Introduction
Renal cell carcinoma (RCC) encompasses a group of
cancers that originate from renal tubular epithelial cells, and
metastatic RCC (mRCC) is currently treated with molecular targeted
therapy (MTT) agents (1). The MTT
agent sorafenib was approved for the treatment of mRCC in Japan in
2008, and targets tyrosine kinases involved in angiogenesis,
including platelet-derived growth factor and vascular endothelial
growth factors (VEGF) (2,3). Prior to its approval, in a period
called the Cytokine Era (4), the
systemic treatment of mRCC was limited to 1st generation
immunotherapy, i.e., treatments with interferon and interleukin 2.
However, new agents were approved in Japan: Sunitinib (5), axitinib (6), pazopanib (7), everolimus (8), and temsirolimus (9). Sunitinib, axitinib, and pazopanib
target similar molecules to sorafenib. Among these, axitinib has
greater selectivity to VEGF receptors. In contrast, everolimus and
temsirolimus inhibit the mammalian target of the rapamycin pathway.
Axitinib, everolimus, and temsirolimus are generally used as 2nd
line MTT. Nivolumab was approved as the first immune checkpoint
inhibitor (2nd generation immunotherapy), and improved survival in
patients with RCC (10).
Our institution, the Saitama Medical University
International Medical Center, is one of the largest volume centers
of high-risk mRCC patients in Japan. As of February 2017, we have
treated 209 patients with MTT agents. Naito et al (4) previously reported predictive factors of
mRCC in the Cytokine Era, and also indicated superior survival in
the Japanese cohort compared to other countries. However, a similar
study in the MTT era was not conducted in Japan. Furthermore, a
novel concept, ‘early tumor shrinkage (eTS)’, was reported to
predict survival under MTT (11–13). In
the present study focused on eTS, we reviewed our MTT experience
and identified predicting factors of mRCC in the MTT era before the
introduction of 2nd generation immunotherapy era.
Materials and methods
Ethics and patient selection
The present study was approved by the Institutional
Review Board of Saitama Medical University International Medical
Center (approval no. 14-049). The clinical and pathological data of
209 patients with advanced RCC (with or without metastasis) treated
with MTT between April 25, 2008 and February 27, 2017, were
retrospectively isolated from electronic medical records using the
terms ‘sorafenib’, ‘sunitinib’, ‘axitinib’, ‘pazopanib’,
‘temsirolimus’, ‘everolimus’, ‘renal tumor’, and ‘renal cell
carcinoma’. Data on known risk factors including the pathology of
nephrectomy and/or tumor biopsy, clinical findings including
laboratory data and radiographic images, and whether patients
underwent nephrectomy were manually isolated from electronic
records. In order to predict the outcomes of mRCC patients treated
with MTT, we stratified patients into three groups using six
factors of 1st line therapy (i.e., time from diagnosis to
treatment, Karnofsky performance status, hemoglobin level,
neutrophil count, platelet count, and corrected calcium) based on
the International Metastatic Renal Cell Carcinoma Database
Consortium (IMDC) model (14)
(Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Total cohort
(n=209) | mRCC patients
(n=194) | mccRCC patients
(n=119) | mRCC patients with
eTS data (n=127) |
|---|
|
|
|
|
|
|
|---|
| Variables | n | % | n | % | n | % | n | % |
|---|
| Age |
| Median,
IQR | 67.2 | 60.0–72.9 | 67.1 | 60.0–72.8 | 67.1 | 60.9–72.6 | 66.9 | 59.2–72.8 |
|
<67 | 106 | 50.7 | 99 | 51.0 | 61 | 51.3 | 69 | 54.3 |
| ≥67 | 103 | 49.3 | 95 | 49.0 | 58 | 48.7 | 58 | 45.7 |
| Sex |
| Male | 134 | 64.1 | 125 | 64.4 | 81 | 68.1 | 85 | 66.9 |
|
Female | 75 | 35.9 | 69 | 35.6 | 38 | 31.9 | 42 | 33.1 |
| Pathology |
|
|
|
| n.a. |
|
|
|
| Pure
clear | 125 | 59.8 | 119 | 61.3 |
|
| 75 | 59.1 |
| Non-pure
clear | 32 | 15.3 | 26 | 13.4 |
|
| 15 | 11.8 |
|
Unknown | 52 | 24.9 | 49 | 25.3 |
|
| 37 | 29.1 |
| IMDC risk
classification | N.A.a |
|
|
|
|
|
|
|
|
Favorable |
|
| 7 | 3.6 | 4 | 3.4 | 5 | 3.9 |
|
Intermediate |
|
| 93 | 47.9 | 63 | 52.9 | 62 | 48.8 |
|
Poor |
|
| 75 | 38.7 | 38 | 31.9 | 51 | 40.2 |
|
Unknown |
|
| 19 | 9.8 | 14 | 11.8 | 9 | 7.1 |
| Nephrectomy |
| Before
MTT | 118 | 56.5 | 117 | 60.3 | 79 | 66.4 | 77 | 60.6 |
| After
MTT (presurgical) | 29 | 13.8 | 18 | 9.3 | 16 | 13.4 | 6 | 4.7 |
| No | 62 | 29.7 | 59 | 30.4 | 24 | 20.2 | 44 | 34.6 |
| Number of MTT
lines |
| 1 | 110 | 52.6 | 98 | 50.5 | 58 | 48.7 | 58 | 45.7 |
| 2 | 44 | 21.1 | 42 | 21.6 | 23 | 19.3 | 29 | 22.8 |
| ≥3 | 55 | 26.3 | 54 | 27.8 | 38 | 31.9 | 40 | 31.5 |
| MTT in the 1st line
setting |
|
Sorafenib | 48 | 23.0 | 47 | 24.2 | 25 | 21.0 | 30 | 23.6 |
|
Sunitinib | 54 | 25.8 | 54 | 27.8 | 33 | 27.7 | 34 | 26.8 |
|
Axitinib | 50 | 23.9 | 45 | 23.2 | 29 | 24.4 | 29 | 22.8 |
|
Pazopanib | 47 | 22.5 | 38 | 19.6 | 30 | 25.2 | 27 | 21.3 |
|
Temsirolimus | 9 | 4.3 | 9 | 4.6 | 2 | 1.7 | 6 | 4.7 |
|
Everolimus | 1 | 0.5 | 1 | 0.5 | 0 | 0.0 | 1 | 0.8 |
| Metastatic
sites |
|
Number |
|
0 | 15 | 7.2 |
|
|
|
|
|
|
|
1 | 73 | 34.9 | 73 | 37.6 | 52 | 43.7 | 43 | 33.9 |
|
2 | 68 | 32.5 | 68 | 35.1 | 43 | 36.1 | 49 | 38.6 |
|
≥3 | 53 | 25.4 | 53 | 27.3 | 24 | 20.2 | 35 | 27.6 |
|
Location |
|
Lung | 150 | 71.8 | 150 | 77.3 | 87 | 73.1 | 107 | 84.3 |
|
Liver | 25 | 12.0 | 25 | 12.9 | 10 | 8.4 | 16 | 12.6 |
|
Bone | 54 | 25.8 | 54 | 27.8 | 34 | 28.6 | 35 | 27.6 |
|
Lymph nodes | 84 | 40.2 | 84 | 43.3 | 47 | 39.5 | 54 | 42.5 |
|
Brain | 10 | 4.8 | 10 | 5.2 | 9 | 7.6 | 6 | 4.7 |
|
Pancreas | 12 | 5.7 | 12 | 6.2 | 5 | 4.2 | 8 | 6.3 |
|
Others | 51 | 24.4 | 51 | 26.3 | 26 | 21.8 | 33 | 26.0 |
| Follow-up duration
(months) |
| Median,
IQR | 14.2 | 6.1–27.5 | 14.1 | 6.0–27.6 | 16.7 | 7.4–27.5 | 16.7 | 6.3–27.5 |
Routine examination and follow-up
In our institution, patients treated with MTT
generally visited our office every 2–4 weeks. Radiographical
evaluations using computed tomography (CT) and/or magnetic
resonance imaging (MRI) were performed every 3–6 months, and
additional CT and bone scintigraphy were conducted when clinically
indicated. Patients without neurological symptoms were generally
not subjected to brain imaging tests.
MTT administration and RDI
Patients generally began MTT at the recommended
starting dose. Relative dose intensity (RDI) was assessed as the
cumulative dose divided by the duration of therapy and optimal
daily doses [sorafenib 800 mg/day, sunitinib 33.3 mg/day, axitinib
10 mg/day, pazopanib 800 mg/day, everolimus 10 mg/day, and
temsirolimus 3.57 mg/day (25 mg infusion/week)].
eTS measurements
Among 194 mRCC patients, the renal tumors or
metastatic lesions of 127 were evaluated by CT or MRI 90 days
before MTT treatment (as baseline scans) as well as between 30 and
90 days after MTT (as first post baseline scans). Two urologists
(HK and TO) and a radiologist (SM) blindly measured the sum of the
longest diameters (SLD) in each case, based on the Response
Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (15). Tumor shrinkage was determined based
on the change in SLD of target lesions at the first post baseline
scan. In order to estimate the power of eTS, cut-off values were
used as follows; −0.2 (20% decrease), −0.15 (15% decrease), −0.1
(10% decrease), −0.05 (5% decrease), 0.0 (no change), and +0.05 (5%
only increase).
Outcomes
The purpose of the present study was to evaluate
overall survival (OS), which was defined as the time from the
initiation of 1st line targeted therapy to the date of death from
any cause or the date of the last follow-up. Radiographic responses
were assessed by radiographic criteria according to RECIST version
1.1. We retrospectively obtained outcome and survival data, while
cause of death was identified by the attending physicians, chart
reviews corroborated by death certificates, or death certificates
alone at other institutions.
Statistical analysis
The variables of different groups were compared by
the Friedman test. Survival curves were constructed via the
Kaplan-Meier method and compared by log-rank test. Cox's
proportional hazards model was used to identify independent factors
associated with clinical outcomes. SPSS version 23.0 software (IBM
Corp., Armonk, NY, USA) was used for analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
In our institution, 209 advanced RCC cases (134
males, 75 females) were treated with MTT between April 2008 and
February 2017, i.e., after the Cytokine Era and before the
initiation of 2nd generation immunotherapy. Some optional therapies
were received in this cohort, such as radiotherapy (n=61, mainly
brain or bone), metasectomy (n=19, mainly lung), or previous
immunotherapies (n=49, interferon or interleukin-2). Median age was
67.2 [interquartile range (IQR): 60.0–72.9] years (Table I). Sorafenib, sunitinib, axitinib,
pazopanib, temsirolimus, and everolimus were administered as 1st
line MTT for 48, 54, 50, 47, 9, and 1 patient, respectively. In the
cohort of all MTT lines (1st-7th lines), the median treatment days
of sorafenib, sunitinib, axitinib, pazopanib, temsirolimus, and
everolimus were 144.5 (n=74), 99.0 (n=79), 130.0 (n=97), 60.5
(n=74), 77.0 (n=21), and 92 days (n=76), respectively. Median RDI
was 0.50, 0.60, 0.61, 0.56, 0.92 and 0.56, respectively.
Nephrectomy was performed on 147 patients (70.3%) before and after
MTT. One, two, and more than three lines of MTTs were performed for
110 (52.6%), 44 (21.1%), and 55 patients (26.3%), respectively.
Impact of eTS on survival outcome in mRCC
patients
Conventional risk factors and survival
outcome
Kaplan-Meier curves evaluating conventional IMDC
risk factors for survival in mRCC patients of our cohort are shown
in Fig. S1. In order
to identify other prognostic predictive factors of OS, a
Kaplan-Meier estimator curve of each factor was generated using the
data of mRCC patients (Table I). Of
note, all cohorts (Table I) included
non-metastatic cases (n=15) treated with MTT for locally advanced
RCC (i.e., presurgical therapy). Sex (male vs. female, Fig. 1A), nephrectomized vs. not
nephrectomized (Fig. 1B), IMDC risk
classification (non-poor risk vs. poor risk, Fig. 1C), and four metastatic sites [the
liver (Fig. 1D), bone (Fig. 1E), lymph nodes (Fig. 1F), and brain (Fig. 1G)] held proportional hazard
assumptions, whereas age and three metastatic sites did not (data
not shown). Among the factors with proportional hazards
assumptions, Cox's proportional hazards model identified five
independent predictors: IMDC risk classification (HR: 1.90), liver
metastasis (HR: 2.08), bone metastasis (HR: 1.83), lymph node
metastasis (HR: 1.75), and brain metastasis (HR: 3.19) (‘mRCC
patients’ in Table II).
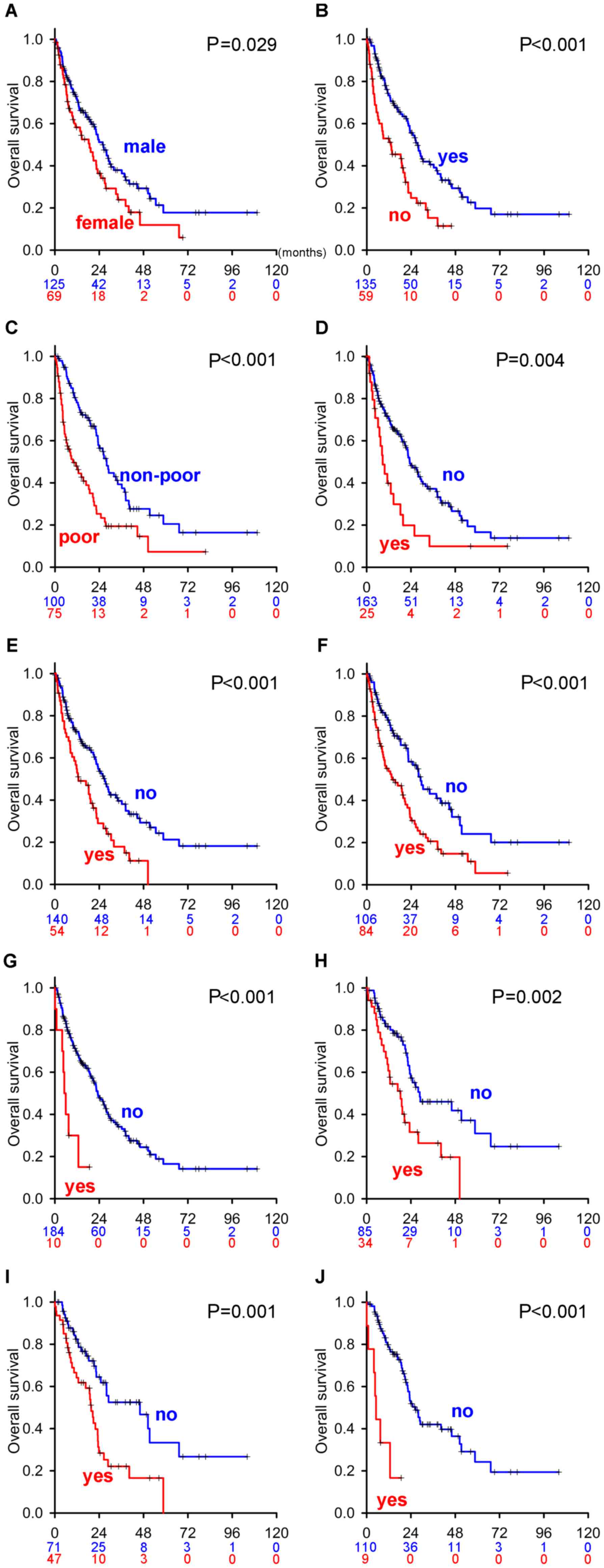 | Figure 1.Kaplan-Meier survival curves of
factors with proportional hazard assumptions in patients with mRCC
and mccRCC. Kaplan-Meier curves of (A) sex (male vs. female), (B)
nephrectomized (yes) vs. not nephrectomized (no), (C) non-poor risk
vs. poor risk in the IMDC classification, (D) with or without liver
metastasis (yes vs. no), (E) with or without bone metastasis (yes
vs. no), (F) with or without lymph node metastasis (yes vs. no),
and (G) with or without brain metastasis (yes vs. no) in all mRCC
patient cohorts as well as (H) with or without bone metastasis (yes
vs. no), (I) with or without lymph node metastasis (yes vs. no),
and (J) with or without brain metastasis (yes vs. no) in the mccRCC
patient cohort. Blue and red numbers below the month in each graph
indicate the number of patients in the groups represented by blue
and red lines, respectively. IMDC, International Metastatic Renal
Cell Carcinoma Database Consortium; mRCC, metastatic renal cell
carcinoma; mccRCC, metastatic clear cell renal cell carcinoma. |
 | Table II.Identification of predictive factors
of overall survival in metastatic renal cell carcinoma
patients. |
Table II.
Identification of predictive factors
of overall survival in metastatic renal cell carcinoma
patients.
|
|
| mRCC patients
(n=194) | mccRCC patients
(n=119) |
|---|
|
|
|
|
|
|---|
|
|
| Univariate |
Multivariatea | Univariate |
Multivariateb |
|---|
|
|
|
|
|
|
|
|---|
| Variables | Comparison | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | <67/≥67 | N.A. |
|
| N.A. |
|
| N.A. |
|
| N.A. |
|
|
| Sex | Female/male | 1.51 | 1.00–2.19 | 0.030 | 1.27 | 0.82–1.94 | 0.283 | N.A. |
|
| N.A. |
|
|
| Nephrectomy | No/yes | 2.23 | 1.52–3.27 | <0.001 | 1.42 | 0.92–2.21 | 0.118 | N.A. |
|
| N.A. |
|
|
| IMDC
classification | Poor/non-poor | 2.28 | 1.56–3.34 | <0.001 | 1.90 | 1.24–2.91 | 0.003 | N.A. |
|
| N.A. |
|
|
| Metastatic
sites |
|
Liver | Yes/no | 2.02 | 1.24–3.29 | 0.005 | 2.08 | 1.22–3.55 | 0.008 | N.A. |
|
| N.A. |
|
|
|
Bone | Yes/no | 1.95 | 1.33–2.86 | 0.001 | 1.83 | 1.19–2.82 | 0.006 | 2.28 | 1.34–3.88 | 0.002 | 2.08 | 1.22–3.56 | 0.007 |
| Lymph
nodes | Yes/no | 1.99 | 1.37–2.88 | <0.001 | 1.75 | 1.16–2.65 | 0.008 | 2.29 | 1.36–3.84 | 0.002 | 2.12 | 1.26–3.58 | 0.005 |
|
Brain | Yes/no | 4.06 | 1.93–8.52 | <0.001 | 3.19 | 1.43–7.10 | 0.005 | 6.65 | 2.85–15.49 | <0.001 | 4.78 | 2.03–11.24 | <0.001 |
|
Lung | Yes/no | N.A. |
|
| N.A. |
|
| N.A. |
|
| N.A. |
|
|
|
Pancreas | Yes/no | N.A. |
|
| N.A. |
|
| N.A. |
|
| N.A. |
|
|
|
Others | Yes/no | N.A. |
|
| N.A. |
|
| N.A. |
|
| N.A. |
|
|
In sub-group analyses of the clear cell mRCC cohort
(mccRCC, Table I), a Kaplan-Meier
estimator curve of three risk factors [bone (Fig. 1H), lymph nodes (Fig. 1I), and brain (Fig. 1J)] held proportional hazards
assumptions, whereas the others did not (data not shown). Cox's
proportional hazards model revealed that all the factors with
proportional hazards assumptions were independent predictors: Bone
metastasis (HR: 2.08), lymph node metastasis (HR: 2.12), and brain
metastasis (HR: 4.78) (‘mccRCC patients’ in Table II).
ETS and survival outcome
In the MTT era, as previously suggested (11,16), eTS
is another predictive factor in mRCC patients. Among mRCC patients,
eTS data was available in 127 patients (Table I). Median days to initial
radiographic evaluation after introduction of the drug were 60.0
days (IQR: 50.0–77.0). In these cases, measurable lesions were
independently assessed by three physicians (HK, TO, and SM), the
eTS value of each was calculated, and average eTS values were used
in statistical analyses. The Friedman test revealed no significant
differences in measurements between the three physicians (P=0.660),
suggesting that these measurements were reliable. In order to
estimate the predictive power of eTS, a Kaplan-Meier estimator
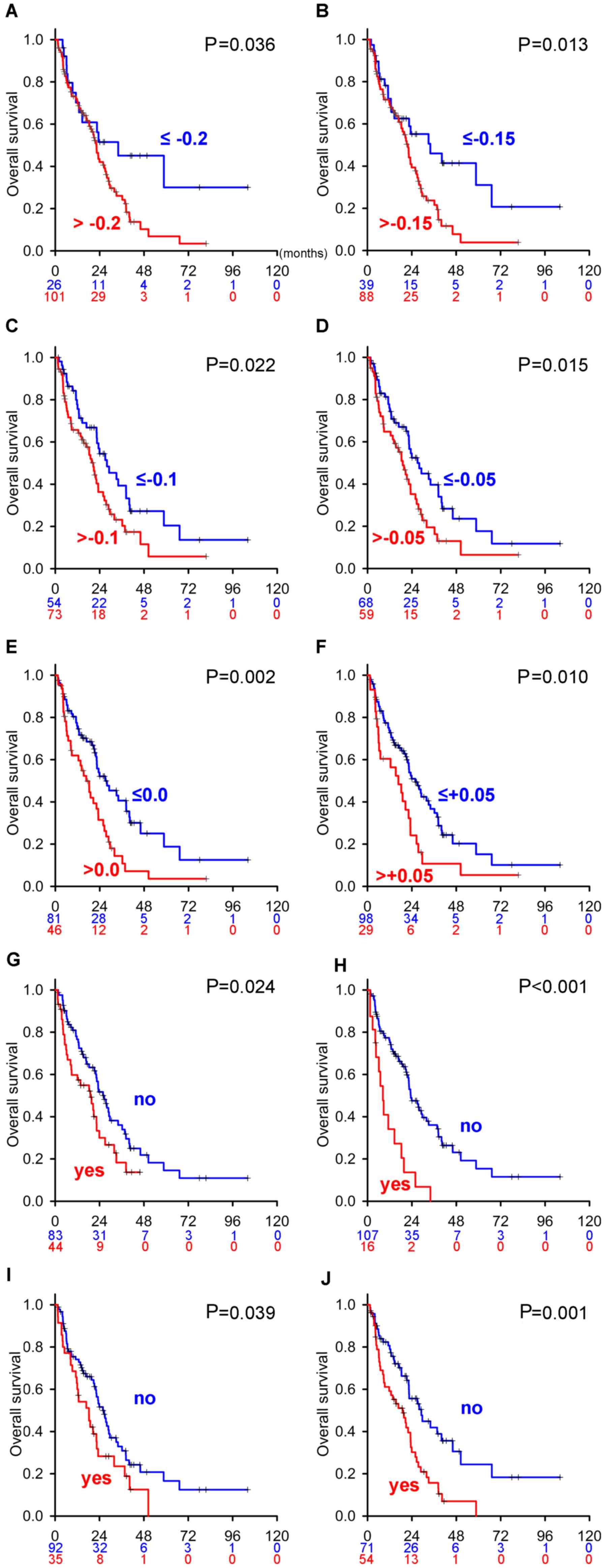
curve of eTS with different cut-off values was generated (Fig. 2). Patients with eTS less than −0.2,
−0.15, −0.1, −0.05, 0.0, and +0.05 had better OS than those with
larger cut-off values, suggesting that eTS is a potent
predictor.
In the cohort of mRCC with eTS data (Table I), a Kaplan-Meier estimator curve of
four risk factors [no preceding nephrectomy (Fig. 2G), liver metastasis (Fig. 2H), bone metastasis (Fig. 2I), and lymph node metastasis
(Fig. 2J)] held proportional hazards
assumptions, whereas age, sex, IMDC classification, brain
metastasis, lung metastasis, pancreatic metastasis, and other
metastases did not (data not shown). In Cox's proportional hazards
models of these factors and eTS with different cut-off values,
metastases of the liver and lymph nodes were independent predictive
factors throughout the analyses (Table S1). As for
eTS, cut-off values of −0.15, −0.05, and 0.0 were significant
predictors. Overall, predictors estimating OS in the present study
were proven to be consistent with those previously reported in a
pre-MTT era cohort.
Difference in predictive factors
between patients treated with different 1st line TKIs
In the cohort of mRCC patients with eTS data
(Table I), sorafenib, sunitinib,
axitinib, pazopanib, temsirolimus, and everolimus were administered
as 1st line MTT to 30 (23.6%), 34 (26.8%), 29 (22.8%), 27 (21.3%),
6 (4.7%), and 1 (0.8%) patient, respectively.
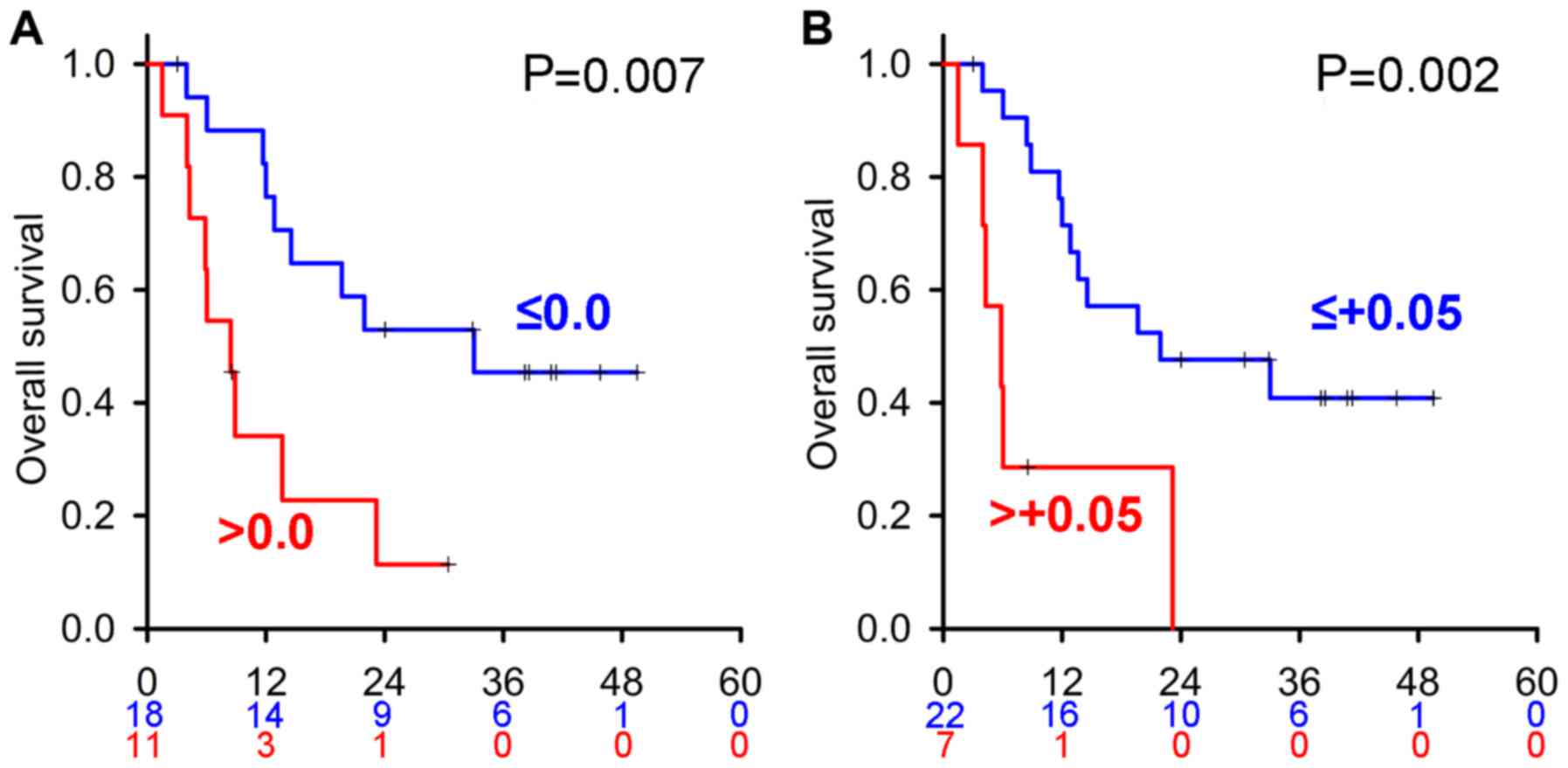
In order to visualize the power of predictors in
each MTT, we delineated the Kaplan-Meier curves of all predictors
in each 1st line MTT treatment cohort. However, due to the small
number of cases in each MTT, no proportional hazards assumption was
observed for any predictor, except eTS with two cut-off values (0.0
and +0.05) in axitinib-treated patients (Fig. 3). Cox's proportional hazards model
analyses revealed these HR values were very high at 3.52 (IQR:
1.32–9.36) and 4.39 (IQR: 1.56–12.37), respectively.
In sub-group analyses, axitinib was administered to
30 cases as 1st line, 12 cases as 2nd line, and 16 cases as 3rd or
more lines, respectively. Median OS of patients treated with 1st
and 2nd lines (n=42) were similar to those treated with 3rd or more
lines (19.7 vs. 18.6 months, P=0.671, respectively). Median
progression free survival (PFS) of patients treated with the 1st
and 2nd lines (n=42) was similar to that of patients treated with
the 3rd or more lines (12.1 vs. 11.4 months, P=0.620,
respectively). In patients treated with 1st and 2nd line axitinib
therapy, eTS less than 0.0 resulted in significantly better OS
(P=0.003) and PFS (P=0.006) than eTS greater than 0.0.
Discussion
In the present study, we retrospectively identified
prognostic predictive factors in several mRCC retrospective
settings: mRCC patients including all pathological types (n=194),
mccRCC (n=119), and mRCC with eTS data (n=127). Four metastatic
sites in the liver, bone, lymph nodes, and brain as well as greater
eTS were potentially independent predictors throughout these
settings. Overall, these results were consistent with previous
findings as described below.
Based on a Pubmed search, several studies were
published on the usefulness of eTS. Rini et al (12) demonstrated that patients with eTS
≤-0.1 and administered axitinib were treatable for longer than 18
months and also had superior OS to those with eTS >-0.1.
Similarly, Grünwald et al (13) reported that an eTS cut-off-0.1 is a
strong predictor of OS and PFS in a large scale (n=4,334) study. In
a Japanese cohort, Miyake et al (11) found that eTS was an independent
predictor of better OS in patients treated with 1st line sunitinib
or sorafenib. Even in 2nd line treatments with axitinib, sunitinib,
everolimus, temsirolimus, and sorafenib, eTS was a significant
predictive factor of OS (13). In
this study, treatment with axitinib resulted in earlier shrinkage
than with other agents, thereby supporting the present results on
axitinib. Overall, eTS appears to be a useful predictor of OS;
however, further analyses are needed in order to select the best
time to measure eTS (17).
The results of the present study suggest that eTS is
advantageous for patients with axitinib. Axitinib is a selective
inhibitor of VEGF receptors (18),
and is currently used as 2nd line MTT (6). Since axitinib exhibits a potent
shrinking ability, it is frequently administered as presurgical
therapy (19). We used axitinib in
many cases as 1st line MTT because we empirically hypothesized that
axitinib shrunk mRCC faster than other TKIs (sorafenib, sunitinib,
and pazopanib), possibly because of its high VEGF receptor
affinity. Oya et al (20)
recently reported that 1st line axitinib treatment is beneficial
for Japanese mRCC patients, which supports our hypothesis. Our
results showed that both OS and PFS of patients treated with 1st
and 2nd lines were similar to those treated with 3rd or more lines.
We suggest that axitinib in any lines may reduce tumors in patients
with mRCC, and that eTS is one of the predictors of OS in
axitinib-treated patients.
There were some limitations in this study including
its retrospective nature, limited number of patients in a single
institution, and incomplete data collection. Our data set used in
the current analysis included patients who received different lines
of treatment and various agents, as well as those with or without
some optional therapies such as cytoreductive nephrectomy,
metasectomy, radiotherapy, and traditional immunotherapy, leading
to heterogeneity in our cohort of patients.
We performed retrospective analyses to identify
prognostic predictive factors of mRCC in the MTT era using a
limited Japanese cohort in our single institution. Four metastatic
sites in the liver, bone, lymph nodes, and brain appear to be
independent predictors of OS throughout several cohorts, as
previously reported. Furthermore, eTS was identified as a potent
prognostic predictive factor, particularly with the administration
of axitinib. Data from analyses using cases just before the immune
checkpoint inhibitor era will add important evidence for MTT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by JSPS
Grants-in-Aid for Scientific Research (grant no. JP17K18062).
Availability of data and materials
The datasets analyzed during the current study are
available in the Figshare repository: All patients:
doi.org/10.6084/m9.figshare.6972782.v1; eTS measurement:
doi.org/10.6084/m9.figshare.6972788.v2.
Authors' contributions
SS and KN conceived and designed the study, and
drafted the manuscript. HK, TO and SM retrospectively obtained the
patient data. SS and RA analyzed the patient data. HK, TO, SM, TK,
GK and MO revised the manuscript critically for important
intellectual content. All authors read and approved the version to
be published.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Saitama Medical University International Medical
Center (approval no. 14-049); the requirement for written informed
consent was waived due to the retrospective nature of this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ratain MJ, Eisen T, Stadler WM, Flaherty
KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, et
al: Phase II placebo-controlled randomized discontinuation trial of
sorafenib in patients with metastatic renal cell carcinoma. J Clin
Oncol. 24:2505–2512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naito S, Yamamoto N, Takayama T, Muramoto
M, Shinohara N, Nishiyama K, Takahashi A, Maruyama R, Saika T,
Hoshi S, et al: Prognosis of Japanese metastatic renal cell
carcinoma patients in the cytokine era: A cooperative group report
of 1463 patients. Eur Urol. 57:317–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Efficacy of everolimus in advanced renal cell
carcinoma: A double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, et al: Temsirolimus, interferon alfa, or both for advanced
renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyake H, Miyazaki A, Imai S, Harada K and
Fujisawa M: Early tumor shrinkage under treatment with first-line
tyrosine kinase inhibitors as a predictor of overall survival in
patients with metastatic renal cell carcinoma: A retrospective
multi-institutional study in Japan. Target Oncol. 11:175–182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Gruenwald V, Jonasch E, Fishman
MN, Tomita Y, Michaelson MD, Tarazi J, Cisar L, Hariharan S, Bair
AH, et al: Long-term duration of first-line axitinib treatment in
advanced renal cell carcinoma. Target Oncol. 12:333–340. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grünwald V, Lin X, Kalanovic D and
Simantov R: Early tumour shrinkage: A tool for the detection of
early clinical activity in metastatic renal cell carcinoma. Eur
Urol. 70:1006–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heng DY, Xie W, Regan MM, Warren MA,
Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: Results from a large, multicenter study. J
Clin Oncol. 27:5794–5799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyake H, Harada K, Ozono S and Fujisawa
M: Prognostic significance of early tumor shrinkage under
second-line targeted therapy for metastatic renal cell carcinoma: A
retrospective multi-institutional study in Japan. Mol Diagn Ther.
20:385–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishihara H, Yagisawa T, Kondo T, Omae K,
Takagi T, Iizuka J, Kobayashi H and Tanabe K: Effect of the timing
of best tumor shrinkage on survival of patients with metastatic
renal cell carcinoma who received first-line tyrosine kinase
inhibitor therapy. Int J Clin Oncol. 22:126–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rixe O, Bukowski RM, Michaelson MD,
Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo
J, et al: Axitinib treatment in patients with cytokine-refractory
metastatic renal-cell cancer: A phase II study. Lancet Oncol.
8:975–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karam JA, Devine CE, Urbauer DL, Lozano M,
Maity T, Ahrar K, Tamboli P, Tannir NM and Wood CG: Phase 2 trial
of neoadjuvant axitinib in patients with locally advanced
nonmetastatic clear cell renal cell carcinoma. Eur Urol.
66:874–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oya M, Tomita Y, Fukasawa S, Shinohara N,
Habuchi T, Rini BI, Fujii Y, Kamei Y, Umeyama Y, Bair AH and Uemura
H: Overall survival of first-line axitinib in metastatic renal cell
carcinoma: Japanese subgroup analysis from phase II study. Cancer
Sci. 108:1231–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|