Introduction
Pancreaticoduodenectomy (PD) is the most common and
effective surgery for the treatment of pancreatic and periampullary
carcinoma (1,2). The evolution of surgical techniques and
perioperative management had significantly decreased the
postoperative complications and mortality in patients (3–5).
Postoperative pancreatic fistula (PF) remains one of the most
dangerous and severe complications following PD (6). Significant risk factors for increased
PF have been demonstrated to be old age, soft pancreatic texture, a
large pancreatic remnant volume, small pancreatic duct diameter,
increased levels of pancreatic fat, pancreatic pathology and longer
surgery times (7–9). Occlusion of the primary pancreatic duct
by pancreatic head and periampullary tumors often leads to
obstructive pancreatitis within a few weeks (10). The pathology of obstructive
pancreatitis is characterized by pancreatic atrophy, fibrosis, and
acute and chronic inflammatory infiltrate that may cause the
morphological and functional destruction of the pancreas (11). Furthermore, previous studies have
demonstrated that the pancreatic inflammatory environment may serve
a critical role in pancreatic cancer progression (12). The magnetic resonance imaging (MRI)
parameters include apparent diffusion coefficient (ADC) value and
signal intensity on T1-weighted fat-suppressed images (rT1), which
are used to quantitatively analyze differences between pancreatic
cancer and pancreatitis (9,13). The T2*-corrected Dixon technique and
intravoxel incoherent motion (IVIM) diffusion-weighted (DW) imaging
have been suggested to assess pancreatic steatosis and fibrosis and
predict postoperative PF (8).
However, at present, it remains unclear whether acute preoperative
obstructive pancreatitis may be a risk factor for fistula
development.
The purpose of the present study was designed to
determine whether patients who have acute obstructive pancreatitis
preoperatively are more likely to develop a PF compared with
matched controls, and if so, to investigate whether the ADC value
and signal intensity on rT1 images may be used as a preoperative
predictive tool for fistula development.
Patients and methods
Patients
The present retrospective study was approved by the
Institutional Review Board of First Affiliated Hospital of Fujian
Medical University (Fuzhou, China), and the requirement for
informed consent was waived. The authors had no access to
information that identified individual participants during or
following data collection. From January 2010 to March 2016, a total
of 124 patients (55 women and 69 men; mean age, 53 years) underwent
PD, performed at the First Affiliated Hospital of Fujian Medical
University by the same surgical team who specialize in pancreatic
surgery. Patients who did not undergo preoperative MRI or had an
MRI at a different hospital were excluded. The final study
population (n=44) consisted of 22 patients [15 women and 7 men,
with a mean age of 53±13 years (range, 26–79 years)] who developed
a PF following PD and 22 control patients [14 women and 8 men, with
a mean age of 56±12 years (range, 35–76 years)] who did not develop
a PF. The two groups (fistula and non-fistula group) of patients
were carefully matched for perioperative parameters, including age,
sex, pancreatic texture, pancreatic duct size at MRI, pancreatic
pathology, and type of operation. Demographic, radiology and
pathological data are summarized in Table I. The pancreatic texture was
evaluated and defined as soft or hard during surgery as described
previously (14,15). Soft pancreatic texture was recorded
as the level of pancreas elasticity preserved.
 | Table I.Demographic, radiology and
pathological data. |
Table I.
Demographic, radiology and
pathological data.
| Characteristics | No fistula group
[n=22] (%) | Fistula group [n=22]
(%) | P-value |
|---|
| Age, mean years | 56±12 | 53±13 | NSa |
| Male: Female | 8:14 | 7:15 | NSb |
| Diagnosis |
|
|
|
|
Pancreas head cancer | 10 (45) | 11 (50) | NSb |
| Ampulla
of Vater cancer | 9 (41) | 6 (27) | NSb |
| Common
bile duct cancer | 2 (9) | 3 (14) | NSb |
|
Duodenal cancer | 1 (5) | 2 (9) | NSb |
| Type of
operation |
|
|
|
|
Conventional
pancreaticoduodenectomy | 17 | 16 | NSb |
|
Pylorus-preserving
pancreatoduodenectomy | 5 | 6 | NSb |
| Pancreatic texture
at surgery |
|
| 0.005b |
|
Soft | 5 (23) | 14 (64) | – |
|
Hard | 17 (77) | 8 (36) | – |
| Pancreatic duct
size on MRI | 3.82±2.31 | 2.56±2.05 | 0.007a |
Surgical technique and postoperative
care
As for the type of surgery performed, conventional
PD and pylorus-preserving pancreatoduodenectomy (PPPD) are
frequently used for the pancreatic and periampullary cancer at the
First Affiliated Hospital of Fujian Medical University. All
patients received standard postoperative treatment. A H2 blocker
(famotidine; 20 mg; trade name, XinFaDing; Shanghai Xinyi
Pharmaceutical Co., Ltd., Shanghai, China) was administered
intravenously every 12 h during the non-oral intake period
following surgery and octreotide (100 µg Sandostatin; Novartis
International AG, Basel, Switzerland) was administered
subcutaneously every 8 h for 5 days. The volume of drained fluids
was recorded daily following surgery. The amylase levels in the
serum and drainage fluid were measured on the first, third, fifth,
seventh and tenth days as described previously (8,16). Serum
C-reactive protein and leukocyte count were measured on
postoperative day 4. A plain computed tomography scan was performed
to detect potential postoperative complications on day 7.
According to the definition of the International
Study Group on Pancreatic Fistula leak criteria, the postoperative
PF was classified into three grades: A, B, and C (17). Tailoring of the treatment strategies
was based on this classification.
Pathological analysis
Pancreatic tissue specimens were fixed in 10%
formalin for 8–24 h at 25°C, dehydrated in an automatic dehydration
processor (Shandon Pathcentre; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and embedded in paraffin, and sectioned serially
at a thickness of 4 µm following surgical removal. Slides were
stained with hematoxylin and eosin (H&E) for 5–20 min at 25°C
and examined under a light microscope (magnification, ×200). The
severity of acute obstructive pancreatitis was qualitatively
assessed by pathological scores as described previously (18,19):
Graded glandular atrophy, scaled from 0–3; intralobular,
interlobular and periductal fibrosis, scaled from 0–3; inflammatory
cell infiltration, scaled from 0–3. Semi-quantitative pathological
scores were obtained by summing up the values for each specimen.
Increased pathological scores indicated an increased severity of
tissue damage.
MRI protocol and image analysis
MRI examinations were performed on a 3-T imager
(Magnetom Verio; Siemens Healthineers, Erlangen, Germany) and a
32-channel phased-array coil. The routine abdominal MRI protocol
included fat-suppressed axial and coronal T2-weighted (T2W) turbo
spin-echo imaging, T1-weighted dual fast gradient recalled echo
sequence (in-phase and out-of-phase sequences) and
diffusion-weighted imaging (DWI) with b values of 0 and 800
s/mm2. The primary scan parameters are summarized in
Table II. Preoperative dynamic MRI
pancreatography following intravenous 0.1 mmol/kg gadolinium
contrast treatment with Multihance® gadobenate
dimeglumine injection was performed using a volumetric interpolated
breath-hold examination with a high-performance phased array
sensitivity coding, and the reduction factor was 2.
 | Table II.Primary magnetic resonance imaging
parameters. |
Table II.
Primary magnetic resonance imaging
parameters.
| Sequence | TR/TE (ms) | FOV (mm) | Matrix slice | Thickness/spacing
between slices | Pixel band width
(KHz) | Flip angle (°) | Echo train
length |
|---|
| Axial fast spin
echo (T2WI) | 2,999/79 | 21/38 | 320×168 | 5/6 | 240 | 140 | 9 |
| Axial single-shot
echo planar imaging (DWI) | 6,000/73 | 21/38 | 128×78 | 5/6 | 2,441 | 90 | 1 |
| 3D fat-suppressed
gradient-echo (VIBE) | 3.9/1.4 | 25/38 | 320×182 | 3/- | 401 | 9 | 1 |
ADC maps were automatically generated on a Syngo
workstation (Syngo Multimodality Workplace; Siemens Healthineers,
Erlangen, Germany) using b-values of 0 and 800 s/mm2.
Regions of interest (ROIs) drawings were performed by consensus
between 2 abdominal radiologists with 16 and 8 years of experience,
respectively, who were blinded to the clinical history and
radiology results. Standard ROI size was 20–45 mm2. A
total of 3 repeated measurements on the ADC maps were calculated in
the nontumorous pancreatic body and tail, which were residual
following PD. Each ROI was manually placed on ADC maps. Special
care was taken to avoid the tumor and the primary pancreatic duct,
which would have affected ADC values.
On the fat-suppressed T2-weighted (rT2) and
unenhanced rT1s, an average signal intensity (SI) of 3 repeated
measurements was also calculated from the nontumorous pancreas and
ipsilateral muscle. The pancreas-muscle SI ratios on rT1 and rT2
images were calculated as follows: Pancreas-muscle SI ratio=(SI
pancreas/SI ipsilateral muscle).
Statistical analysis
Data analysis was performed by using SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA). Two-tailed P<0.05 was
considered to indicate a statistically significant difference. The
mean standard deviation (SD) was used to present continuous
variables. The Chi-square test or Fisher's exact test was used for
comparisons of quantitative parameters, and the Mann-Whitney U test
was used for comparisons of qualitative parameters. The ability of
ADC values and rT1 to predict the PF development was determined
using the receiver-operating characteristic curve (ROC) analysis
and 95% confidence intervals (CI). Point sensitivity and
specificity were calculated using ROC curves.
Results
Patient characteristics
Patient demographic characteristics are summarized
in Table I. No differences were
observed between the pancreatic fistula group and the non-fistula
group in age, sex, pancreatic pathology and type of surgery.
Significant differences in pancreatic texture during surgery and
pancreatic duct size during MRI examination were identified between
the two groups (Table I). In the
pancreatic fistula group, 12 patients (55%) exhibited a grade A
fistula, 7 (31%) exhibited a grade B fistula, and 3 (14%) exhibited
a grade C fistula.
Histopathologic results
Through measuring the grades of severity of
obstructive pancreatitis using pathological scores, the score in
patients within the fistula group was 5.18±1.05, whereas the score
of patients within the non-fistula group was 4.32±1.32 (P=0.021;
Table III). No differences in the
areas of glandular atrophy and fibrosis between these two groups
were observed. The mononuclear inflammatory infiltrates index of
the fistula group was increased compared with the non-fistula
group. An increased number of patients in the pancreatic fistula
group exhibited a higher grade of severity of acute obstructive
pancreatitis.
 | Table III.Comparison of pathological scores
between the fistula and non-fistula groups. |
Table III.
Comparison of pathological scores
between the fistula and non-fistula groups.
| Pathological
markers | Fistula | No fistula |
P-valuea |
|---|
| Glandular
atrophy | 1.77±0.81 | 2.05±0.79 | 0.264 |
| Fibrosis | 1.09±0.68 | 1.05±0.72 | 0.831 |
| Mononuclear
inflammatory infiltrates | 2.09±0.68 | 1.45±0.59 | 0.002 |
| Pathological
score | 5.18±1.05 | 4.32±1.32 | 0.021 |
MRI assessment
The ADC value in the fistula group was
1.14±0.31×10−3 mm2/s, which was significantly
decreased compared with the value in patients in the non-fistula
group, which was 1.48±0.44×10−3 mm2/s
(P=0.005). The value of the rT1 in patients in the fistula group
was 1.71/0.25, which was significantly increased compared with the
value of the rT1 of patients in the non-fistula group, which was
1.25±0.29 (P=0.0001). The value of the rT2 in patients in the
fistula group was 0.72±0.08, whereas the value of the rT2 of
patients in the non-fistula group was 0.62±0.07 (P=0.79). No
difference in the rT2 values was observed between the two groups
(Table IV).
 | Table IV.Signal intensity ratios from
fat-suppressed T1WI and T2WI scans, and ADC values between the
fistula group and non-fistula groups. |
Table IV.
Signal intensity ratios from
fat-suppressed T1WI and T2WI scans, and ADC values between the
fistula group and non-fistula groups.
| Measurements | Fistula | No fistula |
P-valuea |
|---|
| Pancreas-muscle
ratio on fat-suppressed T1WI (rT1) | 1.71±0.25 | 1.25±0.29 | 0.0001 |
| Pancreas-muscle
ratio on fat-suppressed T2WI (rT2) | 0.72±0.08 | 0.62±0.07 | 0.7902 |
| ADC value | 1.14±0.31×10-3 | 1.48±0.44×10-3 | 0.0053 |
Risk factors of pancreatic
fistula
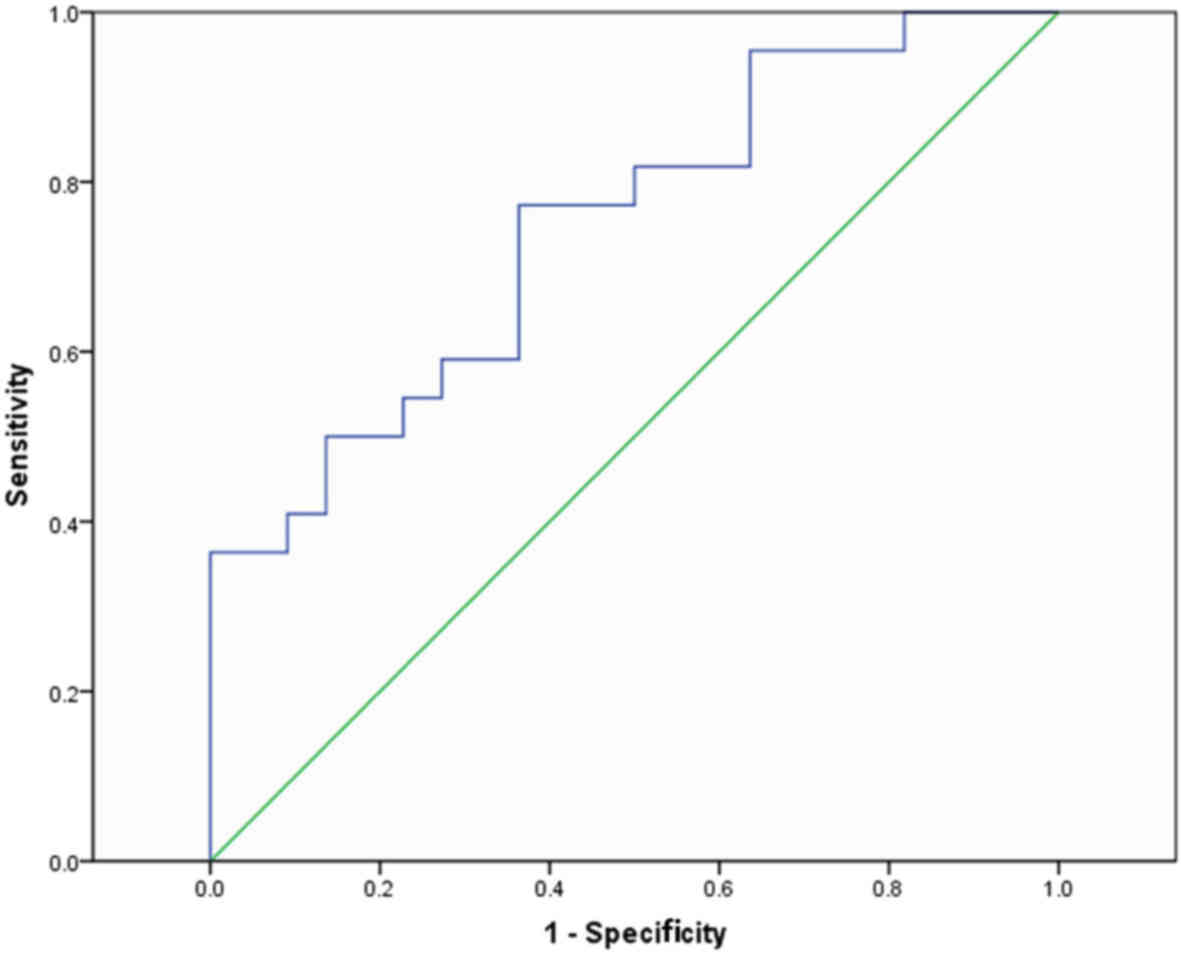
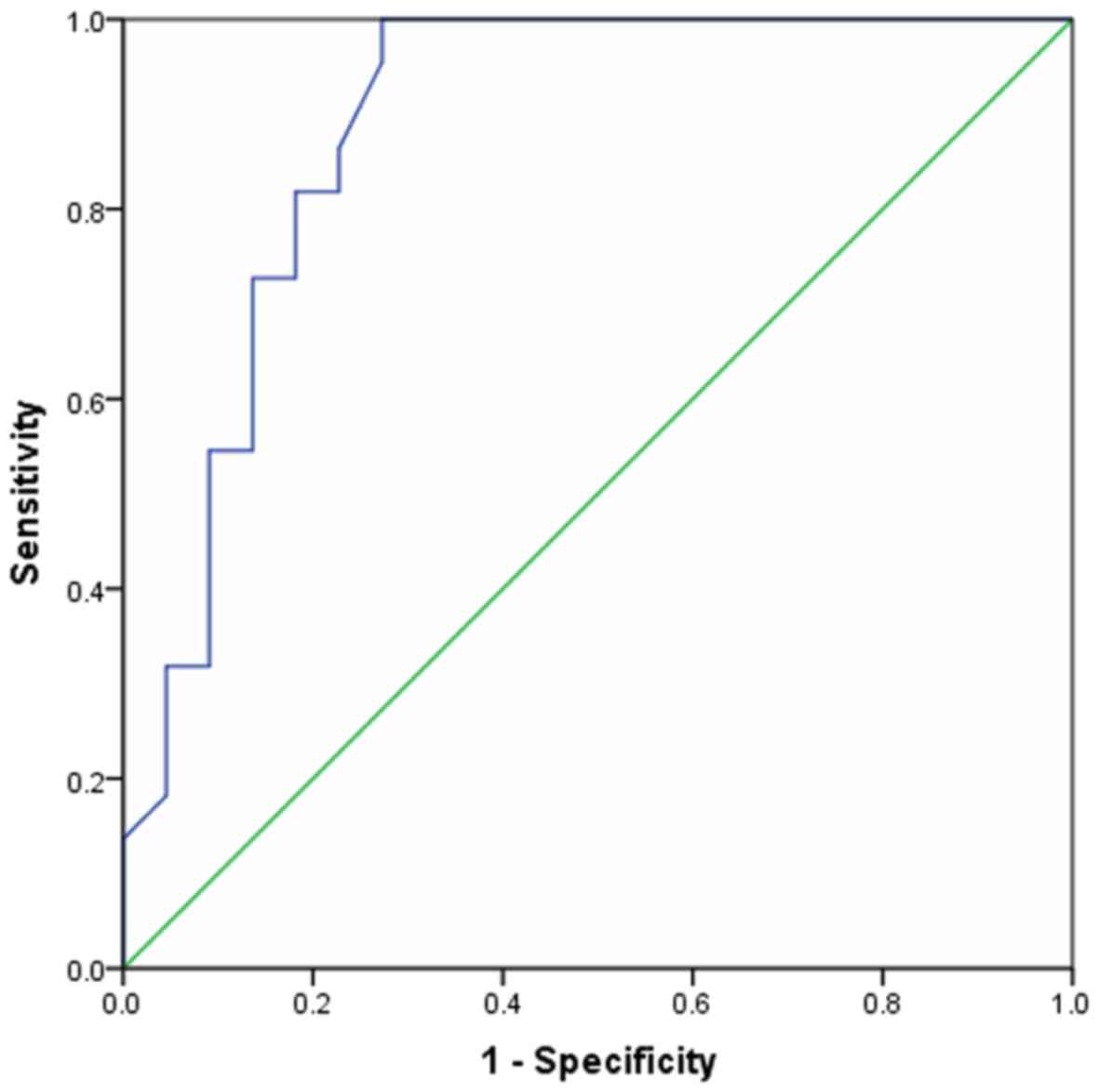
Figs. 1 and 2 demonstrate the diagnostic performance of
ADC values and rT1 parameters for the preoperative prediction of
postoperative PF. Based on the ROC curve, the optimal cut-off value
of ADC as a criterion for the prediction of PF was
1.29×10−3 mm2/s, which yielded a sensitivity
of 77.3% and a specificity of 63.6%; the area under the curve (AUC)
was 0.748 (95% CI: 0.605–0.891). A cut-off value of rT1 was 0.83,
with the AUC ROC value of 0.885 (95% CI: 0.689–0.959). Therefore,
the diagnostic performance of rT1 for the preoperative prediction
of postoperative PF was better compared with the ADC value.
Discussion
PF is the primary cause of morbidity following PD,
and it has been suggested that the risk for PF depends on a number
of variables including age, sex, pancreatic texture and pathology,
pancreatic duct size, blood loss, surgery time and surgical
techniques (9,20–22). The
results of the present study indicated that a significantly
increased number of patients with PF exhibited an increased level
of mononuclear inflammatory infiltrates and more severe tissue
damage of the pancreas due to acute obstructive pancreatitis, as
determined by histological examination, compared with patients
without PF. Therefore, we hypothesized that pancreatic inflammation
and edema were potential risk factors of PF, and the association
between acute obstructive pancreatitis and PF was explored using
routine abdominal MRI protocol.
ADC values derived from DW-MRI as a quantitative
parameter, which demonstrates the Brownian motion of water
molecules, may be used to assess all subgroups of acute
pancreatitis stratified by the Balthazar classification (23–26).
Previous radiology studies have suggested that the ADC value may be
used to quantify pancreatic and liver fibrosis (27–29);
however, it cannot be used to predict the occurrence of PF
(13,30–33). By
contrast, Chang et al (34)
revealed that ADC values of ≤1.3×10−3 mm2/s
may be used to predict the development of PF following PD as a
marker of pancreatic fibrosis. In the present study, the routine
MRI parameters ADC and rT1 were used to evaluate acute obstructive
pancreatitis and determine its predictive ability for PF. These
pancreatic MRI imaging protocols may be used to detect and
characterize pancreatic and periampullary lesions (13,24,35–37). The
present study identified that the optimal cut-off values of ADC and
rT1 as criteria for the prediction of PF was 1.29×10−3
mm2/s and 0.83, respectively. These results were
consistent with those from previous studies (9,33,34). The
diagnostic performance of rT1 (AUC=0.885) for the preoperative
prediction of postoperative PF was better compared with the ADC
value (AUC=0.748).
In the present study, the pathological score and the
mononuclear inflammatory infiltrates of the fistula group, for
example the intense degree of edema and glandular inflammation of
pancreas, were increased compared with that of the non-fistula
group. The likely explanation for the association between acute
obstructive pancreatitis and the risk of fistula development was
that the pancreatic inflammation and edema will cause anastomosis
healing difficulties and the development of leakages. Even a fine
needle will cause small pancreatic juice leakages following
acupunture.
There were certain limitations in the present study.
Firstly, the number of patients was relatively small, and the study
was a retrospective analysis. A prospective diagnostic large-scale
study should be performed to confirm the results obtained.
Secondly, the pathological score, which was calculated in the
pancreatic remnant tissue, may not have been an accurate
representation of the whole pancreas. Thirdly, postoperative
outcomes were not obtained during treatment. Therefore, the
gathering of sufficient data was not possible.
The present study demonstrated that MRI parameters
including ADC value and rt1 were significantly associated with PF,
while rT2 was not. We hypothesize that ADC values and rT1 may
predict the occurrence of PF prior to PD, and the prediction
ability of rT1 was better compared with ADC values.
The rT1 index may be used as a preoperative
predictive tool in PD, similar to the results from a previous study
(9). Therefore, these MRI parameters
may be applied in predicting the possibility of PF preoperatively.
Additional perioperative care should be provided to aid in
decreasing the rate of complications when operating on high-risk
patients.
In conclusion, ADC values and rT1 may be a powerful
tool for evaluating acute pancreatic obstructive pancreatitis and
be useful in predicting the occurrence of PF.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Fujian
Provincial Department of Science and Technology (grant no.
2016Y0039), the Health and Planning Committee of Fujian Province
(grant no. 2016013) and the Health and Planning Committee of Fujian
Province (grant no. 2017-CX-27).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and QC designed the present study. ZS wrote the
manuscript and DC, QC and YL revised the manuscript. RY and XL
performed the statistical analysis. QC performed imaging analysis.
YL and ZS and XL performed MRI scanning procedures. KR and VSL
prepared all figures. XL and ZS performed the region of interest
analysis and illustrations. DC performed imaging analysis.
Ethics approval and consent to
participate
This retrospective study was approved by the
Institutional Review Board of First Affiliated Hospital of Fujian
Medical University, and the requirement for informed consent was
waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Freelove R and Walling AD: Pancreatic
cancer: Diagnosis and management. Am Fam Physician. 73:485–492.
2006.PubMed/NCBI
|
|
2
|
Balcom JH IV, Rattner DW, Warshaw AL,
Chang Y and Fernandez-del Castillo C: Ten-year experience with 733
pancreatic resections: Changing indications, older patients, and
decreasing length of hospitalization. Arch Surg. 136:391–398. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gouma DJ, van Geenen RC, van Gulik TM, de
Haan RJ, de Wit LT, Busch OR and Obertop H: Rates of complications
and death after pancreaticoduodenectomy: Risk factors and the
impact of hospital volume. Ann Surg. 232:786–795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD,
Kaufman HS and Coleman J: One hundred and forty-five consecutive
pancreaticoduodenectomies without mortality. Ann Surg. 217:430–438.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aranha GV, Aaron JM, Shoup M and Pickleman
J: Current management of pancreatic fistula after
pancreaticoduodenectomy. Surgery. 140:561–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bassi C, Butturini G, Molinari E, Mascetta
G, Salvia R, Falconi M, Gumbs A and Pederzoli P: Pancreatic fistula
rate after pancreatic resection. The importance of definitions. Dig
Surg. 21:54–59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathur A, Pitt HA, Marine M, Saxena R,
Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ and Lillemoe KD: Fatty
pancreas: A factor in postoperative pancreatic fistula. Ann Surg.
246:1058–1064. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon JH, Lee JM, Lee KB, Kim SW, Kang MJ,
Jang JY, Kannengiesser S, Han JK and Choi BI: Pancreatic steatosis
and fibrosis: Quantitative assessment with preoperative
multiparametric MR imaging. Radiology. 279:140–150. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim Z, Kim MJ, Kim JH, Jin SY, Kim YB, Seo
D, Choi D, Hur KY, Kim JJ, Lee MH and Moon C: Prediction of
post-operative pancreatic fistula in pancreaticoduodenectomy
patients using pre-operative MRI: A pilot study. HPB (Oxford).
11:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scialpi M, Cagini L, Pierotti L, De Santis
F, Pusiol T, Piscioli I, Magli M, D'Andrea A, Brunese L and Rotondo
A: Detection of small (≤2 cm) pancreatic adenocarcinoma and
surrounding parenchyma: correlations between enhancement patterns
at triphasic MDCT and histologic features. BMC Gastroenterol.
14:162014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukumura Y, Kumasaka T, Mitani K, Karita K
and Suda K: Expression of transforming growth factor beta1, beta2,
and beta3 in chronic, cancer-associated, obstructive pancreatitis.
Arch Pathol Lab Med. 130:356–361. 2006.PubMed/NCBI
|
|
12
|
Kamisawa T, Takuma K, Anjiki H, Egawa N,
Hata T, Kurata M, Honda G, Tsuruta K, Suzuki M, Kamata N and Sasaki
T: Differentiation of autoimmune pancreatitis from pancreatic
cancer by diffusion-weighted MRI. Am J Gastroenterol.
105:1870–1875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muraoka N, Uematsu H, Kimura H, Imamura Y,
Fujiwara Y, Murakami M, Yamaguchi A and Itoh H: Apparent diffusion
coefficient in pancreatic cancer: Characterization and
histopathological correlations. J Magn Reson Imaging. 27:1302–1308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosso E, Casnedi S, Pessaux P,
Oussoultzoglou E, Panaro F, Mahfud M, Jaeck D and Bachellier P: The
role of ‘fatty pancreas’ and of BMI in the occurrence of pancreatic
fistula after pancreaticoduodenectomy. J Gastrointest Surg.
13:1845–1851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH,
Kim MA and Kim SW: Measurement of pancreatic fat by magnetic
resonance imaging: Predicting the occurrence of pancreatic fistula
after pancreatoduodenectomy. Ann Surg. 251:932–936. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molinari E, Bassi C, Salvia R, Butturini
G, Crippa S, Talamini G, Falconi M and Pederzoli P: Amylase value
in drains after pancreatic resection as predictive factor of
postoperative pancreatic fistula: Results of a prospective study in
137 patients. Ann Surg. 246:281–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bassi C, Dervenis C, Butturini G,
Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W
and Buchler M: International Study Group on Pancreatic Fistula
Definition: Postoperative pancreatic fistula: An international
study group (ISGPF) definition. Surgery. 138:8–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Puig-Diví V, Molero X, Vaquero E, Salas A,
Guarner F and Malagelada J: Ethanol feeding aggravates
morphological and biochemical parameters in experimental chronic
pancreatitis. Digestion. 60:166–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puig-Diví V, Molero X, Salas A, Guarner F,
Guarner L and Malagelada JR: Induction of chronic pancreatic
disease by trinitrobenzene sulfonic acid infusion into rat
pancreatic ducts. Pancreas. 13:417–424. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Butturini G, Marcucci S, Molinari E,
Mascetta G, Landoni L, Crippa S and Bassi C: Complications after
pancreaticoduodenectomy: The problem of current definitions. J
Hepatobiliary Pancreat Surg. 13:207–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tajima Y, Kuroki T, Tsuneoka N, Adachi T,
Kosaka T, Okamoto T, Takatsuki M, Eguchi S and Kanematsu T:
Anatomy-specific pancreatic stump management to reduce the risk of
pancreatic fistula after pancreatic head resection. World J Surg.
33:2166–2176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanjay P, Fawzi A, Fulke JL, Kulli C, Tait
IS, Zealley IA and Polignano FM: Late post pancreatectomy
haemorrhage. Risk factors and modern management. JOP. 11:220–225.
2010.PubMed/NCBI
|
|
23
|
Yencilek E, Telli S, Tekesin K, Ozgür A,
Cakır O, Türkoğlu O, Meriç K and Simşek M: The efficacy of
diffusion weighted imaging for detection of acute pancreatitis and
comparison of subgroups according to Balthazar classification. Turk
J Gastroenterol. 25:553–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akisik MF, Aisen AM, Sandrasegaran K,
Jennings SG, Lin C, Sherman S, Lin JA and Rydberg M: Assessment of
chronic pancreatitis: Utility of diffusion-weighted MR imaging with
secretin enhancement. Radiology. 250:103–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shinya S, Sasaki T, Nakagawa Y, Guiquing
Z, Yamamoto F and Yamashita Y: Acute pancreatitis successfully
diagnosed by diffusion-weighted imaging: A case report. World J
Gastroenterol. 14:5478–5480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frozanpor F, Loizou L, Ansorge C,
Segersvärd R, Lundell L and Albiin N: Preoperative pancreas CT/MRI
characteristics predict fistula rate after pancreaticoduodenectomy.
World J Surg. 36:1858–1865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewin M, Poujol-Robert A, Boëlle PY,
Wendum D, Lasnier E, Viallon M, Guéchot J, Hoeffel C, Arrivé L,
Tubiana JM and Poupon R: Diffusion-weighted magnetic resonance
imaging for the assessment of fibrosis in chronic hepatitis C.
Hepatology. 46:658–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshikawa T, Kawamitsu H, Mitchell DG,
Ohno Y, Ku Y, Seo Y, Fujii M and Sugimura K: ADC measurement of
abdominal organs and lesions using parallel imaging technique. AJR
Am J Roentgenol. 187:1521–1530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel J, Sigmund EE, Rusinek H, Oei M,
Babb JS and Taouli B: Diagnosis of cirrhosis with intravoxel
incoherent motion diffusion MRI and dynamic contrast-enhanced MRI
alone and in combination: Preliminary experience. J Magn Reson
Imaging. 31:589–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kah Heng CA, Salleh I, San TS, Ying F and
Su-Ming T: Pancreatic fistula after distal pancreatectomy:
Incidence, risk factors and management. ANZ J Surg. 80:619–623.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe H, Kanematsu M, Tanaka K, Osada
S, Tomita H, Hara A, Goshima S, Kondo H, Kawada H, Noda Y, et al:
Fibrosis and postoperative fistula of the pancreas: Correlation
with MR imaging findings-preliminary results. Radiology.
270:791–799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jimenez RE and Hawkins WG: Emerging
strategies to prevent the development of pancreatic fistula after
distal pancreatectomy. Surgery. 152 (3 Suppl 1):S64–S70. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harada N, Ishizawa T, Inoue Y, Aoki T,
Sakamoto Y, Hasegawa K, Sugawara Y, Tanaka M, Fukayama M and Kokudo
N: Acoustic radiation force impulse imaging of the pancreas for
estimation of pathologic fibrosis and risk of postoperative
pancreatic fistula. J Am Coll Surg. 219:887–894.e5. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang YR, Kang JS, Jang JY, Jung WH, Kang
MJ, Lee KB and Kim SW: Prediction of pancreatic fistula after
distal pancreatectomy based on cross-sectional images. World J
Surg. 41:1610–1617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wiggermann P, Grützmann R, Weissenböck A,
Kamusella P, Dittert DD and Stroszczynski C: Apparent diffusion
coefficient measurements of the pancreas, pancreas carcinoma, and
mass-forming focal pancreatitis. Acta Radiol. 53:135–139. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gallix BP, Bret PM, Atri M, Lecesne R and
Reinhold C: Comparison of qualitative and quantitative measurements
on unenhanced T1-weighted fat saturation MR images in predicting
pancreatic pathology. J Magn Reson Imaging. 21:583–589. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barral M, Sebbag-Sfez D, Hoeffel C, Chaput
U, Dohan A, Eveno C, Boudiaf M and Soyer P: Characterization of
focal pancreatic lesions using normalized apparent diffusion
coefficient at 1.5-Tesla: Preliminary experience. Diagn Interv
Imaging. 94:619–627. 2013. View Article : Google Scholar : PubMed/NCBI
|