Breast cancer (BC) had 2.3 million new cases and
670,000 deaths recorded in 2022 (1). Moreover, projections indicate that
by 2050, new cases will increase by 38%, and deaths will increase
by 68% (1). It is characterized
by high heterogeneity, strong tendency for metastasis and
resistance to treatment (2,3).
The numerous types of cell that constitute the tumor
microenvironment (TME) are not static bystanders within the tumor
but are key drivers of disease progression. These cells secrete and
express key regulatory factors, such as vascular endothelial growth
factor A (VEGFA), transforming growth factor β (TGF-β), matrix
metallopeptidase-9 (MMP-9), C-C motif chemokine ligand-22 and
interleukin-10 (IL-10), which modulate the TME composition, thereby
promoting the invasive progression of tumors, malignancy and
metastasis (4). As the TME
contains specific immune and stromal cell populations with notable
prognostic value, its key role in tumor development is gradually
gaining widespread recognition, making it an emerging field of
research with therapeutic potential (5-7).
As a highly conserved signaling pathway, the Wnt
signaling network serves a key role in core physiological
processes, such as cell proliferation, differentiation, apoptosis,
migration, invasion and maintenance of cellular homeostasis
(8-10). Increasing evidence has revealed
that the abnormal regulation of the Wnt signaling pathway is
related to the occurrence and progression of BC (11,12). In more than half of BC cases, the
tumor cell nucleus has abnormally high β-catenin expression
(13). β-catenin, as a crucial
activation 'hub' in the Wnt signaling pathway and a key
transcriptional driving force in the epithelial-mesenchymal
transition (EMT) process, is associated with the histological
grade, clinical staging and lymph node metastasis and Ki-67
proliferation status of patients with BC, providing a novel
perspective for understanding the pathogenesis and prognostic
mechanisms of BC (14,15). Wnt signaling and the BC TME
exhibit a complex and multifactorial interactive dynamic
bidirectional association. Components of the TME affect the Wnt
signaling pathway within tumor cells, and abnormalities in Wnt
signaling in these cells drive changes in TME components (16-18). For example, abnormal activation of
Wnt signaling can help tumor cells in the TME evade immune system
surveillance, effectively hindering the infiltration process of T
cells, thereby mediating immune tolerance. Furthermore, in the BC
TME, the Wnt signaling pathway is not isolated but exhibits
crosstalk with other key signaling pathways (19). These signaling pathways are
coordinated, jointly regulating the dissemination and metastasis of
tumors, as well as resistance to traditional treatment methods,
revealing the complexity and diversity of BC pathogenesis (20-22).
The present review delves into the complex and
intricate mechanisms of the interaction between the Wnt signaling
pathway and TME in BC, including the functional roles of key
components and their secreted factors in the TME, unique properties
of physical influencing factors, associations with BC-related genes
and intrinsic mechanisms of interactions between cell signaling
pathways. The present review further explores the therapeutic
potential of modulating the Wnt signaling pathway within the BC TME
and its clinical implications, thereby providing new insight for BC
treatment strategies.
Under physiological conditions, the Wnt signaling
system acts as a core regulator of cellular functions through the
canonical Wnt/β-catenin signaling, non-canonical Wnt/planar cell
polarity (PCP) and Wnt-Ca2+ signaling pathways to ensure
the balance of organismal growth, development and homeostasis.
However, when key components of this network undergo mutation,
epigenetic modification or crosstalk with other signaling networks,
they trigger abnormal activation of the Wnt signaling cascade and
its downstream genes (23).
Abnormal activation of Wnt signaling is associated with accelerated
tumor proliferation, extended survival time, enhanced invasiveness
and the maintenance of cancer stem cell (SC) characteristics
(24,25). The canonical Wnt pathway promotes
proliferation by upregulating cyclin D1, enhances anti-apoptotic
capacity through B cell lymphoma-extra large and maintains cancer
SC properties. The non-canonical pathway regulates disruption of
cell polarity and metastasis via ras-related C3 botulinum toxin
substrate guanosine triphosphatases and c-Jun N-terminal kinase
(JNK) signaling (26).
Simultaneously, Wnt signaling combats ferroptosis by activating
glutathione peroxidase 4, while mediating tissue pyroptosis and EMT
via the NOD-like receptor protein 3 inflammasome (27-29). Numerous studies have explored the
diverse mechanisms of Wnt signaling activation in the occurrence
and development of cancer (23,30-32) (Fig.
1).
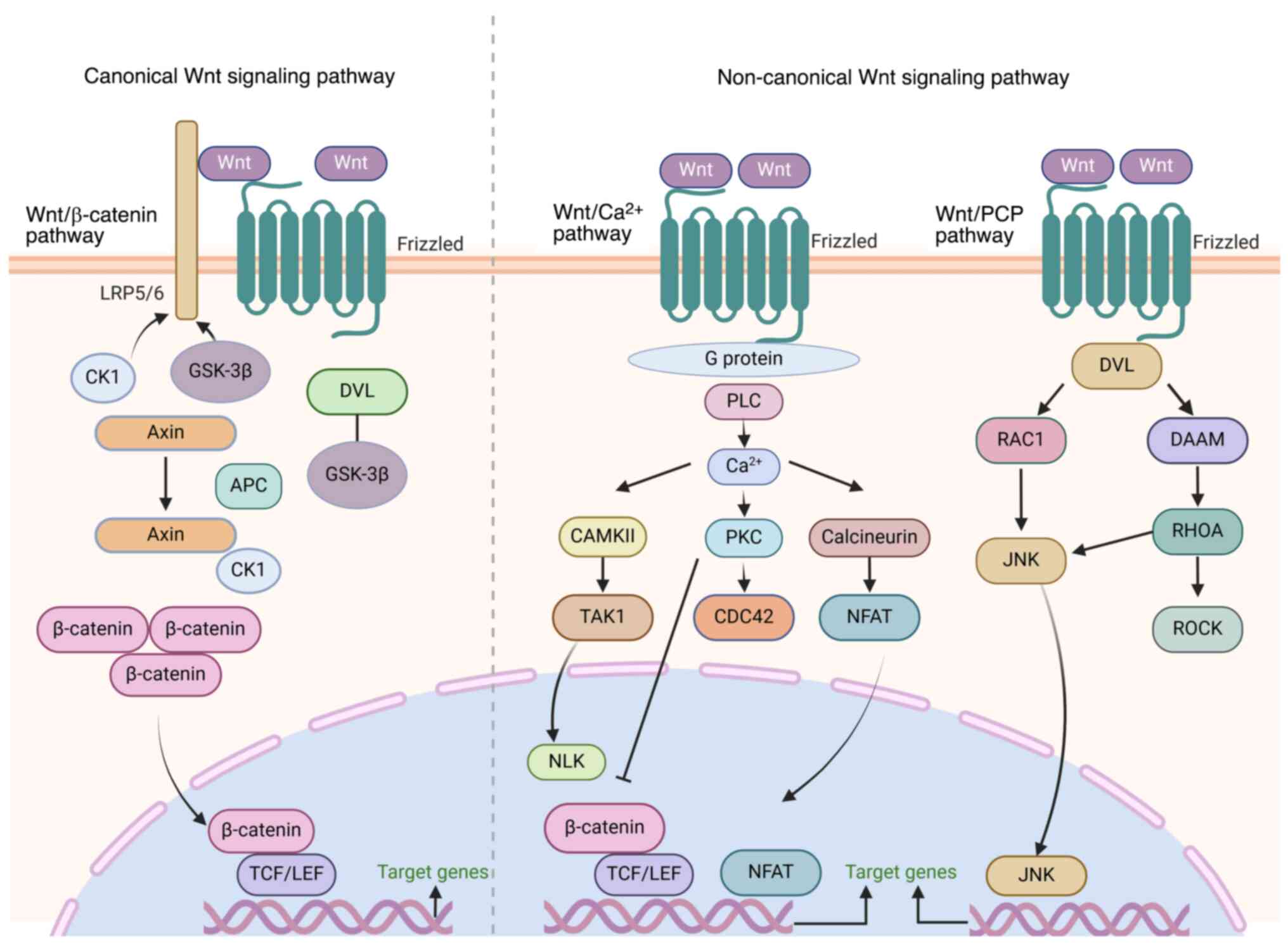
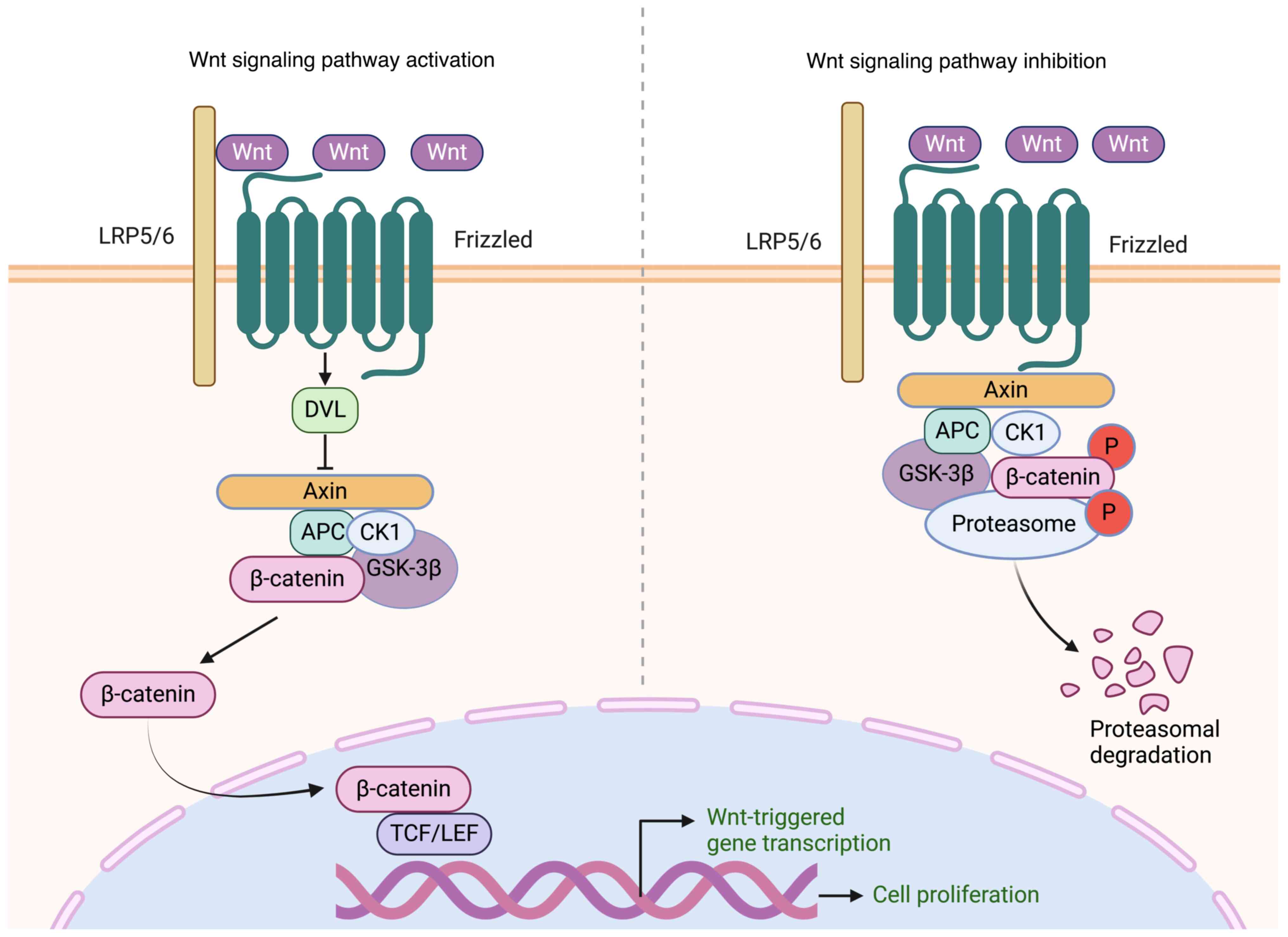
The canonical Wnt/β-catenin pathway is activated by
a cascade of tightly controlled steps. Initially, the Wnt ligand
forms a complex with the frizzled receptor and low-density
lipoprotein receptor-related protein 5 and 6 (LRP5/6) co-receptors,
triggering signaling. This interaction activates dishevelled (DVL),
a membrane-associated protein, which subsequently obstructs the
β-catenin degradation complex, composed of axis inhibition protein
(Axin), glycogen synthase kinase-3β (GSK-3β) and adenomatous
polyposis coli, preventing the breakdown of β-catenin.
Consequently, β-catenin accumulates in the cytoplasm before being
translocated to the nucleus. Upon binding to T cell factor/lymphoid
enhancer-binding factor (TCF/LEF) transcription factors, it induces
the activation of downstream genes. Through the activation of
downstream genes, the Wnt/β-catenin signaling pathway serves a
critical role in regulating cellular functions, such as
proliferation, differentiation, migration and apoptosis (33). Within this pathway, a negative
feedback mechanism maintains signaling equilibrium, preventing both
excessive and insufficient activation, thus decreasing the risk of
pathological conditions, such as cancer (Fig. 2) (34).
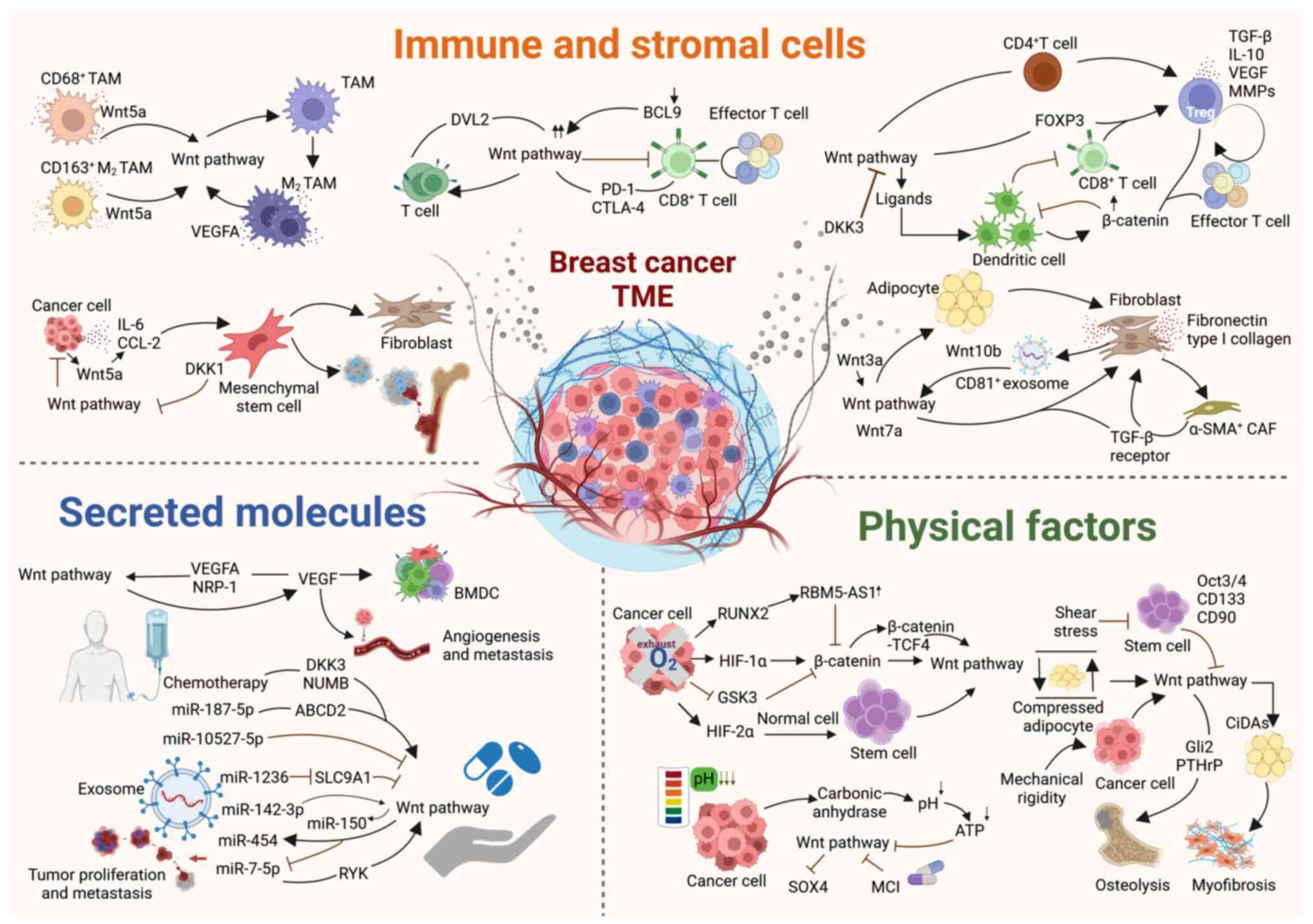
BC progression is a multifactorial process in which
the TME serves a key role. The BC TME is a diverse cellular
ecosystem that includes malignant cells, immune cell populations
and stromal tissue. These components and the factors they release
interact with the Wnt signaling pathway, collectively driving tumor
proliferation, invasion and metastasis, which are a series of
malignant events (17,37). Additionally, physical factors,
such as mechanical stress, hypoxia and acidification in tumor
regions regulate the Wnt signaling pathway and affect the
occurrence and trajectory of BC development. Analysis of the key
elements within the TME that regulate Wnt signaling provides a
theoretical foundation for uncovering the molecular mechanisms of
BC pathogenesis and developing targeted therapeutic drugs (Fig. 3).
TAMs serve a central role in innate and adaptive
immune responses because of their functional diversity and
adaptability. In the complex pathological mechanisms of BC, a
bidirectional interaction exists between Wnt signaling and TAMs,
particularly M2-type TAMs. Wnt signaling drives the polarization of
TAMs toward the M2 phenotype, which promotes the formation of an
immunosuppressive TME, thereby supporting the growth, invasion and
angiogenesis of BC and accelerating the survival and spread of
tumor cells. Inhibiting the Wnt signaling pathway through the use
of β-catenin inhibitors is an effective avenue to block M2
polarization, decreasing the expression levels of M2 markers and
thereby reversing the tumor-promoting effects of TAMs (38,39). Additionally, M2-type TAMs serve
another key role by secreting VEGFA, which activates the
neuropilin-1 (NRP-1)/GTPase-activating protein and VPS9
domain-containing protein 1/Wnt/β-catenin signaling cascade in
triple-negative BC (TNBC) cells, enhancing the self-renewal ability
and metastatic potential of tumor cells (17). Moreover, TAMs serve a critical
role in regulating the activation state of the Wnt signaling
pathway. CD68+ and M2-polarized CD163+ TAMs
release Wnt5a, a signaling molecule that triggers Wnt signaling
activity in malignant and stromal cells, forming a cycle that
continuously drives tumor progression (40-43). Due to the potential of Wnt
signaling to regulate the M2 polarization of TAMs and the
activation of Wnt signaling pathways by factors secreted by M2-type
TAMs, the Wnt-TAM axis may be a therapeutic target in BC.
T lymphocytes are key elements of adaptive immune
responses, and their dysfunction is associated with tumor
progression and the formation of immune escape mechanisms (44,45). Research has revealed the impact of
the Wnt/β-catenin signaling pathway on T cell function, which
reshapes the immune response potential of T cells by regulating the
expression of immune checkpoints, cytokine synthesis and T cell
differentiation status (46).
Specifically, the activation of the Wnt/β-catenin signaling pathway
upregulates immune checkpoint markers on the surface of
CD8+ T cells, such as programmed death receptor-1 and
cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (47-49). This upregulation pushes T cells
toward exhaustion and promotes the formation of an
immunosuppressive microenvironment, facilitating tumor progression
(50). In the immune regulatory
network of BC, DVL2 is a key hub of the Wnt signaling cascade that
controls the expression pattern of Wnt target genes in
HER2+ BC cells by regulating the transcriptional
activity of genes related to immune function and T cell survival
(51). Moreover, the loss of B
cell lymphoma 9, a key transcriptional coactivator of β-catenin,
can effectively weaken abnormal Wnt/β-catenin signaling, thereby
enhancing T cell-mediated tumor immunogenicity, offering potential
for BC immunotherapy (52). In
CD8+ T cells, Wnt signaling inhibits the differentiation
of these cells into effector T cells (TEFFs). This mechanism not
only maintains the self-renewal potential of CD8+ T
cells, but also affects the differentiation fate of T cell
progenitors, contributing to the diversity and complexity of T cell
function (53). Future research
should investigate the interaction network between different T cell
subtypes, their secreted cytokines and Wnt signaling components to
provide more precise strategies for BC immunotherapy.
As regulators of immune homeostasis, Tregs play key
roles in regulating immune response and preventing excessive
autoimmunity (54). Previous
studies have analyzed the mechanisms of the Wnt signaling pathway
and related molecules, including Wnt ligands, forkhead box P3
(FoxP3), poly(C)-binding protein 1 (PCBP1), and dickkopf-related
protein 3 (DKK3) in the TME of Tregs (55-58). Specifically, Wnt ligands activate
β-catenin signaling in dendritic cells, driving the balance between
TEFF and Tregs (55). However,
when β-catenin signaling is overly active, this balance is
disrupted, leading to impaired dendritic cell recruitment,
inhibition of CD8+ T cells, and proliferation of Tregs,
which suppress the antitumor immune response (16,56). By inducing FoxP3 expression, the
Wnt/β-catenin signaling pathway promotes the activation and
differentiation of Tregs, triggering the release of
immunosuppressive factors, such as TGF-β and IL-10. This
facilitates tumor growth and immune evasion (56,59,60). In BC, the role of PCBP1 in
modulating Wnt signaling is key for stimulating adaptive antitumor
immune responses, and its decreased expression is associated with
the imbalance of immune cell populations within tumors, which
promotes the expansion of Tregs and subsequently inhibits the
activation of antitumor immune responses (57). Additionally, DKK3 secreted by
tumors may promote the reprogramming of CD4+ cells into
Tregs by blocking the Wnt signaling pathway, thereby supporting the
survival and spread of tumor cells (56,58). Thus, the association between
Wnt/β-catenin signaling and Tregs in the BC TME may provide a
theoretical foundation for developing targeted treatment strategies
aimed at enhancing antitumor immune responses and improving
clinical outcomes.
CAFs, a highly plastic and heterogeneous stromal
cell population in the BC TME, not only maintain the structural
framework and mechanical stability of tissue but also regulate
biochemical signal transduction processes (61,62). In response to Wnt/β-catenin
signaling, CAFs undergo transformation under the induction of Wnt
proteins, secreting a range of bioactive molecules and generating
exosomes, which facilitate tumor growth and invasion (63,64). There is an association between the
origin of CAFs in BC and Wnt proteins. For example, Wnt7a
transforms quiescent fibroblasts into α-smooth muscle
actin-positive CAFs by binding to TGF-β receptors, thereby
activating their potent tumor-promoting potential (62). Tumor-derived Wnt3a promotes
conversion of adipocytes to CAFs by activating the Wnt/β-catenin
signaling pathway (62,65). Further research has revealed that
CAFs, as a key source of Wnt ligands, actively promote BC
progression by releasing paracrine signals (65,66). These signals maintain tumor
stemness, stimulate the growth of the primary tumor and accelerate
the metastatic colonization process (63,64). For example, Wnt3a activation
enhances the synthesis of key matrix proteins, such as fibronectin
and type I collagen, which further induces synthesis of MMP-9 in BC
cells, thereby enhancing their migration and invasion capability
(65,67,68). CAF-derived exosomes carrying CD81
serve a key role in stimulating BC cell motility due to activation
of the Wnt/PCP signaling pathway. Wnt10b contained within the
exosomes triggers a self-reinforcing cycle by activating the
Wnt/β-catenin pathway, promoting progression and widespread
dissemination of tumors (66,69). Therefore, the interaction between
CAFs and the Wnt signaling pathway underlies the progression of BC
and may provide a basis for development of innovative treatment
strategies.
MSCs have garnered attention for their potential to
differentiate into various cell types, such as adipocytes,
osteoblasts and chondrocytes, which affect tumor angiogenesis,
immune modulation and responses to antitumor therapy (70-72). In regulating interactions with BC
cells, the Wnt signaling pathway serves as a key factor in driving
tumor growth and bone metastasis, while also inhibiting tumor
progression through diverse mechanisms (73). Specifically, the Wnt signaling
pathway connects MSCs with cancer cells, enhancing the
pro-tumorigenic properties of MSCs by inducing their
differentiation into CAFs (74).
Wnt5a released by BC cells triggers the secretion of IL-6 and
monocyte chemoattractant protein-1, which enhances the
phagocytic-like activity of MSCs and accelerates tumor
dissemination. In in vitro models of BC bone metastasis, the
Wnt/β-catenin pathway regulates osteogenic activity in a bone
microenvironment composed of human MSCs, promoting the progression
of BC bone metastasis (75,76). The functions of MSCs are not
limited solely to promoting tumorigenesis; they also have the
ability to inhibit tumor growth (77-79). MSCs effectively limit tumor
progression through a number of biological mechanisms, including
weakening Wnt and AKT signaling, blocking angiogenesis, stimulating
the recruitment of inflammatory cells and inducing cell cycle
arrest and apoptosis (77-82).
In Michigan Cancer Foundation-7 cells, DKK1 derived from MSCs
successfully inhibits tumor cell proliferation by downregulating
β-catenin signaling (65,77). Additionally, in controlled
experimental settings, chondrogenic membrane-derived MSCs inhibit
the proliferation of BC cells, an effect mediated by the regulation
of the Wnt/β-catenin signaling cascade. These findings revealed the
dual nature of MSCs in BC, emphasizing their potential in both
promoting and inhibiting tumor progression through different
signaling pathways. Future studies should clarify the specific
functions and pathways of MSCs in BC.
VEGF is a key factor in regulating angiogenesis,
tumor progression and metastatic spread and promotes the generation
of a tumor immune-suppressive environment (83,84). Wnt signaling influences
endothelial cells and paracrine signaling, further regulating
vascular smooth muscle and vascular network structure (85,86). In BC, the canonical Wnt pathway
not only drives the transcription of VEGF but also interacts with
VEGF and its key receptor NRP-1, whereas the non-canonical Wnt
pathway collaborates with VEGF to affect angiogenesis and cancer
progression. Activation of the Wnt/β-catenin pathway can directly
lead to the transcription of multiple target genes, including VEGF,
by stabilizing β-catenin and promoting its transfer to the nucleus,
thereby driving angiogenesis (87). In the formation of BCSCs and the
induction of tubular angiogenic structures, the binding of VEGFA to
its receptor NRP-1 serves a key role. In the VEGFA/NRP-1 signaling
axis, the Wnt/β-catenin signaling pathway acts as a key downstream
effector; its transmission is triggered by the activation of VEGFA,
thereby facilitating persistence of CSCs (88,89). This cascade enhances the invasive
potential of tumors and accelerates the malignant transformation of
breast tissue. In addition, the non-canonical Wnt pathway often
works together with VEGF, regulating key events in tumor
angiogenesis, such as endothelial cell movement, increased vascular
permeability and extracellular matrix remodeling (90,91). Given the key role of Wnt signaling
in BC angiogenesis, precise therapeutic strategies targeting this
pathway may provide novel avenues for overcoming resistance to
traditional anti-angiogenic therapy, thus holding clinical
translational potential.
Exosomes, as nanoscale vesicles for intercellular
communication, play a key role in tumor progression and acquisition
of therapeutic resistance within the TME (37,92-96). In BC, the Wnt signaling pathway
influences the evolution of tumors, chemoresistance and the
development of resistance reversal strategies by regulating the
content of microRNAs (miRNAs or miRs) in different exosomes
(97,98). Specifically, under the action of
the Wnt signaling pathway, BC cells decrease levels of miR-7-5p in
exosomes, thereby enhancing their migration and invasion capability
and releasing miR-454 to maintain the self-renewal potential of
CSCs (99,100). The roles of exosomal miRNAs in
BC progression vary. Among them, exosomal miR-10527-5p can block
the Wnt/β-catenin signaling pathway to inhibit cancer cell
migration and invasion, whereas miR-7-5p from exosomes derived from
less invasive BC cells inhibits metastasis by targeting the
receptor-like tyrosine kinase gene and the non-canonical Wnt
pathway (99,100). Conversely, upregulated
miR-142-3p expression activates the Wnt pathway and induces miR-150
production, thereby promoting the over-proliferation of BC cells
(101). Exosomes serve a central
role in chemoresistance. Chemotherapy-induced exosomes activate the
Wnt/β-catenin pathway by targeting DKK3 and Notch signaling pathway
inhibitor protein numb homolog, inducing resistance; exosomes
released by paclitaxel-resistant BC cells carry miR-187-5p that
regulates ATP-binding cassette subfamily D member 2 and the
Wnt/β-catenin pathway, affecting proliferation. Exosomes
transported from adipose-derived MSCs transfer miR-1236, which
decreases resistance to cisplatin by inhibiting solute carrier
family 9 member A1 activity and Wnt/β-catenin signaling (101). These findings not only reveal
the dual role of exosomes in BC progression, but also provide a
basis for exploring resistance reversal strategies in BC.
Mechanical stress resulting from the integrated
action of multidimensional factors, such as tissue topography,
matrix stiffness, physical stretching, shear stress and
compression, is closely related to the initiation, development and
dissemination of cancer (102,103). Mechanical stress regulates BC
progression by affecting the response of adipocytes, SCs, cancer
cells and fibroblasts to the Wnt signaling pathway. For example,
shear stress can downregulate the expression of SC marker
molecules, thereby blocking the Wnt/β-catenin pathway, increasing
cellular stiffness and inducing the differentiation of cancer cells
toward a mature state (104).
Adipocytes, an important component of breast tissue, undergo
dedifferentiation transformation via the mechanosensitive response
mechanism of the Wnt/β-catenin pathway when subjected to physical
compression, forming compression-induced dedifferentiated
adipocytes (CiDAs) (105). CiDAs
accelerate myofibroblastosis within the TME, facilitating BC cell
proliferation (105). Matrix
rigidity serves a key role in regulating the invasive potential of
breast tumor cells at the primary site of disease (106-108). The combination of matrix
rigidity and matrix-secreted factors stimulates Gli family zinc
finger protein 2 and parathyroid hormone-related protein through
Wnt signaling in osteolytic breast cancer cells, driving
tumor-induced osteolysis (109).
Intervention strategies targeting physical factors and the Wnt
signaling pathway may provide an effective therapeutic approach to
delay tumor metastasis (103,109).
Hypoxia, a constant and heterogeneous characteristic
of the TME, is pervasive in most types of solid tumor owing to the
abnormally high expression of hypoxia-inducible factors (HIF) under
low-oxygen conditions (110,111). In the hypoxic microenvironment
of BC, HIF regulates the Wnt signaling pathway and stability of
β-catenin while inducing upregulation of runt-related transcription
factor 2 (RUNX2) and inhibiting the activation of GSK3,
facilitating aggressive progression of tumors and the enhancement
of cancer cell invasiveness. Specifically, HIF-1α stabilizes the
structure of β-catenin and enhances its transcriptional activity;
on the other hand, it upregulates the expression of Wnt ligands and
receptors, thereby transcriptionally activating Wnt1-inducible
signaling pathway protein 3. This activates the Wnt pathway,
facilitating tumor growth, metastasis and angiogenesis (112). Concurrently, HIF-2α reprograms
normal cells into a SC-like phenotype by activating the Wnt
signaling pathway in BC cells and induces chemotherapy resistance
(113). This not only drives
metabolic reprogramming and maintains stemness of BC cells but also
enhances glycolysis and glutaminolysis through the Wnt/β-catenin
pathway, ensuring the survival of cancer cells under extreme
hypoxic conditions (114-120).
Hypoxia-induced RUNX2 upregulates RNA binding motif protein
5-antisense RNA 1, preventing the degradation of β-catenin and
promoting the formation of β-catenin-TCF4 transcription complex,
thereby amplifying the effect of the Wnt/β-catenin pathway and
accelerating tumor progression (121). Hypoxic conditions inhibit the
activation of GSK3, decreasing the phosphorylation and subsequent
degradation of β-catenin, further activating the Wnt/β-catenin
pathway, leading to increased expression of snail family
transcriptional repressor 1, and enhancing the invasiveness of
cancer cells (122). These
findings not only reveal the key role of hypoxia in tumor
progression but also provide perspective for the development of
innovative therapy.
A common characteristic of solid tumors is the
acidic metabolic environment, which not only promotes the
proliferation of tumor cells, but also serves a key role in
resistance to radiotherapy and chemotherapy (123). The acidic TME is primarily
shaped by hydrogen ions released from the dissociation of lactate
and carbonic acid. Lactate originates from the anaerobic glycolysis
of tumor cells, whereas carbonic acid is generated by the reaction
of CO2 with H2O catalyzed by carbonic
anhydrase (CA) (123). In BC,
abnormal regulation of CA exacerbates this acidification, further
disrupting the internal pH balance of tumors (124). Intracellular acidification
triggers the activation of the unfolded protein response and ATP
depletion, thereby inhibiting Wnt signaling activity (125). Drugs that induce intracellular
acidification (such as mitochondrial complex I) effectively
suppress the Wnt signaling pathway, decrease the expression of
SRY-box transcription factor 4 and inhibit SC characteristics and
activity of BC cells (125).
These findings not only reveal the potential of targeting the Wnt
pathway in BC treatment but also provide perspective on strategies
that exploit the acidic TME. Therefore, modulating the pH of the
TME may serve as an innovative adjuvant therapy, laying a
foundation for tumor treatment.
In recent years, with the continuous advancement of
oncology research, the association between BC-related genes and the
Wnt signaling pathway has become a research hotspot in this field
(126-128). BC-associated genes, such as
BRCA1, BRCA2 and tumor protein P53 (TP53) are associated with the
Wnt signaling pathway and serve key roles in maintaining normal
physiological function, genomic stability and cell fate
determination.
BRCA1 and BRCA2. BRCA genes, mainly BRCA1 and BRCA2,
are closely associated with the risk of hereditary BC (129,130). They effectively inhibit
excessive cell proliferation and maintain genomic stability by
repairing double-stranded DNA breaks (131). In germline BRCA1 mutation
carriers, the microenvironment harboring heterozygous BRCA1
mutations may promote BC development by creating a tumor-promoting
niche, particularly by affecting the stromal cells residing in the
TME (132,133). Wu et al (134) reported that in basal-like BC,
the Wnt effector Slug epigenetically suppresses BRCA1, resulting in
a negative association between Wnt signaling and BRCA1 expression.
Furthermore, Wu et al (134) indicated that canonical Wnt
signaling drives cancer cell EMT and tissue invasion by modulating
Slug activity, inhibiting the function of BRCA1 and BRCA2 and
rendering breast epithelial cells more vulnerable to
etoposide-mediated DNA damage. Li et al (135) revealed that BRCA1 deficiency may
increase the sensitivity of the active form of β-catenin protein to
H2O2, thereby decreasing its expression. To
the best of our knowledge, however, research on the association
between BRCA genes and the Wnt signaling pathway remains limited,
and more mechanistic studies are needed (135).
TP53 gene mutations serve a key role in BC.
Transcription factor p53, encoded by TP53, serves a crucial role in
regulating a series of cellular activities, including cell cycle
arrest, apoptosis, metabolic regulation, DNA repair and cellular
senescence (136-138). Abnormal activation of the Wnt
signaling pathway effectively suppresses TP53 activity, thereby
facilitating survival of tumor cells and enhancing their resistance
to apoptosis (139). In BC
cells, the absence of TP53 promotes the release of key Wnt factors,
such as Wnt1, Wnt6 and Wnt7a, but also stimulates TAMs to produce
IL-1β, a key inflammatory mediator, through the binding of these
factors with frizzled class receptor 7 (FZD7) and FZD9 receptors on
the surface of TAMs (140).
Moreover, in TP53-deficient basal-like BC tumors, activity of the
Wnt signaling pathway is enhanced and is often accompanied by the
expression of the non-canonical Wnt signaling mediator receptor
tyrosine kinase-like orphan receptor 2 (Ror2). The existence of
Ror2 as an alternative Wnt receptor enhances the diversity of Wnt
signaling and supports Wnt/β-catenin-independent signaling
functions in TP53-deficient model (141). Therefore, the combined
inhibition of TP53 and the Wnt signaling pathway may provide
effective therapeutic options for patients with BC.
Somatic genomic aberrations in breast tumors disrupt
key signaling networks that regulate cell division, survival and
differentiation, highlighting numerous potential biomarkers and
therapeutic targets (142).
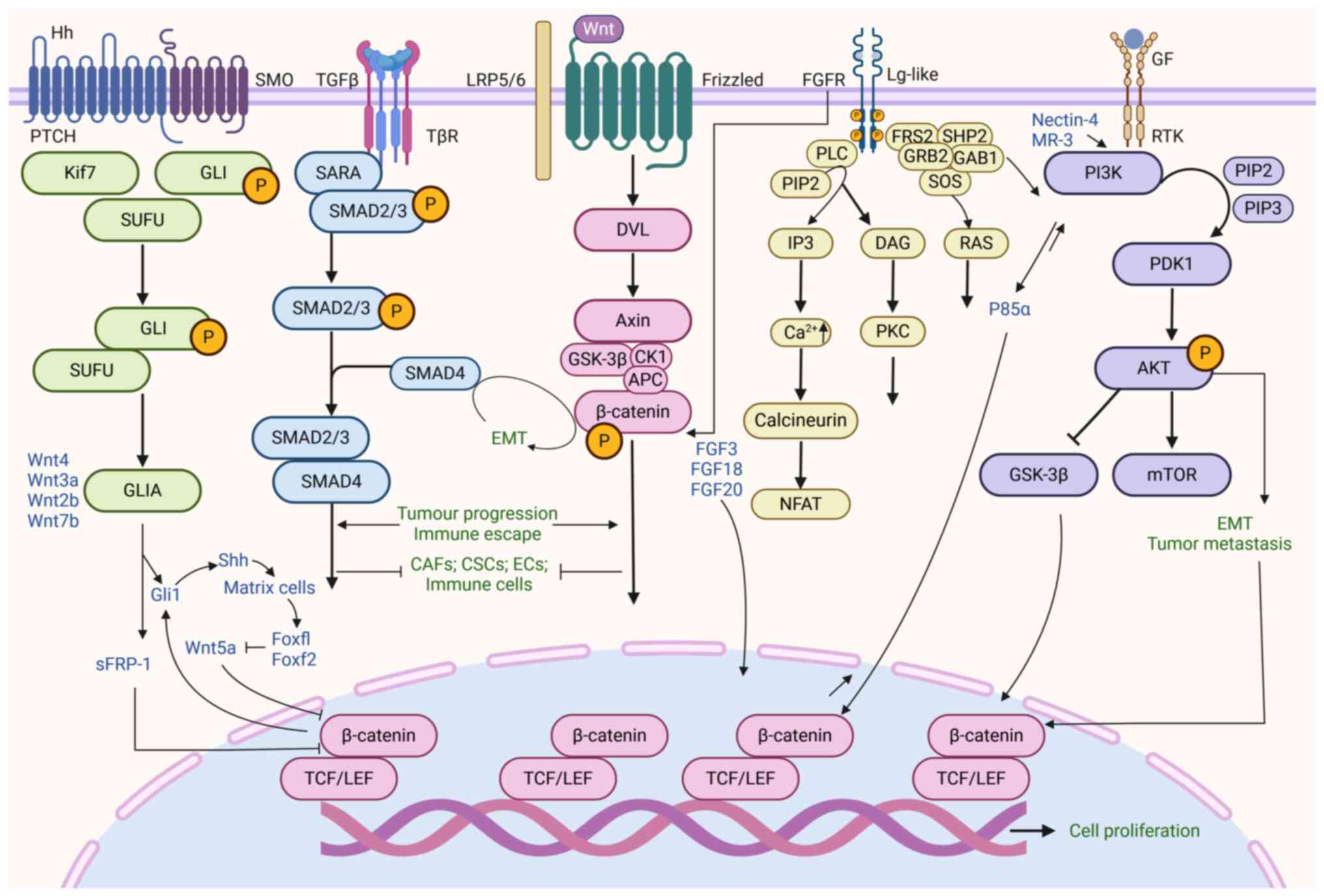
Previous studies have demonstrated crosstalk between the Wnt
signaling network and key pathways within the TME, including TGF-β,
phosphatidylinositol-3 kinase (PI3K)/Akt, fibroblast growth factor
(FGF) and Hedgehog signaling (37,143,144). These signaling networks not only
control tumor cell proliferation and migration, but also serve
indispensable roles in immune evasion, metabolic reprogramming and
therapeutic resistance, which are critical biological processes
(145). The association between
the Wnt signaling pathway and other core pathways, as well as the
underlying biological mechanisms, may serve as potential
therapeutic strategies and biomarkers (Fig. 4).
The Wnt and TGF-β signaling pathways, as
evolutionarily conserved regulatory mechanisms, serve crucial roles
in embryonic differentiation, tissue homeostasis and pathological
processes. Abnormal activation is closely associated with BC
progression, increased potential for metastasis and poor clinical
prognosis (146,147). The mechanisms by which these
pathways influence BC primarily involve driving EMT, inducing
immune evasion and maintaining BC cell stemness (148). EMT, as a key mechanism of tumor
metastasis, is regulated by the Wnt and TGF-β signaling pathways.
TGF-β, acting as a catalyst for EMT, can prompt epithelial cells to
transition into invasive mesenchymal phenotypes (149). Concurrently, the Wnt pathway,
particularly its β-catenin-activated form, promotes EMT. These two
pathways collectively regulate gene expression networks associated
with EMT through interactions between β-catenin and SMAD proteins,
thereby accelerating the invasion and migration of BC cells
(149). In addition to
regulation of EMT, the crosstalk between the Wnt and TGF-β
signaling pathways amplifies their individual effects in promoting
immune evasion. TGF-β signaling activates immune escape mechanisms,
while Wnt signaling is involved in immune modulation, especially in
promoting immune tolerance (32,150). The maintenance of BCSCs is a
component of the functional intersection of Wnt and TGF-β pathways,
which are key for tumor development, metastasis and the emergence
of therapeutic resistance. The Wnt pathway drives the self-renewal
of BCSCs via β-catenin-mediated transcriptional activation, whereas
TGF-β enhances the maintenance of BCSCs by promoting stemness
(151). More specifically,
non-canonical Wnt signaling, by maintaining CSCs in a quiescent
state in association with TGF-β signaling, enhances the EMT
process, thereby promoting the maintenance and expansion of CSCs
(151). Interactions between
these pathways provide valuable insight for the development of
innovative therapeutic interventions. Compared with the inhibition
of a single pathway, targeting both Wnt and TGF-β pathways
simultaneously provides a more efficient approach to improving the
outcomes of BC treatment (152,153).
The PI3K/Akt pathway plays a pivotal role in
regulating core cellular activities, such as proliferation,
division, metabolic adaptation, survival and angiogenesis (154,155). The PI3K/Akt and Wnt pathways are
associated with tumor biological behaviors through regulating CSC
self-renewal, accelerating EMT and metastasis and affecting
angiogenesis, thus facilitating progression of BC. In BC, a key
factor in the combined action of the Wnt and PI3K/Akt signaling
pathways to promote CSC self-renewal is the interaction between
β-catenin and the PI3K regulatory subunit p85α, which enhances the
catalytic activity of PI3K, thereby triggering the phosphorylation
and activation of Akt (156,157). Further research has shown that
the PI3K/Akt pathway intersects with the canonical Wnt signaling
cascade under the regulation of GSK3β, where Akt phosphorylates and
inhibits GSK3β, amplifying the efficiency of Wnt signal
transduction and further promoting the self-renewal of CSCs
(158-160). In terms of promoting EMT and
metastasis, Siddharth et al (161) revealed the overexpression of
nectin-4 drives Wnt/β-catenin signaling through the PI3K/Akt axis,
thereby enhancing the EMT process and promoting metastasis.
Simultaneously, the PI3K/Akt signal phosphorylates and activates
β-catenin; this upregulates the expression spectrum of genes
associated with EMT and enhances its interaction with TCF/LEF
transcription factors, thus advancing the EMT process (162). 3,5,4′-trimethoxystilbene can
affect E-cadherin levels and nuclear localization of β-catenin by
regulating the PI3K/Akt signaling axis and GSK3β, thereby reversing
EMT in BC cells (163). In
addition, the role of Wnt3a and the PI3K/Akt pathway in
angiogenesis has attracted increasing attention (164). Thymoquinone, as an effective
inhibitor, simultaneously impairs the normal function of PI3K and
Wnt3a signaling pathways, thereby inhibiting angiogenesis (164). Arqués et al (165) reported that the combined use of
the PI3K inhibitor BKM120 and the Wnt signaling inhibitor LGK974
can decrease tumor progression and metastasis in BC. These findings
provide a theoretical and experimental basis for the development of
targeted therapeutic strategies for the PI3K/Akt and Wnt signaling
pathways and the formulation of personalized treatment plans.
FGFs are involved in controlling a broad range of
cellular functions, including SC survival, proliferation,
programmed cell death inhibition, drug resistance and angiogenesis
(166,167). The FGF signaling pathway
interacts with the Wnt pathway and regulates the transcriptional
activity of target genes. Members of the FGF family activate the
Wnt pathway through various mechanisms, such as inducing β-catenin
modification and promoting the phosphorylation of low-density LRP6,
and these pathways exhibit synergistic oncogenic effects in mouse
mammary tumor virus (MMTV)-induced tumors. FGF family members FGF18
and FGF20 promote Wnt pathway activation via
β-catenin-TCF/LEF-dependent transcription (168,169). Activation of FGF receptors
induces tyrosine phosphorylation-mediated modification of
β-catenin, leading to the detachment of β-catenin from adherens
junctions and a decrease in its N-terminal serine/threonine
phosphorylation levels. By blocking the proteasomal degradation
pathway of β-catenin, this mechanism enhances the self-reinforcing
loop of the canonical Wnt signaling pathway (170-172). Additionally, FGF signaling
promotes the phosphorylation of LRP6 mediated by cyclin
Y/vertebrate homolog PFTK, further activating the Wnt pathway
during the G2/M transition of the cell cycle (173). The interaction between Wnt-1 and
FGF3 was initially detected in tumors induced by MMTV (174,175). FGF3, as an oncogene, triggers
the occurrence of ~40% of MMTV infection-dependent tumors with
Wnt-1. MMTV insertional mutagenesis studies have demonstrated that
simultaneous activation of Wnt and FGF signaling components is one
of the most common genetic alterations in breast tumors (176,177). Overactivation of the Wnt and FGF
signaling pathways is considered a potential molecular marker for
predicting the response of BC to next-generation eukaryotic
initiation factor 4A RNA helicase inhibitors (178). Therefore, the interaction
between Wnt and FGF signaling in the BC TME is a dynamic and
complex process that affects the behavioral patterns of tumor cells
and promotes BC progression.
The Hedgehog signaling cascade is a highly
conserved process in evolution and serves a crucial role in cell
differentiation, tissue development, homeostasis and EMT (179). Within the complex network of
tumor biology, the crosstalk between the Wnt and Hedgehog signaling
pathways primarily occurs through the transcription factor
Gli1-mediated control of secreted frizzled-related protein 1
(sFRP-1) and sonic hedgehog (Shh) expression, and Gli1 exerts a
synergistic impact with β-catenin, shaping the progression of BC.
Specifically, the key transcription factor Gli1 in the Hedgehog
pathway mediates crosstalk with the Wnt pathway by regulating the
negative regulator sFRP-1 in the Wnt signaling pathway (180). When the function of sFRP-1 is
compromised, the canonical Wnt pathway is abnormally activated,
leading to disorders in the signaling network (180). The regulatory role of Gli1 is
not limited to sFRP-1 but also involves other members of the Wnt
family, such as Wnt2b, Wnt4 and Wnt7b. Furthermore, Gli1
upregulates the expression of the secreted Hedgehog Shh, which
transmits paracrine signals to the surrounding stromal cells,
triggering biological effects. Upon receiving the Shh signal,
stromal cells upregulate the expression of forkhead box F1 (Foxf1)
and Foxf2, thereby inhibiting the production of Wnt5a in the stroma
and indirectly regulating the activity of β-catenin (181). There is an association between
nuclear Gli1 and β-catenin activity. The expression of exogenous
constitutive nuclear β-catenin can enhance the activity of Gli1
reporter genes, indicating that the sustained activation of Wnt
signaling can enhance the transcriptional efficacy of Gli (182). Arnold et al (183) reported that the co-elevation of
nuclear Gli1 and β-catenin activity is associated with the
progression of TNBC stage and poor clinical prognosis (183). Concurrent activation of the
Hedgehog and Wnt pathways is also associated with a decline in
recurrence-free and overall survival rates in patients with TNBC
(183). Thus, the synergistic
activation of the Wnt and Hedgehog pathways may serve as an
important biomarker for the progression and therapeutic response of
BC, providing avenues for optimizing clinical treatment plans and
improving the prognosis of patients with BC.
While exploring the complex mechanisms of the TME,
abnormal regulation of the Wnt signaling pathway has been
identified as a key element in tumor progression, affecting
angiogenesis, immune evasion and metastasis (184). In addition, the Wnt signaling
pathway affects the efficacy of anti-HER2 therapy, chemotherapy and
immunotherapy, influencing the success of BC treatment and patient
prognosis. For example, in HER2-overexpressing BC cells, Wnt3
overexpression activates the Wnt/β-catenin signaling pathway, which
not only leads to the transactivation of EGFR and promotes an
EMT-like transformation but may also be a key cause of trastuzumab
resistance (185,186). This signaling pathway may
regulate CD36 levels, prompting resistant mesenchymal CSCs to
transition to a treatment-sensitive epithelial state, thereby
affecting lapatinib resistance (187,188). In vitro studies by Xu
et al (189) have shown
that β-catenin expression is associated with chemoresistance in
TNBC, as β-catenin knockdown can restore the sensitivity of TNBC
cells to doxorubicin- or cisplatin-mediated cell death. Shetti
et al (190) reported
that the combination of low-dose paclitaxel and XAV939 (a Wnt
signaling inhibitor) can inhibit EMT and angiogenesis by inducing
apoptosis and suppressing Wnt signaling, demonstrating its
therapeutic potential for TNBC. Upregulation of CSC-related Wnt
signaling and activation of β-catenin signaling in PD-L1-high TNBC
suggest that selective Wnt/β-catenin inhibitors may decrease
resistance and re-sensitize TNBC to anti-PD-L1/anti-CTLA-4
monoclonal antibody immunotherapy (191-195).
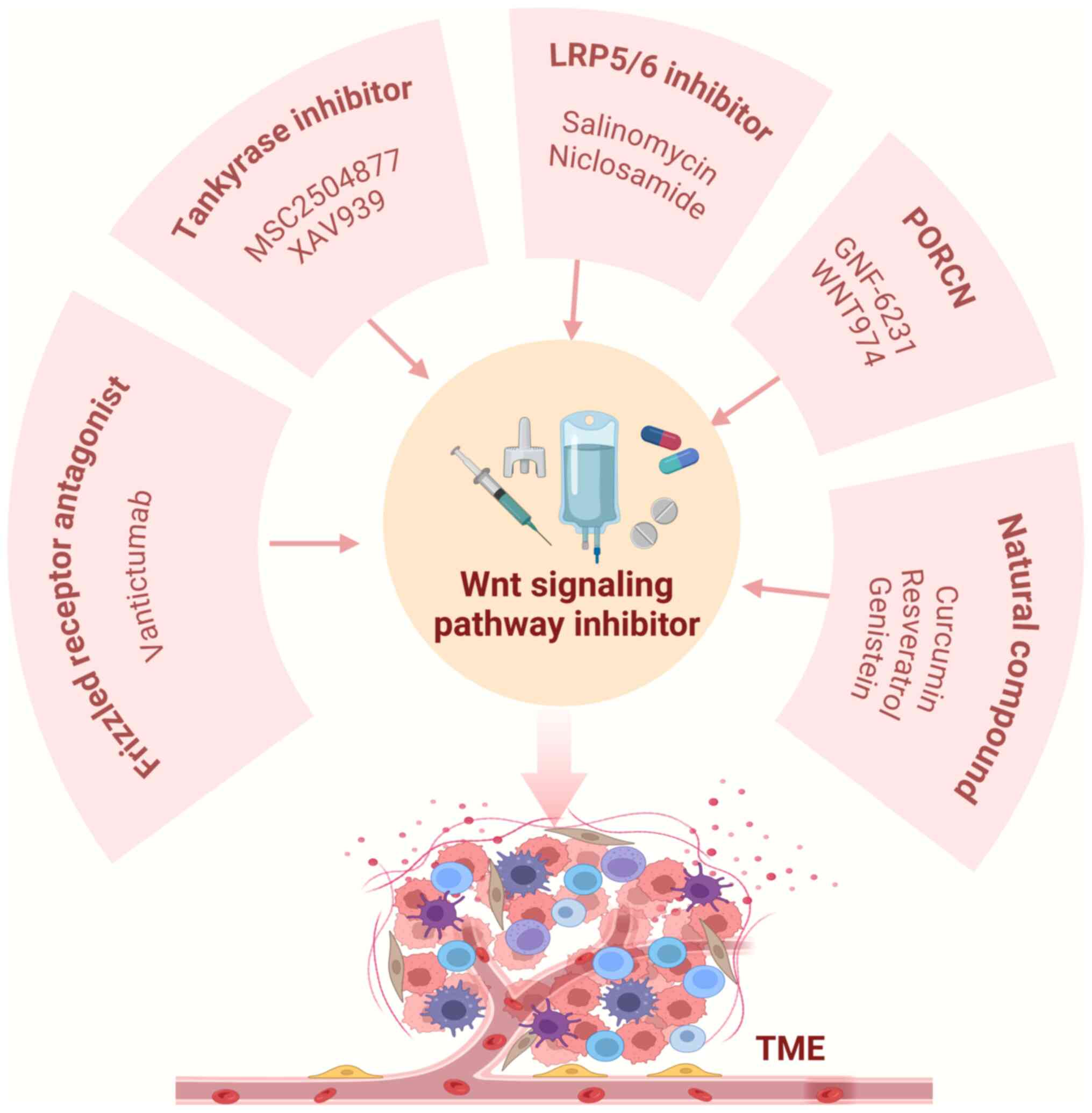
Developing therapeutic strategies targeting both
the canonical and non-canonical Wnt signaling pathways is key for
advancing precision medicine and genome-based disease therapy. Wnt
pathway inhibitors, including porcupine (PORCN) and LRP5/6
inhibitors, frizzled receptor antagonists, tankyrase inhibitors and
natural compounds may be used to target the Wnt signaling pathway
in the BC TME (Fig. 5). These
inhibitors not only modulate the complex signaling network within
the TME but also suggest that precise intervention in the Wnt
pathway may provide new avenues for BC treatment.
PORCN, a member of the membranebound
O-acyltransferase family, catalyzes the palmitoylation of Wnt
ligands, a key step that allows Wnt ligands to be secreted, and
effectively activates the Wnt signaling pathway, thereby regulating
fundamental physiological functions, such as cell differentiation,
proliferation, migration and apoptosis (196-201). To intervene in the
palmitoylation of Wnt proteins within the endoplasmic reticulum,
PORCN inhibitors effectively prevent the secretion of Wnt proteins
and curb the abnormal accumulation of β-catenin to restore normal
cell proliferation and signal transduction (202,203). In a preclinical study of BC
mice, the PORCN inhibitor GNF-6231 showed strong antitumor
activity, stimulating a notable tumor response (204). Similarly, another PORCN
inhibitor, LGK974, not only blocks the secretion of Wnt proteins,
but also effectively inhibits the activation of Wnt signaling
(205). In a preclinical study
of epithelial ovarian cancer, LGK974 in combination with paclitaxel
demonstrates synergistic antitumor effects (206). In BC models induced by Wnt
signaling, such as the MMTV model, LGK974 also performs well at
doses tolerable to animals, achieving tumor regression while
decreasing the phosphorylation levels of LRP6 and the expression of
Axin2 (207,208). LGK974 has entered phase I
clinical trials, and its potential as a monotherapy in combination
with PDR001 for the treatment of Wnt signaling-driven cancer,
including TNBC, is being evaluated (207,209). The aforementioned trials aim to
assess the clinical efficacy and safety of LGK974 and to propose
innovative strategies for Wnt signal inhibition in BC treatment
(30).
LRP5/6, a key co-receptor in the Wnt signaling
pathway, plays a key role in the β-catenin-mediated canonical
signal transduction process. Alkalescel, initially recognized as an
antimicrobial potassium ionophore, inhibits phosphorylation of
LRP5/6 and promotes its degradation, thereby inhibiting BC cell
proliferation and inducing apoptosis (210). Salinomycin can downregulate
LRP5/6 expression, further decreasing the transmission of Wnt
signaling (211,212). By contrast, niclosamide acts on
LRP6 by blocking its phosphorylation and downregulating its protein
synthesis. In BC cell models, niclosamide inhibits cellular
proliferation and promotes apoptosis without affecting DVL2
expression (213,214). Research on BC cells has
confirmed that niclosamide could inhibit tumor sphere formation in
non-obese diabetes/severe combined immunodeficiency mice, promote
apoptosis and effectively curb tumor growth, providing support for
its application in CSC-directed therapy (215). Furthermore, using a mouse BC
model, Ye et al (216)
reported that niclosamide decreases tumor cell proliferation,
migration and invasion, while promoting apoptosis and decreasing
tumor weight. These findings demonstrate the mechanisms of action
of LRP5/6 inhibitors and provide insights for BC treatment
strategies.
Vantictumab is a fully human IgG2 monoclonal
antibody that selectively targets frizzled receptors and
effectively disrupts the canonical Wnt signaling pathway (217). Preclinical experiments involving
human cancer cell cultures and patient-derived BC xenograft models
have shown that vantictumab not only impedes tumor progression but
also exhibits enhanced antitumor efficacy when combined with
paclitaxel compared to monotherapy (217,218). Sequential treatment with
vantictumab and paclitaxel induces mitotic cell death, decreases
tumor proliferation and decreases CSC numbers. Vantictumab
decreases the number of CSCs and downregulates the expression of
EMT-associated genes (219).
Tankyrase regulates Axin stability and directs its
degradation through PARsylation (220). In a BC cell culture, XAV939 or
small interfering RNA-mediated tankyrase knockdown significantly
increases the expression of Axin1 and Axin2 proteins, thereby
inhibiting Wnt-induced transcriptional activity (221). Tankyrase inhibitor MSC2504877
exerts G1 phase cell cycle arrest effects in tumor cells, promotes
cellular senescence and enhances the efficacy of clinically used
CDK4/6 inhibitors, facilitating development of BC treatment
(222).
Natural products are used for the development of
anticancer drugs. The use of these naturally derived compounds and
their derivatives to modulate the TME and target the Wnt signaling
pathway is becoming a highly promising direction in BC treatment
(223-225). Phytochemicals regulate the Wnt
signaling cascade, thereby providing options for future drug
development (Table I).
BC, which poses a threat to lives and safety, has
long been a core focus of medical research and clinical practice
(226,227). The biological research system of
BC is intricate, and the multidimensional network constructed
between the Wnt signaling pathway and TME forms a cornerstone in
this field of study (228-230). The components of the TME are
associated with the Wnt signaling pathway, creating dynamic
regulatory network. Immune cells releasing cytokines to regulate
Wnt signaling, stromal components influencing signal transmission
through chemical induction and secreted molecules directly
interacting with key proteins in the pathway affect the
proliferation, migration and invasion of tumor cells, and serve a
role in promoting immune evasion, metabolic reprogramming and
enhancing therapeutic resistance (231,232).
The hallmark features of the TME, such as hypoxia,
acidity and mechanical stress, are associated with the Wnt
signaling pathway, promoting the continuous progression of tumors
(233,234). The Wnt signaling pathway
interacts with key signaling networks, such as TGF-β, PI3K/Akt, FGF
and Hedgehog, forming a highly interconnected regulatory pattern.
Therefore, single-target treatment strategies for the Wnt signaling
pathway may be insufficient to inhibit development of tumors.
Therefore, developing combination treatment plans that act on
multiple key nodes in these signaling networks is key for more
effective treatment.
Given the role of the Wnt signaling pathway in the
heterogeneous microenvironment of BC and its association with
treatment resistance, it has become a key target for identifying
novel biomarkers (14,235). By exploring the effector
molecules of the Wnt signaling pathway and the association between
Wnt signaling activity and patient prognosis, more precise
predictive biomarkers can be developed (236,237). For example, cyclin D1 is
upregulated following activation of the Wnt pathway and may promote
the proliferation of BC cells (238). Ras GTPase-activating
protein-binding protein 1 (G3BP1) induces β-catenin by inactivating
GSK-3β, thereby increasing the proliferation of BC cells. Blocking
the interaction between G3BP1 and GSK-3β inhibits cell
proliferation (239).
Platelet-activating factor, a co-factor of β-catenin, can induce
Wnt signaling, promote the proliferation of BC cells and increase
the expression levels of CSC markers (240,241). The downregulation of
autophagy-related protein 4A leads to decreased expression of
β-catenin, cyclin D1 and MYC proto-oncogene protein c-Myc, which
impacts the autophagy process and enhances the chemosensitivity of
tamoxifen in BC. Deficiency of fibrous sheath interacting protein 1
promotes autophagy, resulting in decreased Wnt/β-catenin activity
and influencing drug sensitivity in TNBC (242). Kallistatin, a tissue
kallikrein-binding protein and a unique serine proteinase
inhibitor, inhibits the proliferation, migration, invasion, and
tumor progression of BC cells by blocking the Wnt/β-catenin
signaling pathway and inducing autophagy (243). Additionally, the genetic
background of patients, such as the mutation status of BRCA1/2, may
influence Wnt signaling activity, thereby providing a basis for the
development of personalized treatment strategies.
In studies exploring the relationship between the
Wnt signaling pathway and tumor cell death, it has been confirmed
that the Wnt signaling pathway is not only closely related to the
apoptosis and autophagy processes of breast cancer cells, but also
associated with disulfidptosis, a specific type of cell death
caused by disulfide accumulation (244,245). Wnt signaling activation not only
promotes tumor cell self-renewal but also modulates serine
peptidase inhibitor, clade B, member 6B (SERPINB6B) to decrease
immune cell death and interfere with T cell-mediated tumor lysis
(246). Specifically, SERPINB6B
suppresses pyroptosis and immune recognition, thereby inhibiting
apoptosis of immunogenic cells at metastatic sites and facilitating
tumor dissemination (246).
However, knowledge gaps persist in the context of the BC TME,
particularly regarding the interaction between Wnt signaling and
other tumor cell death modalities such as disulfidptosis,
ferroptosis, autophagy and pyroptosis. Future studies should
integrate multidisciplinary approaches, including genomics,
proteomics, bioinformatics, high-throughput screening technology
and precision medicine strategies, to systematically investigate
the crosstalk between Wnt signaling and tumor cell death mechanisms
and identify novel therapeutic targets and clinically actionable
biomarkers.
Small-molecule inhibitors and natural compounds
regulate Wnt pathway activity, effectively inhibiting the
proliferation and migration of tumor cells, while accelerating
apoptosis (247). Existing
research indicates that these inhibitors and compounds may have
clinical translation and application potential in BC (49). However, despite scientific
advancements, the pace of clinical translation is slow, with most
Wnt pathway-targeting drugs in phase I clinical trials, constrained
by thorough safety validation and preliminary efficacy assessment,
and only a few advancing to phase III trials (Table II) (248-250). The clinical safety and efficacy
of Wnt-targeting small molecules and natural compounds must be
validated to confirm their therapeutic potential where toxicity
poses a barrier. For example, salinomycin carries the risk of
neurotoxicity, potentially inducing peripheral neuropathy, whereas
niclosamide is associated with dose-limiting toxicity, posing a
threat to the gastrointestinal system (251,252). Future research should focus on
enhancing the clinical safety and efficacy of these compounds,
which may be achieved through the optimization of chemical
structures or development of innovative drug delivery technology to
enhance bioavailability and reduce adverse reactions (253). This may provide more effective
and safer treatment options for patients with BC and improve
prognosis and overall quality of life.
Not applicable.
CS and RL conceived and designed the study. MS and
HZ wrote and edited the manuscript, constructed the figures and
performed the literature review. YYu, YYa and HL revised the
manuscript. FF and QW constructed figures. All the authors have
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China Key Project (grant no. 82430123), National
Natural Science Foundation of China General Program (grant no.
82174222), Science and Technology Cooperation Project of the
Department of Science and Technology of the Traditional Chinese
Medicine Administration (grant no. GZY-KJS-SD-2023-023), National
Major Scientific and Technological Special Project for the
Prevention and Treatment of Cancer, Cardio-cerebrovascular,
Respiratory, and Metabolic Diseases (grant no. 2024ZD0521406) and
Shandong Province Taishan Scholar Distinguished Expert Reward
Program (grant no. tstp20221166).
|
1
|
Kim J, Harper A, McCormack V, Sung H,
Houssami N, Morgan E, Mutebi M, Garvey G, Soerjomataram I and
Fidler-Benaoudia MM: Global patterns and trends in breast cancer
incidence and mortality across 185 countries. Nat Med. Feb
24–2025.Epub ahead of print.
|
|
2
|
Scholler N, Perbost R, Locke FL, Jain MD,
Turcan S, Danan C, Chang EC, Neelapu SS, Miklos DB, Jacobson CA, et
al: Tumor immune contexture is a determinant of anti-CD19 CAR T
cell efficacy in large B cell lymphoma. Nat Med. 28:1872–1882.
2022.
|
|
3
|
Yu T and Di G: Role of tumor
microenvironment in triple-negative breast cancer and its
prognostic significance. Chin J Cancer Res. 29:237–252. 2017.
|
|
4
|
Rodríguez-Bejarano OH, Parra-López C and
Patarroyo MA: A review concerning the breast cancer-related tumour
microenvironment. Crit Rev Oncol Hematol. 199:1043892024.
|
|
5
|
Park J, Hsueh PC, Li Z and Ho PC:
Microenvironment-driven metabolic adaptations guiding CD8+ T cell
anti-tumor immunity. Immunity. 56:32–42. 2023.
|
|
6
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: new findings and future perspectives. Mol Cancer.
20:1312021.
|
|
7
|
Christofides A, Strauss L, Yeo A, Cao C,
Charest A and Boussiotis VA: The complex role of tumor-infiltrating
macrophages. Nat Immunol. 23:1148–1156. 2022.
|
|
8
|
Soleas JP, D'Arcangelo E, Huang L, Karoubi
G, Nostro MC, McGuigan AP and Waddell TK: Assembly of lung
progenitors into developmentally-inspired geometry drives
differentiation via cellular tension. Biomaterials.
254:1201282020.
|
|
9
|
Salik B, Yi H, Hassan N, Santiappillai N,
Vick B, Connerty P, Duly A, Trahair T, Woo AJ, Beck D, et al:
Targeting RSPO3-LGR4 signaling for leukemia stem cell eradication
in acute myeloid leukemia. Cancer Cell. 38:263–278.e6. 2020.
|
|
10
|
Choi BR, Cave C, Na CH and Sockanathan S:
GDE2-Dependent activation of canonical wnt signaling in neurons
regulates oligodendrocyte maturation. Cell Rep. 31:1075402020.
|
|
11
|
Zhuang X, Zhang H, Li X, Li X, Cong M,
Peng F, Yu J, Zhang X, Yang Q and Hu G: Differential effects on
lung and bone metastasis of breast cancer by Wnt signalling
inhibitor DKK1. Nat Cell Biol. 19:1274–1285. 2017.
|
|
12
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/betacatenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018.
|
|
13
|
Wend P, Runke S, Wend K, Anchondo B,
Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak
MS, et al: WNT10B/β-catenin signalling induces HMGA2 and
proliferation in metastatic triple-negative breast cancer. EMBO Mol
Med. 5:264–279. 2013.
|
|
14
|
Zhu L, Tian Q, Gao H, Wu K, Wang B, Ge G,
Jiang S, Wang K, Zhou C, He J, et al: PROX1 promotes breast cancer
invasion and metastasis through WNT/β-catenin pathway via
interacting with hnRNPK. Int J Biol Sci. 18:2032–2046. 2022.
|
|
15
|
Teng Y, Mei Y, Hawthorn L and Cowell JK:
WASF3 regulates miR-200 inactivation by ZEB1 through suppression of
KISS1 leading to increased invasiveness in breast cancer cells.
Oncogene. 33:203–211. 2014.
|
|
16
|
Mortezaee K: WNT/β-catenin regulatory
roles on PD-(L)1 and immunotherapy responses. Clin Exp Med.
24:152024.
|
|
17
|
Wang L, Zhang L, Zhao L, Shao S, Ning Q,
Jing X, Zhang Y, Zhao F, Liu X, Gu S, et al: VEGFA/NRP-1/GAPVD1
axis promotes progression and cancer stemness of triple-negative
breast cancer by enhancing tumor cell-macrophage crosstalk. Int J
Biol Sci. 20:446–463. 2024.
|
|
18
|
Foldynová-Trantírková S, Sekyrová P,
Tmejová K, Brumovská E, Bernatík O, Blankenfeldt W, Krejcí P,
Kozubík A, Dolezal T, Trantírek L and Bryja V: Breast
cancer-specific mutations in CK1epsilon inhibit Wnt/beta-catenin
and activate the Wnt/Rac1/JNK and NFAT pathways to decrease cell
adhesion and promote cell migration. Breast Cancer Res.
12:R302010.
|
|
19
|
Zhou Y, Xu J, Luo H, Meng X, Chen M and
Zhu D: Wnt signaling pathway in cancer immunotherapy. Cancer Lett.
525:84–96. 2022.
|
|
20
|
Liao Y, Badmann S, Kraus F, Topalov NE,
Mayr D, Kolben T, Hester A, Beyer S, Mahner S, Jeschke U, et al:
PLA2G7/PAF-AH as potential negative regulator of the wnt signaling
pathway mediates protective effects in BRCA1 mutant breast cancer.
Int J Mol Sci. 24:8822023.
|
|
21
|
Liu L, Xiao B, Hirukawa A, Smith HW, Zuo
D, Sanguin-Gendreau V, McCaffrey L, Nam AJ and Muller WJ: Ezh2
promotes mammary tumor initiation through epigenetic regulation of
the Wnt and mTORC1 signaling pathways. Proc Natl Acad Sci USA.
120:e23030101202023.
|
|
22
|
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q,
Deng S and Zhou H: Signaling pathways in cancer-associated
fibroblasts and targeted therapy for cancer. Signal Transduct
Target Ther. 6:2182021.
|
|
23
|
Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu
Y, Dong Q and Wei X: Wnt/β-catenin signaling pathway in
carcinogenesis and cancer therapy. J Hematol Oncol. 17:462024.
|
|
24
|
Liu Y, Zhao C, Wang G, Chen J, Ju S, Huang
J and Wang X: SNORD1C maintains stemness and 5-FU resistance by
activation of Wnt signaling pathway in colorectal cancer. Cell
Death Discov. 8:2002022.
|
|
25
|
Wei B, Cao J, Tian JH, Yu CY, Huang Q, Yu
JJ, Ma R, Wang J, Xu F and Wang LB: Mortalin maintains breast
cancer stem cells stemness via activation of Wnt/GSK3β/β-catenin
signaling pathway. Am J Cancer Res. 11:2696–2716. 2021.
|
|
26
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022.
|
|
27
|
Wang Y, Zheng L, Shang W, Yang Z, Li T,
Liu F, Shao W, Lv L, Chai L, Qu L, et al: Wnt/beta-catenin
signaling confers ferroptosis resistance by targeting GPX4 in
gastric cancer. Cell Death Differ. 29:2190–2202. 2022.
|
|
28
|
Wei L, Ding L, Mo MS, Lei M, Zhang L, Chen
K and Xu P: Wnt3a protects SH-SY5Y cells against 6-hydroxydopamine
toxicity by restoration of mitochondria function. Transl
Neurodegener. 4:112015.
|
|
29
|
Lin TY, Tsai MC, Tu W, Yeh HC, Wang SC,
Huang SP and Li CY: Role of the NLRP3 inflammasome: Insights into
cancer hallmarks. Front Immunol. 11:6104922021.
|
|
30
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13:1652020.
|
|
31
|
Rim EY, Clevers H and Nusse R: The wnt
pathway: From signaling mechanisms to synthetic modulators. Annu
Rev Biochem. 91:571–598. 2022.
|
|
32
|
Katoh M and Katoh M: WNT signaling and
cancer stemness. Essays Biochem. 66:319–331. 2022.
|
|
33
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022.
|
|
34
|
Ozalp O, Cark O, Azbazdar Y, Haykir B,
Cucun G, Kucukaylak I, Alkan-Yesilyurt G, Sezgin E and Ozhan G:
Nradd acts as a negative feedback regulator of Wnt/β-Catenin
signaling and promotes apoptosis. Biomolecules. 11:1002021.
|
|
35
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016.
|
|
36
|
Gao Y, Chen N, Fu Z and Zhang Q: Progress
of wnt signaling pathway in osteoporosis. Biomolecules.
13:4832023.
|
|
37
|
Malla RR and Kiran P: Tumor
microenvironment pathways: Cross regulation in breast cancer
metastasis. Genes Dis. 9:310–324. 2020.
|
|
38
|
Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC,
Han H, Liu WC and Qin HY: Crosstalk between hepatic tumor cells and
macrophages via Wnt/β-catenin signaling promotes M2-like macrophage
polarization and reinforces tumor malignant behaviors. Cell Death
Dis. 9:7932018.
|
|
39
|
Jiang Y, Han Q, Zhao H and Zhang J:
Promotion of epithelial-mesenchymal transformation by
hepatocellular carcinoma-educated macrophages through
Wnt2b/β-catenin/c-Myc signaling and reprogramming glycolysis. J Exp
Clin Cancer Res. 40:132021.
|
|
40
|
Tigue ML, Loberg MA, Goettel JA, Weiss WA,
Lee E and Weiss VL: Wnt signaling in the phenotype and function of
tumor-associated macrophages. Cancer Res. 83:3–11. 2023.
|
|
41
|
Bergenfelz C, Medrek C, Ekström E,
Jirström K, Janols H, Wullt M, Bredberg A and Leandersson K: Wnt5a
induces a tolerogenic phenotype of macrophages in sepsis and breast
cancer patients. J Immunol. 188:5448–5458. 2012.
|
|
42
|
Liu Q, Yang C, Wang S, Shi D, Wei C, Song
J, Lin X, Dou R, Bai J, Xiang Z, et al: Wnt5a-induced M2
polarization of tumor-associated macrophages via IL-10 promotes
colorectal cancer progression. Cell Commun Signal. 18:512020.
|
|
43
|
van Amerongen R: Alternative Wnt pathways
and receptors. Cold Spring Harb Perspect Biol. 4:a0079142012.
|
|
44
|
Spranger S and Gajewski TF: A new paradigm
for tumor immune escape: β-catenin-driven immune exclusion. J
Immunother Cancer. 3:432015.
|
|
45
|
Zebley CC, Zehn D, Gottschalk S and Chi H:
T cell dysfunction and therapeutic intervention in cancer. Nat
Immunol. 25:1344–1354. 2024.
|
|
46
|
Ying J, Li H, Yu J, Ng KM, Poon FF, Wong
SC, Chan AT, Sung JJ and Tao Q: WNT5A exhibits tumor-suppressive
activity through antagonizing the Wnt/beta-catenin signaling, and
is frequently methylated in colorectal cancer. Clin Cancer Res.
14:55–61. 2008.
|
|
47
|
Muto S, Enta A, Maruya Y, Inomata S,
Yamaguchi H, Mine H, Takagi H, Ozaki Y, Watanabe M, Inoue T, et al:
Wnt/β-catenin signaling and resistance to immune checkpoint
inhibitors: From non-small-cell lung cancer to other cancers.
Biomedicines. 11:1902023.
|
|
48
|
Li Q, Wei S, Li Y, Wu F, Qin X, Li Z, Li J
and Chen C: Blocking of programmed cell death-ligand 1 (PD-L1)
expressed on endothelial cells promoted the recruitment of
CD8+IFN-γ+ T cells in atherosclerosis. Inflamm Res. 72:783–796.
2023.
|
|
49
|
Xu X, Zhang M, Xu F and Jiang S: Wnt
signaling in breast cancer: Biological mechanisms, challenges and
opportunities. Mol Cancer. 19:1652020.
|
|
50
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015.
|
|
51
|
Rasha F, Boligala GP, Yang MV,
Martinez-Marin D, Castro-Piedras I, Furr K, Snitman A, Khan SY,
Brandi L, Castro M, et al: Dishevelled 2 regulates cancer cell
proliferation and T cell mediated immunity in HER2-positive breast
cancer. BMC Cancer. 23:1722023.
|
|
52
|
Yang M, Wei Z, Feng M, Zhu Y, Chen Y and
Zhu D: Pharmacological inhibition and genetic knockdown of BCL9
modulate the cellular landscape of cancer-associated fibroblasts in
the tumor-immune microenvironment of colorectal cancer. Front
Oncol. 11:6035562021.
|
|
53
|
Gattinoni L, Zhong XS, Palmer DC, Ji Y,
Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et
al: Wnt signaling arrests effector T cell differentiation and
generates CD8+ memory stem cells. Nat Med. 15:808–813. 2009.
|
|
54
|
Shan F, Somasundaram A, Bruno TC, Workman
CJ and Vignali DAA: Therapeutic targeting of regulatory T cells in
cancer. Trends Cancer. 8:944–961. 2022.
|
|
55
|
Hong Y, Manoharan I, Suryawanshi A,
Majumdar T, Angus-Hill ML, Koni PA, Manicassamy B, Mellor AL, Munn
DH and Manicassamy S: β-catenin promotes regulatory T-cell
responses in tumors by inducing vitamin A metabolism in dendritic
cells. Cancer Res. 75:656–665. 2015.
|
|
56
|
van Loosdregt J, Fleskens V, Tiemessen MM,
Mokry M, van Boxtel R, Meerding J, Pals CE, Kurek D, Baert MR,
Delemarre EM, et al: Canonical wnt signaling negatively modulates
regulatory T cell function. Immunity. 39:298–310. 2013.
|
|
57
|
Yang ZY, Zhang WL, Jiang CW and Sun G:
PCBP1-mediated regulation of WNT signaling is critical for breast
tumorigenesis. Cell Biol Toxicol. 39:2331–2343. 2023.
|
|
58
|
Trotter TN, Dagotto CE, Serra D, Wang T,
Yang X, Acharya CR, Wei J, Lei G, Lyerly HK and Hartman ZC: Dormant
tumors circumvent tumor-specific adaptive immunity by establishing
a Treg-dominated niche via DKK3. JCI Insight. 8:e1744582023.
|
|
59
|
Ding Y, Shen S, Lino AC, Curotto de
Lafaille MA and Lafaille JJ: Beta-catenin stabilization extends
regulatory T cell survival and induces anergy in nonregulatory T
cells. Nat Med. 14:162–169. 2008.
|
|
60
|
Dai W, Liu F, Li C, Lu Y, Lu X, Du S, Chen
Y, Weng D and Chen J: Blockade of Wnt/β-catenin pathway aggravated
silica-induced lung inflammation through tregs regulation on Th
immune responses. Mediators Inflamm. 2016:62356142016.
|
|
61
|
Gunaydin G: CAFs interacting With TAMs in
tumor microenvironment to enhance tumorigenesis and immune evasion.
Front Oncol. 11:6683492021.
|
|
62
|
Hu D, Li Z, Zheng B, Lin X, Pan Y, Gong P,
Zhuo W, Hu Y, Chen C, Chen L, et al: Cancer-associated fibroblasts
in breast cancer: Challenges and opportunities. Cancer Commun
(Lond). 42:401–434. 2022.
|
|
63
|
Xie J, Qi X, Wang Y, Yin X, Xu W, Han S,
Cai Y and Han W: Cancer-associated fibroblasts secrete
hypoxia-induced serglycin to promote head and neck squamous cell
carcinoma tumor cell growth in vitro and in vivo by activating the
Wnt/β-catenin pathway. Cell Oncol (Dordr). 44:661–671. 2021.
|
|
64
|
Aizawa T, Karasawa H, Funayama R, Shirota
M, Suzuki T, Maeda S, Suzuki H, Yamamura A, Naitoh T, Nakayama K
and Unno M: Cancer-associated fibroblasts secrete Wnt2 to promote
cancer progression in colorectal cancer. Cancer Med. 8:6370–6382.
2019.
|
|
65
|
Bochet L, Lehuédé C, Dauvillier S, Wang
YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le
Gonidec S, et al: Adipocyte-derived fibroblasts promote tumor
progression and contribute to the desmoplastic reaction in breast
cancer. Cancer Res. 73:5657–5668. 2013.
|
|
66
|
Chen Y, Zeng C, Zhan Y, Wang H, Jiang X
and Li W: Aberrant low expression of p85α in stromal fibroblasts
promotes breast cancer cell metastasis through exosome-mediated
paracrine Wnt10b. Oncogene. 36:4692–4705. 2017.
|
|
67
|
Liu J, Shen JX, Wu HT, Li XL, Wen XF, Du
CW and Zhang GJ: Collagen 1A1 (COL1A1) promotes metastasis of
breast cancer and is a potential therapeutic target. Discov Med.
25:211–223. 2018.
|
|
68
|
Kim SH, Lee HY, Jung SP, Kim S, Lee JE,
Nam SJ and Bae JW: Role of secreted type I collagen derived from
stromal cells in two breast cancer cell lines. Oncol Lett.
8:507–512. 2014.
|
|
69
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012.
|
|
70
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
|
|
71
|
Cuiffo BG and Karnoub AE: Mesenchymal stem
cells in tumor development: Emerging roles and concepts. Cell Adh
Migr. 6:220–230. 2012.
|
|
72
|
Liang W and Chen X, Zhang S, Fang J, Chen
M, Xu Y and Chen X: Mesenchymal stem cells as a double-edged sword
in tumor growth: Focusing on MSC-derived cytokines. Cell Mol Biol
Lett. 26:32021.
|
|
73
|
Sun Z, Wang S and Zhao RC: The roles of
mesenchymal stem cells in tumor inflammatory microenvironment. J
Hematol Oncol. 7:142014.
|
|
74
|
Shi Y, Du L, Lin L and Wang Y:
Tumour-associated mesenchymal stem/stromal cells: Emerging
therapeutic targets. Nat Rev Drug Discov. 16:35–52. 2017.
|
|
75
|
Kar S, Jasuja H, Katti DR and Katti KS:
Wnt/β-catenin signaling pathway regulates osteogenesis for breast
cancer bone metastasis: Experiments in an in vitro nanoclay
scaffold cancer testbed. ACS Biomater Sci Eng. 6:2600–2611.
2020.
|
|
76
|
Arrigoni C, De Luca P, Gilardi M, Previdi
S, Broggini M and Moretti M: Direct but not indirect co-culture
with osteogenically differentiated human bone marrow stromal cells
increases RANKL/OPG ratio in human breast cancer cells generating
bone metastases. Mol Cancer. 13:2382014.
|
|
77
|
Qiao L, Xu ZL, Zhao TJ, Ye LH and Zhang
XD: Dkk-1 secreted by mesenchymal stem cells inhibits growth of
breast cancer cells via depression of Wnt signalling. Cancer Lett.
269:67–77. 2008.
|
|
78
|
Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao
RC, Ye L and Zhang X: Suppression of tumorigenesis by human
mesenchymal stem cells in a hepatoma model. Cell Res. 18:500–507.
2008.
|
|
79
|
Khakoo AY, Pati S, Anderson SA, Reid W,
Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. 2006.
|
|
80
|
Dasari VR, Velpula KK, Kaur K, Fassett D,
Klopfenstein JD, Dinh DH, Gujrati M and Rao JS: Cord blood stem
cell-mediated induction of apoptosis in glioma downregulates
X-linked inhibitor of apoptosis protein (XIAP). PLoS One.
5:e118132010.
|
|
81
|
Otsu K, Das S, Houser SD, Quadri SK,
Bhattacharya S and Bhattacharya J: Concentration-dependent
inhibition of angiogenesis by mesenchymal stem cells. Blood.
113:4197–4205. 2009.
|
|
82
|
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian
C, Li J, Yan X, Liu Y, Shao C and Zhao RC: Human mesenchymal stem
cells inhibit cancer cell proliferation by secreting DKK-1.
Leukemia. 23:925–933. 2009.
|
|
83
|
Vallée A, Lecarpentier Y, Guillevin R and
Vallée JN: Interactions between TGF-β1, canonical WNT/β-catenin
pathway and PPAR γ in radiation-induced fibrosis. Oncotarget.
8:90579–90604. 2017.
|
|
84
|
Patel SA, Nilsson MB, Le X, Cascone T,
Jain RK and Heymach JV: Molecular mechanisms and future
implications of VEGF/VEGFR in cancer therapy. Clin Cancer Res.
29:30–39. 2023.
|
|
85
|
Zerlin M, Julius MA and Kitajewski J:
Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 11:63–69.
2008.
|
|
86
|
Mankuzhy P, Dharmarajan A, Perumalsamy LR,
Sharun K, Samji P and Dilley RJ: The role of Wnt signaling in
mesenchymal stromal cell-driven angiogenesis. Tissue Cell.
85:1022402023.
|
|
87
|
Xie W, Zhang Y, Zhang S, Wang F, Zhang K,
Huang Y, Zhou Z, Huang G and Wang J: Oxymatrine enhanced anti-tumor
effects of Bevacizumab against triple-negative breast cancer via
abating Wnt/β-Catenin signaling pathway. Am J Cancer Res.
9:1796–1814. 2019.
|
|
88
|
Pagani E, Ruffini F, Antonini Cappellini
GC, Scoppola A, Fortes C, Marchetti P, Graziani G, D'Atri S and
Lacal PM: Placenta growth factor and neuropilin-1 collaborate in
promoting melanoma aggressiveness. Int J Oncol. 48:1581–1589.
2016.
|
|
89
|
Ruffini F, D'Atri S and Lacal PM:
Neuropilin-1 expression promotes invasiveness of melanoma cells
through vascular endothelial growth factor receptor-2-dependent and
-independent mechanisms. Int J Oncol. 43:297–306. 2013.
|
|
90
|
Nilsson LM, Nilsson-Ohman J, Zetterqvist
AV and Gomez MF: Nuclear factor of activated T-cells transcription
factors in the vasculature: The good guys or the bad guys? Curr
Opin Lipidol. 19:483–490. 2008.
|
|
91
|
Reis M and Liebner S: Wnt signaling in the
vasculature. Exp Cell Res. 319:1317–1323. 2013.
|
|
92
|
Roma-Rodrigues C, Fernandes AR and
Baptista PV: Exosome in tumour microenvironment: Overview of the
crosstalk between normal and cancer cells. Biomed Res Int.
2014:1794862014.
|
|
93
|
Graner MW, Schnell S and Olin MR:
Tumor-derived exosomes, microRNAs, and cancer immune suppression.
Semin Immunopathol. 40:505–515. 2018.
|
|
94
|
Ruivo CF, Adem B, Silva M and Melo SA: The
biology of cancer exosomes: Insights and new perspectives. Cancer
Res. 77:6480–6488. 2017.
|
|
95
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020.
|
|
96
|
Samuel P, Fabbri M and Carter DRF:
Mechanisms of drug resistance in cancer: The role of extracellular
vesicles. Proteomics. 17:16003752017.
|
|
97
|
Abd Elmageed ZY, Yang Y, Thomas R, Ranjan
M, Mondal D, Moroz K, Fang Z, Rezk BM, Moparty K, Sikka SC, et al:
Neoplastic reprogramming of patient-derived adipose stem cells by
prostate cancer cell-associated exosomes. Stem Cells. 32:983–997.
2014.
|
|
98
|
Melo SA, Sugimoto H, O'Connell JT, Kato N,
Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et
al: Cancer exosomes perform cell-independent microRNA biogenesis
and promote tumorigenesis. Cancer Cell. 26:707–721. 2014.
|
|
99
|
Liang Z, Liu L, Gao R, Che C and Yang G:
Downregulation of exosomal miR-7-5p promotes breast cancer
migration and invasion by targeting RYK and participating in the
atypical WNT signalling pathway. Cell Mol Biol Lett. 27:882022.
|
|
100
|
Xiao Z, Feng X, Zhou Y, Li P, Luo J, Zhang
W, Zhou J, Zhao J, Wang D, Wang Y, et al: Exosomal miR-10527-5p
inhibits migration, invasion, lymphangiogenesis and lymphatic
metastasis by affecting Wnt/β-catenin signaling via Rab10 in
esophageal squamous cell carcinoma. Int J Nanomedicine. 18:95–114.
2023.
|
|
101
|
Naseri Z, Oskuee RK, Jaafari MR and
Forouzandeh Moghadam M: Exosome-mediated delivery of functionally
active miRNA-142-3p inhibitor reduces tumorigenicity of breast
cancer in vitro and in vivo. Int J Nanomedicine. 13:7727–7747.
2018.
|
|
102
|
Gargalionis AN, Papavassiliou KA, Basdra
EK and Papavassiliou AG: mTOR signaling components in tumor
mechanobiology. Int J Mol Sci. 23:18252022.
|
|
103
|
Liu Q, Luo Q, Ju Y and Song G: Role of the
mechanical microenvironment in cancer development and progression.
Cancer Biol Med. 17:282–292. 2020.
|
|
104
|
Sun J, Luo Q, Liu L and Song G: Low-level
shear stress induces differentiation of liver cancer stem cells via
the Wnt/β-catenin signalling pathway. Exp Cell Res. 375:90–96.
2019.
|
|
105
|
Li Y, Mao AS, Seo BR, Zhao X, Gupta SK,
Chen M, Han YL, Shih TY, Mooney DJ and Guo M: Compression-induced
dedifferentiation of adipocytes promotes tumor progression. Sci
Adv. 6:eaax56112020.
|
|
106
|
Yu H, Mouw JK and Weaver VM: Forcing form
and function: Biomechanical regulation of tumor evolution. Trends
Cell Biol. 21:47–56. 2011.
|
|
107
|
Provenzano PP and Keely PJ: Mechanical
signaling through the cytoskeleton regulates cell proliferation by
coordinated focal adhesion and Rho GTPase signaling. J Cell Sci.
124:1195–1205. 2011.
|
|
108
|
Schrader J, Gordon-Walker TT, Aucot RL,
van Deemter M, Quaas A, Walsh S, Benten D, Forbes SJ, Wells RG and
Iredale JP: Matrix stiffness modulates proliferation,
chemotherapeutic response, and dormancy in hepatocellular carcinoma
cells. Hepatology. 53:1192–1205. 2011.
|
|
109
|
Johnson RW, Merkel AR, Page JM, Ruppender
NS, Guelcher SA and Sterling JA: Wnt signaling induces gene
expression of factors associated with bone destruction in lung and
breast cancer. Clin Exp Metastasis. 31:945–959. 2014.
|
|
110
|
Chen Z, Han F, Du Y, Shi H and Zhou W:
Hypoxic microenvironment in cancer: molecular mechanisms and
therapeutic interventions. Signal Transduct Target Ther.
8:702023.
|
|
111
|
Schito L and Semenza GL: Hypoxia-inducible
factors: Master regulators of cancer progression. Trends Cancer.
2:758–770. 2016.
|
|
112
|
Zhou F, Sun J, Ye L, Jiang T, Li W, Su C,
Ren S, Wu F, Zhou C and Gao G: Fibronectin promotes tumor
angiogenesis and progression of non-small-cell lung cancer by
elevating WISP3 expression via FAK/MAPK/HIF-1α axis and activating
wnt signaling pathway. Exp Hematol Oncol. 12:612023.
|
|
113
|
Yan Y, Liu F, Han L, Zhao L, Chen J,
Olopade OI, He M and Wei M: HIF-2α promotes conversion to a stem
cell phenotype and induces chemoresistance in breast cancer cells
by activating Wnt and Notch pathways. J Exp Clin Cancer Res.
37:2562018.
|
|
114
|
Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren
Y, Li Z, Pang D and Qian C: MiR-23a promotes TGF-β1-induced EMT and
tumor metastasis in breast cancer cells by directly targeting CDH1
and activating Wnt/β-catenin signaling. Oncotarget. 8:69538–69550.
2017.
|
|
115
|
Lopez Almeida L, Sebbagh M, Bertucci F,
Finetti P, Wicinski J, Marchetto S, Castellano R, Josselin E,
Charafe-Jauffret E, Ginestier C, et al: The SCRIB paralog
LANO/LRRC1 regulates breast cancer stem cell fate through
WNT/β-catenin signaling. Stem Cell Rep. 11:1040–1050. 2018.
|
|
116
|
Bhuvanalakshmi G, Basappa, Rangappa KS,
Dharmarajan A, Sethi G, Kumar AP and Warrier S: Breast cancer
stem-like cells are inhibited by diosgenin, a steroidal saponin, by
the attenuation of the wnt β-catenin signaling via the wnt
antagonist secreted frizzled related protein-4. Front Pharmacol.
8:1242017.
|
|
117
|
Lv C, Li F, Li X, Tian Y, Zhang Y, Sheng
X, Song Y, Meng Q, Yuan S, Luan L, et al: MiR-31 promotes mammary
stem cell expansion and breast tumorigenesis by suppressing Wnt
signaling antagonists. Nat Commun. 8:10362017.
|
|
118
|
Xu BS, Chen HY, Que Y, Xiao W, Zeng MS and
Zhang X: ALKATI interacts with c-Myc and promotes cancer
stem cell-like properties in sarcoma. Oncogene. 39:151–163.
2020.
|
|
119
|
Dittmer J: Breast cancer stem cells:
Features, key drivers and treatment options. Semin Cancer Biol.
53:59–74. 2018.
|
|
120
|
Wang F, Chen L, Kong D, Zhang X, Xia S,
Liang B, Li Y, Zhou Y, Zhang Z, Shao J, et al: Canonical Wnt
signaling promotes HSC glycolysis and liver fibrosis through an
LDH-A/HIF-1α transcriptional complex. Hepatology. 79:606–623.
2024.
|
|
121
|
Li X, Yang J, Ni R, Chen J, Zhou Y, Song
H, Jin L and Pan Y: Hypoxia-induced lncRNA RBM5-AS1 promotes
tumorigenesis via activating wnt/β-catenin signaling in breast
cancer. Cell Death Dis. 13:952022.
|
|
122
|
Tirpe AA, Gulei D, Ciortea SM, Crivii C
and Berindan-Neagoe I: Hypoxia: Overview on hypoxia-mediated
mechanisms with a focus on the role of HIF genes. Int J Mol Sci.
20:61402019.
|
|
123
|
Wu J, Chen J, Feng Y, Tian H and Chen X:
Tumor microenvironment as the 'regulator' and 'target' for gene
therapy. J Gene Med. 21:e30882019.
|
|
124
|
Lee S, Toft NJ, Axelsen TV, Espejo MS,
Pedersen TM, Mele M, Pedersen HL, Balling E, Johansen T, Burton M,
et al: Carbonic anhydrases reduce the acidity of the tumor
microenvironment, promote immune infiltration, decelerate tumor
growth, and improve survival in ErbB2/HER2-enriched breast cancer.
Breast Cancer Res. 25:462023.
|
|
125
|
Melnik S, Dvornikov D, Müller-Decker K,
Depner S, Stannek P, Meister M, Warth A, Thomas M, Muley T, Risch
A, et al: Cancer cell specific inhibition of Wnt/β-catenin
signaling by forced intracellular acidification. Cell Discov.
4:372018.
|
|
126
|
Bao L, Wu Y, Ren Z, Huang Y, Jiang Y, Li
K, Xu X, Ye Y and Gui Z: Comprehensive pan-cancer analysis
indicates UCHL5 as a novel cancer biomarker and promotes cervical
cancer progression through the wnt signaling pathway. Biol Direct.
19:1392024.
|
|
127
|
Ghosh A and Gopinath SCB: Molecular
mechanism of breast cancer and predisposition of mouse mammary
tumor virus propagation cycle. Curr Med Chem. May 8–2024.Epub ahead
of print.
|
|
128
|
Wang F, Wang W, Wang M and Chen D: Genetic
landscape of breast cancer subtypes following radiation therapy:
Insights from comprehensive profiling. Front Oncol.
14:12915092024.
|
|
129
|
Wooster R, Neuhausen SL, Mangion J, Quirk
Y, Ford D, Collins N, Nguyen K, Seal S, Tran T, Averill D, et al:
Localization of a breast cancer susceptibility gene, BRCA2, to
chromosome 13q12-13. Science. 265:2088–2090. 1994.
|
|
130
|
Hall JM, Lee MK, Newman B, Morrow JE,
Anderson LA, Huey B and King MC: Linkage of early-onset familial
breast cancer to chromosome 17q21. Science. 250:1684–1689.
1990.
|
|
131
|
Wan A, Zhang G, Ma D, Zhang Y and Qi X: An
overview of the research progress of BRCA gene mutations in breast
cancer. Biochim Biophys Acta Rev Cancer. 1878:1889072023.
|
|
132
|
Weber F, Shen L, Fukino K, Patocs A,
Mutter GL, Caldes T and Eng C: Total-genome analysis of
BRCA1/2-related invasive carcinomas of the breast identifies tumor
stroma as potential landscaper for neoplastic initiation. Am J Hum
Genet. 78:961–972. 2006.
|
|
133
|
Ghosh S, Lu Y, Katz A, Hu Y and Li R:
Tumor suppressor BRCA1 inhibits a breast cancer-associated promoter
of the aromatase gene (CYP19) in human adipose stromal cells. Am J
Physiol Endocrinol Metab. 292:E246–E252. 2007.
|
|
134
|
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG and
Weiss SJ: Canonical Wnt signaling regulates Slug activity and links
epithelial-mesenchymal transition with epigenetic Breast Cancer 1,
Early Onset (BRCA1) repression. Proc Natl Acad Sci USA.
109:16654–16659. 2012.
|
|
135
|
Li H, Sekine M, Tung N and Avraham HK:
Wild-type BRCA1, but not Mutated BRCA1, regulates the expression of
the nuclear Form of beta-catenin. Mol Cancer Res. 8:407–420.
2010.
|
|
136
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012.
|
|
137
|
Kastenhuber ER and Lowe SW: Putting p53 in
context. Cell. 170:1062–1078. 2017.
|
|
138
|
Walerych D, Napoli M, Collavin L and Del
Sal G: The rebel angel: mutant p53 as the driving oncogene in
breast cancer. Carcinogenesis. 33:2007–2017. 2012.
|
|
139
|
Kim NH, Cha YH, Lee J, Lee SH, Yang JH,
Yun JS, Cho ES, Zhang X, Nam M, Kim N, et al: Snail reprograms
glucose metabolism by repressing phosphofructokinase PFKP allowing
cancer cell survival under metabolic stress. Nat Commun.
8:143742017.
|
|
140
|
Wellenstein MD, Coffelt SB, Duits DEM, van
Miltenburg MH, Slagter M, de Rink I, Henneman L, Kas SM, Prekovic
S, Hau CS, et al: Loss of p53 triggers WNT-dependent systemic
inflammation to drive breast cancer metastasis. Nature.
572:538–542. 2019.
|
|
141
|
Roarty K, Pfefferle AD, Creighton CJ,
Perou CM and Rosen JM: Ror2-mediated alternative Wnt signaling
regulates cell fate and adhesion during mammary tumor progression.
Oncogene. 36:5958–5968. 2017.
|
|
142
|
Nolan E, Lindeman GJ and Visvader JE:
Deciphering breast cancer: From biology to the clinic. Cell.
186:1708–1728. 2023.
|
|
143
|
Wang X, Song C, Ye Y, Gu Y, Li X, Chen P,
Leng D, Xiao J, Wu H, Xie S, et al: BRD9-mediated control of the
TGF-β/activin/nodal pathway regulates self-renewal and
differentiation of human embryonic stem cells and progression of
cancer cells. Nucleic Acids Res. 51:11634–11651. 2023.
|
|
144
|
Song X, Wei C and Li X: The signaling
pathways associated with breast cancer bone metastasis. Front
Oncol. 12:8556092022.
|
|
145
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
146
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008.
|
|
147
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017.
|
|
148
|
Luo K: Signaling cross talk between
TGF-β/smad and other signaling pathways. Cold Spring Harb Perspect
Biol. 9:a0221372017.
|
|
149
|
Shi X, Yang J, Deng S, Xu H, Wu D, Zeng Q,
Wang S, Hu T, Wu F and Zhou H: TGF-β signaling in the tumor
metabolic microenvironment and targeted therapies. J Hematol Oncol.
15:1352022.
|
|
150
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015.
|
|
151
|
Scheel C, Eaton EN, Li SH, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL and
Weinberg RA: Paracrine and autocrine signals induce and maintain
mesenchymal and stem cell states in the breast. Cell. 145:926–940.
2011.
|
|
152
|
Funa NS, Mjoseng HK, de Lichtenberg KH,
Raineri S, Esen D, Egeskov-Madsen AR, Quaranta R, Jørgensen MC,
Hansen MS, van Cuyl Kuylenstierna J, et al: TGF-β modulates cell
fate in human ES cell-derived foregut endoderm by inhibiting wnt
and BMP signaling. Stem Cell Reports. 19:973–992. 2024.
|
|
153
|
Liu L, Chen G, Chen T, Shi W, Hu H, Song
K, Huang R, Cai H and He Y: si-SNHG5-FOXF2 inhibits TGF-β1-induced
fibrosis in human primary endometrial stromal cells by the
wnt/β-catenin signalling pathway. Stem Cell Res Ther.
11:4792020.
|
|
154
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001.
|
|
155
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004.
|
|
156
|
Shimura T, Takenaka Y, Tsutsumi S, Hogan
V, Kikuchi A and Raz A: Galectin-3, a novel binding partner of
beta-catenin. Cancer Res. 64:6363–6367. 2004.
|
|
157
|
Luo J, Chen J, Deng ZL, Luo X, Song WX,
Sharff KA, Tang N, Haydon RC, Luu HH and He TC: Wnt signaling and
human diseases: What are the therapeutic implications? Lab Invest.
87:97–103. 2007.
|
|
158
|
Maric G, Annis MG, MacDonald PA, Russo C,
Perkins D, Siwak DR, Mills GB and Siegel PM: GPNMB augments Wnt-1
mediated breast tumor initiation and growth by enhancing
PI3K/AKT/mTOR pathway signaling and β-catenin activity. Oncogene.
38:5294–5307. 2019.
|
|
159
|
Perry JM, He XC, Sugimura R, Grindley JC,
Haug JS, Ding S and Li L: Cooperation between both
Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive
hematopoietic stem cell self-renewal and expansion. Genes Dev.
25:1928–1942. 2011.
|
|
160
|
Mulholland DJ, Dedhar S, Wu H and Nelson
CC: PTEN and GSK3beta: Key regulators of progression to
androgen-independent prostate cancer. Oncogene. 25:329–337.
2006.
|
|
161
|
Siddharth S, Goutam K, Das S, Nayak A,
Nayak D, Sethy C, Wyatt MD and Kundu CN: Nectin-4 is a breast
cancer stem cell marker that induces WNT/β-catenin signaling via
Pi3k/Akt axis. Int J Biochem Cell Biol. 89:85–94. 2017.
|
|
162
|
Bachelder RE, Yoon SO, Franci C, de
Herreros AG and Mercurio AM: Glycogen synthase kinase-3 is an
endogenous inhibitor of Snail transcription: Implications for the
epithelial-mesenchymal transition. J Cell Biol. 168:29–33.
2005.
|
|
163
|
Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH,
Way TD and Chen WJ: 3,5,4′-Trimethoxystilbene, a natural
methoxylated analog of resveratrol, inhibits breast cancer cell
invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin
signaling cascades and reversal of epithelial-mesenchymal
transition. Toxicol Appl Pharmacol. 272:746–756. 2013.
|
|
164
|
Haiaty S, Rashidi MR, Akbarzadeh M,
Bazmani A, Mostafazadeh M, Nikanfar S, Zibaei Z, Rahbarghazi R and
Nouri M: Thymoquinone inhibited vasculogenic capacity and promoted
mesenchymal-epithelial transition of human breast cancer stem
cells. BMC Complement Med Ther. 21:832021.
|
|
165
|
Arqués O, Chicote I, Puig I, Tenbaum SP,
Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J,
Silberschmidt D, et al: Tankyrase inhibition blocks Wnt/β-catenin
pathway and reverts resistance to PI3K and AKT inhibitors in the
treatment of colorectal cancer. Clin Cancer Res. 22:644–656.
2016.
|
|
166
|
Katoh M: Network of WNT and other
regulatory signaling cascades in pluripotent stem cells and cancer
stem cells. Curr Pharm Biotechnol. 12:160–170. 2011.
|
|
167
|
Nyeng P, Norgaard GA, Kobberup S and
Jensen J: FGF10 maintains distal lung bud epithelium and excessive
signaling leads to progenitor state arrest, distalization, and
goblet cell metaplasia. BMC Dev Biol. 8:22008.
|
|
168
|
Shimokawa T, Furukawa Y, Sakai M, Li M,
Miwa N, Lin YM and Nakamura Y: Involvement of the FGF18 gene in
colorectal carcinogenesis, as a novel downstream target of the
beta-catenin/ T-cell factor complex. Cancer Res. 63:6116–6120.
2003.
|
|
169
|
Chamorro MN, Schwartz DR, Vonica A,
Brivanlou AH, Cho KR and Varmus HE: FGF-20 and DKK1 are
transcriptional targets of beta-catenin and FGF-20 is implicated in
cancer and development. EMBO J. 24:73–84. 2005.
|
|
170
|
Pai R, Dunlap D, Qing J, Mohtashemi I,
Hotzel K and French DM: Inhibition of fibroblast growth factor 19
reduces tumor growth by modulating beta-catenin signaling. Cancer
Res. 68:5086–5095. 2008.
|
|
171
|
El-Hariry I, Pignatelli M and Lemoine NR:
FGF-1 and FGF-2 modulate the E-cadherin/catenin system in
pancreatic adenocarcinoma cell lines. Br J Cancer. 84:1656–1663.
2001.
|
|
172
|
Brembeck FH, Rosário M and Birchmeier W:
Balancing cell adhesion and Wnt signaling, the key role of
beta-catenin. Curr Opin Genet Dev. 16:51–59. 2006.
|
|
173
|
Davidson G, Shen J, Huang YL, Su Y,
Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M and
Niehrs C: Cell cycle control of wnt receptor activation. Dev Cell.
17:788–799. 2009.
|
|
174
|
Brennan KR and Brown AM: Wnt proteins in
mammary development and cancer. J Mammary Gland Biol Neoplasia.
9:119–131. 2004.
|
|
175
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
|
|
176
|
Lee FS, Lane TF, Kuo A, Shackleford GM and
Leder P: Insertional mutagenesis identifies a member of the Wnt
gene family as a candidate oncogene in the mammary epithelium of
int-2/Fgf-3 transgenic mice. Proc Natl Acad Sci USA. 92:2268–2272.
1995.
|
|
177
|
Theodorou V, Kimm MA, Boer M, Wessels L,
Theelen W, Jonkers J and Hilkens J: MMTV insertional mutagenesis
identifies genes, gene families and pathways involved in mammary
cancer. Nat Genet. 39:759–769. 2007.
|
|
178
|
Nguyen TM, Kabotyanski EB, Dou Y, Reineke
LC, Zhang P, Zhang XH, Malovannaya A, Jung SY, Mo Q, Roarty KP, et
al: FGFR1-activated translation of WNT pathway components with
structured 5′ UTRs is vulnerable to inhibition of EIF4A-dependent
translation initiation. Cancer Res. 78:4229–4240. 2018.
|
|
179
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014.
|
|
180
|
Chatterjee S and Sil PC: Targeting the
crosstalks of Wnt pathway with Hedgehog and Notch for cancer
therapy. Pharmacol Res. 142:251–261. 2019.
|
|
181
|
Ormestad M, Astorga J, Landgren H, Wang T,
Johansson BR, Miura N and Carlsson P: Foxf1 and Foxf2 control
murine gut development by limiting mesenchymal Wnt signaling and
promoting extracellular matrix production. Development.
133:833–843. 2006.
|
|
182
|
Maeda O, Kondo M, Fujita T, Usami N, Fukui
T, Shimokata K, Ando T, Goto H and Sekido Y: Enhancement of
GLI1-transcriptional activity by beta-catenin in human cancer
cells. Oncol Rep. 16:91–96. 2006.
|
|
183
|
Arnold KM, Pohlig RT and Sims-Mourtada J:
Co-activation of Hedgehog and Wnt signaling pathways is associated
with poor outcomes in triple negative breast cancer. Oncol Lett.
14:5285–5292. 2017.
|
|
184
|
Katoh M and Katoh M: Molecular genetics
and targeted therapy of WNT-related human diseases (Review). Int J
Mol Med. 40:587–606. 2017.
|
|
185
|
Wu Y, Ginther C, Kim J, Mosher N, Chung S,
Slamon D and Vadgama JV: Expression of Wnt3 activates Wnt/β-catenin
pathway and promotes EMT-like phenotype in trastuzumab-resistant
HER2-overexpressing breast cancer cells. Mol Cancer Res.
10:1597–1606. 2012.
|
|
186
|
Zou Y, Yang A, Chen B, Deng X, Xie J, Dai
D, Zhang J, Tang H, Wu T, Zhou Z, et al: crVDAC3 alleviates
ferroptosis by impeding HSPB1 ubiquitination and confers
trastuzumab deruxtecan resistance in HER2-low breast cancer. Drug
Resist Updat. 77:1011262024.
|
|
187
|
Castagnoli L, Tagliabue E and Pupa SM:
Inhibition of the Wnt signalling pathway: An avenue to control
breast cancer aggressiveness. Int J Mol Sci. 21:90692020.
|
|
188
|
Castagnoli L, Franceschini A, Cancila V,
Dugo M, Bigliardi M, Chiodoni C, Toneguzzo P, Regondi V, Corsetto
PA, Pietrantonio F, et al: CD36 enrichment in HER2-positive
mesenchymal stem cells drives therapy refractoriness in breast
cancer. J Exp Clin Cancer Res. 44:192025.
|
|
189
|
Xu J, Prosperi JR, Choudhury N, Olopade OI
and Goss KH: β-Catenin is required for the tumorigenic behavior of
triple-negative breast cancer cells. PLoS One. 10:e01170972015.
|
|
190
|
Shetti D, Zhang B, Fan C, Mo C, Lee BH and
Wei K: Low dose of paclitaxel combined with XAV939 Attenuates
metastasis, angiogenesis and growth in breast cancer by suppressing
Wnt signaling. Cells. 8:8922019.
|
|
191
|
Saleh R, Taha RZ, Sasidharan Nair V,
Alajez NM and Elkord E: PD-L1 blockade by atezolizumab
downregulates signaling pathways associated with tumor growth,
metastasis, and hypoxia in human triple negative breast cancer.
Cancers (Basel). 11:10502019.
|
|
192
|
Castagnoli L, Cancila V, Cordoba-Romero
SL, Faraci S, Talarico G, Belmonte B, Iorio MV, Milani M, Volpari
T, Chiodoni C, et al: WNT signaling modulates PD-L1 expression in
the stem cell compartment of triple-negative breast cancer.
Oncogene. 38:4047–4060. 2019.
|
|
193
|
Merikhian P, Eisavand MR and Farahmand L:
Triple-negative breast cancer: Understanding Wnt signaling in drug
resistance. Cancer Cell Int. 21:4192021.
|
|
194
|
Ke M, Zhu H, Lin Y, Zhang Y, Tang T, Xie
Y, Chen ZS, Wang X and Shen Y: Actin-related protein 2/3 complex
subunit 1B promotes ovarian cancer progression by regulating the
AKT/PI3K/mTOR signaling pathway. J Transl Int Med. 12:406–423.
2024.
|
|
195
|
Tang L, Wang D, Hu T, Lin X and Wu S:
Current applications of tumor local ablation (TLA) combined with
immune checkpoint inhibitors in breast cancer treatment. Cancer
Drug Resist. 7:332024.
|
|
196
|
Torres VI, Godoy JA and Inestrosa NC:
Modulating Wnt signaling at the root: Porcupine and Wnt acylation.
Pharmacol Ther. 198:34–45. 2019.
|
|
197
|
Kabiri Z, Numata A, Kawasaki A, Edison,
Tenen DG and Virshup DM: Wnts are dispensable for differentiation
and self-renewal of adult murine hematopoietic stem cells. Blood.
126:1086–1094. 2015.
|
|
198
|
Takada R, Satomi Y, Kurata T, Ueno N,
Norioka S, Kondoh H, Takao T and Takada S: Monounsaturated fatty
acid modification of Wnt protein: Its role in Wnt secretion. Dev
Cell. 11:791–801. 2006.
|
|
199
|
Hausmann G, Bänziger C and Basler K:
Helping Wingless take flight: How WNT proteins are secreted. Nat
Rev Mol Cell Biol. 8:331–336. 2007.
|
|
200
|
van den Heuvel M, Harryman-Samos C,
Klingensmith J, Perrimon N and Nusse R: Mutations in the segment
polarity genes wingless and porcupine impair secretion of the
wingless protein. EMBO J. 12:5293–5302. 1993.
|
|
201
|
Shah K, Panchal S and Patel B: Porcupine
inhibitors: Novel and emerging anti-cancer therapeutics targeting
the Wnt signaling pathway. Pharmacol Res. 167:1055322021.
|
|
202
|
Resh MD: Palmitoylation of proteins in
cancer. Biochem Soc Trans. 45:409–416. 2017.
|
|
203
|
Madan B, Ke Z, Harmston N, Ho SY, Frois
AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, et al:
Wnt addiction of genetically defined cancers reversed by PORCN
inhibition. Oncogene. 35:2197–2207. 2016.
|
|
204
|
Cheng D, Liu J, Han D, Zhang G, Gao W,
Hsieh MH, Ng N, Kasibhatla S, Tompkins C, Li J, et al: Discovery of
pyridinyl acetamide derivatives as potent, selective, and orally
bioavailable porcupine inhibitors. ACS Med Chem Lett. 7:676–680.
2016.
|
|
205
|
Liu Y, Qi X, Donnelly L,
Elghobashi-Meinhardt N, Long T, Zhou RW, Sun Y, Wang B and Li X:
Mechanisms and inhibition of porcupine-mediated wnt acylation.
Nature. 607:816–822. 2022.
|
|
206
|
Doo DW, Meza-Perez S, Londoño AI,
Goldsberry WN, Katre AA, Boone JD, Moore DJ, Hudson CT, Betella I,
McCaw TR, et al: Inhibition of the Wnt/β-catenin pathway enhances
antitumor immunity in ovarian cancer. Ther Adv Med Oncol.
12:17588359209137982020.
|
|
207
|
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang
T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al: Targeting
Wnt-driven cancer through the inhibition of porcupine by LGK974.
Proc Natl Acad Sci USA. 110:20224–20229. 2013.
|
|
208
|
Nusse R and Varmus H: Three decades of
Wnts: A personal perspective on how a scientific field developed.
EMBO J. 31:2670–2684. 2012.
|
|
209
|
Goswami VG and Patel BD: Recent updates on
Wnt signaling modulators: A patent review (2014-2020). Expert Opin
Ther Pat. 31:1009–1043. 2021.
|
|
210
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009.
|
|
211
|
Lu W and Li Y: Salinomycin suppresses LRP6
expression and inhibits both Wnt/β-catenin and mTORC1 signaling in
breast and prostate cancer cells. J Cell Biochem. 115:1799–1807.
2014.
|
|
212
|
Le PN, McDermott JD and Jimeno A:
Targeting the Wnt pathway in human cancers: Therapeutic targeting
with a focus on OMP-54F28. Pharmacol Ther. 146:1–11. 2015.
|
|
213
|
Lu W, Lin C, Roberts MJ, Waud WR, Piazza
GA and Li Y: Niclosamide suppresses cancer cell growth by inducing
Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin
pathway. PLoS One. 6:e292902011.
|
|
214
|
Londoño-Joshi AI, Arend RC, Aristizabal L,
Lu W, Samant RS, Metge BJ, Hidalgo B, Grizzle WE, Conner M,
Forero-Torres A, et al: Effect of niclosamide on basal-like breast
cancers. Mol Cancer Ther. 13:800–811. 2014.
|
|
215
|
Wang YC, Chao TK, Chang CC, Yo YT, Yu MH
and Lai HC: Drug screening identifies niclosamide as an inhibitor
of breast cancer stem-like cells. PLoS One. 8:e745382013.
|
|
216
|
Ye T, Xiong Y, Yan Y, Xia Y, Song X, Liu
L, Li D, Wang N, Zhang L, Zhu Y, et al: The anthelmintic drug
niclosamide induces apoptosis, impairs metastasis and reduces
immunosuppressive cells in breast cancer model. PLoS One.
9:e858872014.
|
|
217
|
Gurney A, Axelrod F, Bond CJ, Cain J,
Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et
al: Wnt pathway inhibition via the targeting of Frizzled receptors
results in decreased growth and tumorigenicity of human tumors.
Proc Natl Acad Sci USA. 109:11717–11722. 2012.
|
|
218
|
Fischer MM, Cancilla B, Yeung VP,
Cattaruzza F, Chartier C, Murriel CL, Cain J, Tam R, Cheng CY,
Evans JW, et al: WNT antagonists exhibit unique combinatorial
antitumor activity with taxanes by potentiating mitotic cell death.
Sci Adv. 3:e17000902017.
|
|
219
|
Diamond JR, Becerra C, Richards D, Mita A,
Osborne C, O'Shaughnessy J, Zhang C, Henner R, Kapoun AM, Xu L, et
al: Phase Ib clinical trial of the anti-frizzled antibody
vantictumab (OMP-18R5) plus paclitaxel in patients with locally
advanced or metastatic HER2-negative breast cancer. Breast Cancer
Res Treat. 184:53–62. 2020.
|
|
220
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009.
|
|
221
|
Bao R, Christova T, Song S, Angers S, Yan
X and Attisano L: Inhibition of tankyrases induces Axin
stabilization and blocks Wnt signalling in breast cancer cells.
PLoS One. 7:e486702012.
|
|
222
|
Menon M, Elliott R, Bowers L, Balan N,
Rafiq R, Costa-Cabral S, Munkonge F, Trinidade I, Porter R,
Campbell AD, et al: A novel tankyrase inhibitor, MSC2504877,
enhances the effects of clinical CDK4/6 inhibitors. Sci Rep.
9:2012019.
|
|
223
|
Sharma M, Li L, Celver J, Killian C,
Kovoor A and Seeram NP: Effects of fruit ellagitannin extracts,
ellagic acid, and their colonic metabolite, urolithin a, on wnt
signaling. J Agric Food Chem. 58:3965–3969. 2010.
|
|
224
|
Sher A, Tabassum S, Wallace HM, Khan A,
Karim AM, Gul S and Kang SC: In vitro analysis of cytotoxic
activities of monotheca buxifolia targeting WNT/β-catenin genes in
breast cancer cells. Plants (Basel). 12:11472023.
|
|
225
|
Mandal S, Gamit N, Varier L, Dharmarajan A
and Warrier S: Inhibition of breast cancer stem-like cells by a
triterpenoid, ursolic acid, via activation of Wnt antagonist, sFRP4
and suppression of miRNA-499a-5p. Life Sci. 265:1188542021.
|
|
226
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
|
|
227
|
Narod SA: Which genes for hereditary
breast cancer? N Engl J Med. 384:471–473. 2021.
|
|
228
|
Li L, Yang LL, Yang SL, Wang RQ, Gao H,
Lin ZY, Zhao YY, Tang WW, Han R, Wang WJ, et al: Andrographolide
suppresses breast cancer progression by modulating tumor-associated
macrophage polarization through the wnt/β-catenin pathway.
Phytother Res. 36:4587–4603. 2022.
|
|
229
|
Wang M, Zheng Y, Hao Q, Mao G, Dai Z, Zhai
Z, Lin S, Liang B, Kang H and Ma X: Hypoxic BMSC-derived exosomal
miR-210-3p promotes progression of triple-negative breast cancer
cells via NFIX-wnt/β-catenin signaling axis. J Transl Med.
23:392025.
|
|
230
|
Shome R, Sen P, Sarkar S and Ghosh SS:
Single-cell transcriptomics reveals the intra-tumoral heterogeneity
and SQSTM1/P62 and wnt/β-catenin mediated epithelial to mesenchymal
transition and stemness of triple-negative breast cancer. Exp Cell
Res. 438:1140322024.
|
|
231
|
Pitt JM, Marabelle A, Eggermont A, Soria
JC, Kroemer G and Zitvogel L: Targeting the tumor microenvironment:
Removing obstruction to anticancer immune responses and
immunotherapy. Ann Oncol. 27:1482–1492. 2016.
|
|
232
|
Vitale I, Manic G, Coussens LM, Kroemer G
and Galluzzi L: Macrophages and metabolism in the tumor
microenvironment. Cell Metab. 30:36–50. 2019.
|
|
233
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther. 221:1077532021.
|
|
234
|
Jin MZ and Jin WL: The updated landscape
of tumor microenvironment and drug repurposing. Signal Transduct
Target Ther. 5:1662020.
|
|
235
|
Rong Z, Zhang L, Li Z, Xiao Z, Duan Y, Ren
X, Zi Y, Gao J, Mu Y, Guan Y, et al: SIK2 maintains breast cancer
stemness by phosphorylating LRP6 and activating Wnt/beta-catenin
signaling. Oncogene. 41:2390–2403. 2022.
|
|
236
|
Yang Z, Zhang Q, Yu L, Zhu J, Cao Y and
Gao X: The signaling pathways and targets of traditional Chinese
medicine and natural medicine in triple-negative breast cancer. J
Ethnopharmacol. 264:1132492021.
|
|
237
|
Zhong C, Xie Z, Zeng L, Yuan C and Duan S:
MIR4435-2HG is a potential pan-cancer biomarker for diagnosis and
prognosis. Front Immunol. 13:8550782022.
|
|
238
|
Prasad CP, Gupta SD, Rath G and Ralhan R:
Wnt signaling pathway in invasive ductal carcinoma of the breast:
Relationship between beta-catenin, dishevelled and cyclin D1
expression. Oncology. 73:112–117. 2007.
|
|
239
|
Zhang CH, Liu H, Zhao WL, Zhao WX, Zhou HM
and Shao RG: G3BP1 promotes human breast cancer cell proliferation
through coordinating with GSK-3β and stabilizing β-catenin. Acta
Pharmacol Sin. 42:1900–1912. 2021.
|
|
240
|
Jung HY, Jun S, Lee M, Kim HC, Wang X, Ji
H, McCrea PD and Park JI: PAF and EZH2 induce Wnt/β-catenin
signaling hyperactivation. Mol Cell. 52:193–205. 2013.
|
|
241
|
Hashemi M, Hasani S, Hajimazdarany S,
Ghadyani F, Olyaee Y, Khodadadi M, Ziyarani MF, Dehghanpour A,
Salehi H, Kakavand A, et al: Biological functions and molecular
interactions of Wnt/β-catenin in breast cancer: Revisiting
signaling networks. Int J Biol Macromol. 232:1233772023.
|
|
242
|
Liu C, Sun L, Yang J, Liu T, Yang Y, Kim
SM, Ou X, Wang Y, Sun L, Zaidi M, et al: FSIP1 regulates autophagy
in breast cancer. Proc Natl Acad Sci USA. 115:13075–13080.
2018.
|
|
243
|
Li P, Guo Y, Bledsoe G, Yang Z, Chao L and
Chao J: Kallistatin induces breast cancer cell apoptosis and
autophagy by modulating Wnt signaling and microRNA synthesis. Exp
Cell Res. 340:305–314. 2016.
|
|
244
|
Xie J, Deng X, Xie Y, Zhu H, Liu P, Deng
W, Ning L, Tang Y, Sun Y, Tang H, et al: Multi-omics analysis of
disulfidptosis regulators and therapeutic potential reveals
glycogen synthase 1 as a disulfidptosis triggering target for
triple-negative breast cancer. MedComm (2020). 5:e5022024.
|
|
245
|
Song S, Christova T, Perusini S, Alizadeh
S, Bao RY, Miller BW, Hurren R, Jitkova Y, Gronda M, Isaac M, et
al: Wnt inhibitor screen reveals iron dependence of β-catenin
signaling in cancers. Cancer Res. 71:7628–7639. 2011.
|
|
246
|
Kumar D, Gurrapu S, Wang Y, Bae SY, Pandey
PR, Chen H, Mondal J, Han H, Wu CJ, Karaiskos S, et al: LncRNA
Malat1 suppresses pyroptosis and T cell-mediated killing of
incipient metastatic cells. Nat Cancer. 5:262–282. 2024.
|
|
247
|
Liu Y, Wang X, Liu M, Hao X, Peng Y and
Zheng J: Chemical nature of metabolic activation of natural
products in traditional Chinese medicines possibly associated with
toxicities. Acupunct Herb Med. 4:184–196. 2024.
|
|
248
|
OncoMed Pharmaceuticals: A phase 1b dose
escalation study of vantictumab (OMP-18R5) in combination with
paclitaxel in patients with locally recurrent or metastatic breast
cancer. OncoMed Pharmaceuticals, Inc.; 2020
|
|
249
|
Säfholm A, Tuomela J, Rosenkvist J, Dejmek
J, Härkönen P and Andersson T: The Wnt-5a-derived hexapeptide
Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell
motility. Clin Cancer Res. 14:6556–6563. 2008.
|
|
250
|
Curegenix: A phase 1 open-label dose
escalation study of CGX1321 in subjects with advanced solid tumors
with expansion in advanced gastrointestinal tumors and phase 1b
study of CGX1321 in combination with pembrolizumab in subjects with
advanced colorectal cancer or in combination with encorafenib +
cetuximab in subjects with BRAFV600E mutated advanced colorectal
cancer. Curegenix Inc.; 2022
|
|
251
|
Qi D, Liu Y, Li J, Huang JH, Hu X and Wu
E: Salinomycin as a potent anticancer stem cell agent: State of the
art and future directions. Med Res Rev. 42:1037–1063. 2022.
|
|
252
|
Singh S, Weiss A, Goodman J, Fisk M,
Kulkarni S, Lu I, Gray J, Smith R, Sommer M and Cheriyan J:
Niclosamide-A promising treatment for COVID-19. Br J Pharmacol.
179:3250–3267. 2022.
|
|
253
|
Liu L, Li Z and Wu W: Harnessing natural
inhibitors of protein synthesis for cancer therapy: A comprehensive
review. Pharmacol Res. 209:1074492024.
|
|
254
|
Raut D, Vora A and Bhatt LK: The
Wnt/β-catenin pathway in breast cancer therapy: A pre-clinical
perspective of its targeting for clinical translation. Expert Rev
Anticancer Ther. 22:97–114. 2022.
|
|
255
|
Tang C, Gong L, Lvzi Xu, Qiu K, Zhang Z
and Wan L: Echinacoside inhibits breast cancer cells by suppressing
the wnt/β-catenin signaling pathway. Biochem Biophys Res Commun.
526:170–175. 2020.
|
|
256
|
Fatima I, El-Ayachi I, Taotao L, Lillo MA,
Krutilina RI, Seagroves TN, Radaszkiewicz TW, Hutnan M, Bryja V,
Krum SA, et al: The natural compound Jatrophone interferes with
Wnt/ β-catenin signaling and inhibits proliferation and EMT in
human triple-negative breast cancer. PLoS One. 12:e01898642017.
|
|
257
|
Alitongbieke G, Zhang X, Zhu F, Wu Q, Lin
Z, Li X, Xue Y, Lai X, Feng J, Huang R and Pan Y: Glucan from
Oudemansiella raphanipes suppresses breast cancer proliferation and
metastasis by regulating macrophage polarization and the
WNT/β-catenin signaling pathway. J Cancer. 15:1169–1181. 2024.
|
|
258
|
Wang Z, Li B, Zhou L, Yu S, Su Z, Song J,
Sun Q, Sha O, Wang X, Jiang W, et al: Prodigiosin inhibits
Wnt/β-catenin signaling and exerts anticancer activity in breast
cancer cells. Proc Natl Acad Sci USA. 113:13150–13155. 2016.
|
|
259
|
Ahmed RA, Alawin OA and Sylvester PW:
γ-Tocotrienol reversal of epithelial-to-mesenchymal transition in
human breast cancer cells is associated with inhibition of
canonical Wnt signalling. Cell Prolif. 49:460–470. 2016.
|
|
260
|
Lu W, Lin C and Li Y: Rottlerin induces
Wnt co-receptor LRP6 degradation and suppresses both Wnt/β-catenin
and mTORC1 signaling in prostate and breast cancer cells. Cell
Signal. 26:1303–1309. 2014.
|
|
261
|
Li X, Meng Y, Xie C, Zhu J, Wang X, Li Y,
Geng S, Wu J, Zhong C and Li M: Diallyl Trisulfide inhibits breast
cancer stem cells via suppression of Wnt/β-catenin pathway. J Cell
Biochem. 119:4134–4141. 2018.
|
|
262
|
Ahmad A, Sarkar SH, Bitar B, Ali S,
Aboukameel A, Sethi S, Li Y, Bao B, Kong D, Banerjee S, et al:
Garcinol regulates EMT and Wnt signaling pathways in vitro and in
vivo, leading to anticancer activity against breast cancer cells.
Mol Cancer Ther. 11:2193–2201. 2012.
|
|
263
|
Su Z, Wang C, Chang D, Zhu X, Sai C and
Pei J: Limonin attenuates the stemness of breast cancer cells via
suppressing MIR216A methylation. Biomed Pharmacother.
112:1086992019.
|
|
264
|
Kim J, Zhang X, Rieger-Christ KM,
Summerhayes IC, Wazer DE, Paulson KE and Yee AS: Suppression of Wnt
signaling by the green tea compound (-)-epigallocatechin 3-gallate
(EGCG) in invasive breast cancer cells. Requirement of the
transcriptional repressor HBP1. J Biol Chem. 281:10865–10875.
2006.
|
|
265
|
Xu X, Rajamanicham V, Xu S, Liu Z, Yan T,
Liang G, Guo G, Zhou H and Wang Y: Schisandrin A inhibits triple
negative breast cancer cells by regulating Wnt/ER stress signaling
pathway. Biomed Pharmacother. 115:1089222019.
|
|
266
|
Liu X, Wang LL, Duan CY, Rong YR, Liang
YQ, Zhu QX, Hao GP and Wang FZ: Daurisoline inhibits proliferation,
induces apoptosis, and enhances TRAIL sensitivity of breast cancer
cells by upregulating DR5. Cell Biol Int. Apr 2–2024.Epub ahead of
print.
|
|
267
|
Su Y and Simmen RC: Soy isoflavone
genistein upregulates epithelial adhesion molecule E-cadherin
expression and attenuates beta-catenin signaling in mammary
epithelial cells. Carcinogenesis. 30:331–339. 2009.
|
|
268
|
Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou
Y, Zhu J and Mi M: Resveratrol inhibits breast cancer stem-like
cells and induces autophagy via suppressing Wnt/β-catenin signaling
pathway. PLoS One. 9:e1025352014.
|
|
269
|
Huang Y, Zhao K, Hu Y, Zhou Y, Luo X, Li
X, Wei L, Li Z, You Q, Guo Q and Lu N: Wogonoside inhibits
angiogenesis in breast cancer via suppressing Wnt/β-catenin
pathway. Mol Carcinog. 55:1598–1612. 2016.
|
|
270
|
Xiao X, Ao M, Xu F, Li X, Hu J, Wang Y, Li
D, Zhu X, Xin C and Shi W: Effect of matrine against breast cancer
by downregulating the vascular endothelial growth factor via the
Wnt/β-catenin pathway. Oncol Lett. 15:1691–1697. 2018.
|
|
271
|
Chen Y, Chen ZY, Chen L, Zhang JY, Fu LY,
Tao L, Zhang Y, Hu XX and Shen XC: Shikonin inhibits
triple-negative breast cancer-cell metastasis by reversing the
epithelial-to-mesenchymal transition via glycogen synthase kinase
3β-regulated suppression of β-catenin signaling. Biochem Pharmacol.
166:33–45. 2019.
|
|
272
|
Koval A, Pieme CA, Queiroz EF, Ragusa S,
Ahmed K, Blagodatski A, Wolfender JL, Petrova TV and Katanaev VL:
Tannins from Syzygium guineense suppress Wnt signaling and
proliferation of Wnt-dependent tumors through a direct effect on
secreted Wnts. Cancer Lett. 435:110–120. 2018.
|
|
273
|
Wang J, Qi H, Zhang X, Si W, Xu F, Hou T,
Zhou H, Wang A, Li G, Liu Y, et al: Saikosaponin D from Radix
Bupleuri suppresses triple-negative breast cancer cell growth by
targeting β-catenin signaling. Biomed Pharmacother. 108:724–733.
2018.
|
|
274
|
Zhang X, Bao C and Zhang J: Inotodiol
suppresses proliferation of breast cancer in rat model of type 2
diabetes mellitus via downregulation of β-catenin signaling. Biomed
Pharmacother. 99:142–150. 2018.
|
|
275
|
Li X, Wang X, Xie C, Zhu J, Meng Y, Chen
Y, Li Y, Jiang Y, Yang X, Wang S, et al: Sonic hedgehog and
Wnt/β-catenin pathways mediate curcumin inhibition of breast cancer
stem cells. Anticancer Drugs. 29:208–215. 2018.
|
|
276
|
Sun Y, Gu Y, Gao X, Jin X, Wink M,
Sharopov FS, Yang L and Sethi G: Lycorine suppresses the malignancy
of breast carcinoma by modulating epithelial mesenchymal transition
and β-catenin signaling. Pharmacol Res. 195:1068662023.
|
|
277
|
Yang S, Sun S, Xu W, Yu B, Wang G and Wang
H: Astragalus polysaccharide inhibits breast cancer cell migration
and invasion by regulating epithelial-mesenchymal transition via
the Wnt/β-catenin signaling pathway. Mol Med Rep. 21:1819–1832.
2020.
|
|
278
|
Lee HJ, Wang NX, Shi DL and Zheng JJ:
Sulindac inhibits canonical Wnt signaling by blocking the PDZ
domain of the protein Dishevelled. Angew Chem Int Ed Engl.
48:6448–6452. 2009.
|
|
279
|
Maloney D: Phase I study of adoptive
immunotherapy for advanced ROR1+ malignancies with defined subsets
of autologous T cells engineered to express a ROR1-specific
chimeric antigen receptor. Fred Hutchinson Cancer Center; 2022
|
|
280
|
VelosBio Inc., a subsidiary of Merck
&; Co., Inc.: A phase 2 study of VLS-101 in patients with
solid tumors. VelosBio Inc., a subsidiary of Merck &; Co.,
Inc.; Rahway, NJ: 2024
|
|
281
|
NBE-Therapeutics AG: A First-in-Human,
Phase 1/2 Study of NBE-002, an Anti-ROR1 Antibody Drug Conjugate,
in Patients With Advanced Solid Tumors. NBE-Therapeutics AG;
2023
|
|
282
|
Parker B: A phase 1b pilot clinical trial
of cirmtuzumab, an anti-ROR1 monoclonal antibody, in combination
with paclitaxel for the treatment of patients with metastatic, or
locally advanced, unresectable breast cancer. NIH; Bethesda, MD:
2024
|
|
283
|
BioAtla, Inc.: A Phase 1/2 Safety and
Efficacy Dose Escalation/ Dose Expansion Study of a CAB-ROR2-ADC,
Alone and in Combination with a PD-1 Inhibitor, in Patients with
Advanced Solid Tumors (Ph1) and Melanoma and NSCLC Patients (Ph2).
BioAtla, Inc.; 2025
|
|
284
|
Lenz HJ, Argilés G, de Jonge MJA, Yaeger
R, Doi T, El-Khoueiry A, Eskens F, Kuboki Y, Bertulis J,
Nazabadioko S, et al: A phase I dose-escalation study of LRP5/6
antagonist BI 905677 in patients with advanced solid tumors. ESMO
Open. 9:1037292024.
|
|
285
|
Fischer MM, Yeung VP, Cattaruzza F,
Hussein R, Yen WC, Murriel C, Evans JW, O'Young G, Brunner AL, Wang
M, et al: RSPO3 antagonism inhibits growth and tumorigenicity in
colorectal tumors harboring common Wnt pathway mutations. Sci Rep.
7:152702017.
|
|
286
|
Kuroki H, Anraku T, Kazama A, Bilim V,
Tasaki M, Schmitt D, Mazar AP, Giles FJ, Ugolkov A and Tomita Y:
9-ING-41, a small molecule inhibitor of GSK-3beta, potentiates the
effects of anticancer therapeutics in bladder cancer. Sci Rep.
9:199772019.
|
|
287
|
Edenfield WJ, Richards DA, Vukelja SJ,
Weiss GJ, Sirard CA, Landau SB and Ramanathan RK: A phase 1 study
evaluating the safety and efficacy of DKN-01, an investigational
monoclonal antibody (Mab) in patients (pts) with advanced non-small
cell lung cancer. J Chin Oncol. 32:8068. 2014.
|
|
288
|
Children's Oncology Group: A Phase 1/2
Study of Tegavivint (IND#156033, NSC#826393) in Children,
Adolescents, and Young Adults with Recurrent or Refractory Solid
Tumors, Including Lymphomas and Desmoid Tumors. Children's Oncology
Group; 2024
|
|
289
|
McWilliams RR, Ko AH, Chiorean EG, Kwak
EL, Lenz HJ, Nadler PI, Wood DL, Fujimori M, Morita K, Inada T and
Kouji H: A phase Ib dose-escalation study of PRI-724, a
CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with
advanced pancreatic adenocarcinoma (APC) as second-line therapy
after FOLFIRINOX or FOLFOX. J Chin Oncol. 33:e152702015.
|
|
290
|
Rodon J, Argilés G, Connolly RM,
Vaishampayan U, de Jonge M, Garralda E, Giannakis M, Smith DC,
Dobson JR, McLaughlin ME, et al: Phase 1 study of single-agent
WNT974, a first-in-class Porcupine inhibitor, in patients with
advanced solid tumours. Br J Cancer. 125:28–37. 2021.
|
|
291
|
Plummer R, Dua D, Cresti N, Drew Y,
Stephens P, Foegh M, Knudsen S, Sachdev P, Mistry BM, Dixit V, et
al: First-in-human study of the PARP/tankyrase inhibitor E7449 in
patients with advanced solid tumours and evaluation of a novel
drug-response predictor. Br J Cancer. 123:525–533. 2020.
|
|
292
|
Scott A, Call JA, Chandana S, Borazanci E,
Falchook GS, Bordoni R, Richey S, Starodub A, Chung V, Lakhani NJ,
et al: 451O Preliminary evidence of clinical activity from phase I
and Ib trials of the CLK/DYRK inhibitor cirtuvivint (CIRT) in
subjects with advanced solid tumors. Ann Oncol. 33(Suppl 7):
S742–S743. 2022.
|
|
293
|
Redx Pharma Ltd.: A modular multi-arm,
phase 1, adaptive design study to evaluate the safety and
tolerability of RXC004, alone and in combination with anti-cancer
treatments, in patients with advanced malignancies. Redx Pharma
Ltd.; 2024
|
|
294
|
Molenaar RJ, Coelen RJS, Khurshed M, Roos
E, Caan MWA, van Linde ME, Kouwenhoven M, Bramer JAM, Bovée JVMG,
Mathôt RA, et al: Study protocol of a phase IB/II clinical trial of
metformin and chloroquine in patients with IDH1-mutated or
IDH2-mutated solid tumours. BMJ Open. 7:e0149612017.
|
|
295
|
Hattinger CM, Patrizio MP, Magagnoli F,
Luppi S and Serra M: An update on emerging drugs in osteosarcoma:
Towards tailored therapies? Expert Opin Emerg Drugs. 24:153–171.
2019.
|