Diffuse large B-cell lymphoma (BCL) (DLBCL) is the
most common subtype of non-Hodgkin lymphoma, accounting for 30-40%
of all non-Hodgkin lymphoma cases (1). Although the majority of DLBCL cases
are primary, DLBCL can also transform from various indolent
lymphomas. For example, 6.78% of splenic marginal zone lymphomas,
5.55% of follicular lymphomas, 4.05% of nodal marginal zone
lymphomas, 2.22% of lymphoplasmacytic lymphomas/Waldenström's
macroglobulinemias and 1.62% of extra-nodal marginal zone lymphomas
developed into DLBCL (2). Based
on gene expression profiles, DLBCL can be classified into various
subgroups, namely activated B-cell, germinal center B-cell and
unclassified, with each exhibiting a distinct response to treatment
(3). The rituximab
(R)-cyclophosphamide doxorubicin vincristine prednisolone (R-CHOP)
regimen, which includes a monoclonal antibody targeting CD20,
represents an advancement in the initial treatment of patients with
DLBCL (4,5). This regimen has improved the 10-year
progression-free survival (PFS) rate of patients with DLBCL from 20
to 36.5% (6), and the 5-year
overall survival (OS) rate from ~50% to 60-70% (7). However, despite this advancement,
>40% of patients with DLBCL treated with R-CHOP or R-CHOP-like
regimens will experience relapse or refractory disease (5). In addition, the majority of patients
with relapsed or refractory DLBCL will have a shorter survival
period compared with patients without relapsed or refractory
DLBLC.
Previous studies have identified a number of risk
factors for DLBCL, which are outlined in the present review.
Clinical studies indicate that Epstein-Barr virus (EBV), human
immunodeficiency virus (HIV) and hepatitis viral infections are
associated with DLBCL development (8-10).
EBV is a cancer-causing virus that is associated with a number of
lymphoproliferative disorders, such as DLBCL and Burkitt's
lymphoma. Furthermore, in individuals that are HIV-positive, the
risk of EBV-associated cancers, including DLBCL, increases due to
weakened immunity (8,9). Hepatitis C virus (HCV) is also
associated with DLBCL, where co-infection with HCV and HIV further
increases the risk of lymphoproliferative disorders due to combined
immunosuppression and chronic inflammation (10). In addition, numerous studies
indicate a positive association between autoimmune diseases, such
as systemic lupus erythematosus and Sjogren's syndrome, and an
increased risk of DLBCL (11-13). Furthermore, genetic factors are
also considered to serve an important role in the biology and
prognosis of DLBCL, and various studies reveal that DLBCL exhibits
considerable genetic heterogeneity (14,15). The expression and rearrangement of
Myc, Bcl2 and Bcl6 genes are useful for
guiding treatment and predicting the prognosis of DLBCL (16,17). In addition, the tumor
microenvironment (TME) and programmed cell death protein 1
(PD-1)/programmed cell death-ligand 1 (PD-L1) are key factors that
can determine DLBCL treatment outcomes, since they can enable tumor
immune escape, and thus, recurrence or refractory disease (18). Consequently, the management of
DLBCL is currently still challenging.
The influence of dietary nutrients, with a
particular emphasis on vitamin supplementation, in the prevention
and treatment of tumors is gaining interest in the present field of
research. Previous empirical studies reveal that certain vitamins
(vitamin D and E) can exert a substantial effect on tumor
development and progression (19,20). In particular, a number of trials
indicate that high doses of vitamin C (≥1 g/kg) exhibit both direct
and indirect antitumor properties (21-30) (Table
I). As a result, vitamins are regarded as an important agent in
the prevention and therapeutic management of malignancies.
Ascorbic acid (AA), commonly known as vitamin C, is
a water-soluble vitamin with potent antioxidant properties. Vitamin
C is abundant in various fruits and vegetables, with concentrations
differing among species. Citrus and kiwi fruits, mangoes, broccoli,
tomatoes, and peppers have the highest levels of vitamin C
(31). Numerous studies indicate
that high doses of vitamin C (1-4 g/kg) can inhibit tumor cell
proliferation through multiple mechanisms (30,32). These mechanisms include increased
production of reactive oxygen species (ROS), activation of specific
epigenetic regulatory factors [tet methylcytosine dioxygenase 2
(TET2)] and modulation of the antitumor immune response to either
directly or indirectly eliminate tumor cells (30,32,33). Furthermore, previous clinical
trials demonstrate that vitamin C treatment has a high level of
safety and tolerability (34,35). The present review investigated the
role of vitamin C in DLBCL and assessed its effects on the various
pathogenic mechanisms of DLBCL. Additionally, the present review
examined the future prospects and challenges associated with
vitamin C-based antitumor therapy.
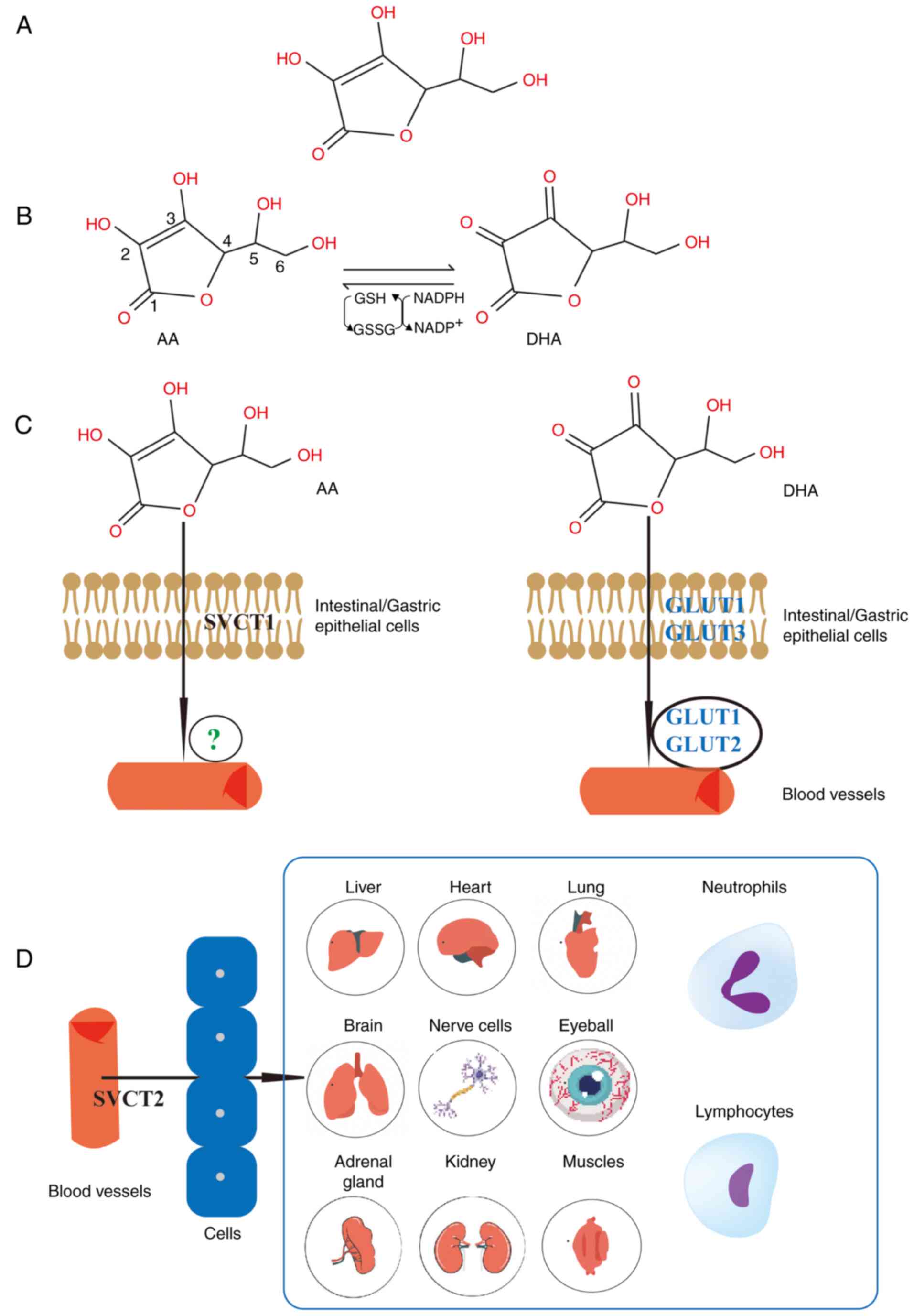
Vitamin C is a weak acid that is comprised of six
carbon atoms and is classified as an α-ketolactone. In the human
body, vitamin C mainly exists in two active forms, dehydroascorbic
acid (DHA) and AA, in an interconvertible manner (Fig. 1A and B) (36). Vitamin C is abundant in a wide
variety of fruits and vegetables and can only be obtained by humans
via ingestion through dietary means, since humans cannot synthesize
it. Following oral intake, vitamin C is primarily absorbed in the
duodenum and upper jejunum, with minimal absorption occurring in
the stomach. It enters the bloodstream through passive and active
transport, facilitating its transmembrane movement (37,38). However, the majority of vitamin C
is absorbed via the sodium-dependent vitamin C transporter (SVCT)1
(39,40). Vitamin C typically moves slowly
across the plasma membrane, even in the presence of a notable
concentration gradient. AA is absorbed in the intestine through
SVCT1 (40), whilst DHA may
undergo transmembrane diffusion and absorption through glucose
transporters (GLUT) (41).
However, the mechanism underlying AA absorption into the
bloodstream is yet to be elucidated (42). Once AA is absorbed into the
bloodstream, it is subsequently transported to various organs
through SVCT2. Vitamin C is widely distributed across various
organs and cells, with particularly high concentrations in the
brain and endocrine glands, such as the pituitary and adrenal
glands (37,43). A schematic diagram illustrating
intestinal vitamin C absorption and distribution is presented in
Fig. 1C and D. However, the
majority of vitamin C entering the body is excreted by the kidneys,
with only a small proportion eliminated through feces (41).
Via the SVCT2, vitamin C accumulates in key organs
and cells, such as the skin, lymphocytes and neutrophils. The skin
serves as the first line of defense for the body. Previous studies
indicate that vitamin C reduces the synthesis and release of
proinflammatory factors, such as IL-1β and TNF-α, by reducing the
transcription of their genes (44,45). Additionally, vitamin C contributes
to the tissue repair processes by promoting collagen synthesis and
maturation, promoting the expression of angiogenic factors (such as
VEGF), enhancing the expression of connective tissue regulators
(such as connective tissue growth factor), increasing fibroblast
activity, and activating important repair enzymes (such as heme
oxygenase 1 and TGF-β) (44-46). In addition, preliminary evidence
suggests that vitamin C can accumulate in neutrophils to an extent.
Following infection, neutrophils can rapidly uptake DHA and convert
it to AA within cells, leading to increases in its concentration.
This concentration increase is calculated to be ~10 mM (36). In vitro and clinical
studies also reveal that vitamin C can enhance the efficacy of
phagocytosis and pathogen killing by promoting neutrophil
chemotaxis (47,48). Lymphocytes, including T and B
cells, are key types of immune cells that utilize SVCT2 for vitamin
C storage. Although the precise mechanism by which vitamin C
regulates lymphocyte physiology remains unclear (49), vitamin C can stimulate lymphocyte
development, differentiation and maturation of immature cells, and
regulate cytokine secretion (36,50,51). Additionally, when vitamin C is
incubated with peripheral blood lymphocytes in vitro, it
reduces the production of various inflammatory cytokines, such as
TNF-α and IFN-γ, induced by lipopolysaccharide, whilst increasing
the production of the anti-inflammatory cytokine IL-10 (50). Immunoregulatory T cells (Tregs), a
subset of CD4+ T cells, serve an important role in
immune regulation. They serve to prevent the hyperactivation of the
immune system and maintain immune homeostasis. A recent study
demonstrates that TET2 enzymes are important for maintaining a
stable population of Treg cells (52). Vitamin C can enhance the activity
of TET enzymes, suggesting that targeting TET enzymes with vitamin
C may improve the efficacy of Treg cell induction (53).
BCL6 is part of the antiapoptotic protein family and
functions as a transcriptional repressor (77). The germinal center reaction
pathway in mature B cells primarily uses BCL6, which serves an
important role in promoting germinal center formation, regulating
the cell cycle and participating in immune response signaling
transduction. BCL6 inhibits the early activation and
differentiation of germinal center B cells, rendering it an
important regulatory factor for effective humoral immunity
(77). In addition, BCL6 is
indispensable for the development and function of T follicular
helper cells and germinal center B cells (78,79). BCL6 was first identified in DLBCL.
High expression of the BCL6 protein in germinal center B cells
markedly inhibits the expression of the tumor suppressor
p53. The inhibition of p53 enables lymphoma cells to
survive in vivo and inhibits tumor cell apoptosis (78).
As an important tumor suppressor gene, p53 regulates
cell division, prevents DNA mutations and the proliferation of
damaged cells, controls apoptosis, and inhibits tumor formation
(90,91). Additionally, p53 halts the cell
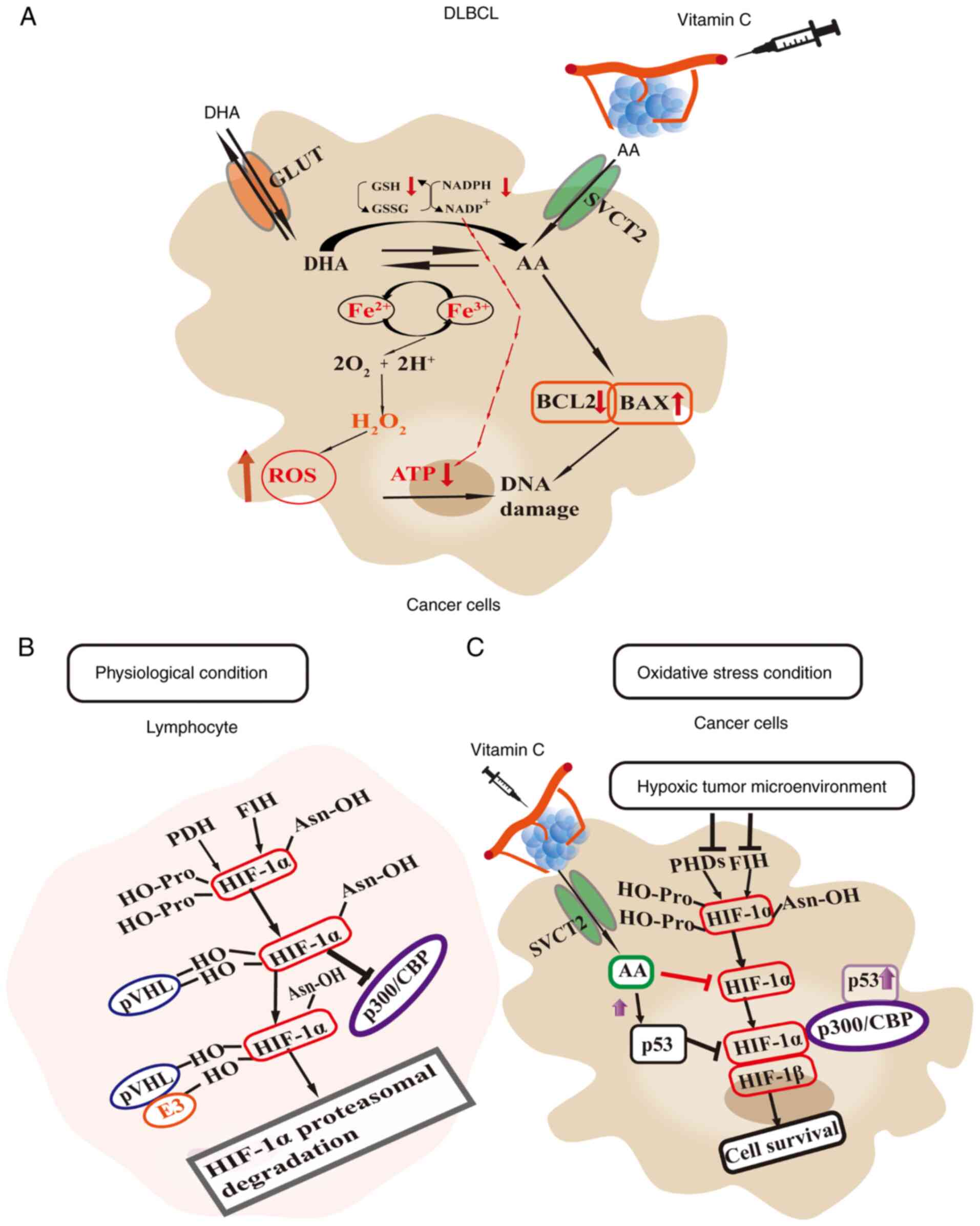
cycle and facilitates DNA repair (92). However, in DLBCL cells, vascular
compression, due to rapid tumor cell proliferation, results in a
hypoxic environment, which subsequently increases the expression of
hypoxia-inducible factor-1 (HIF-1) in tumor cells. However, p53
competes with p300 proteins, which reduces the transcription of
HIF-1α. Under sustained hypoxia, p53 induces the degradation of
HIF-1α and triggers apoptosis, which are both important for
inhibiting tumor growth (93-95). HIF-1 is expressed in most types of
mammalian cell and primarily consists of the oxygen-dependent
HIF-1α and the oxygen-independent HIF-1β. It can regulate the
expression of genes that regulate metastasis, angiogenesis and
tumor cell invasion. In DLBCL, BCL6 primarily inhibits p53, which
in turn inhibits the effects of p53 on cell cycle arrest and
apoptosis. Overexpression of BCL6 reveals reduced wild-type p53
expression levels, resulting in enhanced tumor cell proliferation
and impaired apoptosis (79,96-98).
Vitamin C is a natural antioxidant. In normal cells,
its antioxidant properties dominate because it directly reacts with
superoxide anion radicals (O₂−) and hydroxyl radicals
(OH−), which reduces the level of ROS within the cells
(99). In tumor cells, vitamin C
often acts as a pro-oxidant due to high metal ion concentrations in
the cytoplasm of the cell (such as Fe2+), which leads to
oxidative stress due to reactions such as the Fenton reaction. In
addition, cancer cells also have an increased expression of GLUT1,
which enhances the cellular uptake of oxidized vitamin C (DHA).
This depletes the antioxidants such as glutathione, NADPH and
superoxide dismutase, and increases the levels of ROS in the
cytoplasm of the cell (32).
Previous studies indicate that, at pharmacological concentrations,
vitamin C can function as a pro-oxidant, selectively inducing DNA
damage in cancer cells (71-73). This targeting facilitates the
elimination of cancer cells. In cases of breast and cervical
cancer, studies indicate that vitamin C upregulates the expression
of wild-type p53 and inhibits HIF-1α activity, thus inhibiting
tumor development (Fig. 2B and C)
(74,100,101). Additionally, in DLBCL,
pharmacological doses of vitamin C can increase wild type p53
production, indirectly modulating the impact of BCL6
overexpression. Furthermore, vitamin C can also inhibit HIF-1α
activity, promoting apoptosis in tumor cells (102). In conclusion, vitamin C may be a
potential anticancer therapeutic agent.
c-Myc, a proto-oncogene, is located on chromosome
8q24. When overexpressed, c-Myc promotes cancer cells to re-enter
the cell cycle and proliferate, which promotes tumor growth
(111). Numerous studies
demonstrate that the activation of Myc in cancer cells occurs
through multiple mechanisms (112,113). For example, genetic mutations,
chromosomal translocations and genomic amplifications can induce
elevated Myc expression levels. Furthermore, alterations in an
upstream regulatory pathway (such as Wnt/β-catenin) (114) can also increase Myc oncogene
transcription levels. In addition, post-translational modifications
of the Myc protein such as phosphorylation, proteasomal degradation
and ubiquitination enhances its stability, resulting in the
activation of the Myc pathway (115). As demonstrated in a conditional
knockout mouse model, Myc is essential for hematopoietic system
development (116). It regulates
the balance between hematopoietic stem cell self-renewal and
differentiation, and is also highly expressed in proliferating
cells during lymph node development (117). In DLBCL, Myc is the third most
frequently rearranged oncogene after BCL2 and BCL6.
In total, ~15% of newly diagnosed patients with DLBCL have Myc gene
rearrangements, and these patients are frequently associated with
an increased level of tumor cell invasion and a poorer prognosis
(118-120).
Previous studies demonstrate that vitamin C, acting
as a pro-oxidant, increases the levels of ROS, which inhibits the
growth of tumor cells (74,121,122). Previous studies using tumors
such as clear cell renal carcinoma and esophageal cancer suggest
that the accumulation of ROS inhibits the expression of c-Myc,
induces DNA damage and reduces tumor cell proliferation (123,124). Another study reveals that the
combination of vitamin C with a respiratory complex I inhibitor
effectively eradicated c-Myc-overexpressing cells and enhanced
treatment outcomes in human BCL xenografts (125). However, further research is
required to elucidate the effects of vitamin C on human and
hematological tumors. In summary, vitamin C has potential benefits
in cancer treatment and may promote tumor cell apoptosis via the
ROS/c-Myc axis.
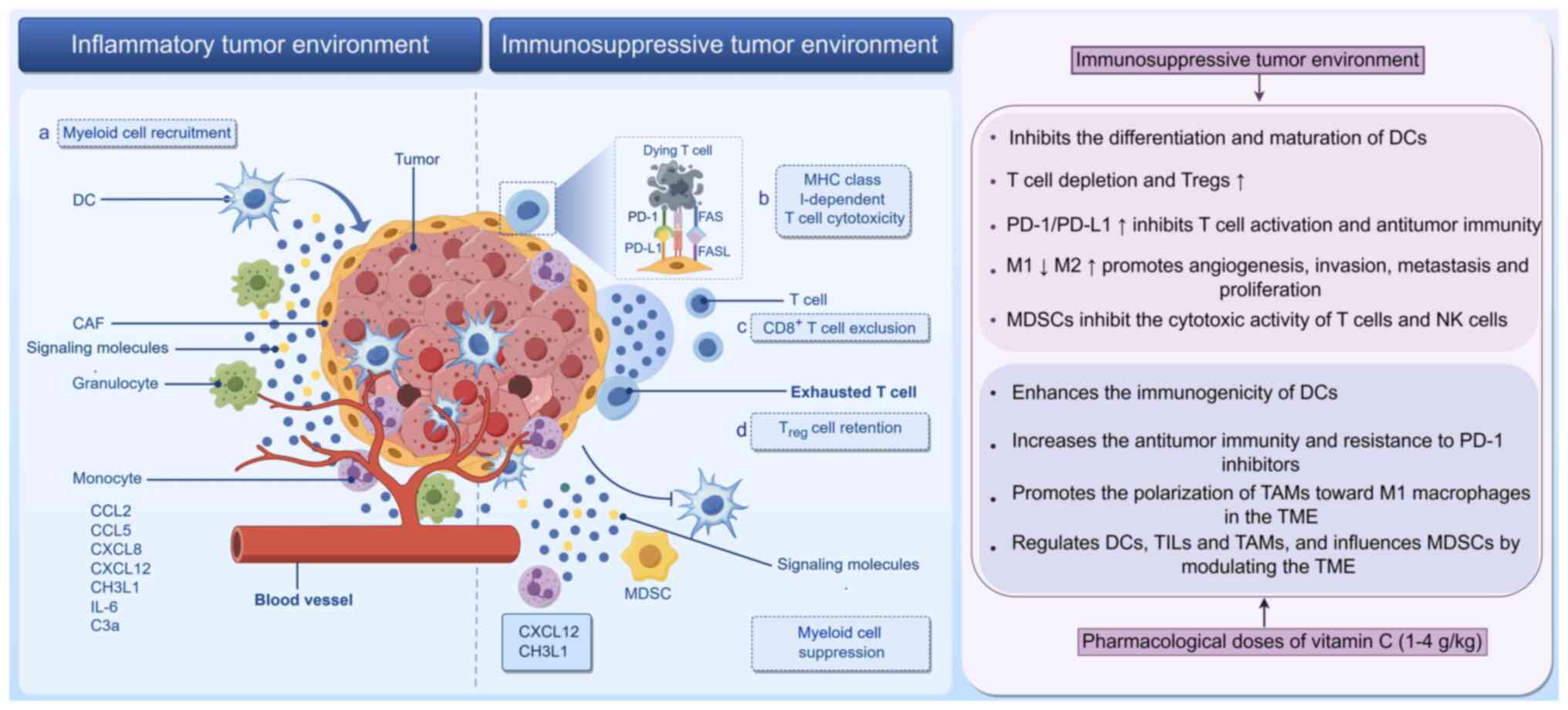
The TME is a dynamic and intricate cellular
landscape in which tumor or cancer stem cells coexist with immune
cells (myeloid and lymphoid), fibroblasts, blood vessels, the
extracellular matrix and a range of signaling molecules (such as
C-X-C motif chemokine ligand 12 and IL-6) (126,127). A previous study indicates that
the TME is adaptable, continuously altering its characteristics
throughout tumor development and serving a role in regulating
cancer progression (128). For
example, immune cells within the TME transition between antitumor
and protumor states in response to cytokines secreted by the TME.
Cells that suppress the antitumor response include Tregs,
myeloid-derived suppressor cells (MDSCs) and M2 phenotype
tumor-associated macrophages (TAMs; Fig. 3) (129). Numerous studies indicate that
the composition of immune cells in the TME, particularly endogenous
immune cells (such as MDSCs, Tregs and M2 macrophages), is directly
associated with unfavorable tumor treatment outcomes (130-132).
DCs are immune cells that originate from pluripotent
hematopoietic stem cells in the bone marrow. These cells can be
categorized into the classical DC (cDC) and lymphoid DC subtypes,
with the latter known as plasma cell-like DCs (pDCs). cDC1 and
cDC2, two subtypes of cDCs, are differentiated by the abundance of
antigen-presenting molecules on their surfaces. These cells are
highly specialized and proficient in stimulating naive
antigen-specific CD4+ and CD8+ T cells
through the processing and presentation of antigens, including
tumor-associated antigens. This function is critical for the
initiation of an adaptive immune response (133).
Previous studies indicate that DCs serve a role in
the antitumor immune response (134,135). Specifically, cDC1 can recognize
tumors, secrete factors that modulate the TME, present
tumor-associated antigens, activate CD8+ T cell
responses and enhance antitumor immune function (136-138). In addition, cDC2 can activate
the CD4+ T cell response, thereby augmenting the ability
of CD8+ T cells to kill tumor cells (139,140). IFN-I produced by pDCs serves an
important role in the activation of stimulator of IFN response
cGAMP interactor 1, which causes cell cycle arrest, regulates cell
death and promotes the expression of proapoptotic proteins such as
Bax and Bak. This increases the permeability of the mitochondrial
membrane, which releases apoptotic factors such as cytochrome C,
which in turn triggers the caspase cascade, leading to apoptosis
(141). However, the TME in
contrast can inhibit the differentiation and maturation of DCs
(128), resulting in functional
defects in their ability to stimulate T cells and drive antitumor
immune responses, thereby promoting tumor growth (142-144). DC dysfunction occurs in various
types of cancer, including colon cancer and melanoma (145,146), and is associated with a poor
prognosis in patients with advanced-stage cancer (147,148). Consequently, activating DCs in
the TME is advantageous for antitumor therapy, making it a
promising target for immunotherapy.
Tumor growth is typically a slow gradual process,
where the interaction between tumor cells and the immune system can
reshape the immune environment, impacting T cell differentiation
(157). In patients with cancer,
a notable number of T lymphocytes migrate to the tumor site to
attack the malignancy. However, prolonged exposure can lead to
exhaustion, T cell depletion, cytokine production and the reduced
ability to destroy tumor cells (157). Contributing to this, Tregs in
the TME obstruct antitumor effects through various mechanisms,
including the secretion of inhibitory cytokines (such as IL-10 and
TGF-β) to reduce the antigen-presenting ability of DCs, inhibiting
CD8+ T cells from targeting tumor cells and promoting
immune evasion (158-160). This is observed in several types
of solid tumors, including melanoma (161), hepatocellular carcinoma
(162), lymphoma (163) and lung cancer (164).
Numerous studies reveal that the dysfunction of
tumor-infiltrating T cells occurs gradually (165,166). Initially, a population of
tumor-specific T cells is depleted during thymic maturation due to
negative selection, limiting the antigen recognition ability of the
remaining auto/tumor-specific T cells (167). Subsequently, in a
non-inflammatory environment, the lack of innate stimulators (such
as lipopolysaccharide) results in the weak activation of
antigen-presenting cells, leading to the poor activation of
tumor-specific T cells (167).
Finally, the TME induces and maintains T cell hypo-responsiveness
(168). PD-1 is a marker of T
cell failure (164).
Tumor-infiltrating CD8+ T cells express PD-1, which
binds to PD-L1 on tumor cells, resulting in the inhibition of T
cell activation and antitumor immunity. Treg cell-mediated
immunosuppression is associated with the upregulation of PD-1
(169). Currently, PD-1
inhibitors are widely used in the treatment of various tumors (such
as in lung and gastric cancer) and can effectively improve disease
prognosis (170,171).
DLBCL is a prevalent type of non-Hodgkin's lymphoma.
Study of the TME in DLBCL is becoming an increasingly popular
research topic in the field of immunotherapeutics. Previous studies
demonstrate immunological dysfunction in TILs and elevated PD-1
expression levels, both of which are associated with poor prognosis
(172,173). Although PD-1 inhibitors (such as
pembrolizumab and nivolumab) do not demonstrate objective remission
rates in DLBCL, PD-1 inhibitors are considered safe, with good
tolerance and promising prospects (174,175). Anti-PD-1 therapy is indicated to
confer notable synergistic effects with vitamin C in a lymphoma
mouse model (30,176,177). Vitamin C boosts antitumor
immunity and improves anti-PD-1 therapy by increasing chemokine
production and TIL (178).
Vitamin C is known to enhance the activation of tumor-specific T
cells by improving the antigen presentation ability of DCs
(152). It also enhances T cell
penetration into the tumor area to increase antitumor efficacy in
combination with anti-PD-1 drugs (179). However, research on the role of
Tregs in the antitumor effects of vitamin C remains warranted.
Macrophages serve a key role in both innate and
adaptive immunity. They are primarily derived from bone
marrow-derived monocytes and their phenotype and function depend on
the surrounding microenvironment. There are two types of
macrophages: Classical activation (M1 macrophages) and alternative
activation (M2 macrophages) (180). In different tissue environments,
macrophages can differentiate into specific subtypes. M1
macrophages function to reduce inflammation, as well as to
phagocytose and kill target cells (181). By contrast, M2 macrophages are
involved in angiogenesis, tissue repair, and tumor progression
(182).
TAMs are the most common immune cells that
infiltrate the TME. Their role in promoting tumor growth has been
extensively studied (183).
Recent studies have shown that macrophages in the TME are
continuously transitioning between two states: M1 and M2 (184,185). During the early stages of tumor
growth, macrophages primarily differentiate into tumor infiltrating
M1 macrophages to promote the antitumor immune response. However,
in the latter stages, they primarily differentiate into the M2
subtype to counteract the antitumor effect. In the TME, TAMs are
predominantly of the M2 variety (178). They primarily promote
angiogenesis, invasion, metastasis and tumor cell proliferation
(180). TAMs exert their
antitumor effects through three main mechanisms: i) Secretion of
inhibitory cytokines, which inhibit CD8+ T lymphocytes
from destroying tumor cells, such as TGF-β and IL-10 (181); ii) regulation of the
epithelial-mesenchymal transition (EMT) and extracellular matrix
remodeling, which contributes to the malignant biological
characteristics and migration of tumor cells (182,183); and iii) tumor angiogenesis, in
which growth factors (such as platelet-derived growth factor and
VEGF) released by TAMs aid in tumor angiogenesis, creating blood
vessels that promote tumor cell spread. These effects were
indicated in several malignant types of tumor, including prostate,
bladder and breast cancer (186). Therefore, TAMs serve a critical
role in tumor development.
In DLBCL, TAMs are associated with disease
progression. Their expression is inversely associated with DLBCL
prognosis (187). A number of
experimental studies reveal that vitamin C can induce the
transformation of M2 macrophages into M1 macrophages to markedly
enhance the oxidative toxicity of M1 macrophages, promoting the
infiltration of tumor-killing T cells and enhancing antitumor
effects (188-190). In summary, pharmacological doses
of vitamin C, acting as a pro-oxidant, can promote the polarization
of TAMs towards the M1 subtype in the TME. This promotes antitumor
effects and facilitates TME remodeling.
MDSCs form a heterogeneous population of cells that
suppress the immune system within the TME. They primarily inhibit
the cytotoxic activity of T cells and natural killer cells, serving
a role in tumor-mediated immune escape (127,191). MDSCs are primarily immature
myeloid cells, consisting mainly of monocytes (M-MDSC) and
polymorphonuclear cells (PMN-MDSC). Studies demonstrate that M-MDSC
and PMN-MDSC are expanded in the majority of solid tumors, such as
hepatocellular carcinoma (192)
and melanoma (193). Although
PMN-MDSC forms the predominant subgroup, a study by Azzaoui et
al (194) demonstrates that
M-MDSC is the most inhibitory subgroup, with the absence of
PMN-MDSC not altering the tumor incidence. In colorectal and breast
cancer, monocytes/M-MDSC can differentiate into TAMs within the TME
and interact with TAMs to inhibit antitumor effects (195-197). This suggests that targeting
MDSCs may enhance antitumor effects. It has been observed that a
notable number of MDSCs can effectively impair T cell responses in
DLBCL (194). Previous studies
suggest that MDSCs serve a key role in early drug resistance
following R-CHOP treatment for DLBCL (198,199). At present, to the best of our
knowledge, the direct impact of vitamin C on MDSCs has not been
studied. However, it is likely that vitamin C can mediate
regulatory effects on DCs, TILs and TAMs within the TME to
influence MDSCs by modulating the TME (200).
AA is a water-soluble vitamin with various chemical
and physical properties. It should be stored in a shaded
environment. When administered at high doses (1-1.5 g/kg), vitamin
C exhibits pro-oxidant properties and may be beneficial in
antitumor therapy (201,202). Previous studies indicate that
when taken orally, vitamin C reaches lower plasma concentrations
compared with intravenous (IV) administration (33,74). The majority of existing in
vivo studies indicate that high doses of AA (1-4 g/kg)
administered through IV or intraperitoneal injection can inhibit
tumor growth by 40-60%.
The present review investigated the role of vitamin
C in treating DLBCL, but several challenges remain that limit its
clinical application. These challenges include determining the
optimal dose and blood concentration of vitamin C, in addition to
its concentration at the tumor site. High-dose vitamin C has
demonstrated antitumor effects in various studies, with doses
ranging from 1.5 g/kg (32,202), 10 g/day (203) and 50-100 g per dose (2-3 times a
week) (204). However, no
conclusive data support an ideal dose and/or frequency of
administration that would maximize its antitumor benefits. It is
also unclear whether the antitumor effect is dose-independent or
associated with the appropriate blood concentration (205,206). Therefore, further research is
needed to determine the optimal vitamin C treatment strategy to
maximize cellular uptake whilst preventing cytotoxicity.
Another challenge is understanding the role of
SVCT2 in the transport of vitamin C. SVCT2 has low saturation and
high affinity (207), but its
involvement is crucial for effective drug distribution. In
addition, the growth and hypoxic environment of tumor blood vessels
in DLBCL can reduce the permeability and concentration of antitumor
drugs in the TME (208),
blocking the access of vitamin C to the tumor site. SVCT2 protein
cannot be detected in the cell membranes of renal cell carcinoma
and breast cancer cells, but it is unclear whether this affects the
transport of vitamin C (209).
There is insufficient information on how SVCT2 is distributed and
expressed in tumor cells. Therefore, further research is needed to
elucidate how SVCT2 is expressed and distributed in tumor cells to
enhance the concentration of vitamin C in the TME. Numerous studies
have reported that vitamin C has anticancer properties and can
enhance the efficacy of antitumor treatments (33,153). However, relevant in vitro
and in vivo experiments involving DLBCL are currently
lacking. Therefore, further research is needed to clarify the
anticancer efficacy of vitamin C in DLBCL, its associated signaling
pathways and its impact on the TME. The present paper aimed to
establish a theoretical and empirical foundation for the clinical
application of vitamin C as a supplementary agent in cancer
therapy.
DLBCL is the most common subtype of non-Hodgkin's
lymphoma and is distinguished by its aggressive nature and
unfavorable prognosis. The present review investigated the
mechanisms through which vitamin C may influence the efficacy of
DLBCL treatment, proposing a potential association between vitamin
C and DLBCL, which may enhance the antitumor effectiveness of
pharmacological therapies through various mechanisms or pathways.
However, further research is required to clarify the specific
mechanisms involved and to investigate the potential therapeutic
implications within the framework of DLBCL management.
Not applicable.
CR took part in the conceptualization, figure
curation and writing of the original draft. YW and ML took part in
the conceptualization, supervision and editing and reviewing the
manuscript. YL took part in the conceptualization and figure
curation. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
Supported by the Graduate Student Research Grant from Weifang
Medical University (Weifang Science and Technology Development
Program; grant no. 2024YX045).
|
1
|
Zhao P, Zhao S, Huang C, Li Y, Wang J, Xu
J, Li L, Qian Z, Li W, Zhou S, et al: Efficacy and safety of
polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin
and prednisone for previously untreated diffuse large B-cell
lymphoma: A real-world, multi-center, retrospective cohort study.
Hematol Oncol. 43:e700172025. View Article : Google Scholar
|
|
2
|
Vaughn JL, Ramdhanny A, Munir M,
Rimmalapudi S and Epperla N: A comparative analysis of transformed
indolent lymphomas and de novo diffuse large B-cell lymphoma: A
population-based cohort study. Blood Cancer J. 14:2122024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmitz R, Wright GW, Huang DW, Johnson
CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer
AL, et al: Genetics and pathogenesis of diffuse large B-cell
lymphoma. N Engl J Med. 378:1396–1407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bantilan KS, Smith AN, Maurer MJ,
Teruya-Feldstein J, Matasar MJ, Moskowitz AJ, Straus DJ, Noy A,
Palomba ML, Horwitz SM, et al: Matched control analysis suggests
that R-CHOP followed by (R)-ICE may improve outcome in non-GCB
DLBCL compared with R-CHOP. Blood Adv. 8:2172–2181. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
García-Sancho AM, Cabero A and Gutiérrez
NC: Treatment of relapsed or refractory diffuse large B-cell
lymphoma: New approved options. J Clin Med. 13:702023. View Article : Google Scholar
|
|
6
|
Coiffier B, Thieblemont C, Van Den Neste
E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M,
Sebban C, et al: Long-term outcome of patients in the LNH-98.5
trial, the first randomized study comparing rituximab-CHOP to
standard CHOP chemotherapy in DLBCL patients: a study by the Groupe
d'Etudes des Lymphomes de l'Adulte. Blood. 116:2040–2045. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pfreundschuh M, Trümper L, Osterborg A,
Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani
PL, et al: CHOP-like chemotherapy plus rituximab versus CHOP-like
chemotherapy alone in young patients with good-prognosis diffuse
large-B-cell lymphoma: A randomised controlled trial by the
MabThera international trial (MInT) group. Lancet Oncol. 7:379–391.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinzone MR, Berretta M, Cacopardo B and
Nunnari G: Epstein-barr virus- and Kaposi sarcoma-associated
herpesvirus-related malignancies in the setting of human
immunodeficiency virus infection. Semin Oncol. 42:258–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chapman JR, Bouska AC, Zhang W, Alderuccio
JP, Lossos IS, Rimsza LM, Maguire A, Yi S, Chan WC, Vega F and Song
JY: EBV-positive HIV-associated diffuse large B cell lymphomas are
characterized by JAK/STAT (STAT3) pathway mutations and unique
clinicopathologic features. Br J Haematol. 194:870–878. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gianella S, Anderson CM, Var SR, Oliveira
MF, Lada SM, Vargas MV, Massanella M, Little SJ, Richman DD, Strain
MC, et al: Replication of human herpesviruses is associated with
higher HIV DNA levels during antiretroviral therapy started at
early phases of HIV infection. J Virol. 90:3944–3952. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SS: Epidemiology and etiology of
diffuse large B-cell lymphoma. Semin Hematol. 60:255–266. 2023.
View Article : Google Scholar
|
|
12
|
Ekström Smedby K, Vajdic CM, Falster M,
Engels EA, Martínez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori
Costantini A, Bracci PM, et al: Autoimmune disorders and risk of
non-Hodgkin lymphoma subtypes: A pooled analysis within the
InterLymph consortium. Blood. 111:4029–4038. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Liang Y, Pu J, Cai L, Gao R, Han
F, Chang K, Pan S, Wu Z, Zhang Y, et al: Dysregulated serum lipid
profile is associated with inflammation and disease activity in
primary Sjögren's syndrome: A retrospective study in China. Immunol
Lett. 267:1068652024. View Article : Google Scholar
|
|
14
|
Almasmoum HA: Molecular complexity of
diffuse large B-cell lymphoma: A molecular perspective and
therapeutic implications. J Appl Genet. 65:57–72. 2024. View Article : Google Scholar
|
|
15
|
Serganova I, Chakraborty S, Yamshon S,
Isshiki Y, Bucktrout R, Melnick A, Béguelin W and Zappasodi R:
Epigenetic, metabolic, and immune crosstalk in
germinal-center-derived B-cell lymphomas: Unveiling new
vulnerabilities for rational combination therapies. Front Cell Dev
Biol. 9:8051952022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horn H, Ziepert M, Becher C, Barth TF,
Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML, et
al: MYC status in concert with BCL2 and BCL6 expression predicts
outcome in diffuse large B-cell lymphoma. Blood. 121:2253–2263.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riedell PA and Smith SM: Double hit and
double expressors in lymphoma: Definition and treatment. Cancer.
124:4622–4632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cioroianu AI, Stinga PI, Sticlaru L,
Cioplea MD, Nichita L, Popp C and Staniceanu F: Tumor
microenvironment in diffuse large B-cell lymphoma: role and
prognosis. Anal Cell Pathol (Amst). 2019:85863542019.
|
|
19
|
Momivand M, Razaghi M, Mohammadi F,
Hoseinzadeh E and Najafi-Vosough R: The status of serum 25(OH)D
levels is related to breast cancer. Cancer Treat Res Commun.
42:1008702024. View Article : Google Scholar
|
|
20
|
Jang Y and Kim CY: The role of vitamin e
isoforms and metabolites in cancer prevention: Mechanistic insights
into sphingolipid metabolism modulation. Nutrients. 16:41152024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paller CJ, Zahurak ML, Mandl A, Metri NA,
Lalji A, Heath E, Kelly WK, Hoimes C, Barata P, Taksey J, et al:
High-dose intravenous vitamin C combined with docetaxel in men with
metastatic castration-resistant prostate cancer: A randomized
placebo-controlled phase II trial. Cancer Res Commun. 4:2174–2182.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gillberg L, Ørskov AD, Nasif A, Ohtani H,
Madaj Z, Hansen JW, Rapin N, Mogensen JB, Liu M, Dufva IH, et al:
Oral vitamin C supplementation to patients with myeloid cancer on
azacitidine treatment: Normalization of plasma vitamin C induces
epigenetic changes. Clin Epigenetics. 11:1432019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Leary BR, Alexander MS, Du J, Moose DL,
Henry MD and Cullen JJ: Pharmacological ascorbate inhibits
pancreatic cancer metastases via a peroxide-mediated mechanism. Sci
Rep. 10:176492020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furqan M, Abu-Hejleh T, Stephens LM,
Hartwig SM, Mott SL, Pulliam CF, Petronek M, Henrich JB, Fath MA,
Houtman JC, et al: Pharmacological ascorbate improves the response
to platinum-based chemotherapy in advanced stage non-small cell
lung cancer. Redox Biol. 53:1023182022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bodeker KL, Smith BJ, Berg DJ,
Chandrasekharan C, Sharif S, Fei N, Vollstedt S, Brown H, Chandler
M, Lorack A, et al: A randomized trial of pharmacological
ascorbate, gemcitabine, and nab-paclitaxel for metastatic
pancreatic cancer. Redox Biol. 77:1033752024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su X, Shen Z, Yang Q, Sui F, Pu J, Ma J,
Ma S, Yao D, Ji M and Hou P: Vitamin C kills thyroid cancer cells
through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways
via distinct mechanisms. Theranostics. 9:4461–4473. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Chen C, Chen X, Fei Y, Jiang L and
Wang G: Vitamin C promotes apoptosis and cell cycle arrest in oral
squamous cell carcinoma. Front Oncol. 10:9762020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su X, Li P, Han B, Jia H, Liang Q, Wang H,
Gu M, Cai J, Li S, Zhou Y, et al: Vitamin C sensitizes
BRAFV600E thyroid cancer to PLX4032 via inhibiting the
feedback activation of MAPK/ERK signal by PLX4032. J Exp Clin
Cancer Res. 40:342021. View Article : Google Scholar
|
|
29
|
Zhao X, Liu M, Li C, Liu X, Zhao J, Ma H,
Zhang S and Qu J: High dose vitamin C inhibits PD-L1 by ROS-pSTAT3
signal pathway and enhances T cell function in TNBC. Int
Immunopharmacol. 126:1113212024. View Article : Google Scholar
|
|
30
|
Lv H, Zong Q, Chen C, Lv G, Xiang W, Xing
F, Jiang G, Yan B, Sun X, Ma Y, et al: TET2-mediated tumor cGAS
triggers endothelial STING activation to regulate vasculature
remodeling and anti-tumor immunity in liver cancer. Nat Commun.
15:62024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Williams DJ, Edwards D, Pun S, Chaliha M,
Burren B, Tinggi U and Sultanbawa Y: Organic acids in Kakadu plum
(Terminalia ferdinandiana): The good (ellagic), the bad (oxalic)
and the uncertain (ascorbic). Food Res Int. 89:237–244. 2016.
View Article : Google Scholar
|
|
32
|
Böttger F, Vallés-Martí A, Cahn L and
Jimenez CR: High-dose intravenous vitamin C, a promising
multi-targeting agent in the treatment of cancer. J Exp Clin Cancer
Res. 40:3432021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ngo B, Van Riper JM, Cantley LC and Yun J:
Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev
Cancer. 19:271–282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang JE, Voorhees PM, Kolesar JM, Ahuja
HG, Sanchez FA, Rodriguez GA, Kim K, Werndli J, Bailey HH and Kahl
BS: Phase II study of arsenic trioxide and ascorbic acid for
relapsed or refractory lymphoid malignancies: A wisconsin oncology
network study. Hematol Oncol. 27:11–16. 2009. View Article : Google Scholar
|
|
35
|
Kawada H, Sawanobori M, Tsuma-Kaneko M,
Wasada I, Miyamoto M, Murayama H, Toyosaki M, Onizuka M, Tsuboi K,
Tazume K, et al: Phase I clinical trial of intravenous l-ascorbic
acid following salvage chemotherapy for relapsed B-cell
non-Hodgkin's lymphoma. Tokai J Exp Clin Med. 39:111–115.
2014.PubMed/NCBI
|
|
36
|
Carr AC and Maggini S: Vitamin C and
immune function. Nutrients. 9:12112017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Padayatty SJ and Levine M: Vitamin C: The
known and the unknown and goldilocks. Oral Dis. 22:463–493. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhoot HR, Zamwar UM, Chakole S and
Anjankar A: Dietary sources, bioavailability, and functions of
ascorbic acid (vitamin C) and its role in the common cold, tissue
healing, and iron metabolism. Cureus. 15:e493082023.PubMed/NCBI
|
|
39
|
Bowry SK: Dialysis membranes today. Int J
Artif Organs. 25:447–460. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bürzle M, Suzuki Y, Ackermann D, Miyazaki
H, Maeda N, Clémençon B, Burrier R and Hediger MA: The
sodium-dependent ascorbic acid transporter family SLC23. Mol
Aspects Med. 34:436–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lykkesfeldt J and Tveden-Nyborg P: The
pharmacokinetics of vitamin C. Nutrients. 11:24122019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Doseděl M, Jirkovský E, Macáková K,
Krčmová LK, Javorská L, Pourová J, Mercolini L, Remião F, Nováková
L, Mladěnka P, et al: Vitamin C-sources, physiological role,
kinetics, deficiency, use, toxicity, and determination. Nutrients.
13:6152021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hasselholt S, Tveden-Nyborg P and
Lykkesfeldt J: Distribution of vitamin C is tissue specific with
early saturation of the brain and adrenal glands following
differential oral dose regimens in guinea pigs. Br J Nutr.
113:1539–1549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mohammed BM, Fisher BJ, Kraskauskas D,
Ward S, Wayne JS, Brophy DF, Fowler AA III, Yager DR and Natarajan
R: Vitamin C promotes wound healing through novel pleiotropic
mechanisms. Int Wound J. 13:572–584. 2016. View Article : Google Scholar
|
|
45
|
Moores J: Vitamin C: A wound healing
perspective. Br J Community Nurs. Suppl:S6S8–S11. 2013. View Article : Google Scholar
|
|
46
|
Fisher BJ, Kraskauskas D, Martin EJ,
Farkas D, Puri P, Massey HD, Idowu MO, Brophy DF, Voelkel NF,
Fowler AA III and Natarajan R: Attenuation of sepsis-induced organ
injury in mice by vitamin C. JPEN J Parenter Enteral Nutr.
38:825–839. 2014. View Article : Google Scholar
|
|
47
|
Carr AC, Shaw GM, Fowler AA and Natarajan
R: Ascorbate-dependent vasopressor synthesis: A rationale for
vitamin C administration in severe sepsis and septic shock? Crit
Care. 19:4182015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bozonet SM, Carr AC, Pullar JM and Vissers
MC: Enhanced human neutrophil vitamin C status, chemotaxis and
oxidant generation following dietary supplementation with vitamin
C-rich SunGold kiwifruit. Nutrients. 7:2574–2588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hong JM, Kim JH, Kang JS, Lee WJ and Hwang
YI: Vitamin C is taken up by human T cells via sodium-dependent
vitamin C transporter 2 (SVCT2) and exerts inhibitory effects on
the activation of these cells in vitro. Anat Cell Biol. 49:88–98.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Molina N, Morandi AC, Bolin AP and Otton
R: Comparative effect of fucoxanthin and vitamin C on oxidative and
functional parameters of human lymphocytes. Int Immunopharmacol.
22:41–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van Gorkom GNY, Klein Wolterink RGJ, Van
Elssen CHMJ, Wieten L, Germeraad WTV and Bos GMJ: Influence of
vitamin C on lymphocytes: An overview. Antioxidants (Basel).
7:412018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang X, Zhang Y, Wang C and Wang X: TET
(Ten-eleven translocation) family proteins: Structure, biological
functions and applications. Signal Transduct Target Ther.
8:2972023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yue X, Trifari S, Äijö T, Tsagaratou A,
Pastor WA, Zepeda-Martínez JA, Lio CW, Li X, Huang Y, Vijayanand P,
et al: Control of Foxp3 stability through modulation of TET
activity. J Exp Med. 213:377–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng X, Gao F and May WS Jr: Bcl2 retards
G1/S cell cycle transition by regulating intracellular ROS. Blood.
102:3179–3185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Güler A, Yardımcı BK and Özek NŞ: Human
anti-apoptotic Bcl-2 and Bcl-xL proteins protect yeast cells from
aging induced oxidative stress. Biochimie. 229:69–83. 2025.
View Article : Google Scholar
|
|
58
|
Yu X, Wang Y, Tan J, Li Y, Yang P, Liu X,
Lai J, Zhang Y, Cai L, Gu Y, et al: Inhibition of NRF2 enhances the
acute myeloid leukemia cell death induced by venetoclax via the
ferroptosis pathway. Cell Death Discov. 10:352024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sies H: Oxidative stress: A concept in
redox biology and medicine. Redox Biol. 4:180–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Assi M: The differential role of reactive
oxygen species in early and late stages of cancer. Am J Physiol
Regul Integr Comp Physiol. 313:R646–R653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hayes JD, Dinkova-Kostova AT and Tew KD:
Oxidative stress in cancer. Cancer Cell. 38:167–197. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sahoo BM, Banik BK, Borah P and Jain A:
Reactive oxygen species (ROS): Key components in cancer therapies.
Anticancer Agents Med Chem. 22:215–222. 2022. View Article : Google Scholar
|
|
63
|
Ebrahim AS, Sabbagh H, Liddane A, Raufi A,
Kandouz M and Al-Katib A: Hematologic malignancies: Newer
strategies to counter the BCL-2 protein. J Cancer Res Clin Oncol.
142:2013–2022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ashkenazi A, Fairbrother WJ, Leverson JD
and Souers AJ: From basic apoptosis discoveries to advanced
selective BCL-2 family inhibitors. Nat Rev Drug Discov. 16:273–284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sermer D, Bobillo S, Dogan A, Zhang Y,
Seshan V, Lavery JA, Batlevi C, Caron P, Hamilton A, Hamlin P, et
al: Extra copies of MYC, BCL2, and BCL6 and outcome in patients
with diffuse large B-cell lymphoma. Blood Adv. 4:3382–3390. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cerón R, Martínez A, Ramos C, De la Cruz
A, García A, Mendoza I, Palmeros G, Montaño Figueroa EH, Navarrete
J, Jiménez-Morales S, et al: Overexpression of BCL2, BCL6, VEGFR1
and TWIST1 in circulating tumor cells derived from patients with
DLBCL decreases event-free survival. Onco Targets Ther.
15:1583–1595. 2022. View Article : Google Scholar
|
|
67
|
Low IC, Kang J and Pervaiz S: Bcl-2: A
prime regulator of mitochondrial redox metabolism in cancer cells.
Antioxid Redox Signal. 15:2975–2987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Krishna S, Low ICC and Pervaiz S:
Regulation of mitochondrial metabolism: Yet another facet in the
biology of the oncoprotein Bcl-2. Biochem J. 435:545–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Eno CO, Zhao G, Olberding KE and Li C: The
Bcl-2 proteins Noxa and Bcl-xL co-ordinately regulate oxidative
stress-induced apoptosis. Biochem J. 444:69–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lagadinou ED, Sach A, Callahan K, Rossi
RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer
KM, et al: BCL-2 inhibition targets oxidative phosphorylation and
selectively eradicates quiescent human leukemia stem cells. Cell
Stem Cell. 12:329–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lv H, Wang C, Fang T, Li T, Lv G, Han Q,
Yang W and Wang H: Vitamin C preferentially kills cancer stem cells
in hepatocellular carcinoma via SVCT-2. NPJ Precis Oncol. 2:12018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ghanem A, Melzer AM, Zaal E, Neises L,
Baltissen D, Matar O, Glennemeier-Marke H, Almouhanna F, Theobald
J, Abu El Maaty MA, et al: Ascorbate kills breast cancer cells by
rewiring metabolism via redox imbalance and energy crisis. Free
Radic Biol Med. 163:196–209. 2021. View Article : Google Scholar
|
|
73
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fan D, Liu X, Shen Z, Wu P, Zhong L and
Lin F: Cell signaling pathways based on vitamin C and their
application in cancer therapy. Biomed Pharmacother. 162:1146952023.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
El-Garawani IM, El-Nabi SH, El-Shafey S,
Elfiky M and Nafie E: Coffea arabica bean extracts and vitamin C: A
novel combination unleashes MCF-7 cell death. Curr Pharm
Biotechnol. 21:23–36. 2020. View Article : Google Scholar
|
|
76
|
Chen MS, Zhao HK, Cheng YY, Yuan ZH and
Zhang YL: Toxic effects of vitamin C combined with temozolomide on
glioma cells and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za
Zhi. 36:616–621. 2020.In Chinese.
|
|
77
|
Liongue C, Almohaisen F and Ward AC: B
cell lymphoma 6 (BCL6): A conserved regulator of immunity and
beyond. Int J Mol Sci. 25:109682024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Basso K and Dalla-Favera R: Roles of BCL6
in normal and transformed germinal center B cells. Immunol Rev.
247:172–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Phan RT and Dalla-Favera R: The BCL6
proto-oncogene suppresses p53 expression in germinal-centre B
cells. Nature. 432:635–639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kerckaert JP, Deweindt C, Tilly H, Quief
S, Lecocq G and Bastard C: LAZ3, a novel zinc-finger encoding gene,
is disrupted by recurring chromosome 3q27 translocations in human
lymphomas. Nat Genet. 5:66–70. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Johnston RJ, Poholek AC, DiToro D, Yusuf
I, Eto D, Barnett B, Dent AL, Craft J and Crotty S: Bcl6 and
Blimp-1 are reciprocal and antagonistic regulators of T follicular
helper cell differentiation. Science. 325:1006–1010. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zan H, Wu X, Komori A, Holloman WK and
Casali P: AID-dependent generation of resected double-strand DNA
breaks and recruitment of Rad52/Rad51 in somatic hypermutation.
Immunity. 18:727–738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu Y, Feng J, Yuan K, Wu Z, Hu L, Lu Y,
Li K, Guo J, Chen J, Ma C and Pang X: The oncoprotein BCL6 enables
solid tumor cells to evade genotoxic stress. Elife. 11:e692552022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
McLachlan T, Matthews WC, Jackson ER,
Staudt DE, Douglas AM, Findlay IJ, Persson ML, Duchatel RJ, Mannan
A, Germon ZP and Dun MD: B-cell lymphoma 6 (BCL6): From master
regulator of humoral immunity to oncogenic driver in pediatric
cancers. Mol Cancer Res. 20:1711–1723. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Louwen F, Kreis NN, Ritter A, Friemel A,
Solbach C and Yuan J: BCL6, a key oncogene, in the placenta,
pre-eclampsia and endometriosis. Hum Reprod Update. 28:890–909.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Leeman-Neill RJ and Bhagat G: BCL6 as a
therapeutic target for lymphoma. Expert Opin Ther Targets.
22:143–152. 2018. View Article : Google Scholar
|
|
87
|
Huang C and Melnick A: Mechanisms of
action of BCL6 during germinal center B cell development. Sci China
Life Sci. 58:1226–1232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Krull JE, Wenzl K, Hartert KT, Manske MK,
Sarangi V, Maurer MJ, Larson MC, Nowakowski GS, Ansell SM, McPhail
E, et al: Somatic copy number gains in MYC, BCL2, and BCL6
identifies a subset of aggressive alternative-DH/TH DLBCL patients.
Blood Cancer J. 10:1172020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ting CY, Chang KM, Kuan JW, Sathar J, Chew
LP, Wong OJ, Yusuf Y, Wong L, Samsudin AT, Pana MNBM, et al:
Clinical significance of BCL2, C-MYC, and BCL6 genetic
abnormalities, epstein-barr virus infection, CD5 protein
expression, germinal center B Cell/non-germinal center B-cell
subtypes, co-expression of MYC/BCL2 proteins and co-expression of
MYC/BCL2/BCL6 proteins in diffuse large B-cell lymphoma: a clinical
and pathological correlation study of 120 patients. Int J Med Sci.
16:556–566. 2019. View Article : Google Scholar :
|
|
90
|
Ferretti GDS, Quarti J, Dos Santos G,
Rangel LP and Silva JL: Anticancer therapeutic strategies targeting
p53 aggregation. Int J Mol Sci. 23:110232022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bieging KT, Mello SS and Attardi LD:
Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev
Cancer. 14:359–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Williams AB and Schumacher B: p53 in the
DNA-damage-repair process. Cold Spring Harb Perspect Med.
6:a0260702016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sermeus A and Michiels C: Reciprocal
influence of the p53 and the hypoxic pathways. Cell Death Dis.
2:e1642011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Miyata S, Ishii T and Kitanaka S:
Reduction of HIF-1α/PD-L1 by catalytic topoisomerase inhibitor
induces cell death through caspase activation in cancer cells under
hypoxia. Anticancer Res. 44:49–59. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Madan E, Parker TM, Pelham CJ, Palma AM,
Peixoto ML, Nagane M, Chandaria A, Tomás AR, Canas-Marques R,
Henriques V, et al: HIF-transcribed p53 chaperones HIF-1α. Nucleic
Acids Res. 47:10212–10234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ismail S, Elshimali Y, Daoud A and
Alshehabi Z: Immunohistochemical expression of transcription
factors PAX5, OCT2, BCL6 and transcription regulator P53 in
Non-Hodgkin lymphomas: A diagnostic cross-sectional study. Ann Med
Surg (Lond). 78:1037862022.PubMed/NCBI
|
|
97
|
Choi SH, Koh DI, Cho SY, Kim MK, Kim KS
and Hur MW: Temporal and differential regulation of
KAISO-controlled transcription by phosphorylated and acetylated p53
highlights a crucial regulatory role of apoptosis. J Biol Chem.
294:12957–12974. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Margalit O, Amram H, Amariglio N, Simon
AJ, Shaklai S, Granot G, Minsky N, Shimoni A, Harmelin A, Givol D,
et al: BCL6 is regulated by p53 through a response element
frequently disrupted in B-cell non-Hodgkin lymphoma. Blood.
107:1599–1607. 2006. View Article : Google Scholar
|
|
99
|
Kaźmierczak-Barańska J, Boguszewska K,
Adamus-Grabicka A and Karwowski BT: Two faces of vitamin
C-antioxidative and pro-oxidative agent. Nutrients. 12:15012020.
View Article : Google Scholar
|
|
100
|
Xiong Y, Xu S, Fu B, Tang W, Zaky MY, Tian
R, Yao R, Zhang S, Zhao Q, Nian W, et al: Vitamin C-induced
competitive binding of HIF-1α and p53 to ubiquitin E3 ligase CBL
contributes to anti-breast cancer progression through p53
deacetylation. Food Chem Toxicol. 168:1133212022. View Article : Google Scholar
|
|
101
|
Kim J, Lee SD, Chang B, Jin DH, Jung SI,
Park MY, Han Y, Yang Y, Il Kim K, Lim JS, et al: Enhanced antitumor
activity of vitamin C via p53 in cancer cells. Free Radic Biol Med.
53:1607–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Huang K: Chemical inducers of proximity:
Precision tools for apoptosis in transcriptional regulation. Signal
Transduct Target Ther. 9:3642024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Duffy MJ, O'Grady S, Tang M and Crown J:
MYC as a target for cancer treatment. Cancer Treat Rev.
94:1021542021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Das SK, Lewis BA and Levens D: MYC: A
complex problem. Trends Cell Biol. 33:235–246. 2023. View Article : Google Scholar :
|
|
105
|
Ellenbroek BD, Kahler JP, Arella D, Lin C,
Jespers W, Züger EA, Drukker M and Pomplun SJ: Development of
DuoMYC: A synthetic cell penetrant miniprotein that efficiently
inhibits the oncogenic transcription factor MYC. Angew Chem Int Ed
Engl. 64:e2024160822025. View Article : Google Scholar :
|
|
106
|
Baluapuri A, Wolf E and Eilers M: Target
gene-independent functions of MYC oncoproteins. Nat Rev Mol Cell
Biol. 21:255–267. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu Z, Chen SS, Clarke S, Veschi V and
Thiele CJ: Targeting MYCN in pediatric and adult cancers. Front
Oncol. 10:6236792021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Massó-Vallés D, Beaulieu ME and Soucek L:
MYC, MYCL, and MYCN as therapeutic targets in lung cancer. Expert
Opin Ther Targets. 24:101–114. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Stoelzle T, Schwarb P, Trumpp A and Hynes
NE: c-Myc affects mRNA translation, cell proliferation and
progenitor cell function in the mammary gland. BMC Biol. 7:632009.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Felipe I, Martínez-de-Villarreal J, Patel
K, Martínez-Torrecuadrada J, Grossmann LD, Roncador G, Cubells M,
Farrell A, Kendsersky N, Sabroso-Lasa S, et al: BPTF cooperates
with MYCN and MYC to link neuroblastoma cell cycle control to
epigenetic cellular states. bioRxiv [Preprint]: 2024.02.11.579816.
2024.
|
|
111
|
Mahdavi P, Panahipoor Javaherdehi A,
Khanjanpoor P, Aminian H, Zakeri M, Zafarani A and Razizadeh MH:
The role of c-Myc in Epstein-Barr virus-associated cancers:
Mechanistic insights and therapeutic implications. Microb Pathog.
197:1070252024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yoon J, Jeon T, Kwon JA and Yoon SY:
Characterization of MYC rearrangements in multiple myeloma: An
optical genome mapping approach. Blood Cancer J. 14:1652024.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jakobsen ST and Siersbæk R:
Transcriptional regulation by MYC: An emerging new model. Oncogene.
44:1–7. 2025. View Article : Google Scholar
|
|
114
|
Bisso A, Filipuzzi M, Gamarra Figueroa GP,
Brumana G, Biagioni F, Doni M, Ceccotti G, Tanaskovic N, Morelli
MJ, Pendino V, et al: Cooperation between MYC and β-catenin in
liver tumorigenesis requires Yap/Taz. Hepatology. 72:1430–1443.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dhanasekaran R, Deutzmann A,
Mahauad-Fernandez WD, Hansen AS, Gouw AM and Felsher DW: The MYC
oncogene-the grand orchestrator of cancer growth and immune
evasion. Nat Rev Clin Oncol. 19:23–36. 2022. View Article : Google Scholar
|
|
116
|
Baena E, Ortiz M, Martínez-A C and de
Alborán IM: c-Myc is essential for hematopoietic stem cell
differentiation and regulates Lin(-)Sca-1(+)c-Kit(-) cell
generation through p21. Exp Hematol. 35:1333–1343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sewastianik T, Prochorec-Sobieszek M,
Chapuy B and Juszczyński P: MYC deregulation in lymphoid tumors:
Molecular mechanisms, clinical consequences and therapeutic
implications. Biochim Biophys Acta. 1846:457–467. 2014.PubMed/NCBI
|
|
118
|
Susanibar-Adaniya S and Barta SK: 2021
Update on diffuse large B cell lymphoma: A review of current data
and potential applications on risk stratification and management.
Am J Hematol. 96:617–629. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Candelaria M, Cerrato-Izaguirre D,
Gutierrez O, Diaz-Chavez J, Aviles A, Dueñas-Gonzalez A and Malpica
L: Characterizing the mutational landscape of diffuse large B-cell
lymphoma in a prospective cohort of mexican patients. Int J Mol
Sci. 25:93282024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hashmi AA, Iftikhar SN, Nargus G, Ahmed O,
Asghar IA, Shirazi UA, Afzal A, Irfan M and Ali J: Double-expressor
phenotype (BCL-2/c-MYC co-expression) of diffuse large B-cell
lymphoma and its clinicopathological correlation. Cureus.
13:e131552021.PubMed/NCBI
|
|
121
|
Wu G, Liu T, Li H, Li Y, Li D and Li W:
c-MYC and reactive oxygen species play roles in tetrandrine-induced
leukemia differentiation. Cell Death Dis. 9:4732018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Koo JI, Sim DY, Lee HJ, Ahn CH, Park J,
Park SY, Lee D, Shim BS, Kim B and Kim SH: Apoptotic and
anti-Warburg effect of Morusin via ROS mediated inhibition of
FOXM1/c-Myc signaling in prostate cancer cells. Phytother Res.
37:4473–4487. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Meng L, Gao J, Mo W, Wang B, Shen H, Cao
W, Ding M, Diao W, Chen W, Zhang Q, et al: MIOX inhibits autophagy
to regulate the ROS-driven inhibition of STAT3/c-Myc-mediated
epithelial-mesenchymal transition in clear cell renal cell
carcinoma. Redox Biol. 68:1029562023. View Article : Google Scholar
|
|
124
|
Yao L, Wu P, Yao F, Huang B, Zhong F and
Wang X: ZCCHC4 regulates esophageal cancer progression and
cisplatin resistance through ROS/c-myc axis. Sci Rep. 15:51492025.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Donati G, Nicoli P, Verrecchia A,
Vallelonga V, Croci O, Rodighiero S, Audano M, Cassina L, Ghsein A,
Binelli G, et al: Oxidative stress enhances the therapeutic action
of a respiratory inhibitor in MYC-driven lymphoma. EMBO Mol Med.
15:e169102023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Firouzjaei AA and Mohammadi-Yeganeh S: The
intricate interplay between ferroptosis and efferocytosis in
cancer: Unraveling novel insights and therapeutic opportunities.
Front Oncol. 14:14242182024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ahn M, Ali A and Seo JH: Mitochondrial
regulation in the tumor microenvironment: Targeting mitochondria
for immunotherapy. Front Immunol. 15:14538862024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Shen M and Kang Y: Complex interplay
between tumor microenvironment and cancer therapy. Front Med.
12:426–439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Manea AJ and Ray SK: Advanced
bioinformatics analysis and genetic technologies for targeting
autophagy in glioblastoma multiforme. Cells. 12:8972023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pitt JM, Marabelle A, Eggermont A, Soria
JC, Kroemer G and Zitvogel L: Targeting the tumor microenvironment:
Removing obstruction to anticancer immune responses and
immunotherapy. Ann Oncol. 27:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tong X, Tang R, Xiao M, Xu J, Wang W,
Zhang B, Liu J, Yu X and Shi S: Targeting cell death pathways for
cancer therapy: Recent developments in necroptosis, pyroptosis,
ferroptosis, and cuproptosis research. J Hematol Oncol. 15:1742022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Babar Q, Saeed A, Tabish TA, Sarwar M and
Thorat ND: Targeting the tumor microenvironment: Potential strategy
for cancer therapeutics. Biochim Biophys Acta Mol Basis Dis.
1869:1667462023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Worbs T, Hammerschmidt SI and Förster R:
Dendritic cell migration in health and disease. Nat Rev Immunol.
17:30–48. 2017. View Article : Google Scholar
|
|
134
|
Wculek SK, Cueto FJ, Mujal AM, Melero I,
Krummel MF and Sancho D: Dendritic cells in cancer immunology and
immunotherapy. Nat Rev Immunol. 20:7–24. 2020. View Article : Google Scholar
|
|
135
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Noubade R, Majri-Morrison S and Tarbell
KV: Beyond cDC1: Emerging roles of DC crosstalk in cancer immunity.
Front Immunol. 10:10142019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Böttcher JP and Reis e Sousa C: The role
of type 1 conventional dendritic cells in cancer immunity. Trends
Cancer. 4:784–792. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wculek SK, Amores-Iniesta J, Conde-Garrosa
R, Khouili SC, Melero I and Sancho D: Effective cancer
immunotherapy by natural mouse conventional type-1 dendritic cells
bearing dead tumor antigen. J Immunother Cancer. 7:1002019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Binnewies M, Mujal AM, Pollack JL, Combes
AJ, Hardison EA, Barry KC, Tsui J, Ruhland MK, Kersten K,
Abushawish MA, et al: Unleashing type-2 dendritic cells to drive
protective antitumor CD4+ T cell immunity. Cell.
177:556–571.e16. 2019. View Article : Google Scholar
|
|
140
|
Ruhland MK, Roberts EW, Cai E, Mujal AM,
Marchuk K, Beppler C, Nam D, Serwas NK, Binnewies M and Krummel MF:
Visualizing synaptic transfer of tumor antigens among dendritic
cells. Cancer Cell. 37:786–799.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Holicek P, Guilbaud E, Klapp V, Truxova I,
Spisek R, Galluzzi L and Fucikova J: Type I interferon and cancer.
Immunol Rev. 321:115–127. 2024. View Article : Google Scholar
|
|
142
|
Chrisikos TT, Zhou Y, Slone N, Babcock R,
Watowich SS and Li HS: Molecular regulation of dendritic cell
development and function in homeostasis, inflammation, and cancer.
Mol Immunol. 110:24–39. 2019. View Article : Google Scholar
|
|
143
|
Veglia F and Gabrilovich DI: Dendritic
cells in cancer: The role revisited. Curr Opin Immunol. 45:43–51.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Han SH and Ju MH: Characterizing the tumor
microenvironment and its correlation with cDC1-related gene
expression in gastric cancer. J Immunol Res. 2024:44681452024.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Kießler M, Plesca I, Sommer U, Wehner R,
Wilczkowski F, Müller L, Tunger A, Lai X, Rentsch A, Peuker K, et
al: Tumor-infiltrating plasmacytoid dendritic cells are associated
with survival in human colon cancer. J Immunother Cancer.
9:e0018132021. View Article : Google Scholar
|
|
146
|
Aspord C, Leccia MT, Charles J and Plumas
J: Melanoma hijacks plasmacytoid dendritic cells to promote its own
progression. Oncoimmunology. 3:e274022014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
De Sá Fernandes C, Novoszel P, Gastaldi T,
Krauß D, Lang M, Rica R, Kutschat AP, Holcmann M, Ellmeier W,
Seruggia D, et al: The histone deacetylase HDAC1 controls dendritic
cell development and anti-tumor immunity. Cell Rep. 43:1143082024.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Verneau J, Sautés-Fridman C and Sun CM:
Dendritic cells in the tumor microenvironment: Prognostic and
theranostic impact. Semin Immunol. 48:1014102020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Ennishi D: The biology of the tumor
microenvironment in DLBCL: Targeting the 'don't eat me' signal. J
Clin Exp Hematop. 61:210–215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Jeong YJ, Kim JH, Hong JM, Kang JS, Kim
HR, Lee WJ and Hwang YI: Vitamin C treatment of mouse bone
marrow-derived dendritic cells enhanced CD8(+) memory T cell
production capacity of these cells in vivo. Immunobiology.
219:554–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kim HW, Cho SI, Bae S, Kim H, Kim Y, Hwang
YI, Kang JS and Lee WJ: Vitamin C up-regulates expression of CD80,
CD86 and MHC class II on dendritic cell line, DC-1 via the
activation of p38 MAPK. Immune Netw. 12:277–283. 2012. View Article : Google Scholar
|
|
152
|
Morante-Palacios O, Godoy-Tena G,
Calafell-Segura J, Ciudad L, Martínez-Cáceres EM, Sardina JL and
Ballestar E: Vitamin C enhances NF-κB-driven epigenomic
reprogramming and boosts the immunogenic properties of dendritic
cells. Nucleic Acids Res. 50:10981–10994. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Magrì A, Germano G, Lorenzato A, Lamba S,
Chilà R, Montone M, Amodio V, Ceruti T, Sassi F, Arena S, et al:
High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med.
12:eaay87072020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chopp L, Redmond C, O'Shea JJ and Schwartz
DM: From thymus to tissues and tumors: A review of T-cell biology.
J Allergy Clin Immunol. 151:81–97. 2023. View Article : Google Scholar
|
|
155
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar
|
|
156
|
Kina E, Larouche JD, Thibault P and
Perreault C: The cryptic immunopeptidome in health and disease.
Trends Genet. 41:162–169. 2025. View Article : Google Scholar
|
|
157
|
Zhao Y, Shao Q and Peng G: Exhaustion and
senescence: Two crucial dysfunctional states of T cells in the
tumor microenvironment. Cell Mol Immunol. 17:27–35. 2020.
View Article : Google Scholar :
|
|
158
|
Knez J, Kovačič B and Goropevšek A: The
role of regulatory T-cells in the development of endometriosis. Hum
Reprod. deae1032024.Epub ahead of print. PubMed/NCBI
|
|
159
|
Sawant DV, Yano H, Chikina M, Zhang Q,
Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, et al: Adaptive
plasticity of IL-10+ and IL-35+
Treg cells cooperatively promotes tumor T cell
exhaustion. Nat Immunol. 20:724–735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Yi M, Niu M, Wu Y, Ge H, Jiao D, Zhu S,
Zhang J, Yan Y, Zhou P, Chu Q and Wu K: Combination of oral STING
agonist MSA-2 and anti-TGF-β/PD-L1 bispecific antibody YM101: A
novel immune cocktail therapy for non-inflamed tumors. J Hematol
Oncol. 15:1422022. View Article : Google Scholar
|
|
161
|
Naulaerts S, Datsi A, Borras DM, Antoranz
Martinez A, Messiaen J, Vanmeerbeek I, Sprooten J, Laureano RS,
Govaerts J, Panovska D, et al: Multiomics and spatial mapping
characterizes human CD8+ T cell states in cancer. Sci
Transl Med. 15:eadd10162023. View Article : Google Scholar
|
|
162
|
Li S, Li K, Wang K, Yu H, Wang X, Shi M,
Liang Z, Yang Z, Hu Y, Li Y, et al: Low-dose radiotherapy combined
with dual PD-L1 and VEGFA blockade elicits antitumor response in
hepatocellular carcinoma mediated by activated intratumoral
CD8+ exhausted-like T cells. Nat Commun. 14:77092023.
View Article : Google Scholar
|
|
163
|
Opinto G, Vegliante MC, Negri A, Skrypets
T, Loseto G, Pileri SA, Guarini A and Ciavarella S: The tumor
microenvironment of DLBCL in the computational era. Front Oncol.
10:3512020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Thommen DS, Koelzer VH, Herzig P, Roller
A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C,
et al: A transcriptionally and functionally distinct
PD-1+ CD8+ T cell pool with predictive
potential in non-small-cell lung cancer treated with PD-1 blockade.
Nat Med. 24:994–1004. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
van der Leun AM, Thommen DS and Schumacher
TN: CD8+ T cell states in human cancer: Insights from
single-cell analysis. Nat Rev Cancer. 20:218–232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Dolina JS, Van Braeckel-Budimir N, Thomas
GD and Salek-Ardakani S: CD8+ T cell exhaustion in
cancer. Front Immunol. 12:7152342021. View Article : Google Scholar
|
|
167
|
Baitsch L, Fuertes-Marraco SA, Legat A,
Meyer C and Speiser DE: The three main stumbling blocks for
anticancer T cells. Trends Immunol. 33:364–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Thinyakul C, Sakamoto Y, Shimoda M, Liu Y,
Thongchot S, Reda O, Nita A, Sakamula R, Sampattavanich S, Maeda A,
et al: Hippo pathway in cancer cells induces
NCAM1+αSMA+ fibroblasts to modulate tumor
microenvironment. Commun Biol. 7:13432024. View Article : Google Scholar
|
|
169
|
Sarhan D, Hippen KL, Lemire A, Hying S,
Luo X, Lenvik T, Curtsinger J, Davis Z, Zhang B, Cooley S, et al:
Adaptive NK cells resist regulatory T-cell suppression driven by
IL37. Cancer Immunol Res. 6:766–775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Li L, Nong J, Li J, Fang L, Pan M, Qiu H,
Huang S, Li Y, Wei M and Yin H: Dendrobine suppresses tumor growth
by regulating the PD-1/PD-L1 checkpoint pathway in lung cancer.
Curr Cancer Drug Targets. Sep 18–2024.Epub ahead of print.
View Article : Google Scholar
|
|
171
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y,
Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of
immune-checkpoint inhibitors in human cancers. Front Immunol.
13:9644422022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ma J, Pang X, Li J, Zhang W and Cui W: The
immune checkpoint expression in the tumor immune microenvironment
of DLBCL: Clinicopathologic features and prognosis. Front Oncol.
12:10693782022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Roussel M, Le KS, Granier C, Llamas
Gutierrez F, Foucher E, Le Gallou S, Pangault C, Xerri L, Launay V,
Lamy T, et al: Functional characterization of PD1+TIM3+
tumor-infiltrating T cells in DLBCL and effects of PD1 or TIM3
blockade. Blood Adv. 5:1816–1829. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Sheikh S and Kuruvilla J: Pembrolizumab
for the treatment of diffuse large B-cell lymphoma. Expert Opin
Biol Ther. 19:1119–1126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Luchtel RA, Bhagat T, Pradhan K, Jacobs WR
Jr, Levine M, Verma A and Shenoy N: High-dose ascorbic acid
synergizes with anti-PD1 in a lymphoma mouse model. Proc Natl Acad
Sci USA. 117:1666–1677. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Bedhiafi T, Inchakalody VP, Fernandes Q,
Mestiri S, Billa N, Uddin S, Merhi M and Dermime S: The potential
role of vitamin C in empowering cancer immunotherapy. Biomed
Pharmacother. 146:1125532022. View Article : Google Scholar
|
|
178
|
Xu YP, Lv L, Liu Y, Smith MD, Li WC, Tan
XM, Cheng M, Li Z, Bovino M, Aubé J and Xiong Y: Tumor suppressor
TET2 promotes cancer immunity and immunotherapy efficacy. J Clin
Invest. 129:4316–4331. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li H, Li Y, Zhang T, Liu S, Song C, Wang
K, Yan W, Wang Z, Yang Q, Yang X and Wang H: Genome-wide CRISPR
screen reveals specific role of type I interferon signaling pathway
in Newcastle disease virus establishment of persistent infection.
Vet Microbiol. 300:1102882025. View Article : Google Scholar
|
|
180
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Perry CJ, Muñoz-Rojas AR, Meeth KM,
Kellman LN, Amezquita RA, Thakral D, Du VY, Wang JX, Damsky W,
Kuhlmann AL, et al: Myeloid-targeted immunotherapies act in synergy
to induce inflammation and antitumor immunity. J Exp Med.
215:877–893. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Ngambenjawong C, Gustafson HH and Pun SH:
Progress in tumor-associated macrophage (TAM)-targeted
therapeutics. Adv Drug Deliv Rev. 114:206–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Afra F, Eftekhar SP, Farid AS and Ala M:
Non-coding RNAs in cancer immunotherapy: A solution to overcome
immune resistance. Prog Mol Biol Transl Sci. 209:215–240. 2024.
View Article : Google Scholar
|
|
184
|
Zhu W, Liu L, Wu J, Gao R, Fu L, Yang X,
Zou Y, Zhang S and Luo D: SMYD3 activates the TCA cycle to promote
M1-M2 conversion in macrophages. Int Immunopharmacol.
127:1113292024. View Article : Google Scholar
|
|
185
|
Fang C, Zhong R, Lu S, Yu G, Liu Z, Yan C,
Gao J, Tang Y, Wang Y, Zhao Q and Feng X: TREM2 promotes macrophage
polarization from M1 to M2 and suppresses osteoarthritis through
the NF-κB/CXCL3 axis. Int J Biol Sci. 20:1992–2007. 2024.
View Article : Google Scholar :
|
|
186
|
Basak U, Sarkar T, Mukherjee S,
Chakraborty S, Dutta A, Dutta S, Nayak D, Kaushik S, Das T and Sa
G: Tumor-associated macrophages: an effective player of the tumor
microenvironment. Front Immunol. 14:12952572023. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Daetwyler E, Wallrabenstein T, König D,
Cappelli LC, Naidoo J, Zippelius A and Läubli H:
Corticosteroid-resistant immune-related adverse events: A
systematic review. J Immunother Cancer. 12:e0074092024. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ma Z, Yang M, Foda MF, Zhang K, Li S,
Liang H, Zhao Y and Han H: Polarization of tumor-associated
macrophages promoted by vitamin C-loaded liposomes for cancer
immunotherapy. ACS Nano. 16:17389–17401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Xu Y, Guo X, Wang G and Zhou C: Vitamin C
inhibits metastasis of peritoneal tumors by preventing spheroid
formation in ID8 murine epithelial peritoneal cancer model. Front
Pharmacol. 11:6452020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Yao H, Xu J, Wang J, Zheng N, Yue J, Mi J,
Zheng L, Dai B, Huang W, Yung S, et al: Combination of magnesium
ions and vitamin C alleviates synovitis and osteophyte formation in
osteoarthritis of mice. Bioact Mater. 6:1341–1352. 2020.PubMed/NCBI
|
|
191
|
Najjar YG and Finke JH: Clinical
perspectives on targeting of myeloid derived suppressor cells in
the treatment of cancer. Front Oncol. 3:492013. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Hashimoto A, Sarker D, Reebye V, Jarvis S,
Sodergren MH, Kossenkov A, Sanseviero E, Raulf N, Vasara J,
Andrikakou P, et al: Upregulation of C/EBPα inhibits suppressive
activity of myeloid cells and potentiates antitumor response in
mice and patients with cancer. Clin Cancer Res. 27:5961–5978. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Holtzhausen A, Harris W, Ubil E, Hunter
DM, Zhao J, Zhang Y, Zhang D, Liu Q, Wang X, Graham DK, et al: TAM
family receptor kinase inhibition reverses MDSC-mediated
suppression and augments anti-PD-1 therapy in melanoma. Cancer
Immunol Res. 7:1672–1686. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Azzaoui I, Uhel F, Rossille D, Pangault C,
Dulong J, Le Priol J, Lamy T, Houot R, Le Gouill S, Cartron G, et
al: T-cell defect in diffuse large B-cell lymphomas involves
expansion of myeloid-derived suppressor cells. Blood.
128:1081–1092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Sadeghi M, Dehnavi S, Sharifat M, Amiri AM
and Khodadadi A: Innate immune cells: Key players of orchestra in
modulating tumor microenvironment (TME). Heliyon. 10:e274802024.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Johnson B: Targeting myeloid-derived
suppressor cell trafficking as a novel immunotherapeutic approach
in microsatellite stable colorectal cancer. Cancers (Basel).
15:54842023. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Wu Y, Yi M, Niu M, Mei Q and Wu K:
Myeloid-derived suppressor cells: An emerging target for anticancer
immunotherapy. Mol Cancer. 21:1842022. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Dhar S, Chakravarti M, Ganguly N, Saha A,
Dasgupta S, Bera S, Sarkar A, Roy K, Das J, Bhuniya A, et al: High
monocytic MDSC signature predicts multi-drug resistance and cancer
relapse in non-Hodgkin lymphoma patients treated with R-CHOP. Front
Immunol. 14:13039592024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wu C, Wu X, Liu X, Yang P, Xu J, Chai Y,
Guo Q, Wang Z and Zhang L: Prognostic significance of monocytes and
monocytic myeloid-derived suppressor cells in diffuse large B-cell
lymphoma treated with R-CHOP. Cell Physiol Biochem. 39:521–530.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Ali Y, Monini C, Russeil E, Létang JM,
Testa E, Maigne L and Beuve M: Estimate of the biological dose in
hadrontherapy using GATE. Cancers (Basel). 14:16672022. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Adibi A, Tokat ÜM, Özgü E, Aydın E,
Demiray İ and Demiray M: PARP inhibitor combinations with high-dose
vitamin C in the treatment of Ewing sarcoma: Two case reports and
mechanistic overview. Ther Adv Med Oncol. 15:175883592312138412023.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Wang F, He MM, Xiao J, Zhang YQ, Yuan XL,
Fang WJ, Zhang Y, Wang W, Hu XH, Ma ZG, et al: A randomized,
open-label, multicenter, phase 3 study of high-dose vitamin c plus
FOLFOX ± bevacizumab versus FOLFOX ± bevacizumab in unresectable
untreated metastatic colorectal cancer (VITALITY study). Clin
Cancer Res. 28:4232–4239. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Zasowska-Nowak A, Nowak PJ and
Ciałkowska-Rysz A: High-dose vitamin C in advanced-stage cancer
patients. Nutrients. 13:7352021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Polireddy K, Dong R, Reed G, Yu J, Chen P,
Williamson S, Violet PC, Pessetto Z, Godwin AK, Fan F, et al: High
dose parenteral ascorbate inhibited pancreatic cancer growth and
metastasis: Mechanisms and a phase I/IIa study. Sci Rep.
7:171882017. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Wang L and Lou X: A predictive model for
gastric cancer-specific death after gastrectomy: A competing-risk
nomogram. Iran J Public Health. 53:2350–2361. 2024.PubMed/NCBI
|
|
206
|
Chen P, Reed G, Jiang J, Wang Y, Sunega J,
Dong R, Ma Y, Esparham A, Ferrell R, Levine M, et al:
Pharmacokinetic evaluation of intravenous vitamin C: A classic
pharmacokinetic study. Clin Pharmacokinet. 61:1237–1249. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Linowiecka K, Foksinski M and Brożyna AA:
Vitamin C transporters and their implications in carcinogenesis.
Nutrients. 12:38692020. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Cheng YQ, Wang SB, Liu JH, Jin L, Liu Y,
Li CY, Su YR, Liu YR, Sang X, Wan Q, et al: Modifying the tumour
microenvironment and reverting tumour cells: New strategies for
treating malignant tumours. Cell Prolif. 53:e128652020. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Praditi C, Bozonet SM, Dachs GU and
Vissers M: Ascorbate uptake and retention by breast cancer cell
lines and the intracellular distribution of sodium-dependent
vitamin C transporter 2. Antioxidants (Basel). 12:19292023.
View Article : Google Scholar : PubMed/NCBI
|