Introduction
To the best of our knowledge, the functional
relationship between inflammation and cancer has not been recently
investigated. Karl Virchow hypothesized that cancer originates at
sites of chronic inflammation (1).
Although a causal relationship for inflammation and innate immunity
of cancer is more widely accepted today, the precise cell
mechanisms mediating this relationship have not been elucidated.
Th17 cells were identified in 2005 (2–4) and
in humans, the cytokines that direct Th17 cell lineage development
likely include IL-6, IL-21, IL-23 and IL-1-β. In addition, TGF-β
plays a potentially synergistic role through its ability to
suppress Th1 cell lineage commitment (5,6).
Although the IL-17 cytokine family includes six members, Th17 cells
are considered to produce only the proinflammatory cytokines IL-17A
and IL-17F, which are 55% identical. IL-17A and IL-17F combine to
form a heterodimer (7). It was
previously reported that IL-17 plays an important role in the
pathogenesis of inflammatory bowel diseases (IBDs), including
Crohn’s disease and ulcerative colitis (8,9).
Myeloid-derived suppressor cells (MDSCs) have been
identified in the majority of patients and experimental mice with
tumours and inflammation, based on their ability to suppress T-cell
activation (10). In mice, MDSCs
are uniformly characterised by expression of the cell surface
molecules detected by antibodies to Gr1 and CD11b (11). Variations in the MDSC phenotype are
consistent with the concept that MDSCs are a diverse family of
cells that are in various intermediate stages of myeloid cell
differentiation.
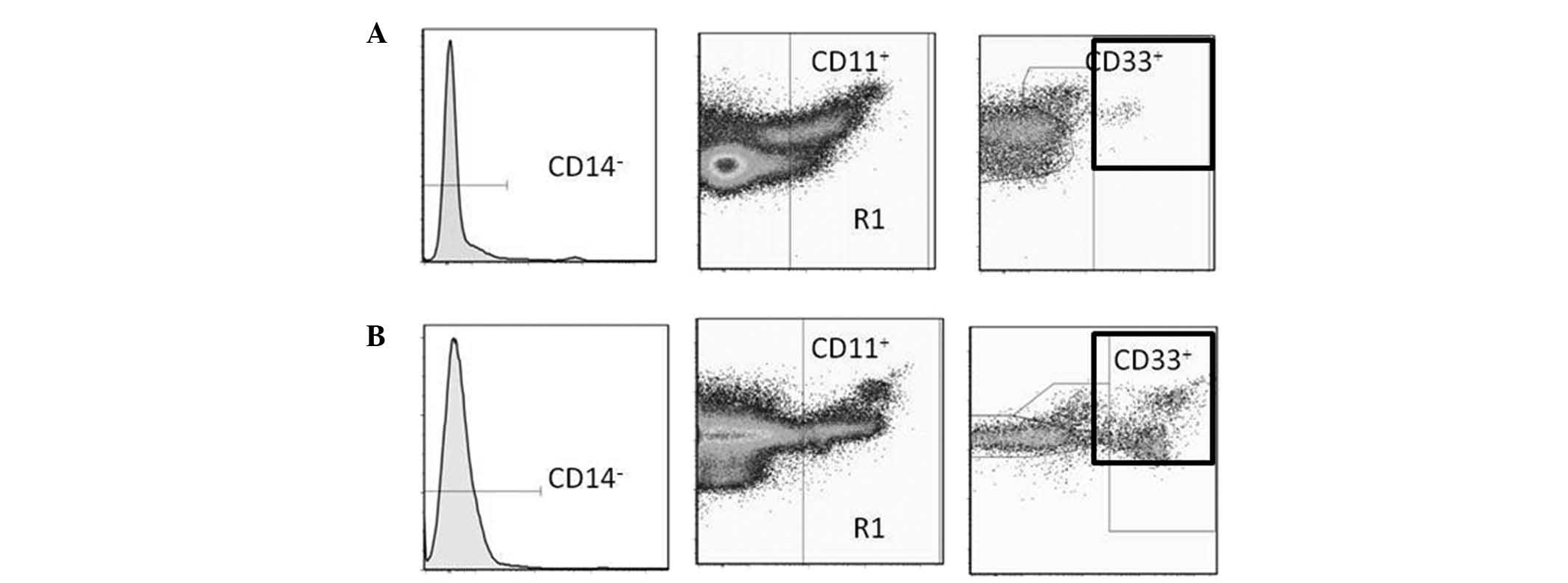
In humans, MDSCs are most commonly defined as
CD14−CD11b+ cells or, more narrowly, as cells
that express the common myeloid marker CD33 but not the markers of
mature myeloid or lymphoid cells, or the MHC class II molecule
HLA-DR (12). For the purposes of
this study, MDSCs were defined as
CD14−CD11b+CD33 cells.
Tumour development and growth occurs as a result of
interactions between tumour and host immune/inflammatory cells and
chronic inflammation plays an important role in cancer development
and progression (13,14). Inflammatory parameters based on
differential white cell counts, such as the neutrophil/lymphocyte
ratio (NLR), may be simple and readily available biomarkers for
tracking inflammation and cancer development. The results of the
present study demonstrate the correlation of IL-17 production
levels with MDSCs and other markers for nutritional status, immune
suppression and chronic inflammation in patients with a variety of
gastrointestinal cancers.
Materials and methods
Study subjects
Blood samples were collected from 60 patients with
various types of gastrointestinal cancer, that were as follows: 7
esophageal (2 stage II, 2 stage III and 3 stage IV); 14 gastric (5
stage I, 3 stage II, 1 stage III and 5 stage IV); 20 colorectal (1
stage I, 7 stage II, 4 stage III and 8 stage IV); 5 hepatocellular
(2 stage II and 3 stage III); 7 cholangiocellular (1 stage I, 2
stage III and 4 stage IV); and 7 pancreatic (2 stage II, 1 stage
III and 4 stage IV) cancer patients. In addition, samples from 18
healthy volunteers of similar age and gender distributions were
used as controls. The enrolled patients underwent surgery or
chemotherapy for the treatment of histologically confirmed cancer
in the departments of Organ Regulatory Surgery and Regenerative
Surgery of Fukushima Medical University from January, 2011 to
March, 2012. The patients were 41–85 years of age and newly
diagnosed. Blood samples were collected prior to the intitiation of
any treatment.
The study protocol was approved by the Ethics
Committee of Fukushima Medical University (2010–2014) and written
informed consent was obtained from the enrolled patients and normal
donors.
Blood samples
Peripheral blood mononuclear cells (PBMCs) were
separated on Ficoll-Hypaque (Pharmacia-Biotech, Uppsala, Sweden)
columns. The isolated PBMCs were washed twice with RPMI-1640 (Wako
Pure Chemical Industries Ltd., Osaka, Japan) and maintained at
−80°C in freezing medium (BLC-1; Juji-Field Co. Ltd., Tokyo, Japan)
until used.
Flow cytometry
Cells were labelled with fluorescent isothiocyanate
(FITC), phycoerythrin (PE) and phycoerythrin cyanin 5.1 (PC5).
Antibodies used included those directed against FITC-conjugated
CD14 (Abcam, Cambridge, UK), PE-conjugated CD11b (Beckman Coulter,
Inc., Marseille, France) and PC5-conjugated CD33 (Beckman Coulter),
diluted in phosphate-buffered saline (PBS) to 10 and 50
μg/ml. Cells were incubated with the antibodies for 20 min
at 4°C and then washed with PBS. Data acquisition and analysis were
performed using a FACSAria II flow cytometer (BD Biosciences,
Mountain View, CA, USA) accompanied by Flow Jo software (TreeStar,
Inc., Ashland, OR, USA). Typical expression patterns are shown in
Fig. 1.
Cytokine production by PBMCs
Samples (20 ml) of blood collected directly from
heparinized collection tubes were subjected to Ficoll-density
gradient centrifugation in order to isolate the PBMCs,
106 of which were incubated in 1 ml of RPMI-1640 medium
supplemented with 10% heat-inactivated fetal calf serum (Gibco BRL,
Rockville, MD, USA) and 20 μg/ml phytohemagglutinin (PHA)
(Sigma, St. Louis, MO, USA) in 5% CO2 at 37°C for 24 h.
Aliquots of these supernatants were then frozen and maintained at
−80°C until use. Supernatant samples subsequently were thawed and
used for measurement of IL-17 and IL-12 concentrations using ELISA
test kits (R&D Systems, Minneapolis, MN, USA). Each sample was
used only once after thawing.
Lymphocyte proliferation assay
Lymphocyte proliferation assays were performed using
PBMC suspended in RPMI-1640 (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) containing 10% fetal calf serum (Sigma). Following
the addition of 10 μg/ml PHA into PBMC culture wells kept at
37°C in a 5% CO2 atmosphere, PHA mitogenesis was
observed for 80 h. 3H-thymidine (Japan Radioisotope
Association, Tokyo, Japan) was added to the wells for the last 8 h
of incubation. Cells were harvested and 3H-thymidine
incorporation was determined using a liquid scintillation counter
(PerkinElmer, Inc., Waltham, MA, USA) and expressed as counts per
minute (cpm). The stimulation index (SI) was obtained by
calculating total cpm/control cpm. The controls were defined as
PBMCs that had not been subjected to PHA addition.
Markers for nutritional status and
chronic inflammation
To evaluate the nutritional status of the subjects,
serum concentrations of albumin (determined by nephelometry) and
prealbumin (determined using a turbidimetric immunoassay) were
measured using standard protocols. Neutrophil and lymphocyte
counts, as well as their ratios (NLR) in peripheral blood samples,
were used as indicators of inflammation in this study.
Statistical analysis
Differences between groups were determined by
Student’s t-tests. Correlations between two variables were
quantified by determining the Spearman’s rank correlation
coefficients. P<0.05 was considered to indicate a statistically
significant difference. Inadequate amounts of blood were obtained
from some patients and in these cases, certain measurements were
not possible.
Results
Factors affecting IL-7 production
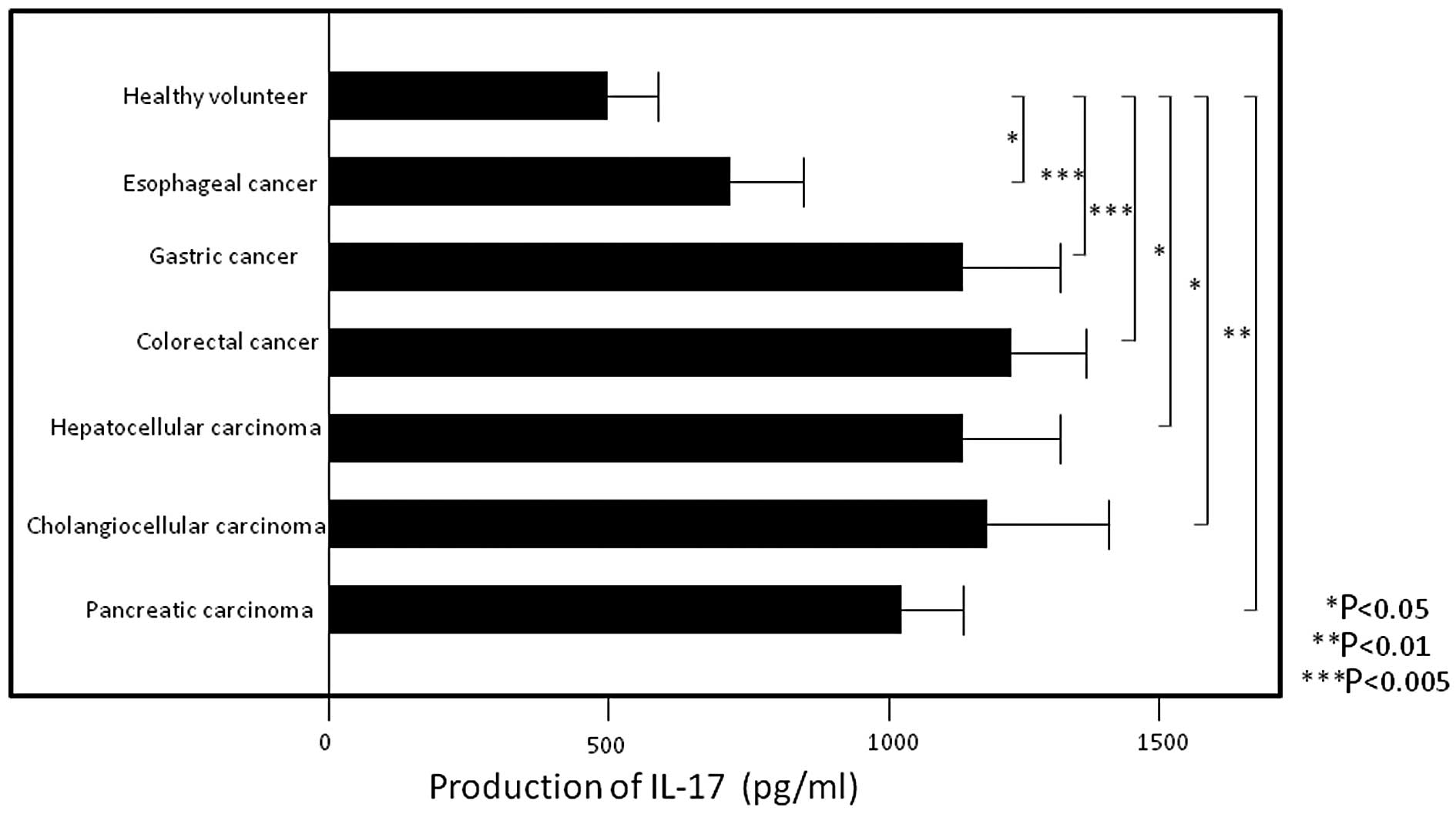
PBMC IL-17 production levels were significantly
higher in patients with esophageal (705.9±164.0, P<0.05),
gastric (1167.1±135.6, P<0.005), colorectal (1231.4±145.5,
P<0.005), hepatocellular (1133.6±212.6, P<0.05)
cholangiocellular (1181.5±261.6, P<0.05) and pancreatic
(1033.3±84.0, P<0.01) carcinoma compared to those in healthy
volunteers (544.6±133.4) (Fig. 2)
(all values are expressed as pg/ml). In addition, IL-17 production
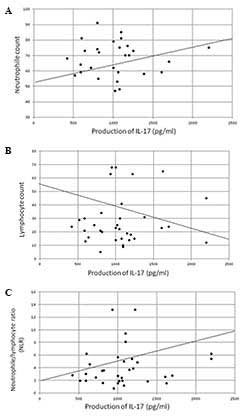
was significantly correlated with the neutrophil count (P<0.005,
r=0.436) and NLR (P<0.005, r=0.535) and was significantly
inversely correlated with the lymphocyte count (P<0.01,
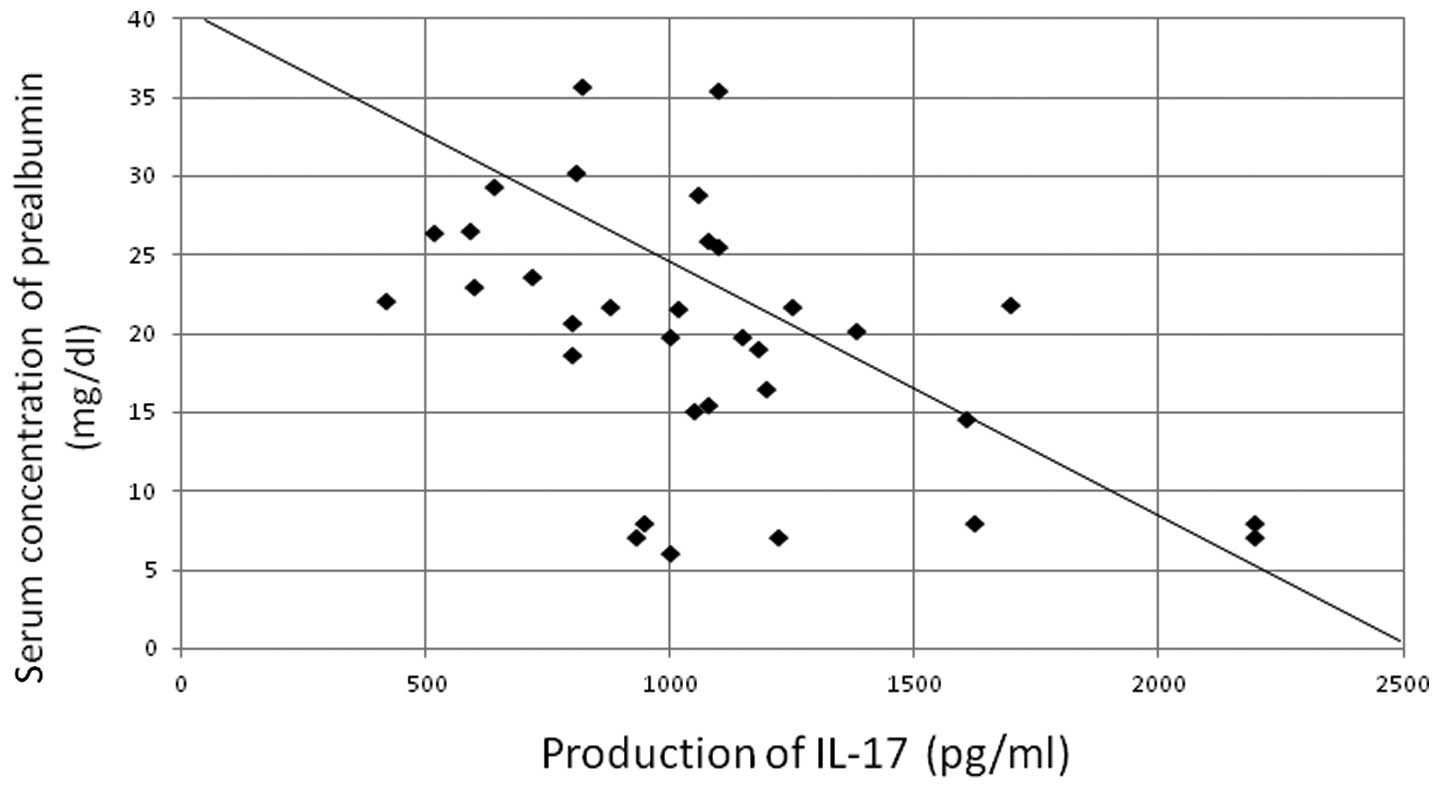
r=−0.420) (Fig. 3) and serum
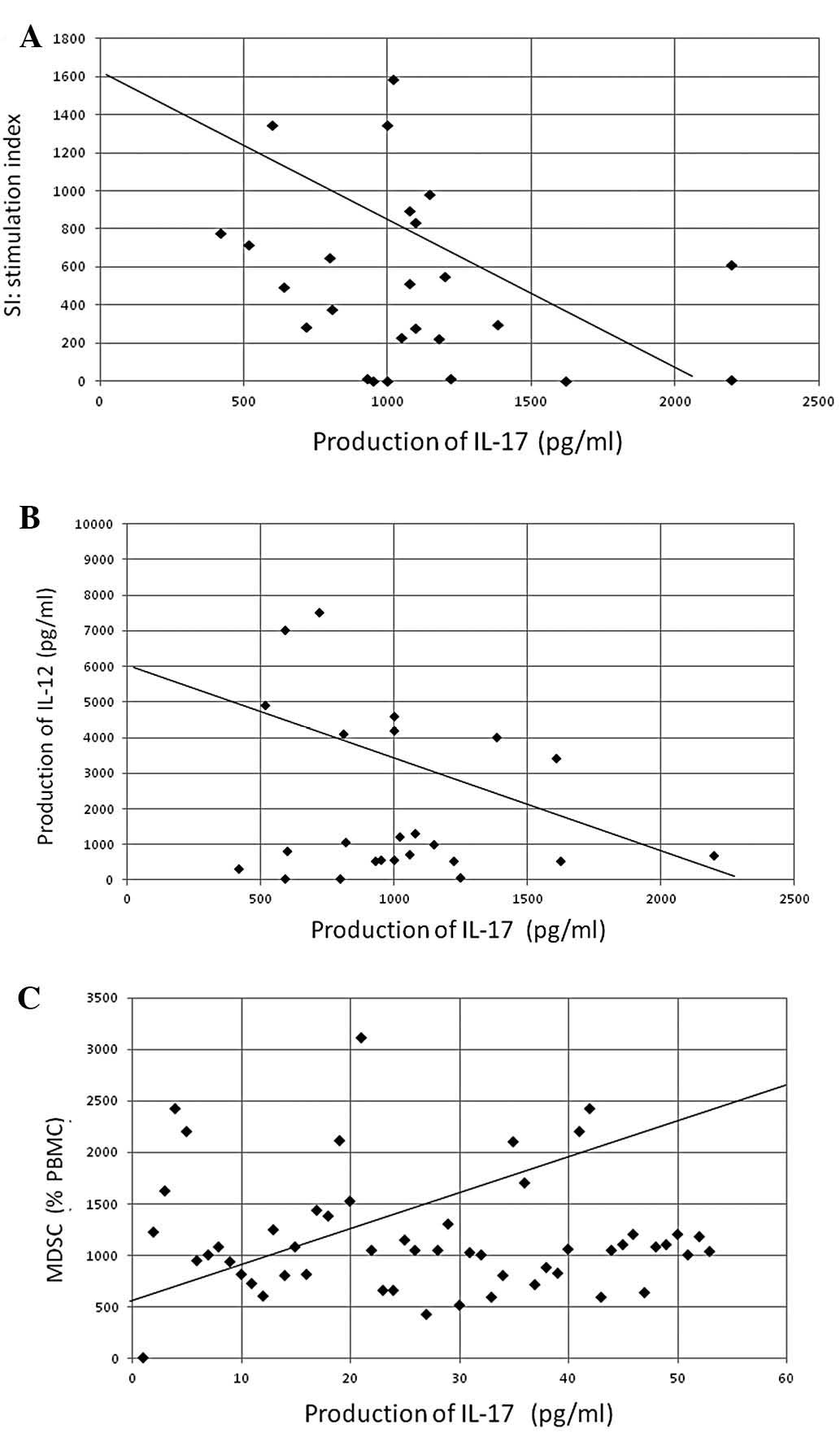
prealbumin concentration (P<0.05, r=−0.387) (Fig. 4). The production of IL-17
demonstrated a significant inverse correlation with SIs (found by
assessing lymphocyte PHA-blastogenesis) (P<0.05, r=−0.302) and
IL-12 production (P<0.01, r=−0.411), as well as a significant
positive correlation with circulating levels of MDSCs (P<0.001,
r=0.492) (Fig. 5).
Discussion
To the best of our knowledge, this study is the
first to describe an important role for IL-17 in the induction of
immune suppression and nutritional impairment during systemic
inflammation. IL-17 production was significantly higher in patients
with various types of gastrointestinal cancer compared to that in
normal volunteers. Production levels were significantly correlated
with neutrophil counts and NLRs. By contrast, they were
significantly inversely correlated with cell-mediated immune
responses, including lymphocyte PHA-blastogenesis and Th1
induction, as reflected by IL-12 production and compromised patient
nutritional status, as reflected by prealbumin levels. Circulating
levels of MDSCs were also significantly correlated with IL-17
production levels.
Over the last decade, there has been an expansion of
scientific knowledge regarding the pathogenesis of IBDs, including
Crohn’s disease and ulcerative colitis. IBDs have been reported to
arise due to a combination of genetic variations and alterations in
intestinal microflora, which may subsequently promote an
uncontrolled immune response and result in chronic intestinal
inflammation (8,9). IL-17 is considered to stimulate
various types of cells to produce proinflammatory mediators that
amplify intestinal inflammation. It has also been reported that in
humans, Th17 cells play an essential role in protective immunity
against certain microorganisms (15). Th17 cells have a close
developmental link with FOXP3+CD4+ regulatory
T cells. Th17 cells have been reported to transiently express FOXP3
during their development (16). In
the present study, the inflammation induced by IL-17 appeared to
cause the production of MDSCs that may potentially inhibit
maturation of dendritic cells, and cell-mediated immunity may be
suppressed through Th2 dominant conditions driven by the depressed
production of IL-12. Thus, Th17 cells may play important roles in
the development of immune suppression in patients with malignant
diseases.
The NLR was reported to be a marker of systemic
inflammatory response and an independent predictor of clinical
benefit, good prognosis and survival in patients receiving cancer
chemotherapy (17). The likelihood
of the association of IL-17 production with nutritional impairment
is high, due to the role of IL-17 as a marker of systemic
inflammation. It was previously reported that the key mechanisms
leading to cancer cachexia, in which nutritional impairment is a
major clinical issue, are mostly immune reactions caused by chronic
inflammation and that treatment with a COX-2 inhibitor or a
specific nutrient formula is effective (18,19).
Thus, in human cancers, chronic inflammation
involving IL-17 is considered to be important in the development of
disease-advancement indicators, such as immune suppression or
cachexia. In order to suppress Th17, several candidates have been
experimentally used. Since it was reported that Th17 cells are
induced by TGF-β, IL-1, IL-6, IL-21 or IL-23, antibodies targeting
IL-6 or IL-23 have been considered strong candidates for the
development as treatments for autoimmune diseases, including IBD
and rheumatoid arthritis (1,20).
Further investigations are required to explore this possibility and
gain more insight into this field of medicine.
Acknowledgements
The authors would like to thank Mr.
Shunichi Saito, Department of Blood Transfusion and Transplantation
Immunology, for his technical assistance in preparatory experiments
with MDSCs, Professor Hideharu Sekine, Department of Immunology,
Fukushima Medical University, for organizing flow cytometric study
for MDSCs and to Dr Mineyuki Haruta, Nihon University College of
Engineering, for processing the ELISA data.
References
|
1.
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Harrington LE, Hatton RD, Mangan PR, et
al: Interleukin 17-producing CD4+effector T cells
develop via a lineage distinct from the T helper type 1 and type 2
lineages. Nat Immunol. 6:1123–1132. 2005.PubMed/NCBI
|
|
3.
|
Langrish CL, Chen Y, Blumenschein WM, et
al: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Med. 201:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Park H, Li Z, Yang XO, et al: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chen Z, Tato CM, Muul L, Laurence A and
O’Shea JJ: Distinct regulation of interleukin-17 in human T helper
lymphocytes. Arthritis Rheum. 56:2936–2946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Santarlasci V, Maggi L, Capone M, et al:
TGF-beta indirectly favors the development of Th17 cells by
inhibiting Th1 cells. Eur J Immunol. 39:207–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kolls JK and Linden A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Brand S: Crohn’s disease: Th1, Th17 or
both? The change of a paradigm: new immunological and genetic
insights implicate Th17 cells in the pathogenesis of Crohn’s
disease. Gut. 58:1152–1167. 2009.
|
|
9.
|
Maynard CL and Weaver CL: Intestinal
effector T cells in health and disease. Immunity. 31:389–400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kao J, Ko EC, Einstein S, Sikora AT, Fu S
and Chen SH: Targeting immune suppressing myeloid-derived
suppressor cells in oncology. Crit Rev Oncol Hematol. 77:12–19.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kusmartsev S, Nefedova Y, Yoder D and
Gabrilovich DI: Antigen-specific inhibition of CD8+T
cell response by immature myeloid cells in cancer is mediated by
reactive oxygen species. J Immunol. 172:989–999. 2004.PubMed/NCBI
|
|
12.
|
Almand B, Clark J I, Nikitina E, et al:
Increased production of immature myeloid cells in cancer patients:
a mechanism of immunosuppression in cancer. J Immunol. 166:678–689.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Balkwill F and Mantovani A: Cancer and
inflammation: implications for pharmacology and therapeutics. Clin
Phamacol Ther. 87:401–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zielinski CE, Mele F, Aschenbrenner D, et
al: Pathogen-induced human TH17 cells produce IFNγ or IL-10 and are
regulated by IL-1β. Nature. 484:514–518. 2012.
|
|
16.
|
Zhou L, Lopes JE, Chong MM, et al:
TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by
antagonizing RORgammat function. Nature. 453:236–240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chua W, Charles KA, Baracos VE and Clarke
SJ: Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in
patients with advanced colorectal cancer. Br J Cancer.
104:1288–1295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mantovani G and Madeddu C:
Cyclooxygenase-2 inhibitors and antioxidants in the treatment of
cachexia. Curr Opin Support Palliat Care. 2:275–281. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Fearon KJC, von Meyenfeldt MF, Moses AG,
van Geenen R, Roy A, Gouma DJ, et al: Effect of a protein and
energy dense: N-3 fatty acid enriched oral supplement on loss of
weight and lean tissue in cancer cachexia: a randomized double
blind trial. Gut. 52:1479–1486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Marwaha AK, Leung NJ, McMurchy AN and
Levings MK: TH17 cells in autoimmunity and immunodeficiency:
Protective or pathogenic? Front Immunol. 3:1292012. View Article : Google Scholar : PubMed/NCBI
|