The dysregulation of apoptosis is a key factor in cancer resistance
to chemotherapy.
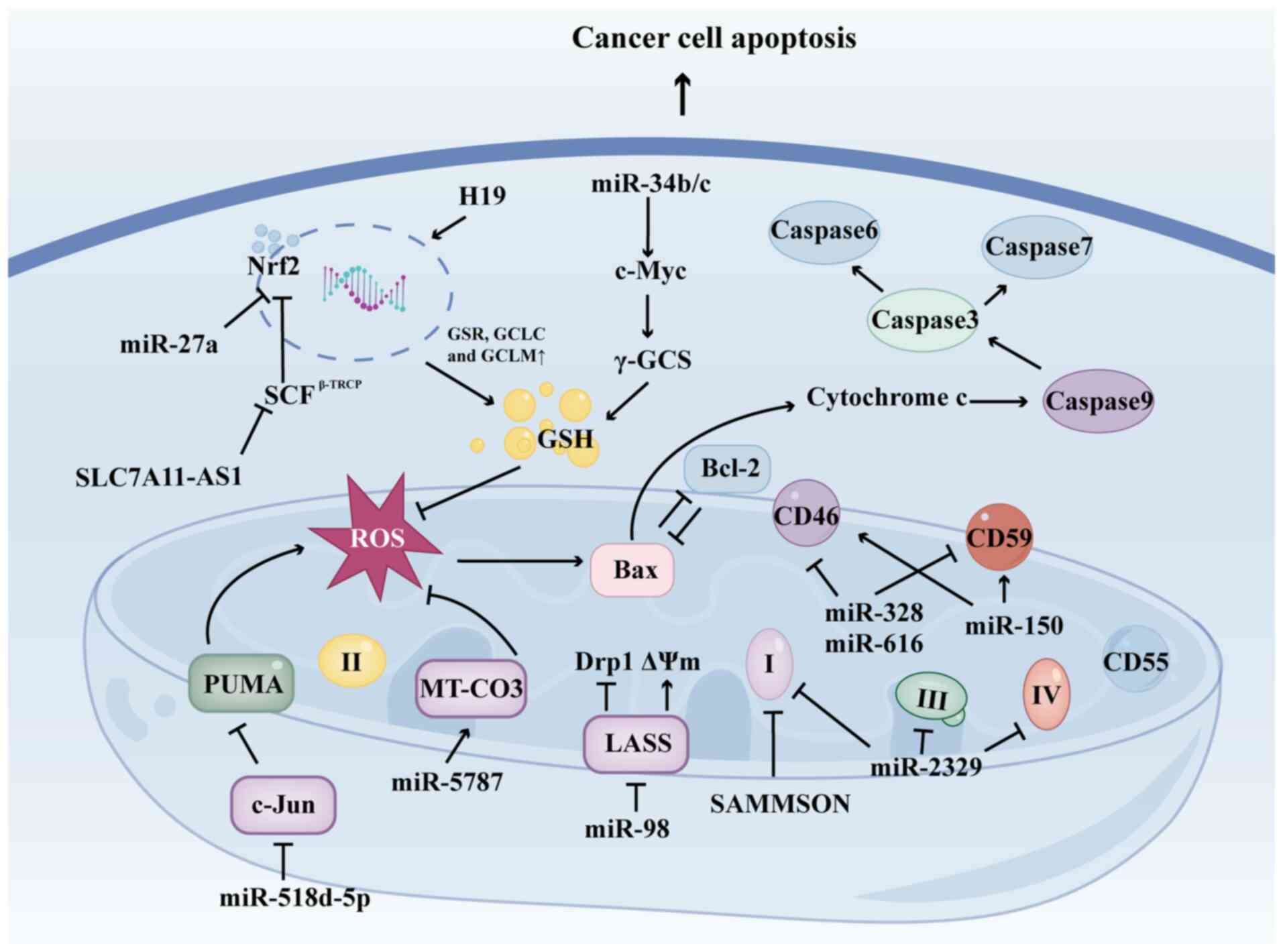
Dysregulated ncRNAs within mitochondria modulate
the activation of mitochondrial-mediated signaling pathways,
promoting tumor growth and drug resistance. miR-125b is associated
with increased release of mitochondrial cytochrome c, the induction
of apoptotic protease activating factor 1 and the activation of
caspase-3 and poly (ADP-ribose) polymerase (201). Furthermore, the overexpression
of miR-125b can inhibit the expression of BCL-2 while
simultaneously increasing the expression of Bax (201).
Chemotherapy agents, including cisplatin,
doxorubicin and PTX, are known pro-oxidants that induce cell death
through mitochondrial dysfunction and increased ROS production
(202). This has intensified
interest in the role of ncRNAs in modulating ROS generation within
mitochondria (203,204). Sorafenib, for example, induces
mitochondrial dysfunction and ROS production via the
C-Jun/p53-upregulated modulator of apoptosis signaling pathway,
while miR-518d-5p impedes this process by targeting and inhibiting
C-Jun, resulting in sorafenib resistance (205). Mitochondrial miRNA
(mitomiRNA)-2329 is significantly upregulated in
cisplatin-resistant cells. It inhibits the activity of
mitochondrial complexes I, III and IV, leading to elevated
mitochondrial ROS and decreased ATP production. Additionally,
mitomiR-2329 partially regulates mtDNA transcription in an
Argonaute2 (AGO2)-dependent manner, promoting glycolysis and
lactate production (206).
Similarly, the lncRNA SAMMSON increases ROS production in BC cells
by inhibiting mitochondrial complex I (207). These findings underscore the key
role of ncRNAs targeting mitochondrial complexes in regulating
mitochondrial function. Furthermore, Hillman et al (208) demonstrated that miR-150, miR-328
and miR-616 target membrane complement regulatory factors CD46,
CD55 and CD59, thereby rendering mitochondria resistant to
complement lysis and enhancing cellular tolerance to
complement-dependent cytotoxicity. miR-98 regulates mitochondrial
fusion and fission by directly targeting Longevity Assurance
Homologue 2, promoting mitochondrial fission and increasing
mitochondrial membrane potential, which contributes to resistance
to cisplatin and doxorubicin (209). Notably, mitomiR-5787 inhibits
oxidative stress and glycolysis in mitochondria by binding
mitochondrial cytochrome c oxidase subunit 3 (MT-CO3), restoring
cisplatin sensitivity (205).
Conversely, the binding of mitomiR-5787 to MT-CO3 does not suppress
MT-CO3 expression but enhances its translation. This may be due to
the absence of the RNA-induced silencing complex (RISC)-associated
factor GW182, which is responsible for mRNA cleavage, within
mitochondria (210).
ncRNAs serve a key role in mitigating intracellular
ROS and oxidative stress by promoting the production of GSH. For
example, in prostate cancer, miR-34b/c facilitates the generation
of GSH and Bcl-2 via the c-Myc/γ-glutamyl-cysteine synthetase axis,
which decreases the cytotoxic effects of oxidative stress and
enhances cellular tolerance to ROS (211). lncRNA H19 is overexpressed in
cisplatin-resistant high-grade serous ovarian cancer (HGSC), and
its overexpression enhances cisplatin resistance. H19 upregulates
Nrf2 expression, promoting the transcription of GSH-associated
proteins such as Glutathione reductase (GSR), Glutamate-cysteine
ligase catalytic subunit (GCLC) and Glutamate-cysteine ligase
modifier subunit (GCLM), which increases intracellular GSH
accumulation (212). Conversely,
miR-27a targets GSH-associated genes such as cystathionine
gamma-lyase, xCT and Nrf2, reducing cellular resistance to ROS and
increasing sensitivity to chemotherapy (213). Moreover, Nrf2 is regulated by
its upstream regulatory factor SKP1-Cul1-Rbx1
(SCFβ−TRCP). SLC7A11-AS1 blocks
SCFβ−TRCP-mediated Nrf2 ubiquitination, resulting in low
intracellular ROS levels (214).
In conclusion, ncRNAs regulate mitochondrial
complexes, apoptosis-related proteins and other factors, playing a
key role in mediating mitochondrial dysfunction in cancer cells.
Targeting ncRNAs can modify the ROS/GSH balance within
mitochondria, diminishing the tolerance of cancer cells to
drug-induced ROS (Fig. 6,
Table IV).
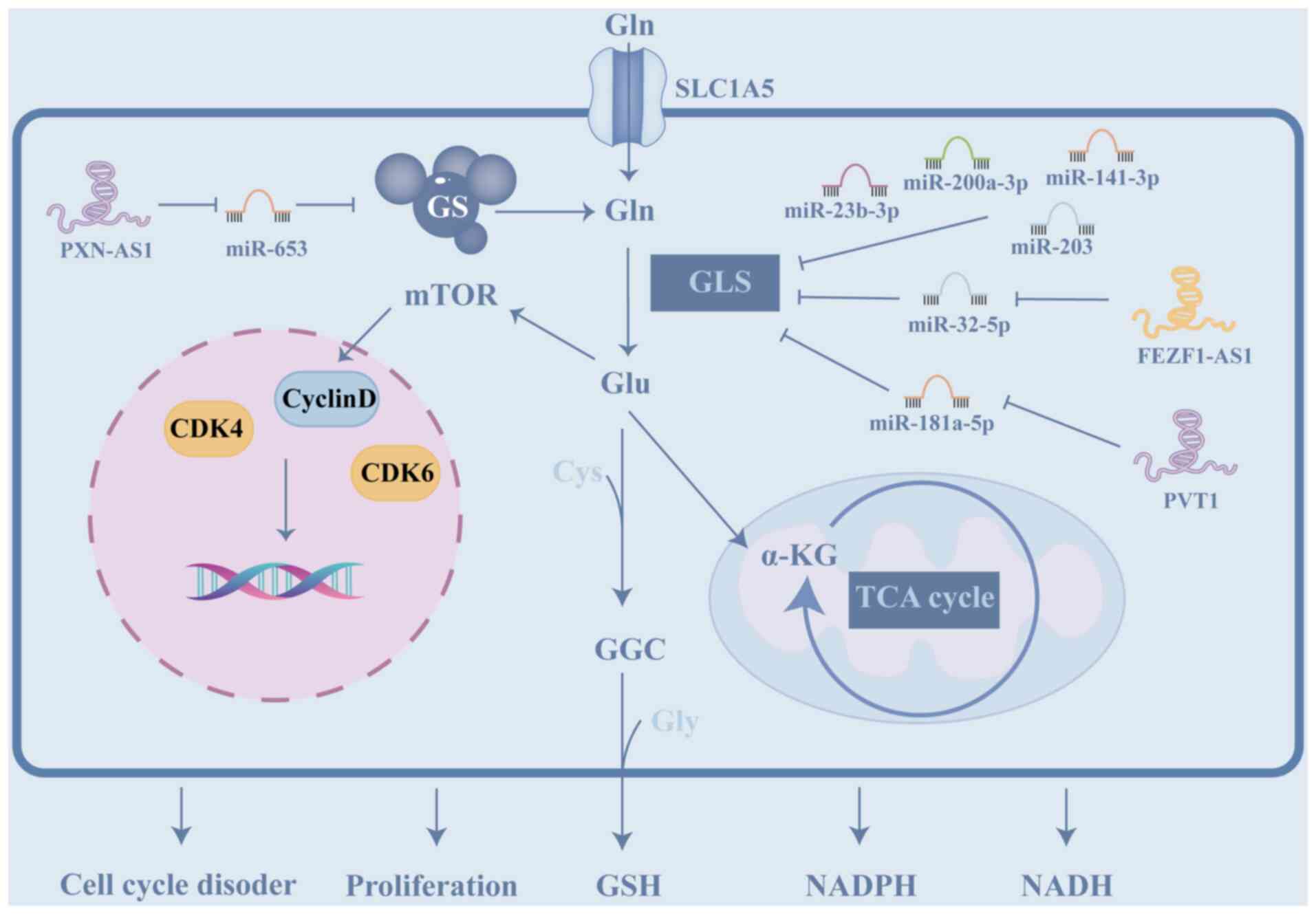
Glutamine (Gln), a non-essential amino acid, serves
a key role in cancer initiation and progression. Cancer cells
depend on glutamine for its involvement in the TCA, as well as for
the biosynthesis of nucleotides, GSH and other non-essential amino
acids, thus providing essential nutrients for cancer drug
resistance (10). Numerous
studies (215-220) have demonstrated that ncRNAs
regulate GSH metabolism in cancer cells primarily by targeting
glutaminase (GLS), a mitochondrial enzyme responsible for
catalyzing the deamidation of glutamine (Fig. 7; Table V). GLS is a key player in cancer
drug resistance, tumor growth and metastasis (221). In cancers such as HCC and NSCLC,
numerous miRNAs have been identified as tumor suppressors that
inhibit glutamine metabolism by targeting the 3′UTR of GLS
(215-218). Overexpression of these miRNAs,
which suppress GLS, enhances cancer cell sensitivity to drugs and
promotes apoptosis (214).
lncRNAs, such as PVT1 and FEZF1-AS1, restore GLS expression by
serving as sponges for miR-181a-5p and miR-32-5p, respectively,
which target GLS, thereby promoting cell resistance to cisplatin
(219,220). These findings highlight the key
role of the ncRNA network in regulating GLS expression and
contributing to cellular drug resistance. Additionally, in
imatinib-resistant chronic myelogenous leukemia cells, lncRNA
PXN-AS1 indirectly upregulates glutamine synthetase by modulating
miR-653, promoting glutamine synthesis. Increased glutamine
activates the mTOR signaling pathway, leading to elevated
expression of CDK4, Cyclin D and CDK6 in the nucleus, thus
promoting cell proliferation and cell cycle dysregulation (222).
NSCLC is characterized by a strong dependence on
aerobic glycolysis, with elevated expression of glycolytic enzymes
such as GLUT1 and PKM2, which promote rapid proliferation and
confer resistance to targeted therapy, such as Osimertinib
(64,84). In NSCLC, circRNA-0002130 has been
shown to promote glycolysis and osimertinib resistance by serving
as a sponge for miR-498, leading to the upregulation of GLUT1 and
enhanced glucose uptake (64).
Similarly, miR-326 directly targets HNRNPA1, a key regulator of PKM
splicing, to suppress PKM2 expression and reverse cisplatin
resistance (84). Additionally,
lncRNA PCIF1 competitively binds miR-326, promoting PKM2 expression
and glycolysis, thereby enhancing cisplatin resistance (84). Other ncRNAs, such as miR-200a-3p,
target GLS to suppress glutamine metabolism. Concurrently
inhibiting miR-200a-3p may represent an effective strategy to
prevent the compensatory increase of glutamine in cancer cells
(214). Therapeutic strategies
targeting these axes, such as silencing circRNA-0002130 or
restoring miR-326, may sensitize NSCLC cells to chemotherapy.
CRC cells also exhibit enhanced glycolysis, which
supports stemness and drug resistance (78). In CRC, circ-SAMD4A drives
glycolysis and 5-FU resistance by sponging miR-545-3p, leading to
the upregulation of PFKFB3 and enhanced glycolytic flux (78). miR-488 also directly targets
PFKFB3 (77). Furthermore, lncRNA
LINC01852 promotes PKM2 splicing via SRSF5, enhancing glycolysis
and 5-FU resistance (86).
miR-143 inhibits HK2, reversing 5-FU resistance by suppressing
glycolysis (122). Inhibiting
glycolysis-related ncRNAs, such as circ-SAMD4A or miR-143, may
disrupt energy supply and stemness, thereby overcoming
chemoresistance.
HCC cells exploit abnormal lipid metabolism and
resistance to ferroptosis, a key mechanism of sorafenib-induced
cell death (170,187). In HCC, miR-23a-3p is upregulated
by sorafenib and suppresses ACSL4, a key enzyme in lipid
peroxidation, thereby inhibiting ferroptosis (170). lncRNA PVT1 promotes PLAG1
expression by sponging miR-195-5p, enhancing GPX4 activity
(187), while miR-128-3p
directly inhibits GPX4, promoting ferroptosis and overcoming
sorafenib resistance (188).
Additionally, LINC01056 inhibits PPARα, suppressing FAO and
restoring sorafenib sensitivity (163). Other ncRNAs, such as miR-374b,
target HNRNPA1 to reduce PKM2 expression and sensitize HCC cells to
sorafenib (91). Restoring lipid
peroxidation, for example by inhibiting miR-23a-3p or PVT1, may
enhance sorafenib efficacy in patients with HCC.
OC cells exhibit enhanced glycolysis and
antioxidant capacity, which contribute to cisplatin resistance
(93,210). In OC, lncRNA CTSLP8 binds to
PKM2, forming a dimer that transcriptionally activates c-Myc,
driving glycolysis and cisplatin resistance (93). Another key player, lncRNA H19,
upregulates Nrf2, enhancing GSH synthesis and oxidative stress
resistance (210). Additionally,
miR-21-5p targets PDHA1, suppressing mitochondrial metabolism and
promoting cisplatin resistance (105). Targeting CTSLP8 or H19 may
disrupt glycolysis and redox balance, sensitizing OC cells to
platinum-based therapy.
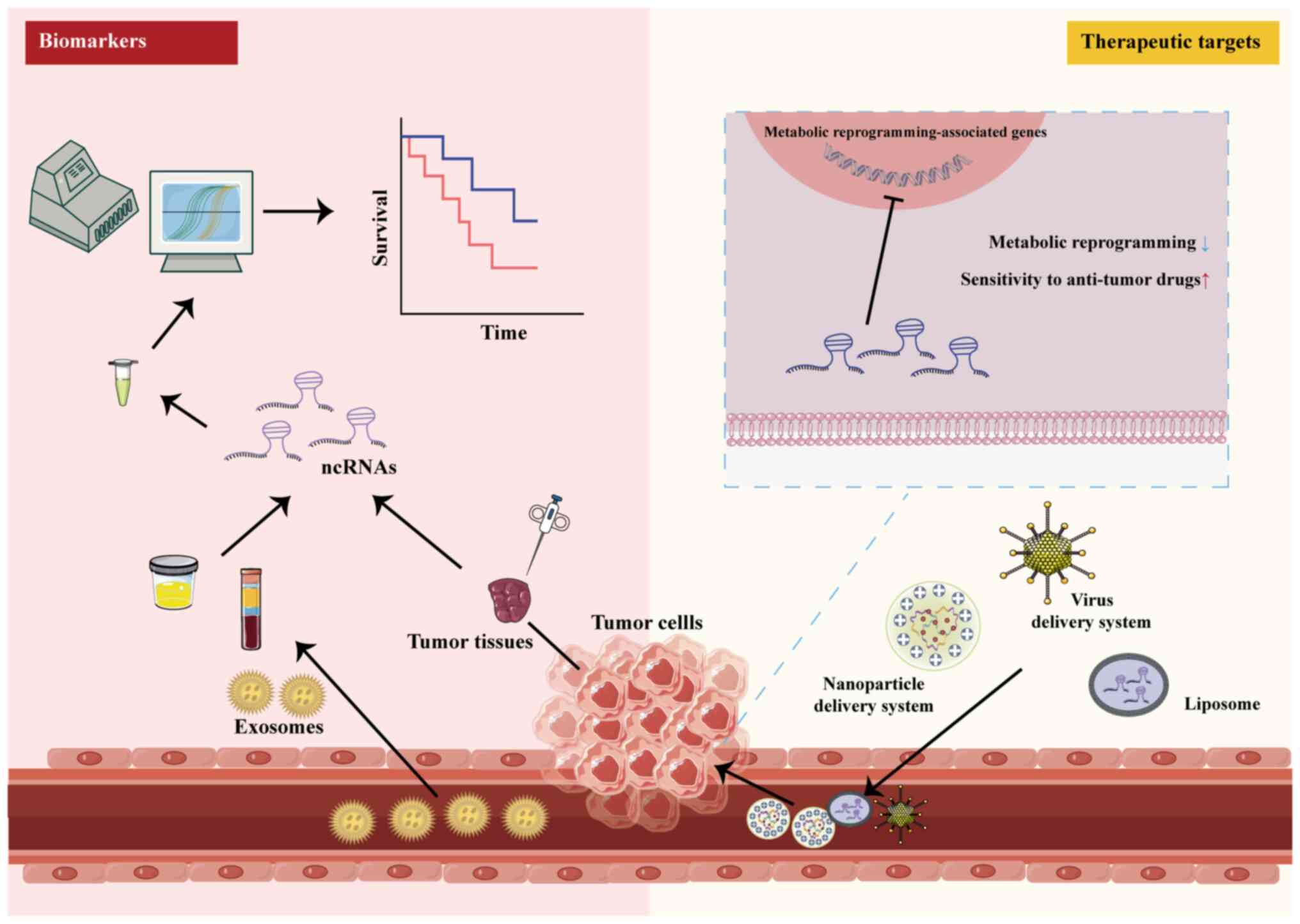
Early detection, diagnosis and treatment are
critical for improving cancer prognosis. However, many patients
with cancer experience disease progression due to drug resistance,
which delays treatment opportunities. ncRNAs, particularly
circulating miRNAs, are found in bodily fluids such as blood, urine
and saliva and exhibit high stability when stored under appropriate
conditions (223). These ncRNAs
are specifically expressed in cancer cells and enter bodily fluids
after binding proteins or being encapsulated by exosomes, making
them potential biomarkers for cancer (Fig. 8) (224). Numerous studies have
demonstrated that ncRNAs can predict patient responses to treatment
(67,93,225). For example, the upregulation of
circ-0002130 in serum exosomes from patients with
osimertinib-resistant NSCLC can predict sensitivity to osimertinib
(67). In OC, patients with high
CTSLP8 expression have notably reduced overall survival (93). Liu et al (225) used changes in the levels of
eight lncRNAs to predict the response of patients with HGSC to
platinum-based chemotherapy.
Additionally, ncRNAs reflect the association
between drug resistance and metabolic changes in cancer cells
(226). AIs, the first-line
endocrine therapy for postmenopausal patients with ER+
BC, have resistance closely linked to glycolysis (225). High baseline expression of
miR-155 is associated with increased glycolysis and poor response
to AI treatment (227). Changes
in the expression of lncRNAs associated with cholesterol and
sphingolipids predict the prognosis of HCC and pancreatic cancer
and assess their sensitivity to immunotherapy (228,229). A study used 11 lncRNAs
associated with FA metabolism to construct a risk prediction model
for skin melanoma, aimed at predicting melanoma sensitivity to
immunotherapy (230). Ongoing
research efforts are being made to establish ncRNAs as biomarkers
of tumor drug resistance (231-233). For example, a clinical trial
involving 144 patients with BC found that lncRNA MEG3 is associated
with the response and toxicity of PTX and cisplatin chemotherapy
(231).
The regulation of metabolism in combination with
anti-tumor therapy is a promising strategy to overcome tumor drug
resistance (234). Numerous
studies have demonstrated that blocking key metabolic pathways in
cancer cells contributes to the eradication of recurrent or
drug-resistant cancer cells (234,235). However, non-cancerous and immune
cells exhibit metabolic fragility; targeting the intrinsic
metabolism of cancer cells can also affect these essential cells,
leading to severe adverse reactions (12). The advantage of targeting ncRNAs
to regulate metabolism is the specific abnormal expression of these
ncRNAs in cancer cells. This specificity allows more targeted
ncRNA-based therapy. miRNAs promoting glycolysis are notably
elevated in drug-resistant cancer cells. When anti-miR drugs are
delivered to these cells, specific miRNAs are notably inhibited
(236). Although these miRNAs
are inhibited in normal cells as well, the impact on normal cells,
where these miRNAs are expressed at low levels, is smaller compared
with that on cancer cells. Furthermore, certain ncRNAs bind to
multiple sites simultaneously. For example, miR-498 binds GLUT1,
HK2 and LDHA to inhibit cellular glycolysis (64). In mitochondria, miR-634 binds to
multiple sites, including optic atrophy 1, lysosomal-associated
membrane 2 gene and Nrf2, thereby regulating mitochondrial
homeostasis, autophagy and the production of apoptosis-related
proteins (237).
The inherent instability of ncRNAs in the body
makes them highly susceptible to degradation by RNases, which
presents a notable challenge for therapeutic application. To
overcome this, the development of effective delivery systems that
ensure targeting specificity, decrease immunogenicity and maintain
the stability of ncRNAs is crucial (242). Various approaches have been
explored to address these challenges. Lin et al (243) developed a pH- and GSH-sensitive
nanocarrier for the co-delivery of docetaxel and the miR-34
activator rubbone (RUB). This carrier demonstrates good stability
in vitro, targets cancer cells efficiently and rapidly
releases docetaxel and RUB into the cytoplasm, enhancing the
sensitivity of tumor cells to docetaxel (243). Another study (244) designed GSH-sensitive
nanoparticles. These nanoparticles lead to micellar degradation and
facilitating drug release by reducing GSH to sulfhydryl groups.
This strategy is used for the targeted delivery of GEM and miR-519c
to pancreatic cancer tissue with elevated GSH levels, improving GEM
resistance. Additionally, miR-519c decreases glycolysis and
angiogenesis by inhibiting HIF-1α (244). In melanoma, Guo et al
(245) employed liposomes
containing miR-21-3p to regulate lipid metabolism. The
nanoparticles deliver miR-21-3p into melanoma cells, promoting
lipid peroxidation and lipid ROS production by inhibiting TXNRD1,
which enhances the response of melanoma cells to immunotherapy
(245). Similarly, lipid-coated
calcium carbonate nanoparticles are used to deliver 5-FU and
miR-375-3p to tumor cells in a weakly acidic environment (246). miR-375-3p interferes with
thymidylate metabolism by targeting thymidylate synthase, enhancing
the chemotherapy effect of 5-FU (246). Targeting mitochondrial
dysfunction in cancer, nanoparticles modified with
mitochondria-targeting peptides and tumor-targeting ligands
efficiently deliver miR-125 to mitochondria within cancer cells.
This approach regulates mitochondrial dynamics-associated proteins
and suppresses intracellular ATP production (247). Bioengineered miRNAs targeting
metabolic pathways have shown promise in cancer therapy (248,249). For example, the bioengineered
tRNA/miR-328-3p enables controlled release of miR-328-3p in human
osteosarcoma cells, inhibiting glycolysis and cell proliferation by
targeting GLUT1 (249).
Exosomes serve a key role in intercellular
communication and can be easily engineered with tumor-targeting
properties through molecular biology techniques, making them a
promising vehicle for ncRNA delivery (250). For example, exosomes targeting
HER2 have been used to deliver oncomiR-21 inhibitors to CRC cells,
successfully reversing 5-FU resistance in these cells (251). However, current research is
mainly focused on encapsulating both antitumor drugs and ncRNAs
within nanoparticles for synergistic antitumor effects (252), while the use of exosomes for
concurrent delivery of drugs and ncRNAs remains underexplored.
Stability issues surrounding dual delivery via exosomes need to be
addressed in future studies. In addition to nanoparticle-based
approaches, inhibition of miRNAs using antisense DNA oligomers that
incorporate locked nucleic acids (LNAs) offers a promising strategy
for gene therapy. A clinical trial investigated a 13-mer LNA
inhibitor targeting miR-221 (LNA-i-miR-221) and found that, among
17 patients treated with this therapy, 16 demonstrated good
tolerance, with eight achieving stable disease and one experiencing
partial response (253).
Furthermore, gene delivery systems based on viral vectors and
clustered regularly interspaced short palindromic repeat-associated
protein9 (Cas9) genome editing system have been explored for
editing ncRNAs to hinder tumor progression (254).
In summary, ncRNAs exhibit specificity in targeting
key pathways in tumor cell metabolism, serving a pivotal role in
tumor growth and drug resistance. As such, ncRNAs represent
valuable targets for metabolic regulation in treating
drug-resistant cancer. Interfering with ncRNAs in tumor cells via
targeted delivery systems, such as nanoparticles, can effectively
modulate tumor metabolism and enhance the efficacy of anti-cancer
therapy (Fig. 8).
The aforementioned studies underscored the
importance of ncRNA-mediated metabolic regulation in combating drug
resistance, yet limitations persist. Studies (97,189,205,213) often focus on individual ncRNAs
in specific cancer models, neglecting the tissue-specific and
context-dependent roles of ncRNAs and rarely account for
spatiotemporal variations, which are key for predicting therapeutic
outcomes. For example, miR-181a exhibits oncogenic properties in GC
(255) but acts as a tumor
suppressor in NSCLC (256).
These fragmented views impede the identification of the complex
ncRNA regulatory network in cancer. Metabolic reprogramming
involves interconnected pathways, such as glycolysis, lipid and
glutamine metabolism. yet most studies (66,215) investigate ncRNAs targeting a
single pathway, which may trigger compensatory mechanisms. For
example, inhibition of glycolysis can enhance glutamine dependency
(257).
The regulatory mechanisms by which ncRNA influences
metabolic reprogramming pathways require further investigation. The
JAK/STAT pathway facilitates rapid signal transduction from the
cell membrane to the nucleus and induces the production of
oncogenic factors, serving as a key pathway regulating cancer cell
proliferation, metabolism and immune responses (258). The JAK/STAT3 pathway promotes
glycolysis and FAO, thereby contributing to drug resistance
(259,260). Several ncRNAs are effective
regulators of the JAK/STAT3 pathway (261,262). However, further research is
needed to elucidate the mechanisms by which these ncRNAs regulate
JAK/STAT3 in the context of metabolism and cancer drug resistance.
In addition, the circadian clock and cancer cell stemness are key
phenotypes that profoundly influence metabolic reprogramming
(263,264). ncRNAs serve a regulatory role in
the circadian gene and stemness driving pathways (265-267). For example, in gastrointestinal
tumors, miR-494-3p and miR-135b directly target the 3′UTR of brain
and muscle arnt-like protein 1 (BMAL1) to inhibit its expression.
BMAL1 interacts with Enhancer of zeste homolog 2 to suppress
glycerol-3-phosphate acyltransferase mitochondrial, resulting in a
reduction of LPA levels. By targeting BMAL1, miR-494-3p promotes
LPA metabolism and proliferation of HCC cells (259). Meanwhile, miR-135b disrupts the
tumor-suppressive circadian rhythm in pancreatic cancer via the
BMAL1/Yin Yang-1 axis, thereby promoting pancreatic cancer
progression and GEM resistance (268). ncRNAs mediate the occurrence and
progression of EC by disrupting circadian rhythm in EC cells
through the regulation of Zinc Finger and BTB Domain Containing 7A,
p21) and Neuronal PAS domain protein 2 expression (266). Regarding tumor stemness,
multiple ncRNAs enhance the expression of transcription factors
such as NF-κB and STAT3 in tumor cells to evade the toxic effects
of chemotherapy and radiotherapy (267). Mechanistically, these ncRNAs
activate key signaling pathways that promote the properties of
cancer stem cells. These pathways include Wnt/β-catenin,
PI3K/AKT/mTOR, Notch and TGF-β (269). This process not only promotes
cancer stemness but also facilitates the formation of feedback
loops, which in turn leads to cancer metastasis and drug resistance
(270). Exploring these ncRNAs,
particularly their modulation of circadian rhythm and
stemness-mediated metabolic alterations and drug resistance, offers
a promising and valuable option for future research.
Targeting ncRNAs to regulate metabolism presents a
promising strategy for personalized cancer therapy. However,
several challenges must be overcome before this approach can be
widely applied in clinical practice. A notable obstacle is the
toxicity of ncRNA-targeting drugs. For example, the phase 1 trial
of the liposomal miR-34a mimic MRX34 in patients with advanced
solid tumors showed some clinical activity but was terminated due
to four cases of immune-mediated serious adverse events (271). As methods for modulating ncRNA
expression in cells, common vectors such as liposomes and chitosan
and polymeric nanoparticles are relatively safe and effective for
large gene transfer, but they suffer from limitations such as low
transfection efficiency and poor transgene expression (272). Based on a mesenchymal stem cell
(MSC)-based gene delivery system, tumor suppressor genes are
introduced to enhance the specific expression of MSCs and improve
their homing effect. This positions MSC-based therapy for cancer as
a potential and effective strategy (273). By contrast, viral vectors,
including lentiviruses and adenoviruses, offer high infection
efficiency and sustained transgene expression but are hampered by
high immunogenicity and limited payload capacity (274). Future research may unlock the
potential of ncRNA-based therapy to combat drug-resistant cancer.
Collaborative efforts among molecular biologists, bioengineers and
clinicians will be essential to translate these insights into
clinically viable solutions.
The adaptability of tumor cells has led to a rising
prevalence of drug resistance, notably decreasing the overall
survival of patients with cancers (275). When resistance to first-line
chemotherapy drugs arises, adjustments to the chemotherapy regimen
or the addition of other agents are often required, which can
result in suboptimal therapeutic outcomes or more severe adverse
reactions. Previous studies (10,17) have demonstrated the pivotal role
of metabolic reprogramming in tumor drug resistance. The present
review systematically summarizes the mechanisms by which metabolic
reprogramming contributes to drug resistance in tumors, with a
focus on how ncRNAs mediate this process through the regulation of
glycolysis, lipid and glutamine metabolism and mitochondrial
dysfunction, as well as the potential of ncRNAs as biomarkers and
therapeutic targets. The specific expression patterns of ncRNAs in
tumor cells serve as indicators of both the metabolic state and
drug sensitivity of these cells. Targeting ncRNAs that regulate
metabolism may offer innovative strategies to combat tumor drug
resistance in the future.
Not applicable.
JL wrote the manuscript and constructed figures and
tables. YL wrote the manuscript. LF constructed figures. HC, ZW and
FD revised the manuscript. YZ and YH revised the manuscript. YX and
JM conceived the study. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bukhari SNA: Emerging nanotherapeutic
approaches to overcome drug resistance in cancers with update on
clinical trials. Pharmaceutics. 14:8662022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tufail M, Hu JJ, Liang J, He CY, Wan WD,
Huang YQ, Jiang CH, Wu H and Li N: Hallmarks of cancer resistance.
iScience. 27:1099792024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubik J, Humeniuk E, Adamczuk G,
Madej-Czerwonka B and Korga-Plewko A: Targeting energy metabolism
in cancer treatment. Int J Mol Sci. 23:55722022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Metab.
34:355–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paul S, Ghosh S and Kumar S: Tumor
glycolysis, an essential sweet tooth of tumor cells. Semin Cancer
Biol. 86:1216–1230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoo HC, Yu YC, Sung Y and Han JM:
Glutamine reliance in cell metabolism. Exp Mol Med. 52:1496–1516.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stine ZE, Schug ZT, Salvino JM and Dang
CV: Targeting cancer metabolism in the era of precision oncology.
Nat Rev Drug Discov. 21:141–162. 2022. View Article : Google Scholar
|
|
13
|
Landau BR, Laszlo J, Stengle J and Burk D:
Certain metabolic and pharmacologic effects in cancer patients
given infusions of 2-deoxy-D-glucose. J Natl Cancer Inst.
21:485–494. 1958.PubMed/NCBI
|
|
14
|
Slack FJ and Chinnaiyan AM: The role of
non-coding RNAs in oncology. Cell. 179:1033–1055. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nemeth K, Bayraktar R, Ferracin M and
Calin GA: Non-coding RNAs in disease: From mechanisms to
therapeutics. Nat Rev Genet. 25:211–232. 2024. View Article : Google Scholar
|
|
16
|
Sanchez-Mejias A and Tay Y: Competing
endogenous RNA networks: Tying the essential knots for cancer
biology and therapeutics. J Hematol Oncol. 8:302015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin W, Zhou Q, Wang CQ, Zhu L, Bi C, Zhang
S, Wang X and Jin H: LncRNAs regulate metabolism in cancer. Int J
Biol Sci. 16:1194–1206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shih JW, Wang LY, Hung CL, Kung HJ and
Hsieh CL: Non-coding RNAs in castration-resistant prostate cancer:
Regulation of androgen receptor signaling and cancer metabolism.
Int J Mol Sci. 16:28943–28978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu S, Wang L, Zhao Y, Mo T, Wang B, Lin J
and Yang H: Metabolism-regulating non-coding RNAs in breast cancer:
Roles, mechanisms and clinical applications. J Biomed Sci.
31:252024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keyvani-Ghamsari S, Khorsandi K, Rasul A
and Zaman MK: Current understanding of epigenetics mechanism as a
novel target in reducing cancer stem cells resistance. Clin
Epigenetics. 13:1202021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng Z, Fu M, Hu Y, Wei Y, Wei X and Luo
M: Regulation and signaling pathways in cancer stem cells:
Implications for targeted therapy for cancer. Mol Cancer.
22:1722023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan Y, Li H, Pu W, Chen L, Guo D, Jiang
H, He B, Qin S, Wang K, Li N, et al: Cancer metabolism and tumor
microenvironment: Fostering each other? Sci China Life Sci.
65:236–279. 2022. View Article : Google Scholar
|
|
23
|
Lin X, Wu Z, Hu H, Luo ML and Song E:
Non-coding RNAs rewire cancer metabolism networks. Semin Cancer
Biol. 75:116–126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang D, Guo Q, You K, Zhang Y, Zheng Y
and Wei T: m6A-modified circARHGAP12 promotes the
aerobic glycolysis of doxorubicin-resistance osteosarcoma by
targeting c-Myc. J Orthop Surg Res. 19:332024. View Article : Google Scholar
|
|
25
|
Zhang Q, Wu J, Zhang X, Cao L, Wu Y and
Miao X: Transcription factor ELK1 accelerates aerobic glycolysis to
enhance osteosarcoma chemoresistance through miR-134/PTBP1
signaling cascade. Aging (Albany NY). 13:6804–6819. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He W, Liang B, Wang C, Li S, Zhao Y, Huang
Q, Liu Z, Yao Z, Wu Q, Liao W, et al: MSC-regulated lncRNA
MACC1-AS1 promotes stemness and chemoresistance through fatty acid
oxidation in gastric cancer. Oncogene. 38:4637–4654. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ge X, Pan MH, Wang L, Li W, Jiang C, He J,
Abouzid K, Liu LZ, Shi Z and Jiang BH: Hypoxia-mediated
mitochondria apoptosis inhibition induces temozolomide treatment
resistance through miR-26a/Bad/Bax axis. Cell Death Dis.
9:11282018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding C, Yi X, Chen X, Wu Z, You H, Chen X,
Zhang G, Sun Y, Bu X, Wu X, et al: Warburg effect-promoted exosomal
circ_0072083 releasing up-regulates NANGO expression through
multiple pathways and enhances temozolomide resistance in glioma. J
Exp Clin Cancer Res. 40:1642021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun X, Sun G, Huang Y, Hao Y, Tang X,
Zhang N, Zhao L, Zhong R and Peng Y: 3-Bromopyruvate regulates the
status of glycolysis and BCNU sensitivity in human hepatocellular
carcinoma cells. Biochem Pharmacol. 177:1139882020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z,
Li W, Hu J, Lu C and Liu Y: PI3K/AKT pathway as a key link
modulates the multidrug resistance of cancers. Cell Death Dis.
11:7972020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castellví A, Pequerul R, Barracco V,
Juanhuix J, Parés X and Farrés J: Structural and biochemical
evidence that ATP inhibits the cancer biomarker human aldehyde
dehydrogenase 1A3. Commun Biol. 5:3542022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou GX, Liu PP, Zhang S, Yang M, Liao J,
Yang J, Hu Y, Jiang WQ, Wen S and Huang P: Elimination of stem-like
cancer cell side-population by auranofin through modulation of ROS
and glycolysis. Cell Death Dis. 9:892018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giddings EL, Champagne DP, Wu MH, Laffin
JM, Thornton TM, Valenca-Pereira F, Culp-Hill R, Fortner KA, Romero
N, East J, et al: Mitochondrial ATP fuels ABC transporter-mediated
drug efflux in cancer chemoresistance. Nat Commun. 12:28042021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu S, Tian H, Li L, Li B, Yang M, Zhou L,
Jiang H, Li Q, Wang W, Nice EC, et al: Nanoengineering a zeolitic
imidazolate framework-8 capable of manipulating energy metabolism
against cancer chemo-phototherapy resistance. Small.
18:e22049262022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roberts DJ and Miyamoto S: Hexokinase II
integrates energy metabolism and cellular protection: Akting on
mitochondria and TORCing to autophagy. Cell Death Differ.
22:248–257. 2015. View Article : Google Scholar :
|
|
36
|
Zhang Z, Deng X, Liu Y, Liu Y, Sun L and
Chen F: PKM2, function and expression and regulation. Cell Biosci.
9:522019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jang M, Kang HJ, Lee SY, Chung SJ, Kang S,
Chi SW, Cho S, Lee SC, Lee CK, Park BC, et al:
Glyceraldehyde-3-phosphate, a glycolytic intermediate, plays a key
role in controlling cell fate via inhibition of caspase activity.
Mol Cells. 28:559–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong Q, Niu W, Mu M, Ye C, Wu P, Hu S and
Niu C: Lycorine hydrochloride interferes with energy metabolism to
inhibit chemoresistant glioblastoma multiforme cell growth through
suppressing PDK3. Mol Cell Biochem. 480:355–369. 2025. View Article : Google Scholar
|
|
39
|
Yalcin A, Clem BF, Imbert-Fernandez Y,
Ozcan SC, Peker S, O'Neal J, Klarer AC, Clem AL, Telang S and
Chesney J: 6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle
progression and suppresses apoptosis via Cdk1-mediated
phosphorylation of p27. Cell Death Dis. 5:e13372014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi L, Pan H, Liu Z, Xie J and Han W:
Roles of PFKFB3 in cancer. Signal Transduct Target Ther.
2:170442017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cantelmo AR, Conradi LC, Brajic A, Goveia
J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen
LA, et al: Inhibition of the glycolytic activator PFKFB3 in
endothelium induces tumor vessel normalization, impairs metastasis,
and improves chemotherapy. Cancer Cell. 30:968–985. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morais-Santos F, Granja S,
Miranda-Gonçalves V, Moreira AH, Queirós S, Vilaça JL, Schmitt FC,
Longatto-Filho A, Paredes J, Baltazar F and Pinheiro C: Targeting
lactate transport suppresses in vivo breast tumour growth.
Oncotarget. 6:19177–19189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hraběta J, Belhajová M, Šubrtová H, Merlos
Rodrigo MA, Heger Z and Eckschlager T: Drug sequestration in
lysosomes as one of the mechanisms of chemoresistance of cancer
cells and the possibilities of its inhibition. Int J Mol Sci.
21:43922020. View Article : Google Scholar
|
|
44
|
Bogdanov A, Bogdanov A, Chubenko V, Volkov
N, Moiseenko F and Moiseyenko V: Tumor acidity: From hallmark of
cancer to target of treatment. Front Oncol. 12:9791542022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mahoney BP, Raghunand N, Baggett B and
Gillies RJ: Tumor acidity, ion trapping and chemotherapeutics. I.
Acid pH affects the distribution of chemotherapeutic agents in
vitro. Biochem Pharmacol. 66:1207–1218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alfarouk KO, Stock CM, Taylor S, Walsh M,
Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO,
Harguindey S, et al: Resistance to cancer chemotherapy: Failure in
drug response from ADME to P-gp. Cancer Cell Int. 15:712015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dong Q, Zhou C, Ren H, Zhang Z, Cheng F,
Xiong Z, Chen C, Yang J, Gao J, Zhang Y, et al: Lactate-induced
MRP1 expression contributes to metabolism-based etoposide
resistance in non-small cell lung cancer cells. Cell Commun Signal.
18:1672020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li J, Gao N, Gao Z, Liu W, Pang B, Dong X,
Li Y and Fan T: The emerging role of exosomes in cancer
chemoresistance. Front Cell Dev Biol. 9:7379622021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brown TP and Ganapathy V: Lactate/GPR81
signaling and proton motive force in cancer: Role in angiogenesis,
immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther.
206:1074512020. View Article : Google Scholar
|
|
50
|
Sandforth L, Ammar N, Dinges LA, Röcken C,
Arlt A, Sebens S and Schäfer H: Impact of the monocarboxylate
transporter-1 (MCT1)-mediated cellular import of lactate on
stemness properties of human pancreatic adenocarcinoma cells †.
Cancers (Basel). 12:5812020. View Article : Google Scholar
|
|
51
|
Takada T, Takata K and Ashihara E:
Inhibition of monocarboxylate transporter 1 suppresses the
proliferation of glioblastoma stem cells. J Physiol Sci.
66:387–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang XY, Zhang M, Cong Q, Zhang MX, Zhang
MY, Lu YY and Xu CJ: Hexokinase 2 confers resistance to cisplatin
in ovarian cancer cells by enhancing cisplatin-induced autophagy.
Int J Biochem Cell Biol. 95:9–16. 2018. View Article : Google Scholar
|
|
53
|
Thirusangu P, Ray U, Sarkar Bhattacharya
S, Oien DB, Jin L, Staub J, Kannan N, Molina JR and Shridhar V:
PFKFB3 regulates cancer stemness through the hippo pathway in small
cell lung carcinoma. Oncogene. 41:4003–4017. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Amin S, Yang P and Li Z: Pyruvate kinase
M2: A multifarious enzyme in non-canonical localization to promote
cancer progression. Biochim Biophys Acta Rev Cancer. 1871:331–341.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee SH, Golinska M and Griffiths JR:
HIF-1-independent mechanisms regulating metabolic adaptation in
hypoxic cancer cells. Cells. 10:23712021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tirpe AA, Gulei D, Ciortea SM, Crivii C
and Berindan-Neagoe I: Hypoxia: Overview on hypoxia-mediated
mechanisms with a focus on the role of HIF genes. Int J Mol Sci.
20:61402019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nagao A, Kobayashi M, Koyasu S, Chow CCT
and Harada H: HIF-1-dependent reprogramming of glucose metabolic
pathway of cancer cells and its therapeutic significance. Int J Mol
Sci. 20:2382019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Delgado JM and Shoemaker CJ: An unexpected
journey for BNIP3. Autophagy. 20:1447–1448. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Geng Y, Zheng X, Zhang D, Wei S, Feng J,
Wang W, Zhang L, Wu C and Hu W: CircHIF1A induces cetuximab
resistance in colorectal cancer by promoting HIF1α-mediated
glycometabolism alteration. Biol Direct. 19:362024. View Article : Google Scholar
|
|
60
|
Xu G, Li M, Wu J, Qin C, Tao Y and He H:
Circular RNA circ-NRIP1 sponges microRNA-138-5p to maintain
hypoxia-induced resistance to 5-fluorouracil through
HIF-1α-dependent glucose metabolism in gastric carcinoma. Cancer
Manag Res. 12:2789–2802. 2020. View Article : Google Scholar :
|
|
61
|
Li Q, Sun H, Luo D, Gan L, Mo S, Dai W,
Liang L, Yang Y, Xu M, Li J, et al: Lnc-RP11-536 K7.3/SOX2/HIF-1α
signaling axis regulates oxaliplatin resistance in patient-derived
colorectal cancer organoids. J Exp Clin Cancer Res. 40:3482021.
View Article : Google Scholar
|
|
62
|
Chen F, Chen J, Yang L, Liu J, Zhang X,
Zhang Y, Tu Q, Yin D, Lin D, Wong PP, et al: Extracellular
vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated
macrophages regulates aerobic glycolysis of breast cancer cells.
Nat Cell Biol. 21:498–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu F, Huang M, Chen Q, Niu Y, Hu Y, Hu P,
Chen D, He C, Huang K, Zeng Z, et al: LncRNA HIF1A-AS1 promotes
gemcitabine resistance of pancreatic cancer by enhancing glycolysis
through modulating the AKT/YB1/HIF1α pathway. Cancer Res.
81:5678–5691. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zeng Z, Zhao Y, Chen Q, Zhu S, Niu Y, Ye
Z, Hu P, Chen D, Xu P, Chen J, et al: Hypoxic exosomal
HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic
pancreatic cancer cells via enhancing glycolysis. Oncogene.
40:5505–5517. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Meng Y, Xu X, Luan H, Li L, Dai W, Li Z
and Bian J: The progress and development of GLUT1 inhibitors
targeting cancer energy metabolism. Future Med Chem. 11:2333–2352.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dong P, Wang F, Taheri M, Xiong Y, Ihira
K, Kobayashi N, Konno Y, Yue J and Watari H: Long non-coding RNA
TMPO-AS1 promotes GLUT1-mediated glycolysis and paclitaxel
resistance in endometrial cancer cells by interacting with miR-140
and miR-143. Front Oncol. 12:9129352022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma J, Qi G and Li L: A novel serum
exosomes-based biomarker hsa_circ_0002130 facilitates
osimertinib-resistance in non-small cell lung cancer by sponging
miR-498. Onco Targets Ther. 13:5293–5307. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li P, Yang X, Cheng Y, Zhang X, Yang C,
Deng X, Li P, Tao J, Yang H, Wei J, et al: MicroRNA-218 increases
the sensitivity of bladder cancer to cisplatin by targeting Glut1.
Cell Physiol Biochem. 41:921–932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tu MJ, Duan Z, Liu Z, Zhang C, Bold RJ,
Gonzalez FJ, Kim EJ and Yu AM: MicroRNA-1291-5p sensitizes
pancreatic carcinoma cells to arginine deprivation and chemotherapy
through the regulation of arginolysis and glycolysis. Mol
Pharmacol. 98:686–694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zuo J, Tang J, Lu M, Zhou Z, Li Y, Tian H,
Liu E, Gao B, Liu T and Shao P: Glycolysis rate-limiting enzymes:
Novel potential regulators of rheumatoid arthritis pathogenesis.
Front Immunol. 12:7797872021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xu J, Xu Y, Ye G and Qiu J: LncRNA-SNHG1
promotes paclitaxel resistance of gastric cancer cells through
modulating the miR-216b-5p-hexokianse 2 axis. J Chemother.
35:527–538. 2023. View Article : Google Scholar
|
|
72
|
Shi H, Li K, Feng J, Liu G, Feng Y and
Zhang X: LncRNA-DANCR interferes with miR-125b-5p/HK2 axis to
desensitize colon cancer cells to cisplatin vis activating
anaerobic glycolysis. Front Oncol. 10:10342020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang H, Zhao L, Ren P and Sun X: LncRNA
MBNL1-AS1 knockdown increases the sensitivity of hepatocellular
carcinoma to tripterine by regulating miR-708-5p-mediated
glycolysis. Biotechnol Genet Eng Rev. 40:1407–1424. 2024.
View Article : Google Scholar
|
|
74
|
Deng Y, Li X, Feng J and Zhang X:
Overexpression of miR-202 resensitizes imatinib resistant chronic
myeloid leukemia cells through targetting Hexokinase 2. Biosci Rep.
38:BSR201713832018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang JX, Gao S, Pan YZ, Yu C and Sun CY:
Overexpression of microRNA-125b sensitizes human hepatocellular
carcinoma cells to 5-fluorouracil through inhibition of glycolysis
by targeting hexokinase II. Mol Med Rep. 10:995–1002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang Y, Liu Y and Xu X: Knockdown of
LncRNA-UCA1 suppresses chemoresistance of pediatric AML by
inhibiting glycolysis through the microRNA-125a/hexokinase 2
pathway. J Cell Biochem. 119:6296–6308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shi H, Li K, Feng J and Zhang X:
Overexpression of long non-coding RNA urothelial carcinoma
associated 1 causes paclitaxel (Taxol) resistance in colorectal
cancer cells by promoting glycolysis. J Chemother. 33:409–419.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Deng X, Li D, Ke X, Wang Q, Yan S, Xue Y,
Wang Q and Zheng H: Mir-488 alleviates chemoresistance and
glycolysis of colorectal cancer by targeting PFKFB3. J Clin Lab
Anal. 35:e235782021. View Article : Google Scholar
|
|
79
|
Gao Y, Liu C, Xu X, Wang Y and Jiang Y:
Circular RNA sterile alpha motif domain containing 4A contributes
to cell 5-fluorouracil resistance in colorectal cancer by
regulating the
miR-545-3p/6-phosphofructo-2-kinase/fructose-2,6-bisphosphataseisotype
3 axis. Anticancer Drugs. 33:553–563. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yang W and Lu Z: Pyruvate kinase M2 at a
glance. J Cell Sci. 128:1655–1660. 2015.PubMed/NCBI
|
|
81
|
Dayton TL, Jacks T and Vander Heiden MG:
PKM2, cancer metabolism, and the road ahead. EMBO Rep.
17:1721–1730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yu Y, Liang Y, Xie F, Zhang Z, Zhang P,
Zhao X, Zhang Z, Liang Z, Li D, Wang L, et al: Tumor-associated
macrophage enhances PD-L1-mediated immune escape of bladder cancer
through PKM2 dimer-STAT3 complex nuclear translocation. Cancer
Lett. 593:2169642024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yao Y, Chen X, Wang X, Li H, Zhu Y, Li X,
Xiao Z, Zi T, Qin X, Zhao Y, et al: Glycolysis related lncRNA
SNHG3/miR-139-5p/PKM2 axis promotes castration-resistant prostate
cancer (CRPC) development and enzalutamide resistance. Int J Biol
Macromol. 260:1296352024. View Article : Google Scholar
|
|
84
|
Zhong W, Wang C and Sun Y: LncRNA PCIF1
promotes aerobic glycolysis in A549/DDP cells by competitively
binding miR-326 to regulate PKM expression. Mol Cell Probes.
77:1019772024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wu H, Du J, Li C, Li H, Guo H and Li Z:
Kaempferol can reverse the 5-Fu resistance of colorectal cancer
cells by inhibiting PKM2-mediated glycolysis. Int J Mol Sci.
23:35442022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bian Z, Yang F, Xu P, Gao G, Yang C, Cao
Y, Yao S, Wang X, Yin Y, Fei B and Huang Z: LINC01852 inhibits the
tumorigenesis and chemoresistance in colorectal cancer by
suppressing SRSF5-mediated alternative splicing of PKM. Mol Cancer.
23:232024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhu Z, Tang G and Yan J: MicroRNA-122
regulates docetaxel resistance of prostate cancer cells by
regulating PKM2. Exp Ther Med. 20:2472020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pan C, Wang X, Shi K, Zheng Y, Li J, Chen
Y, Jin L and Pan Z: MiR-122 reverses the doxorubicin-resistance in
hepatocellular carcinoma cells through regulating the tumor
metabolism. PLoS One. 11:e01520902016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang X, Zhang H, Yang H, Bai M, Ning T,
Deng T, Liu R, Fan Q, Zhu K, Li J, et al: Exosome-delivered circRNA
promotes glycolysis to induce chemoresistance through the
miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 14:539–555.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhu S, Guo Y, Zhang X, Liu H, Yin M, Chen
X and Peng C: Pyruvate kinase M2 (PKM2) in cancer and cancer
therapeutics. Cancer Lett. 503:240–248. 2021. View Article : Google Scholar
|
|
91
|
Zhang M, Zhang H, Hong H and Zhang Z:
MiR-374b re-sensitizes hepatocellular carcinoma cells to sorafenib
therapy by antagonizing PKM2-mediated glycolysis pathway. Am J
Cancer Res. 9:765–778. 2019.PubMed/NCBI
|
|
92
|
Jiang CF, Xie YX, Qian YC, Wang M, Liu LZ,
Shu YQ, Bai XM and Jiang BH: TBX15/miR-152/KIF2C pathway regulates
breast cancer doxorubicin resistance via promoting PKM2
ubiquitination. Cancer Cell Int. 21:5422021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li X, Zhang Y and Wang X, Lin F, Cheng X,
Wang Z and Wang X: Long non-coding RNA CTSLP8 mediates ovarian
cancer progression and chemotherapy resistance by modulating
cellular glycolysis and regulating c-Myc expression through PKM2.
Cell Biol Toxicol. 38:1027–1045. 2022. View Article : Google Scholar :
|
|
94
|
Van Wilpe S, Koornstra R, Den Brok M, De
Groot JW, Blank C, De Vries J, Gerritsen W and Mehra N: Lactate
dehydrogenase: A marker of diminished antitumor immunity.
Oncoimmunology. 9:17319422020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pei LJ, Sun PJ, Ma K, Guo YY, Wang LY and
Liu FD: LncRNA-SNHG7 interferes with miR-34a to de-sensitize
gastric cancer cells to cisplatin. Cancer Biomark. 30:127–137.
2021. View Article : Google Scholar
|
|
96
|
Hua G, Zeng ZL, Shi YT, Chen W, He LF and
Zhao GF: LncRNA XIST contributes to cisplatin resistance of lung
cancer cells by promoting cellular glycolysis through sponging
miR-101-3p. Pharmacology. 106:498–508. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li G, Li Y and Wang DY: Overexpression of
miR-329-3p sensitizes osteosarcoma cells to cisplatin through
suppression of glucose metabolism by targeting LDHA. Cell Biol Int.
45:766–774. 2021. View Article : Google Scholar
|
|
98
|
Hu J, Huang L, Ding Q, Lv J and Chen Z:
Long noncoding RNA HAGLR sponges miR-338-3p to promote 5-Fu
resistance in gastric cancer through targeting the LDHA-glycolysis
pathway. Cell Biol Int. 46:173–184. 2022. View Article : Google Scholar
|
|
99
|
Shao X, Zheng X, Ma D, Liu Y and Liu G:
Inhibition of lncRNA-NEAT1 sensitizes 5-Fu resistant cervical
cancer cells through de-repressing the microRNA-34a/LDHA axis.
Biosci Rep. 41:BSR202005332021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shi L, Duan R, Sun Z, Jia Q, Wu W, Wang F,
Liu J, Zhang H and Xue X: LncRNA GLTC targets LDHA for
succinylation and enzymatic activity to promote progression and
radioiodine resistance in papillary thyroid cancer. Cell Death
Differ. 30:1517–1532. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Purhonen J, Klefström J and Kallijärvi J:
MYC-an emerging player in mitochondrial diseases. Front Cell Dev
Biol. 11:12576512023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang H, Wang L, Pan H, Wang Y, Shi M, Yu
H, Wang C, Pan X and Chen Z: Exosomes derived from macrophages
enhance aerobic glycolysis and chemoresistance in lung cancer by
stabilizing c-Myc via the inhibition of NEDD4L. Front Cell Dev
Biol. 8:6206032021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jiang X, Guo S, Wang S, Zhang Y, Chen H,
Wang Y, Liu R, Niu Y and Xu Y: EIF4A3-induced circARHGAP29 promotes
aerobic glycolysis in docetaxel-resistant prostate cancer through
IGF2BP2/c-Myc/LDHA signaling. Cancer Res. 82:831–845. 2022.
View Article : Google Scholar
|
|
104
|
Anwar S, Shamsi A, Mohammad T, Islam A and
Hassan MI: Targeting pyruvate dehydrogenase kinase signaling in the
development of effective cancer therapy. Biochim Biophys Acta Rev
Cancer. 1876:1885682021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhuang L, Zhang B, Liu X, Lin L, Wang L,
Hong Z and Chen J: Exosomal miR-21-5p derived from
cisplatin-resistant SKOV3 ovarian cancer cells promotes glycolysis
and inhibits chemosensitivity of its progenitor SKOV3 cells by
targeting PDHA1. Cell Biol Int. 45:2140–2149. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Qian Y, Wu X, Wang H, Hou G, Han X and
Song W: MicroRNA-4290 suppresses PDK1-mediated glycolysis to
enhance the sensitivity of gastric cancer cell to cisplatin. Braz J
Med Biol Res. 53:e93302020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Xie Y, Shi Z, Qian Y, Jiang C, Liu W, Liu
B and Jiang B: HDAC2- and EZH2-mediated histone modifications
induce PDK1 expression through miR-148a downregulation in breast
cancer progression and adriamycin resistance. Cancers (Basel).
14:36002022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ding Y, Gao S, Zheng J and Chen X:
Blocking lncRNA-SNHG16 sensitizes gastric cancer cells to 5-Fu
through targeting the miR-506-3p-PTBP1-mediated glucose metabolism.
Cancer Metab. 10:202022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen X, Luo R, Zhang Y, Ye S, Zeng X, Liu
J, Huang D, Liu Y, Liu Q, Luo ML and Song E: Long noncoding RNA
DIO3OS induces glycolytic-dominant metabolic reprogramming to
promote aromatase inhibitor resistance in breast cancer. Nat
Commun. 13:71602022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yang GJ, Tao F, Zhong HJ, Yang C and Chen
J: Targeting PGAM1 in cancer: An emerging therapeutic opportunity.
Eur J Med Chem. 244:1147982022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu Z, Zheng N, Li J, Li C, Zheng D, Jiang
X, Ge X, Liu M, Liu L, Song Z, et al: N6-methyladenosine-modified
circular RNA QSOX1 promotes colorectal cancer resistance to
anti-CTLA-4 therapy through induction of intratumoral regulatory T
cells. Drug Resist Updat. 65:1008862022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Poliaková M, Aebersold DM, Zimmer Y and
Medová M: The relevance of tyrosine kinase inhibitors for global
metabolic pathways in cancer. Mol Cancer. 17:272018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li C, Liu FY, Shen Y, Tian Y and Han FJ:
Research progress on the mechanism of glycolysis in ovarian cancer.
Front Immunol. 14:12848532023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fontana F, Giannitti G, Marchesi S and
Limonta P: The PI3K/Akt pathway and glucose metabolism: A dangerous
liaison in cancer. Int J Biol Sci. 20:3113–3125. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang L, Jiang P, Li J, Huang Y, Wen J, Wu
Z, Chen Y and Hu J: Loss of MiR-155 sensitizes
FLT3-ITD+AML to chemotherapy and FLT3 inhibitors via
glycolysis blocking by targeting PIK3R1. J Cancer. 14:99–113. 2023.
View Article : Google Scholar :
|
|
117
|
Chen X, Song Y, Tian Y, Dong X, Chang Y
and Wang W: miR-149-3p enhances drug sensitivity of AML cells by
inhibiting warburg effect through PI3K/AKT pathway. Cell Biochem
Biophys. 82:3287–3296. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lim SO, Li CW, Xia W, Lee HH, Chang SS,
Shen J, Hsu JL, Raftery D, Djukovic D, Gu H, et al: EGFR signaling
enhances aerobic glycolysis in triple-negative breast cancer cells
to promote tumor growth and immune escape. Cancer Res.
76:1284–1296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Concha-Benavente F and Ferris RL:
Reversing EGFR mediated immunoescape by targeted monoclonal
antibody therapy. Front Pharmacol. 8:3322017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gao SJ, Ren SN, Liu YT, Yan HW and Chen
XB: Targeting EGFR sensitizes 5-Fu-resistant colon cancer cells
through modification of the lncRNA-FGD5-AS1-miR-330-3p-Hexokinase 2
axis. Mol Ther Oncolytics. 23:14–25. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wen JF, Jiang YQ, Li C, Dai XK, Wu T and
Yin WZ: LncRNA-SARCC sensitizes osteosarcoma to cisplatin through
the miR-143-mediated glycolysis inhibition by targeting Hexokinase
2. Cancer Biomark. 28:231–246. 2020. View Article : Google Scholar
|
|
122
|
Chen W, Chen Y and Hui T: microRNA-143
interferes the EGFR-stimulated glucose metabolism to re-sensitize
5-FU resistant colon cancer cells via targeting hexokinase 2. J
Chemother. 35:539–549. 2023. View Article : Google Scholar
|
|
123
|
Cheng WL, Feng PH, Lee KY, Chen KY, Sun
WL, Van Hiep N, Luo CS and Wu SM: The role of EREG/EGFR pathway in
tumor progression. Int J Mol Sci. 22:128282021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
He M, Jin Q, Chen C, Liu Y, Ye X, Jiang Y,
Ji F, Qian H, Gan D, Yue S, et al: The miR-186-3p/EREG axis
orchestrates tamoxifen resistance and aerobic glycolysis in breast
cancer cells. Oncogene. 38:5551–5565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Asl ER, Amini M, Najafi S, Mansoori B,
Mokhtarzadeh A, Mohammadi A, Lotfinejad P, Bagheri M, Shirjang S,
Lotfi Z, et al: Interplay between MAPK/ERK signaling pathway and
MicroRNAs: A crucial mechanism regulating cancer cell metabolism
and tumor progression. Life Sci. 278:1194992021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
He Z, Zhong Y, Lv T, Wang J, Jin Y, Li F
and Hu H: PP4R1 promotes glycolysis and gallbladder cancer
progression through facilitating ERK1/2 mediated PKM2 nuclear
translocation. Cancer Lett. 586:2166772024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li X, Tsauo J, Geng C, Zhao H, Lei X and
Li X: Ginsenoside Rg3 Decreases NHE1 expression via inhibiting
EGF-EGFR-ERK1/2-HIF-1 α pathway in hepatocellular carcinoma: A
novel antitumor mechanism. Am J Chin Med. 46:1915–1931. 2018.
View Article : Google Scholar
|
|
128
|
Li M, Cai O, Yu Y and Tan S: Paeonol
inhibits the malignancy of Apatinib-resistant gastric cancer cells
via LINC00665/miR-665/MAPK1 axis. Phytomedicine. 96:1539032022.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
cellular energy sensing and restoration of metabolic balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li YZ, Deng J, Zhang XD, Li DY, Su LX, Li
S, Pan JM, Lu L, Ya JQ, Yang N, et al: Naringenin enhances the
efficacy of ferroptosis inducers by attenuating aerobic glycolysis
by activating the AMPK-PGC1α signalling axis in liver cancer.
Heliyon. 10:e322882024. View Article : Google Scholar
|
|
131
|
Lyu X, Wang J, Guo X, Wu G, Jiao Y, Faleti
OD, Liu P, Liu T, Long Y, Chong T, et al: EBV-miR-BART1-5P
activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to
promote glycolysis and angiogenesis in nasopharyngeal carcinoma.
PLoS Pathog. 14:e10074842018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Barisciano G, Colangelo T, Rosato V,
Muccillo L, Taddei ML, Ippolito L, Chiarugi P, Galgani M,
Bruzzaniti S, Matarese G, et al: miR-27a is a master regulator of
metabolic reprogramming and chemoresistance in colorectal cancer.
Br J Cancer. 122:1354–1366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Vallée A, Lecarpentier Y and Vallée JN:
The key role of the WNT/β-catenin pathway in metabolic
reprogramming in cancers under normoxic conditions. Cancers
(Basel). 13:55572021. View Article : Google Scholar
|
|
134
|
Chen L, Hu N, Wang C and Zhao H: HOTAIRM1
knockdown enhances cytarabine-induced cytotoxicity by suppression
of glycolysis through the Wnt/β-catenin/PFKP pathway in acute
myeloid leukemia cells. Arch Biochem Biophys. 680:1082442020.
View Article : Google Scholar
|
|
135
|
Zhang Z, Tan X, Luo J, Yao H, Si Z and
Tong JS: The miR-30a-5p/CLCF1 axis regulates sorafenib resistance
and aerobic glycolysis in hepatocellular carcinoma. Cell Death Dis.
11:9022020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Yi Q, Wei J and Li Y: Effects of
miR-103a-3p targeted regulation of TRIM66 axis on docetaxel
resistance and glycolysis in prostate cancer cells. Front Genet.
12:8137932022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang Z, Wang Y, Li Z, Xue W, Hu S and Kong
X: Lipid metabolism as a target for cancer drug resistance:
Progress and prospects. Front Pharmacol. 14:12743352023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Qin J, Ye L, Wen X, Zhang X, Di Y, Chen Z
and Wang Z: Fatty acids in cancer chemoresistance. Cancer Lett.
572:2163522023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ladanyi A, Mukherjee A, Kenny HA, Johnson
A, Mitra AK, Sundaresan S, Nieman KM, Pascual G, Benitah SA, Montag
A, et al: Adipocyte-induced CD36 expression drives ovarian cancer
progression and metastasis. Oncogene. 37:2285–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Guo Y, Yang L, Guo W, Wei L and Zhou Y:
FV-429 enhances the efficacy of paclitaxel in NSCLC by
reprogramming HIF-1α-modulated FattyAcid metabolism. Chem Biol
Interact. 350:1097022021. View Article : Google Scholar
|
|
141
|
Ventura R, Mordec K, Waszczuk J, Wang Z,
Lai J, Fridlib M, Buckley D, Kemble G and Heuer TS: Inhibition of
de novo palmitate synthesis by fatty acid synthase induces
apoptosis in tumor cells by remodeling cell membranes, inhibiting
signaling pathways, and reprogramming gene expression.
EBioMedicine. 2:808–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liu S, Sun Y, Hou Y, Yang L, Wan X, Qin Y,
Liu Y, Wang R, Zhu P, Teng Y and Liu M: A novel lncRNA
ROPM-mediated lipid metabolism governs breast cancer stem cell
properties. J Hematol Oncol. 14:1782021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Varghese E, Samuel SM, Líšková A, Samec M,
Kubatka P and Büsselberg D: Targeting glucose metabolism to
overcome resistance to anticancer chemotherapy in breast cancer.
Cancers (Basel). 12:22522020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Eytan GD, Regev R, Oren G and Assaraf YG:
The role of passive transbilayer drug movement in multidrug
resistance and its modulation. J Biol Chem. 271:12897–12902. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang X, He S, Gu Y, Wang Q, Chu X, Jin M,
Xu L, Wu Q, Zhou Q, Wang B, et al: Fatty acid receptor GPR120
promotes breast cancer chemoresistance by upregulating ABC
transporters expression and fatty acid synthesis. EBioMedicine.
40:251–262. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kuan CY, Walker TH, Luo PG and Chen CF:
Long-chain polyunsaturated fatty acids promote paclitaxel
cytotoxicity via inhibition of the MDR1 gene in the human colon
cancer Caco-2 cell line. J Am Coll Nutr. 30:265–273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Royo-García A, Courtois S, Parejo-Alonso
B, Espiau-Romera P and Sancho P: Lipid droplets as metabolic
determinants for stemness and chemoresistance in cancer. World J
Stem Cells. 13:1307–1317. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Huang KB, Pan YH, Shu GN, Yao HH, Liu X,
Zhou M, Wei JH, Chen ZH, Lu J, Feng ZH, et al: Circular RNA
circSNX6 promotes sunitinib resistance in renal cell carcinoma
through the miR-1184/GPCPD1/lysophosphatidic acid axis. Cancer
Lett. 523:121–134. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang X, Xu Y, Ma L, Yu K, Niu Y, Xu X,
Shi Y, Guo S, Xue X, Wang Y, et al: Essential roles of exosome and
circRNA_101093 on ferroptosis desensitization in lung
adenocarcinoma. Cancer Commun (Lond). 42:287–313. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Nan Y, Luo Q, Wu X, Liu S, Zhao P, Chang
W, Zhou A and Liu Z: DLGAP1-AS2-mediated phosphatidic acid
synthesis activates YAP signaling and confers chemoresistance in
squamous cell carcinoma. Cancer Res. 82:2887–2903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Huang X, Taeb S, Jahangiri S, Emmenegger
U, Tran E, Bruce J, Mesci A, Korpela E, Vesprini D, Wong CS, et al:
miRNA-95 mediates radioresistance in tumors by targeting the
sphingolipid phosphatase SGPP1. Cancer Res. 73:6972–6986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kopecka J, Trouillas P, Gašparović AČ,
Gazzano E, Assaraf YG and Riganti C: Phospholipids and cholesterol:
Inducers of cancer multidrug resistance and therapeutic targets.
Drug Resist Updat. 49:1006702020. View Article : Google Scholar
|
|
153
|
Palma GBH and Kaur M: miRNA-128 and
miRNA-223 regulate cholesterol-mediated drug resistance in breast
cancer. IUBMB Life. 75:743–764. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wolfe AR, Bambhroliya A, Reddy JP, Debeb
BG, Huo L, Larson R, Li L, Ueno NT and Woodward WA: MiR-33a
decreases high-density lipoprotein-induced radiation sensitivity in
breast cancer. Int J Radiat Oncol Biol Phys. 95:791–799. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta
P, Wei L, Ashby CR Jr, Yang DH and Chen ZS: Modulating ROS to
overcome multidrug resistance in cancer. Drug Resist Updat.
41:1–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kennedy L, Sandhu JK, Harper ME and
Cuperlovic-Culf M: Role of glutathione in cancer: From mechanisms
to therapies. Biomolecules. 10:14292020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Castelli S, De Falco P, Ciccarone F,
Desideri E and Ciriolo MR: Lipid catabolism and ROS in cancer: A
bidirectional liaison. Cancers (Basel). 13:54842021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang Y, Lu JH, Wang F, Wang YN, He MM, Wu
QN, Lu YX, Yu HE, Chen ZH, Zhao Q, et al: Inhibition of fatty acid
catabolism augments the efficacy of oxaliplatin-based chemotherapy
in gastrointestinal cancers. Cancer Lett. 473:74–89. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Farge T, Saland E, de Toni F, Aroua N,
Hosseini M, Perry R, Bosc C, Sugita M, Stuani L, Fraisse M, et al:
Chemotherapy-resistant human acute myeloid leukemia cells are not
enriched for leukemic stem cells but require oxidative metabolism.
Cancer Discov. 7:716–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
De Oliveira MP and Liesa M: The role of
mitochondrial fat oxidation in cancer cell proliferation and
survival. Cells. 9:26002020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Luo J, Hong Y, Tao X, Wei X, Zhang L and
Li Q: An indispensable role of CPT-1a to survive cancer cells
during energy stress through rewiring cancer metabolism. Tumour
Biol. 37:15795–15804. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Snaebjornsson MT, Janaki-Raman S and
Schulze A: Greasing the wheels of the cancer machine: The role of
lipid metabolism in cancer. Cell Metab. 31:62–76. 2020. View Article : Google Scholar
|
|
163
|
Chan YT, Wu J, Lu Y, Li Q, Feng Z, Xu L,
Yuan H, Xing T, Zhang C, Tan HY, et al: Loss of lncRNA LINC01056
leads to sorafenib resistance in HCC. Mol Cancer. 23:742024.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wu H, Liu B, Chen Z, Li G and Zhang Z:
MSC-induced lncRNA HCP5 drove fatty acid oxidation through
miR-3619-5p/AMPK/PGC1α/CEBPB axis to promote stemness and
chemo-resistance of gastric cancer. Cell Death Dis. 11:2332020.
View Article : Google Scholar
|
|
165
|
Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W and
Wang J: Molecular mechanisms of ferroptosis and its role in cancer
therapy. J Cell Mol Med. 23:4900–4912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Dos Santos AF, Fazeli G, Xavier da Silva
TN and Friedmann Angeli JP: Ferroptosis: Mechanisms and
implications for cancer development and therapy response. Trends
Cell Biol. 33:1062–1076. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar :
|
|
168
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Qu S, Qi S, Zhang H, Li Z, Wang K, Zhu T,
Ye R, Zhang W, Huang G and Yi GZ: Albumin-bound paclitaxel augment
temozolomide treatment sensitivity of glioblastoma cells by
disrupting DNA damage repair and promoting ferroptosis. J Exp Clin
Cancer Res. 42:2852023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Lu Y, Chan YT, Tan HY, Zhang C, Guo W, Xu
Y, Sharma R, Chen ZS, Zheng YC, Wang N and Feng Y: Epigenetic
regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates
sorafenib resistance in human hepatocellular carcinoma. J Exp Clin
Cancer Res. 41:32022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Pope LE and Dixon SJ: Regulation of
ferroptosis by lipid metabolism. Trends Cell Biol. 33:1077–1087.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Zhong S, Wang Z, Yang J, Jiang D and Wang
K: Ferroptosis-related oxaliplatin resistance in multiple cancers:
Potential roles and therapeutic implications. Heliyon.
10:e376132024. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Zou Y, Zheng S, Xie X, Ye F, Hu X, Tian Z,
Yan SM, Yang L, Kong Y, Tang Y, et al: N6-methyladenosine regulated
FGFR4 attenuates ferroptotic cell death in recalcitrant
HER2-positive breast cancer. Nat Commun. 13:26722022. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Baird L and Dinkova-Kostova AT: The
cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol.
85:241–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Sun X, Niu X, Chen R, He W, Chen D, Kang R
and Tang D: Metallothionein-1G facilitates sorafenib resistance
through inhibition of ferroptosis. Hepatology. 64:488–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Zhang W, Li Q, Zhang Y, Wang Z, Yuan S,
Zhang X, Zhao M, Zhuang W and Li B: Multiple myeloma with high
expression of SLC7A11 is sensitive to erastin-induced ferroptosis.
Apoptosis. 29:412–423. 2024. View Article : Google Scholar
|
|
178
|
Xu X, Zhang X, Wei C, Zheng D, Lu X, Yang
Y, Luo A, Zhang K, Duan X and Wang Y: Targeting SLC7A11
specifically suppresses the progression of colorectal cancer stem
cells via inducing ferroptosis. Eur J Pharm Sci. 152:1054502020.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zheng Y, Li L, Chen H, Zheng Y, Tan X,
Zhang G, Jiang R, Yu H, Lin S, Wei Y, et al: Luteolin exhibits
synergistic therapeutic efficacy with erastin to induce ferroptosis
in colon cancer cells through the HIC1-mediated inhibition of GPX4
expression. Free Radic Biol Med. 208:530–544. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Yin LB, Li ZW, Wang JL, Wang L, Hou L, Hu
SY, Chen H, Luo P, Cui XB and Zhu JL: Sulfasalazine inhibits
esophageal cancer cell proliferation by mediating ferroptosis. Chem
Biol Drug Des. 102:730–737. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Yang J, Zhou Y, Xie S, Wang J, Li Z, Chen
L, Mao M, Chen C, Huang A, Chen Y, et al: Metformin induces
Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J
Exp Clin Cancer Res. 40:2062021. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Sui X, Zhang R, Liu S, Duan T, Zhai L,
Zhang M, Han X, Xiang Y, Huang X, Lin H and Xie T: RSL3 drives
ferroptosis through GPX4 inactivation and ROS production in
colorectal cancer. Front Pharmacol. 9:13712018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Gaschler MM, Andia AA, Liu H, Csuka JM,
Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E,
et al: FINO2 initiates ferroptosis through GPX4
inactivation and iron oxidation. Nat Chem Biol. 14:507–515. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Cheff DM, Huang C, Scholzen KC, Gencheva
R, Ronzetti MH, Cheng Q, Hall MD and Arnér ESJ: The ferroptosis
inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4
but of TXNRD1. Redox Biol. 62:1027032023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Wang Y, Yuan X, Ren M and Wang Z:
Ferroptosis: A new research direction of artemisinin and its
derivatives in anti-cancer treatment. Am J Chin Med. 52:161–181.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Chen GQ, Benthani FA, Wu J, Liang D, Bian
ZX and Jiang X: Artemisinin compounds sensitize cancer cells to
ferroptosis by regulating iron homeostasis. Cell Death Differ.
27:242–254. 2020. View Article : Google Scholar :
|
|
187
|
Li J, Li Y, Wang D, Liao R and Wu Z: PLAG1
interacts with GPX4 to conquer vulnerability to sorafenib induced
ferroptosis through a PVT1/miR-195-5p axis-dependent manner in
hepatocellular carcinoma. J Exp Clin Cancer Res. 43:1432024.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Nalla LV and Khairnar A: Empagliflozin
drives ferroptosis in anoikis-resistant cells by activating
miR-128-3p dependent pathway and inhibiting CD98hc in breast
cancer. Free Radic Biol Med. 220:288–300. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Li X, Li Y, Lian P, Lv Q and Liu F:
Silencing lncRNA HCG18 regulates GPX4-inhibited ferroptosis by
adsorbing miR-450b-5p to avert sorafenib resistance in
hepatocellular carcinoma. Hum Exp Toxicol. 42:96032712211428182023.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhang H, Deng T, Liu R, Ning T, Yang H,
Liu D, Zhang Q, Lin D, Ge S, Bai M, et al: CAF secreted miR-522
suppresses ferroptosis and promotes acquired chemo-resistance in
gastric cancer. Mol Cancer. 19:432020. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhao J, Shen J, Mao L, Yang T, Liu J and
Hongbin S: Cancer associated fibroblast secreted miR-432-5p targets
CHAC1 to inhibit ferroptosis and promote acquired chemoresistance
in prostate cancer. Oncogene. 43:2104–2114. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Zong WX, Rabinowitz JD and White E:
Mitochondria and cancer. Mol Cell. 61:667–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Li X, Zhong Y, Lu J, Axcrona K, Eide L,
Syljuåsen RG, Peng Q, Wang J, Zhang H, Goscinski MA, et al: MtDNA
depleted PC3 cells exhibit Warburg effect and cancer stem cell
features. Oncotarget. 7:40297–40313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Gonzalez-Sanchez E, Marin JJ and Perez MJ:
The expression of genes involved in hepatocellular carcinoma
chemoresistance is affected by mitochondrial genome depletion. Mol
Pharm. 11:1856–1868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Lee W, Choi HI, Kim MJ and Park SY:
Depletion of mitochondrial DNA up-regulates the expression of MDR1
gene via an increase in mRNA stability. Exp Mol Med. 40:109–117.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Guerra F, Arbini AA and Moro L:
Mitochondria and cancer chemoresistance. Biochim Biophys Acta
Bioenerg. 1858:686–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Yang H, Villani RM, Wang H, Simpson MJ,
Roberts MS, Tang M and Liang X: The role of cellular reactive
oxygen species in cancer chemotherapy. J Exp Clin Cancer Res.
37:2662018. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Calcium and mitochondria in the regulation of cell death. Biochem
Biophys Res Commun. 460:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Seong JB, Kim B, Kim S, Kim MH, Park YH,
Lee Y, Lee HJ, Hong CW and Lee DS: Macrophage peroxiredoxin 5
deficiency promotes lung cancer progression via ROS-dependent
M2-like polarization. Free Radic Biol Med. 176:322–334. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE,
Bok S, Oh JM, Gwak SH, Yoo MY, Lee MS, et al: Tumor-associated
macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer
Res. 79:795–806. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Shi L, Zhang S, Feng K, Wu F, Wan Y, Wang
Z, Zhang J, Wang Y, Yan W, Fu Z and You Y: MicroRNA-125b-2 confers
human glioblastoma stem cells resistance to temozolomide through
the mitochondrial pathway of apoptosis. Int J Oncol. 40:119–129.
2012.
|
|
202
|
Kuo CL, Ponneri Babuharisankar A, Lin YC,
Lien HW, Lo YK, Chou HY, Tangeda V, Cheng LC, Cheng AN and Lee AY:
Mitochondrial oxidative stress in the tumor microenvironment and
cancer immunoescape: Foe or friend? J Biomed Sci. 29:742022.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Liu JS, Yeh CA, Huang IC, Huang GY, Chiu
CH, Mahalakshmi B, Wen SY, Huang CY and Kuo WW: Signal transducer
and activator of transcription 3 mediates apoptosis inhibition
through reducing mitochondrial ROS and activating Bcl-2 in
gemcitabine-resistant lung cancer A549 cells. J Cell Physiol.
236:3896–3905. 2021. View Article : Google Scholar
|
|
204
|
Katopodi V, Marino A, Pateraki N,
Verheyden Y, Cinque S, Jimenez EL, Adnane S, Demesmaeker E,
Scomparin A, Derua R, et al: The long non-coding RNA ROSALIND
protects the mitochondrial translational machinery from oxidative
damage. Cell Death Differ. 32:397–415. 2025. View Article : Google Scholar
|
|
205
|
Fernández-Tussy P, Rodríguez-Agudo R,
Fernández-Ramos D, Barbier-Torres L, Zubiete-Franco I, Davalillo
SL, Herraez E, Goikoetxea-Usandizaga N, Lachiondo-Ortega S, Simón
J, et al: Anti-miR-518d-5p overcomes liver tumor cell death
resistance through mitochondrial activity. Cell Death Dis.
12:5552021. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Fan S, Tian T, Chen W, Lv X, Lei X, Zhang
H, Sun S, Cai L, Pan G, He L, et al: Mitochondrial miRNA determines
chemoresistance by reprogramming metabolism and regulating
mitochondrial transcription. Cancer Res. 79:1069–1084. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Orre C, Dieu X, Guillon J, Gueguen N,
Ahmadpour ST, Dumas JF, Khiati S, Reynier P, Lenaers G, Coqueret O,
et al: The long non-coding RNA SAMMSON is a regulator of
chemosensitivity and metabolic orientation in MCF-7
doxorubicin-resistant breast cancer cells. Biology (Basel).
10:11562021.PubMed/NCBI
|
|
208
|
Hillman Y, Mardamshina M, Pasmanik-Chor M,
Ziporen L, Geiger T, Shomron N and Fishelson Z: MicroRNAs affect
complement regulator expression and mitochondrial activity to
modulate cell resistance to complement-dependent cytotoxicity.
Cancer Immunol Res. 7:1970–1983. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Luan T, Fu S, Huang L, Zuo Y, Ding M, Li
N, Chen J, Wang H and Wang J: MicroRNA-98 promotes drug resistance
and regulates mitochondrial dynamics by targeting LASS2 in bladder
cancer cells. Exp Cell Res. 373:188–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Chen W, Wang P, Lu Y, Jin T, Lei X, Liu M,
Zhuang P, Liao J, Lin Z, Li B, et al: Decreased expression of
mitochondrial miR-5787 contributes to chemoresistance by
reprogramming glucose metabolism and inhibiting MT-CO3 translation.
Theranostics. 9:5739–5754. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Benassi B, Marani M, Loda M and Blandino
G: USP2a alters chemotherapeutic response by modulating redox. Cell
Death Dis. 4:e8122013. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Zheng ZG, Xu H, Suo SS, Xu XL, Ni MW, Gu
LH, Chen W, Wang LY, Zhao Y, Tian B and Hua YJ: The essential role
of H19 contributing to cisplatin resistance by regulating
glutathione metabolism in high-grade serous ovarian cancer. Sci
Rep. 6:260932016. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Ueda S, Takanashi M, Sudo K, Kanekura K
and Kuroda M: miR-27a ameliorates chemoresistance of breast cancer
cells by disruption of reactive oxygen species homeostasis and
impairment of autophagy. Lab Invest. 100:863–873. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Yang Q, Li K, Huang X, Zhao C, Mei Y, Li
X, Jiao L and Yang H: lncRNA SLC7A11-AS1 promotes chemoresistance
by blocking SCFβ−TRCP-mediated degradation of NRF2 in
pancreatic cancer. Mol Ther Nucleic Acids. 19:974–985. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Kaur R, Kanthaje S, Taneja S, Dhiman RK
and Chakraborti A: miR-23b-3p modulating cytoprotective autophagy
and glutamine addiction in sorafenib resistant HepG2, a
hepatocellular carcinoma cell line. Genes (Basel). 13:13752022.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Liu X, Chen L and Wang T: Overcoming
cisplatin resistance of human lung cancer by sinomenine through
targeting the miR-200a-3p-GLS axis. J Chemother. 35:357–366. 2023.
View Article : Google Scholar
|
|
217
|
Zhou X, Wei P, Wang X, Zhang J and Shi Y:
miR-141-3p promotes the cisplatin sensitivity of osteosarcoma cell
through targeting the glutaminase [GLS]-mediated glutamine
metabolism. Curr Mol Med. 23:177–184. 2023. View Article : Google Scholar
|
|
218
|
Chang X, Zhu W, Zhang H and Lian S:
Sensitization of melanoma cells to temozolomide by overexpression
of microRNA 203 through direct targeting of glutaminase-mediated
glutamine metabolism. Clin Exp Dermatol. 42:614–621. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Chen Z, Wang Q, Huang L, Xu G and Hu J:
LncRNA PVT1 confers cisplatin resistance of esophageal cancer cells
through Modulating the miR-181a-5p-Glutaminase (GLS) axis. Nutr
Cancer. 75:1646–1657. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Lin W, Wu WC, Liang Z, Zhang JH and Fang
SP: LncRNA FEZF1-AS1 facilitates cisplatin resistance in non-small
cell lung cancer through modulating the miR-32-5p-glutaminase axis.
Am J Cancer Res. 14:3153–3170. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
De Los Santos-Jiménez J, Campos-Sandoval
JA, Alonso FJ, Márquez J and Matés JM: GLS and GLS2 glutaminase
isoenzymes in the antioxidant system of cancer cells. Antioxidants
(Basel). 13:7452024. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Li Y, Yuan S, Zhou Y, Zhou J, Zhang X,
Zhang P, Xiao W, Zhang Y, Deng J and Lou S: Long non-coding RNA
PXN-AS1 promotes glutamine synthetase-mediated chronic myeloid
leukemia BCR::ABL1-independent resistance to Imatinib via cell
cycle signaling pathway. Cancer Cell Int. 24:1862024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Montani F and Bianchi F: Circulating
cancer biomarkers: The macro-revolution of the micro-RNA.
EBioMedicine. 5:4–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Sarfi M, Abbastabar M and Khalili E: Long
noncoding RNAs biomarker-based cancer assessment. J Cell Physiol.
234:16971–16986. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Liu R, Zeng Y, Zhou CF, Wang Y, Li X, Liu
ZQ, Chen XP, Zhang W and Zhou HH: Long noncoding RNA expression
signature to predict platinum-based chemotherapeutic sensitivity of
ovarian cancer patients. Sci Rep. 7:182017. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Wang Q, Zheng C, Hou H, Bao X, Tai H,
Huang X, Li Z, Li Z, Wang Q, Pan Q, et al: Interplay of
sphingolipid metabolism in predicting prognosis of GBM patients:
Towards precision immunotherapy. J Cancer. 15:275–292. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Bacci M, Giannoni E, Fearns A, Ribas R,
Gao Q, Taddei ML, Pintus G, Dowsett M, Isacke CM, Martin LA, et al:
miR-155 drives metabolic reprogramming of ER+ breast cancer cells
following long-term estrogen deprivation and predicts clinical
response to aromatase inhibitors. Cancer Res. 76:1615–1626. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Lei H, Xiang T, Zhu H and Hu X: A novel
cholesterol metabolism-related lncRNA signature predicts the
prognosis of patients with hepatocellular carcinoma and their
response to immunotherapy. Front Biosci (Landmark Ed). 29:1292024.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
He X, Xu Z, Ren R, Wan P, Zhang Y, Wang L
and Han Y: A novel sphingolipid metabolism-related long noncoding
RNA signature predicts the prognosis, immune landscape and
therapeutic response in pancreatic adenocarcinoma. Heliyon.
10:e236592023. View Article : Google Scholar
|
|
230
|
Wang X, Yang X, Zhang Y, Guo A, Luo S,
Xiao M, Xue L, Zhang G and Wang H: Fatty acid metabolism-related
lncRNAs are potential biomarkers for predicting prognoses and
immune responses in patients with skin cutaneous melanoma. Clin
Cosmet Investig Dermatol. 16:3595–3614. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Bayarmaa B, Wu Z, Peng J, Wang Y, Xu S,
Yan T, Yin W, Lu J and Zhou L: Association of LncRNA MEG3
polymorphisms with efficacy of neoadjuvant chemotherapy in breast
cancer. BMC Cancer. 19:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Xu W, Hua Y, Deng F, Wang D, Wu Y, Zhang W
and Tang J: MiR-145 in cancer therapy resistance and sensitivity: A
comprehensive review. Cancer Sci. 111:3122–3131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Hedayat S, Cascione L, Cunningham D,
Schirripa M, Lampis A, Hahne JC, Tunariu N, Hong SP, Marchetti S,
Khan K, et al: Circulating microRNA analysis in a prospective
co-clinical trial identifies MIR652-3p as a response biomarker and
driver of regorafenib resistance mechanisms in colorectal cancer.
Clin Cancer Res. 30:2140–2159. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Gonçalves AC, Richiardone E, Jorge J,
Polónia B, Xavier CPR, Salaroglio IC, Riganti C, Vasconcelos MH,
Corbet C and Sarmento-Ribeiro AB: Impact of cancer metabolism on
therapy resistance-clinical implications. Drug Resist Updat.
59:1007972021. View Article : Google Scholar
|
|
235
|
Abdel-Wahab AF, Mahmoud W and Al-Harizy
RM: Targeting glucose metabolism to suppress cancer progression:
Prospective of anti-glycolytic cancer therapy. Pharmacol Res.
150:1045112019. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Sun Z, Zhang Q, Yuan W, Li X, Chen C, Guo
Y, Shao B, Dang Q, Zhou Q, Wang Q, et al: MiR-103a-3p promotes
tumour glycolysis in colorectal cancer via hippo/YAP1/HIF1A axis. J
Exp Clin Cancer Res. 39:2502020. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Fujiwara N, Inoue J, Kawano T, Tanimoto K,
Kozaki K and Inazawa J: miR-634 activates the mitochondrial
apoptosis pathway and enhances chemotherapy-induced cytotoxicity.
Cancer Res. 75:3890–3901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Chen B, Dragomir MP, Yang C, Li Q, Horst D
and Calin GA: Targeting non-coding RNAs to overcome cancer therapy
resistance. Signal Transduct Target Ther. 7:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Kara G, Calin GA and Ozpolat B: RNAi-based
therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv
Rev. 182:1141132022. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Yadav DN, Ali MS, Thanekar AM, Pogu SV and
Rengan AK: Recent advancements in the design of nanodelivery
systems of siRNA for cancer therapy. Mol Pharm. 19:4506–4526. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Garbo S, Maione R, Tripodi M and
Battistelli C: Next RNA therapeutics: The mine of non-coding. Int J
Mol Sci. 23:74712022. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Lin F, Wen D, Wang X and Mahato RI: Dual
responsive micelles capable of modulating miRNA-34a to combat
taxane resistance in prostate cancer. Biomaterials. 192:95–108.
2019. View Article : Google Scholar
|
|
244
|
Xin X, Kumar V, Lin F, Kumar V, Bhattarai
R, Bhatt VR, Tan C and Mahato RI: Redox-responsive nanoplatform for
codelivery of miR-519c and gemcitabine for pancreatic cancer
therapy. Sci Adv. 6:eabd67642020. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Guo W, Wu Z, Chen J, Guo S, You W, Wang S,
Ma J, Wang H, Wang X, Wang H, et al: Nanoparticle delivery of
miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by
promoting ferroptosis. J Immunother Cancer. 10:e0043812022.
View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Xu F, Ye ML, Zhang YP, Li WJ, Li MT, Wang
HZ, Qiu X, Xu Y, Yin JW, Hu Q, et al: MicroRNA-375-3p enhances
chemosensitivity to 5-fluorouracil by targeting thymidylate
synthase in colorectal cancer. Cancer Sci. 111:1528–1541. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Lo YL, Wang CS, Chen YC, Wang TY, Chang
YH, Chen CJ and Yang CP: Mitochondrion-directed nanoparticles
loaded with a natural compound and a microRNA for promoting cancer
cell death via the modulation of tumor metabolism and mitochondrial
dynamics. Pharmaceutics. 12:7562020. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Yi WR, Tu MJ, Yu AX, Lin J and Yu AM:
Bioengineered miR-34a modulates mitochondrial inner membrane
protein 17 like 2 (MPV17L2) expression toward the control of cancer
cell mitochondrial functions. Bioengineered. 13:12489–12503. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Yi W, Tu MJ, Liu Z, Zhang C, Batra N, Yu
AX and Yu AM: Bioengineered miR-328-3p modulates GLUT1-mediated
glucose uptake and metabolism to exert synergistic
antiproliferative effects with chemotherapeutics. Acta Pharm Sin B.
10:159–170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Vahabi M, Comandatore A, Franczak MA,
Smolenski RT, Peters GJ, Morelli L and Giovannetti E: Role of
exosomes in transferring chemoresistance through modulation of
cancer glycolytic cell metabolism. Cytokine Growth Factor Rev.
73:163–172. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si
K, Sun B, Chen B and Xiao Z: Engineered exosomes for targeted
co-delivery of miR-21 inhibitor and chemotherapeutics to reverse
drug resistance in colon cancer. J Nanobiotechnology. 18:102020.
View Article : Google Scholar : PubMed/NCBI
|
|
252
|
El Moukhtari SH, Garbayo E, Amundarain A,
Pascual-Gil S, Carrasco-León A, Prosper F, Agirre X and
Blanco-Prieto MJ: Lipid nanoparticles for siRNA delivery in cancer
treatment. J Control Release. 361:130–146. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Tassone P, Di Martino MT, Arbitrio M,
Fiorillo L, Staropoli N, Ciliberto D, Cordua A, Scionti F, Bertucci
B, Salvino A, et al: Safety and activity of the first-in-class
locked nucleic acid (LNA) miR-221 selective inhibitor in refractory
advanced cancer patients: a first-in-human, phase 1, open-label,
dose-escalation study. J Hematol Oncol. 16:682023. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Yang S, Wang X, Zhou X, Hou L, Wu J, Zhang
W, Li H, Gao C and Sun C: ncRNA-mediated ceRNA regulatory network:
Transcriptomic insights into breast cancer progression and
treatment strategies. Biomed Pharmacother. 162:1146982023.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Yu J, Qi J, Sun X, Wang W, Wei G, Wu Y,
Gao Q and Zheng J: MicroRNA-181a promotes cell proliferation and
inhibits apoptosis in gastric cancer by targeting RASSF1A. Oncol
Rep. 40:1959–1970. 2018.PubMed/NCBI
|
|
256
|
Zhang LX, Gao J, Long X, Zhang PF, Yang X,
Zhu SQ, Pei X, Qiu BQ, Chen SW, Lu F, et al: The circular RNA
circHMGB2 drives immunosuppression and anti-PD-1 resistance in lung
adenocarcinomas and squamous cell carcinomas via the
miR-181a-5p/CARM1 axis. Mol Cancer. 21:1102022. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Lehuédé C, Dupuy F, Rabinovitch R, Jones
RG and Siegel PM: Metabolic plasticity as a determinant of tumor
growth and metastasis. Cancer Res. 76:5201–5208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Hu X, Li J, Fu M, Zhao X and Wang W: The
JAK/STAT signaling pathway: From bench to clinic. Signal Transduct
Target Ther. 6:4022021. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Xiao C, Zhang W, Hua M, Chen H, Yang B,
Wang Y and Yang Q: RNF7 inhibits apoptosis and sunitinib
sensitivity and promotes glycolysis in renal cell carcinoma via the
SOCS1/JAK/STAT3 feedback loop. Cell Mol Biol Lett. 27:362022.
View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Wang T, Fahrmann JF, Lee H, Li YJ,
Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, et al:
JAK/STAT3-regulated fatty acid β-oxidation is critical for breast
cancer stem cell self-renewal and chemoresistance. Cell Metab.
27:136–150.e5. 2018. View Article : Google Scholar
|
|
261
|
Yin YZ, Zheng WH, Zhang X, Chen YH and Tuo
YH: LINC00346 promotes hepatocellular carcinoma progression via
activating the JAK-STAT3 signaling pathway. J Cell Biochem.
121:735–742. 2020. View Article : Google Scholar
|
|
262
|
Hu H, Zhang Q, Chen W, Wu T, Liu S, Li X,
Luo B, Zhang T, Yan G, Lu H and Lu Z: MicroRNA-301a promotes
pancreatic cancer invasion and metastasis through the JAK/STAT3
signaling pathway by targeting SOCS5. Carcinogenesis. 41:502–514.
2020. View Article : Google Scholar
|
|
263
|
Sulli G, Lam MTY and Panda S: Interplay
between circadian clock and cancer: New frontiers for cancer
treatment. Trends Cancer. 5:475–494. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Varadharaj V, Petersen W, Batra SK and
Ponnusamy MP: Sugar symphony: Glycosylation in cancer metabolism
and stemness. Trends Cell Biol. Oct 26–2024.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Yang Y, Yang T, Zhao Z, Zhang H, Yuan P,
Wang G, Zhao Z, An J, Lyu Z, Xing J and Li J: Down-regulation of
BMAL1 by MiR-494-3p promotes hepatocellular carcinoma growth and
metastasis by increasing GPAM-mediated lipid biosynthesis. Int J
Biol Sci. 18:6129–6144. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Zheng LT, Chen SR, Zhou LY, Huang QY, Chen
JM, Chen WH, Lin S and Shi QY: Latest advances in the study of
non-coding RNA-mediated circadian rhythm disorders causing
endometrial cancer. Front Oncol. 13:12775432023. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Alsayed RKME, Sheikhan KSAM, Alam MA,
Buddenkotte J, Steinhoff M, Uddin S and Ahmad A: Epigenetic
programing of cancer stemness by transcription factors-non-coding
RNAs interactions. Semin Cancer Biol. 92:74–83. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Jiang W, Zhao S, Shen J, Guo L, Sun Y, Zhu
Y, Ma Z, Zhang X, Hu Y, Xiao W, et al: The MiR-135b-BMAL1-YY1 loop
disturbs pancreatic clockwork to promote tumourigenesis and
chemoresistance. Cell Death Dis. 9:1492018. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Flores-Huerta N, Silva-Cázares MB,
Arriaga-Pizano LA, Prieto-Chávez JL and López-Camarillo C: LncRNAs
and microRNAs as essential regulators of stemness in breast cancer
stem cells. Biomolecules. 11:3802021. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Jiao X, Qian X, Wu L, Li B, Wang Y, Kong X
and Xiong L: microRNA: The impact on cancer stemness and
therapeutic resistance. Cells. 9:82019. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Hong DS, Kang YK, Borad M, Sachdev J,
Ejadi S, Lim HY, Brenner AJ, Park K, Lee JL, Kim TY, et al: Phase 1
study of MRX34, a liposomal miR-34a mimic, in patients with
advanced solid tumours. Br J Cancer. 122:1630–1637. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Patil S, Gao YG, Lin X, Li Y, Dang K, Tian
Y, Zhang WJ, Jiang SF, Qadir A and Qian AR: The development of
functional non-viral vectors for gene delivery. Int J Mol Sci.
20:54912019. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Oggu GS, Sasikumar S, Reddy N, Ella KKR,
Rao CM and Bokara KK: Gene delivery approaches for mesenchymal stem
cell therapy: Strategies to increase efficiency and specificity.
Stem Cell Rev Rep. 13:725–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Yahya EB and Alqadhi AM: Recent trends in
cancer therapy: A review on the current state of gene delivery.
Life Sci. 269:1190872021. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Aleksakhina SN, Kashyap A and Imyanitov
EN: Mechanisms of acquired tumor drug resistance. Biochim Biophys
Acta Rev Cancer. 1872:1883102019. View Article : Google Scholar : PubMed/NCBI
|