Introduction
Both etiology and pathogenesis of odontogenic
lesions are poorly understood. Therefore, studying odontogenic
lesions is important in order to update the knowledge of these
lesions. For example, after a long period of its discovery, WHO in
the year 2005 re-classified odontogenic keratocyst from odontogenic
cyst into an odontogenic tumor. Then after, and in its 4th edition,
the WHO in the year 2017 re-classified keratocystic odontogenic
tumor into odontogenic keratocysts (OKC). This controversies in the
classification of OKC reflects the limitation in the knowledge and
molecular basis of this lesion. OKCs are associated with unerupted
tooth in 25–40% of cases, which results in a clinicoradiographical
diagnosis of dentigerous cyst (1).
The treatment of OKC remains also controversial. It ranges from as
simple as marsupialization and decompression (2) to radical resection with subsequent bone
graft reconstruction (3).
Dentigerous cysts (DCs) are odontogenic lesions
arising from the crown of impacted, embedded, or unerupted teeth.
Enucleation of the cyst and extraction of the cyst-associated tooth
is the current standard treatment for a dentigerous cyst (4).
Ameloblastoma (AB) is a rare, benign, slow-growing
but locally invasive neoplasm of odontogenic origin involving
mainly the mandible and less frequently the maxilla. It has a high
recurrence rate if treated in a conservative manner (5). The radical surgical option is the
current standard of care for ameloblastoma and includes en bloc
resection with 1–2 cm bone margins and immediate bone
reconstruction to help return function (6).
Keratin 15 (K15) is a type I cytoskeletal protein.
Although its main function is providing structural support to the
cells, other functions of K15 in both adult stratified epithelia
and different pathological lesions is not fully understood. While
K15 was proposed to be a stem cell marker that presents in the
basal keratinocytes of all stratified epithelia, it also has been
reported to be expressed in suprabasal epithelial layers of normal
and diseased epithelial tissues. This different location of K15
expression questions the status of K15 as an actual stem cell
marker (7).
The expression and type of keratin is highly
affected by epithelial cell differentiation and lineage (8). Therefore, expression of the keratins 1,
2, 4, 5, 6, 7, 8, 10, 13, 14, 16, 17, 18, 19 and 20 was
investigated in odontogenic cysts and tumors (9–14). To
the best of our knowledge, there is only one study that discussed
the expression of K15 in AB (15),
and no presence of any previous literature that deals with K15
expression in other odontogenic cysts. The aim of the current study
was to evaluate the expression of K15 in DC, OKC, and AB.
Materials and methods
A total of 41 formalin fixed paraffin embedded
(FFPE) samples were retrieved from the archive of the Oral
Pathology Department of Tongji Hospital, Huazhong University of
Science and Technology (Wuhan, China). These samples were used
retrospectively and were categorized into three groups, which are
DC (n=13), OKC (n=12), and AB (n=16). No true tissue equivalent
exists to serve as a control group, as odontogenic lesions
encompass reactive tissues and tumors which replace the healthy
bone. This study was approved by the Institutional Review Board of
Tongji Medical College, Huazhong University of Science and
Technology and followed the protocol of the World Medical
Association Declaration of Helsinki.
Standard streptavidin-biotin peroxidase complex
immunostaining method was used (Wuhan Boster Biological Technology,
Ltd., Wuhan, China). Samples were cut as 5-µm tissue sections, then
dewaxed before rehydration. After that, 3%
H2O2 solution was used to quench the
endogenous peroxidase activity before the antigen unmasking step in
a microwave using 0.01 M citrate buffer heated to boiling point.
After that, goat serum (Wuhan Boster Biological Technology, Ltd.)
was used to treat the samples for 50 min at room temperature and
then samples were incubated with 1:100 diluted primary mouse
monoclonal antibody at 4°C overnight (BM0783; Clone: 6E7, Wuhan
Boster Biological Technology, Ltd.). This step was followed by
using 10 µg/ml biotinylated secondary antibody for 2 h at room
temperature (goat anti-mouse; BA1001 Wuhan Biological Technology,
Ltd.). Then, the slides were stained with 20 µg/ml
streptavidin-biotin-peroxidase complex. Finally, the sections were
developed with 3,3′-diaminobenzidine substrate and counter-stained
with Mayer's hematoxylin. Negative controls were passed in the same
procedure, but instead the step of the primary antibodies, the
sample is incubated with phosphate-buffered saline.
K15 expression was recognized as yellowish to brown
cytoplasmic staining of the positive cells. Samples were
semi-quantitatively scored using a standard light microscope. The
positively stained cells were counted and scored as the percentage
of positive cells from the total epithelial cells in 10 continuous
and representative high power (magnification, ×400) fields. The
scoring was 0 (absent), when there was no identified staining of
the odontogenic epithelium or when the staining was questionable; 1
(weak) for ≤20%; 2 (mild) for 21–40%; and 3 (strong) for >40%
positivity rate of the odontogenic epithelium.
Data were analyzed using the SPSS 19.0 software (IBM
Corp., Armonk, NY, USA) and are presented as the mean ± standard
deviation (SD). Comparison of K15 protein expression among the
studied groups was analyzed using Kruskal-Wallis statistical test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The study composed of 41 samples
Males were 23 (56.1%) and females were 18 (43.9%).
Fifteen (36.6%) of the cases were located in the maxilla, and 26
(63.4%) cases were in the mandible. The mean age was 37.24 with SD
of ±18.269.
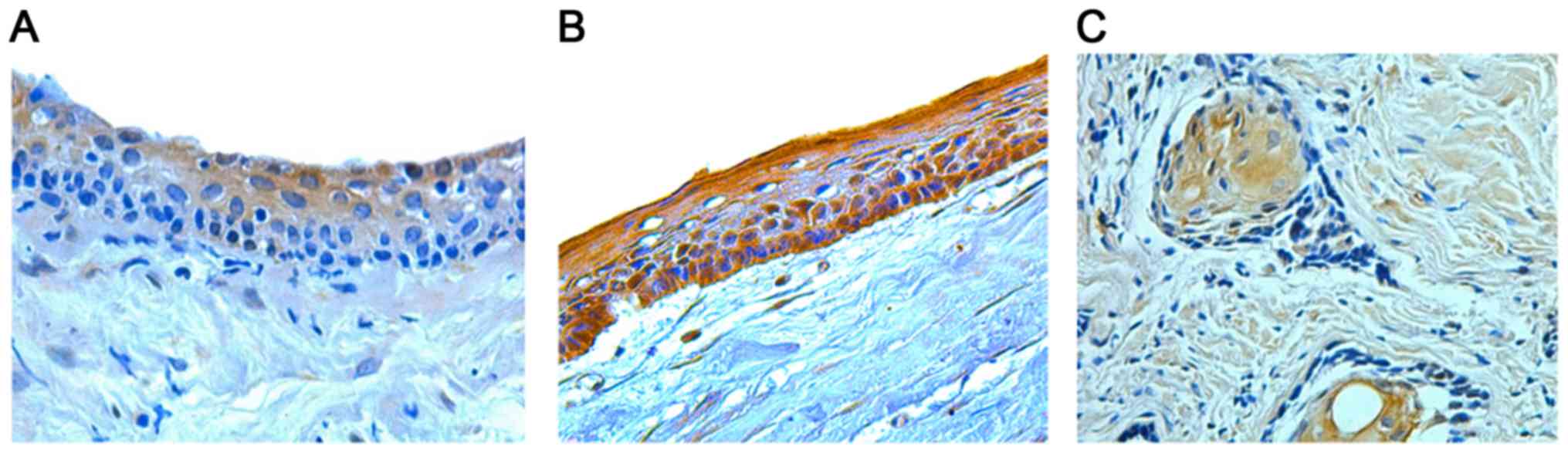
K15 protein expression was determined as yellowish
to brown cytoplasmic staining in the odontogenic epithelial cells
of DCs, OKCs and ABs (Fig. 1). In
the DCs, positive cells were distributed mainly through the
suprabasal layers and K15 expression was weak in 30.8% and strong
in 69.2% of the cases (Table I). In
OKC samples, K15 expression involved mostly all epithelial layers.
It was absent in 16.7%, mild in 8.3%, and strong in 75% of the
total OKC cases (Table I). The
distribution of positive K15 stained epithelial cells in AB was
more through the central stellate reticulum-like cells than the
peripheral columnar cells. It was absent in 6.3%, weak in 25%, mild
in 25% and strong in 43.8% of AB samples (Table I). All samples were analyzed and the
photos in (Fig. 1) were the best
representative of all the samples. Kruskal-Wallis test showed
non-significant differences in the expression of K15 among the
three odontogenic lesions (P=0.380).
 | Table I.Semi-quantitative analysis of K15
protein expression in the studied samples. |
Table I.
Semi-quantitative analysis of K15
protein expression in the studied samples.
| Cases | No. of cases | Absent expression of
K15, n (%) | Weak expression of
K15, n (%) | Mild expression of
K15, n (%) | Strong expression
of K15, n (%) |
|---|
| Dentigerous
cyst | 13 | 0 (0) | 4 (30.8) | 0 (0) | 9 (69.2) |
| Odontogenic
keratocyst | 12 | 2 (16.7) | 0 (0) | 1 (8.3) | 9 (75) |
| Ameloblastoma | 16 | 1 (6.3) | 4 (25) | 4 (25) | 7 (43.8) |
Discussion
The immunohistochemical detection of different
keratins has been made in many epithelial diseases. Specifically,
the expression of keratins type 1, 2, 4, 5, 6, 7, 8, 10, 13, 14,
16, 17, 18, 19 and 20 was investigated in odontogenic cysts and
tumors (9–14). However, very little is known about
the expression of K15 in these lesions. As far to our knowledge,
there is only one study that discussed the expression of K15 in AB
(15), and no presence of any
previous literature that deals with K15 expression in other
odontogenic cysts. This is the first study that determines the
expression of K15 in DC and OKC in addition to AB.
Keratins form intermediate filament proteins of the
epithelial cells. They show molecular diversity and are categorized
into acidic type I keratins, which include (K9, K10, K11, K12, K13,
K14, K15, K16, K17, K18, K19, and K20), while the basic or neutral
type II keratins involves (K1, K2, K3, K4, K5, K6, K7, and K8).
Specific pairing of types I and II molecules of keratin leads to
the formation of the heteropolymeric filaments. An exception is
K15, which lacks a natural co-expression partner (7,8).
Keratins function as an important cytoskeleton that provides
mechanical integrity of the epithelium. Furthermore, some keratins
are involved in intracellular signaling pathways like during wound
healing, protection from stress, and apoptosis (8). Meanwhile, K15 function in both adult
stratified epithelia and different pathological lesions is not
fully understood. Our results showed that K15 was expressed in most
of the studied cases of DC, OKC, and AB and in different epithelial
layers. The high expression of K15 in the current study could
indicate the importance of K15 protein for the mechanical stability
of odontogenic lesions which usually exist and extend inside strong
jaw bone like the mandible in addition to the maxilla.
We observed a non-significant difference in K15
expression among DC, OKC, and AB. This interprets the same limit of
extensions that necessitate the almost same method of treatment
which is surgical removal of these different pathologically
classified lesions. On the other hand, the non-significant
difference in the expression of K15 among the studied lesions is
precluding the utility of the quantity of K15 expression as an
indicator to differentiate between these lesions. However, the
location and distribution of K15 expression could be helpful in
differentiating DC in which K15 positive cells were mostly located
suprabasal than OKC which showed K15 expression throughout all
epithelial layers. In the same subject, Pal et al (15) used K15 to differentiate between
different AB histopathological types. Wassem et al (16) concluded that activation and
proliferation of keratinocytes result in downregulation of K15. Our
previous studies showed the non-different expression of
proliferating epithelial cells in OKC and AB (17,18).
This may explain the similar expression of K15 in odontogenic
epithelium of both OKC and AB in the current study.
Tumors are hierarchically organized tissue in which
tumor stem cells are responsible for the uncontrolled self-renewal
and abnormal differentiation of the tumor tissues. Tumor stem cells
confer the heterogeneity of tumor cellular composition. The
differences in properties and response to external stimuli of
benign tumor stem cells from their counterparts of normal tissue
are not well known (19). The role
of tumor stem cells in odontogenic lesions is essential for the
better understanding of the biology and management of these
lesions. Several benign and malignant odontogenic neoplasms have
been reported to originate from the remnants of dental stem cells
(20). In AB, different studies
confirmed the expressions of stem cell markers in different
locations. While the tumor stem cells are suggested to be located
in the peripheral layers (20),
another study demonstrate considerable expression of the P75NTR
stem cell marker mostly in regions resembling the stellate
reticulum (21). Further studies
observed the stem cell indicators in both the stellate reticulum
like cells and the peripheral basal cells of ABs (22,23). A
recent study performed by Monroy et al (23), demonstrated a high expression of a
tumor stem cell marker, namely Oct-4 in the suprabasal layers of
OKC and in both stellate reticulum and peripheral columnar cells of
AB, while the CD44, which is another stem cell marker used in the
same study was highly expressed in all epithelium of OKC and AB
(23).
K15 is mainly known to be expressed in hair follicle
bulge stem cells (24). Currently,
no consensus about considering K15 as a marker for stem cell
population in a tissue. There are different studies indicating that
K15 is a reliable marker for stem cells in a normal (24–26) and
pathological tissues (27–29). Recently, a study showed that K15
marks multipotent, long-lived, and injury-resistant crypt cells.
These cells may function as a cell of origin in intestinal cancer
(30). Furthermore, K15 was
predominantly detected in the basal cell zone of the normal oral
mucosa (31). However, other studies
have shown that K15 is not always restricted to the basal layers of
the epithelium, but it has a variant distribution in different
normal tissues. K15 expressed in the suprabasal layer in
IFN-γ-treated ex vivo skin samples (32), esophagus (33), and in pathological conditions
(34). K15 also present in
uninterrupted pattern in the basal layers of oral epithelium
(35). Therefore, these studies
excluded the reliability of K15 to be a marker for stem cell. Bose
et al (7), concluded that K15
could be expressed in the stem cells but also in differentiated
cells. So that K15 marker may not be regarded as an indicator of
the stem cell population in a tissue (7).
In the epithelium, the stem cells should represent a
limited number of the total epithelial cells and usually have basal
layer distribution (36). Contrary,
the results of our study demonstrated high expression of K15 in
different epithelial layers, including the suprabasal layer of DC,
OKC, and AB. This high expression in addition to the suprabasal
distribution of the K15 positive cells in our study lead to the
conclusion which excludes the reliability of K15 to be a stem cell
marker in the studied odontogenic cysts and tumor. This conclusion
is in agreement with other studies that doubt the use of K15 as a
stem cell marker in other tissue types (7,34)
The high expression of K15 in AB could contribute to
its benign activity and inability for metastasis. This suggestion
is in correspondence with previous findings that connected the
metastatic ability of squamous cell carcinoma to the downregulation
of K15 (37). In the same line, a
more pronounced expression of K15 in normal esophageal tissue
occurs, compared with carcinoma tissue (38). Another observation that agree with
our suggestion found a downregulation of K15 in the keratinocyte
which become more mobile during wound healing (39).
Instead of being a stem cell marker, K15 staining of
cells in the present study may reflect abnormal cell
differentiation as the studied samples represent pathological
tissues. This attribution is in accordance with a study performed
by Troy et al (34). That
study suggested that K15 expression in basal-like cells of
epithelium could reflect their loss of homeostasis and probably
undergoing abnormal epidermal differentiation program.
The current study, as many previous reports
(9–12,15,17,18)
excluded the use of control group samples in the study of
odontogenic lesions. This is because no true tissue equivalent
exists to serve as a negative control, as odontogenic lesions
encompass reactive tissues and tumors which replace the healthy
bone. The present study is limited to description of one molecular
expression, which is K15. However, it is the first study to
investigate and compare the expression of K15 in DC and OKC. This
study will open the door for subsequent clinical applications to
evaluate the pathways and related factors of K15 expression in
odontogenic cysts and tumors. At the time of the diagnosis of the
odontogenic cysts and tumors, investigators do not know the exact
time for initiation of these lesions. Therefore, it is irrational
to correlate the radiographical size of the odontogenic cysts and
tumors to the biomarkers, like K15. Furthermore, correlating
immunohistochemical marker, like K15 in our study to the site, age,
or sex of the patient needs a relatively large study samples which
is usually not present in a study of odontogenic lesions. For all
these reasons, the present study did not correlate the K15
expression with the size of lesion, site, sex, or age of the
patients.
The current standard management of the DC, OKC, and
AB is the surgery which usually results in significant morbidity
especially in large lesions and high recurrence rate as in OKC and
AB. Additionally, surgery may necessitate the need for graft
tissues. Overexpression of K15 in the studied odontogenic lesions
could be helpful for the understanding the disease process in these
pathogenically poorly understood lesions and could lead to the
development of an adjuvant non-surgical treatment modality to
control the disease process. One of those modalities could include
the radiolabelling mAb against K15 as a tool for the therapy of DC,
OKC, and AB. This suggestion is supported by several studies that
formulated the initiation of radioimmunotherapy against different
keratins like K19 in the management of cervical cancer (40), and against K8 in head and neck
squamous cell carcinoma (41).
The present study confirmed the high expression of
K15 in different epithelial layers of DC, OKC, and AB. This manner
of expression excludes the reliability of regarding K15 as a stem
cell marker in DC, OKC, and AB. But rather, K15 may reflect
abnormal differentiation of pathological epithelial cells in these
lesions.
Acknowledgements
The authors would like to thank Professor Wei Min
Chen and Professor Xu Jin Tao (Department of Oral and Maxillofacial
Surgery, Tongji Hospital of Tongji Medical College, Huazhong
University of Science and Technology, Wuhan, China) for their help
with clinical sample collection.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAA designed the study, collected the samples,
performed immunohistochemical analyses and microscopical studies,
conducted statistical analysis and wrote the manuscript. AMA
designed the study, performed microscopical studies and wrote the
manuscript. SZ designed the study, collected the samples and wrote
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China) and followed the protocol of
the World Medical Association Declaration of Helsinki. Due to the
retrospective nature of the present study the requirement for
informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
K15
|
keratin 15
|
|
DC
|
dentigerous cyst
|
|
OKC
|
odontogenic keratocyst
|
|
AB
|
ameloblastoma
|
References
|
1
|
Chirapathomsakul D, Sastravaha P and
Jansisyanont P: A review of odontogenic keratocysts and the
behavior of recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:5–9; discussion 10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Molon RS, Verzola MH, Pires LC,
Mascarenhas VI, da Silva RB, Cirelli JA and Barbeiro RH: Five years
follow-up of a keratocyst odontogenic tumor treated by
marsupialization and enucleation: a case report and literature
review. Contemp Clin Dent. 6 Suppl 1:S106–S110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Moraissi EA, Dahan AA, Alwadeai MS,
Oginni FO, Al-Jamali JM, Alkhutari AS, Al-Tairi NH, Almaweri AA and
Al-Sanabani JS: What surgical treatment has the lowest recurrence
rate following the management of keratocystic odontogenic tumor? A
large systematic review and meta-analysis. J Craniomaxillofac Surg.
45:131–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Q, Xu GZ, Yang C, Yu CQ, He DM and
Zhang ZY: Dentigerous cysts associated with impacted supernumerary
teeth in the anterior maxilla. Exp Ther Med. 2:805–809. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McClary AC, West RB, McClary AC, Pollack
JR, Fischbein NJ, Holsinger CF, Sunwoo J, Colevas AD and Sirjani D:
Ameloblastoma: A clinical review and trends in management. Eur Arch
Otorhinolaryngol. 273:1649–1661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sham E, Leong J, Maher R, Schenberg M,
Leung M and Mansour AK: Mandibular ameloblastoma: clinical
experience and literature review. ANZ J Surg. 79:739–744. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bose A, Teh MT, Mackenzie IC and Waseem A:
Keratin k15 as a biomarker of epidermal stem cells. Int J Mol Sci.
14:19385–19398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moll R, Divo M and Langbein L: The human
keratins: biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meara JG, Pilch BZ, Shah SS and Cunningham
MJ: Cytokeratin expression in the odontogenic keratocyst. J Oral
Maxillofac Surg. 58:862–865; discussion 866. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stoll C, Stollenwerk C, Riediger D,
Mittermayer C and Alfer J: Cytokeratin expression patterns for
distinction of odontogenic keratocysts from dentigerous and
radicular cysts. J Oral Pathol Med. 34:558–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aragaki T, Michi Y, Katsube K, Uzawa N,
Okada N, Akashi T, Amagasa T, Yamaguchi A and Sakamoto K:
Comprehensive keratin profiling reveals different histopathogenesis
of keratocystic odontogenic tumor and orthokeratinized odontogenic
cyst. Hum Pathol. 41:1718–1725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuji K, Wato M, Hayashi T, Yasuda N,
Matsushita T, Ito T, Gamoh S, Yoshida H, Tanaka A and Morita S: The
expression of cytokeratin in keratocystic odontogenic tumor,
orthokeratinized odontogenic cyst, dentigerous cyst, radicular cyst
and dermoid cyst. Med Mol Morphol. 47:156–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heikinheimo K, Kurppa KJ, Laiho A,
Peltonen S, Berdal A, Bouattour A, Ruhin B, Catón J, Thesleff I,
Leivo I, et al: Early dental epithelial transcription factors
distinguish ameloblastoma from keratocystic odontogenic tumor. J
Dent Res. 94:101–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Safadi RA, Quda BF and Hammad HM:
Immunohistochemical expression of K6, K8, K16, K17, K19, maspin,
syndecan-1 (CD138), α-SMA, and Ki-67 in ameloblastoma and
ameloblastic carcinoma: diagnostic and prognostic correlations.
Oral Surg Oral Med Oral Pathol Oral Radiol. 121:402–411. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pal SK, Sakamoto K, Aragaki T, Akashi T
and Yamaguchi A: The expression profiles of acidic epithelial
keratins in ameloblastoma. Oral Surg Oral Med Oral Pathol Oral
Radiol. 115:523–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waseem A, Dogan B, Tidman N, Alam Y,
Purkis P, Jackson S, Lalli A, Machesney M and Leigh IM: Keratin 15
expression in stratified epithelia: downregulation in activated
keratinocytes. J Invest Dermatol. 112:362–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alsaegh MA, Miyashita H and Zhu SR:
Expression of human papillomavirus is correlated with Ki-67 and
COX-2 expressions in keratocystic odontogenic tumor. Pathol Oncol
Res. 21:65–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alsaegh MA, Miyashita H, Taniguchi T and
Zhu SR: Odontogenic epithelial proliferation is correlated with
COX-2 expression in dentigerous cyst and ameloblastoma. Exp Ther
Med. 13:247–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin H, Bao D, Tong X, Hu Q, Sun G and
Huang X: The role of stem cells in benign tumors. Tumour Biol. Sep
21–2016.(Epub ahead of print). View Article : Google Scholar
|
|
20
|
Harada H, Mitsuyasu T, Toyono T and
Toyoshima K: Epithelial stem cells in teeth. Odontology. 90:1–6.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silva FP, Dias A, Coelho CA, Guerra EN,
Marques AE, Decurcio DA, Mantesso A, Cury SE and Silva BS:
Expression of CD90 and P75NTR stem cell markers in ameloblastomas:
a possible role in their biological behavior. Braz Oral Res.
30:e1092016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Juuri E, Jussila M, Seidel K, Holmes S, Wu
P, Richman J, Heikinheimo K, Chuong CM, Arnold K, Hochedlinger K,
et al: Sox2 marks epithelial competence to generate teeth in
mammals and reptiles. Development. 140:1424–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monroy EAC, de Andrade Santos PP, de Sousa
Lopes MLD, Mosqueda-Taylor A, Pinto LP and de Souza LB: Oct-4 and
CD44 in epithelial stem cells like of benign odontogenic lesions.
Histochem Cell Biol. 150:371–377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lyle S, Christofidou-Solomidou M, Liu Y,
Elder DE, Albelda S and Cotsarelis G: The C8/144B monoclonal
antibody recognizes cytokeratin 15 and defines the location of
human hair follicle stem cells. J Cell Sci. 111:3179–3188.
1998.PubMed/NCBI
|
|
25
|
Inoue K, Aoi N, Sato T, Yamauchi Y, Suga
H, Eto H, Kato H, Araki J and Yoshimura K: Differential expression
of stem-cell-associated markers in human hair follicle epithelial
cells. Lab Invest. 89:844–856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garza LA, Yang CC, Zhao T, Blatt HB, Lee
M, He H, Stanton DC, Carrasco L, Spiegel JH, Tobias JW, et al: Bald
scalp in men with androgenetic alopecia retains hair follicle stem
cells but lacks CD200-rich and CD34-positive hair follicle
progenitor cells. J Clin Invest. 121:613–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S, Park H, Trempus CS, Gordon D, Liu Y,
Cotsarelis G and Morris RJ: A keratin 15 containing stem cell
population from the hair follicle contributes to squamous papilloma
development in the mouse. Mol Carcinog. 52:751–759. 2013.PubMed/NCBI
|
|
28
|
Quist SR, Eckardt M, Kriesche A and
Gollnick HP: Expression of epidermal stem cell markers in skin and
adnexal malignancies. Br J Dermatol. 175:520–530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HC, Sohng SH, Shin DH, Choi JS and Bae
YK: Immunohistochemical expression of cytokeratin 15, cytokeratin
19, follistatin, and Bmi-1 in basal cell carcinoma. Int J Dermatol.
55:36–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giroux V, Stephan J, Chatterji P, Rhoades
B, Wileyto EP, Klein-Szanto AJ, Lengner CJ, Hamilton KE and Rustgi
AK: Mouse intestinal Krt15+ crypt cells are radio-resistant and
tumor initiating. Stem Cell Reports. 10:1947–1958. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barakat SM and Siar CH: Differential
expression of stem cell-like proteins in normal, hyperplastic and
dysplastic oral epithelium. J Appl Oral Sci. 23:79–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Radoja N, Stojadinovic O, Waseem A,
Tomic-Canic M, Milisavljevic V, Teebor S and Blumenberg M: Thyroid
hormones and gamma interferon specifically increase K15 keratin
gene transcription. Mol Cell Biol. 24:3168–3179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leube RE, Bader BL, Bosch FX, Zimbelmann
R, Achtstaetter T and Franke WW: Molecular characterization and
expression of the stratification-related cytokeratins 4 and 15. J
Cell Biol. 106:1249–1261. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Troy TC, Arabzadeh A and Turksen K:
Re-assessing K15 as an epidermal stem cell marker. Stem Cell Rev.
7:927–934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Köse O, Lalli A, Kutulola AO, Odell EW and
Waseem A: Changes in the expression of stem cell markers in oral
lichen planus and hyperkeratotic lesions. J Oral Sci. 49:133–139.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alonso L and Fuchs E: Stem cells of the
skin epithelium. Proc Natl Acad Sci USA. 100 Suppl 1:11830–11835.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abbas O and Bhawan J: Expression of stem
cell markers nestin and cytokeratin 15 and 19 in cutaneous
malignancies. J Eur Acad Dermatol Venereol. 25:311–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen YH, Xu CP, Shi ZM, Zhang YJ, Qiao YG
and Zhao HP: Cytokeratin 15 is an effective indicator for
progression and malignancy of esophageal squamous cell carcinomas.
Asian Pac J Cancer Prev. 17:4217–4222. 2016.PubMed/NCBI
|
|
39
|
Werner S and Munz B: Suppression of
keratin 15 expression by transforming growth factor beta in vitro
and by cutaneous injury in vivo. Exp Cell Res. 254:80–90. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang CH, Tsai LC, Chen ST, Yuan CC, Hung
MW, Hsieh BT, Chao PL, Tsai TH and Lee TW: Radioimmunotherapy and
apoptotic induction on CK19-overexpressing human cervical carcinoma
cells with Re-188-mAbCx-99. Anticancer Res. 25:2719–2728.
2005.PubMed/NCBI
|
|
41
|
Andratschke M, Luebbers CW, Johannson V,
Schmitt B, Mack B, Zeidler R, Lang S, Wollenberg B and Gildehaus
FJ: Biodistribution of 131I-labeled anti-CK8 monoclonal antibody in
HNSCC in xenotransplanted SCID mice. Anticancer Res. 31:3315–3321.
2011.PubMed/NCBI
|